A liraglutide in-situ gel preparation and a preparing method thereof
A technology of in-situ gel and liraglutide, which is applied in the fields of pharmaceutical formulation, aerosol delivery, peptide/protein composition, etc., can solve problems such as poor patient compliance, short drug action time, peak and valley phenomenon of blood drug concentration, etc. , achieve pain relief, clear skin and connective tissue structure, and reduce the number of medications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
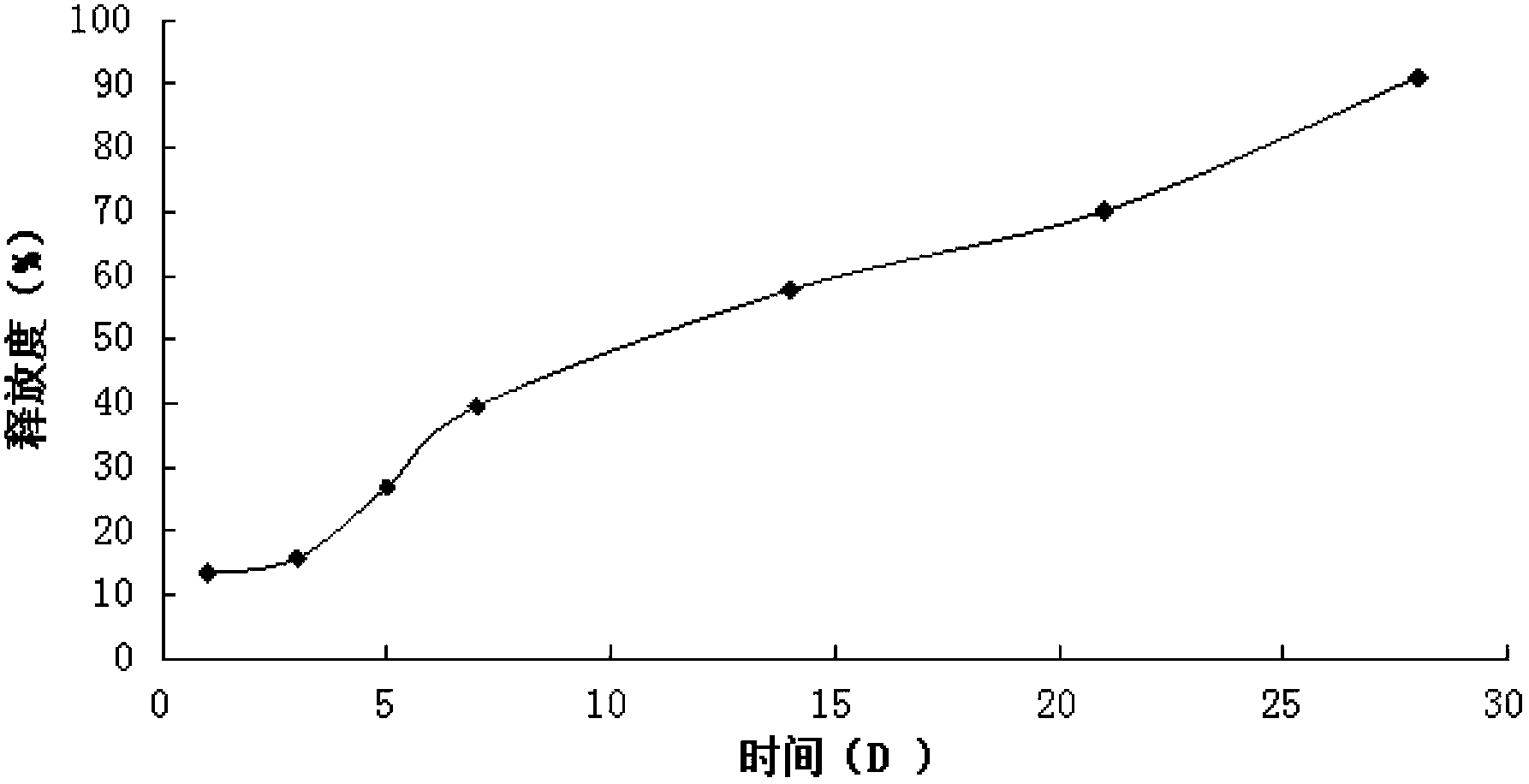
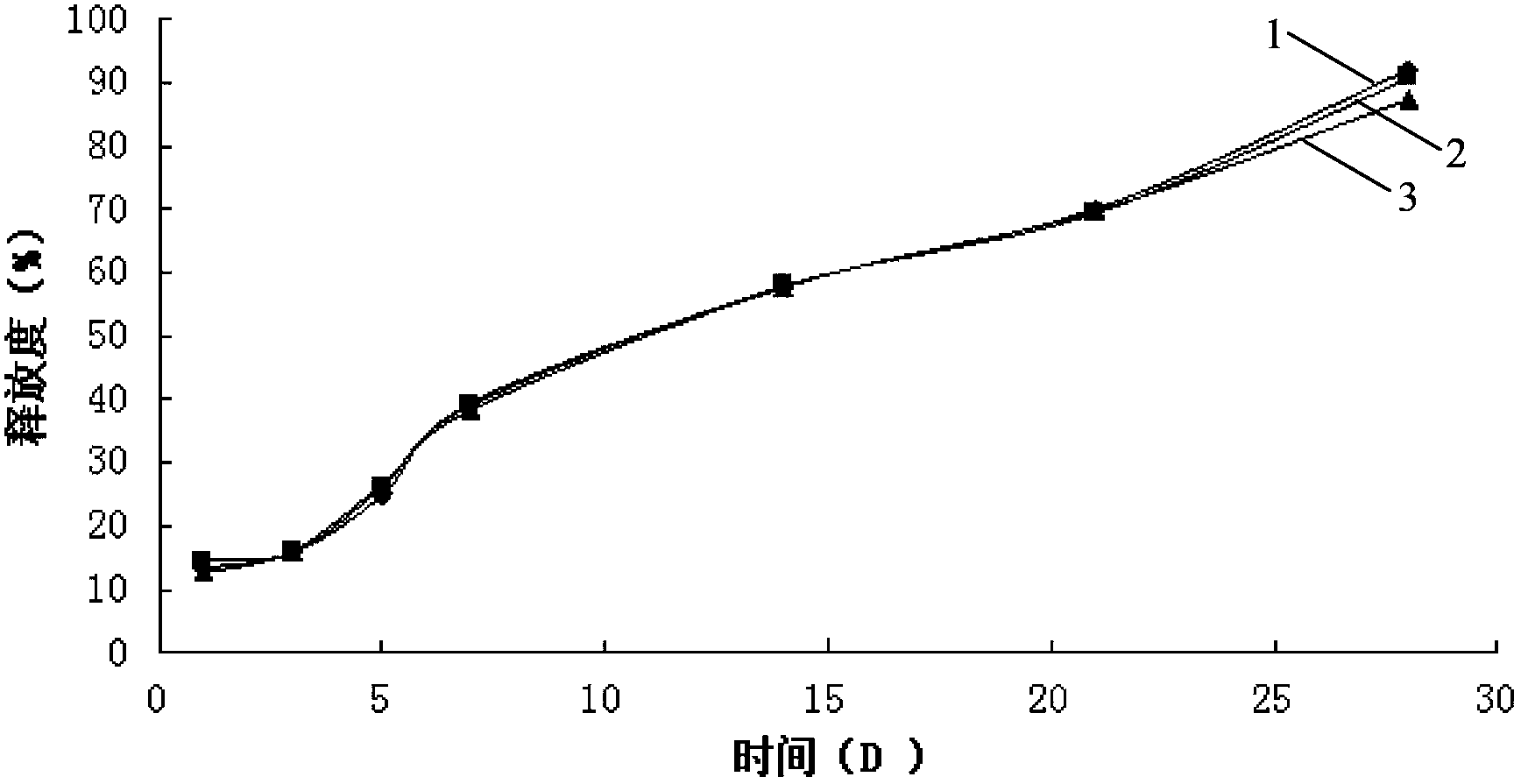
[0054] Example 1 Preparation of liraglutide in situ gel preparation
[0055] Weigh 25g of PLGA (Mn=10000, LA:GA=75:25), add it into 100mL NMP solvent, mix well, and prepare a polymer solution, then add 7.0g of liraglutide raw material, heat and stir at constant temperature (temperature 30 ℃, 200rpm) for 30 minutes to form a translucent drug-loaded sol system, which is sterilized by γ-ray radiation.
Embodiment 2
[0056] Example 2 Preparation of liraglutide in situ gel preparation
[0057] Weigh 30g of PLGA (Mn=15000, LA:GA=50:50), add it into 100mL NMP solvent, mix well, and prepare a polymer solution, then add 7.5g of liraglutide raw material, heat and stir at constant temperature (temperature 30 ℃, 250rpm) for 30 minutes to form a translucent drug-loaded sol system, which is sterilized by γ-ray radiation.
Embodiment 3
[0058] Example 3 Preparation of liraglutide in situ gel preparation
[0059] Weigh 25g PLGA (Mn=20000, LA:GA=50:50) and 4g poloxamer 407, dissolve in 110mL NMP, mix well, and prepare a polymer solution, then add 6.5g liraglutide, keep the temperature Heat and stir (temperature 30°C, 150rpm) for 35 minutes to form a translucent drug-loaded sol system, which is sterilized by gamma-ray radiation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com