Crystalline form of hydrochlorothiazide and application thereof
A technology of hydrochlorothiazide and crystal form, which is applied in the field of medicinal chemistry, can solve the problems of slow dissolution of hydrochlorothiazide and poor production utilization, and achieve the effects of improving dissolution effect, solving slow dissolution speed and effective therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
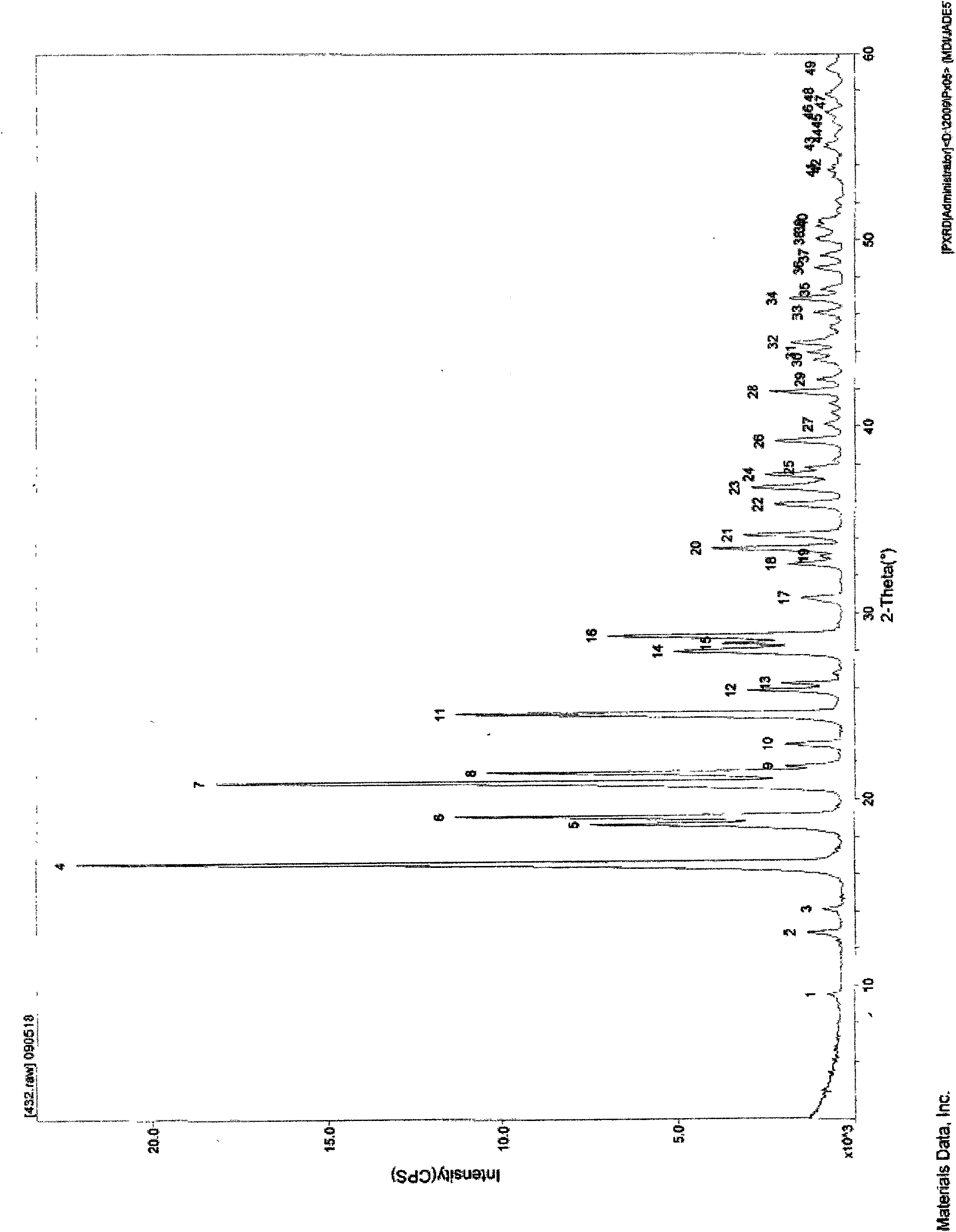
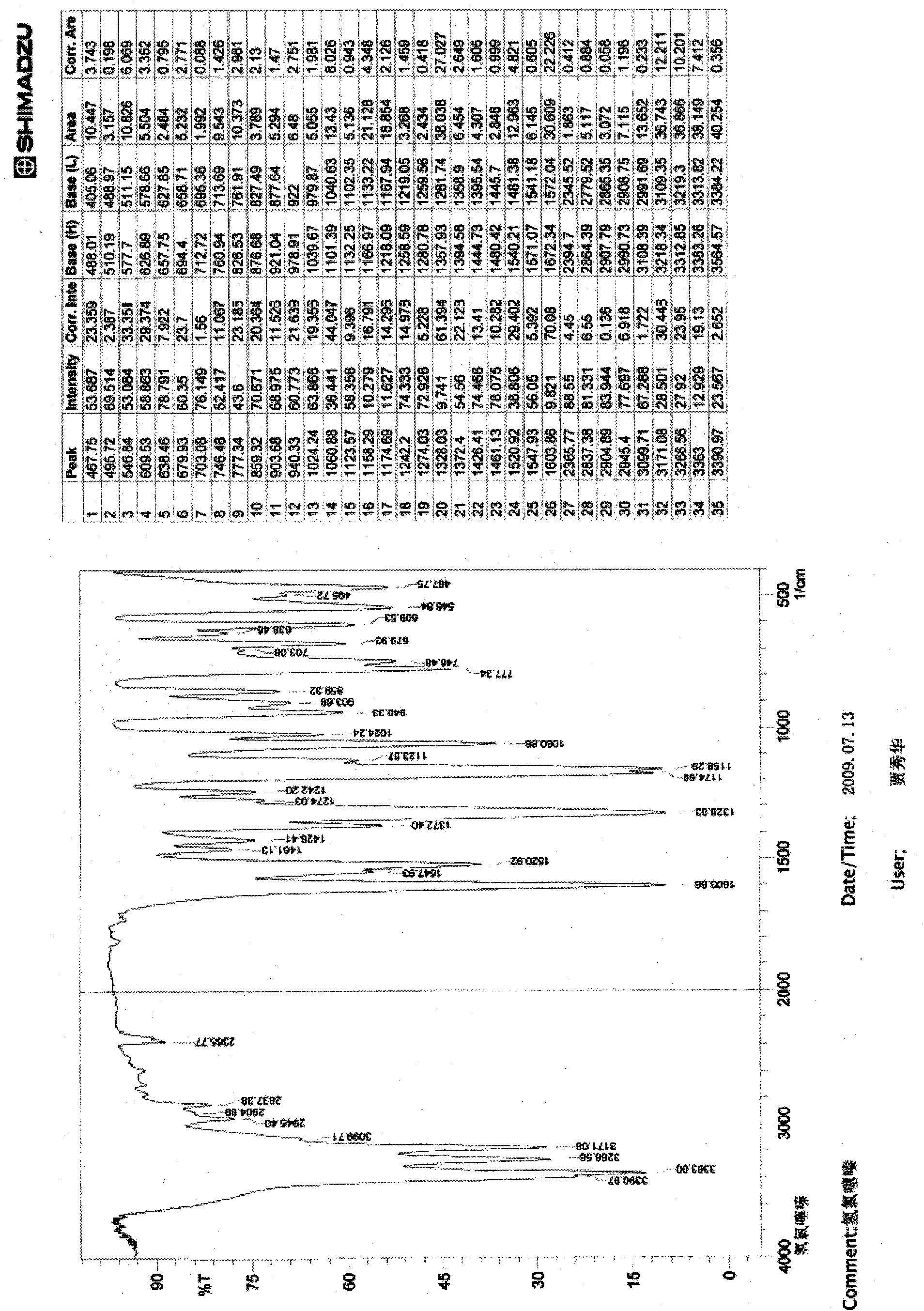
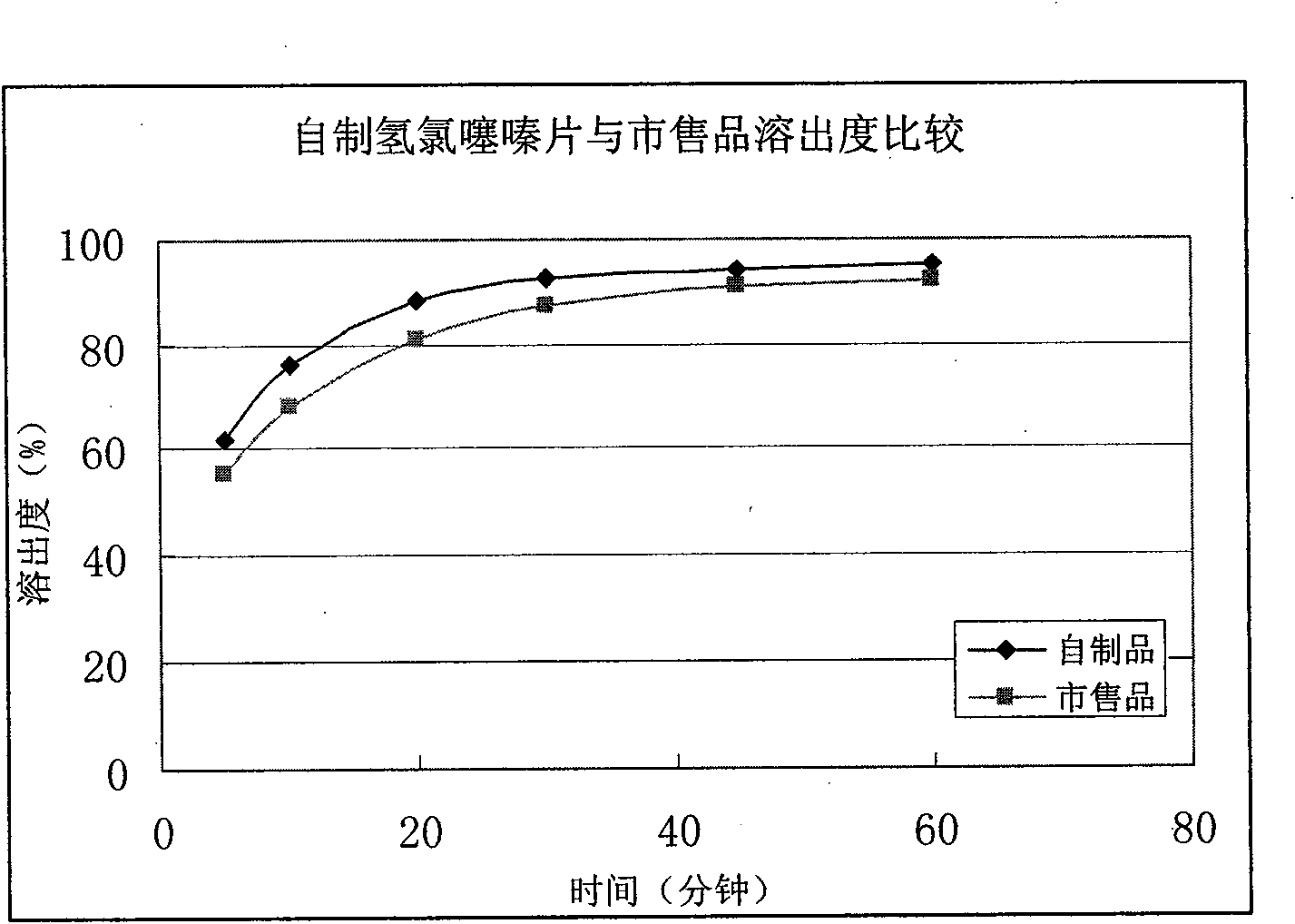
Image
Examples
Embodiment 1
[0039] Hydrochlorothiazide Tablets
[0040] components
quantity
25g
60g
48.5g
Low-substituted hydroxypropyl cellulose
7g
povidone k30
8g
Micropowder silica gel
0.75g
0.75g
[0041] Preparation method: sieve the raw and auxiliary materials for later use; take hydrochlorothiazide, microcrystalline cellulose, starch, mannitol, sodium carboxymethyl starch, and low-substituted hydroxypropyl cellulose and mix evenly; then add micronized silica gel and magnesium stearate Mix well; tablet that is.
