Patents
Literature
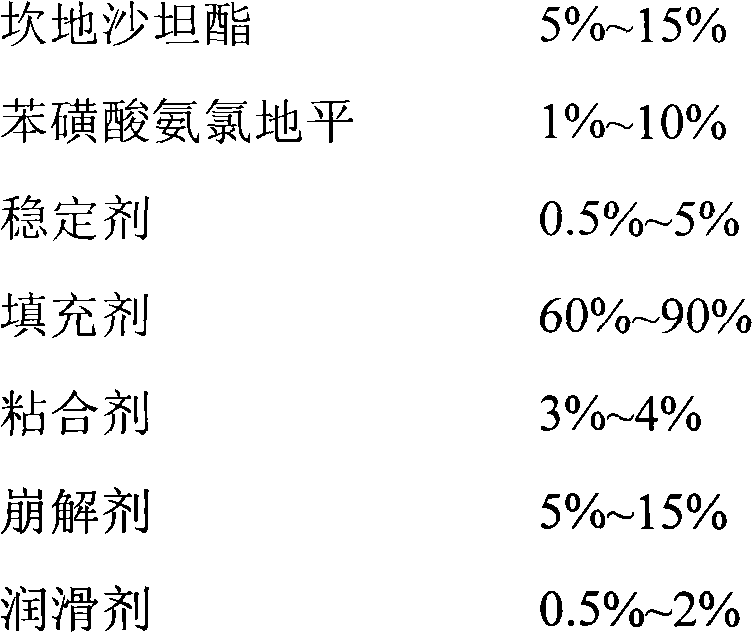
160 results about "Candesartan" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Candesartan is used to treat high blood pressure (hypertension).
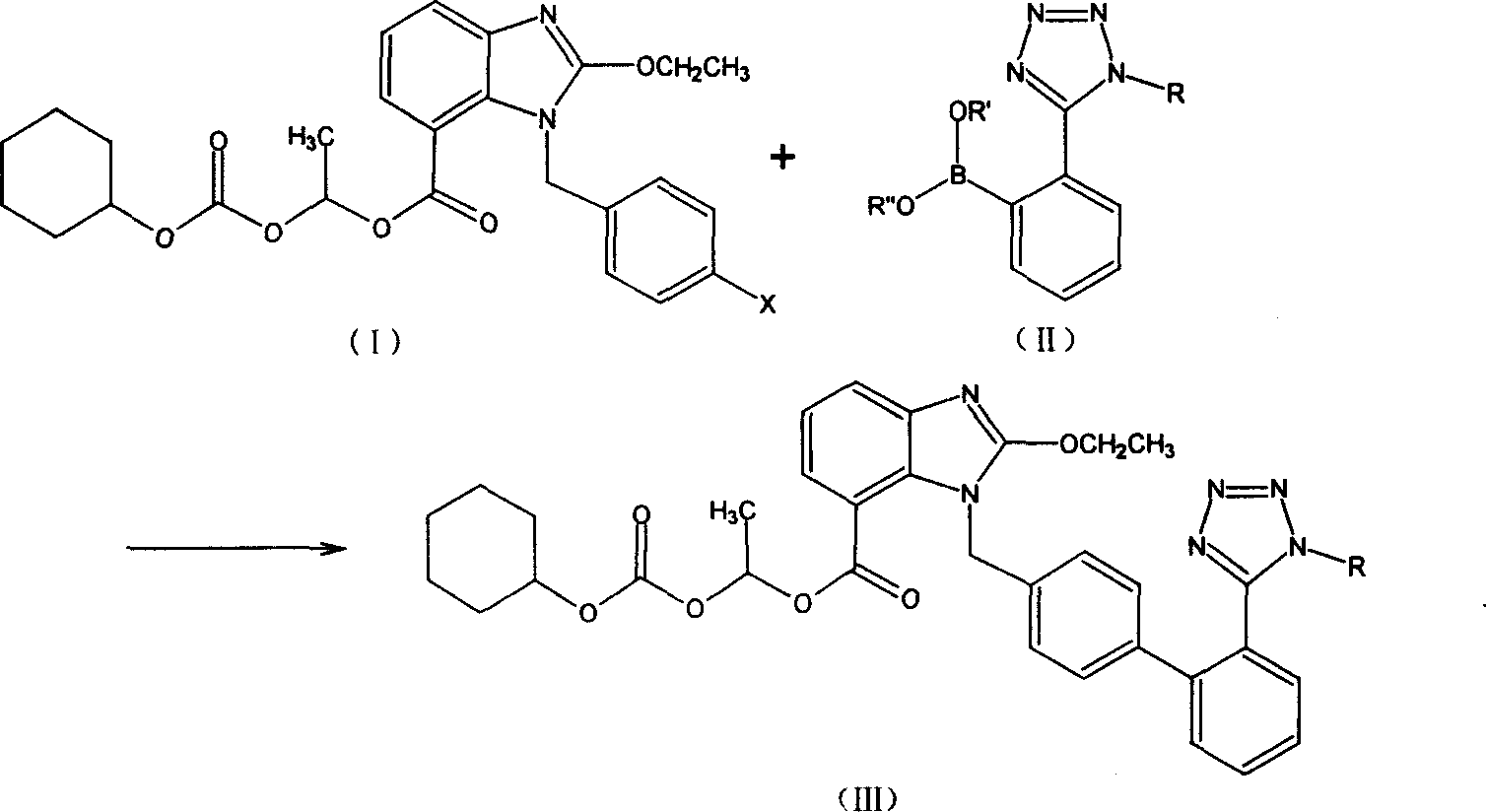
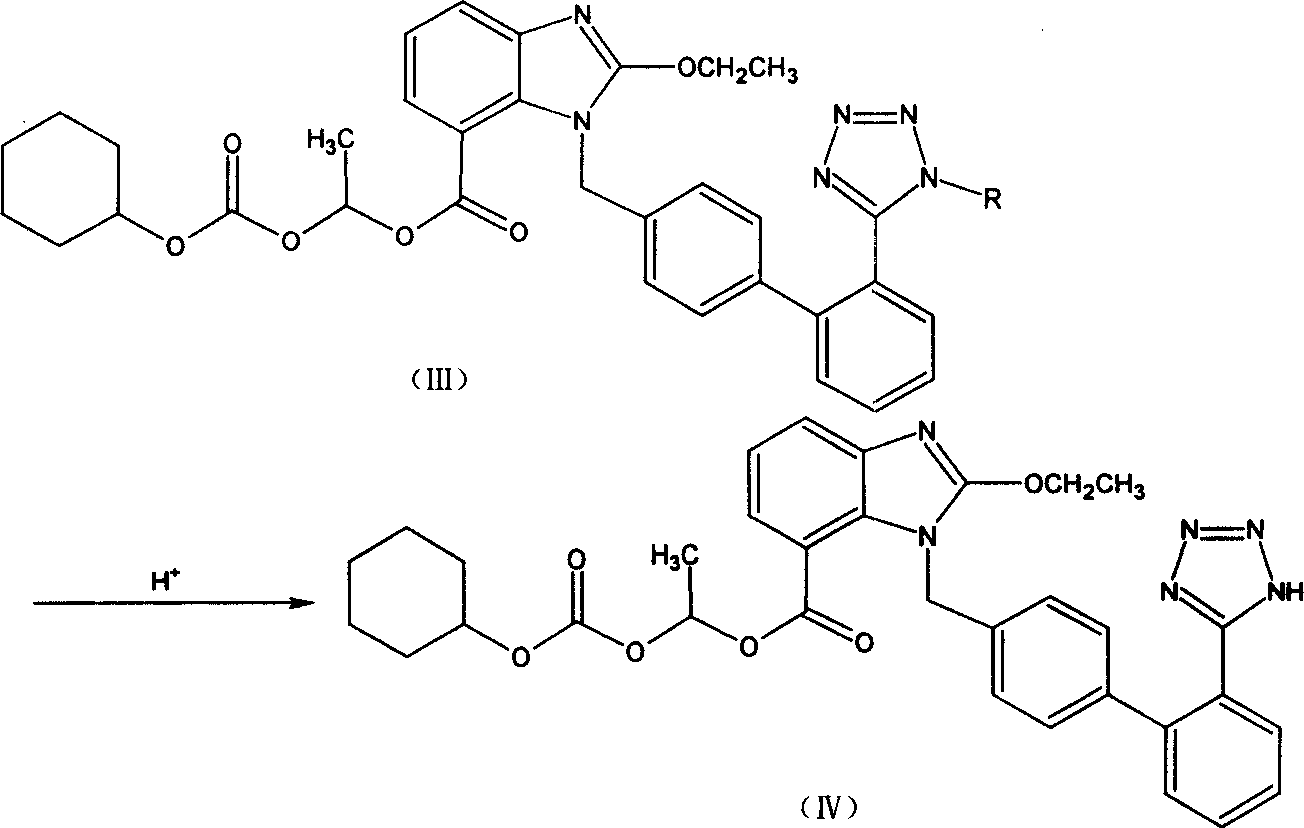
Candesartan Cilexetil and its precursor compound preparation method
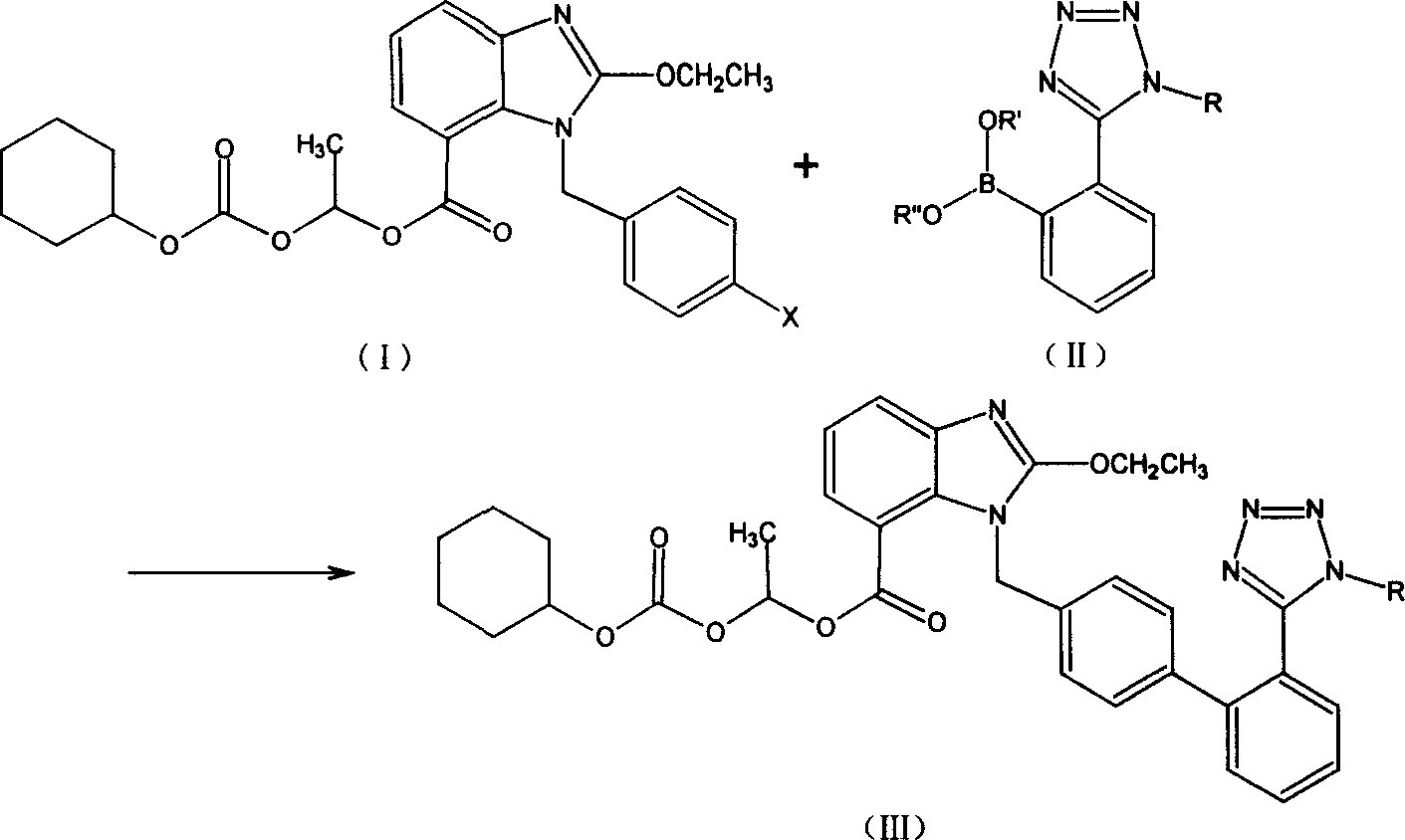
The invention discloses a ridge-ground shatan ester and making method of prosoma compound, wherein the prosoma compound is 2-ethoxy-1-[[2'-(1-R-tetrazole-5-base) [1, 1'-biphenyl]-4-base] methyl]-1H-benzimidazole-7- carboxyl acid1-(cyclohexaoxy carbonyloxygen) carbethoxy (III), which is reacted by 2-ethoxy-1-(4'-halogenated phenyl) methyl-1H-benzimidazole-7-carboxyl acid1-[[(cyclohexaoxy) carbonyl] oxygen] carbethoxy (I) and 2-(1-R-1H-tetrazoline-5-base) borophenylic acid or 2-(1-R-1H-tetrazoline-5-base) borophenylic acid ester (II) in the organic dielectric and catalyst allowed by Suzuki reaction under soluble alkaline compound condition; hydrolyzing the prosoma compound under acid condition to obtain the medical product.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD
Medicament composition containing amlodipine besylate and candesartan cilexetil and medicine box
ActiveCN101371834AGood synergyOrganic active ingredientsCardiovascular disorderCompounding drugsCoronary artery disease
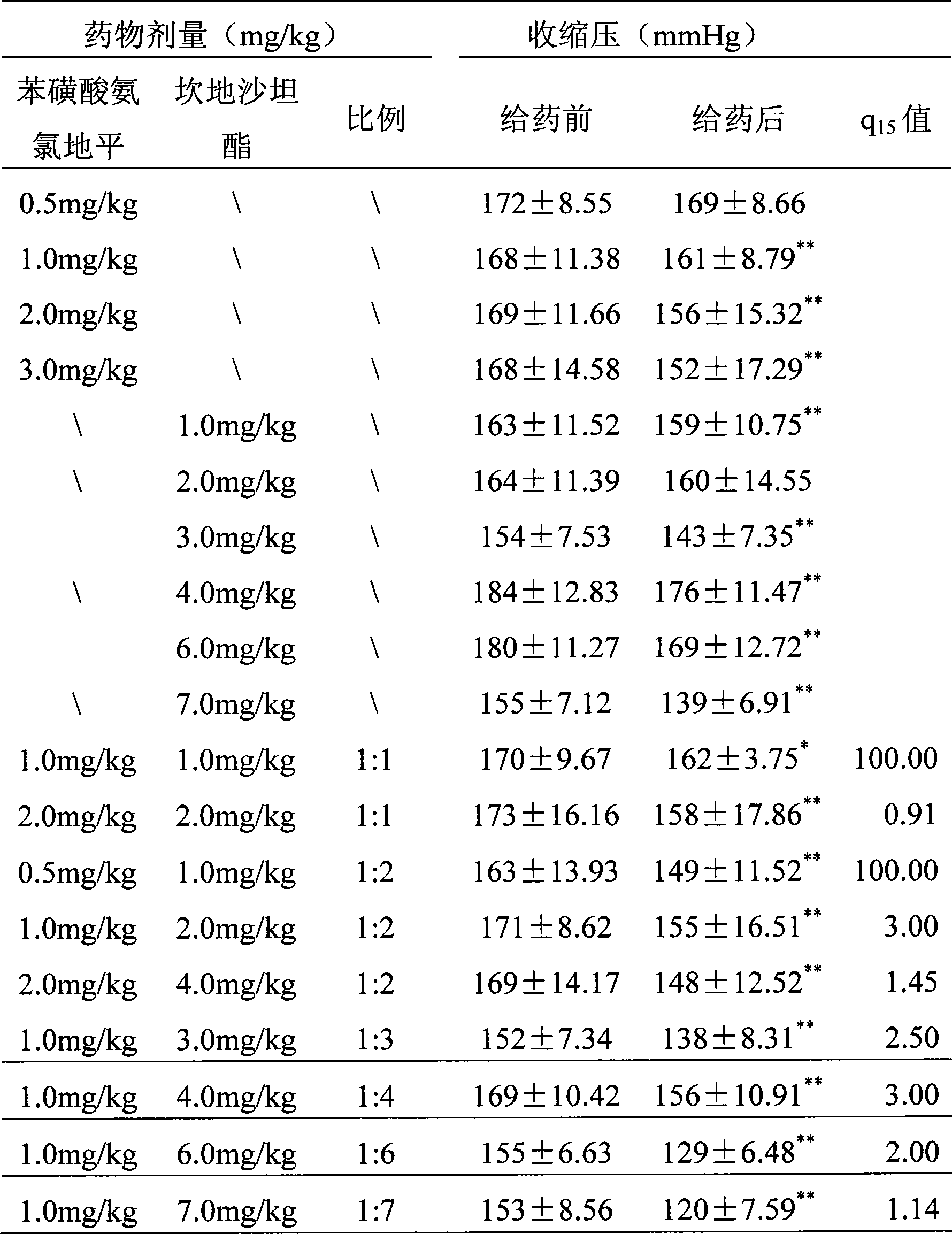
The invention discloses a drug composition containing amlodipine besylate and candesartan cilexetil and a drug box thereof, wherein, the weight ratio of the amlodipine besylate to the candesartan cilexetil is 1:1-6. The compound drug has a better synergistic effect in reducing blood pressure and extending blood vessels, and can be used for curing cardiovascular diseases such as hypertension and coronary heart disease.
