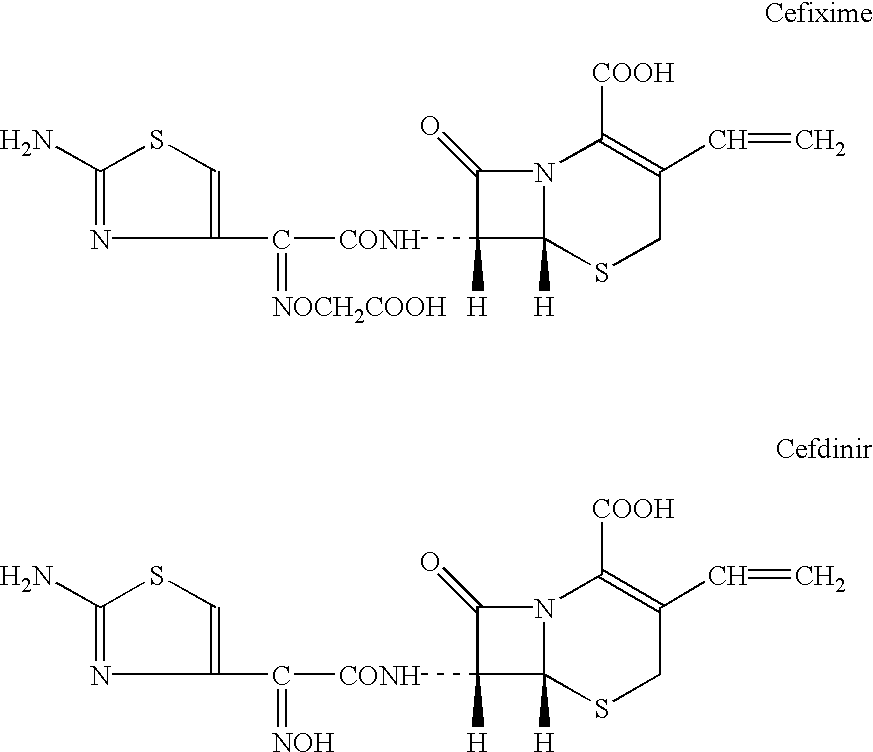
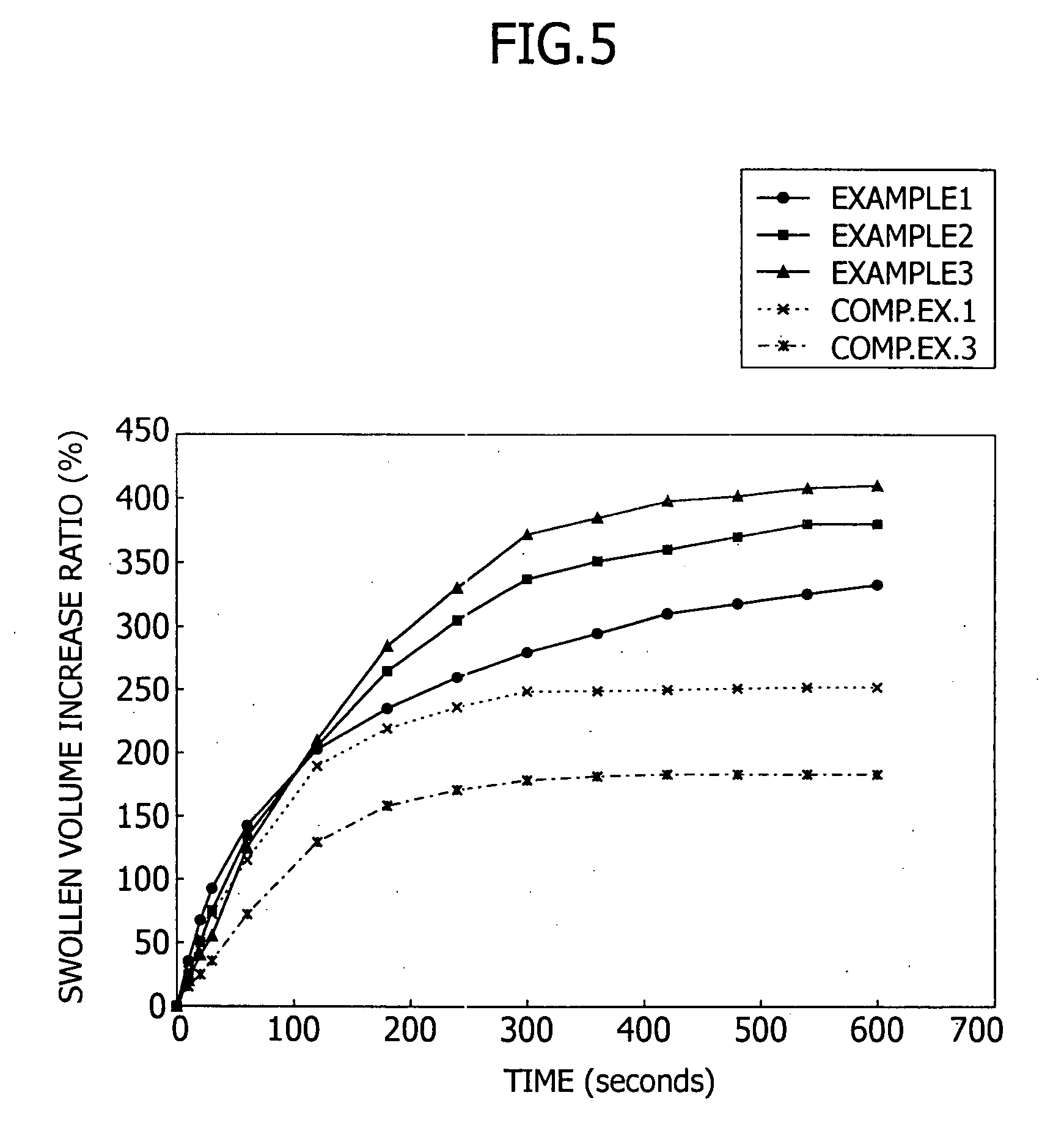
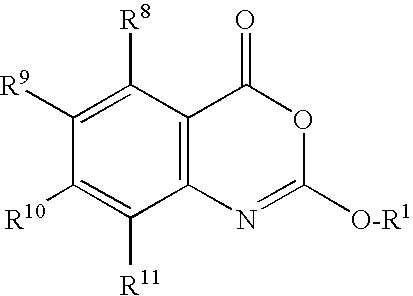
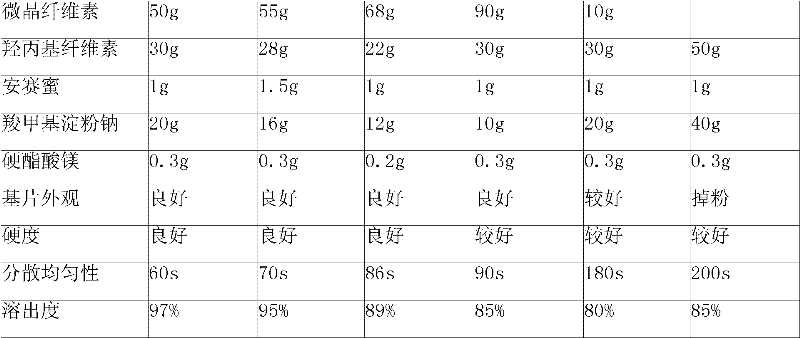
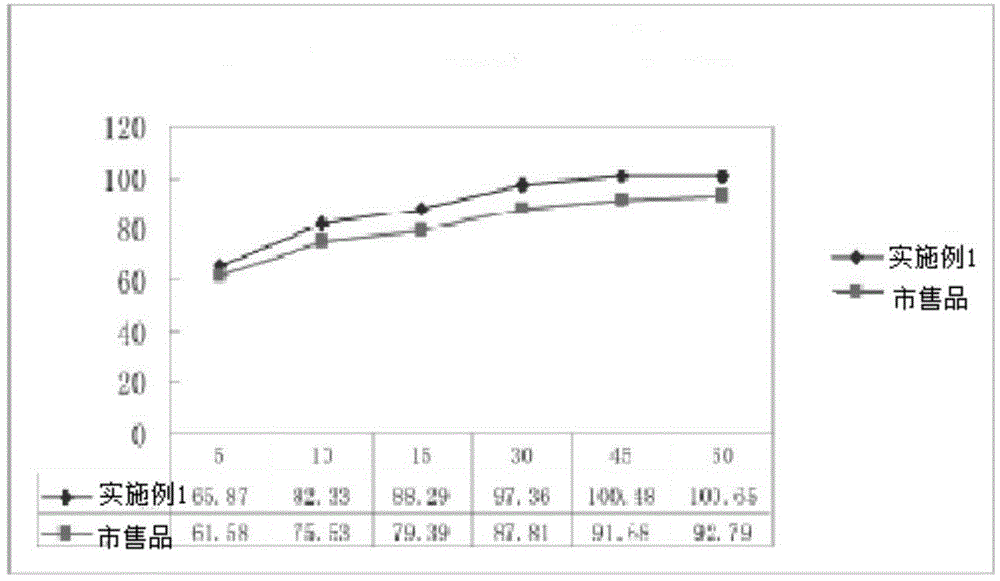
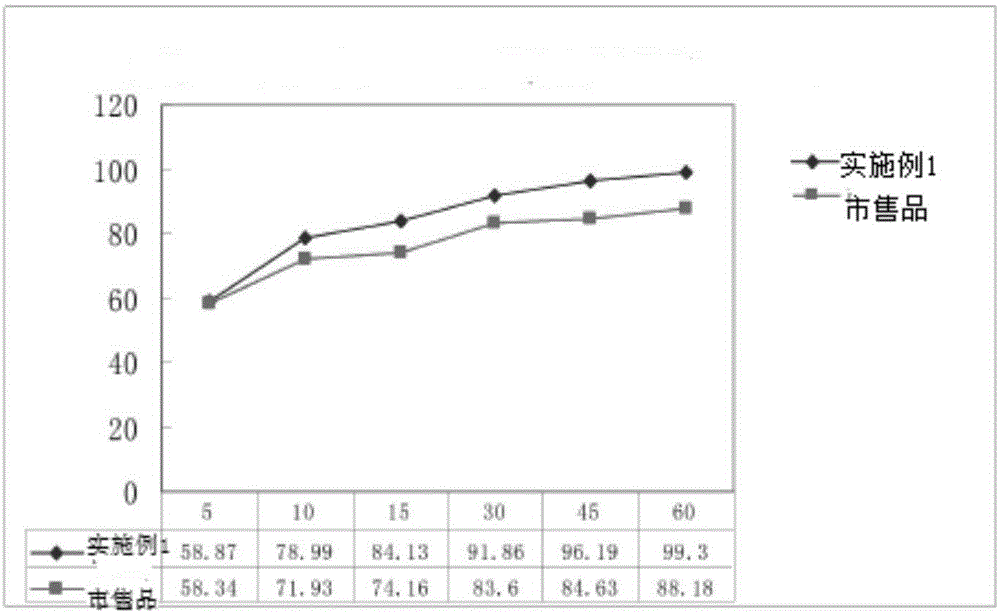
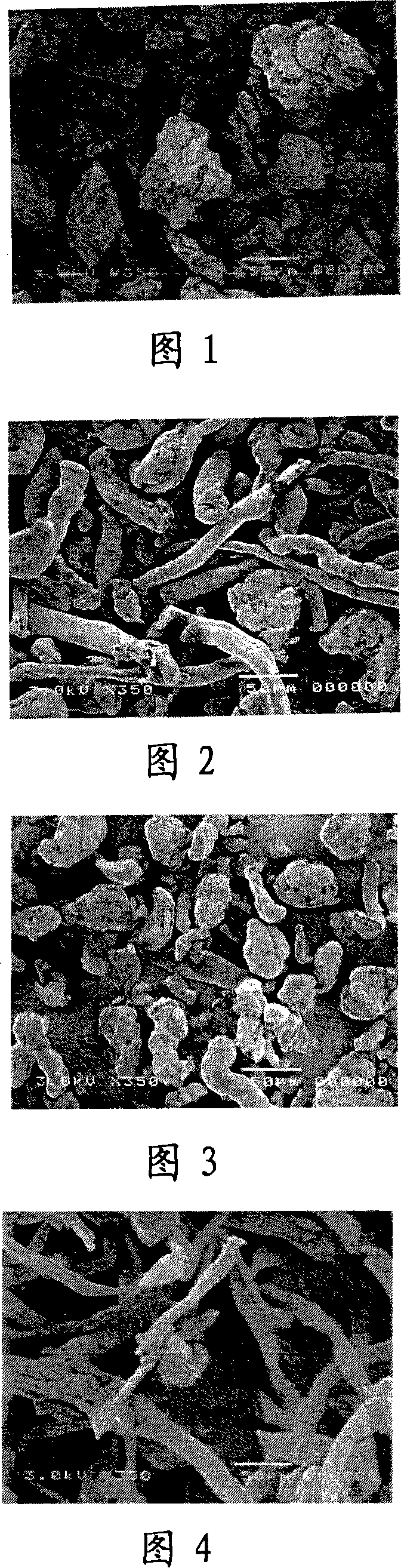
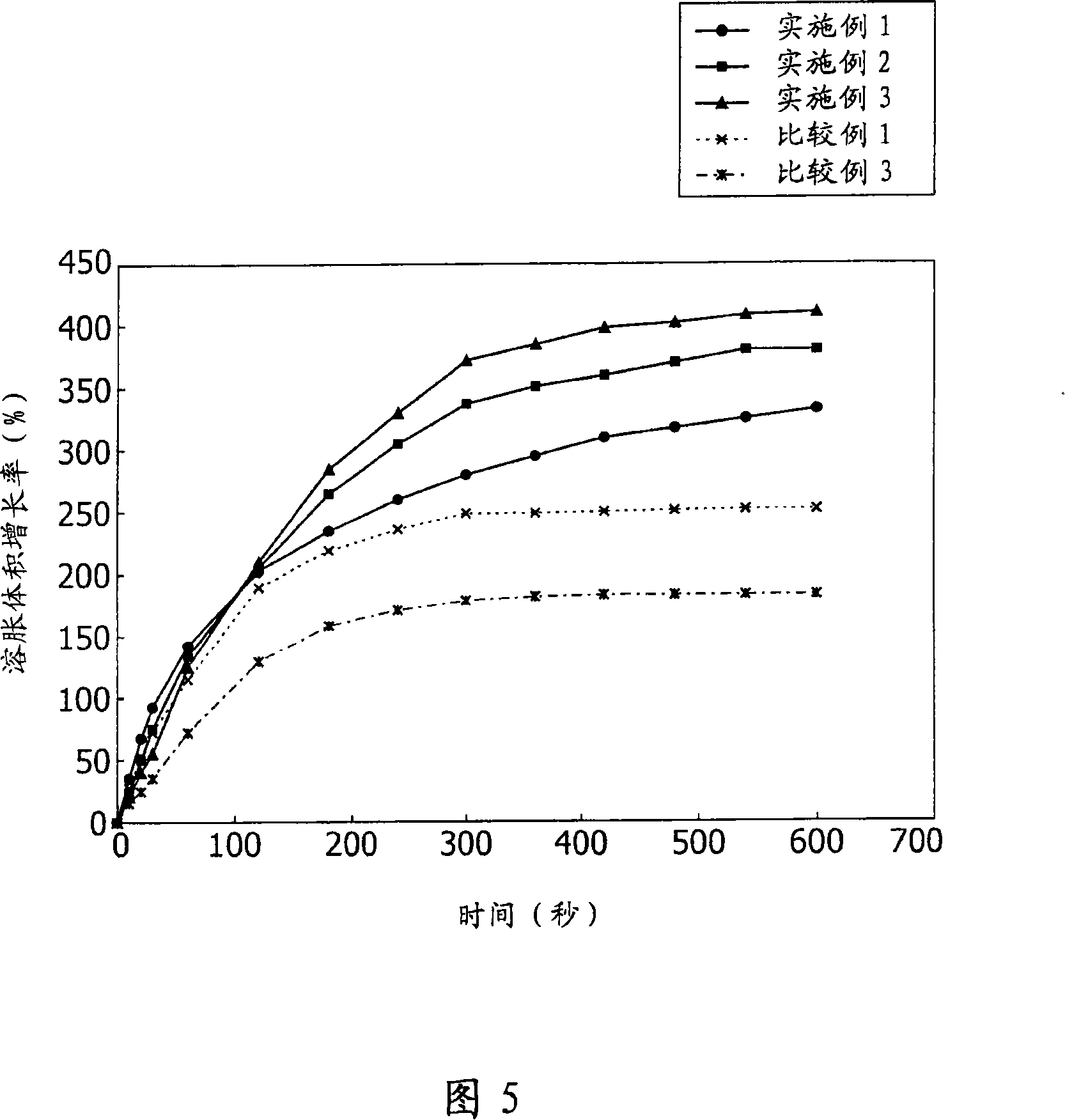
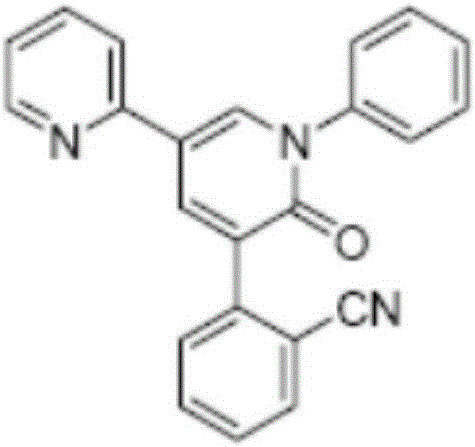
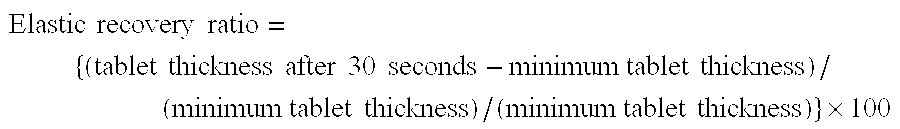Patents
Literature
346 results about "Low-substituted hydroxypropylcellulose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Oral cavity disintegrating tablet and method of producing the same
ActiveUS20100278930A1Easy to produceDisintegrates quicklyBiocideOrganic active ingredientsSucroseOrally disintegrating tablet
The invention provides an orally disintegrating tablet containing (a) one or more saccharides or sugar alcohols selected from the group consisting of mannitol, lactose, xylitol, sucrose, erythritol and glucose and (b) low substituted hydroxypropylcellulose and substantially free of a starch disintegrant, which tablet is produced by steps of granulating a composition containing the above-mentioned components (a) and (b) by an agitation granulation method, and compression-molding the obtained granulation product. The invention also provides a method of producing an orally disintegrating tablet substantially free of a starch disintegrant, including steps of granulating a composition containing the above-mentioned components by an agitation granulation method, and compression-molding the obtained granulation product.
Owner:SAWAI PHARMA
Solid pharmaceutical preparation
