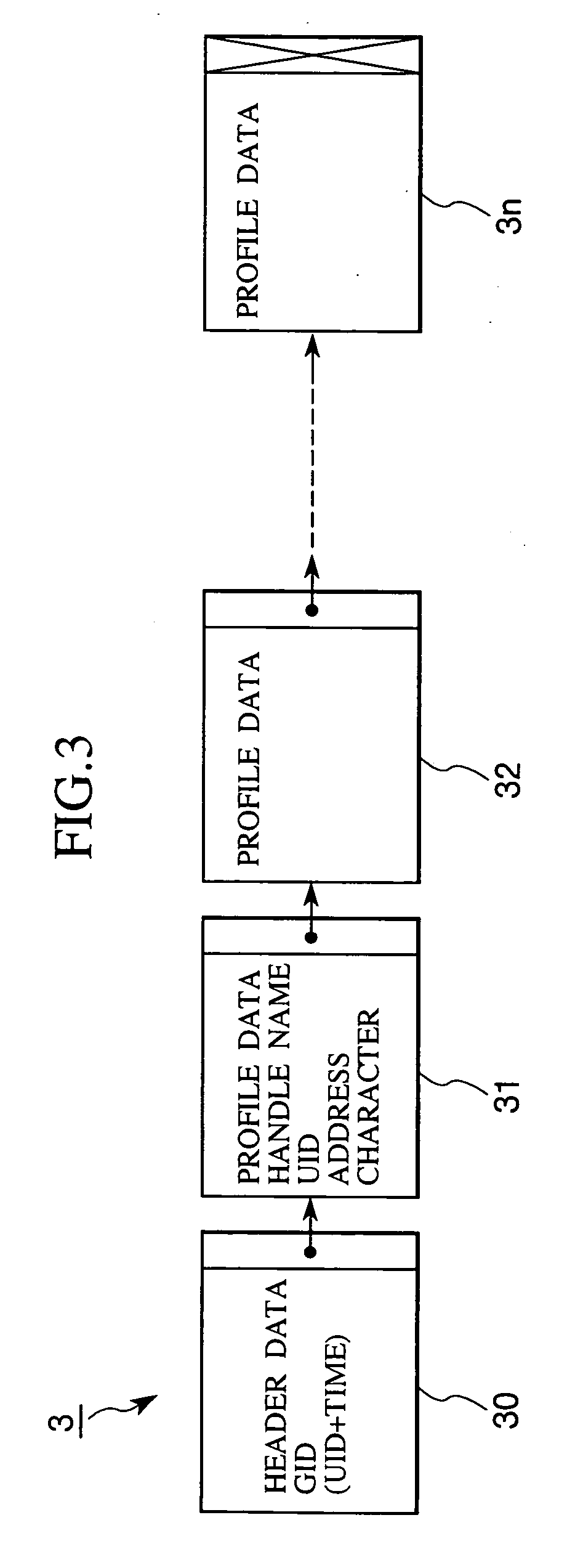
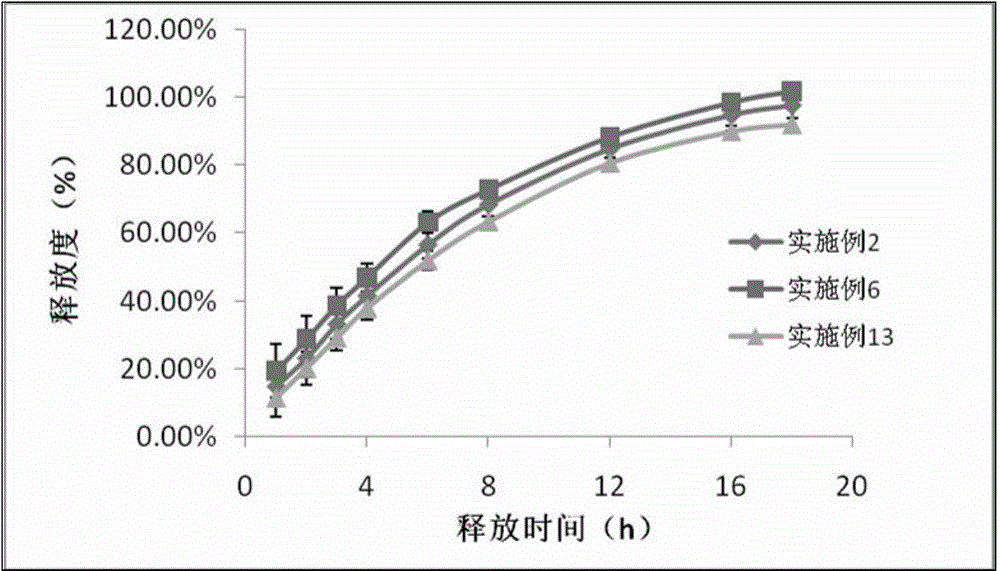
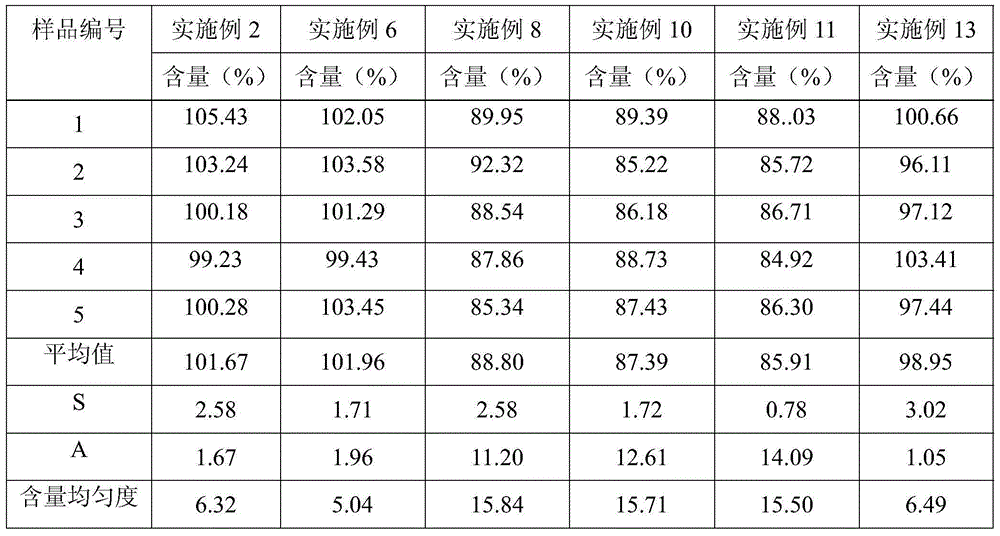
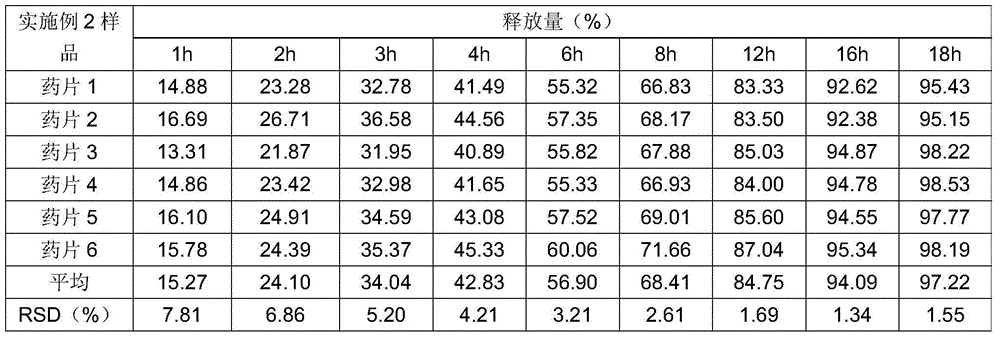
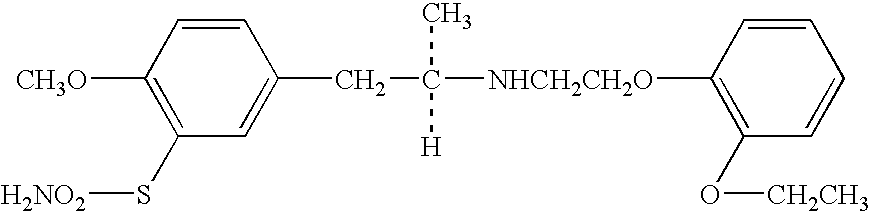
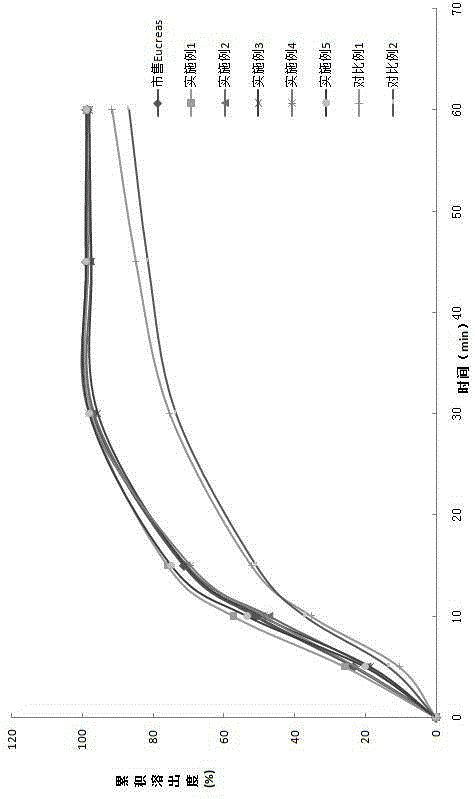
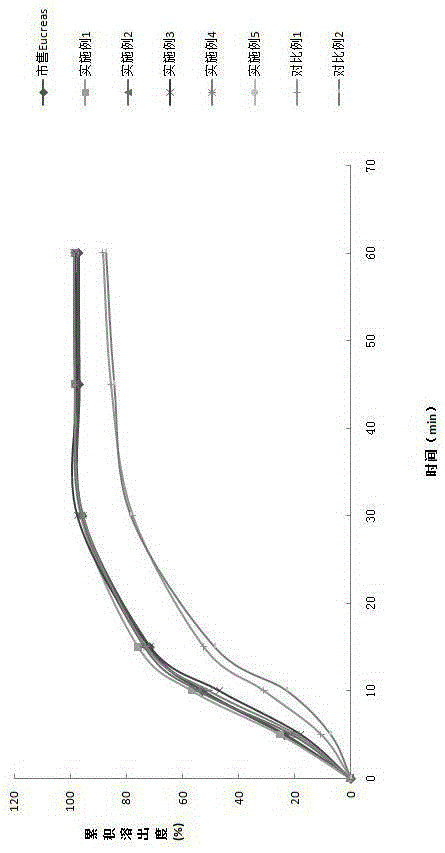
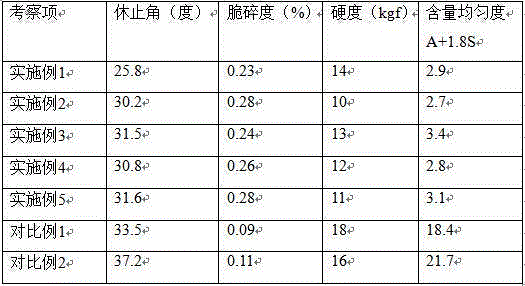
Patents
Literature
355results about How to "Uniform content" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
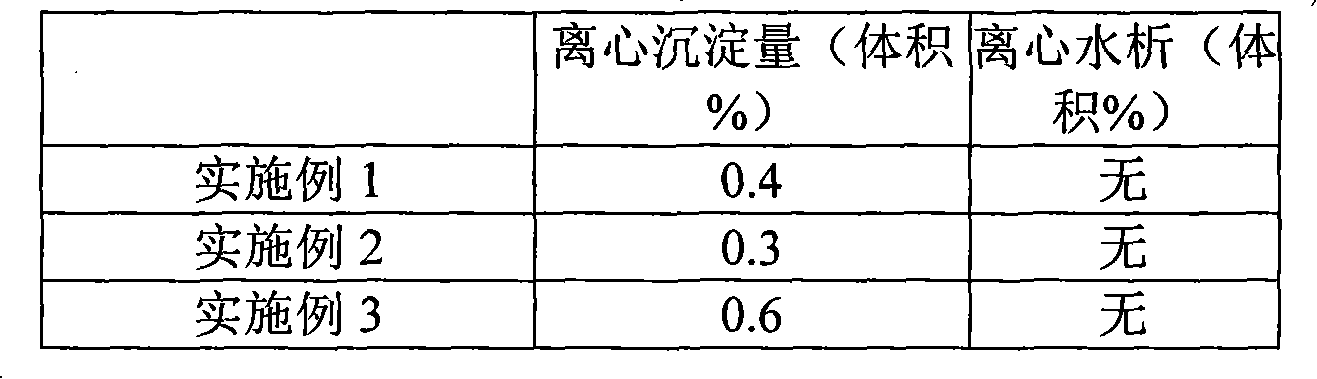
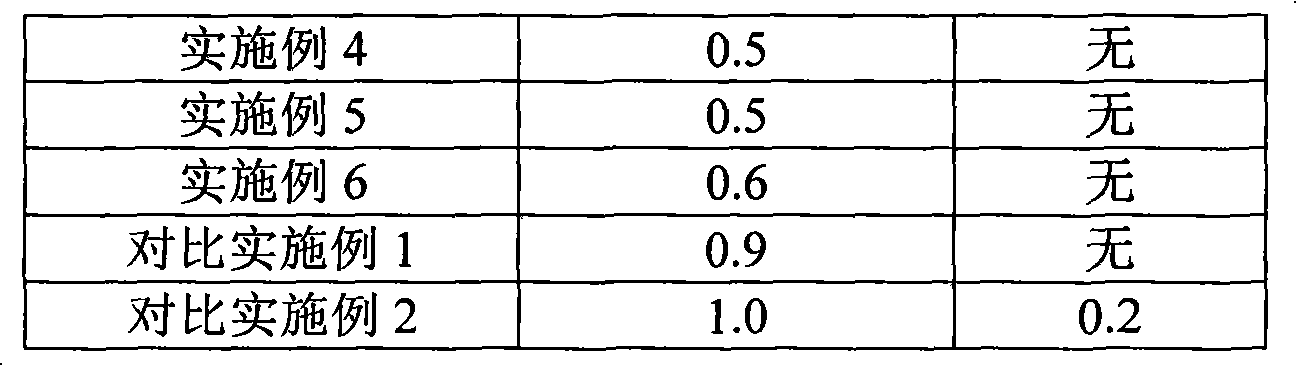
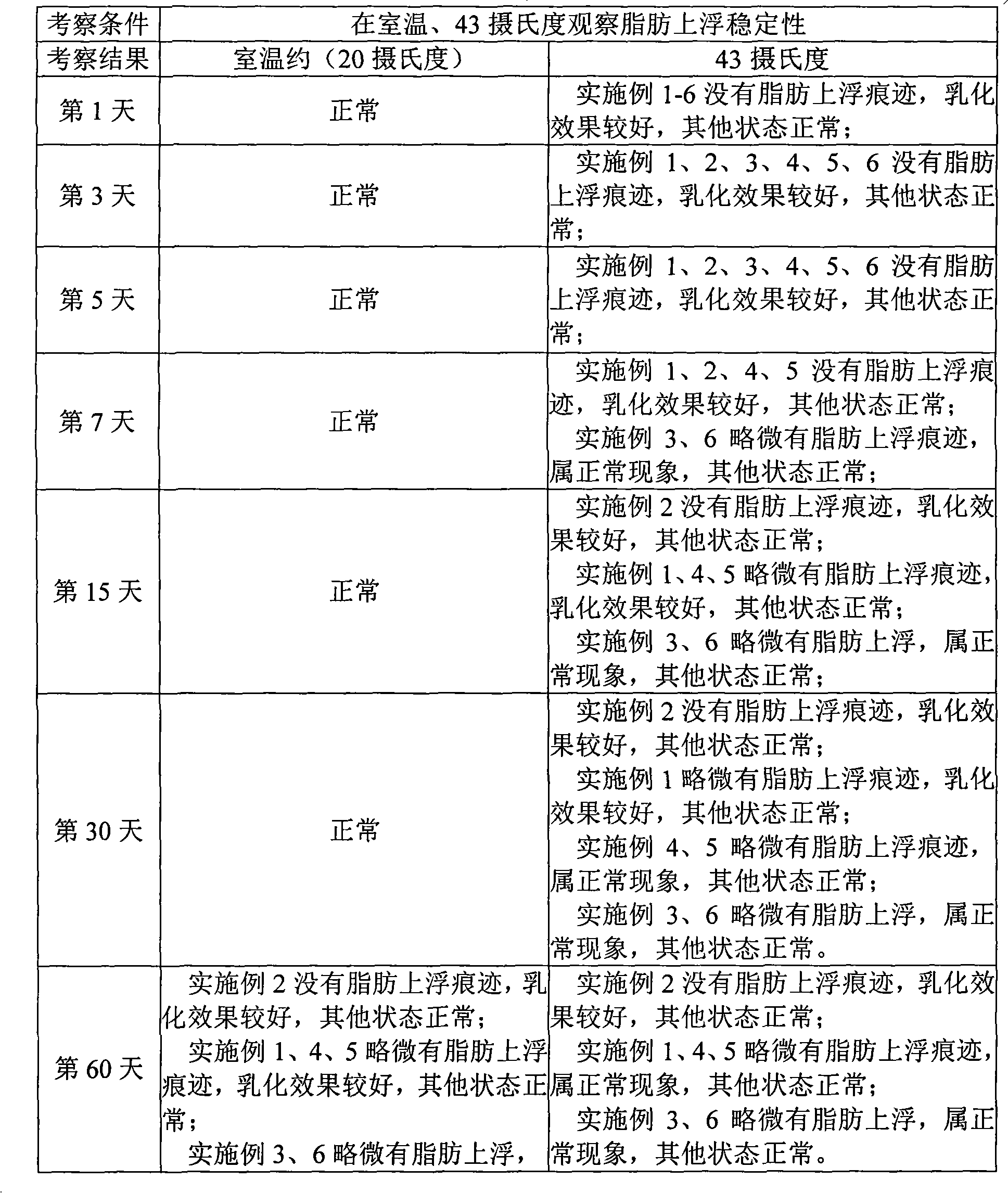
Stable low digestive enzyme content formulation
ActiveUS20120201875A1Good content uniformityMinimal loss of enzymatic activityPeptide/protein ingredientsMetabolism disorderMedicineDigestive enzyme
The present invention is directed to a pharmaceutical composition or dosage form having a stable, low (diluted) digestive enzyme content comprising at least one digestive enzyme and at least one carrier, or a dosage form thereof. The invention is also directed to a process of preparation of the composition or the dosage form. In addition the invention is directed to the treatment and prevention of disorders or conditions associated with a digestive enzyme deficiency in a patient in need thereof, comprising administering to said patient a pharmaceutically acceptable amount of the composition having a stable low digestive enzyme content or dosage form thereof.
Owner:SOC DES PROD NESTLE SA
Process for producing microcapsule enclosing electrophoretic particle dispersion, microcapsule enclosing electrophoretic particle dispersion and reversible display medium containing the same
InactiveUS20050179983A1Increase display contrastReduce production efficiencyLiposomal deliveryMicroballoon preparationElectrophoresisPolymer chemistry
Owner:TOYO INK SC HOLD CO LTD
Fermented milk beverage containing red date particles and preparation method thereof
ActiveCN101874520AUniform suspension distributionSmooth online mixingMilk preparationGellan gumFlavor
The invention relates to the field of liquid milk, in particular to a fermented milk beverage containing red date particles and a preparation method thereof. The milk beverage comprises the following components in percentage by weight: 24 to 80 percent of milk, 0.0025 to 0.005 percent of lactic acid bacteria baking agent, 0.25 to 1 percent of stabilizer, 1 to 20 percent of red date particles, 0.1 to 0.4 percent of acidity regulator and the balance of water, wherein the stabilizer consists of a thickener