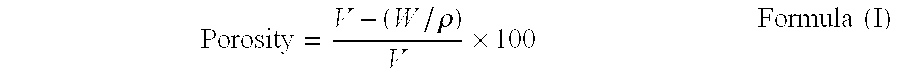Patents
Literature
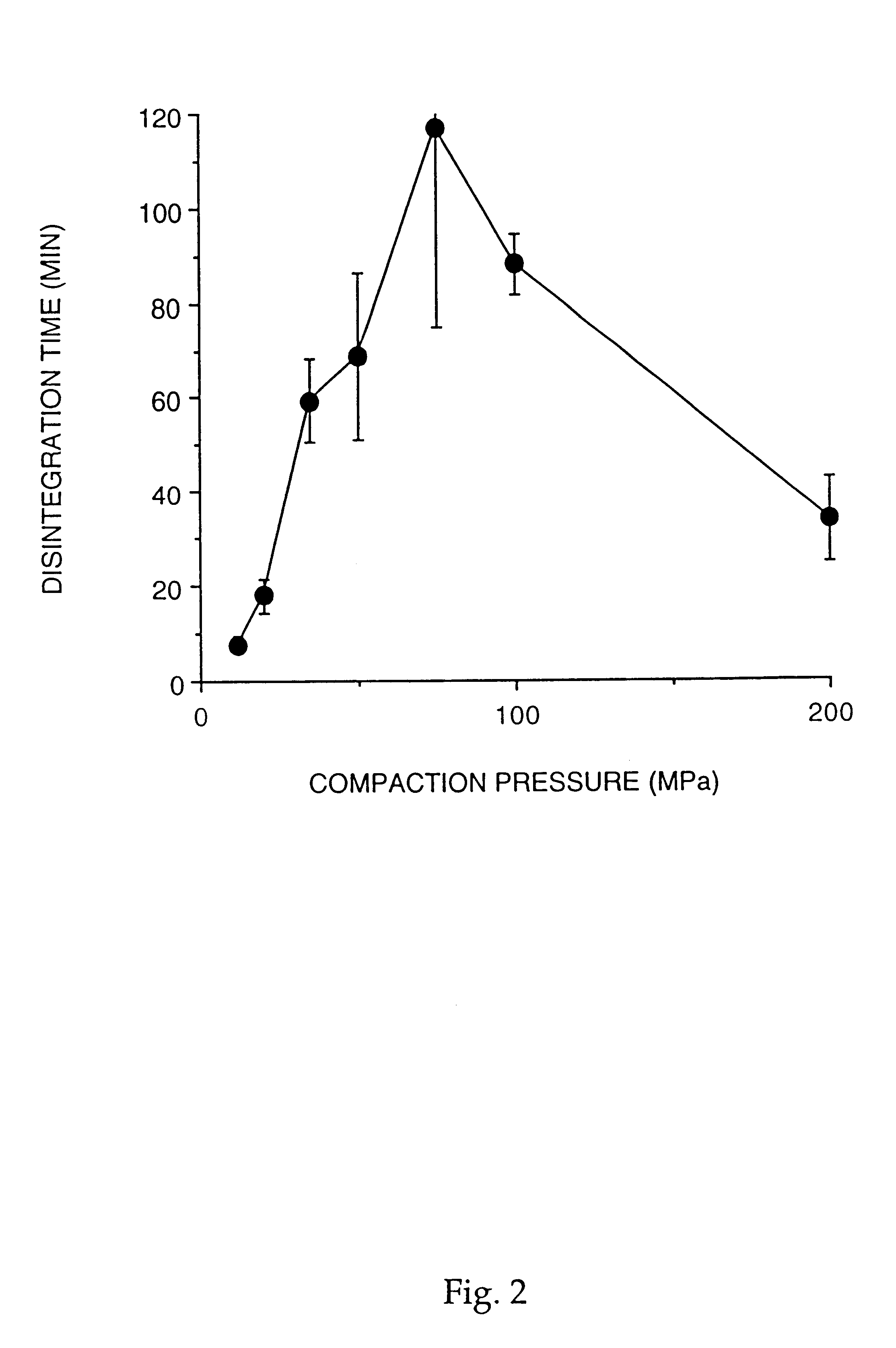
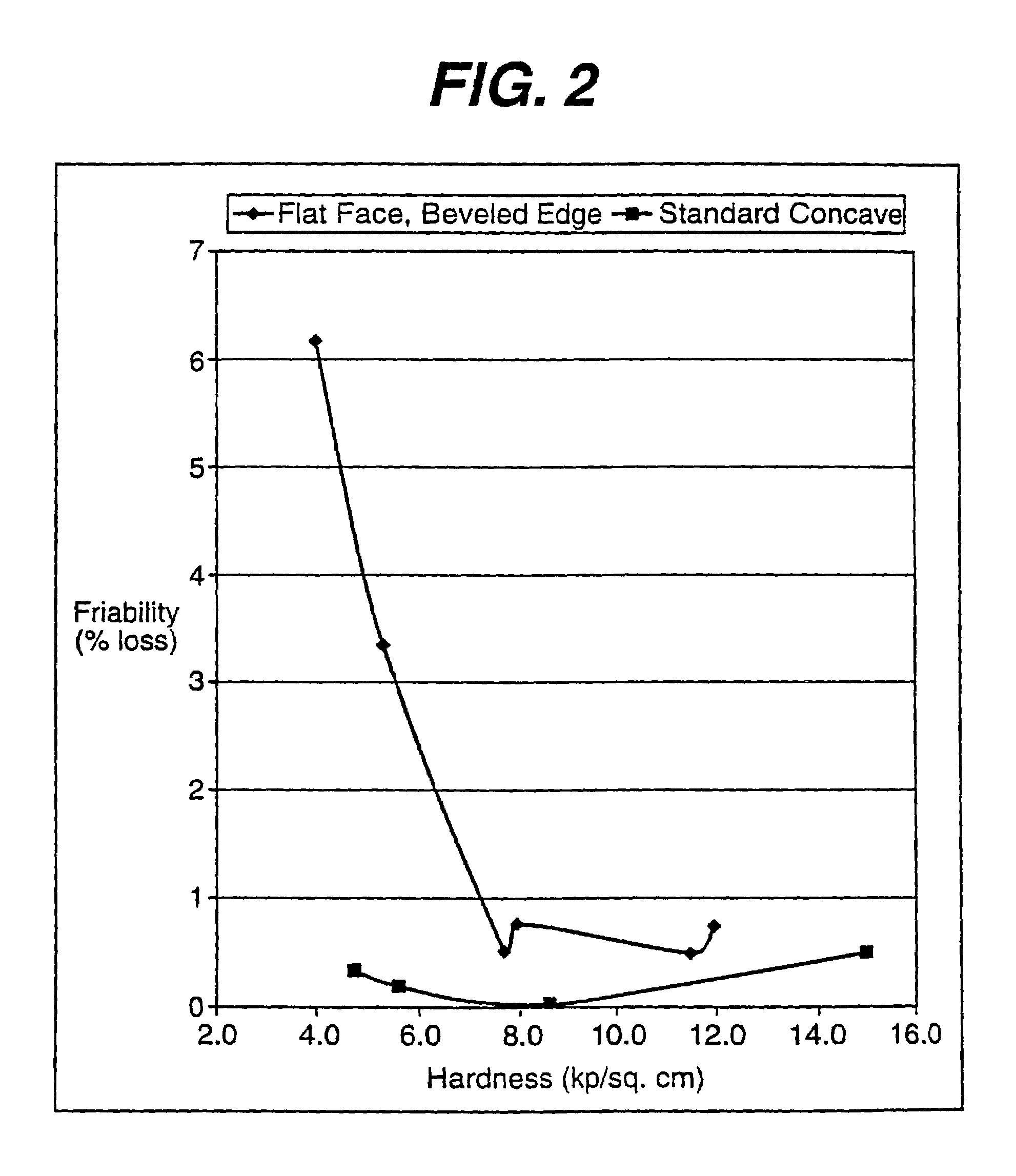
293 results about "Friability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Friability (/ˌfraɪəˈbɪləti/ FRY-ə-BIL-ə-tee), the condition of being friable, describes the tendency of a solid substance to break into smaller pieces under duress or contact, especially by rubbing. The opposite of friable is indurate.
Solid form
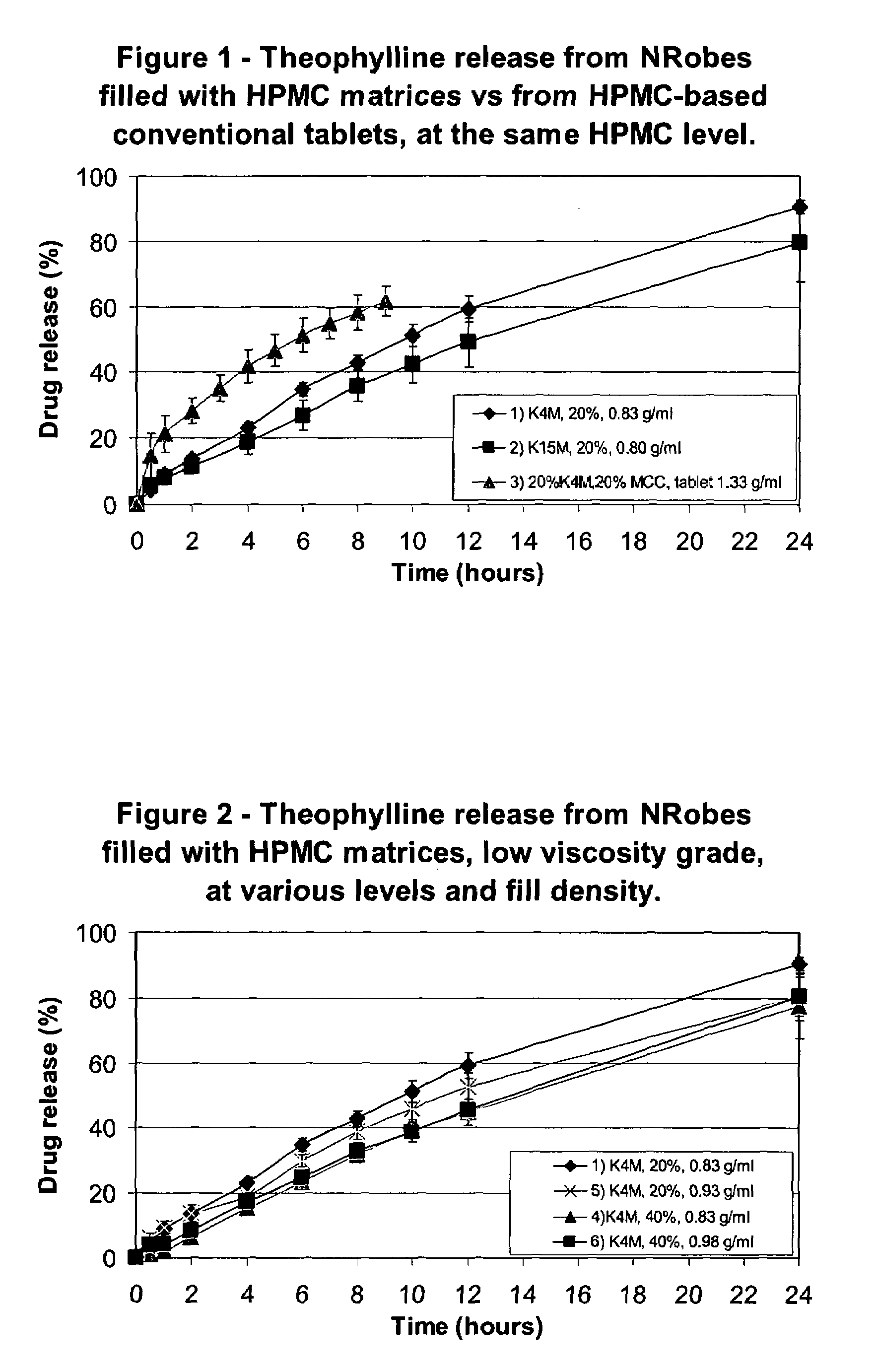
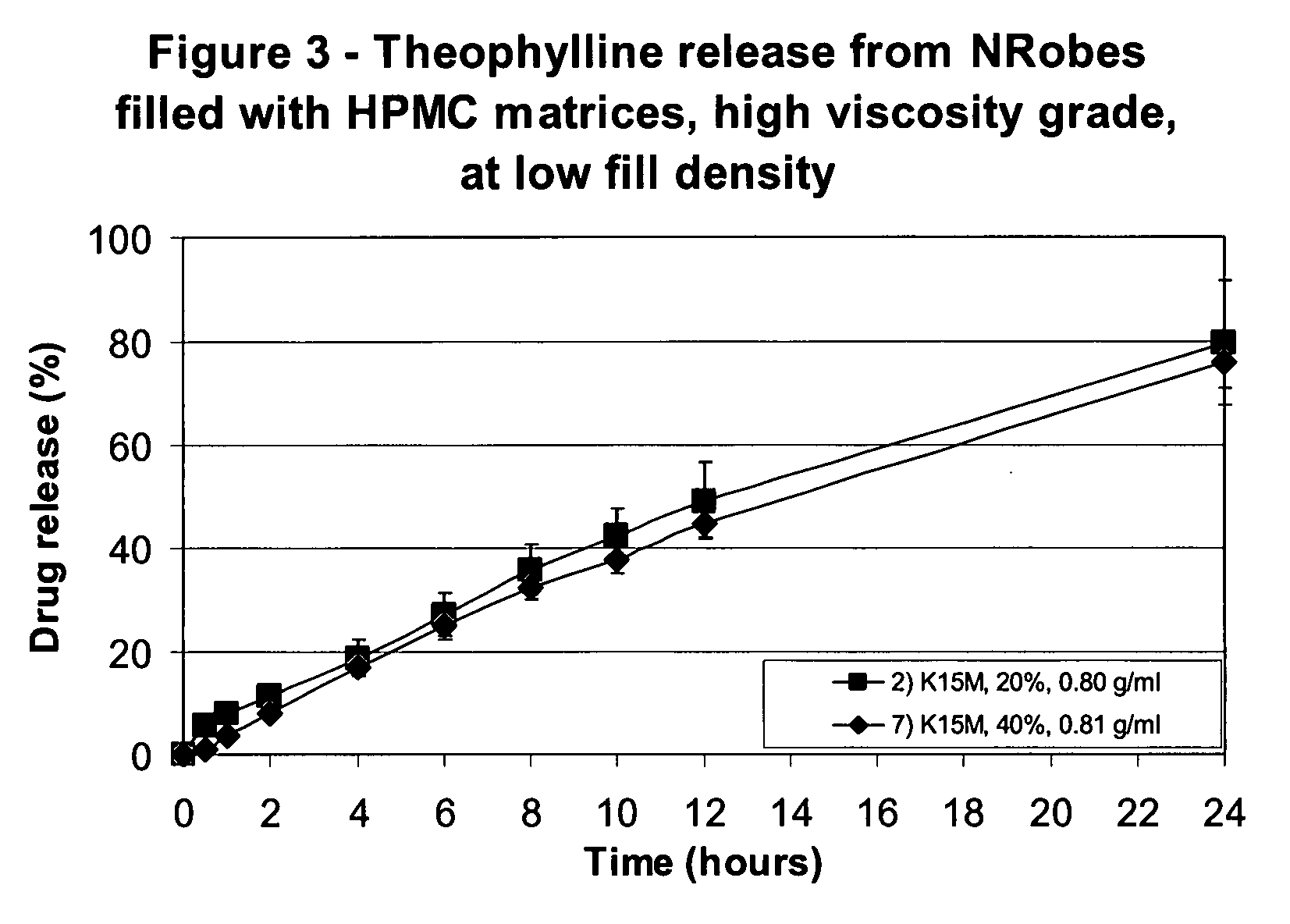
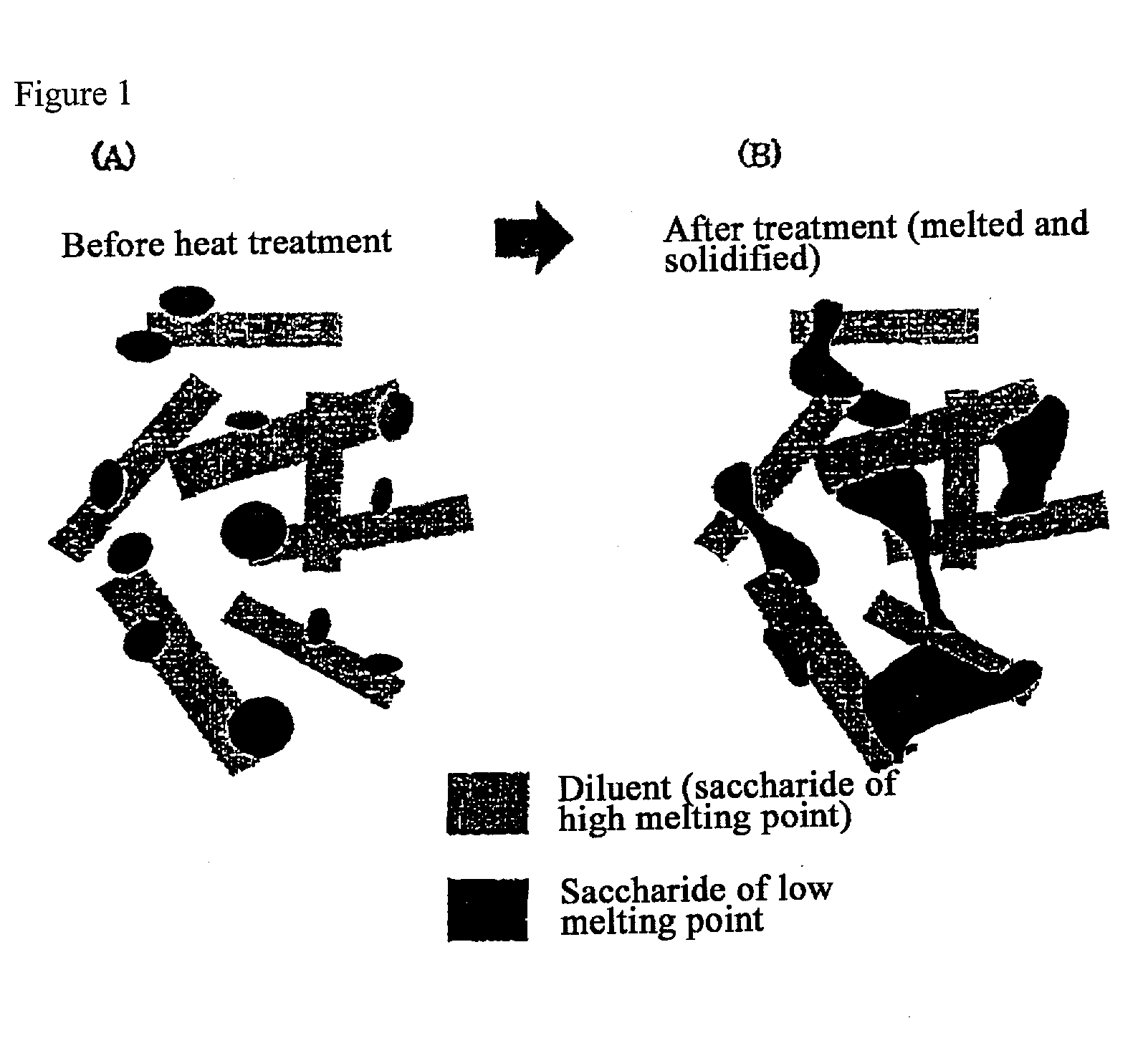
InactiveUS20080286344A1Strong enoughAvoids and reduces processing and product drawbackPowder deliveryNervous disorderFilling materialsVolumetric Mass Density
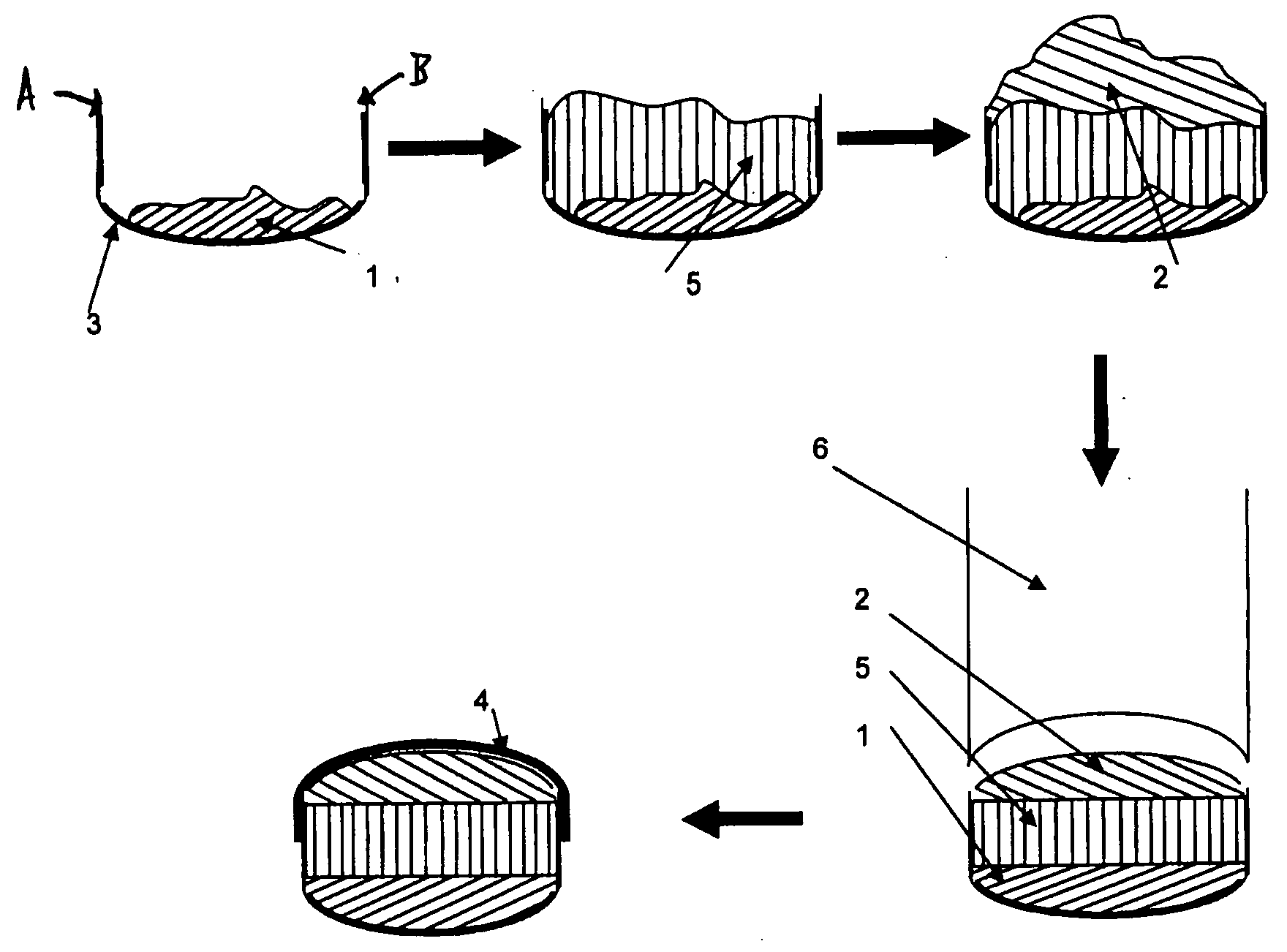
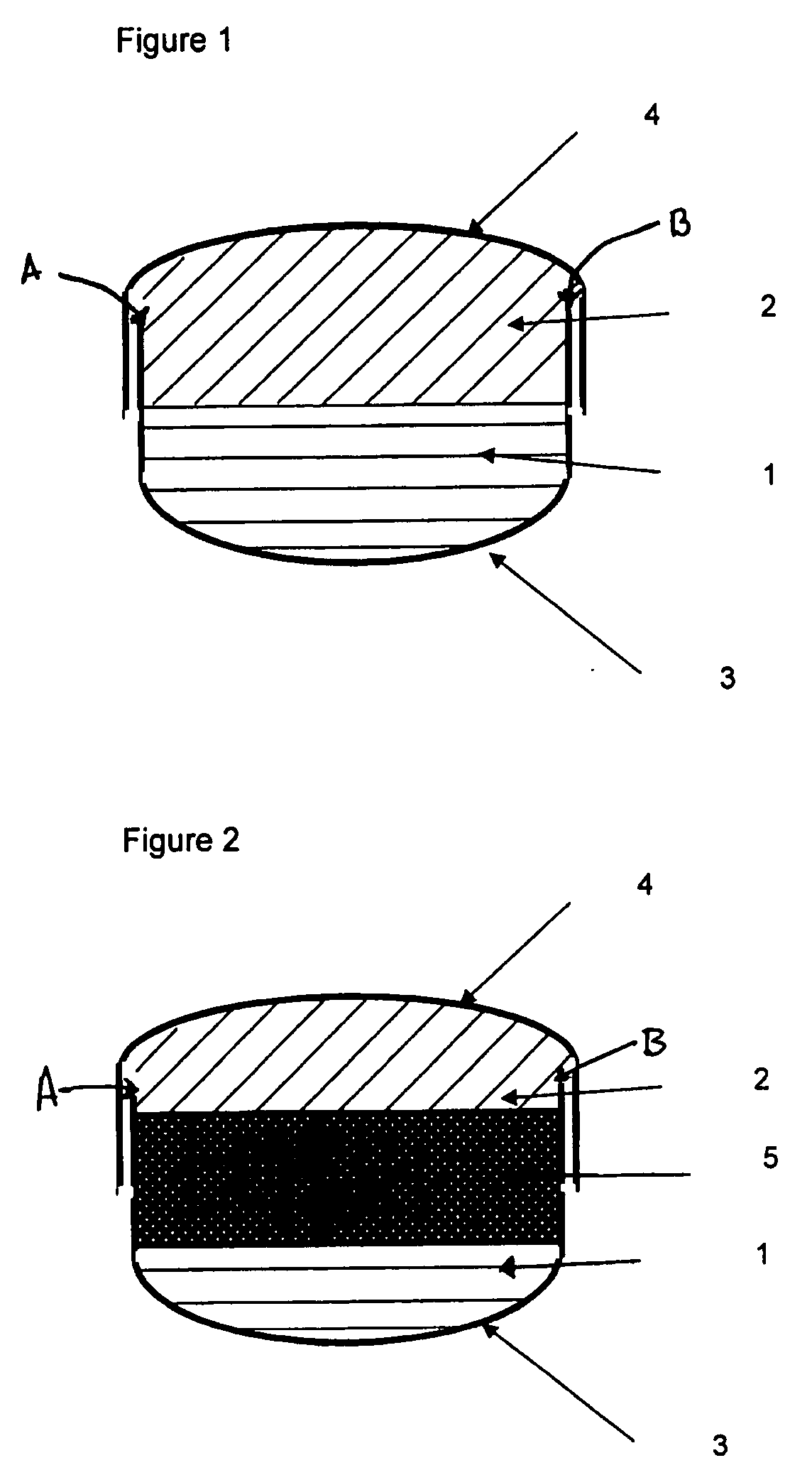
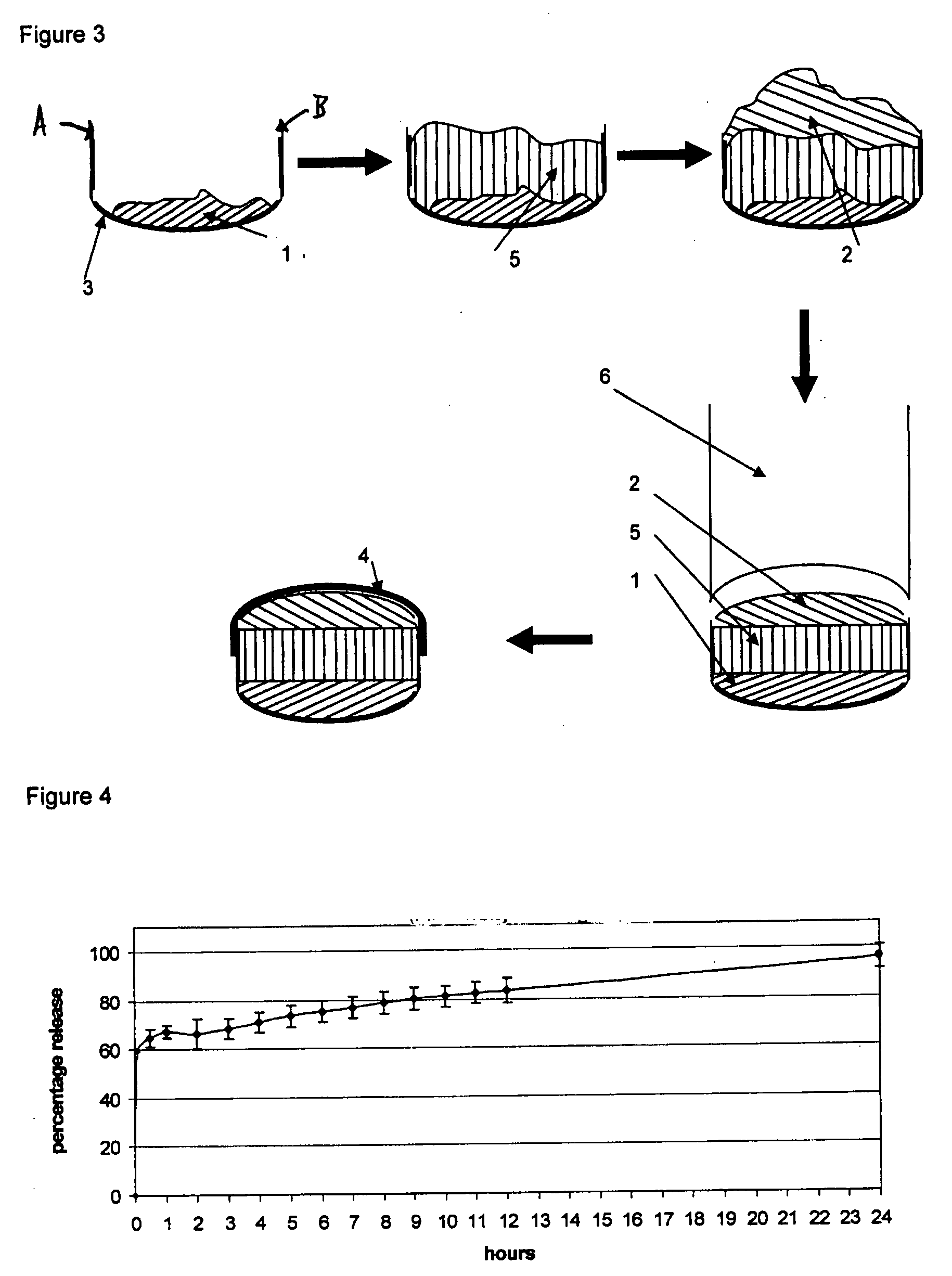
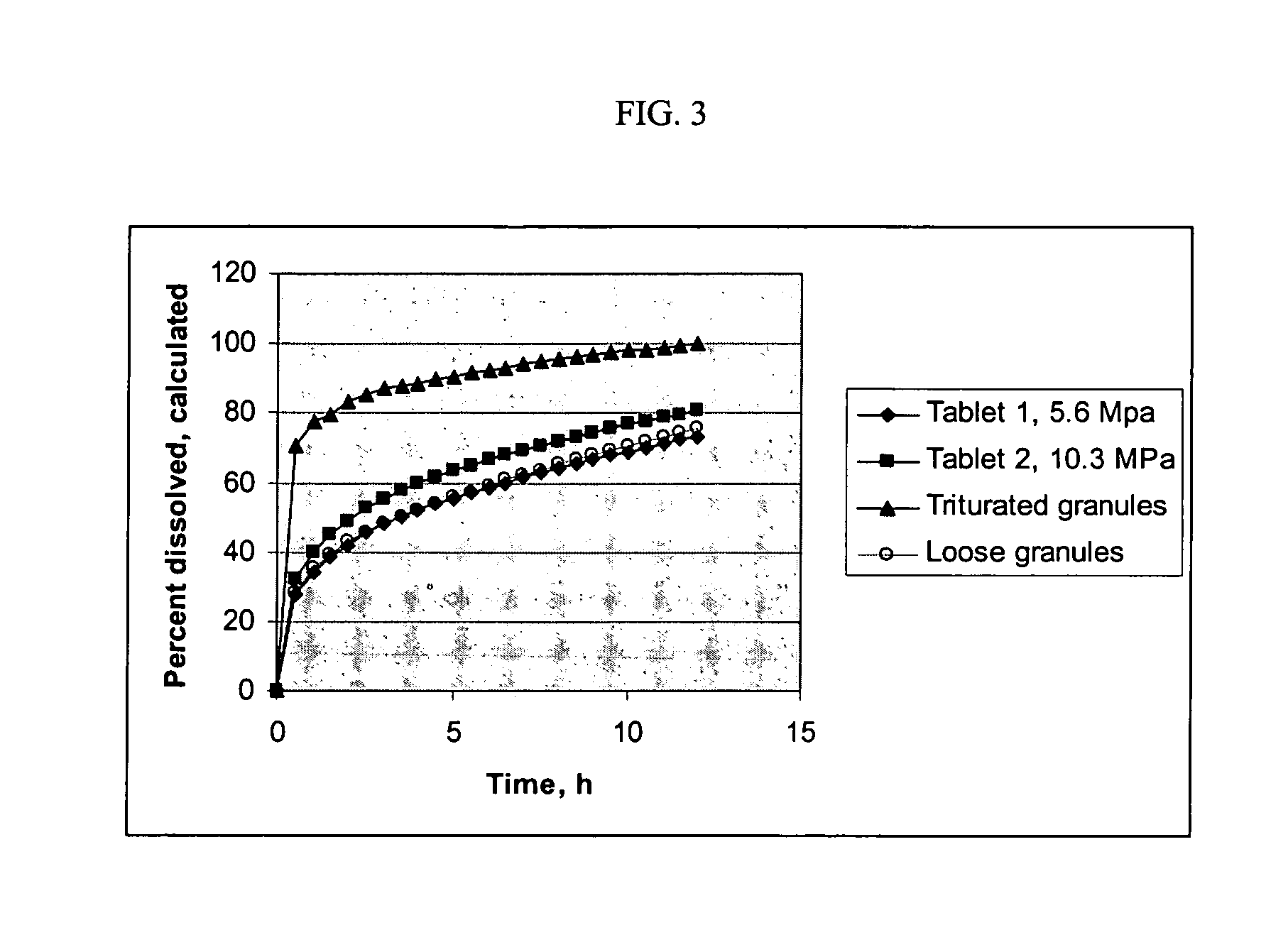
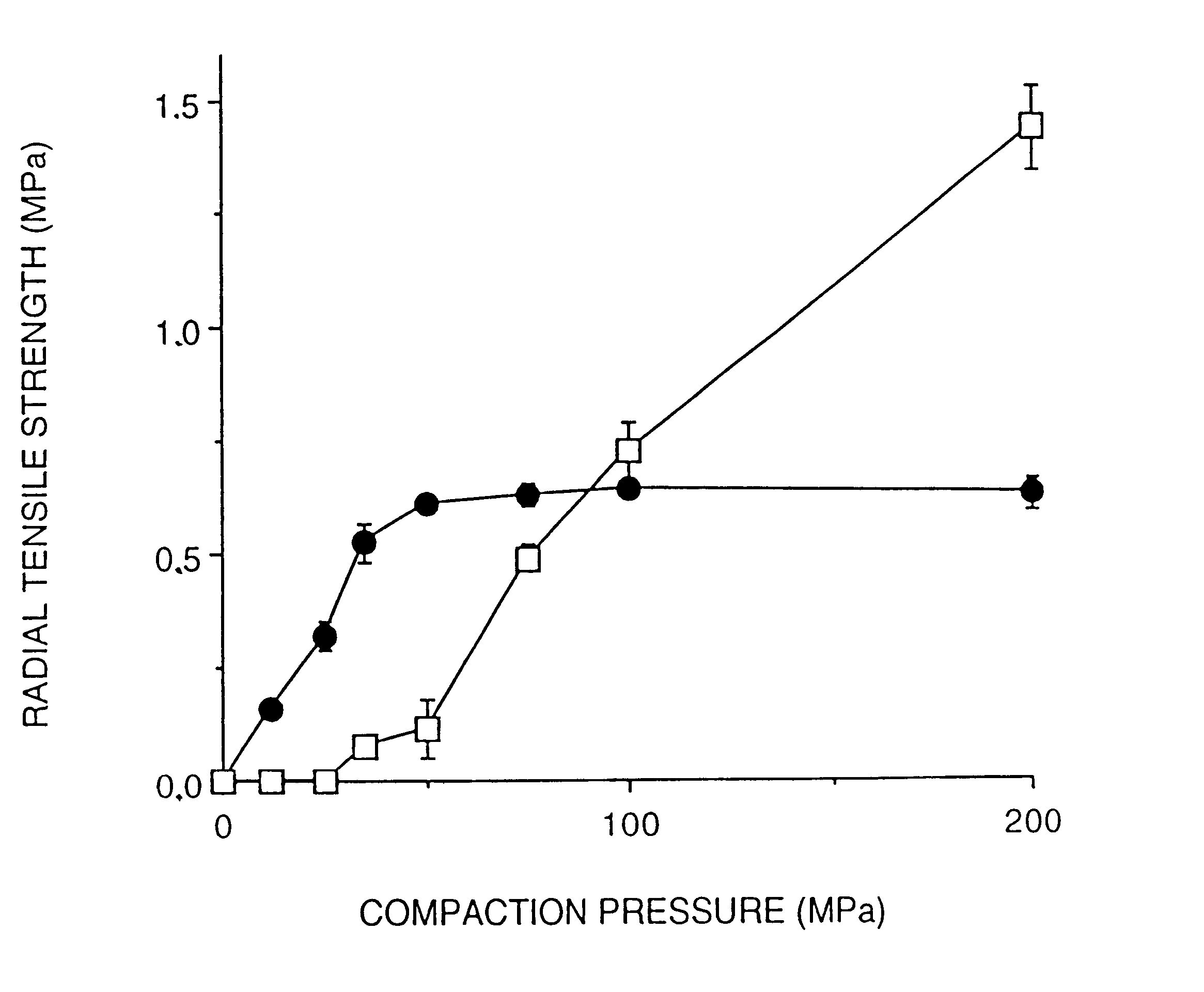
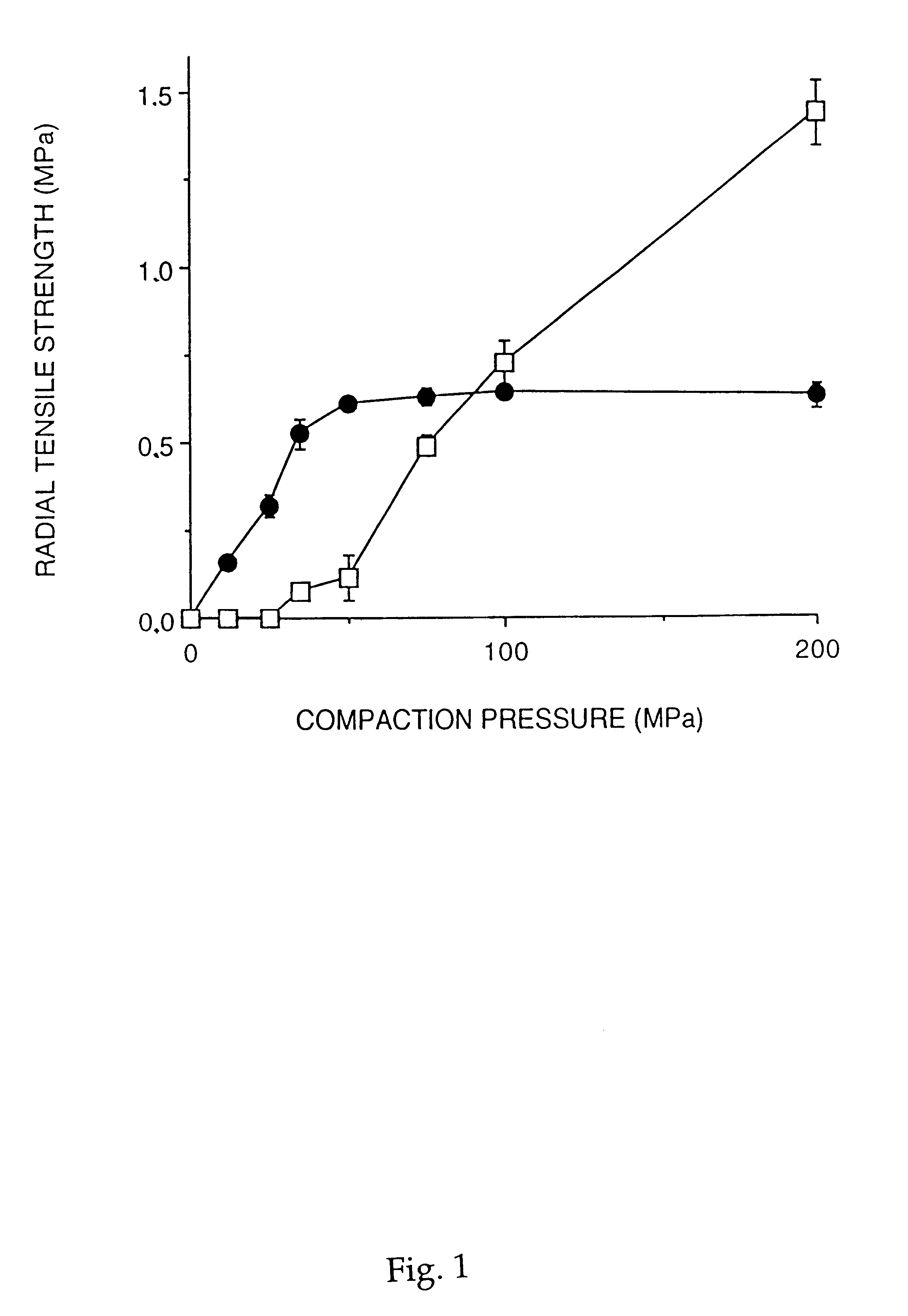
A solid form comprising at least one film enrobing a compacted fill material wherein:i) the compacted fill material comprises at least one active material;ii) the solid form shows a weight loss that is less than 1% during a 30 minutes USP friability test United States Pharmacopeia (USP) 29 Test Number 1216 (page 3046);iii) the compacted fill material has a density of at least 0.5 g / ml based on the total solid volume of the solid form and a tensile strength of less than 0.9 MPa; andiv) the compacted fill material is present in the solid form in at least a first zone and a second zone and the active material is present in at least one of the zones.
