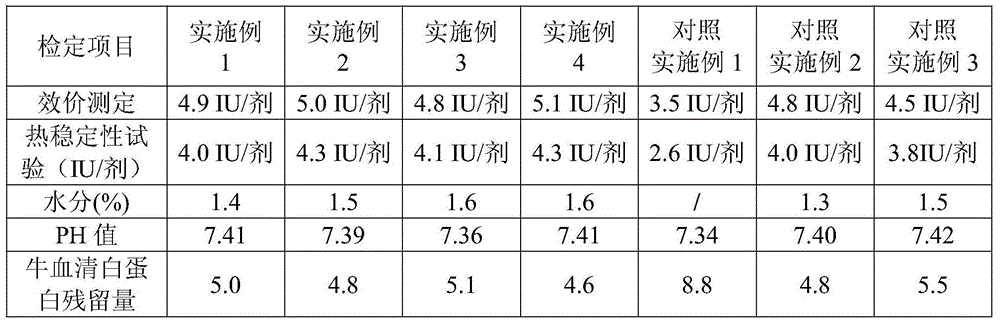
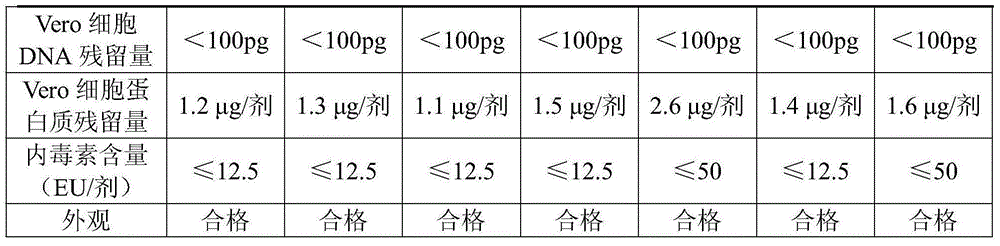
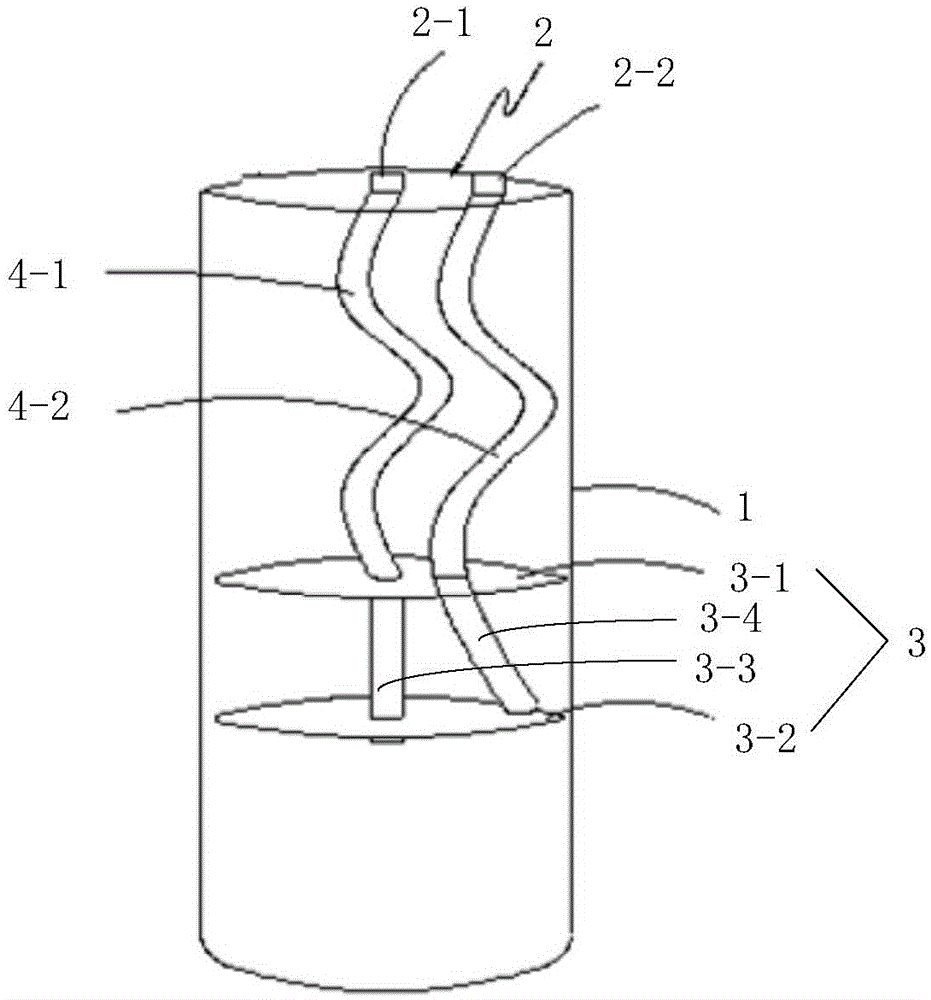
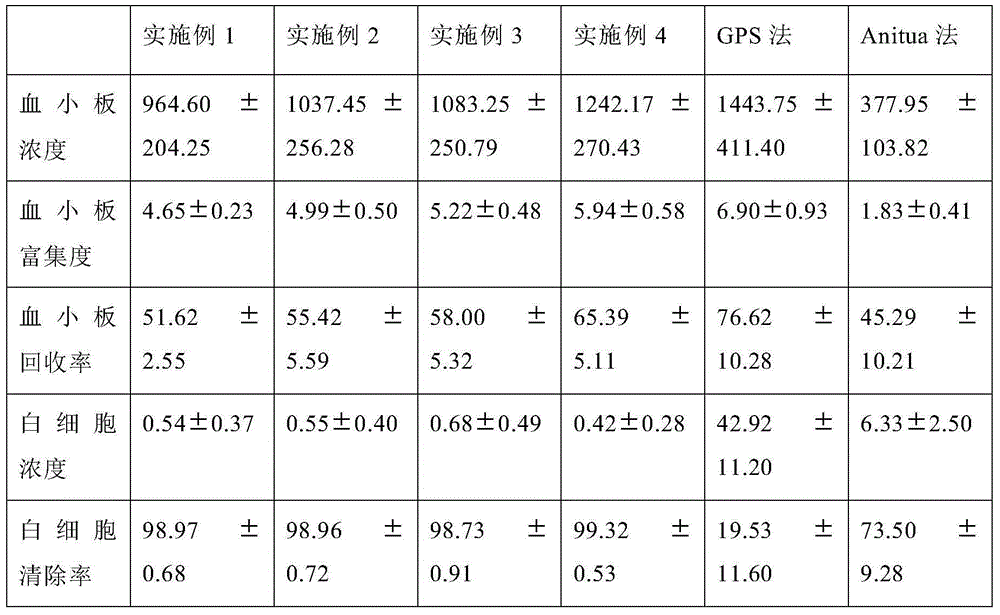
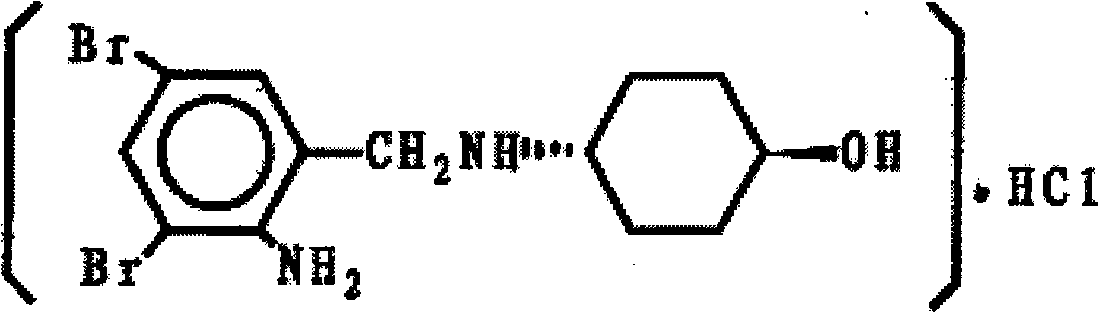
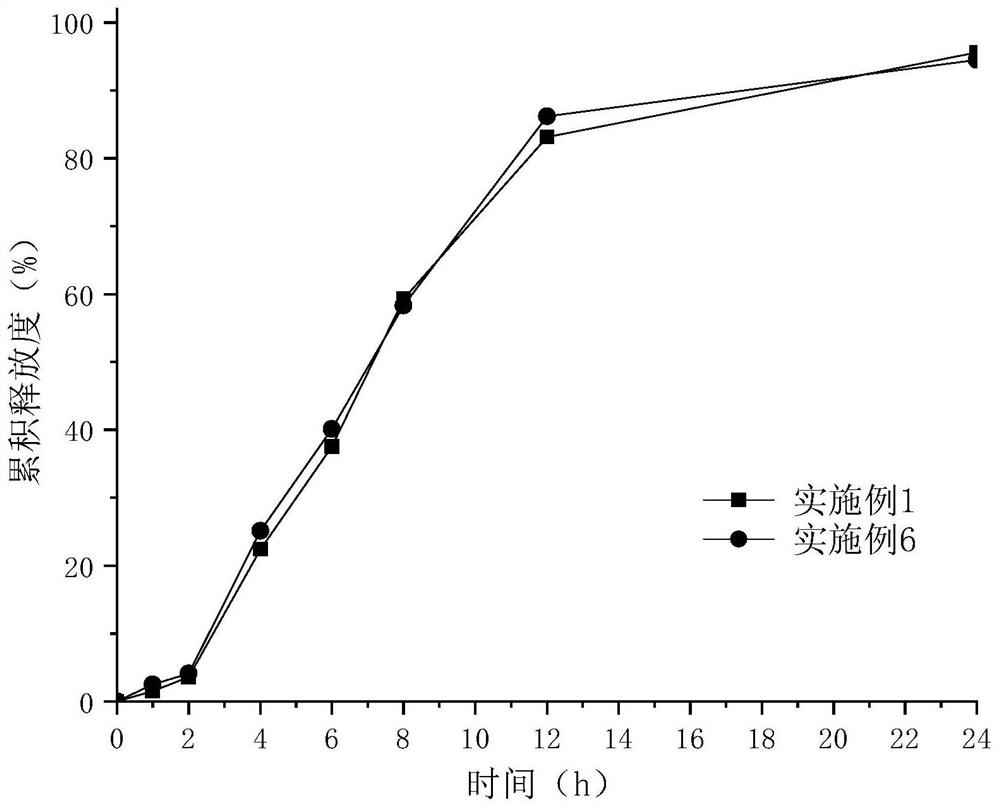
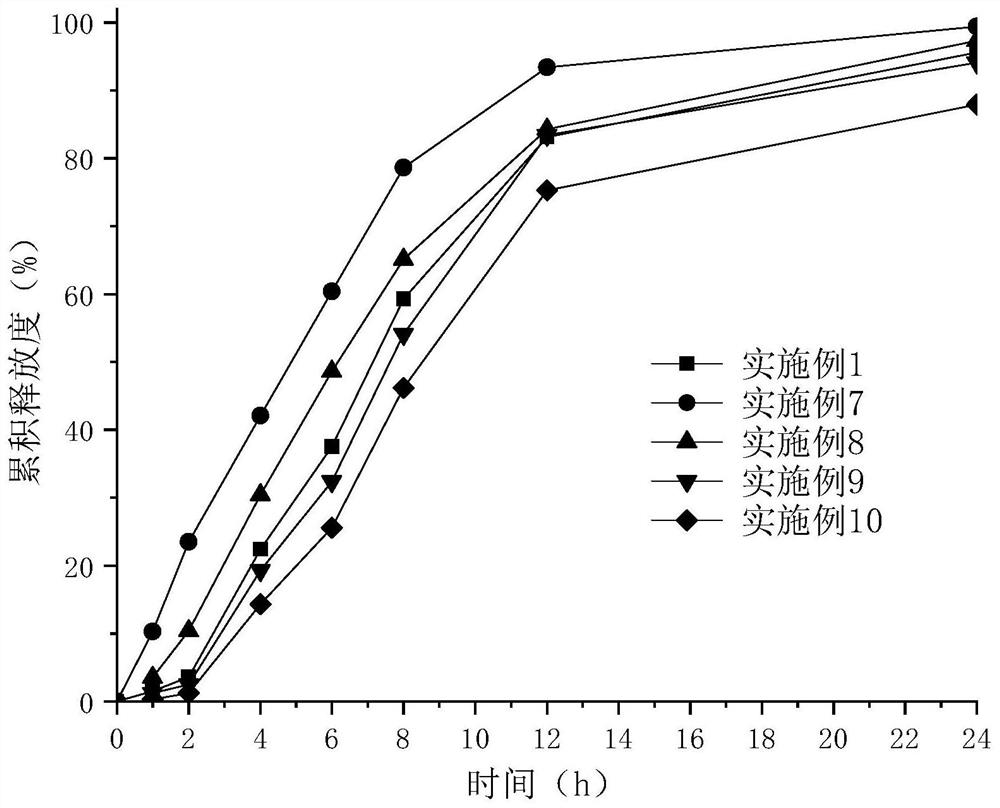
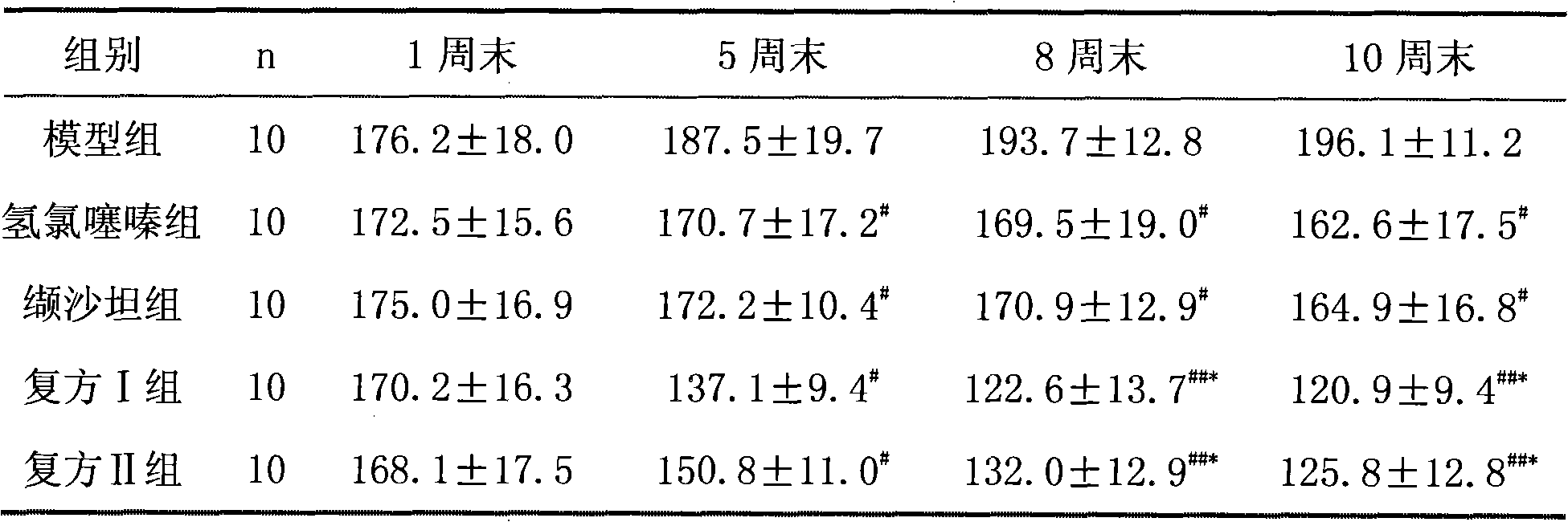
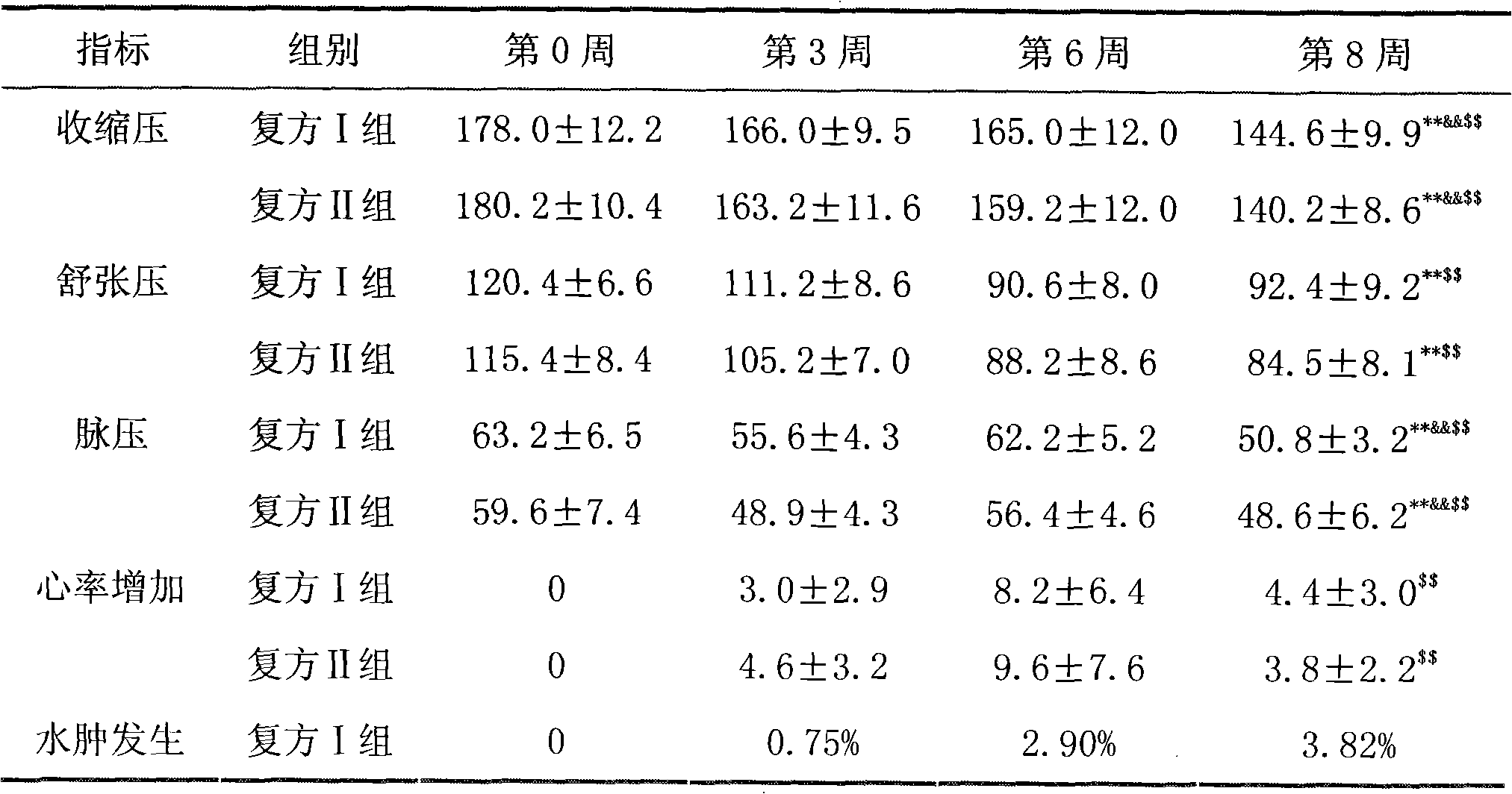
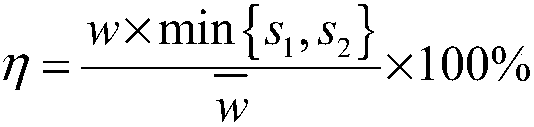Patents
Literature
414results about How to "Low incidence of adverse reactions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Oral medicine for treating cardio-cerebral vascular disease and preparation process thereof
InactiveCN1502337AReduce the number of daily dosesMedication convenienceOrganic active ingredientsPharmaceutical delivery mechanismOral medicineDisease
The present invention discloses a slowly-released oral medicine preparation which is made up by using breviscapine as main raw material and has the functions of promoting blood circulation and removing blood stasis, removing obstruction in the channels to relieve pain for curing angiocardiopathy and cerebrovascular disease with obvious therapeutic effect and its preparation method. Said slowly-released tablet (by one tablet) contains 60-120 mg of breviscapine, 20-75 mg of diluent, 50-150 mg of filling agent and 10-50 mg of slow release material. Said invention also provides its preparation method and concrete steps.
Owner:CHENGDU LIST PHARMA
Taxol medicinal compositions and preparation method thereof
ActiveCN101658516ANo degradationGood chemical stabilityOrganic active ingredientsPowder deliveryFreeze-dryingCarrier protein
The invention provides medicinal compositions of taxol medicaments and pharmaceutically acceptable biological carriers and preparation method thereof. The medicinal compositions of the taxol medicaments and carrier proteins are nano mixed suspensions prepared from the carrier proteins, organic phases, stabilizers, freeze drying protective agents and the taxol medicaments. The weight volume percentconcentration of the taxol medicaments is 0.075 to 1.0. The taxol medicament nano particles prepared by using high-pressure homogenization can reduce adverse reaction aroused by the taxol medicamentsand improve the safety of the clinical administration of the taxol medicaments. The preparation process of the nano mixed suspensions is simple and feasible and is suitable for large-scale preparation and industrial production.
Owner:QILU PHARMA HAINAN
Slow-releasing preparation containing metformin hydrochloride and glipizide and its preparation method
InactiveCN101057849AEvenly distributedReduce local irritationOrganic active ingredientsMetabolism disorderMedicineMetformin Hydrochloride