and / or an emulsifier, and the thickener contains 0.1 to 0.15 weight percent of high acyl gellan gum. The milk beverage containing the red date particles ensures that the red date particles can more completely, uniformly and stably suspend in milk for long time while the nutrition of yogurt, the flavor of the red date and the chewing sense of the red date particles are effectively combined, and enables consumers to truly feel the real existence of the red date particles in taste and vision of the product.
Owner:INNER MONGOLIA YILI INDUSTRIAL GROUP CO LTD
Method of manufacturing drug granules, the drug granules and pharmaceutical preparation containing the drug granules
InactiveUS7192608B2High densityImprove stabilityPowder deliveryPill deliveryHigh densityWater soluble
The present invention provides coated granules using drug granules containing a water soluble drug as an active ingredient at a high density, which is superior in uniform content and stability, and which is capable of providing a pharmaceutical preparation superior in drug release control and having a smaller size than conventional preparations, and a production method of the granules, and further, a pharmaceutical preparation using the drug granules.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Special fertilizer for cultivating kiwi
InactiveCN104876676APromote photosynthesisRich in organic matterFertilizer mixturesNutrientChemistry
The invention discloses special fertilizer for cultivating kiwi. The special fertilizer comprises the following ingredients in parts by weight: 50 to 80 parts of urea, 60 to 100 parts of ammonium dihydrogen phosphate, 10 to 35 parts of monopotassium phosphate, 30 to 50 parts of potassium sulfate, 20 to 40 parts of potassium nitrate, 15 to 25 parts of calcium nitrate, 5 to 20 parts of magnesium sulfate, 120 to 200 parts of an organic fertilizer, 0.5 to 1.2 parts of copper sulfate, 0.5 to 2 parts of ferrous sulfate, 0.2 to 1 parts of magnesium sulfate, 5 to 15 parts of sodium borate, 0.6 to 2 parts of zinc sulfate, 0.8 to 1.5 parts of sodium selenate, 0.8 to 2 parts of ammonium molybdate, 10 to 35 parts of astragalus smicus, 5 to 10 parts of amino acid and 1 to 5 parts of soil conditioner. The special fertilizer is scientific in formula, complete in nutrients, capable of improving the growth of kiwi, increasing the yield of the kiwi, improving the quality of kiwi fruits and preventing soil hardening, high in fertilizer utilization rate and capable of protecting the environment.
