Patents
Literature
102 results about "Esophageal sphincter" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
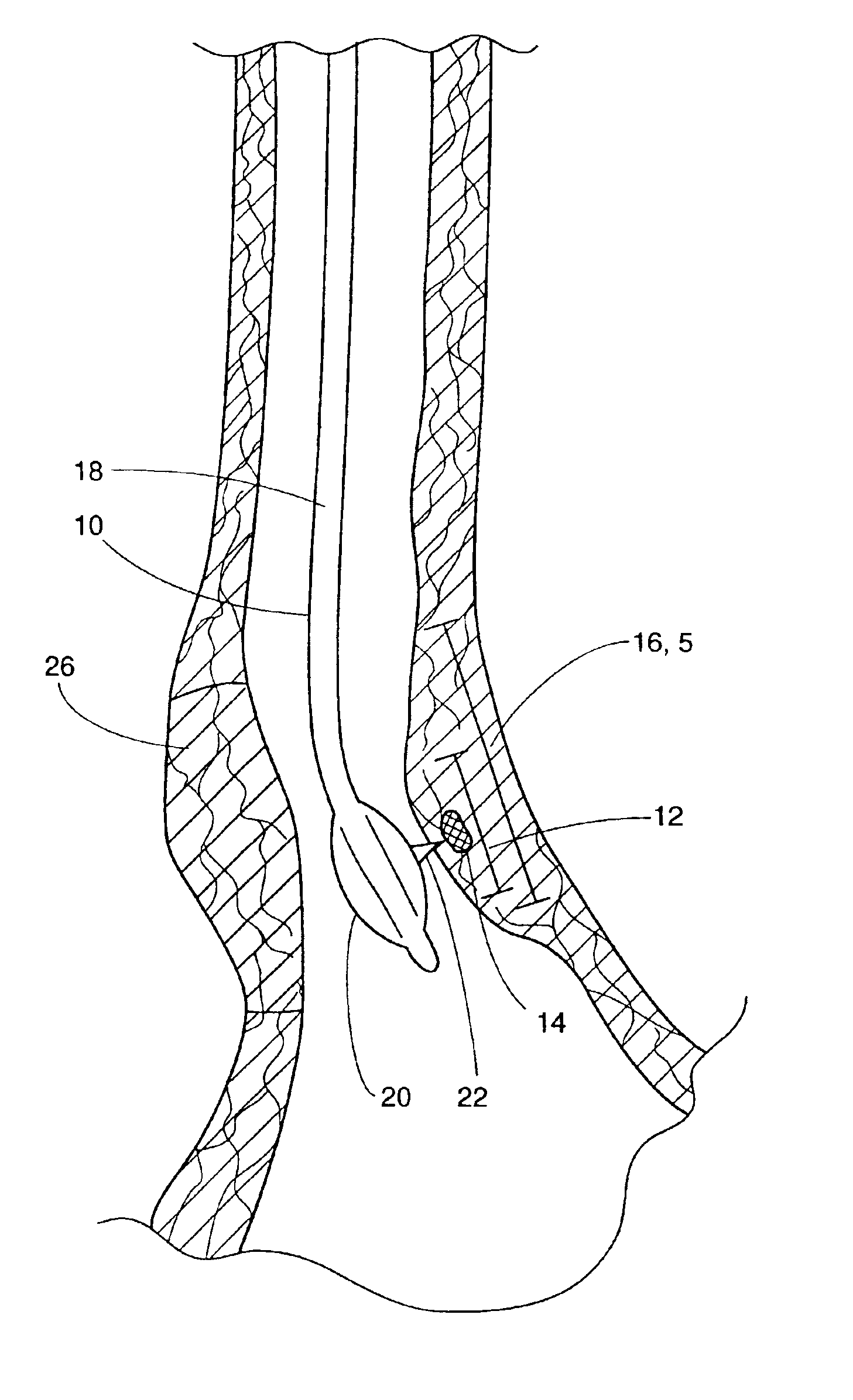
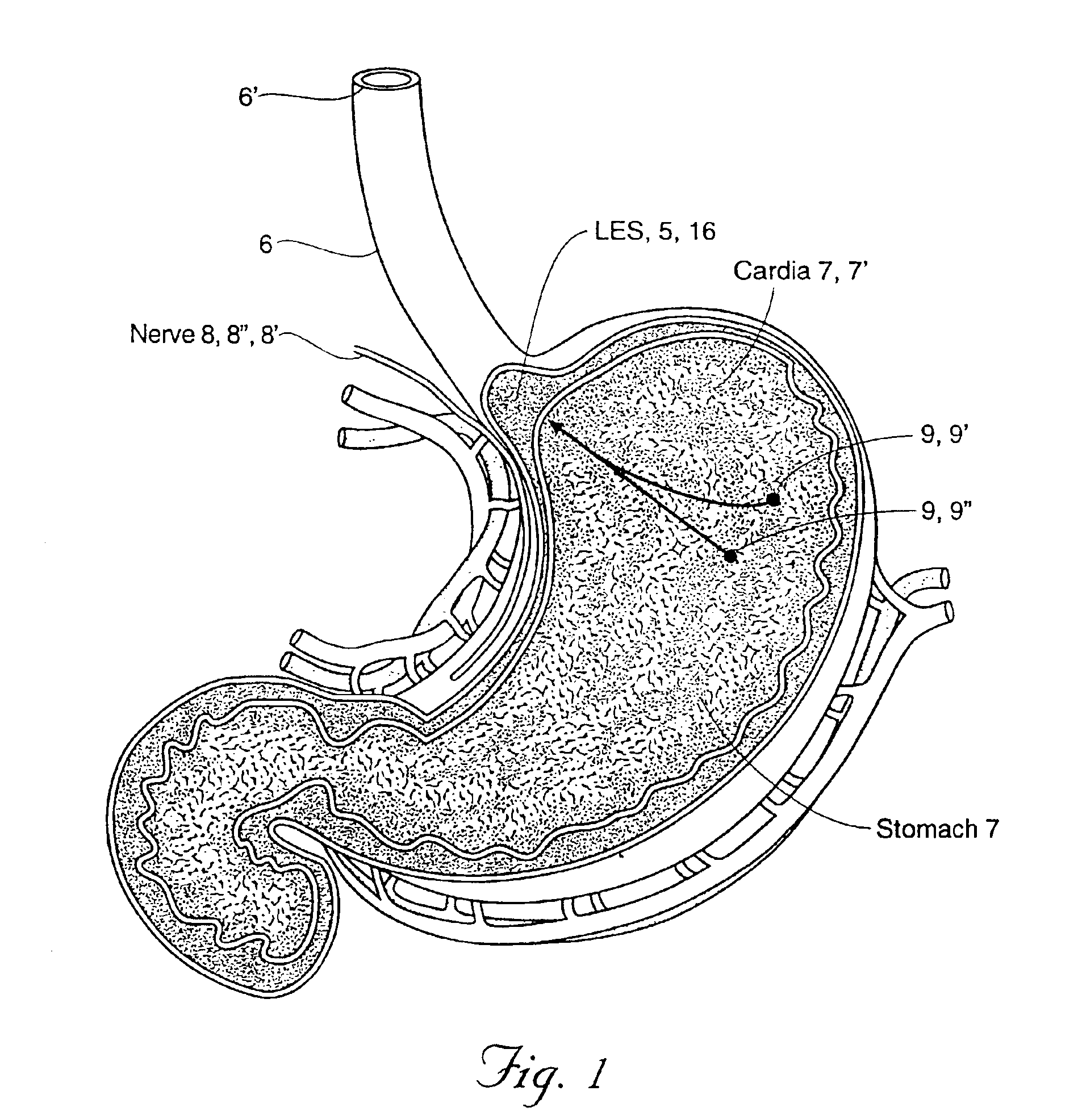
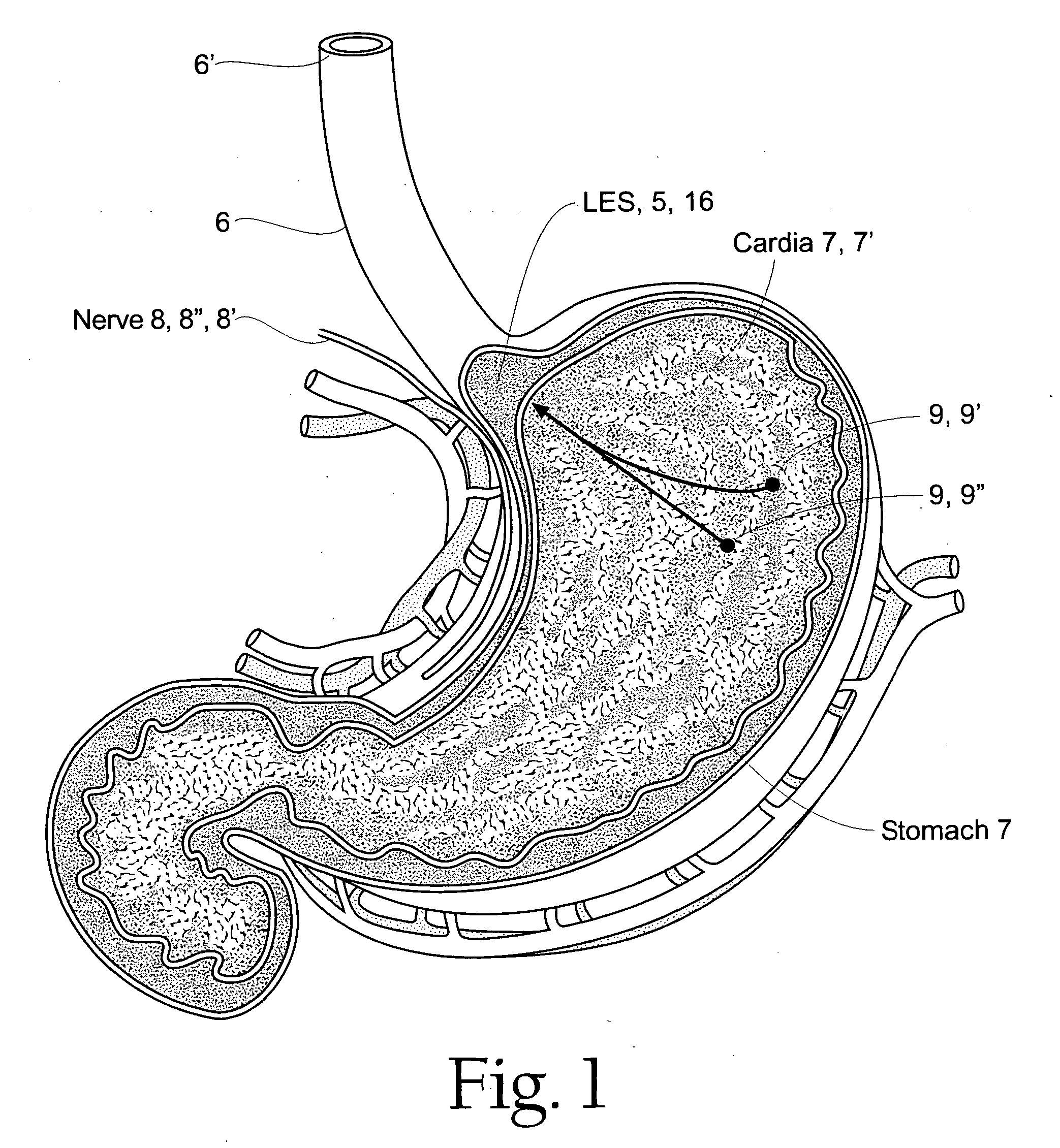
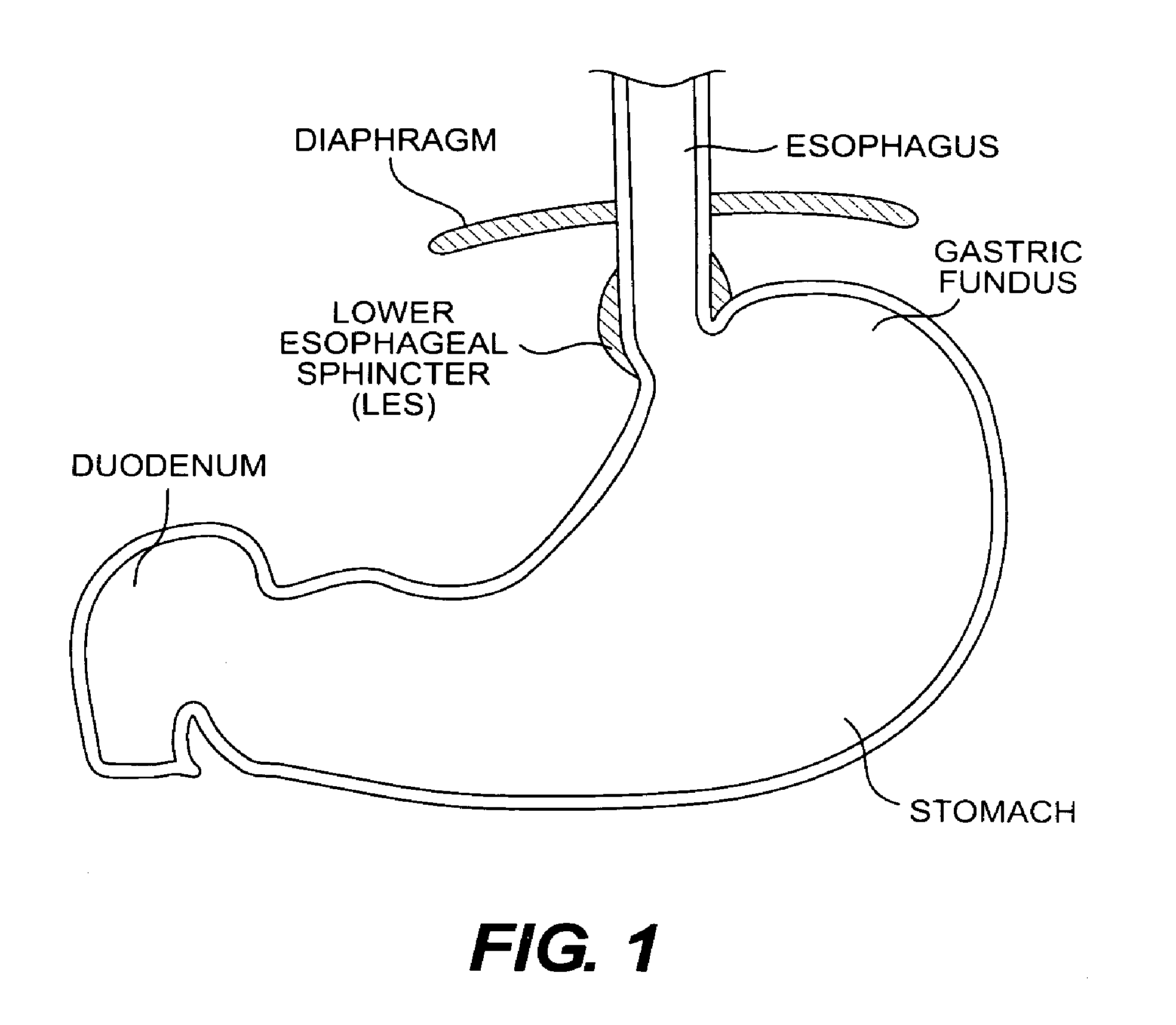
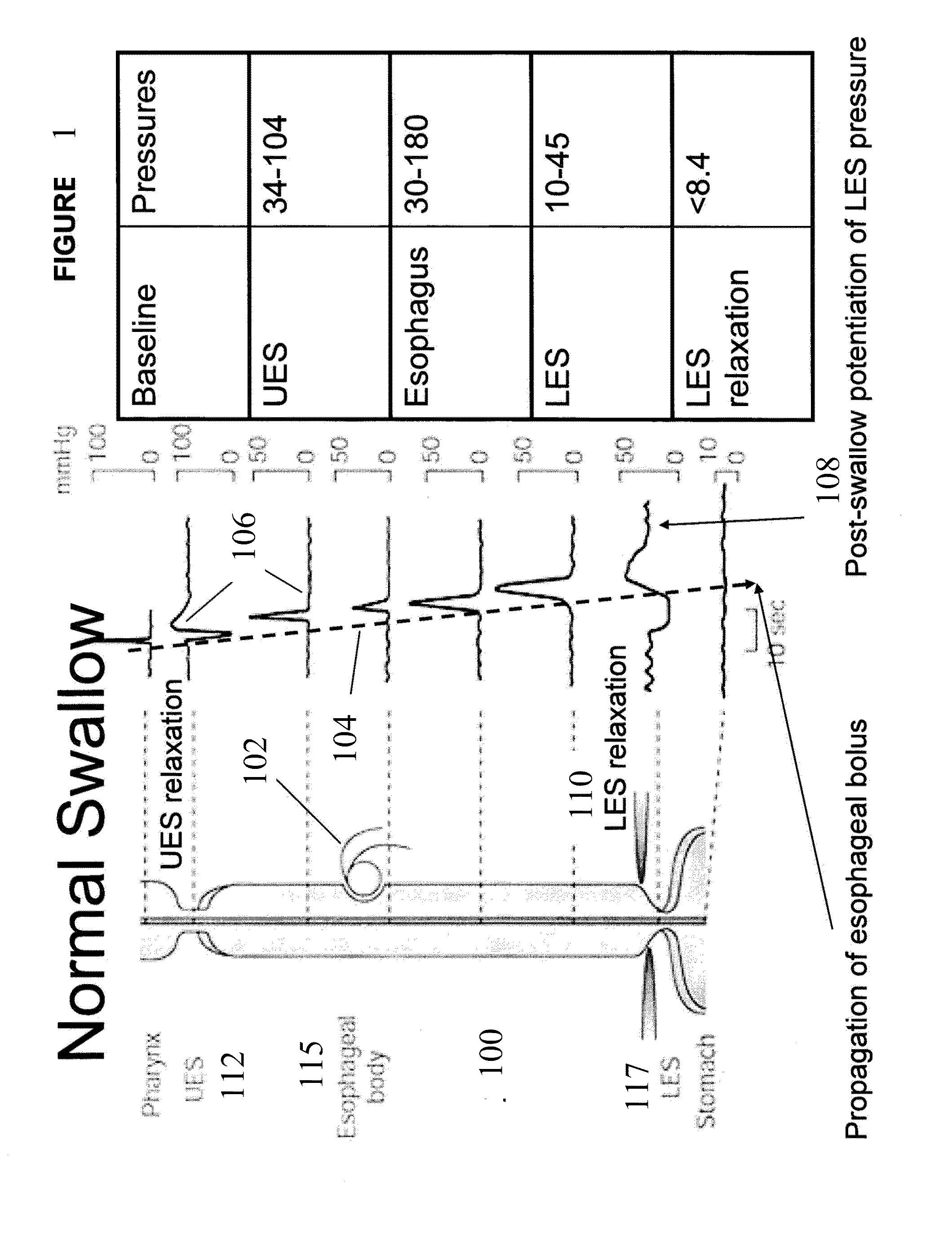
Esophageal sphincter. A circular band of muscle that closes the last few centimeters of the esophagus and prevents the backward flow of stomach contents. sphincter. a circular muscle that constricts a passage or closes a natural orifice. When relaxed, a sphincter allows materials to pass through the opening. When contracted, it closes the opening.
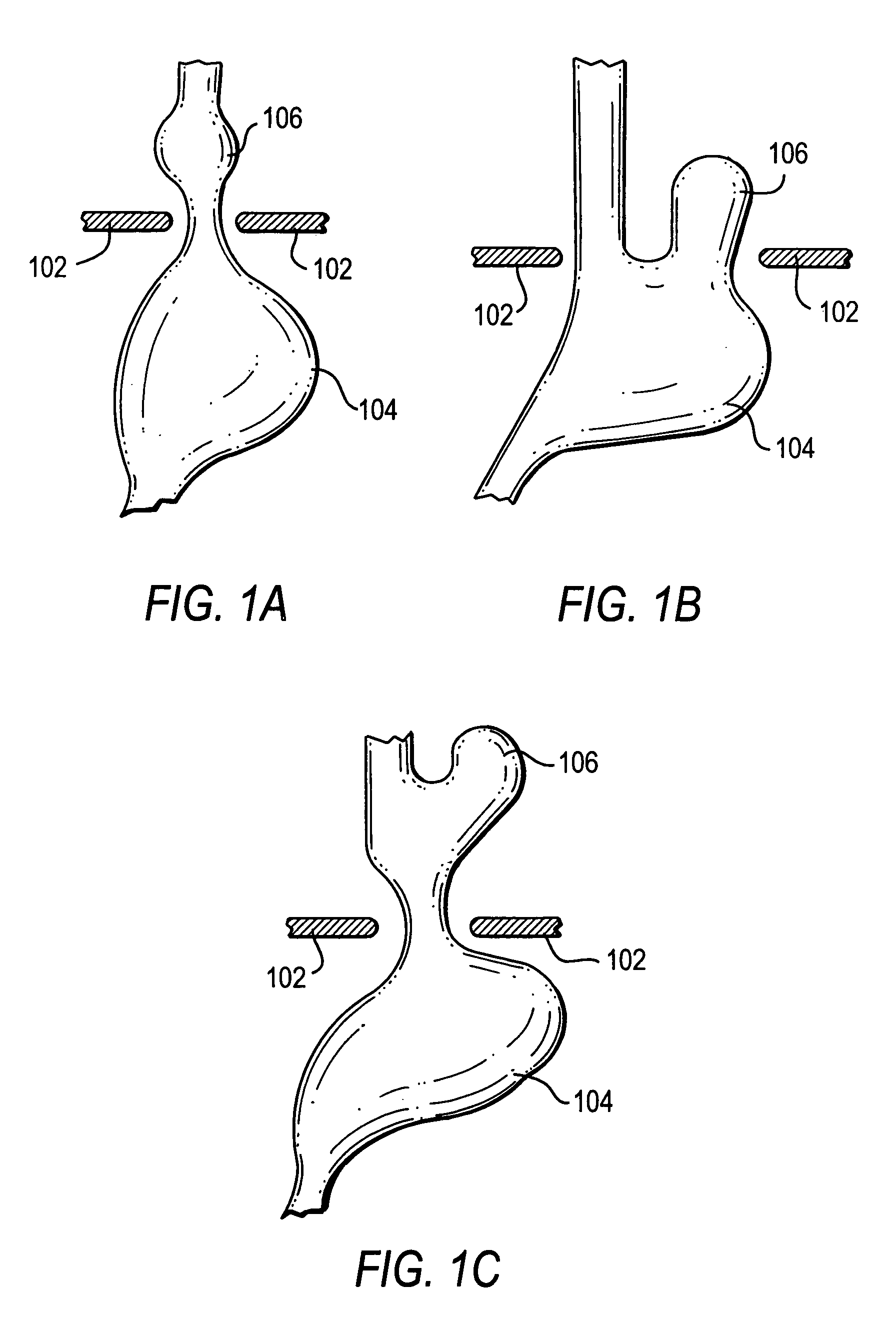
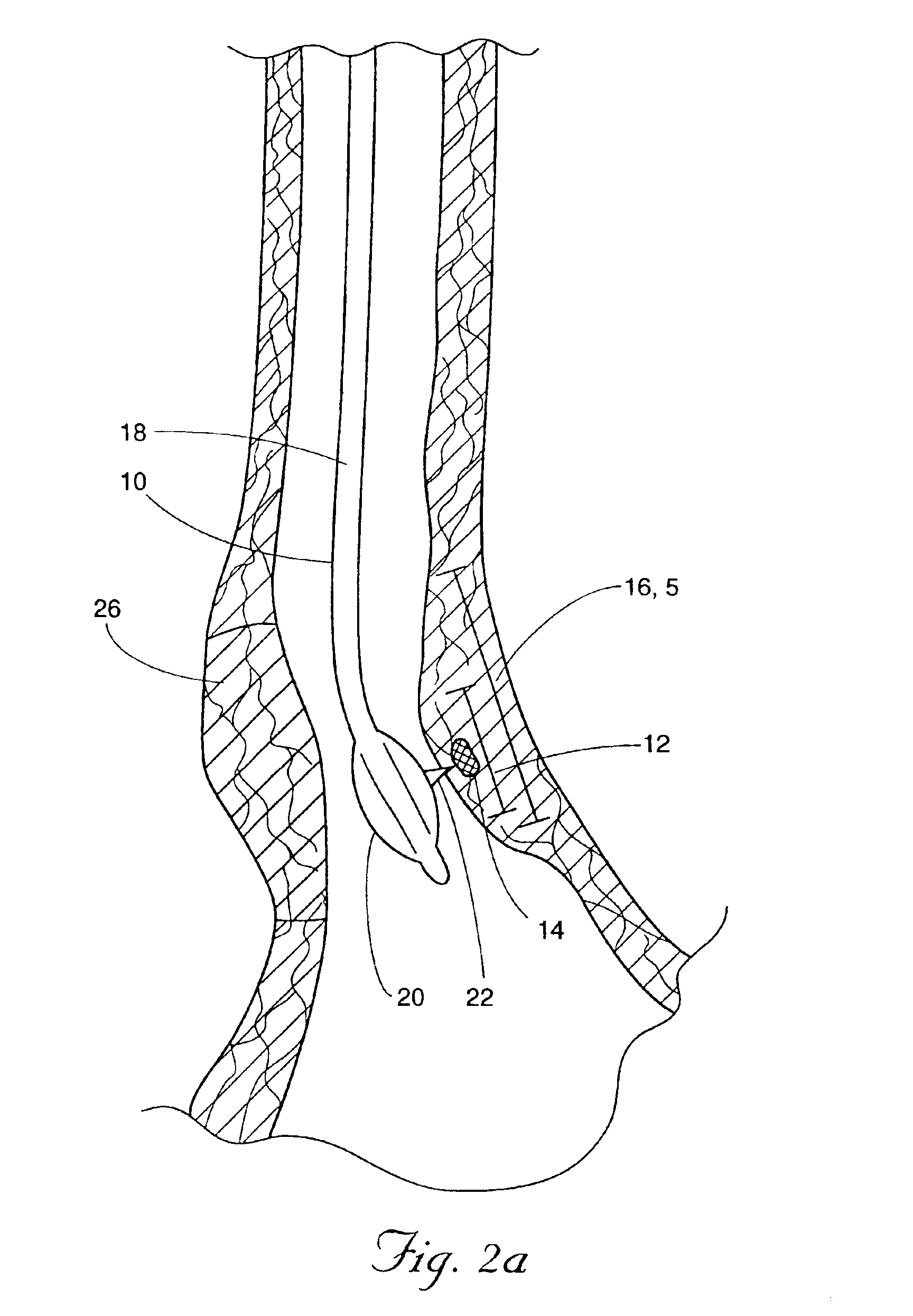
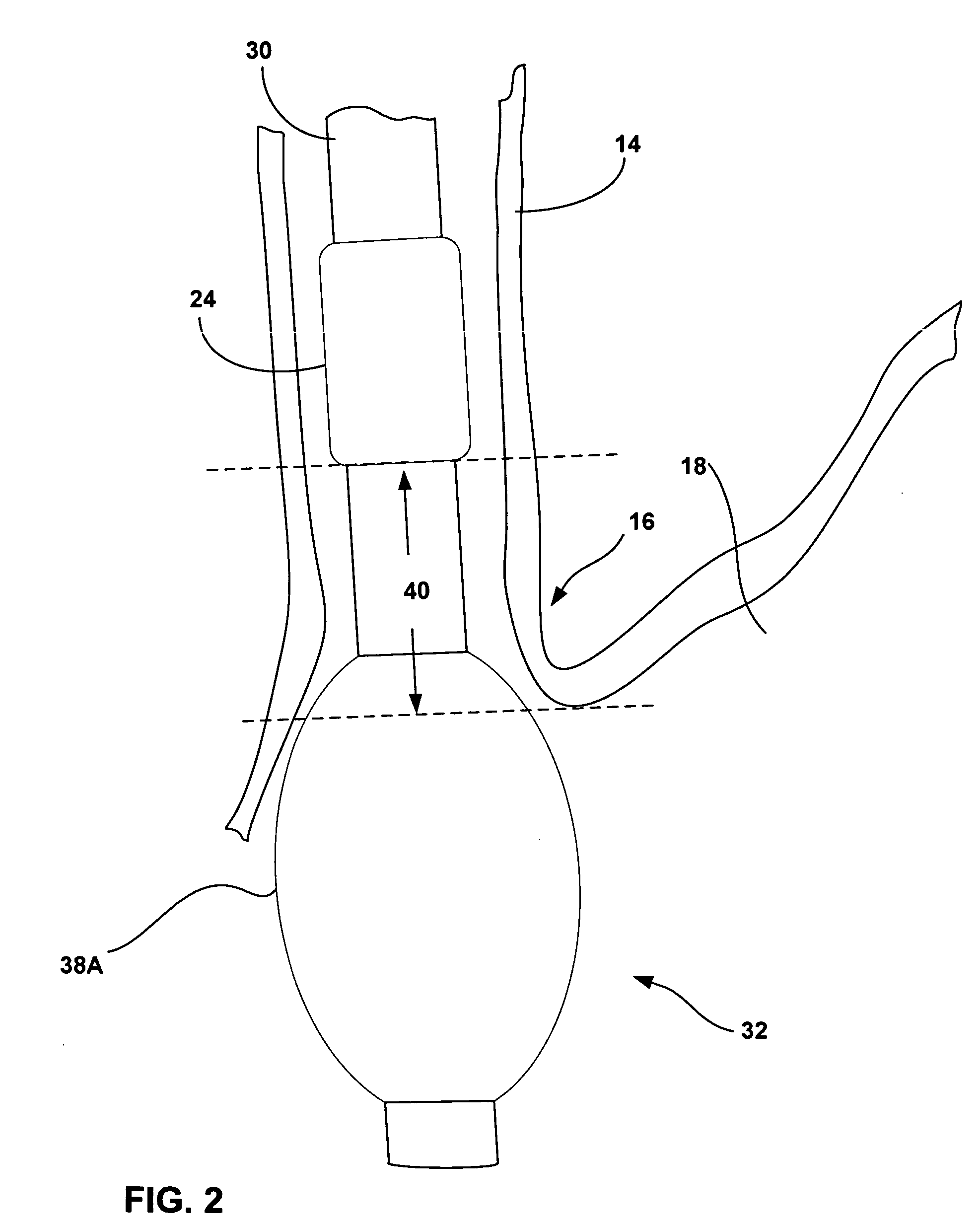
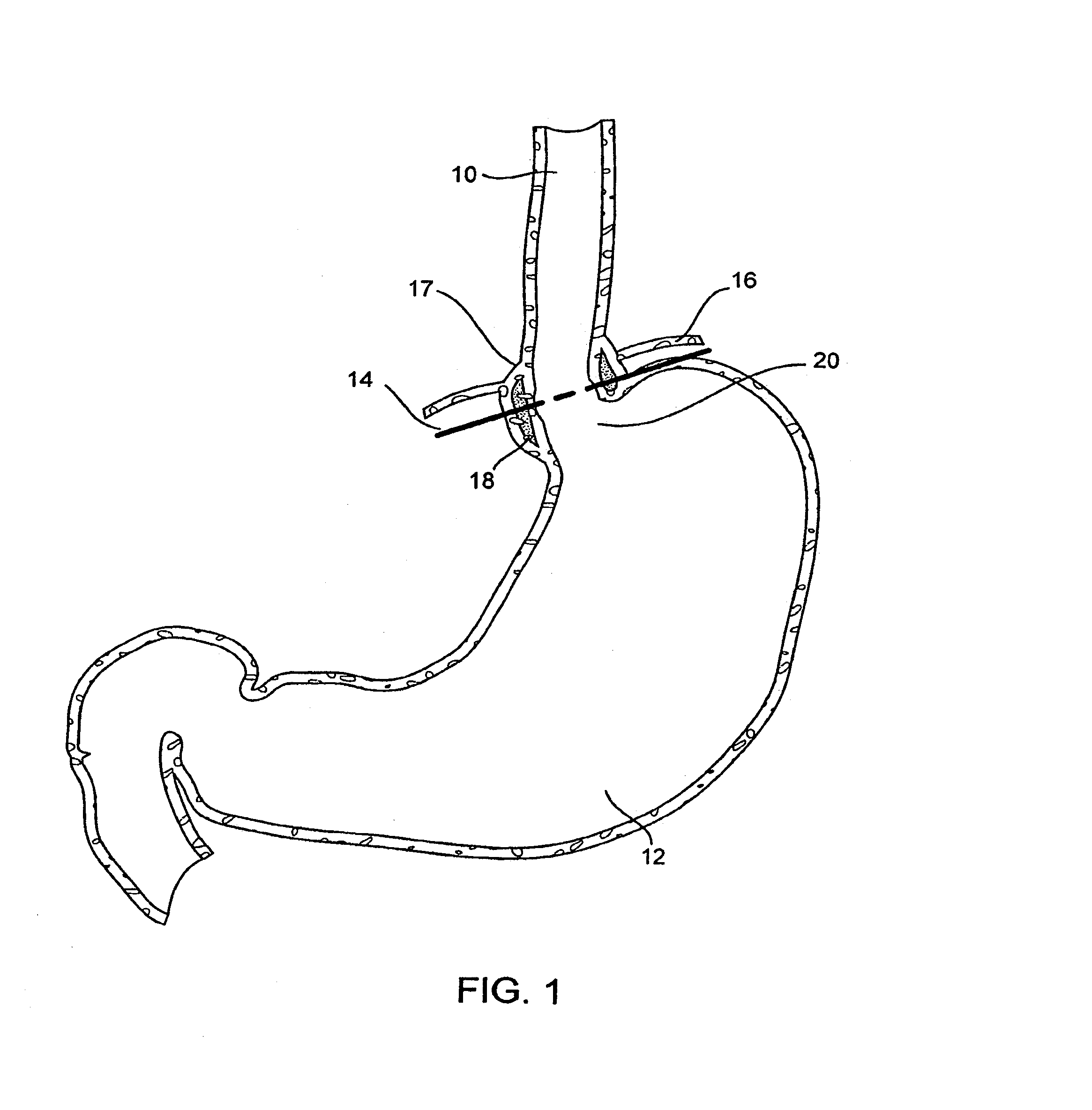
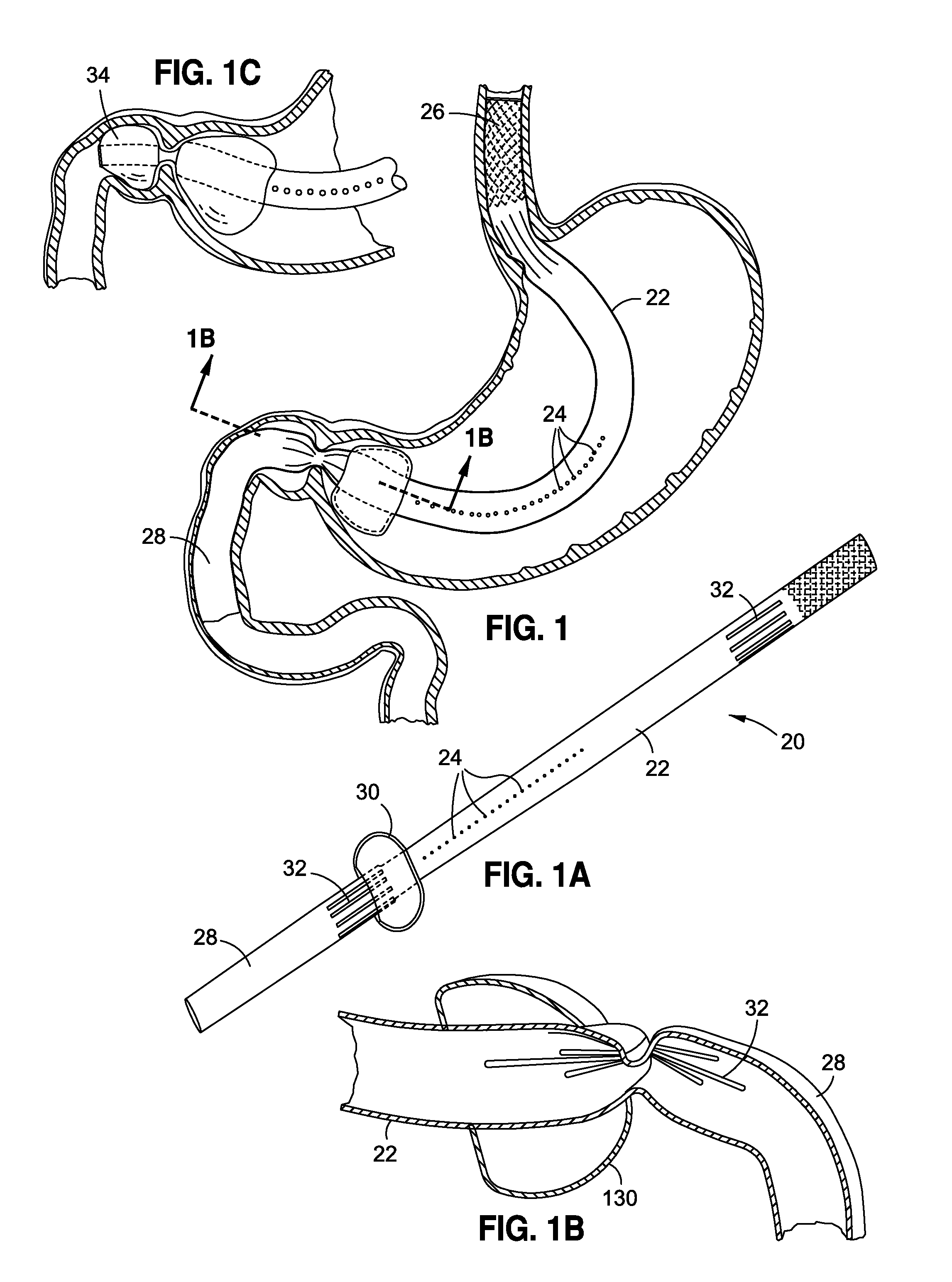
Methods and apparatus for improving the function of biological passages
ActiveUS6960233B1Reduces the hiatal herniaStrengthens the function of the lower esophageal sphincterTubular organ implantsInsertion stentEsophageal wall
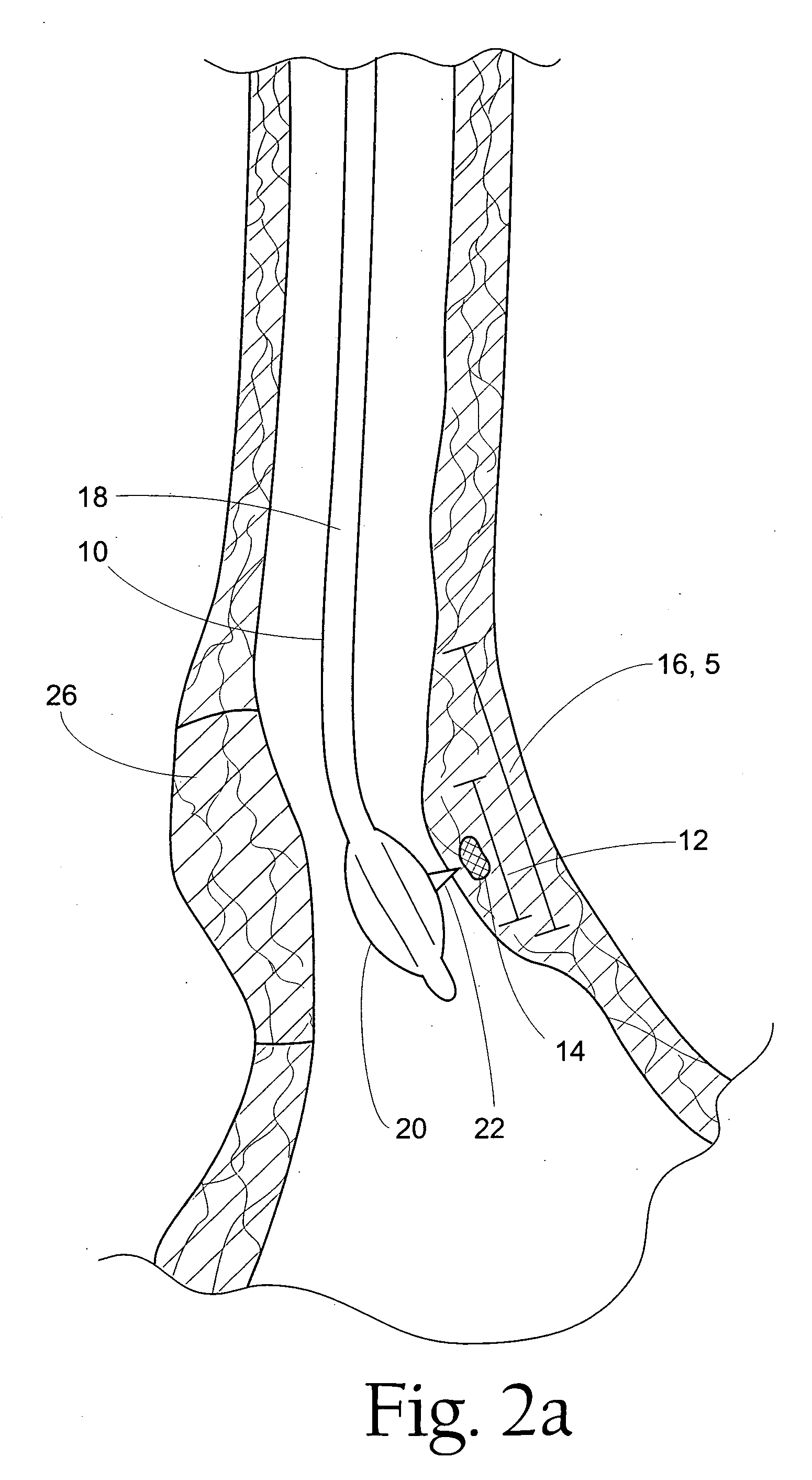
Several methods and apparatus are available for treating patients that suffer from both gastro-esophageal reflux disorder and hiatal hernias. In order to treat these patients, an endoscopic probe may be used to push the herniated stomach below the diaphragm. In some embodiments, a two-part stent comprising a funnel stent and a reverse stent may be deployed to prevent a future re-herniation of the stomach and to reduce the annulus of the lower esophageal sphincter. In some embodiments, a reverse stent with perforating barbs may be deployed, in which the barbs penetrate the esophageal wall and attach to the diaphragm. In some embodiments, the crus muscles may be sutured together to reduce the hiatus size and a reverse stent may be deployed to reduce the annulus of the lower esophageal sphincter.