InactiveUS6299904B1Good disintegrationPromote dissolutionOrganic active ingredientsBiocideLow-substituted hydroxypropylcelluloseMaltitol
A solid preparation which comprises (i) a pharmaceutically active ingredient, (ii) one or more water-soluble sugar alcohols selected from the group consisting of sorbitol, maltitol, reduced starch saccharide, xylitol, reduced palatinose and erythritol, and (iii) low-substituted hydroxypropylcellulose having hydroxypropoxyl group contents of 7.0 to 9.9 percent by weight; which exhibits excellent buccal disintegration and dissolution and also appropriate strength.
Owner:TAKEDA PHARMA CO LTD
Beta-lactam antibiotic-containing tablet and production thereof
InactiveUS6423341B1Antibacterial agentsOrganic active ingredientsLow-substituted hydroxypropylcelluloseEngineering
This invention provides beta-lactam antibiotic-containing tablets capable of being orally taken either as such owing to their being small-sized, hence still easily swallowable, or, in the case of administration to the aged encountering some difficulty in swallowing, in the form of dispersions resulting from easy self-disintegration upon being dropped into water in a glass as well as a method of producing the same. The tablets of this invention comprise, on the per-tablet basis, 60-85% by weight of a beta-lactam antibiotic, 1-10% by weight of low-substituted hydroxypropylcellulose and / or crosslinked polyvinylpyrrolidone as a disintegrator, and 0.5-2% by weight of a binder. Granules to be compressed for tableting are prepared using water or an aqueous solution of ethanol or the like.
