Composite medicine containing flucloxacillin magnesium and amoxicillin sodium
A technology of amoxicillin sodium and flucloxacillin magnesium, applied in the field of novel combination drugs, can solve the problems of poor antibacterial activity of Staphylococcus aureus, increased clinical adverse reactions, increased allergic reactions, etc., and achieves low incidence of adverse reactions, Low cost of excipients and the effect of expanding indications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
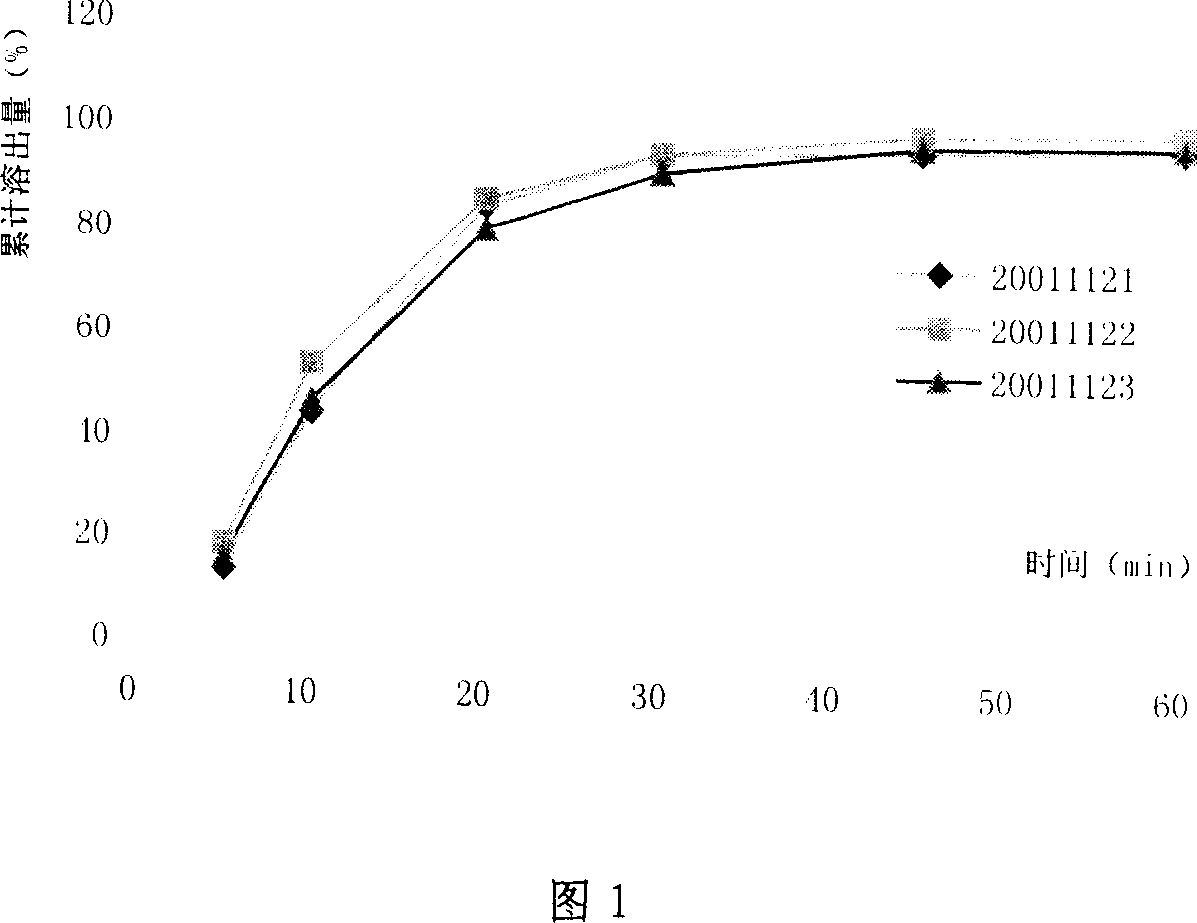
Image
Examples
Embodiment 1
[0047] prescription
[0048] Flucloxacillin magnesium (calculated as flucloxacillin) 125g
[0049] Amoxicillin sodium (as amoxicillin) 125g
[0051] Proper amount of 50% ethanol solution of 10% povidone K30 (just make the first three raw materials into soft materials, the same below)
[0052] Makes 1000 capsules
[0053] Preparation process
[0054] Pass the main ingredient and auxiliary materials through an 80-mesh sieve for later use. Weigh povidone K30, use 50% ethanol solution to prepare a solution with a concentration of 10%, and set aside. Weigh the main drug in the prescription amount, use 10% povidone K30 alcohol solution to make soft material, granulate through 60 mesh, blast dry below 50°C for 1-2 hours, granulate in 60 mesh, add the prescription amount of auxiliary materials, mix well , measure the content of the main drug in the granules, calculate the average loading, fill in capsules, and pack.
[0055] Selection of excipient...
Embodiment 2
[0072] prescription
[0073] Flucloxacillin magnesium (calculated as flucloxacillin) 75g
[0074] Amoxicillin sodium (as amoxicillin) 500g
[0076] Makes 1000 capsules
[0077] Preparation process
[0078] Due to the large amount of the main drug, in order to reduce the volume of the capsule contents, the main drug and auxiliary materials are directly passed through an 80-mesh sieve for future use. Weigh the main drug in the prescribed amount, add the auxiliary materials in the prescribed amount, mix well, measure the content of the main drug, calculate the average loading, fill in capsules, and pack.
[0079] Influencing factor test
[0080] The two kinds of capsules that will make are placed under strong light (4500 ± 500LX), high temperature (60 ℃), high humidity (relative humidity 92.5%) conditions respectively, respectively in 5 days, 10 days sampling, investigate outward appearance, content color and luster, Content, related substanc...
Embodiment 3
[0087] prescription:
[0088] Flucloxacillin magnesium (calculated as flucloxacillin) 500g
[0089] Amoxicillin sodium (as amoxicillin) 125g
[0090] Starch 20g
[0091] Microcrystalline Cellulite (MCC) 35g
[0092] Sodium Carboxymethyl Cellulose 20g
[0094] 2.0% HPMC 15CP aqueous solution appropriate amount
[0095] Pressed into 1000 pieces
[0096] Preparation Process:
[0097] (1) Preparation of solution
[0098] Weigh 40g HPMC 15CP, measure 2000ml water to dissolve HPMC K4M completely, set aside.
[0099] (2) Ingredients and mixed powder: take the prescribed amount of medicine and lactose and stir and mix, pass through a 60-mesh sieve to obtain mixed powder A; take the prescribed amount of MCC and CMS-Na, stir and mix, pass through a 60-mesh sieve to obtain mixed powder B ; After stirring and mixing the above two mixed powders, pass through a 60-mesh sieve twice.
[0100] (3) Preparation of soft material and wet granules: Take the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com