The invention discloses a diabecron and glipizide -containing slow-release agent and the method for preparing the same. The glipizide micro-pill takes blank micro-pill as carrier, and combines glipizide and other medical findings with it. The diabecron-containing slow-release micro-pill comprises diabetosan pill, slow-release coating membrane material or other medical findings. The method for preparing diabecron-containing slow-release micro-pill takes extrusion rolling method or blank micro-pill loading method. The product is characterized by safety, high efficient, low toxicity and convenient usage. It can be used to treat non-insulin-dependent diabetes mellitus.
Owner:QIQIHAR MEDICAL UNIVERSITY
Ornidazole injection
ActiveCN102552127ALow impurity contentImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismSuperficial phlebitisOrnidazole
The invention provides an ornidazole injection. The ornidazole injection has the advantages of high stability, low impurity content, pH value that is closer to the human physiological pH value, low incidence rate of infusion pain, infusion phlebitis and other adverse reactions, etc.
Owner:HEBEI RENHE YIKANG PHARMA
Controlled-release colon targeting drug administration preparation and preparation method thereof
InactiveCN101780055AConvenient treatmentImprove complianceOrganic active ingredientsDigestive systemIntestinal tract diseasesColonic segment
The invention relates to a controlled-release colon targeting drug adminitration preparation. The forms of the preparation are colon site-specific coated tablets or colon targeting pellets. The preparation consists of a tablet core or pellet core, an isolating layer and a controlled-release coating layer, wherein the controlled-release coating layer comprises an internal coating layer and an external coating layer. By adopting the multilayer coating technology, enteric soluble acrylic resin water dispersion and osmotic acrylic resin water dispersion are used as main coating materials for carrying out coating, thereby obtaining the controlled-release colon targeting drug adminitration preparation. The preparation of the invention enables drugs to be released at a constant rate at a colon section, realizes accurate site-specific drug release, increases the concentration of the drugs at some parts of positions with pathological changes, is beneficial to treating ulcerative colitis and carcinoma of colon, avoids the stimulation of the drugs on stomaches and small intestines, achieves the goal of colon site-specific drug release, enhances the targeting site-specific curative effect on colon diseases and reduces the toxic and side effect. Compared with the common oral preparations, under the condition of the same drug adminitration dosage, the preparation of the invention can enhance the curative effect and reduce the incidence rate of untoward reactions. Compared with the enemas or the rectal suppositories, the preparation has the advantages of uniform drug distribution in the colon and good patient compliance.
