Tablet containing valsartan and hydrochlorothiazide
A technology of hydrochlorothiazide and valsartan granules, applied in the field of medicine, can solve the problems of low bulk density, adverse reactions, and large drugs of valsartan oral dosage forms, and achieve the effect of reducing the incidence of adverse reactions and the incidence of adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 180
[0033] Embodiment 180mg / 12.5mg bilayer tablet
[0034] Composition Weight / g Content / %
[0035] Valsartan layer
[0036] A Valsartan 80.00 8.70
[0037] B Microcrystalline cellulose 312.00 33.91
[0038] C Crospovidone 60.00 6.52
[0039] D Colloidal silicon dioxide 6.00 0.65
[0040] E Magnesium stearate (I) 12.00 1.30
[0041] F Magnesium (II) stearate 6.00 0.65
[0042] Subtotal 476.00 51.74
[0043] Hydrochlorothiazide layer
[0044] G Hydrochlorothiazide 12.50 1.36
[0045] H Microcrystalline cellulose 400.40 43.52
[0046] I Sodium starch glycolate 28.00 3.04
[0047] G Iron oxide 0.20 0.02
[0048] K Magnesium (III) stearate 1.30 0.14
[0049] L Magnesium (IV) stearate 1.60 0.17
[0050] Subtotal 444.00 48.26
[0051] Total 920.00 100
[0052] Preparation Process:
[0053] First, combine ingredients A-E in a diffusion blender for valsartan granulation; sieve the blended mat...
Embodiment 2
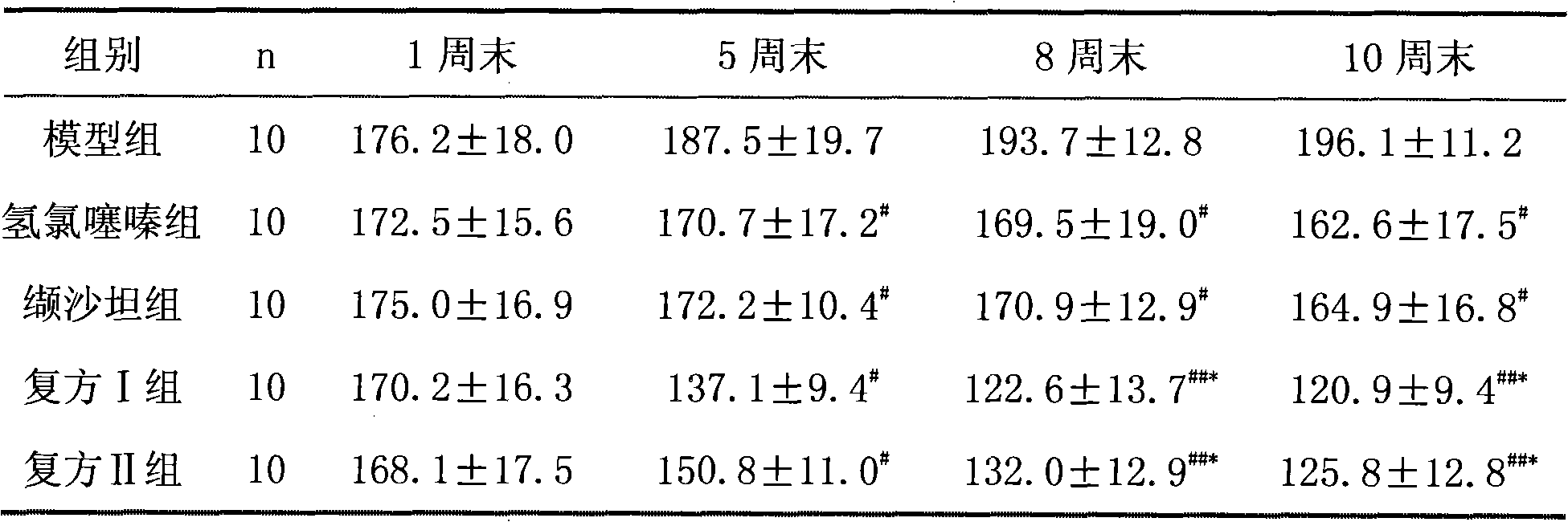
[0057] Embodiment 2 The effect of valsartan / hydrochlorothiazide compound on hypertensive model rats
[0058] 1. Grouping
[0059] A total of 50 10-week-old spontaneously hypertensive rats (SHR) were randomly divided into model group, valsartan group, hydrochlorothiazide group, compound I group, and compound II group, with 10 rats in each group, half male and half male.
