High-efficient oral silibinin sustained-release preparation and preparation method thereof
A technology for silibinin and sustained-release preparations, which is applied in the field of preparation of high-efficiency oral sustained-release preparations for poorly soluble drugs, can solve the problems of not providing in vivo measurement results, and reducing the number of times of medication, and achieves faster release speed, improved solubility, and improved release. constant rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
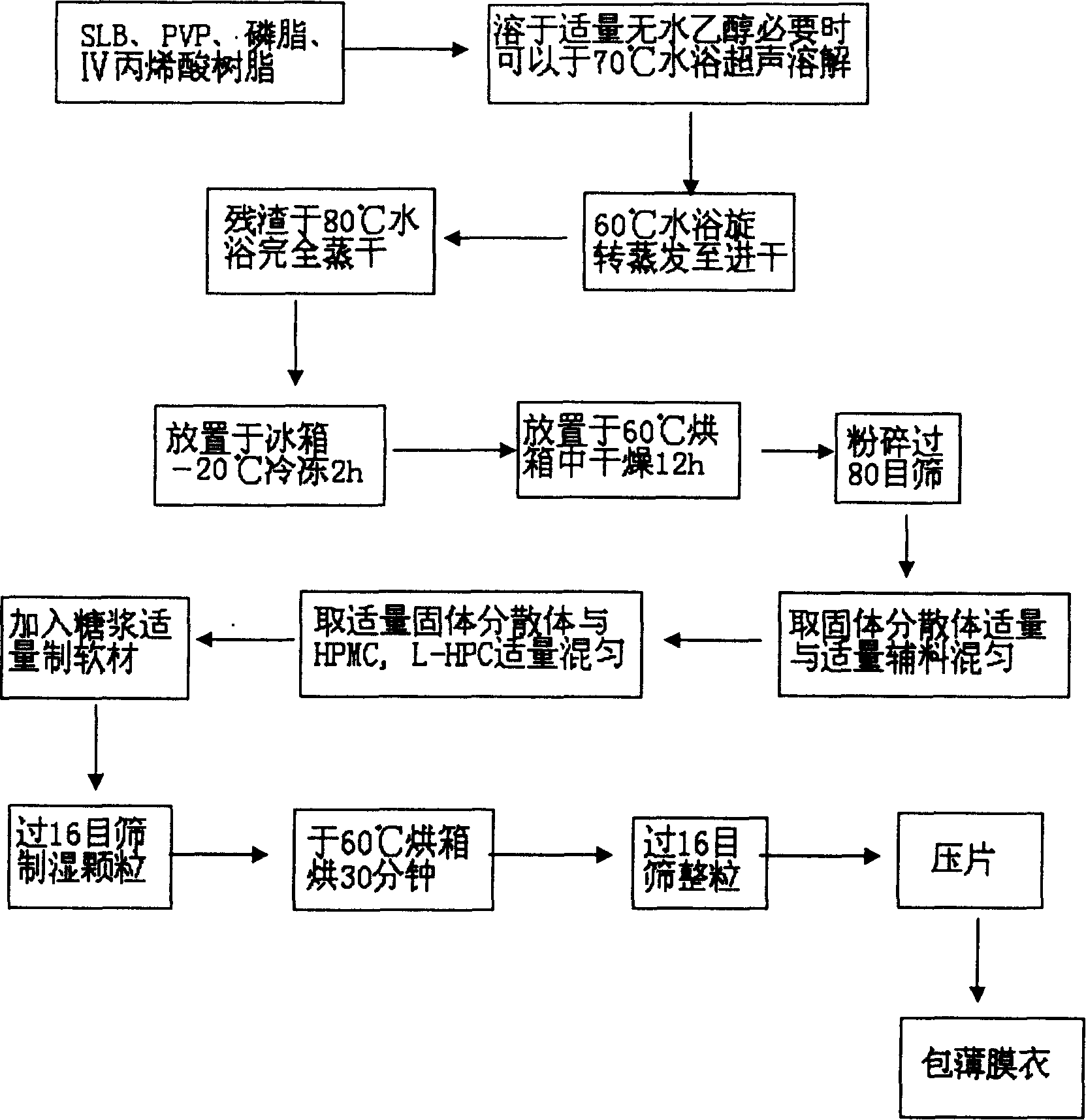