Owner:TORAX MEDICAL
Surgical instruments for treating gastro-esophageal reflux
InactiveUS6740082B2Augment objectInexpensive and disposableElectrotherapyEndoscopesSensor arrayFiber
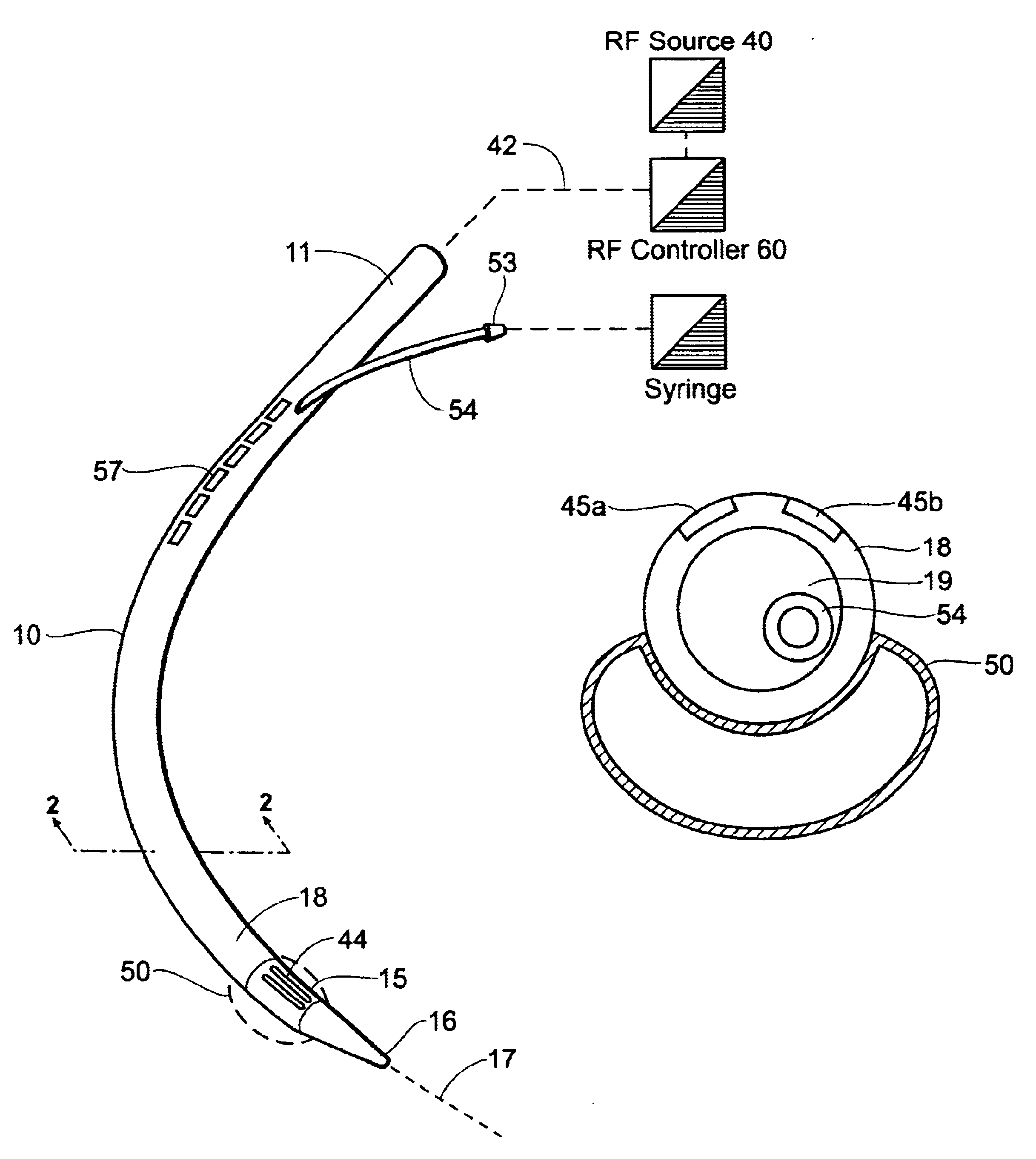
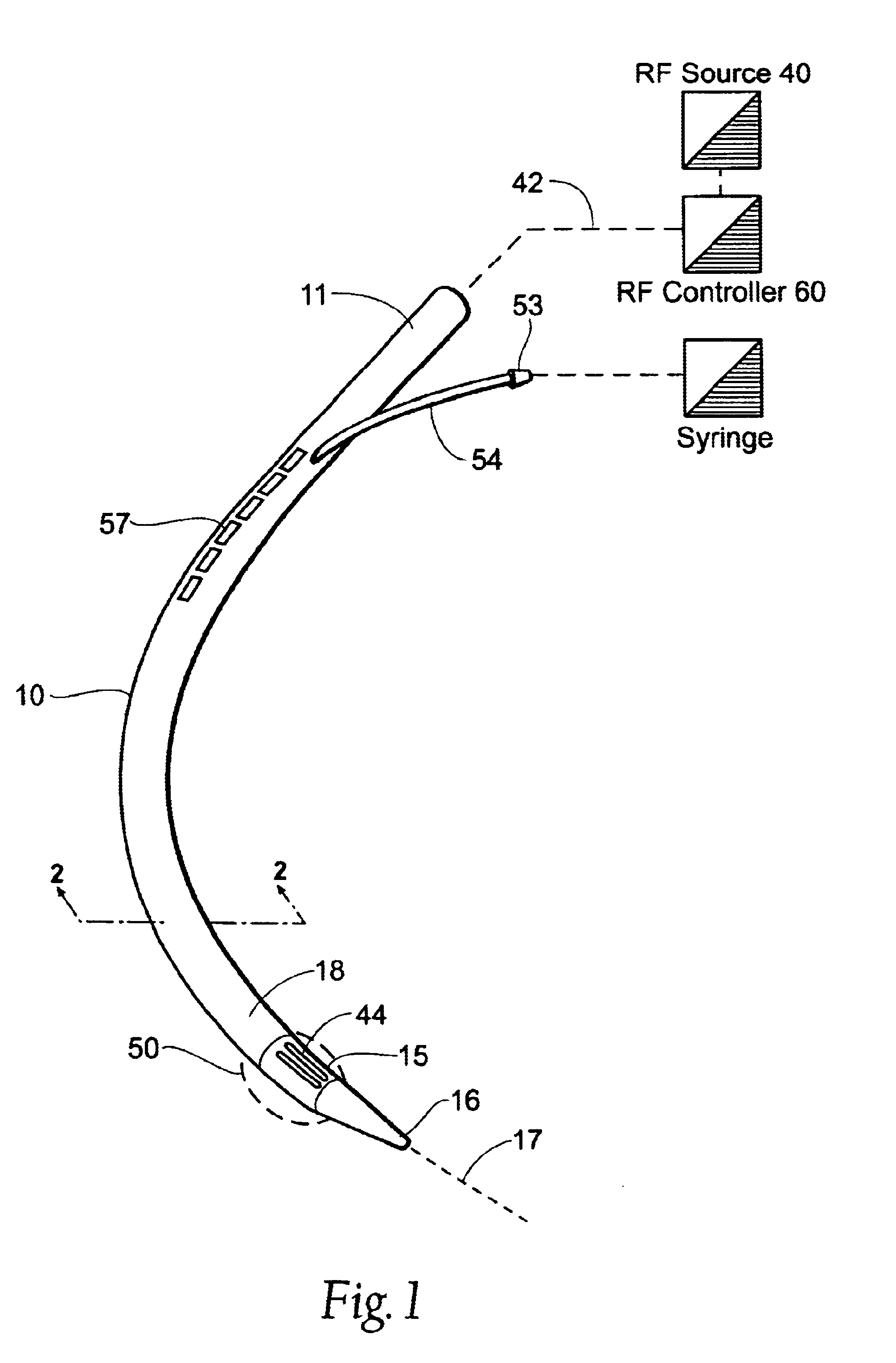
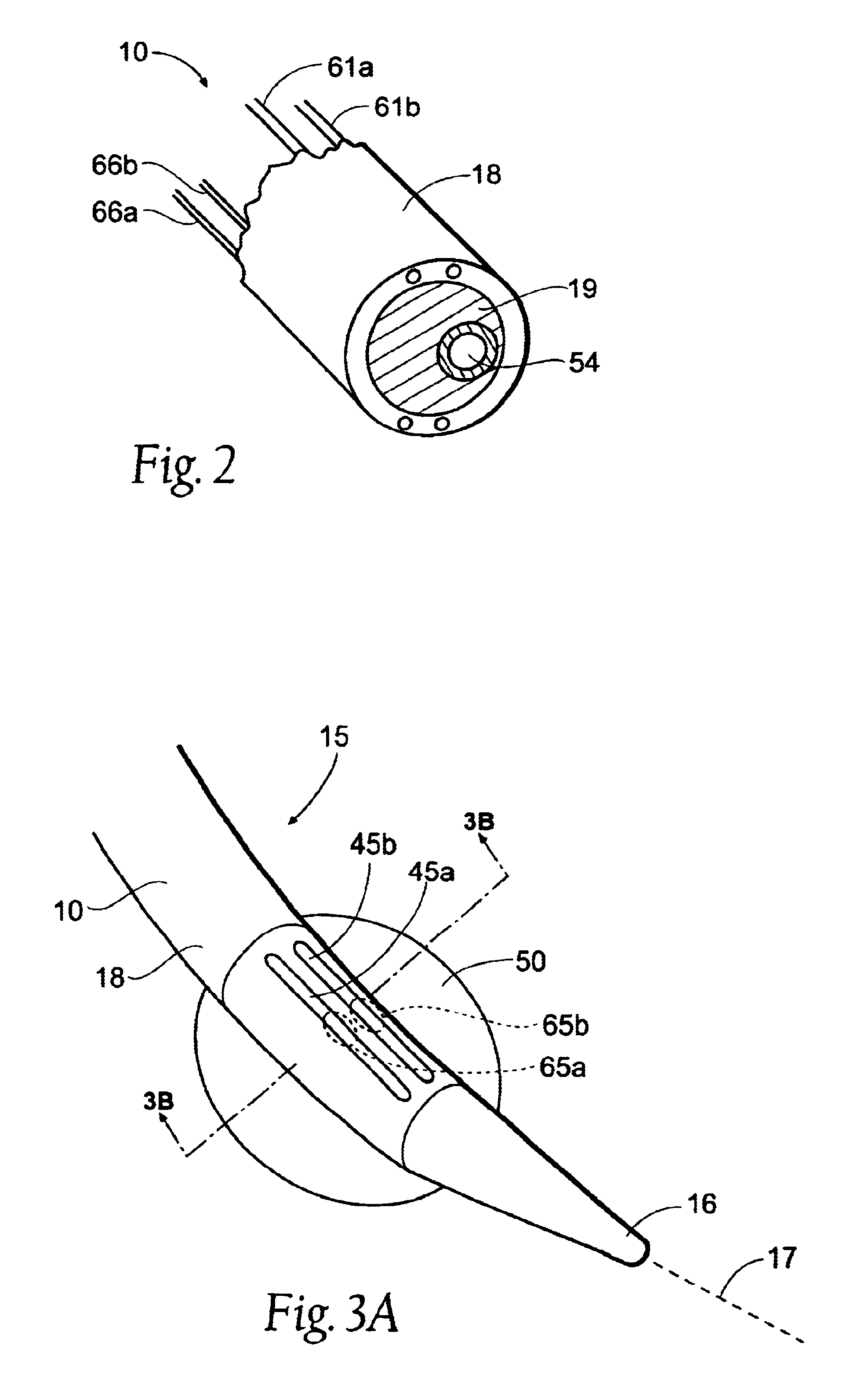
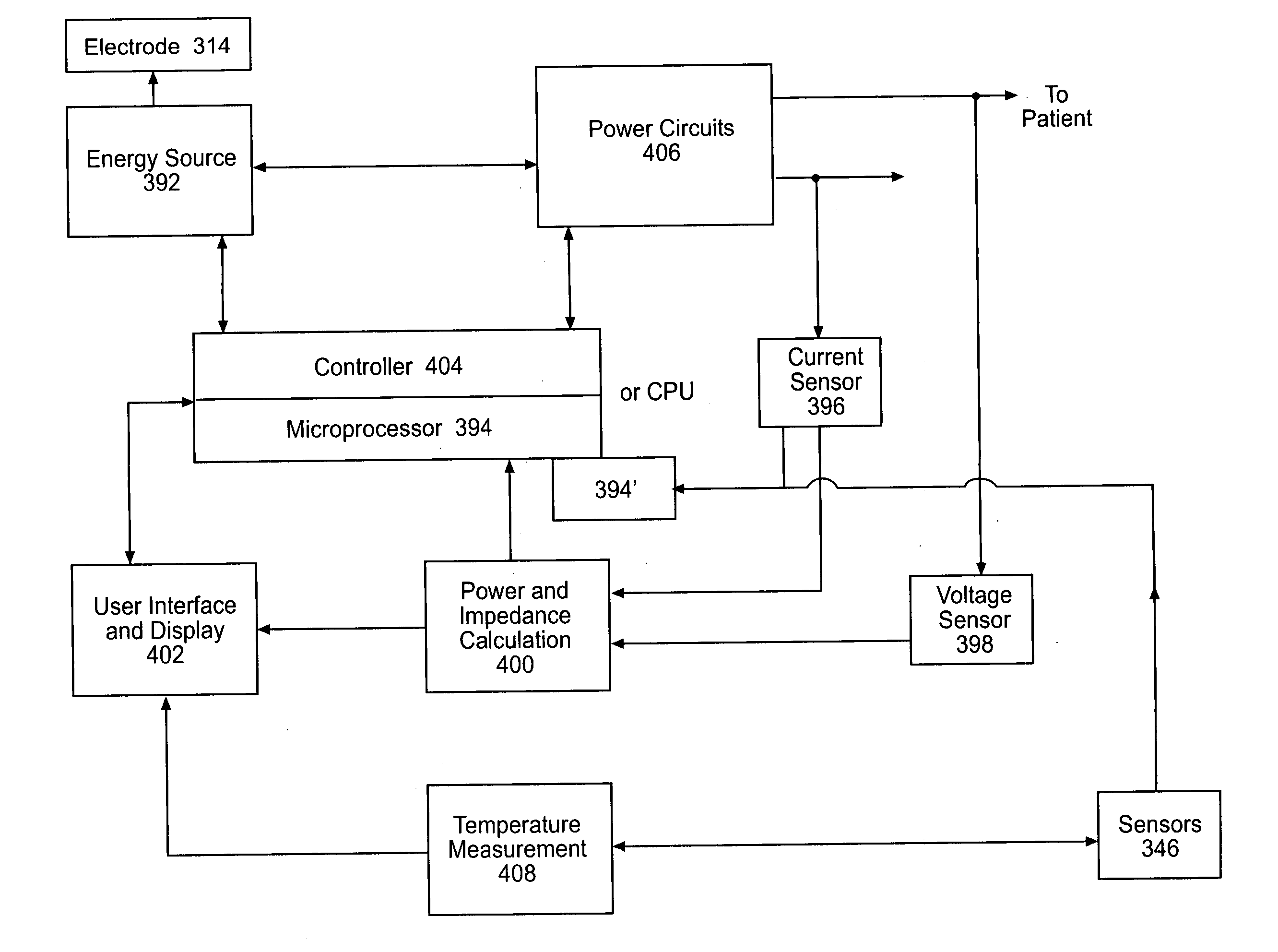
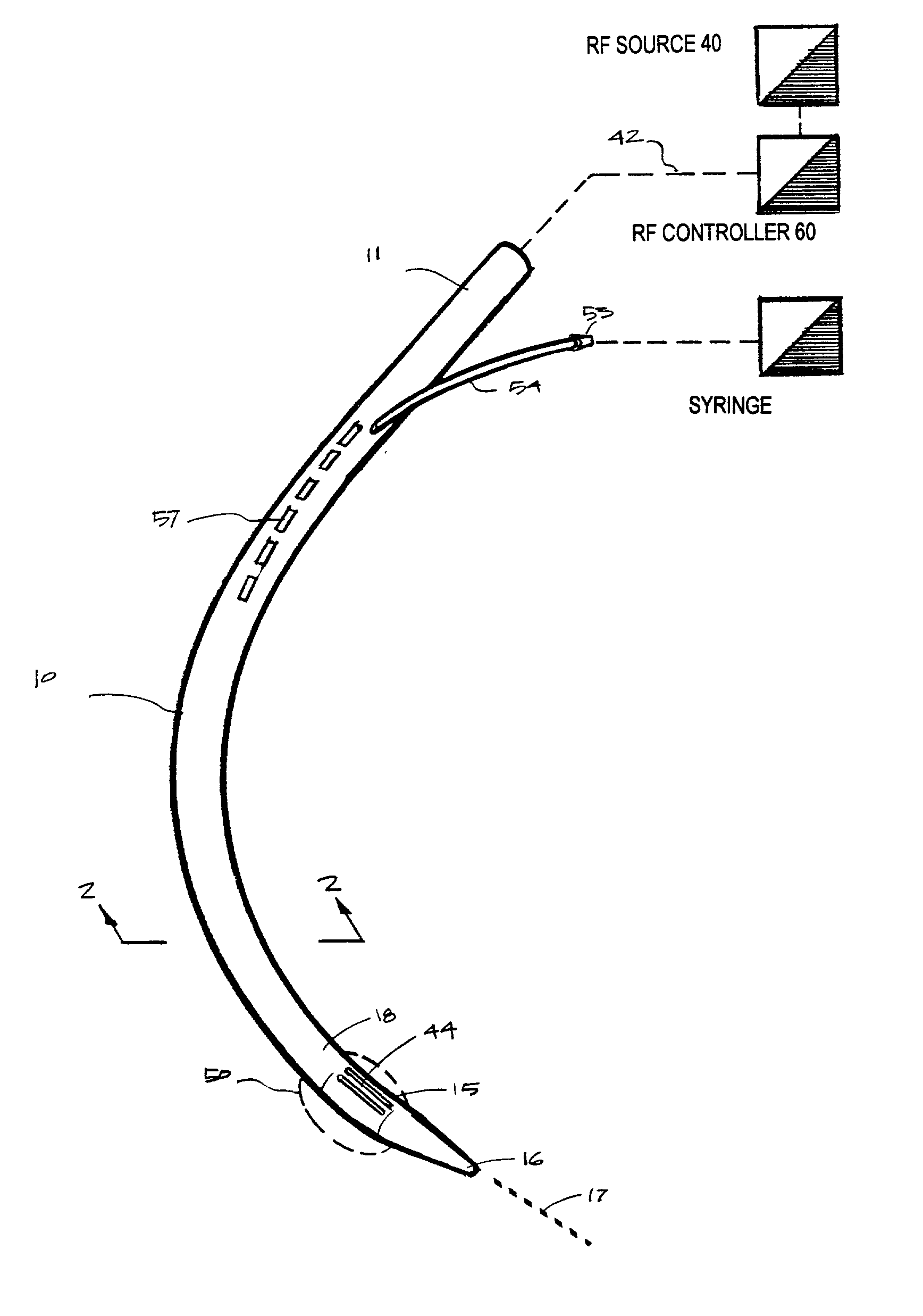
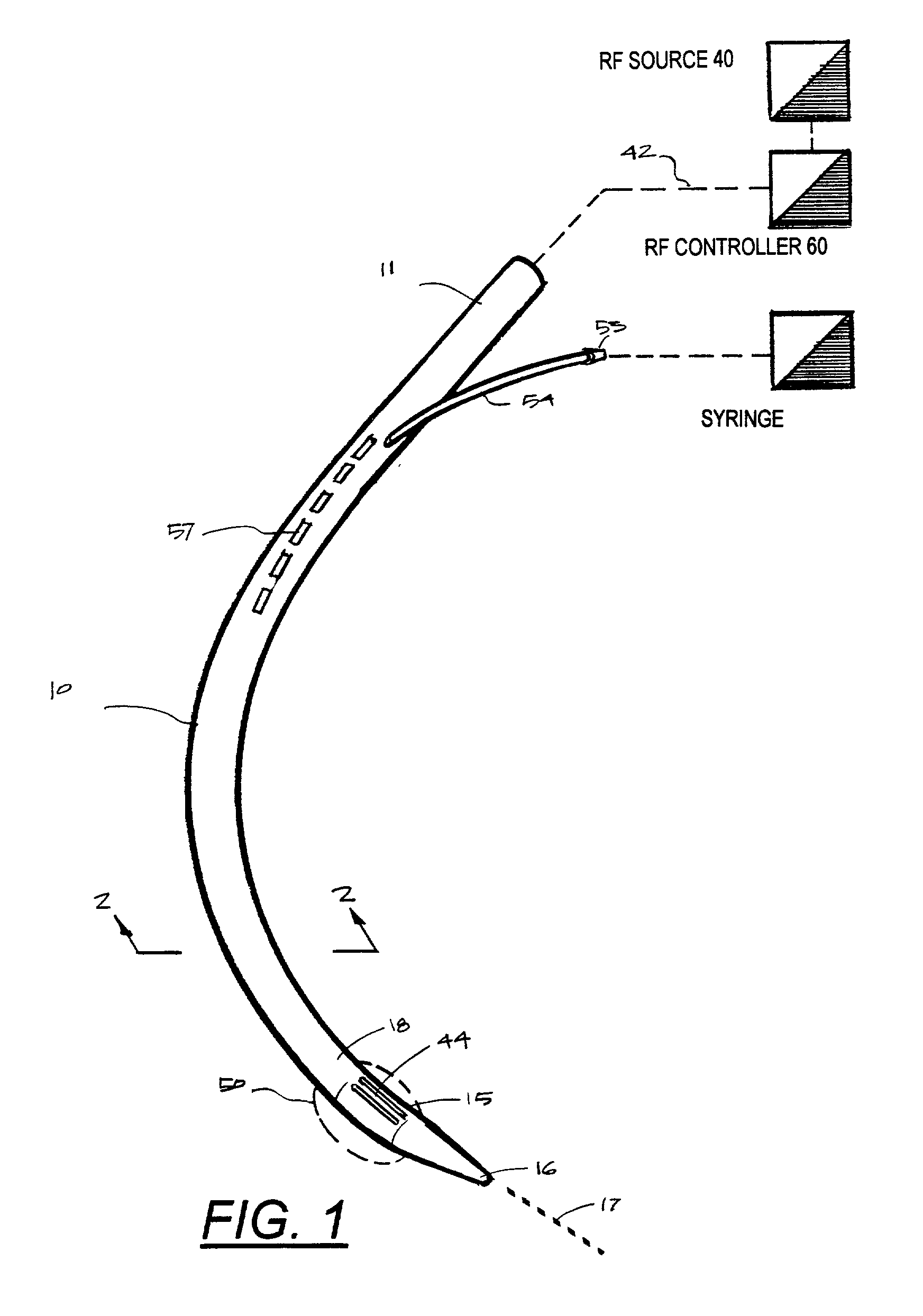
Instruments for thermally-mediated treatment of a patient's lower esophageal sphincter (LES) to induce an injury healing response to thereby populate the extracellular compartment of walls of the LES with collagen matrices to altere the biomechanics of the LES to provide an increased intra-esophageal pressure for preventing acid reflux. A preferred embodiment is a bougie-type device for trans-esophageal introduction that carries conductive electrodes for delivering Rf energy to walls of the LES (i) to induce the injury healing response or (ii) to "model" collagenous tissues of the LES by shrinking collagen fibers therein. Typically, an Rf source is connected to at least one conductive electrode that may be operated in a mono-polar or bi-polar fashion. A sensor array of individual sensors is provided in the working end. A computer controller is provided, which together with feedback circuitry, is capable of full process monitoring and control of: (i) power delivery; (ii) parameters of a selected therapeutic cycle, (iii) mono-polar or bi-polar energy delivery, and (iv) multiplexing of current flow between various paired electrodes. The controller can determine when the treatment is completed based on time, temperature, tissue impedance or any combination thereof.
