Patents
Literature
47 results about "Clonidine Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
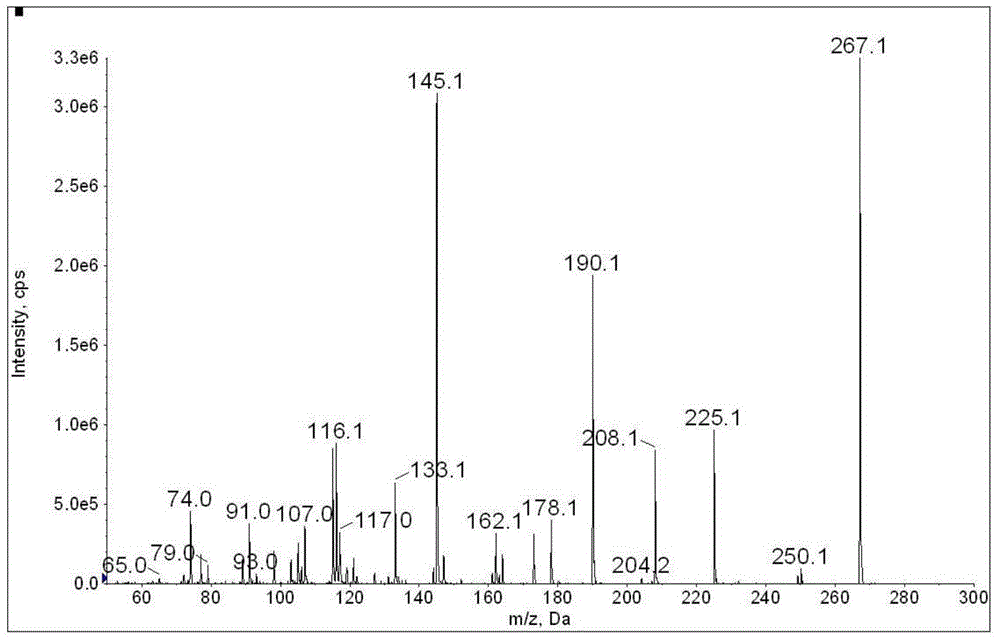
The hydrochloride salt form of clonidine, an imidazoline derivate and centrally-acting alpha-adrenergic agonist as well as antagonist with antihypertensive activity. Clonidine hydrochloride binds to and stimulates central alpha-2 adrenergic receptors, thereby decreasing sympathetic outflow to the heart, kidneys, and peripheral vasculature. The reduction in sympathetic outflow, leads to decreased peripheral vascular resistance, decreased blood pressure, and decreased heart rate.
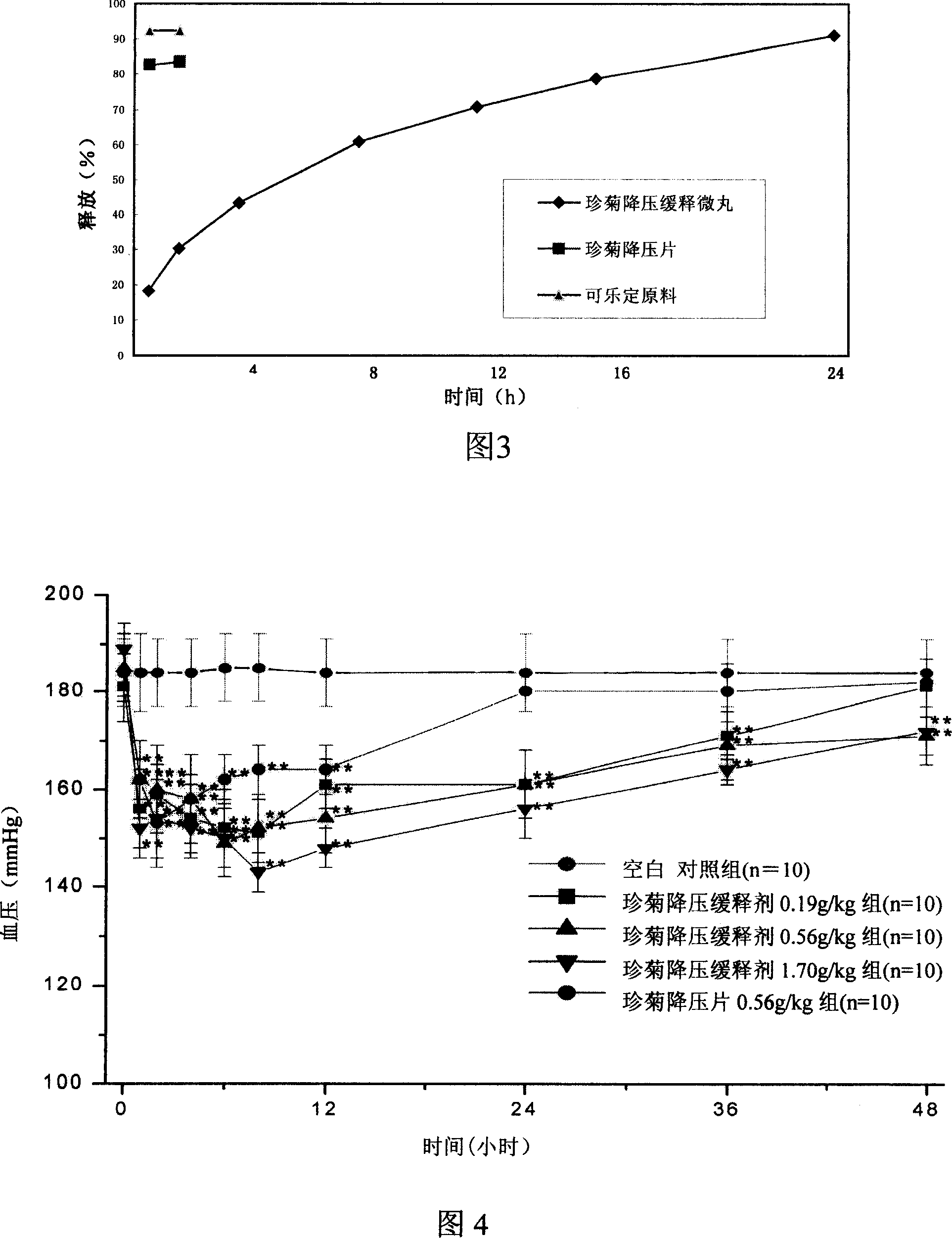
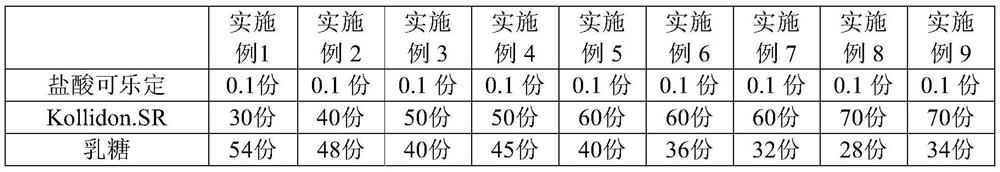
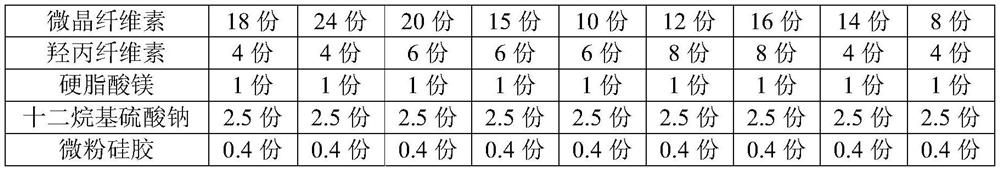
Clonidine hydrochloride sustained release tablets and preparation method thereof
InactiveCN104352473AImprove stabilityDefinite curative effectOrganic active ingredientsPharmaceutical product form changeExtended release tabletsPlastic packaging
The invention provides clonidine hydrochloride sustained release tablets. The clonidine hydrochloride sustained release tablets are prepared from 0.2 part of clonidine hydrochloride, 70-90 parts of sustained release skeleton material and 10-20 parts of lubricating agent by weight. A preparation method of the clonidine hydrochloride sustained release tablets comprises the steps of material preparing, blending, granulating, blending, tabletting and aluminium-plastic packaging. The clonidine hydrochloride sustained release tablets and the preparation method have the beneficial effects that the clonidine hydrochloride sustained release tablets have good stability and definite curative effects and can be used for effectively treating hypertension; the novel sustained release preparations are adopted; sustained release refers to reducing the medicine release rates of medicines from the dosage forms and reducing the absorption rates of the medicines into bodies, thus achieving more stable treatment effects; compared with oral liquids, the clonidine hydrochloride sustained release tablets have the advantages of good medicine stability, convenience in packaging, transportation and storage, and the like; the preparation method is simple and practicable and is suitable for industrial production.
Owner:HARBIN SHENGJI PHARMA
Clonidine hydrochloride multivesicular liposome and preparation method thereof
InactiveCN101536981AHigh encapsulation efficiencyGood sustained release effectOrganic active ingredientsAntipyreticLipid formationCholesterol
The invention discloses clonidine hydrochloride multivesicular liposome and a preparation method thereof. The clonidine hydrochloride multivesicular liposome comprises the following components by weight portion: 1 portion of active ingredient (clonidine hydrochloride), 1 to 10 portions of lipid component, 0.1 to 3.5 portions of acid regulator, 10 to 50 portions of osmotic pressure regulator, and 1.0 to 6.0 portions of lysine, wherein the lipid component comprises neutral phospholipid and cholesterol in a mol ratio of 1: 0.5-1: 2, and neutral lipid which accounts for 1 to 2 mol percent of the weight of the lipid component. The clonidine hydrochloride multivesicular liposome has high encapsulation rate and stability, and is shown in vivo and in vitro experiments to have a good slow release effect.
