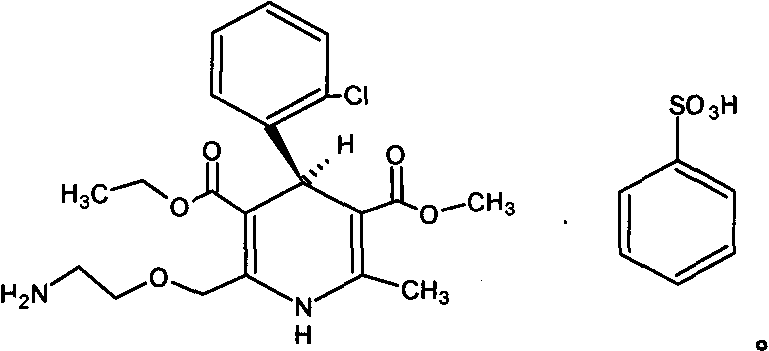
Levamlodipine beaylate tablets and preparation method thereof
A technology of levamlodipine besylate and levo-amlodipine besylate, applied in the field of levamlodipine besylate tablets and its preparation, can solve tablet compressibility, friability, tablet weight difference, and content uniformity , Dissolution is difficult to control, poor stability and other problems, to achieve the effect of good tablet weight difference, good compressibility and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Levoamlodipine Besylate 3.5g
[0041] 70g pregelatinized starch
[0042] Low-substituted hydroxypropyl cellulose 30g
[0043] Cross-linked polyvinylpyrrolidone 8.5g
[0045]
[0046] Makes 1000 pieces
[0047] Preparation
[0048] Weigh respectively levamlodipine besylate, pregelatinized starch, low-substituted hydroxypropyl cellulose, cross-linked polyvinylpyrrolidone, magnesium stearate according to the prescription quantity, mix well, pulverize, pass through an 80 mesh sieve, and mix well , placed in a powder feeder for a new type of tablet press, the dry powder is directly pressed into tablets, packed, and ready to be obtained.
Embodiment 2
[0050] Levoamlodipine Besylate 3.5g
[0051] Lactose 70g
[0052] Low-substituted hydroxypropyl cellulose 30g
[0053] Cross-linked polyvinylpyrrolidone 8.5g
[0055]
[0056] Makes 1000 pieces
[0057] Preparation
[0058] Weigh respectively levamlodipine besylate, lactose, low-substituted hydroxypropyl cellulose, cross-linked polyvinylpyrrolidone, and magnesium stearate according to the prescription amount, mix well, pulverize, pass through a 80-mesh sieve, mix well, and place in a new In the powder feeder for the tablet machine, the dry powder is directly compressed into tablets, packed, and ready to be obtained.
Embodiment 3
[0060] Levoamlodipine Besylate 3.5g
[0061] Mannitol 70g
[0062] Low-substituted hydroxypropyl cellulose 30g
[0063] Cross-linked polyvinylpyrrolidone 8.5g
[0065]
[0066] Makes 1000 pieces
[0067] Preparation
[0068] Weigh respectively levamlodipine besylate, mannitol, low-substituted hydroxypropyl cellulose, cross-linked polyvinylpyrrolidone, and magnesium stearate according to the prescription amount, mix well, pulverize, pass through an 80-mesh sieve, mix well, and place In the powder feeder for the new tablet press machine, the dry powder is directly compressed into tablets, packaged, and ready to be obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com