Owner:FMC CORP
Solid form
InactiveUS20080014228A1Reduce deliveryImprove robustnessCosmetic preparationsNervous disorderFilling materialsImmediate release
An enrobed solid form comprising a film enrobing a compacted fill material having at least one active material, the solid form shows a weight loss that is less than 1% during a 30 minutes USP Friability Test, the fill material having a density of at least 0.5 g / ml based on the total solid volume of the solid form and a tensile strength less than 0.9 MPa, and the at least one active material within the solid form has an immediate release profile. The solid form is useful in effective delivery of high dose levels of active material.
Owner:FMC CORP
Hemostatic Bioabsorbable Device with Polyethylene Glycol Binder
ActiveUS20130149343A1Improves friabilityImprove wettabilityNon-adhesive dressingsPeptide/protein ingredientsFibrinogen DusardPolyethylene glycol
A hemostatic pad comprising a bioabsorbable scaffolding material; a lyophilized thrombin powder, a lyophilized fibrinogen powder, and a meltable binder powder, with all powders disposed on the bioabsorbable scaffolding material. A meltable binder such as PEG bonds the lyophilized thrombin powder and the lyophilized fibrinogen powder to the bioabsorbable scaffolding material for improved friability, wettability and performance in a use, such as for hemostatic treatment or sealing at a wound site.