Owner:SHANGHAI INST OF PHARMA IND
Preparation method of clonidine hydrochloride sustained-release tablet
InactiveCN104138362AWell mixedIncrease in sizeOrganic active ingredientsNervous disorderSustained Release TabletFiller Excipient
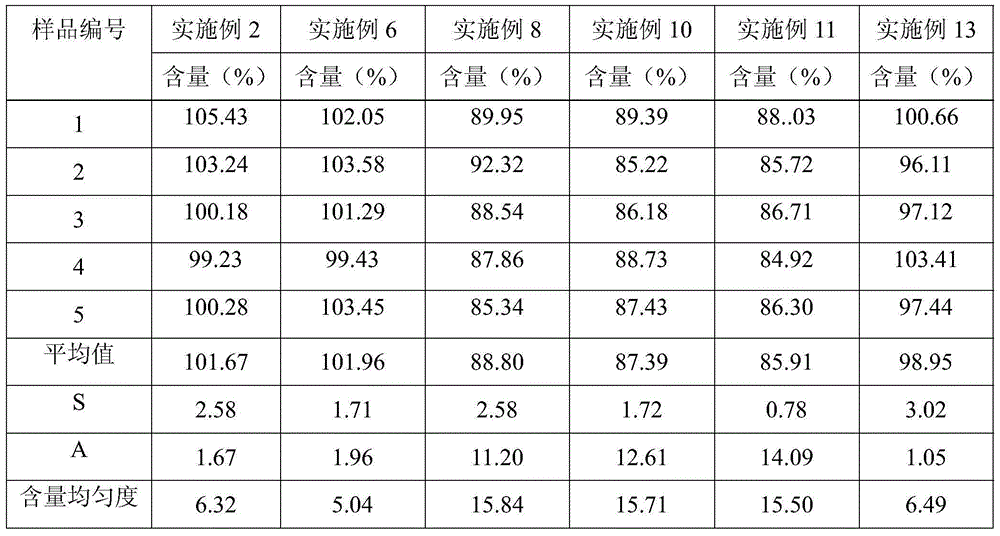
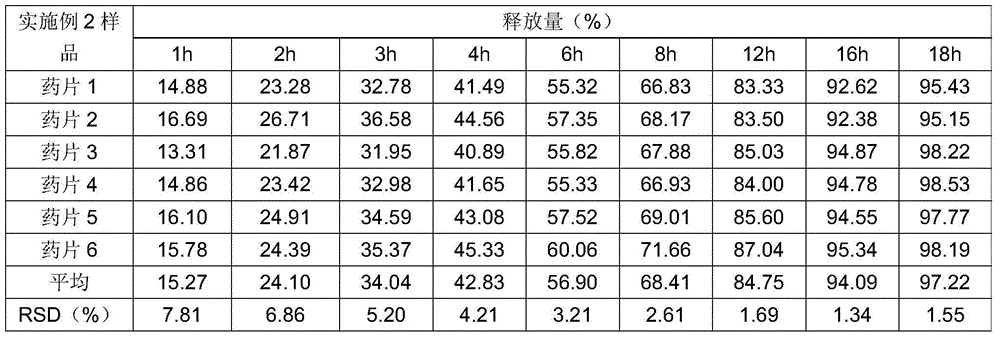
The invention relates to a preparation method of a clonidine hydrochloride sustained-release tablet. The preparation method comprises the steps of dissolving a clonidine hydrochloride raw material into a proper amount of wetting agent by using a solvent dispersion method to prepare a solution containing 10-28.57mg / ml clonidine; adding the solution into a granulation pan filled with a filling agent and a binder at constant speed of 5-40ml / min to granulate; drying the prepared granules, screening by using a 50-100-mesh sieve, mixing the granules, a framework material, a flow aid and a lubricating agent, and tabletting to obtain the clonidine hydrochloride sustained-release tablet. By using the preparation method, the problem of non-uniform content generated in a preparation process of the clonidine hydrochloride sustained-release tablet can be effectively solved, the prepared clonidine hydrochloride sustained-release tablet is uniform in content, and the release behavior is in accord with the sustained-release characteristic. All process parameters in the preparation method can be correspondingly amplified according to the industrial production scale, so that the requirement for large-scale industrial production can be met.
Owner:LP PHARM (XIAMEN) CO LTD
Method for determination of illegally added substances in traditional Chinese medicines and health-care products
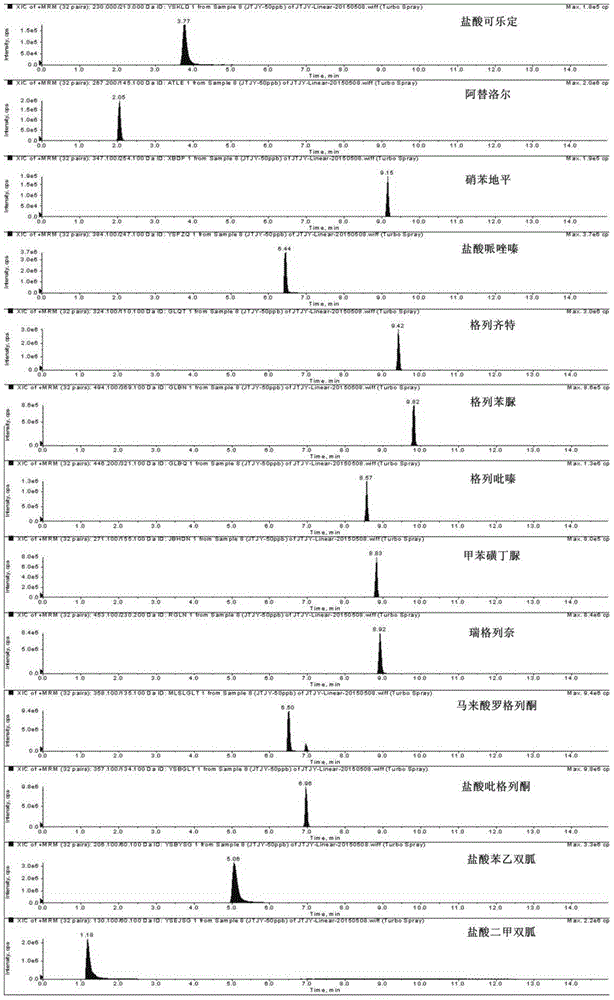
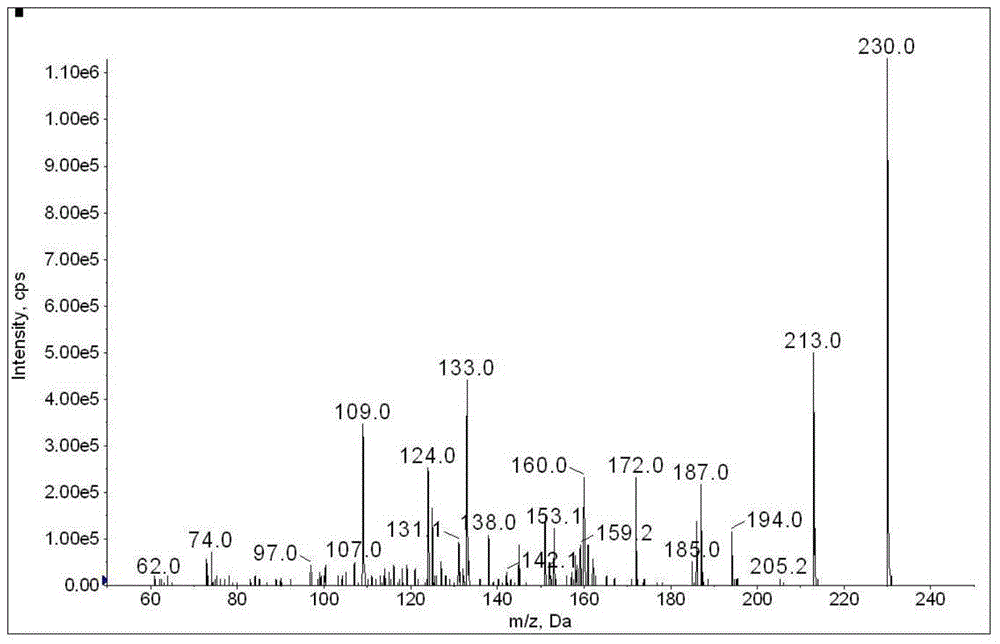
The invention discloses a method for determination of illegally added substances in traditional Chinese medicines and health-care products; with use of high performance liquid chromatography-tandem quadrupole linear ion trap mass spectrometry, the method which is capable of simultaneously qualitative and quantitative detection (multiple reaction monitoring sMRM-information dependent acquisition IDA-enhanced product ion scanning EPI detection) of 13 kinds of illegally added hypoglycemic hypotensive chemical drugs such as clonidine hydrochloride and gliclazide in the traditional Chinese medicines and the health-care products is developed. And through one-time sampling detection, not only can a quantitative result obtained, but also occurrence of a false positive result is effectively avoided through qualitative database screening. The establishment of the method provides a technical support for enacting detection standards of the illegally added hypoglycemic hypotensive chemical drugs in the traditional Chinese medicine and the health-care products.
