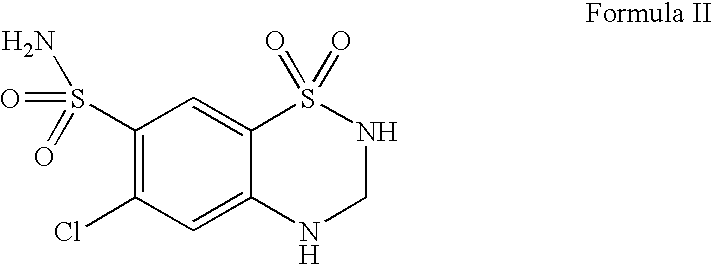
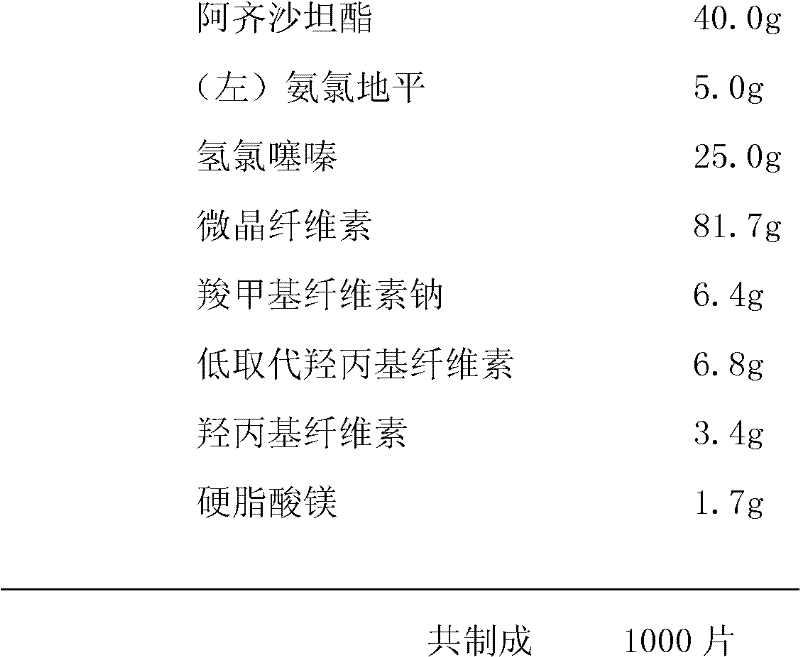
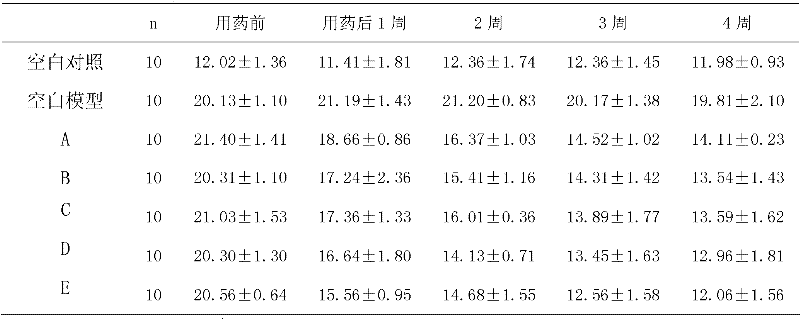
Patents
Literature
297 results about "Hydrochlorothiazide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat high blood pressure.
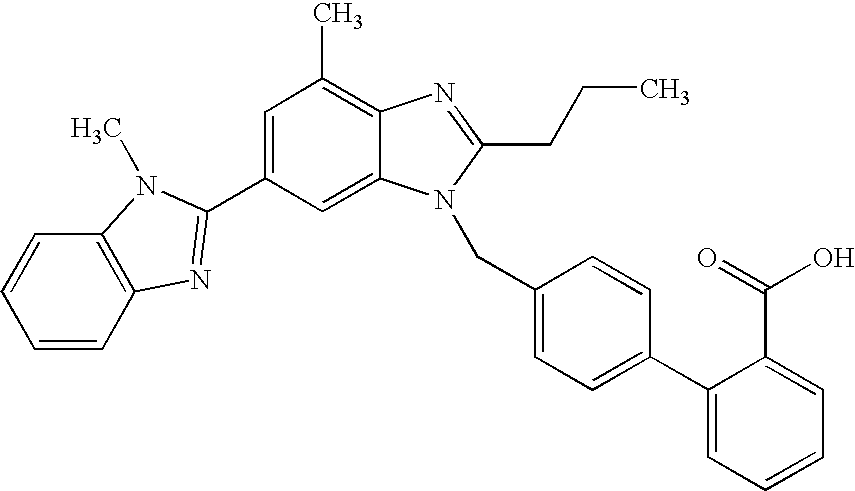
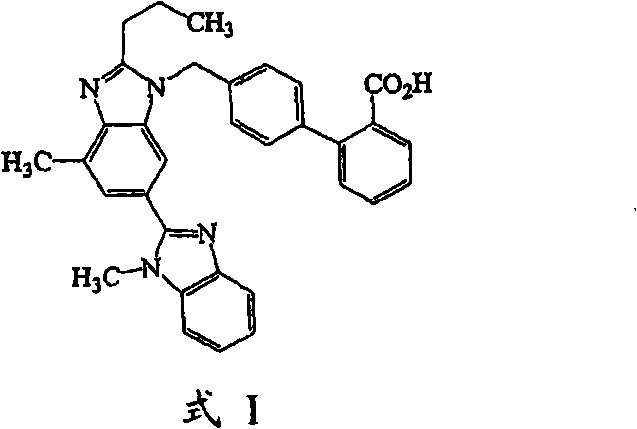
Pharmaceutical co-crystal compositions of drugs such as carbamazepine, celecoxib, olanzapine, itraconazole, topiramate, modafinil, 5-fluorouracil, hydrochlorothiazide, acetaminophen, aspirin, flurbiprofen, phenytoin and ibuprofen
A pharmaceutical composition comprising a co-crystal of an API and a co-crystal former; wherein the API has at least one functional group selected from ether, thioether, alcohol, thiol, aldehyde, ketone, thioketone, nitrate ester, phosphate ester, thiophosphate ester, ester, thioester, sulfate ester, carboxylic acid, phosphinic acid, phosphonic acid, sulfonic acid, amide, primary amine, secondary amine, ammonia, tertiary amine, imine, thiocyanate, cyanamide, oxime, nitrile diazo, organohalide, nitro, S-heterocyclic ring, thiophene, N-heterocyclic ring, pyrrole, 0-heterocyclic ring, furan, epoxide, peroxide, hydroxamic acid, imidazole, pyridine and the co-crystal former has at least one functional group selected from amine, amide, pyridine, imidazole, indole, pyrrolidine, carbonyl, carboxyl, hydroxyl, phenol, sulfone, sulfonyl, mercapto and methyl thio, such that the API and co-crystal former are capable of co-crystallizing from a solution phase under crystallization conditions.
Owner:UNIV OF SOUTH FLORIDA +3
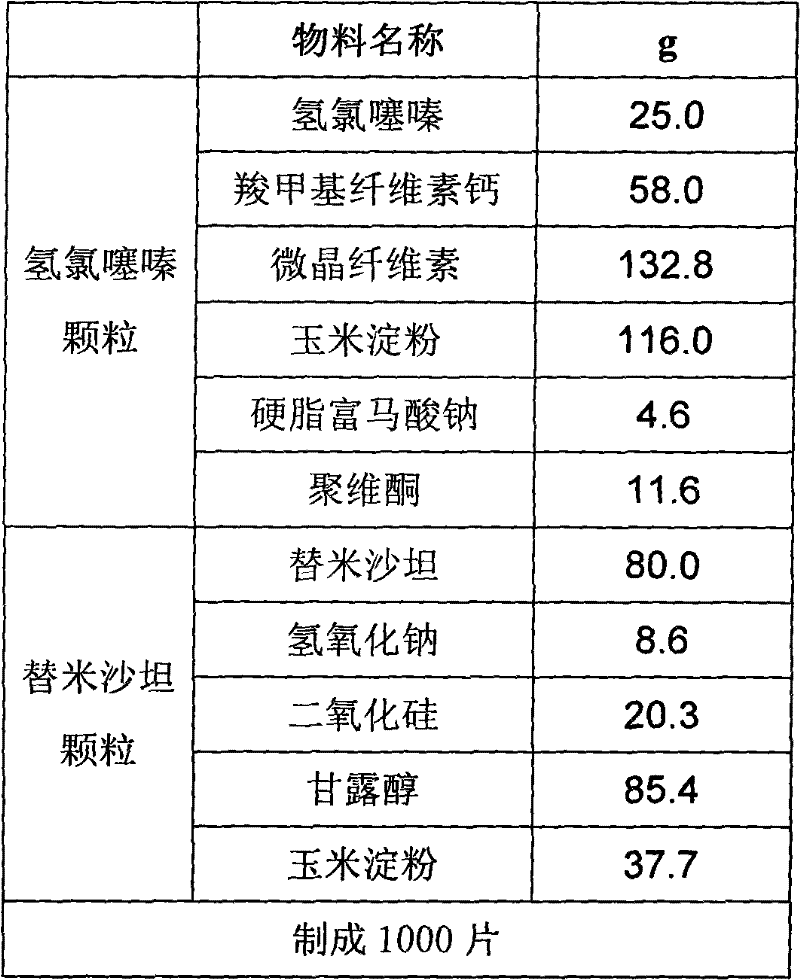
Bilayer pharmaceutical tablet comprising telmisartan and a diuretic and preparation thereof
InactiveUS20050089575A1Low dissolution rateOvercome problemsMaterial analysis by electric/magnetic meansPharmaceutical non-active ingredientsHydrochlorothiazideImmediate release
The present invention relates to a bilayer pharmaceutical tablet comprising a first layer formulated for immediate release of the angiotensin II receptor antagonist telmisartan from a dissolving tablet matrix which contains telmisartan in substantially amorphous form, and a second layer formulated for immediate release of a diuretic like hydrochlorothiazide from a fast disintegrating tablet matrix. A method of producing the bilayer tablet is also disclosed.
