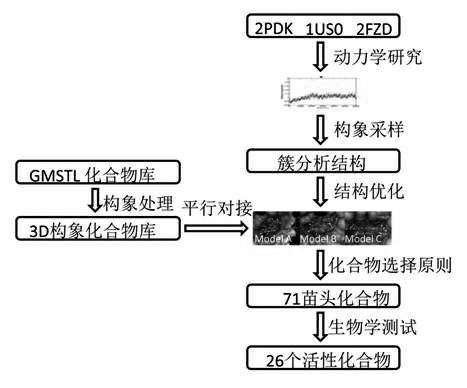
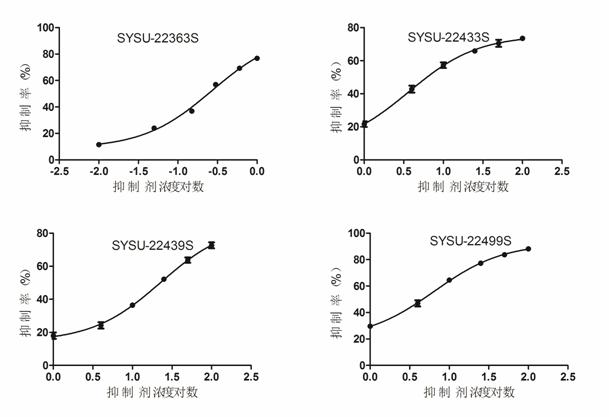
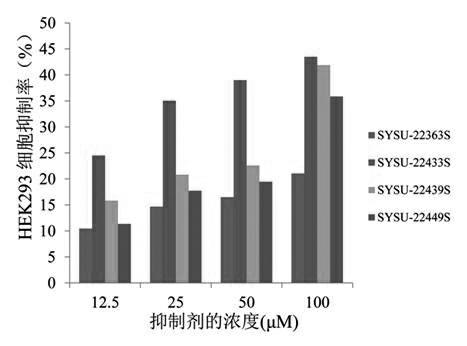
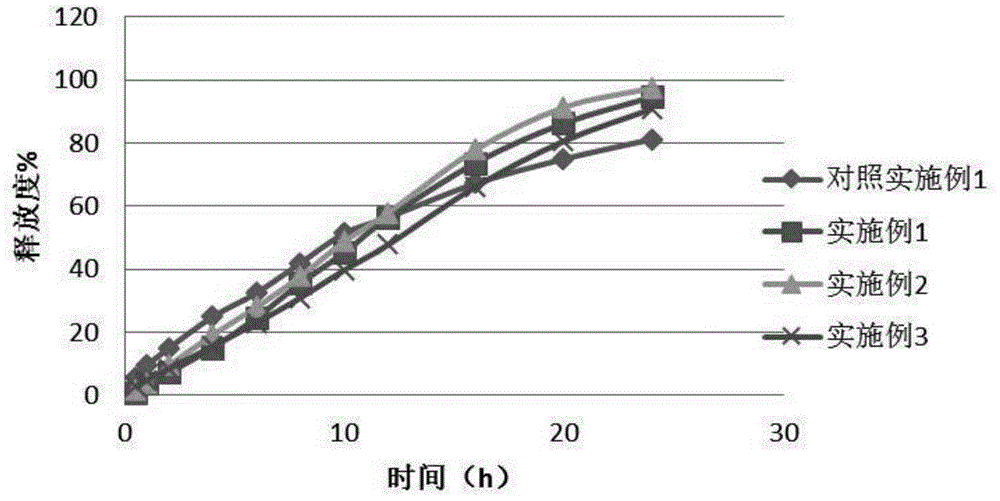
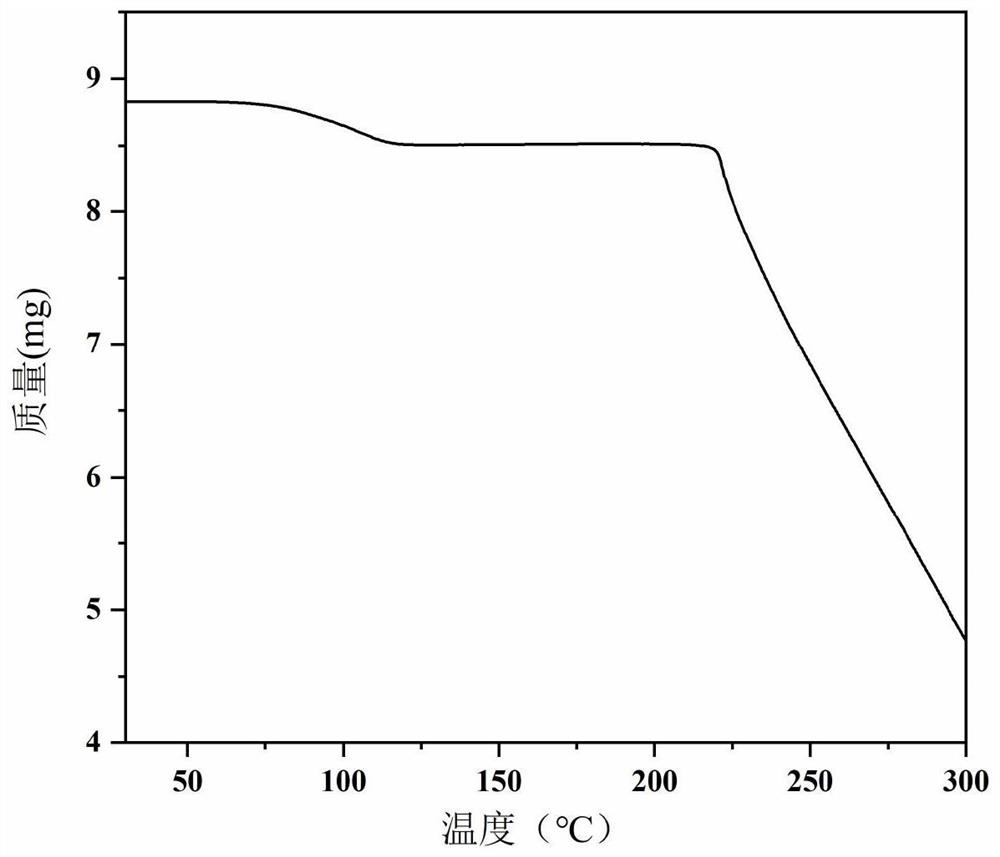
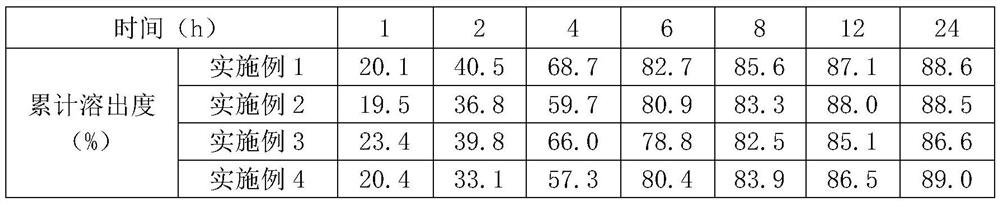
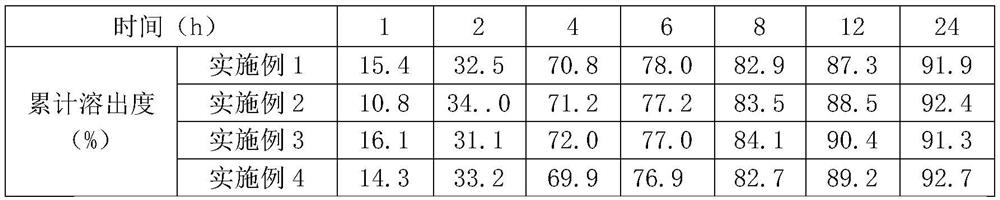
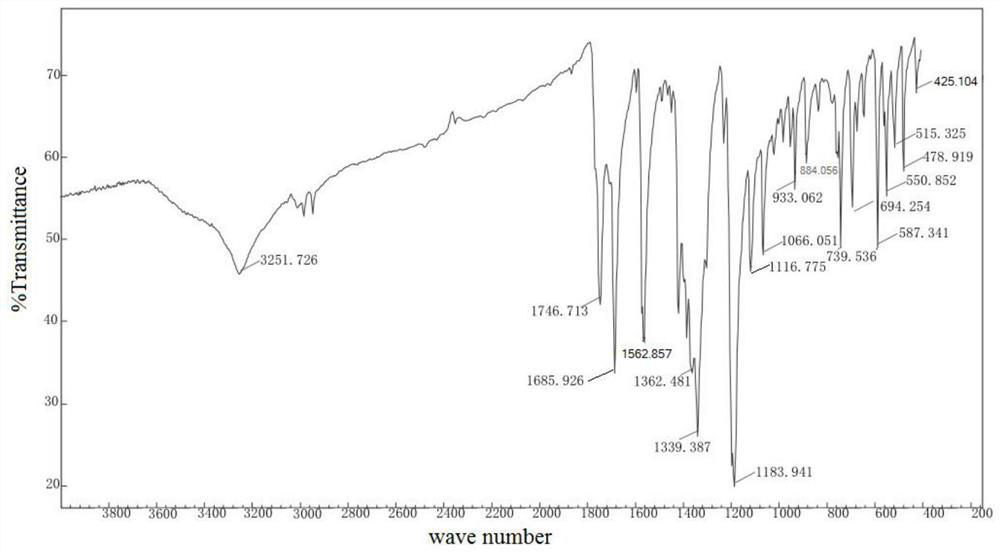
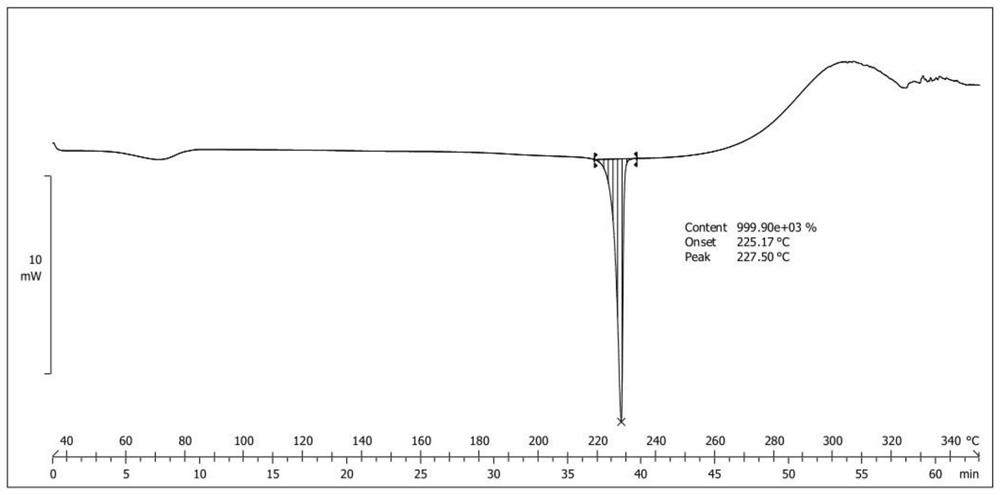
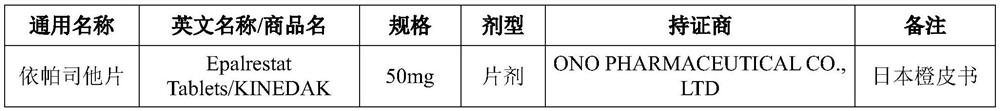
Patents
Literature
58 results about "Epalrestat" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
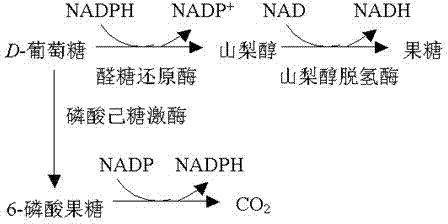
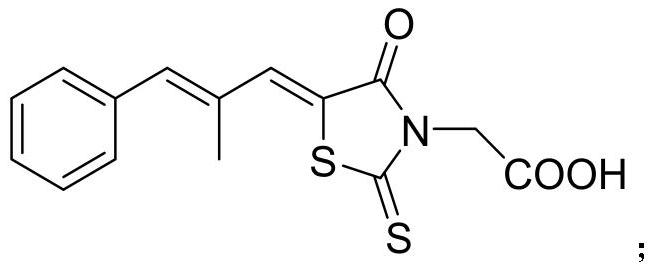
Epalrestat is a carboxylic acid derivative and a noncompetitive and reversible aldose reductase inhibitor used for the treatment of diabetic neuropathy, which is one of the most common long-term complications in patients with diabetes mellitus. It reduces the accumulation of intracellular sorbitol which is believed to be the cause of diabetic neuropathy, retinopathy and nephropathy It is well tolerated, with the most commonly reported adverse effects being gastrointestinal issues such as nausea and vomiting, as well as increases in certain liver enzymes. Chemically, epalrestat is unusual in that it is a drug that contains a rhodanine group. Aldose reductase is the key enzyme in the polyol pathway whose enhanced activity is the basis of diabetic neuropathy. Aldose reductase inhibitors (ARI) target this enzyme. Out of the many ARIs developed, ranirestat and fidarestat are in the trial stage. Others have been discarded due to unacceptable adverse effects or weak efficacy. Epalrestat is the only ARI commercially available. It is easily absorbed into the neural tissue and inhibits the enzyme with minimum side effects.
Application of utilizing beta-phenylalanine compounds as aldose reductase inhibitors
InactiveCN102512407AHigh selectivityLow toxicityOrganic active ingredientsSenses disorderDiabetic complicationPhenylalanine
The invention discloses an application of utilizing beta-phenylalanine compounds as aldose reductase inhibitors. The beta-phenylalanine compounds are used as aldose reductase (ALR2) inhibitors, have the similar enzymology activity of medicine Epalrestat on the market, and show the high selectivity to aldose reductase (EC1.1. 1.2, ALR1) which has very high homology to the ALR2, the cytotoxicity test shows that the compounds have small toxicity. The compounds can be used for preparing medicines for curing or preventing diabetic complications (diabetic cataract, peripheral neuropathy, retinopathy, kidney disease and arterial atherosclerosis). The general formula of the skeleton structure of the beta-phenylalanine compounds is shown in the description: wherein R1, R4 and R5 are hydrogen; R2 and R3 are hydrogen, alkyl, alkoxy, sulfhydryl or halogen; and R6 is naphth, pyrimidyl, pyrazinyl, phenyl, tetrahydro naphthyl or indolyl.