Owner:SHANDONG ANALYSIS & TEST CENT
Clonidine hydrochloride sustained release micropill preparation
ActiveCN102138906AExtended half-lifeExtension of timeOrganic active ingredientsPharmaceutical non-active ingredientsSustained release pelletsPharmaceutical formulation
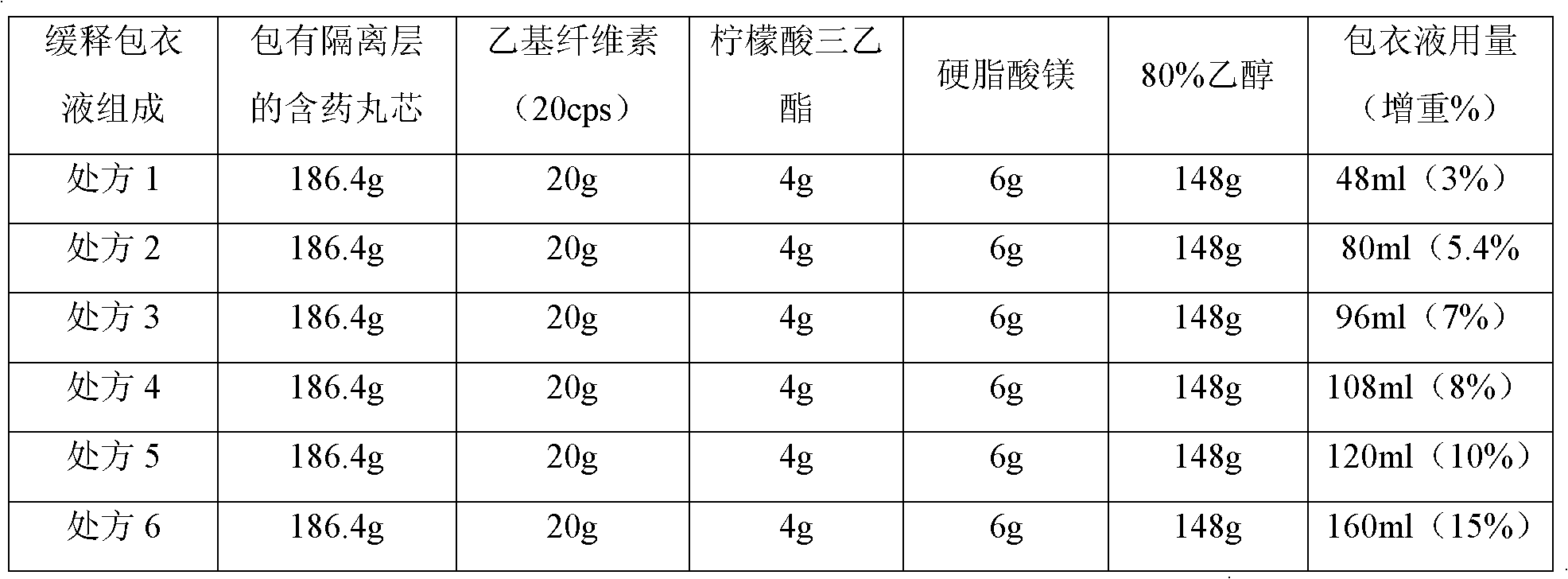
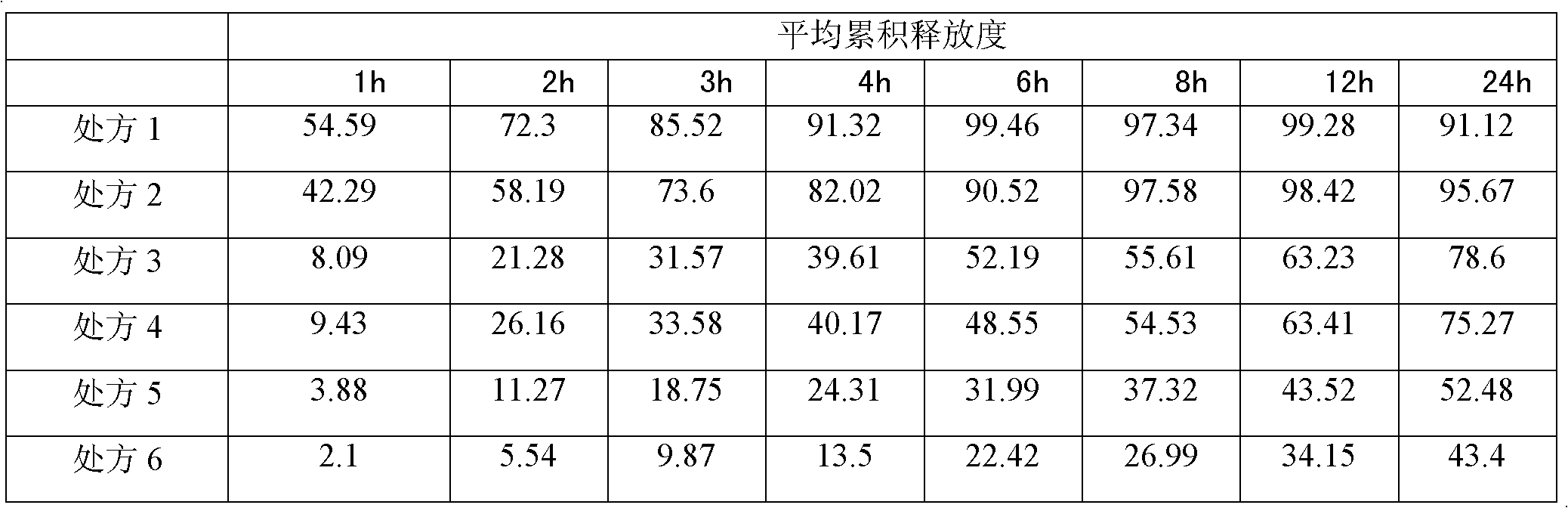
The invention relates to the field of medicinal preparations, in particular to a clonidine hydrochloride sustained release preparation and a preparation method thereof. The clonidine hydrochloride sustained release preparation is characterized in that: a sustained release coating membrane is divided into two layers, wherein a sustained release coating membrane of an inner layer contains a pore-forming agent, and a sustained release coating membrane on an outer layer does not contain the pore-forming agent; and a weight ratio of a sustained release coating on the inner layer to a sustained release coating of the outer layer is (1:1)-(2:5), and the weight of sustained release coatings on inner and outer layers are increased by 7 to 8 percent. In the sustained release preparation, two sustained release layers are coated, so that medicaments are released slowly, safely and effectively.
Owner:HEFEI COSOURCE PHARMA CO LTD
External preparation for treating abdominal pain and women's menorrhalgia, preparation method thereof, and quality control method thereof
InactiveCN101732645ANo side effectsImprove effectivelyOrganic active ingredientsComponent separationTreatment effectRhizome
Owner:徐朋 +1
Clonidine hydrochloride sustained release pellets
InactiveCN104352447AReduce absorption rateStable absorptionOrganic active ingredientsPharmaceutical non-active ingredientsSustained release pelletsAdhesive
The invention provides clonidine hydrochloride sustained release pellets. The clonidine hydrochloride sustained release pellets comprise medicine-containing pellets and enteric coating layers, wherein the medicine-containing pellets are coated by the enteric coating layers; the medicine-containing pellets comprise 0.1mg of clonidine hydrochloride, 180mg of hollow pellet cores and 10mg of adhesive; the enteric coating layers comprise 45-225mg of Eudragit NE30D and 7-68mg of talcum powder. A preparation method of the clonidine hydrochloride sustained release pellets comprises the following processes: 1. material preparation; 2. pellet preparation; 3. preparation of an enteric coating agent; 4. coating; 5. filling; 6. aluminium-plastic packaging and preparation of finished products. The sustained release pellets with clonidine hydrochloride as an active ingredient are mainly used for treating hypertension and have the beneficial effects that as the two kinds of advanced technologies, namely novel sustained release preparations and pellet preparations, are adopted, the clonidine hydrochloride sustained release pellets have stable treatment effects and higher bioavailability and have the advantages of good medicine stability, convenience in packaging, transportation and storage, and the like. The preparation method is simple and practicable and is suitable for industrial production.
Owner:HARBIN SHENGJI PHARMA
Slow-released preparation containing hydrochlorothiazide and clonidine hydrochloride and its preparing method
ActiveCN101023951ASustained antihypertensive effectBlood drug concentration in the body is stableOrganic active ingredientsPharmaceutical non-active ingredientsHydrochlorothiazideMedicine
The present invention belongs to the field of medicine preparation. In the concrete, it relates to a compound medicine slow release preparation containing hydrochlorothiazide and clonidine hydrochloride. It is characterized by that said slow release preparation is composed of two portions of quick-released portion and slow-released portion. Besides, said invention also provides its preparation method and concrete steps.
Owner:北京合源汇丰医药科技有限公司
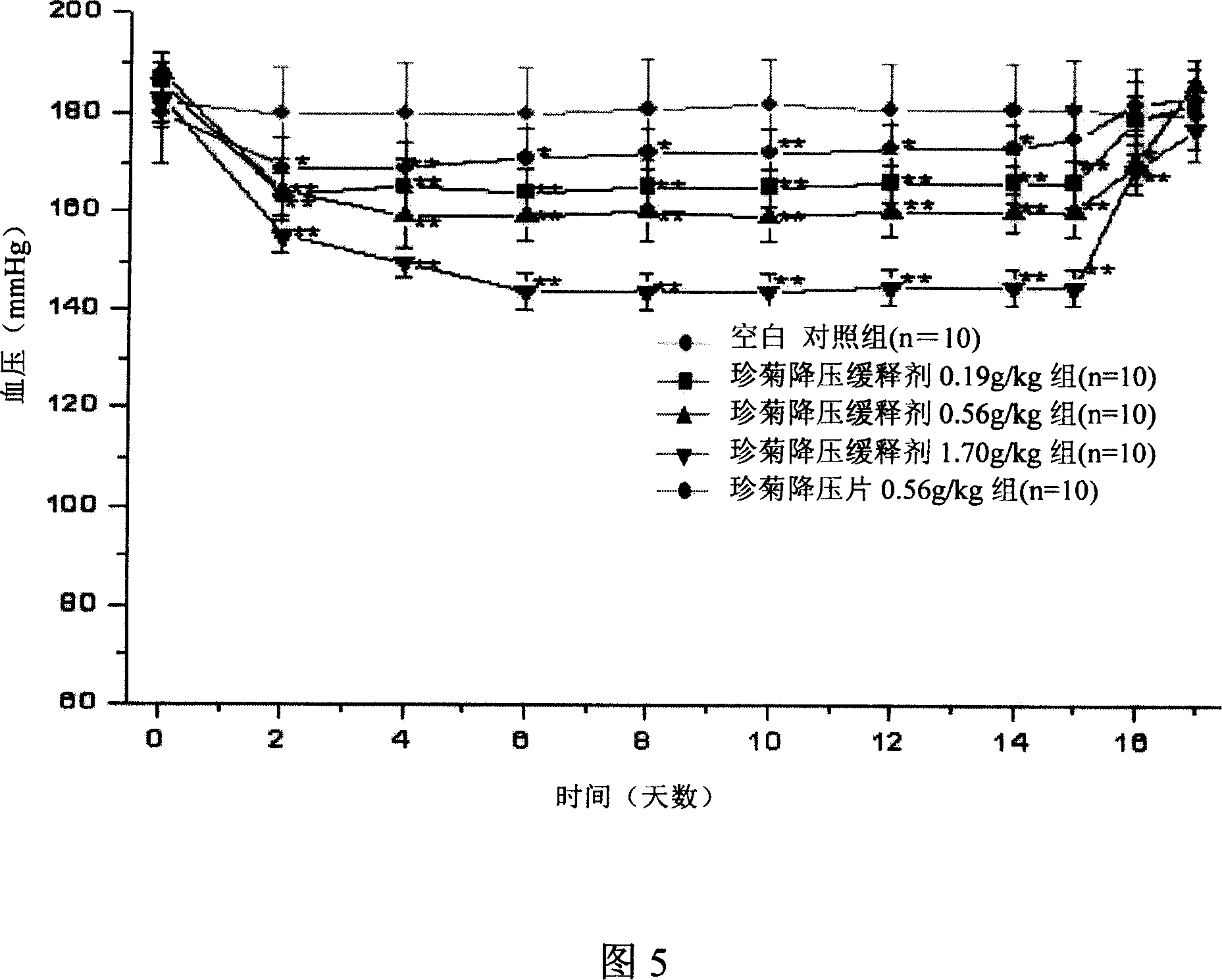
Blood pressue lowering sustained-release preparation with chrysanthemum flower and pearl
InactiveCN101099762ADrug release behaviorAccurate predictionOrganic active ingredientsPharmaceutical delivery mechanismWestern medicineHydrochlorothiazide
The present invention discloses a Zhenju Jiangya slow release preparation for stably reducing blood pressure. It is a Chinese medicine and Western medicine combined compound slow release preparation made up by using hydrochlorothiazide, clonidine hydrochloride, wild chrysanthemum flower extract, pearl layer powder and sophora flower extract through a certain preparation process. Said invention also provides the concrete steps of its preparation process.
