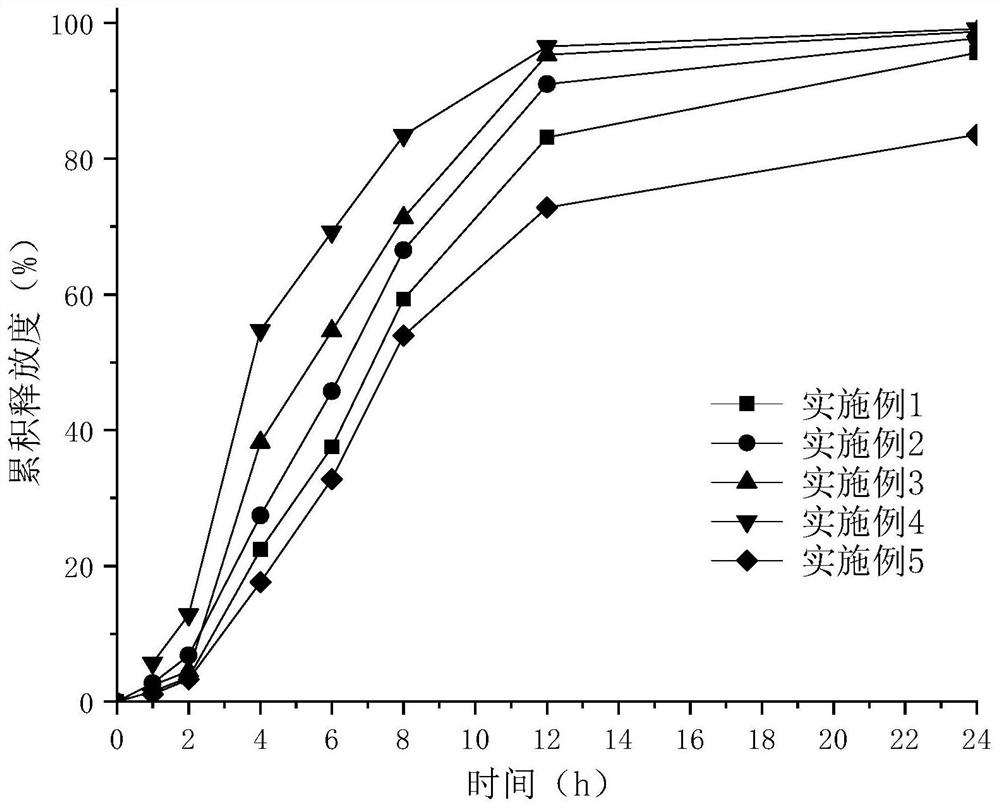
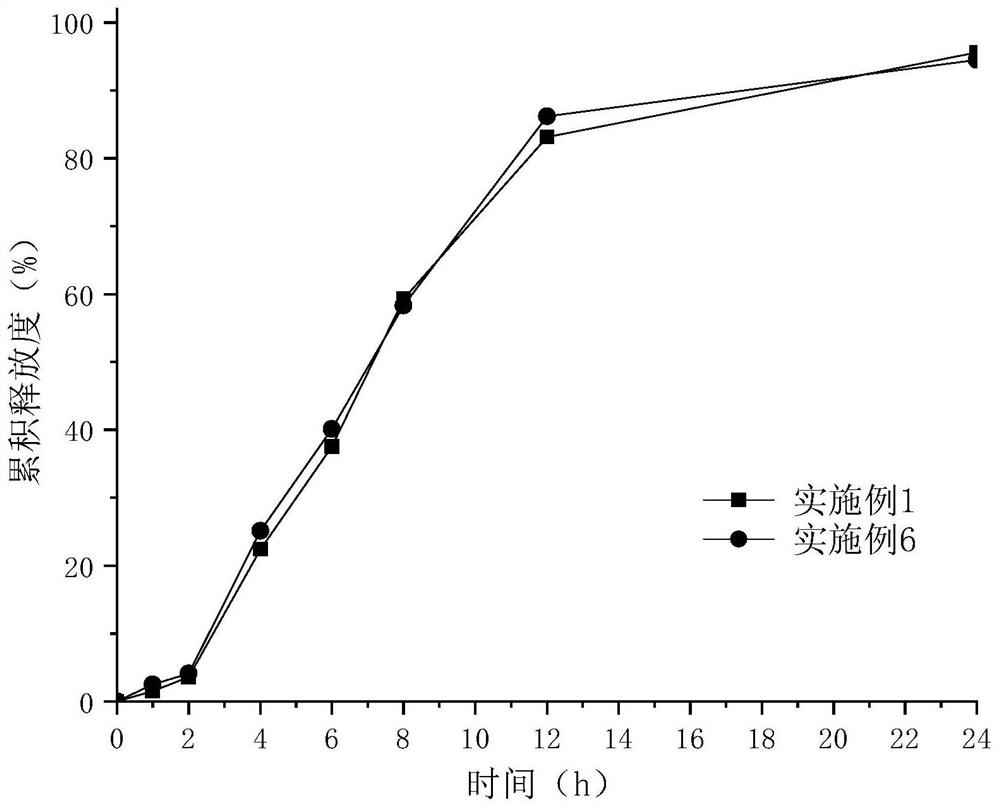
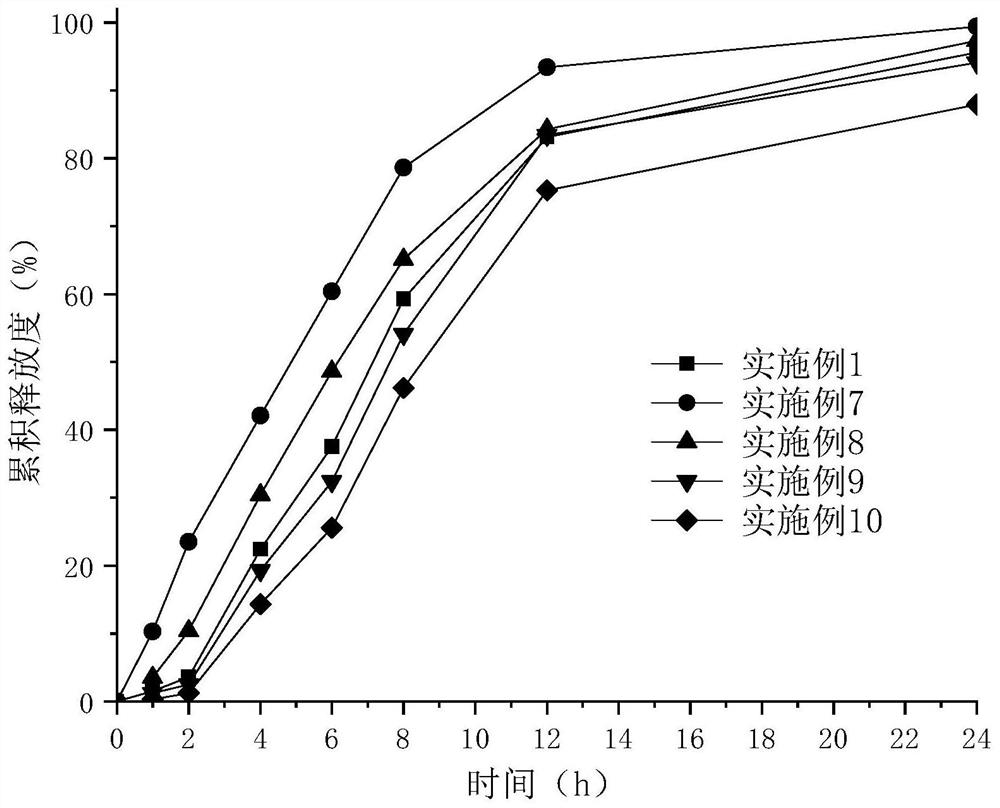
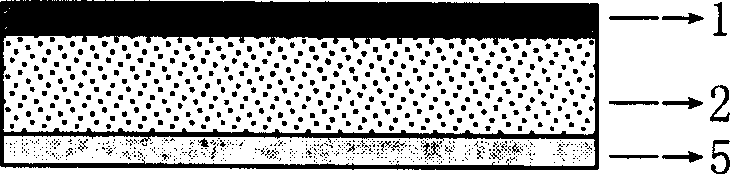
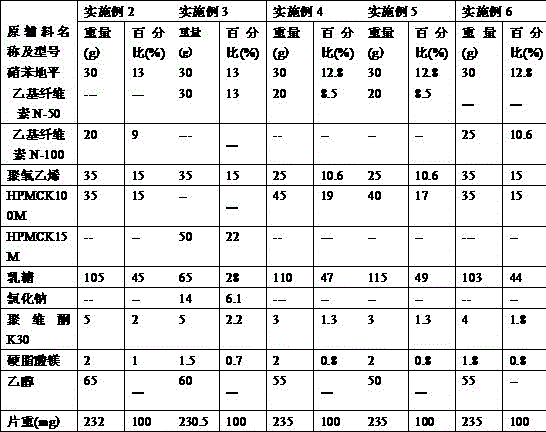
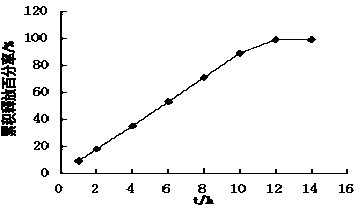
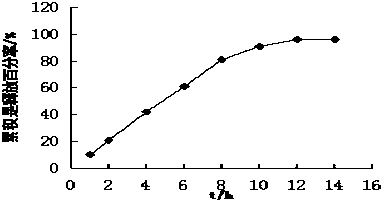
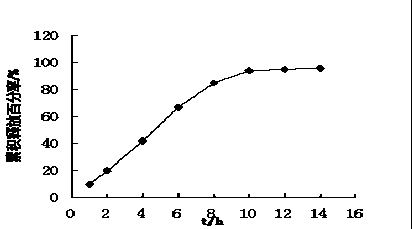
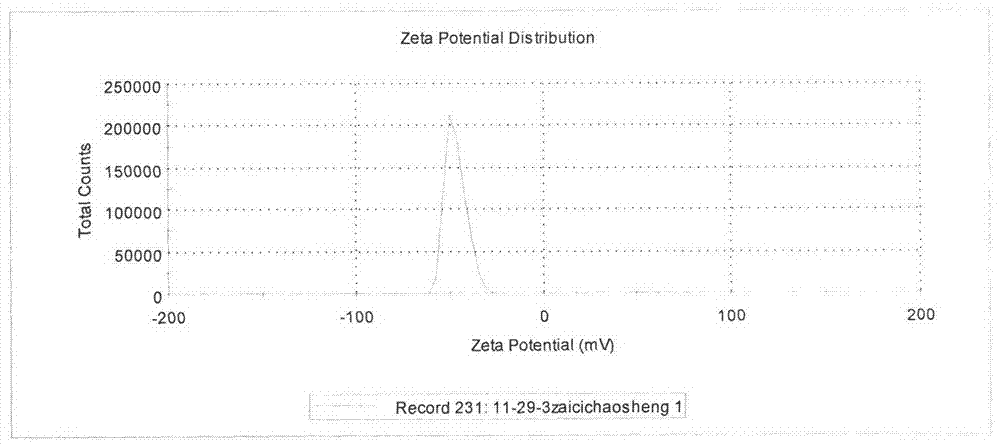
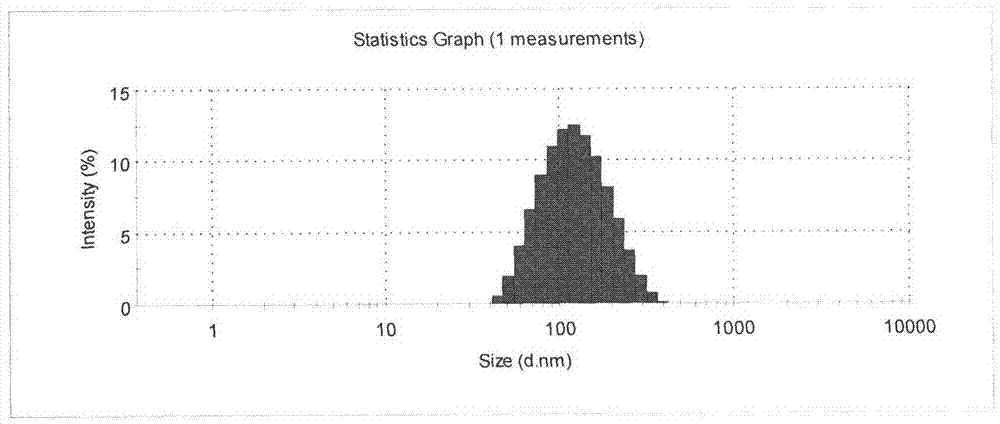
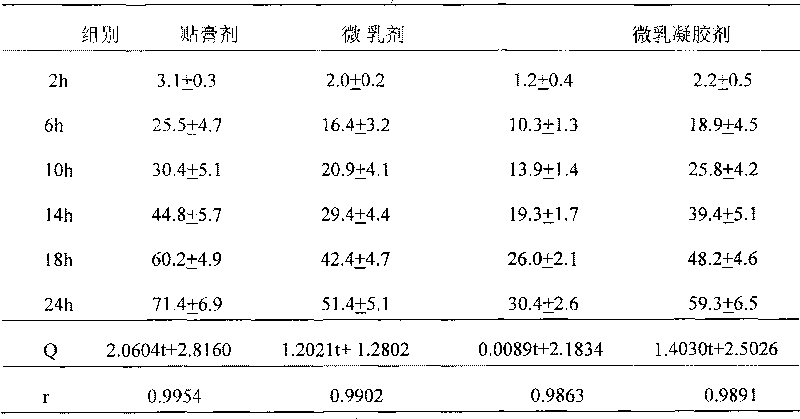
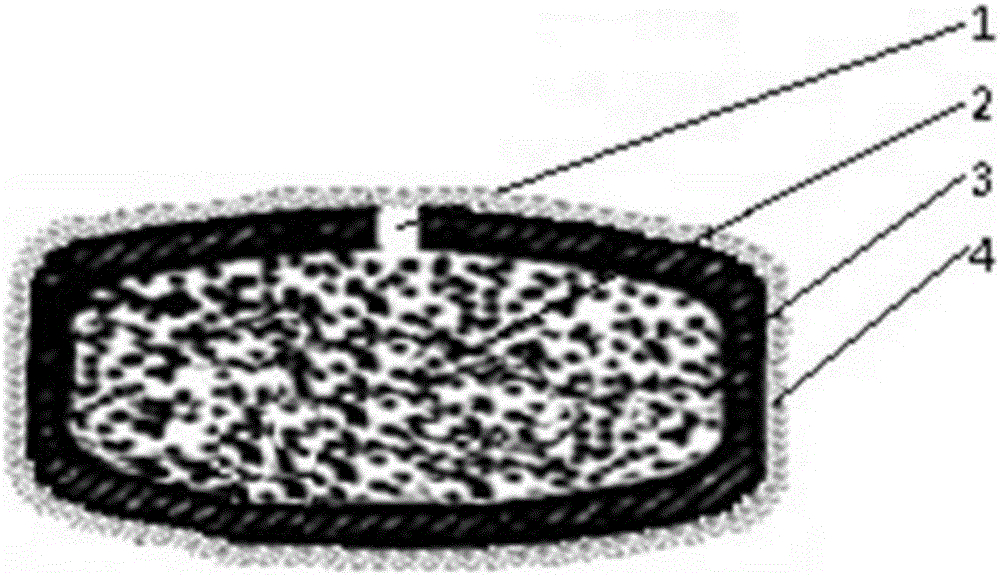
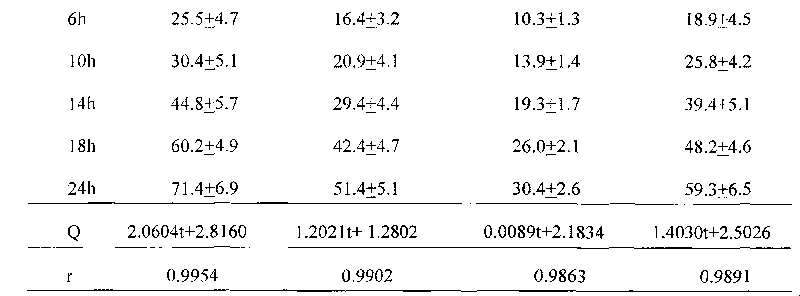Patents
Literature
77results about How to "Avoid peaks and valleys in blood concentration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Transdermal plaster of rivastigmine and preparation process thereof
InactiveCN1994290AEasy to useClear curative effectOrganic non-active ingredientsEster active ingredientsTransdermal patchRivastigmine
The invention relates to a method for preparing kabalatin adhere agent for treating senile dementia, wherein said adhere agent can stably hold blood drug density and reduce feeding time, with high safety.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Tripterine nano structure lipid carrier and preparation method and application thereof
InactiveCN102225205AReduce systemic side effectsImprove bioavailabilityOrganic active ingredientsAntipyreticNano structuringTreatment effect
The invention relates to the field of Chinese medicine preparation, in particular to a method for preparing tripterine nano structure lipid carrier containing traditional Chinese medicine monomer and application of the tripterine nano structure lipid carrier in preparation of transdermal drugs used for treating psoriasis, rheumatoid arthritis, skin cancer and breast cancer. The tripterine nano structure lipid carrier is characterized by comprising the following components in parts by weight: 1 part of tripterine, 5-100 parts of mixed lipid, 0.5-10 parts of phospholipid, 0.1-15 parts of poloxamer-188 and 0.5-10 parts of vitamin E and tocopherol polyethylene glycol succinate, wherein the mixed lipid is composed of solid lipid monoglycerine and liquid lipid octylic acid / caprin according to the weight ratio of 1: 0.1-10: 1. Tripterine is prepared into the nano structure lipid carrier, the tripterine nano structure lipid carrier in a semi-solid or liquid preparation form is applied in a transdermal way, bioavailability of the tripterine can be improved, toxic response of tripterine can be reduced, and the nano structure lipid carrier provided by the invention has great clinical application value in the improvement of the treatment effect of tripterine.
