Favipiravir tablets and preparation method thereof
A technology of pravir tablets and favipiravir, which is applied in the field of medicine, can solve the problems of decreased drug compliance, low fluidity, and lack of compression molding properties, and achieves simple preparation process, good drug compliance, and good dissolution Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
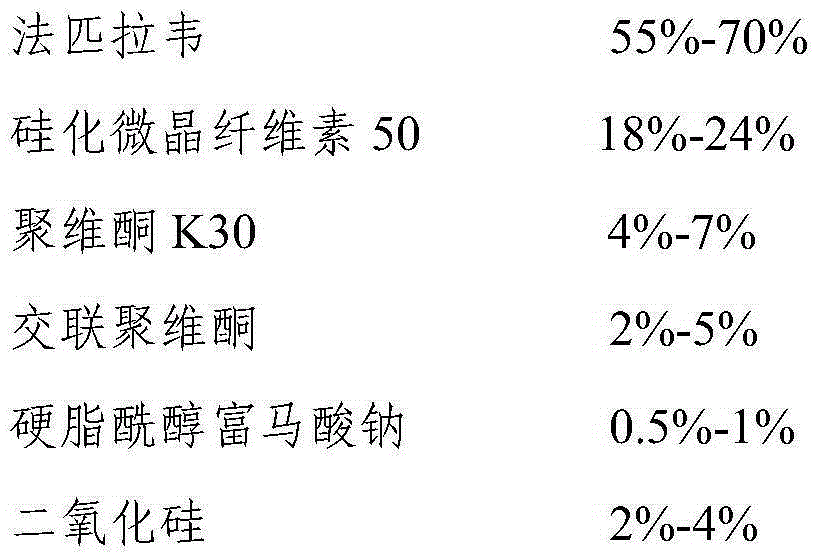
[0036] The selection of embodiment 1 favipiravir tablet excipient
[0037] Weigh a certain amount of Favipiravir and each auxiliary material listed in Table 1 according to the prescription ratio, sieve and mix 10 times, add water to make a soft material, granulate with a 32-mesh sieve, dry at 50°C, and granulate with a 32-mesh sieve , add the converted sodium stearyl fumarate, mix well, and compress into tablets.
[0038] Table 1 Favipiravir tablet test results
[0039]
[0040] Remarks: The slash " / " in the above table indicates that the indicator has not been measured.
[0041] From the test results in the above table, it can be known that using microcrystalline cellulose as an excipient and disintegrant, if tablets with acceptable quality (such as suitable hardness, low friability and dissolution qualification) are to be prepared, the tablet size to be prepared should be Very large; using silicified microcrystalline cellulose 50 and low-substituted hydroxypropyl cellul...
Embodiment 4-13
[0042] Embodiment 4-13 favipiravir tablet binder, excipient consumption and sheet shape selection
[0043] Weigh a certain amount of Favipiravir listed in Table 2 and Table 3 according to the prescription ratio and the dosage of each auxiliary material, sieve and mix 10 times, add water to make a soft material, granulate with a 32-mesh sieve, dry at 50 ° C, 32-mesh Sieve for granulation, add the converted sodium stearyl fumarate, mix well, and press into tablets.
Embodiment 14-18
[0054] Embodiment 14-18 and comparative example 2-3 favipiravir tablet raw material particle size selection
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com