Owner:上海雷允上科技发展有限公司 +1
Medicinal composition for treating glaucoma and application thereof
InactiveCN101648006ASalicyclic acid active ingredientsSenses disorderSalicylic acidPharmaceutical medicine
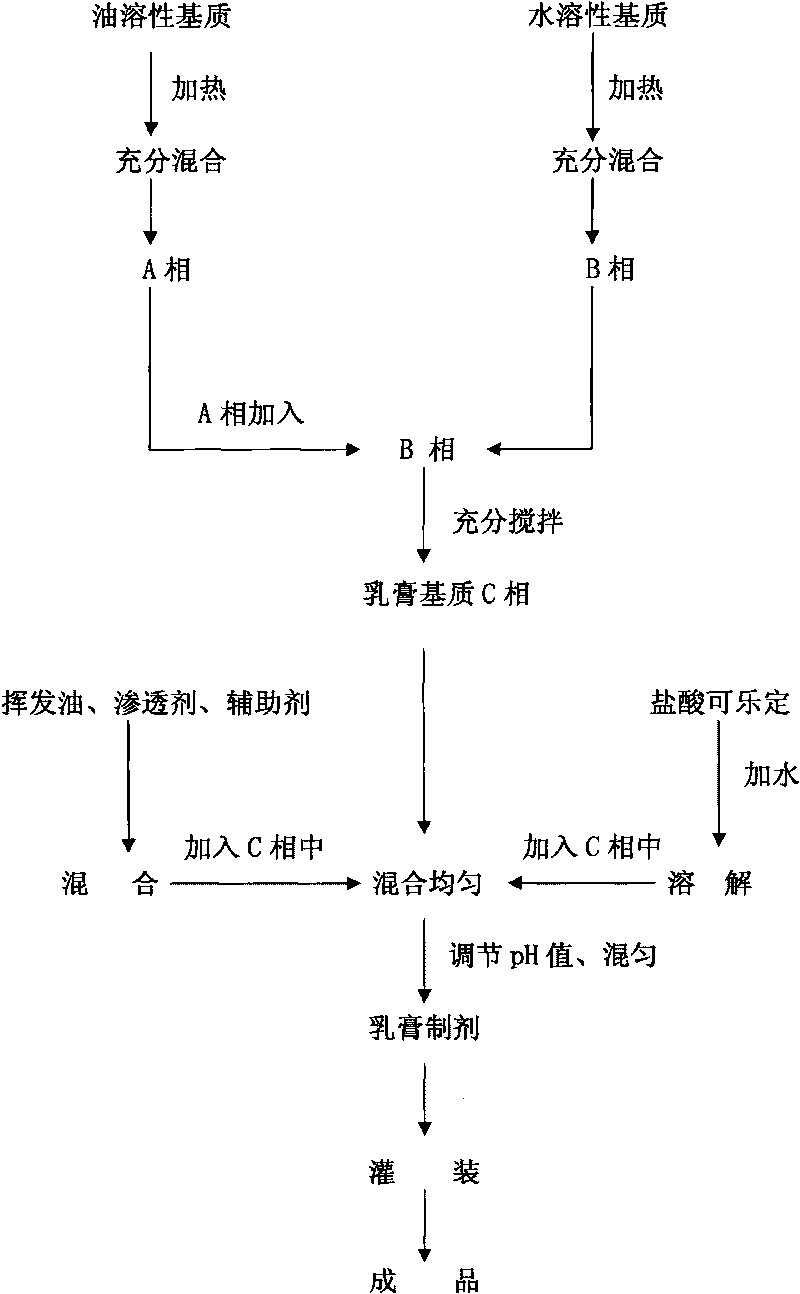
The invention discloses a medicinal composition for treating glaucoma, which consists of collagen and medicaments for miosis and blood pressure reduction, and belongs to the field of pharmacy. Raw material medicaments of effective components of the medicinal composition comprise collagen and medicaments for miosis and blood pressure reduction such as clonidine hydrochloride, physostigmine salicylate, pilocarpine nitrate and the like which are prepared into various eye preparations by combining the prior art. The technical scheme creatively combines and applies the collagen and the effective components of the medicaments in the eye preparations so as to enlarge the application range of the collagen and achieve better effect in the aspect of treating various glaucomas.
Owner:BEIJING HERUN INNOVATION PHARMA TECH DEV
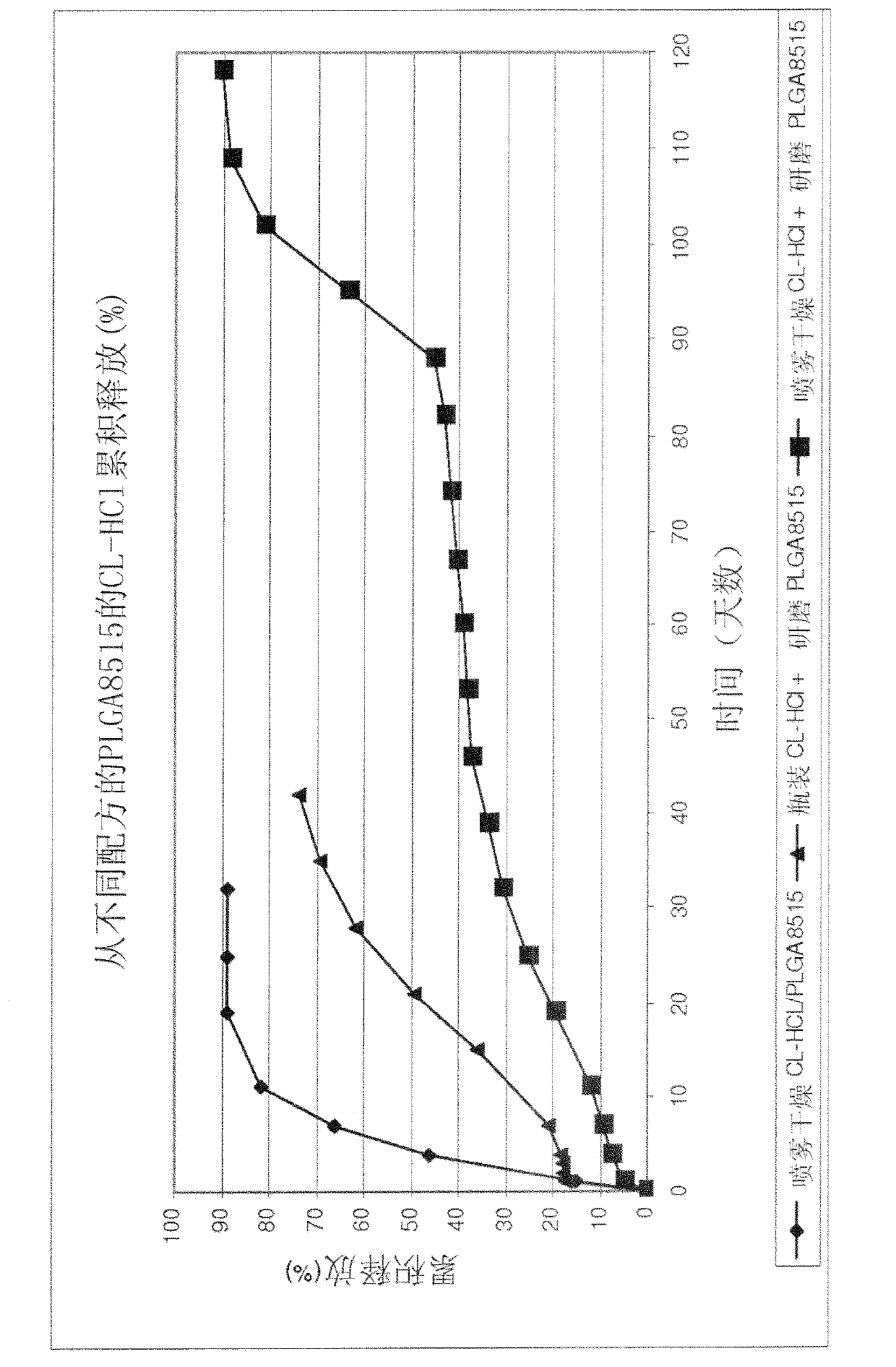
Medical devices and methods including polymers having biologically active agents therein
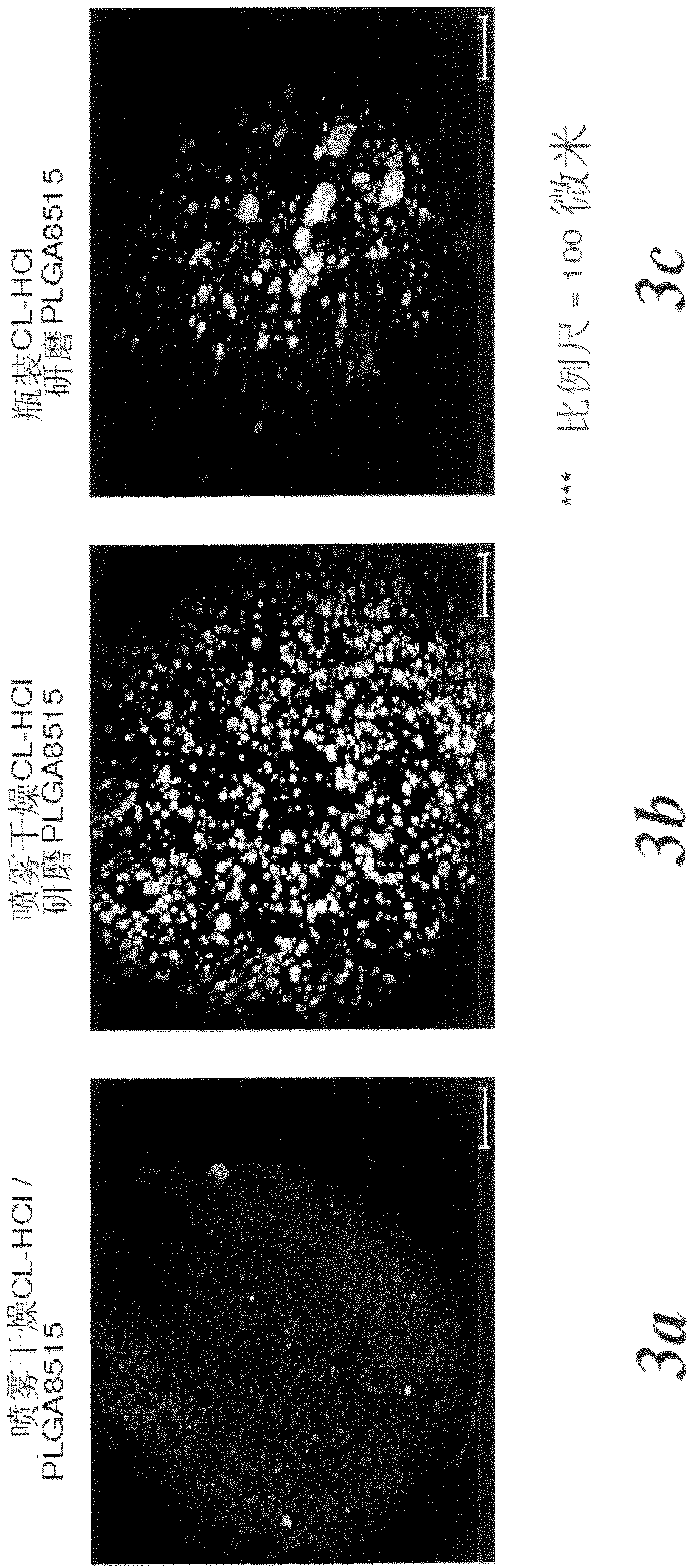
The implant design is a drug loaded polymer device, such as a rod, designed to control the release of a biologically active agent, such as clonidine or its derivatives, such as clonidine HCl for a prolonged period of time, such as 2 months, 3 months, 4 months, and even 4.5 months. The polymer is preferably a biodegradable polymer, such as poly(lactide-co-glycolide) or polylactic acid / polylactide. The challenge in using the HCl salt forms of drugs such as clonidine, is controlling the release of the highly water soluble drug for up to 4.5 months. It has been found that by controlling the particle size distribution of the drug powder, the drug distribution within the polymer matrix is more uniform and can be controlled. Therefore, the large aggregates, which cause rapid drug release can be eliminated.
Owner:MEDTRONIC INC
A kind of clonidine hydrochloride freeze-dried orally disintegrating tablet and preparation method thereof
ActiveCN107362150BEasy to takeGreat tasteOrganic active ingredientsNervous disorderDry mouthOrally disintegrating tablet