Owner:ETHICON INC
Preparation method of pellet-type formula granules
InactiveCN107744510ASimple preparation processImprove compliancePharmaceutical product form changeCoatingsMedicineMoisture absorption
The invention discloses a preparation method of pellet-type formula granules. The method comprises the following steps: (1) selecting at least one of the traditional Chinese medicinal materials; (2) respectively extracting the medicinal materials selected in step (1) for obtaining extracts, or respectively grinding the medicinal materials, or respectively carrying out partial extraction and partial grinding, thus obtaining pretreated materials; and (3) preparing the pretreated materials obtained in step (2) into pellets, thus obtaining the pellet-type formula granules. According to the preparation method provided by the invention, on the basis of the prior art, the preparation process of the formula granules is innovated, the formula granules are innovated into pellets, the fluidity and moisture absorption resistance are good, the friability is small, the shape is round and uniform as well as regular, the content uniformity is small, and the divided dose is accurate.
Owner:GUANGDONG LUOFUSHAN SINOPHARM
Oral care products comprising silica
A rapidly disintegrating oral care tablet is provided. The tablet comprises: a silica; a super disintegrant; and a sugar alcohol. When immersed in water the tablet has a friability of less than about 2% and disintegrates in less than about 60 seconds.
Owner:J M HUBER CORP
Rapidly disintegrating tablets comprising calcium carbonate
Solid-form, orally-administered, rapidly disintegrating pharmaceutical products and oral care tablets are provided. The tablet comprises: a calcium carbonate; a super disintegrant; and a sugar alcohol. When immersed in water the tablet has a friability of less than about 2% and disintegrates in less than about 60 seconds.
Owner:J M HUBER CORP
Rapidly dissolving tablets comprising low surface area calcium phosphates
InactiveUS20070196477A1Effective quick dissolving resultReduce brittlenessCosmetic preparationsToilet preparationsCalcium biphosphateMedicine
This invention pertains to the ability to provide rapidly disintegrating tablets through the inclusion of a calcium phosphate material in combination with other common tablet components. Such a calcium phosphate material must exhibit a sufficiently low surface area in order to boost the ability of the table to separate quickly when introduced into a user's mouth cavity. Such a tablet is dimensionally stable prior to use (low friability) and, when immersed in water the tablet disintegrates therein in less than about 60 seconds.