Owner:广州市爱菩新医药科技有限公司
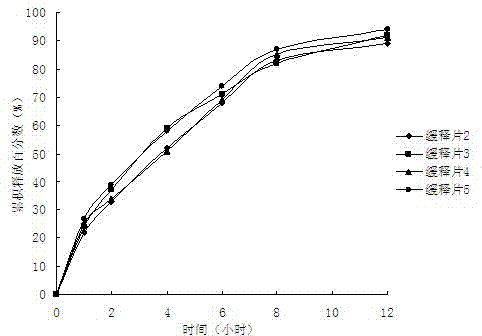
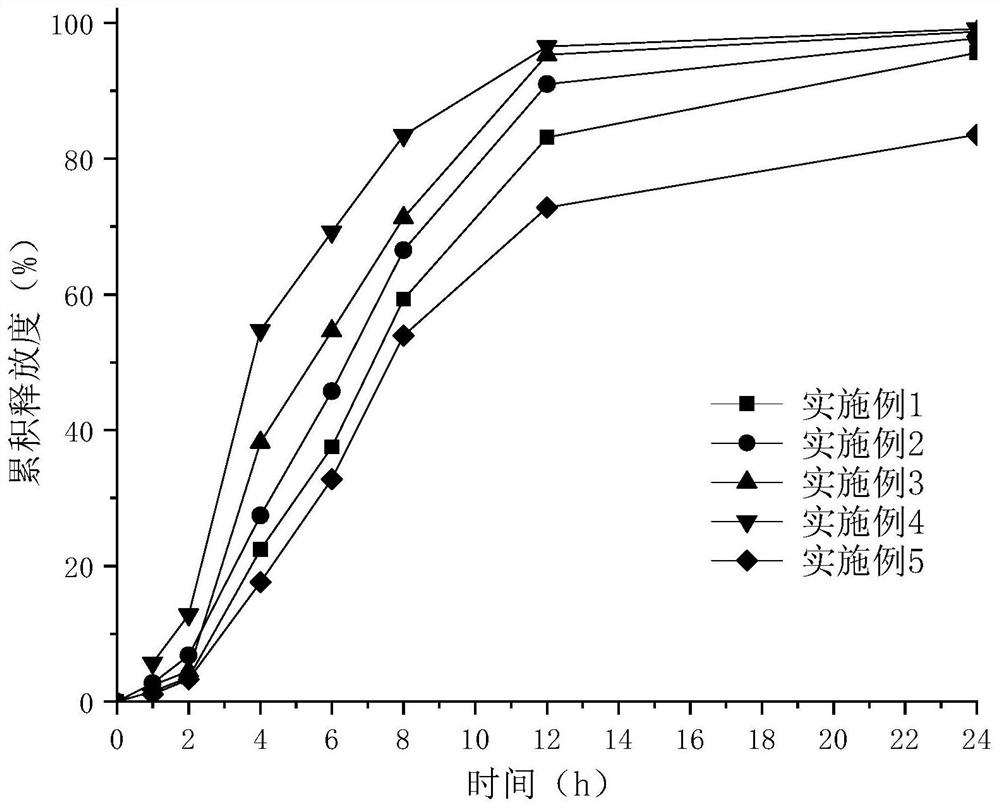
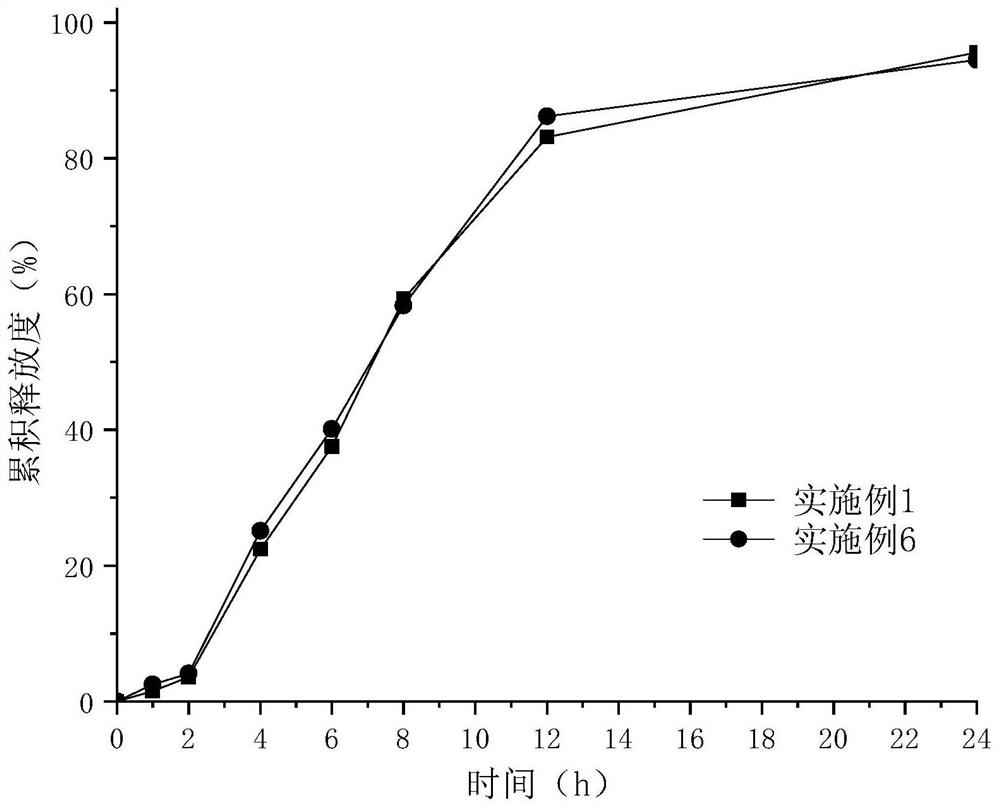
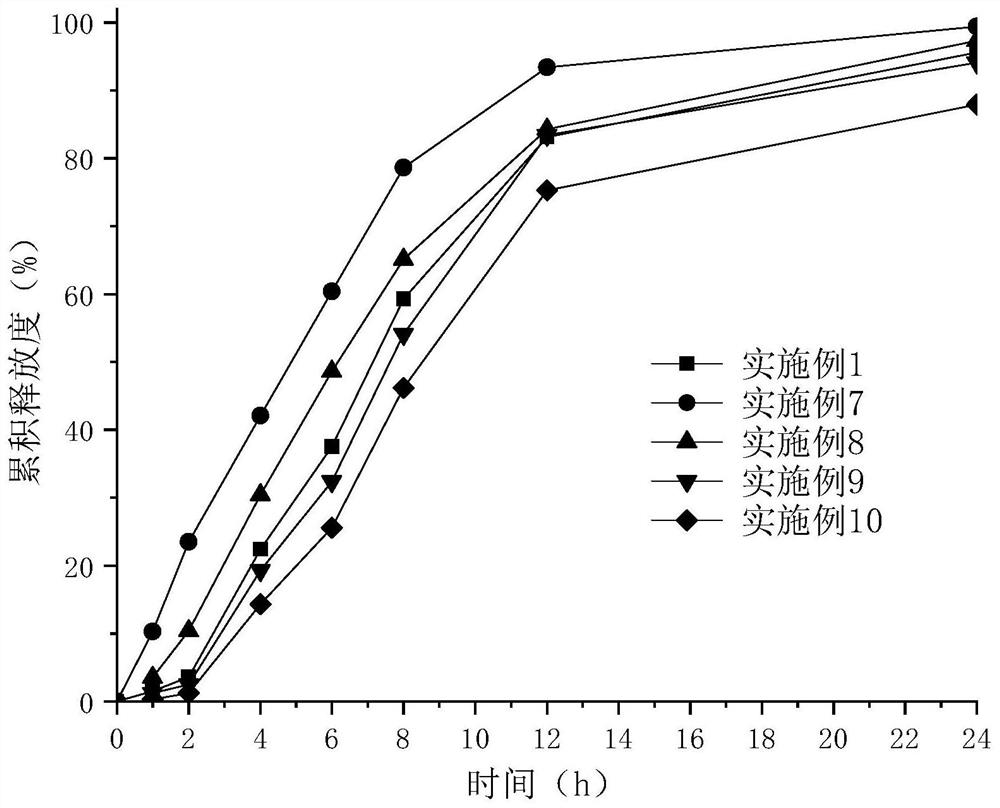
Epalrestat enteric-coated and sustained-release tablets and preparation method thereof
ActiveCN104940156AGood slow releaseReduce typesOrganic active ingredientsMetabolism disorderOral medicationTherapeutic effect
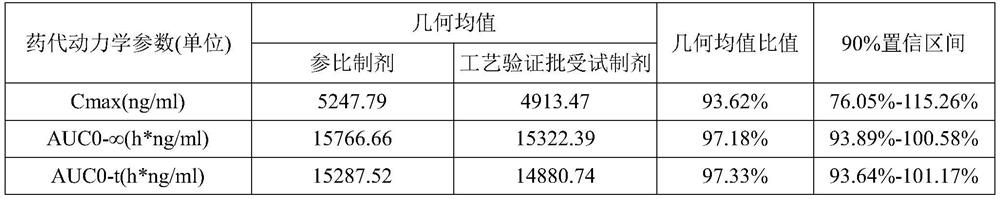
The invention discloses an epalrestat enteric-coated and sustained-release preparation and a preparation method thereof. The preparation comprises epalrestat or pharmaceutically acceptable salts of epalrestat, single-layer tablet cores of a sustained-release framework material and an enteric coating. The invention further provides a preparation method of epalrestat enteric-coated and sustained-release tablets. The Epalrestat enteric-coated and sustained-release tablets are simple in production process and stable in quality; compared with the common epalrestat preparation, the Epalrestat enteric-coated and sustained-release tablets provided by the invention have the advantages that drug release can be more smoothly and slowly achieved after oral administration, in-vivo drug release can be smoother and more consistent, and the therapeutic effect can be kept for a longer time.
Owner:YANGTZE RIVER PHARM GRP NANJING HAILING PHARM CO LTD +1
Epalrestat tablet, and its prepn. method
InactiveCN1692903ASimple preparation processHigh hardnessOrganic active ingredientsNervous disorderCarboxymethyl starchLow-substituted hydroxypropylcellulose
A tablet for epalrestat for suppressing the transmission speed decreasement of sciatic nerve and coccygeal nerve and the increasement of sorbitol in red cell and sciatic nerve is prepared from lactose, microcrystalline cellulose, low-substituent hydroxypropyl cellulose, carboxymethyl starch sodium magnesium stearate and epalrestat through sequential mixing, granulating, drying, coating and tabletting.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Novel choline cocrystal of epalrestat
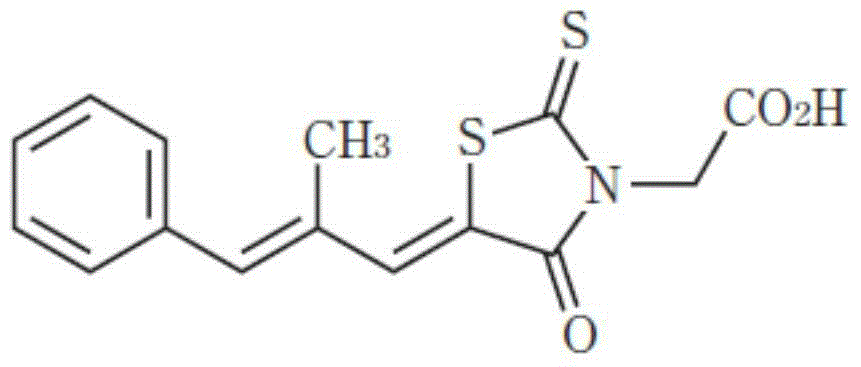
The invention relates to a novel choline cocrystal of 5-[(lZ.2E)-2-methyl-3-phenylpropenylidene]-4-oxo-2-thioxo-3-thiazolidineacetic acid. The preparation and characterization of the novel choline cocrystal according to various embodiments of the invention is described. The invention also relates to pharmaceutical compositions containing the novel choline cocrystal and the therapeutic use of the novel choline cocrystal to treat and / or prevent various conditions, including treating and / or preventing diabetic complications, treating and / or preventing homocystinuria reducing levels of homocysteine in blood serum, inhibiting aldose reductase, and affording cardioprotection in non-diabetic patients.
Owner:BIONEVIA PHARMACEUTICALS INC
Refining method of crude epalrestat product
The invention discloses a refining method of a crude epalrestat product, which comprises the following steps of: firstly, dissolving the crude epalrestat product into a tetrahydrofuran solvent, heating to completely dissolve the crude epalrestat product, and stopping heating after the crude epalrestat product is completely dissolved; then adding isopropyl ether so that a small amount of red solids are separated out, further heating to completely dissolve the red solids, then stopping heating, naturally cooling, crystallizing and filtering, and then drying to obtain initially refined epalrestat; and then adding the tetrahydrofuran solvent, heating till the initially refined epalrestat is completely dissolved, stopping heating, then slowly adding petroleum ether so that a small amount of red solids are separated out, continuously heating till the red solids are completely dissolved, then stopping heating, naturally cooling, crystallizing and filtering, and then drying to obtain a refined epalrestat product. The refining method disclosed by the invention can be used for obtaining the high-purity epalrestat and has the advantages that the solvent raw material is available, reaction condition is mild, operation is simple and yield is high, and is suitable for industrial production.
Owner:KAIFENG MINGREN PHARMA
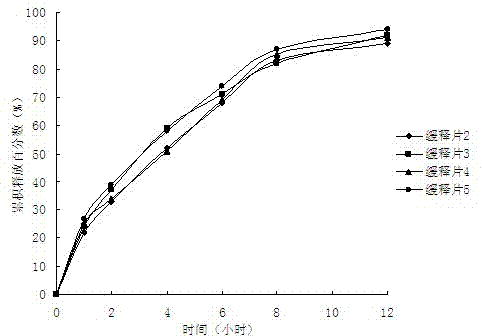
Epalrestat slow-release tablet and preparation method thereof
InactiveCN102440976ASimple processSimple and fast operationOrganic active ingredientsMetabolism disorderCelluloseOral medication
The invention provides an epalrestat slow-release tablet and a preparation method thereof. The slow-release tablet is prepared from the following components in parts by weight: 1-40 parts of epalrestat, 5-60 parts of slow-release material, 0-90 parts of filler, 0.1-2 parts of lubricant and right amount of adhesive. The slow-release material comprises hydroxypropyl methylcellulose or a mixture of hydroxypropyl methylcellulose and ethyl cellulose. The preparation method comprises the following steps: evenly mixing the epalrestat, slow-release material and filler according to the prescription amounts, adding the adhesive to obtain a soft material, granulating the soft material, drying, finishing, adding the lubricant, and tabletting. Compared with the conventional oral epalrestat common preparation, the epalrestat slow-release tablet provided by the invention can smoothly and slowly release the medicines after being orally dosed, and can maintain therapeutic action for a longer time.
Owner:NANJING HAILING TRADITIONAL CHINESE MEDICINE RES CO LTD +1
Refining method of high-purity epalrestat
The invention provides a refining method of high-purity epalrestat. The refining method comprises the following steps of: adding the epalrestat into a good solvent, keeping out of light at the temperature of 110 DEG C, stirring, refluxing, and dissolving; after dissolving, adding a bad solvent into the mixture, maintaining the temperature, standing for crystallization, carrying out suction filtration, cleaning a filter cake for one time by using the bad solvent, drying at the temperature of 60 DEG C and obtaining a finished epalrestat product. The structure of the epalrestat is as shown in the specification. Compared with the prior art, the refining method provided by the invention has the advantages that the used refining solvent is low in cost and easy to obtain, the reaction condition is mild, the production cost is low, the pollution is less, the operation is simple, heat filtration is not needed, only once recrystallization is needed, the purity and the yield of the obtained finished epalrestat product are high, the quality of the product is improved, and the suitability for massive industrial production is achieved.
Owner:KANGYA OF NINGXIA PHARMA
Epalrestat sustained release preparation and preparation method thereof
InactiveCN112137990AAvoid peaks and valleys in blood concentrationImprove complianceOrganic active ingredientsNervous disorderSustained release pelletsDrug utilisation
The invention belongs to the field of pharmaceutical preparations, and provides an epalrestat sustained release preparation and a preparation method thereof. The epalrestat sustained release preparation is composed of 64%-92% of drug-containing sustained-release pellets, 1.0%-8.0% of a gastric-soluble isolation coating layer, 5.0%-20% of an enteric-soluble coating layer, and 2.0%-8.0% of a protective coating layer. The epalrestat sustained release preparation disclosed by the invention can effectively avoid the peak valley phenomenon of the blood concentration of a common preparation, reducesthe medicine taking frequency, reduces the occurrence rate of adverse reactions, and increases the compliance of a patient; and can avoid a possible drug burst release effect of a monobasic sustainedrelease preparation, reduces the toxicity and side effects of the drug, and further ensures the safety of clinical medication. The selected auxiliary materials are common, the preparation process is simple, the technical difficulty is low, the industrial mass production is easy to realize, and the market potential is large.
Owner:南京康川济医药科技有限公司
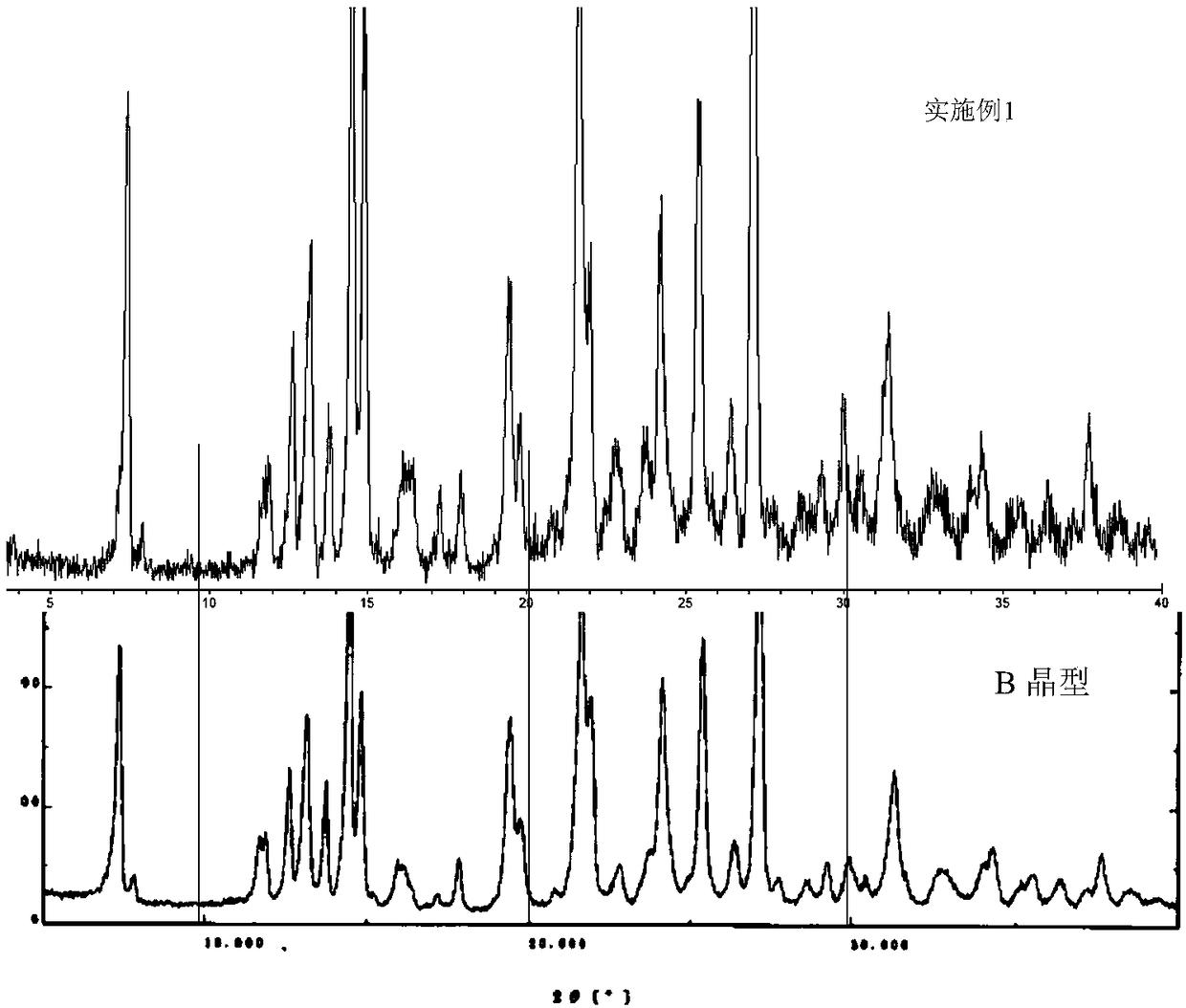
B crystal form epalrestat and preparation method thereof
The invention provides B crystal form epalrestat and a preparation method thereof. The method comprises the following steps: taking rhodanine acetic acid and alpha-methylcinnamaldehyde as raw materials and reacting to generate epalrestat crude product suspension; adding a first acid solution into the epalrestat crude product suspension; then heating and melting, carrying out centrifugal treatment,washing a filter cake and drying to obtain an epalrestat crude fine product, wherein the mole ratio of an acidic substance in the first acid solution to the rhodanine acetic acid is (3 to 4.5) to 1;adding low-carbon alcohol into the epalrestat crude fine product; after heating and melting, keeping for pre-set time; then cooling to 20 to 30 DEG C; carrying out the centrifugal treatment and dryinga filter cake to obtain an epalrestat fine product. According to the B crystal form epalrestat and the preparation method thereof, provided by the invention, the yield and purity of B crystal form epalrestat products are easy to improve and the content of 2Z-isomer impurities is reduced.
