Clonidine hydrochloride multi-vesicular liposome and preparation method thereof
A clonidine hydrochloride and liposome technology, which is applied in liposome delivery, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the problems of preparation encapsulation rate and drug release differences, and reduce the total dosage , good sustained-release effect, good analgesic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Step 1: Precisely weigh 200 mg of lecithin, 50 mg of cholesterol, 40 mg of phosphatidyl glycerol and 17.4 mg of triolein, dissolve with 5 ml of dichloromethane, and use it as the lipid phase;
[0033] Step 2: Precisely weigh 5 mg of clonidine hydrochloride and 100 mg of sucrose, dissolve with an appropriate amount of hydrochloric acid solution (0.1 mol / L) ultrasonically, and dilute to 5 ml, as the inner water phase, slowly add the upper layer of the lipid phase;
[0034] Step 3: use a high-speed shearing homogenizer (Fluko, ATS Industrial Systems Co., Ltd.) to act on the mixture obtained in step 2 at a rotational speed of 10,000 rpm for 9 minutes to prepare W / O type colostrum;
[0035] Step 4: To prepare the dichloromethane microsphere suspension, add 20 mL of the outer water phase containing 3.2 g / 100 ml of glucose and 40 mM lysine to the upper layer of the W / O colostrum, and act at 4500 rpm for 15 seconds clock to form a suspension of dichloromethane microspheres;
...
Embodiment 2
[0039] Step 1: Accurately weigh 200 mg of lecithin, 50 mg of cholesterol, 10 mg of phosphatidic acid and 15.6 mg of glyceryl triolein, and dissolve them in 5 ml of dichloromethane as the lipid phase;
[0040] Step 2: Accurately weigh 50 mg of clonidine hydrochloride and 200 mg of sucrose, ultrasonically dissolve with an appropriate amount of hydrochloric acid solution (0.1 mol / L), and set the volume to 5 ml, as the inner water phase, slowly add to the upper layer of the lipid phase;
[0041] Step 3: with embodiment 1;
[0042] Step 4: To prepare the dichloromethane microsphere suspension, add 20mL of the external aqueous phase containing 6.0g / 100ml glucose and 40mM lysine to the upper layer of W / O colostrum, and act for 15 seconds at a speed of 4500rpm Clock, form the suspension of dichloromethane microsphere;
[0043] Step 5: Same as Example 1.
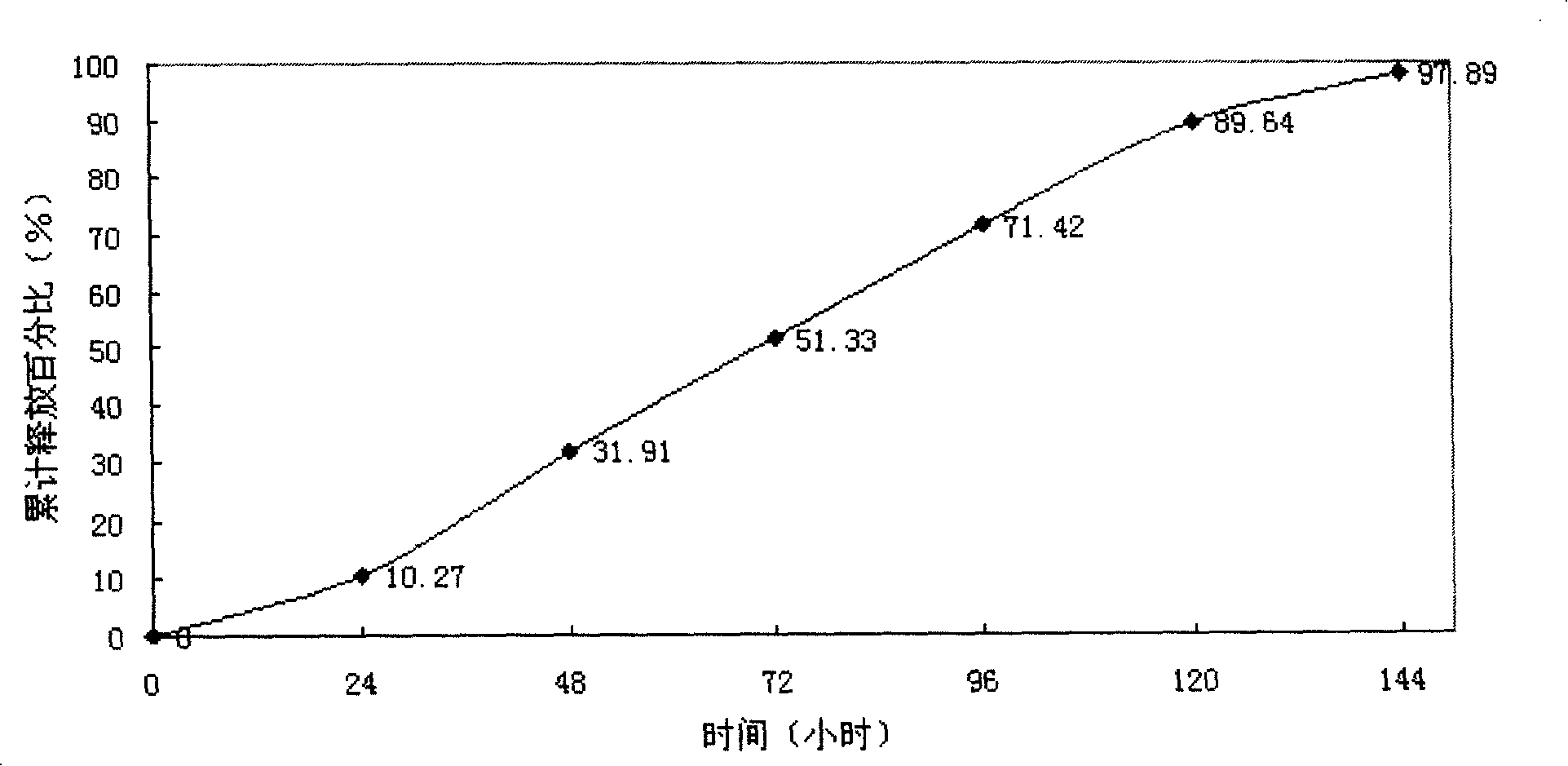
[0044] The particle size of clonidine hydrochloride multivesicular liposomes was measured to be 5-50 μm; the encapsulation effici...
Embodiment 3
[0046] Step 1: Accurately weigh 100 mg of dioleoylphosphatidylcholine, 50 mg of cholesterol, 20 mg of phosphatidylserine and 3.4 mg of glyceryl trioleate, dissolve them in 5 ml of chloroform-ether mixture (1:1 by volume), and use them as lipid Mutually;
[0047] Step 2: Accurately weigh 2.5mg of clonidine hydrochloride, dissolve it ultrasonically with an appropriate amount of hydrochloric acid solution (0.1mol / L), and set the volume to 5ml, as the inner water phase, slowly add to the upper layer of the lipid phase;
[0048] Step 3: with embodiment 1;
[0049] Step 4: To prepare the chloroform-ether microsphere suspension, add 60mL of the external aqueous phase containing 3.2g / 100ml glucose and 40mM lysine to the upper layer of W / O colostrum, and act for 15 seconds at a speed of 4500rpm Clock, form the suspension of chloroform-ether microsphere;
[0050] Step 5: Same as Example 1.
[0051] The particle size of clonidine hydrochloride multivesicular liposomes was measured to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com