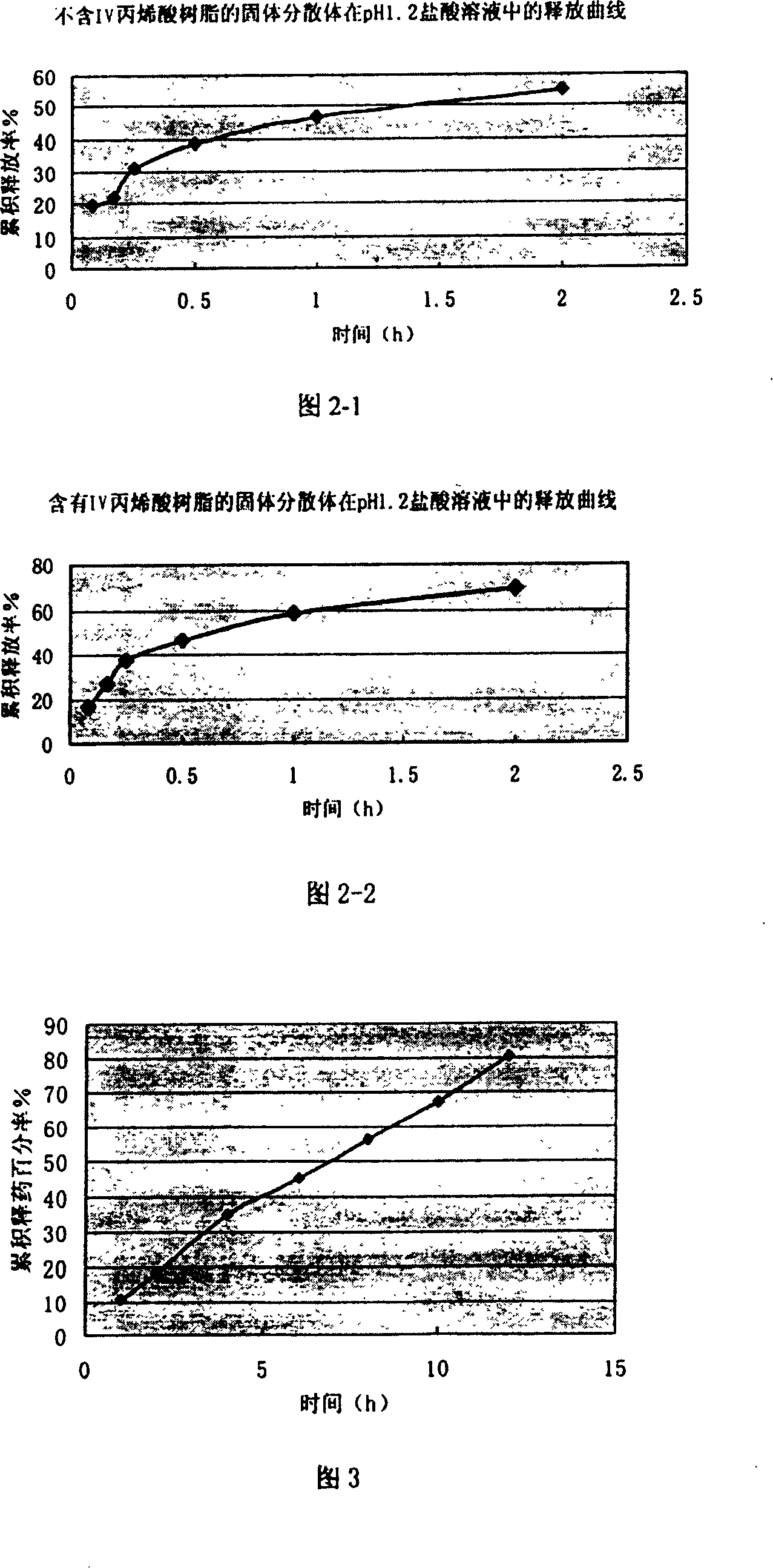
[0032] 1. Weigh 2g of SLB, 4g of PVP-K30, 1g of phospholipid, 0.6g of IV acrylic resin, add 20ml of absolute ethanol to dissolve (if necessary, put it in a 70°C water bath to accelerate the dissolution), put it in a 60°C water bath, and evaporate at 90rpm When it is nearly dry, evaporate the solvent completely in a water bath at 70°C, put it in a refrigerator at -20°C for 2 hours, then put it in an oven at 60°C for 12 hours, pulverize it, and pass it through an 80-mesh sieve to obtain a solid dispersion, which is set aside.