Owner:SHIJIAZHUANG NO 4 PHARMA
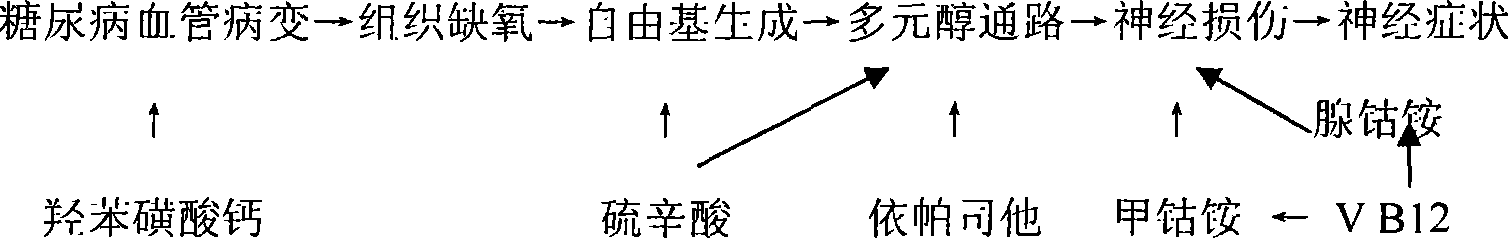
Medicinal composition for treating diabetes chronic complication and preparation method thereof
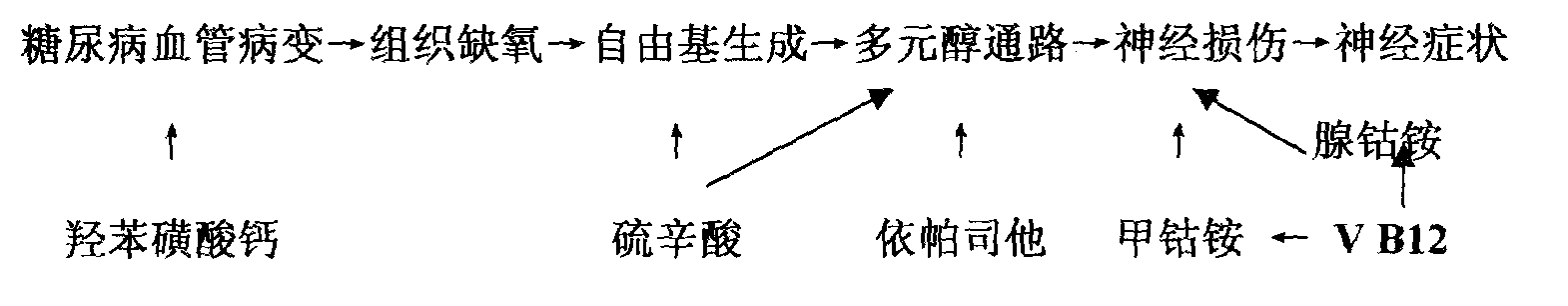
InactiveCN101214257AReduced mechanical pain thresholdImprove neurological symptomsNervous disorderMetabolism disorderAdditive ingredientVitamin B12
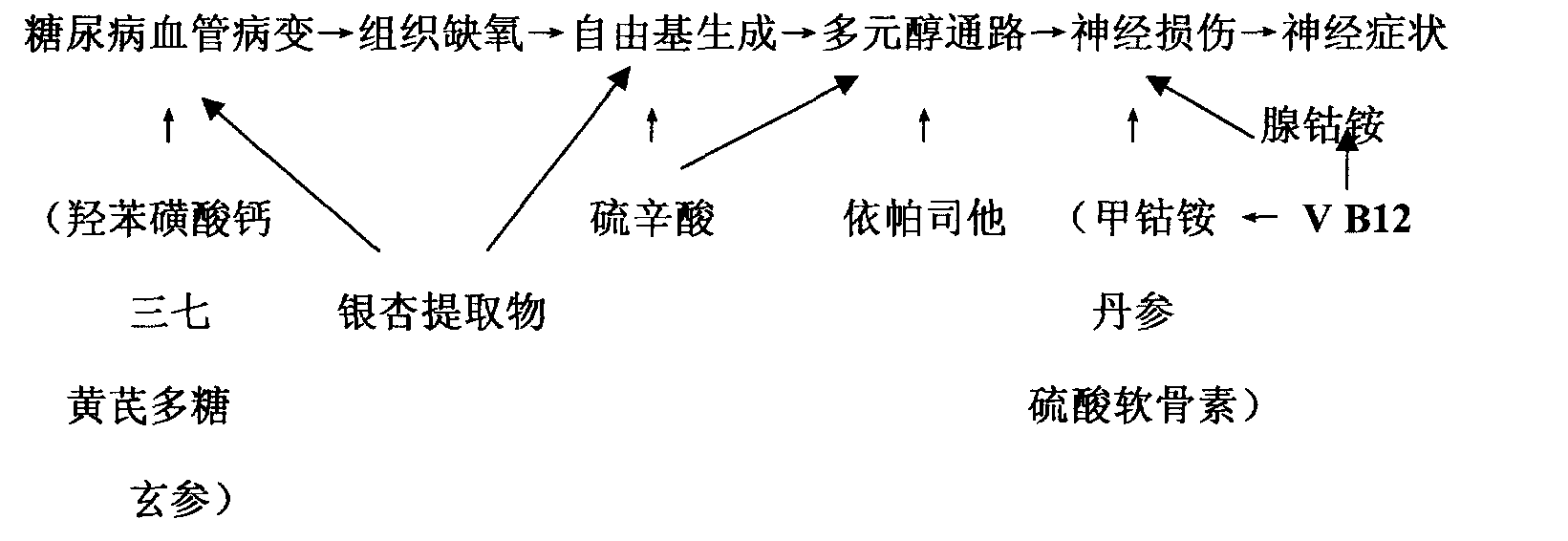
The present invention relates to a series of medicine combinations for remedying diabetic chronic complication and a preparation method thereof, which are compound oral preparations (comprising troche, dispersible tablet, effervescent tablet, capsule preparation, soft capsule and granule preparation) which are mainly made from at least two active components of mecobalamin, cobamamide, vitamin B12, epalrestat, lipoic acid and calcium dobesilate and pharmic acceptable auxiliary materials. The preferential combinations of the present invention are the mecobalamin plus the lipoic acid, the mecobalamin and the lipoic acid plus the calcium dobesilate, the mecobalamin and the epalrestat plus the calcium dobesilate, the mecobalamin, the lipoic acid and the epalrestat plus the calcium dobesilate, and the mecobalamin and the vitamin B12 plus cobamamide. The present invention is mainly used for remedying the nervous lesion of the diabetic chronic complication.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Epalrestat dropping pills and preparation thereof
InactiveCN101239046ATake effect quicklyImprove bioavailabilityOrganic active ingredientsMetabolism disorderAntioxidantPolyethylene glycol
The invention discloses an Epalrestat pill, which is characterized in that the invention comprises an Epalrestat, a Carmowax carrier, an antioxidant and a surfactant. The pill has favorable stability and high bioavailability.
Owner:HAINAN JINXING PHARMA
Epalrestat double-layered osmotic pump controlled release tablet and preparation method thereof
ActiveCN110548014ASmall toxicityAvoid peaks and valleys in blood concentrationOrganic active ingredientsNervous disorderSide effectDrug release
The invention provides an epalrestat double-layered osmotic pump controlled release tablet and a preparation method thereof. An osmotic pump comprises a drug-containing layer, a boosting layer and a coating film; the drug-containing layer comprises the following components in percentages by weight: 30%-40% of epalrestat, 30%-50% of a swelling agent, 1%-10% of a pH regulator, 5%-15% of a solubilizer and 1%-3% of a lubricant; the boosting layer comprises the following components in percentages by weight: 60%-75% of a swelling agent, 5%-30% of an osmotically active substance and 1%-3% of a lubricant; and the semi-permeable coating film for 100 tablets comprises the following components: 5 g-15 g of a semi-permeable polymer material and 0.5 g-3 g of a water-soluble pore-forming agent. By the epalrestat double-layered osmotic pump controlled release tablet, drugs can be released in vivo at a constant speed, and the drug release behavior is not influenced by factors such as the pH value of amedium environment, enzyme, gastrointestinal peristalsis and food, so that the stability of blood concentration can be maintained, the toxic and side effects of the drugs can be relieved, the administration times can be reduced, and the compliance of patients can be improved.
Owner:南京康川济医药科技有限公司
Chemical medicine and natural medicine composition for treating diabetes chronic complication and preparation method thereof
The invention relates to a series of combinations of chemical drug and natural drug used for treating diabetic chronic complications and the preparation method thereof, which are the combinations formed by one or a plurality of chemical drugs and one or a plurality of natural drugs, wherein, the chemical drug is any of mecobalamin, cobamamide, vitamin B12, epalrestat, lipoic acid and calcium dobesilate, and the natural drug is any of chondroitin sulfate, ginkgo biloba extract, astragalus polysaccharide, salvia miltiorrhiza, panax notoginseng and scrophularia. The preferred combination is: mecobalamin + lipoic acid + ginkgo biloba extract, mecobalamin + lipoic acid + ginkgo biloba extract + astragalus polysaccharide + salvia miltiorrhiza + panax notoginseng + scrophularia, mecobalamin + lipoic acid + calcium dobesilate + ginkgo biloba extract, mecobalamin + lipoic acid + chondroitin sulfate+ epalrestat + calcium dobesilate + ginkgo biloba extract, mecobalamin + lipoic acid + chondroitin sulfate + epalrestat + calcium dobesilate + ginkgo biloba extract + astragalus polysaccharide + salvia miltiorrhiza + panax notoginseng + scrophularia. The combinations are used for treating the microvessel and nerve lesions of diabetic chronic complications.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
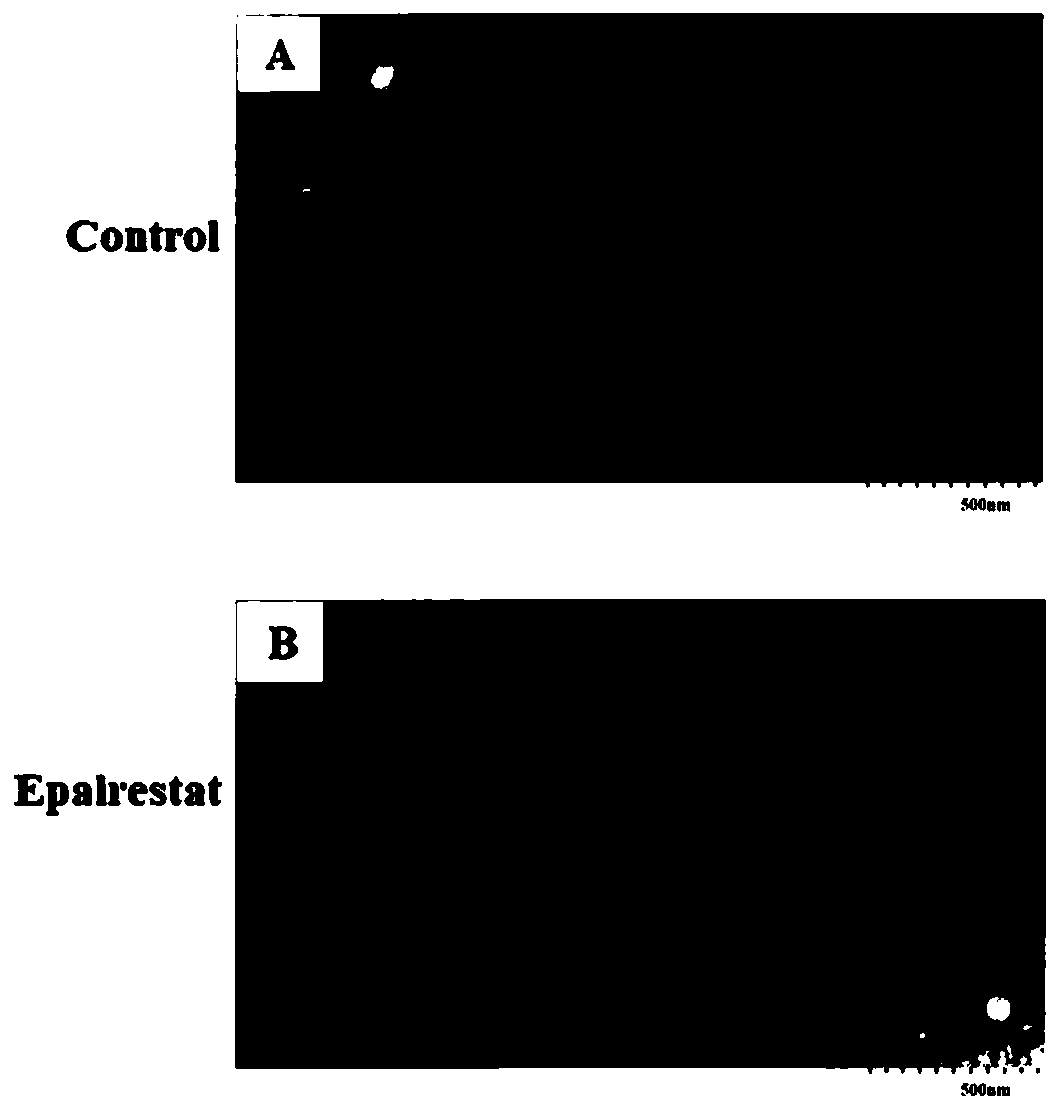
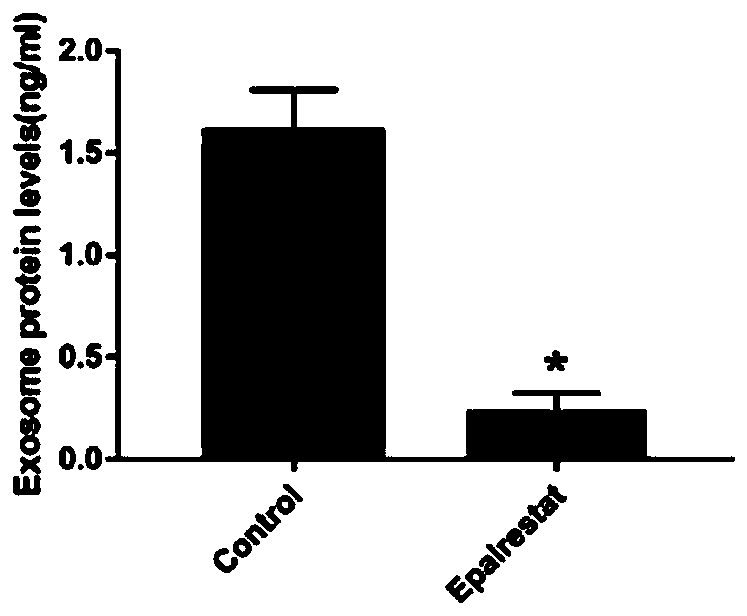
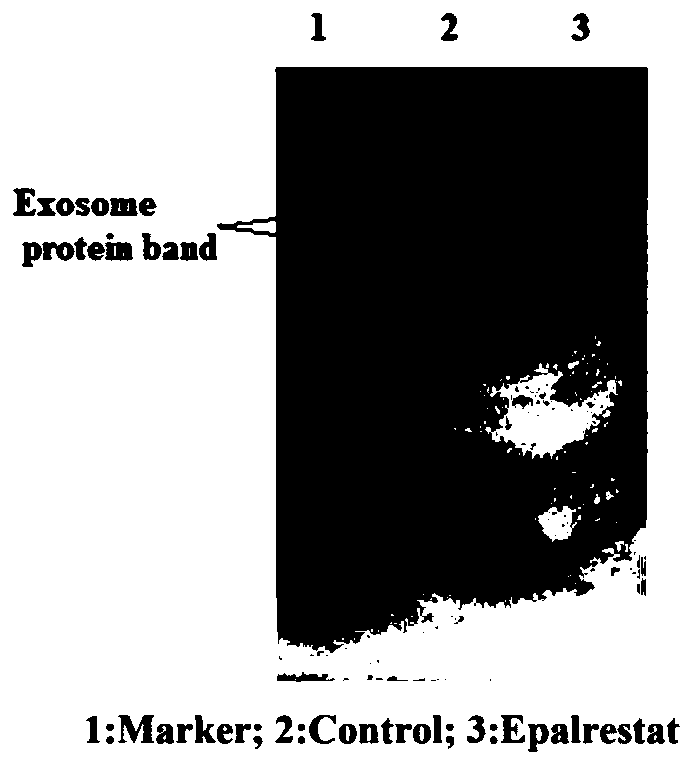
Application of epalrestat in preparation of pancreatic cancer drugs and method for verifying inhibiting effect on exosome secretion of pancreatic cancer cells
InactiveCN110051668AGreat application potentialInhibition of secretionOrganic active ingredientsMaterial analysis using wave/particle radiationPancreas CancersTumor therapy
The invention provides an application of epalrestat in preparation of pancreatic cancer drugs. The pancreatic cancer drugs are used for inhibiting exosome secretion of pancreatic cancer cells. A pharmaceutical composition for treating pancreatic cancer contians epalrestat. The pharmaceutical composition is used for inhibiting exosome secretion of pancreatic cancer cells. The embodiment of the invention also provides a method for verifying the inhibition effect of epalrestat on exosome secretion by pancreatic cancer cells. The method comprises the following steps: extracting cell supernatant exosomes by using a low-temperature ultracentrifugation method; lysing the collected exosomes, using a BCA kit to quantify protein, and using the measured amount of the protein to reflect the amount ofthe exosomes; using a transmission electron microscope to verify a double-layer lipid membrane wrapped cup-shaped structure of the exosomes; detecting exosome protein concentration by protein polyacrylamide gel electrophoresis with Coomassie brilliant blue. The invention provides a novel application of epalrestat, namely the inhibition of exosome secretion. The epalrestat has great application potential in clinical tumor treatment.