Owner:BOEHRINGER INGELHEIM PHARM KG
Telmisartan and hydrochlorothiazide combination therapy
InactiveUS20060159747A1Increased riskGood blood pressureBiocidePill deliveryHydrochlorothiazideCombination therapy
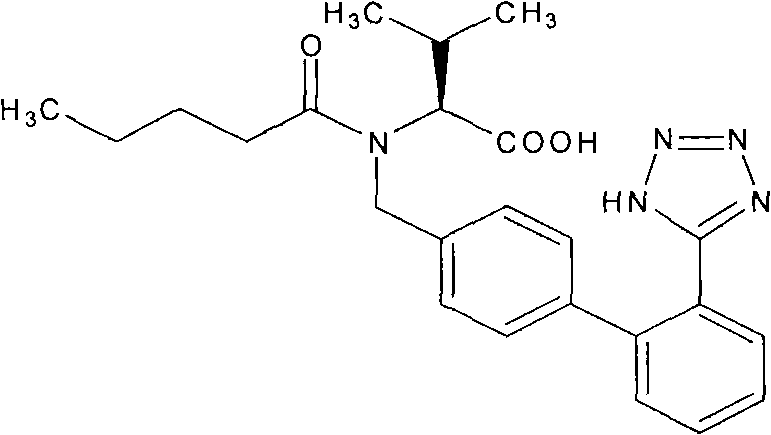
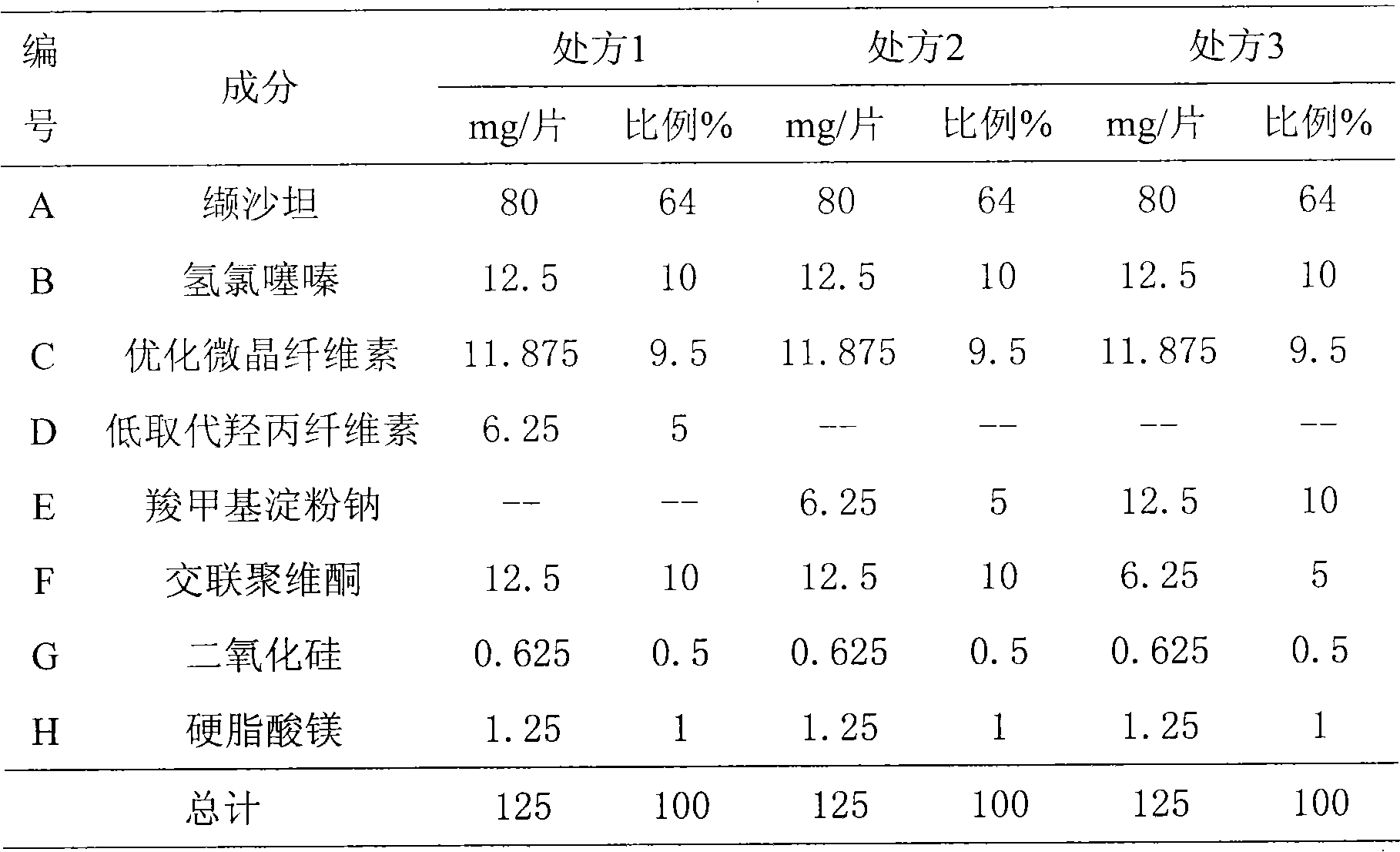
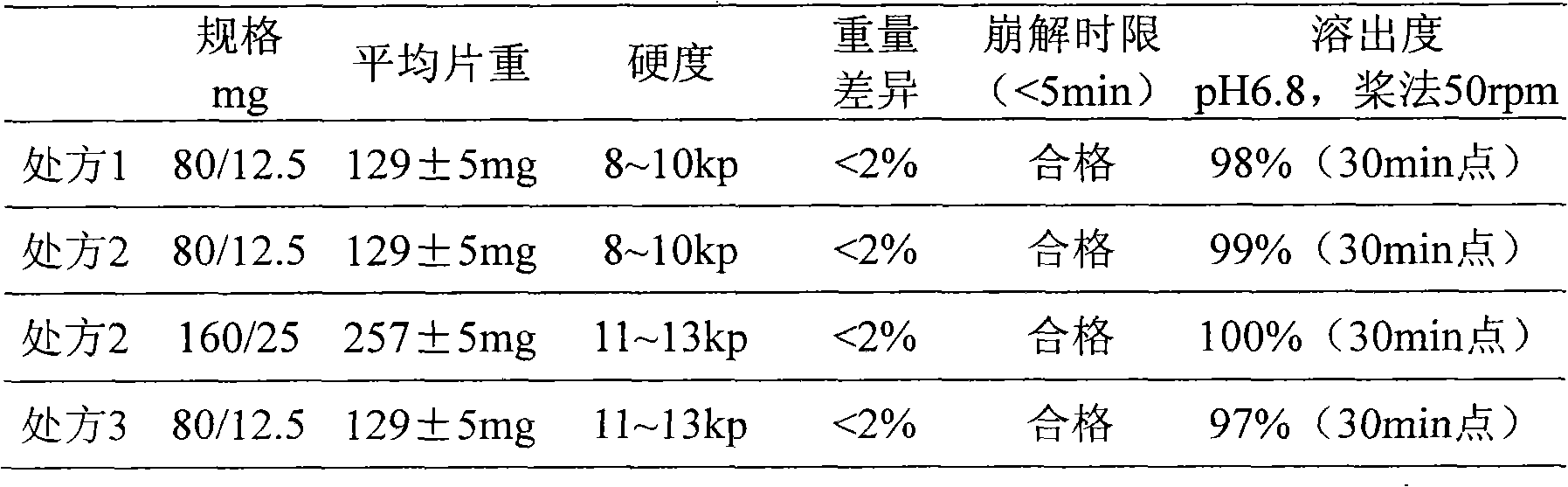
A pharmaceutical composition comprising about 80 mg of telmisartan or a salt thereof and about 25 mg of hydrochlorothiazide or about 160 mg of telmisartan or a salt thereof and about 50 mg of hydrochlorothiazide, and methods of treating hypertension in patients with such combination.
Owner:BOEHRINGER INGELHEIM INT GMBH
Solid Pharmaceutical Dosage Form
InactiveUS20110028456A1High drug loadingEasy to manufacturePowder deliveryBiocideValsartanTrenbolone
A pharmaceutical composition comprising a solid unit dosage form comprising: one or more of pharmaceutically active ingredients selected from