Owner:广德市箐箐果业专业合作社
System for exchanging mail among members belonging to group
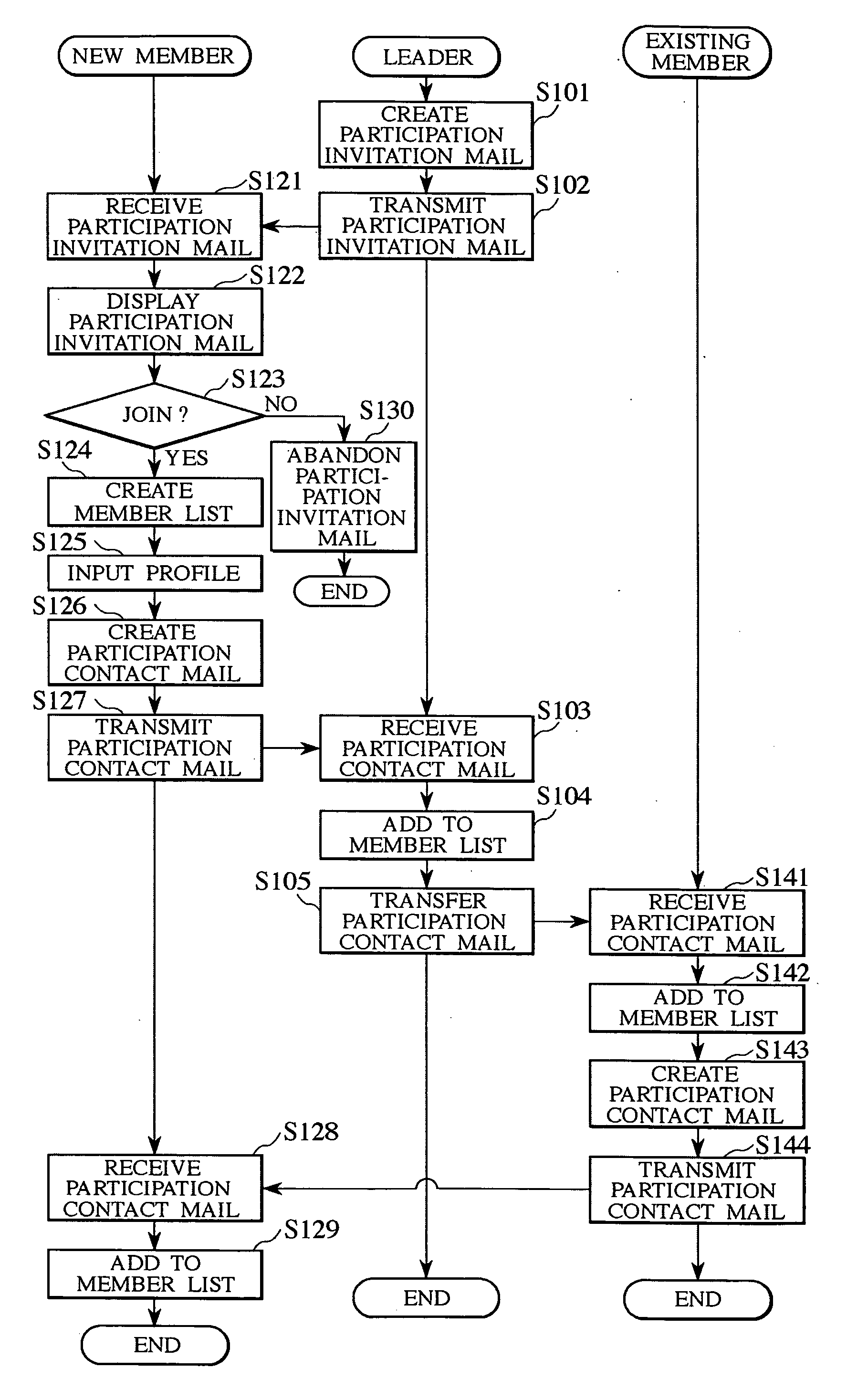
ActiveUS20040234045A1Uniform contentSpecial service for subscribersSubstation equipmentCellular telephoneComputer science
A cellular phone of a member, who newly joins a group, creates a member list including a group ID and profile of a leader, and transmits a participation contact mail including a profile of the corresponding new member to a cellular phone of the leader. The cellular phone of the leader adds the profile of the new member to a member list after receiving the participation contact mail. The participation contact mail is transferred to the cellular phones of other existing members registered in the member list. The cellular phones of the existing members add the profile of the new member to their member lists in response to the received contact mail and transmit their profiles to the cellular phone of the new member. The cellular phone of the new member adds the profiles of the existing members to the member list in response to the received participation contact mail.
Owner:SQUARE ENIX HLDG CO LTD
Preparation method of clonidine hydrochloride sustained-release tablet
InactiveCN104138362AWell mixedIncrease in sizeOrganic active ingredientsNervous disorderSustained Release TabletFiller Excipient
The invention relates to a preparation method of a clonidine hydrochloride sustained-release tablet. The preparation method comprises the steps of dissolving a clonidine hydrochloride raw material into a proper amount of wetting agent by using a solvent dispersion method to prepare a solution containing 10-28.57mg / ml clonidine; adding the solution into a granulation pan filled with a filling agent and a binder at constant speed of 5-40ml / min to granulate; drying the prepared granules, screening by using a 50-100-mesh sieve, mixing the granules, a framework material, a flow aid and a lubricating agent, and tabletting to obtain the clonidine hydrochloride sustained-release tablet. By using the preparation method, the problem of non-uniform content generated in a preparation process of the clonidine hydrochloride sustained-release tablet can be effectively solved, the prepared clonidine hydrochloride sustained-release tablet is uniform in content, and the release behavior is in accord with the sustained-release characteristic. All process parameters in the preparation method can be correspondingly amplified according to the industrial production scale, so that the requirement for large-scale industrial production can be met.
Owner:LP PHARM (XIAMEN) CO LTD
Process for producing microcapsule enclosing electrophoretic particle dispersion, microcapsule enclosing electrophoretic particle dispersion and reversible display medium containing the same
InactiveUS7488513B2Increase display contrastReduce production efficiencyLiquid surface applicatorsRotary stirring mixersElectrophoresisPolymer chemistry
Owner:TOYO INK SC HOLD CO LTD
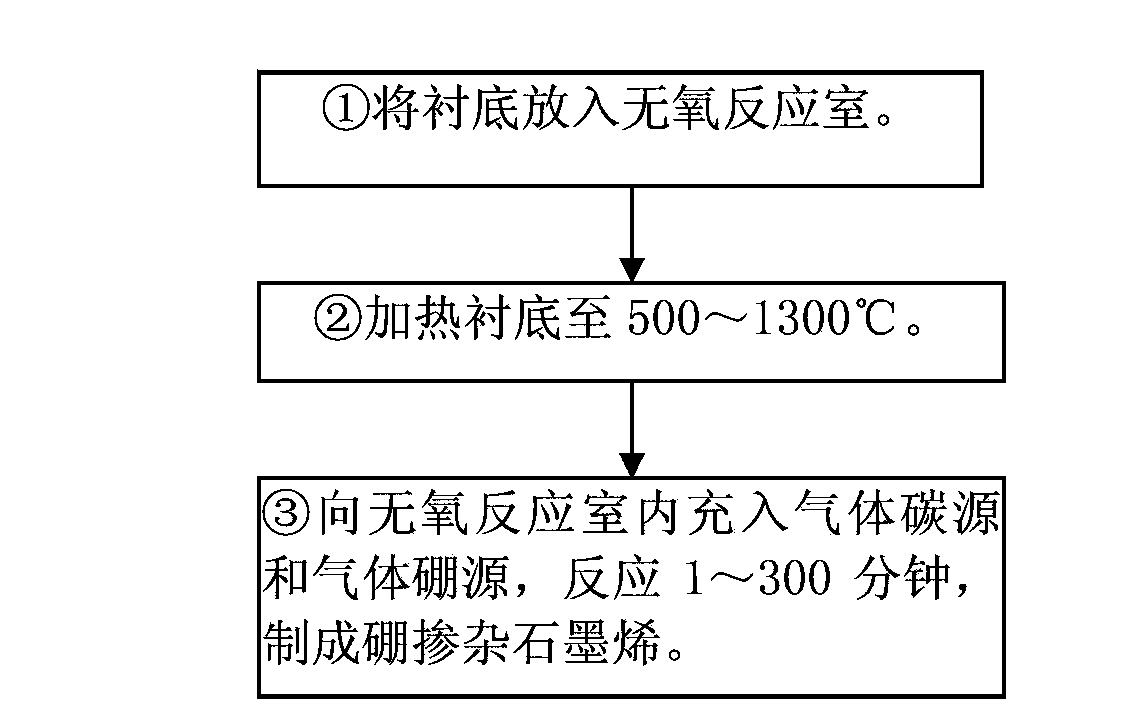
Boron-doped graphene and preparation method thereof
The invention discloses a boron-doped graphene and a preparation method thereof. A chemical vapor deposition method is used for preparing the boron-doped graphene. The preparation method comprises the following steps: (1) placing a substrate in an oxygen-free reaction chamber; (2) heating the substrate to a temperature of 500 to 1300 DEG C; and (3) introducing a gaseous carbon source and a gaseous boron source into the oxygen-free reaction chamber and carrying out a reaction for 1 to 300 min so as to prepare the boron-doped graphene. The preparation method provided by the invention has the advantages of simple equipment, easy and practicable operation, low production cost, suitability for batch production and no usage of a metal catalyst; the prepared boron-doped graphene has the advantages of high purity, high and easily controllable boron content, uniform doping of boron, a high theoretical specific surface area, excellent mechanical strength, good flexibility and high conductivity and has good application prospects in photoelectron fields like illumination, display, laser and information and in energy fields.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +1
Method for reeling silk from mulberry silkworm fresh cocoons by virtue of cocoon cooking
The invention discloses a method for reeling silk from mulberry silkworm fresh cocoons by virtue of cocoon cooking. The method is characterized by comprising the process steps of cold storage preservation, cocoon selection, vacuum infiltration, cocoon cooking, silk reeling, treatment of fresh basin residues, and preservation of fresh silkworm chrysalis. The method is simple and convenient, quality indexes such as strength, cohesion, cleanness and purity of raw silk are effectively improved by virtue of the cocoon cooking, and the raw silk is in a grade of being over 4A; silkworm chrysalis can be separated from basin residues through a scutcher to obtain high-quality fresh silkworm chrysalis and silk floss, and therefore the utilization value of byproducts is increased.
Owner:GUANGXI GUIHUA SILK CO LTD
Drug composite containing limaprost and preparation method thereof
ActiveCN101862337ADoes not affect dissolutionDoes not affect disintegrationOrganic active ingredientsPharmaceutical product form changeCoated tabletsMANNITOL/SORBITOL
The invention provides a drug composite containing limaprost and a preparation method thereof, wherein the drug composite of the invention contains 0.01-1% (weight) of the cyclodextrin inclusion compound of limaprost, 0.5-10% (weight) of freeze-drying stabilizer and other pharmaceutically acceptable excipients, wherein the free-drying stabilizer contains mannitol. The drug composite adopts a freeze drying and dry granulating combined preparation technology, greatly overcomes the defects of easy moisture adsorption and extremely bad stability of limaprost which is the main drug, and simultaneously cannot influence the slaking characteristic and the dissolution of the main drug, in addition, the product is not a coated tablet, and thereby the invention prevents the defect of slower dissolution of the conventional coated tablets.
Owner:BEIJING TIDE PHARMA
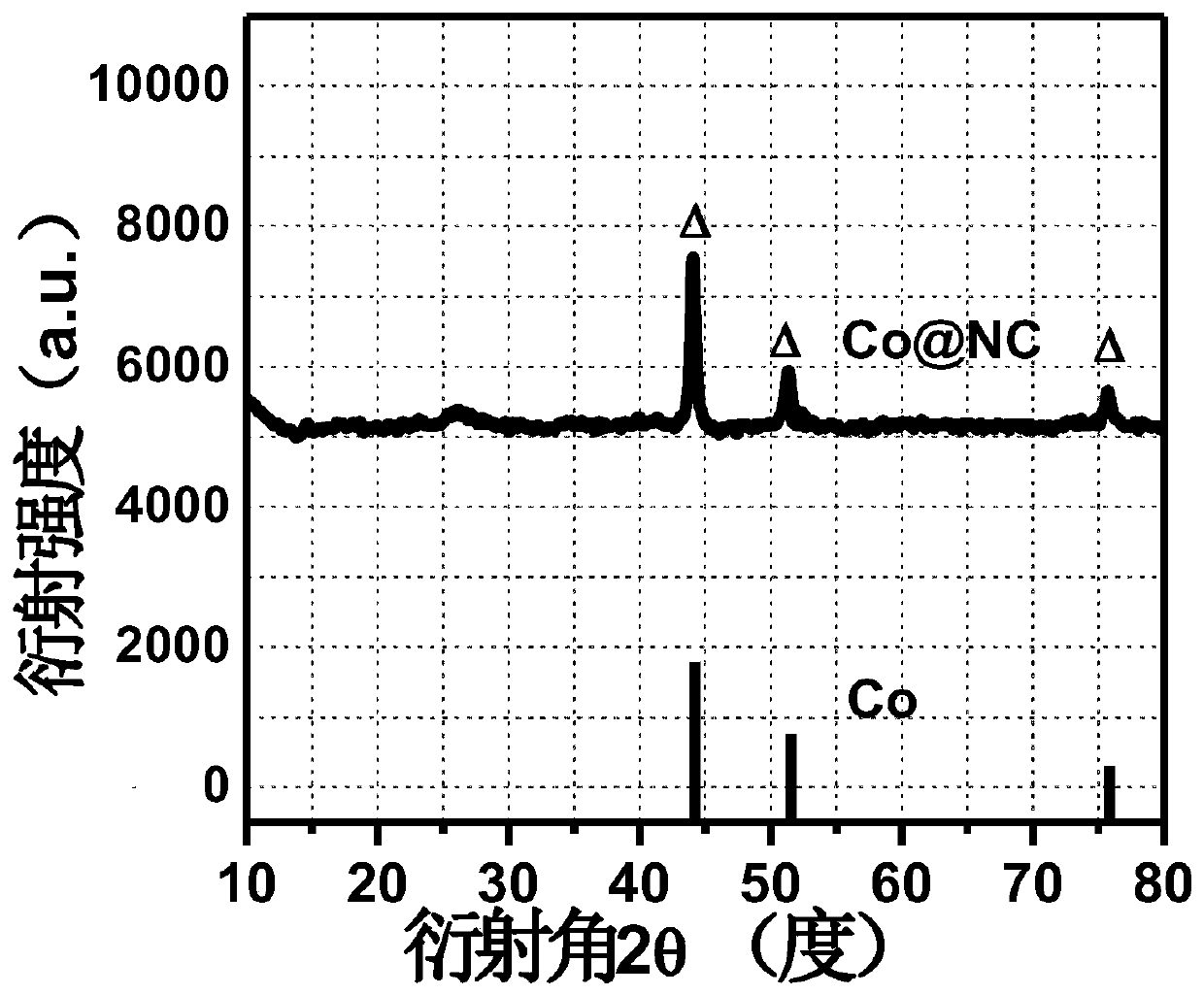
Cobalt-nitrogen co-doped carbon-based electrocatalyst material and preparation method thereof
ActiveCN111001427ASimplify the experimental stepsFast preparationFuel and primary cellsCell electrodesDoped carbonElectro catalyst
The invention discloses a cobalt-nitrogen co-doped carbon-based electrocatalyst material and a preparation method thereof, and belongs to the field of electrochemistry and new energy, wherein the cobalt-nitrogen co-doped carbon-based nano material is prepared by taking an anionic metal organic framework encapsulated with metal cobalt ions as a precursor through a high-temperature pyrolysis methodin a nitrogen atmosphere, and has excellent oxygen reduction, oxygen evolution and hydrogen evolution catalytic performances under an alkaline condition. According to the invention, a rechargeable zinc-air battery and a full water splitting device assembled by utilizing the material have good charging and discharging performance and long-term stability; and the preparation process is simple, and the catalyst is good in performance, economical and capable of being prepared on a large scale.