The invention belongs to the field of medicine, in particular to a clonidine hydrochloride freeze-dried orally disintegrating tablet and a preparation method thereof. The technical solution adopted by the present invention is a clonidine hydrochloride freeze-dried orally disintegrating tablet, which is characterized in that it contains the following components by weight: 2-10 parts of clonidine hydrochloride, 80-98 parts of filler, and 1-10 parts of binder 5 parts, 0-3 parts of pH regulator, 0-3 parts of flavoring agent, 0-2 parts of preservative. The clonidine hydrochloride freeze-dried orally disintegrating tablet prepared by the invention is convenient to take, has a good taste, and is beneficial to improving the compliance of patients taking medicine; it disintegrates rapidly in the oral cavity, is beneficial to the dissolution of the medicine, accelerates the absorption of the medicine and exerts the curative effect; the content is uniform and stable Good sex. The production process of the clonidine hydrochloride freeze-dried orally disintegrating tablet is simple in technology, easy to control, suitable for industrial production, and has achieved unexpected technical effects.
Owner:CP PHARMA QINGDAO CO LTD
Pharmaceutical composition for relieving or eliminating protracted opioid abstinence syndrome and application of pharmaceutical composition
ActiveCN112494486AImprove Medication AdherenceImprove experienceOrganic active ingredientsNervous disorderWithdrawal syndromeUse medication
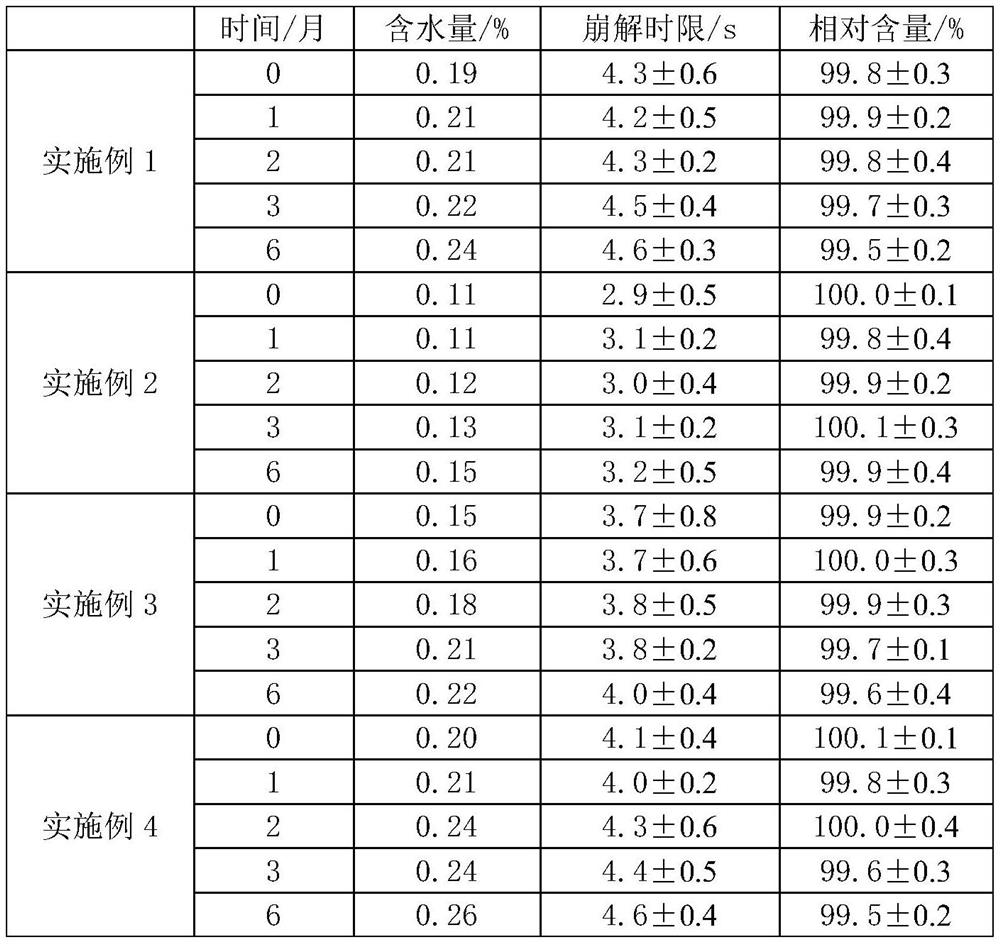
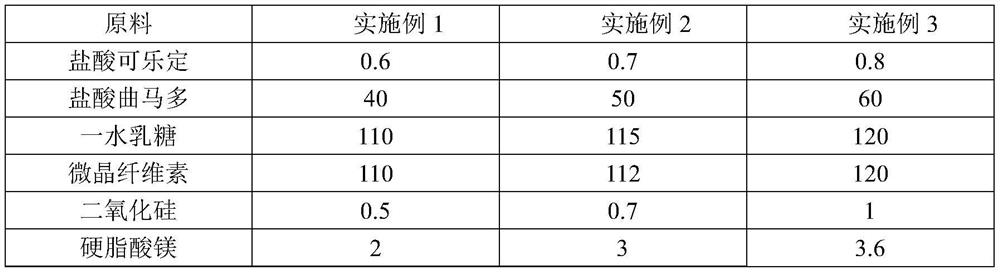
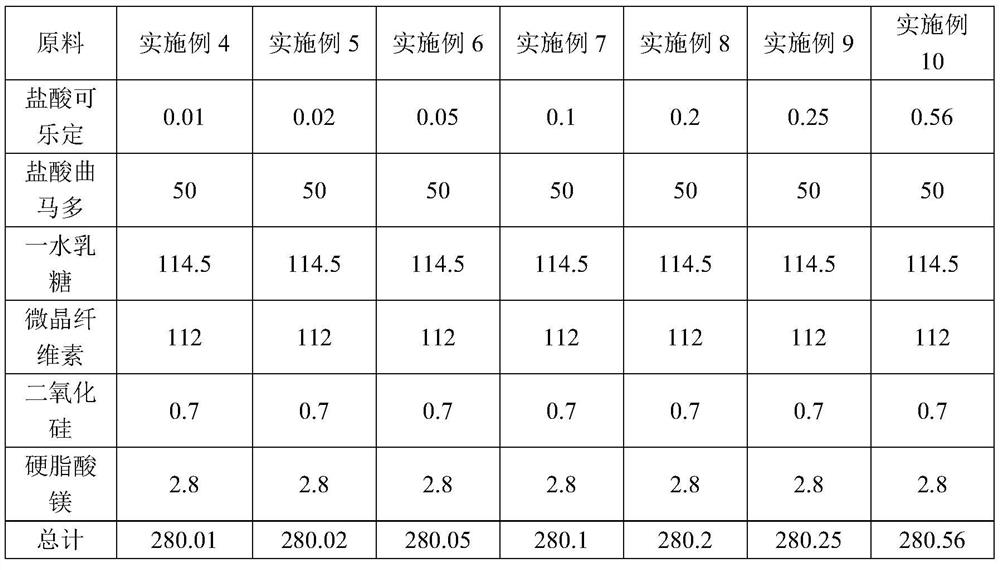
The invention discloses a pharmaceutical composition for relieving or eliminating protracted opioid abstinence syndrome and application of the pharmaceutical composition. The pharmaceutical composition comprises clonidine hydrochloride and tramadol hydrochloride in a mass ratio of (0.01-0.8):(40-60), and the application of the pharmaceutical composition in preparation of drugs for relieving or eliminating the protracted opioid abstinence syndrome is also disclosed. The pharmaceutical composition is more suitable for detoxification of opioid abuse patients, has the advantages of good effect, short time, completion in 3-5 days, safety, effectiveness and convenience in operation, has obvious clinical advantages and curative effects, can improve the medication compliance and medication experience of the patients, and prevents abuse.