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE
Thyroid nodule eliminating paste preparation for treating benign non-toxic thyroid nodules and preparation method thereof
InactiveCN104162098AImprove complianceDefinite curative effectUnknown materialsDrug compositionsMedicinal herbsDioscorea bulbifera
The invention discloses a thyroid nodule eliminating paste preparation for treating benign non-toxic thyroid nodules. The preparation is composed of the following medicinal raw materials in parts by weight: 6 to 11 parts of radix bupleuri, 10 to 15 parts of curcuma rhizome processed with vinegar, 15 to 20 parts of dioscorea bulbifera, 15 to 20 parts of fried semen brassicae, 6 to 11 parts of processed rhizoma cyperi, 30 to 35 parts of ternate buttercup root, 20 to 25 parts of prunella vulgaris, 10 to 15 parts of salt cake, 10 to 15 parts of rhizoma pinelliae preparata, 15 to 20 parts of laminaria, 15 to 20 parts of alga, and 30 to 35 parts of live oyster.
Owner:DONGZHIMEN HOSPITAL OF BEIJING UNIV OF CHINESE MEDICINE +1
Chinese-medicinal suppository for treating lumbar vertebrae disease
InactiveCN1895600AReduce toxic and side effectsEnhance immune functionAmphibian material medical ingredientsHeavy metal active ingredientsDiseaseLumbar intervertebral disc
A Chinese medicine in the form of suppository for treating lumbar vertebra disease, lumbar intervertebral disc protrusion, tonic rachitis and hyperosteogeny is prepared from 9 Chinese-medicinal materials including astragalus root, capejasmine fruit, musk, cinnamon twig, etc through pulverizing, extracting in alcohol, filtering, concentrating by distilling, to obtain concentrated liquid, immersing catgut suture in physiologic saline, baking, shearing short, putting them in bottle, filling said concentrated liquid, sealing and magnetizing.
Owner:方焕生
Method for preparing hollow fiber with temperature sensitive medicinal hydrogel and application thereof
ActiveCN1961959AAvoid first pass effectAvoid degradationHollow filament manufacturePharmaceutical non-active ingredientsHollow fibreFiber
The invention relates to a method for preparing temperature-sensitive aquagel hollow fiber, wherein it uses hollow fiber as carrier, whose chamber contains temperature-sensitive aquagel; the aquagel will change phase at human temperature to release the drug. And the preparation comprises a, holding the holes of hollow fiber; b, putting temperature-sensitive monomer solution into the chamber of hollow fiber; c, compositing temperature-sensitive aquagel in the chamber; d, using said aquagel to absorb drub. The invention has better temperature-sensitive property and slow-release property.