Owner:SHANXI UNIV
Wet friction material
InactiveUS20060223907A1Increase coefficient of frictionExcellent compression fatigue propertySynthetic resin layered productsSpecial tyresPHENOL LIQUIDPaper based
The invention provides a wet friction material having high friction coefficient, excellent compression fatigue property and positive gradient of a μ-V property. The wet friction material contains a paper base material and a binder, in which the binder contains a cured material of a liquid resin composition obtained by mixing a hydrolyzed solution of a silane coupling agent represented by the following formula (1) and a resol-type phenol resin, and a weight ratio (S / R) between respective non-volatile components of the hydrolyzed solution (S) of the silane coupling agent and the resol-type phenol resin (R) is in the range of from 80 / 20 to 20 / 80: (X)(R1)nSi(OR2)3-n (1), in which X represents an alkylamino group having a primary amine at a terminal; R1 and R2 each independently represent an alkyl group having from 1 to 3 carbon atoms; and n represents an integer of 0 or 1.
Owner:NSK WARNER
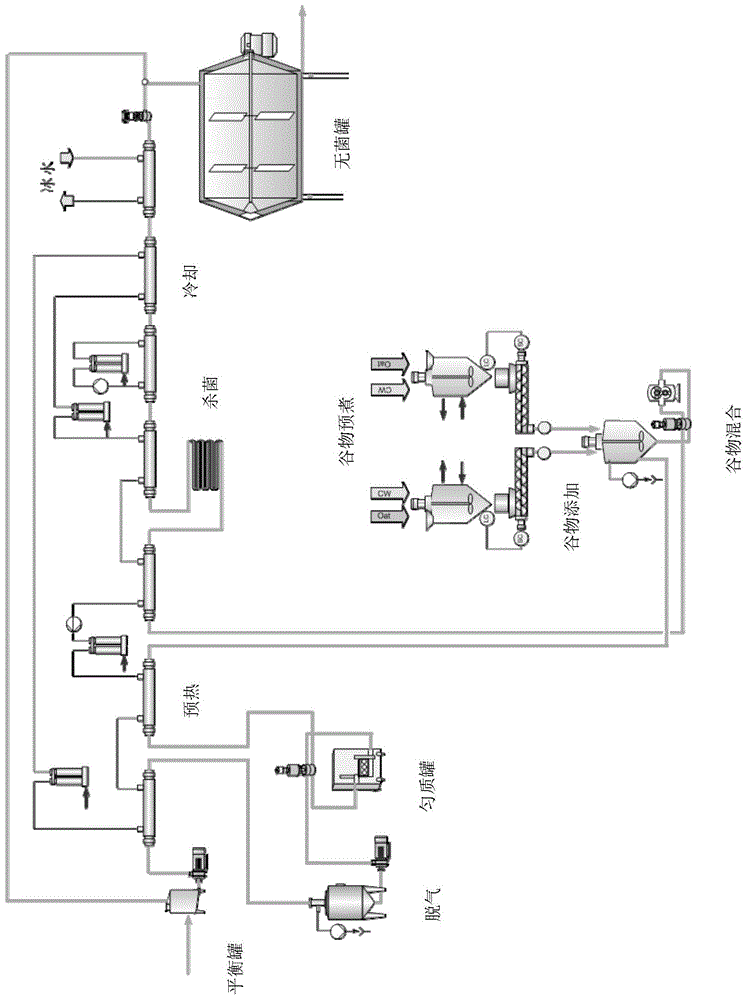
Continuous production process and devices of milk drink containing cereal grains
The present invention discloses a continuous production process and devices of milk drink containing cereal grains. The devices include an aseptic tank, a heat exchange sterilization conveying pipeline, and a grain adding device. The devices are characterized in that: the grain adding device includes a plurality of precooking tanks, mixing tanks and quantitative putting mechanisms. Cereals are put into the precooking tanks, water washing, draining, precooking, and cooling are carried out, the precooked cereal grains are continuously and quantitatively added into the mixing tanks through the quantitative putting mechanisms from the precooking tanks, at the same time, milk or drink is injected into the mixing tanks to be stirred and mixed together, then the mixture is subjected to heat sterilization, the sterilized mixture is cooled, the cooled mixture is injected into the aseptic tank to be stored and stirred, and finally the finished products are obtained by filling. The existing sterilization machine and aseptic tank structures are utilized, which achieves the production of the drink containing cereal grains or milk, online adding and continuous automatic filling, avoids pipeline blockage, improves productivity and reduces production costs.
Owner:GEA PROCESS ENG ASIA LTD
Smelting process for K418 mother alloy
The invention relates to a smelting process for a K418 mother alloy. The smelting process for the K418 mother alloy is characterized by including the following operation steps that firstly, water in raw materials is removed, and the raw materials comprise smaller than or equal to 0.14% of carbon (C), 13.2% of chromium (Cr), 4.2% of molybdenum (Mo), 2.1% of niobium (Nb), 6.30% of aluminum (Al), 0.85% of titanium (Ti), 0.01% of boron (B), 0.11% of zirconium (Zr) and the balance nickel (Ni); secondly, the raw materials with the water removed is contained into a vacuum intermediate frequency induction smelting furnace; thirdly, the raw materials are smelted in the vacuum intermediate frequency induction smelting furnace; fourthly, molten steel is subjected to refining in the vacuum intermediate frequency induction smelting furnace to achieve alloying; and fifthly, the molten steel after refining alloying of the fourth step is subjected to casting molding, so that the K418 mother alloy can be obtained. By means of the smelting process for the K418 mother alloy, the K418 mother alloy is resistant to high temperature and stretching, high in staying power and excellent in performance.
Owner:卞兴来
Hydrogel dressing for repairing soft tissue wounds and scars and preparation method thereof
InactiveCN110478519APromote growth and healingShorten healing timeAbsorbent padsBandagesCuticleGINSENG EXTRACT
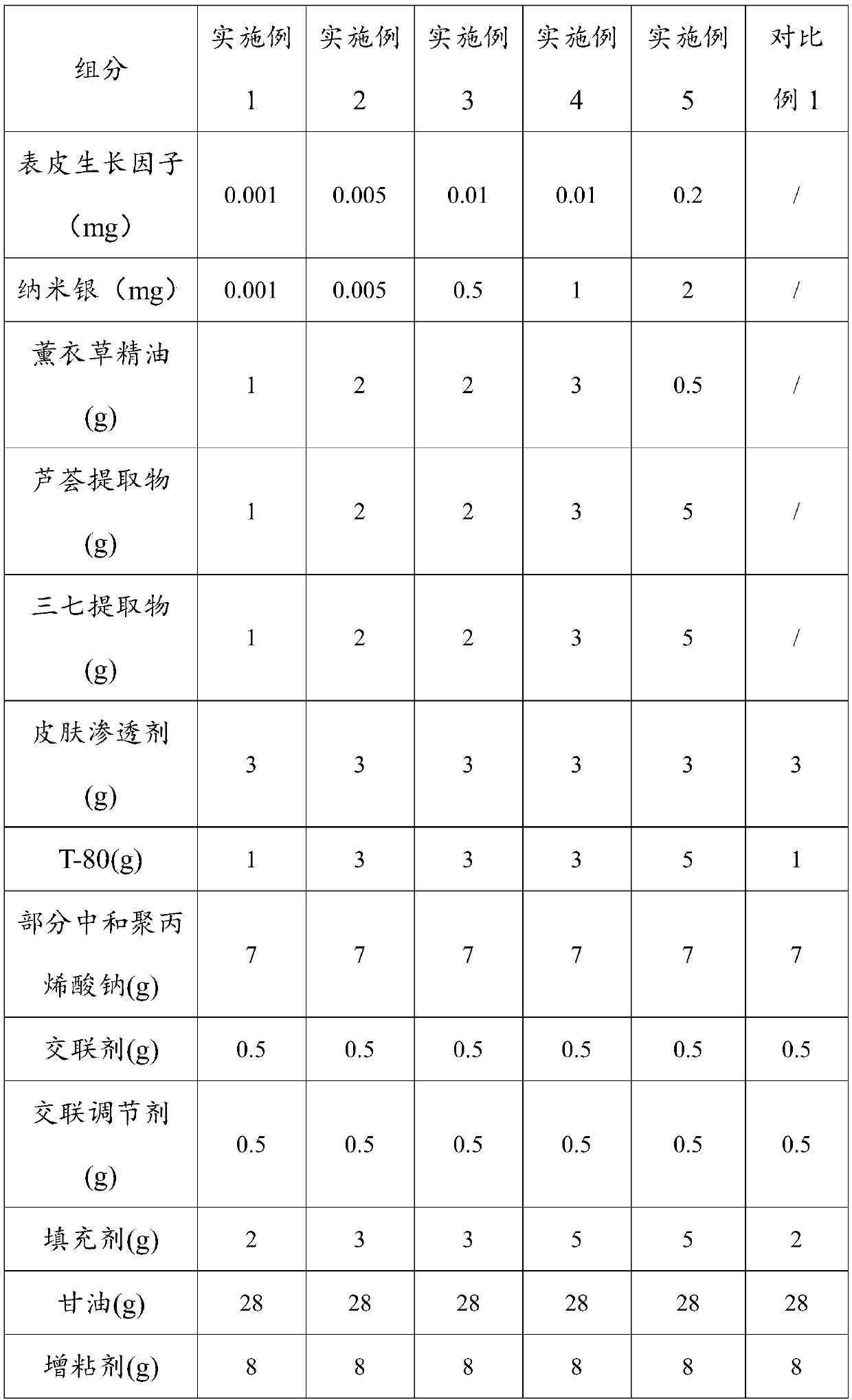
The invention belongs to the technical field of medicines, and discloses a hydrogel dressing for repairing soft tissue wounds and scars and a preparation method thereof. The dressing is made of the following components in percentage by weight: 0.0001-0.02% of epidermal growth factor, 0.0001-2% of nano silver, 0.5-5% of lavender essential oil, 0.5-5% of aloe extract, 0.5-5% of pseudo-ginseng extract, 1-5% of skin penetrant, 0.5-5% of penetration enhancer, 6-15% of partially neutralized sodium polyacrylate, 0.5-3% of cross-linking agent, 0.5-3% of cross-linking regulator, 2-5% of filler, 25.0-35.0% of humectant, 3-8% of tackifier and 4-59% of deionized water. The preparation method of the dressing includes the following steps: preparing phase I, preparing phase II, mixing the phase I and thephase II, and preparing a gel patch. The dressing of the invention promotes healing and growth of the wounds, has good breathability, convenience of use, inhibition of wound infection and a wide range of applications.