Owner:ZHEJIANG YONGNING PHARMA
Novel preparation of trityl group candesartan cilexetil intermediate
ActiveCN101323610AThe synthesis process is simpleLow investment costOrganic chemistryBenzoic acidCandesartan
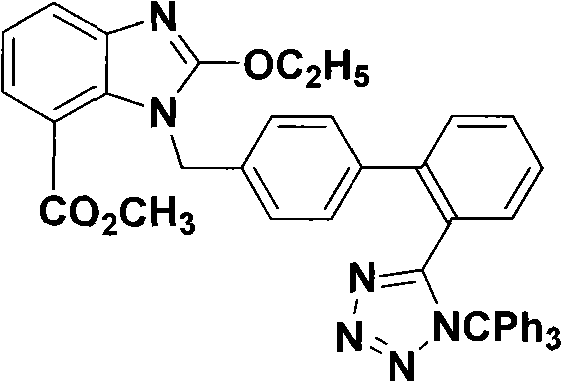
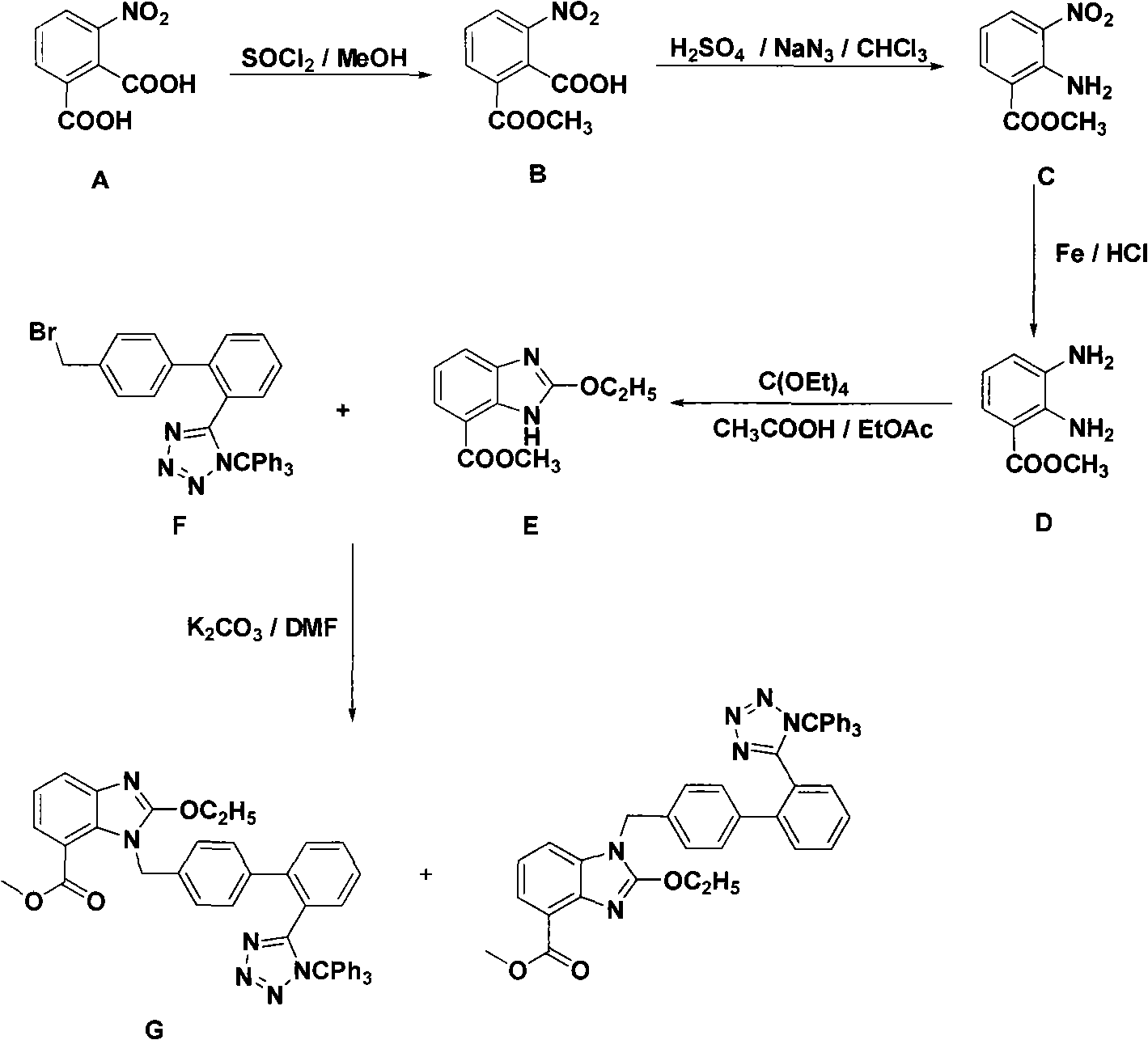
The invention discloses a novel technology for synthesizing an intermediate of trityl candesartan; the synthesis steps thereof comprise: (1) a preparation method of 2-menthyl formate-6-nitryl-benzoic acid; (2) a preparation method of 2-amido-3-nitryl-methyl benzoate; (3) a preparation method of 2, 3-diaminobenzene menthyl formate; (4) a preparation method of 2-oxethyl-4-menthyl formate-3-H-benzimidazole; and (5) the preparation method of the intermediate of trityl candesartan.
Owner:APELOA PHARM CO LTD +1
Calldesartan ci1exetil medicine compounds
ActiveCN101229157AAvoid decompositionLong validity periodOrganic active ingredientsPharmaceutical non-active ingredientsCandesartanPhospholipid
The invention discloses a candesartan ester drug composition which consists of candesartan ester and phospholipids; wherein, the weight of the phospholipid is 0.5 to 10 times that of the candesartan ester; the composition of the invention inserts or wraps the candesartan ester into a central hydrophobic layer of a bimolecular layer of the phospholipid to prepare the candesartan into a candesartan ester liposome composition and the candesartan ester can be prepared into candesartan preparations with suitable pharmaceutical excipient. The composition can prevent the decomposition of the candesartan ester and keep long stability of the candesartan ester so as to prolong the period of validity of the candesartan ester preparations.