Owner:ZHEJIANG UNIV
Sirolimos sustained and controlled release preparation and preparation method thereof
InactiveCN101361703AOvercome the problems of poor water solubility and narrow therapeutic windowImprove securityOrganic active ingredientsPharmaceutical delivery mechanismSolubilityCyclodextrin
The invention provides a Sirolimus slow controlled release preparation and a preparation method thereof. By adopting the solubilizing methods including a solid dispersion technology or cyclodextrin inclusion technology or adding one or a plurality of surface active agents after micronizing drugs and the like, the solubility is dramatically improved and further the bioavailability is improved, therefore, a matrix type slow controlled release preparation is made by adding one or a plurality of framework materials and other accessories, or a diaphragm-controlled or osmotic pump type slow controlled release preparation is made by adopting slow controlled release materials for coating. The Sirolimus slow controlled release preparation has better solubility and dissolution rate, high bioavailability as well as slow controlled release effect, thus being capable of maintaining steady blood concentration, bringing down the concentration of peak, reducing the occurrence of adverse reaction and improving the safety of clinical medicines, in addition, the raw materials can be acquired easily, the preparation process is simple and feasible with high yield rate and low cost, and the large-scale industrial production can be realized and the economic benefit is marked.
Owner:宋洪涛
Antiblocking hernia patch and preparation method thereof
InactiveCN101579540AReduce the chance of adverse reactionsOutstanding FeaturesSurgeryWater solubleAbsorbent material
The present invention discloses an antiblocking hernia patch and a preparation method thereof. A polypropylene net is combined with an absorptive material chitosan antiblocking piece to prepare a partial absorptive composite mesh. The invention is characterized in that the absorptive material chitosan antiblocking piece is prepared from water-soluble chitosan by a tap casting method. The chitosan phlegm is arranged between the polypropylene net and the absorptive material chitosan antiblocking piece. The preparation method comprises the steps of preparing the chitosan antiblocking piece, uniformly coating one layer of chitosan on the polypropylene woven mesh, then placing the chitosan antiblocking piece on the polypropylene woven mesh, placing the polypropylene woven mesh between the moulds after compressing, and obtaining the antiblocking hernia patch after drying by a film isolation method in the environment at a temperature of between 15 DEG C below zero and 150 DEG C and pressure of 0 to 0.6MPa. The antiblocking hernia patch of the invention has the advantages of effectively preventing the blocking after surgery, increasing the anti-inflection effect, sustaining the excellent repairing strength, reducing the recurrence rate and complication after surgery, reducing the probability of adverse effect caused by the unthorough elimination of acid, simply controlling preparation process and eliminating the adverse effect which may be caused by the chemical process.
Owner:都本立
Method for extracting active constituent from Tibetan capillary
ActiveCN101053589ANo pollutionReduce consumptionDigestive systemPlant ingredientsSide effectTherapeutic effect
The invention discloses a method for extracting anagallidium effective components with a producing steps of extracting, removing the impurity, primary separation, absorbing separation, concentrating, drying, smashing, secondary refining and etc. The extracting method extracts by solvent water and ethanol which can be recovered and has no pollution. The ultrasonic accessory extraction can shorten the extraction period, improves the efficiency, reduces energy consumption. The centrifugation and large-hole resin absorption treatments can keep the effective components, remove the impurity in a high limit, refine the Chinese traditional medicine, reduce dosage and facilitate to dispose a plurality of preparation formulation containing injection (intravenous injection ), oral reagent, external use regent, and also can be the material of the compound preparation. Meanwhile, it can reduce the side effects and bad reaction incidence rate because the impurity is removed throughly.
Owner:TIBENTAN RIKEZE ZANGNUO PHARMA CO LTD
Medicinal composition containing caffeic acid ester and scutellarin, preparation method and application thereof
ActiveCN104586911AAvoid quality problemsReduce adverse reactionsOrganic active ingredientsPharmaceutical delivery mechanismDiseaseCaffeic acid
The invention provides a medicinal composition containing caffeic acid ester and scutellarin. The composition is a composition for injection, and comprises, by weight, 45-95% of caffeic acid ester and 5-25% of scutellarin. The invention also provides application of the composition in preparation of drugs for treating cardiovascular and cerebrovascular diseases.
Owner:YUNNAN BIOVALLEY PHARMA CO LTD
Lyophilized viper antivenin and preparation method thereof
ActiveCN101816789AHigh potencyGuaranteed curative effectAntinoxious agentsMammal material medical ingredientsMass ratioBlood plasma
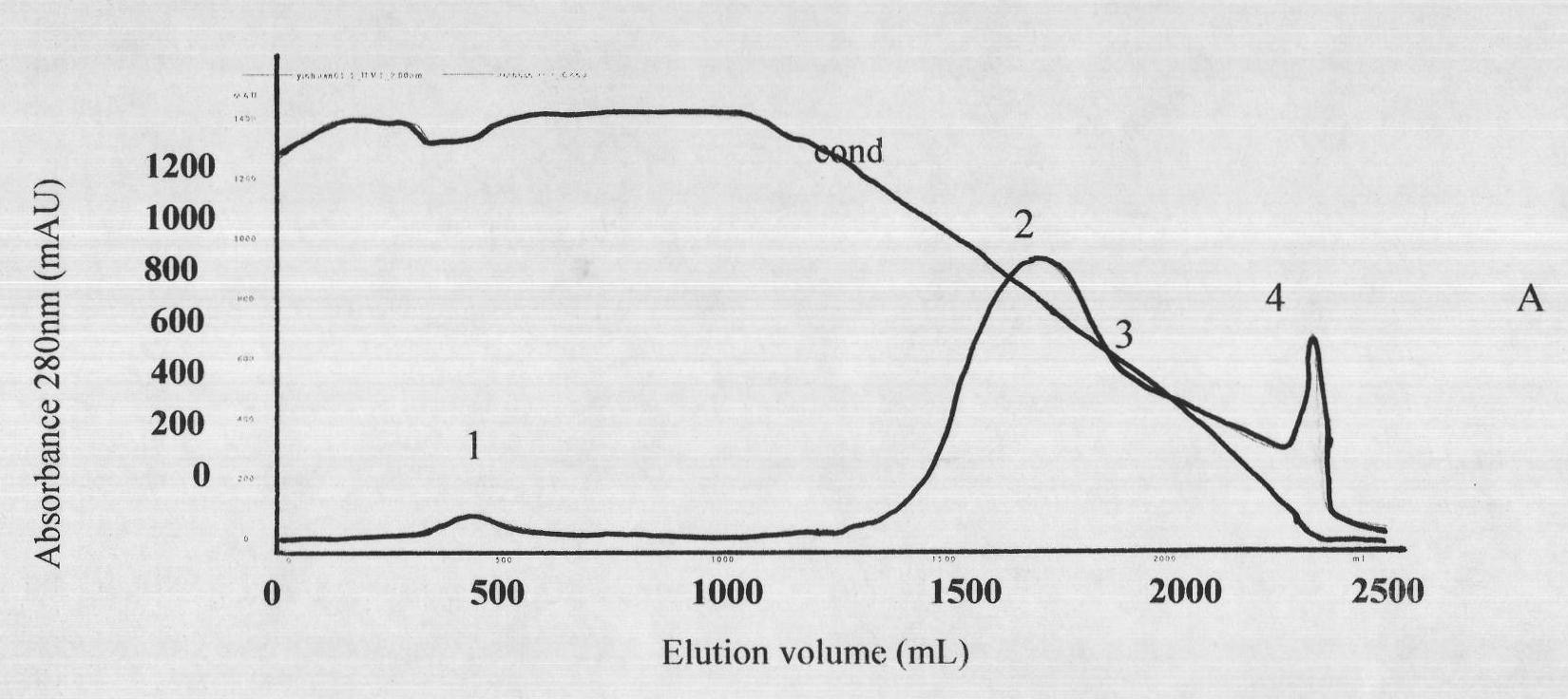
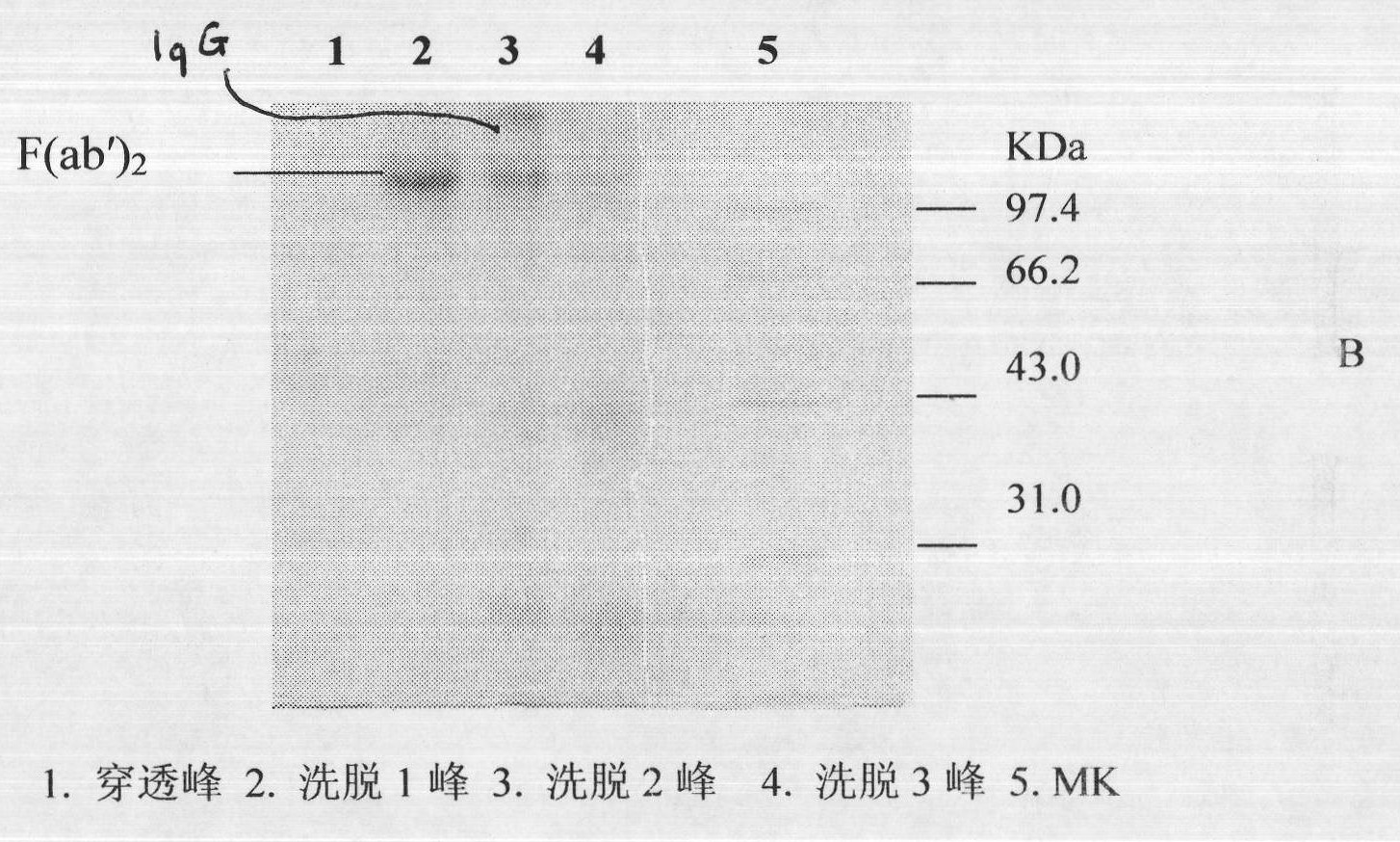
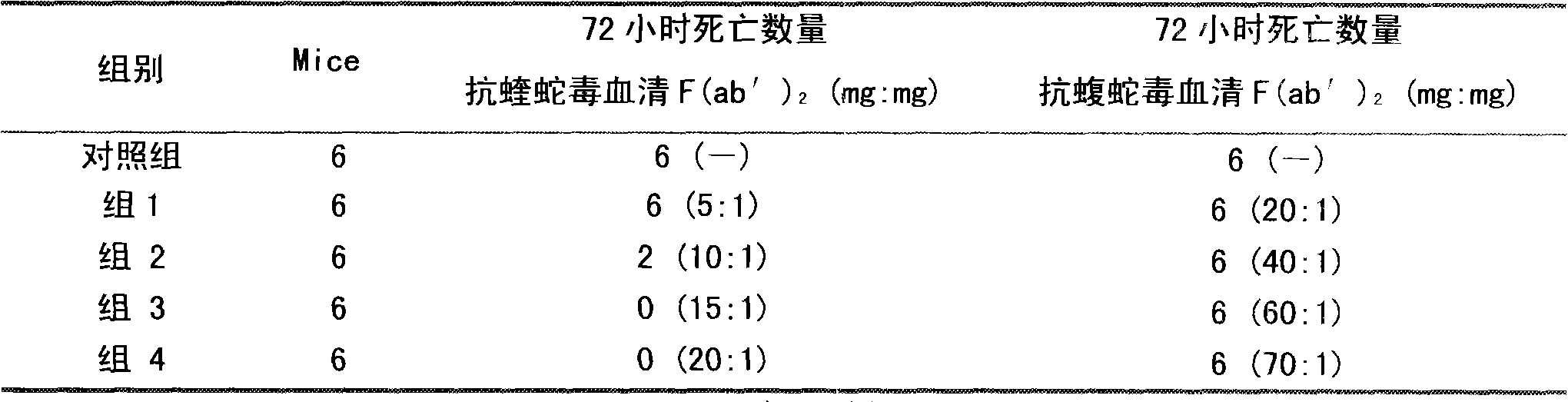
The invention discloses a lyophilized viper antivenin and a preparation method thereof, belonging to biochemical products, more particularly relating to an antivenin and a preparation technology thereof. The mass ratio of the antivenin and viper venom is 15:1, which can specifically neutralize the viper venom injected to mouse; as determined by immunodiffusion, the immunoprecipitation line appears in case that the ratio of viper antivenin F(ab')2 in lyophilized form to viper venom is 8:1; and other detected items conform to the quality standard of antivenin in Chinese Pharmacopoeia 2010. The detection of Phenyl-Sepharose (low-sub) FF column chromatography result shows that: activity is centralized at eluting peak 1, micromolecule impurity proteins are centralized at penetration peal, eluting peak 2 and eluting peak 3. According to the technology of the invention, immune blood plasma is resulted from viper venom immune horse, IgG is prepared by salting out the immune blood plasma, and F(ab')2 active fragment is obtained after the IgG is subject to enzymolysis and purification by a hydrophobic column. The lyophilized viper antivenin has strong specificity, high potency and more than 85% of the purity of antivenin F(ab')2 in lyophilized form.