Owner:ASTELLAS PHARMA INC
Gastrointestinal mucosa-adherent pharmaceutical composition
InactiveUS6428813B1Improve security featuresEnhanced adhesion mucosaAntibacterial agentsPowder deliveryLow-substituted hydroxypropylcelluloseEfficacy
In order to provide a composition having a long gastroduodenal residence time and exhibiting an improved efficacy, is provided a gastrointestinal mucosa-adherent composition comprising an active ingredient and a material which swells a viscogenic agent capable of being viscous with water a (e.g. curdlan and / or a low-substituted hydroxypropylcellulose etc.).
Owner:TAKEDA PHARMA CO LTD
Compound danshen oral disintegrant tablet and its preparation method
The present invention relates to a compound salvia oral disintegrant tablet capable of being disintegrated quickly in oral cavity to release medicine. It composition includes the salvia root extract, notoginseng total saponin, borneol and excipient, and its disintegration time is within 40 sec, in which the excipient includes filling agent, disintegrant, glidant and corrective. Its filling agent is of microcrystalline cellulose, its dose is 40%-90% of total weight of the prescription, and the disintegrant is selected from sodium carboxymethylstarch, cross-linked carboxymethylcellulose sodium, cross-linked polyvinylpyrrolidone and low-substituted hydroxypropyl cellulose, and its dose is 5%-25% of total weight of prescription.
Owner:BEIJING BOERDA BIO TECH DEV
Low-substituted hydroxypropylcellulose powder and method for producing the same
ActiveUS20080039621A1Improve compression performanceGood lowabilityPill deliveryGranular deliveryAqueous sodium hydroxideLow-substituted hydroxypropylcellulose
Provided are a low-substituted hydroxypropylcellulose powder having high compressibility, good flowability and excellent disintegration, and a method for producing the same. More specifically, provided is a method for producing a low-substituted hydroxypropylcellulose powder having a molar substitution number per anhydrous glucose unit of 0.05 to 1.0, which is insoluble in water and swollenable by absorbing water, comprising the steps of: adding an aqueous sodium hydroxide solution to powdered pulp in such a manner that weight ratio of sodium hydroxide with respect to anhydrous cellulose is 0.1 to 0.3 so as to produce alkali cellulose; etherifying the obtained alkali cellulose to obtain a crude product; neutralizing the sodium hydroxide contained in the obtained crude reaction product; washing the resultant; drying; and pulverizing using by compaction-grinding.
Owner:SHIN ETSU CHEM IND CO LTD
Solid preparation
InactiveUS20010009678A1Good disintegrationPromote dissolutionBiocideMetabolism disorderLow-substituted hydroxypropylcelluloseMaltitol
A solid preparation which comprises (i) a pharmaceutically active ingredient, (ii) one or more water-soluble sugar alcohol selected from the group consisting of sorbitol, maltitol, reduced starch saccharide, xylitol, reduced palatinose and erythritol, and (iii) low-substituted hydroxypropylcellulose having hydroxypropoxyl group contents of 7.0 to 9.9 percent by weight; which exhibits excellent buccal disintegration and dissolution and also appropriate strength.
Owner:TAKEDA PHARMA CO LTD
Benzene sulfonic acid levo-amlodipine pill and preparation method thereof
InactiveCN101559043AHigh dissolution rateReasonable designOrganic active ingredientsPill deliveryBenzeneMagnesium stearate
The invention discloses benzene sulfonic acid levo-amlodipine pills and a preparation method thereof. On the basis of 1000 pills, the benzene sulfonic acid levo-amlodipine pill comprises 1-10g of benzene sulfonic acid levo-amlodipine, preferably 2.5g; 50-100g of lactose, preferably 67-87g and most preferably 80g; 5-55g of low-substituted hydroxy propyl cellulose, preferably 20-40g and particularly preferred 30g; 2-20g of crosslinked polyethylene ketopyrrolidine, preferably 5g; and 0.5-2.5g of magnesium stearate, preferably 1.5g. The benzene sulfonic acid levo-amlodipine pills have more than 95 percent of dissolution rate and good production stability.
Owner:NANCHANG HELIOEAST PHARMA
Dispersible tablet of colloid petcin
InactiveCN1634132AHeavy metal active ingredientsDigestive systemLow-substituted hydroxypropylcelluloseCross-linked polyethylene
The invention discloses a dispersible tablet of colloid pectin, which comprises (by weight ratio, in mg) colloid pectine bismuth 25-100 (by bismuth), filling agent 60-400, crumbling agent 42-280, flow aid 1-6, lubricant 0.25-5, the filling agent can be selected from lactose, white dextrine, pregelatine starch or / and starch. The crumbling agent can be selected from cross bonding polyvinylpyrrolidone, crystalline cellulose, cross-linked sodium carboxymethylstarch, low substituted methylcellulose propylene glycol ether, sodium carboxymethylstarch, the glidant can be selected from miropowdered silica gel, the lubricant can be selected from magnesium stearate or / and talcum powder.
Owner:HUNAN WARRANT PHARMA
Orally disintegrating tablets
InactiveUS20110053942A1Solve the lack of hardnessDisintegrates quicklyBiocideDigestive systemMANNITOL/SORBITOLMedicine
The present invention relates to an orally disintegrating tablet containing (1) an active ingredient, (2) mannitol, (3) crystalline cellulose and (4) at least two kinds of particular ingredients selected from the group consisting of low-substituted hydroxypropylcellulose, cornstarch and carmellose, wherein the blending ratio of each ingredient relative to 100 wt % of the disintegrating tablet is (1) 0.01 to 50 wt %, (2) 20 to 86 wt %, (3) 10 to 30 wt %, and (4) 1 to 20 wt % for each particular ingredient and 3 to 60 wt % as the total of the particular ingredients to be blended, and an assembly of (3) crystalline cellulose to be blended has a bulk density of not more than 0.18 g / cm3, and can provide an orally disintegrating tablet having both suitable hardness and rapid disintegrability in oral cavity, which maintains orally disintegrability even under moist conditions, and hardness of not less than a predetermined level necessary for using in an automatic packaging machine.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Oral disintegrant tablet and its preparation method
InactiveCN1476826AGreat tasteShort disintegration timePill deliveryPharmaceutical non-active ingredientsCross-linkOrally disintegrating tablet
The present invention relates to an oral disintegration tablet and its preparation method. It includes the medicinal active component and auxiliary material. Auxiliary material includes diluting agent and disintegrant, in which the diluting agent is mannite and lactose and the disintegrant is microcrystal cellulose, low-substituted hydroxypropyl cellulose and cross-linked polyvinylpyrrolidone, the weight of disintegrant is less than 25% of total weight of the auxiliary material, the weight ratio of medicinal active component and auxiliary material is 1:2-75. Said disintegration tablet has enough hardness (strength).
Owner:北京中西经纬释药技术有限公司
Voglibose dispersible tablet, capsule and method for preparing the same
InactiveCN101219127APromote dissolutionPromote absorptionOrganic active ingredientsMetabolism disorderSucroseLow-substituted hydroxypropylcellulose
The invention relates to a voglibose dispersible tablet and capsule and a preparation method thereof. The voglibose dispersible tablet comprises: 0.01-5 percent of voglibose, 1-99 percent of disintegrating agent, 0-98 percent of diluting agent; 0.5-20 percent of lubricant and fluidizer, 0.1-20 percent of bonding agent; wherein, the disintegrating agent is one or more selected from starch, modified starch, cellulose, microcrystalline cellulose, cross-linked polyvinyl pyrrolidone, sodium carboxymethyl starch, cross-linked sodium carboxymethyl cellulose, low-substituted hydroxypropyl cellulose, alginic acid and colloid magnesium aluminum silicate; the diluting agent is one or more selected from lactose, mannitol, sorbitol, sucrose, calcium sulfate, kaolin, dextrine and sodium chloride. The capsule preparation does not contain the bonding agent. The invention has the advantages that the voglibose dispersible tablet disintegrates swiftly and disperses evenly, the voglibose dispersible tablet can be disintegrated swiftly into fine particles and scattered evenly after being taken orally, which is beneficial for the dissolution and the absorption of the voglibose dispersible tablet with the short onset time. Disintegrated swiftly in three minutes; after being taken orally, the capsule shell quickly swells and splits, which conceals the discomfort caused by the capsule taken in mouth.