Owner:湖北兵兵药业(集团)有限公司 +1
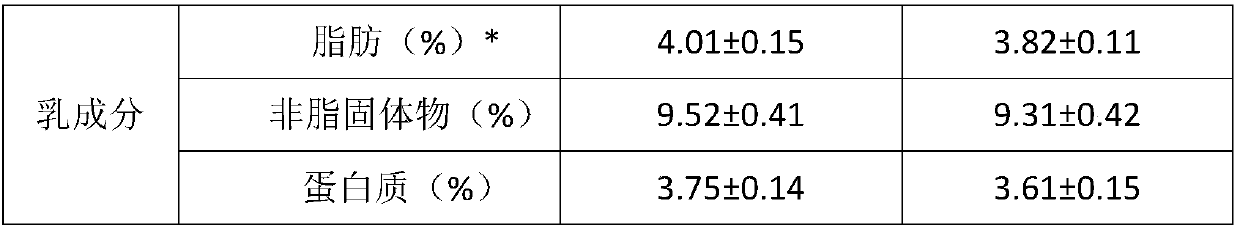
Composite calcium carbonate and vitamin D3 chewable tablet for children and preparation method thereof
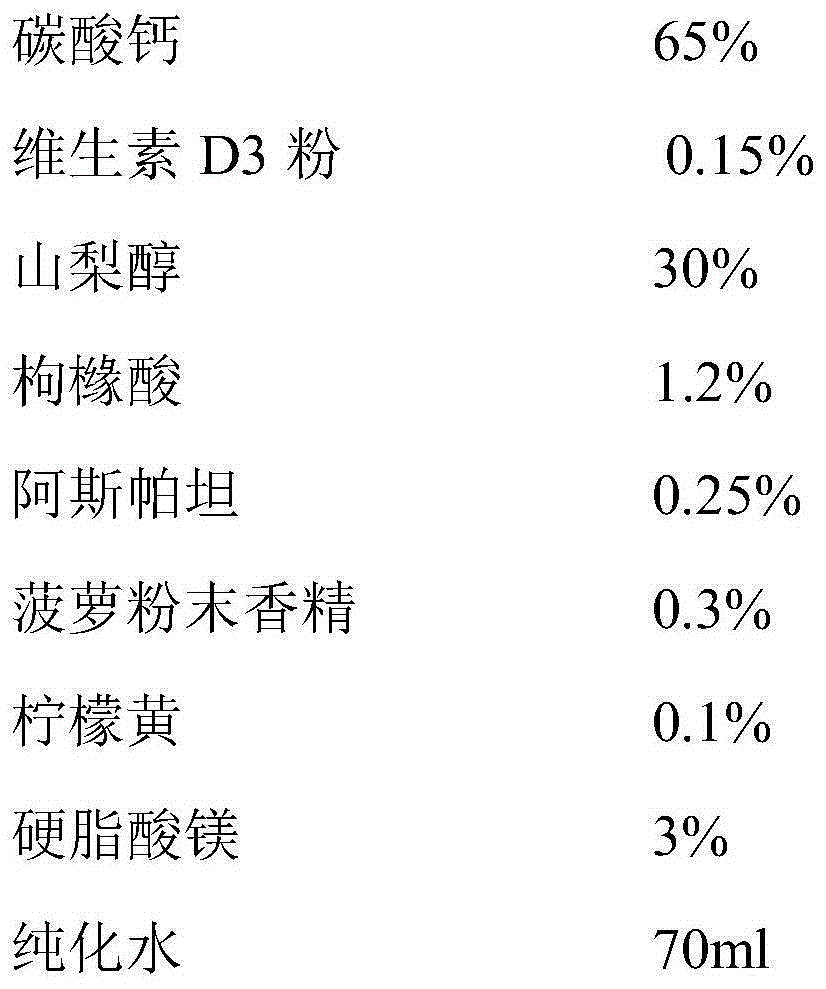
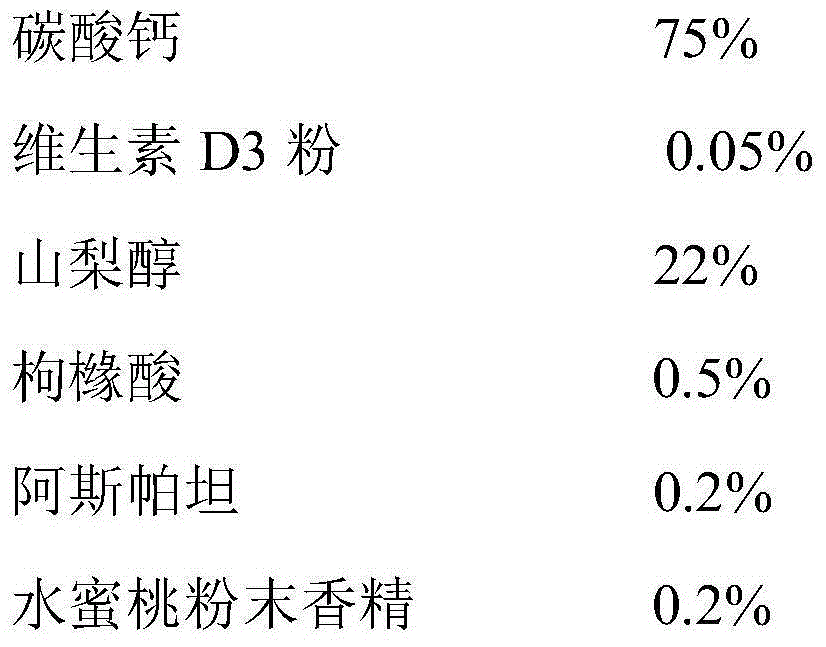
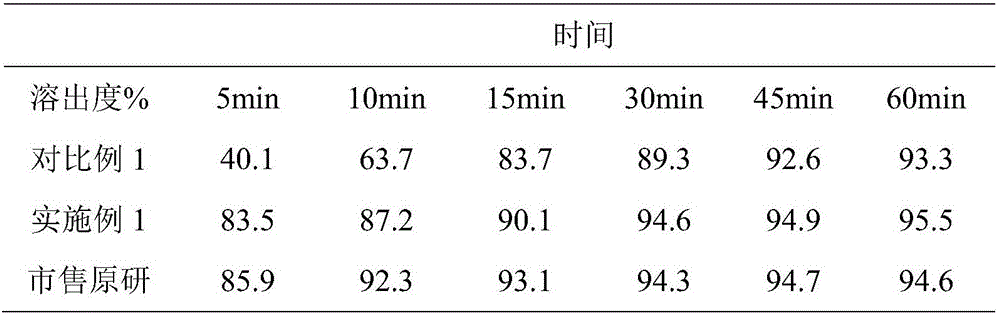
InactiveCN105168243AUniform particle size distributionImprove liquidityOrganic active ingredientsMetabolism disorderVitaminCitric acid
The invention relates to the field of pharmaceuticals, in particular to a composite calcium carbonate and vitamin D3 chewable tablet for children and a preparation method thereof. The chewable tablet is composed of, by weight, 50-75% of calcium carbonate, 0.05-0.2% of vitamin D3 powder, 22-40% of sorbitol, 0.6-2.4% of citric acid, 0.1-0.6% of aspartame, 0.2-0.6% of essence, 0.05-0.2% of pigment and 2-6% of magnesium stearate. The chewable tablet is suitable for large-scale industrial production, and taste, color and smell of the chewable tablet are adjusted by reasonably proportioning the components and content, so that medication compliance of the children is improved.
Owner:GUANGZHOU BAIYUSN GUANGHUA PHARMA
Pharmaceutical composition containing mecobalamin and preparation method thereof
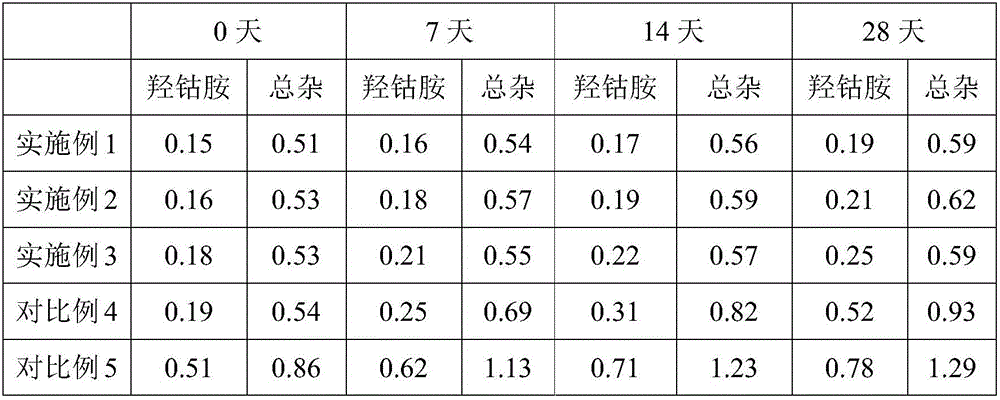
ActiveCN106236719AReduce degradationUniform contentOrganic active ingredientsNervous disorderMethylcobalaminPharmaceutical formulation
The invention relates to the technical field of pharmaceutical preparations, in particular to a pharmaceutical composition containing mecobalamin and a preparation method thereof. The pharmaceutical composition containing mecobalamin is composed of, by mass percentage, 0.01-1% of methylcobalamin, 1%-10% of a stabilizer, 40-95% of a diluent, 0-20% of a disintegrant, and 0.1-10% of a lubricant. The preparation method consists of: premixing mecobalamine and part of the diluents, then mixing the pre-mixture with the stabilizer, the diluent and the disintegrant to obtain mixed powder, mixing the mixed powder with the lubricant for a certain period of time to obtain mixed raw material powder, and subjecting the mixed raw material powder to direct tabletting and coating. The method provided by the invention adopts a dry granulation process, involves fewer steps, avoids the influence of moisture and heat to the main drug, further improves the quality of mecobalamin tablets, lowers the cost, and is beneficial to industrial production.
Owner:YANGTZE RIVER PHARM GRP NANJING HAILING PHARM CO LTD
Method for manufacturing a pharmaceutical composition for controlled release of an active substance
InactiveUS20090035372A1High viscosityGood content uniformityBiocideUrinary disorderPolyethylene oxideControl release
The present invention pertains to a sized product, which contains a drug, polyethylene oxide with a molecular weight of 2,000,000 or higher, and a specific size controlling agent for polyethylene oxide (substance with the appropriate plasticity and binding force) and wherein at least the above-mentioned specific size controlling agent is uniformly dispersed in the above-mentioned polyethylene oxide, a controlled-release pharmaceutical composition containing this sized product, and a method of manufacturing a controlled-release pharmaceutical composition containing this sized product.A controlled-release pharmaceutical composition with good uniformity of content can be presented by using powder particles of polyethylene oxide with powder properties suitable for tableting, which is obtained by uniform dispersion of the specific size controlling agent for polyethylene oxide of the present invention.