valacyclovir, olanzapine, voriconazole, topotecan, artesunate, amodiaquine, guggulosterone, ramipril, telmisartan, tibolone, atorvastatin, simvastatin, amlodipine, ezetimibe, fenofibrate, tacrolimus, valgancyclovir, valsartan, clopidrogel, estradiol, trenbolone, efavirenz, metformin, pseudoephedrine, verapamil, felodipine, valproic acid / sodium valproate, mesalamine, hydrochlorothiazide, levosulpiride, nelfinavir, cefixime and cefpodoxime proxetil in combination with a water insoluble polymer and / or a water soluble polymer. Methods for making the pharmaceutical composition are also disclosed.
Owner:CIPLA LTD
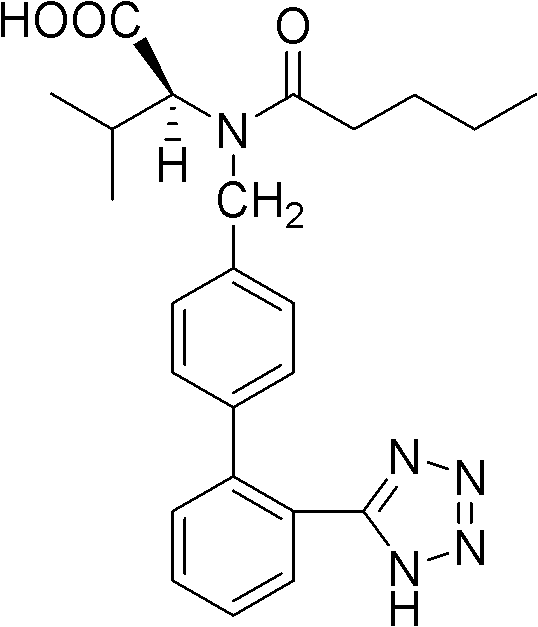
Crystalline form of hydrochlorothiazide and application thereof
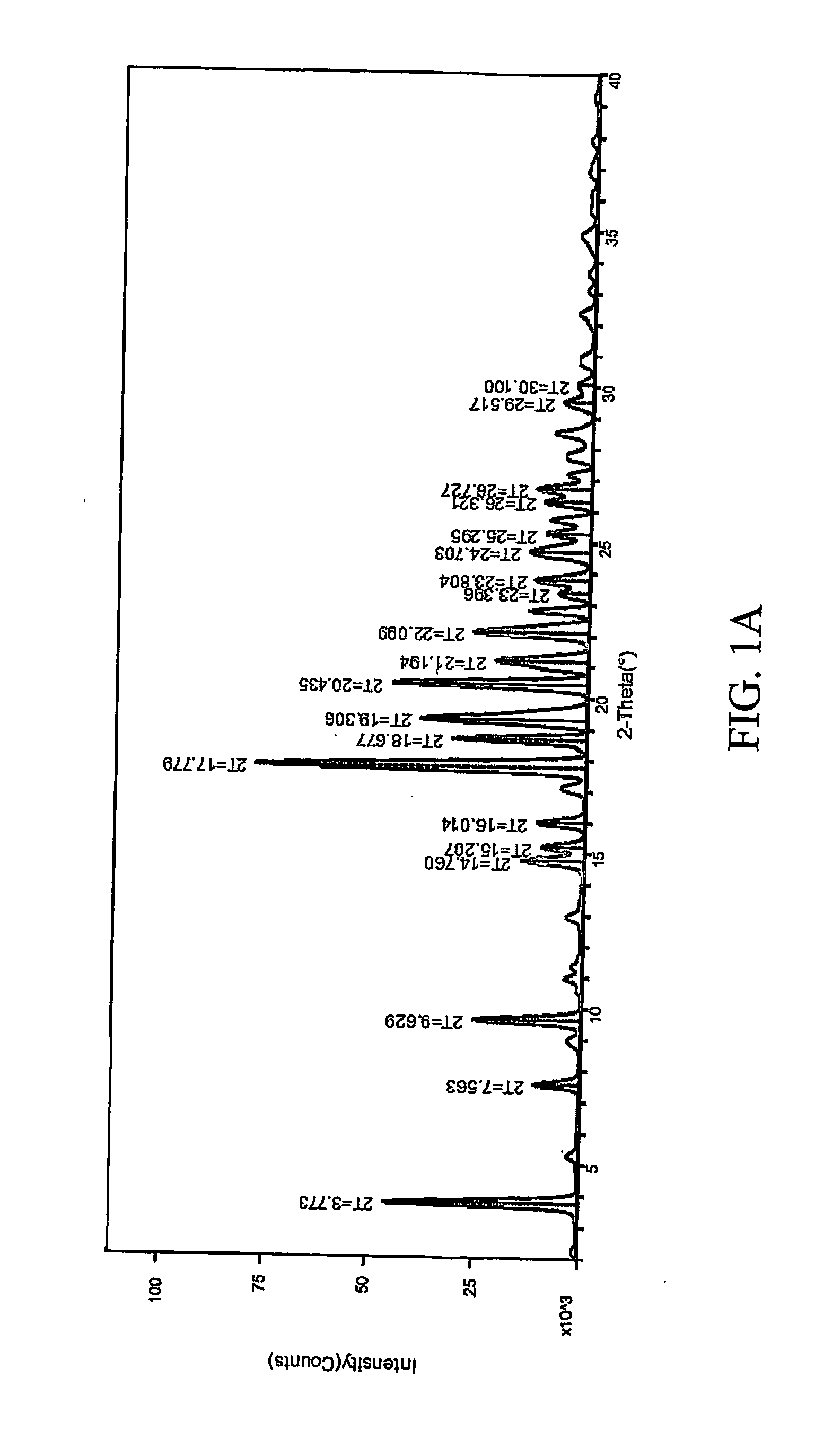
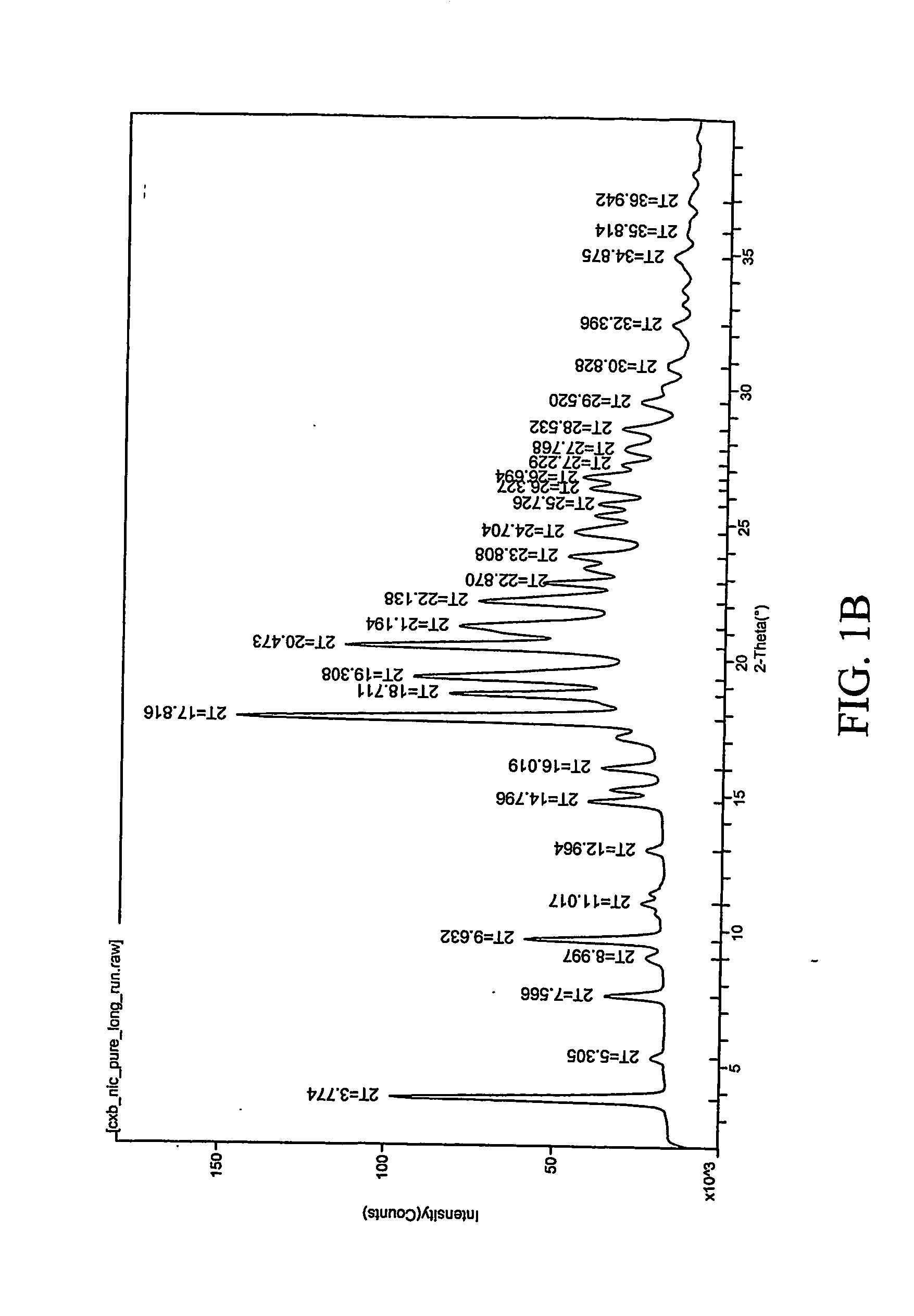
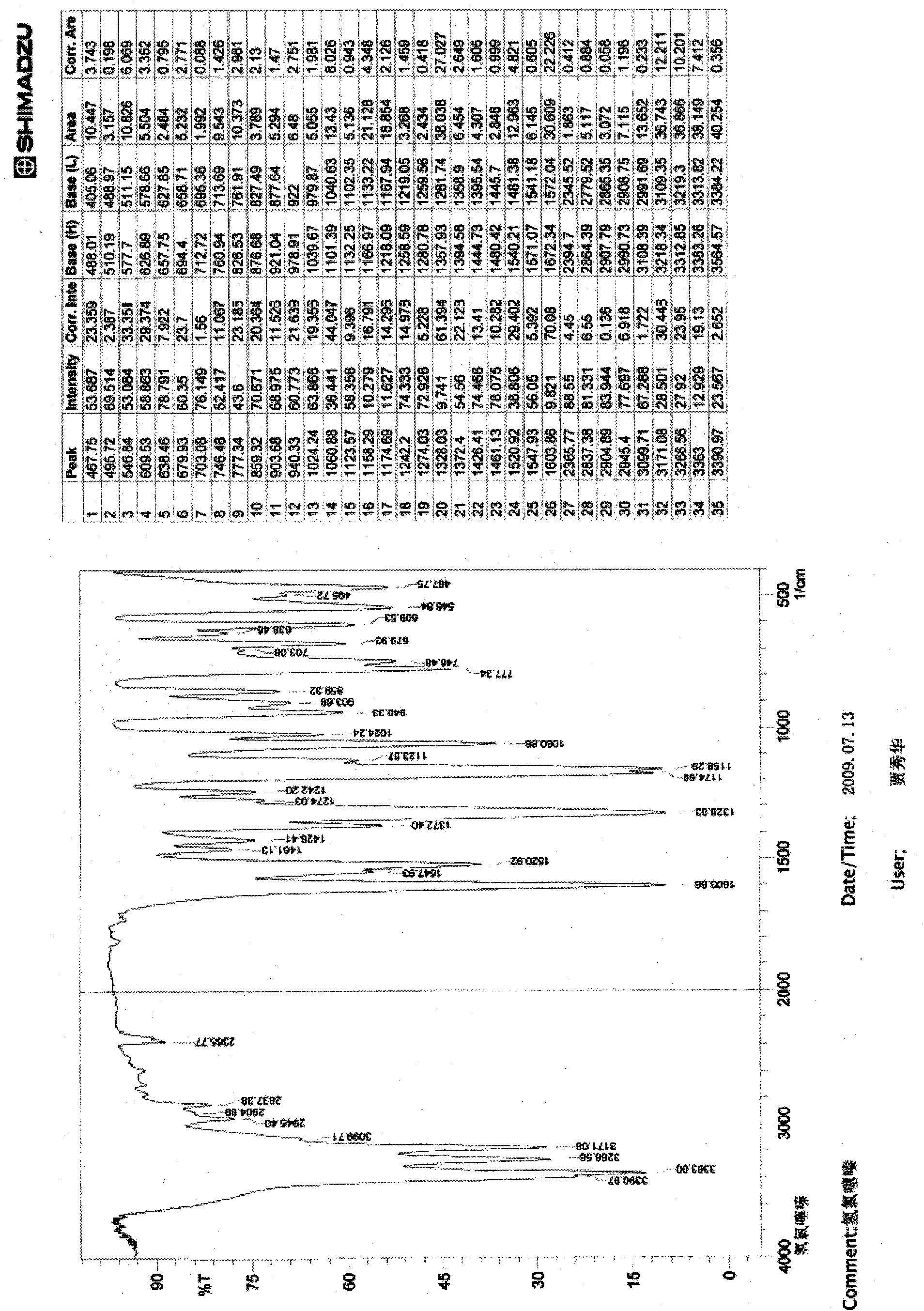
InactiveCN101659643AGood dissolution effectSolve the problems of slow dissolution rate and low bioavailabilityOrganic chemistryDipeptide ingredientsHydrochlorothiazideChemistry
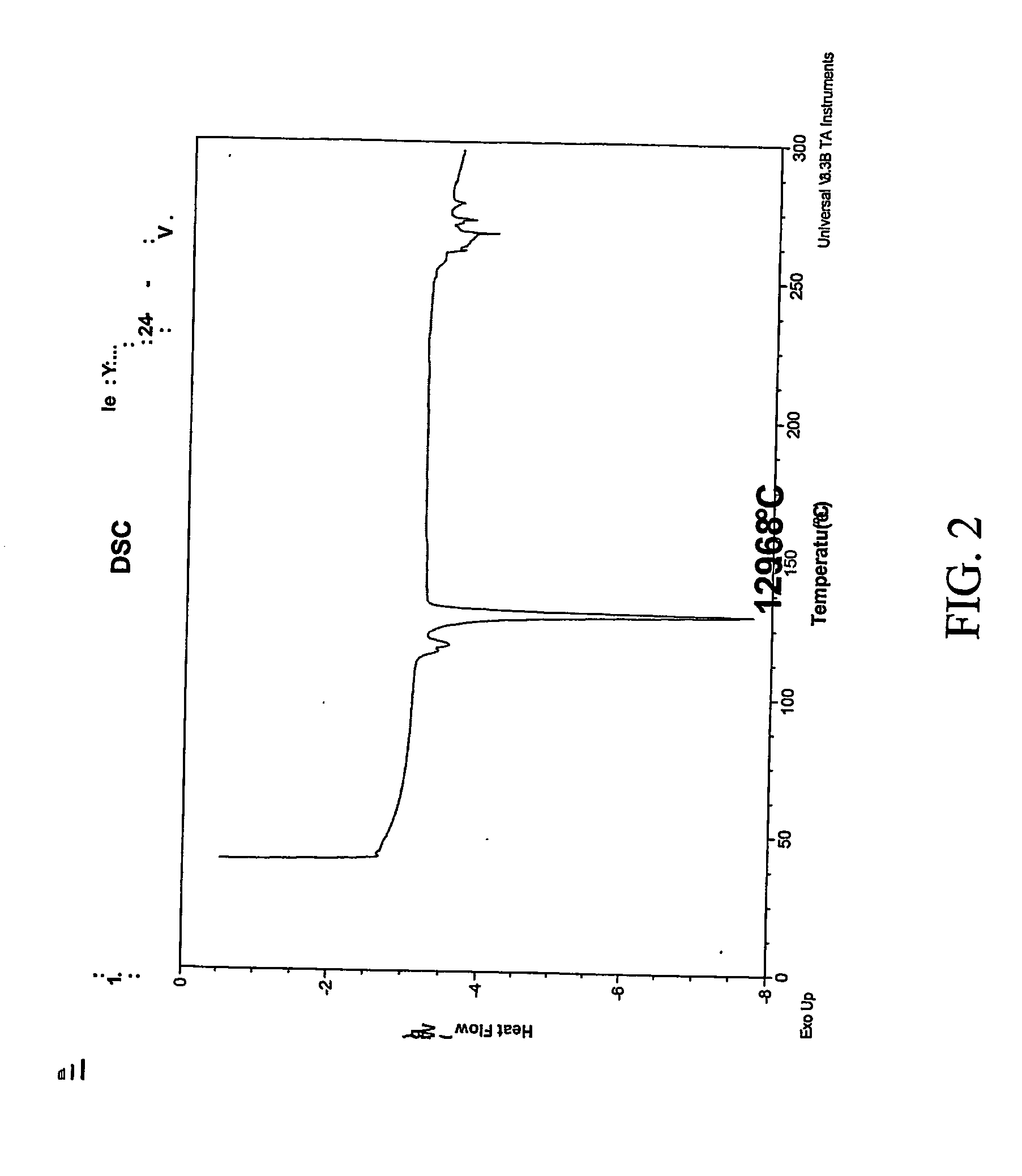