Owner:MEDERI RF LLC
Method to treat gastric reflux via the detection and ablation of gastro-esophageal nerves and receptors
Owner:MEDERI RF LLC
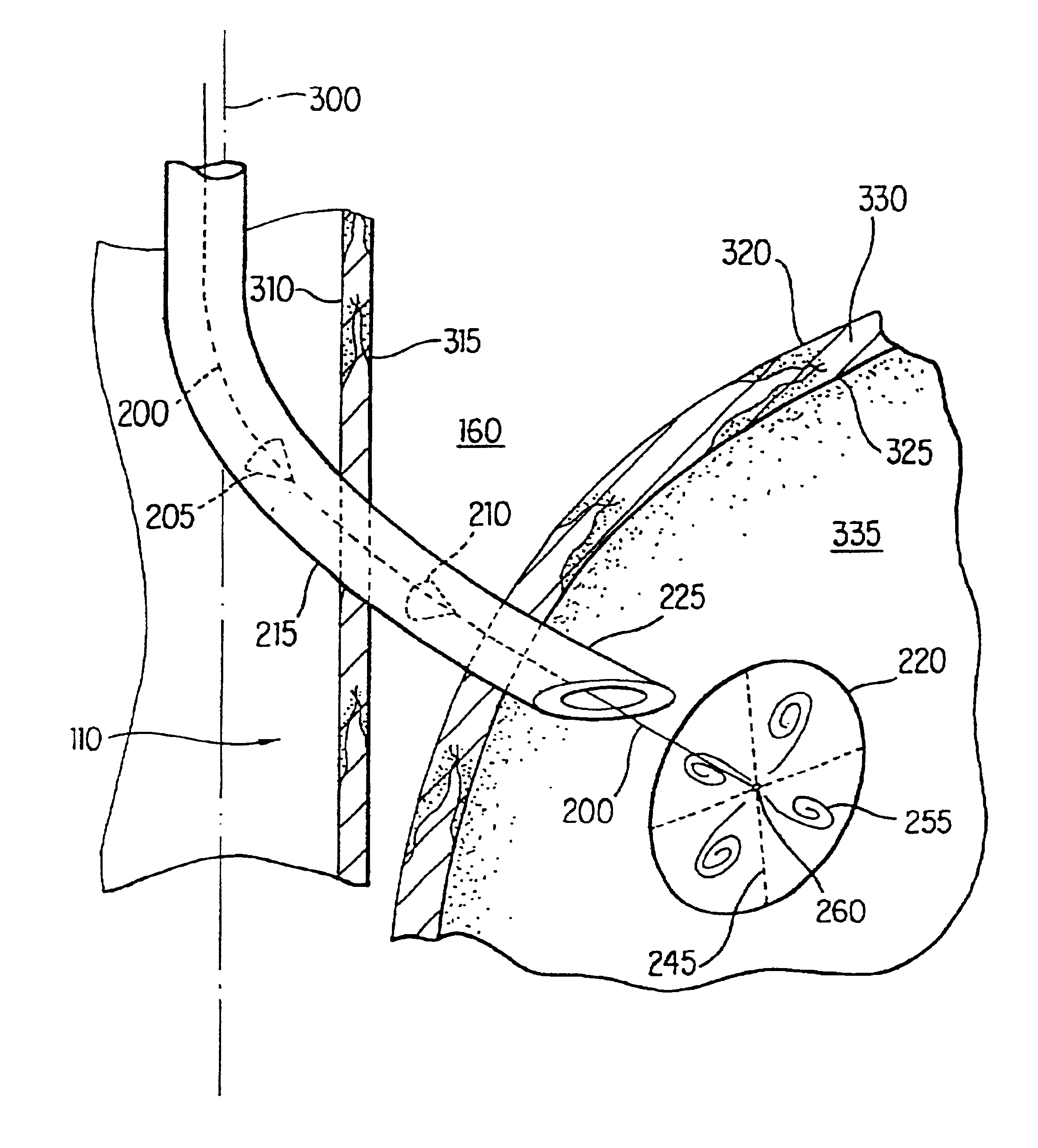
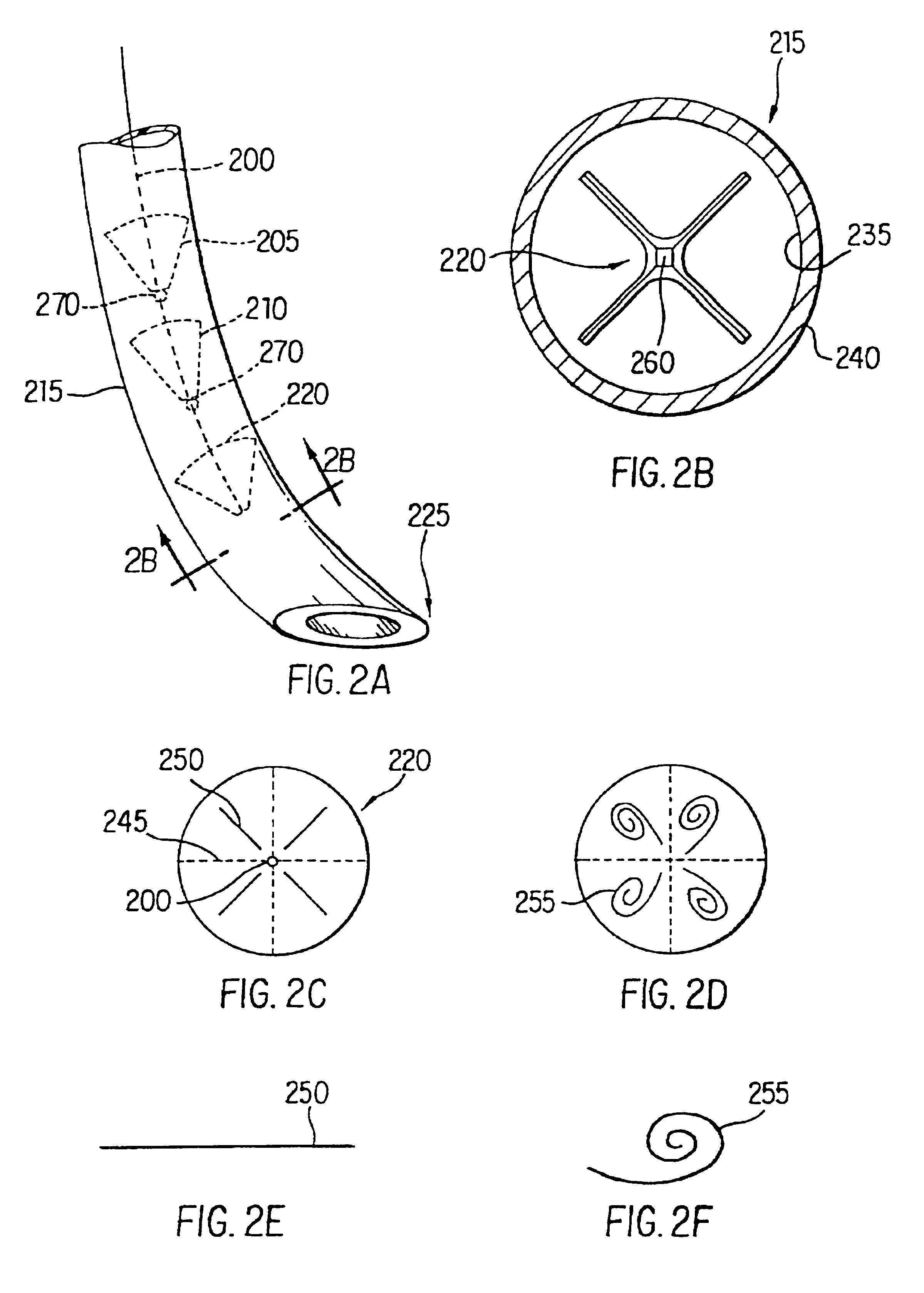
Method and apparatus for endoscopic repair of the lower esophageal sphincter
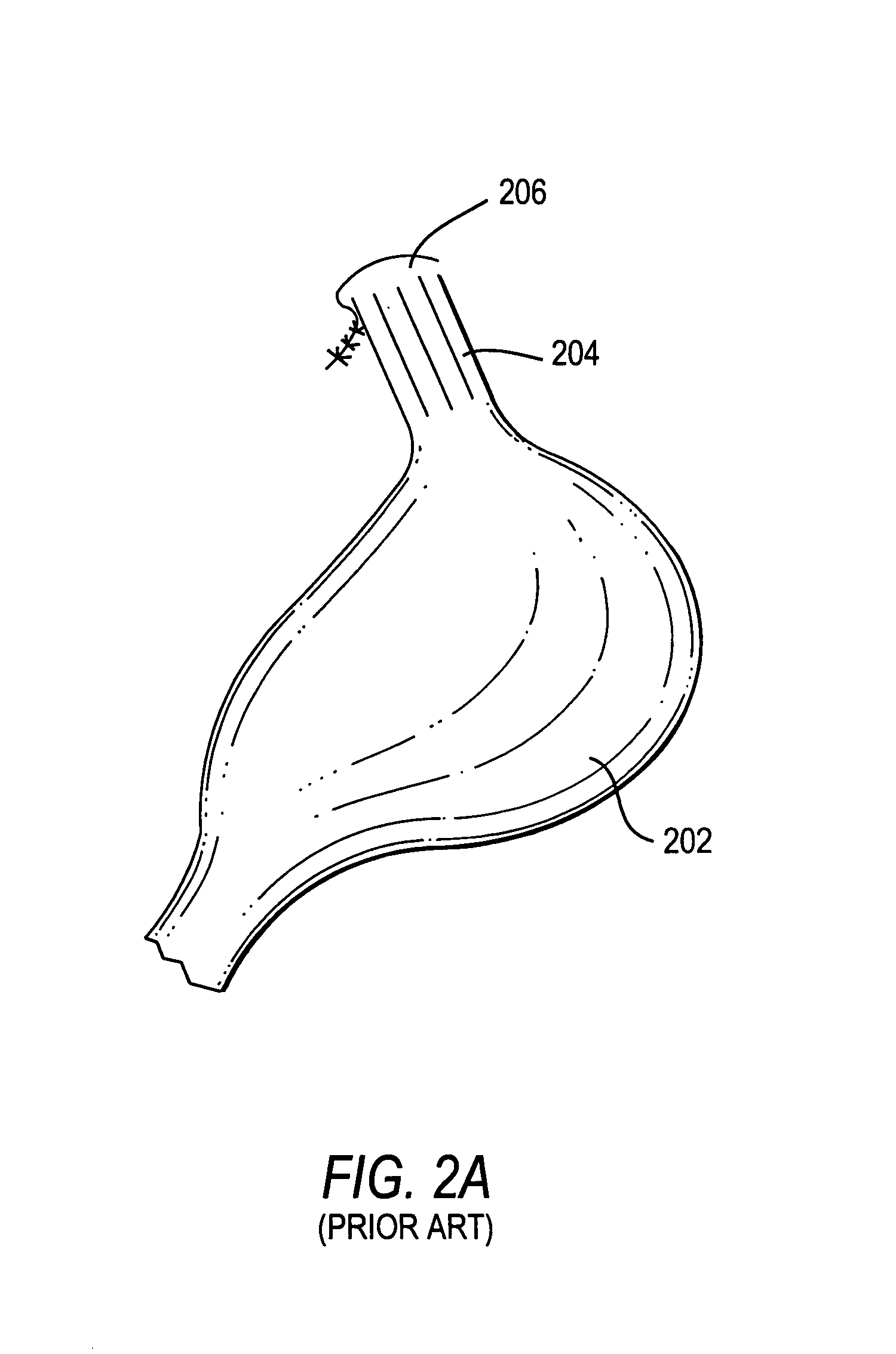
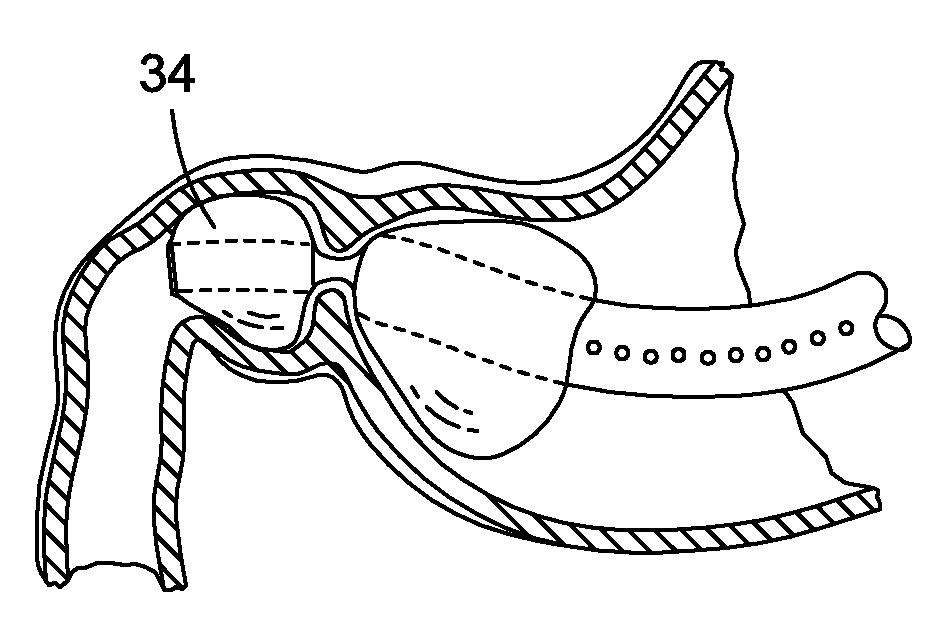
The present invention includes a method and apparatus for adhering tissue to one another. In an embodiment of the present invention the two tissues to be joined, for example the lower esophagus and the fundus of the stomach, are first placed adjacent to one another. Next a first restraint is placed near the outside surface of one of the tissues and a second restraint is placed near the outside surface of the other tissue. An irritant is then placed between the two adjacent tissues. The restraints, and consequently the tissue surfaces, are then drawn together. As the touching irritated tissue surfaces heal they will become bonded to one another and their need for the mechanical fastening of the restraints, to secure them together, will be diminished.
Owner:SCI MED LIFE SYST
Method to treat gastric reflux via the detection and ablation of gastro-esophageal nerves and receptors
A method of treating a sphincter provides a sphincter electropotential mapping device with at least one of a mapping electrode or a treatment electrode. The sphincter electropotential mapping device is introduced into at least a portion of the sphincter, the lower esophageal sphincter, stomach, the cardia or the fundus. Bioelectric activity causing a relaxation of the sphincter is detected and energy is delivered from either the mapping electrode or the treatment electrode to treat the bioelectric activity. A method of treating a sphincter provides a sphincter electropotential mapping device with at least one of a mapping electrode or a treatment electrode. The sphincter electropotential mapping device is introduced into at least a portion of the sphincter, the lower esophageal sphincter, stomach, the cardia or the fundus. Bioelectric activity causing a relaxation of the sphincter is detected and energy is delivered from either the mapping electrode or the treatment electrode to treat the bioelectric activity.
Owner:MEDERI RF LLC
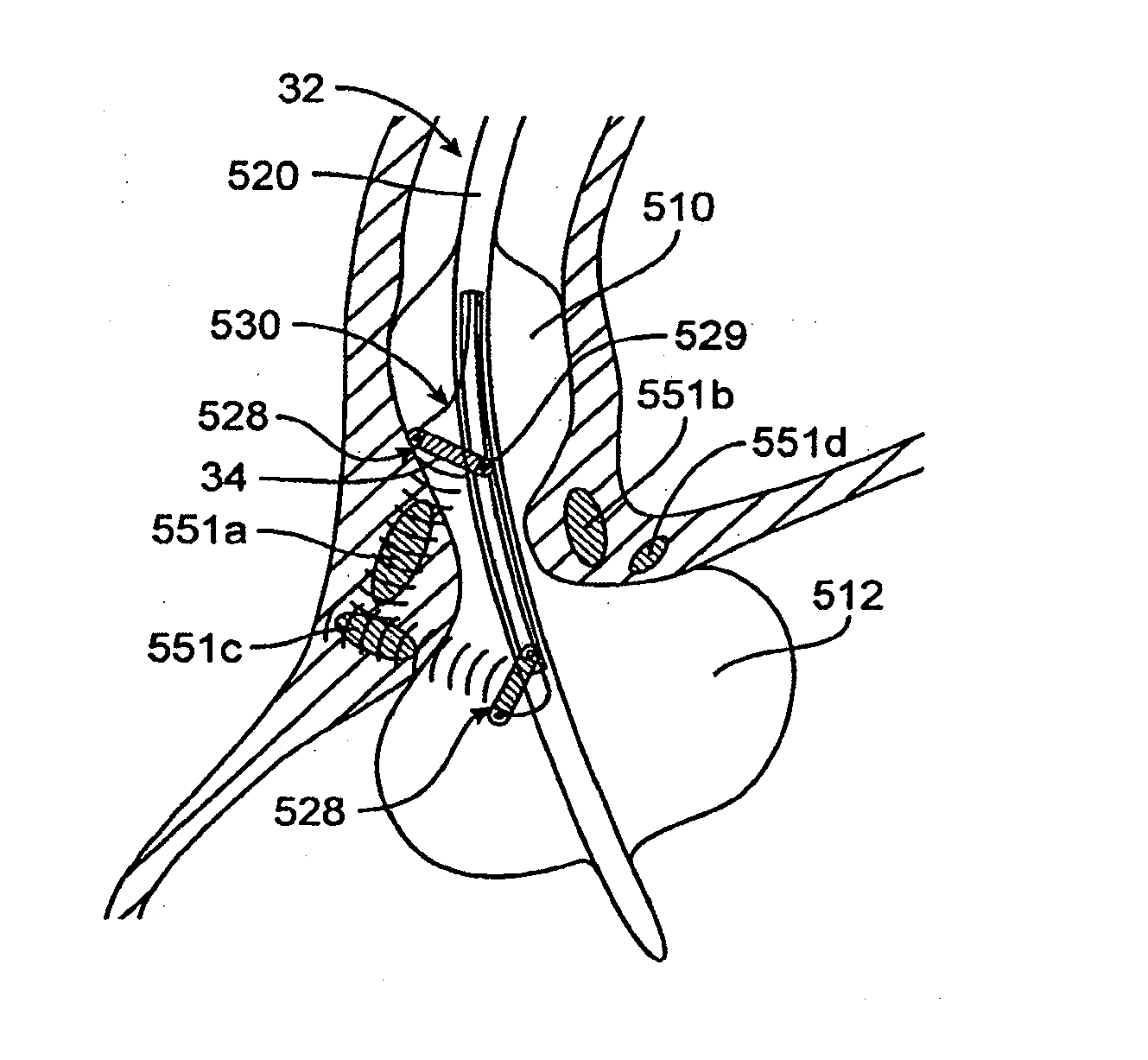
Methods and devices for folding and securing tissue
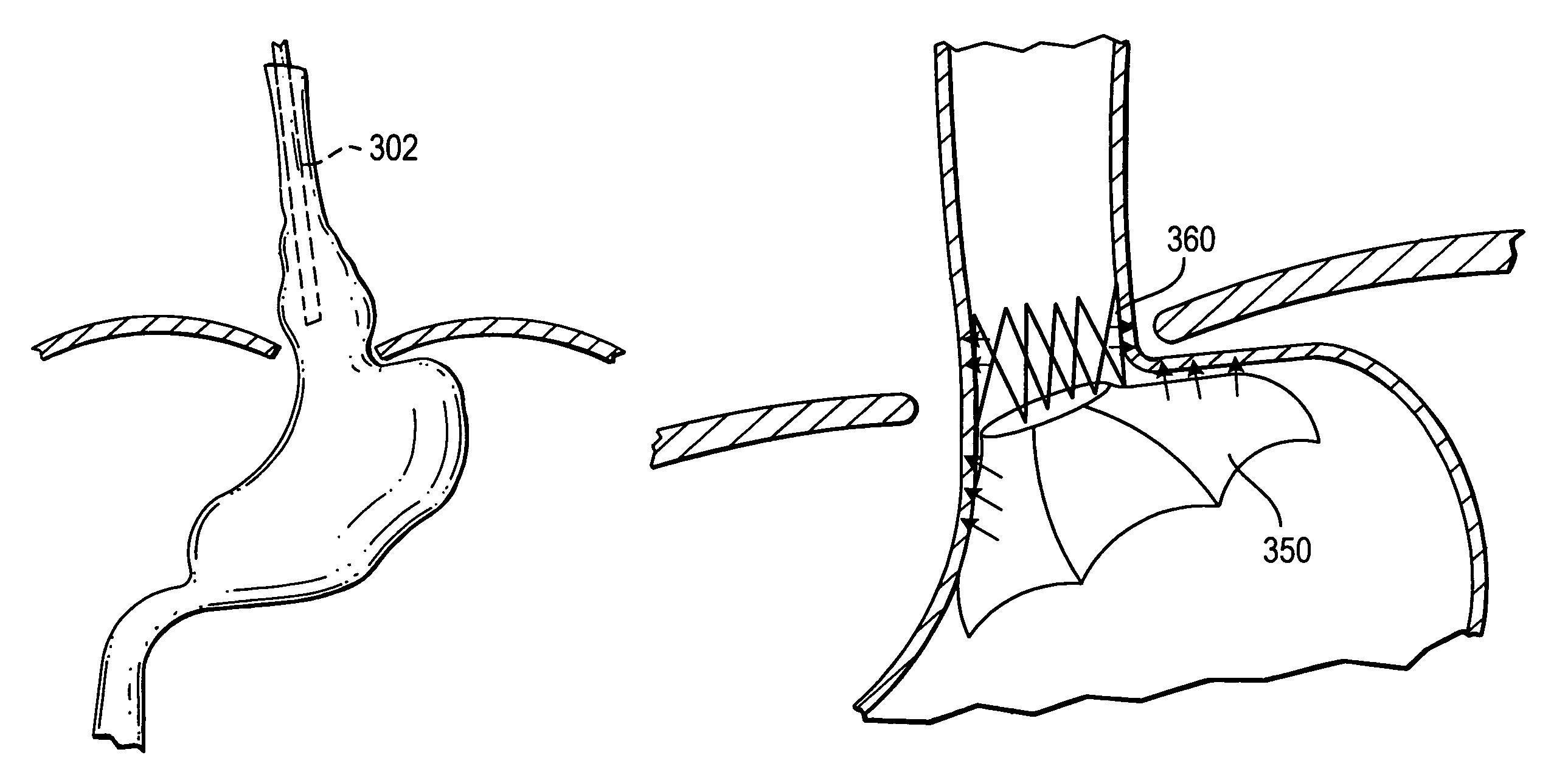
InactiveUS9173656B2Without compromising safetyHigh intrusion performanceStaplesNailsEsophagus wallMedical treatment
The present invention relates to devices, and methods for using the devices, to create and secure a tissue fold during an endoluminal medical procedure. The devices and methods may be used for folding and securing, for example, a fundus wall onto an esophagus wall or esophageal tissue in the region of the lower esophageal sphincter (LES) to reduce the diameter of the esophagus opening in that region. One aspect of the invention includes forming the tissue fold by closing a grasping arm that is pivotably connected to an overtube that has been positioned at the juncture of the fundus wall and esophagus wall. A further aspect of the invention includes tissue clips configured to be inserted and positioned through an endoluminal device.
Owner:BOSTON SCI SCIMED INC
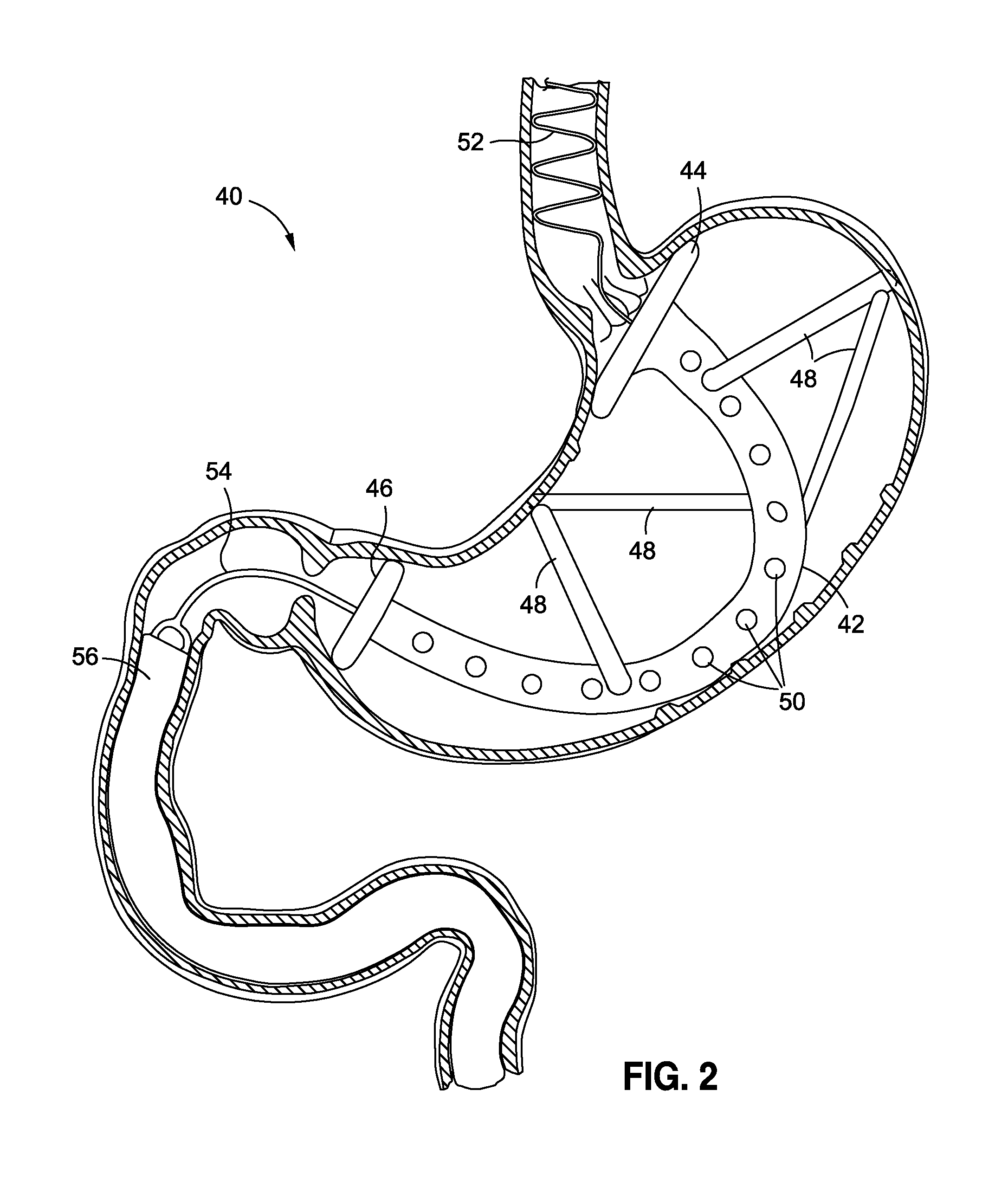
Device and method for treatment of gastroesophageal reflux disease
A lower esophageal sphincter tightening device for treating gastroesophageal reflux disease which includes an insertion device, an energy source, and an energy transmitting device. The insertion device, by insertion through a body opening, positions the energy transmitting device in the proximity of the lower esophageal sphincter. The energy source generates and transmits energy via the insertion device to the energy transmitting device which directs the transmitted energy onto the lower esophageal sphincter which is comprised largely of collagen. The energy source transmits energy at a level sufficient to cause heating of the sphincter's collagen resulting in a shrinkage of the collagen and a tightening of the sphincter.