Owner:ASTELLAS PHARMA INC
Activated high-modulus low-shrinkage polyester industrial yarn and preparation method of same
InactiveCN102995155AHigh strengthImprove wear resistanceArtificial thread manufacturing machinesFibre treatmentYarnPolyester
The invention relates to an activated high-modulus low-shrinkage polyester industrial yarn and a preparation method of the same. The activated high-modulus low-shrinkage polyester industrial yarn is formed in a way that high-adhesion PET (polyester) slices are taken as raw materials and then are subjected to melt spinning, a proper amount of spinning oil is added, the drawing formation is carried out, a proper quantity of activators are added and then the winding is carried out. A formula contains the following components by weight parts: 100 parts of Halead (HLD) 301 high-adhesion PET, 0.2-0.6 part of Sanyo 911 spinning oil and 0.2-0.5 part of a Syma TC12 activator. The preparation method comprises the steps of: solid-phase polymerizing, melting, metering, cooling forming, oiling, drawing, activator feeding and winding. According to the yarn, the product breaking strength is over 7.0CN / dtex, the yarn has the advantages of good abrasion resistance, small index of dimensional stability, uniform content of the activator, and the like, and can be applied to the field of tyre cord fabrics, and the manufacturing cost of post processing is saved remarkably.
Owner:ZHEJIANG HAILIDE NEW MATERIAL
Lysine hydrochloride/zinc gluconate composite particle and preparation method of composite particle
InactiveCN103976992ASuitable for a wide range of peopleLittle complianceOrganic active ingredientsMetabolism disorderFiller ExcipientGluconates
The invention discloses a lysine hydrochloride / zinc gluconate composite particle and a preparation method of the composite particle. The lysine hydrochloride / zinc gluconate composite particle is prepared from the following components in parts by weight: 10-15 parts of lysine hydrochloride, 3-4 parts of zinc gluconate, 0.5-2 parts of sweetening agent, 1.0-2.5 parts of citric acid, 60-90 parts of filling agent, 3-5 parts of povidone K30 and 0.5-1.0 part of essence. The composite particle is free of sugar and especially suitable for patients with diabetes mellitus. In addition, the composite particle is small in difference of weight ratios between raw material amount and accessory material amount; the prepared particle is uniform; after packaging, the content in each bag is relatively uniform; the granularity is changed from 10-13% to 5-8%; due to the adoption of the povidone K30, the strength of the particle is changed from 8-13N to 15-20N; the particle is good in pressure resistance and has a small possibility of being crushed in the packaging process and the transportation process; meanwhile, the particle is excellent in mobility; a repose angle is changed from 35-45 degrees to 25-30 degrees; carr index is changed from 20-30% to 10-20%.
Owner:海南慧谷药业有限公司
Preparation method of vildagliptin and metformin hydrochloride compound preparation
InactiveCN106580960AAvoid contactImprove stabilityMetabolism disorderPharmaceutical non-active ingredientsMetformin HydrochlorideCompressibility
The invention discloses a preparation method of a vildagliptin and metformin hydrochloride compound preparation. The method includes: subjecting metformin hydrochloride, an adhesive and vildagliptin to sieving mixing, performing dry granulation, adding a lubricant, conducting total blending, carrying out sampling detection, tabletting and other processes. The tablet prepared by the process solves the content uniformity problem of vildagliptin, also improves the tablet compressibility and fluidity, the friability and hardness are accord with the quality standards of Pharmacopoeia, and the stability is good. Also the method has the characteristics of simple operation and low production cost, and can realize industrialization.
Owner:NANJING YOKO PHARMA +2
Removable horizontal glue removing furnace for hard alloy production
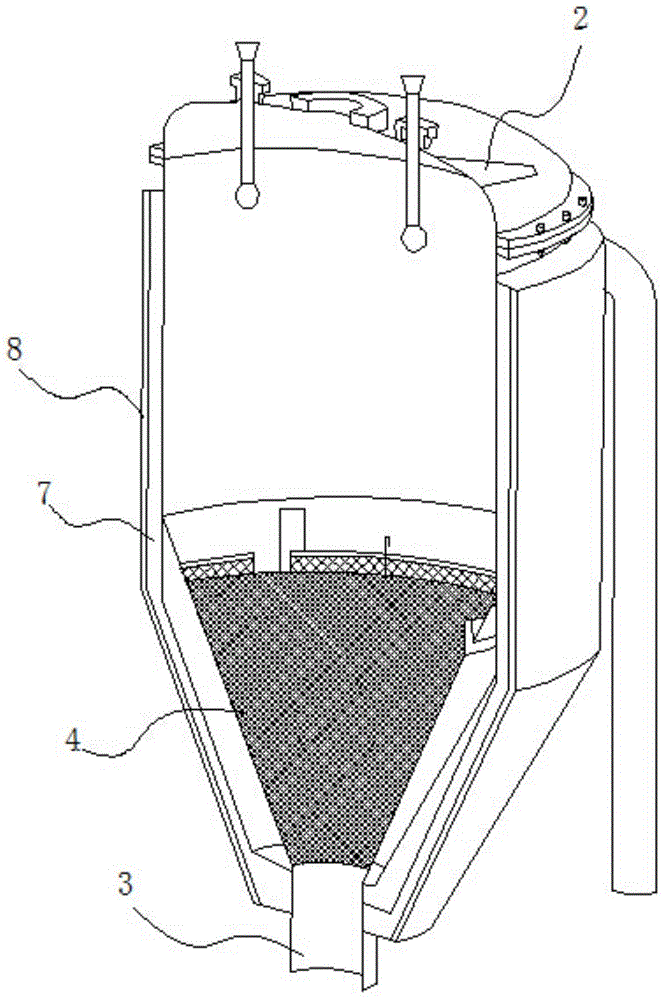
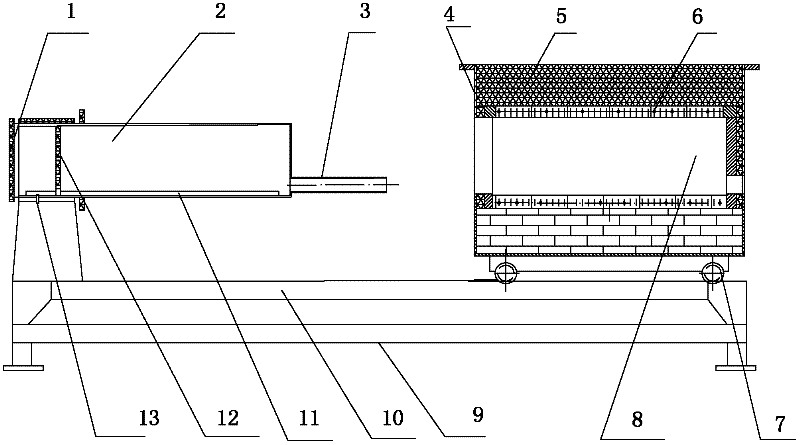
InactiveCN102410726AEven carbon contentReduce Wiring DevicesLighting and heating apparatusGastric tube feedingElectric heating
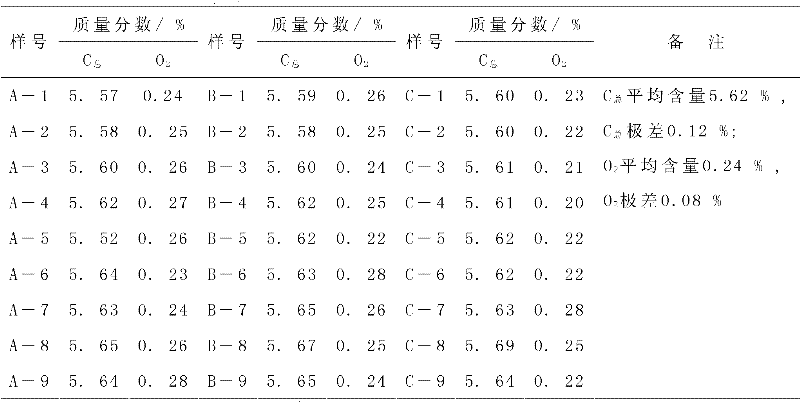
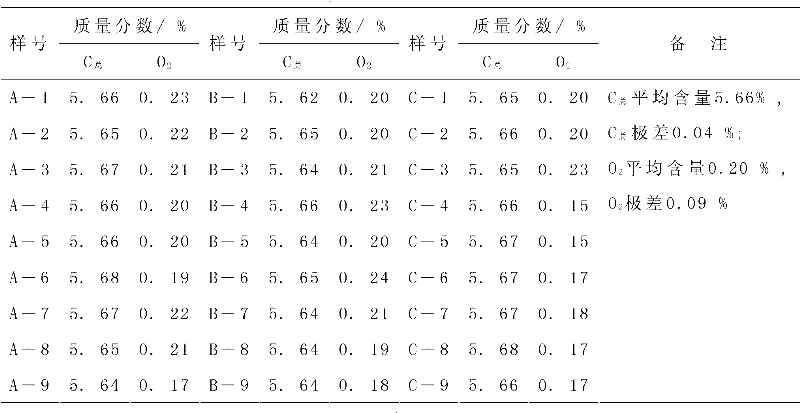
The invention discloses a removable horizontal glue removing furnace for hard alloy production. One end of a furnace cradle (9) is fixedly provided a horizontal furnace tube (2); a furnace door (1), a hydrogen feeding tube (13) and a hydrogen discharging tube (3) are arranged on the horizontal furnace tube (2); a guide rail (10) is arranged on the furnace cradle (9); a horizontal furnace hearth (8) and a heat-insulating material (5) are arranged on a furnace body (4); an electric heating element is arranged in the horizontal furnace hearth (8); rollers (7) are arranged at the bottom of the furnace body (4); the furnace body (4) is arranged on a guide rail (10) through the rollers (7) and can move back and forth; and by moving the furnace body (4) back and forth on the guide rail (10), the horizontal furnace tube (2) is made to enter the horizontal furnace hearth (8) or be separated from the horizontal furnace hearth (8). The removable horizontal glue removing furnace for the hard alloy production in the invention is used for removing a forming agent, can be used for ensuring the uniformity in oxygen content and carbon content inside and outside as well as on the upper part and the lower part of a green compact, and has a long service life.
Owner:CENT SOUTH UNIV +1
Extended release formulation of pramipexole dihydrochloride
InactiveUS20060110454A1Reduce doseHighly photosensitiveBiocideOrganic active ingredientsMulti unitActive agent
An extended release composition of Pramipexole or a pharmaceutical acceptable salt thereof, wherein the active agent is coated on a non pareil inert core, the drug loaded core is further coated with a polymeric layer which enables the release of the active agent over an extended period and optionally the extended release pellets being further blended with suitable excipients and compressed into a multi unit tablet and processes for the preparation of the said composition.