Owner:SHENZHEN SCIENCARE MEDICAL INDUSTRIES CO. LTD.
Clonidine hydrochloride multi-vesicular liposome and preparation method thereof
InactiveCN101190183AHigh encapsulation efficiencyGood sustained release effectOrganic active ingredientsLiposomal deliveryLipid formationOrganic solvent
The invention discloses a clonidine hydrochloride bubbly liposome and a preparation method of the clonidine hydrochloride bubbly liposome. The preparation method includes the following steps: (1) lipid comprising neutral phospholipids, cholesterin and neutral lipid is dissolved in organic solvent, and the content of the neutral phospholipids is controlled to be from 20 to 40 mg / ml as a lipid phase; (2) the clonidine hydrochloride is dissolved in injection-used water and concentration of the clonidine hydrochloride is regulated to be from 20 to 10,000Mug / ml as an internal-water phase; (3) the equal-volume internal-water phase is added to the upper layer of the lipid to be mixed and emulsified to get water-in-oil type initial emulsion; (4) external-water containing osmotic pressure regulator is added into the upper layer of the water-in-oil type initial emulsion to be stirred to form water-in-oil-in-water type compound emulsion; (5) organic solvent in the compound emulsion is removed to get the clonidine hydrochloride bubbly liposome. The clonidine hydrochloride bubbly liposome has higher encapsulation rate, better slow release effect and better pain-killing effect.
Owner:SHANGHAI INST OF PHARMA IND
Clonidine pamoate and preparation method thereof
The invention relates to clonidine pamoate and a preparation method thereof. The clonidine pamoate is formed by clonidine and pamoate in a solvent in a molar ratio of 2:1. The clonidine pamoate provided by the invention exists in three crystal forms, the solubility of each crystal form is lower, the solubility in water is about 0.02mg / ml and is equivalent to one hundredth of the solubility of clonidine (2.19mg / ml) and one thousandth of the solubility of clonidine hydrochloride (79.4mg / ml), the slow-release effect can be achieved without a complex preparation technology and is very stable, and crystal transformation does not occur under hot and humid and strong light conditions and under an accelerated test condition; and a production process is simple, and granularity can be easily controlled, so that the clonidine pamoate is applicable to enlarged production. The clonidine pamoate is applicable to being made into a long-acting slow-release preparation, can reduce mediation frequency, improves the medication compliance of a patient, can enable blood drug concentration to be balanced, avoids a peak valley phenomenon and reduces adverse reactions during medication.
Owner:力赛生物医药科技(厦门)有限公司 +1
Clonidine hydrochloride freeze-dried orally disintegrating tablet and preparation method thereof
ActiveCN107362150AEasy to takeGreat tasteOrganic active ingredientsNervous disorderTreatment effectOrally disintegrating tablet
The invention belongs to the field of medicines, and particularly relates to a clonidine hydrochloride freeze-dried orally disintegrating tablet and a preparation method thereof. According to the technical scheme of the invention, the clonidine hydrochloride freeze-dried orally disintegrating tablet is characterized by comprising the following components in parts by weight: 2-10 parts of clonidine hydrochloride, 80-98 parts of a filling agent, 1-5 parts of an adhesive, 0-3 parts of a pH value adjusting agent, 0-3 parts of a corrigent and 0-2 parts of a preservative. The clonidine hydrochloride freeze-dried orally disintegrating tablet provided by the invention is convenient to take, good in taste, beneficial to improvement of compliance of patients taking the medicine, rapid in disintegration in mouths, beneficial to medicine dissolution, rapid in medicinal absorption and treatment effect taking, uniform in content and good in stability. The clonidine hydrochloride freeze-dried orally disintegrating tablet provided by the invention is simple in production process, easy to control and applicable to industrial production, and technical effects beyond expectation are achieved.
Owner:CP PHARMA QINGDAO CO LTD
Micro-powder encapsulating and material mixing device for improving content uniformity of clonidine hydrochloride in zhenju antihypertensive tablets and material mixing method
ActiveCN109092191AGood content uniformityImprove mixing uniformityOrganic active ingredientsTransportation and packagingMedicineProduct tablet
The invention discloses a micro-powder encapsulating and material mixing device for improving the content uniformity of clonidine hydrochloride in zhenju antihypertensive tablets and a material mixingmethod. The material mixing method comprises the following steps: firstly, a clonidine hydrochloride solution is prepared by dissolving clonidine hydrochlorid with an ethanol solution, and is uniformly sprayed to a medicine mixing container containing other auxiliary materials while rotating at high speed in a dosing nozzle, furthermore, a transverse cutting blade and a longitudinal cutting bladeopposite to the dosing nozzle in the rotation direction are arranged in the medicine mixing container, and the medicine mixing container synchronously rotates at the high speed reversely while the materials are mixed until the solution is completely sprayed, the ethanol solution is volatilized in the high-speed rotating container, and the clonidine hydrochloride and the other raw materials form amicro-powder encapsulating state to complete the micro-powder encapsulating and material mixing process; and the micro-powder encapsulating and material mixing device for improving the content uniformity of the clonidine hydrochloride in the zhenju antihypertensive tablets and the material mixing method disclosed by the invention effectively improve the mixing uniformity and the bonding firmnessdegree of the clonidine hydrochloride, enhance the content uniformity of the clonidine hydrochloride in finished product tablets of the zhenju antihypertensive tablets, lower errors in the proceduresof artificial compounding, material mixing and the like, reduce the workload and improve the work efficiency.
Owner:ZHONGXING PHARM CO LTD JIANGSU
Method for preparing clonidine hydrochloride
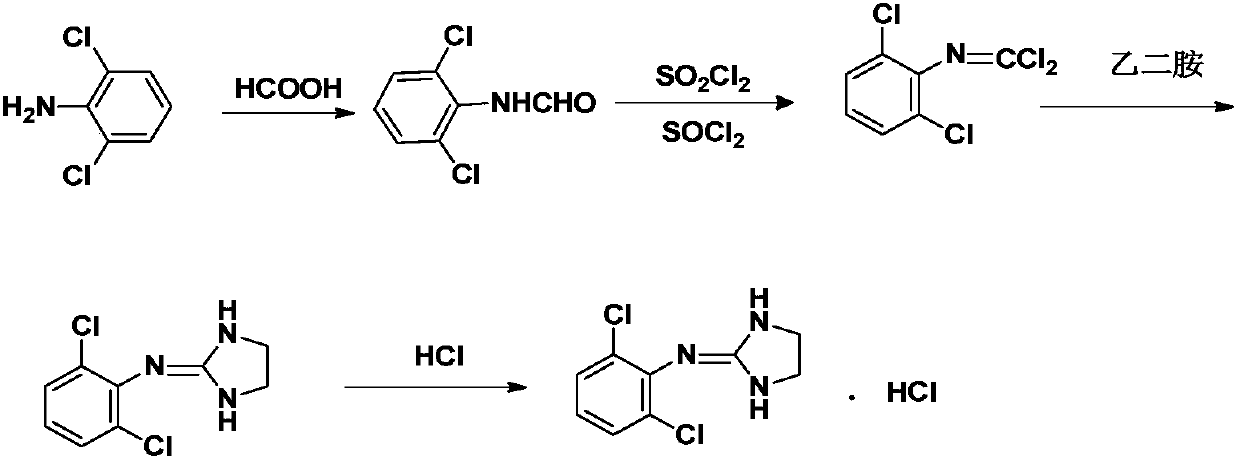
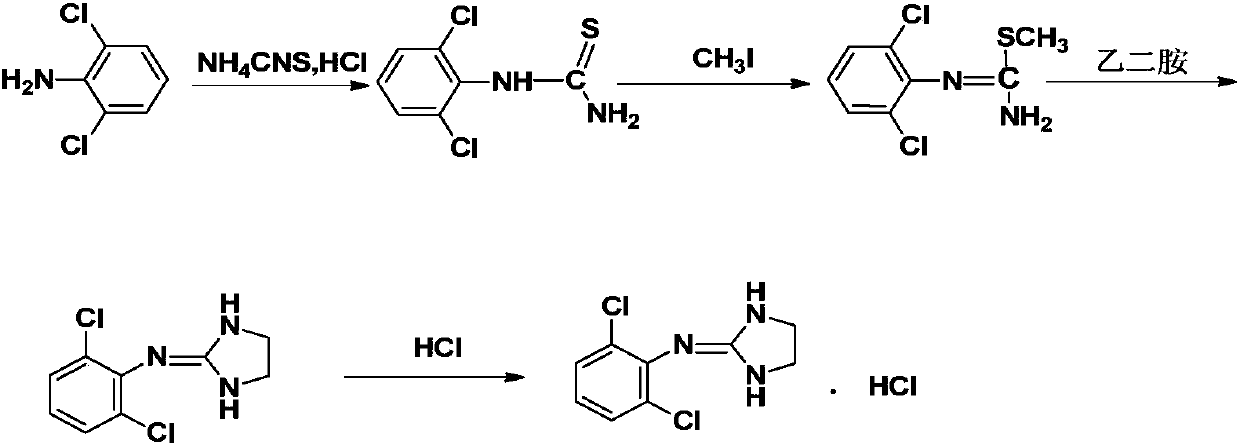
The invention discloses a preparation method of clonidine hydrochloride, which includes three steps of synthesis of intermediate 1, synthesis of intermediate 2 and synthesis of clonidine hydrochloride. Acylation reaction to obtain intermediate 1, "one-pot method" to obtain clonidine free base, and finally salify with hydrogen chloride ethanol solution to obtain clonidine hydrochloride. A preparation method of high-purity clonidine hydrochloride is obtained by processing the synthesis process of the raw materials, firstly salifying and then adjusting the base in the purification of free base, and using ethyl acetate beating for the purification of clonidine hydrochloride. The method not only improves the product purity and yield, but also can effectively control impurities, greatly reduces the cost, simplifies the process, and is more suitable for industrial production.