Owner:BOSTON SCI SCIMED INC
Gastro-esophageal reflux disease (GERD) treatment method and apparatus
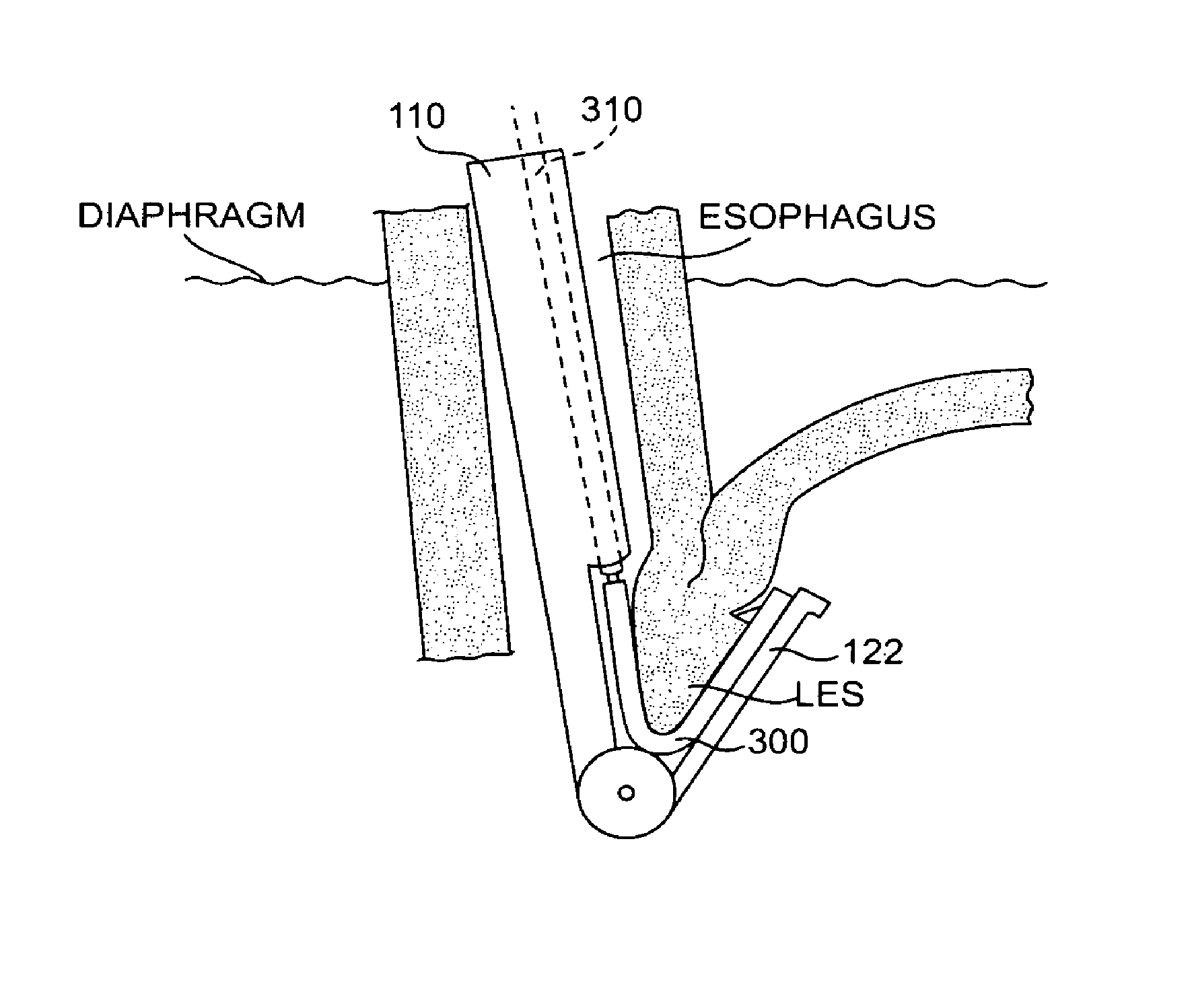
The method of the invention includes accessing a juncture of an esophagus and a stomach of the patient on a distal side of a diaphragm. The esophagus and a fundus of the stomach intersect at a cardiac notch located at an original cardiac notch position. A reducing element is placed at the junction with the reducing element selected to reposition the cardiac notch to a position more distal to a lower esophageal sphincter of the patient.
Owner:RESHAPE LIFESCIENCES INC
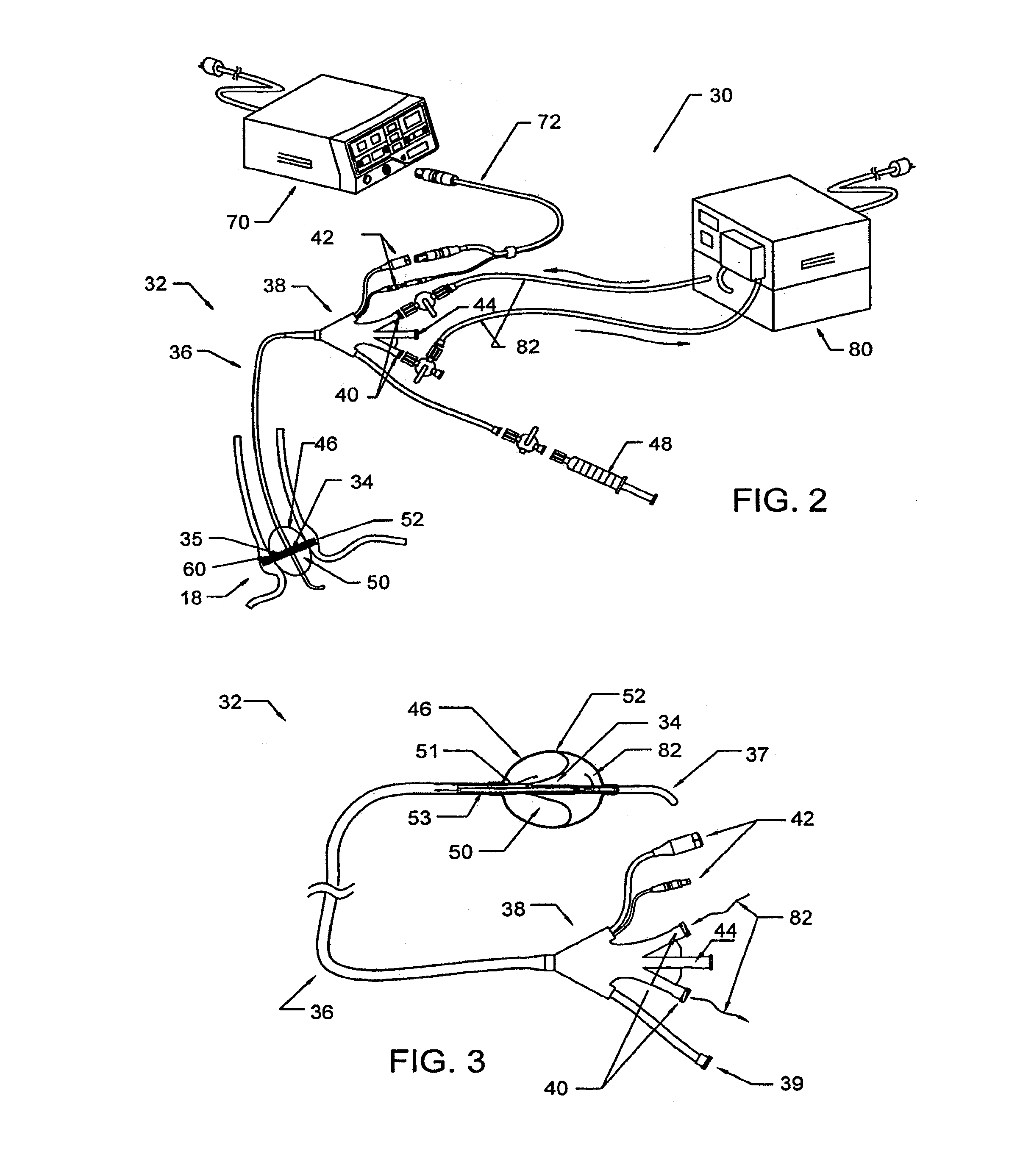
Device and implantation system for electrical stimulation of biological systems
ActiveUS20110307027A1Increase pressureKeep the pressureImplantable neurostimulatorsDiagnostic recording/measuringElectricityPhysical therapy
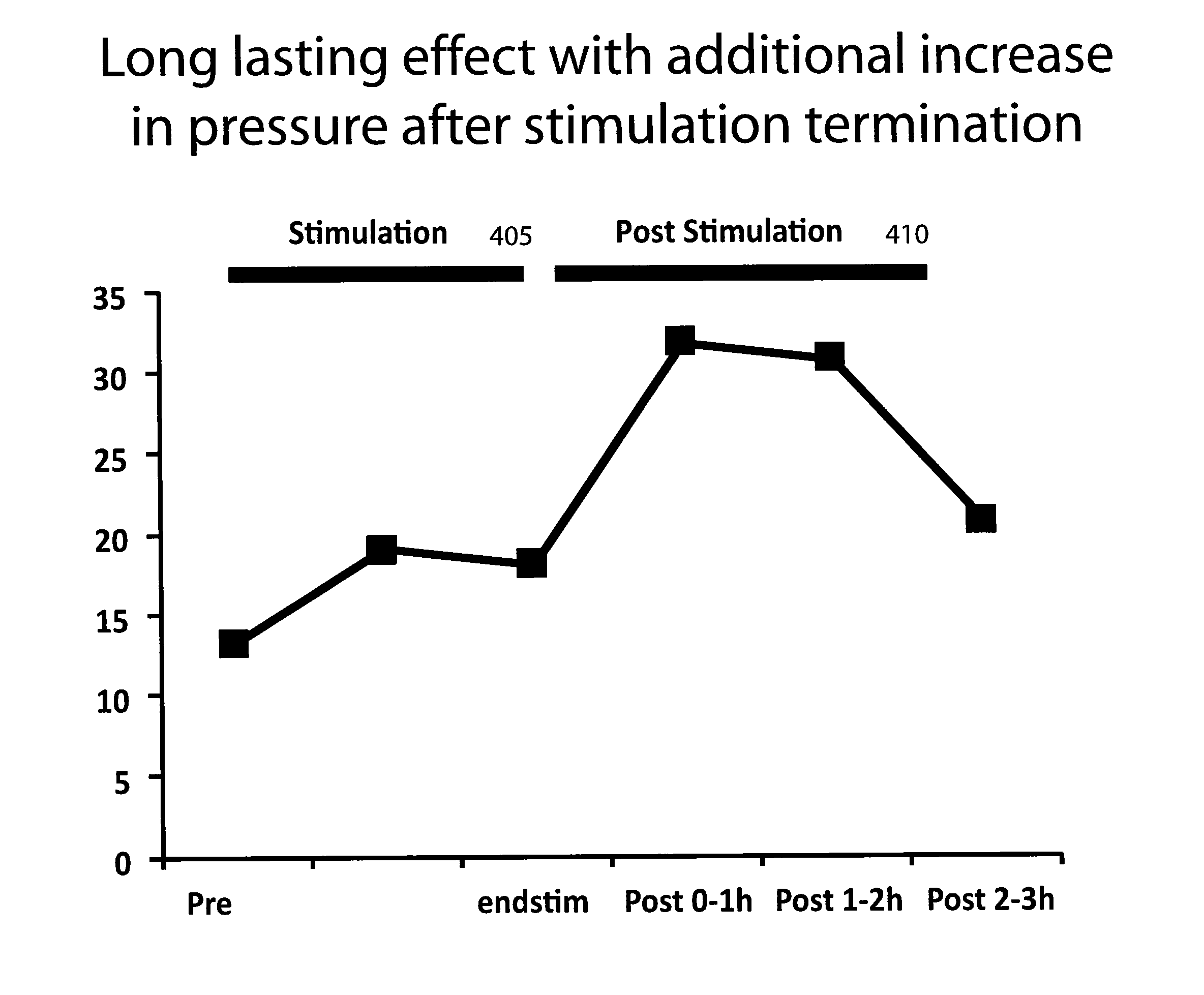
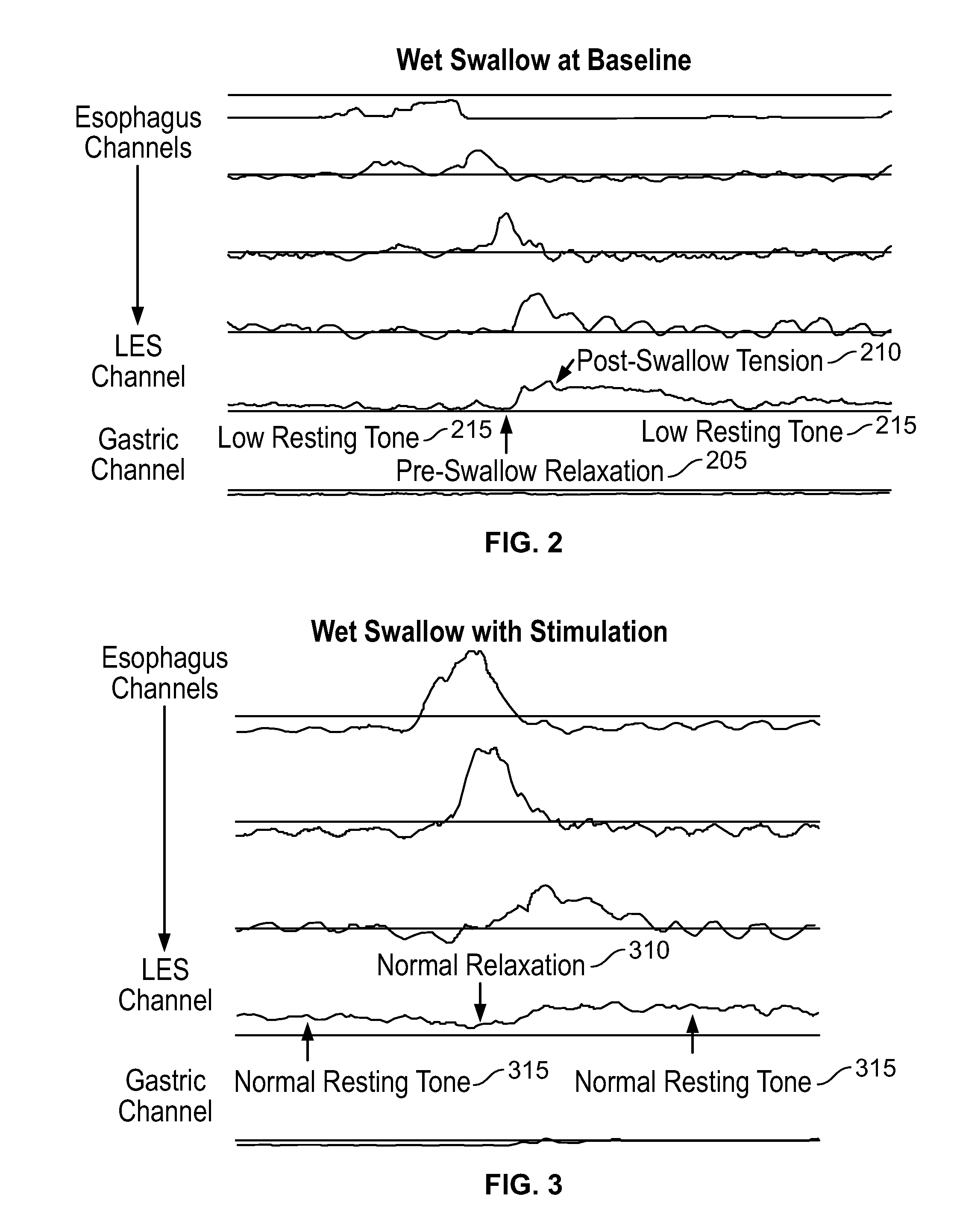
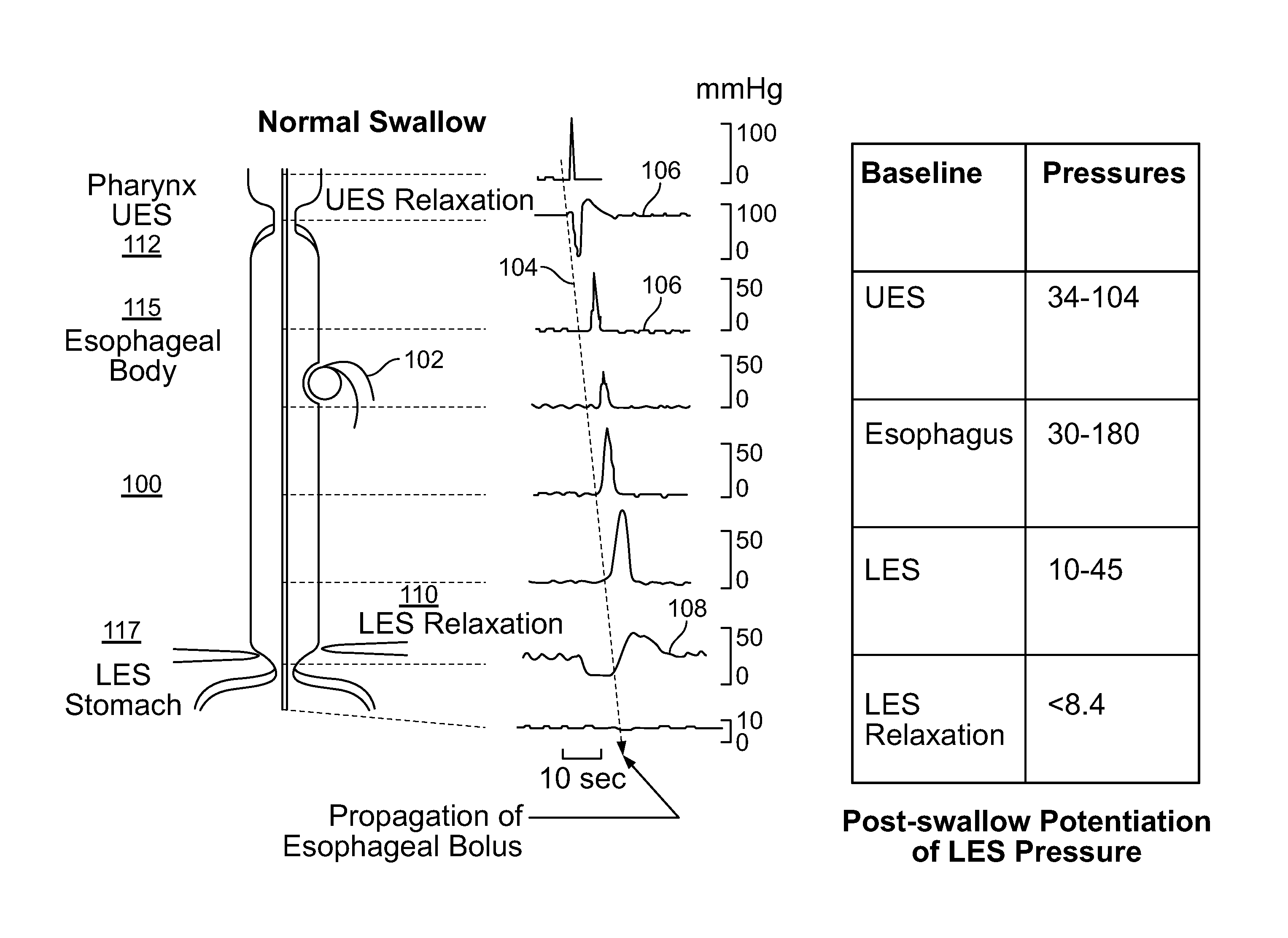
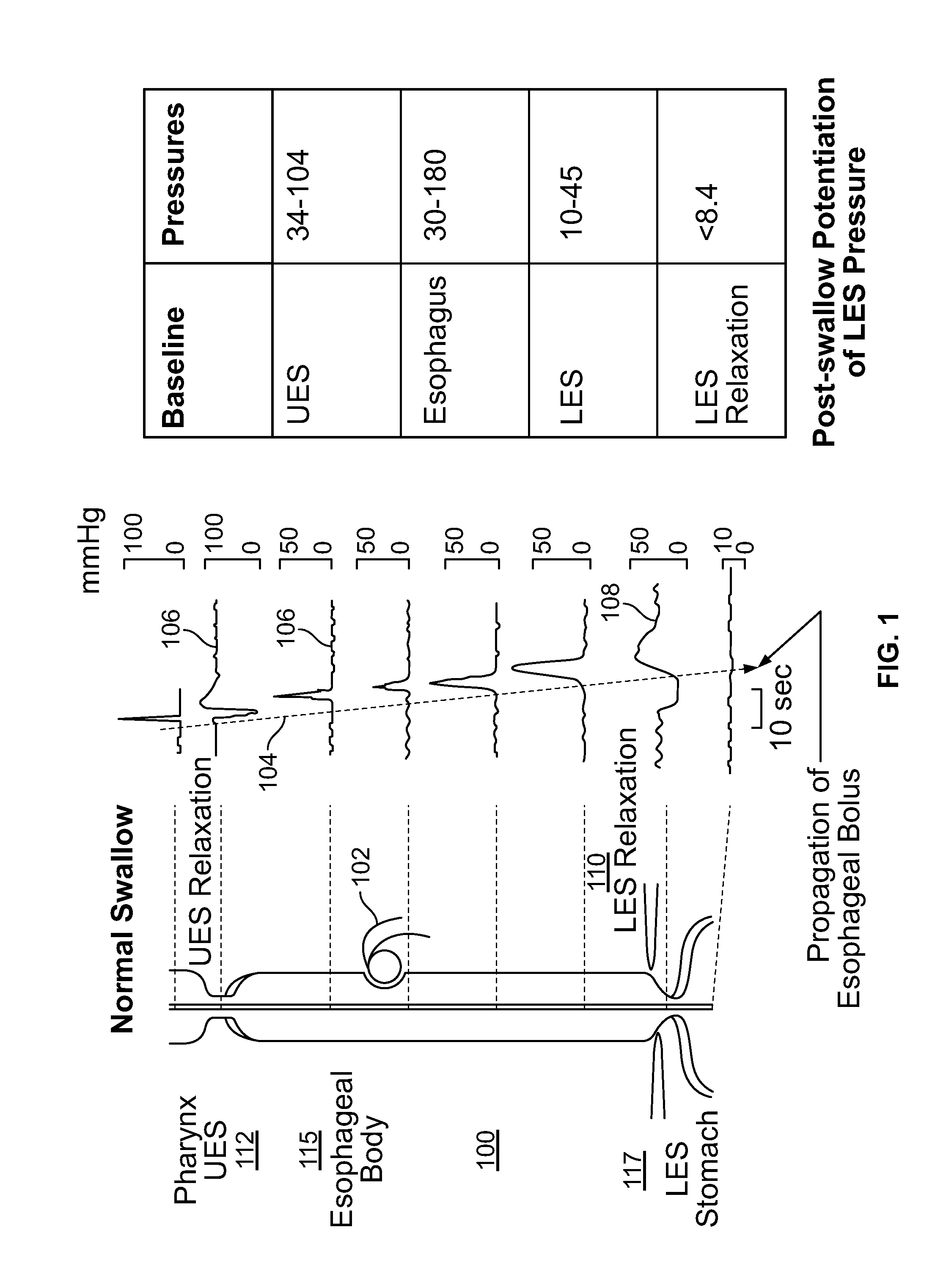
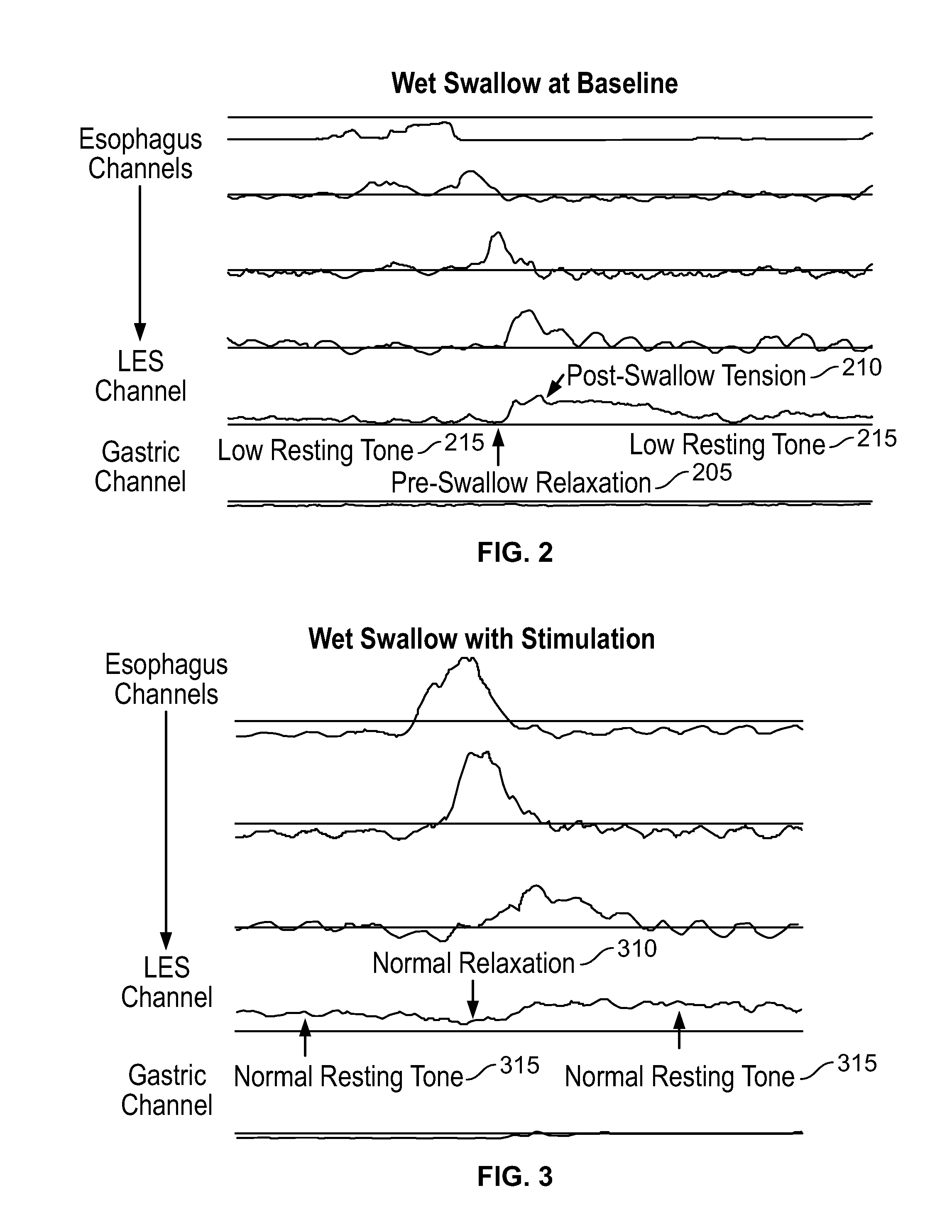
The present specification discloses devices and methodologies for the treatment of GERD. Individuals with GERD may be treated by implanting a stimulation device within the patient's lower esophageal sphincter and applying electrical stimulation to the patient's lower esophageal sphincter, in accordance with certain predefined protocols. The presently disclosed devices have a simplified design because they do not require sensing systems capable of sensing when a person is engaged in a wet swallow and have improved energy storage requirements.
Owner:PARAS HLDG LLC
Device and implantation system for electrical stimulation of biological systems
ActiveUS20110295336A1Less energyImproved LES functionImplantable neurostimulatorsDiagnostic recording/measuringElectricityPhysical therapy
The present specification discloses devices and methodologies for the treatment of diurnal GERD. Individuals with GERD may be treated by implanting a stimulation device within the patient's lower esophageal sphincter and applying electrical stimulation to the patient's lower esophageal sphincter, in accordance with certain predefined protocols. The presently disclosed devices have a simplified design because they do not require sensing systems capable of sensing when a person is engaged in a wet swallow and have improved energy storage requirements.