Owner:ALEMBIC LTD
Pramipexole dihydrochloride sustained-release preparation and preparing method thereof
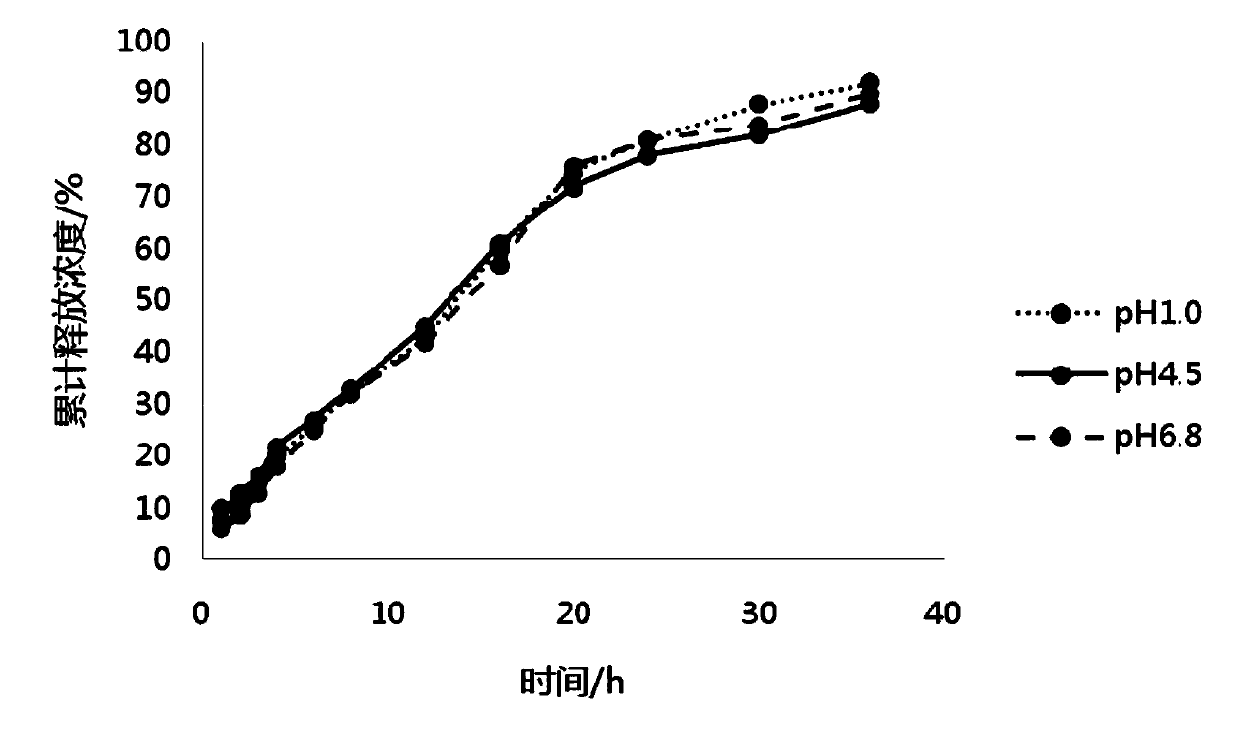
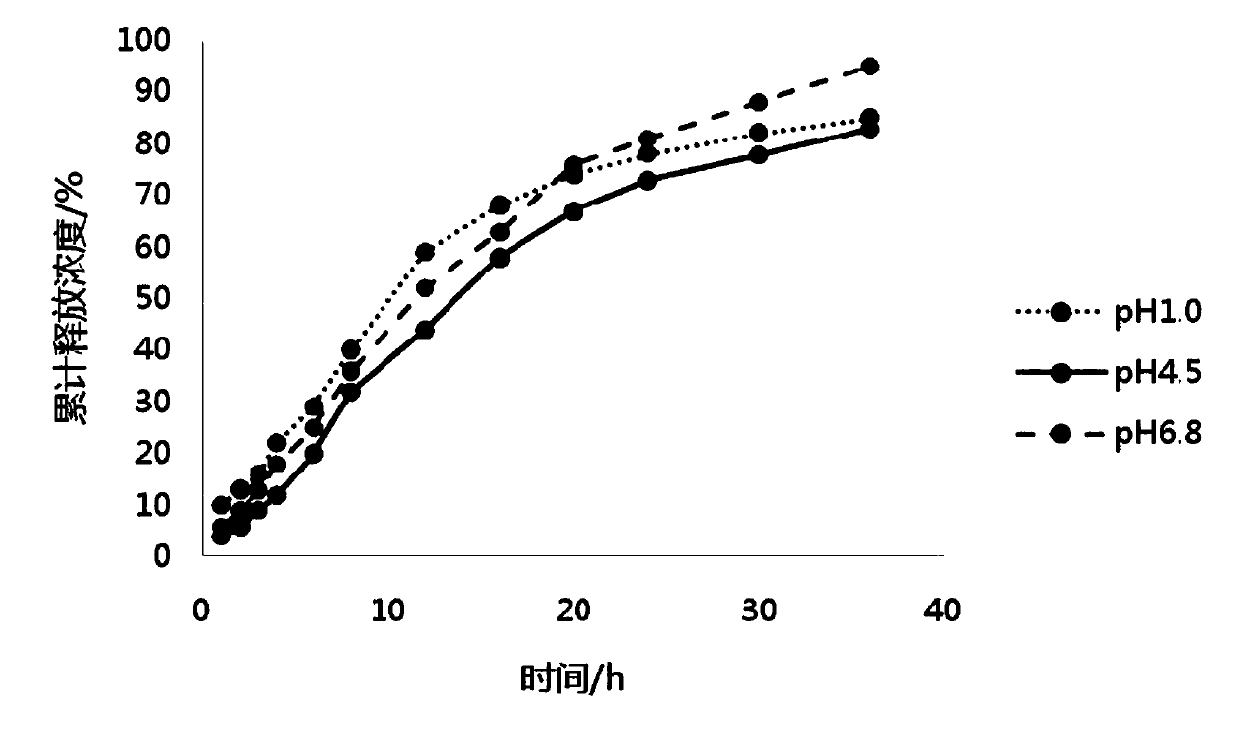
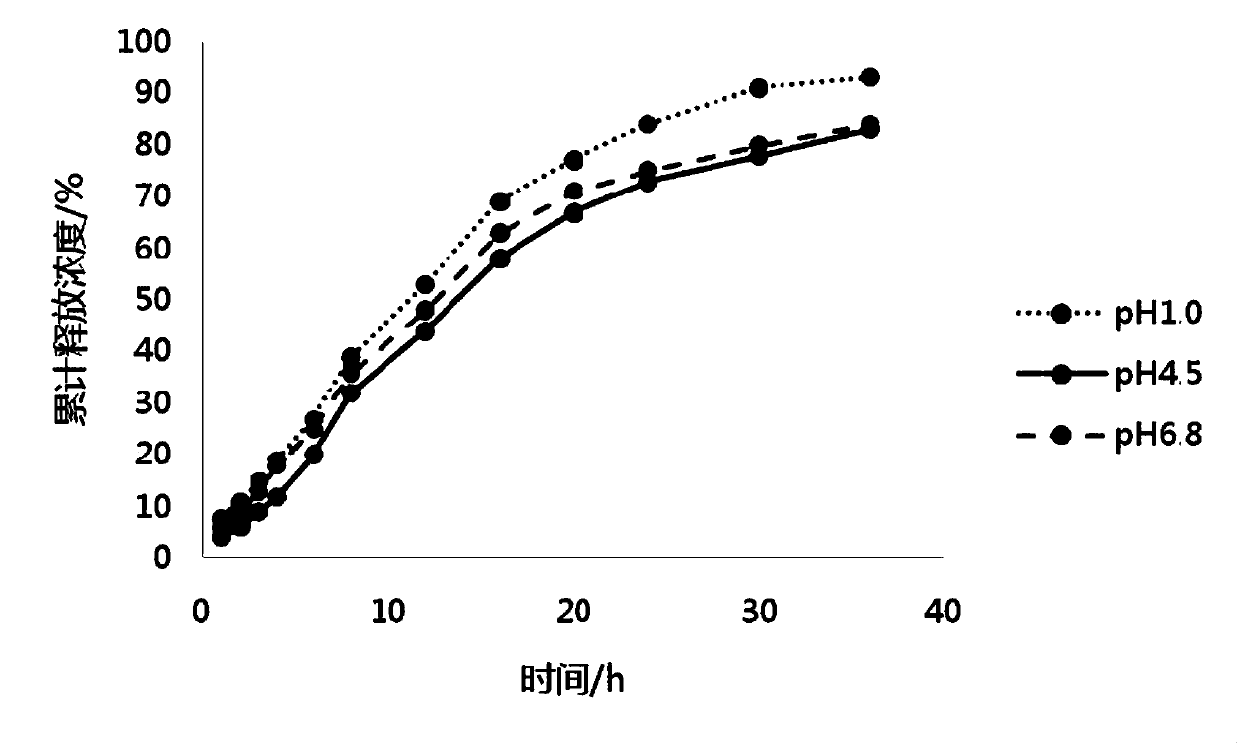
ActiveCN108159007AUniform and stable contentAvoid the defect of different release volumeOrganic active ingredientsNervous disorderOral medicationMedicine
The invention discloses a pramipexole dihydrochloride sustained-release preparation and a preparing method thereof, and belongs to the field of pharmaceutical preparations. The invention provides thepramipexole dihydrochloride sustained-release preparation which is prepared from the following raw and auxiliary materials by weight: 1-5 parts of pramipexole dihydrochloride, 35-140 parts of a sustained-release material, 130-400 parts of a skeleton material, 300-750 parts of a diluent, 10-20 parts of a flow aid, and 5-15 parts of a lubricant. The invention also provides the preparing method of the sustained-release preparation, wherein the preparing method includes the following steps: (1) taking pramipexole dihydrochloride, and crushing; (2) mixing the sustained-release material and pramipexole dihydrochloride evenly; (3) adding the skeleton material and the diluent, and mixing evenly; (4) making the mixture obtained in the step (3) into dry particles; (5) mixing the dry particles with the lubricant and the flow aid, to obtain an intermediate; and (6) tabletting. Under the specific ratio and granulating process, the sustained-release preparation has the advantages of uniform content,stable release and reliable quality, can be released evenly in various dissolving media with different pH, and is suitable for oral administration once a day.
Owner:CHENGDU BAIYU PHARMA CO LTD
Soil saline-alkali prevention and control equipment and prevention and control method
InactiveCN112740853APrevent evaporationEasy to sampleSpadesTransportation and packagingSoil scienceAlkali soil
The invention discloses soil saline-alkali prevention and control equipment and a prevention and control method, and belongs to the technical field of saline-alkali soil prevention and control. The soil saline-alkali prevention and control equipment comprises a base, a box body, a crushing mechanism, a stirring mechanism, a pH value detector and a second driving mechanism, wherein the box body is fixedly connected to the base, the crushing mechanism is arranged at the bottom of the base, the pH value detector is arranged in the base, a suction cavity is formed in the base, a second fan is arranged in the suction cavity, a feeding pipe and a discharging pipe are sequentially and fixedly connected to the bottom of the suction cavity from left to right, the second fan is matched with the feeding pipe and the discharging pipe, and the feeding pipe is matched with the crushing mechanism. When the equipment is used for preventing and treating the saline-alkali soil, the alkali content of the soil can be automatically measured, liquid medicine with different concentrations can be prepared without manual sampling, and the equipment can adjust the concentration of the required liquid medicine according to the real-time condition.
Owner:谢超
External pharmaceutical composition for treating mammary gland diseases and preparation method thereof
InactiveCN102552818AUniform contentImprove stabilitySexual disorderSheet deliveryMammary gland diseaseDissolution
The invention relates to an external pharmaceutical composition for treating mammary gland diseases and a preparation method of the external pharmaceutical composition, and belongs to the field of pharmaceutical preparation. The external pharmaceutical composition for treating mammary gland disease is uniform in content, good in stability, high in dissolution rate, simple and convenient in preparation method and suitable for industrial production.
