Domperidone particles, domperidone preparation and preparation method thereof
A technology of domperidone and microparticles, applied in the direction of pill delivery, digestive system, diseases, etc., to achieve the effect of easy industrial production, low cost and good dissolution performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
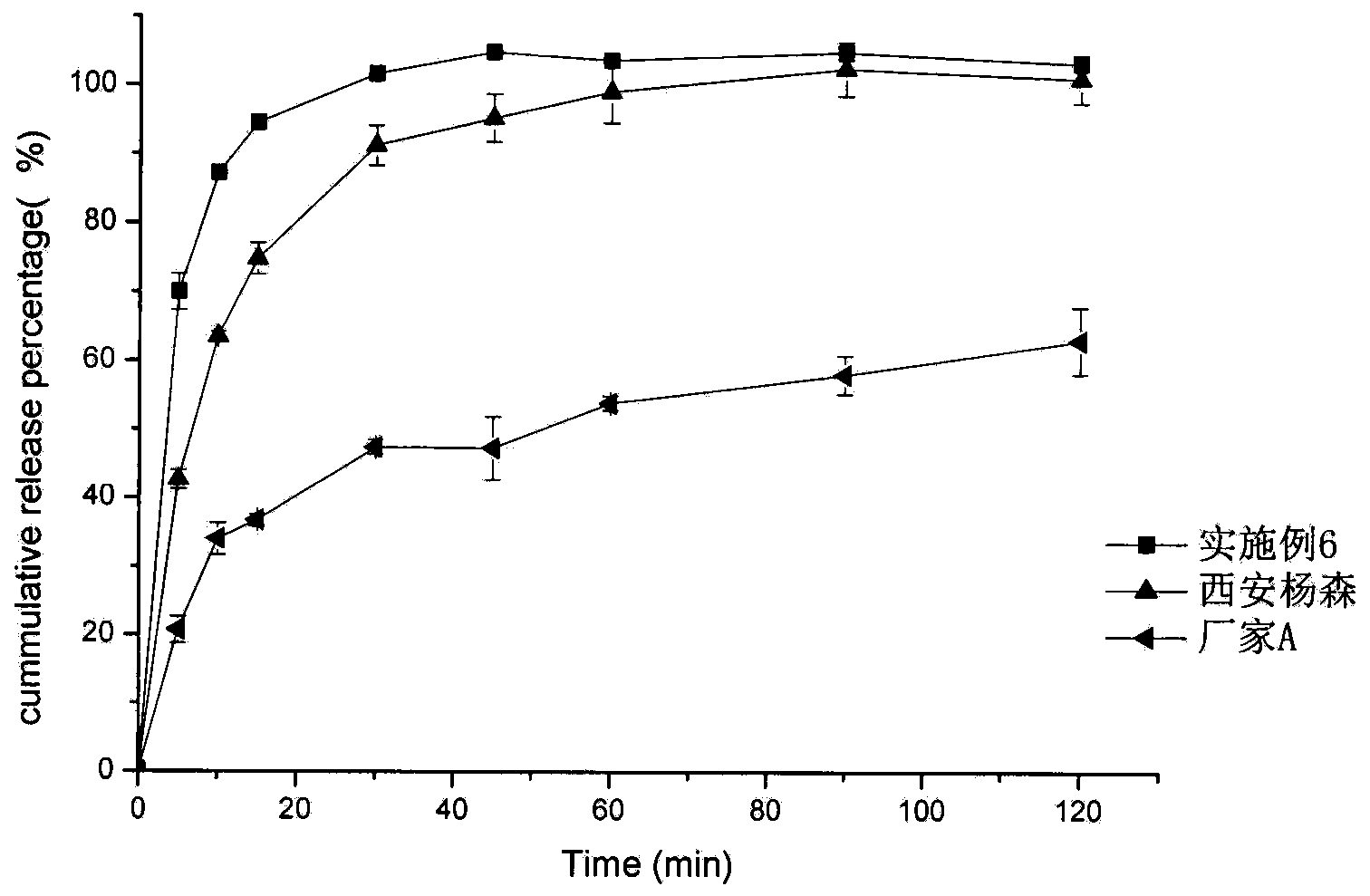
Embodiment 1
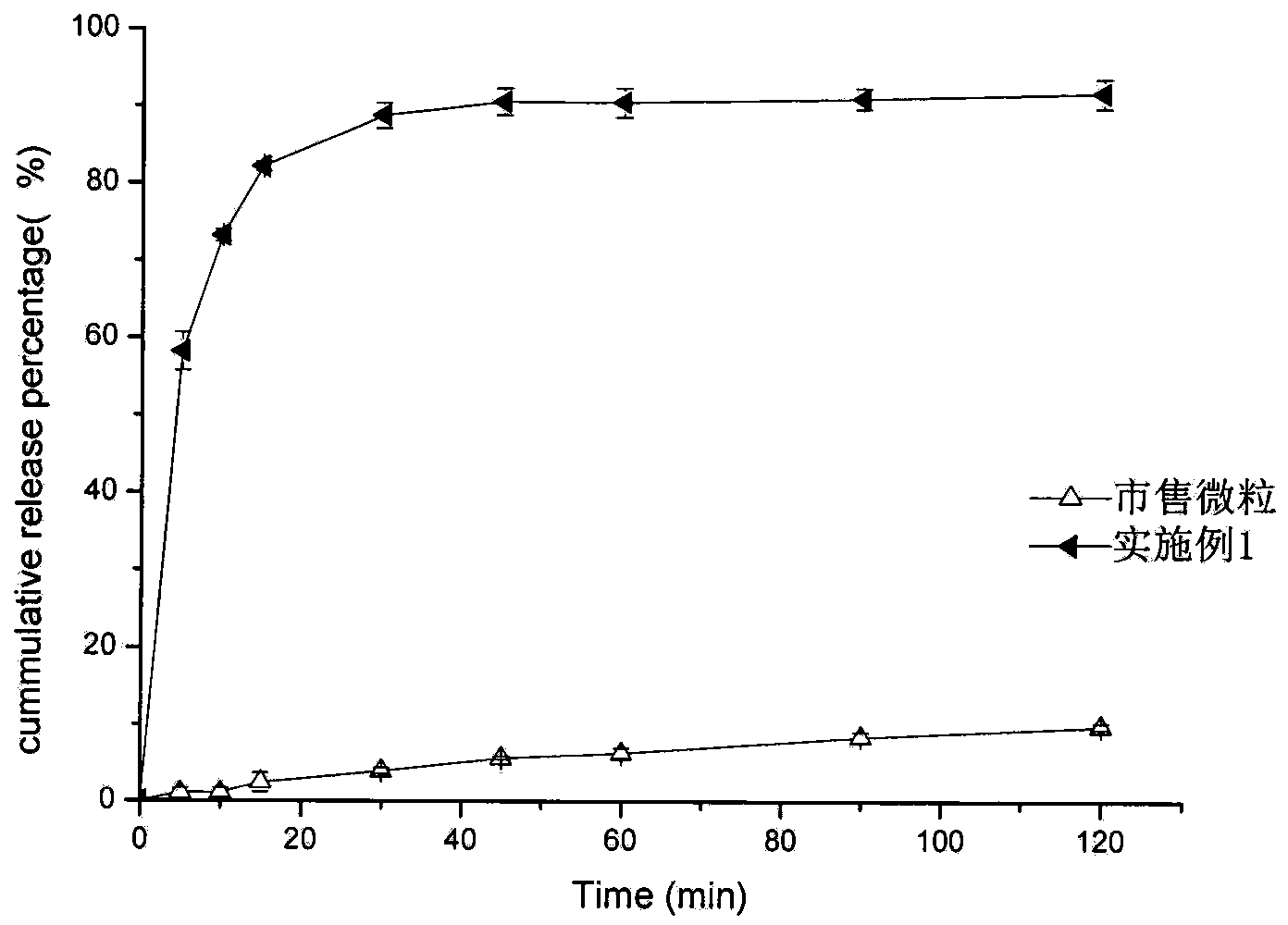
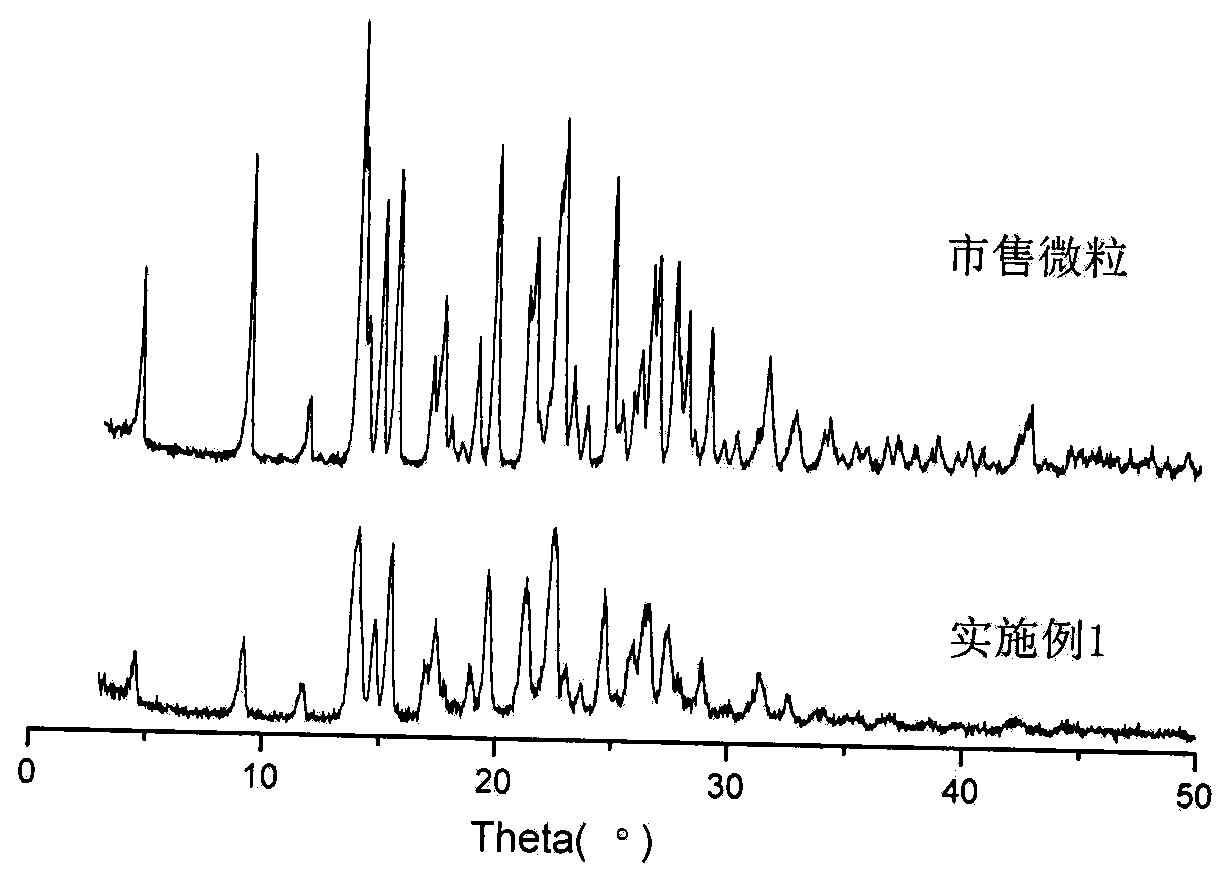
[0040] Embodiment 1 prepares domperidone microparticles
[0041] (1) Add 10 grams of domperidone coarse crystals and 2 grams of poloxamer 188 into 60 grams of 70% ethanol aqueous solution, stir to disperse, add 4.7 grams of 20% hydrochloric acid and stir until completely dissolved to obtain solution I.
[0042] (2) Add about 11 grams of 10% sodium hydroxide aqueous solution to a pH value of 7.3 under stirring at room temperature (stirring linear velocity 100 m / min), continue stirring for 5 minutes, add 50 grams of water and stir for another 5 minutes, and