Owner:AFFILIATED HOSPITAL OF NANTONG UNIV
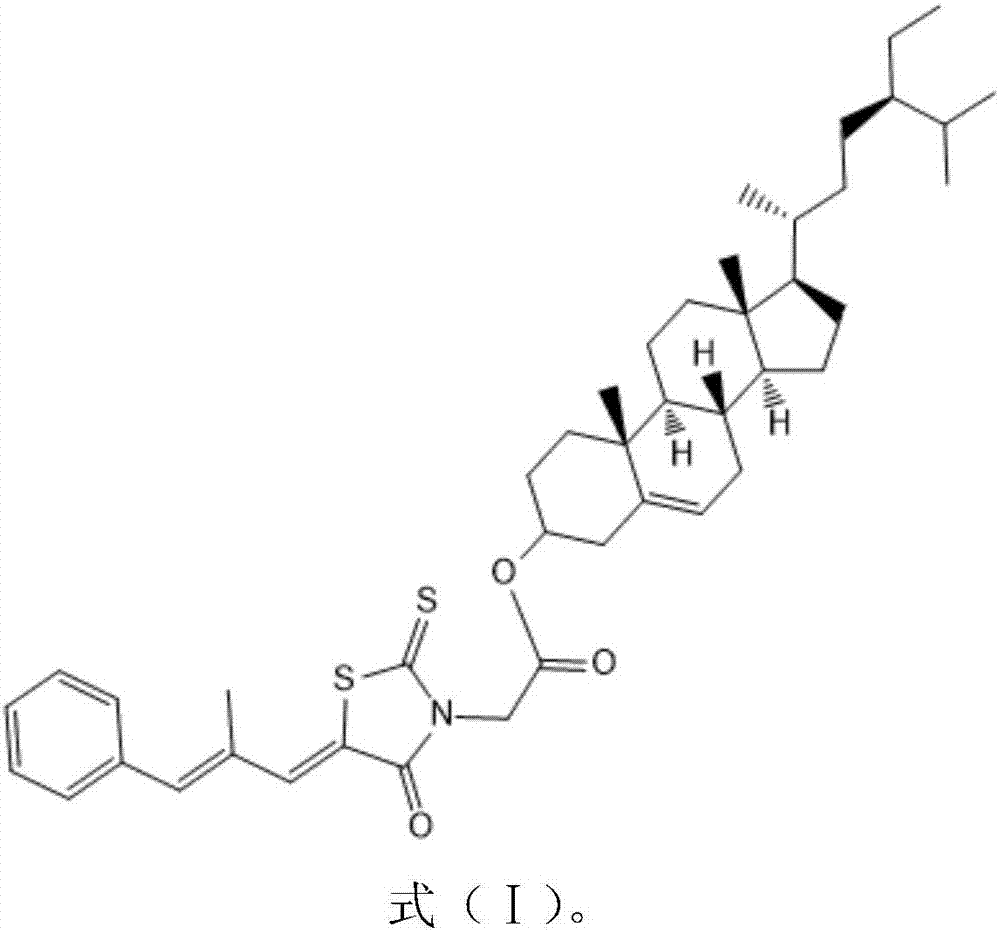
Beta-sitosterol and epalrestat conjugate, preparation method and application of conjugate
InactiveCN107383147AEnhanced inhibitory effectOrganic active ingredientsAntipyreticCancer cellApoptosis
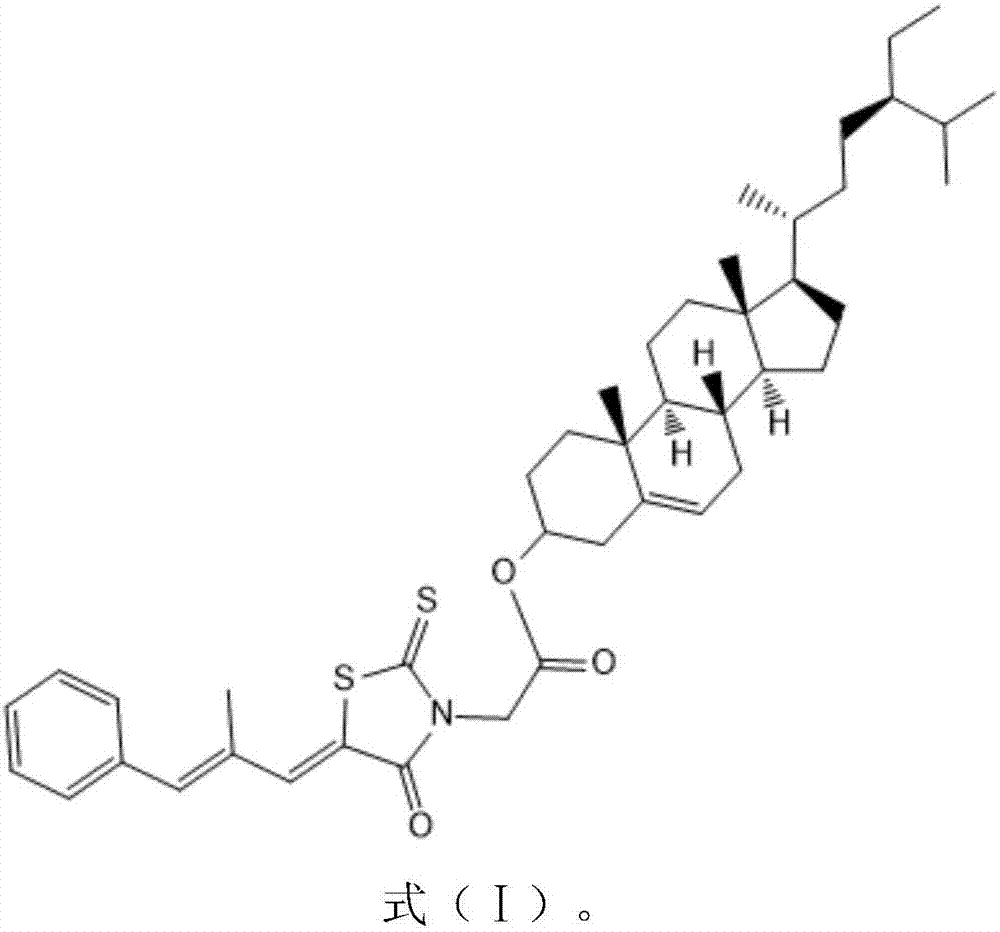
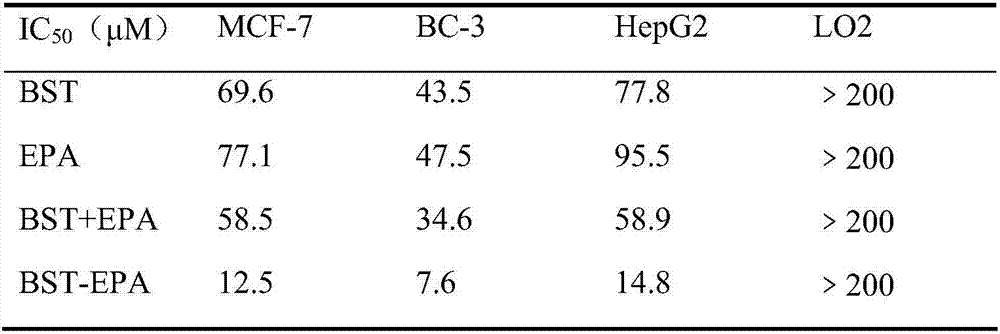
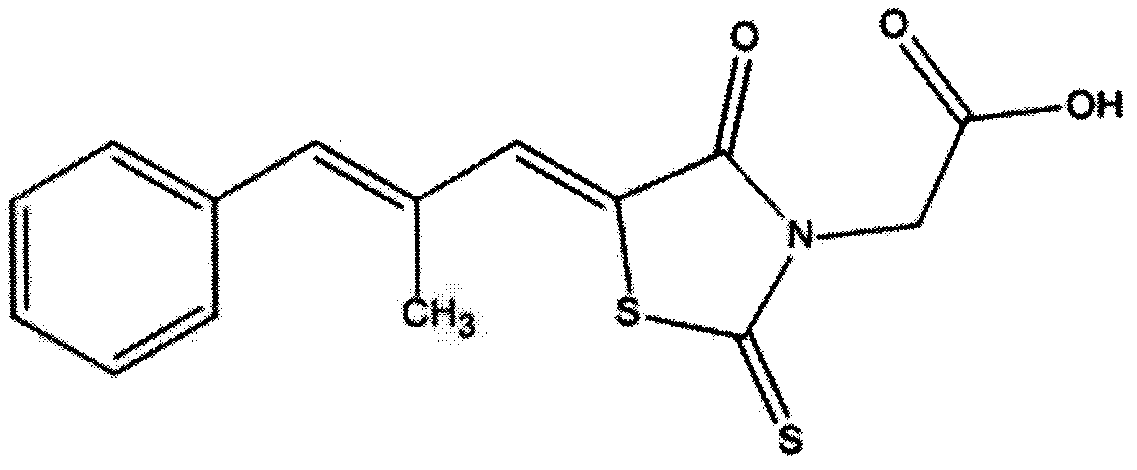
The invention belongs to the field of natural medicines and discloses a beta-sitosterol and epalrestat conjugate, a preparation method and application of the conjugate. The conjugate is formed by coupling beta-sitosterol with epalrestat. The structure is shown as formula (I) described in the specification. The preparation method comprises the following steps: dissolving beta-sitosterol and epalrestat in anhydrous dichloromethane, reacting under room temperature overnight under the catalytic effect of pyridine and 4-dimethylamino pyridine, filtering, pouring onto a silica gel chromatographic column, performing rotary steaming and drying, thereby acquiring a target compound. An in vitro MTT detection experiment proves that the beta-sitosterol and epalrestat conjugate has an excellent restraining effect on tumor cell lines, has an IC50 value being 3-6 times that of beta-sitosterol but has low toxicity to normal liver cells L-O2. The compound is used for treating tumor in the manner of conjugating two drug molecules into prodrug, the problems of insufficient antitumor capacity of beta-sitosterol and synergic targeted inhibition for cancer cell aldose reductase target are solved and the effect of clearing away tumor cells in the manner of enhancing cell apoptosis can be achieved.
Owner:GUANGDONG FOOD & DRUG VOCATIONAL COLLEGE
Application of epalrestat in preparation of drug for treating diabetic nephropathy
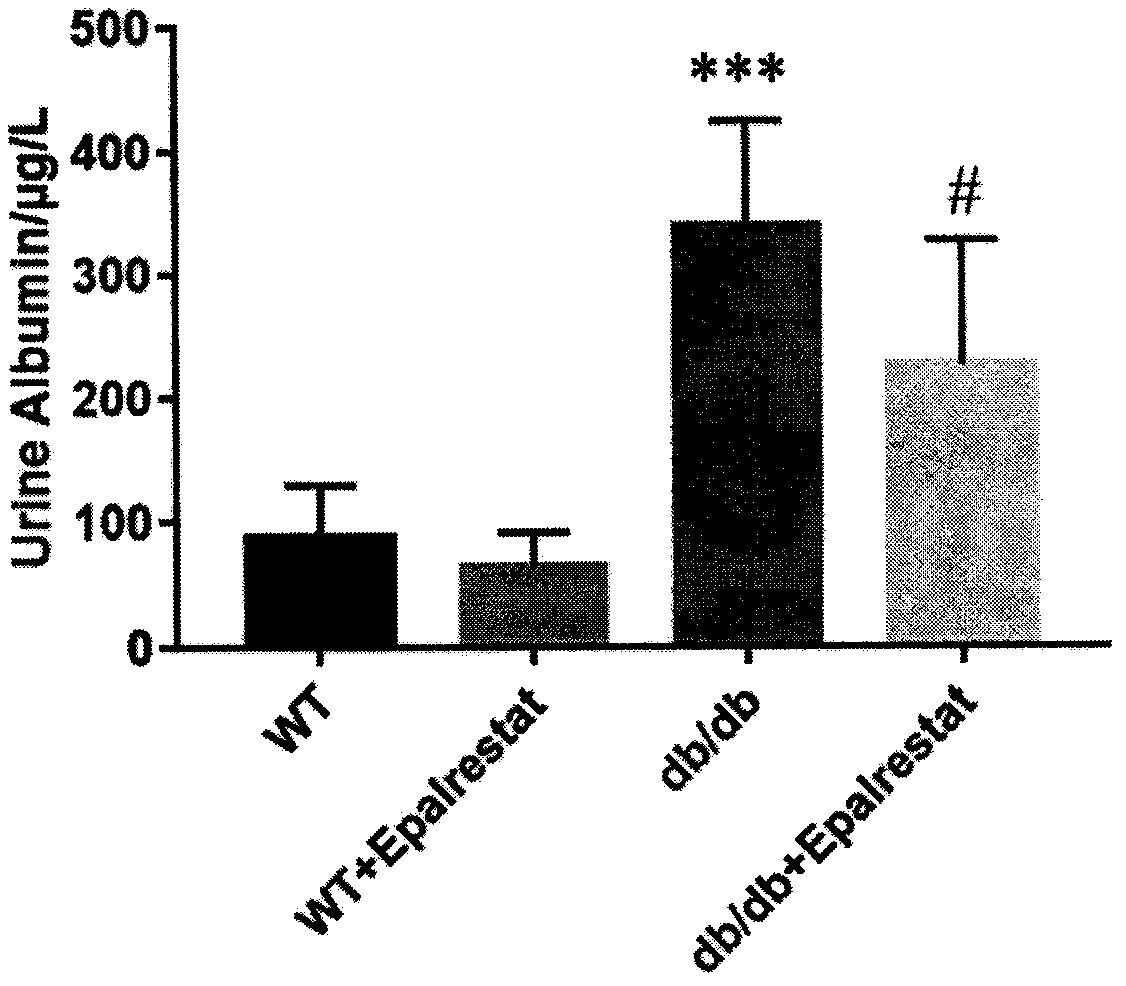
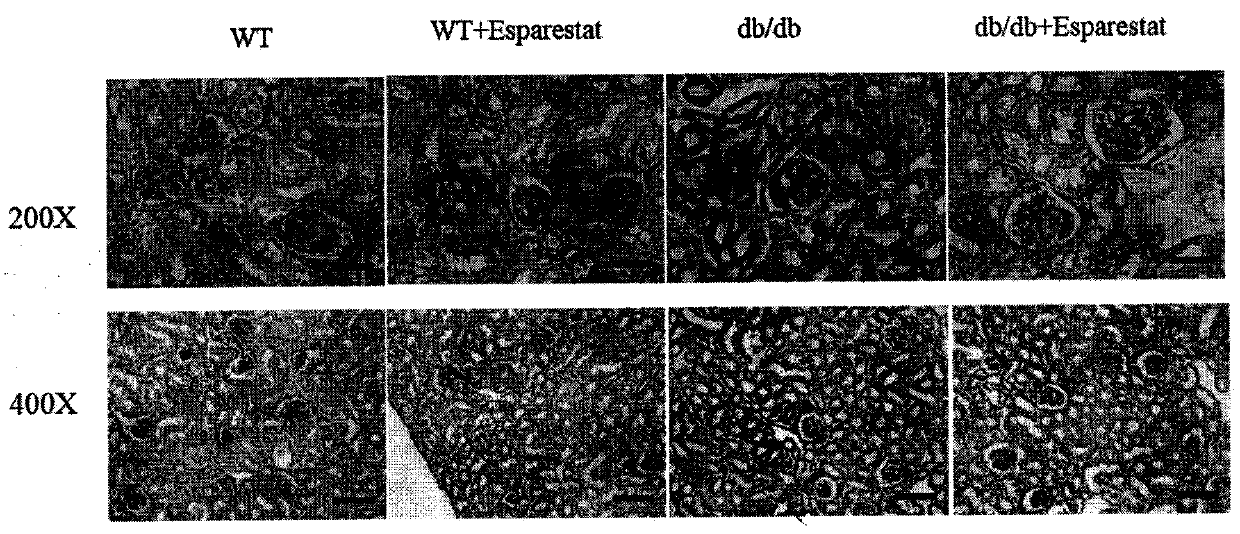
The invention discloses an application of epalrestat in the preparation of a drug for treating diabetic nephropathy, relating o the technical field of biological medicine. The epalrestat is an only on-sale aldose reductase inhibitor in China and is administered to a db / db mouse through gavage, so that the urinary albumin excretion of the db / db mouse is remarkably reduced, and the pathological injury of the kidney is improved. The orally administered epalrestat disclosed by the invention has a good kidney protection effect, and a candidate drug is provided for the kidney protection of crowds with clinic diabetic nephropathy.