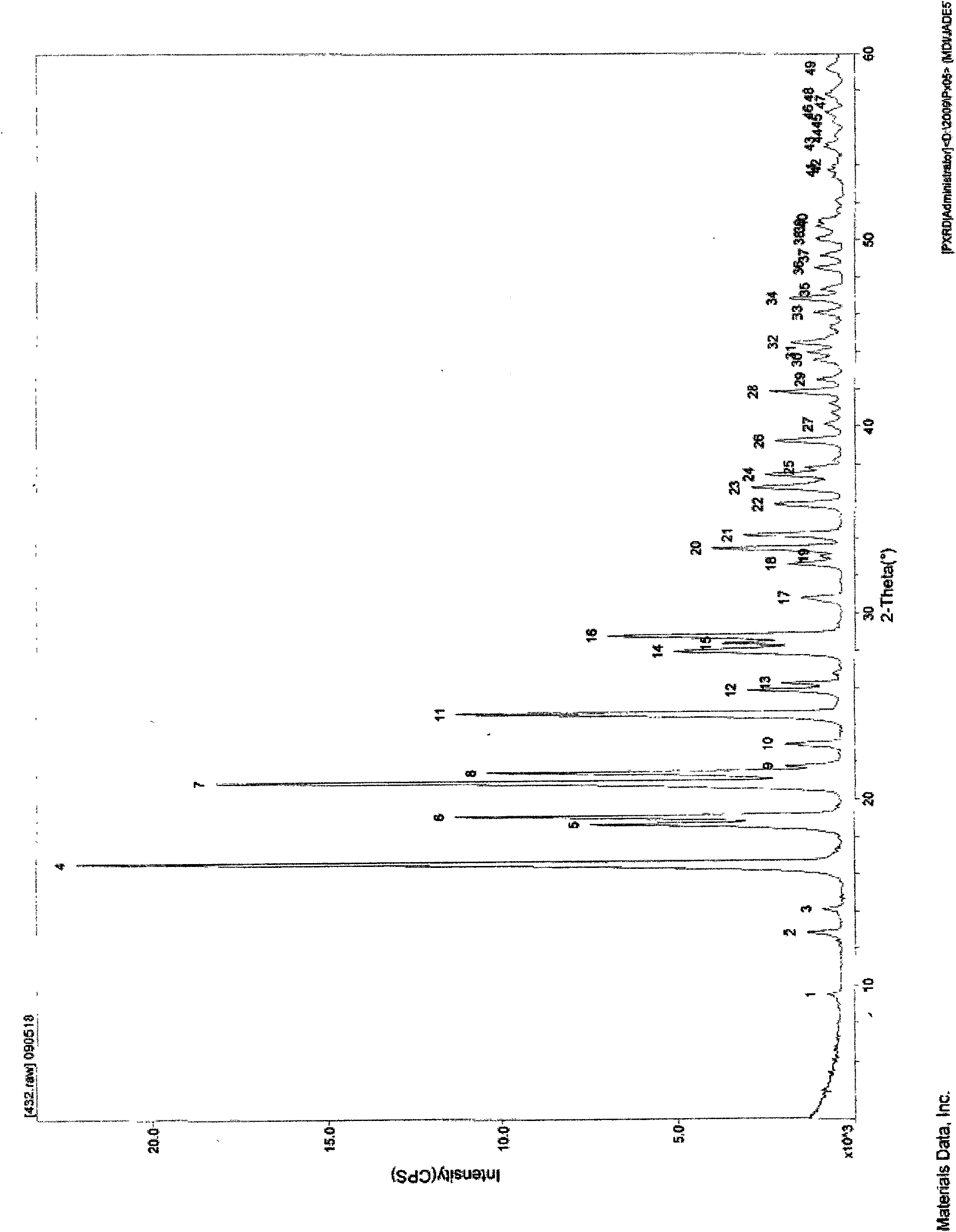
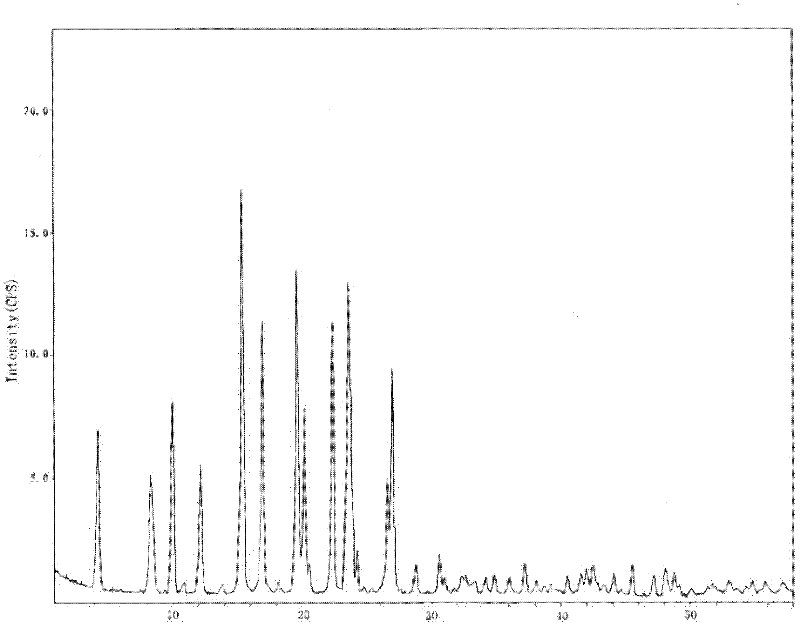
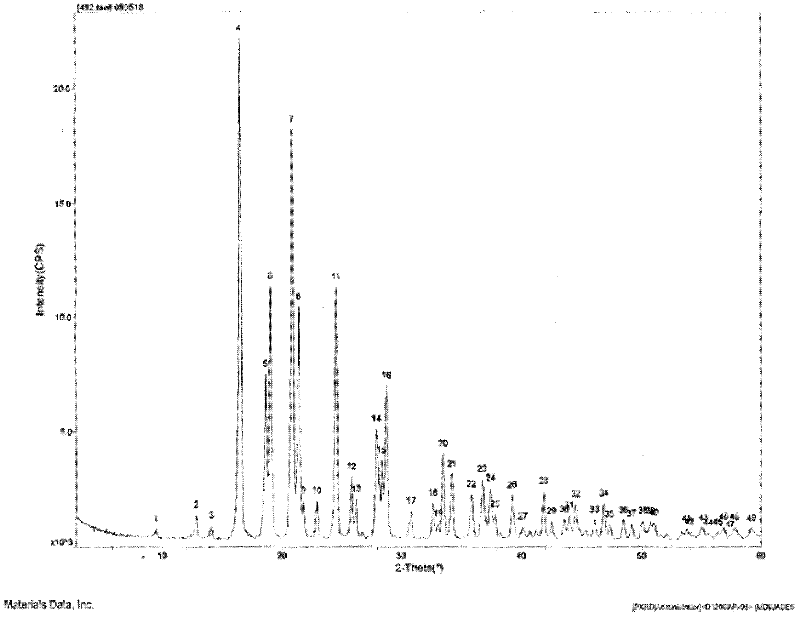
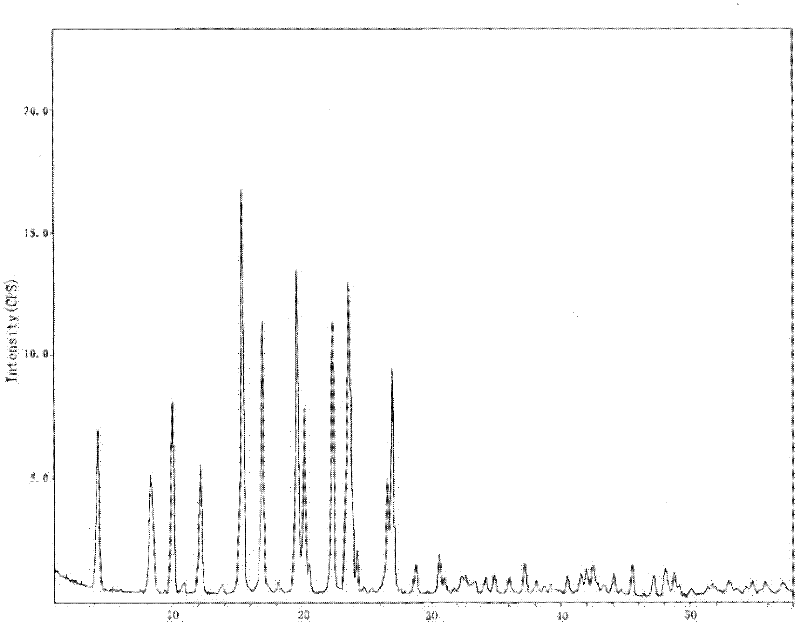
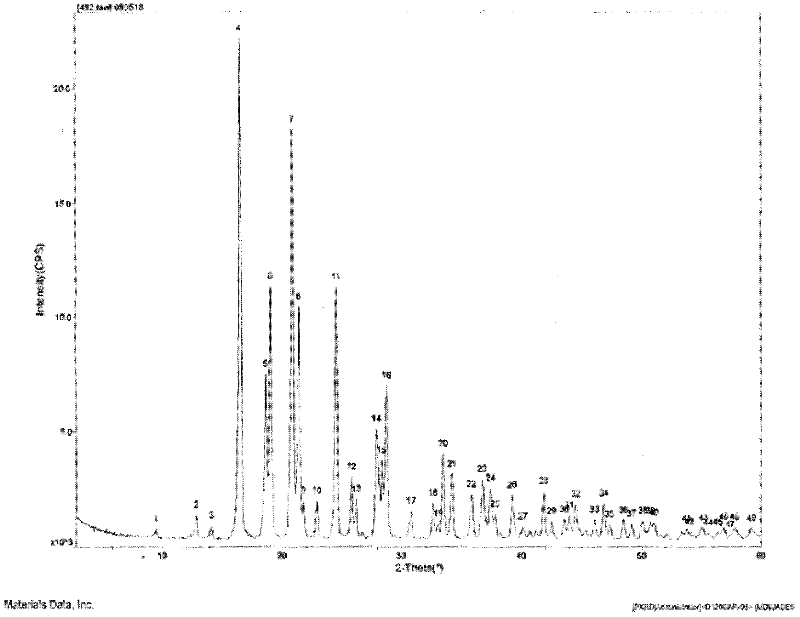
The invention relates to a crystalline form of hydrochlorothiazide and an application thereof; the crystalline form of hydrochlorothiazide has the following diffraction angles of powder X diffractionapproximately: 16.581 degrees, 18.641 degrees, 19.060 degrees, 20.879 degrees, 21.401 degrees, 24.598 degrees, 25.880 degrees, 26.279 degrees, 27.981 degrees, 28.402 degrees, 28.819 degrees, 33.480 degrees, 34.199 degrees and 41.879 degrees; the crystalline form of hydrochlorothiazide has the infrared peaks: 3390.97 cm <-1>, 3363.00cm <-1>, 3266.56 cm <-1>, 3171.08 cm <-1>, 1603.86cm <-1>, 1520.92cm <-1>, 1372.40 cm <-1>, 1328.03 cm <-1>, 1174.69 cm <-1>, 1158.29 cm <-1>, 1060.88 cm <-1>, 777.34 cm <-1> and 546.84 cm <-1>; and the crystalline form of hydrochlorothiazide has the melting pointof 263-266 DEG C.