Owner:PARAS HLDG LLC
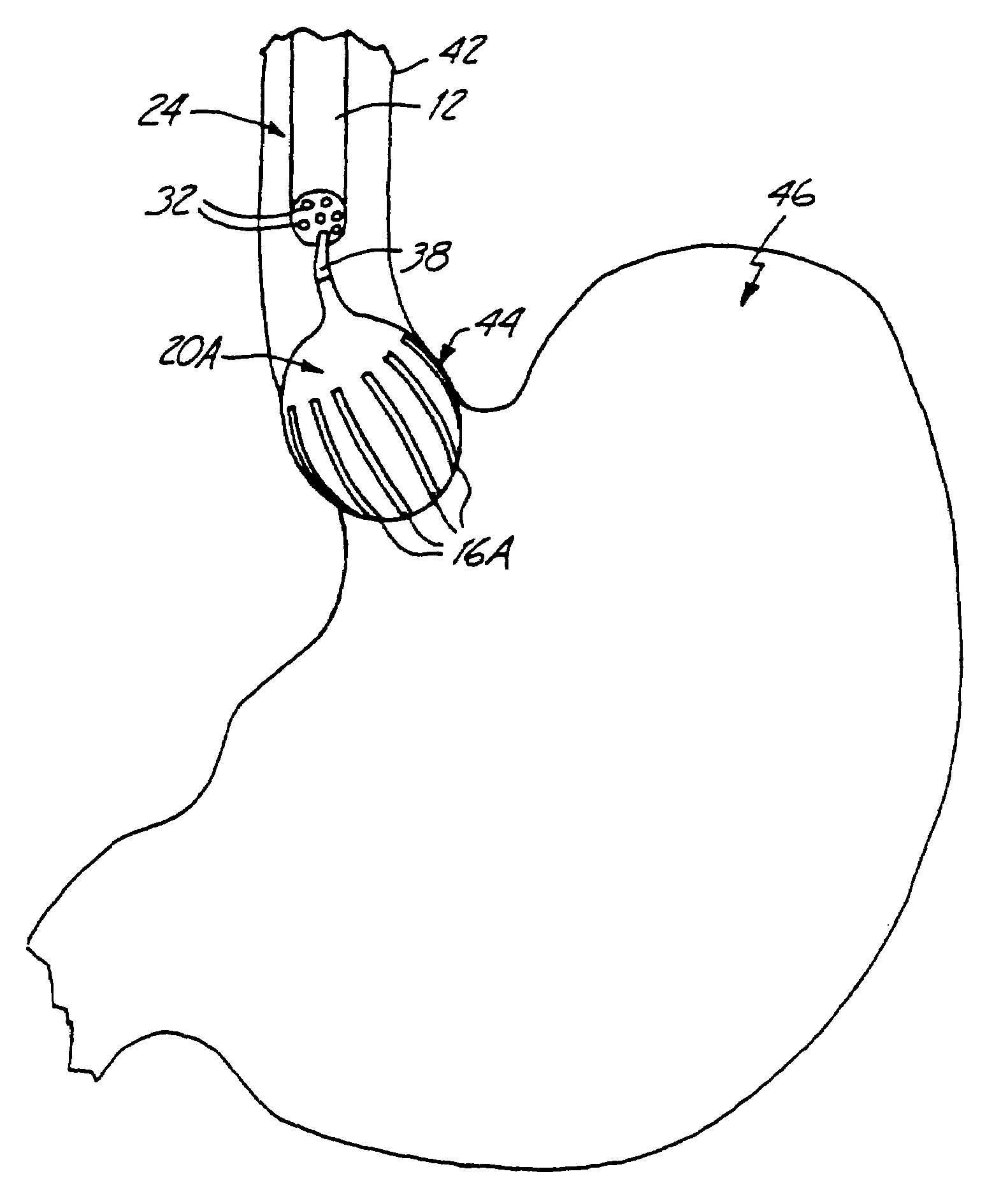
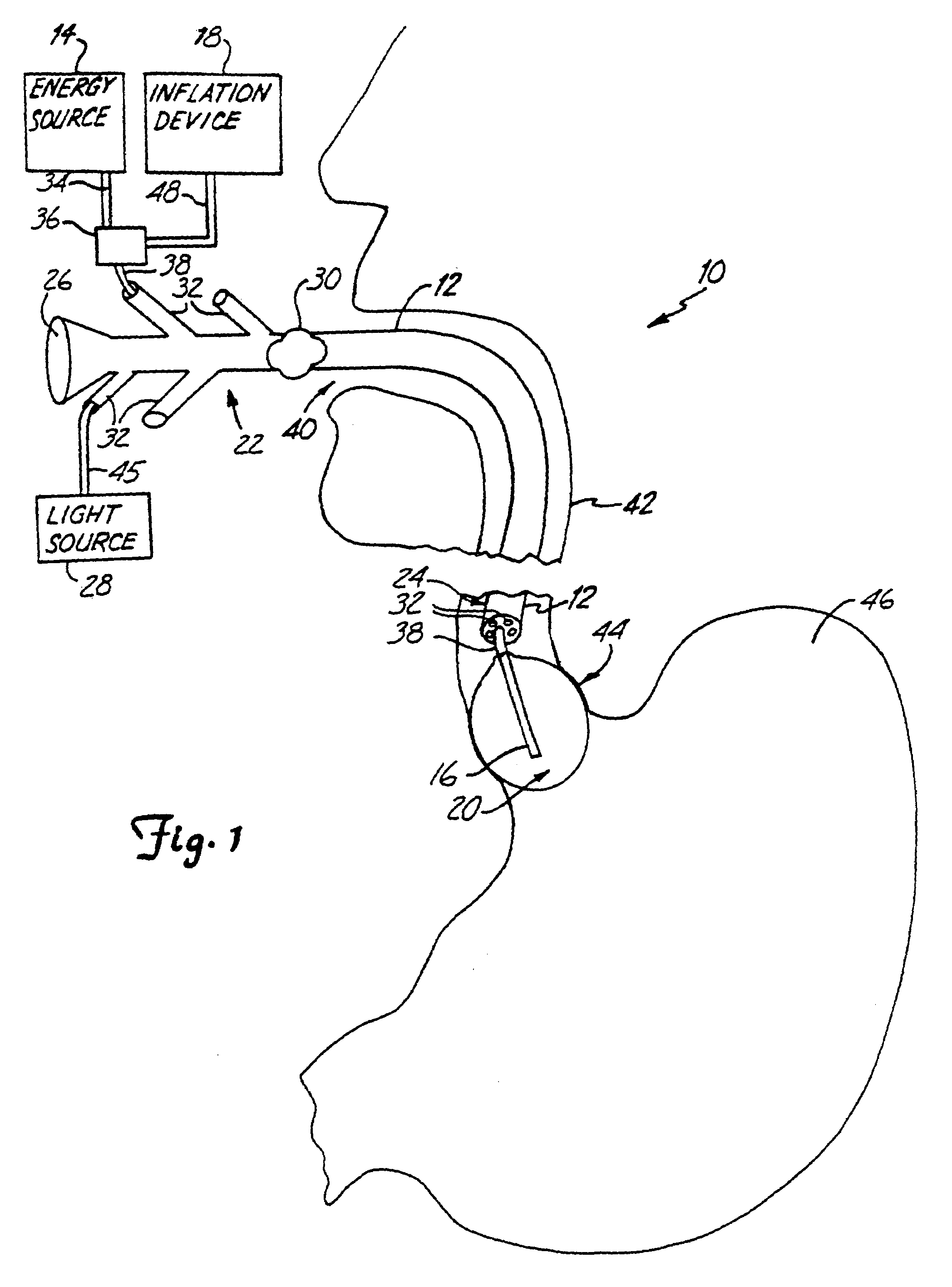
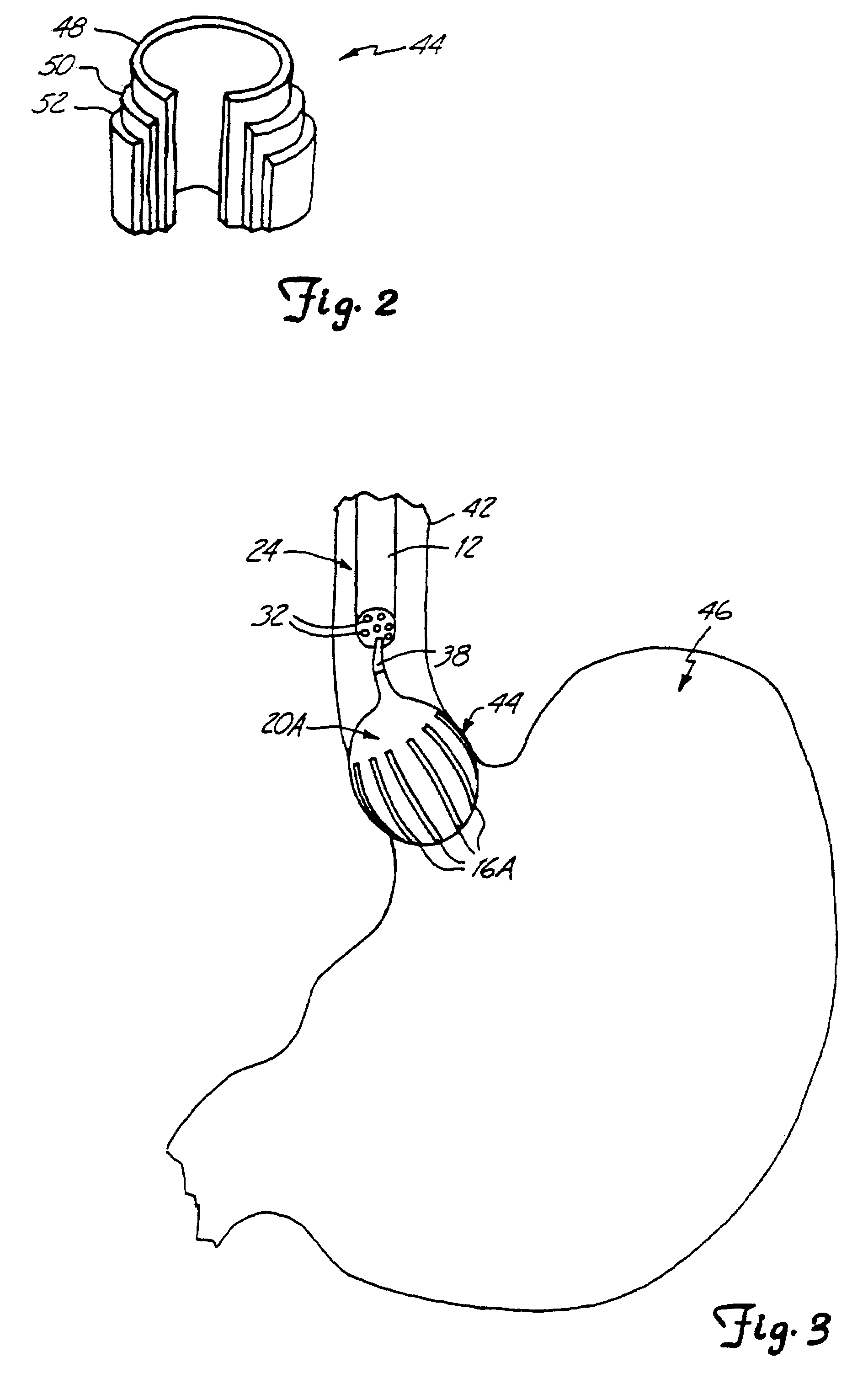
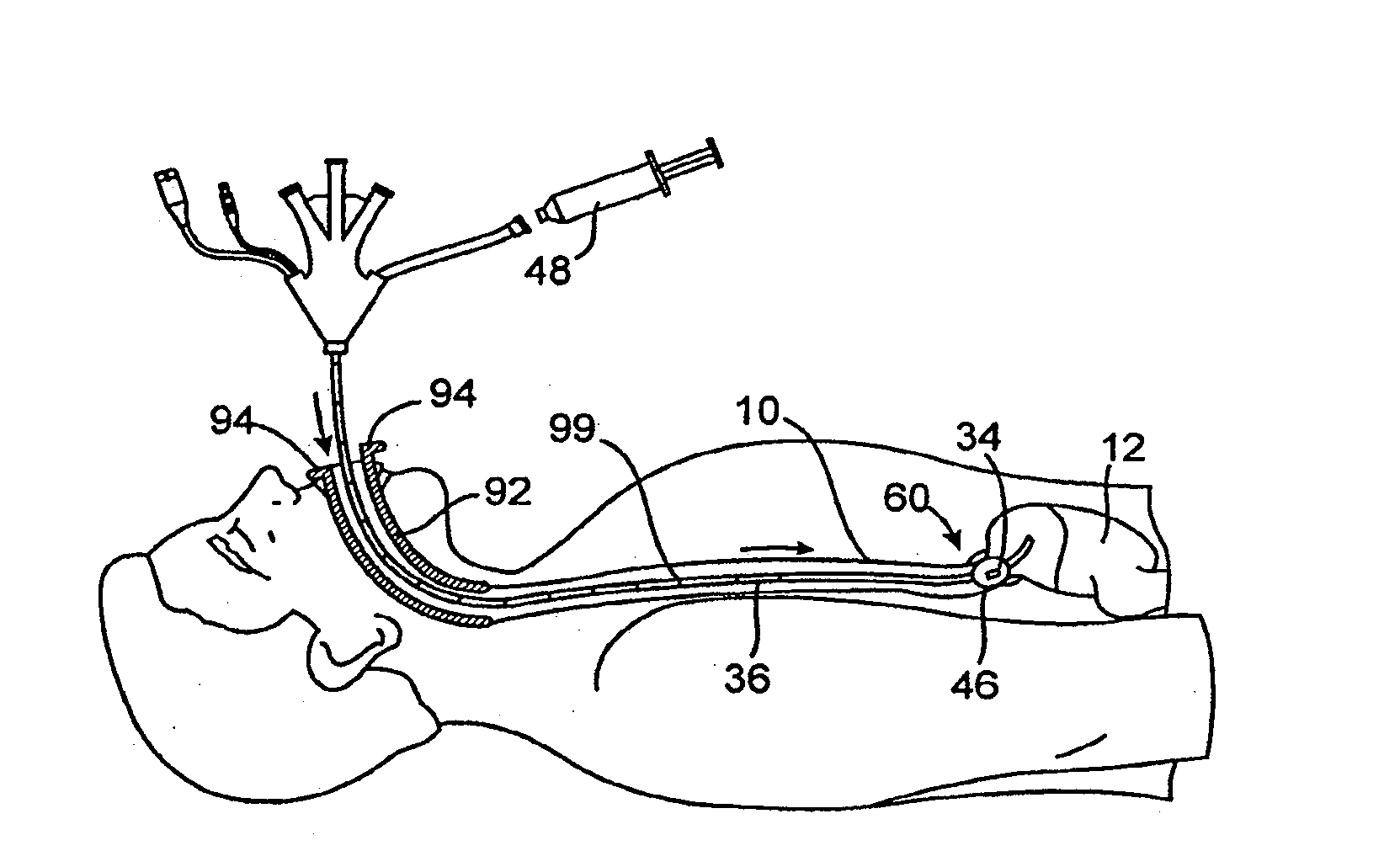
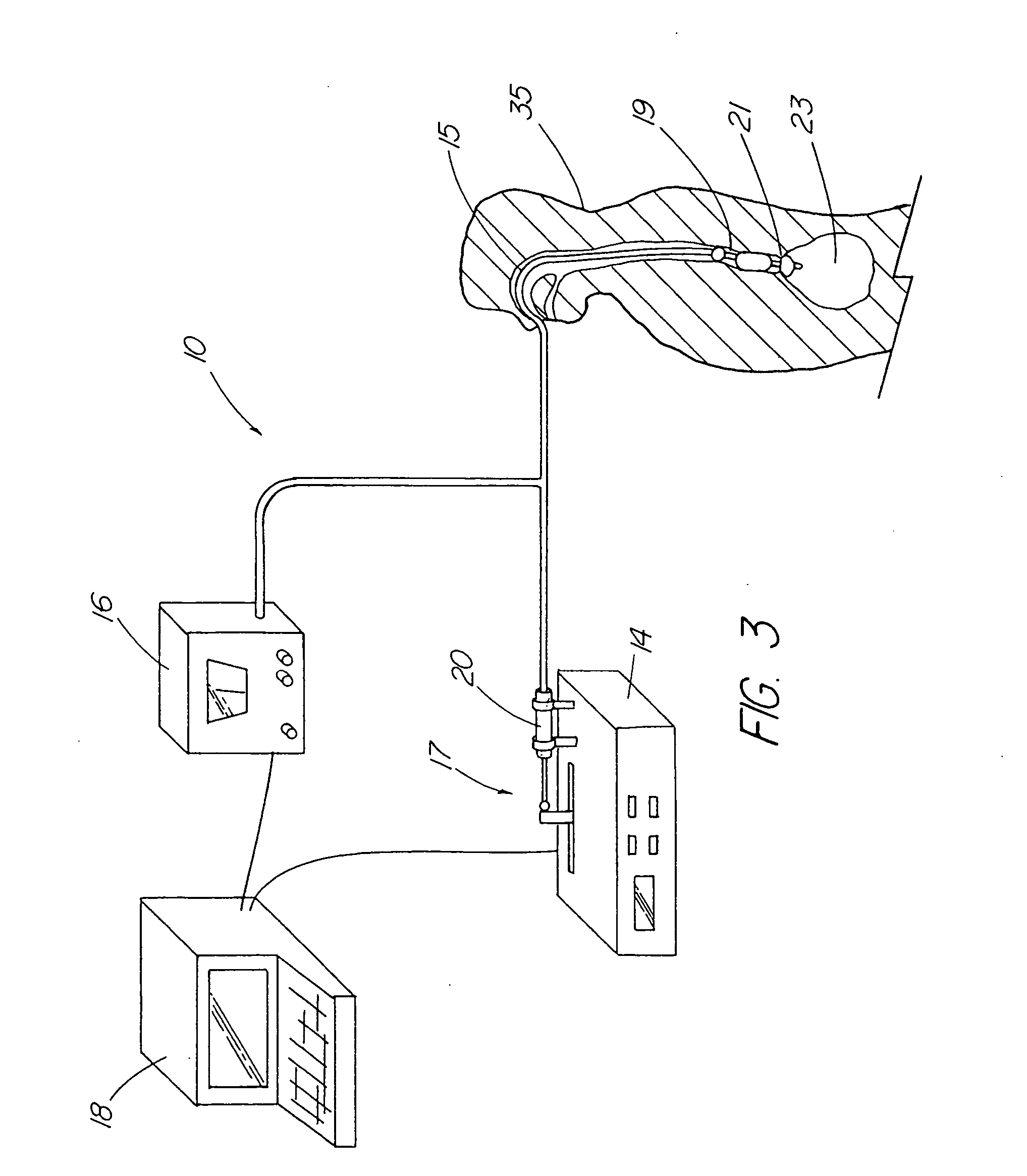
Esophageal delivery system and method with position indexing
InactiveUS20050245788A1Precise positioningQuick and convenient and accurate placementGastroscopesOesophagoscopesImaging equipmentEndoscope
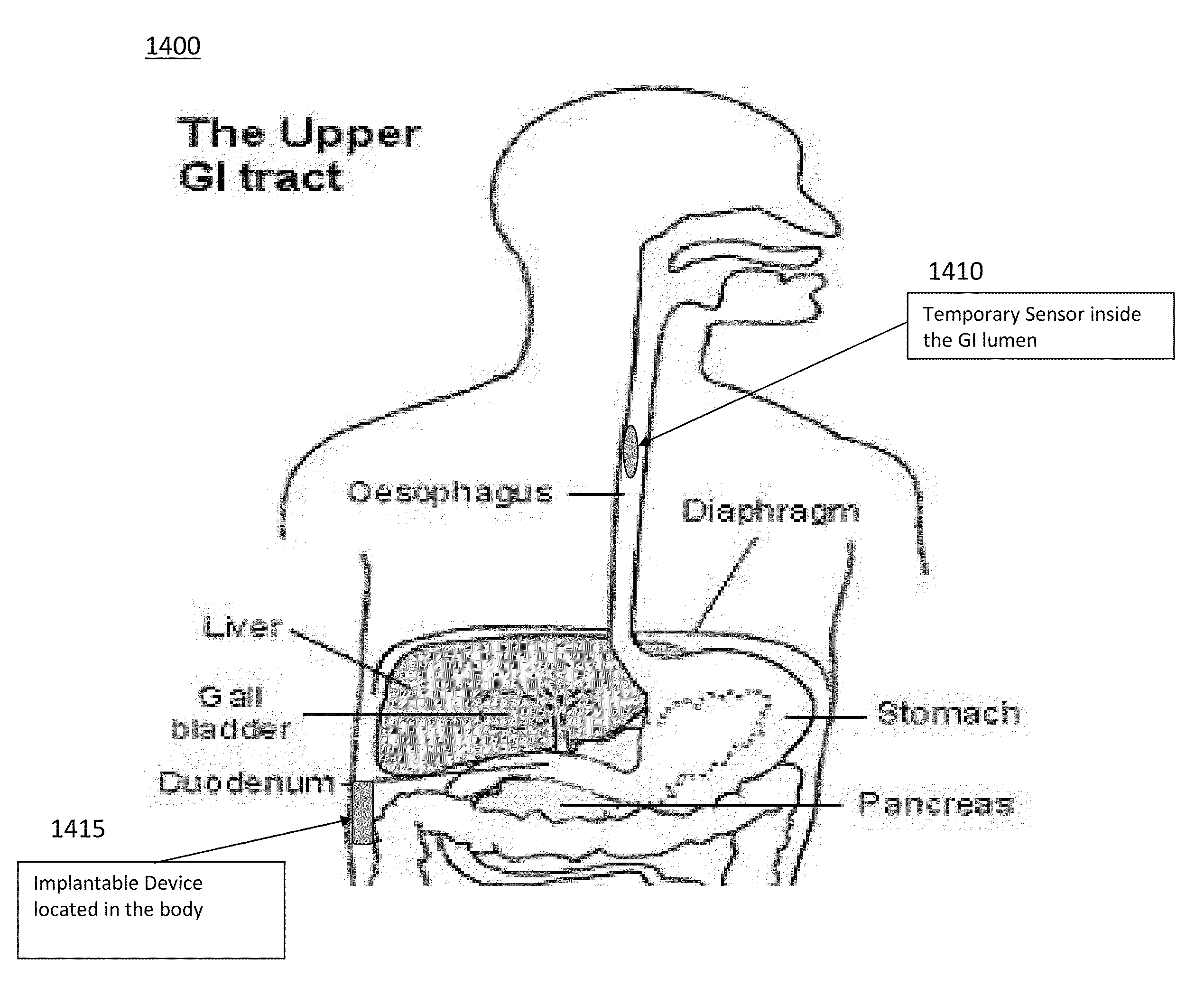
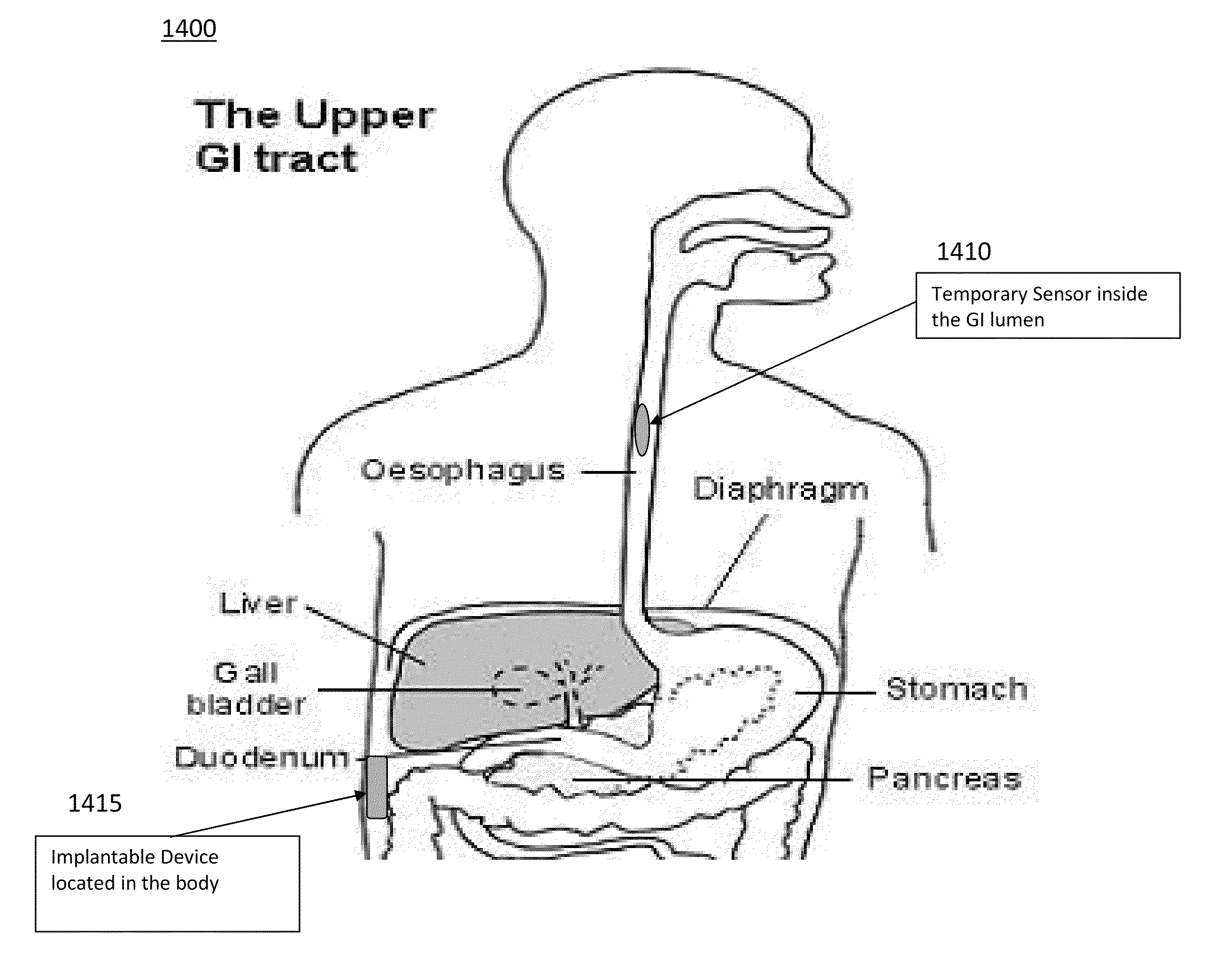
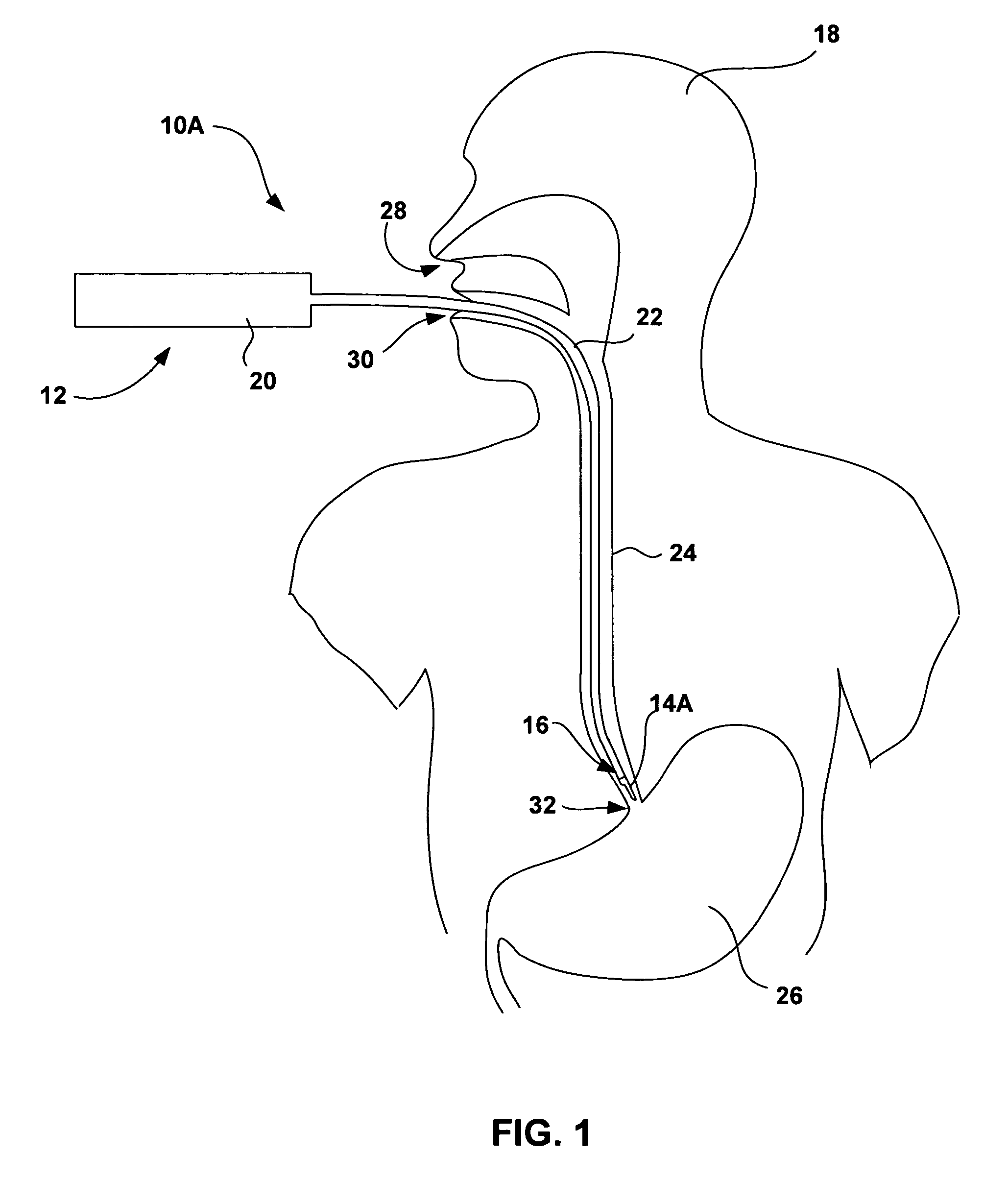
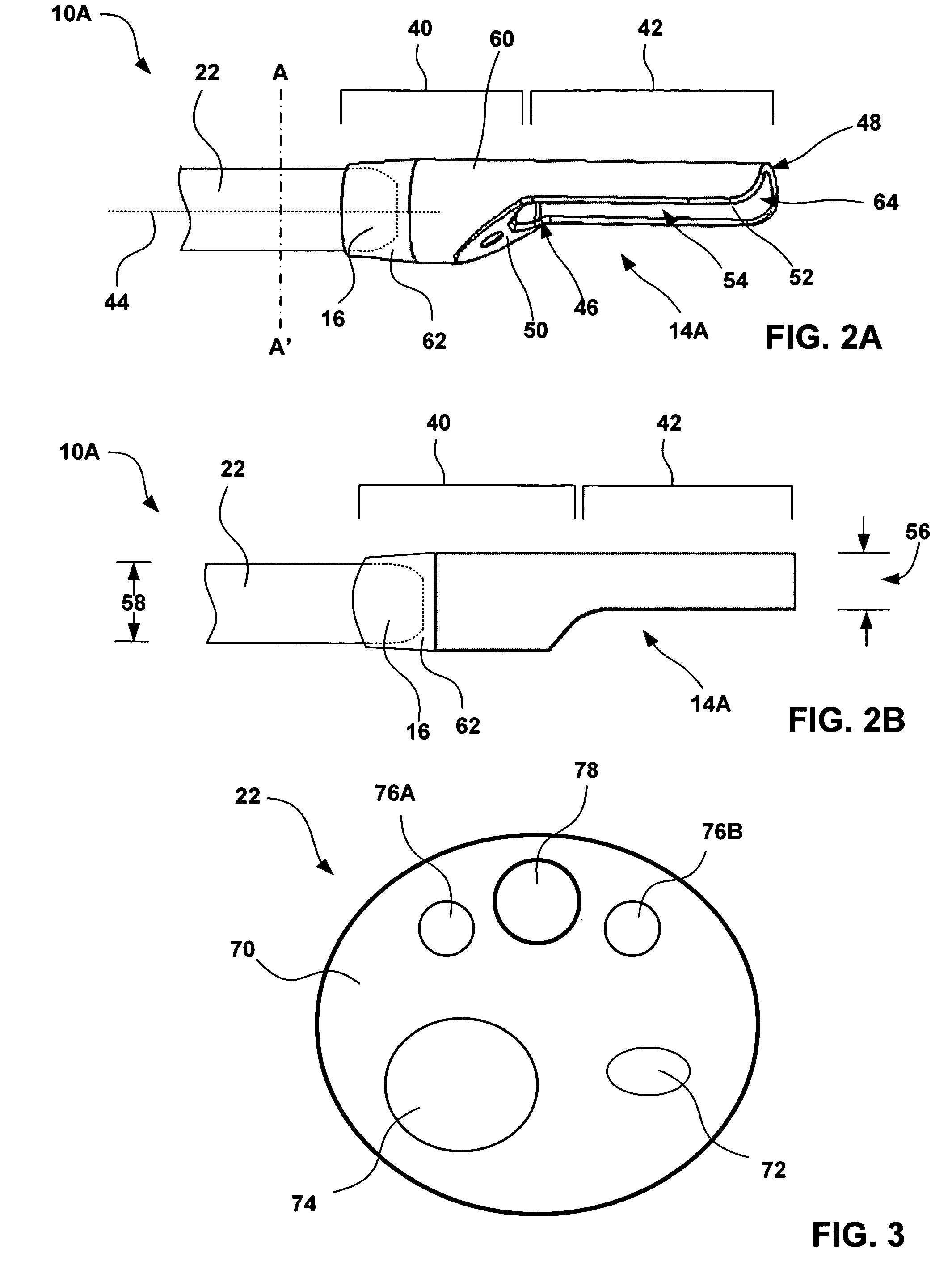
An esophageal delivery system includes features that facilitate the precise positioning of a medical device within the gastrointestinal tract. The system supports indexed positioning without relying solely on endoscopic viewing or external imaging equipment to identify the location of the medical device. A fixation element holds an elongated delivery device at a selected position within the gastrointestinal tract. The medical device is carried at a position that is a fixed and known distance away from the fixation element. Once the fixation element is positioned at a known location, precise positioning of the medical device within the esophagus can be achieved with greater certainty. As an illustration, the fixation element may take the form of a balloon an expandable frame or other device capable of engaging the lower esophageal sphincter (LES) to provide a reference position for a medical device to be placed at a precise distance above the LES.