Owner:CHINA PHARM UNIV
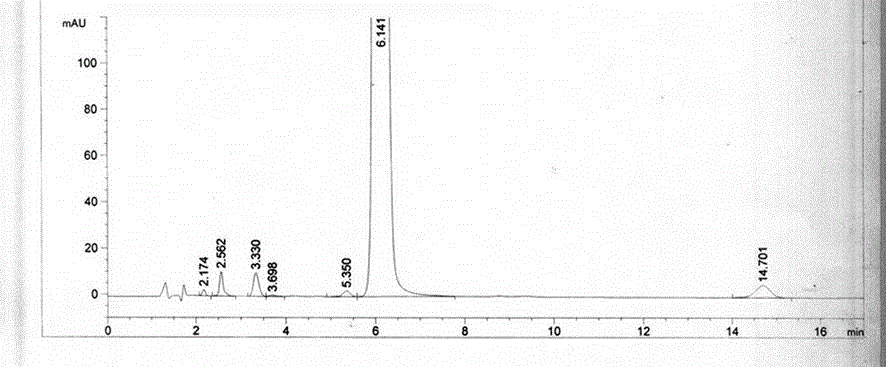
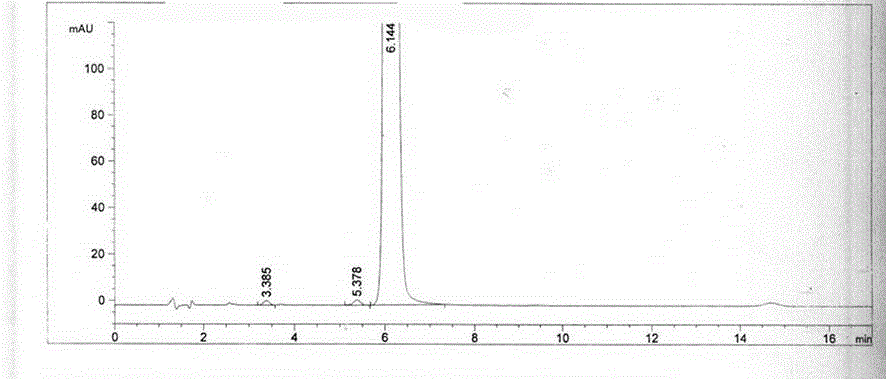
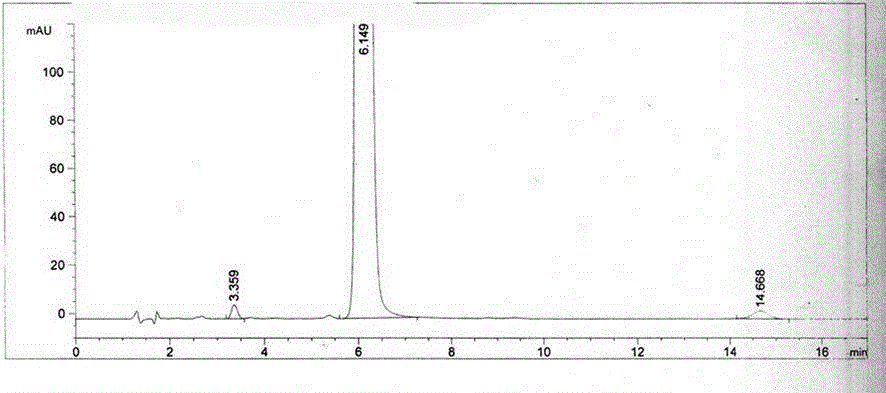
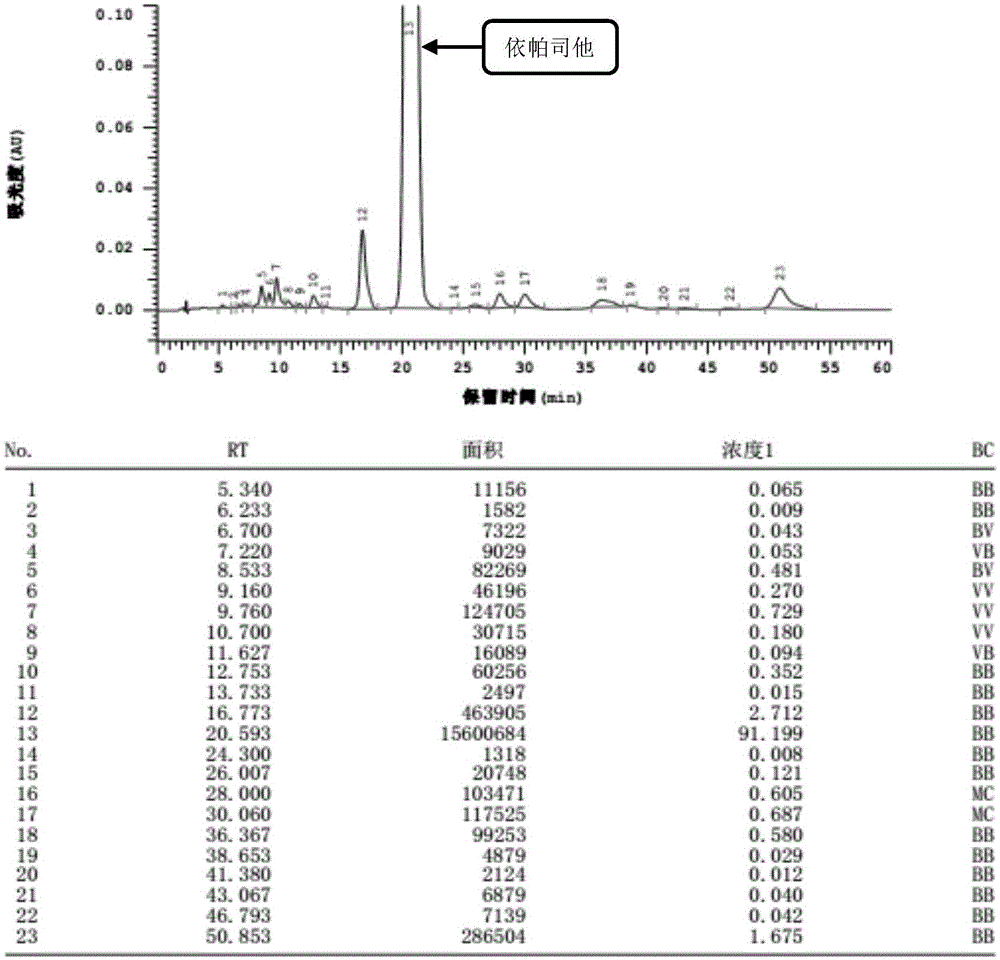
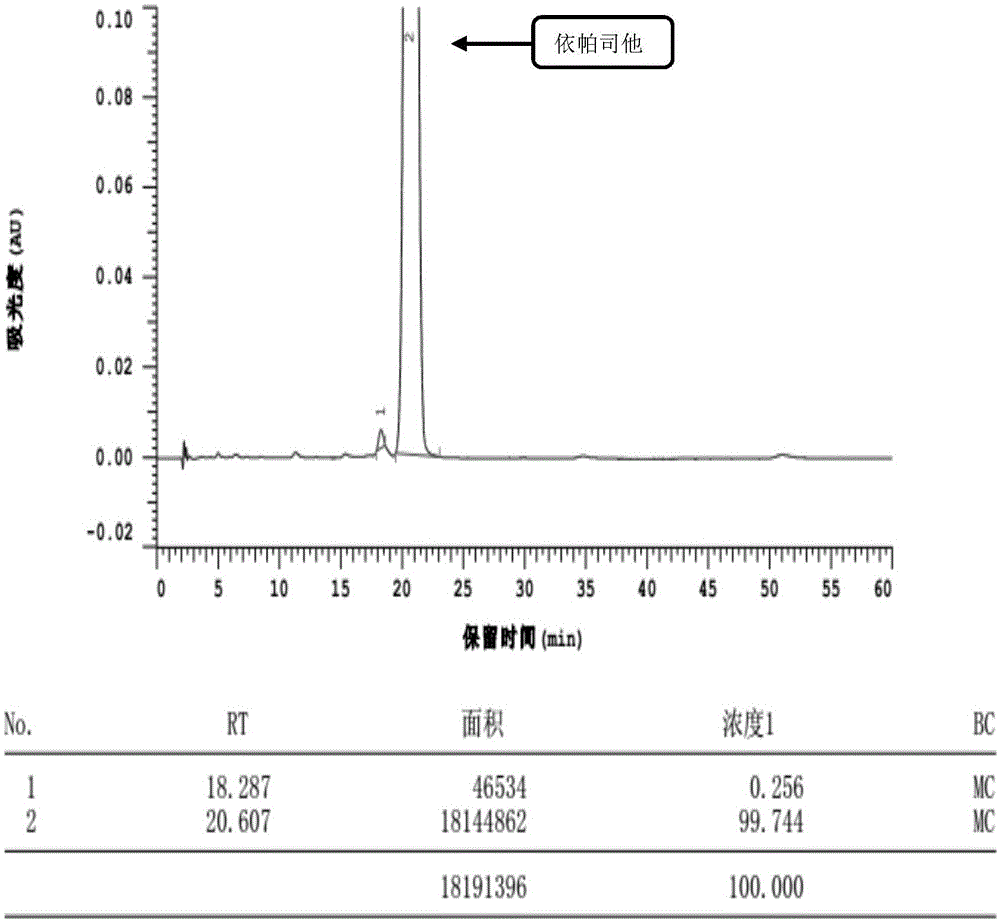
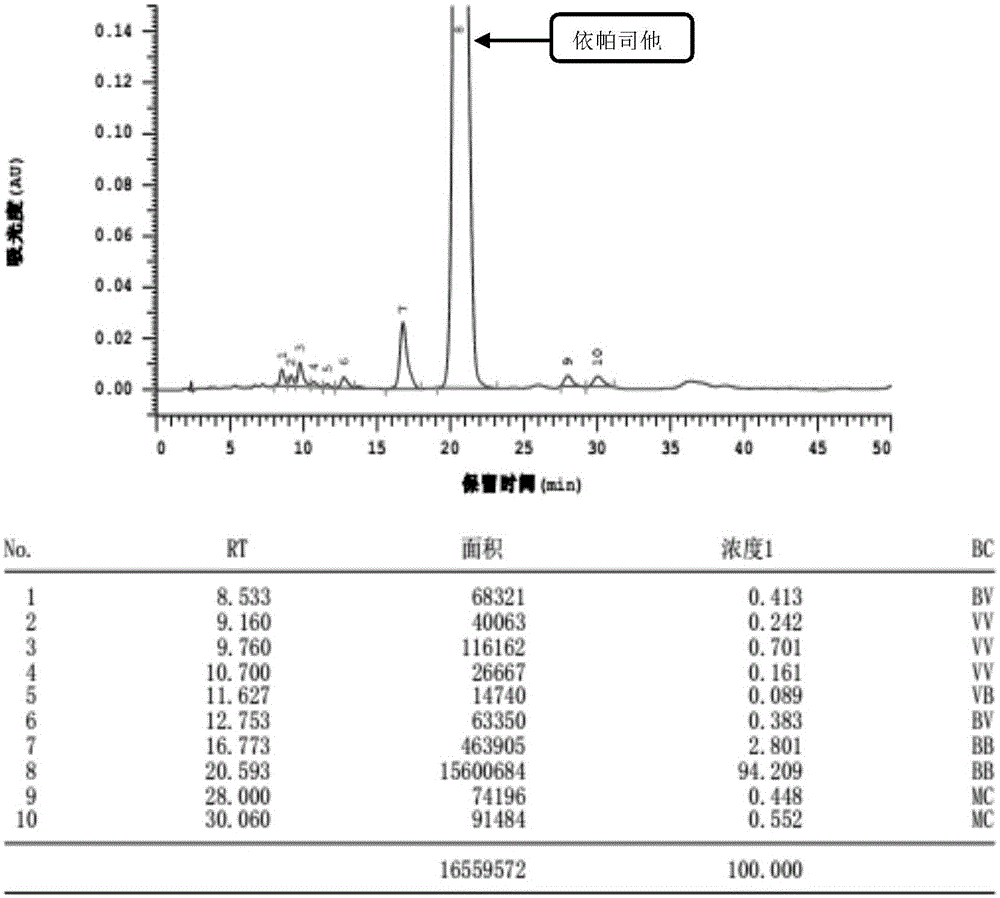
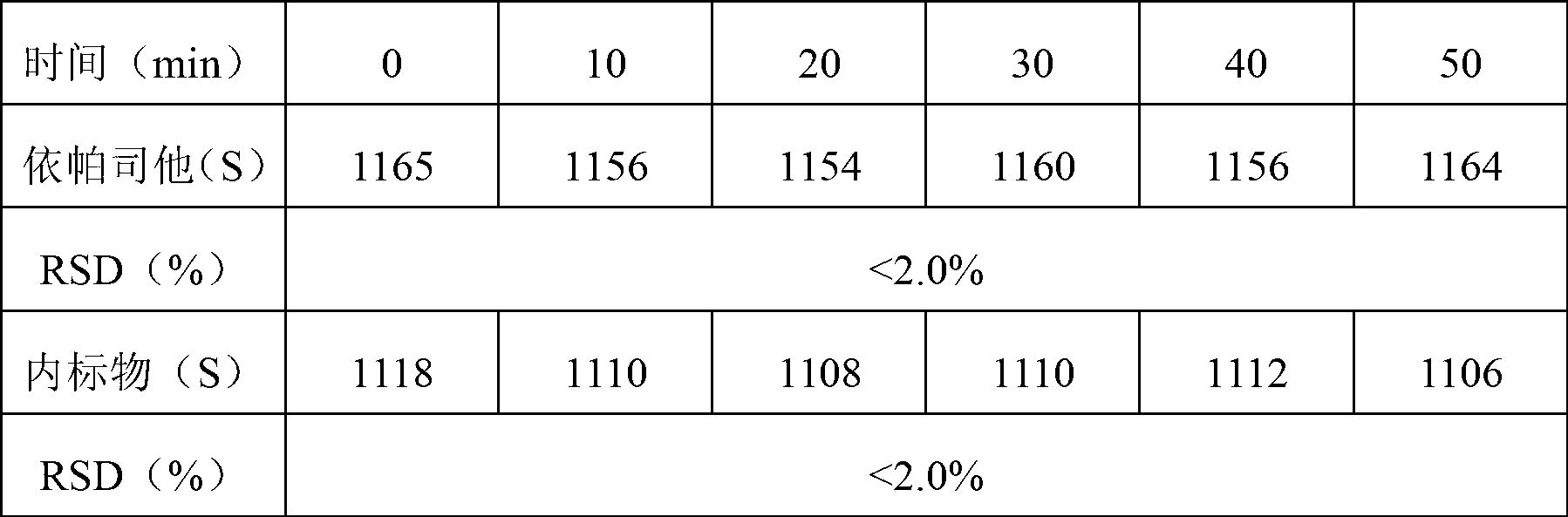
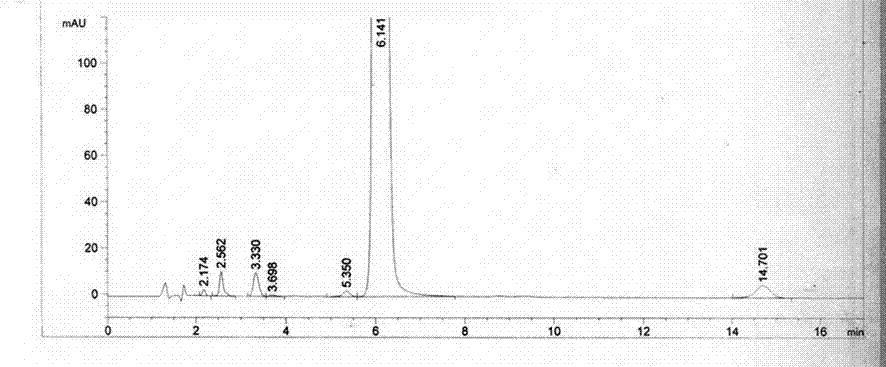
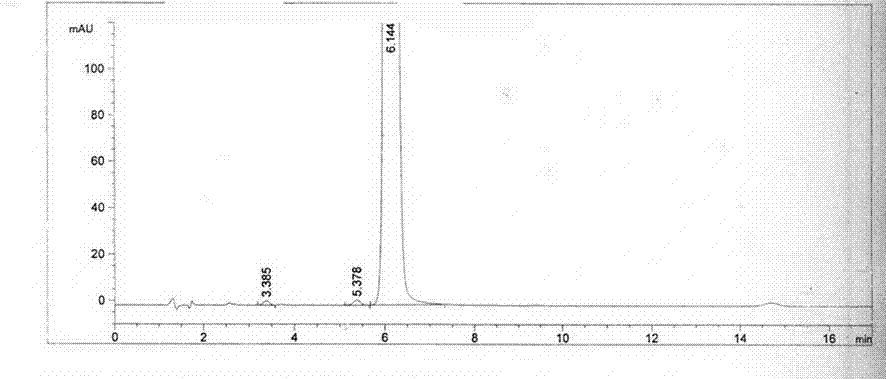
Content detecting and control method of epalrestat tablets
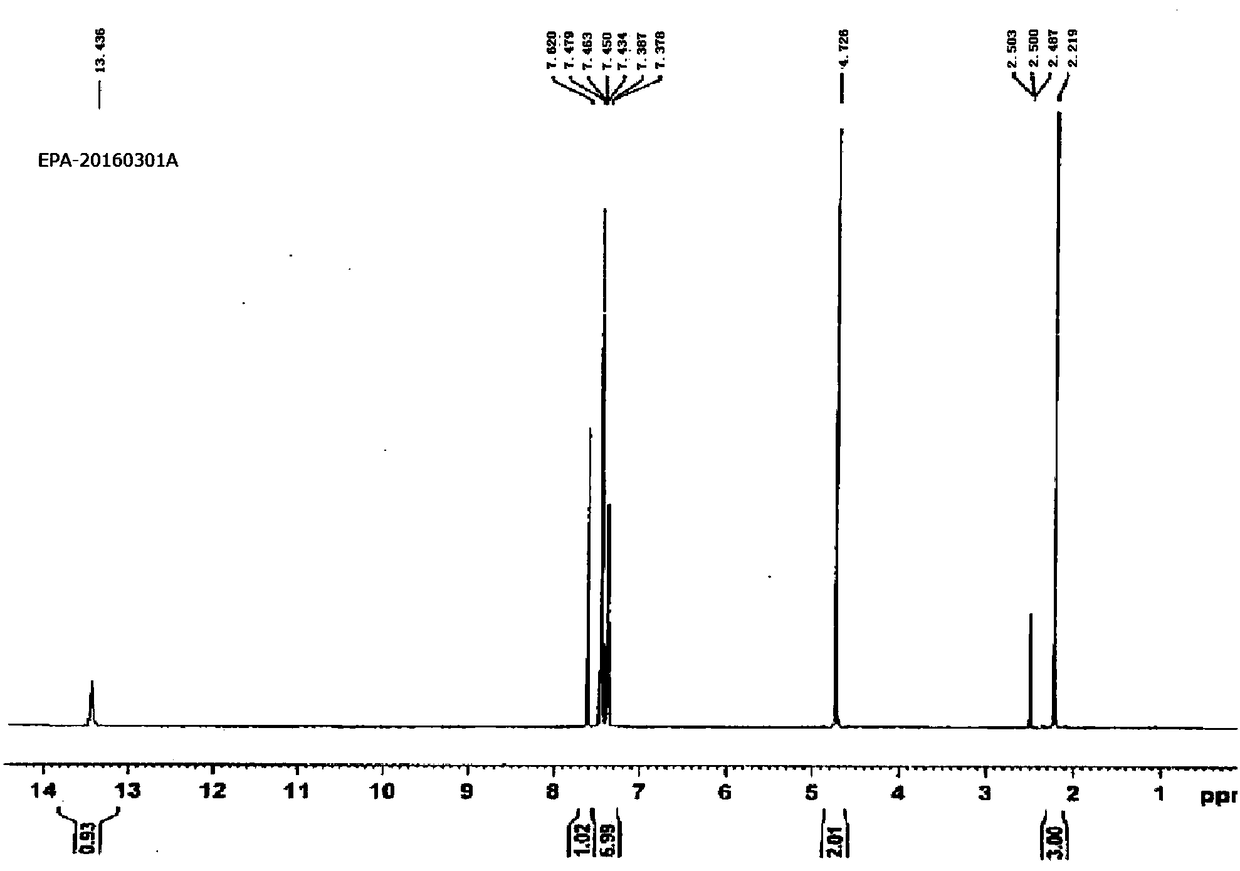
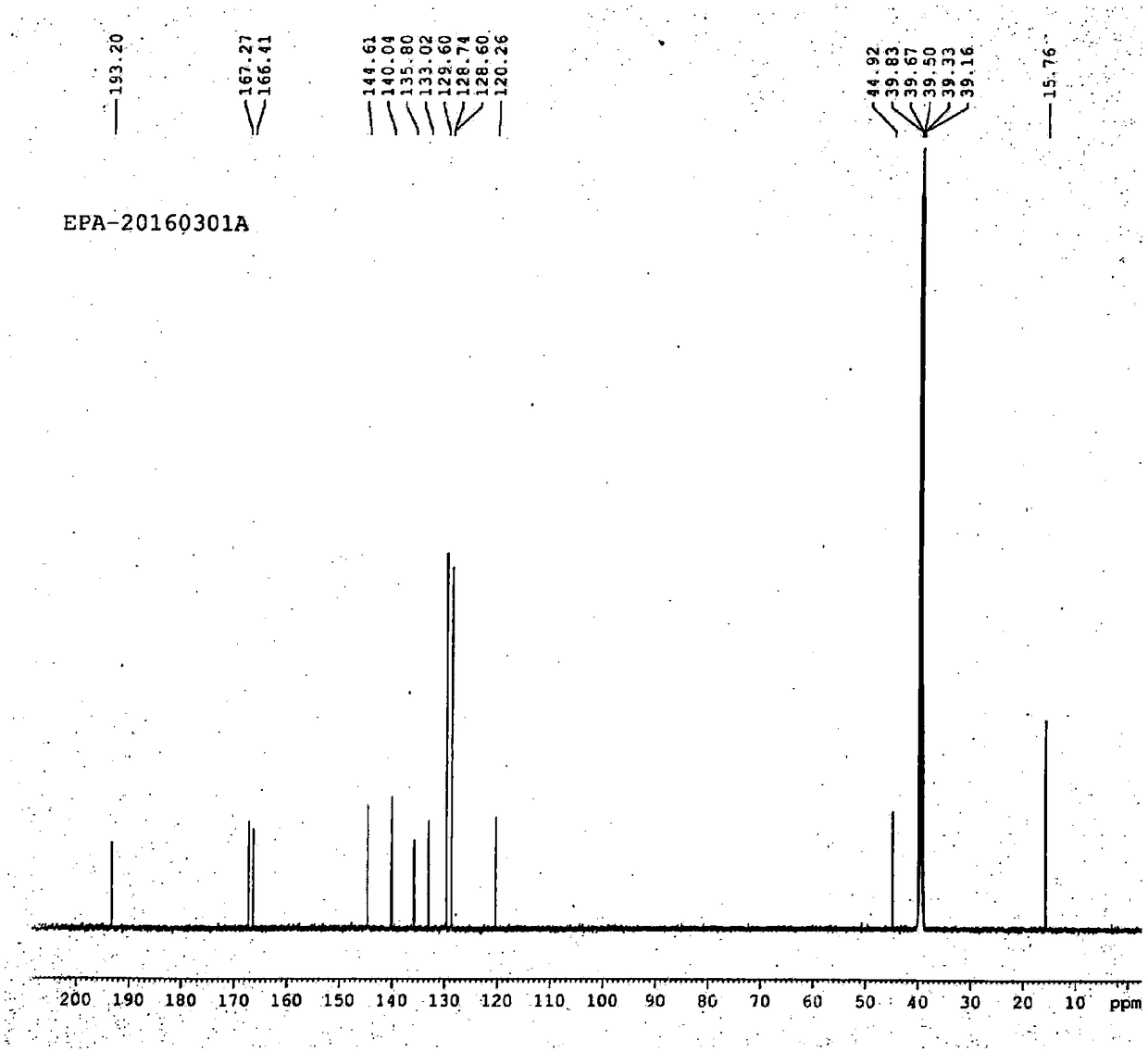
The invention discloses a content detecting and control method of epalrestat tablets, according to the method, high-performance liquid chromatography is used for detecting the epalrestat tablets. Analytical conditions such as an optimal internal standard compound, mobile phase composition, an elution program, elution flow velocity, a detecting wavelength and a chromatographic column are optimized through a large number of experiments. Many times of experimental verification indicates that the content detecting and control method of the epalrestat tablets is good in stability and repeatability, high in analysis efficiency, good in resolution and capable of detecting epalrestat sensitively and accurately in a qualitative and quantitative mode, so that the quality of the epalrestat tablets can be evaluated objectively, comprehensively and accurately, and the content detecting and control method of the epalrestat tablets is of great significance in controlling of the quality of the epalrestat tablets and ensuring of clinical effects.
Owner:YANGTZE RIVER PHARM GRP NANJING HAILING PHARM CO LTD
Novel choline cocrystal of epalrestat
The invention relates to a novel choline cocrystal of 5-[(1Z,2E)-2-methyl-3-phenylpropenylidene]-4-oxo-2-thioxo-3-thiazolidineacetic acid. The preparation and characterization of the novel choline cocrystal according to various embodiments of the invention is described. The invention also relates to pharmaceutical compositions containing the novel choline cocrystal and the therapeutic use of the novel choline cocrystal to treat and / or prevent various conditions, including treating and / or preventing diabetic complications, treating and / or preventing homocystinuria reducing levels of homocysteine in blood serum, inhibiting aldose reductase, and affording cardioprotection in non-diabetic patients.
Owner:BIONEVIA PHARMACEUTICALS INC
Preparation method of epalrestat
The invention relates to the technical field of medicines, in particular to a preparation method of epalrestat. The preparation method provided by the invention comprises the steps of mixing 3-carboxymethyl rhodanine, alpha-methylcinnamyl aldehyde, a catalyst and water, and sequentially carrying out condensation reaction and acid neutralization to obtain an epalrestat crude product, wherein the catalyst comprises a basic catalyst and a phase transfer catalyst; and mixing the epalrestat crude product with an alcohol organic solvent, and recrystallizing to obtain the epalrestat. The preparation method adopts water as a reaction solvent, and is safe and environment-friendly; the existing three procedures such as crude product preparation, acidification dissociation and recrystallization are simplified into two procedures such as crude product preparation and recrystallization, and the two procedures such as crude product preparation and acidification dissociation are combined into one, so that the procedures are simplified; and an alcohol organic solvent is adopted for recrystallization, so that recycling is facilitated.