Owner:QINGDAO HUANGHAI PHARM CO LTD +1
Medicine compound containing candesartan cilexetil
ActiveCN101862325AAvoid hydrolysisImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsCandesartanEssential hypertension
The invention discloses a medicine compound containing candesartan cilexetil, and a preparation method thereof; and the medicine compound improves the stability of the base medicine and improves the dissolution rate of the medicine by preparing solid dispersion. The medicine compound is used for curing primary hypertension.
Owner:AVENTIS PHARMA HAINAN
Capsule containing candesartan cilexetil and preparation method thereof
InactiveCN101623275AOvercome degenerationOvercoming Dissolution DeclinePharmaceutical non-active ingredientsCapsule deliveryMedicineCandesartan
The invention discloses a capsule containing candesartan cilexetil and the contents of the capsules comprise candesartan cilexetil or other active ingredients compounded with the candesartan cilexetil, a low melting-point oiliness compound, at least one disintegrating agent and at least one lubricating agent; and a capsule shell thereof is a hydroxypropyl methyl cellulose capsule shell. The invention also discloses a preparation method of the capsule containing candesartan cilexetil. The capsule containing candesartan cilexetil uses the capsule shell containing hydroxypropyl methyl cellulose and a large quantity of disintegrating agents, thereby achieving stable dissolution performance; moreover, the dissolution situation is almost invariant after long period (two years) of storage, the dissolution rate has no significant difference compared with newly prepared capsules.
Owner:JIANGSU TIANYISHI PHARMA
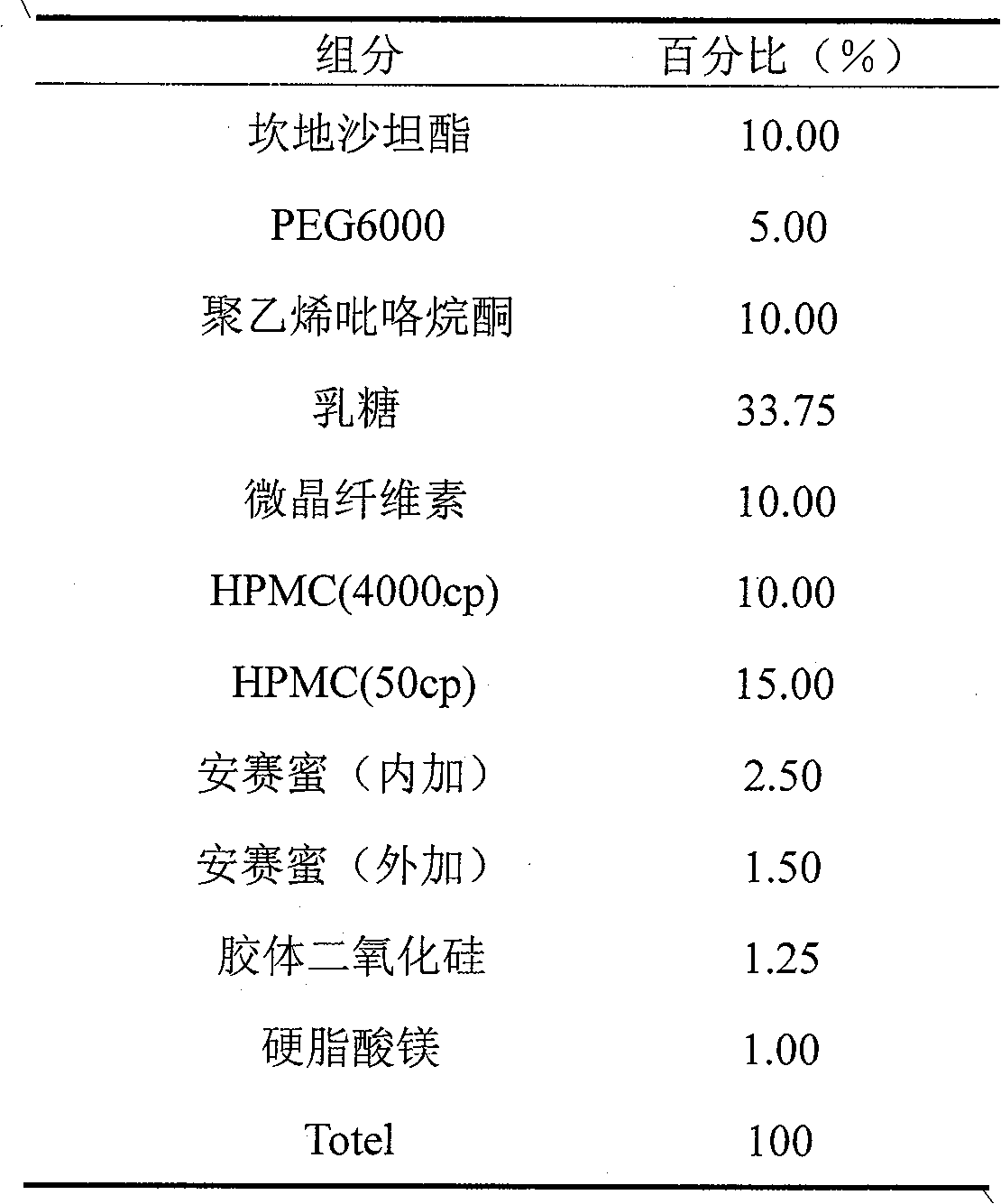
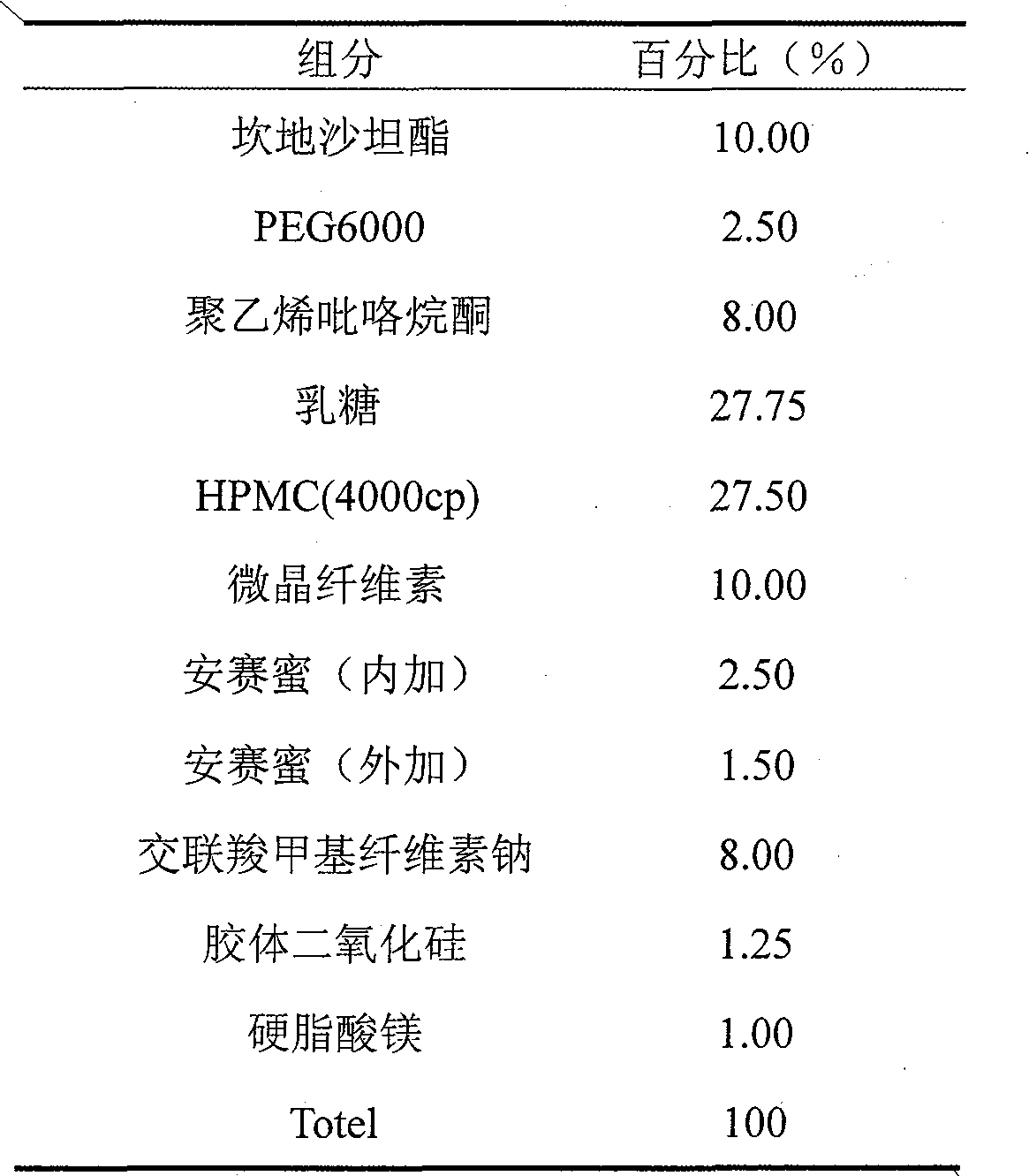
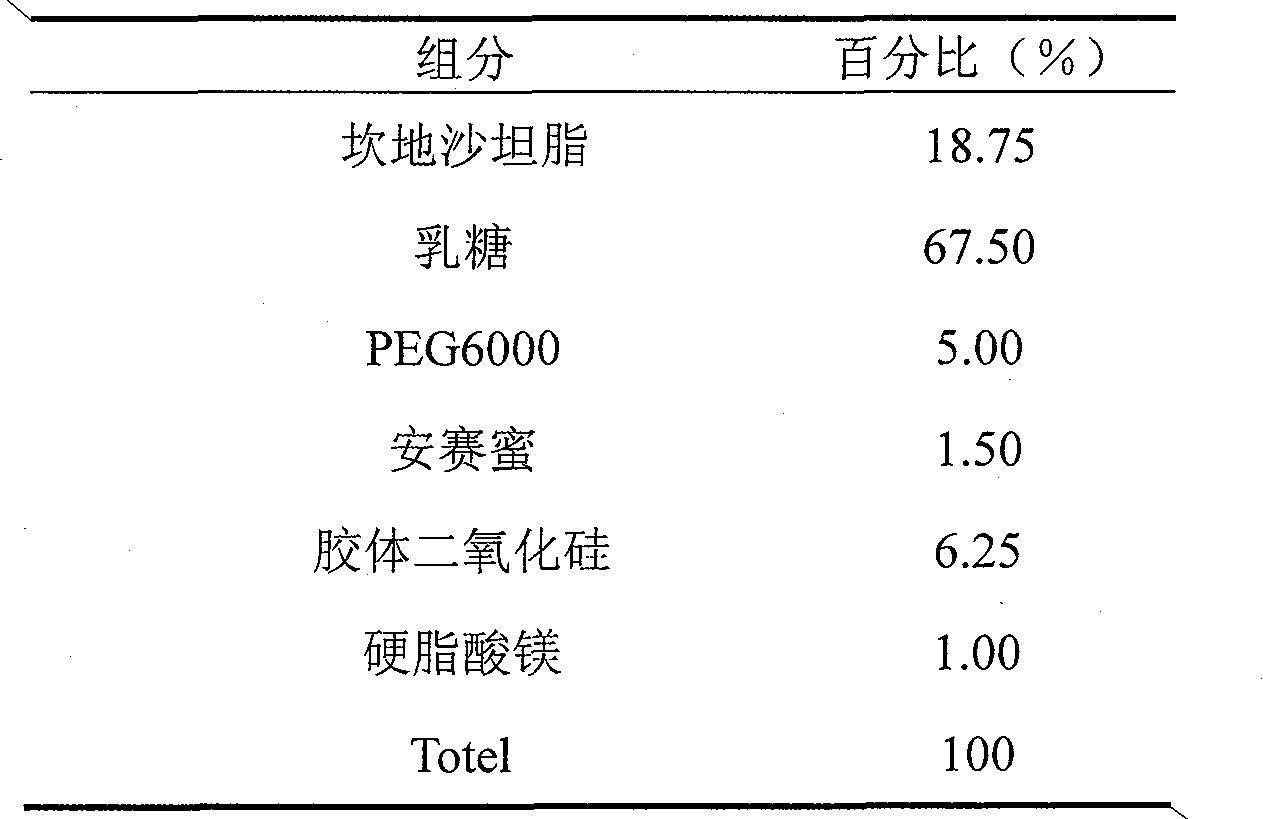
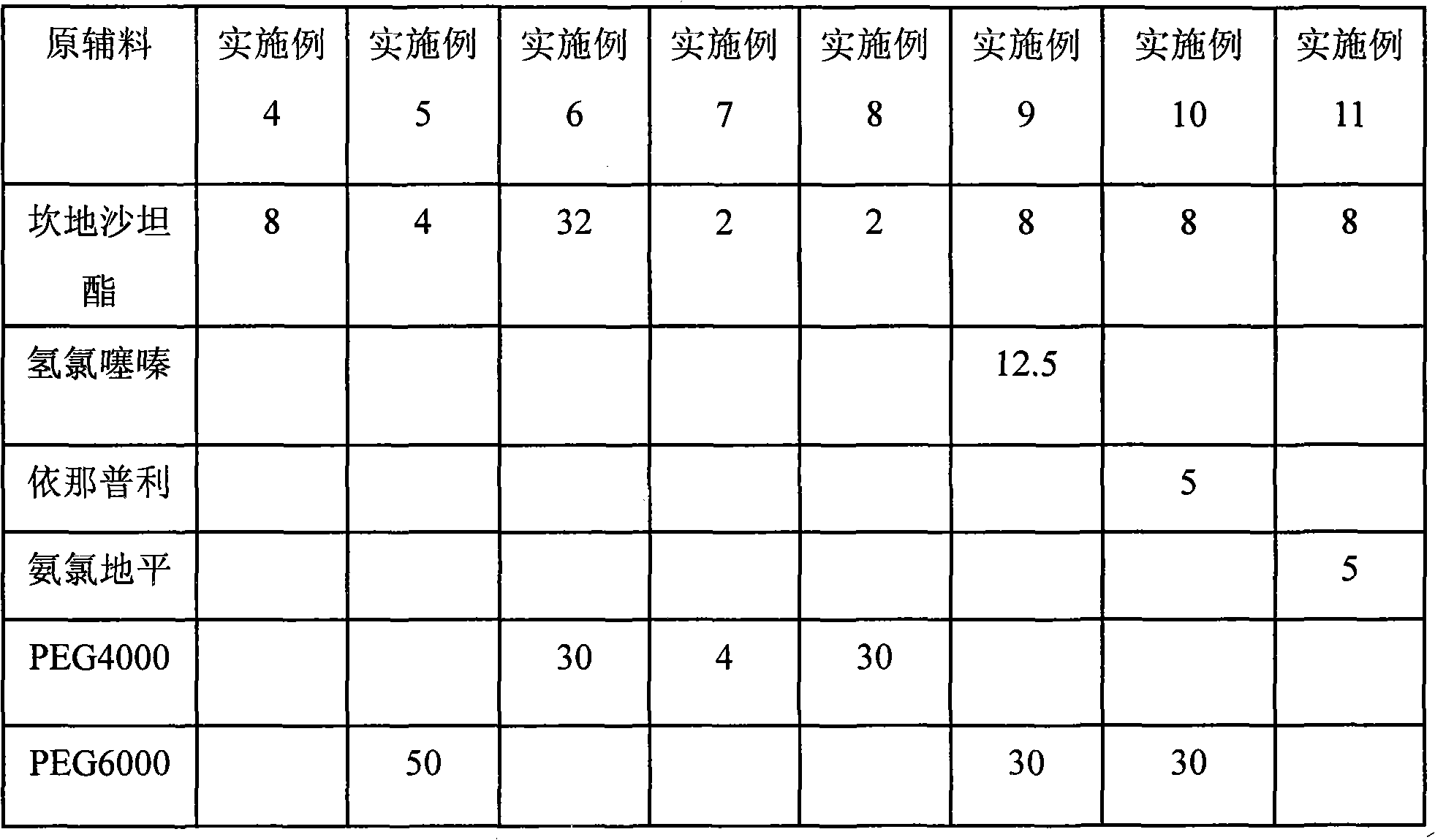
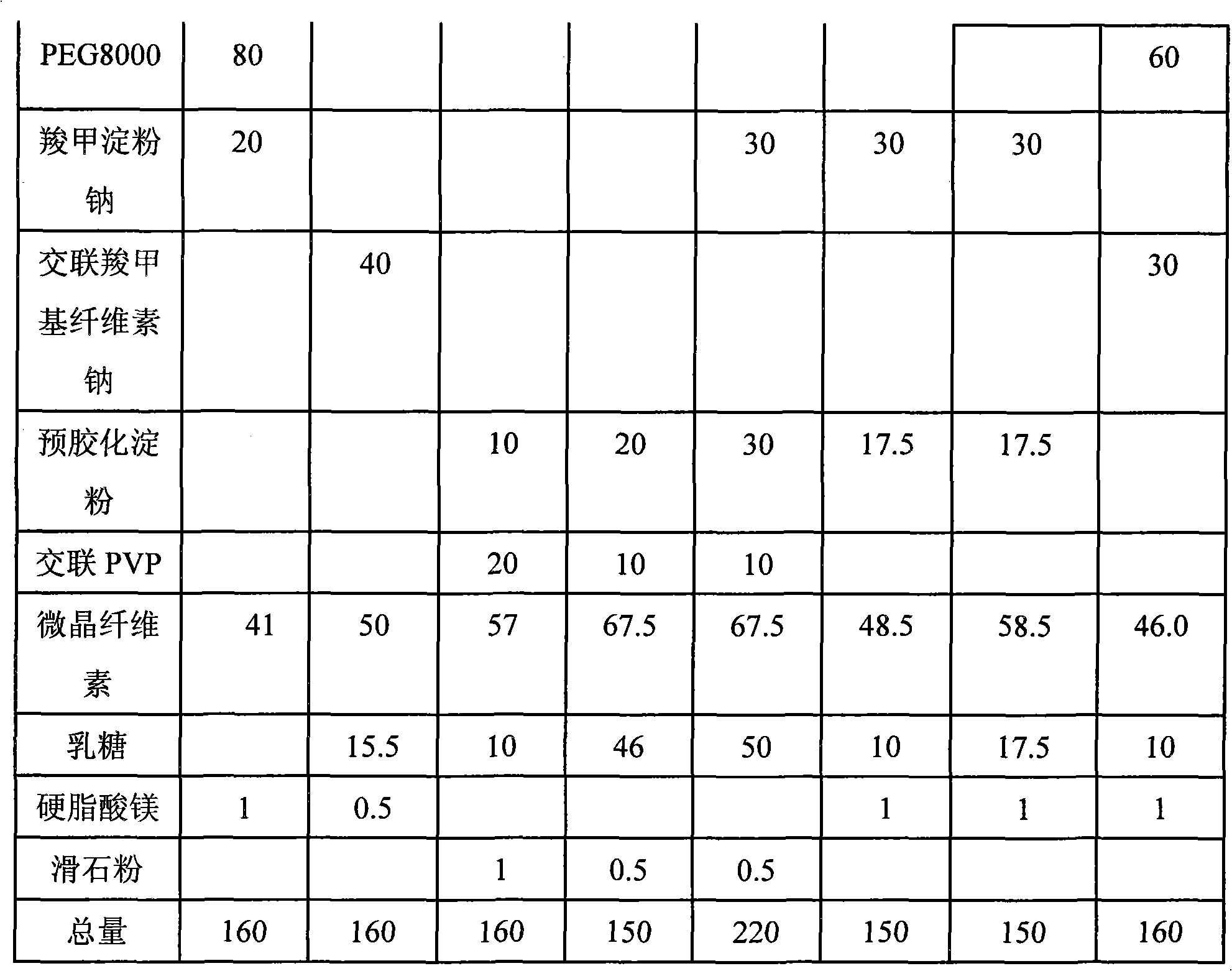
Candesartan cilexetil and amlodipine tablet composition and preparation method thereof
ActiveCN102688236AImprove stabilityReduce disorderOrganic active ingredientsPharmaceutical non-active ingredientsCandesartanMedicine
The invention discloses a candesartan cilexetil and amlodipine besylate tablet composition. According to the formula, the tablet composition contains 2 to 4 weight parts of polyethylene glycol (PEG) 6000 serving as a stabilizer; and the active ingredients of the composition, namely the candesartan cilexetil and the amlodipine besylate, have high stability.