Owner:CHINA RESOURCES SAIKE PHARMA
Pharmaceutical formulations comprising telmisartan and hydrochlorothiazide
InactiveUS20100247649A1Lower blood pressureReduce liquid volumePowder deliverySolution deliveryHydrochlorothiazideImmediate release
Pharmaceutical tablets comprising a first layer formulated for immediate release of telmisartan from a dissolving matrix and a second layer formulated for immediate release of hydrochlorothiazide from a dissolving matrix, methods for producing tablets and methods of use for treating hypertension.
Owner:DR REDDYS LAB LTD
Compound dispersible tablet for treating hypertension
The invention relates to a medicinal oral preparation containing Valsartan and Hydrochlorothiazide, more specifically the dispersible tablets for enhancing the beneficial effects of Valsartan and Hydrochlorothiazide.
Owner:江苏万高药业股份有限公司
Pharmaceutical composition used for lowering blood pressure
InactiveCN102225203AExplain the curative effect advantageOrganic active ingredientsDipeptide ingredientsAngiotensin-converting enzymeHydrochlorothiazide
The invention relates to a pharmaceutical composition used for lowering blood pressure. The pharmaceutical composition with azilsartan medoxomil and another one or two blood pressure lowering substances as active ingredients mainly comprises calcium ion antisticking agent, angiotensin converting enzyme inhibitor (ACEI) and hydrochlorothiazide. The composition provided by the invention can be prepared into an oral preparation and is used for treating vascular hypertension.
Owner:FUKANGREN BIO PHARMA
Liposome solid preparation of losartan potassium hydrochlorothiazide pharmaceutical composition
InactiveCN101797230AMitigate counter-regulationGood blood pressure effectOrganic active ingredientsPharmaceutical non-active ingredientsYolkSide effect
The invention discloses a liposome solid preparation of a losartan potassium hydrochlorothiazide pharmaceutical composition and a preparation method thereof. In the invention, active components of losartan potassium and hydrochlorothiazide and specific combined hydrogenated yolk lecithin, cholesterol and poloxamer 188 are prepared into a liposome which is then mixed with other pharmaceutical accessories to prepare the solid preparation, thereby greatly improving the pharmaceutical stability and bioavailability and having stable and lasting effect, small side effect and obvious curative effect.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Compound solid preparation of valsartan and hydrochlorothiazide, and preparation method thereof
ActiveCN102247376AEasy to operateLow equipment requirementsDrageesHeterocyclic compound active ingredientsHydrochlorothiazideLubricant
The invention belongs to the field of pharmaceutical preparation, and relates to a compound solid preparation of valsartan or pharmaceutically acceptable salts thereof and hydrochlorothiazide, and a preparation method thereof. The pharmaceutical composition provided by the invention is processed from the following raw and auxiliary materials in percentage by weight: 25-35% of valsartan or pharmaceutically acceptable salts thereof, 2-20% of hydrochlorothiazide, 25-71.4% of microcrystalline cellulose, 1-5.3% of partially pregelatinized starch, 0-20% of disintegrant, 0.5-5% of flow aid and 0.1-5% of lubricant, wherein the weight ratio of the microcrystalline cellulose to the partially pregelatinized starch is requested not to be less than 5:1.
Owner:CHINA RESOURCES SAIKE PHARMA
Dispersible tablet for treating hypertension
The invention relates to a medicinal oral preparation containing Irbesartan and Hydrochlorothiazide, more specifically the dispersible tablets for enhancing the beneficial effects of Irbesartan and Hydrochlorothiazide.
Owner:江苏万高药业股份有限公司
Brand-new oral solid medicinal composition and preparation method thereof
ActiveCN102335176AGuaranteed curative effectHigh content of the main drugOrganic chemistryCapsule deliveryCross-linkValsartan
The invention discloses a brand-new oral solid medicinal composition. The medicinal composition is an oral preparation prepared from hydrochlorothiazide, levamlodipine, valsartan and pharmaceutically acceptable auxiliaries, and the oral preparation comprises but is not limited to tablets or capsules. The composition comprises the following raw materials in parts by weight: 5-25 parts of the hydrochlorothiazide, 2.5-5 parts of the levamlodipine, 80-160 parts of the valsartan, 40-120 parts of microcrystalline cellulose, 30-90 parts of compressible starch, 5-25 parts of cross-linked sodium carboxymethylcellulose, 3-8 parts of silicon dioxide and 1-2 parts of stearic acid. The medicinal composition disclosed by the invention has the advantages of scientific and reasonable prescription, low auxiliary content and high bioavailability, and is a first choice of medicine for treating hypertension.
Owner:HAINAN JINRUI PHARMA
Compound tablet containing telmisartan and hydrochlorothiazide
ActiveCN102512423AEvenly mixedAvoid delamination during tablet compressionOrganic active ingredientsPharmaceutical delivery mechanismHydrochlorothiazideMedicine
The invention discloses a compound tablet containing telmisartan and hydrochlorothiazide. The tablet is prepared by uniformly mixing telmisartan particles and hydrochlorothiazide particles, and tabletting the mixture. According to the weight of the hydrochlorothiazide particles, the hydrochlorothiazide particles contain 6 to 50 percent of disintegrant. The compound tablet is a single-layer tablet. The telmisartan / hydrochlorothiazide tablet is quickly dissolved and is stable in quality. The invention also provides a preparation method for the compound tablet, which is simple in process, low in cost and suitable for industrial mass production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
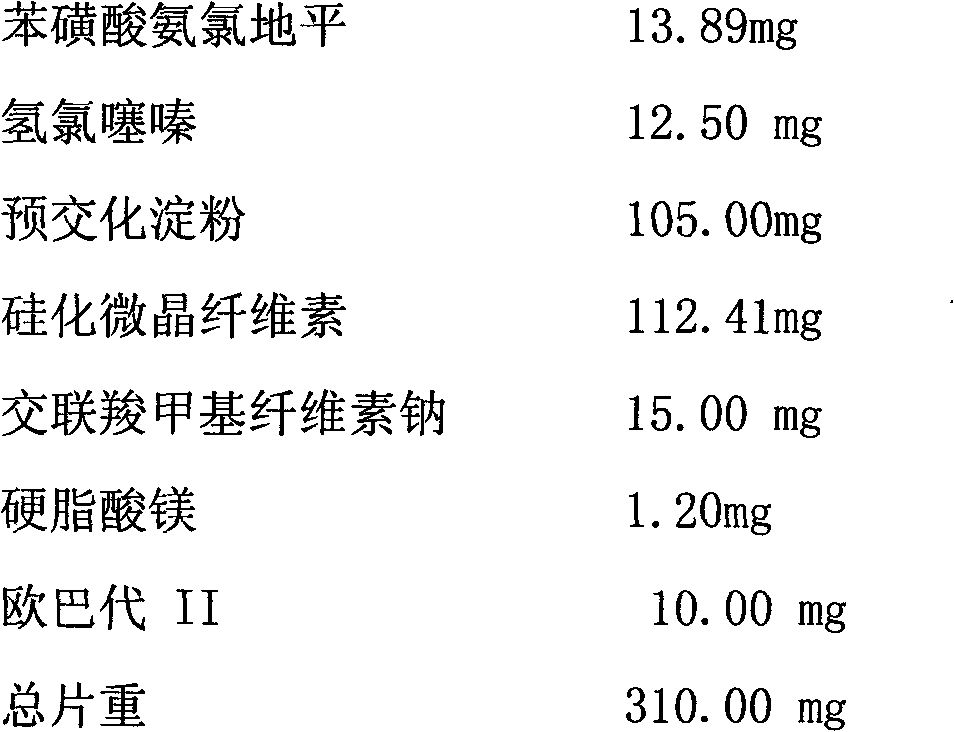
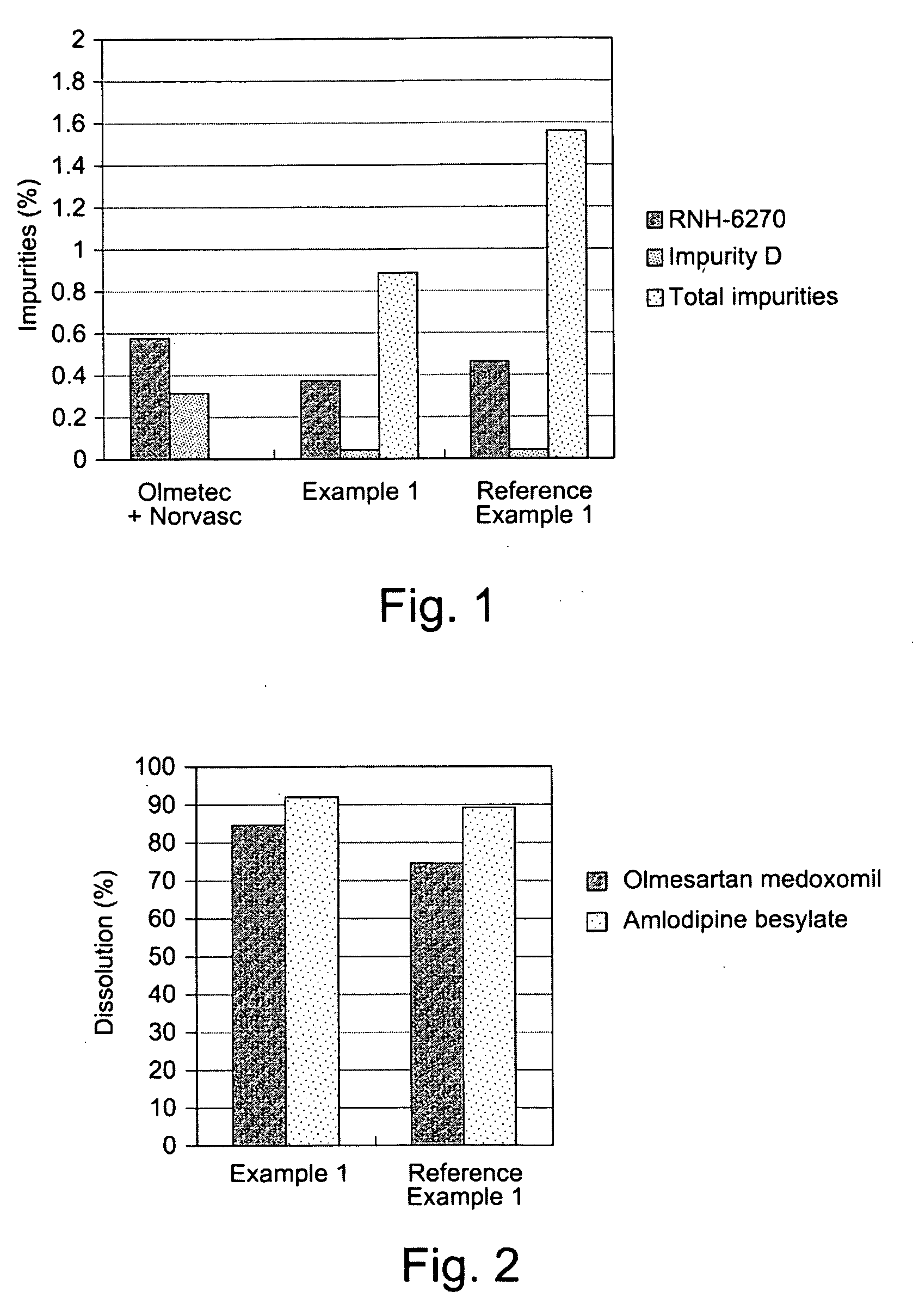
Medicament compound preparation formed by mixing olmesartan medoxomil with benzene sulfonic acid amlodipine and hydrochlorothiazide
The invention relates to a medicament compound preparation formed by mixing olmesartan medoxomil with benzene sulfonic acid amlodipine and hydrochlorothiazide. Both the olmesartan medoxomil and amlodipine are unstable compounds, and are difficultly prepared into mixed medicaments with stable active ingredients. According to the invention, the adjuvant of the olmesartan medoxomil and amlodipine compound preparation is regulated, so that the amounts of degradation products and impurities of the olmesartan medoxomil are effectively reduced. The invention provides a method for preparing the fixed-dose compound preparation which takes the olmesartan medoxomil, the amlodipine and hydrochlorothiazide as the active ingredients.