[0060] 2 Administration method
[0061] Rats in each group were administered intragastrically for 12 weeks, and the doses were as follows:
[0062] Model group: intragastric administration of 0.9% normal saline with the same volume;
[0063] Valsartan group: 8.0mg / (kg.d) valsartan;
[0064] Hydrochlorothiazide group: 1.25mg / (kg.d) hydrochlorothiazide;
[0065] Compound group I: 8.0mg / (kg.d) valsartan+1.25mg / (kg.d) hydrochlorothiazide, the preparation process is the same as in Example 1, valsartan / hydrochlorothiazide 80mg / 12.5mg;
[0066] Compound group II: 8.0mg / (kg.d) valsartan + 1.25mg / (kg.d) hydrochlor...
Embodiment 3
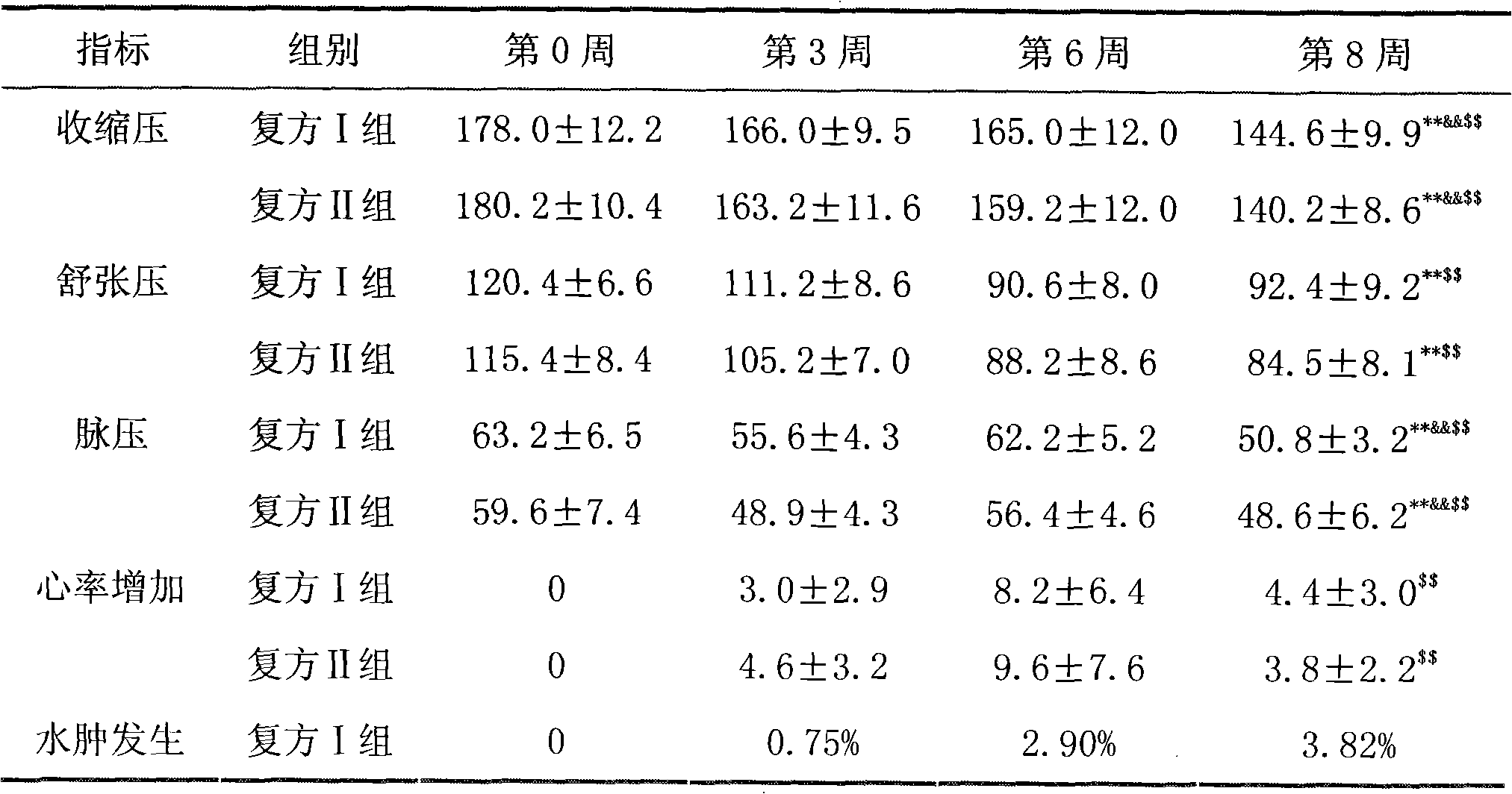
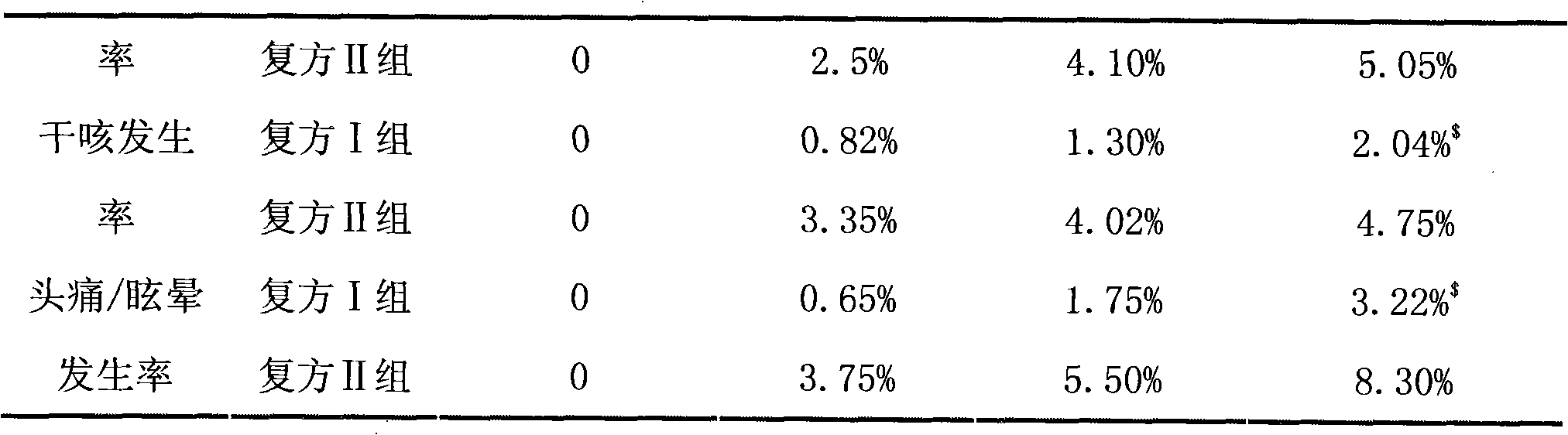
[0078] Embodiment 3 valsartan / hydrochlorothiazide compound antihypertensive clinical trial research
[0079] 1. Case selection
[0080] Elderly hypertensive patients between 60 and 70 years old, without other obvious history of cardiovascular and cerebrovascular diseases, and patients with poor clinical efficacy after traditional antihypertensive treatment are eligible to participate in this trial.
[0081] 2. Medication regimen
[0082] A total of 160 patients participated in this test, and all patients participating in this test were randomly divided into two groups according to the level of systolic blood pressure and age, that is, compound group I and compound group II, with 80 cases in each group. The compound group I is administered the double-layer tablet valsartan / hydrochlorothiazide 80mg / 12.5mg prepared in Example 1 provided by the present invention, once a day for 8 consecutive weeks; the compound group II is administered orally by Nanbeite Pharmaceutical Co., Ltd. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com