Owner:SHANDONG DYNE MARINE BIOTECHCAL PHARM HLDG CO LTD +1
Diabetes treatment pharmaceutical composite and preparation method thereof
ActiveCN105998012AHigh average dissolution rateQuality improvementOrganic active ingredientsNervous disorderActive agentPlasticizer
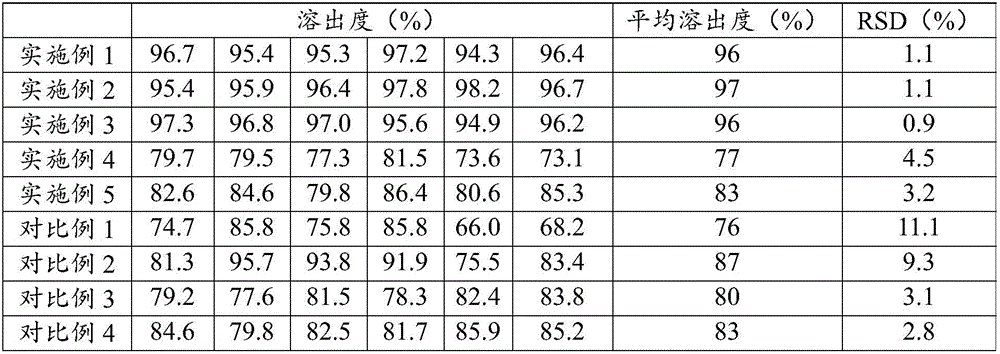
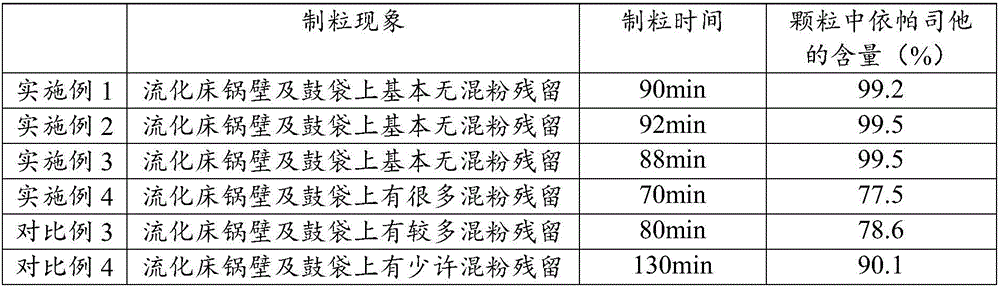
The invention relates to an epalrestat tablet and a preparation method and application thereof. The epalrestat medicine composite comprises the following raw materials and auxiliary materials: 30-70 parts by weight of epalrestat, 30-70 parts by weight of filler, 5-25 parts by weight of binder, 1-10 parts by weight of surfactant, 1-10 parts by weight of disintegrant, 0.1-5 parts by weight of lubricant, 1-30 parts by weight of film-forming material, 1-10 parts by weight of sunscreen and 1-3 parts by weight of plasticizer. The epalrestat tablet prepared from the preparation method has a high average dissolution rate (more than 95%), the inter-tablet difference of the dissolution rate is small (RSD (Relative Standard Deviation) is smaller than 3%), and quality is obviously improved. The preparation method obviously reduces mixed powder residues on a fluidized bed pot wall and a drum bag, improves the contents and the yield of the epalrestat in particles, improves the average dissolution rate of the prepared epalrestat tablet and lowers the inter-tablet difference of the dissolution rate.
Owner:SUZHOU CHUNGHWA CHEM & PHARMA IND
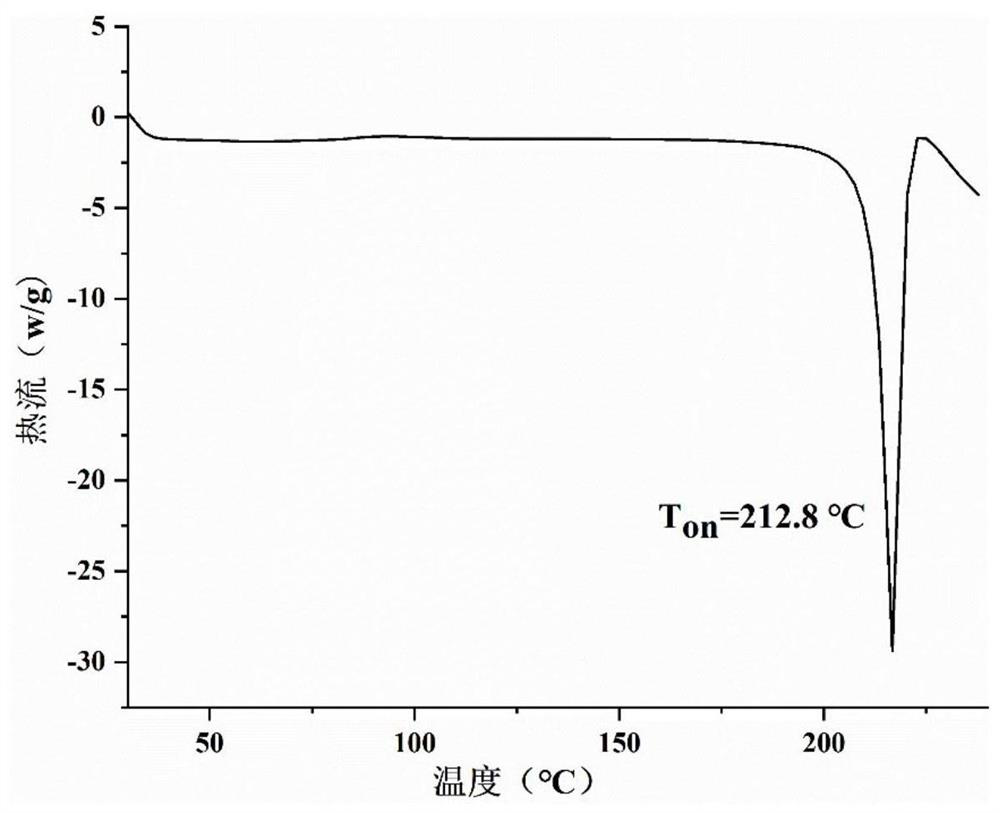
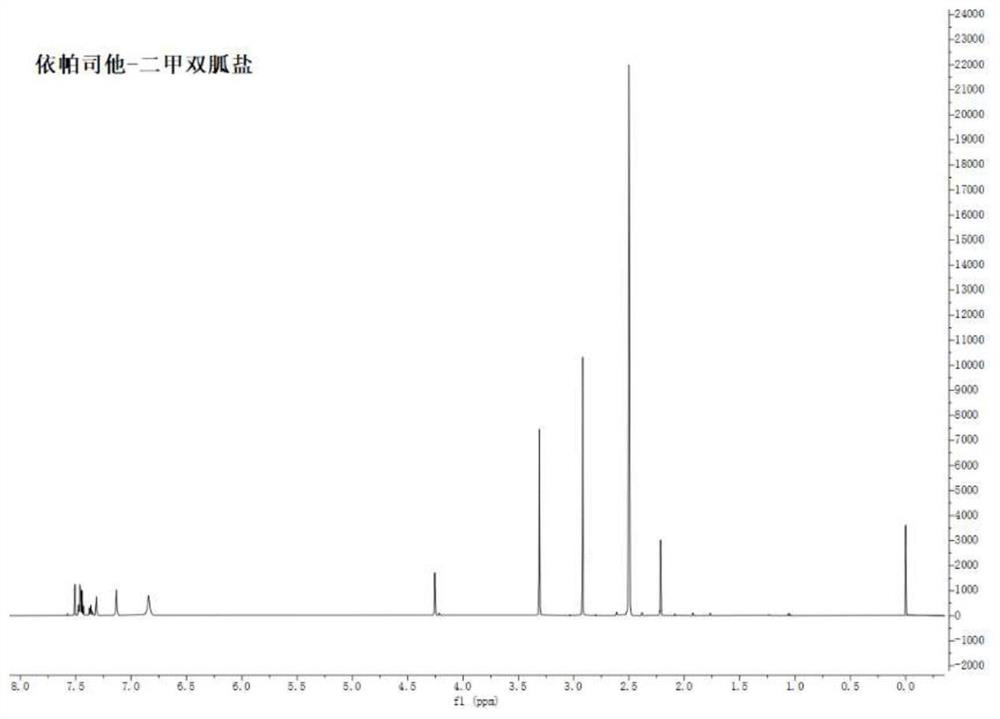
Epalrestat-metformin salt hydrate as well as preparation method and application thereof
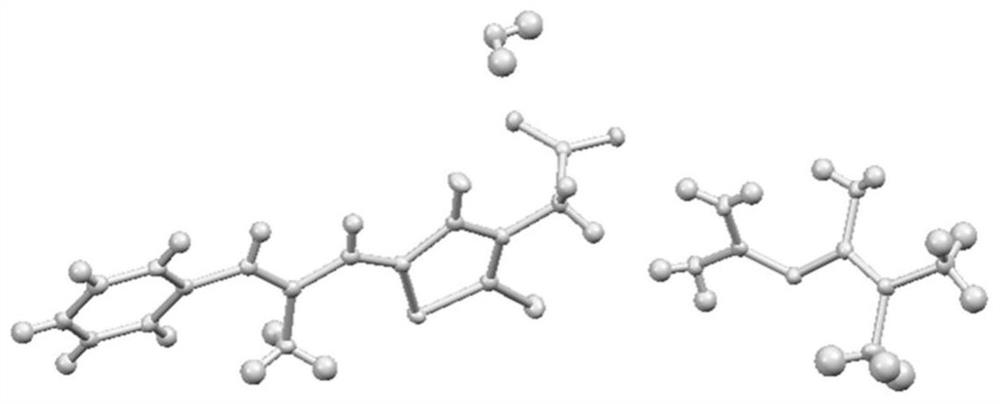
ActiveCN113277962AReduce humidityGood reproducibilityMetabolism disorderOrganic compound preparationSolubilityMedicine
The invention relates to an epalrestat-metformin salt hydrate crystal form and a preparation method thereof. The molecular formula of the hydrate is C19H26N6O4S2, and the molecular weight is 466.6. The crystallographic characteristics of the hydrate are as follows: the bond length a is 7.8711 (4), the bond length b is 8.3789 (4), the bond length c is 18.3489 (8), the bond angle alpha is 77.437 (4), the bond angle beta is 82.244 (4), the bond angle gamma is 66.896 (4), and V is 1084.7 (6). Compared with epalrestat, the epalrestat-metformin salt hydrate provided by the invention has the advantages that the dissolution rate and solubility are greatly improved, the problem of high hygroscopicity of metformin at the present stage is well solved, meanwhile, the preparation method is simple to operate, the crystallization process is easy to control, and the epalrestat-metformin salt hydrate is suitable for industrialization.
