Clonidine hydrochloride sustained release micropill preparation
A technology of clonidine hydrochloride and sustained-release pellets, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, cardiovascular system diseases, etc., and can solve obstacles affecting curative effect, missed doses, and medication compliance To achieve the effect of reducing the number of medications, keeping the blood concentration high and stable, and reducing the incidence of adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
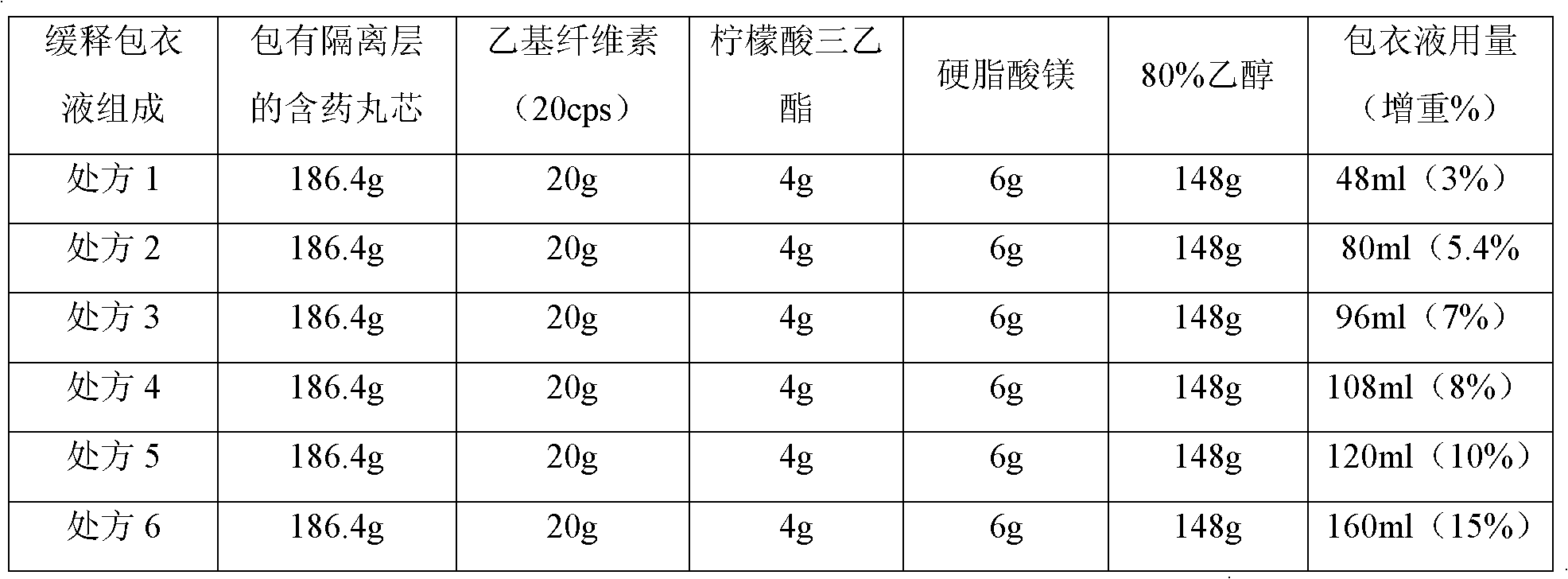
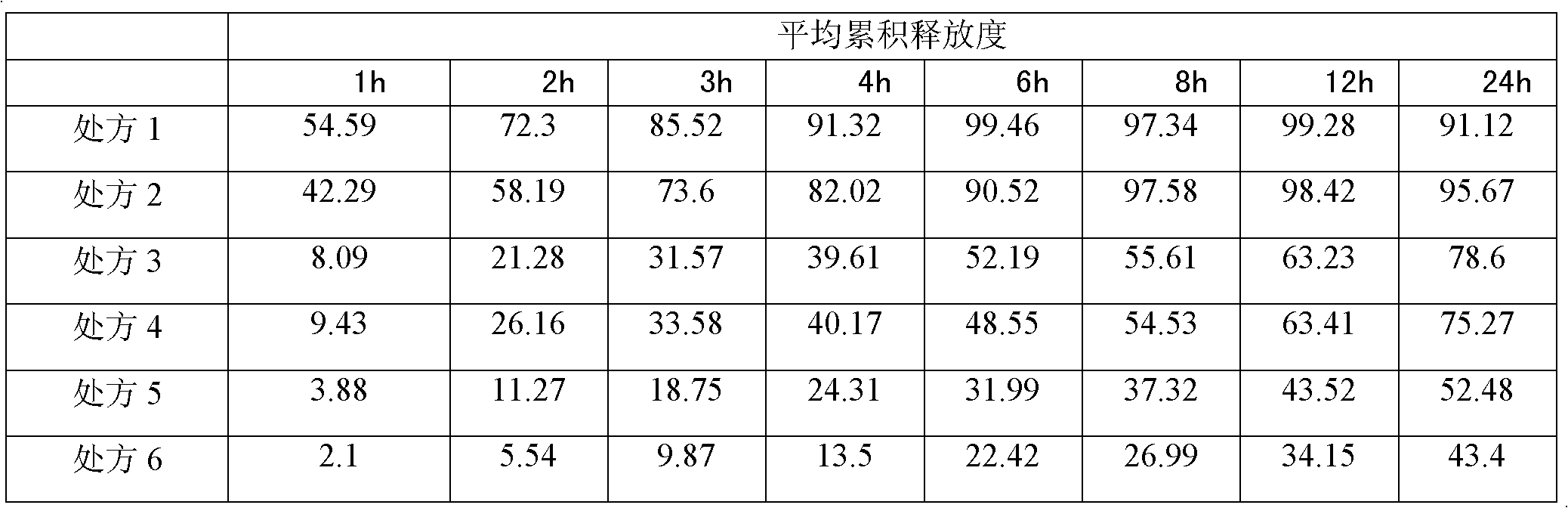
[0046] (1) Preparation of drug-containing pellet core (A)
[0047] Clonidine Hydrochloride
4.0g
Blank ball core (sucrose type)
1600g
[0048] Opadry (CLEAN)
40.0g
760.0g
[0049] Preparation Process:
[0050]Weigh Opadry (CLEAN), add to purified water and stir to dissolve, pass through a 100-mesh sieve before use, then add the prescribed amount of clonidine hydrochloride raw material, stir and dissolve to obtain a coating solution for the drug layer. Weigh blank ball cores and add them to a fluidized bed coating machine (WBF-5G, Chongqing Yingge Granulation Coating Technology Co., Ltd.), and use a low-spray coating system (Wurster system) for coating. The coating parameters of the equipment are : Air intake 250m 3 / hr, air inlet temperature 50℃, atomization pressure 2kg / cm 2 , coating liquid flow rate 7rpm. After the spraying of the coating solution is finished, the material is discharged aft...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com