Owner:石药集团中诺药业(石家庄)有限公司 +1
Composition containing candesartan and amlodipine, preparation process, testing process and application thereof
ActiveCN102670604AUnique formulaReduce manufacturing costOrganic active ingredientsComponent separationCandesartanAngina
The invention relates to a composition and a preparation process, a testing process and an application thereof. The composition comprises a therapeutically effective amount of candesartan or pharmaceutically acceptable ester or salt, a therapeutically effective amount of amlodipine or other pharmaceutically acceptable salt, 70-150 parts of mannitol or lactose, 2-5 parts of croscarmellose sodium or cross-calcium carboxymethyl cellulose and 5-40 parts of microcrystalline cellulose or corn starch. The composition is easy to prepare, low in production cost, utilizes accessories of unique prescription, and the like, can be prepared into oral preparation in forms of tablets, capsules, granules and the like by means of pharmaceutics, can be checked by different checking processes, has high quality, has functions of treating hypertension, angina pectoris and kidney diseases and has effect of myocardial protection.
Owner:SICHUAN SUNNYHOPE PHARM CO LTD
Amlodipine and candesartan pharmaceutical composition and preparation method thereof
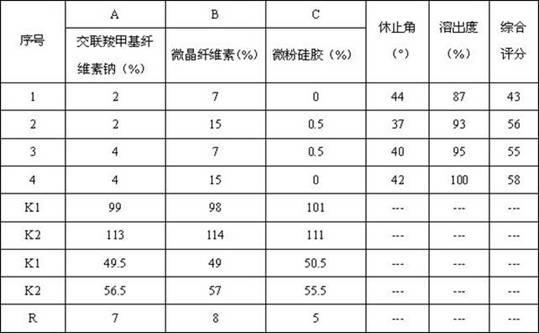
ActiveCN102342937ALarge specific surface areaIncrease dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsCross-linkCandesartan
The invention relates to an amlodipine and candesartan pharmaceutical composition. The pharmaceutical composition comprises the following components in parts by weight: 2.5-5 parts of amlodipine hydrate crystal, 4-16 parts of candesartan, 5-50 parts of compressible starch, 10-60 parts of microcrystalline cellulose, 15-40 parts of low-substituted hydroxypropyl cellulose, 10-45 parts of cross-linked polyvinyl pyrrolidone and 1-3 parts of magnesium stearate, wherein the amlodipine is amlodipine maleate hydrate crystal with a molecular formula of C24H29ClN2O9.1.5H2O. The pharmaceutical composition has the advantages that: amlodipine maleate has rapid and stable action, and can be stably released within 24 hours; and the pharmaceutical composition has strong synergism, accumulation and complementation effects, and has high bioavailability.