Owner:ZHUHAI EBANG PHARMA
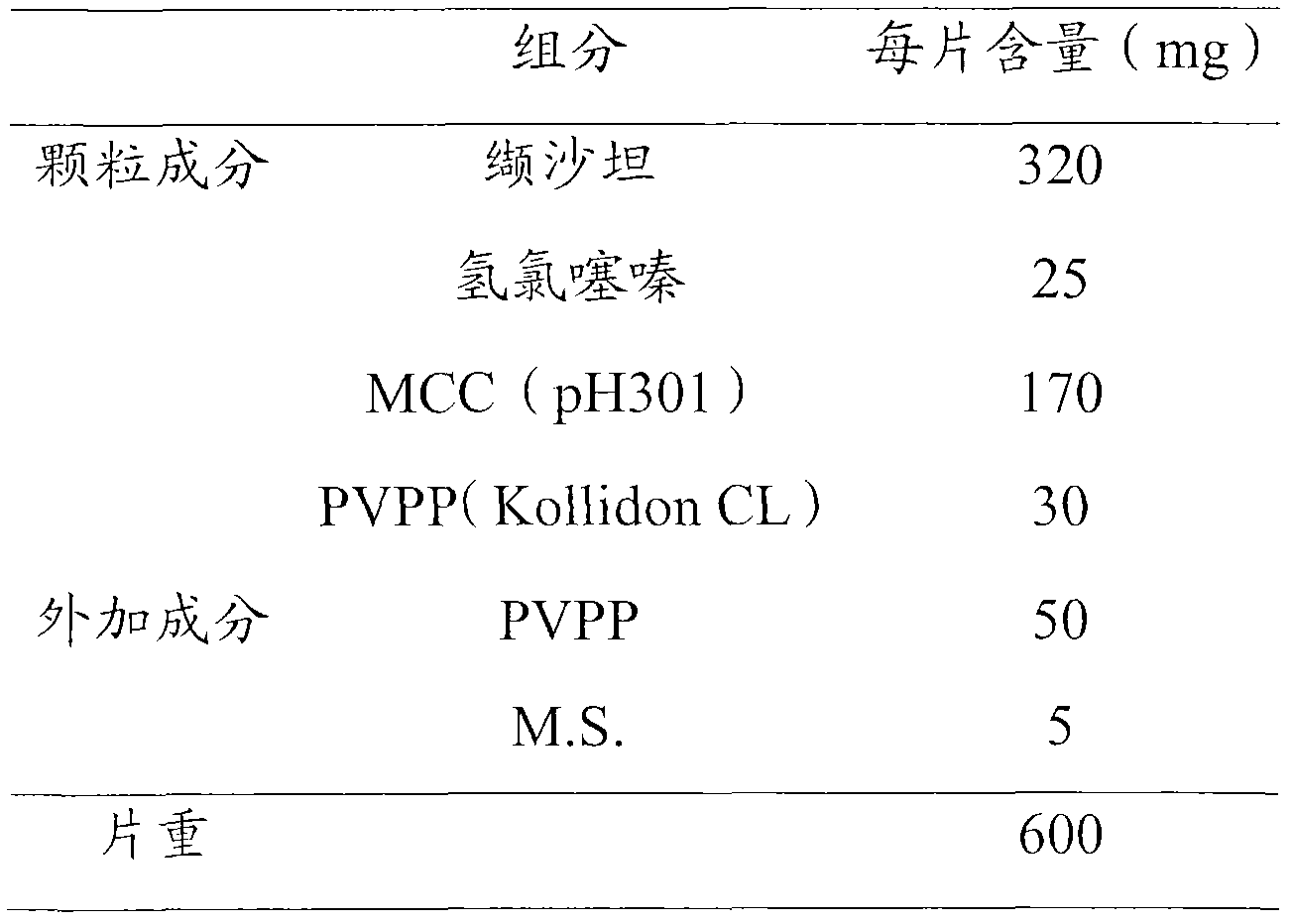
Valsartan and hydrochlorothiazide composition, and its preparation method
The invention relates to a valsartan and hydrochlorothiazide composition, and its preparation method. According to the invention, valsartan and hydrochlorothiazide are taken as active components, an auxiliary material comprises a disintegrating agent, a filling agent and a lubricant, the component composition is simple, less amount of disintegrating agent is capable of acquiring satisfied disintegrating time limit and performing good dissolution effect; an employed wet granulating process enables wide industrialized production.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
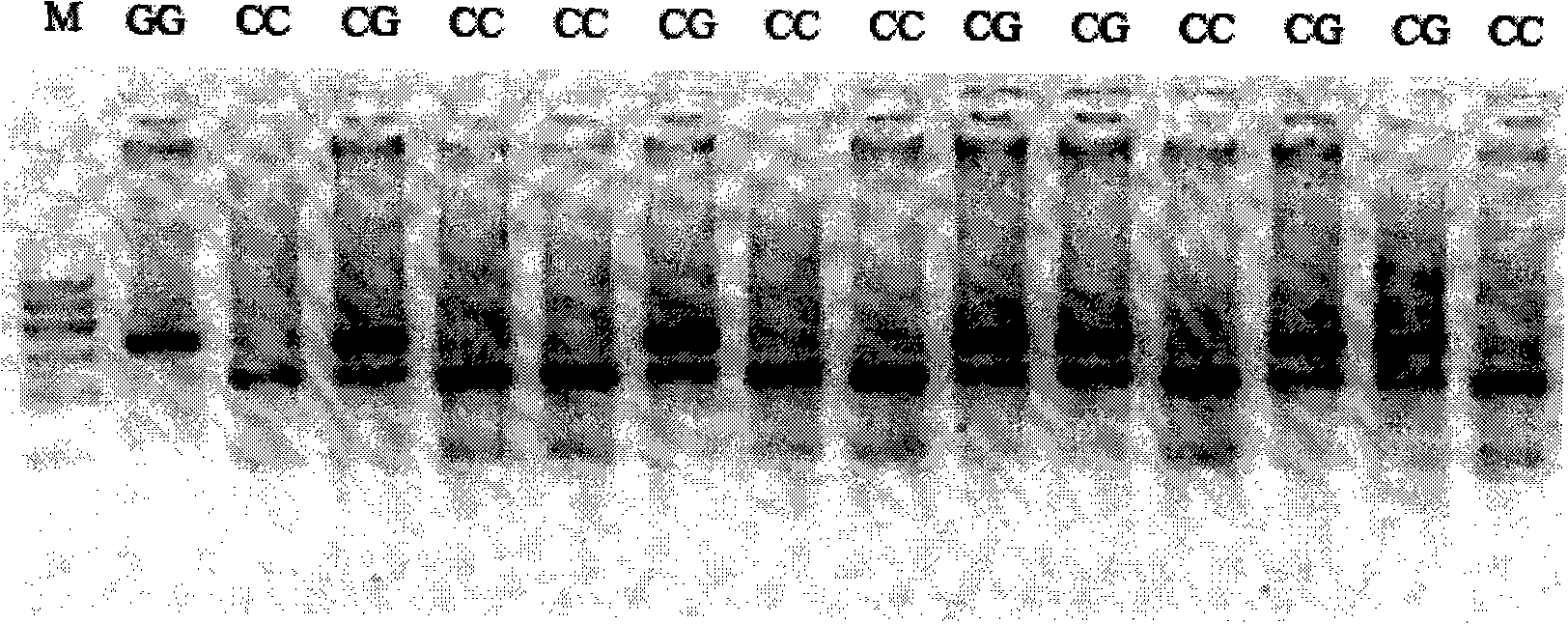
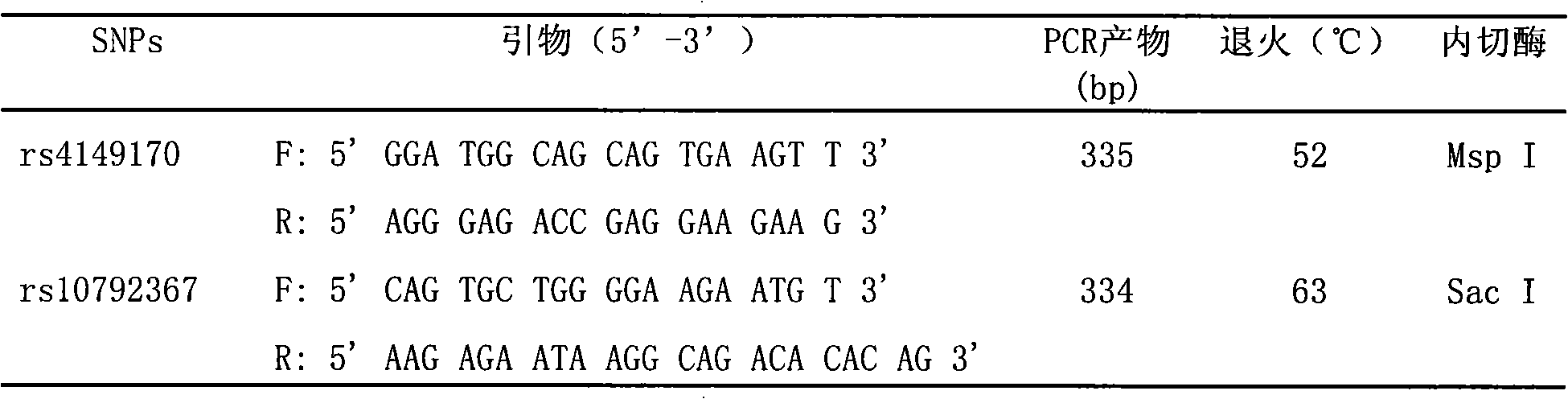
Method and reagent box for predicting dihydrochlorothiazide antihypertensive efficacy
InactiveCN101328502APredictableImprove representationMicrobiological testing/measurementDNA/RNA fragmentationHydrochlorothiazideCurative effect
The invention discloses a method for predicting the blood pressure reducing effect of hydrochlorothiazide and a kit, belonging to the medical molecular biology field. The method for predicting the blood pressure reducing effect of hydrochlorothiazide is realized by measuring and determining SLC22A6 gene polymorphism site. The method has the advantage of providing scientific basis for the individual therapy of the patient with high blood pressure disease in the real sense.
Owner:FUWAI HOSPITAL OF CARDIOVASCULAR DESEASE CHINESE ACAD OF MEDICAL SCI
Preparation method of compound preparation for treating high blood pressure
InactiveCN103479643AReasonable prescriptionSimple processCapsule deliveryHeterocyclic compound active ingredientsValsartanAdjuvant
The invention discloses a preparation method of a compound preparation for treating high blood pressure. The compound preparation is prepared by making valsartan and / or amlodipine and / or hydrochlorothiazide into nanometer or micrometer powder with an average particle size of 100 nanometers-60 micrometers by means of air flow crushing or grinding. According to an adopted solid dispersion technique, valsartan and / or amlodipine and / or hydrochlorothiazide and at least one pharmaceutic adjuvant are made into a solid dispersion, which is obtained by carrying out spray drying or grinding on the drugs and the adjuvant. The drugs are dispersed in the adjuvant in a nanometer or micrometer state. The prepared valsartan, amlodipine and hydrochlorothiazide micropowder or the solid dispersion material can be individually encapsuled or mixed with a pharmaceutically acceptable adjuvant and then encapsuled, or can be made into particles by a preparation technology and then encapsuled.