Owner:LANGSHA KNITTING
Chinese-medicinal suppository for treating cervical spondylosis and its preparation
InactiveCN1895633ANo side effectsSignificant effectAmphibian material medical ingredientsHeavy metal active ingredientsDiseaseCervical spondylosis
A Chinese medicine in the form of suppository for treating lumbar vertebra disease, lumbar intervertebral disc protrusion, tonic rachitis and hyperosteogeny is prepared from 10 Chinese-medicinal materials including astragalus root, capejasmine fruit, musk, cinnamon twig, etc through pulverizing, extracting in alcohol, filtering, concentrating by distilling, to obtain concentrated liquid, immersing catgut suture in physiologic saline, baking, shearing short, putting them in bottle, filling said concentrated liquid, sealing and magnetizing.
Owner:方焕生
Clonidine paster for treating children's hyperkinetic symptom, twitch symptom and its preparation method
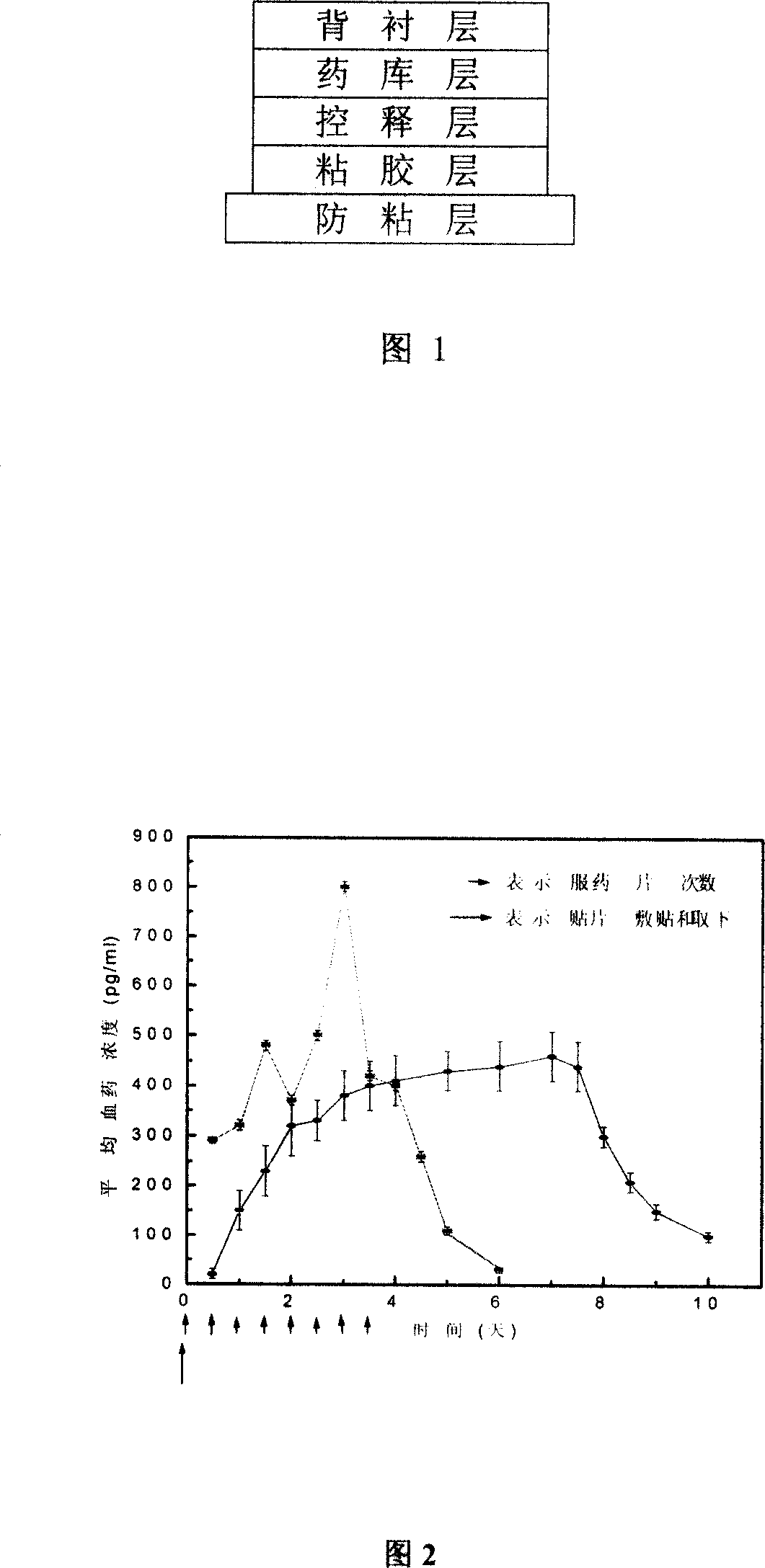
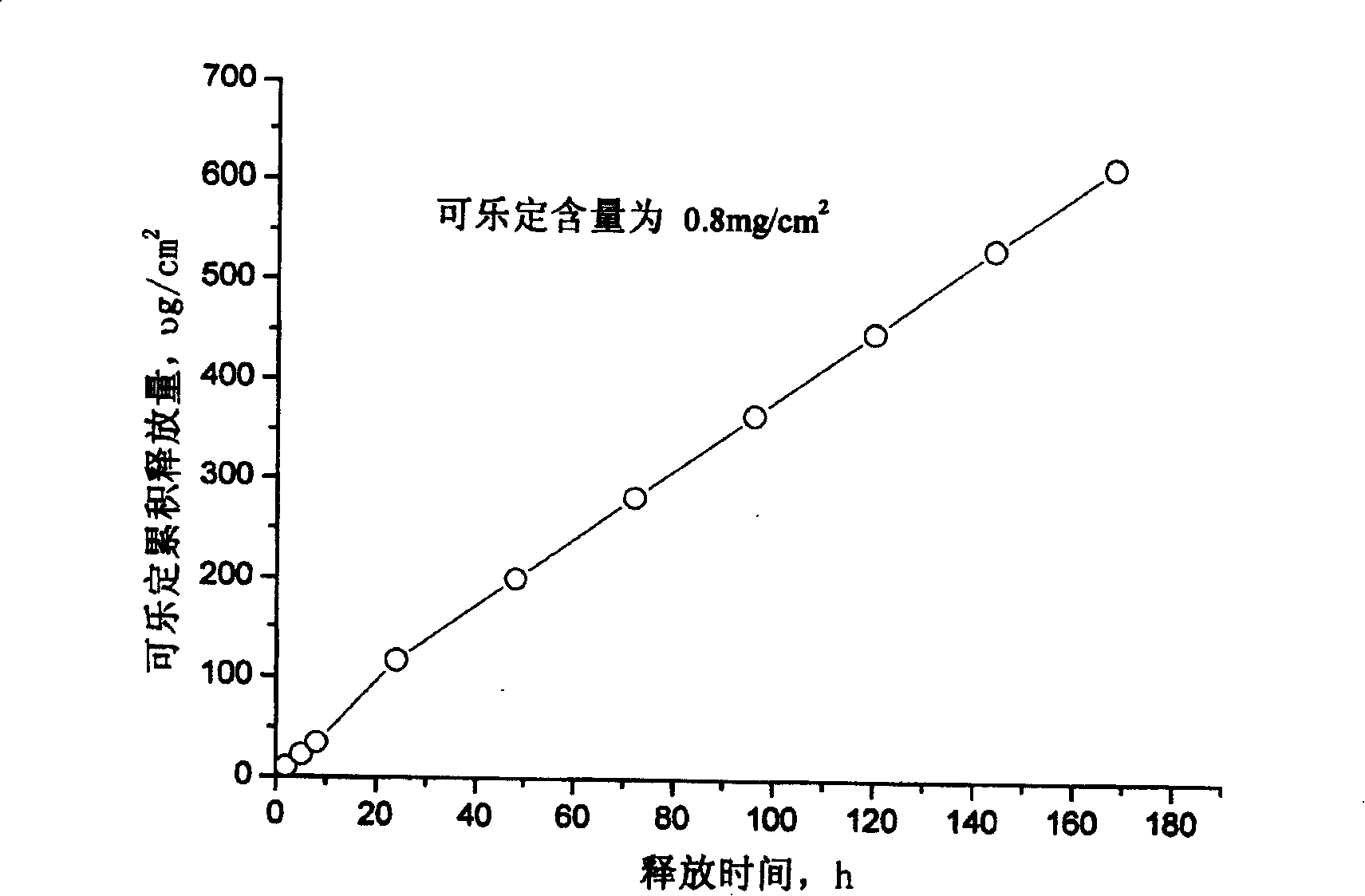
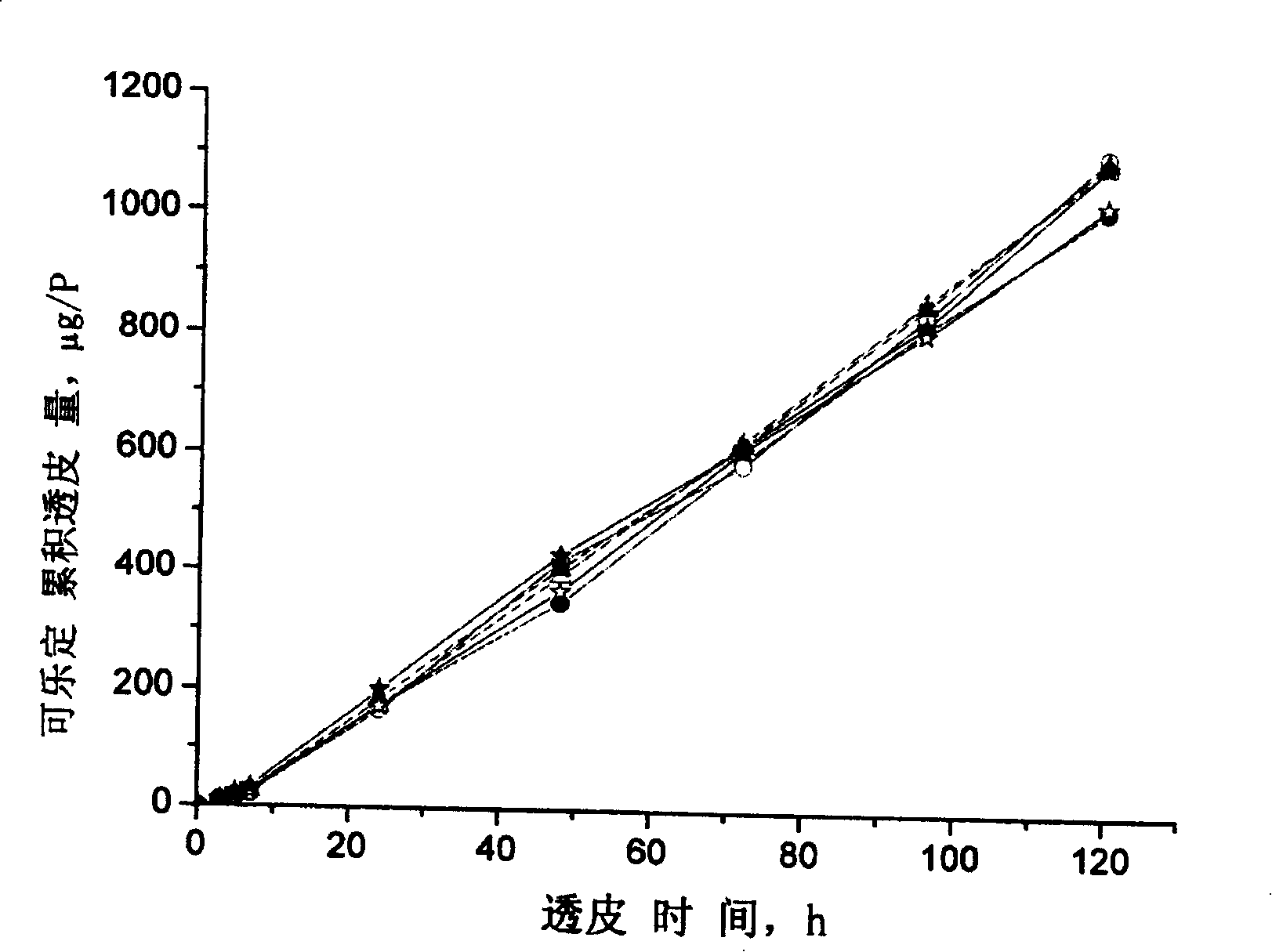
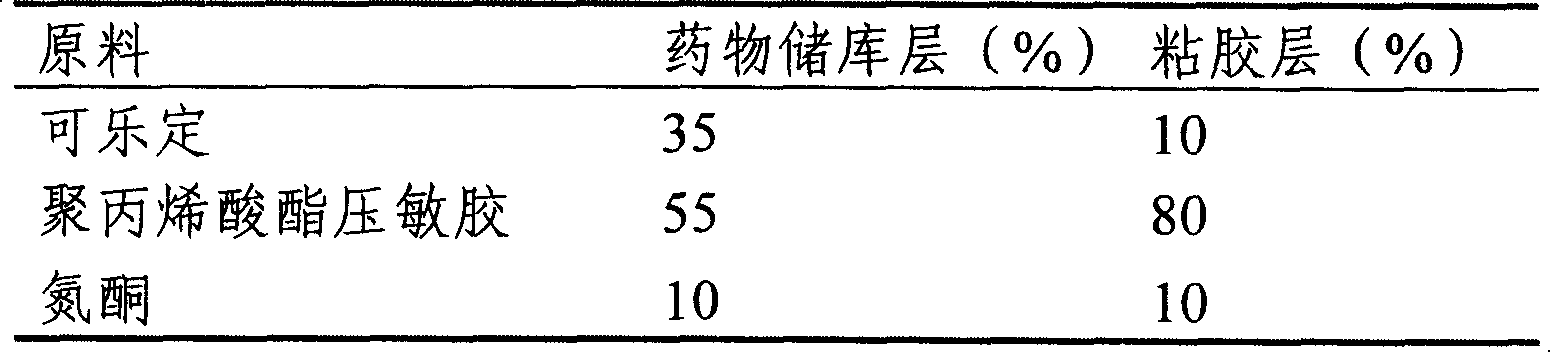
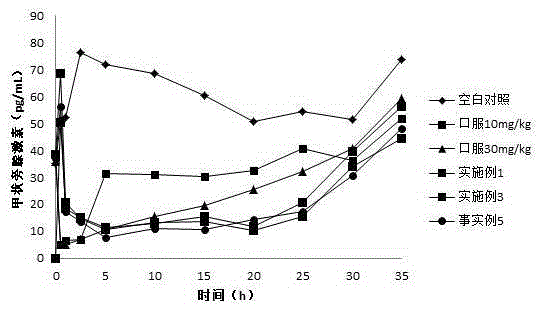
InactiveCN101011391AImprove bioavailabilityAvoid peaks and valleys in blood concentrationOrganic active ingredientsNervous disorderDrug contentControl layer
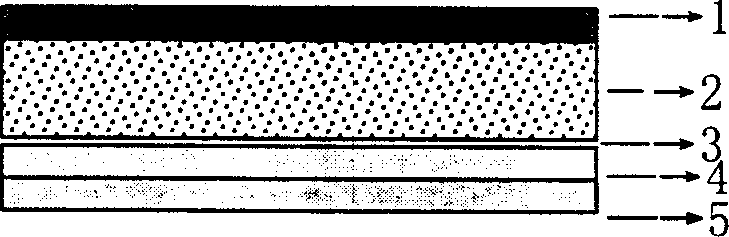
The invention relates to a cola stable paster which can treat hyperkinetic symptom of children, which comprises back liner, drug layer, release-control layer, paste layer, and anti-paste layer. The invention is characterized in that the back liner is aluminized film; the drug layer is cola stable drug and polyacrylacid ester pressure-sensitive gel; the release-control layer is modified EVA film or nuclear track porous film; the paste layer is cola stable drug and polyacrylacid ester pressure-sensitive gel; the cola stable drug content of drug layer is higher than the cola stable drug content of paste layer. The invention can avoid oral taking, with high biological utilization, stable effect, continuous release, and high safety. The invention also provides relative production.