Owner:浙江健博生物科技股份有限公司
Low-concentration tropane drug eye drops and preparation method
InactiveCN108338969AStrong adhesionIncrease contact areaSenses disorderPharmaceutical delivery mechanismSodium hyaluronateIrritation
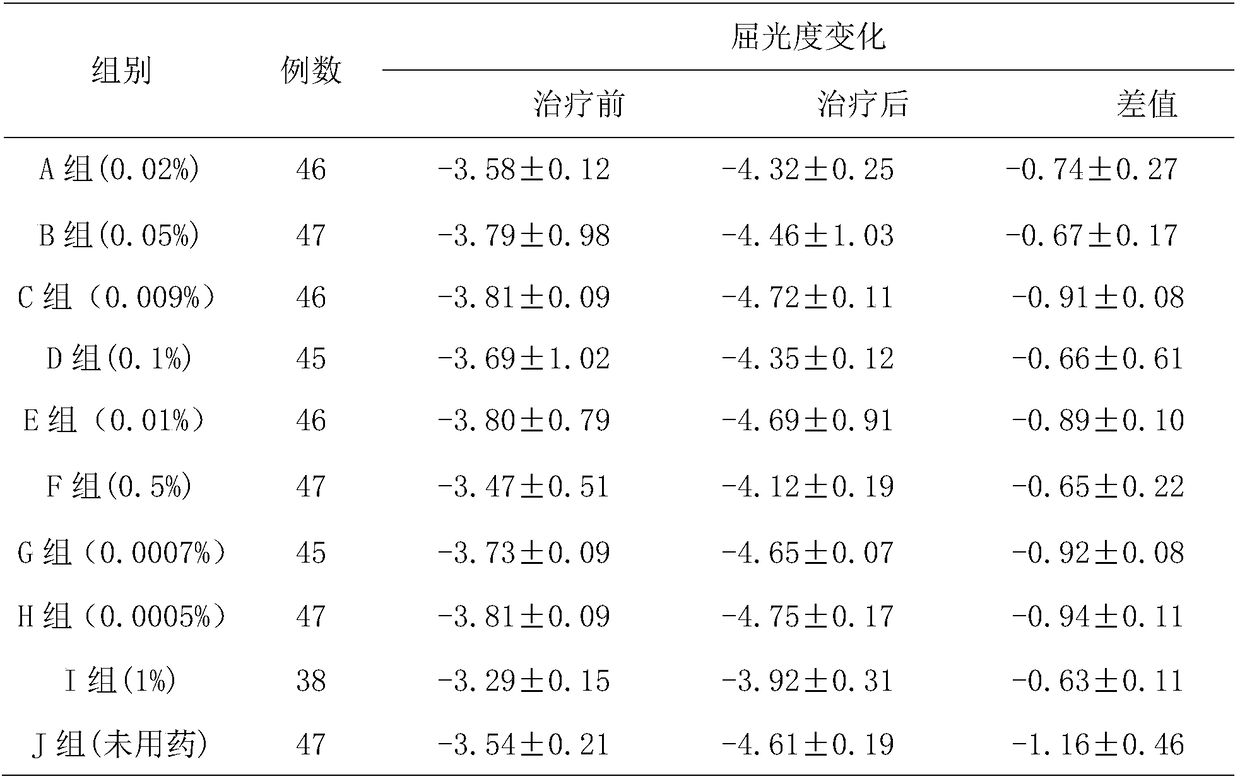
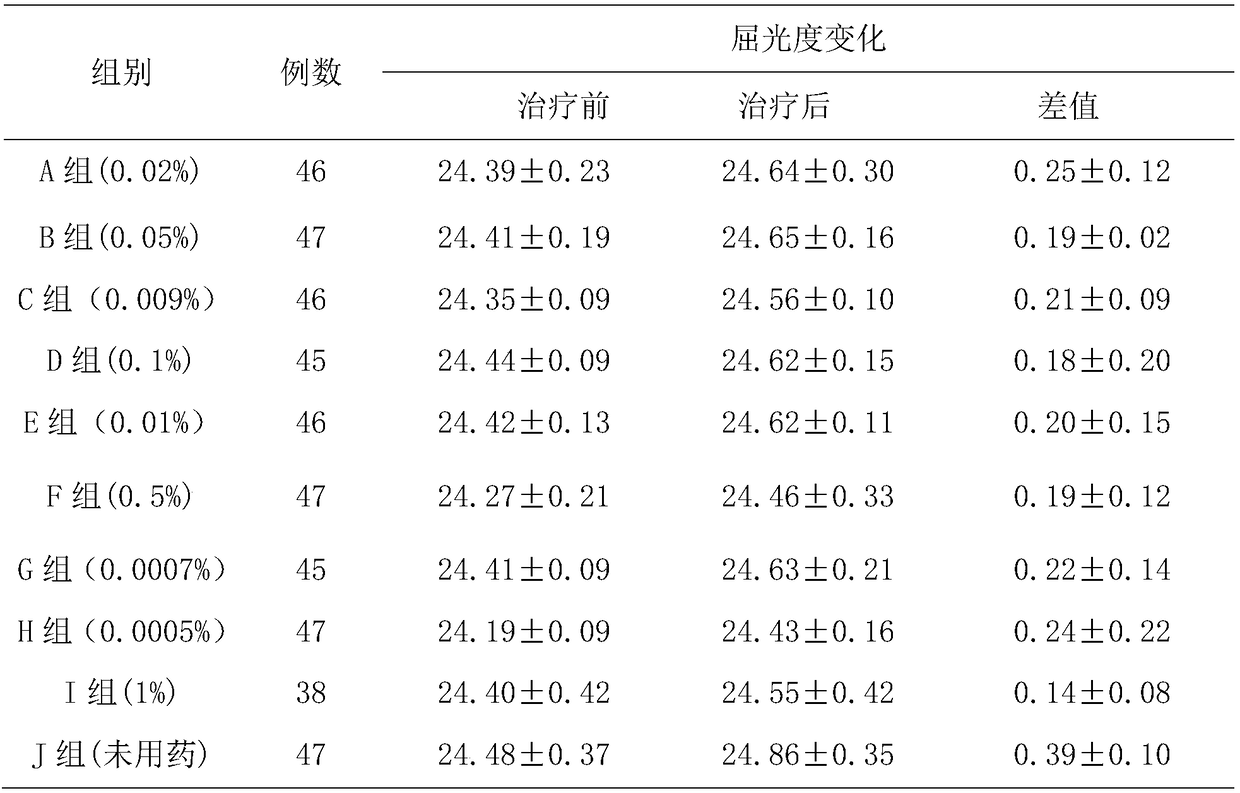
The invention belongs to the technical field of drugs, and particularly relates to low-concentration tropane drug eye drops. The low-concentration tropane drug eye drops are prepared from 0.0005-0.5%of tropane drug, 0-10% of solute which achieves inclusion and is capable of reducing irritation, 0.01-5% of solute which increases the viscosity of drug liquid, an acidifying or alkalizing agent, an osmotic regulation agent, 0.01-0.05% of bacteriostatic agent and the balance water for injection, wherein the use amount of the acidifying or alkalizing agent enables the pH to be adjusted to be 4.0-6.5, and the use amount of the osmotic regulation agent enables the osmotic pressure to be regulated to 280-330 mOsm / L. The preparation method comprises the steps of S1, the solute which achieves inclusion and is capable of reducing irritation is weighed in proportion and solved into water, the tropane drug is added, and ultrasonic mixing is conducted for 30 minutes in an ultrasonic shake cleaning machine, so that the tropane drug is included by the solute which achieves inclusion and is capable of reducing irritation; S2, ethylparaben, sodium chloride and sodium hyaluronate are weighed in proportion and solved into the water for injection at 40-75 DEG C; S3, the solutions obtained in S1 and S2 are mixed; S4, filtration, sterilization and subpackage are conducted, and the low-concentration tropane drug eye drops are obtained.
