Clonidine pamoate and preparation method thereof
A kind of technology of pamoate and pamoic acid, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
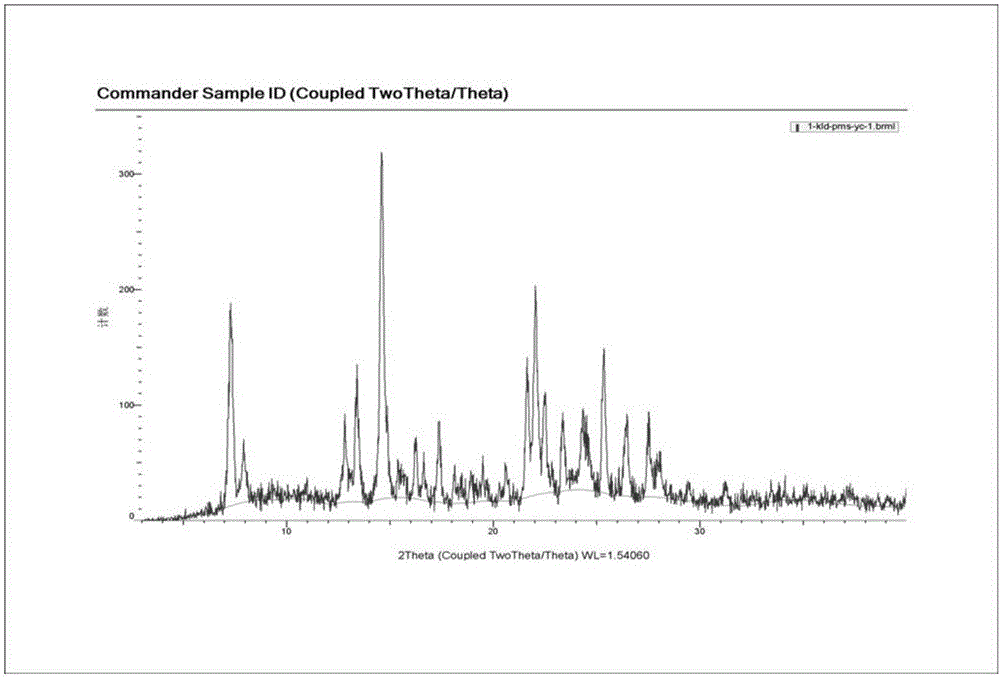
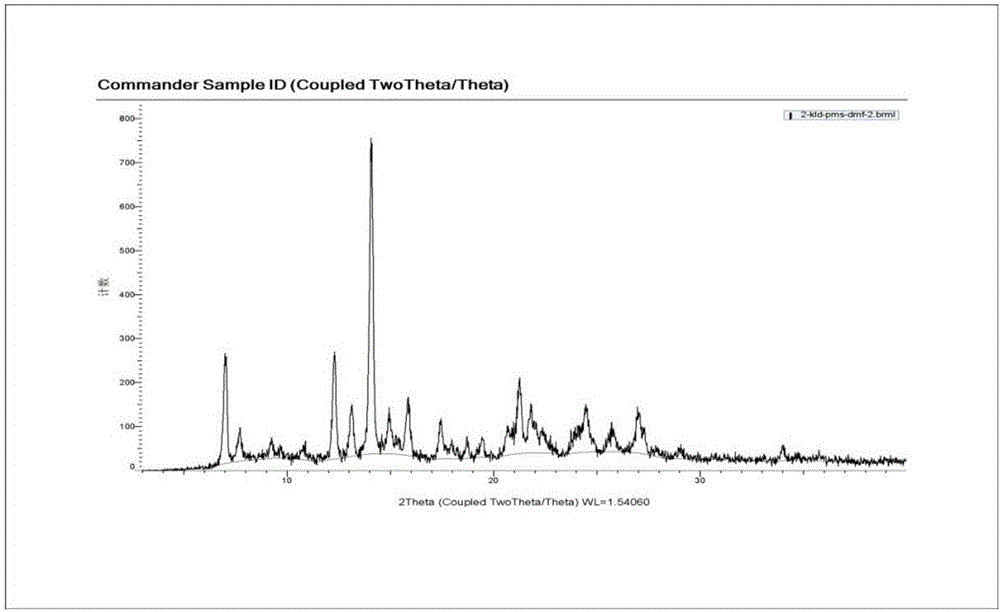
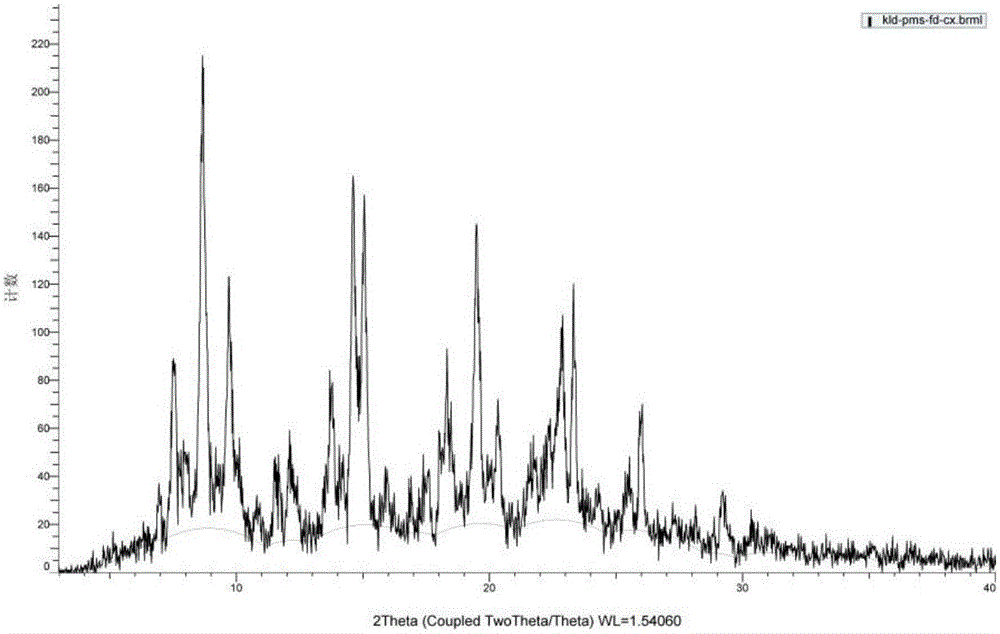
[0079] Example 1 Screening the preparation conditions of clonidine pamoate with different solvent systems and clonidine free base and pamoic acid molar ratio
[0080] Screen the preparation conditions of clonidine pamoate with different solvent systems and molar ratios of clonidine free base and pamoic acid, as shown in the table below.
[0081]
[0082]
[0083] As can be seen from the table above, the solvent used to prepare the clonidine pamoate and the molar ratio of clonidine free base and pamoic acid have a great influence on the preparation of clonidine pamoate, and many solvent systems form salts The crystal form of is a solvate and is relatively unstable. Clonidine free base and pamoic acid carry out feeding reaction at a molar ratio of 1:1, and the solvent is removed by suction filtration to obtain a solid, which is measured by XRD and HPLC, and the measurement results show that the solid is clonidine pamoate (2:1) The mixture of clonidine free base and pamoic...
Embodiment 2
[0084] Embodiment 2 Preparation of clonidine pamoate crystal form I
[0085] Add 10.0 mg of clonidine into 1 ml of DMSO, stir to obtain a clear solution, add 8.4 mg of pamoic acid, stir to dissolve at 25°C, continue stirring for 1 hour, slowly add 5 ml of water, and obtain a light yellow solid by suction filtration. Vacuum-dried at room temperature in a drying oven to obtain about 12.1 mg of light yellow crystals.
Embodiment 3
[0086] Embodiment 3 Preparation of clonidine pamoate crystal form I
[0087] Add 20mg of clonidine into 3ml of ethanol, stir to obtain a clear solution, add 8.4mg of pamoic acid, suspend and stir at 20°C for 2 hours, filter with suction to obtain a light yellow solid, place it in a drying oven and dry it under vacuum at room temperature to obtain About 10.5 mg of light yellow crystals.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com