Owner:北京克莱斯瑞控释药业有限公司
Sustained-release suspension with lurasidone and preparation method of sustained-release suspension
InactiveCN104983679AReduce releaseMask bad smellOrganic active ingredientsNervous disorderBlood concentrationLurasidone Hydrochloride
The invention belongs to the field of pharmaceutic preparations, and particularly relates to a sustained-release suspension composition with lurasidone or salt of the lurasidone and a preparation method of the sustained-release suspension composition. A sustained-release suspension comprises the lurasidone or the salt, capable of being medically accepted, of the lurasidone and a sustained-release micro-capsule coating material medicine composition. According to the preparation, the release time of lurasidone hydrochloride is effectively prolonged, and the blood concentration peak valley phenomenon caused by quick release preparations is avoided. The sustained-release suspension with the lurasidone is low in production cost and has the good taste and medicine release character, the preparation process of the sustained-release suspension with the lurasidone is convenient, and the aim of providing the preparation with the lurasidone or the salt, capable of being medically accepted, of the lurasidone for a patient is achieved, wherein the preparation is convenient to take, high in bioavailability and mild in medicine release.
Owner:AVENTIS PHARMA HAINAN
Epalrestat sustained release preparation and preparation method thereof
InactiveCN112137990AAvoid peaks and valleys in blood concentrationImprove complianceOrganic active ingredientsNervous disorderSustained release pelletsDrug utilisation
The invention belongs to the field of pharmaceutical preparations, and provides an epalrestat sustained release preparation and a preparation method thereof. The epalrestat sustained release preparation is composed of 64%-92% of drug-containing sustained-release pellets, 1.0%-8.0% of a gastric-soluble isolation coating layer, 5.0%-20% of an enteric-soluble coating layer, and 2.0%-8.0% of a protective coating layer. The epalrestat sustained release preparation disclosed by the invention can effectively avoid the peak valley phenomenon of the blood concentration of a common preparation, reducesthe medicine taking frequency, reduces the occurrence rate of adverse reactions, and increases the compliance of a patient; and can avoid a possible drug burst release effect of a monobasic sustainedrelease preparation, reduces the toxicity and side effects of the drug, and further ensures the safety of clinical medication. The selected auxiliary materials are common, the preparation process is simple, the technical difficulty is low, the industrial mass production is easy to realize, and the market potential is large.
Owner:南京康川济医药科技有限公司
Compound control-released percutaneous medicine plaster for treating high blood pressure and its preparation method
InactiveCN1633994AImprove treatment efficiencyImprove securityPharmaceutical active ingredientsSheet deliveryBlood drugTreatment hypertension
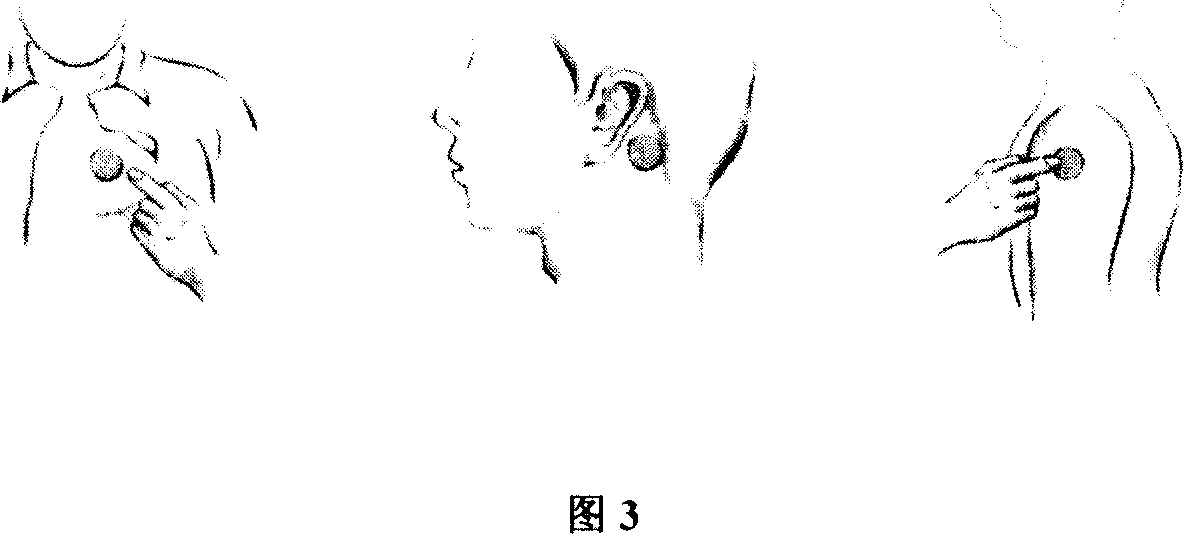
The invention relates to a compound control-released percutaneous medicine plaster for treating high blood pressure and its preparation method, wherein the paste comprises laminated back lining layer, a pressure-sensitive adhesive framework form medicament-containing layer and a protective film, wherein the compound medicament comprises diuretics, central alpha-acceptor excitant and cirumferential alpha-receptor blocking agent, beta-receptor blocking agent, calcium antagonist and any two of the five categories of medicament for influencing angiotensin II formation and treating high blood pressure. The invention may realize medicine administration once in front of chest or behind the ears, wherein homeostasis blood concentration can be reached.
Owner:王睿 +3
Patch of abstention containing cola stationary medicament and preparing method thereof
InactiveCN101199495AAvoid interferenceAvoid degradationOrganic active ingredientsNervous disorderAdhesiveNonwoven fabric
The invention discloses a detoxification plaster with clonidine and the related preparation method used for curing opioid and tobacco-dependentance. The detoxification plaster is structurally composed of a protective layer, an adhesive layer, a control release membrane layer, a medicine storehouse layer and a back lining layer. The ingredients of the preparation are that: the medicine storehouse layer contains 10 to 40 percent of clonidine and 0 to 20 percent of transdermal enhancer and the rest are soluble or non-soluble substrates; the adhesive layer contains 0 to 30 percent of clonidine and 2 to 20 percent of transdermal enhancer and the rest is pressure-sensitive adhesive; the back lining layer is a PET membrane or a non-woven fabric; the protective layer is a PET film processed with antisticking treatment; the control release membrane is an EVA polymer membrane. The area of each plaster is 1-5square centimeters; each plaster contains 1mg to 5mg of clonidine. The invention is not addictive but fast in effect, high in cure rate and safe and convenient. The external release of the invention reaches zero-degree rate process.