Owner:李新民
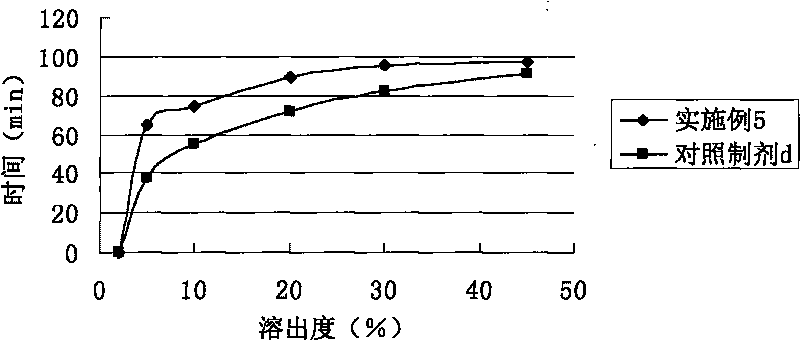
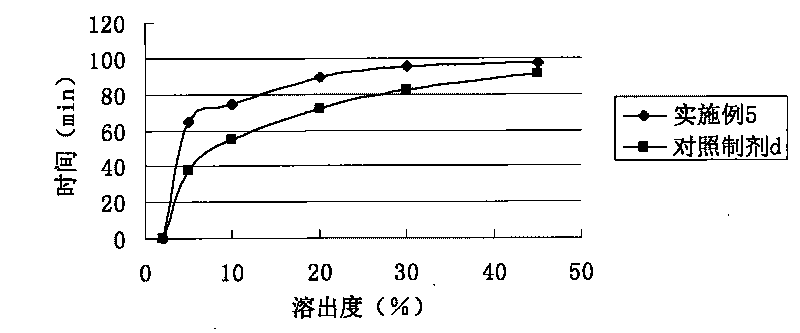
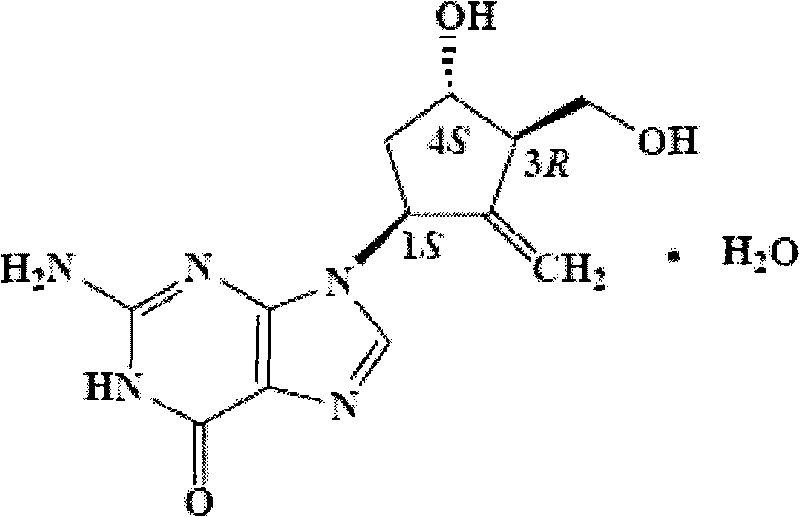
Entecavir soft capsule
The invention relates to a soft capsule with an active component inhibiting hepatitis virus, in particular to an Entecavir soft capsule with high dissolubility, low penetration rate and stable property. The Entecavir soft capsule comprises a soft capsule shell and a content composition, wherein the content composition comprises the following components by 100 percent of total weight of the composition: 0.05-1.0 percent of Entecavir, 80-99.85 percent of filling solvent and 0.10-5.0 percent of povidone; and the total weight of all components in the composition is 100 percent. The invention improves the dissolubility and stability of the Entecavir and can maintain a stable composition system under various conditions without precipitation; the Entecavir active component can not be changed along with the change of time and has uniform and consistent content and high storage stability, and impurities of the Entecavir active component can not increase by long-time storage. Compared with propylene glycol, the Entecavir soft capsule has low penetration rate and more stable composition system without sediment precipitation of the active component for long-time storage under a certain condition.
Owner:杭州浙中医药科技有限公司
Complete granular feed for milk goats, as well as preparation method and application of complete granular feed
InactiveCN107647124ANutritional diversityGuarantee the nutritional needs of milk productionFodderFiberPhosphate
The invention discloses a complete granular feed for milk goats, as well as a preparation method and application of the complete granular feed, and solves the problems that a feed for milk goats in the prior art is threatened by aflatoxin, so that the quantity of crude fibers and the quantity of neutral washing fibers are out of control and the palatability of the feed is poor. The complete granular feed disclosed by the invention is prepared from the following raw materials in definite parts by weight: corn, soybean meal, cottonseed meal, rapeseed meal, alfalfa meal, gunited corn peel, applepomace, rice bran, soybean hull, rumen fat, ruminant yeast, stone powder, dicalcium phosphate, baking soda, sodium chloride, calcium sulfate, a mildewproof agent, montmorillonite, Runiule, a trace element additive and composite vitamins. The complete granular feed disclosed by the invention can directly replace an original concentrated feed and an original coarse feed to be used for feeding the milk goats in a mixed manner, can provide sufficient and comprehensive nutrients for the milk goats and can meet nutrition requirements of the milk goats for milk production.
Owner:SICHUAN TQLS IND
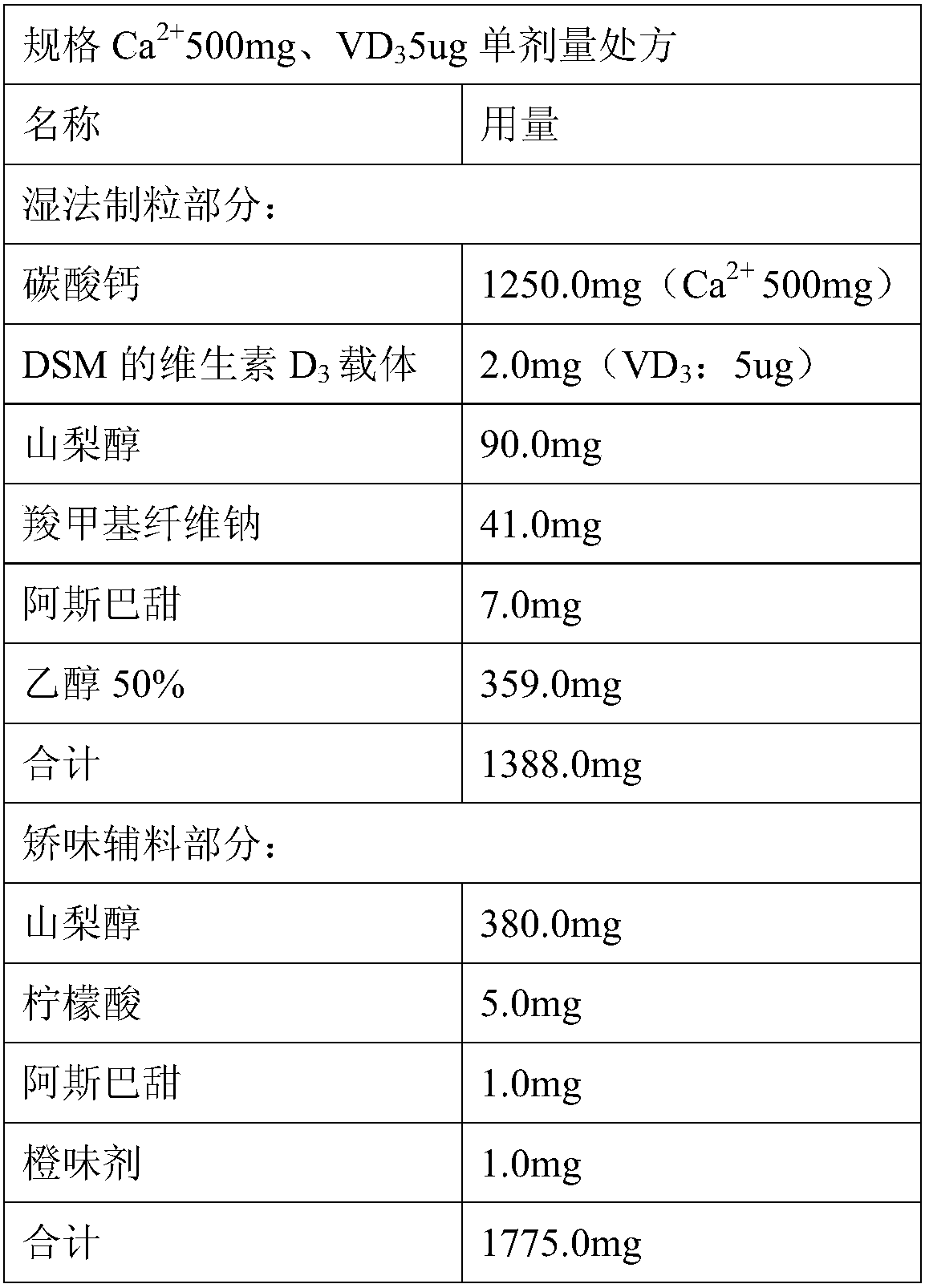
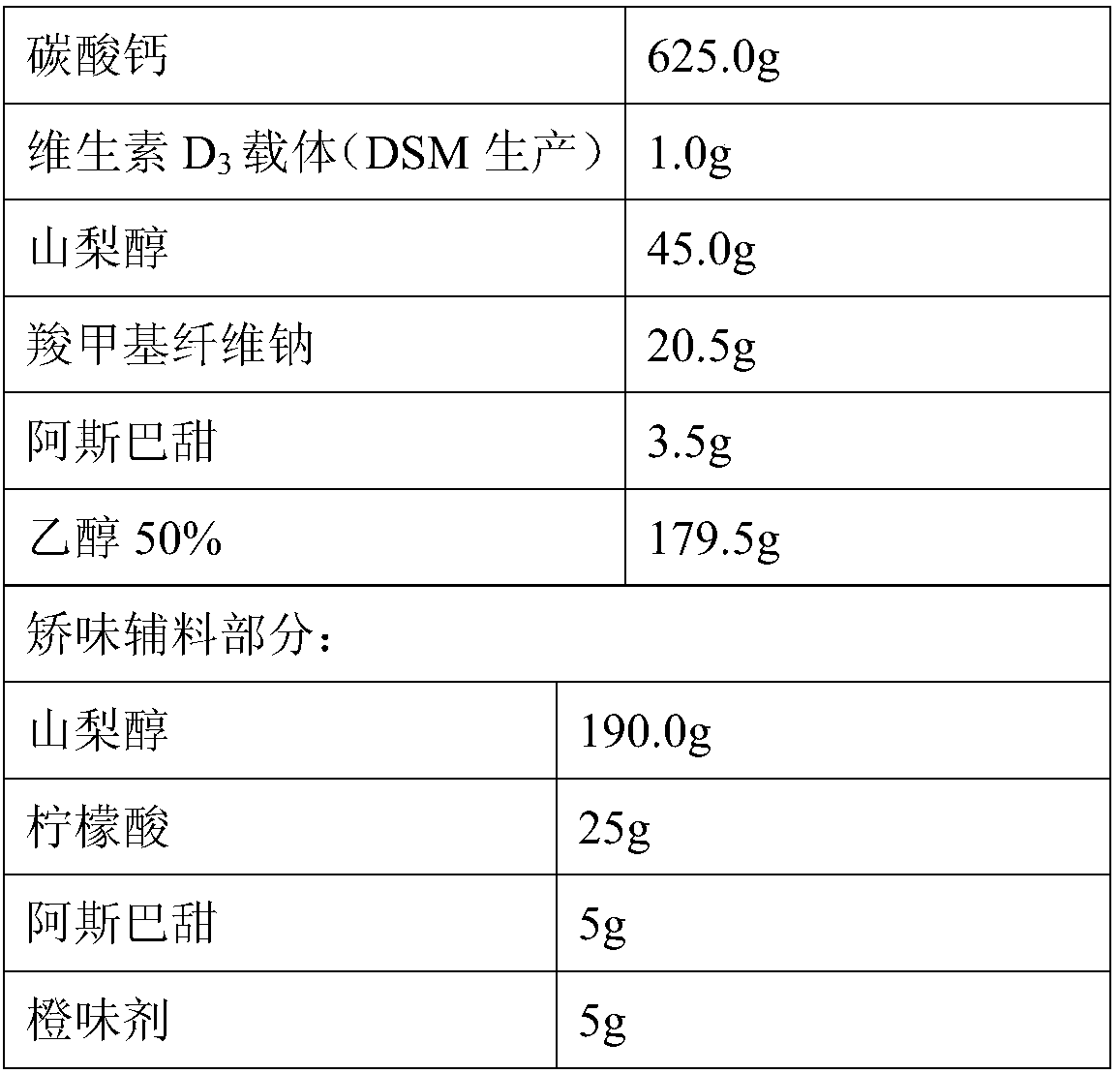
Calcium carbonate vitamin D3 composition and water-free-swallowing granules thereof
PendingCN107625786ASolve the problem of poor content uniformityEasy to swallowOrganic active ingredientsPharmaceutical non-active ingredientsVitamin d 3Cam
The present invention relates to a calcium carbonate vitamin D3 composition and water-free-swallowing granules thereof, and belongs to the field of pharmaceutical preparations. According to the present invention, in the calcium carbonate vitamin D3 composition and the calcium carbonate vitamin D3 water-free-swallowing granules containing the composition or prepared from the composition, vitamin D3is fed in a vitamin D3 carrier manner; compared to the granules in the prior art, the granules of the present invention have the following advantages that the rapid water-free swallowing of the granules in the oral cavity cam be achieved, the taste is good, and the compliance of the user can be improved, such that the problems of poor content uniformity and poor stability of vitamin D3 in the preparation can be solved; and particularly compared to the existing calcium carbonate vitamin D3 preparation, the preparation of the present invention has the following advantages that the calcium carbonate content in the unit preparation is increased while the loading is lower than the loading of the traditional dosage form.
Owner:山东科成医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com