Owner:JIANGSU WANBANG BIOPHARMLS +1
Phosphate-binding polymer and tablets using the same
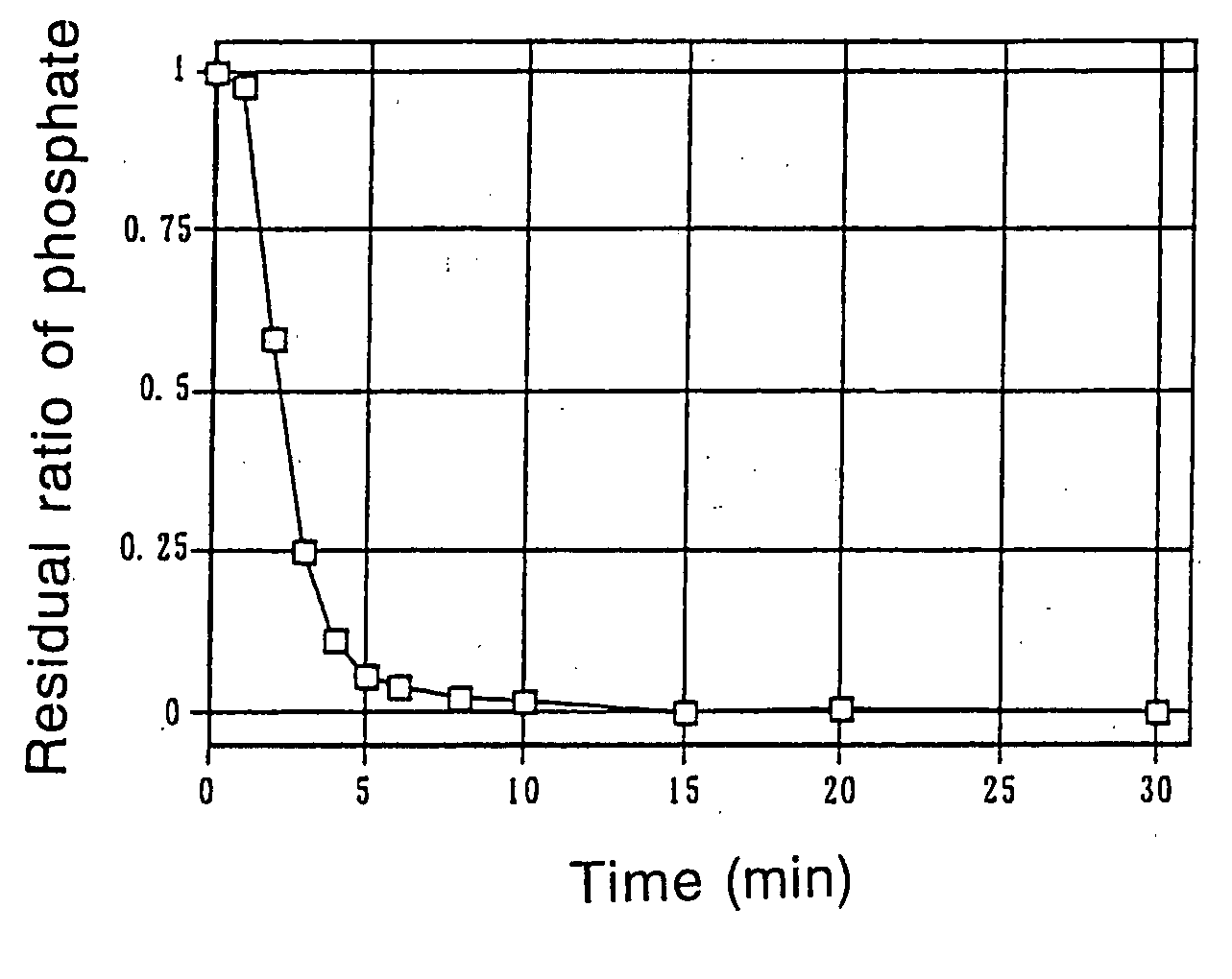
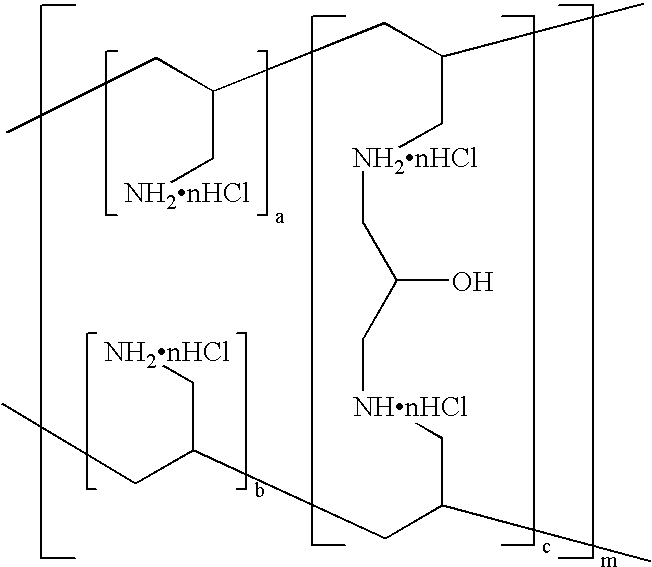
InactiveUS20070190135A1Rapid disintegrabilityGood dispersibilityUrinary disorderPill deliveryHigh phosphateLow-substituted hydroxypropylcellulose
Disclosed are a phosphate-binding polymer having a true specific gravity of 1.18-1.24, tablets that solely consist of the particles of a phosphate-binding polymer having an average particle size of no more than 400 μm, with at least 90% being occupied by particles no larger than 500 μm, and having a true specific gravity of 1.18-1.24 and a water content of 1-14%, or tablets that contain both the particles and crystalline cellulose and / or low substituted hydroxypropyl cellulose, and a process for producing such tablets. The phosphate-binding polymer can be formulated as tablets either alone or in combination with specified additives. Whichever the case, the tablets have satisfactory hardness, contain the active ingredient in high proportion, have high phosphate-binding capability and exhibit rapid disintegrability in an acidic to neutral region while having little sensitivity to the strength of agitation. The tablets are excellent pharmaceutical preparations that undergo reduced variations in bioavailability in spite of movements within the digestive tracts and pH changes.
Owner:CHUGAI PHARMA CO LTD
Solid dosage form of enteric solid dispersion and method for producing the same
Provided are a solid dosage form comprising an enteric solid dispersion that allows a drug in the preparation to be rapidly dissolved without compromising the solubility of the solid dispersion, and a method for producing the same. More specifically, provided is a solid dosage form comprising an enteric solid dispersion comprising a poorly soluble drug, an enteric polymer and a disintegrant, wherein the disintegrant is low-substituted hydroxypropylcellulose having an average particle size of 10 to 100 μm and a specific surface area measured by BET method of at least 1.0 m2 / g. Moreover, provided is a method for producing a solid dosage form comprising an enteric solid dispersion, the method comprising steps of: spraying an enteric polymer solution in which a poorly soluble drug has been dispersed or dissolved, on a powder of low-substituted hydroxypropylcellulose having an average particle size of 10 to 100 μm and a specific surface area measured by BET method of at least 1.0 m2 / g and serving as a disintegrant; and granulating the resultant; and drying.
Owner:SHIN ETSU CHEM IND CO LTD
Solid preparation
InactiveUS20090208584A1Low melting pointSuperior in disintegration property propertyPowder deliveryOrganic active ingredientsOral medicationLow-substituted hydroxypropylcellulose
The solid preparation of the present invention aims at providing a solid preparation superior in the stability during production and preservation even when a poorly water-soluble substance having a low melting point is contained in a large amount, and also superior in the disintegration property and release property of a poorly water-soluble substance having a low melting point, after oral administration, and is characterized by the following 1) to 3): 1) containing a poorly water-soluble substance having a low melting point, a saccharide, and a cellulose selected from a crystalline cellulose and a low-substituted hydroxypropylcellulose, 2) a saccharide / cellulose weight ratio exceeding 2, and 3) a cellulose content of not less than 5 wt %.
Owner:NORGINE BV
Erdosteine composition and preparation method thereof
ActiveCN101606931BImprove dispersion uniformityImprove dissolution efficiencyOrganic active ingredientsPharmaceutical product form changeCarboxymethyl starchAlcohol
The invention provides an Erdosteine composition which consists of the following components by weight portions: 130 to 170 portions of Erdosteine, 35 to 65 portions of lactose, 30 to 70 portions of microcrystalline cellulose, 20 to 40 portions of low substituted hydroxypropyl cellulose, 1 to 5 portions of acesulfame, 10 to 30 portions of sodium carboxymethyl starch and 0.1 to 0.5 portions of magnesium stearate; the invention also provides a preparation method of the Erdosteine composition, comprising the following steps of: preparing materials and pelleting; mixing the Erdosteine and the low substituted hydroxypropyl cellulose evenly, then grinding, adding the sodium carboxymethyl starch, adding absolute ethyl alcohol, wet-mixing, cutting, drying, granulation and tabletting; mixing granules and microcrystalline cellulose evenly, adding the lactose, the acesulfame and the magnesium stearate for pelleting, subpackaging and obtaining the Erdosteine composition. The Erdosteine compositionhas good dispersible uniformity and high drug dissolution efficiency.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
Ticagrelor tablets and preparation method thereof
ActiveCN104523640AIncrease dissolution ratePromote absorptionOrganic active ingredientsPharmaceutical non-active ingredientsMANNITOL/SORBITOLTicagrelor
The invention discloses ticagrelor tablets and a preparation method thereof and belongs to the technical field of medicines. According to the ticagrelor tablets disclosed by the invention, mannitol, microcrystalline cellulose, low-substituted hydroxypropyl cellulose, croscarmellose sodium and magnesium stearate are selected as auxiliary materials and are compounded with the raw material medicine ticagrelor tablets so as to obtain the ticagrelor tablets, and each auxiliary material and the raw material medicine are synergetic in the defined amount range, so that the dissolution rate of the prepared ticagrelor tablets is higher than that of the conventional commercially available tablets. Moreover, the ticagrelor tablets have the same dissolution behavior as the commercially available medicines, the ticagrelor tablets have good absorption effects, and the bioavailability of the ticagrelor tablets is improved. The ticagrelor tablets disclosed by the invention are small in impurity content and are stable in performance under high-temperature illumination conditions. The method for preparing the ticagrelor tablets disclosed by the invention is simple in process flow, easy to operate and implement and suitable for industrial popularization and application.
Owner:HENAN RUNHONG PHARMA
Dual-phase capsule for preventing and treating chronic pelvic inflammation and preparation method and detection method thereof
ActiveCN103784664AStrong specificityFully extractedComponent separationCapsule deliveryClinical efficacyLow-substituted hydroxypropylcellulose
The invention provides a dual-phase capsule for preventing and treating chronic pelvic inflammation and a preparation method and a detection method thereof. The dual-phase capsule for preventing and treating chronic pelvic inflammation is prepared by extracting 20g of Chinese pulsatilla root, 20g of nutgrass galingale rhizome, 12g of twotooth achyranthes, 12g of Chinese atractylodes, 8g of amur corktree bark and 8g of selinum japenious seed serving as bulk pharmaceuticals, adding a proper amount of superfine silica powder and low-substituted hydroxypropyl cellulose, and adding 80 percent ethanol as a wetting agent. Aiming at the defects in the prior art, a preparation process of the dual-phase capsule for preventing and treating chronic pelvic inflammation is optimized, so that the curative effect of the dual-phase capsule on chronic pelvic inflammation is more remarkable. A systematic, complete and effective component identification and content determination method is established, so that the quality of the dual-phase capsule can be controlled effectively, and the clinical curative effect is ensured.
Owner:GUIZHOU NORMAL UNIVERSITY
Complex acetaminophen vitamin C dispersion tablet and preparing method thereof
InactiveCN1507862AQuick effectDefinite curative effectOrganic active ingredientsAntipyreticSorbyl alcoholCross-link
The present invention relates to compound paracetamol vitamin C disperser tablet and its preparation method. It is formed from paracetamol, vitamin C, disintegrant, filling agent, correctives, adhesive and lubricating agent according to the ratio of 200-400:200-400:1-60:1-100:1-60:2-50:1-10. The disintegrant is carboxy methyl starch sodium, hydroxypropyl starch, low-substituted hydroxypropyl cellulose, calcium cellulose glycollate, cross-linked sodium cellulose glycollate, sodium lauryl sulfate, and the filling agent is lactose, mannitol, sorbyl alcohol, starch, modified starch, beta-dextrin, calcium sulfate dihydrate, microcrystal cellulose, and the corrective is wintergreen oil, peperitol, aspatan, citric acid, cane sugar, sterioside, the adhesive is polyvidone, hydroxypropyl cellulose, gelling starch and ethyl cellulose, and the lubricating agent is magnesium stearate, calcium stearate, polyglycol 6000 and talcum powder.