Owner:SHENYANG PHARMA UNIVERSITY +2
Medicinal composition capsules of valsartan and hydrochlorothiazide and preparation method for capsules
ActiveCN102670630ARegulate the time of peak plasma concentrationStrong absorption capacityCapsule deliveryGranular deliveryValsartanHydrochlorothiazide
The invention discloses medicinal composition capsules of valsartan and hydrochlorothiazide. The capsules are prepared by mixing instant granules made of the valsartan and slowly dissolved pellets made of the hydrochlorothiazide and filling. The medicinal composition capsules are strong in absorption capability of active ingredients, have a good treatment effect on primary hypertension, and have effects of protecting liver and treating left ventricular hypertrophy caused by hypertension.
Owner:CHONGQING CONQUER PHARML
Medicinal composition and preparation method thereof
InactiveCN102526061AReduce hydrophobicityReduce viscosityPharmaceutical non-active ingredientsCardiovascular disorderValsartanHydrochlorothiazide
The invention discloses a medicinal composition. The medicinal composition consists of the following ingredients in percentage by weight: 1 to 5 percent of amlodipine and pharmaceutically acceptable salt thereof, 20 to 50 percent of valsartan, 1 to 10 percent of hydrochlorothiazide, 20 to 50 percent of filling agent, 8 to 24 percent of disintegrating agent, 0.01 to 0.06 percent of adhesive 1, 0.01 to 0.2 percent of adhesive 2, 0.3 to 2 percent of flow aid and 0.5 to 2.5 percent of lubricating agent. The medicinal composition has the advantages of simple preparation process, low equipment requirement, simplicity in operation and small dust pollution, guarantees the quality of medicines and contributes to industrialized production.
Owner:BEIJING D VENTUREPHARM TECH DEV
Valsartan and hydrochlorothiazide oral solid preparation with high medicament loading capacity
The invention relates to a valsartan and hydrochlorothiazide oral solid preparation with high medicament loading capacity. The oral solid preparation contains valsartan and hydrochlorothiazide which serve as active ingredients and a pharmaceutically acceptable additive which is suitable for preparing a solid oral preparation form by a pressing method. The oral solid preparation is characterized in that: the quantity of the active ingredients of the preparation is 65 to 80 percent of the total weight of the oral solid preparation, preferably 65 to 75 percent. The invention also relates to a preparation method for the oral solid preparation. The preparation method has the characteristics of simple process, low cost, good product stability and the like, improves the production efficiency to a certain extent, and is suitable for industrialized production. By detecting the produced sample according to the quality standards, various indexes accord with the specification.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Prescription of low-dose condensed type Mai jun an tablet or capsule and preparation thereof
InactiveCN101297884ASignificant antihypertensive effectSignificant effectOrganic active ingredientsPill deliverySide effectBULK ACTIVE INGREDIENT
The invention provides maijun'an tablets or capsules for lowering blood pressure which have more significant effect, safety, and no toxicity or side effect, thus achieving the purpose of effectively lowering the blood pressure under the situation of taking smaller dose. The adopted method is to respectively carry out separation and purification of active ingredients for lowering the blood pressure in kudzuvine root and gambir plant, thus obtaining total flavonoids of the kudzuvine root with the purity of over 80 percent and total alkaloids of the gambir plant with the purity of over 50 percent. 8g / 500g of hydrochlorothiazide, 260g / 500g of the total flavonoids of the kudzuvine root, 120g / 50g of total alkaloids of the gambir plant and 112g / 500g of auxiliary agents are added for preparing tablets, pills or capsules. The concentrated maijun'an tablets or capsules of the invention do not only have small administration dose, but also have significant efficacy, controllable quality and easily quantified indicators, thus facilitating the standardized production of the pharmaceutical composition preparation for lowering the blood pressure.
Owner:HUBEI UNIV
Losartan potassium and hydrochrothiazide dropping pills and their preparing methods
InactiveCN1981766ARapid dissolutionHigh dissolution rateOrganic active ingredientsPill deliveryHydrochlorothiazideTreatment hypertension
Owner:陈茜
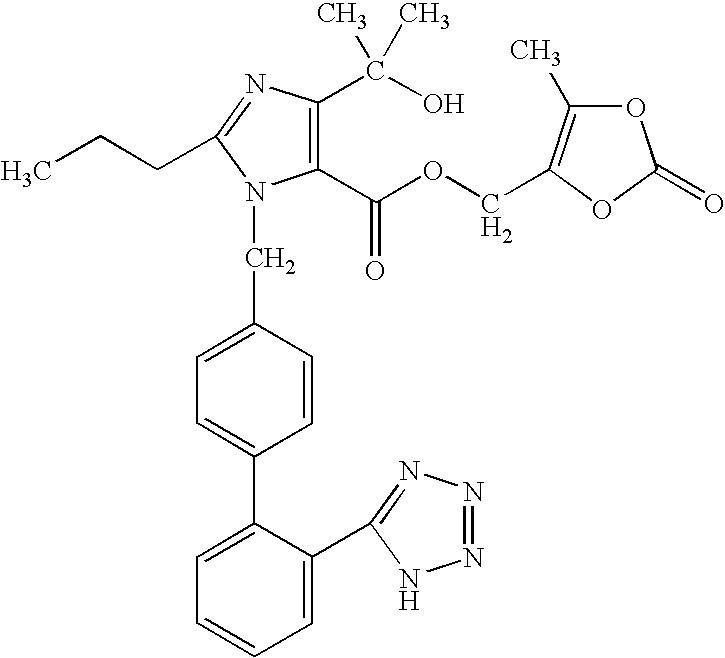
Solid Dosage Form of Olmesartan Medoxomil And Amlodipine
InactiveUS20090175942A1Improve stabilityReduce weightBiocideAnimal repellantsHydrochlorothiazideOlmesartan
The invention relates to a stable solid dosage form comprising olmesartan medoxomil and amlodipine or a pharmacologically acceptable salt thereof. In particular, it relates to solid dosage forms free from reducing sugars. The stable solid dosage form may optionally further comprise hydrochlorothiazide or a pharmacologically acceptable salt thereof.
Owner:DAIICHI SANKYO CO LTD
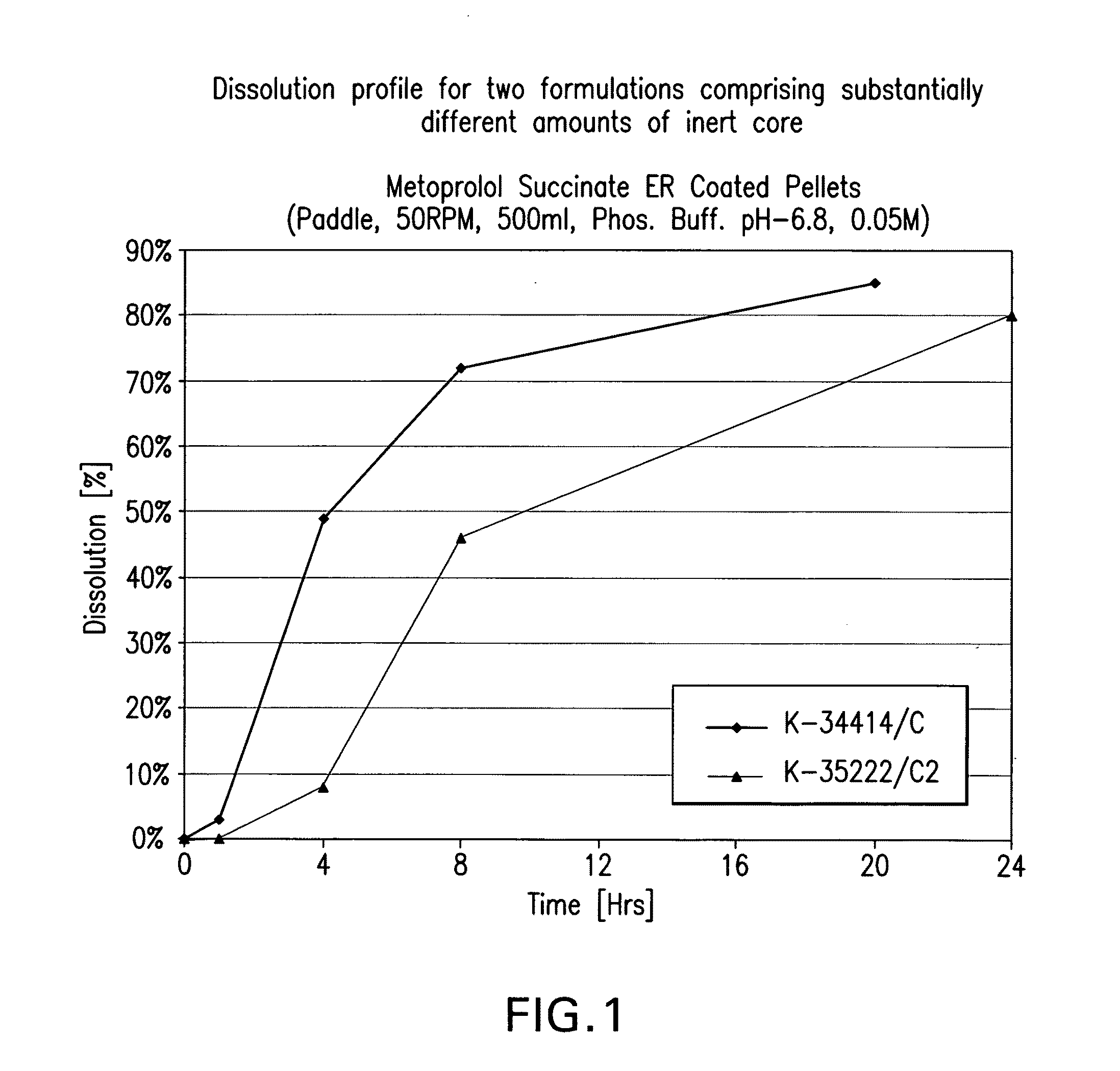
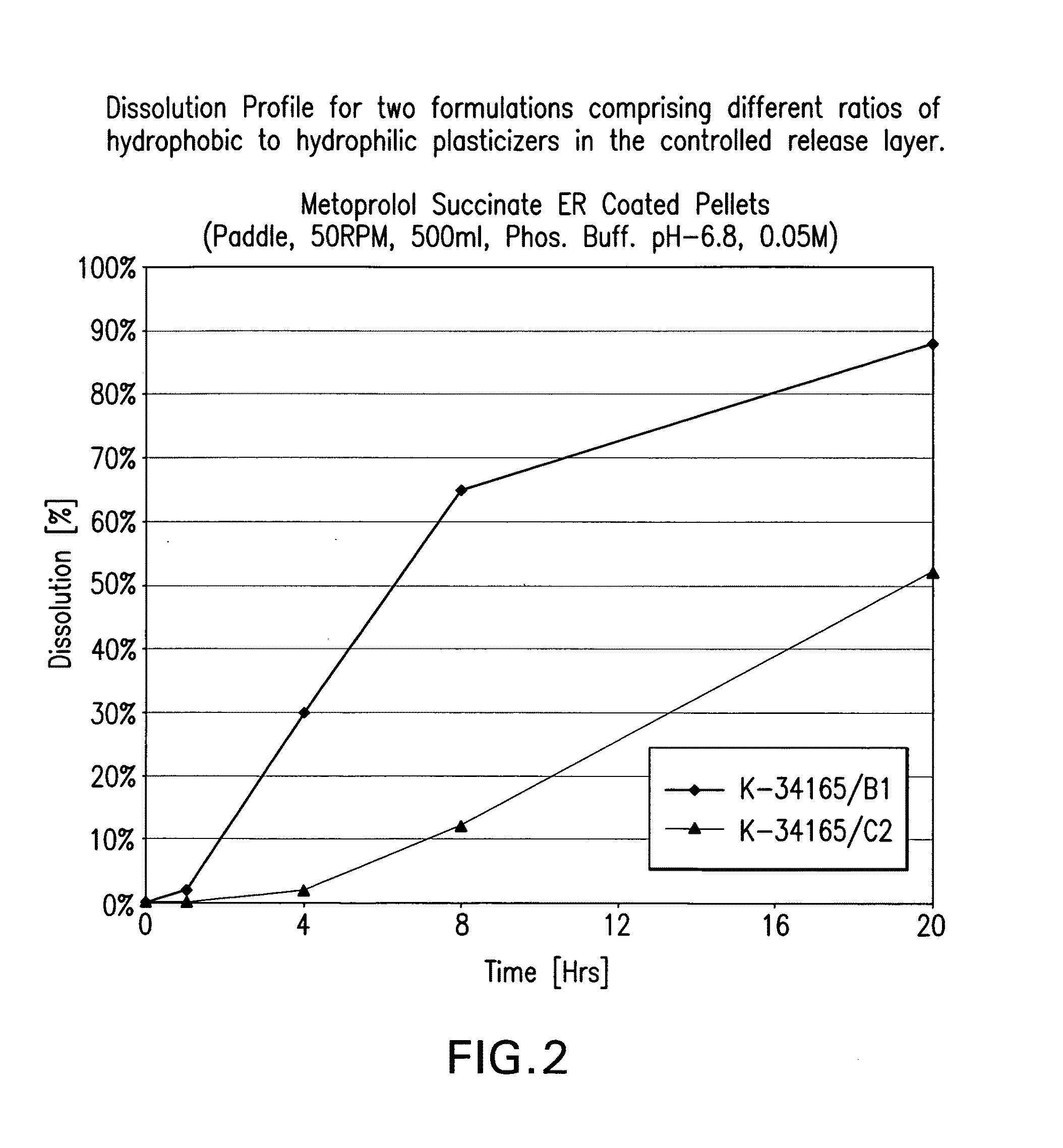
Beta-1-selective adrenoceptor blocking agent compositions and methods for their preparation
InactiveUS20090068260A1Low production costShorten the timeOrganic active ingredientsPretreated surfacesAdrenergicBeta blocker
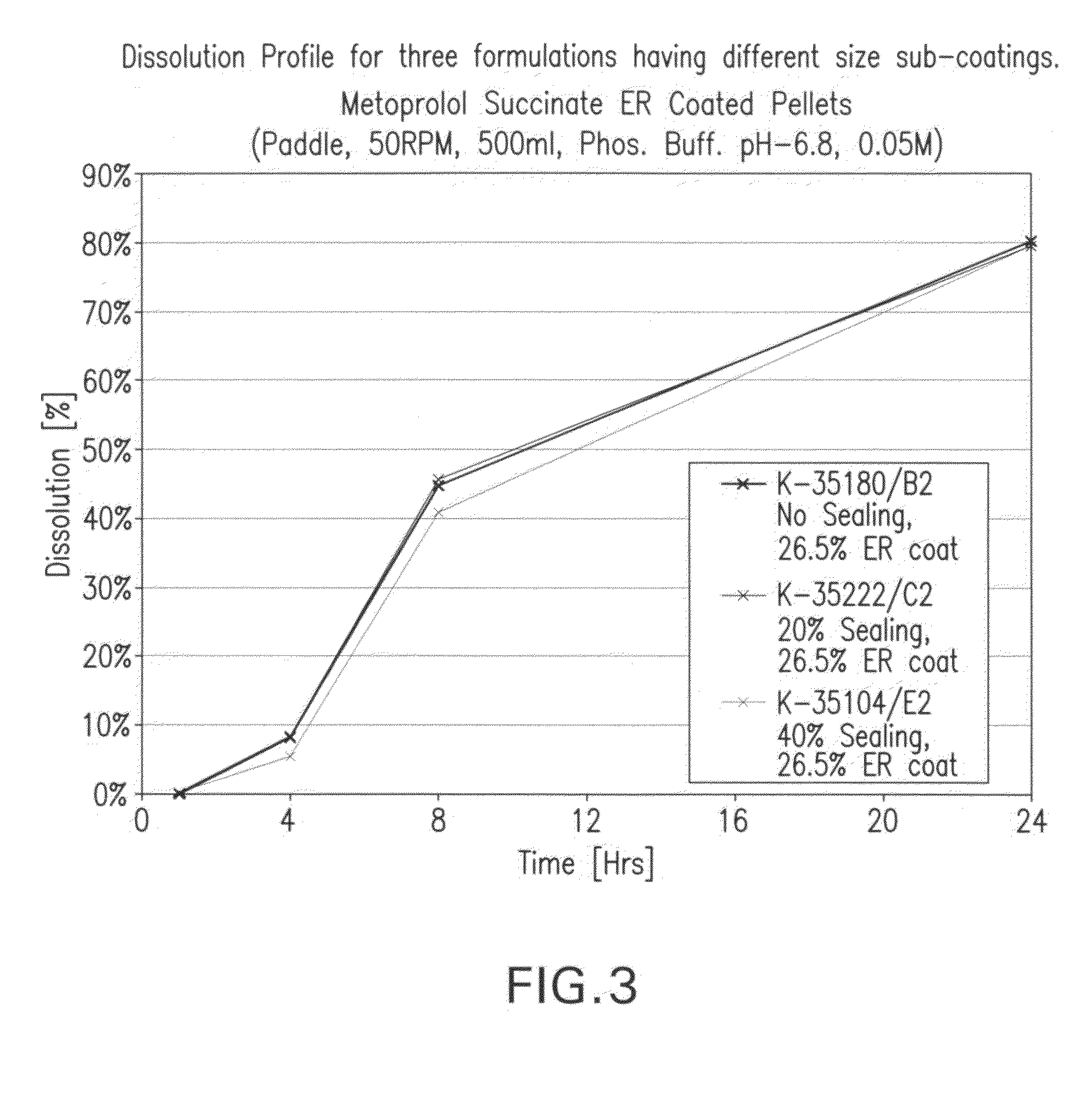
The present invention provides extended release pharmaceutical compositions of a beta blocker such as, but not limited to, metoprolol succinate as the active ingredient, optionally also comprising a diuretic such as but not limited to hydrochlorothiazide, and methods of preparing such extended release pharmaceutical compositions.
Owner:TEVA PHARM USA INC
Double-element preparation containing telmisartan and hydrochlorothiazide