Owner:HAINAN JINRUI PHARMA
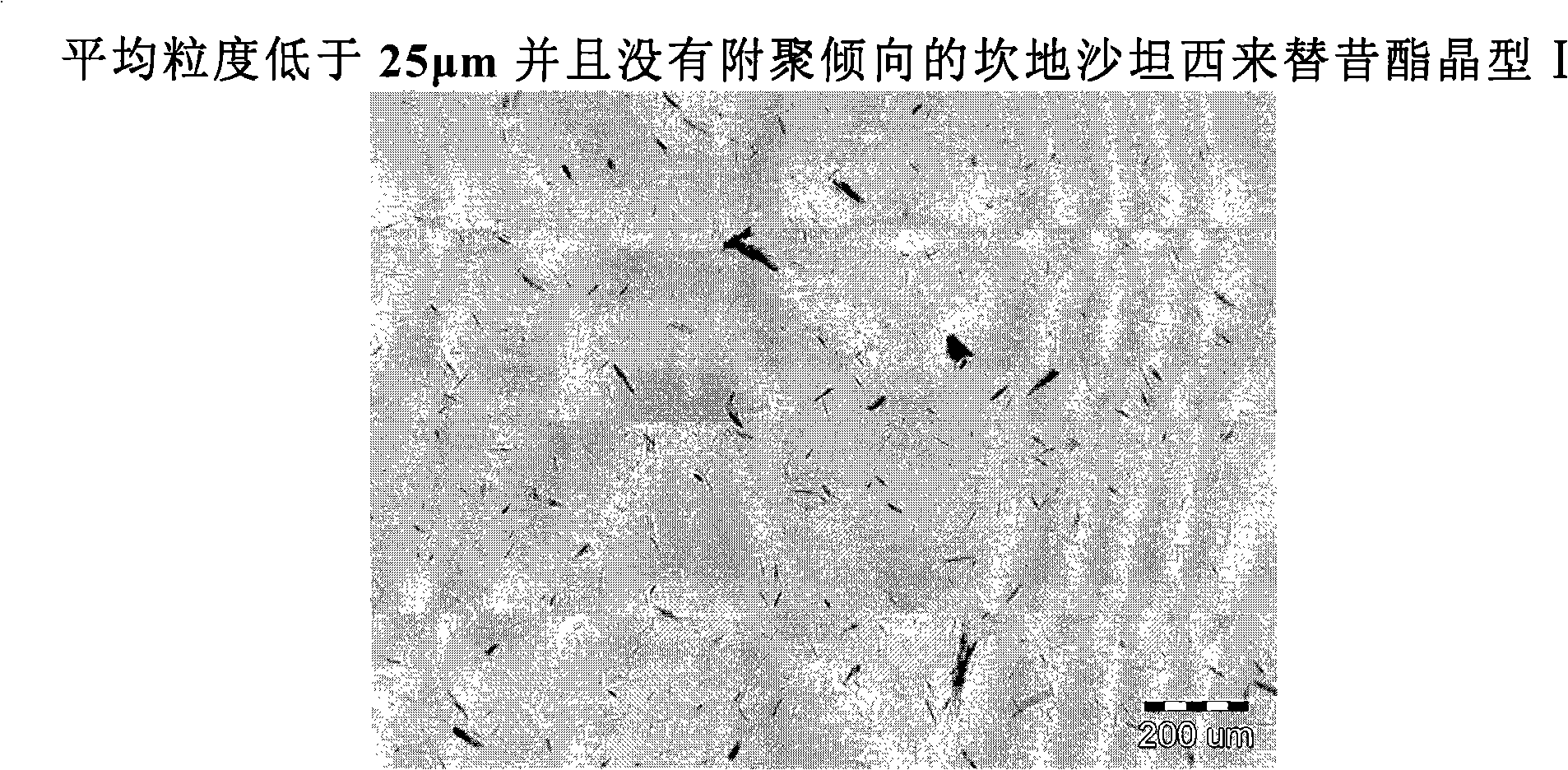
Process for the preparation of candesartan cilexetil form i
InactiveCN101516864ANo tendency to agglomerateOrganic chemistryCardiovascular disorderCandesartanCarbonate
The present invention provides an improved process for the manufacture of candesartan cilexetil form I. Further the present invention discloses a candesartan cilexetil preparation containing less than 0.15%, preferably less than 0.10%, of (+-)-1-Hydroxyethyl candesartan oxanyl carbonate (ester) of the following formula (V) wherein one of A, B and C is an oxygen atom and the other two are-CH2-groups.
Owner:新梅斯托克尔卡托瓦纳兹德拉韦尔公司
Pharmaceutical composition containing calcium blocker, AII receptor blocker and statins
InactiveCN101618215AReduce morbidityImprove complianceSenses disorderMetabolism disorderCandesartanLacidipine
The invention relates to a pharmaceutical composition containing a calcium channel blocker (CCB) or the mixture thereof, an angiotonin 3II receptor blocker (ARB) or the mixture thereof, statins or the mixture thereof and a pharmaceutically acceptable carrier, wherein the CCB is selected from l-amlodipine, amlodipine, lacidipine, nitrendipine or the mixture thereof; the angiotonin II receptor blocker is selected from telmisartan, losartan, irbesartan, candesartan or the mixture thereof; and the statins are selected from atorvastatin, simvastatin, ruishufatadine, fluvastatin or the mixture thereof. The pharmaceutical composition is used for treating various high blood pressures and preventing or treating cardiovascular and cerebrovascular diseases relevant to the hypertension, reduces the disease rate and / or mortality rate of the cardiovascular and cerebrovascular diseases and also improves the adaptability for a sufferer taking medicine.
Owner:王丽燕
Preparation method of C-type candesartan cilexetil crystal
The invention discloses a preparation method of a C-type candesartan cilexetil crystal to solve the problem that an existing candesartan cilexetil crystal form and crystal grains are difficult to process. The preparation method comprises the following steps of: (1) adding a candesartan cilexetil crude product K6 into an ethanol solution, and stirring and heating until completely dissolving; (2) reducing the temperature of the completely dissolved solution to 30-35 DEG C, stirring at the temperature until a crystal is separated out, dropwise adding water, and filtering after complete crystallization to obtain a K6 fine product; and (3) adding absolute ethyl alcohol into the K6 fine product, pulping by adopting an emulsifying agent, conveying by using a peristaltic pump, and performing spray drying to obtain the C-type candesartan cilexetil crystal of which the particle size is of nano-scale. According to the preparation method, a spray drying method is adopted, so that the problem of product agglomeration in the conventional drying is solved, and then a dried product meets requirements on the crystal form and particle size once.
Owner:EMEISHAN HONGSHENG PHARMA
Preparation method of candesartan cilexetil
The invention relates to a preparation method of candesartan cilexetil. The method takes candesartan acid as a starting material to acquire trityl candesartan cilexetil by a one-pot process, and then the trityl is removed from the trityl candesartan cilexetil in lower alcohol, thus obtaining the candesartan cilexetil. The candesartan cilexetil product prepared by the method has purity up to more than 99.80%. The method reduces the operation steps of reaction and solvent consumption, shortens the reaction period, and greatly improves the yield of trityl candesartan cilexetil, thus being more suitable for industrial production.
Owner:迪嘉药业集团股份有限公司
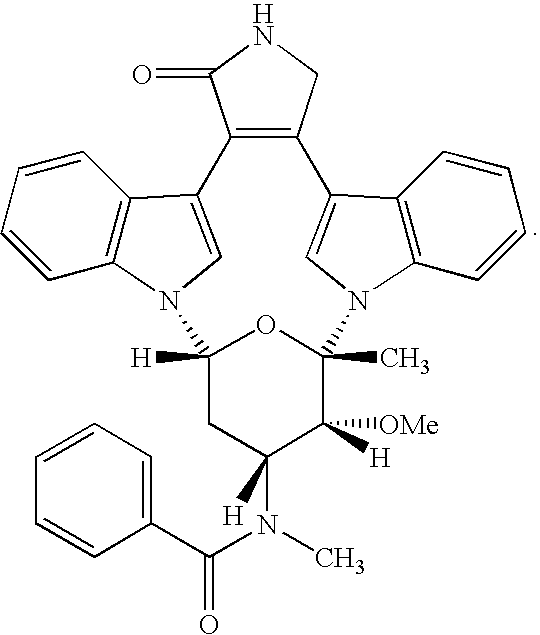
Methods For Diagnosing And Treating Diabetic Microvascular Complications
The present invention relates to a method for treating one or more diabetic microvascular complications in a patient in need of said treatment comprising: (a) diagnosing the severity of at least three different microvascular complications in said patient by calculating a diabetes microvascular complications score with a diabetes microvascular complications scoring tool; and (b) administering to said patient in need thereof a therapeutic amount of a compound selected from the group consisting of ruboxistaurin, enzastaurin, PKC 412, candesartan cilexetil, fidarestat, lidorestat, pyridoxamine and pegaptanib, or a pharmaceutically acceptable salt thereof, and ranibizumab; in an amount that is effective in treating one or more diabetic microvascular complications in said diabetic patient.
Owner:ELI LILLY & CO
Oral tablet containing candesartan cilexetil and benzene sulfonate amlodipine and preparation method for oral tablet
ActiveCN102670603AAvoid stickingSimple processOrganic active ingredientsPill deliveryCandesartanAmlodipine besilate
The invention discloses an oral tablet containing candesartan cilexetil and benzene sulfonate amlodipine. The oral tablet consists of benzene sulfonate amlodipine, candesartan cilexetil and medicinal auxiliary materials, wherein the medicinal auxiliary materials contain a stabilizing agent and other auxiliary materials such as one or more of a filling agent, a binding agent and a lubricating agent. The tablet containing candesartan cilexetil and benzene sulfonate amlodipine is attractive in surface and stable in quality. The invention also provides a preparation method for the oral tablet; and the process is simple, low in cost and suitable for commercial production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Method for preparing candesartan cilexetil
ActiveCN101781286AReduce usageHigh yieldOrganic chemistryBulk chemical productionCandesartanTetrazole
The invention provides a method for preparing candesartan cilexetil, which can solve the problems of longer reaction route, easy remaining of toxic substances in medicines and lower total yield existing in the prior art. In the method, the candesartan cilexetil is finally prepared by using 2-amino-3-nitrobenzoic acid as an initial raw material through esterification reaction, N-alkylation reaction, nitro reduction reaction, cyclization reaction, hydrolysis reaction, esterification reaction and tetrazole protecting group deprotection reaction. The synthetic method has simple steps and high yield, greatly reduces the participation and generation of toxic products in the reaction process, reduces the release of waste and is beneficial to clean production.