Owner:KUNMING YUANRUI PHARMA
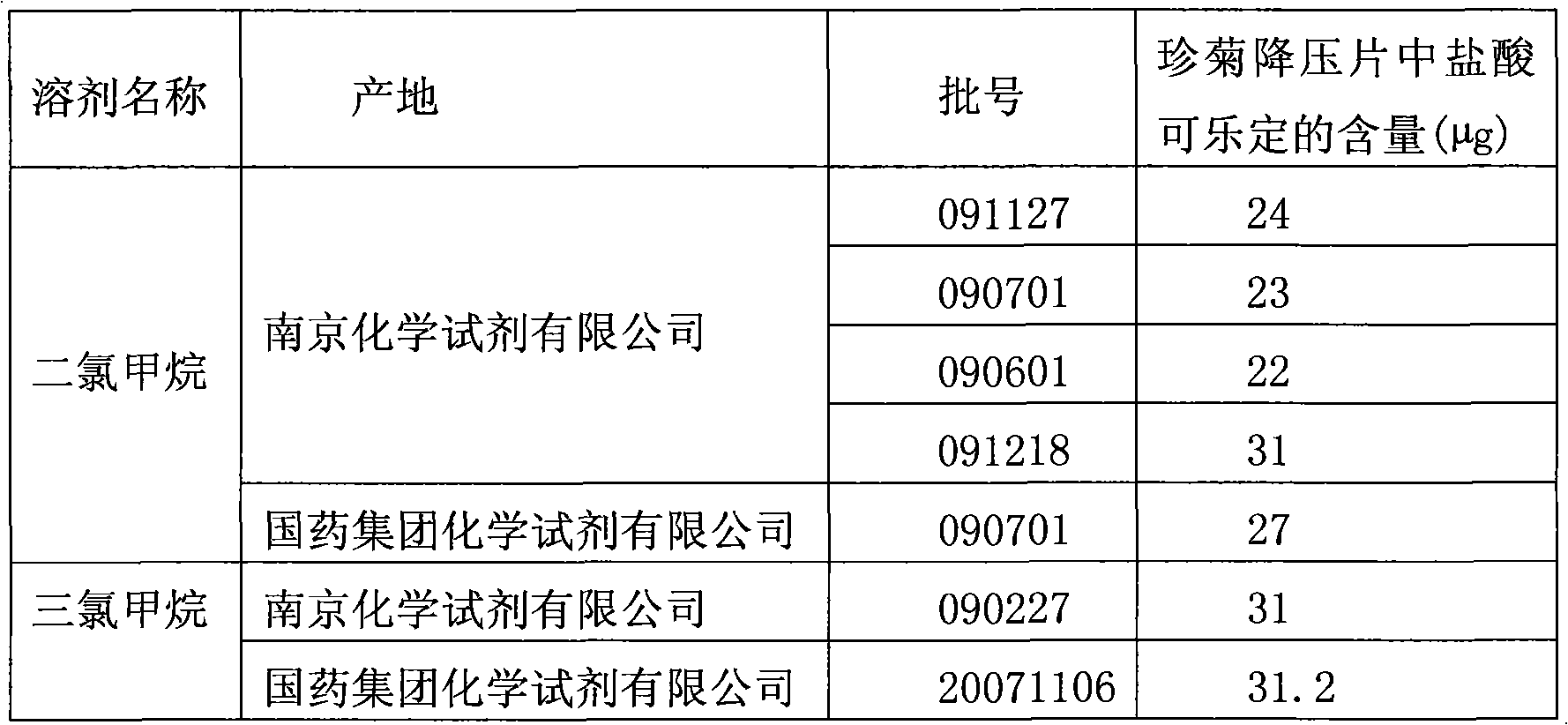
Method for determining clonidine hydrochloride content in Zhenju Jiangya Tablet
ActiveCN101782561AGood reproducibilityReliable test resultsComponent separationTheoretical plateSilanes
The invention discloses a method for determining clonidine hydrochloride content in a Zhenju Jiangya Tablet, which comprises the following steps that: a reference substance solution is prepared; a sample solution is specially prepared; finally high performance liquid chromatography is used to determine; octadecyl silane bonded silica serves as the filler; methanol and 0.01mol / L sodium dihydrogen phosphate in the volume ratio of 30:70 is mobile phase; the detection wavelength is 210nm; the flowing speed is 1.0ml / min; calculated by the clonidine hydrochloride peak, the theoretical plate number is no less than 2000; and when in determination, respective 20mu l of the reference substance solution and the sample solution are precisely absorbed, a liquid chromatograph is injected, and the content of clonidine hydrochloride is determined. The method specially prepares the sample, and then uses the high performance liquid chromatography to determine the clonidine hydrochloride content in the Zhenju Jiangya Tablet, can improve the reoccurrence, and meanwhile, the inspection results are more reliable, and the product quality can be better controlled.
Owner:ZHONGXING PHARM CO LTD JIANGSU
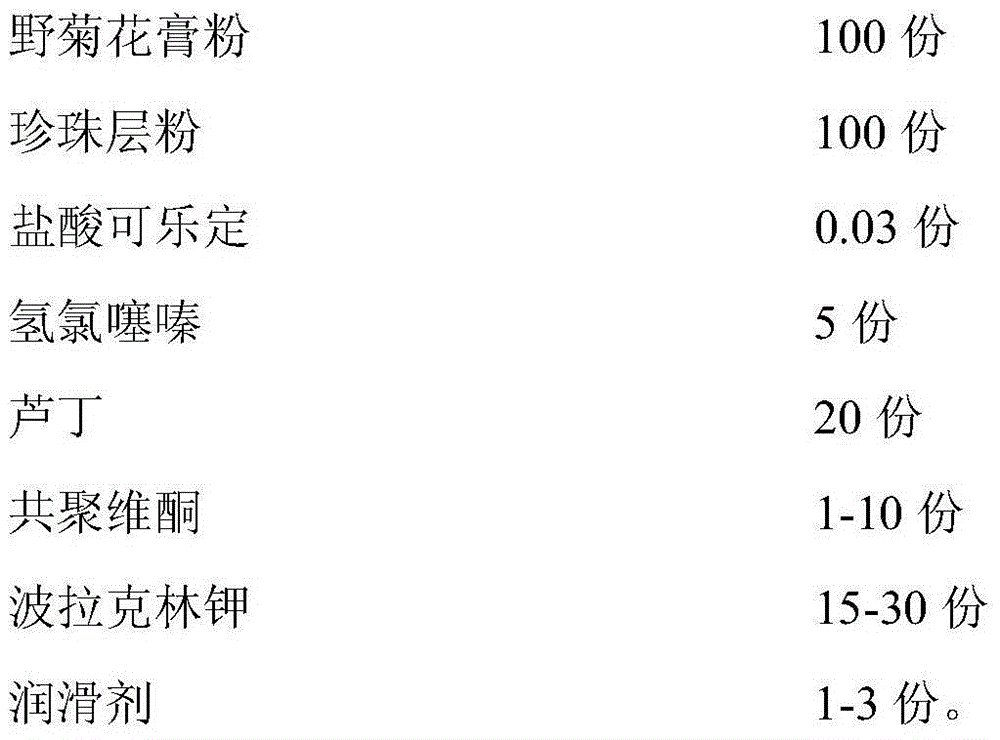
Rapidly-dissolved Zhenju antihypertension tablet and preparation process thereof
InactiveCN104784239AGood compressibilityDisintegrates quicklyOrganic active ingredientsPill deliveryDissolutionCompressibility
The invention discloses a rapidly-dissolved Zhenju antihypertension tablet and a preparation process thereof. The preparation contains copovidone and polacrilin potassium. The preparation process comprises the following steps: (1) placing the copovidone into pure water, stirring the pure water until the copovidone is dissolved, then adding hydrochlorothiazide and clonidine hydrochloride, and stirring until the hydrochlorothiazide and clonidine hydrochloride are dissolved for standby use; (2) weighing wild chrysanthemum flower cream powder, pearl layer powder, rutin and polacrilin potassium, filtering and uniformly mixing to obtain a mixture, spraying a mixed solution prepared in the step (1) by adopting a fluidized bed spray method, and carrying out the granulation and drying; and (3) weighing dry particles prepared in the step (2), adding the lubricating agent, uniformly mixing, and tabletting. The rapidly-dissolved Zhenju antihypertension tablet is good in compressibility, rapid to disintegrate and high in dissolution rate and has the advantages of simple preparation process and easiness in operation.
Owner:THE FIRST AFFILIATED HOSPITAL OF XINXIANG MEDICAL UNIV
Slow-released preparation containing hydrochlorothiazide and clonidine hydrochloride and its preparing method
ActiveCN100493512CSustained antihypertensive effectTo achieve a sustained release effectOrganic active ingredientsPharmaceutical non-active ingredientsHydrochlorothiazidePharmaceutical formulation
The present invention belongs to the field of medicine preparation. In the concrete, it relates to a compound medicine slow release preparation containing hydrochlorothiazide and clonidine hydrochloride. It is characterized by that said slow release preparation is composed of two portions of quick-released portion and slow-released portion. Besides, said invention also provides its preparation method and concrete steps.