Owner:TIANJIN ZHONGBAO PHARMACY
Medicinal herbals fiber soft cushion and production method thereof
InactiveCN101361931AAvoid degradationAvoid degradation, drug absorption is not affected by gastrointestinal factorsNervous disorderStuffed mattressesDiseaseFiber
The invention relates to a medical herbal fiber spongy pad body and a preparation method thereof, the medicinal herbal fiber spongy pad body is shaped by mixing the fibers and medicinal components extracted from such herbal plants as Carex meyeriana, ramuli mori, rhizoma homalonemae, phyllostachys pubescens, cortex eucommiae, radices dipsaci and Gynostemma pentaphylla, and the natural latex. The preparation method comprises the steps of: (1) ingredient cutting; (2) rolling and slicing; (3) digesting and splitting; (4) hydrolyzation; (5) neutralization and degreasing; and (6) processing and molding of the pad body. According to the principles of 'external treatment to internal diseases' and 'lower treatment to upper diseases', the pad body can be made into the shape of mattress, insole, knee pad, belly bank, and shoulder periphery or other needed shapes, which can be conveniently used and replace sponge as pad bodies widely used in sound insulation and heat insulation for health care.
Owner:黄 建华
Preparation method for solid implantation catgut for treating spinal diseases
InactiveCN102188758AAvoid peaks and valleys in blood concentrationReduce adverse reactionsSurgerySkeletal disorderAcupuncture pointDisease
The invention relates to a preparation method for a solid implantation catgut for treating spinal diseases, which is characterized by comprising the following steps of: extracting active ingredients from Chinese medicinal compositions such as musk, rosewood heart wood, frankincense, sappan wood, storax, costustoot, root of red-rooted salvia and the like to obtain concentrated solution of the Chinese medicinal compositions; and adding hyaluronidase into the concentrated solution of the Chinese medicinal compositions to obtain mixed soak solution, and soaking a catgut with the length of between 2.5 and 3.5 centimeters in the mixed soak solution for 10 to 20 days to obtain the solid implantation catgut. The solid implantation catgut for treating spinal diseases can be used for being implanted into an ashi point or acupuncture points to treat spinal diseases. By the solid implantation catgut, medicaments can enter in bodies at constant speed within long time to maintain constant and effective blood concentration, so the phenomenon of the peak valley of the blood concentration due to other administration modes is avoided, adverse reactions can be reduced, and the double effects of the medicaments and the acupuncture points are expressed simultaneously to achieve the optimum pharmacological effect.
Owner:袁明省
Doractin dashing agent and method of preparing the same
InactiveCN101401782APromote transdermal absorptionImprove solubilityOrganic active ingredientsPharmaceutical delivery mechanismAntioxidantSolvent
The invention provides a Doramectin dash agent and a preparation method thereof. The Doramectin dash agent consists of propylene glycol, anhydrous ethyl alcohol, azone, Tween-80 and 2, 6-ditertbutyl-p-cresol, wherein the azone is a percutaneous absorption accelerant for accelerating percutaneous absorption of Doramectin; the propylene glycol is a solvent, is used with the azone and has synergism for accelerating the percutaneous absorption of the Doramectin, the Tween-80 is a solubilizing agent for making the Doramectin dissolved easily, the anhydrous ethyl alcohol is an assistant agent for reducing the viscosity of the dash agent and making the dash agent permeate the skin through the clothing hair, and the 2, 6-ditertbutyl-p-cresol is an oxidation inhibitor. The Doramectin dash agent has the advantages that the Doramectin dash agent has little toxicity, lower cost and convenient use, and can be used for killing internal eelworms and external parasites of animals.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Skin external-use patch containing calcium-sensing receptor stimulant
InactiveCN106806893AEnsure safetyImprove securityOrganic active ingredientsSheet deliveryOral medicationAdditive ingredient
The invention discloses a skin external-use patch containing a calcium-sensing receptor stimulant. For the patch disclosed by the invention, a medicinal storage layer, a backing layer and a protection layer are adopted as the necessary ingredients of the patch, and meanwhile, other pharmaceutically acceptable excipients also can be added. The patch is administrated through the skin, so that the first-pass effect of the liver and the stimulation to the gastrointestinal tract are avoided, the untoward effects of the medicines are effectively reduced, the medication to patients is safe, meanwhile, compared with the tablets for oral administration, the patch provided by the invention has the permanent efficacies, and the bioavailability is obviously improved.
Owner:BEIJING TIDE PHARMA
Nifedipine controlled-release tablet composition and preparation method thereof
ActiveCN106344531AReduce usageAvoid peaks and valleys in blood concentrationOrganic active ingredientsPill deliveryPOLYOXYETHYLENE ETHERFineness
The invention relates to a nifedipine controlled-release tablet composition, comprising the following components in percentage by weight: 4.0-35.0% of nifedipine, 5.0-20.0% of polyoxyethylene, 5.0-30.0% of high-viscosity hydrophilic matrix material, 5.0-20.0% of a hydrophobic retarder, 20.0-60.0% of a filling agent, 0-10.0% of an adhesive solution, and 0.5-1.0% of a lubricant. A preparation method of the composition comprises the following steps: micronizing raw materials under a dark condition until the fineness ensures the powder to pass through a 200-mesh sieve, and screening an auxiliary material through a sieve with 80-100 meshes; adding the medicinal raw materials into the adhesive solution, and stirring uniformly for later use; and weighing a formula amount of polyoxyethylene, hydroxypropyl methylcellulose, the retarder namely ethyl cellulose and the filling agent, mixing uniformly, adding the prepared adhesive solution to prepare a soft material, and performing granulation, drying, straightening, addition of the lubricant, total blending, tabletting and package to obtain the composition. The nifedipine controlled-release tablet composition has the advantages that the preparation process is simple and easy, the production cost is low, and the production cycle is short.
Owner:浙江为康制药有限公司
Transdermal plaster of rivastigmine and preparation process thereof
InactiveCN1994290BEasy to useClear curative effectOrganic non-active ingredientsEster active ingredientsTransdermal patchRivastigmine
The invention relates to a method for preparing kabalatin adhere agent for treating senile dementia, wherein said adhere agent can stably hold blood drug density and reduce feeding time, with high safety.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Trimetazidine hydrochloride bi-layer osmotic pump controlled release tablet and preparation method thereof
ActiveCN103735528ARelease constant speedSmall toxicityOrganic active ingredientsPharmaceutical delivery mechanismSide effectMotility
The invention provides a trimetazidine hydrochloride bi-layer osmotic pump controlled release tablet and a preparation method thereof. The osmotic pump controlled release tablet is formed by a drug-containing layer, a booster layer and a coating film, wherein the drug-containing layer comprises the following components in percentage by weight: 10-50% of trimetazidine hydrochloride, 30-80% of suspension agent and the balance of other auxiliary materials; the booster layer comprises the following components in percentage by weight: 20-90% of swelling agent, 5-70% of osmotic active substance and 0.5-5% of lubricating agent; the semipermeable coating film comprises 10-20g of semipermeable high polymer material and 1-5g of water-soluble pore-forming agent every 100 tablets. The trimetazidine hydrochloride bi-layer osmotic pump controlled release tablet can realizes constant release of drug in the body of a patient without being affected by pH value of a medium environment, enzyme, gastrointestinal motility and food, and is capable of maintaining the stability of plasma concentration of drug, reducing toxic and side effects of drug, decreasing dosing frequency and improving compliance of the patient.
Owner:SHENYANG PHARMA UNIVERSITY
Tamoxifen citrate emplastrum and preparation method thereof
InactiveCN101711754AConvenient treatmentStabilize local blood drug concentrationOrganic active ingredientsSexual disorderTransdermal patchAdditive ingredient
The invention relates to a tamoxifen citrate emplastrum and a preparation method thereof. A tamoxifen citrate micro emulsion is prepared into a transdermal emplastrum, and the transdermal emplastrum is divided into four layers: an anti-adhesion layer, a viscose layer, a back lining layer and a medicine storage layer. The tamoxifen citrate micro emulsion is used as a drug effect ingredient and comprises tamoxifen citrate used as an active ingredient, polysorbate 80 used as a surfactant, propylene glycol used as a cosurfactant, pure water or distilled water, and the like. Besides the drug effect ingredient, some non-polar polymers, a plasticizer, a tackifier, transdermal enhancer and an antioxidant are also added to the emplastrum. Compared with the traditional dosage forms such as tablets, solutions, oral cavity disintegrants, dispersants and external micro emulsion preparations, the tamoxifen citrate emplastrum has the prominent advantages of safety, low toxicity, convenient and simple use and accurate drug dosage.