Owner:MEDTRONIC INC
Method and apparatus for treatment of the gastrointestinal tract
InactiveUS7738961B2Increasing UES, esophageal or LES sphincter toneReduce delaysElectrotherapyArtificial respirationUpper esophageal sphincterSmall intestine
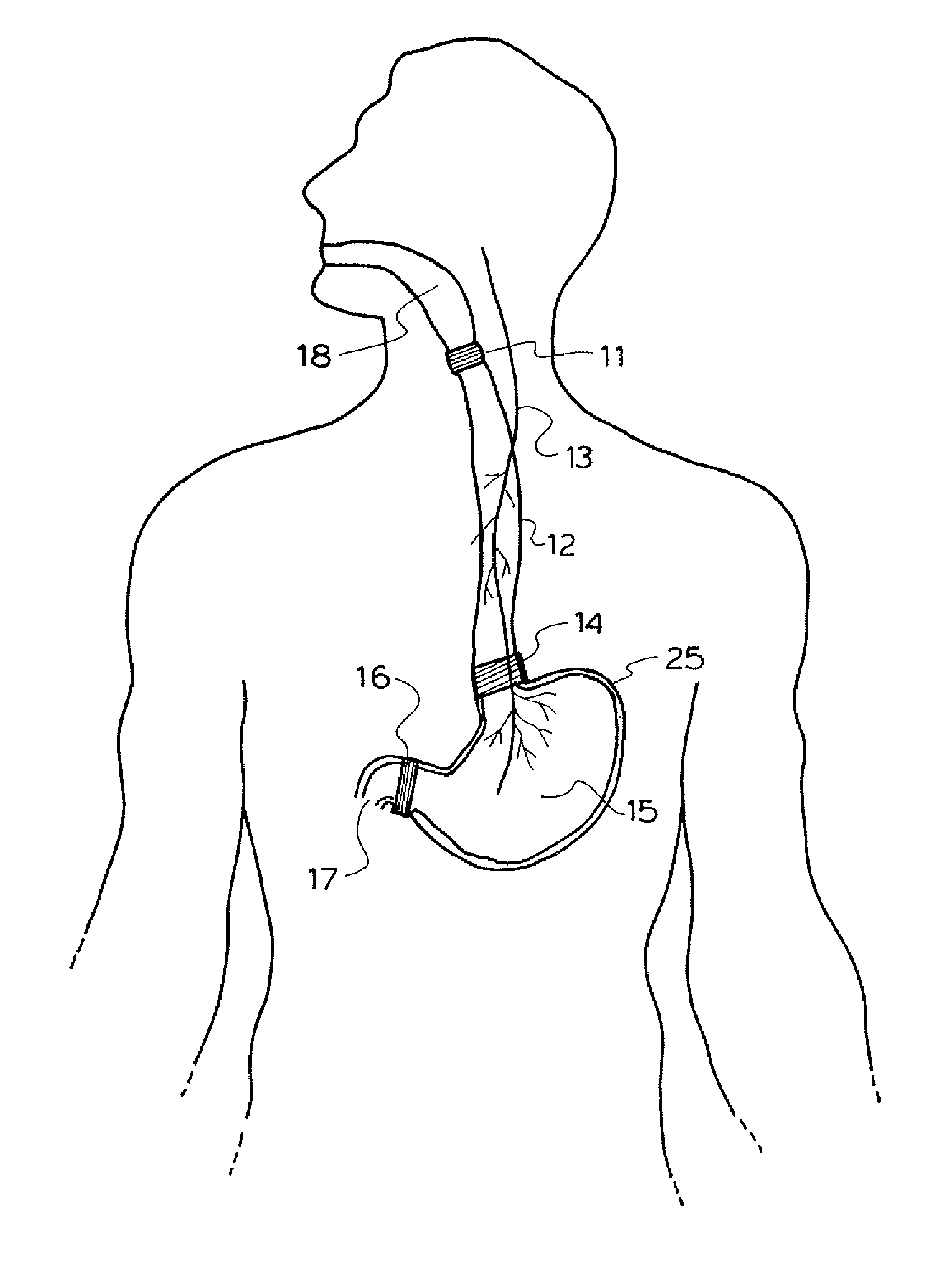
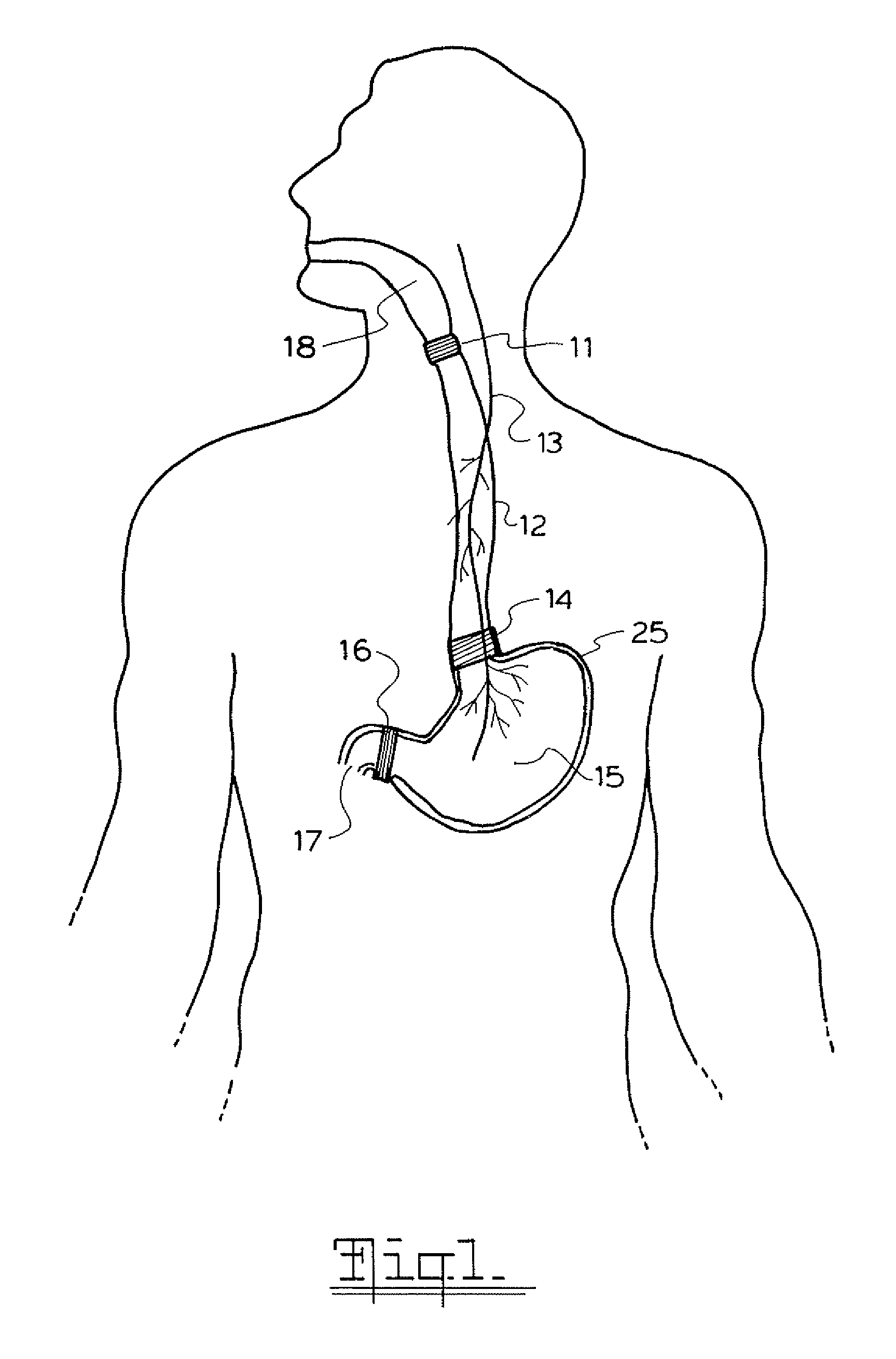
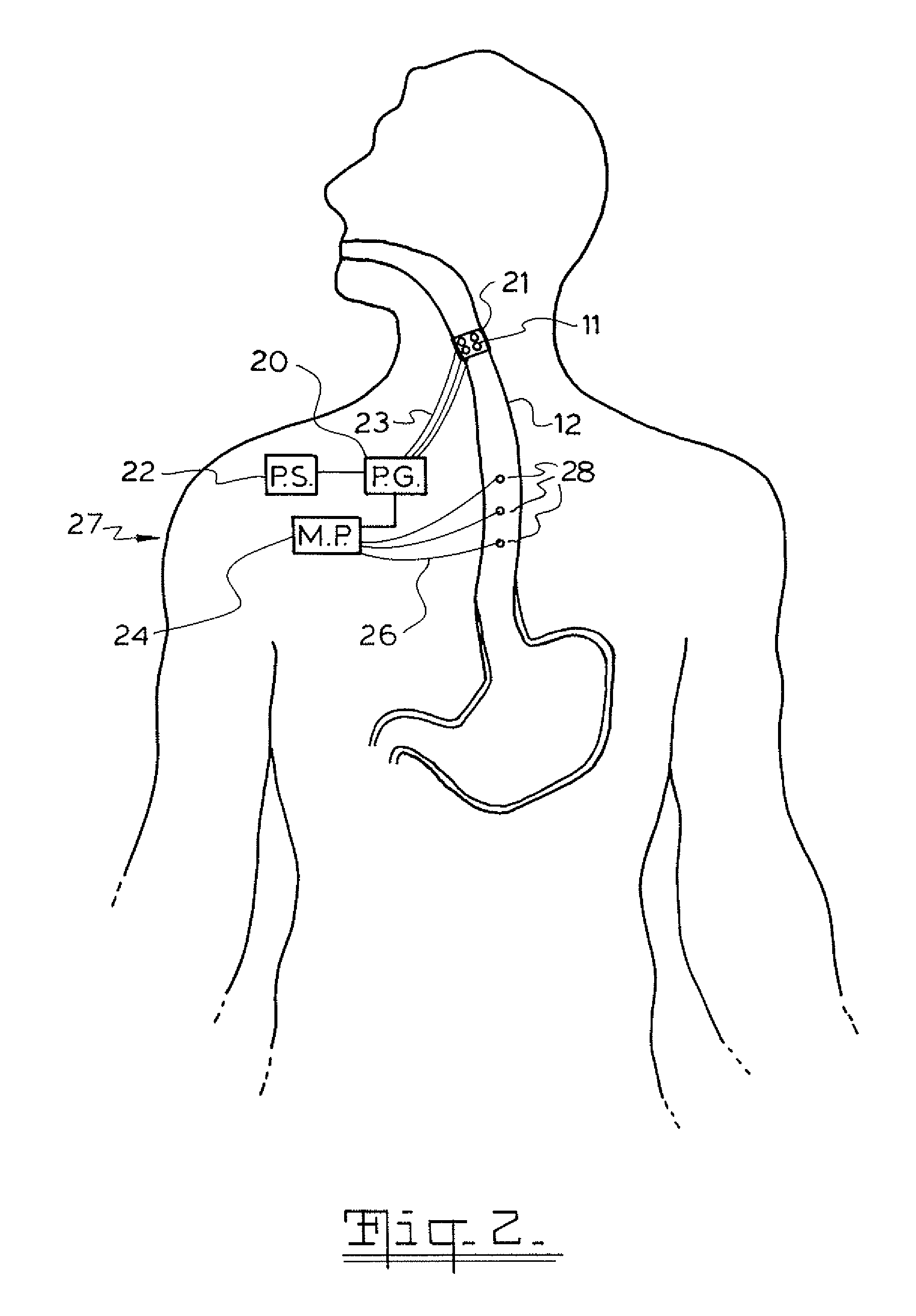
A method and device for electrically stimulating one or more structures in the gastrointestinal tract as described. The one or more structures are preferably selected from the upper esophageal sphincter, the esophagus and gastric fundus. The method may involve the step of arranging a plurality of stimulating electrodes adjacent one or more structures which may further include the lower esophageal sphincter, the stomach, the pyloric sphincter, the small intestine, the colon and the vagus. The method and device may further include sensing electrodes to detect change in one or more physiological parameters in the gastrointestinal tract and modulate the stimulating electrodes in response to the change. The device comprises a pulse generator, a power source, a plurality of stimulating electrode set, one or more sensing electrodes and means for varying activity of the stimulating electrodes in response to change detected in the gastrointestinal tract. The method and device may be used to treat obesity and / or GERD.
Owner:PARAS HLDG LLC +1
Surgical instruments for treating gastro-esphageal reflux
Instruments for thermally-mediated treatment of a patient's lower esophageal sphincter (LES) to induce an injury healing response to thereby populate the extracellular compartment of walls of the LES with collagen matrices to altere the biomechanics of the LES to provide an increased intra-esophageal pressure for preventing acid reflux. A preferred embodiment is a bougie-type device for trans-esophageal introduction that carries conductive electrodes for delivering Rf energy to walls of the LES (i) to induce the injury healing response or (ii) to "model" collagenous tissues of the LES by shrinking collagen fibers therein. Typically, an Rf source is connected to at least one conductive electrode that may be operated in a mono-polar or bi-polar fashion. A sensor array of individual sensors is provided in the working end. A computer controller is provided, which together with feedback circuitry, is capable of full process monitoring and control of: (i) power delivery; (ii) parameters of a selected therapeutic cycle, (iii) mono-polar or bi-polar energy delivery, and (iv) multiplexing of current flow between various paired electrodes. The controller can determine when the treatment is completed based on time, temperature, tissue impedance or any combination thereof.
Owner:MEDERI RF LLC
Device and implantation system for electrical stimulation of biological systems
ActiveUS8447403B2Less energyFunction increaseImplantable neurostimulatorsDiagnostic recording/measuringLower esophagusPhysical medicine and rehabilitation
The present specification discloses devices and methodologies for the treatment of nocturnal GERD. Individuals with nocturnal GERD may be treated by implanting a stimulation device within the patient's lower esophageal sphincter and applying electrical stimulation to the patient's lower esophageal sphincter, in accordance with certain predefined protocols. The presently disclosed devices have a simplified design because they do not require sensing systems capable of sensing when a person is engaged in a wet swallow and have improved energy storage requirements.
Owner:PARAS HLDG LLC
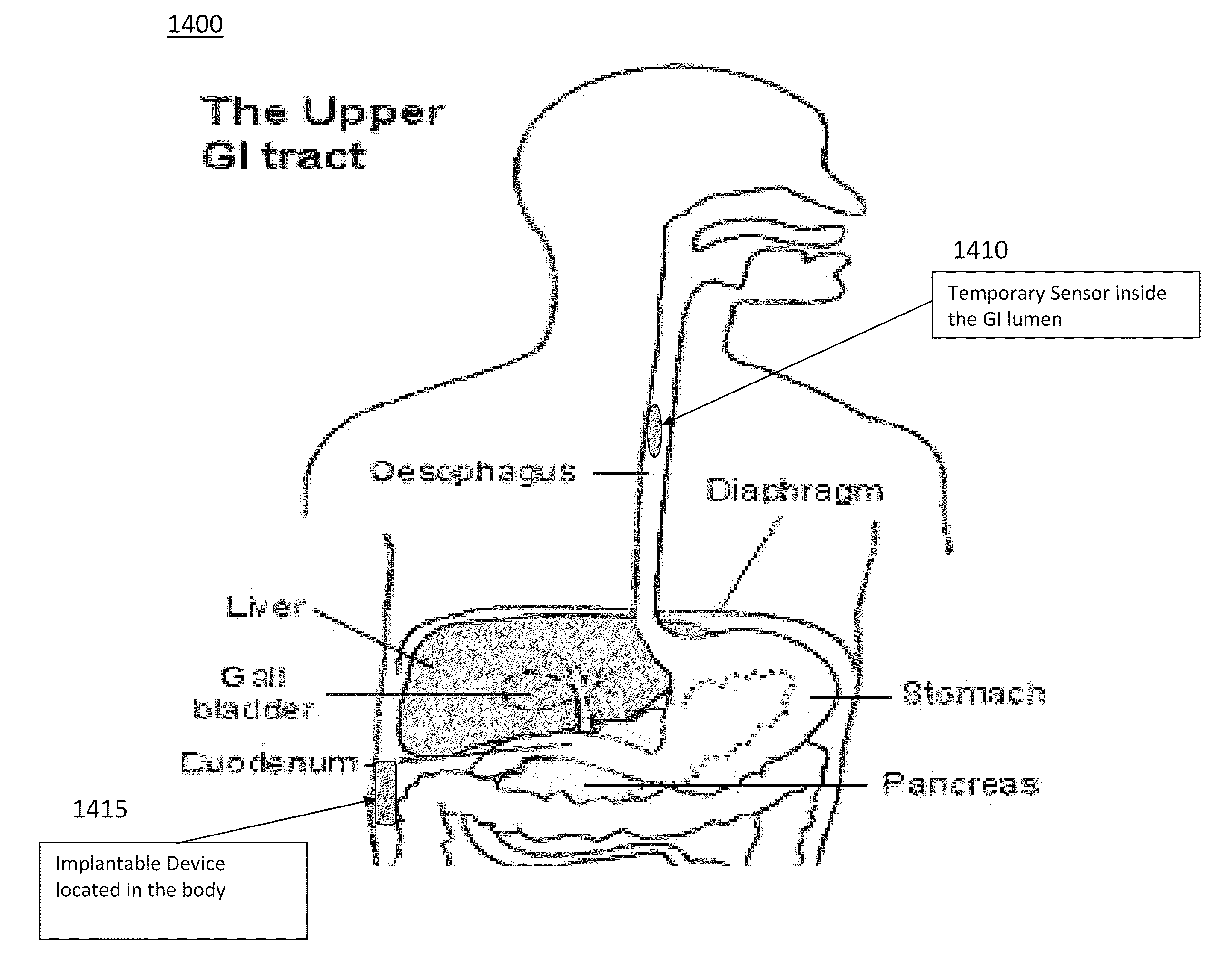
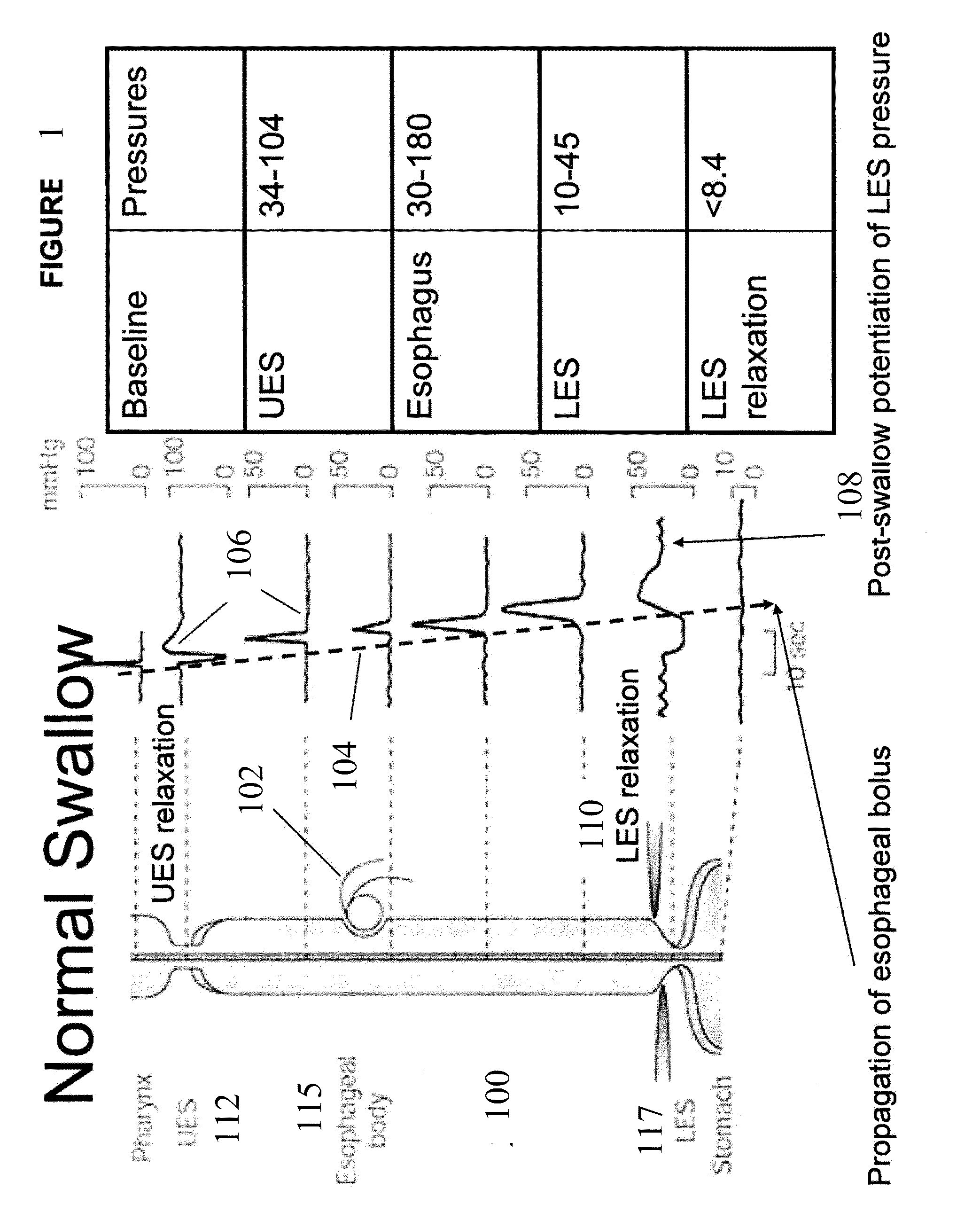
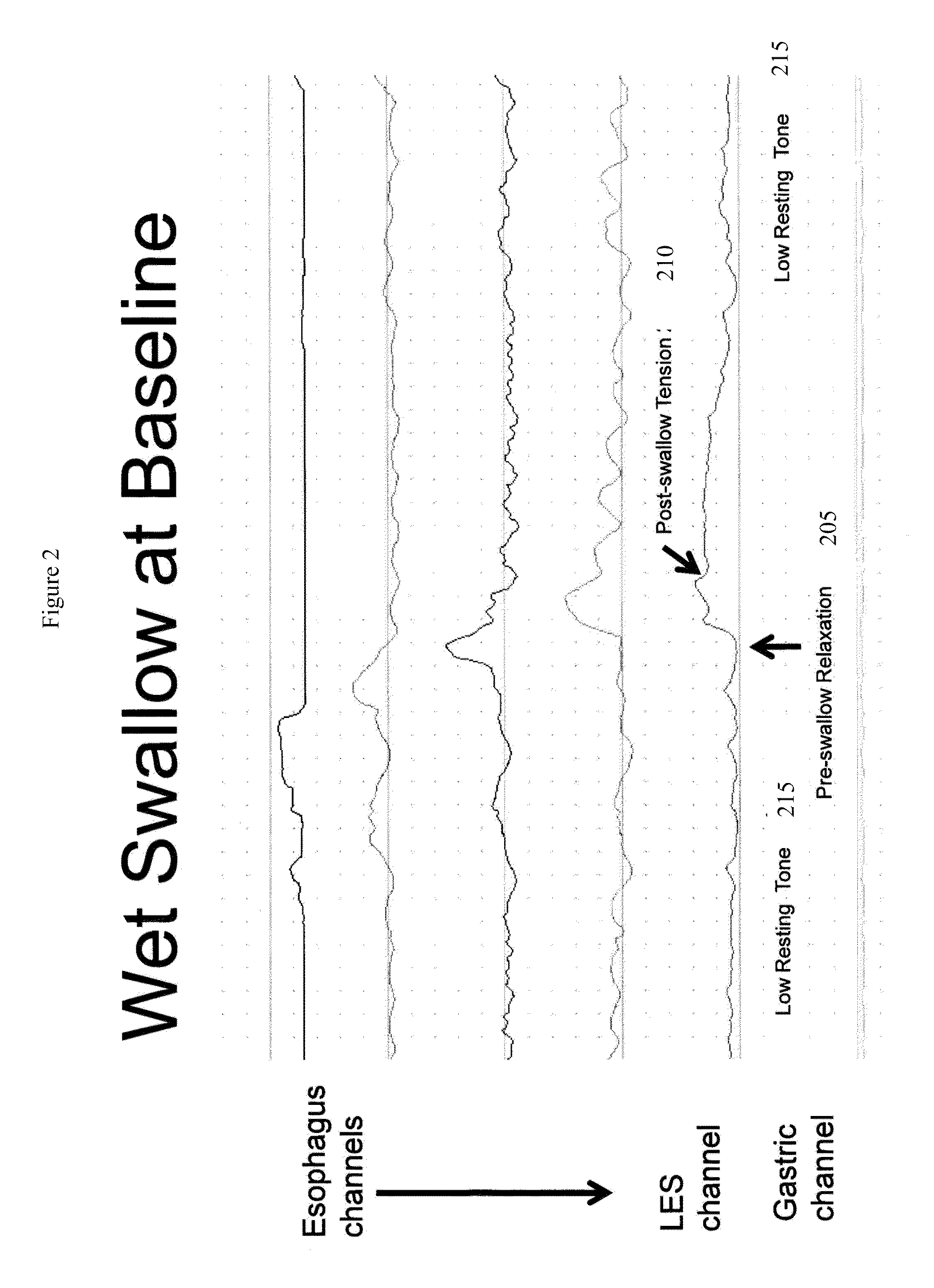
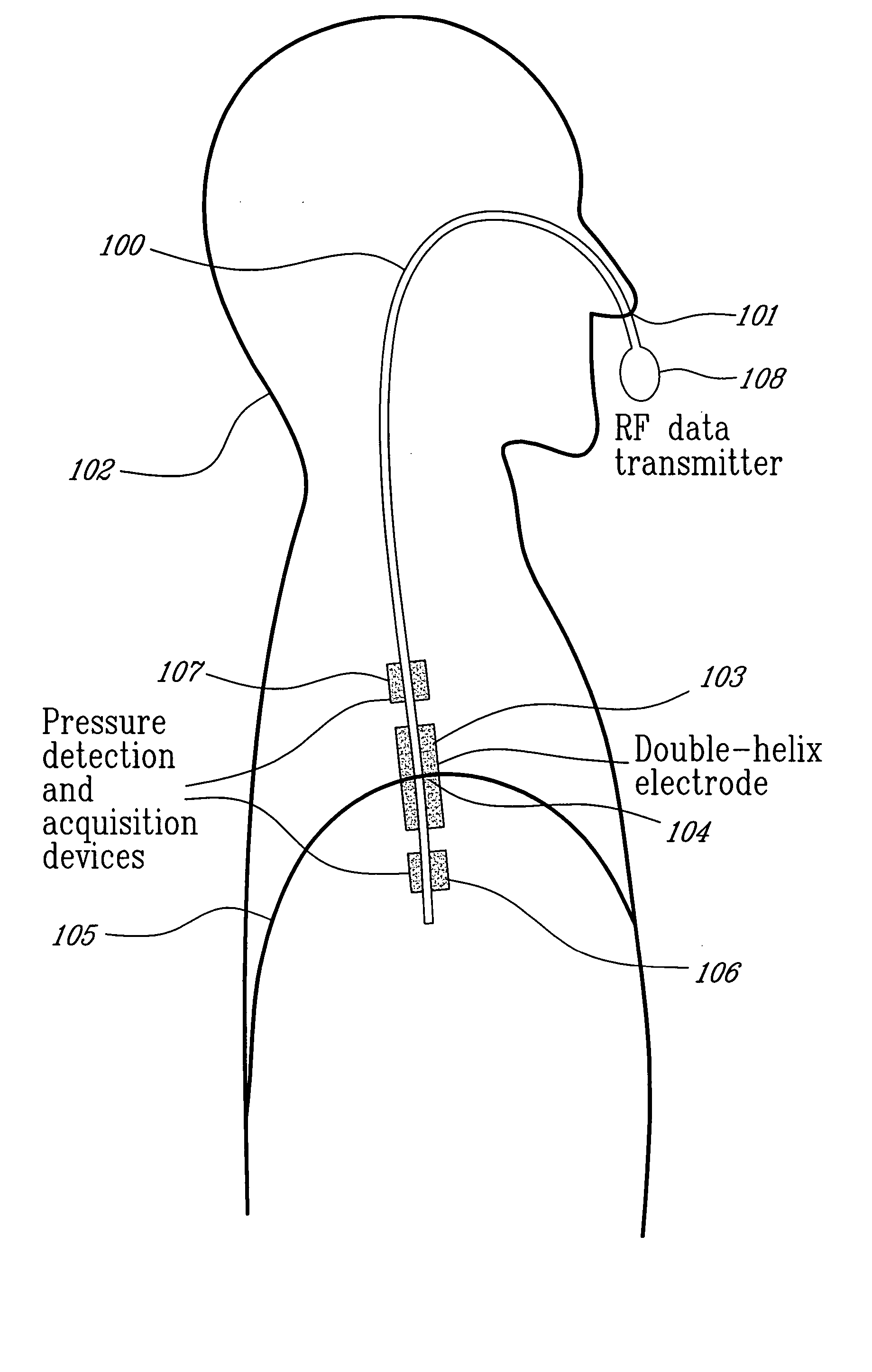
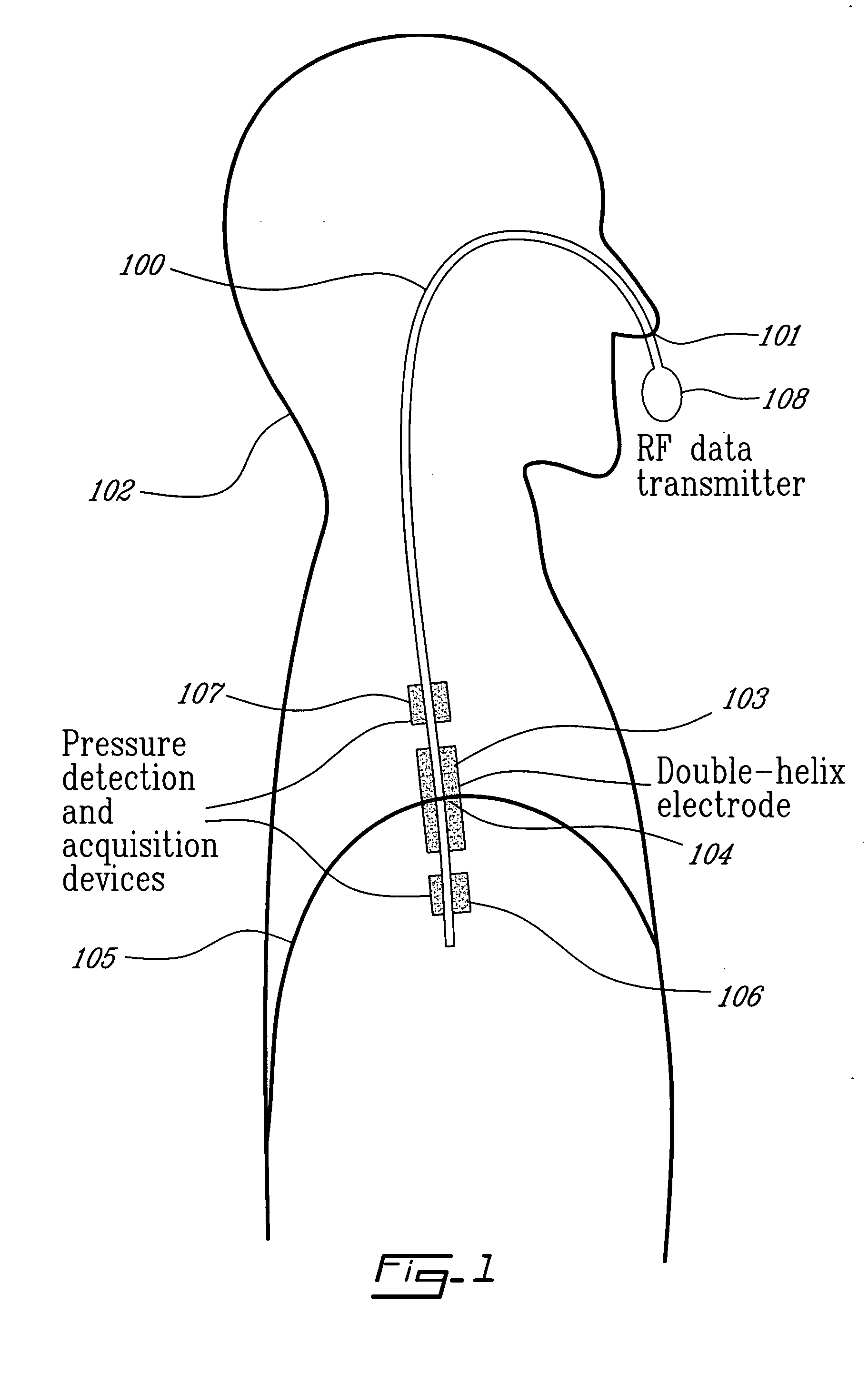
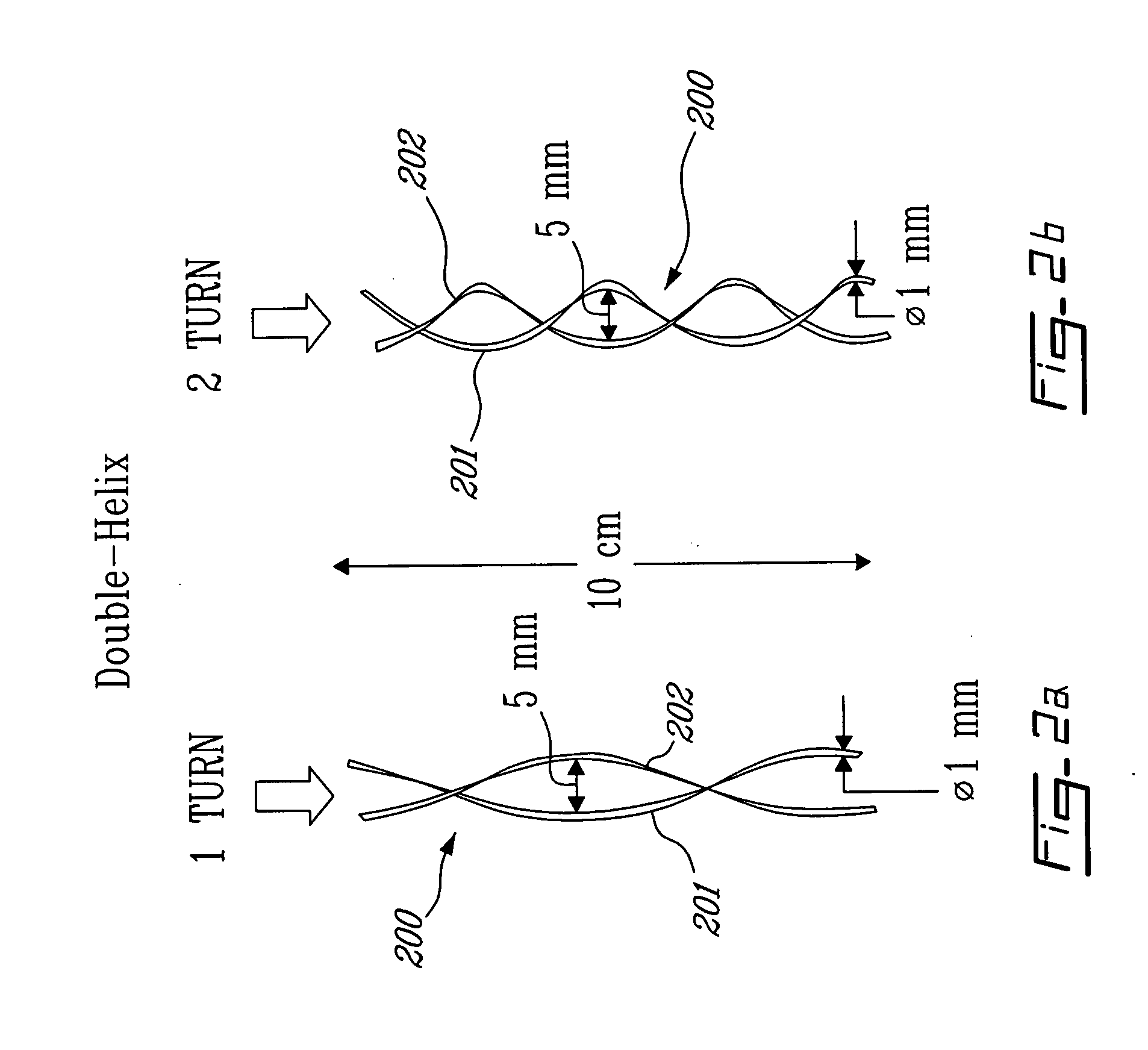
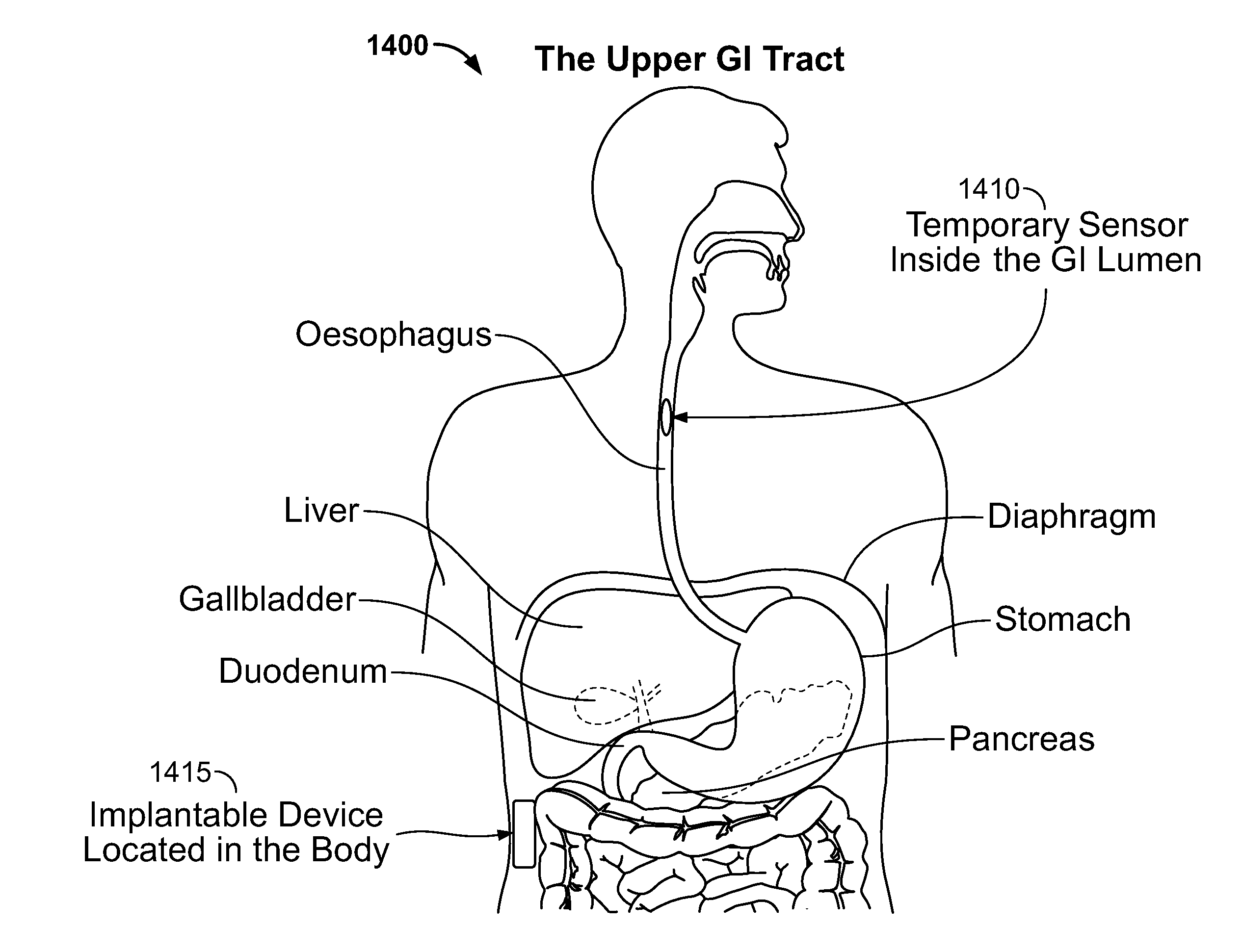
Catheter for transdiaphragmatic pressure and diaphragm electromyogram recording using helicoidal electrodes
A first aspect of the present invention is concerned with a double-helix electrode structure for sensing electrical activity of a patient's diaphragm, comprising first and second helical electrodes disposed in a double-helix arrangement for being positioned in the gastro-esophageal sphincter of the patient's diaphragm in view of sensing electrical activity of the patient's diaphragm. According to a second aspect, the present invention provides a pressure detection and acquisition device comprising a semiconductor substrate, a pressure sensor implemented on the semiconductor substrate and producing, when subjected to an external pressure, a pressure representative signal, and a signal acquisition and transmission circuit integrated to the semiconductor substrate, connected to the pressure sensor, and supplied with the pressure representative signal. Other aspects of the present invention relate to an EMGdi signal and pressure acquisition catheter.