Owner:TIANJIN UNIV
Compound sustained-release tablet of epalrestat and sitagliptin or pharmaceutically acceptable salt thereof and preparation method thereof
ActiveCN113925838AExtend the time of synergyReduce releaseOrganic active ingredientsNervous disorderProlonged-release tabletPatient compliance
The invention belongs to the field of pharmaceutical preparations, and particularly relates to a compound sustained-release tablet of epalrestat and sitagliptin or pharmaceutically acceptable salt thereof and a preparation method thereof. The compound sustained-release tablet contains epalrestat, sitagliptin or pharmaceutically acceptable salt thereof and / or hydrate of the salt, anhydrous calcium hydrophosphate, a framework sustained-release material and other pharmaceutical excipients. The compound sustained-release tablet can be a single-layer tablet or a double-layer tablet, and is used for treating type 2 diabetes, especially peripheral neuropathy caused by the type 2 diabetes. The compound composition is prepared into the sustained-release tablet, so that the synergistic interaction time of the two active ingredients can be exerted and prolonged, the stimulation effect of the medicine on gastrointestinal tracts can be reduced, the compliance of patients can be improved, the peak valley phenomenon of a common preparation in blood after the common preparation is taken is avoided, the occurrence of adverse reactions is reduced, and the medication safety is improved.
Owner:乐普制药科技有限公司
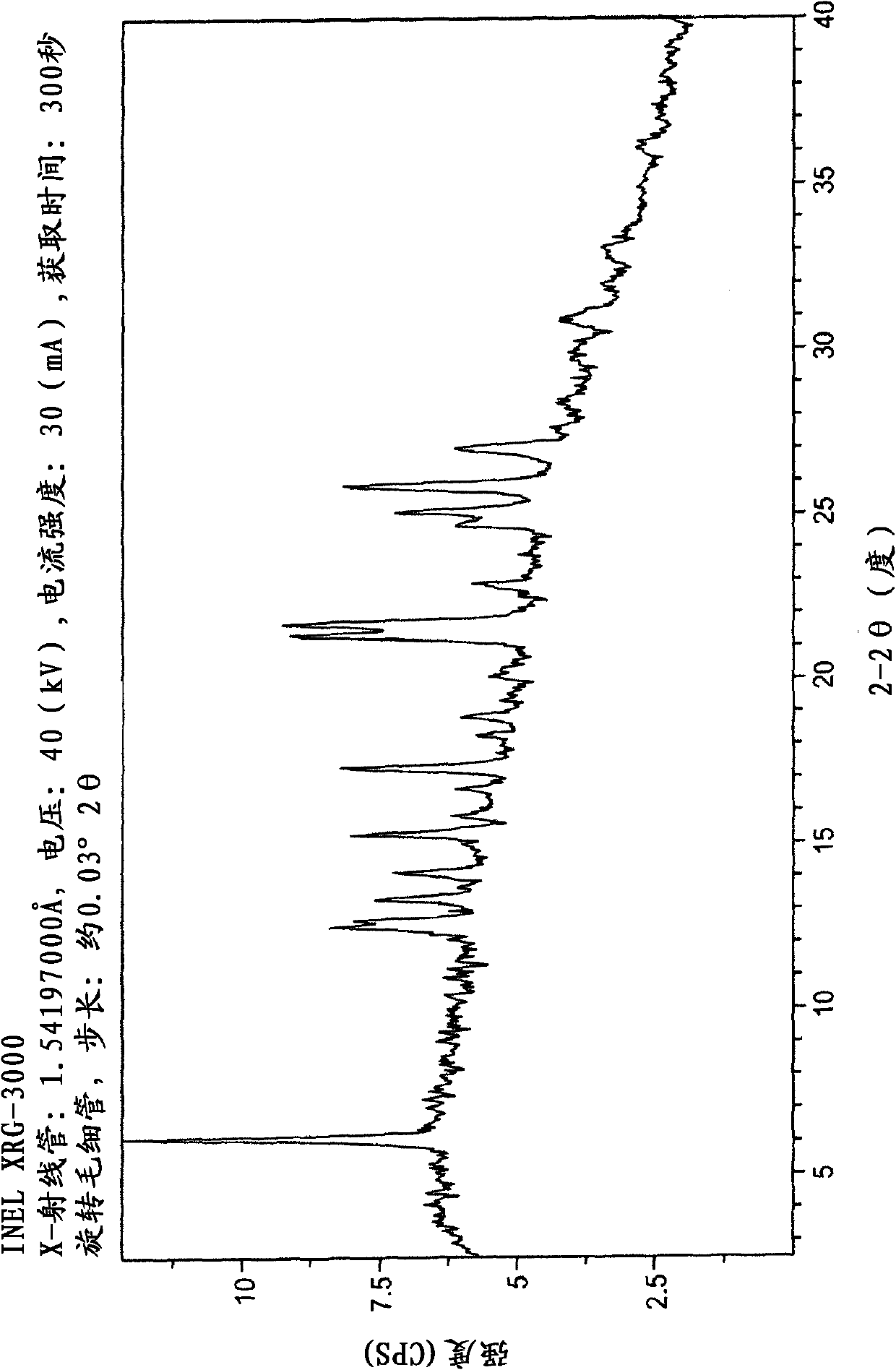
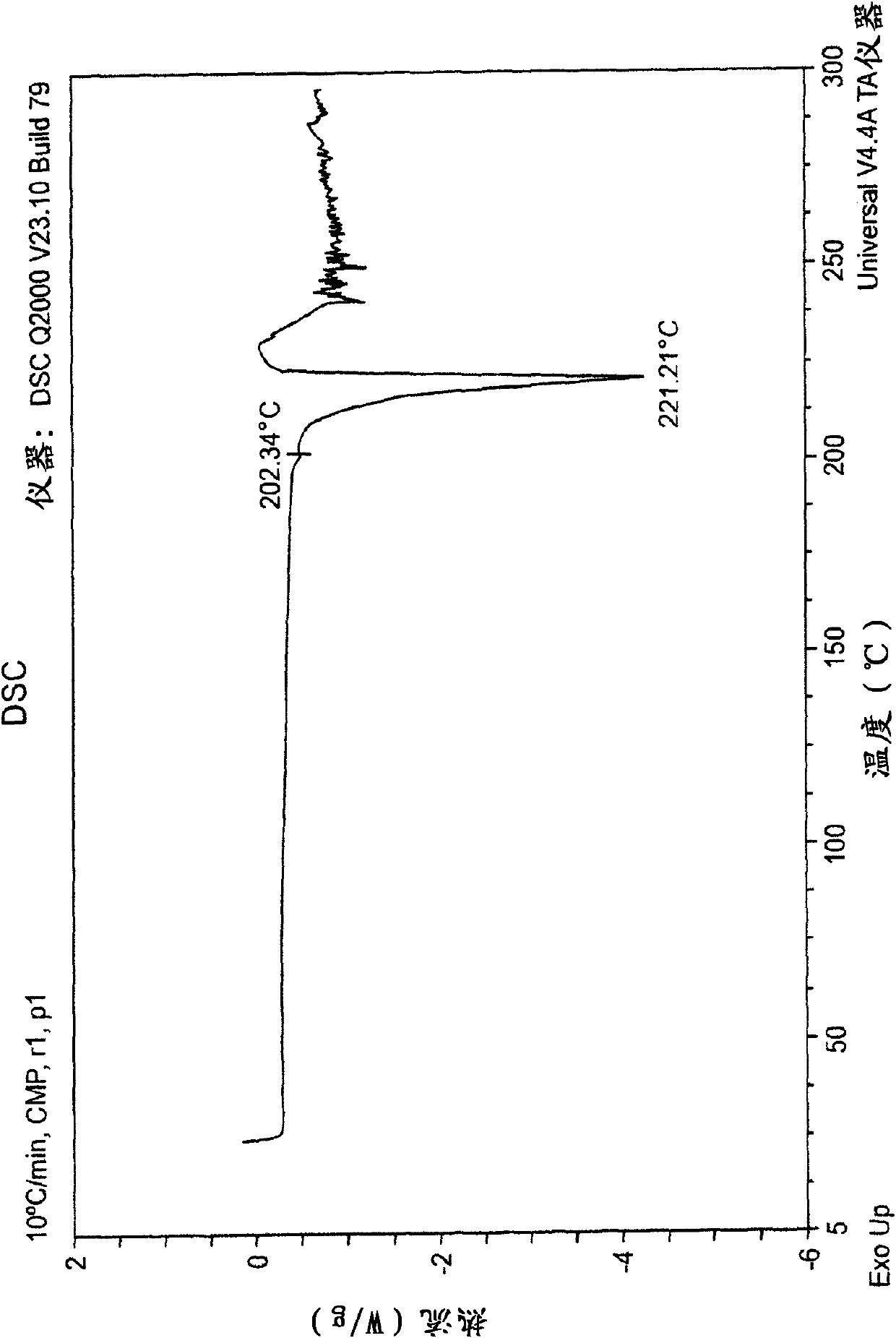
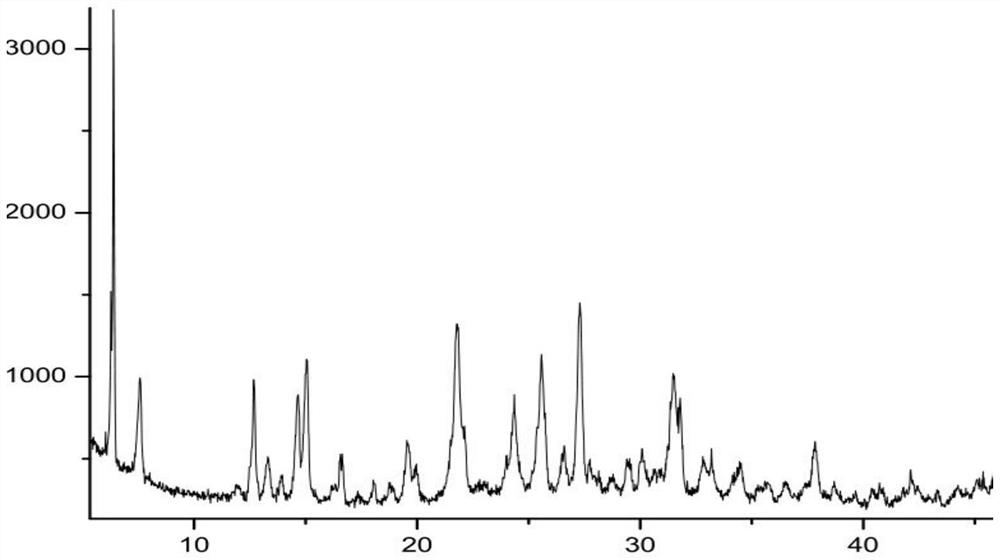
New epalrestat crystal form as well as preparation method and application thereof
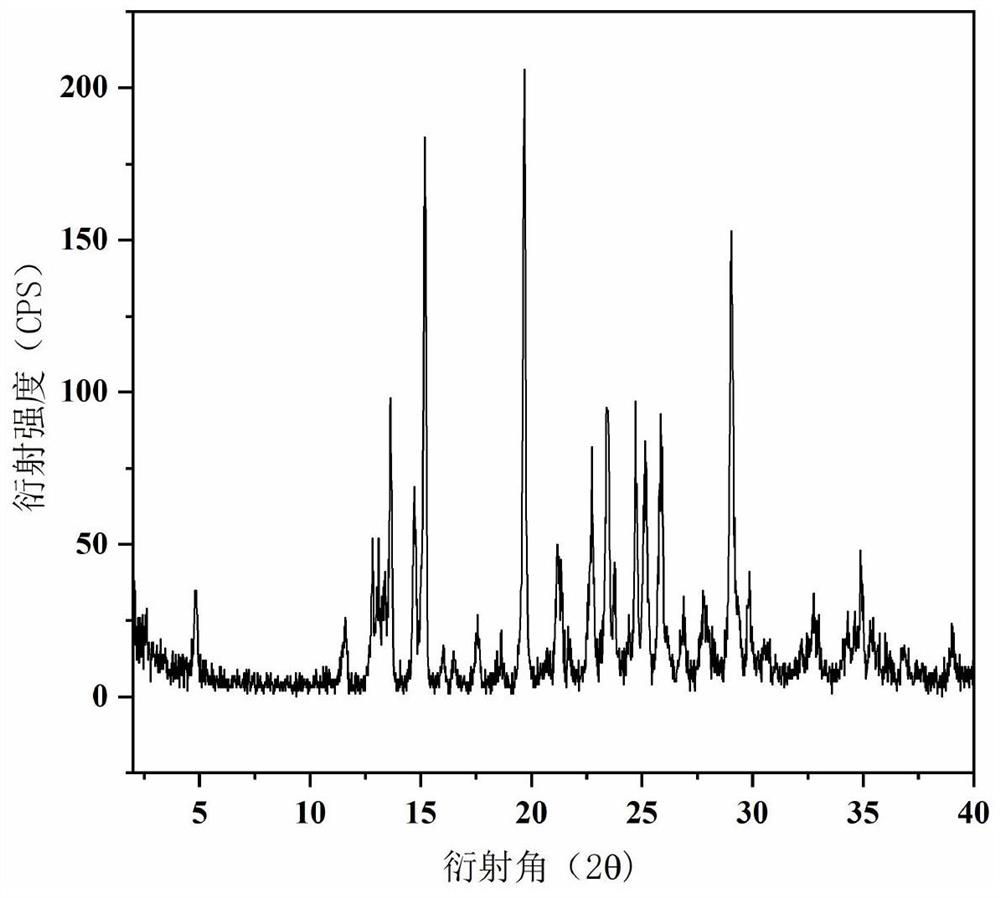
ActiveCN113651770AGood dissolution effectOrganic active ingredientsSenses disorderDrugs preparationsPharmaceutical drug
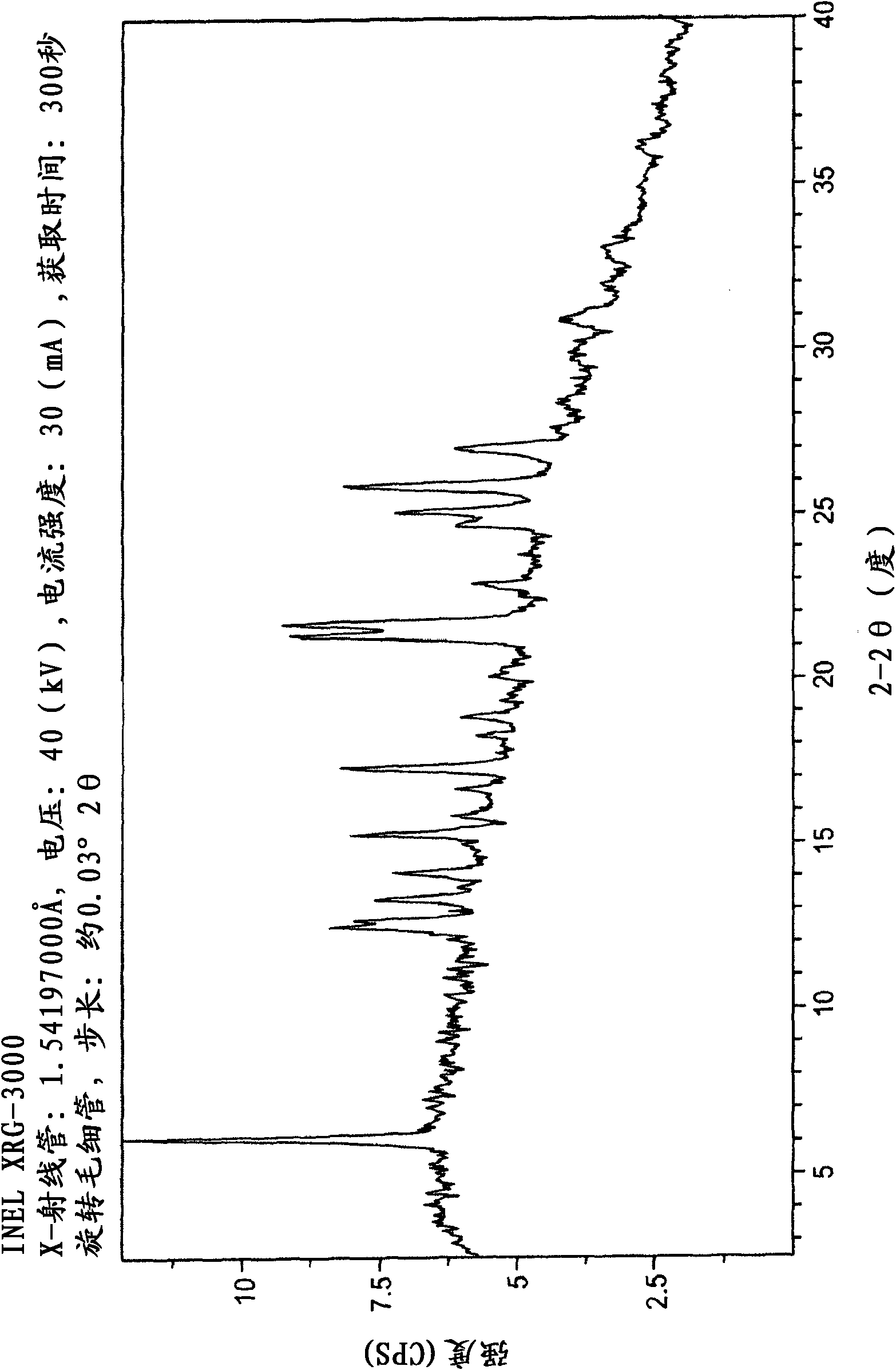
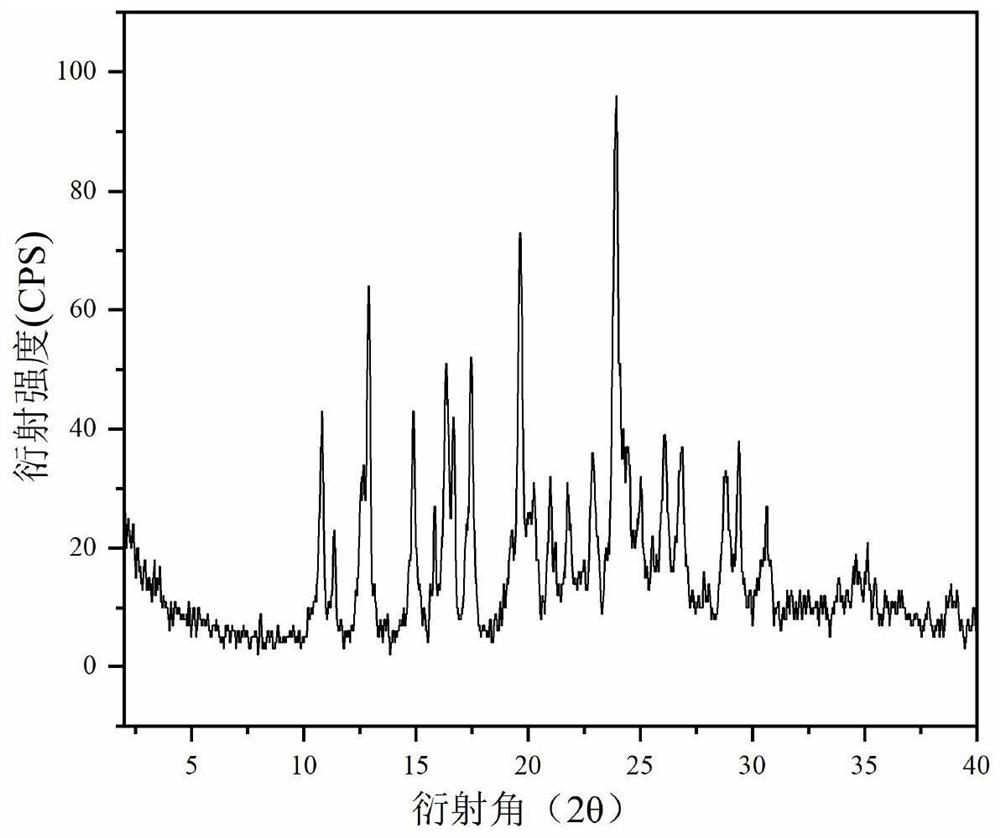
The invention belongs to the technical field of medicines, and provides a novel epalrestat crystal form as well as a preparation method and application thereof. The X-ray powder diffraction pattern of the epalrestat new crystal form has characteristic diffraction peaks when the diffraction angles 2 theta are 6.39 + / -0.2 degrees, 7.57 + / -0.2 degrees, 12.69 + / -0.2 degrees, 14.67 + / -0.2 degrees, 15.04 + / -0.2 degrees, 21.79 + / -0.2 degrees, 24.36 + / -0.2 degrees, 25.59 + / -0.2 degrees, 27.30 + / -0.2 degrees and 31.48 + / -0.2 degrees. The epalrestat novel crystal form has excellent dissolution performance and water solubility, and the bioavailability of the epalrestat novel crystal form is improved; moreover, the epalrestat new crystal form has excellent flowability, the angle of repose can reach 26 degrees or below, the compression coefficient is smaller than 10%, the Hausner ratio is 1.00-1.11, and the epalrestat new crystal form is suitable for being used as a raw material medicine for production of prodrugs or pharmaceutical preparations of the epalrestat new crystal form.
Owner:SHANDONG DYNE MARINE BIOTECHCAL PHARM HLDG CO LTD
Pharmaceutical composition for treating diabetic neuropathy and preparation method thereof
PendingCN114762683ALarge specific surface areaFacilitated releaseOrganic active ingredientsNervous disorderPharmaceutical drugEpalrestat
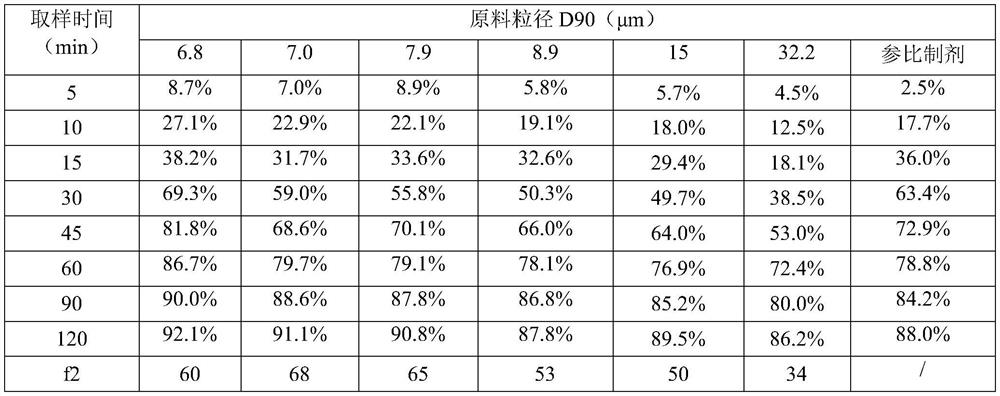
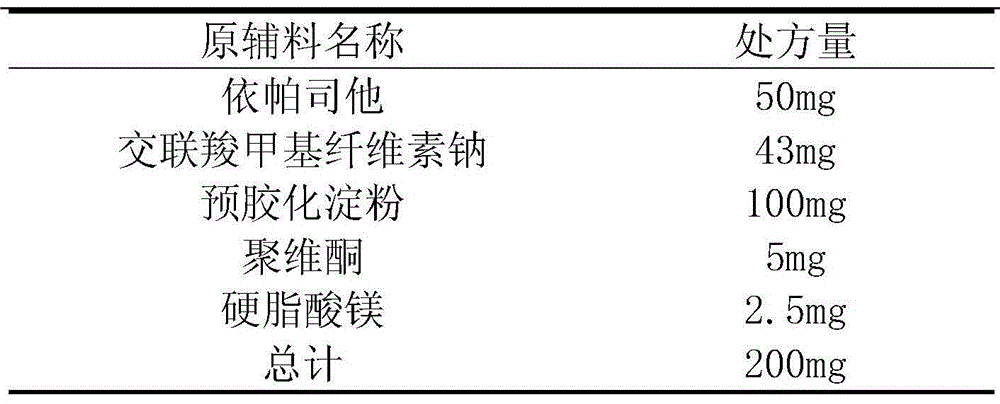
The invention discloses a pharmaceutical composition for treating diabetic neuropathy and a preparation method thereof, and particularly relates to an epalrestat tablet and a preparation method thereof, the epalrestat tablet comprises 40-50% of epalrestat, 45-50% of mannitol, 1-5% of a disintegrating agent, 1-5% of an adhesive and 0.5-2% of a lubricant, and the particle size D90 of the epalrestat tablet is less than or equal to 15 microns. According to the epalrestat pharmaceutical composition disclosed by the invention, epalrestat is crushed until the particle size (D90) is less than 15 microns, so that the specific surface area of raw material medicine particles is increased, the release and absorption of effective components are accelerated, the average dissolution rate and bioavailability of prepared epalrestat tablets are improved, and the dissolution rate difference between the tablets is reduced.
Owner:YANGTZE RIVER PHARM GRP NANJING HAILING PHARM CO LTD +1
Preparation method of epalrestat dispersible tablet
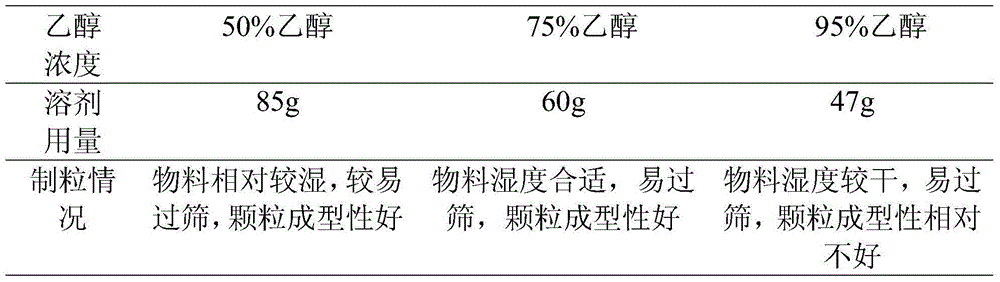
InactiveCN106265549AContent Uniformity GuaranteeSmall particle sizeOrganic active ingredientsNervous disorderDrug contentAdhesive
The invention provides a preparation method of an epalrestat dispersible tablet. The method comprises the following steps: a, weighing raw materials and auxiliary materials according to a weight ratio, wherein the raw materials and auxiliary materials comprise 15-65 parts of epalrestat, 20-150 parts of a filling agent, 5-10 parts of a disintegrating agent, 1-10 parts of an adhesive, and 1-3 parts of a lubricant; b, dissolving epalrestat and the disintegrating agent in ethanol of 50-95% to prepare a drug-containing adhesive; c, taking a mixture of the filling agent, the lubricant and the adhesive for sieving and mixing; d, adding the drug-containing adhesive obtained in the step b into the mixture prepared in the step c, and performing granulation, drying, size grading, whole blending, and tabletting to prepare the tablet. Through the preparation method, the uniformity of the drug content is ensured, and micronization of raw materials for particle size control is not required. Dissolution of indissolvable drugs is improved, and technological operation steps are reduced through co-dissolution. The energy is saved, and the cost is reduced.