The invention provides a double-element preparation containing telmisartan and hydrochlorothiazide, comprising telmisartan containing pellets and hydrochlorothiazide containing solubilization carriers, wherein the telmisartan pellets comprise blank pellet cores, drug layers containing the active component telmisartan and expanded layers; and the hydrochlorothiazide solubilization carriers comprise solubilization carrier materials which are selected from polyethylene glycol, polyvidone, polyoxyethylene group containing surfactants, water-soluble cellulose derivatives, organic acids, saccharides and alcohols. The double-element preparation containing telmisartan and hydrochlorothiazide can be used for preparing antihypertensive medicaments, can accelerate disintegration of medicaments and promote dissolution and diffusion of active components, thereby improving the bioavailability of the medicaments.
Owner:上海中邦斯瑞生物药业技术有限公司
Pharmaceutical composition for treating cardiovascular diseases and preparation method and use thereof
The invention relates to a pharmaceutical composition containing eprosartan, amlodipine and hydrochlorothiazide and a prepration method and a use thereof, the composition consists of the eprosartan, the amlodipine and the hydrochlorothiazide, and the composition is used for treating patients with moderate hypertension, severe hypertension, coronary heart disease and angina and the patients with the hypertension, the coronary heart disease and the angina, wherein blood pressure of the patients can not be fully controlled after using an angiotensin II receptor antagonist or a calcium antagonist for treating.
Owner:GUANGXI FANGLUE GROUP LONGZHOU PHARMA
Stable formulation comprising a combination of a moisture sensitive drug and a second drug and manufacturing procedure thereof
InactiveUS20080008751A1Process stabilityPill deliveryHeterocyclic compound active ingredientsAngiotensin-converting enzymeHydrochlorothiazide
The present invention provides stable pharmaceutical compositions comprising a combination of active pharmaceutical ingredients. The pharmaceutical composition of the present invention comprises a moisture sensitive drug, in particular an angiotensin converting enzyme (ACE) inhibitor such as Cilazapril, as an active ingredient, a second pharmaceutically active ingredient such as for example Hydrochlorothiazide, and at least one pharmaceutical excipient, wherein the moisture sensitive active pharmaceutical ingredient is wet granulated with a solution of at least one pharmaceutical excipient, and methods for preparing such stable pharmaceutical compositions.
Owner:TEVA PHARM USA INC
Novel blood pressure reducing composition
InactiveCN102247344AExplain the curative effect advantageHeterocyclic compound active ingredientsCardiovascular disorderAngiotensin-converting enzymeHydrochlorothiazide
The invention relates to a novel blood pressure reducing composition. The blood pressure reducing composition is a medicinal composition of which active ingredients are any one or two of aliskiren and a medicinal salt thereof, a hydrate, a calcium channel blocker (CCB), an angiotensin type 1 (AT1) receptor blocker (ARB), an angiotensin-converting enzyme inhibitor (ACEI), hydrochlorothiazide and the like. The composition can be prepared into oral tablets or capsules and is used for treating hypertension, and the curative effect is superior to the blood pressure reducing effect of the independent aliskiren.
Owner:FUKANGREN BIO PHARMA
Candesartan cilexetil/hydrochlorothiazide capsule and preparation method thereof
The invention provides a candesartan / hydrochlorothiazide capsule and a preparation method thereof. The invention has the obvious advantages as follows: on the one hand, the invention can obviously reduce the blood pressure of a hypertensive patient, effectively protect the heart, the brain and the kidney organ and reduce the incidence of complications and has safety, effectiveness, high tolerance,and the like; on the other hand, the invention also has the advantages of less tablet process link, short production period, less quality control points of production process, and the like besides keeping the advantages of accurate dosage, convenient application, stable quality, convenience for carrying, transport and storage, high degree of production mechanization and automatization, large yield, and the like of the prior tablet / dispersible tablet and can simplify the industrial production, thereby being beneficial to the reduction of drug production and management costs and the selection of doctors and patients for cheap and good drugs.
Owner:北京瑞伊人科技发展有限公司 +1
Novel oral solid medicinal composition and preparation method thereof
ActiveCN102342942AReduce dosageSolving Quality Control IssuesOrganic active ingredientsOrganic chemistryCandesartanLevamlodipine
The invention discloses a novel oral solid medicinal composition. The novel oral solid medicinal composition is an oral preparation prepared from hydrochlorothiazide, levamlodipine besylate, candesartan cilexetil and pharmaceutically acceptable auxiliary materials. The novel oral solid medicinal composition can be processed into tablets, capsules and the like. Specifically, the novel oral solid medicinal composition comprises: be weight, 5 to 25 parts of hydrochlorothiazide, 2.5 to 5 parts of levamlodipine besylate, 4 to 20 parts of candesartan cilexetil, 30 to 60 parts of microcrystalline cellulose, 30 to 60 parts of compressible starch, 30 to 50 parts of crosslinked polyvinylpyrrolidone, 1 to 2 parts of silica and 0.5 to 2 parts of magnesium stearate. The novel oral solid medicinal composition has a scientific and reasonable formula, low auxiliary material content and high bioavailability. Therefore, the novel oral solid medicinal composition is a drug of first choice for the treatment of hypertension.
Owner:HAINAN JINRUI PHARMA
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com