Owner:严洁
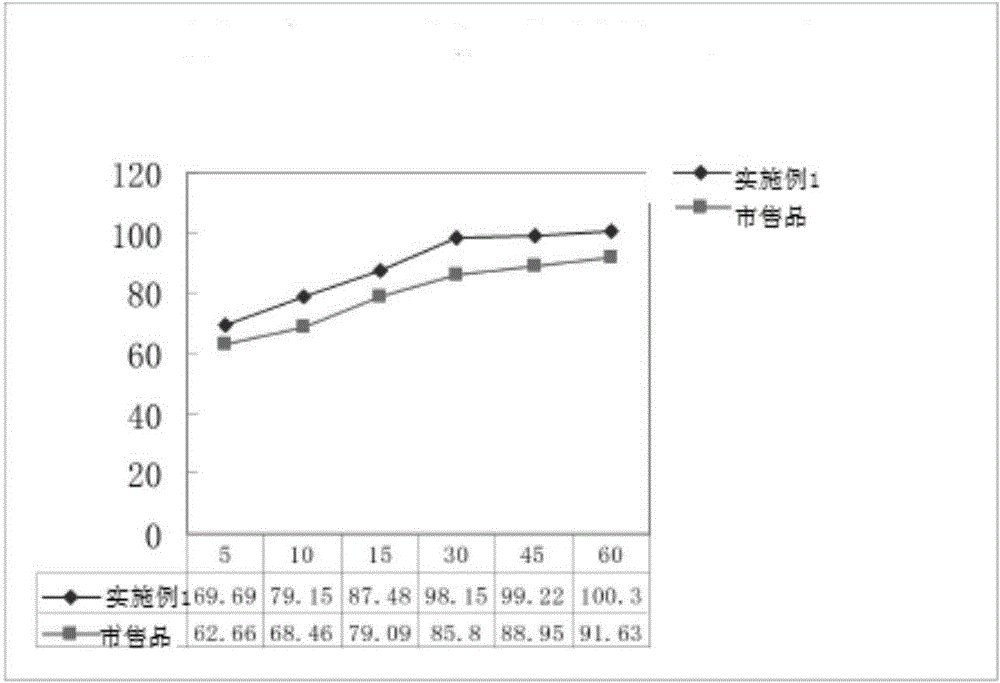
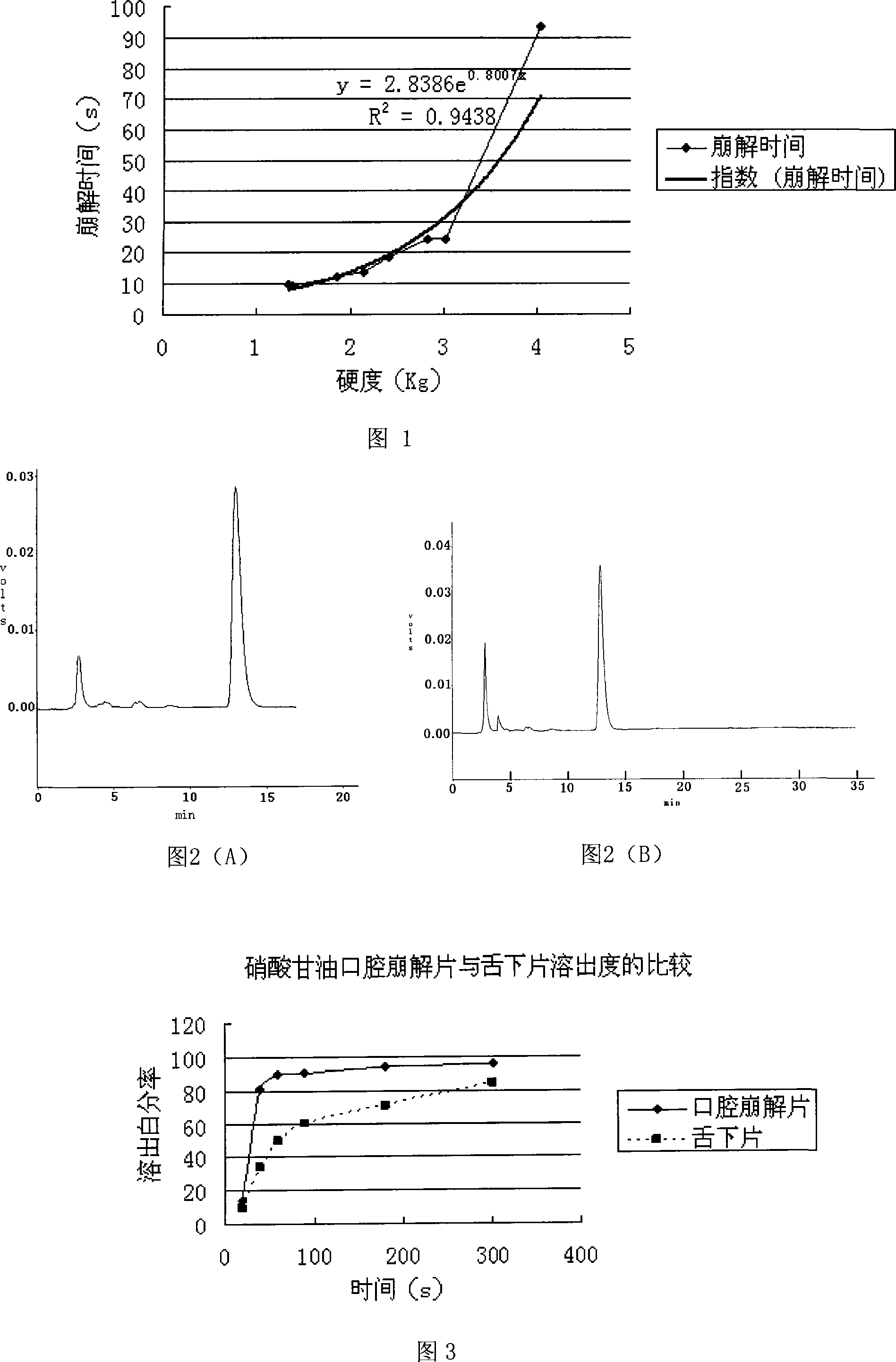
Glonoin Orally disintegrating tablets preparation of and preparing method thereof
InactiveCN101229148AImprove stabilityFast disintegration timePharmaceutical non-active ingredientsPill deliveryOrally disintegrating tabletLow-substituted hydroxypropylcellulose
The invention relates to a nitroglycerin cavity (hypoglossis) disintegrating tablet and a preparation method which mainly adopts water-soluble excipient. When coming across saliva, a disintegrating tablet can be disintegrated rapidly and most of the excipient is dissolved, which can be absorbed into body to recycle by hypoglossis mucosa. The main indigent of the invention is nitroglycerin ethanol solution occupying 10 percent in weight, and the containing weight uses the mixture of lactose and xylitol in every 10g is 0.8 to 0.9ml. The excipient comprises microcrystalline cellulose 101, low-substituted hydroxypropyl cellulose, disintegrant, lactose, xylitol, alcoholic solution of PVP30 and magnesium stearate. The invention adopts a wet method of preparing particle and tablet, combines with blank particle method to mix the blank particles and the particles containing medicine after being made into particles, which changes the traditional method of spraying medicine layer to blank particles and raises the stability of medicine. The pressure of a tabletting machine is adjusted to 1 to 3kg and the disintegration of the pressed tablets is speeded up.
Owner:TIANJIN MEDICAL UNIV
Epalrestat tablet, and its prepn. method
InactiveCN1692903ASimple preparation processHigh hardnessOrganic active ingredientsNervous disorderCarboxymethyl starchLow-substituted hydroxypropylcellulose
A tablet for epalrestat for suppressing the transmission speed decreasement of sciatic nerve and coccygeal nerve and the increasement of sorbitol in red cell and sciatic nerve is prepared from lactose, microcrystalline cellulose, low-substituent hydroxypropyl cellulose, carboxymethyl starch sodium magnesium stearate and epalrestat through sequential mixing, granulating, drying, coating and tabletting.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Low-substituted hydroxypropylcellulose powder and method for producing the same
Provided are a solid dosage form comprising an enteric solid dispersion that allows a drug in the preparation to be rapidly dissolved without compromising the solubility of the solid dispersion, and a method for producing the same. More specifically, provided is a solid dosage form comprising an enteric solid dispersion comprising a poorly soluble drug, an enteric polymer and a disintegrant, wherein the disintegrant is low-substituted hydroxypropylcellulose having an average particle size of 10 to 100 [mu]m and a specific surface area measured by BET method of at least 1.0 m 2 / g. Moreover, provided is a method for producing a solid dosage form comprising an enteric solid dispersion, the method comprising steps of: spraying an enteric polymer solution in which a poorly soluble drug has been dispersed or dissolved, on a powder of low-substituted hydroxypropylcellulose having an average particle size of 10 to 100 [mu] m and a specific surface area measured by BET method of at least 1.0 m 2 / g and serving as a disintegrant; and granulating the resultant; and drying.
Owner:SHIN ETSU CHEM IND CO LTD
Perampanel coated tablet pharmaceutical composition
ActiveCN106389367AHigh hardnessGood disintegrationOrganic active ingredientsNervous disorderSucrosePolyvinyl alcohol
The invention relates to a perampanel coated tablet pharmaceutical composition, in particular to a pharmaceutical composition in the form of tablets. The pharmaceutical composition comprises perampanel, an excipient, a disintegrating agent, a binder and a lubricant, wherein the excipient is selected from lactose, sucrose, glucose, corn starch, mannitol, sorbitol, starch, calcium hydrogen phosphate and the like, the disintegrating agent is selected from agar, calcium citrate, dextrin, low-substituted hydroxypropyl cellulose, carboxymethyl cellulose, calcium carboxymethyl cellulose, croscarmellose sodium, carboxymethyl starch, carboxymethyl starch sodium and the like, the binder is selected from polyvinyl alcohol, methyl cellulose, ethyl cellulose, hydroxypropyl methyl cellulose, hydroxy propyl cellulose, sodium carboxymethyl cellulose, polyvinylpyrrolidone, polyethylene glycol and the like, and the lubricant is selected from magnesium stearate, calcium stearate, talcum powder, polyethylene glycol, colloidal silicon dioxide and the like. The invention further relates to a preparation method and application of the composition. The pharmaceutical composition has excellent properties as described in the description.