Owner:胡敏
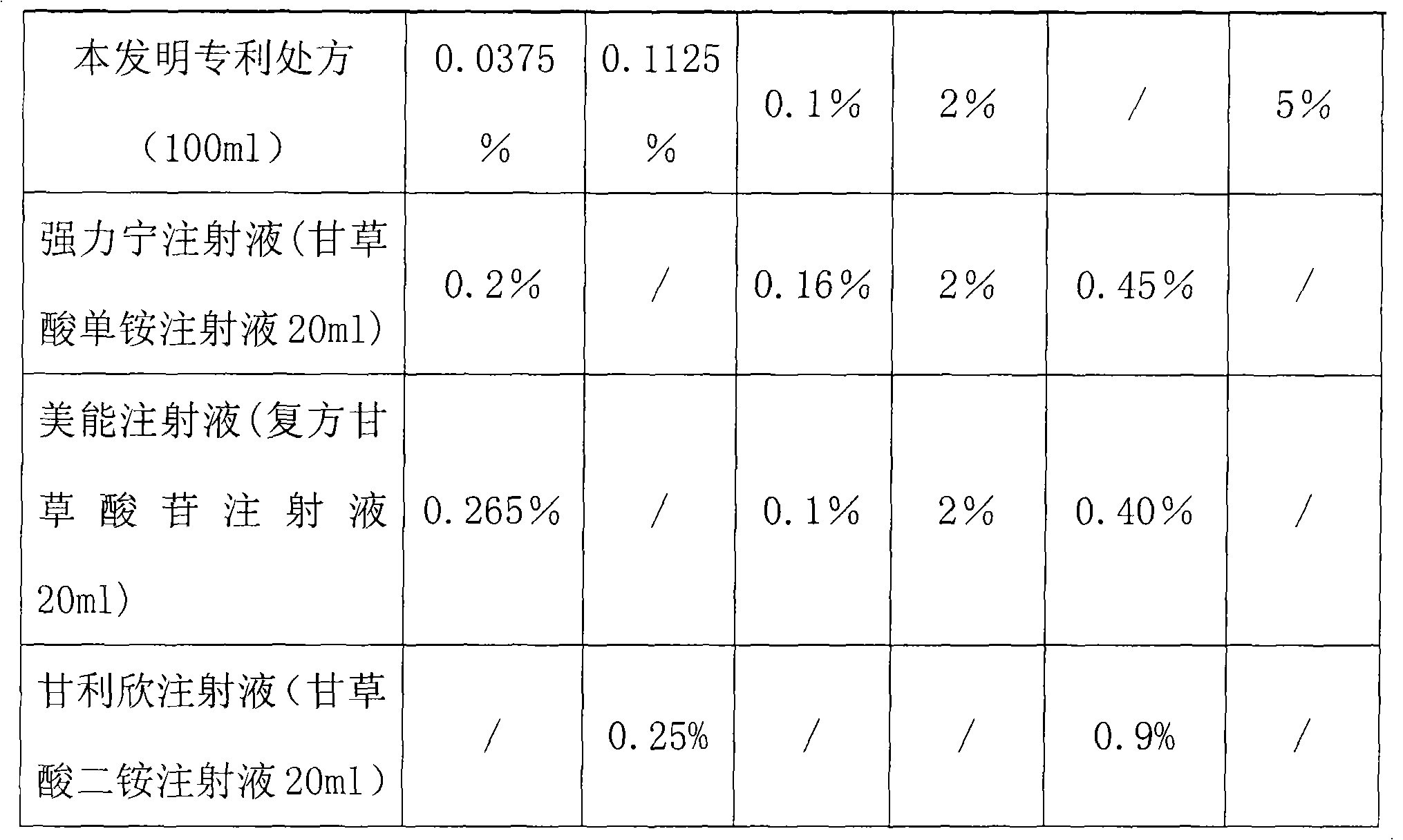
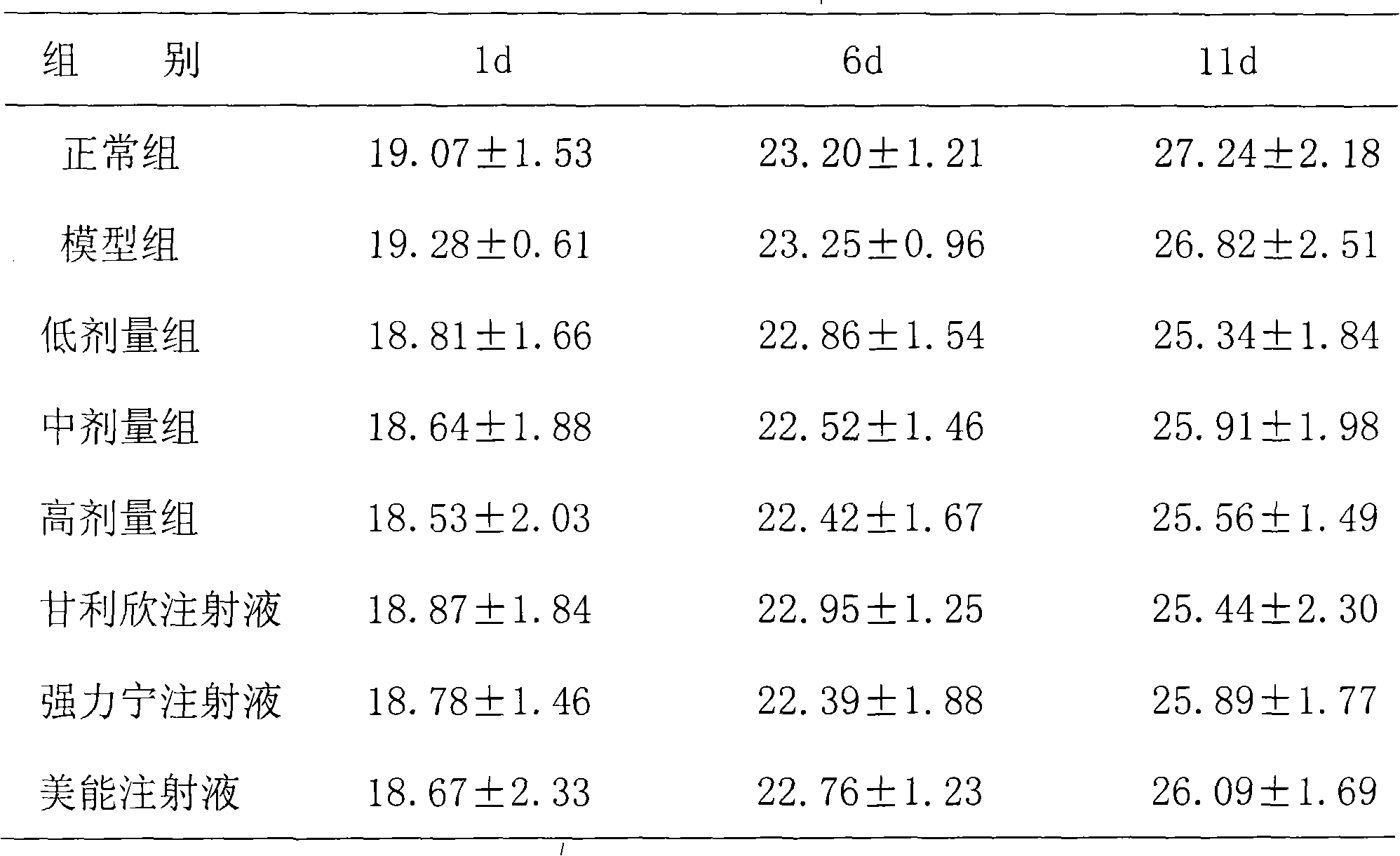
Composite glycyrrhizic acid amino acid injection, and preparation method as well as applications thereof
InactiveCN101669962AReduced functionalityImprove functionalityAntipyreticMetabolism disorderAmino Acid InjectionDrug product
The invention belongs to the technical field of medicines, in particular to a composite glycyrrhizic acid amino acid injection, and simultaneously, the invention also relates to a method for preparingthe injection and applications thereof in medicines. The invention aims at providing the composite glycyrrhizic acid amino acid injection which has obvious functions of anti-inflammation, antianaphylaxis, oxidation resistance, antiatheroscloresis, immune regulation and detoxication, and also has the characteristics of good product quality, safe and reliable taking and the like; and the method hasthe characteristics of simple technological process and high product yield. The technical scheme provides the composite glycyrrhizic acid amino acid injection which is characterized by being preparedby following crude drug by weight percent: 0.05-0.4% of composite glycyrrhizic acid, 1.05-3.2% of acid amino, 3-8% of xylitol, 0.01-0.05% of pH modifier, and the balance of water for injection.
Owner:杭州市第六人民医院
Freeze-dried rabies vaccine for humans and preparation method of vaccine
ActiveCN104826101AThe process steps are simpleEasy to operateInactivation/attenuationAntiviralsHuman useSide effect
The invention relates to a freeze-dried rabies vaccine for humans and a preparation method of the vaccine, relates to the field of vaccine production preparation technologies and aims at solving the problems that effective virus antigen expression content is low, the side effect rate of a vaccinator is high and vaccine yield and quality can not meet standard requirements as only a biological reactor is adopted for producing a rabies vaccine. The freeze-dried rabies vaccine for humans is obtained by inoculating aG strain rabies virus on Vero cells and sequentially carrying out ultrafiltration and concentration, separation and purification as well as freeze drying, wherein the packing volume of the freezed-dried rabies vaccine for human use is 0.5ml / dose, and during freeze drying, the adopted vaccine freeze-drying protecting agent comprises the following ingredients: 60-90g / l of trehalos, 6-14g / l of sodium glutamate, 3-6g / l of urea, 2-3g / l of L-arginine and 10g / l of 199 culture medium, and the vaccine freeze-drying protecting agent does not contain gelatin, human serum albumin or dextran. The freeze-dried rabies vaccine for humans has the advantages that cost is low, operation is easy, pollution is hardly produced, vaccine quality and yield are greatly improved, the content of impurities in a vaccine is reduced, allergy reactions are hardly caused, and vaccine safety is greatly improved.
Owner:江生(深圳)生物技术研发中心有限公司
Preparation method and device for leukocyte-depleted platelet rich plasma
InactiveCN105107233ALow incidence of adverse reactionsEasy to operateCentrifugesLiquid separationWhole blood productWhite blood cell
The invention belongs to the blood product technology field, and discloses a preparation method for leukocyte-depleted platelet rich plasma. The method comprises the following steps: firstly, anticoagulants and blood are drawn and mixed, and whole blood to be separated is obtained; secondly, first centrifugation is carried out, a centrifugate divided into three layers from bottom to top is obtained, and platelet and plasma components in the upper layer are obtained; thirdly, the obtained upper layer components are centrifuged again, and platelet deposition at the bottom and a plasma supernatant are formed; part of the plasma supernatant is drawn, the platelet deposition is subjected to resuspension by the left plasma supernatant, and leukocyte-depleted platelet rich plasma is prepared. The method achieves elimination of leukocytes and enrichment of platelets at the same time, operation is convenient, consumed time is short, the technical indexes of the prepared plasma product are better than technical indexes of present products with the same kind. Centrifugation for two times in the method can be carried out in two centrifuge tubes respectively, or be carried out in one centrifugal device. The invention provides a centrifugal device used for performing the preparation method.
Owner:SHANGHAI SIXTH PEOPLES HOSPITAL
Ambroxol hydrochloride liquid preparation and preparation method thereof
ActiveCN101627967AImprove solubilityOvercome the defect of being slightly soluble in waterOrganic active ingredientsPharmaceutical delivery mechanismWater useBULK ACTIVE INGREDIENT
The invention discloses an ambroxol hydrochloride liquid preparation and a preparation method thereof. The method comprises the steps: dissolving ambroxol hydrochloride, stabilizing agent and osmotic pressure regulator into water used for injection, and evenly mixing together to obtain solution I; then, filtering the solution I, and obtaining the ambroxol hydrochloride liquid preparation. The preparation method does not introduce active carbon, so as to avoid the danger of hurting human body since active carbon particle is introduced into the preparation; meanwhile, the active ingredients in the preparation is ensured to be stable, and the safety (namely, the chemical stability of the ambroxol hydrochloride can be effectively improved, the particle content in the preparation is reduced, and the purity of the preparation is improved) of the finished product can be guaranteed.