Owner:QINGDAO HUANGHAI PHARM CO LTD
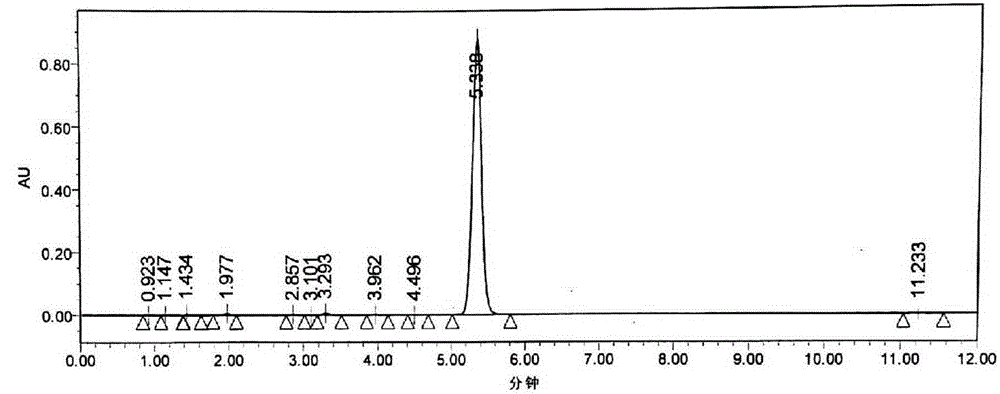
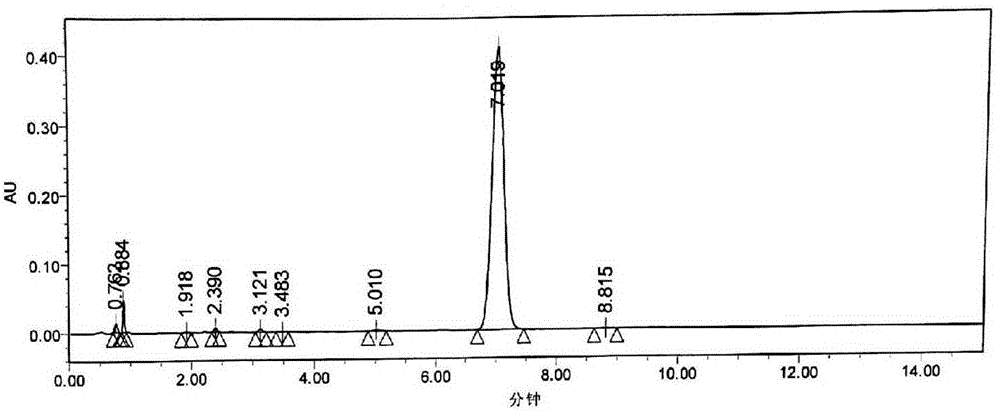
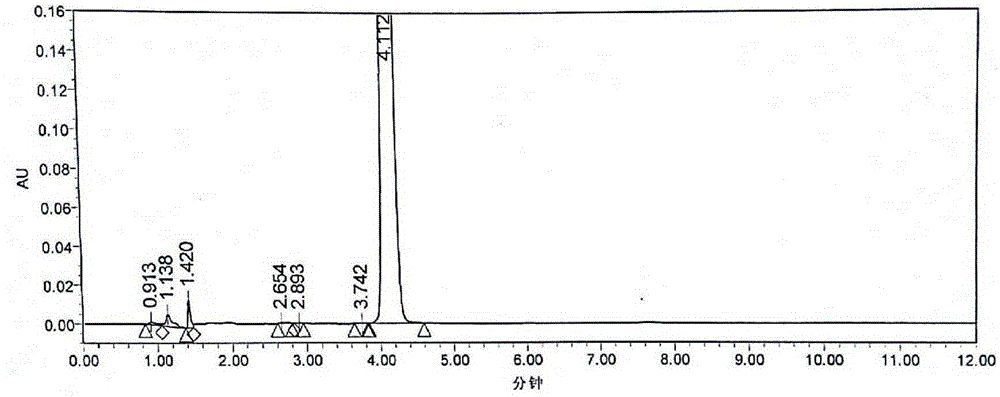
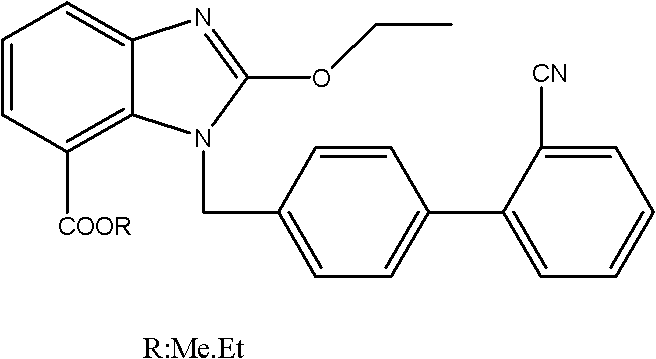
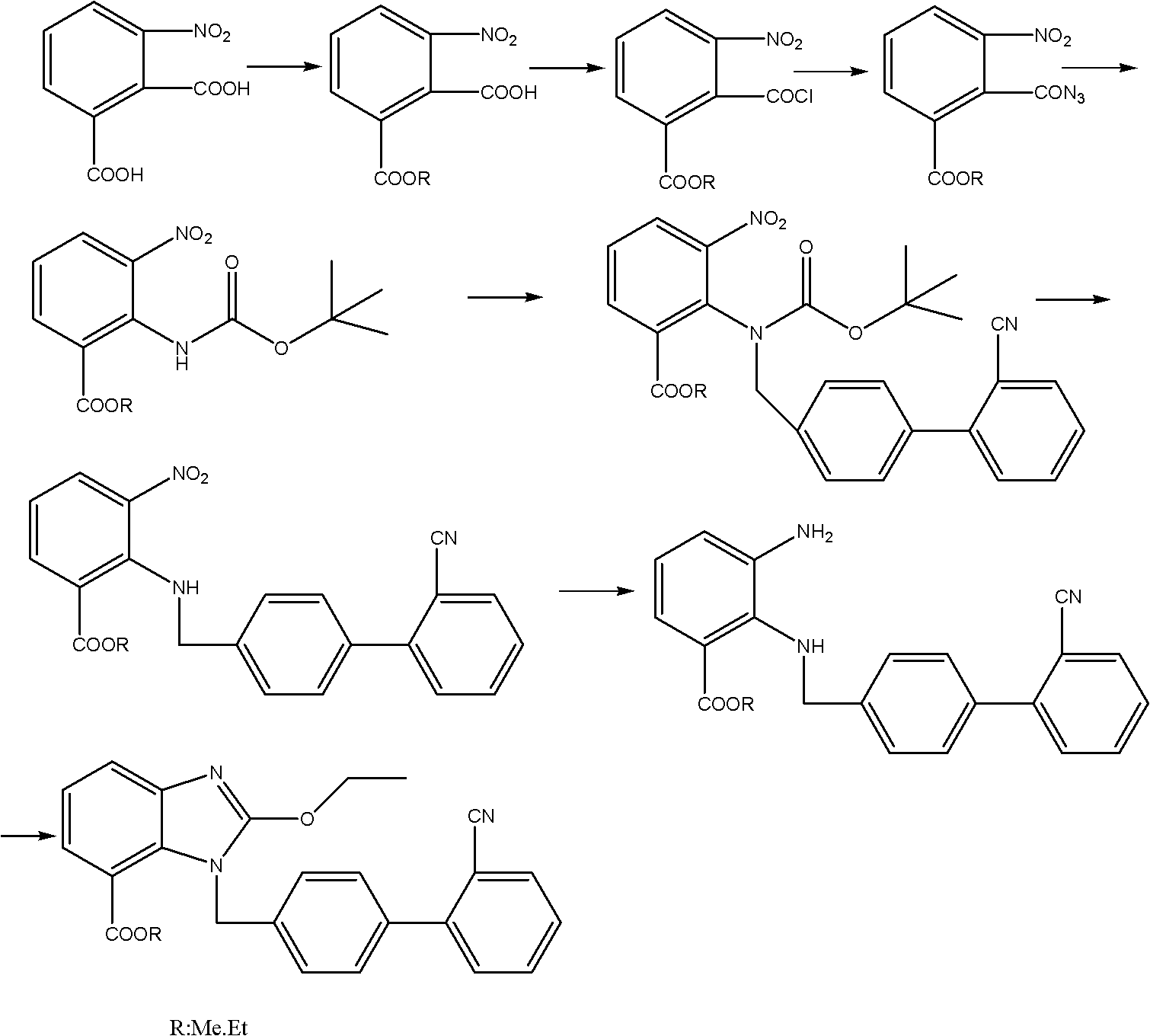