Owner:HANGZHOU ZHUYANGXIN PHARMA
Live-keeping transport box for aquatic products
ActiveCN104920281ASimple structureLittle stressPisciculture and aquariaAdditive ingredientLow-substituted hydroxypropylcellulose
The invention discloses a live-keeping transport box for aquatic products. The live-keeping transport box comprises a box body with an open top, wherein an oxygenating agent layer, a sponge layer and a pressing plate are sequentially arranged in the box body from bottom to top, a closed cavity is formed in the middle of the pressing plate, a cushion chamber is formed by the closed cavity, circulation holes which are uniformly distributed are formed in the top face of the pressing plate, capillary holes which are uniformly distributed are formed in the bottom face of the pressing plate, the circulation holes and the capillary holes are communicated with the cushion chamber, and an oxygenating agent in the oxygenating agent layer is prepared from the following ingredients in percentage by mass: 5-10% of vermiculite powder, 10-15% of microcrystalline cellulose, 2-5% of low-substitution hydroxypropyl cellulose, 2-5% of lactose and the balance of calcium peroxide. The live-keeping transport box for the aquatic products is simple in structure and convenient to transport; and the oxygenating agent is adopted to oxygenate water in the transport box, and a formula of the oxygenating agent is subjected to optimization and improvement, so that the oxygenating effect is good, the stress effect on the aquatic products is low, and thus the increase of the survival rate of the aquatic products is facilitated.
Owner:MARINE FISHERIES RES INST OF ZHEJIANG
High-efficient oral silibinin sustained-release preparation and preparation method thereof
ActiveCN101164537AImprove solubilityRapid drug releaseOrganic active ingredientsDigestive systemMedicineLow-substituted hydroxypropylcellulose
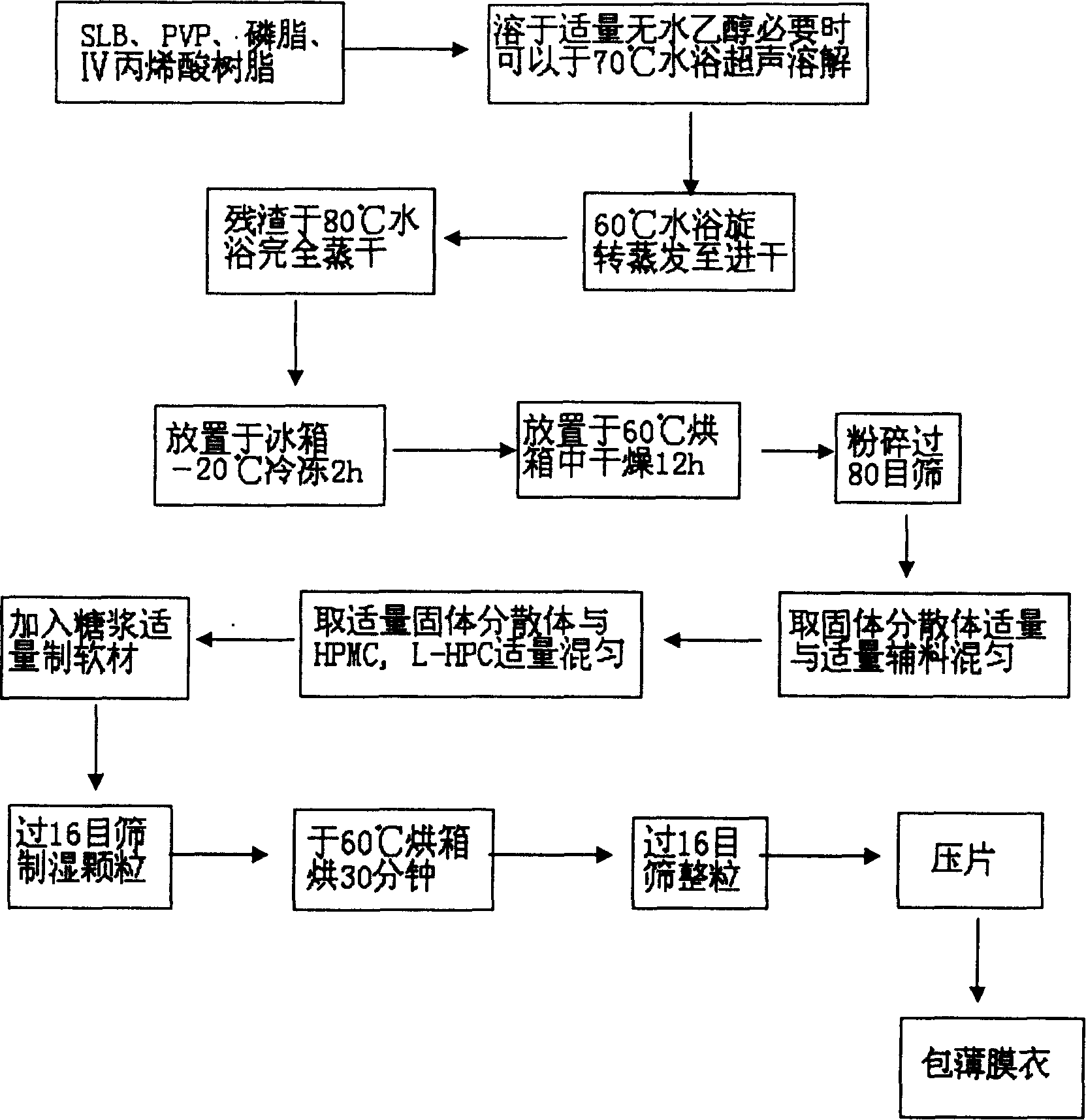
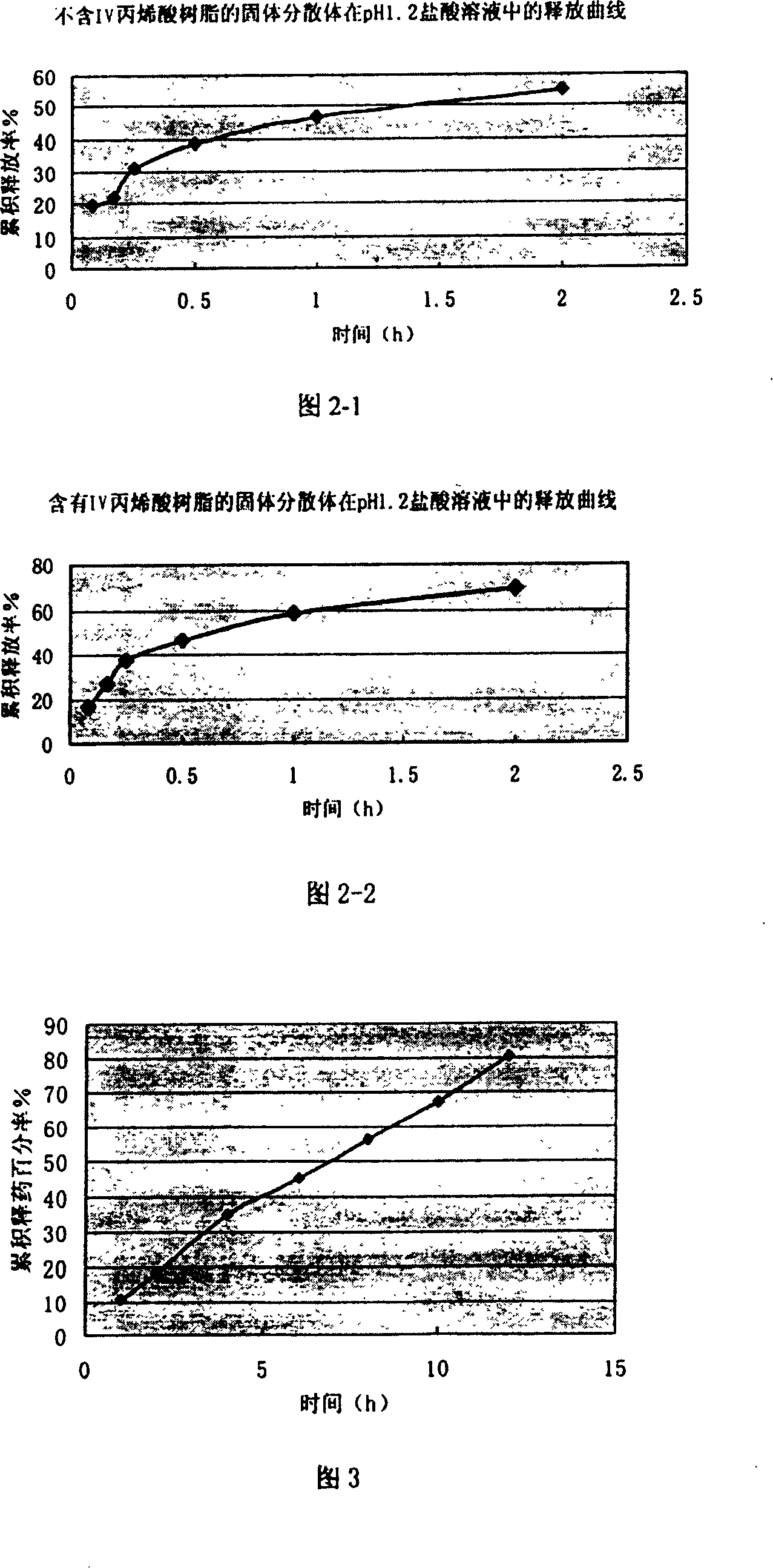
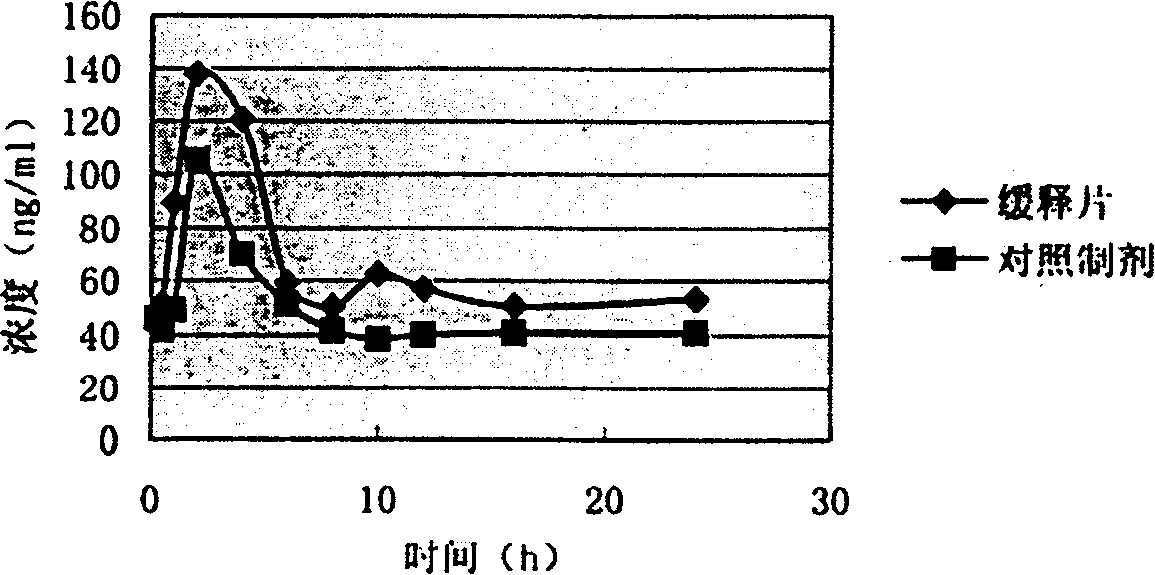
The present invention relates to a high-effective oral silibinin (SLB) slow-released preparation. Its composition includes (by mass component portion) 1 portion of silibinin, 1.5-2.5 portions of polyvidone-K30, 0.23-0.58 portion of hydroxypropyl methyl cellulose 4000cPa.S, 0.46-1.38 portions of low-substituted hydroxypropyl cellulose. Said invention adopts the combination of solid dispersion technique and slow-released hydrophilic gel skeleton technique to raise the dissolubility of silibinin.