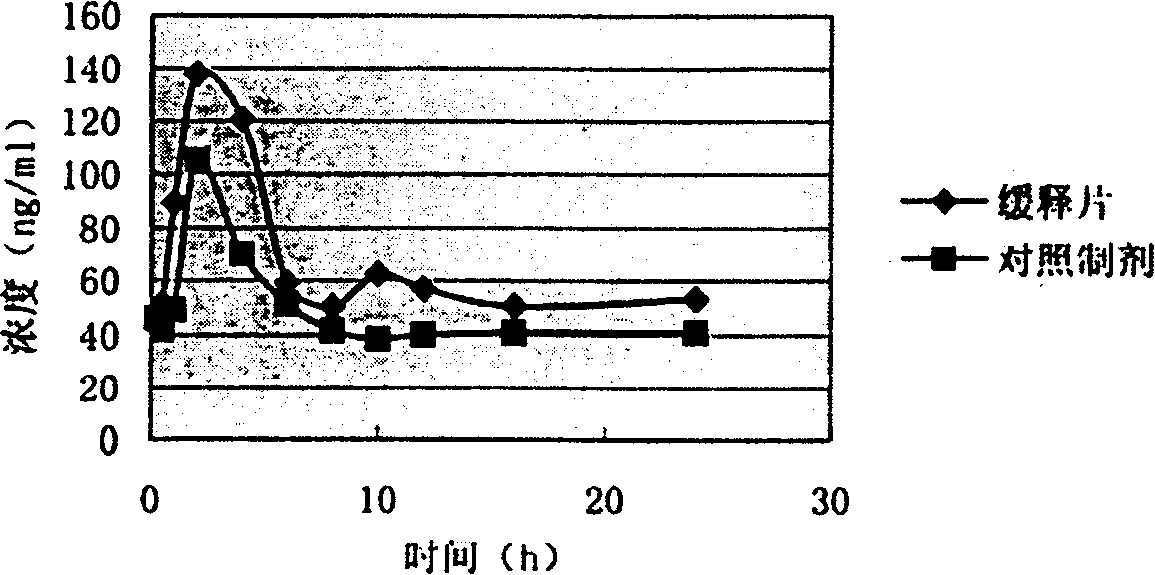
[0033] 2. Take 7g of solid dispersion, mix with HPMC4000cPa·s 0.5g, L-HPC 1g, after mixing, add an appropriate amount of 70% syrup to prepare soft material, pass through a 16-mesh sieve to obtain wet granules, bake at 60°C for 30 minutes, and take out , passed through a 16-mesh sieve for sizing, tableting, and the pressure was controlled at 40-60N to obtain 34 nude sustained-release tablets.
[0034] 3. Put 0.058g of IV acrylic resin into a beaker, add 1....
Embodiment 2
[0036] 1. Weigh 2g of SLB, 4g of PVP-K30, 1g of phospholipid, 0.6g of IV acrylic resin, add 20ml of absolute ethanol to dissolve (if necessary, put it in a 70°C water bath to accelerate the dissolution), put it in a 60°C water bath, and evaporate at 90rpm When it is nearly dry, evaporate the solvent completely in a water bath at 70°C, put it in a refrigerator at -20°C for 2 hours, then put it in an oven at 60°C for 12 hours, pulverize it, and pass it through an 80-mesh sieve to obtain a solid dispersion, which is set aside.
[0037] 2. Take 7g of solid dispersion, mix with 2g of HPMC4000cPa·s and 3g of L-HPC, after mixing, add an appropriate amount of 70% syrup to prepare soft material, pass through a 16-mesh sieve to obtain wet granules, bake at 60°C for 30 minutes, and then take it out. Sieve through a 16-mesh sieve for granulation, press into tablets, and control the pressure at 40-60N to obtain 48 bare sustained-release tablets.
[0038] 3. Put 0.07g of IV acrylic resin in...
Embodiment 3
[0040] 1. Weigh 2g of SLB, 3g of PVP-K30, 0.8g of phospholipid, 0.4g of IV acrylic resin, add 20ml of absolute ethanol to dissolve (if necessary, put it in a water bath of 70°C to accelerate the dissolution), and then rotate in a water bath of 60°C at 90rpm Evaporate to nearly dryness, evaporate the solvent completely in a water bath at 70°C, place in a refrigerator at -20°C for 2 hours, then place in an oven at 60°C for 12 hours, pulverize, pass through an 80-mesh sieve to obtain a solid dispersion, and set aside.
[0041] 2. Take 6.3g of solid dispersion, mix with HPMC4000cPa·s 0.9g, L-HPC 1.35g, after mixing, add an appropriate amount of 70% syrup to prepare soft material, pass through a 16-mesh sieve to obtain wet granules, and bake at 60°C for 30 minutes Then take it out, pass through a 16-mesh sieve for granulation, and press into tablets with a pressure controlled at 40-60N to obtain 34 bare tablets of sustained-release tablets.
[0042] 3. Put 0.054g of IV acrylic resi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com