Owner:北京合源汇丰医药科技有限公司
Clonidine hydrochloride sustained-release tablet composition and preparation method thereof
InactiveCN111991361ALow friabilityHigh hardnessOrganic active ingredientsNervous disorderPolyvinyl acetateProlonged-release tablet
The invention provides a clonidine hydrochloride-containing pharmaceutical composition and a preparation method thereof, particularly relates to a clonidine hydrochloride sustained-release tablet preparation and a preparation method thereof, and belongs to the field of pharmaceutical preparations. According to the invention, a polyvinyl acetate-povidone mixture is used as a sustained-release matrix material, and a sustained-release matrix tablet is prepared by combining the matrix material with clonidine hydrochloride and auxiliary materials in a specific ratio and adopting a powder direct compression method. The sustained-release matrix tablet prepared from the polyvinyl acetate-povidone mixture is high in hardness and low in friability, and the sustained-release matrix tablet can uniformly release drugs within 24 hours. The sustained-release tablet prepared by the powder direct compression method is uniform in content and stable in quality. The preparation process is simple, good inreproducibility and suitable for industrial production.
Owner:SHANDONG DYNE MARINE BIOTECHCAL PHARM HLDG CO LTD
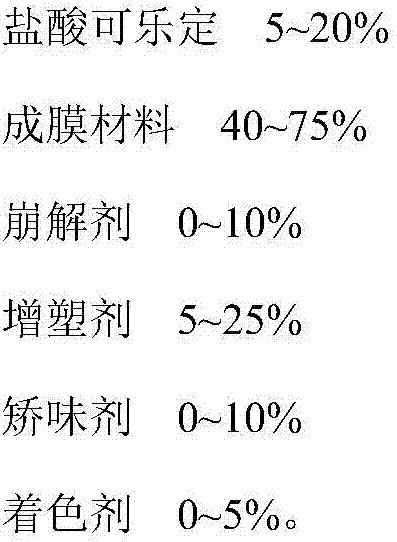
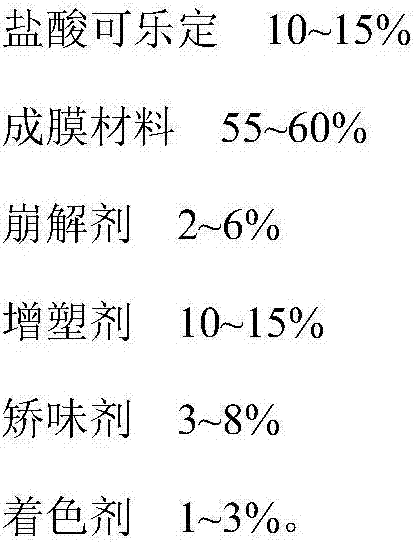
A kind of clonidine hydrochloride oral film and preparation method thereof
ActiveCN107441068BEasy to takeGreat tasteOrganic active ingredientsNervous disorderPlasticizerDrugs preparations
The invention belongs to the field of pharmaceutical preparations, in particular to a clonidine hydrochloride oral film and a preparation method thereof. The technical solution adopted by the present invention is a clonidine hydrochloride oral film, which is characterized in that it comprises the following components: clonidine hydrochloride, film-forming material, disintegrant, plasticizer, flavoring agent and coloring agent. The clonidine hydrochloride oral film prepared by the invention is convenient to take, has a good taste, and is beneficial to improve the safety and compliance of patients taking medicine; it dissolves quickly in the oral cavity, has high bioavailability, and accelerates drug absorption and curative effect; the content is uniform, Good stability. The preparation process of the clonidine hydrochloride oral film of the invention is simple, easy to control, suitable for industrial production, and has achieved unexpected technical effects.
Owner:CP PHARMA QINGDAO CO LTD
Clonidine hydrochloride oral membrane and preparation method thereof
ActiveCN107441068AEasy to takeGreat tasteOrganic active ingredientsNervous disorderPlasticizerCurative effect
The invention belongs to the field of pharmaceutical preparations and in particular relates to a clonidine hydrochloride oral membrane and a preparation method thereof. According to the adopted technical scheme, the clonidine hydrochloride oral membrane is characterized by containing the following components: clonidine hydrochloride, a membrane forming material, a disintegrating agent, a plasticizer, a corrective agent and a coloring agent. The prepared clonidine hydrochloride oral membrane is easy to take and good in taste and can beneficially improve safety and compliance of a patient in a process of taking a medicine; the clonidine hydrochloride oral membrane is rapidly dissolved in an oral cavity, bioavailability is high, and medicine absorption and play of curative effect are sped up; and content is uniform, and stability is good. The clonidine hydrochloride oral membrane provided by the invention has the advantages that preparation technique is simple, control is easy, the clonidine hydrochloride oral membrane is applicable to industrial production, and unexpected technical effect is obtained.
Owner:CP PHARMA QINGDAO CO LTD
Clonidine hydrochloride sustained-release tablet and preparation method thereof
ActiveCN109364035AGood blood pressure effectAchieve synergyOrganic active ingredientsPharmaceutical non-active ingredientsSustained Release TabletPolyvinyl alcohol
The invention relates to a clonidine hydrochloride sustained-release tablet and a preparation method thereof, and belongs to the field of pharmaceutical preparations. The clonidine hydrochloride sustained-release tablet is prepared from the following raw materials in parts by weight: 0.1 to -0.5 part of an active constituent, 8 to -18 parts of a composite polymer skeleton system, 10 to -18 parts of a disintegrating agent, 12 to -18 parts of a binder, 15 to -30 parts of a diluent, and 0.1 to -2 parts of a lubricant; and the active constituent is constituted by clonidine hydrochloride and scutellarin, and the composite polymer skeleton system is constituted by methylcellulose and polyvinyl alcohol. The clonidine hydrochloride and the scutellarin are jointly applied to preparation of the sustained-release tablet for depressurization for the first time, by optimizing and screening a formula, an effect experiment study finds that through joint application of the clonidine hydrochloride andthe scutellarin, the depressurization effect can be improved remarkably, and the synergistic interaction effect is achieved.
Owner:CP PHARMA QINGDAO CO LTD
Transdermal patch of clonidine hydrochloride and preparation method thereof
InactiveCN110354104AImprove ease of medicationOrganic active ingredientsNervous disorderTransdermal patchMass ratio
The invention provides a transdermal patch of clonidine hydrochloride. The patch comprises a liner layer and a paste layer, wherein the liner layer comprises an identification code with a unique code,the identification code comprises a product display information module and a user text and / or photo information input module, the paste layer is prepared from, by weight, 5-15% of clonidine hydrochloride, 2-5% of triethylamine, 3-7% of diethanol amine, 0.3-0.6% of an antioxidant, 42-64% of TPE-S resin and 10-22% of terpene resin, wherein the mass ratio of the triethylamine to the diethanol amineis (1:1)-(1:1.3), and the mass ratio of the TPE-S resin to the terpene resin is (3:1)-(4.5:1). The transdermal patch can effectively improve the smoothness of medication for patients through the management of the Internet of Things, and can optimize the subsequent medication treatment course through the management of the Internet of Things.
Owner:苏州盈得来医药科技有限公司
Clonidine hydrochloride sustained-release micro-tablets as well as preparation method and application thereof
InactiveCN112043677AExtended stayLittle side effectsOrganic active ingredientsSenses disorderUse medicationPharmaceutical drug
The invention relates to the technical field of sustained-release drugs, in particular to clonidine hydrochloride sustained-release micro-tablets as well as a preparation method and application thereof. The clonidine hydrochloride sustained-release micro-tablets comprise the following components in percentage by weight of 3-6% of clonidine hydrochloride, 68-86% of a gel skeleton, 0.5-2% of a lubricant and 10-25% of a filler. The preparation method comprises the following steps of irradiating and sterilizing all the raw materials by gamma-rays before use, and performing the following operationsin a sterile environment: uniformly mixing and grinding all the raw materials by using a mortar, and performing pressing into small pieces with the diameter of 3mm through an anisotropic tabletting machine under the maximum pressure by a direct tabletting method. Compared with clonidine hydrochloride eye drops, the ophthalmic clonidine hydrochloride sustained-release micro-tablets disclosed by the invention have a good controlled-release effect, can prolong the residence time of drugs in eyes, reduce the administration times, improve the compliance of patients, improve the bioavailability ofthe drugs and reduce the toxic and side effects of a central nervous system, are simple in preparation process and have good practical application value.
Owner:SHANDONG UNIV
Clonidine pamoate and preparation method thereof
ActiveCN106831594BReduce solubilityImprove Medication AdherenceOrganic chemistry methodsSolubilityGranularity
Owner:力赛生物医药科技(厦门)有限公司 +1
Blood pressure lowering dripping pills with chryanthemum flower and pearl and its preparation process
InactiveCN100375612CIncrease surface areaHas a wetting effectPill deliveryCardiovascular disorderHydrochlorothiazideChrysanthemum Flower
Disclosed is a dripping pill for treating hypertension. The objective of the invention is to provide a medicinal composition having the advantages of high biological availability, quick-speed medicine release, quick-speed effect, higher medicinal content, easy administration, low price, and causing no pollution during production. The drop pill is prepared from powdered wild chrysanthemum flower, pearl powder, clonidine hydrochloride, hydrochlorothiazidum, globularicitrin as raw material, and medicinal carrying agent as the base material.
Owner:广西宝瑞坦广明制药有限公司
A kind of clonidine hydrochloride dry suspension and preparation method thereof
ActiveCN104523683BGood effectEvenly distributedPowder deliveryOrganic active ingredientsBioavailabilityClonidine Hydrochloride
The invention provides a clonidine hydrochloride dry suspension and a preparation method thereof. The clonidine hydrochloride dry suspension comprises 40-90 parts of clonidine hydrochloride, 500-2000 parts of a filler, 50-180 parts of a corrigent, 40-100 parts of a suspending aid and 10-40 parts of a flocculant. The clonidine hydrochloride dry suspension provided by the invention is uniform in distribution, good in stability, large in distribution area in the stomach and intestines, fast to absorb and high in bioavailability, fast takes effect, and has medicinal effect superior to that of a clonidine hydrochloride premix.
Owner:CP PHARMA QINGDAO CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com