Owner:WITHIAM MICHAEL C +2
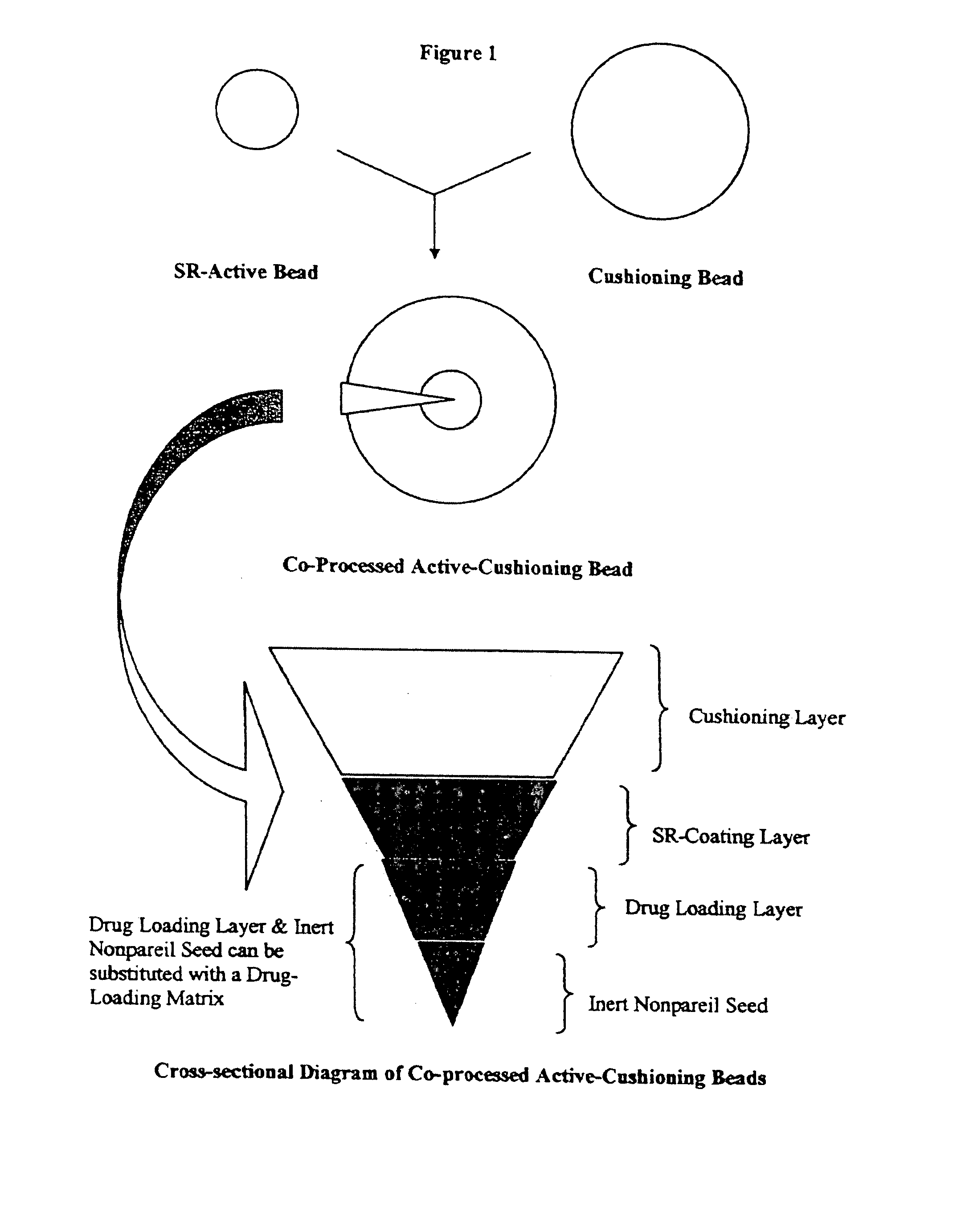
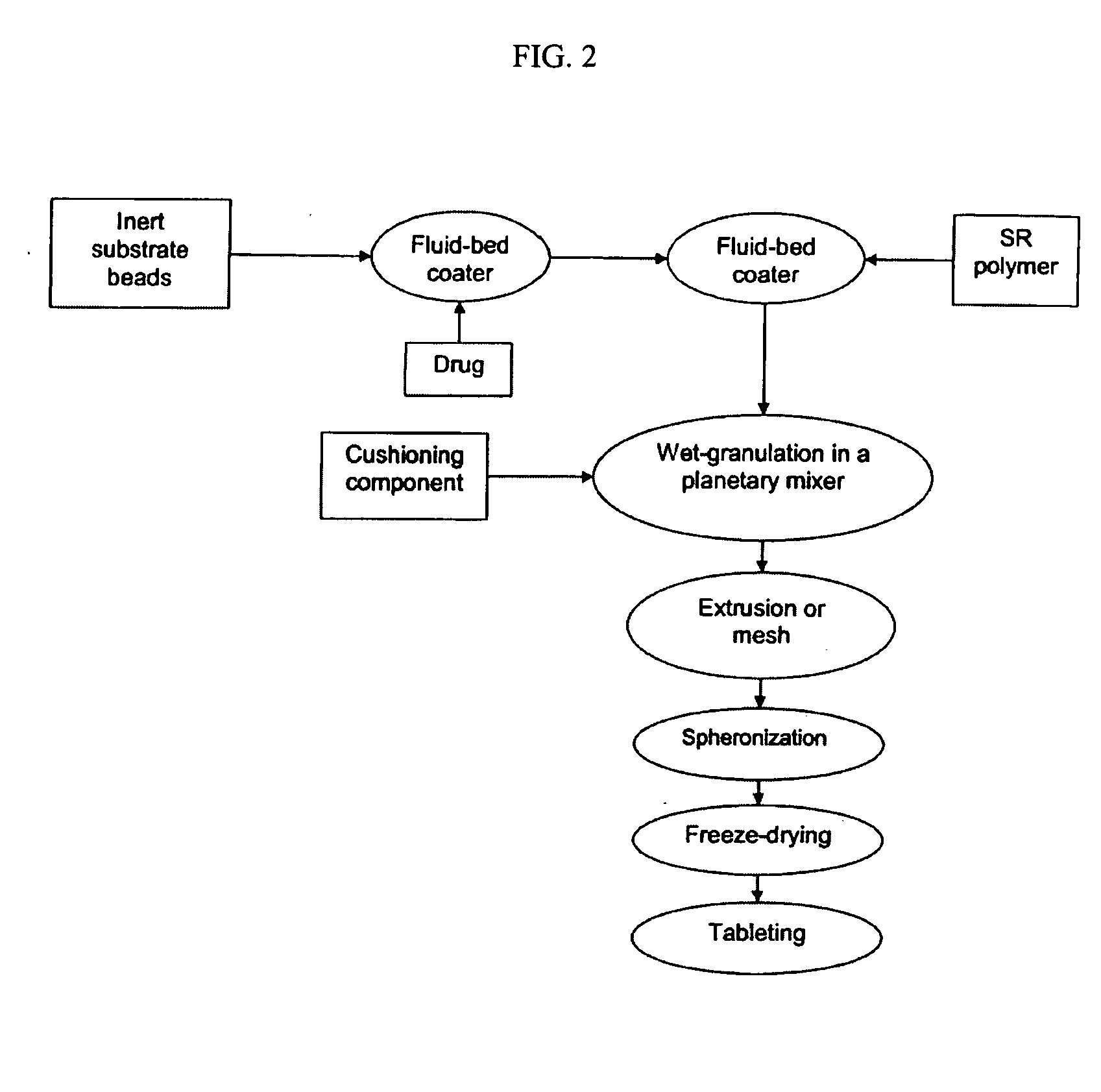
Methods for making pharmaceutical dosage forms containing active cushioning components
InactiveUS20050019393A1Uniform compositionPowder deliveryOrganic active ingredientsCushioningDosage form
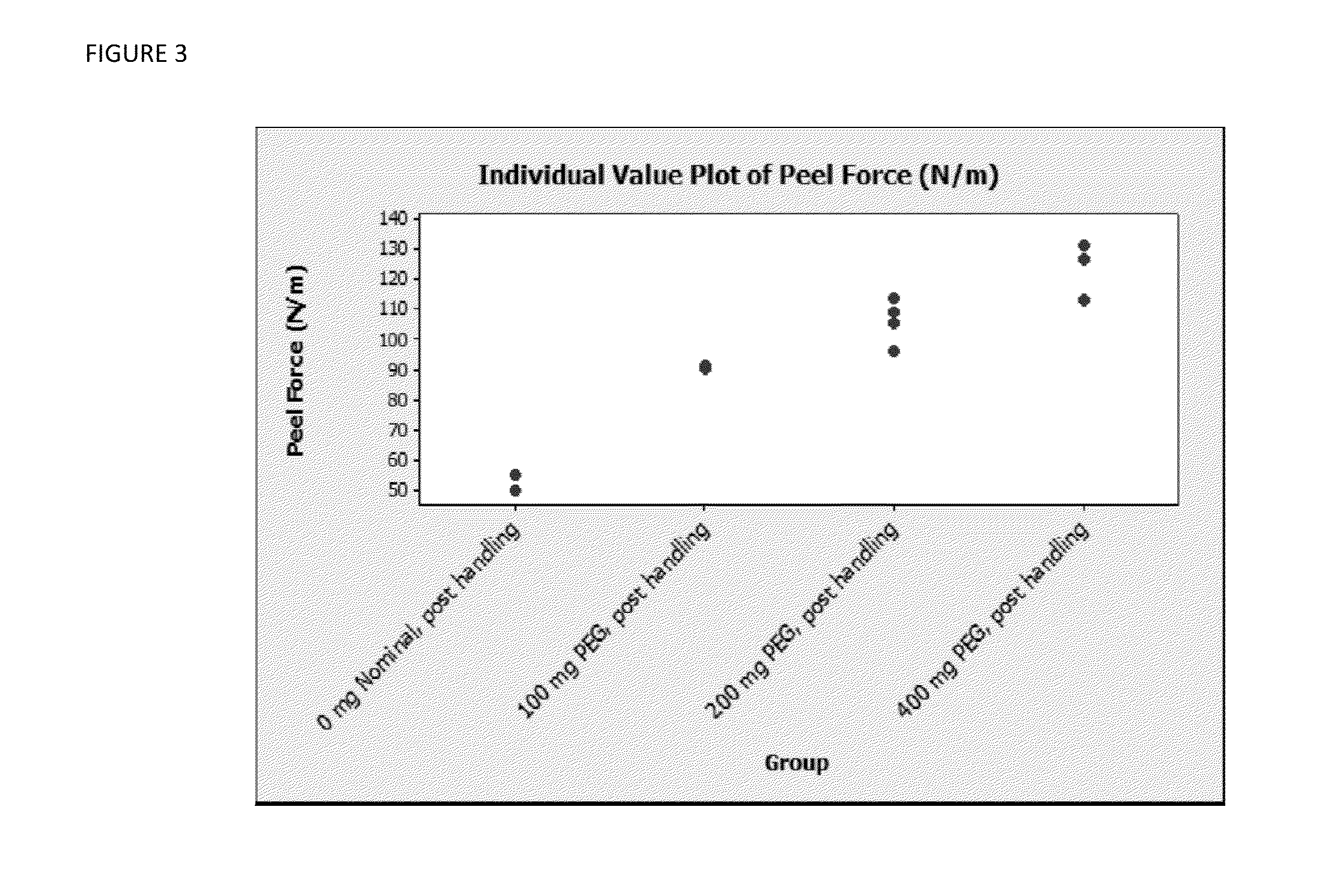
Novel methods for making dosages form comprising a cushioning component. The methods of the present invention provides dosage forms which can be compressed to form compressed dosage forms that are substantially uniform in composition and robust and exhibit reduced friability. The invention also relates to methods for making fast-disintegrating dosage forms.
Owner:FOTONATION VISION +1
Solid form
InactiveUS20080311162A1Promote absorptionMinimize side effectsPowder deliveryBiocideSolid massFilling materials
A solid form comprising at least one film enrobing a compacted fill material comprising a pressure sensitive multiparticulate and at least one cushioning agent, in which the multiparticulate and / or the cushioning agent comprises at least one active material, having low friability and wherein the compacted fill material has a density of at least 0.5 g / ml based on the total solid volume of the solid form and a tensile strength of less than 0.9 MPa.