Owner:JIANGSU UNIV
Cefditoren pivoxil tablet and its preparation method
ActiveCN102949359AHigh dissolution rateImprove bioavailabilityAntibacterial agentsOrganic active ingredientsMedicineLow-substituted hydroxypropylcellulose
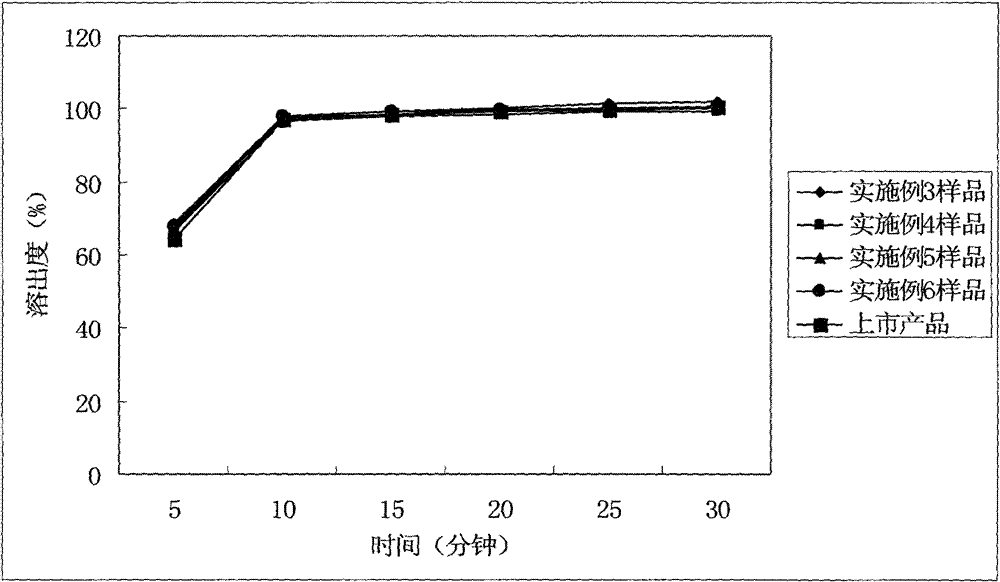
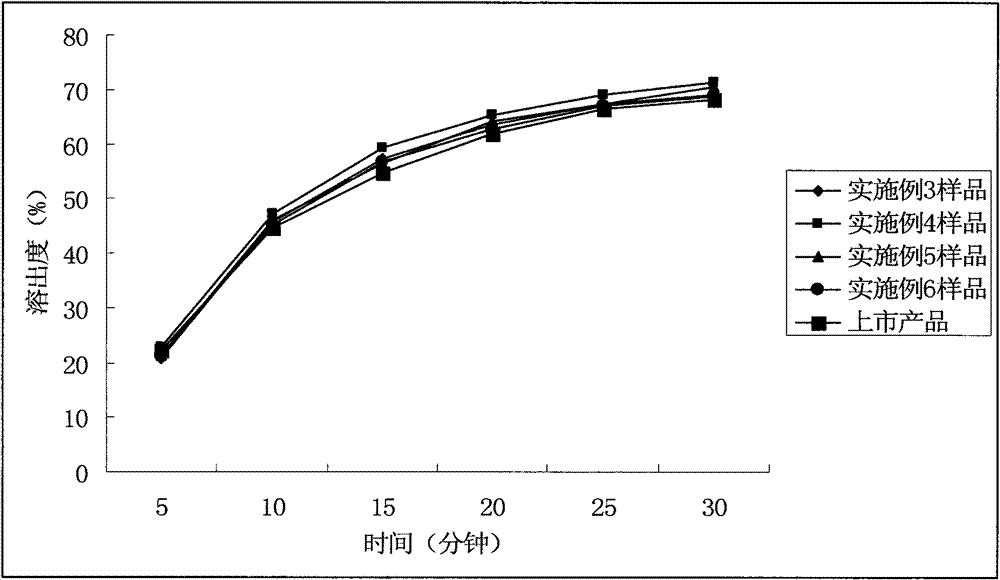
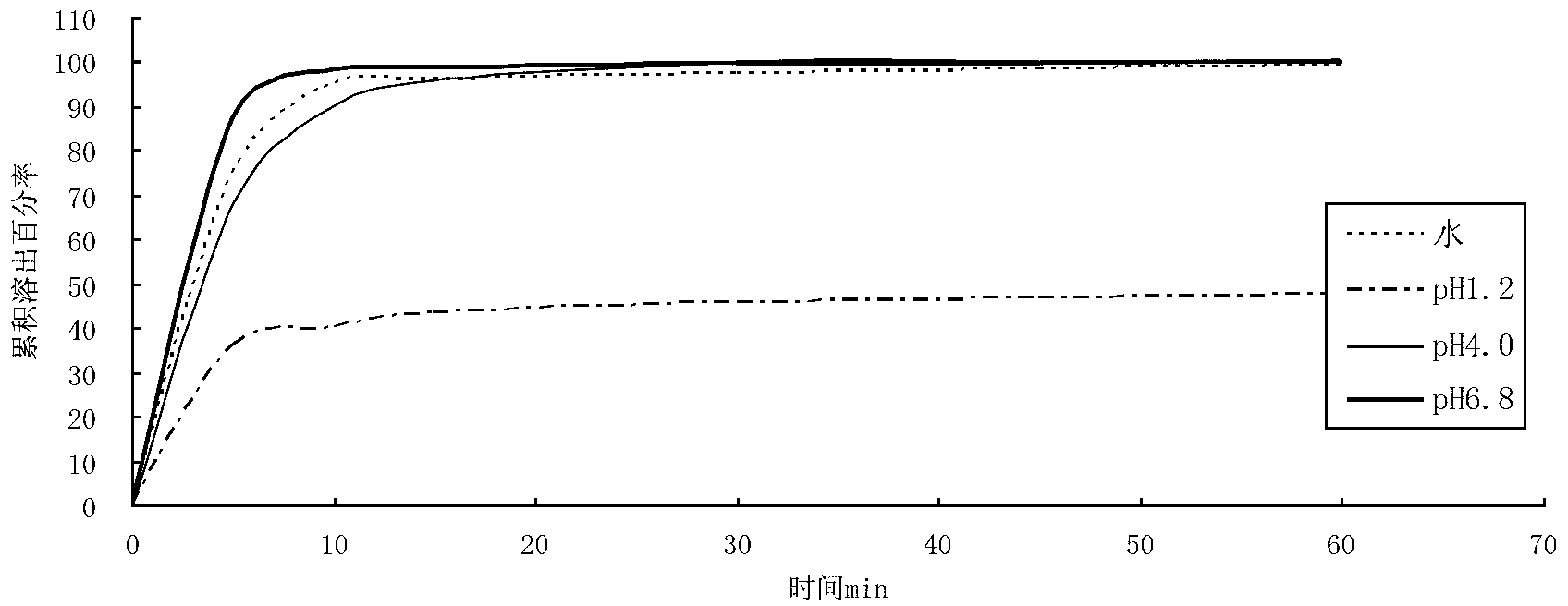
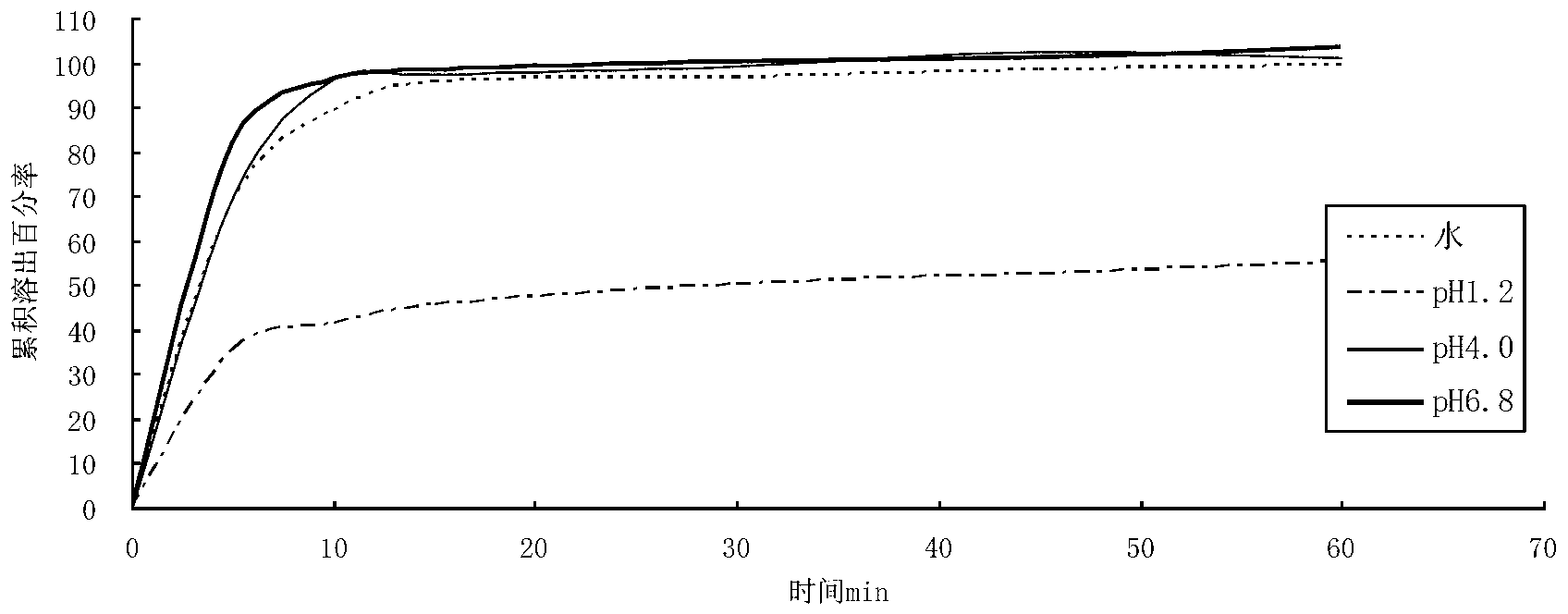
The invention relates to a cefditoren pivoxil tablet. The tablet comprises a tablet core and a coating layer, wherein the tablet core contains amorphous cefditoren pivoxil, a filler, a disintegrating agent, a solubilizer and a lubricant, and the filler is a mixture of D-mannitol and beta-cyclodextrin and / or low-substituted hydroxy propyl cellulose. The cefditoren pivoxil tablet avoids the conversion of amorphous cefditoren pivoxil to crystals and has good disintegration and dissolve-out properties under four dissolve-out conditions, and the dissolve-out curve of the cefditoren pivoxil tablet is consistent with the dissolve-out curve of a listed product Meiact.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Berberine hydrochloride tablet and preparation method thereof
ActiveCN103301084ANo dependencyHigh hardnessOrganic active ingredientsMetabolism disorderFiller ExcipientLow-substituted hydroxypropylcellulose
The invention discloses a preparation method of a berberine hydrochloride tablet, and the preparation method is applied to the field of pharmaceutical preparation. The berberine hydrochloride tablet consists of a tablet core and a coating material, wherein the tablet core consists of berberine hydrochloride and auxiliary materials, each tablet contains 300mg of the berberine hydrochloride, and the auxiliary materials contains one or a plurality of a filling agent, a disintegrating agent, a lubricant, a surfactant and a wetting agent; a coating layer consists of the coating material and water; the coating layer further contains a plasticizer or an antiadherent, wherein the filling agent contains one or a random composition of starch, lactose, pregelatinized starch and microcrystalline cellulose; the disintegrating agent contains one or a random composition of crosslinking sodium carboxymethyl cellulose, polyvinylpolypyrrolidone, sodium carboxymethyl starch, hydroxypropyl starch and low-substitution hydroxyl propyl cellulose; and the lubricant contains one or a random composition of aerosol, talc powder and magnesium stearate. The method is suitable for the large-scale and long-time production of production departments, and has the advantages of stable technology, no dependence on tablet machines, good particle liquidity and formation, controllable tablet weight difference, stable quality, high tablet core hardness, difficulty in wearing and coating suitability.