Owner:河南省生物工程技术研究中心
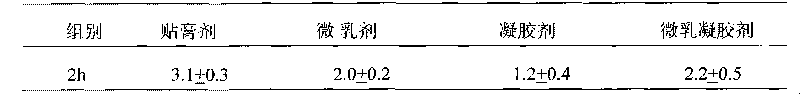
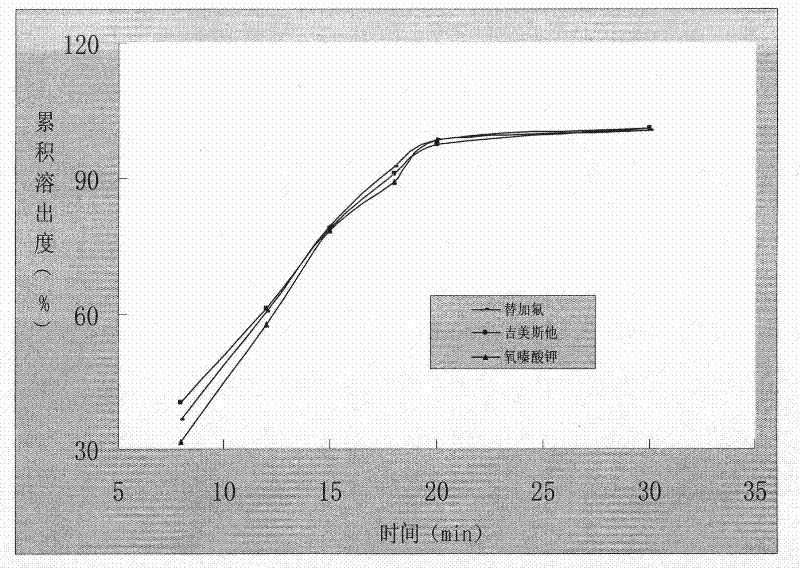
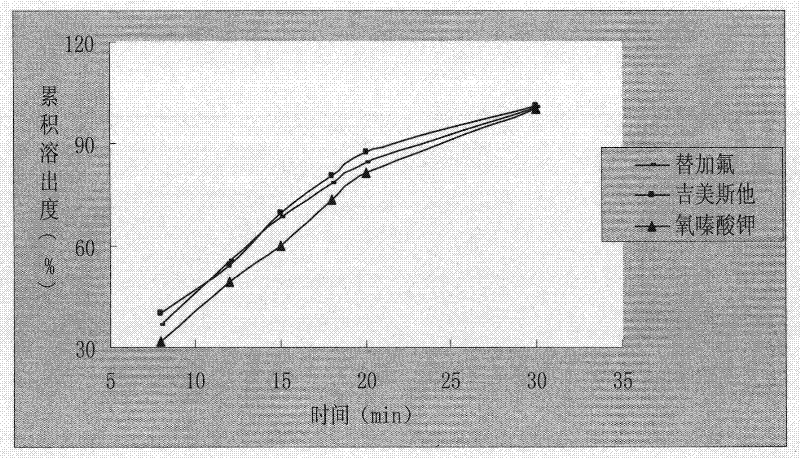
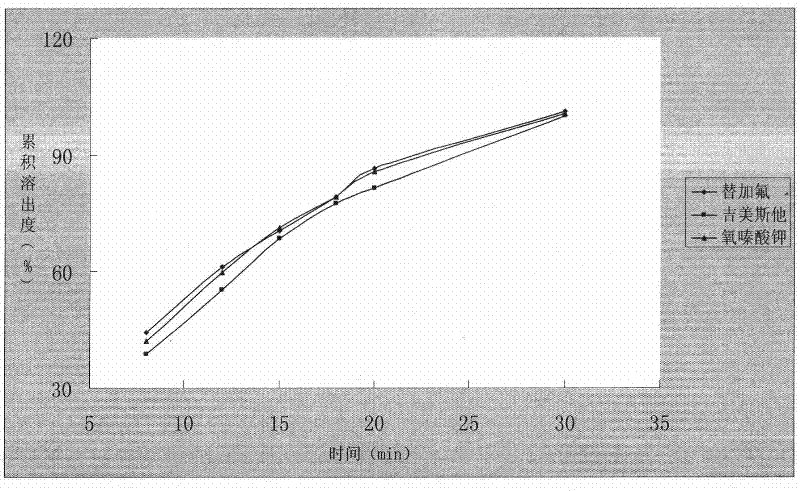
A kind of capsule preparation containing tegafur, gemesta and oxonate potassium and preparation method thereof
ActiveCN101574326BWidely evenly distributedLess irritatingCapsule deliveryMacromolecular non-active ingredientsAdditive ingredientPotassium
The invention relates to a capsule preparation containing tegafur, gemestat and oxonate potassium and a preparation method thereof, which is characterized in that the capsule preparation contains tegafur pellets, gemesta pellets and oxonate potassium pellets , wherein the pellets are composed of active pharmaceutical ingredients, diluents, binders and surfactants; the pellets are prepared by extrusion-spheronization method, centrifugal-fluidization method and coating pot pan-pellet method, and then other excipients are added, Finally the capsules are filled. The capsule prepared by the invention can release the drug stably and quickly in the body, improves the stability of the drug, has better dissolution rate and bioavailability, and reduces the adverse reaction of the drug.
Owner:LUNAN PHARMA GROUP CORPORATION
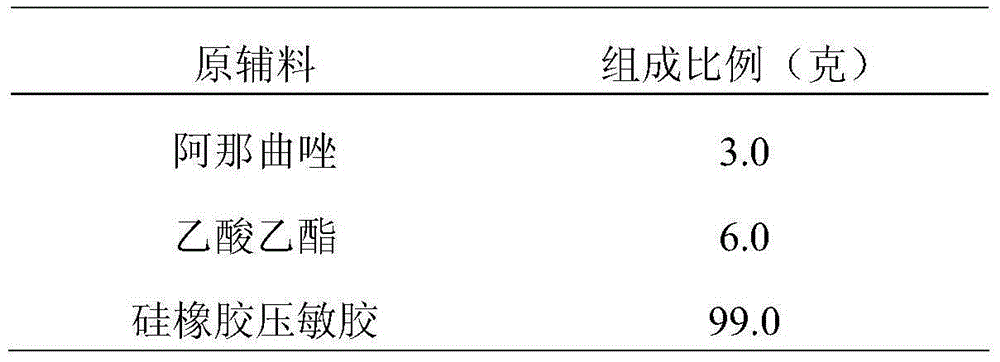
External patch containing anastrozole and application thereof
InactiveCN105055379ASmall toxicityAvoid first pass effectOrganic active ingredientsAntineoplastic agentsContinuous useAnastrozole
The invention relates to an external patch containing anastrozole and a preparation method thereof. The external preparation uses anastrozole as a main drug, and is used for treating breast cancer. The external patch containing anastrozole is absorbed in a percutaneous manner, is uniform in drug release and mild and durable in action. One patch can be continuously used for 7 days. The patch directly acts on skin without passing through gastrointestinal tracts, so that the first pass effect of liver can be avoided, adverse response is reduced, and the occurrence rate of adverse responses is reduced. The external patch has good patient compliance, and is convenient to use and carry.
Owner:徐静 +1
External-applied traditional Chinese medicine preparation for treating rheumatism pain disease and preparing method
InactiveCN101099854AReduce toxic and side effectsAvoid first pass effecAnthropod material medical ingredientsHydroxy compound active ingredientsDiseaseMonkshoods
The invention is concerned with a kind of Chinese traditional medicine for external use and its preparetion method to cure spondylopathy and lumbar illness and pain from rheumatism. It relates to 10 g chinese clematis, 10 g prepared dog button, 10 g long-nosed pitviper, 10 g prepared Sichuan aconite root, 10 g prepared wild aconite root, 10 g prepared common monkshood Daughter root, 10 g cassia, 10 g pubescent angelica root, 20 g common flowering quince fruit, 10 g dried argyi leaf, 6 g white mustard seed, 20 g sevenlobed yam rhizome, 10 g hairy birthwort, 10 gherb a lycopodii, 10 g earth worm, 10 g burreed tuber, 10 g zedoary, 10 g prepared eucommia bark, 10 g cloves, 20 g asarum, 10 g bornel , 10 g anisodus acutangucus, 6 g short-pedicel aconite root and 20 g dried ginger. It has perfect effect for neck and vertebra illness, lumbar intervertebral disc protrusion, spondylitis ankylopoietica, hyperosteogeny, pain from rheumatism, lumbar muscle strain and arthropathy.