Owner:FMC CORP
Solid form
InactiveUS20080286343A1Reduce deliveryPowder deliveryNervous disorderControl releaseFilling materials
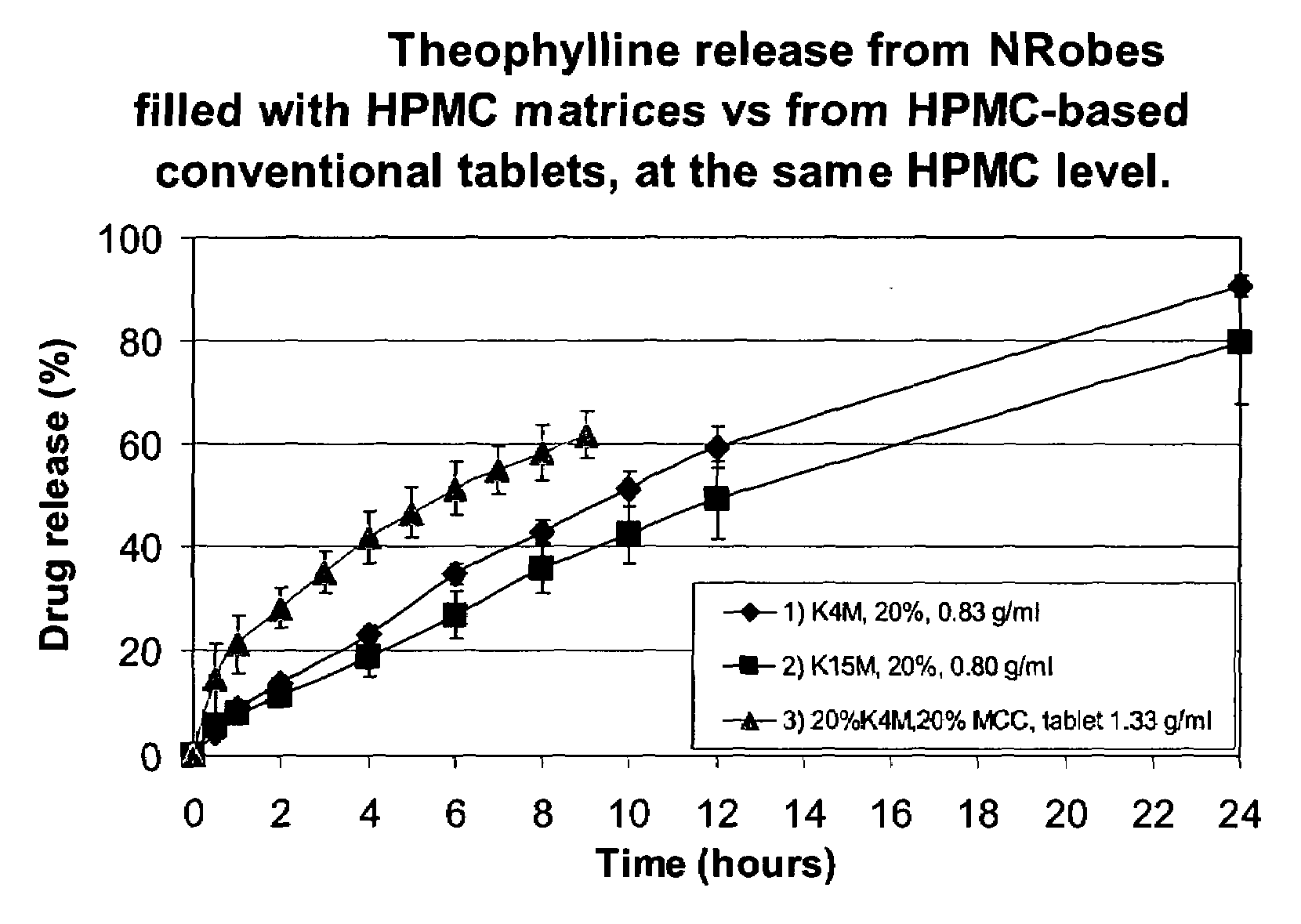
A solid form comprising at least one film enrobing a compacted fill material having at least one active material contained in a matrix and having low friability, a density of at least 0.5 g / ml based on the total solid volume of the solid form and a tensile strength less than 0.9 MPa and which exhibits a controlled release profile for release of the active material. Zero order release may be achieved.
Owner:FMC CORP
Quick-disintegrating tablet in buccal cavity and manufacturing method thereof
InactiveUS20030099701A1Good molding effectEasily crystallizesBiocideOrganic active ingredientsMedicineDiluent
The present invention relates to a quick-disintegrating tablet in the buccal cavity comprising a drug, a diluent, and a saccharide with a relatively lower melting point than the drug and the diluent, which is obtained by uniformly mixing the saccharide with a low melting point in the tablet so that a bridge will be formed between said drug and / or said diluent particles by the product of melting and then solidification of this saccharide with a low melting point. Moreover, the present invention relates to a method of manufacturing a quick-disintegrating tablet in the buccal cavity comprising a drug, a diluent and a saccharide with a relatively lower melting point than the drug and the diluent, which comprises (a) the process whereby tablet starting materials including a drug, a diluent, and a saccharide with a relatively lower melting point than the drug and the diluent are molded under the low pressure necessary for retaining the shape of a tablet, (b) the process whereby the molded product obtained in process (a) is heated to at least the temperature at which this saccharide with a low melting point will melt, and (c) the process whereby the molded product obtained in process (b) is cooled to at least the temperature at which the molten saccharide with a low melting point solidifies. The present invention presents a quick-disintegrating tablet in the buccal cavity that can be used for practical purposes in that it has almost the same properties as conventional oral pharmaceutical tablets, that is, it has sufficient tablet strength that it can be used with automatic unit dosing machines, and it is produced by conventional tableting machines, and a manufacturing method thereof. Moreover, the present invention presents a quick-disintegrating tablet in the buccal cavity which, in comparison to conventional quick-disintegrating tablets in the buccal cavity, has increased tablet strength and an improved friability without prolonging the disintegration time in the buccal cavity, and a manufacturing method thereof.
Owner:ASTELLAS PHARMA INC
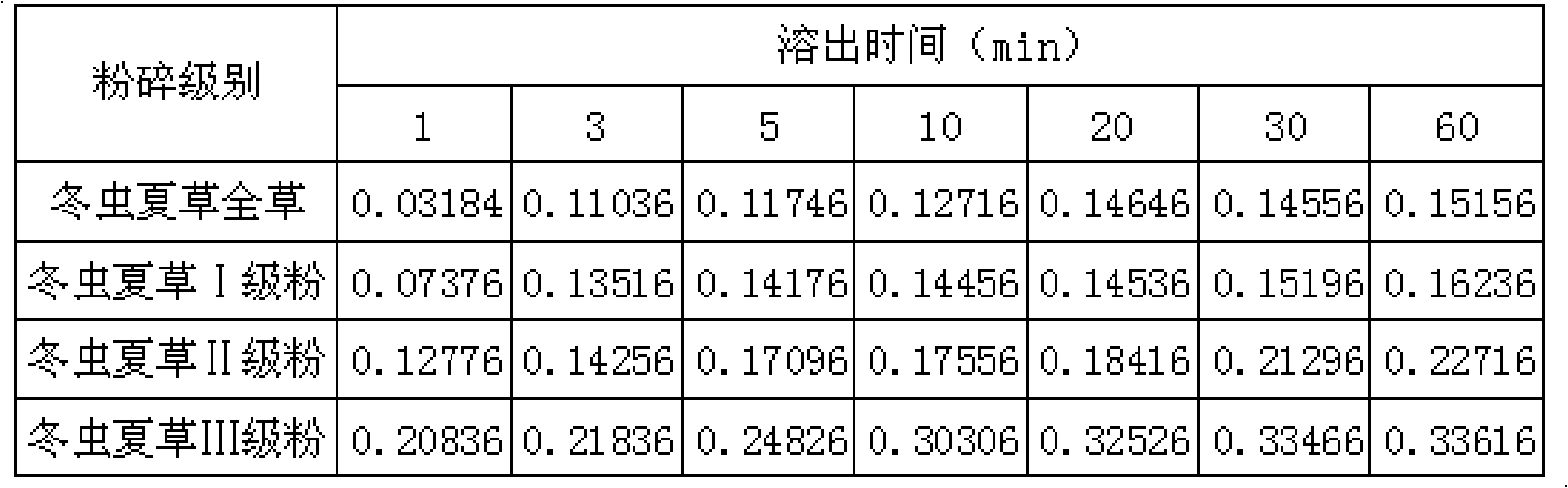
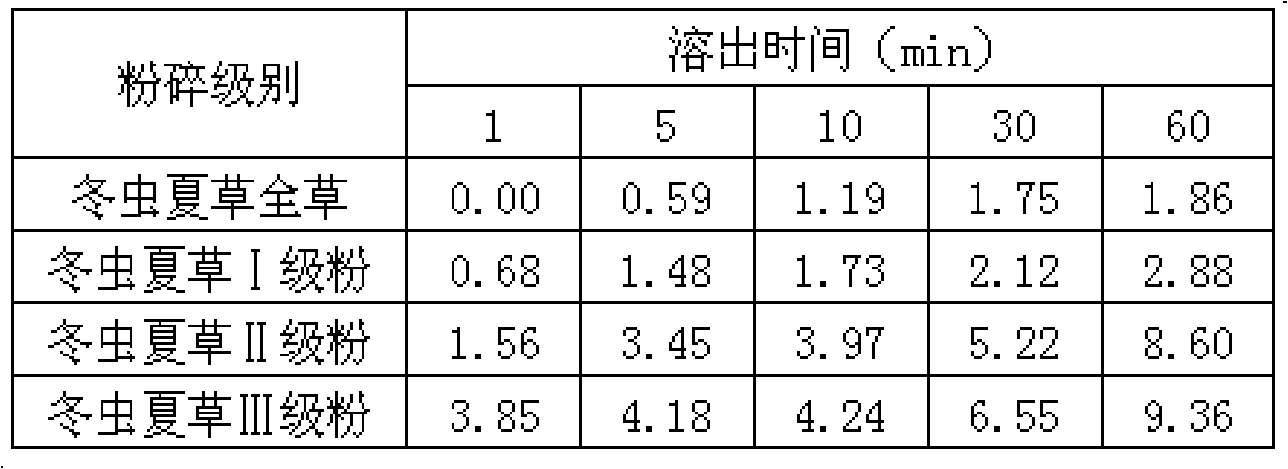
Aweto micropowder tablet and preparation method thereof
ActiveCN101332212AIncrease concentrationFully activePill deliveryImmunological disordersMedicineTableting
The present invention relates to a cordyceps sinensis powder tablet and a preparation method thereof, which pertains to the field of medicines and health products. The technical problem the present invention aims at providing a tablet containing cordyceps sinensis medicinal powder and no auxiliary material; the appearance, shape and harness of the tablet is consistent with the tablet quality standard. The cordyceps sinensis powder tablet only adopts cordyceps sinensis powder with the water content of 8 to 18 percent and the granule diameter of 1 to 150mum; no auxiliary material is added in the preparation process and the cordyceps sinensis powder is the only component. The aim of direct tablet forming can be achieved by controlling the water of the powder or by the process of second tabletting or dry granule tabletting. The process ensures that the tablet appearance is good; pockmark surface rate and fracture rate are low; tablet harness, disintegration rate and friability are consistent with the tablet quality requirement; the harness can also ensure that the tablet can not fracture in the preparation process when coating and film covering is carried out in the later stage.
Owner:QINGHAI SPRING MEDICINAL RESOURCES TECHNOLOGY CO LTD
Pellets having an active compound matrix and a polymer coating, and a process for the production of the pellets
InactiveUS20080206324A1Easy to preparePowder deliveryPharmaceutical non-active ingredientsPolymer sciencePolymer coatings
An active compound-containing pellet has a polymer coating of an anionic (meth)acrylate copolymer and a pharmaceutically active substance, embedded in a polymer matrix of one or more polymers, a particle size in the range from 300 to 1100 μm, a friability of at most 0.1%, measured using 200 g of pellets in a screening machine having a 200 μm screen, a screening diameter of 20 cm and 1.5 mm shaking amplitude at a shaking frequency of 50 l / sec for 10 min in the presence of six rubber cubes having a 1.8 cm edge length, with the proviso that the pellet releases no more than 10% of the active compound in a release test according to USP in artificial gastric juice at pH 1.2 after 120 min.
Owner:EVONIK ROEHM GMBH
Deodorizing composition and method of forming thereof
An odor absorbing composition is provided comprising zinc salt of ricinoleic acid, a solubility promoter including sodium iminodisuccinate, water and optionally, other ingredients such as perfumes and antifungal agents or bactericides. The zinc ricinoleate can be completely solubilized in water, yet the solution will exhibit low foaming and friability. In addition, the end product may be in the form of a sprayable liquid, a thick liquid capable of clinging to a vertical surface, a gel or solid tablet, a powder, or any other form. The present invention further relates to a method for forming an odor absorbing composition comprising zinc salt of ricinoleate acid, sodium iminodisuccinate and water.
Owner:ZORBX
Natural dietary supplement tablet
InactiveUS20090197974A1BiocidePharmaceutical non-active ingredientsAdditive ingredientDietary supplement
An all-natural or substantially all-natural dietary supplement in a tablet form that comprises at least 95 percent Certified Organic ingredients by weight and exhibits desirable hardness, friability and release of the active dietary supplement is formulated with naturally occurring saccharides that exhibit tablet binder, tablet disintegrant, or both tablet binder and tablet disintegrant functionality. The resulting tablets may be labeled as “natural” dietary supplements.
Owner:ENZYMATIC THERAPY
Robust rapid disintegration tablet formulation
InactiveUS20080305166A1Disintegrates quicklyReduce brittlenessOrganic active ingredientsNervous disorderAlcohol sugarsWater soluble
A rapidly disintegrating, orally administered tablet or compressed dosage form, comprising ethylcellulose (EC) as a directly compressible binder which enhances tablet robustness as manifested by improved strength, lower friability, lower hygroscopicity and yet, hydrophobic nature notwithstanding, does not retard disintegration, but shortens disintegration time or is disintegration time neutral when co-formulated with disintegrants and other water-soluble excipients such as sugar alcohols.