Owner:CHENGDU BAOKE BIOTECH
Epalorestat capsule, and its prepn. method
InactiveCN1692904AImprove liquiditySimple preparation processOrganic active ingredientsNervous disorderCarboxymethyl starchAlcohol
A capsule of epalrestat is proportionally prepared from epalrestat, lactose, microcrystalline cellulose, starch, carboxymethyl starch sodium, and silica gel, micropowder through sequential mixing, adding alcohol, granulating, drying, adding silica gel micropowder, stirring and loading in capsules.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Epalrestat-metformin salt as well as preparation method and application thereof
ActiveCN113336718AIncrease dissolution rateImprove solubilityMetabolism disorderOrganic compound preparationSolubilityFluid phase
The invention discloses epalrestat-metformin salt as well as a preparation method and application thereof. The molecular formula of the epalrestat-metformin salt is C19H24N6O3S2, and the molecular weight of the epalrestat-metformin salt is 448.6; the epalrestat-metformin salt is in a crystalline state. The preparation method of the epalrestat-metformin salt comprises the following steps: dissolving epalrestat and metformin in a solvent according to a molar ratio of 1: 0.8-1: 1.2 to obtain a mixture; reacting and crystallizing the mixture for 12-48 hours under the condition of 15-60 DEG C; and carrying out solid-liquid phase separation on the obtained product, and drying to obtain the epalrestat-metformin salt. Compared with a metformin single product, the epalrestat-metformin salt form provided by the invention has the advantages that the strong hygroscopicity is greatly improved, the solubility is greatly improved compared with an epalrestat single product, meanwhile, the preparation method is simple to operate, the crystallization process is easy to control, and the reproducibility of the salt form is good.
Owner:TIANJIN UNIV
Refining method of crude epalrestat product
The invention discloses a refining method of a crude epalrestat product, which comprises the following steps of: firstly, dissolving the crude epalrestat product into a tetrahydrofuran solvent, heating to completely dissolve the crude epalrestat product, and stopping heating after the crude epalrestat product is completely dissolved; then adding isopropyl ether so that a small amount of red solids are separated out, further heating to completely dissolve the red solids, then stopping heating, naturally cooling, crystallizing and filtering, and then drying to obtain initially refined epalrestat; and then adding the tetrahydrofuran solvent, heating till the initially refined epalrestat is completely dissolved, stopping heating, then slowly adding petroleum ether so that a small amount of red solids are separated out, continuously heating till the red solids are completely dissolved, then stopping heating, naturally cooling, crystallizing and filtering, and then drying to obtain a refined epalrestat product. The refining method disclosed by the invention can be used for obtaining the high-purity epalrestat and has the advantages that the solvent raw material is available, reaction condition is mild, operation is simple and yield is high, and is suitable for industrial production.
Owner:KAIFENG MINGREN PHARMA
A kind of refining method of high-purity epalrestat
The invention provides a refining method of high-purity epalrestat. The refining method comprises the following steps of: adding the epalrestat into a good solvent, keeping out of light at the temperature of 110 DEG C, stirring, refluxing, and dissolving; after dissolving, adding a bad solvent into the mixture, maintaining the temperature, standing for crystallization, carrying out suction filtration, cleaning a filter cake for one time by using the bad solvent, drying at the temperature of 60 DEG C and obtaining a finished epalrestat product. The structure of the epalrestat is as shown in the specification. Compared with the prior art, the refining method provided by the invention has the advantages that the used refining solvent is low in cost and easy to obtain, the reaction condition is mild, the production cost is low, the pollution is less, the operation is simple, heat filtration is not needed, only once recrystallization is needed, the purity and the yield of the obtained finished epalrestat product are high, the quality of the product is improved, and the suitability for massive industrial production is achieved.
Owner:KANGYA OF NINGXIA PHARMA
Medicinal composition for treating diabetes chronic complication and preparation method thereof
The invention relates to a series of medical compositions used for treating chronic complications of diabetes and preparation methods thereof; the invention are oral compound preparations (including tablet, dispersible tablet, effervescent tablet, capsule, soft capsule and granule) prepared by at least two active components of mecobalamine, cobamamide, vitamine B12, epalrestat, lipoic acid and calcium dobesilate and the pharmaceutically accepted excipients. The preferential compositions of the invention are mecobalamine+ lipoic acid, mecobalamine+ lipoic acid+ calcium dobesilate, mecobalamine+epalrestat+ calcium dobesilate, mecobalamine+ lipoic acid+ epalrestat+ calcium dobesilate, and mecobalamine+ vitamine B12+ cobamamide. The invention is mainly used for treating neuropathy in chronic complications of diabetes.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com