Owner:上海华源药业(宁夏)沙赛制药有限公司
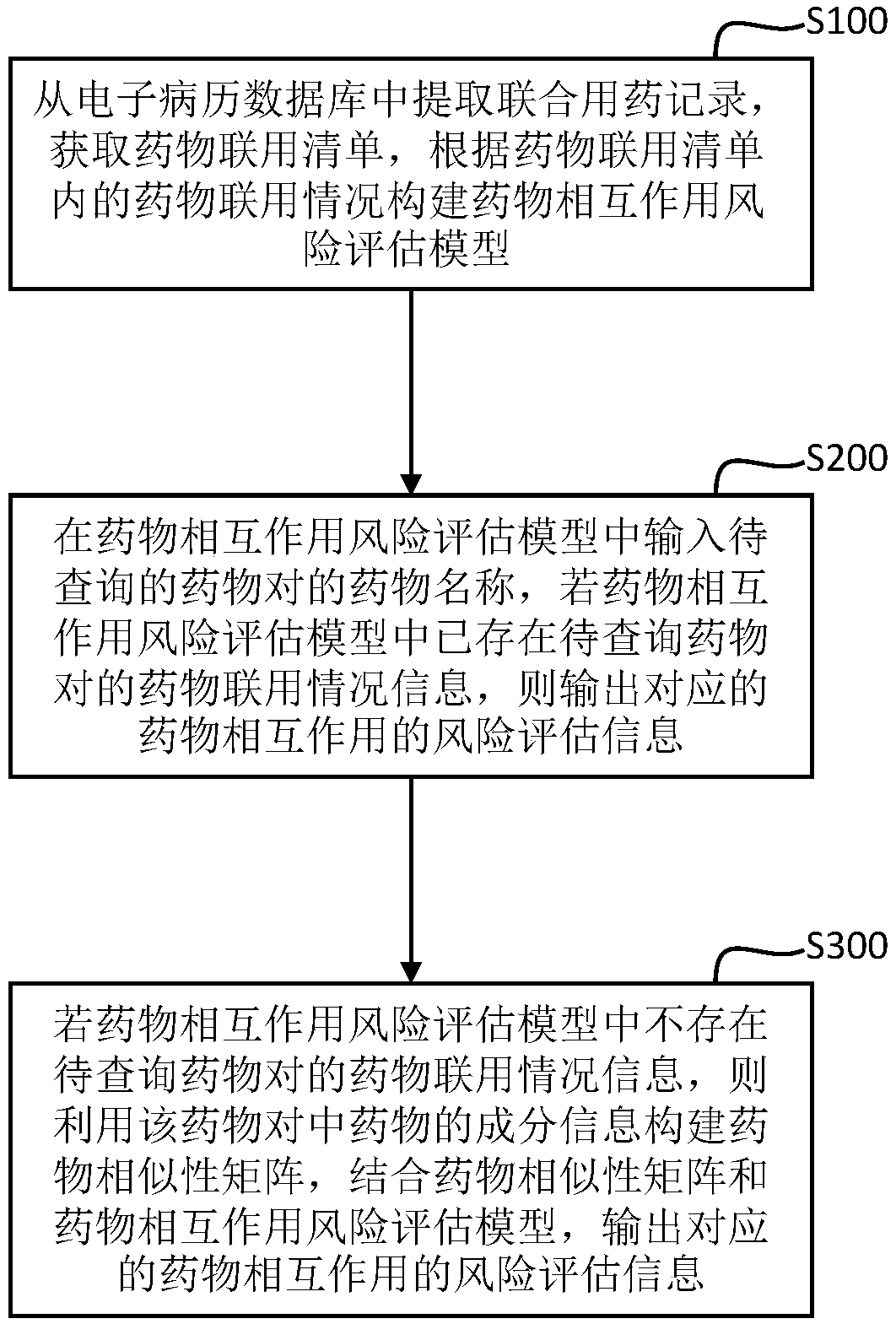
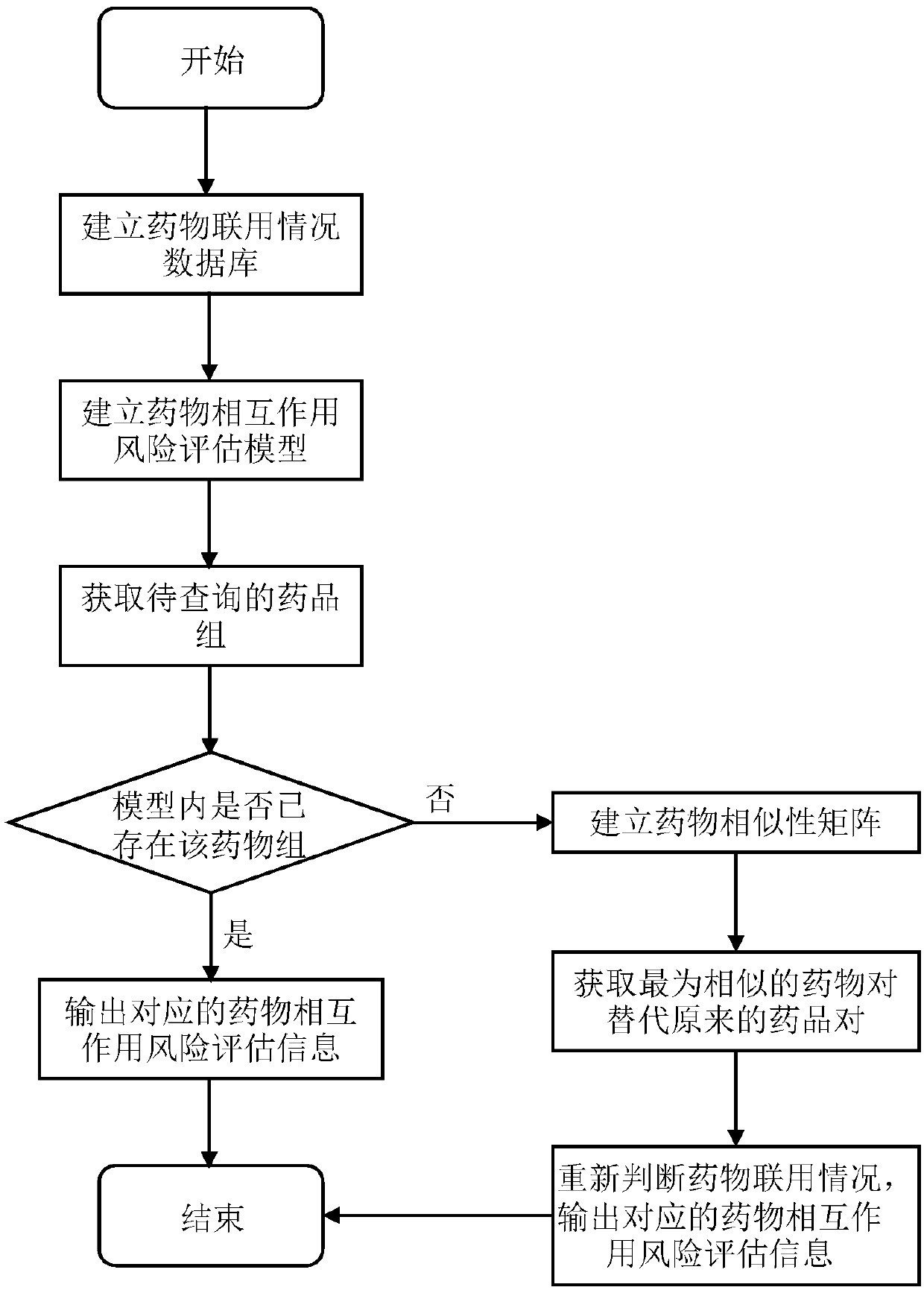
Drug interaction modeling and risk evaluation method, terminal equipment and storage medium
ActiveCN108630322ALow incidence of adverse reactionsEnsure medication safetyMedical data miningDrug referencesDrug groupMedical emergency
Owner:XIAMEN UNIV
Muscular amino acid and peptide nucleoside powder injection and its preparation method
InactiveCN1522757AImprove stabilityHigh preparation process yieldPowder deliveryPeptide/protein ingredientsMedicineFreeze-drying
The present invention relates to a sarcopeptidoglucoside powder injection and its preparation method. It is formed from polypeptide, hydroxanthine and excipient. Said invention adopts the modern biological extraction technique to prepare sarcopeptidoglucoside solution, and utilizes the modern low-temp. freeze-drying preparation technique to obtain the invented sterile freeze-dried powder injection. When it is used, it can be dissolved by injection water or infusion fluid, then can be injected. Said invention has good stability, long storage time and high safety, etc.
Owner:黑龙江江世药业有限公司
Bacteria inhibiting and balancing type gynecological gel and preparation method thereof
InactiveCN107243017AImprove immunityEnhance metabolismAntibacterial agentsAntimycoticsMicrobiologyPolysaccharide
The invention discloses bacteria inhibiting and balancing type gynecological gel and a preparation method thereof. The bacteria inhibiting and balancing type gynecological gel comprises the following components in percentage by weight: 0.1-2.0% of cationic biopolysaccharides, 5.0-20% of a humectant, 0.1-2.0% of a thickening agent, 5-25% of a balancing flora composite extract, 0.1-2.0% of an acidity regulator, and the balance purified water. According to the bacteria inhibiting and balancing type gynecological gel and the preparation method thereof, the components with bacteria inhibiting effect are mixed to coordinately act to inhibit pernicious bacteria and promoting the growth of probiotics; the thickening agent and cationic biopolysaccharides are mixed to coordinately act to improve the product viscosity. Therefore, the product can be conveniently used; the concentration of effective components is properly increased; moreover, the humectant is used. Therefore, a protective shield is provided for the skin, and the lubrication level of the skin is improved.
Owner:成都珂萝瑞诗化妆品有限公司
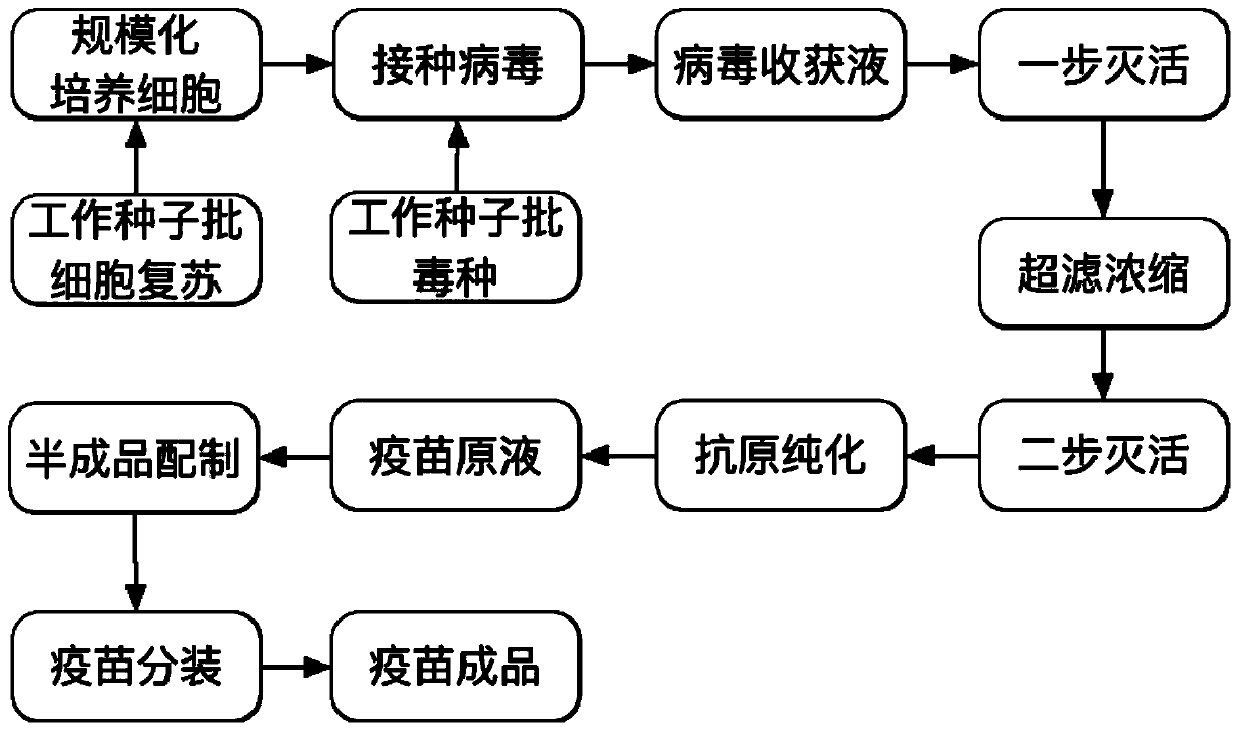
SARS-CoV-2 inactivated vaccine and preparation method of vaccine
ActiveCN111569058AReduce Biosecurity RisksLow impurity contentSsRNA viruses positive-senseViral antigen ingredientsVirus inactivationTGE VACCINE
The invention relates to a SARS-CoV-2 inactivated vaccine and a preparation method of the vaccine. The preparation method comprises the following steps: step (1) recovery and large-scale culture of Vero cells; step (2) inoculation of working seeds, batch of virus seeds, virus culture and one-time harvest virus liquid, namely, the virus harvest liquid; step (3) obtaining an inactivated virus concentrated solution after a first virus inactivation, concentration and a second virus inactivation; step (4) obtaining a vaccine stock solution after purifying the inactivated virus concentrated solution; and step (5) preparation of semi-finished product and sub-package after the stock solution is verified to be qualified.