Owner:HERCULES INC
Levamlodipine beaylate tablets and preparation method thereof
InactiveCN101721384AGood compressibilityPromote dissolutionOrganic active ingredientsPharmaceutical delivery mechanismMedicineFiller Excipient
The invention discloses levamlodipine beaylate tablets which is prepared by comprising the following raw materials in parts by weight: 1-20 parts of levamlodipine beaylate, 20-150 parts of filling agent, 10-100 parts of disintegrating agent and 1-10 parts of lubricant. The preparation method of the levamlodipine beaylate tablets comprises the following steps of: evenly mixing the raw materials, crushing, screening with a 60-100 mesh sieve, evenly mixing, and preparing the levamlodipine beaylate tablets in a novel powder feeder for a tablet machine by using a direct dry powder tablet compressing method. Compared with the traditional tablet production technology, the levamlodipine beaylate tablets of the invention have the advantages of favorable compressibility, friability and tablet weight variation, uniform content and stable quality.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Rapidly disintegrating tablets comprising titanium dioxide
An orally-administered, rapidly disintegrating tablet is provided. The tablet comprises: a titanium dioxide; a super disintegrant; and a sugar alcohol. When immersed in water the tablet has a friability of less than about 2% and disintegrates in less than about 60 seconds.
Owner:J M HUBER CORP
Flexible-paper-base friction material
ActiveCN101024760AGood flexibilityImprove friction and wear performanceNon-fibrous pulp additionOther chemical processesFiberCarbon fibers
The invention discloses a soft paper based friction material that contains 3-7wt% flexibility reinforcement fiber, 5-20wt% carbon fiber, 4-25wt% other fiber, 2-20wt% flexibility adhesive, 20-44wt% friability adhesive, 10-25wt% frictional properties regulator, and 15-30wt% filling material. The invention has good flexibility, and improved frictional wear ability of the paper base friction material. The minimum bending radius of curvature is 6.0-6.8cm, and good mechanical strength and thermal endurance.
Owner:陕西航沣新材料有限公司
Fast water-dispersible domperidone tablets
InactiveUS20060051414A1Improves Structural IntegrityPleasant tasteBiocideDispersion deliverySolubilityWater dispersible
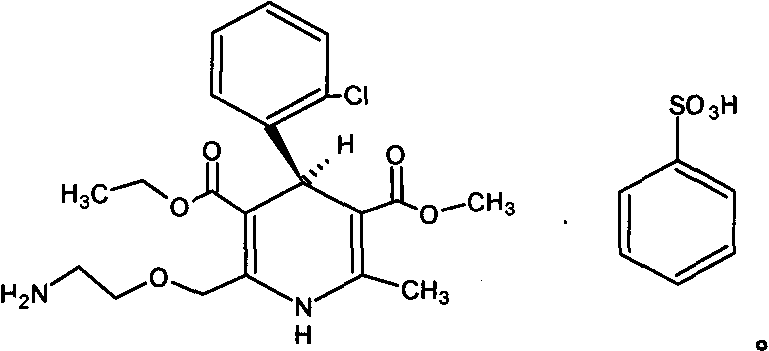
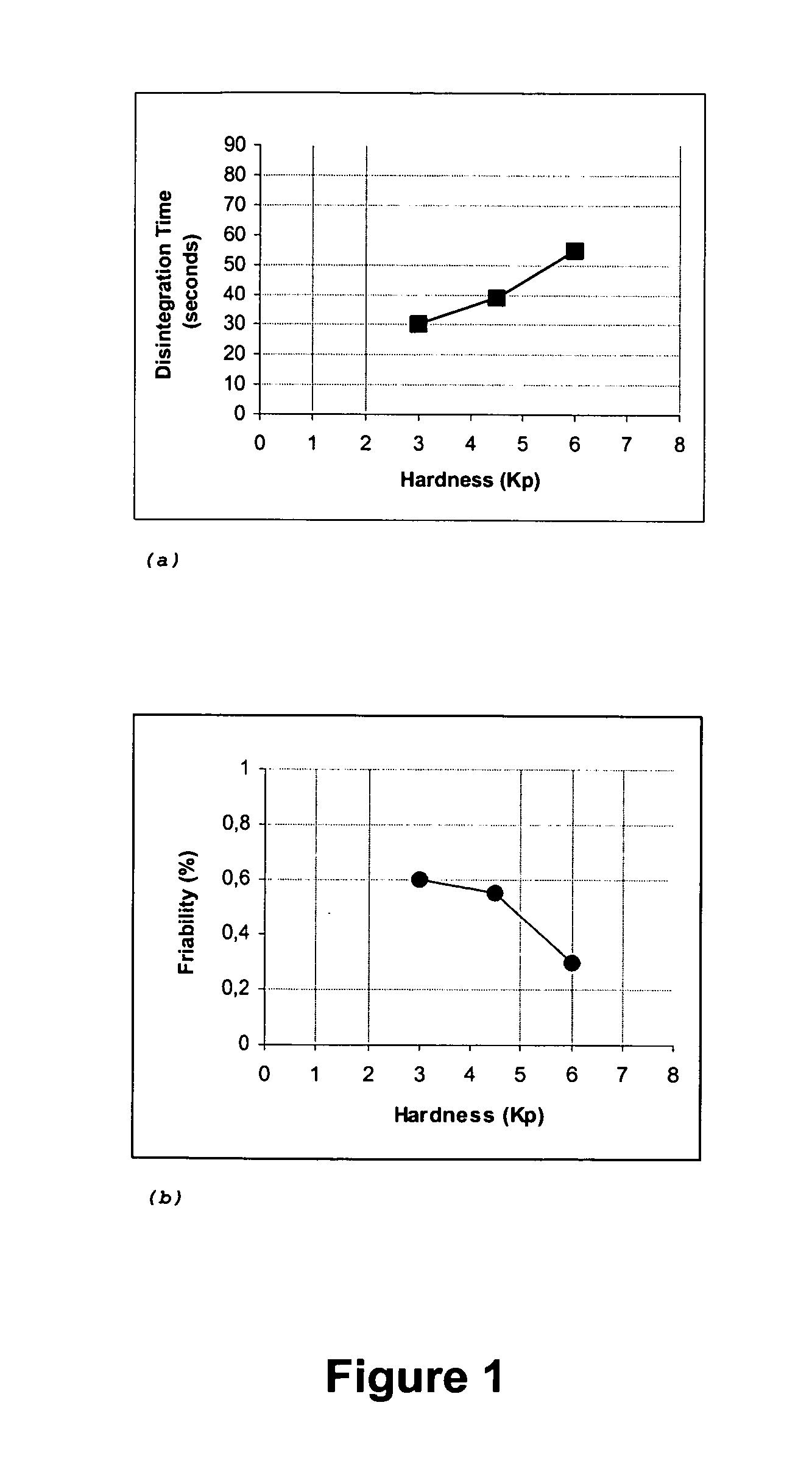
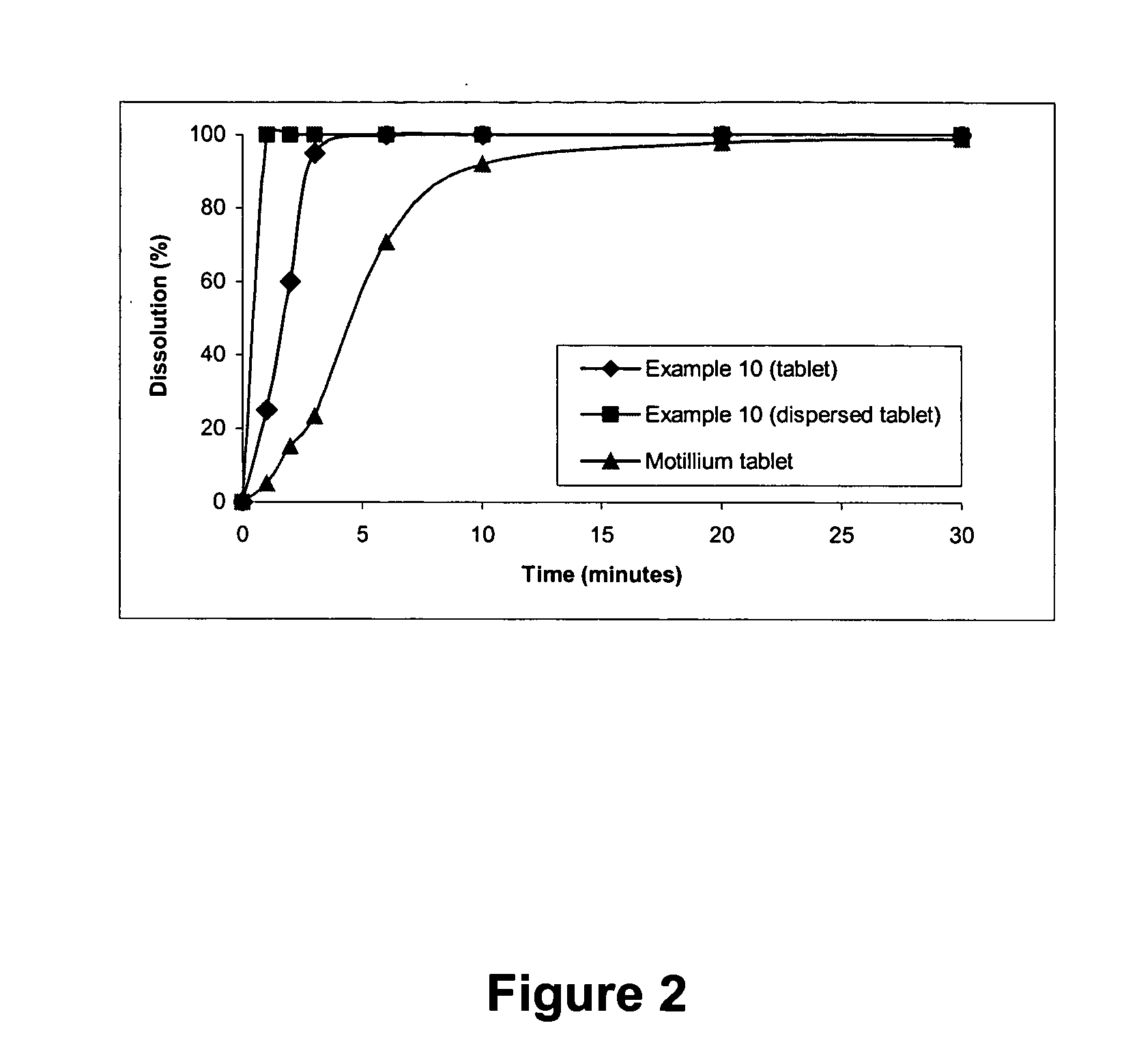
The present invention relates to fast water-dispersible tablets containing domperidone for oral administration. The formulations comprise domperidone or pharmaceutically acceptable salts thereof, about 60-80% of a “auxiliary” granulate (w / w), and about 10-30% of microcrystalline cellulose (w / w), expressed in relation to the total weight of the tablets, a sweetener, a flavouring agent and a lubricant. The “auxiliary” granulate is obtained by wet granulation of D-mannitol and maize starch gum in a high shear granulator, it facilitates the flowability and the compressibility of the mixture and, because of its high solubility in water, contributes to the fast dispersion of the tablet. The formulations have an enhanced structural integrity, for instance having a friability lower than 1.0% and hardness values between 3 and 6 Kp, and are able to disperse in water within 3 minutes, preferably within 2 minutes and most preferably within 1 minute, to provide a dispersion that passes through a 710 μm diameter mesh size sieve and presents a pleasant taste and the absence of perceptible granules in the mouth. This invention also refers to the process for the preparation of said pharmaceutical preparations.
Owner:LAB MEDINFAR PROD FARMS
Rapidly disintegrating low friability tablets comprising calcium carbonate
InactiveUS20070196474A1Effective quick dissolving resultReduce brittlenessOrganic active ingredientsBiocideMedicineBrittleness
This invention pertains to the ability to provide rapidly disintegrating tablets through the inclusion of a calcium carbonate material in combination with other common tablet components. Such a calcium carbonate material must exhibit a sufficiently low surface area in order to boost the ability of the tablet to separate quickly when introduced into a user's mouth cavity. Such a tablet is dimensionally stable prior to use (low friability) and, when immersed in water the tablet disintegrates therein in less than about 60 seconds.