Owner:SAWAN MOHAMAD +3
Device and implantation system for electrical stimulation of biological systems
ActiveUS20110295335A1Less energyImproved LES functionImplantable neurostimulatorsDiagnostic recording/measuringPhysical medicine and rehabilitationSphincter
The present specification discloses devices and methodologies for the treatment of nocturnal GERD. Individuals with nocturnal GERD may be treated by implanting a stimulation device within the patient's lower esophageal sphincter and applying electrical stimulation to the patient's lower esophageal sphincter, in accordance with certain predefined protocols. The presently disclosed devices have a simplified design because they do not require sensing systems capable of sensing when a person is engaged in a wet swallow and have improved energy storage requirements.
Owner:PARAS HLDG LLC
Method and apparatus employing ultrasound energy to treat body sphincters
InactiveUS20130072928A1Prevent and delay openingDecreased tissue complianceUltrasonic/sonic/infrasonic diagnosticsUltrasound therapyAcoustic energySphincter
Methods and apparatus for treating gastroesophageal reflex and other luminal conditions provide for delivering acoustic energy to a body lumen to remodel tissue surrounding the body lumen. In the case of treating GERD, a catheter carrying an ultrasonic or other vibrational transducer is introduced to the lower esophageal sphincter, and acoustic energy is delivered to the sphincter in order to tighten or bulk the sphincter such that reflex is reduced.
Owner:RECOR MEDICAL INC
Intraluminal methods of ablating nerve tissue
InactiveUS20130131668A1Prevent and delay openingReduce complianceUltrasonic/sonic/infrasonic diagnosticsUltrasound therapyAcoustic energySphincter
Methods and apparatus for treating gastroesophageal reflex and other luminal conditions provide for delivering acoustic energy to a body lumen to remodel tissue surrounding the body lumen. In the case of treating GERD, a catheter carrying an ultrasonic or other vibrational transducer is introduced to the lower esophageal sphincter, and acoustic energy is delivered to the sphincter in order to tighten or bulk the sphincter such that reflex is reduced.
Owner:RECOR MEDICAL INC
Device and implantation system for electrical stimulation of biological systems
ActiveUS20110307028A1Not induce dysphagiaIncrease pressureImplantable neurostimulatorsDiagnostic recording/measuringLower esophagusPhysical medicine and rehabilitation
The present specification discloses devices and methodologies for the treatment of transient lower esophageal sphincter relaxations (tLESRs). Individuals with tLESRs may be treated by implanting a stimulation device within the patient's lower esophageal sphincter and applying electrical stimulation to the patient's lower esophageal sphincter, in accordance with certain predefined protocols. The presently disclosed devices have a simplified design because they do not require sensing systems capable of sensing when a person is engaged in a wet swallow and have improved energy storage requirements.
Owner:PARAS HLDG LLC
Gastro-esophageal reflux disease (GERD) treatment method and apparatus
The method of the invention includes accessing a juncture of an esophagus and a stomach of the patient on a distal side of a diaphragm. The esophagus and a fundus of the stomach intersect at a cardiac notch located at an original cardiac notch position. A reducing element is placed at the junction with the reducing element selected to reposition the cardiac notch to a position more distal to a lower esophageal sphincter of the patient.
Owner:RESHAPE LIFESCIENCES INC
High specific gravity intragastric device
The teachings are directed to an intragastric device comprising a flexible and expandable bladder having a predetermined shape upon expansion for contacting the antrum of the stomach of a subject. The device is designed to avoid passage of any part of the device beyond the pylorus and lower esophageal sphincter while the bladder is expanded during use. In these embodiments, the bladder can contain a high specific gravity material when expanded; wherein, the high specific gravity material contributes to an in vivo specific gravity of the device that ranges from about 1.2 g / ml to about 2.1 g / ml and functions to direct the device to the pyloric antrum of the subject during use of the device. Moreover, these embodiments can include a filling material comprising a biocompatible fluid component and a hydrogel component to make the device substantially leakproof and contribute to the in vivo specific gravity of the device.
Owner:HANCOCK JOHN
Distal portion of an endoscopic delivery system
InactiveUS7780592B2Facilitate easier and more comfortable advancementConvenient verificationSurgical needlesEndoscopesVacuum pressureProximate
The disclosure describes an endoscopic delivery system that includes an endoscope and a tissue-receiving member. The tissue-receiving member includes an opening to receive the distal end of the endoscope. The tissue-receiving member also defines a tissue-receiving space that receives tissue when vacuum pressure is applied to the space through the endoscope. A tool and / or material may be delivered to the tissue drawn into the tissue-receiving space. The tissue-receiving member may be cap-like, and only receive the distal end of the endoscope, or include an overtube that receives a substantial portion of the endoscope. The system may be used to, for example, deliver tissue bulking devices to a location proximate to the lower esophageal sphincter (LES) for treatment of gastroesophageal reflux disease (GERD).
Owner:MEDTRONIC INC
Device and Implantation System for Electrical Stimulation of Biological Systems
ActiveUS20140228911A1Not induce dysphagiaIncrease pressureImplantable neurostimulatorsCatheterLower esophagusSphincter
The present specification discloses devices and methodologies for the treatment of transient lower esophageal sphincter relaxations (tLESRs). Individuals with tLESRs may be treated by implanting a stimulation device within the patient's lower esophageal sphincter and applying electrical stimulation to the patient's lower esophageal sphincter, in accordance with certain predefined protocols. The presently disclosed devices have a simplified design because they do not require sensing systems capable of sensing when a person is engaged in a wet swallow and have improved energy storage requirements.