separate out by suction filtration The microparticles were washed twice with 10 grams of water and suction-filtered respectively, and dried under reduced pressure at 60° C. for 4 hours to obtain 9.31 grams of domperidone microparticles, with a yield of 93.1% and a content of 99.1%.
Embodiment 2
[0043] Embodiment 2 prepares domperidone microparticles
[0044] (1) Add 10 grams of domperidone coarse crystals and 3 grams of Tween 80 into 50 grams of 80% ethanol aqueous solution, stir to disperse, add 4.7 grams of 20% hydrochloric acid and stir until completely dissolved to obtain solution I.
[0045] (2) Add about 10.5 grams of 10% sodium hydroxide aqueous solution to a pH value of 6.8 under stirring at room temperature (stirring linear velocity 100 m / min), continue stirring for 5 minutes, add 50 grams of water and stir for another 5 minutes, and separate out by suction filtration The microparticles were washed twice with 10 grams of water and suction-filtered respectively, and dried under normal pressure at 60°C for 6 hours to obtain 9.06 grams of domperidone microparticles, with a yield of 90.6% and a content of 98.9%.
Embodiment 3
[0046] Embodiment 3 prepares domperidone microparticles
[0047] (1) Add 10 grams of domperidone coarse crystals to 60 grams of 50% ethanol aqueous solution, stir to disperse, add 4.7 grams of 20% hydrochloric acid and stir, warm at 50°C until completely dissolved, add 2 grams of poloxamer 188 to dissolve, and obtain solution I.
[0048] (2) Add about 11 grams of 10% sodium hydroxide aqueous solution to a pH value of 7.0 under stirring (stirring line speed 100 m / min), continue stirring for 5 minutes, add 50 grams of water and stir for another 5 minutes, set high-speed milk to homogenize After spinning at 10,000 rpm for 10 minutes, the precipitated particles were suction filtered, washed twice with 10 grams of water and filtered separately, and dried at 50°C under normal pressure for 7 hours to obtain 9.12 grams of domperidone particles, with a yield of 91.2% and a content of 99.3%. .
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com