Owner:WITHIAM MICHAEL C +2
Pulullan polysaccharide empty hard capsule and preparation method thereof
InactiveCN101579326AReduce moisture contentSmall range of molecular weight variationPharmaceutical non-active ingredientsCapsule deliveryPlasticizerHard Capsule
The invention discloses a polullan polysaccharide empty hard capsule and a preparation method thereof. The preparation method comprises the following steps: mainly adopting polullan polysaccharide, gel agent, plasticizer, emulsifying agent, latent solvent, water, and the like as raw materials; stirring and dissolving the raw materials in a heating-stirring tank which is vacuumized, heating to form glue, filtering, conveying glue solution into a capsule making machine for making a capsule and finally obtaining the polullan polysaccharide empty hard capsule. The empty hard capsule has the advantages of good quality stability, uneasy friability, stable breakdown index, difficult hydrolysis, no misty and yellowing appearance on the surface while in long-term deposition and can hold solids, oily liquid, semisolids and pasty materials; especially, as the water content of the capsule is lower than that of a gelatine capsule, the capsule is more suitable for being filled with moisture-absorption medicines and aldehydes-containing medicines and has wide market prospect and practical value.
Owner:金斐嘉
Starch hollow capsule and preparation method thereof
ActiveCN103070845ALow priceQuality improvementPharmaceutical non-active ingredientsCapsule deliverySODIUM METAPHOSPHATECross-link
The invention provides a starch hollow capsule and a preparation method thereof. The starch hollow capsule comprises components in parts by weight as follows: 60 parts to 90 parts of cross-linking high amylose, 3 parts to 15 parts of tributyl citrate, 5 parts to 15 parts of guar gum, 1 part to 5 parts of span-80 and 1 part to 5 parts of inulin. The cross-linking high amylose is prepared by cross-linking natural high direct connection starch through a cross-linking agent consisting of sodium metaphosphate and adipic acid. The starch hollow capsule has the advantages that the price is low, the quality is good, and the strength is high, standards of Chinese Pharmacopoeia are achieved, the friability is 5%, the disintegration time is five minutes, and obvious effects are achieved in practical application.
Owner:北京爱特康医疗科技有限公司
Starch-base plant empty capsules and preparation technology thereof
ActiveCN104784150APrevent regenerationImprove performancePharmaceutical non-active ingredientsCapsule deliveryPlasticizerHumectant
The invention discloses starch-base plant empty capsules used in the fields of food, health care products and medicine, and a preparation technology of the empty capsules. The starch-base plant empty capsules mainly comprise starch, starch derivatives, water, a solidification system as well as other optional auxiliary materials of a humectant, a plasticizer, and the like, and from the preparation technology comprises the procedures of batching, solating, billet producing, cutting, embedding. Compared with the products in the prior art, the starch-based plant empty capsules has the advantages of being safe, reliable, high in friability and humectancy, convenient to store, and high in stability.
Owner:湖南素囊健康科技有限公司
Method for making chicken bean curd
The invention discloses a method for making chicken bean curd. The method comprises the following steps of: (1) preparing raw materials of the chicken bean curd in parts by weight: 50-60 parts of chicken breast meat, 4-6 parts of chicken skin, 1-3 parts of isolated soybean protein, 5-8 parts of corn starch, 1-1.5 parts of common salt, 0.01 part of sodium erythorbate, 2-3 parts of white sugar, 0.1part of trimeric sodium phosphate, 4-6 parts of egg white, 0.04-0.06 part of five-spice powder, 0.2-0.3 part of enzymolytic chicken meal, 0.04-0.06 part of ground pepper powder, 0.1 part of carrageenan and 24-28 parts of ice water; (2) sousing the chicken breast meat; (3) chopping, mixing and emulsifying the soused chicken breast meat and chicken skin; and (4) forming, cooking, frying and quicklyfreezing. According to the method for making the chicken bean curd, disclosed by the invention, the taste of the chicken breast meat is full of elasticity, friability and toughness; the gold chicken bean curd comprises more comprehensive animal protein, vegetable protein, fat and carbohydrate, and has more comprehensive nutrition constituents compared with the chicken breast meat.
Owner:河南省淇县永达食业有限公司
Spraying powder used for household appliance evaporator and preparation method thereof
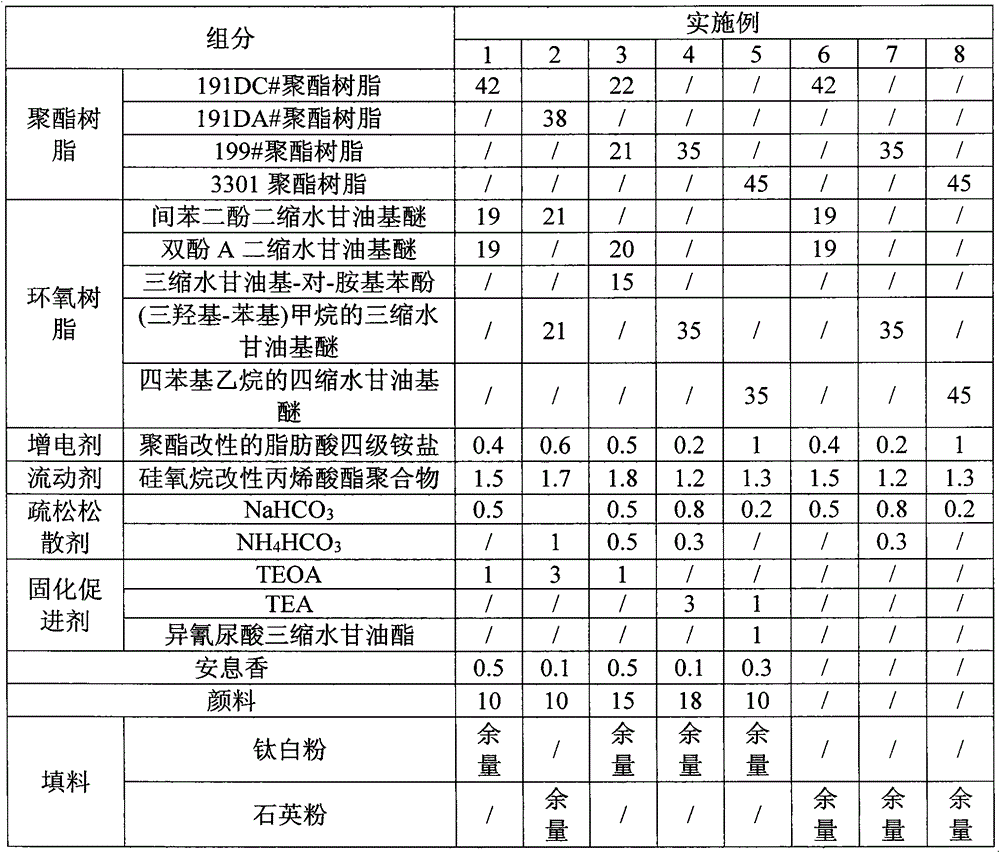
InactiveCN104087135AImprove liquidityImprove loosenessPowdery paintsEpoxy resin coatingsEpoxyPolyester resin
The invention discloses spraying powder used for a household appliance evaporator and a preparation method thereof. The spraying powder comprises the following components in percentage by weight: 35%-45% of polyester resin, 35%-45% of epoxy resin, 0.2%-1% of charging additive, 1.2%-1.8% of flowable agent, 0.2%-1.1% of loose agent and filler. The preparation method comprises the following steps of: (1), uniformly mixing the components, and extruding and tableting under the condition of 135-155 DEG C to obtain tablets; (2), crushing the tablets and preparing powder with grain size of 10-80 mu m by cyclone separation. By improving components and content thereof, the mobility, friability and physical impact performance of the spraying powder disclosed by the invention are improved, the powder applying rate is improved and the spraying thickness is reduced.
Owner:蒋炳
Compact member, method of manufacturing and use thereof
The invention relates to a compact member comprising a plurality of porous cellulose matrices (PCMs), and providing extended release of an active compound located in the pores of said PCMs together with a release modifying agent. The friability of the compact member is less than 2.1%, and the disintegration time in-vitro of said compact member is less than 240 minutes. The compact member is manufactured by exposing a plurality of PCMs to an active compound and a release modifying agent, in optional sequence or mixture, for a time sufficient for said active compound and release modifying agent to fill the pores in said PCMs to a preselected level. The PCMs are subsequently compacted to a desired shape. The invention further relates to use of the compact members for administration of a drug.
Owner:PHARMACIA AB
Soft chewable tablets
InactiveUS7029699B2Reduce fracturesTaste of may become dullPowder deliveryPharmaceutical containersBULK ACTIVE INGREDIENTActive ingredient
The present invention relates to a compressed, chewable tablet containing at least one active ingredient, a water-disintegratable, compressible carbohydrate and a binder. These components are dry blended and compressed into convex-shaped tablet having a hardness of about 2 to about 11 kp / cm2 and friability less than 1%.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Highly compactable and durable direct compression excipients and excipient systems
ActiveUS8617588B2Reduce brittlenessHigh hardnessOrganic active ingredientsAntipyreticSolubilityMedicine
The present invention relates to solid dispersions including, but not limited to, co-processed carbohydrates with different solubilities and concentrations, which have a microcrystalline plate structure. The solid dispersions, excipient systems and formulations of the present invention are highly compactable and durable and when compressed into solid dosage forms demonstrate uniform densification, low friability at low pressures, and / or relatively constant low disintegration times at various hardnesses. The solid dosage forms of the present invention demonstrate superior organoleptics, disintegration, and / or robustness.
Owner:SPI PHARMA
Fluorine-substituted apatite coating on surface of biologic medical magnesium or alloy thereof and preparation method
ActiveCN101703797AReduce solubilityGood biocompatibilityImpression capsDentistry preparationsApatiteHydroxylapatite
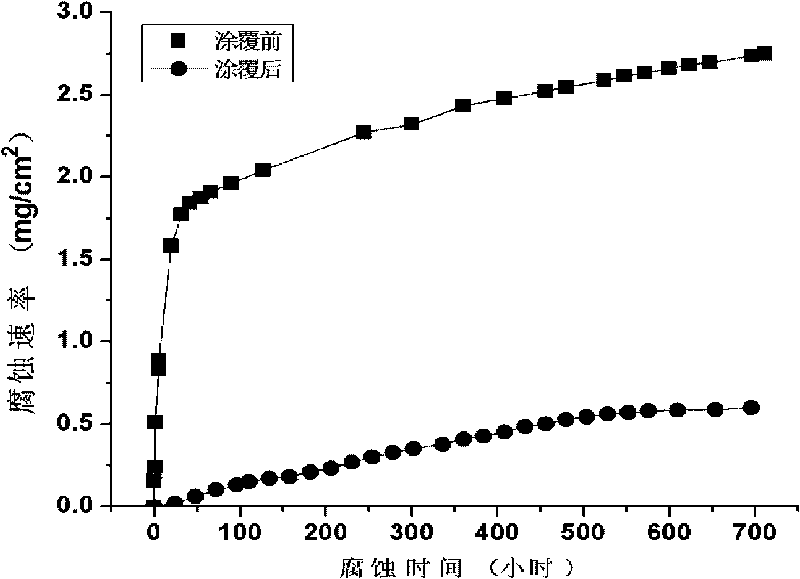
The invention relates to a fluorine-substituted apatite coating on the surface of biologic medical magnesium or alloy thereof in the technical field of the medicine and a preparation method; the components of the coating are shown as the following molecular formula (1) or (2): (1) (Ca, M) 10 (PO4) 6 (OH) 2-xFx, 0<x<=2; (2) (Ca, N) 10 (PO4) 6-2y (CO3) 3y (OH) 2-zFz, 0<y<=3, 0<z<=2; and the preparation method of the coating comprises the following steps: preparing deposition liquid; immersing the medical magnesium or the alloy thereof in the deposition liquid, regulating the pH of the deposition liquid to 3.0-7.4 and the temperature of the deposition liquid to 30-90 DEG C and carrying out spontaneous deposition on the fluorine-substituted apatite coating; and after completing the deposition, heating to obtain the fluorine-substituted apatite coating. The coating overcomes the defects of large friability and short degradation period of a pure hydroxylapatite implantation material and has favorable thermal stability and biologic stability.
Owner:CHANGSHU MICROTUBE TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com