Owner:方焕生
A kind of ivabradine hydrochloride osmotic pump controlled-release tablet and preparation method thereof
ActiveCN104398486BSmall toxicityControl releasePharmaceutical delivery mechanismPharmaceutical non-active ingredientsPatient complianceBULK ACTIVE INGREDIENT
The invention provides an ivabradine hydrochloride osmotic pump controlled-release tablet and a preparation method thereof. The osmotic pump controlled-release tablet of the invention is characterized in that it contains the active ingredient of ivabradine hydrochloride, and is composed of a double-layer tablet core compressed by a polymer material, an osmotic pressure active substance and the like, and a semi-permeable coating film. The preparation enables ivabradine hydrochloride to achieve a constant-speed slow release within 0-24 hours, and the release rate exceeds 90%, and has the characteristics of long-acting zero-order drug release. The invention is used for treating patients with chronic angina pectoris, can stabilize blood drug concentration, reduce adverse reactions caused by large fluctuation of blood drug concentration, and improve drug safety; meanwhile, it reduces the number of patients taking the drug and improves the patient's compliance.
Owner:WUHAN WUYAO SCI & TECH
Trimetazidine hydrochloride double-layer osmotic pump controlled-release tablet and preparation method
ActiveCN103735528BSmall toxicityAvoid peaks and valleys in blood concentrationOrganic active ingredientsPharmaceutical delivery mechanismSide effectTramadol Hydrochloride
The invention provides a trimetazidine hydrochloride bi-layer osmotic pump controlled release tablet and a preparation method thereof. The osmotic pump controlled release tablet is formed by a drug-containing layer, a booster layer and a coating film, wherein the drug-containing layer comprises the following components in percentage by weight: 10-50% of trimetazidine hydrochloride, 30-80% of suspension agent and the balance of other auxiliary materials; the booster layer comprises the following components in percentage by weight: 20-90% of swelling agent, 5-70% of osmotic active substance and 0.5-5% of lubricating agent; the semipermeable coating film comprises 10-20g of semipermeable high polymer material and 1-5g of water-soluble pore-forming agent every 100 tablets. The trimetazidine hydrochloride bi-layer osmotic pump controlled release tablet can realizes constant release of drug in the body of a patient without being affected by pH value of a medium environment, enzyme, gastrointestinal motility and food, and is capable of maintaining the stability of plasma concentration of drug, reducing toxic and side effects of drug, decreasing dosing frequency and improving compliance of the patient.
Owner:SHENYANG PHARMA UNIVERSITY
Lasofoxifene tartrate emplastrum
InactiveCN103830207AConvenient treatmentStabilize local blood drug concentrationSkeletal disorderSexual disorderTransdermal patchAntioxidant
The invention discloses a lasofoxifene tartrate emplastrum preparation and a preparation method for the same. According to the preparation method disclosed by the invention, lasofoxifene tartrate micro-emulsion is prepared into a transdermal emplastrum, and the transdermal emplastrum is divided into four layers, that is, an anti-sticking layer, an adhesive layer, a backing layer and a medicine storage layer; the medicinal ingredient is lasofoxifene tartrate micro-emulsion, and is composed of lasofoxifene tartrate used as an active ingredient, polysorbate 80 used as a surfactant, propylene glycol used as a cosurfactant, pure water or distilled water and the like. Some non-polar polymers, a plasticizer, a tackifier, a transdermal accelerant and an antioxidant are further added in the emplastrum preparation disclosed by the invention besides the medicinal ingredients above. The lasofoxifene tartrate emplastrum preparation disclosed by the invention has the positive significance that compared with the existing dosage forms and tablets, the lasofoxifene tartrate emplastrum preparation has the prominent advantages of being lasting in efficacy, safe and low-toxicity, convenient, simple and sanitary to use, and accurate in medication dose.
Owner:崔书豪
Tripterine nano structure lipid carrier and preparation method and application thereof
InactiveCN102225205BReduce systemic side effectsImprove bioavailabilityOrganic active ingredientsAntipyreticNano structuringPhospholipid
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE
Method for preparing medicinal lining for preventing and treating mammary glands hyperplasia and application thereof
ActiveCN1951243AMedication safetyAvoid peaks and valleys in blood concentrationOrganic active ingredientsMedical devicesMammary gland structureBiomedical engineering
The invention relates to a method for preparing the drug material used to treat mammary gland hyperplasia. Wherein, it fixes the drug micro capsule whose corn is the said treatment drug and the drug transparent absorber micro capsule whose corn is the drug transparent absorber, while their weight ratio is 100-10:1-10. The preparation comprises that 1, preparing the drug micro capsule and the drug transparent absorber micro capsule; 2, mixing them at 100-10:1-10 into the micro capsule.
Owner:ZHEJIANG LANGSHA UNDERWEAR
Raloxifene emplastrum preparation and preparation method thereof
InactiveCN101700241AConvenient treatmentStabilize local blood drug concentrationOrganic active ingredientsSkeletal disorderTransdermal patchAdditive ingredient
The invention relates to a raloxifene emplastrum and a preparation method thereof. Raloxifene microemulsion is made into a transdermal emplastrum which is divided into an anti-adhesion layer, a viscose layer, a backlining layer and a medicine storage layer. The raloxifene microemulsion taken as a medical ingredient of the raloxifene emplastrum comprises the following ingredients: raloxifene taken as an active ingredient, polysorbate taken as surfactant, propanediol taken as cosurfactant, pure water or distilled water, and the like. Except for the medical ingredient, a plurality of nonpolar polymers, plasticizing agent, caking agent, transdermal enhancing agent and antioxidant are also added in the emplastrum. Compared with the prior dosage forms, such as tablets, the invention has the remarkable advantages of durable medical effect, safety, low toxicity, simple, convenient and sanitary, and accurate medical dosage.
Owner:河南省生物工程技术研究中心
Kurarinone osmotic pump controlled release tablets and preparation method thereof
ActiveCN105769797AAvoid peaks and valleys in blood concentrationThe peak and valley phenomenon of blood drug concentration is alleviatedOrganic active ingredientsDigestive systemTectorial membraneMagnesium stearate
The invention belongs to the technical field of medicine controlled release preparations, particularly discloses kurarinone osmotic pump controlled release tablets, and further discloses a preparation method of the kurarinone osmotic pump controlled release tablets. The kurarinone osmotic pump controlled release tablets are prepared from kurarinone tablets and protective films outside the tablets, wherein the tablets are prepared from core tablets and semipermeable films outside the core tablets; a medicine release hole is formed in each semipermeable film; the core tablets are prepared from the following raw materials in percentage by weight: 40.00%-60.00% of kurarinone, 15.00%-25.00% of sodium chloride, 20.00%-30.00% of lactose, 2.00%-5.00% of hydroxypropyl methylcellulose, 1.00%-3.50% of polyvinylpyrrolidone and 0.50%-2.00% of magnesium stearate. The kurarinone osmotic pump controlled release tablets provided by the invention can keep favorable zero-order medicine release within 20 hours, the final total release amount exceeds 70%, and part of the final total release amount exceeds 80%, so that the kurarinone osmotic pump controlled release tablets can be clinically used for treating Chronic Hepatitis B.
Owner:CHANGZHOU ORFAMA PHARM TECH CO LTD
Centella total glycoside cataplasm and preparation method thereof
InactiveCN103356508ALarge drug loading capacity in matrixComfortable to useMacromolecular non-active ingredientsDermatological disorderChemistryKeloid
The invention aims to disclose centella total glycoside cataplasm and a preparation method thereof. The centella total glycoside cataplasm comprises the following components in percentage by weight: 5.0%-8.0% of sodium polyacrylate, 0.5%-0.8% of gelatin, 7.0%-10.0% of sorbitol, 1.5%-2.5% of PVP (polyvinylpyrrolidone), 20.0%-40.0% of glycerinum, 0.3%-0.6% of titanium dioxide, 2.0%-4.0% of beta-cyclodextrin, 0.15%-0.4% of tartaric acid, 2.0%-5.0% of centella glycoside powder, 0.05%-0.25% of dihydroxyaluminium aminoacetate and 61.5%-28.45% of purified water. Compared with the prior art, the centella total glycoside cataplasm has the characteristics of being large in drug loading capacity, convenient to use, etc, overcomes the inconvenience caused by pollution to clothes in use, and is suitable for treating trauma, operative wound, burn, keloid and scleroderma, therefore, the aim of the invention is realized.
Owner:上海卫生材料厂有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com