Owner:SHENYANG NO 1 PHARMA FACTORY DONGBEI PHARMA GRP
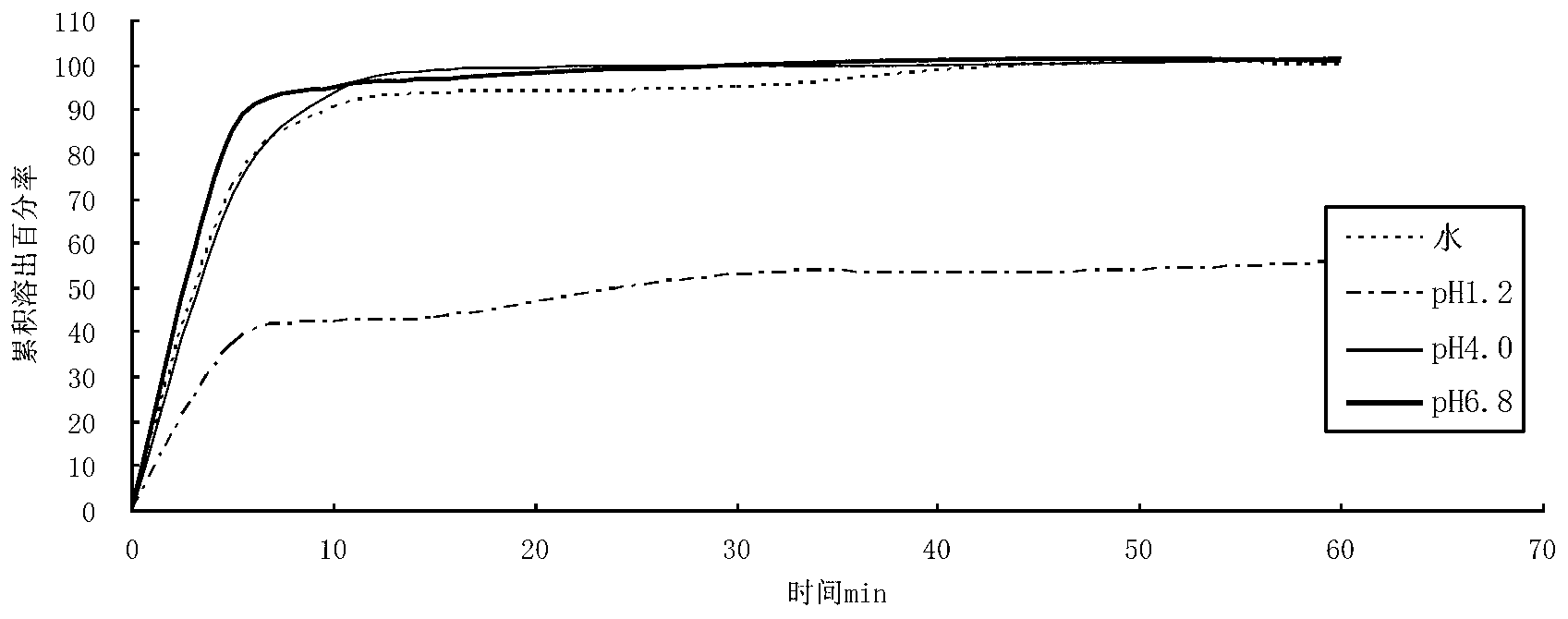
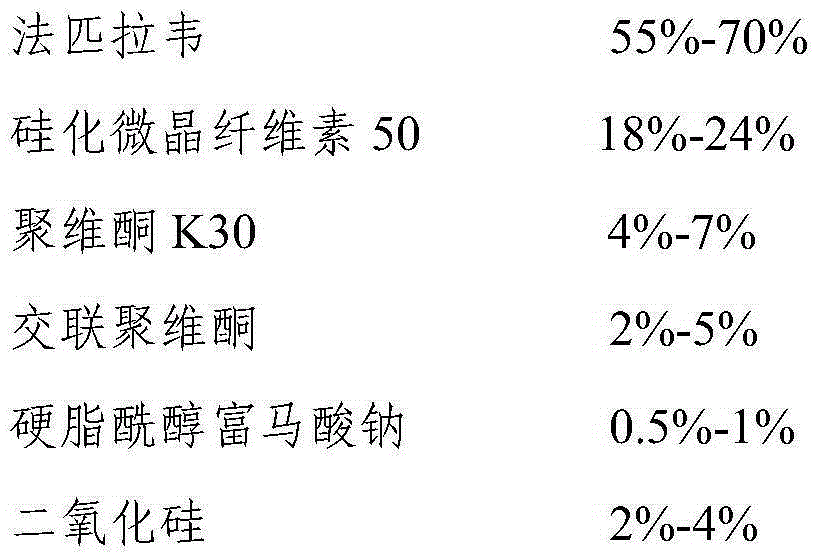
Favipiravir tablets and preparation method thereof
ActiveCN106667926APrescription costs are lowReduce sizeOrganic active ingredientsAntiviralsMedicineAdhesive
The invention relates to favipiravir tablets. The tablets comprise favipiravir, silicified microcrystalline cellulose and adhesive; the silicified microcrystalline cellulose is lower in price, proper in size and high in medicine compliance when being compared with low-substituted hydroxypropyl cellulose; the defective rate is lower and the dissolution rate is higher. The invention further relates to a preparation method of the tablets. The preparation method is simple in preparation process and suitable for industrialized large-scale production.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Asiabell polysaccharide tablet of asiabell effective ingredient
InactiveCN1733050AExpand drug sourceSufficient sources of medicineOrganic active ingredientsDigestive systemAdditive ingredientLow-substituted hydroxypropylcellulose
Disclosed is an asiabell polysaccharide tablet of asiabell effective ingredient, wherein the main ingredients include codonopsis pilosula polysaccharides 100-600 parts, the auxiliary materials include low substituted methylcellulose propylene glycol ether or crystalline cellulose 2-4 parts, and magnesium stearate 0.5-1.5 parts, cocoa powder or mannitol.
Owner:叶美曾
Solid instant preparation extracted from ginkgo leaf and its preparing process
InactiveCN1623593APromote dissolutionGood dispersionNervous disorderDigestive systemFreeze-dryingMedicine
An instant solid preparation of gingko leaf extract is disclosed, which includes the dispersing tablet prepared by freeze drying, spray drying, or direct tabletting press and coating, tabletting press methods, and the oral disintegrating tablet prepared from gingko leaf extract, disintegrant, filler, adhesive, lubricant and flavouring.
Owner:BEIJING BOERDA BIO TECH DEV
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com