[0042] The preparation method of hydrochlorothiazide is as follows: dissolve 100 g of hydrochlorothiazide in 500 ml of 4% sodium hydroxide aqueous solution, add dropwise 10% hydrochloric acid, precipitate hydrochlorothiazide crystals, suction filter, and dry the product to obtain hydrochlorothiazide cryst...
Embodiment 2
[0044] Valsartan Hydrochlorothiazide Tablets
[0045] components
quantity
80g
Hydrochlorothiazide
12.5g
60g
30g
10g
1g
10% povidone k30
20ml
[0046] Preparation method: take valsartan, hydrochlorothiazide, starch, and microcrystalline cellulose and sieve separately for later use; the above-mentioned raw and auxiliary materials are mixed and mixed evenly when passing through a 100-mesh sieve; Granulate with a mesh sieve; dry the granules in a drying oven at 50°C; add sodium carboxymethyl starch and magnesium stearate to mix, pass through a 18-mesh sieve for granulation; compress into tablets; coat plain tablets to obtain the product.
[0047] The preparation method of hydrochlorothiazide is as follows: dissolve 100 g of hydrochlorothiazide in 250 ml of 5% sodium hydroxide aq...
Embodiment 3
[0049] Irbesartan Hydrochlorothiazide Capsules
[0050] components
quantity
150g
Hydrochlorothiazide
12.5g
starch
30g
40g
25g
Crospovidone
10g
Magnesium stearate
1.5g
5% povidone k30 aqueous solution
40ml
[0051] Preparation method: sieve each raw and auxiliary material for later use; take irbesartan, hydrochlorothiazide, starch, microcrystalline cellulose, lactose, and cross-linked povidone and mix evenly; add 5% povidone solution in the prescribed amount to make a soft material ; Sieve through a 40-mesh granulation; dry the granules in a drying oven at 50°C; add magnesium stearate to the dry granules, pass through a 30-mesh granule; pack the granules into capsules.
[0052] The preparation method of hydrochlorothiazide is as follows: dissolve 100 g of hydrochlorothiazide in 1000 ml of an aqueous solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com