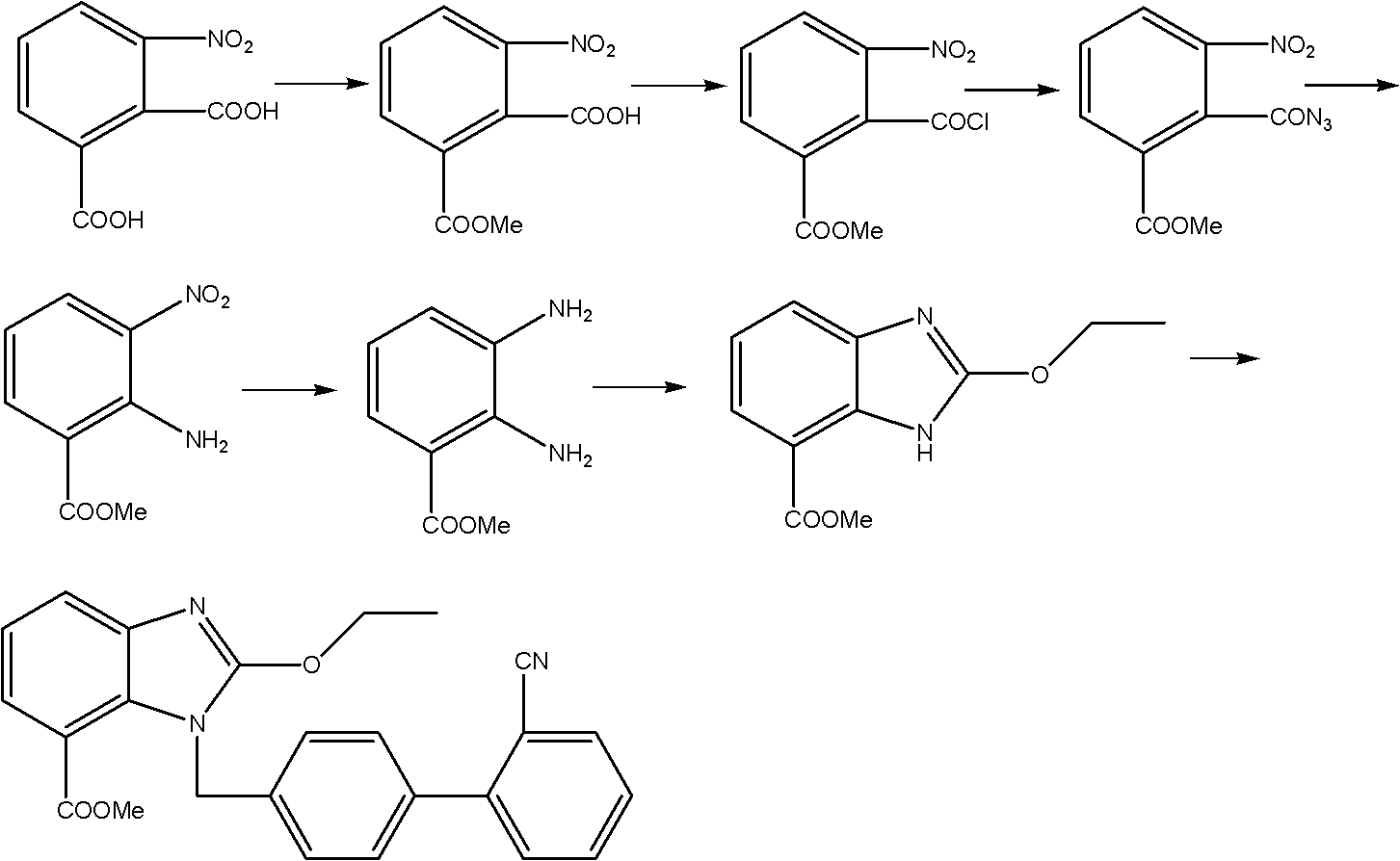
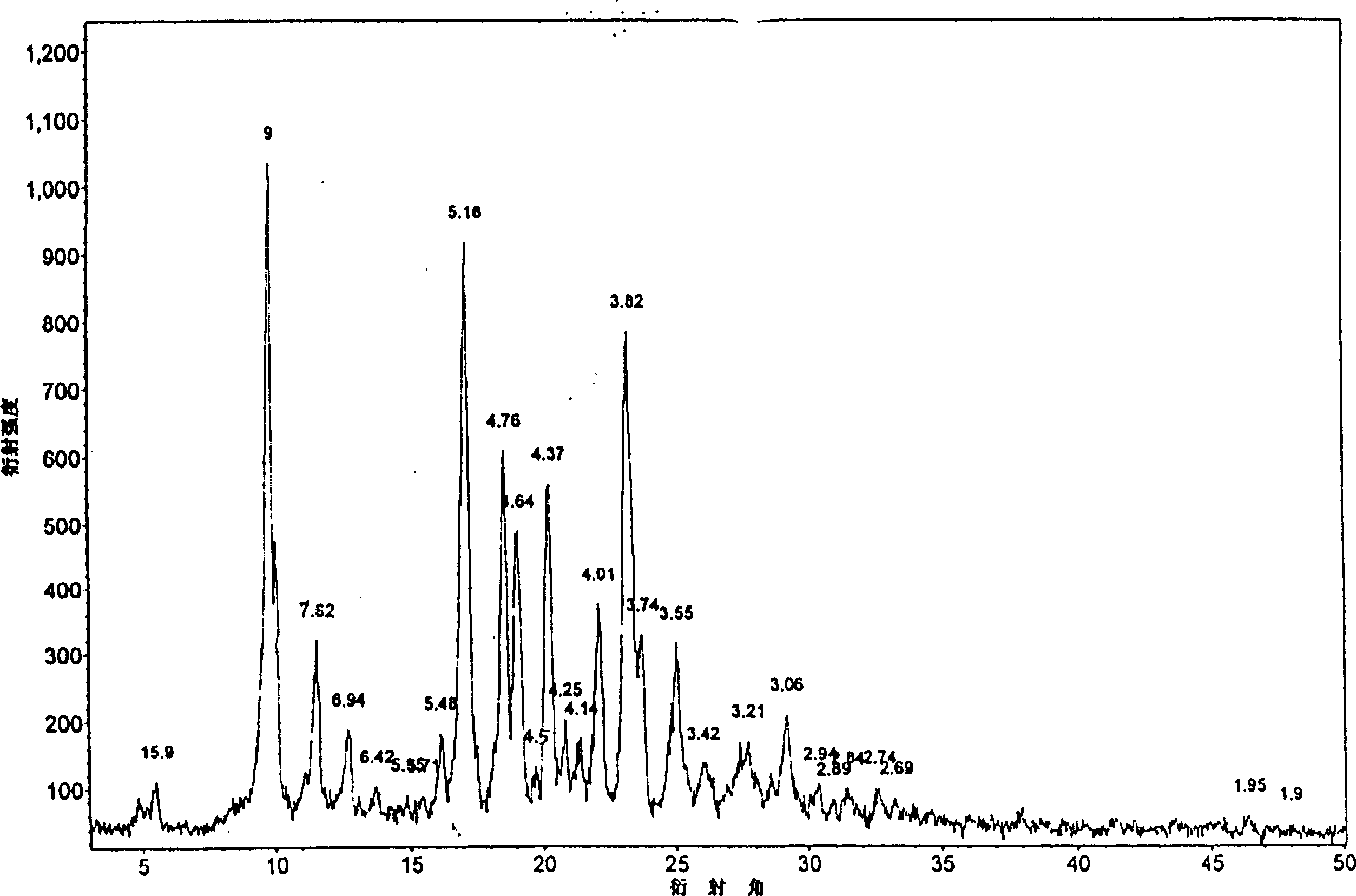
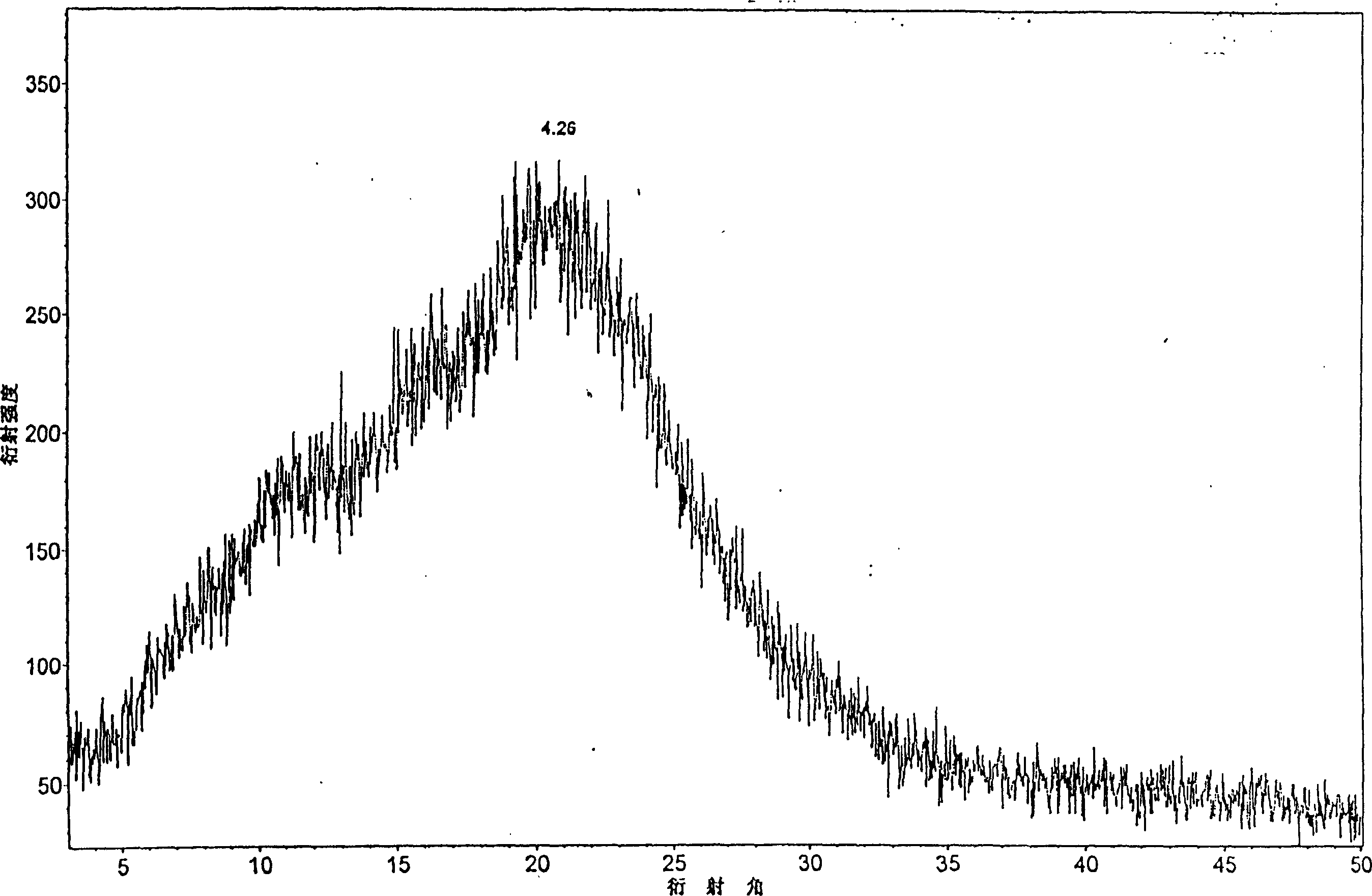
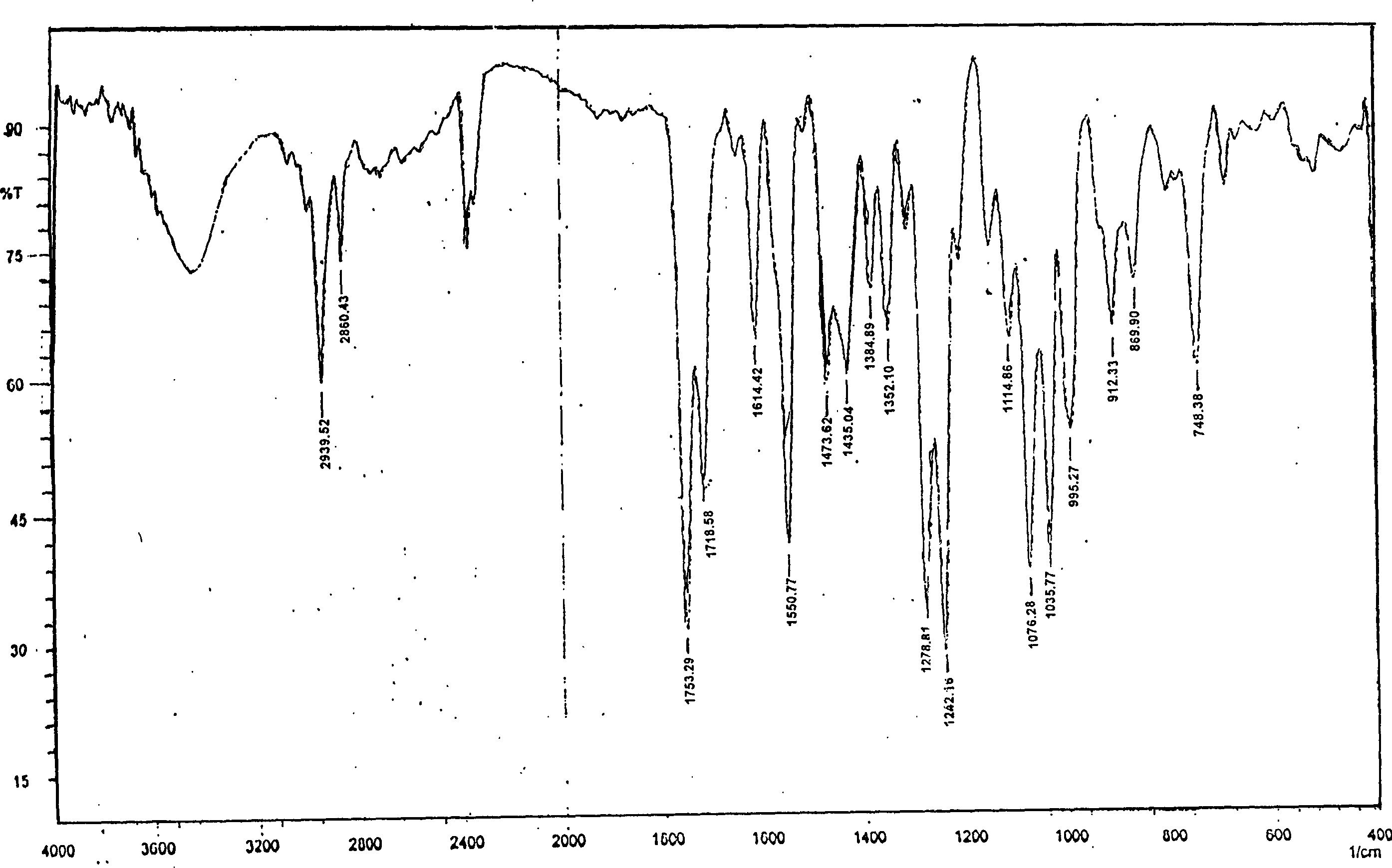
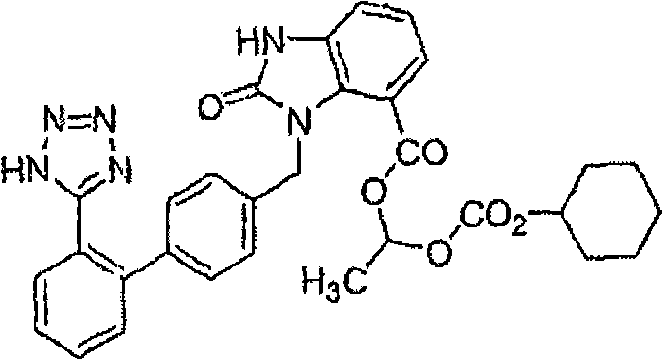
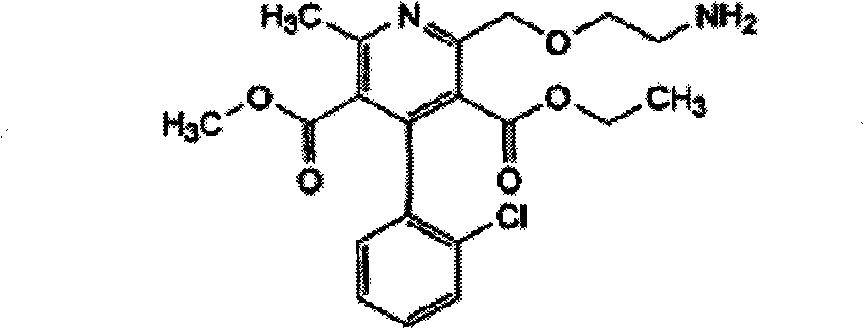