Owner:中国生物技术股份有限公司 +1
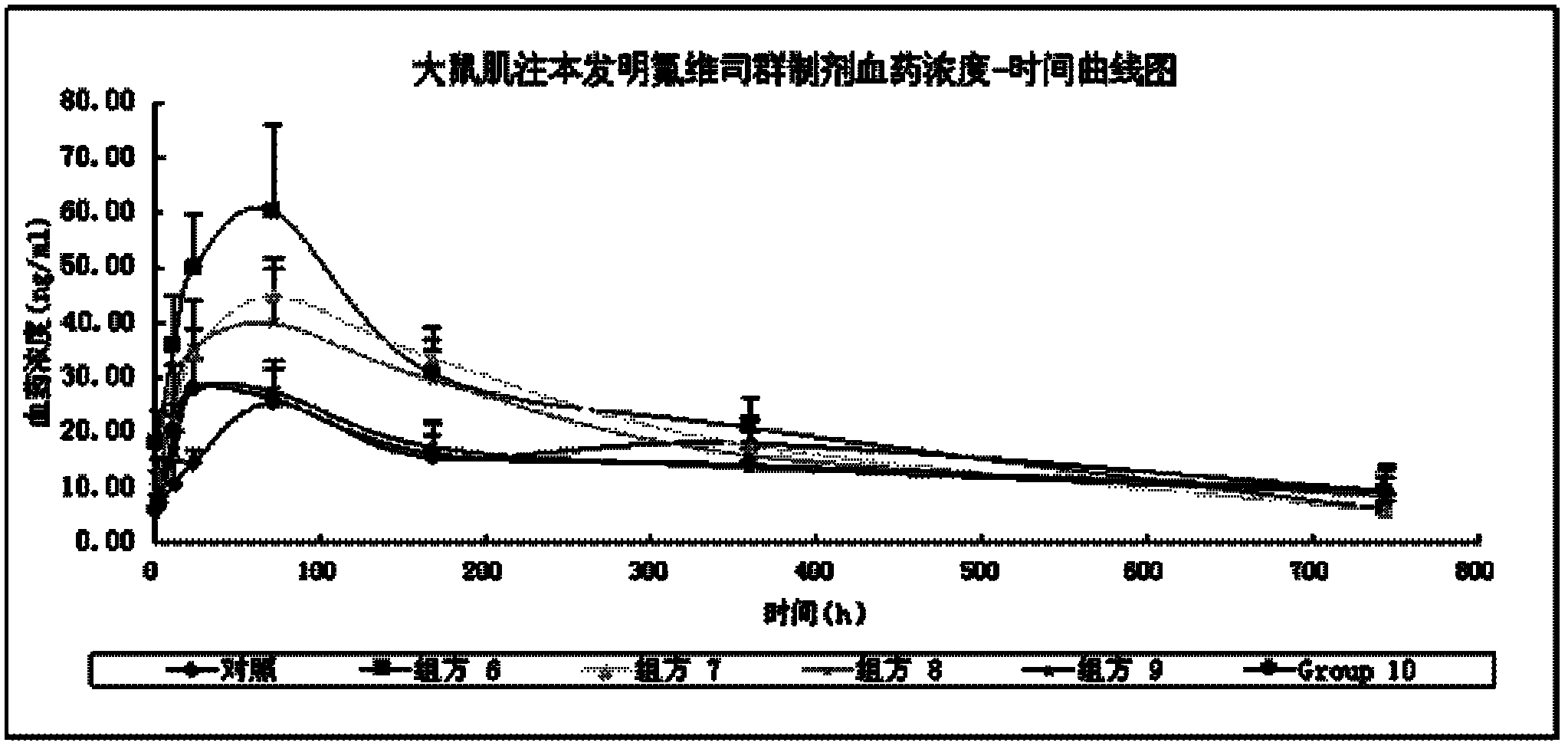
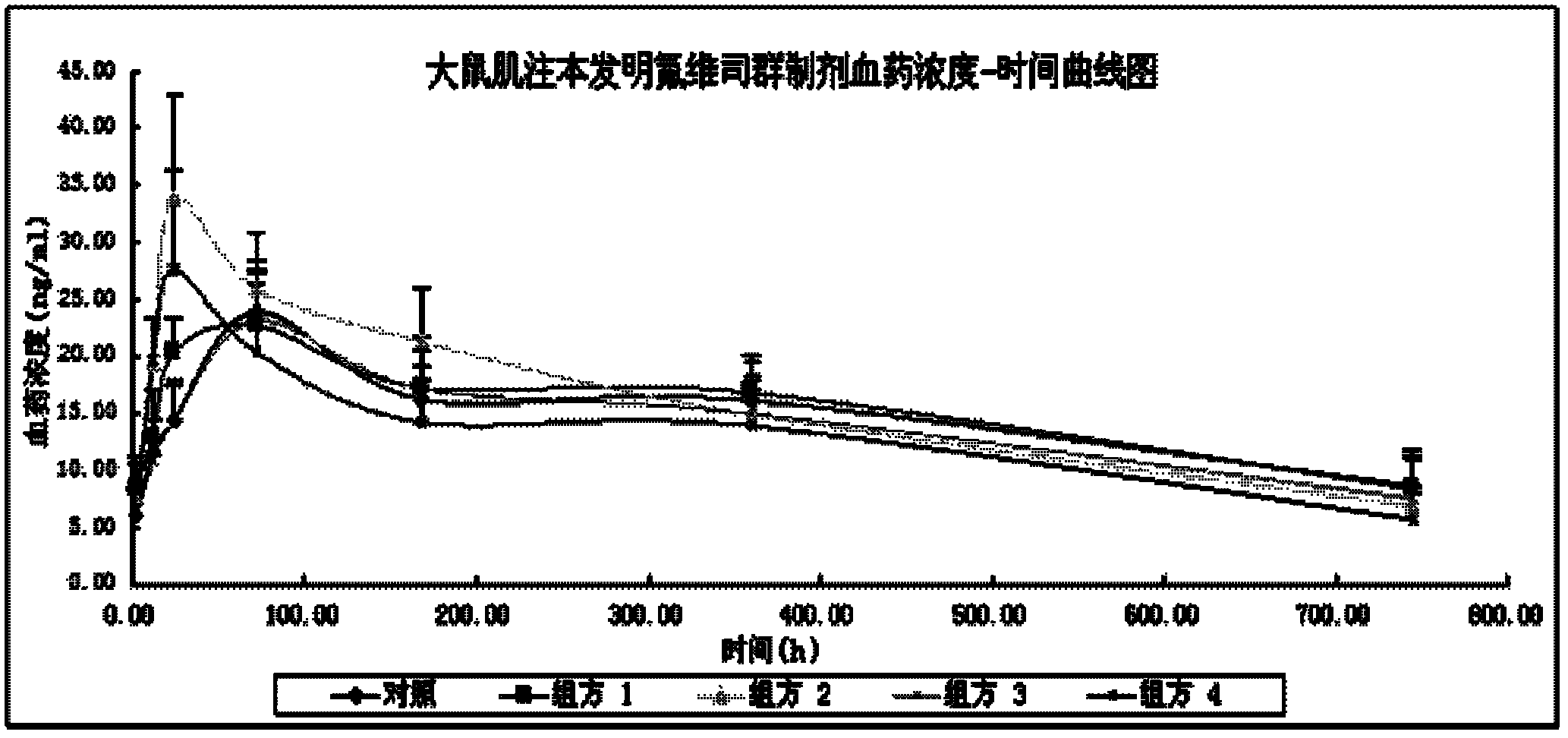
Fulvestrant or fulvestrant derivative sustained release preparation and preparation method thereof
InactiveCN102600064ALow incidence of adverse reactionsGrowth inhibitionOrganic active ingredientsOrganic non-active ingredientsVegetable oilAntioxidant
The invention relates to a long-acting fulvestrant or fulvestrant derivative sustained release preparation and preparation method of the long-acting fulvestrant or fulvestrant derivative sustained release preparation. The formula of the preparation provided by the invention comprises (1) 10mg / ml to 500mg / ml fulvestrant or fulvestrant derivative served as the active compound based on the content of preparation, (2) 3% to 80% of ketone compound or dimethyl sulfoxide served as the cosolvent based on the total weight of the preparation, (3) dispersing agent using vegetable oil or synthetic grease (ester), (4) analgesic, and (5) optional antioxidant.
Owner:XIAN LIBANG PHARMA
Traditional Chinese medicine leech extract product and its application
ActiveCN103251926AImprove bioavailabilityImprove utilizationPowder deliveryHydrolysed protein ingredientsDiseaseUltrafiltration
The invention discloses a traditional Chinese medicine leech extract product and its application and belongs to the field of traditional Chinese medicines. The invention is characterized in that the traditional Chinese medicine leech extract product is prepared by the following steps: carrying out enzymatic hydrolysis on leech by a double-enzyme continuous enzymatic hydrolysis method (leech is firstly subjected to enzymatic hydrolysis under the acidic condition by pepsin, and an enzymolysis suspension is subjected to enzymatic hydrolysis under the alkaline condition by trypsin), carrying out ultrafiltration and nanofiltration, refining by cation exchange gel and the like, and purifying so as to obtain the leech extract product. Purity of the leech extract product reaches more than 90%. Pharmaceutical adjuncts can be added into the leech extract product to prepare a freeze-dried powder injection according to a conventional method. The traditional Chinese medicine leech extract product has a good effect of invigorating blood circulation, is used for treating cardiovascular and cerebrovascular diseases, and especially has a good effect of treating ischemia apoplexy.
Owner:山东禹泽药康产业技术研究院有限公司
Medicine for treating gastroesophageal reflux disease and functional dyspepsia
InactiveCN101143143AImprove toleranceHigh synergistic effectDigestive systemSolution deliveryLansoprazoleRabeprazole
A combination preparation for remedying the gastroesophageal reflux disease (GERD) and the functional dyspepsia is characterized in that the prescription of the combination preparation consists of a proton pump depressor and a gastrointestinal power drug of itopride; the proton pump depressor is selected from one of a Pantoprazole, a Omeprazole, a Esomeprazole, a Lansoprazole, a Rabeprazole, a Tenatoprazole and a Leminorazole, wherein the Pantoprazole is preferential, and at the same time the neutral form of the basic salt of the proton pump depressor is also included, such as Naplus, Mg2plus, Ca2plus, Kplus or Li plus salt and a pure optical stereoisomer of the proton pump depressor or an active metabolite of the proton pump depressor; the gastrointestinal power drug is the itopride and a ramification of the itopride or one of the medicinal salts of the itopride; in the combination preparation, the weight ratio of the Pantoprazole and the itopride is 2 to 5 to 2 to 7. The invention has important affect for remedying the gastroesophageal reflux disease and the functional dyspepsia, and the preparation method of the invention is simple and convenient; the cost is low; the invention is fit for being orally taken by the patient; the invention has good conformance performance, high curative effect, low recrudescence rate and little adverse reaction.
Owner:沈阳东宇药业有限公司
Butylphthalide- and edaravone-containing compound injection and preparation method thereof
ActiveCN102526036ALow incidence of adverse reactionsGood curative effectOrganic active ingredientsNervous disorderAlcoholAntioxidant
The invention relates to a butylphthalide- and edaravone-containing compound injection and a preparation method thereof. The injection of every 100 ml contains 25-50 mg of active ingredient butylphthalide, 12.5-50 mg of edaravone, as well as absolute ethyl alcohol, hydroxypropyl-beta-cyclodextrin, an osmotic pressure regulator, an antioxidant and the like. With the adoption of the butylphthalide- and edaravone-containing compound injection, the administration dosage is greatly lowered, the burden of liver metabolism is reduced, and the incidence rate of liver adverse reactions is lowered. According to determination, with the adoption of the butylphthalide- and edaravone-containing compound injection, the administration dosage of the edaravone can be reduced by at least 20%, besides, the compound injection has a better curative effect, and the incidence rate of the liver adverse reactions is also remarkably lowered.
Owner:SHIJAZHUANG ZHONGSHUO PHARMA CO LTD
Medicinal composition for treating psoriasis
InactiveCN1478478ASignificant effectLow incidence of adverse reactionsOrganic active ingredientsDermatological disorderMedicineAdrenal gland
A composite medicine in the form of ointment and gels for treating psoriasis contains Tazhuluoding, betamethasone dipropionate and pharmacologically receptable auxiliary. Its advantages are high curative effect and high safety.
Owner:CHONGQING HUAPONT PHARMA
Epalrestat sustained release preparation and preparation method thereof
InactiveCN112137990AAvoid peaks and valleys in blood concentrationImprove complianceOrganic active ingredientsNervous disorderSustained release pelletsDrug utilisation
The invention belongs to the field of pharmaceutical preparations, and provides an epalrestat sustained release preparation and a preparation method thereof. The epalrestat sustained release preparation is composed of 64%-92% of drug-containing sustained-release pellets, 1.0%-8.0% of a gastric-soluble isolation coating layer, 5.0%-20% of an enteric-soluble coating layer, and 2.0%-8.0% of a protective coating layer. The epalrestat sustained release preparation disclosed by the invention can effectively avoid the peak valley phenomenon of the blood concentration of a common preparation, reducesthe medicine taking frequency, reduces the occurrence rate of adverse reactions, and increases the compliance of a patient; and can avoid a possible drug burst release effect of a monobasic sustainedrelease preparation, reduces the toxicity and side effects of the drug, and further ensures the safety of clinical medication. The selected auxiliary materials are common, the preparation process is simple, the technical difficulty is low, the industrial mass production is easy to realize, and the market potential is large.