Owner:PARAS HLDG LLC
Device and Implantation System for Electrical Stimulation of Biological Systems
InactiveUS20140222106A1Increase pressureKeep the pressureCatheterImplantable neurostimulatorsElectricityPhysical therapy
The present specification discloses devices and methodologies for the treatment of GERD. Individuals with GERD may be treated by implanting a stimulation device within the patient's lower esophageal sphincter and applying electrical stimulation to the patient's lower esophageal sphincter, in accordance with certain predefined protocols. The presently disclosed devices have a simplified design because they do not require sensing systems capable of sensing when a person is engaged in a wet swallow and have improved energy storage requirements.
Owner:ENDOSTIM INC
Magnetic device and method to prevent gastroesophageal reflux, fecal incontinence and urinary incontinence
The invention consists in a couple of magnets in form of small plaques of variable dimensions and magnetic force, covered by a bio-compatible material, that are positioned by means of a special endoluminal delivery device or by means of a surgical intervention, close to the sphincters that are to be reinforced, with the opposite polarities face to face, so that, attracting to each other, they close the visceral lumen, preventing the reflux or the incontinence.This method may be applied to the lower esophageal sphincter in order to prevent gastroesophageal reflux, to the anal sphincter to prevent fecal incontinence, and to the urethral sphincter to prevent urinary incontinence.
Owner:BORTOLOTTI MAURO
Device and implantation system for electrical stimulation of biological systems
ActiveUS9020597B2Less energyFunction increaseDiagnostic recording/measuringSensorsElectricityPhysical therapy
Owner:PARAS HLDG LLC
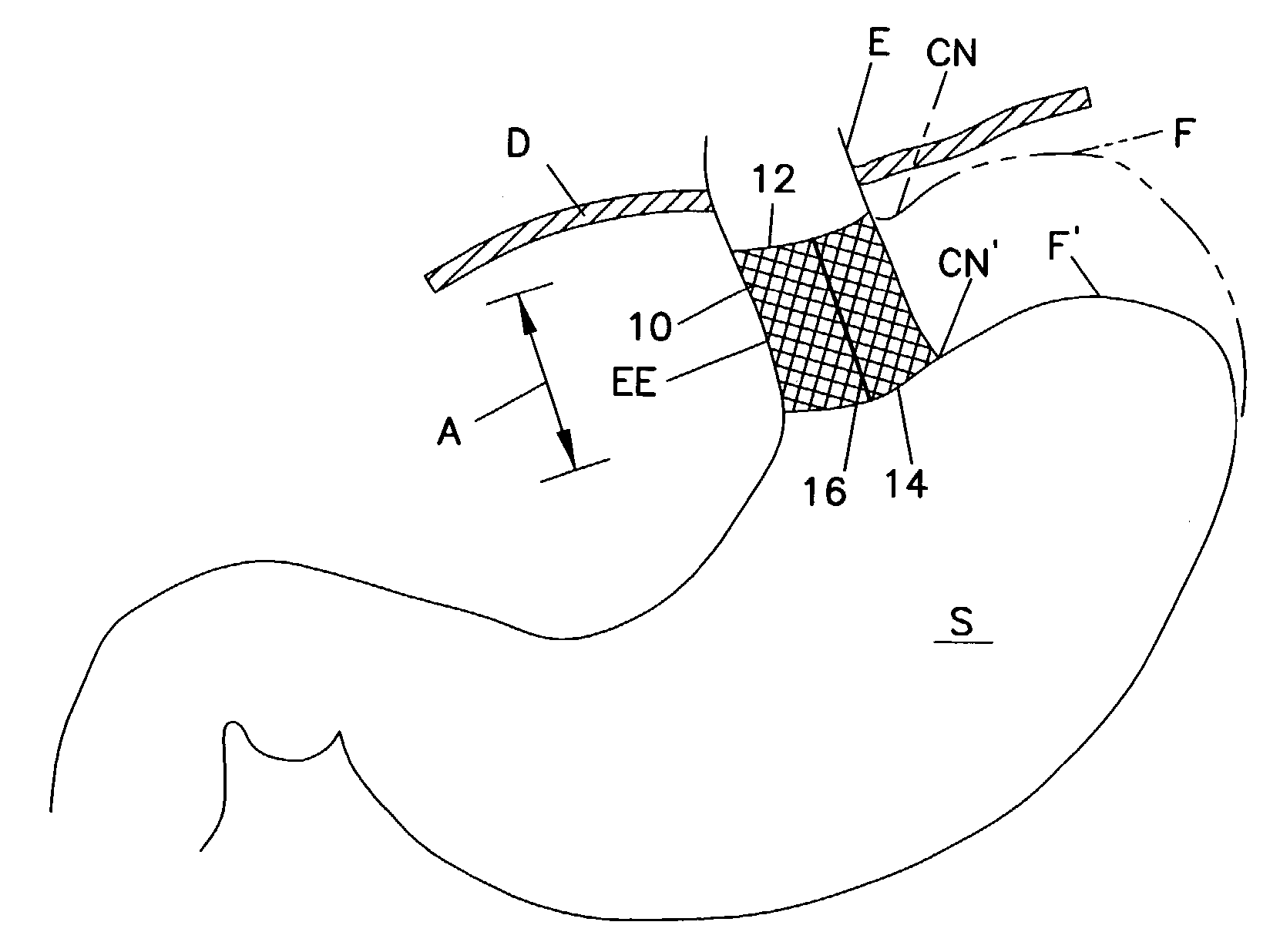
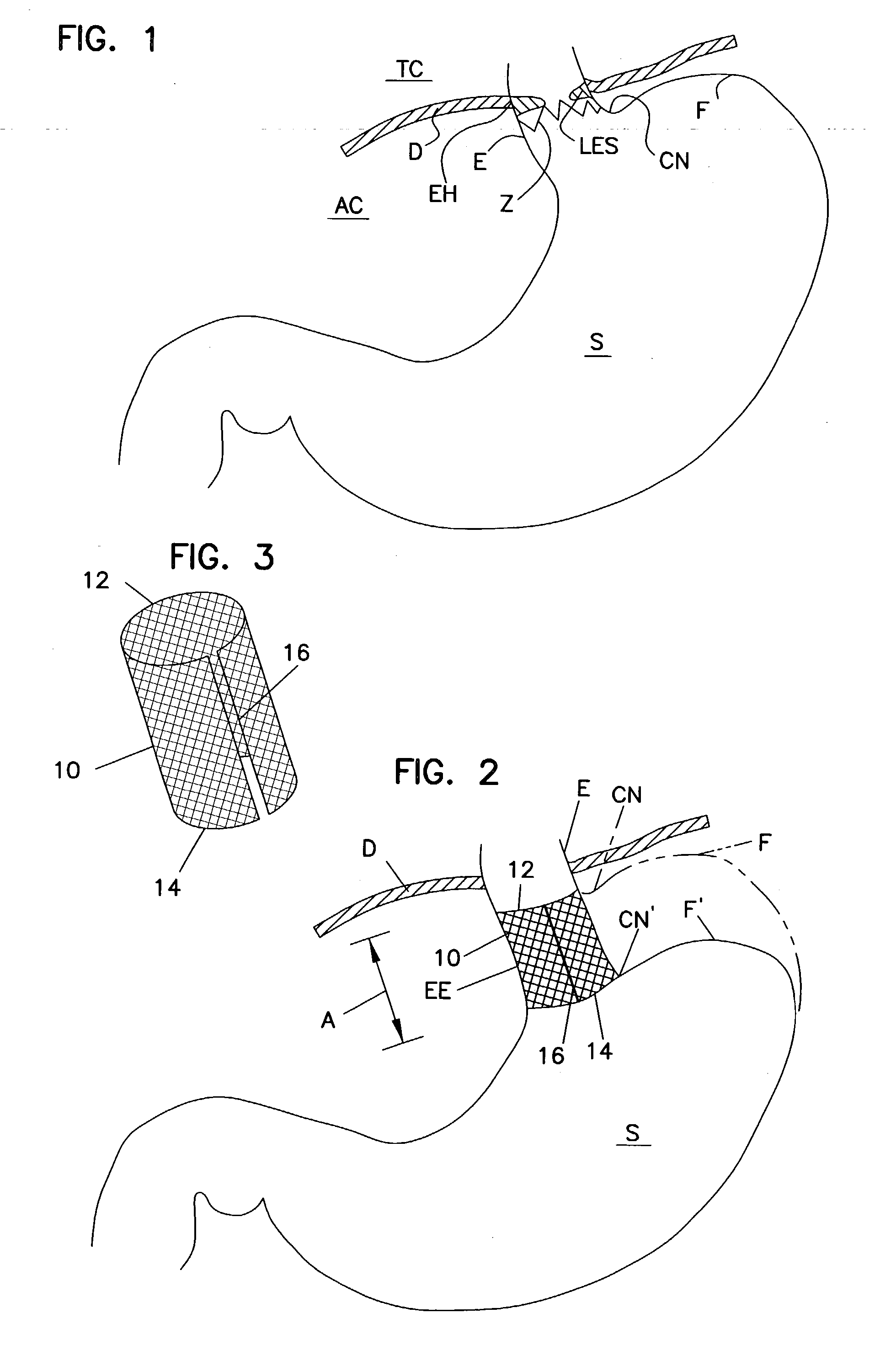
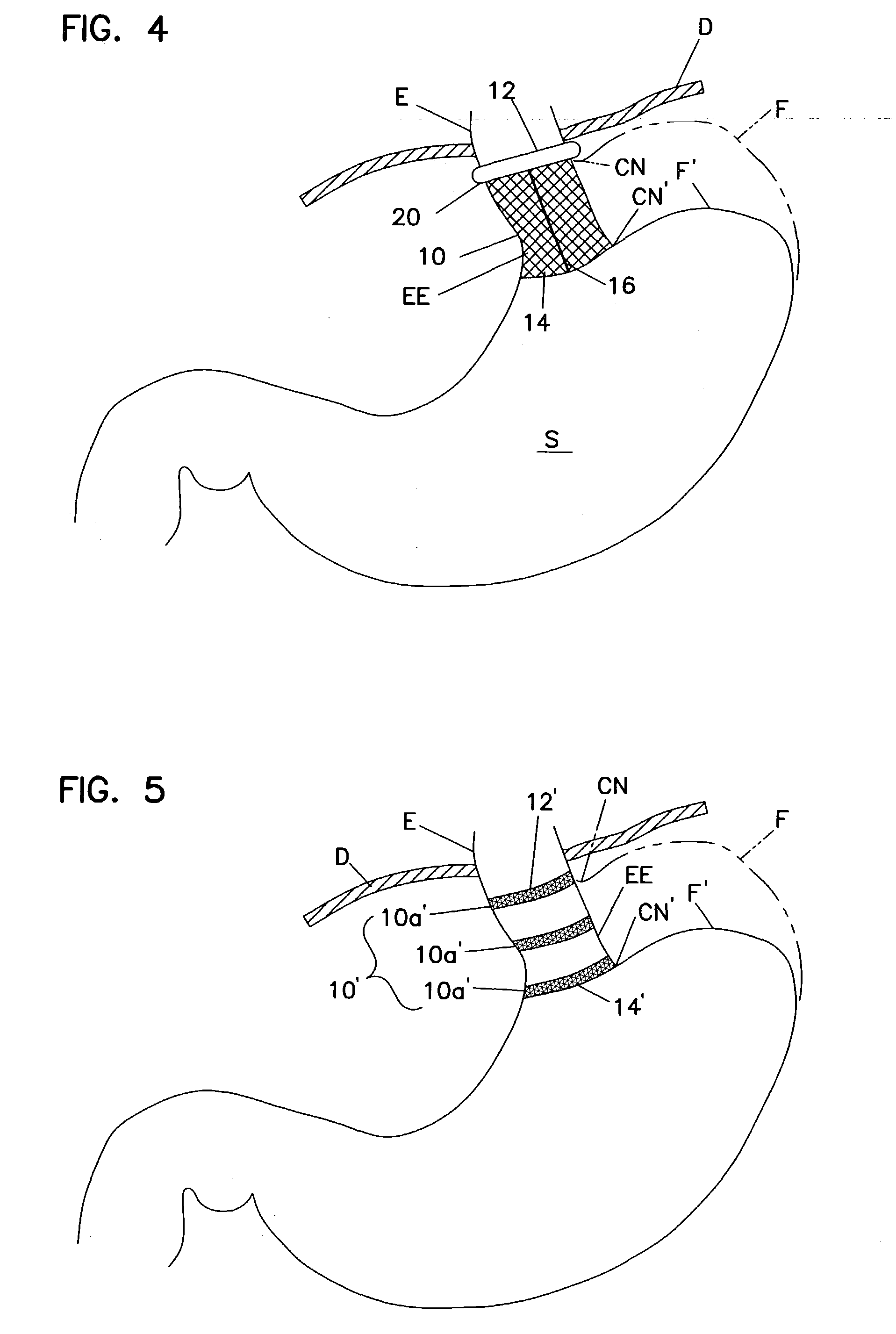
Method for measuring esophageal sphincter compliance
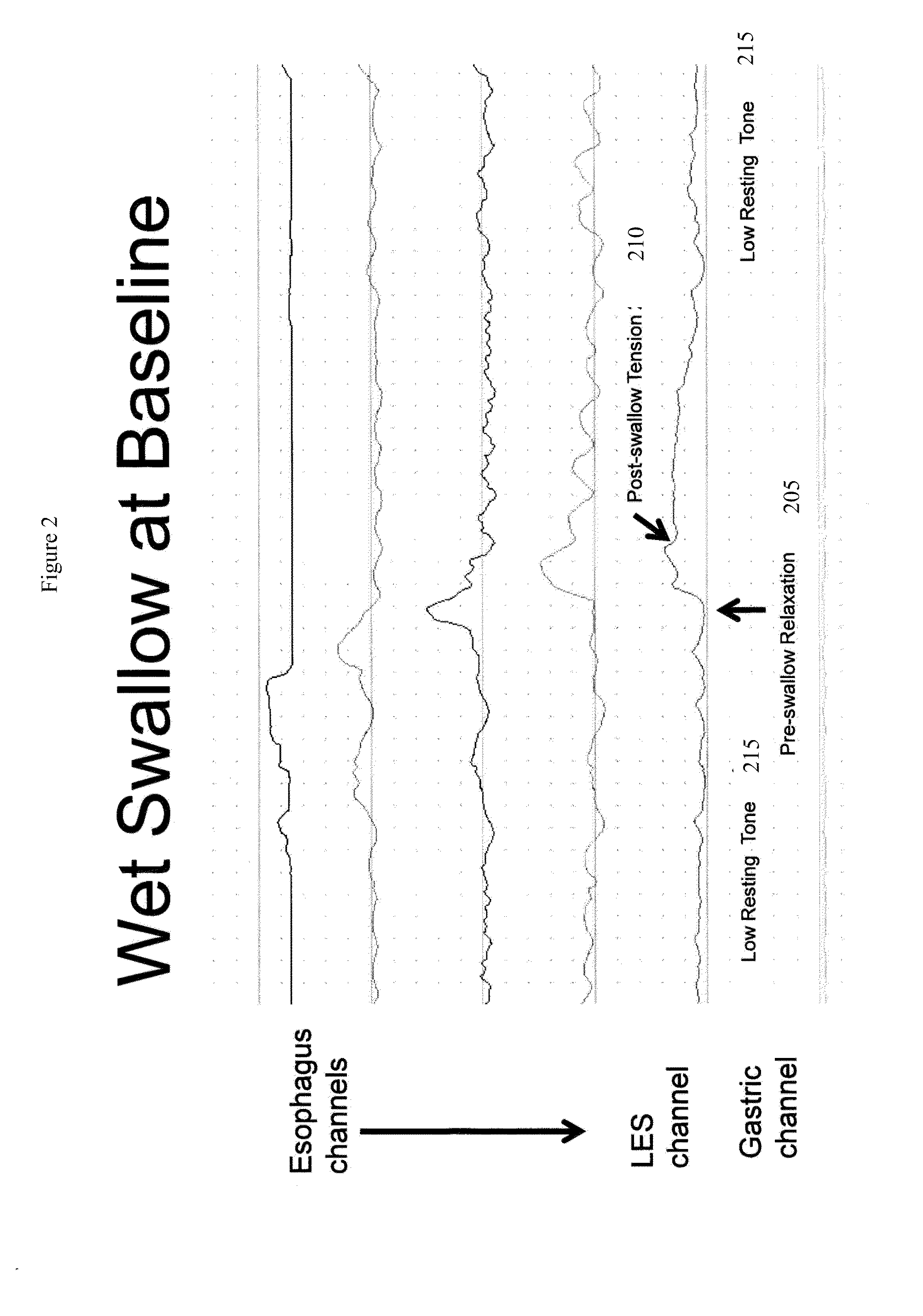
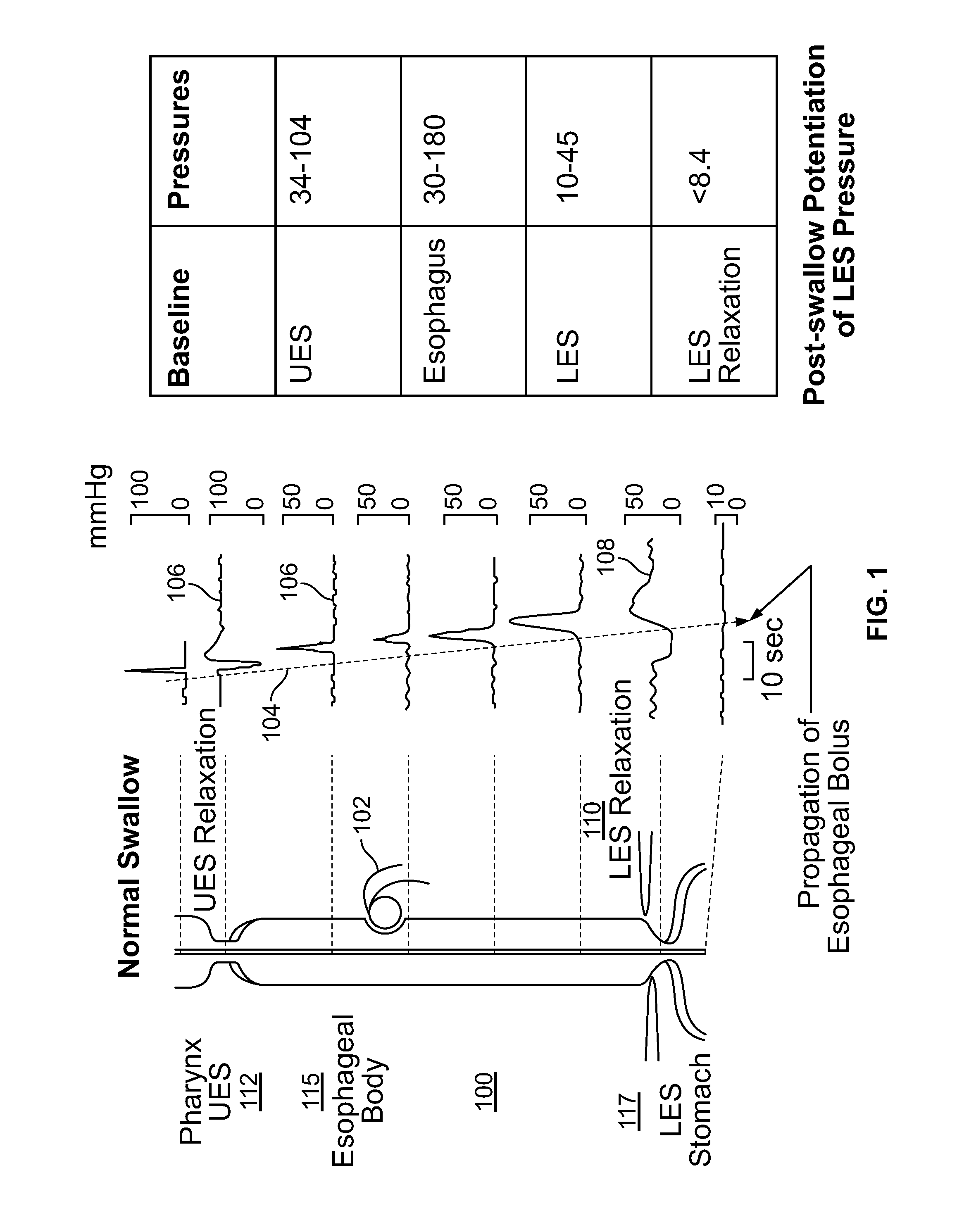
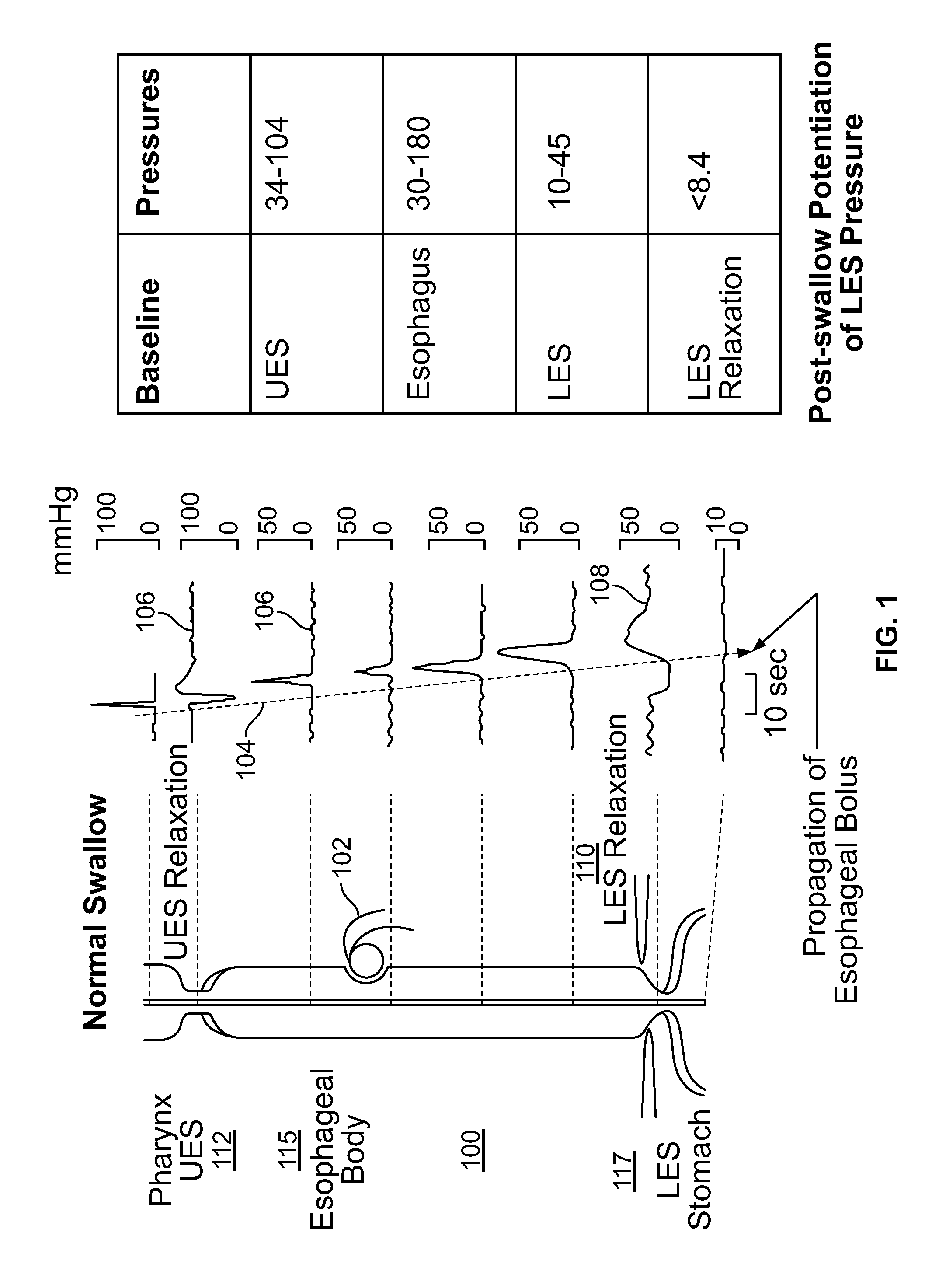
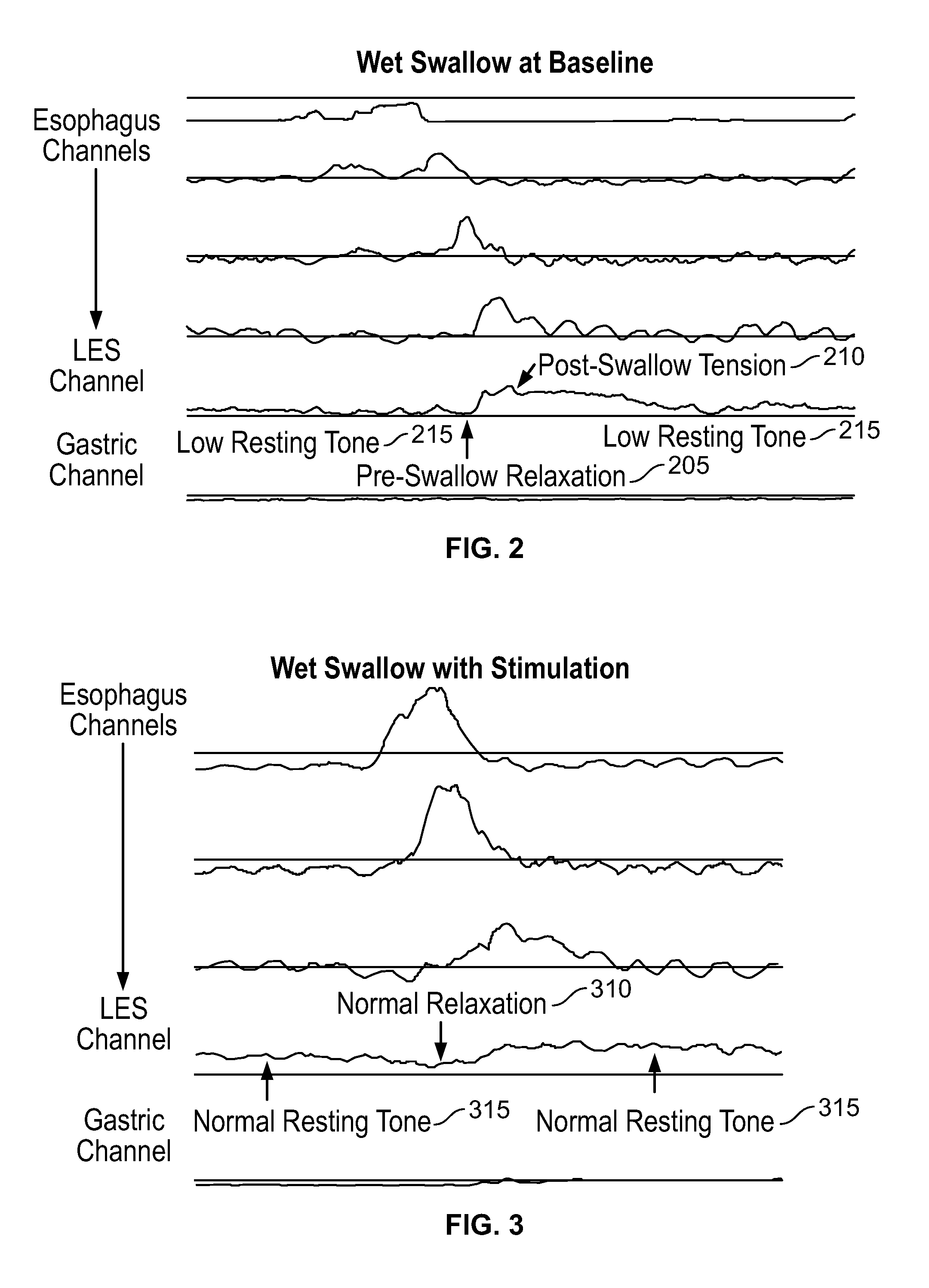
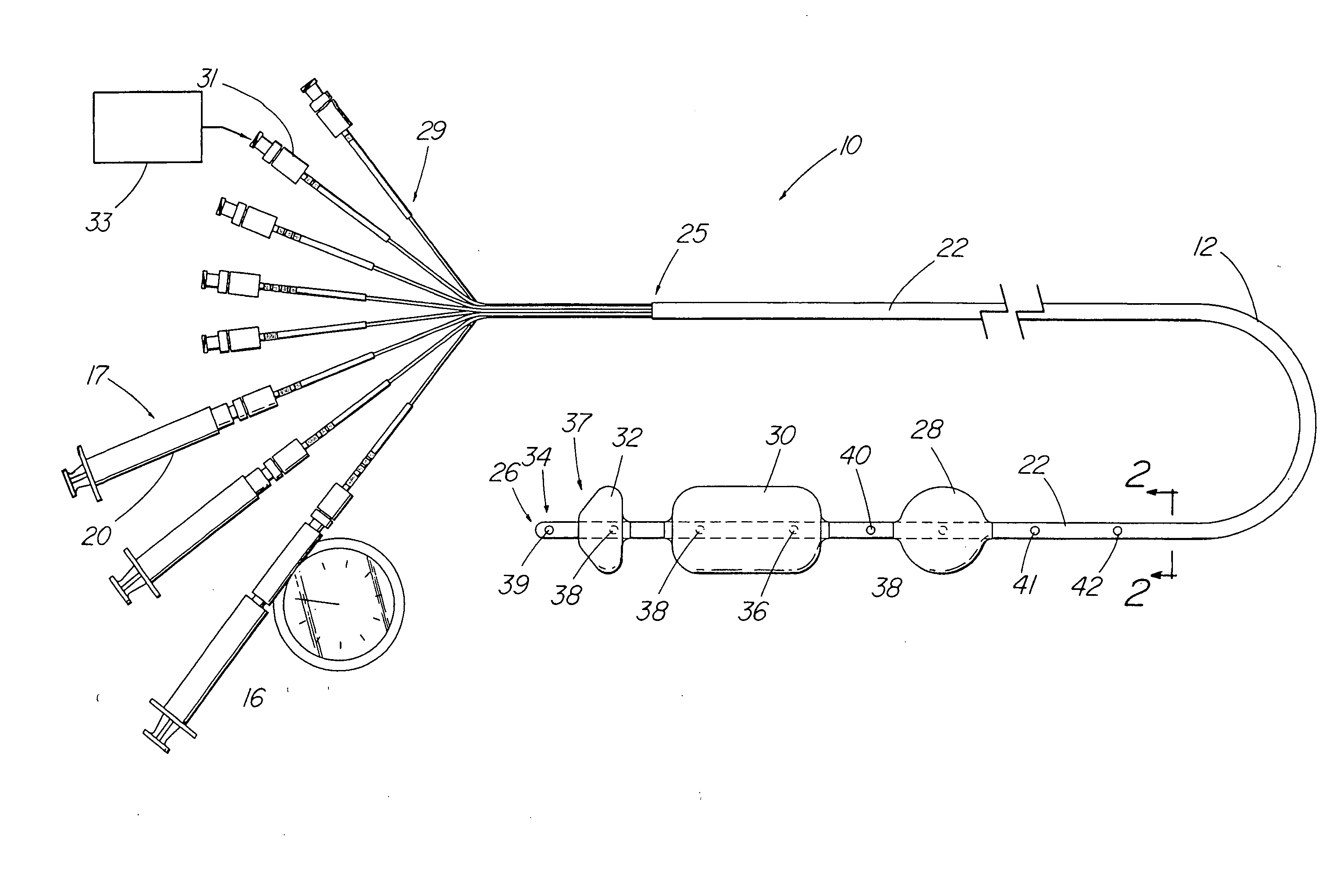
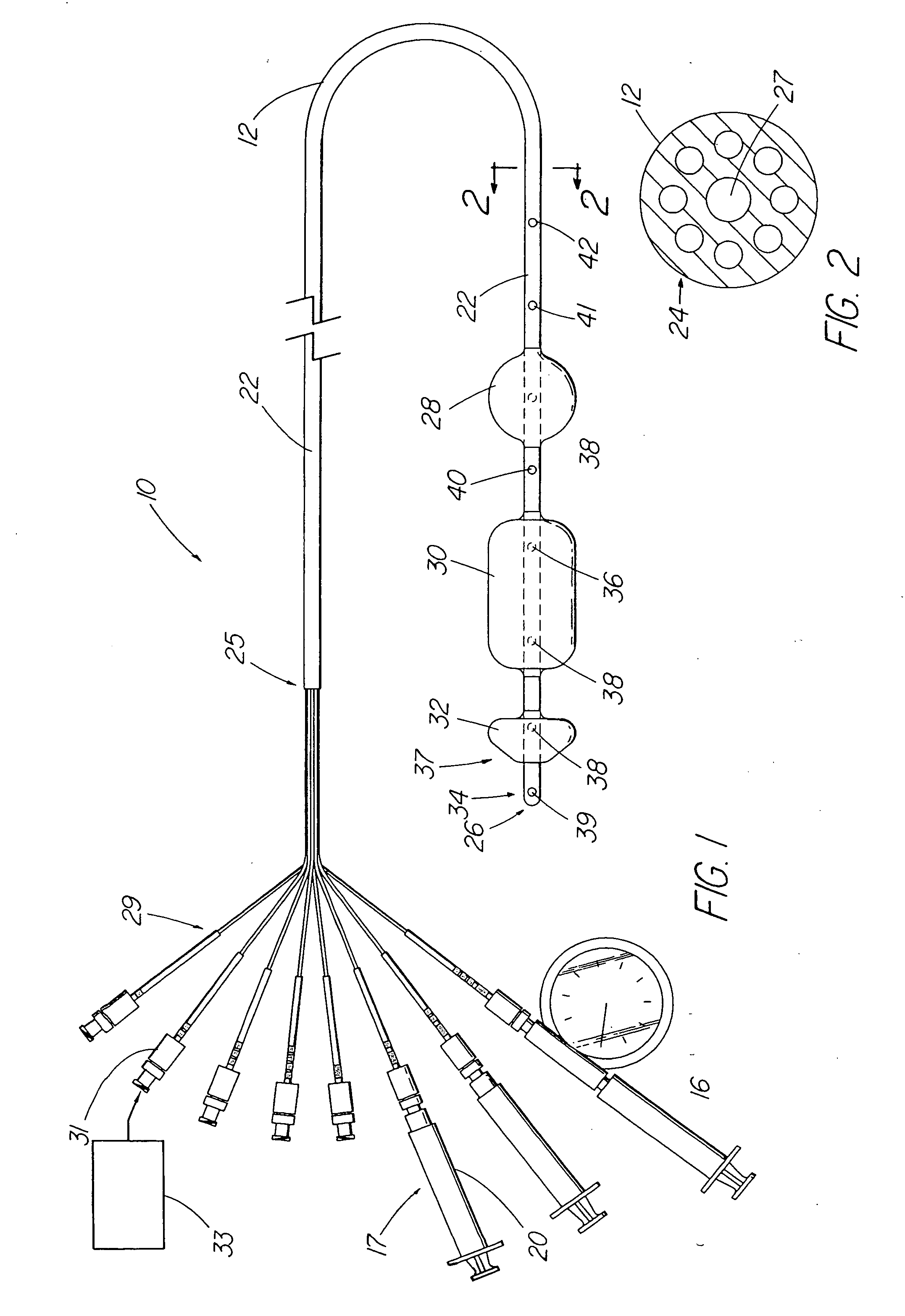
An apparatus and method are disclosed for measuring the compliance of the lower esophageal sphincter (LES), the apparatus comprises a catheter having a plurality of extendable members disposed therealong for both measuring the compliance of the LES and stimulating the esophagus which relaxes the LES to obtain a more clinically relevant measurement of LES compliance. In one embodiment, the plurality of extendable members include a first balloon which is located and inflated within the esophagus to trigger esophageal motility, a second, non-elastic balloon that is positioned within the LES, and a third balloon which is inflated within the stomach to help align the second balloon with LES. Incrementally increasing volumes of air are introduced into the second balloon, with the resulting pressures exerted by the LES calculated and compared to normative values to assess the condition of the LES.
Owner:SHAKER REZA
Non-inflatable gastric implants and systems
A variety of passive intragastric implant devices for obesity treatment are disclosed. Such passive devices do not autonomously change shape, but instead react within the stomach to induce satiety. The devices may take up volume within the stomach, thus reducing the intake capacity. Additionally, the devices may contact areas within the stomach, such as the cardia surrounding the esophageal sphincter, or the greater and lesser curvatures in the middle of the stomach, to stimulate satiety-inducing nerves. Some devices may combine two or more of these satiety-inducing features. Methods of implant are disclosed including compressing the devices within a delivery tube and transorally advancing the devices through the esophagus to be deployed within the stomach. Removal of the devices occurs in the reverse.
Owner:APOLLO ENDOSURGERY INC
Stomach-spanning gastric implants
A variety of passive intragastric implant devices for obesity treatment are disclosed. Such passive implants do not autonomously change shape, but instead react within the stomach to induce satiety. The implants may take up volume within the stomach, thus reducing the digestive capacity. Additionally, the implants may contact areas within the stomach, such as the cardia surrounding the esophageal sphincter, to stimulate satiety-inducing nerves. Also, a number of implants slow gastric emptying by blocking or otherwise impeding flow through the pyloric sphincter. Other implants delay digestion by providing a duodenal sleeve. A number of implants combine two or more of these satiety-inducing features. Methods of implant are disclosed including compressing the implants within a delivery tube and transorally advancing the implants through the esophagus to be deployed within the stomach. Removal of the implants occurs in the reverse.
Owner:ALLERGAN INC
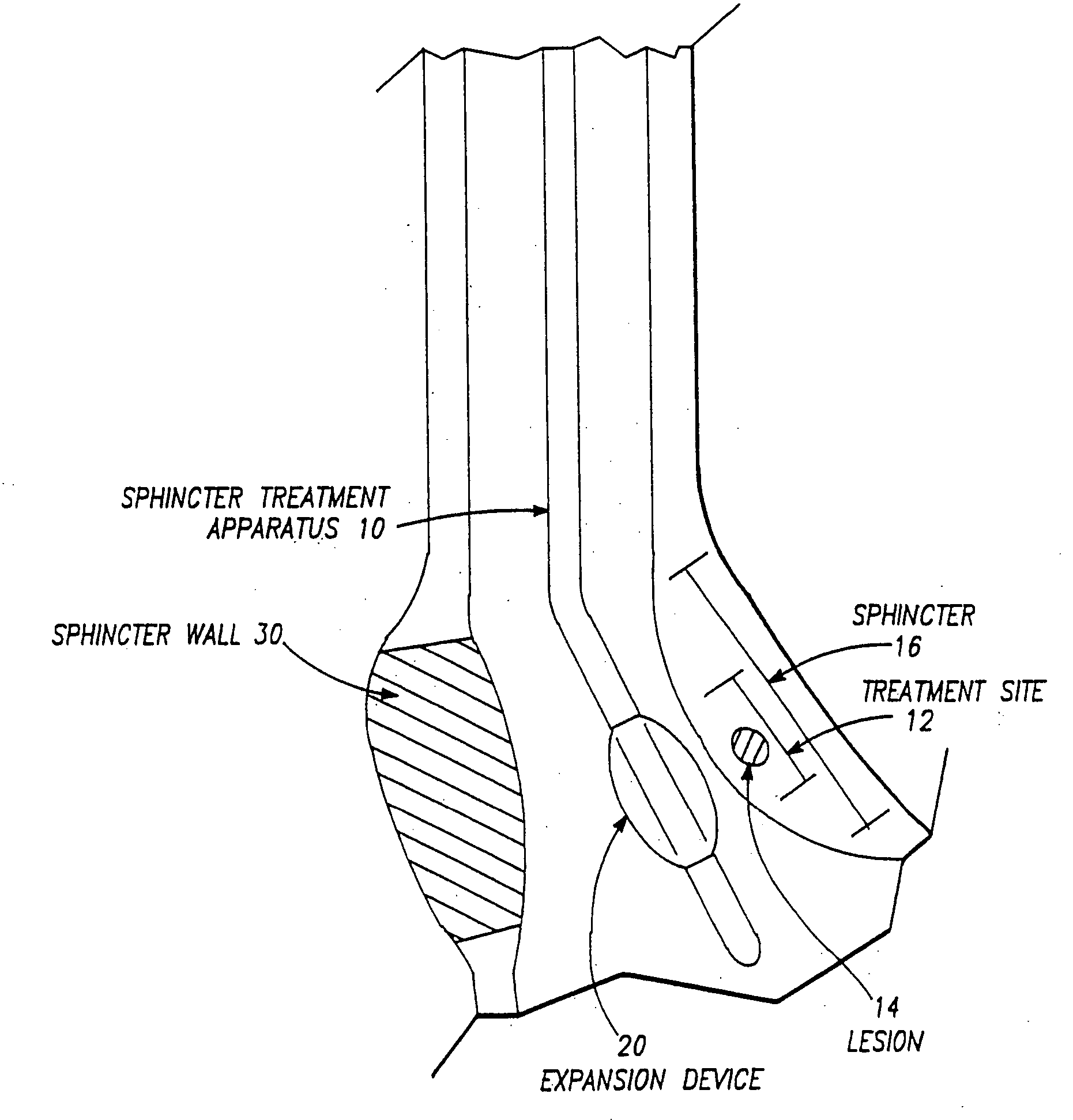
Apparatus to treat esophageal sphincters
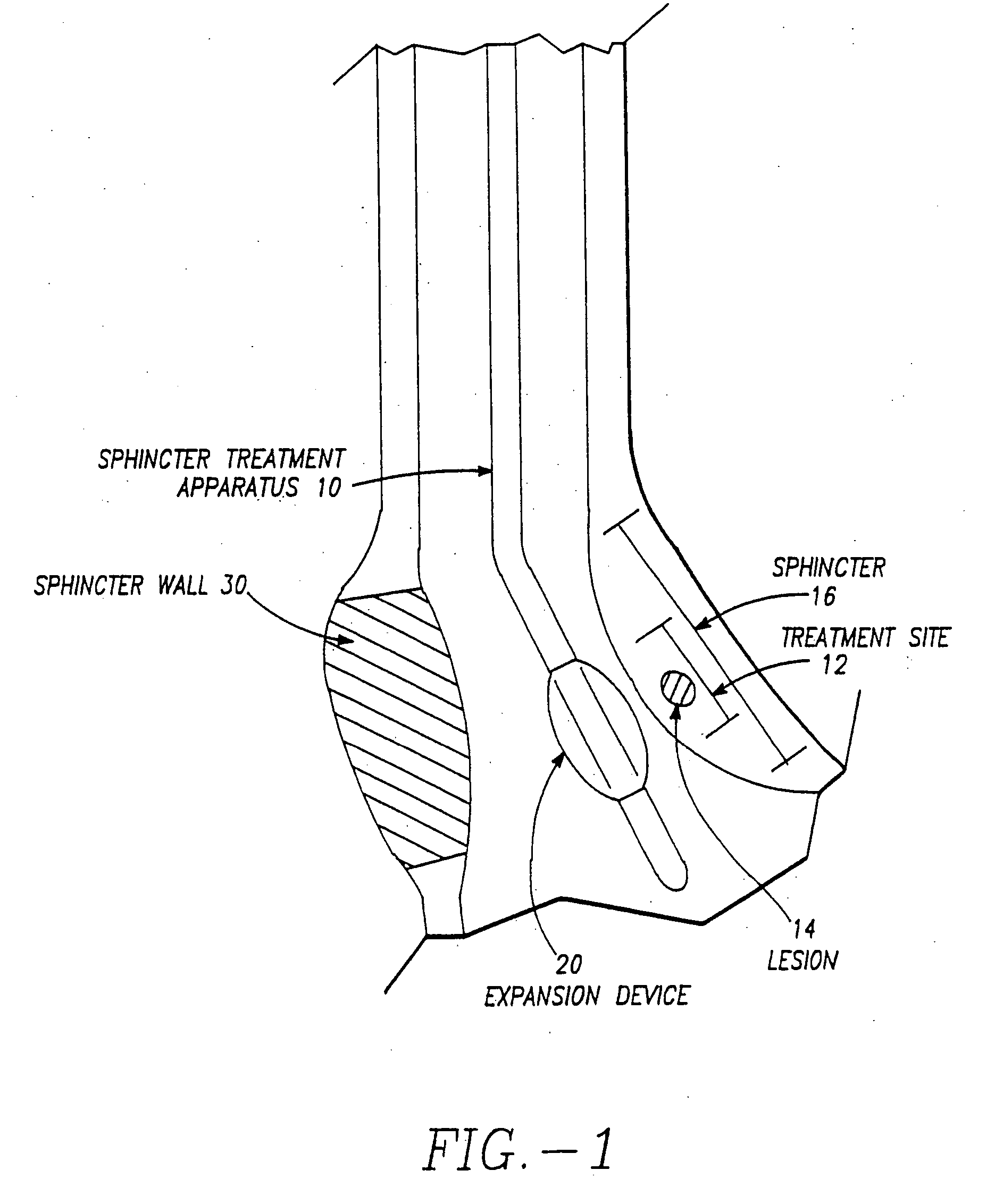
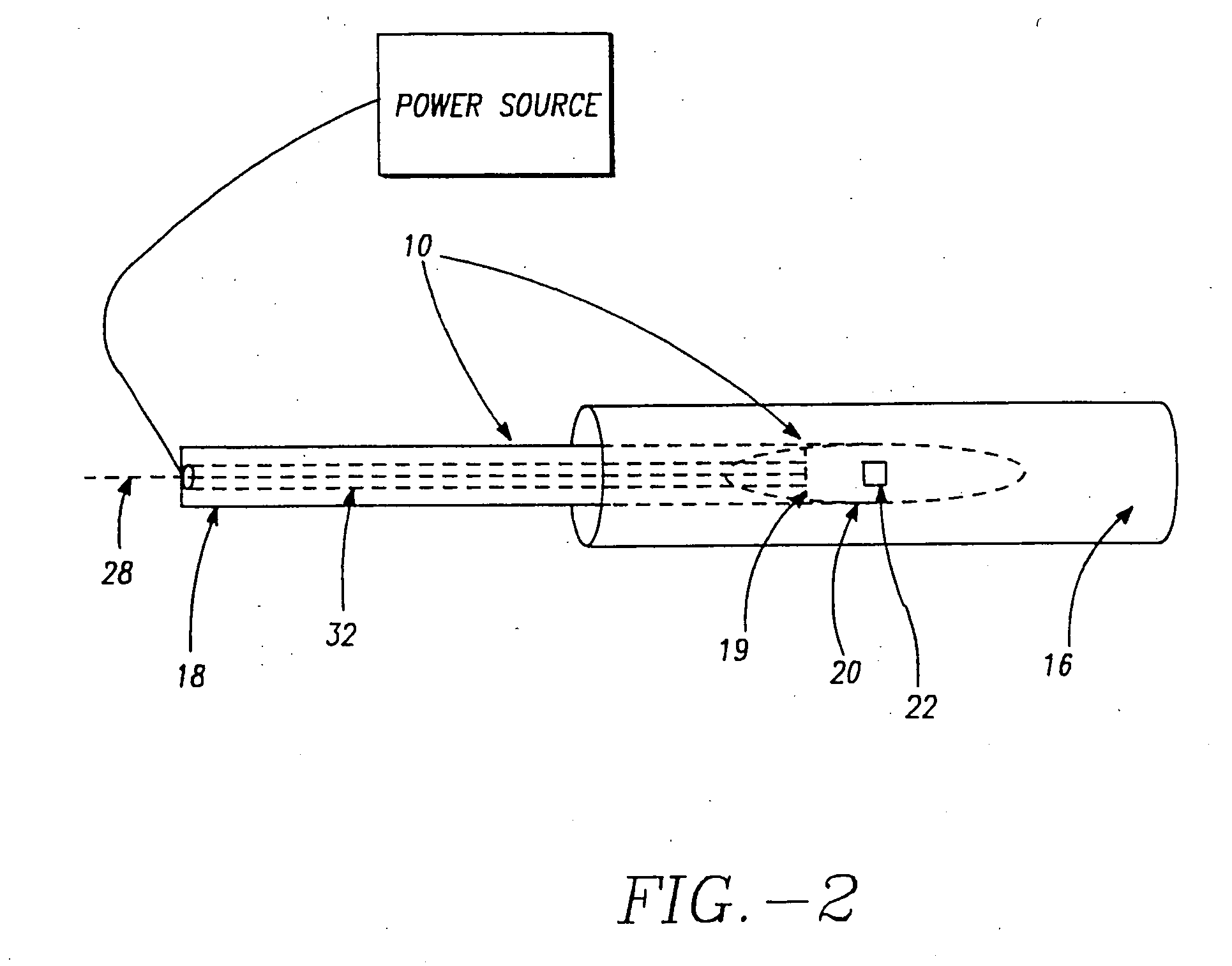
InactiveUS20070093809A1Reduce frequencySufficient energyCannulasSurgical needlesSphincterDistal portion
A sphincter treatment apparatus has an introducer means including a distal portion means. An expandable device means includes a plurality of arm means. Each arm means of the plurality has a distal section means and a proximal section means. Each of distal sections means of the arm means are coupled and each of the proximal sections means of the arm means are coupled to the introducer means distal portion means. The expandable device means is configured to at least partially dilate a sphincter in a deployed state. An energy delivery device means is introduceable from the introducer means into a selected site of the sphincter. The energy delivery device means is configured to deliver sufficient energy to reduce a frequency of relaxation of the sphincter.
Owner:MEDERI THERAPEUTICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com