Owner:南京康川济医药科技有限公司
Tablet containing valsartan and hydrochlorothiazide
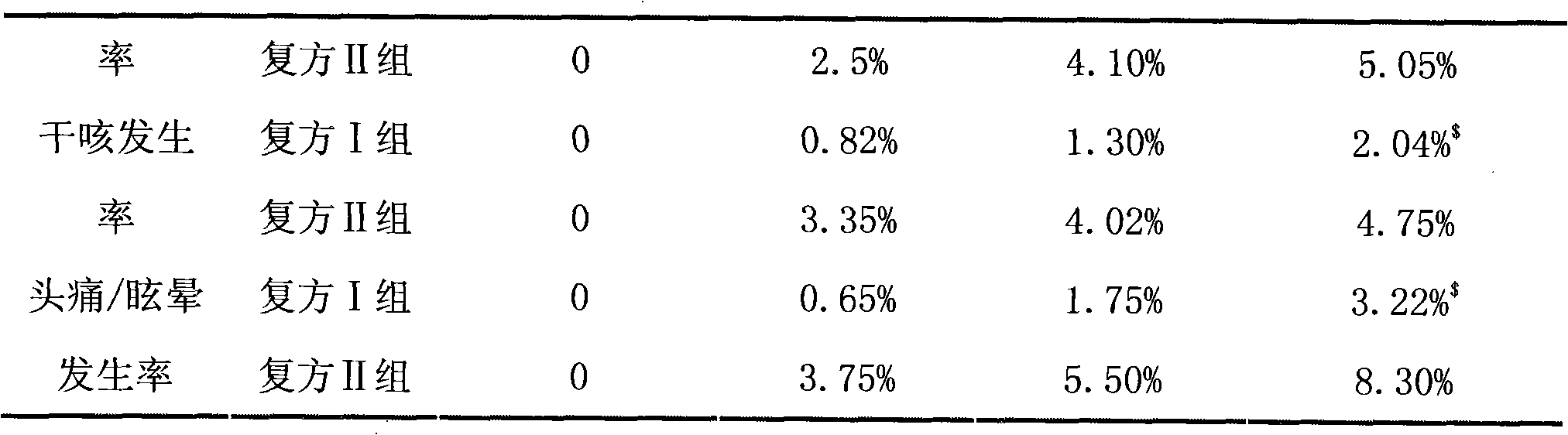
ActiveCN101780090ALow incidence of adverse reactionsGood antihypertensiveDrageesCardiovascular disorderCurative effectHeadaches
The invention belongs to the filed of medicines, in particular relates to a tablet containing valsartan and hydrochlorothiazide. The double-layer tablet with synergy antihypertension function provided by the invention contains 80mg / 12.5mg of the valsartan and hydrochlorothiazide. Compared with a compound unilayer tablet and a capsule containing 80mg / 12.5mg of the valsartan / hydrochlorothiazide, the curative effect of the tablet provided by the invention is improved, and the incidence rates of adverse effects such as hacking cough, headache and dizziness are notably reduced, and p is less than 0.05.
Owner:LUNAN BETTER PHARMA
Folic acid pellet and preparation method thereof
InactiveCN101444488AGood dispersionQuick effectOrganic active ingredientsMetabolism disorderDissolutionBioavailability
The invention provides a folic acid pellet preparation composed of folic acid and medical supplementary materials. The folic acid pellet preparation is characterized in that the medical supplementary materials are excipients and binders; and in the pellet preparation, the content of folic acid is 0.02-20 percent by weight, the content of excipients is 75-99.98 percent by weight, and the content of binders is 1-5 percent by weight. The pellet preparation has high dissolution rate and high bioavailability, and the preparation method is simple, convenient and easy for operation.
Owner:JF PHARMALAND TECH DEV
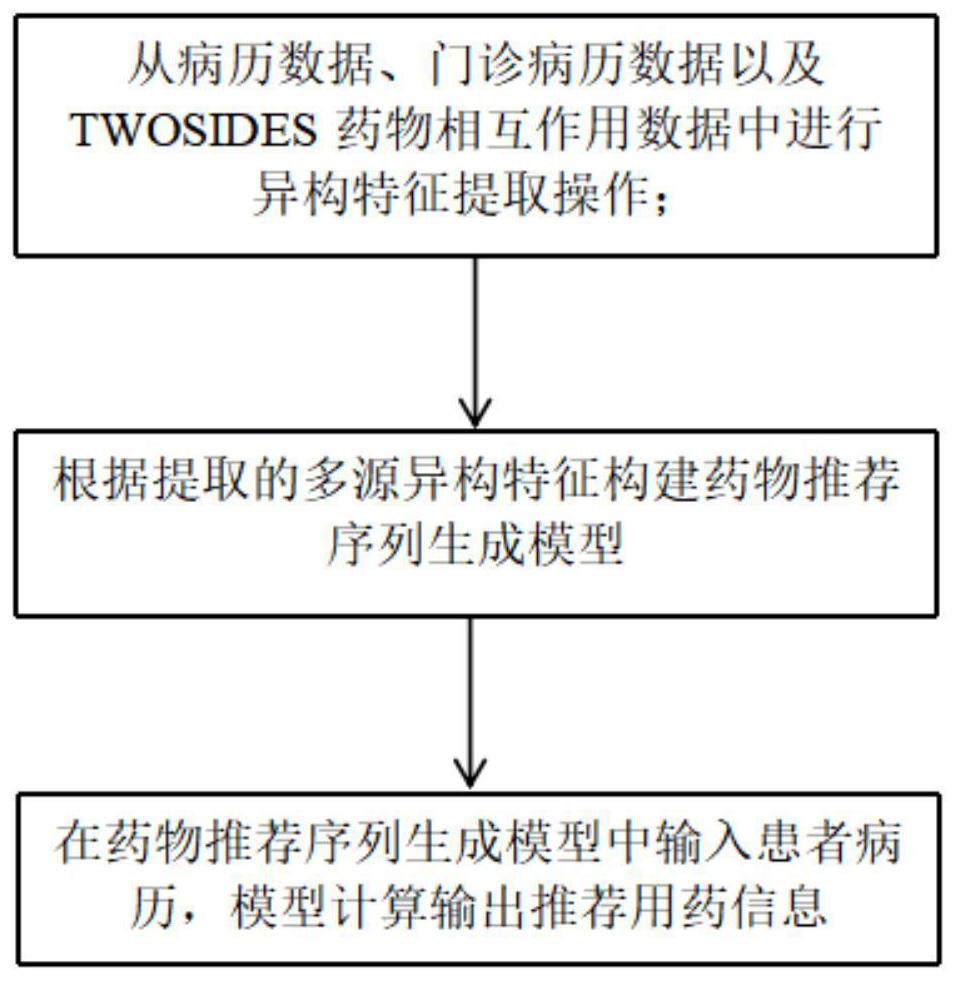
Drug recommendation modeling method based on medical record data and drug interaction risks
ActiveCN111968715AReduce the incidence of adverse drug reactionsMedication Safety GuaranteeDrug and medicationsBiological neural network modelsDrug adverse reactionsMedication information
The invention discloses a drug recommendation modeling method based on medical record data and drug interaction risk. The method specifically comprises the following steps: S100, performing heterogeneous feature extraction operation on medical record data, outpatient medical record data and TWOSIDES drug interaction data; S200, constructing a drug recommendation sequence generation model accordingto extracted multi-source heterogeneous features; and S300, inputting patient medical records into the drug recommendation sequence generation model, and calculating and outputting recommended medication information by the model. According to the drug recommendation modeling method based on medical record data and drug interaction risks in the invention, automatic learning and modeling are performed based on massive historical case data and drug interaction information, drug recommendation information is given, an accurate and effective decision basis is provided for reasonable combination ofclinical drugs, the occurrence rate of adverse drug reactions is reduced, and effective guarantee is provided for drug use safety of users.
Owner:XIAMEN UNIV
Composite medicine containing flucloxacillin magnesium and amoxicillin sodium
ActiveCN100346787CReduce outputImprove stabilityAntibacterial agentsPill deliveryFlucloxacillinFlucloxacillin magnesium
Owner:SHANXI C&Y PHARMACEUTICAL GROUP CO LTD
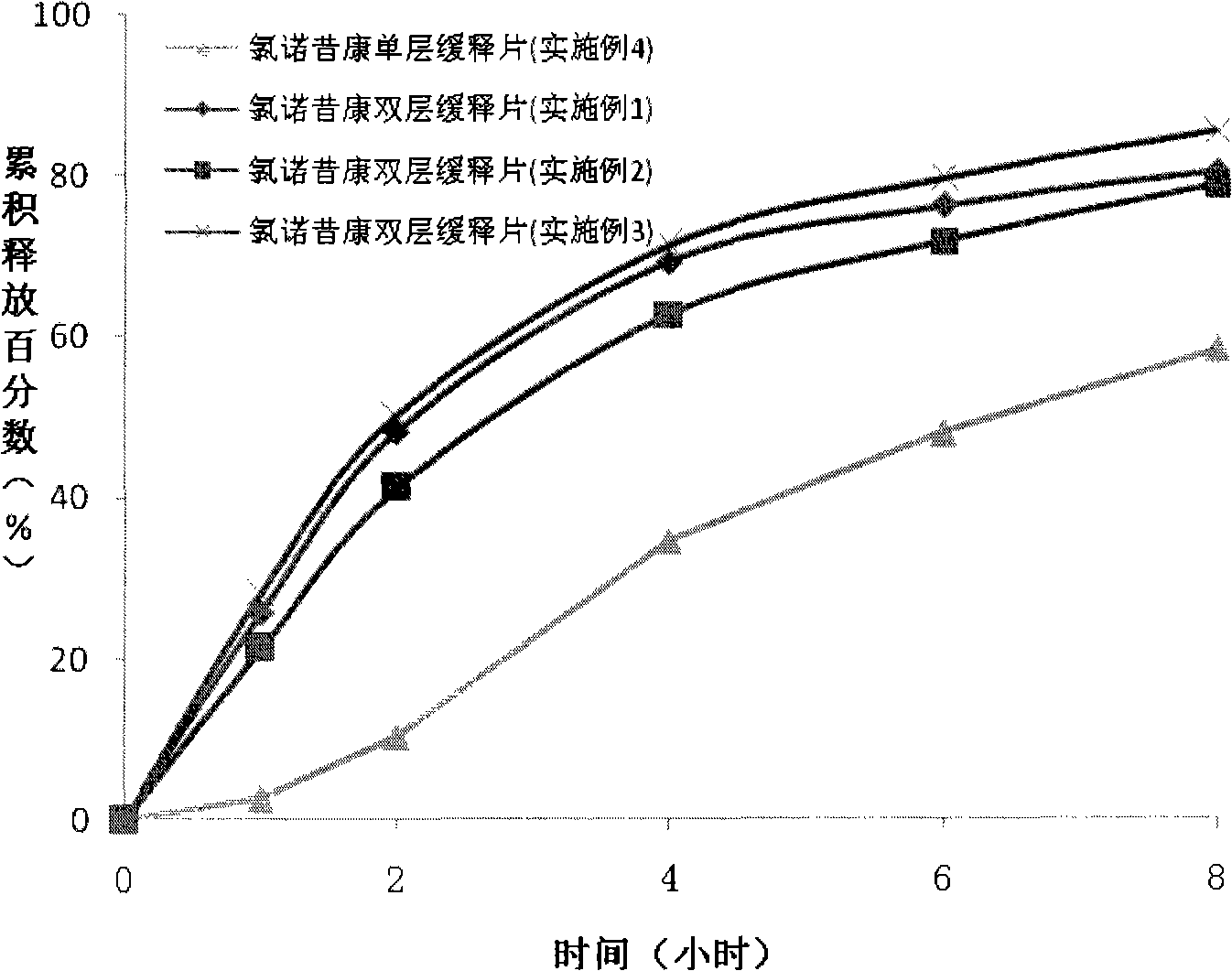
Lornoxicam double-layer sustained release tablets
ActiveCN101342177AReduce the number of dosesBlood concentration is effectiveOrganic active ingredientsAntipyreticPharmaceutical preservativesSustained-Release Preparations
The invention relates to a double-layer sustained release lornoxicam tablet comprising (a) a quick release layer and (b) a sustained release layer, wherein, the quick release layer comprises (a1) the lornoxicam, (a2) alkaline matter and (a3) an other optional carrier or excipient acceptable to pharmacy and the sustained release layer comprises the (b1) lornoxicam, (b2) sustained release substance and (b3) the other optional carrier or the excipient acceptable to the pharmacy. The weight proportion of (a1) and (b1) is 1:50 to 50:1. The invention also provides a preparation method of the double-layer sustained release lornoxicam tablet. The double-layer sustained release preparation of the invention has the advantages of the quick effect of the quick release preparation and the sustained effect of sustained release preparation. In addition, the double-layer sustained release preparation can maintain the effect of effective blood concentration continuously and stably after the effective blood concentration is reached rapidly.
Owner:CHINA PHARM UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com