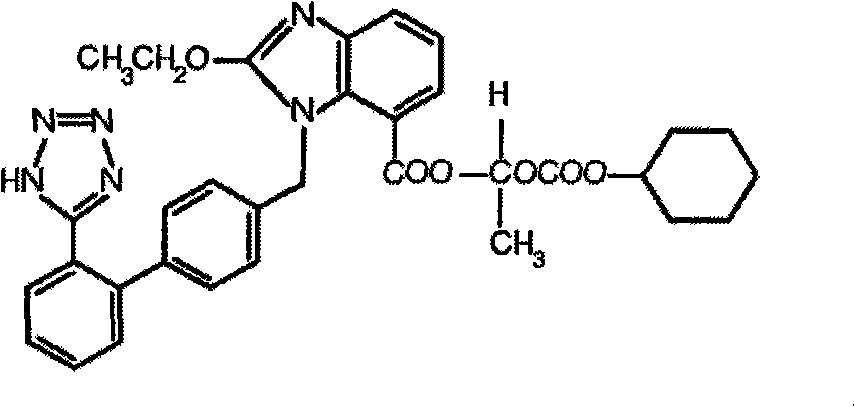
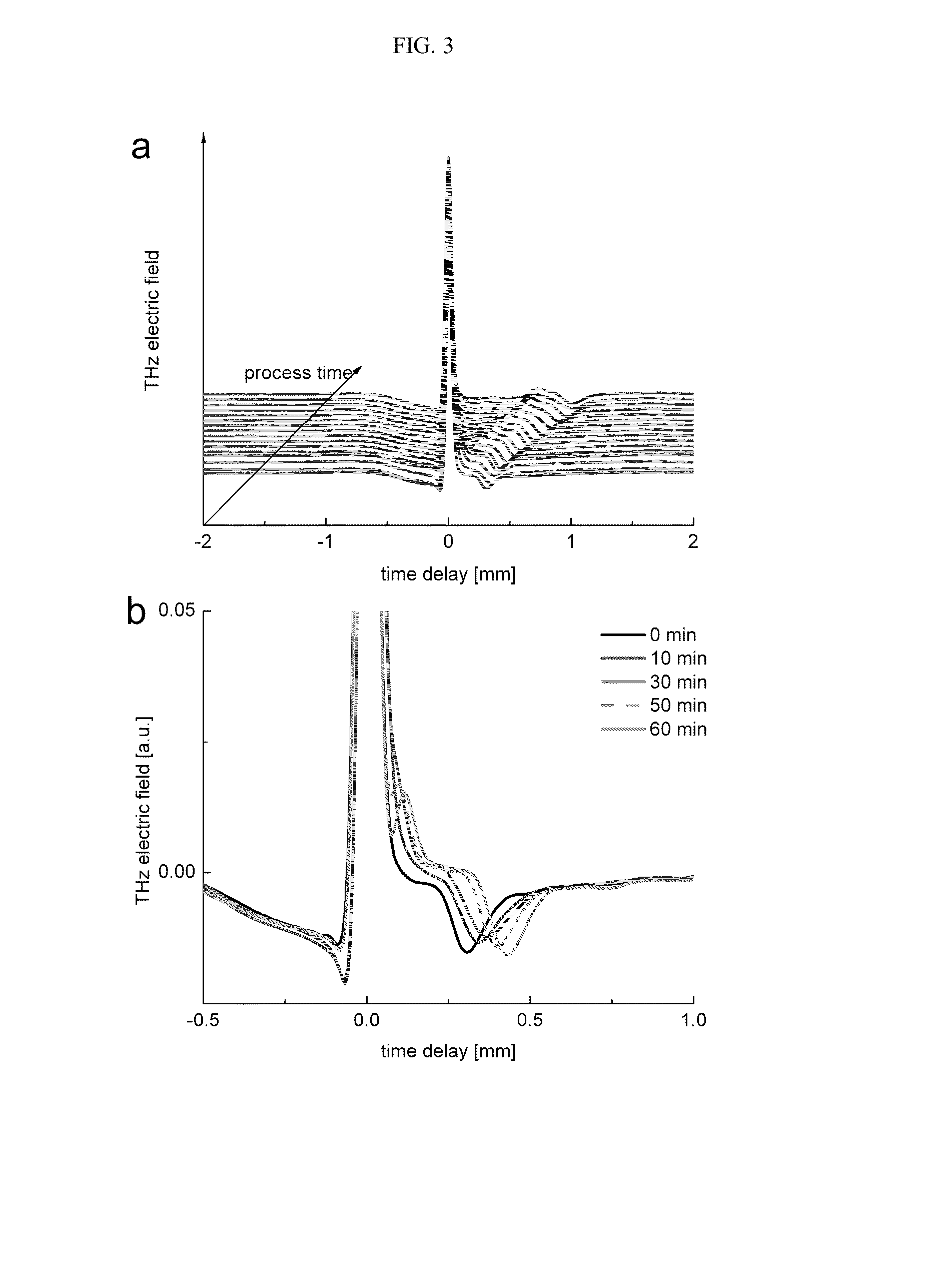
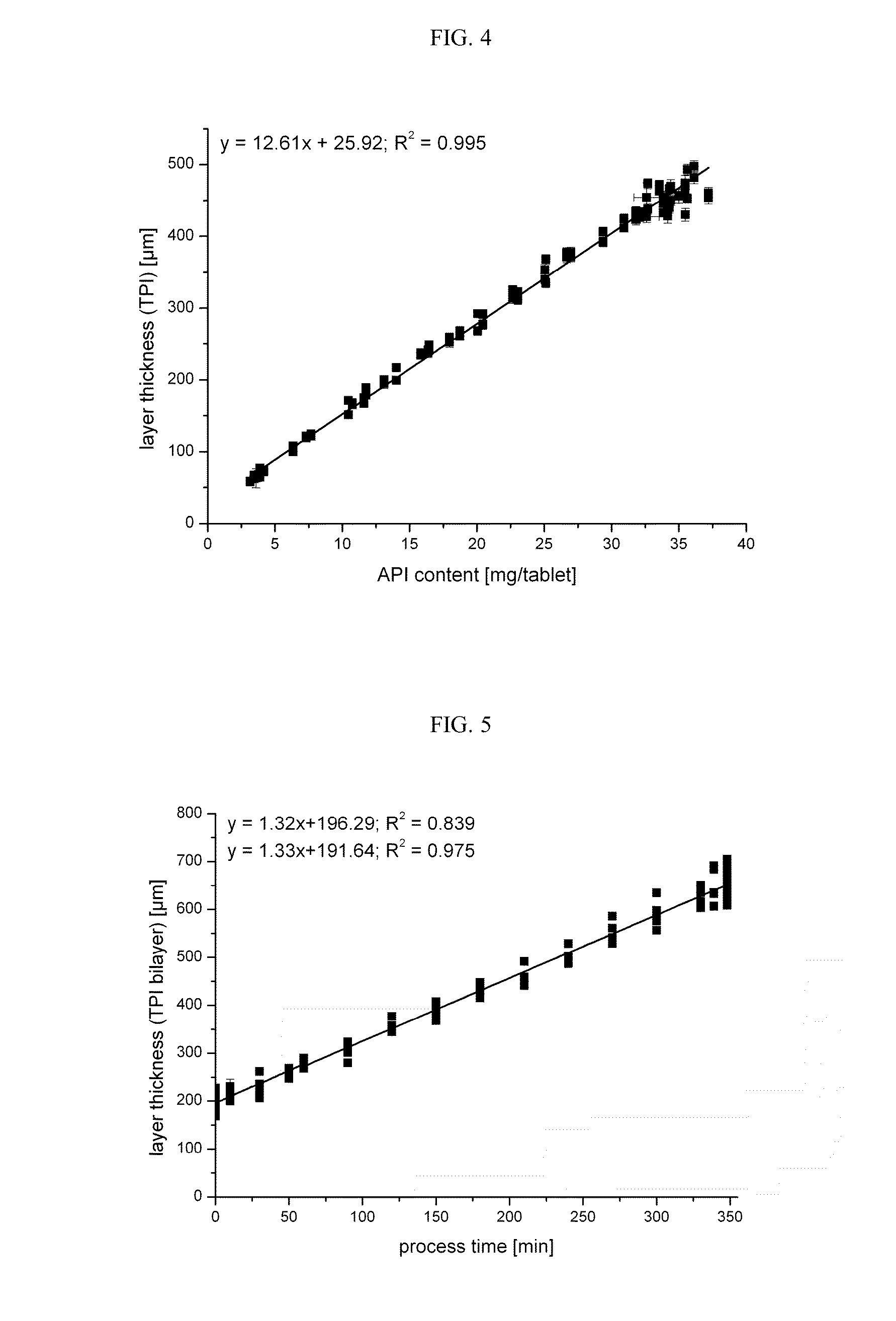
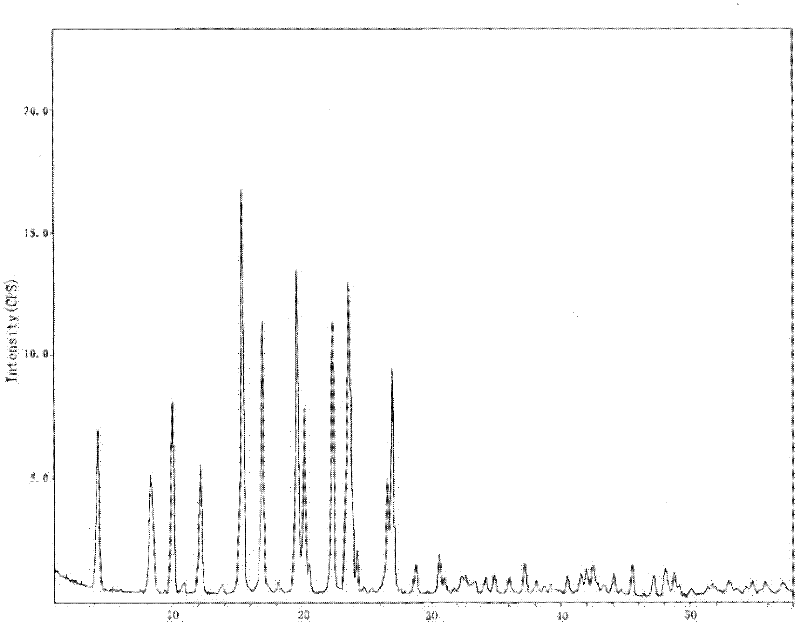
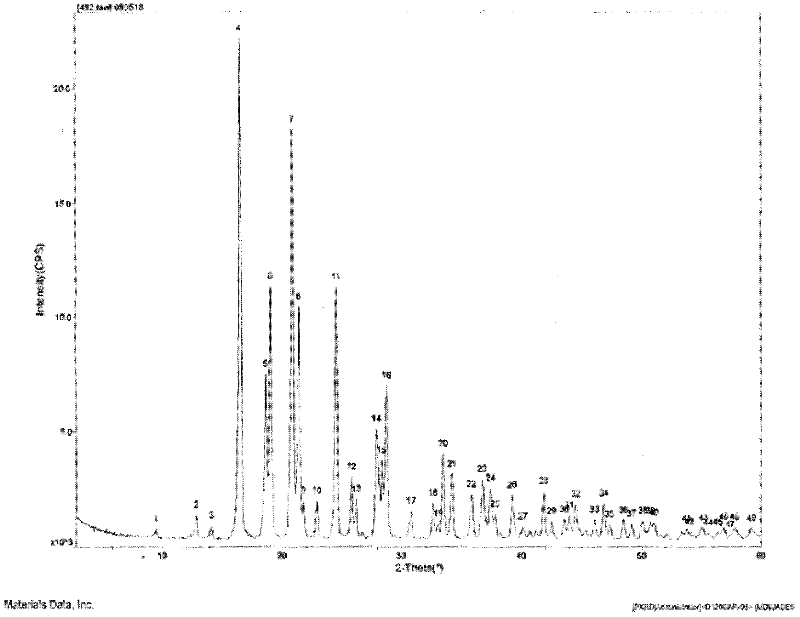
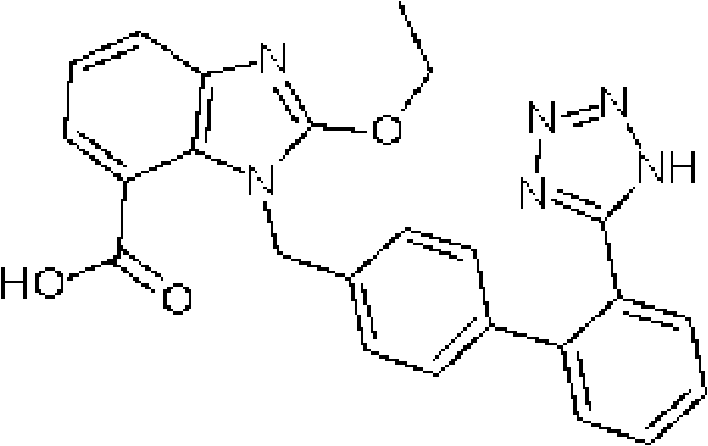
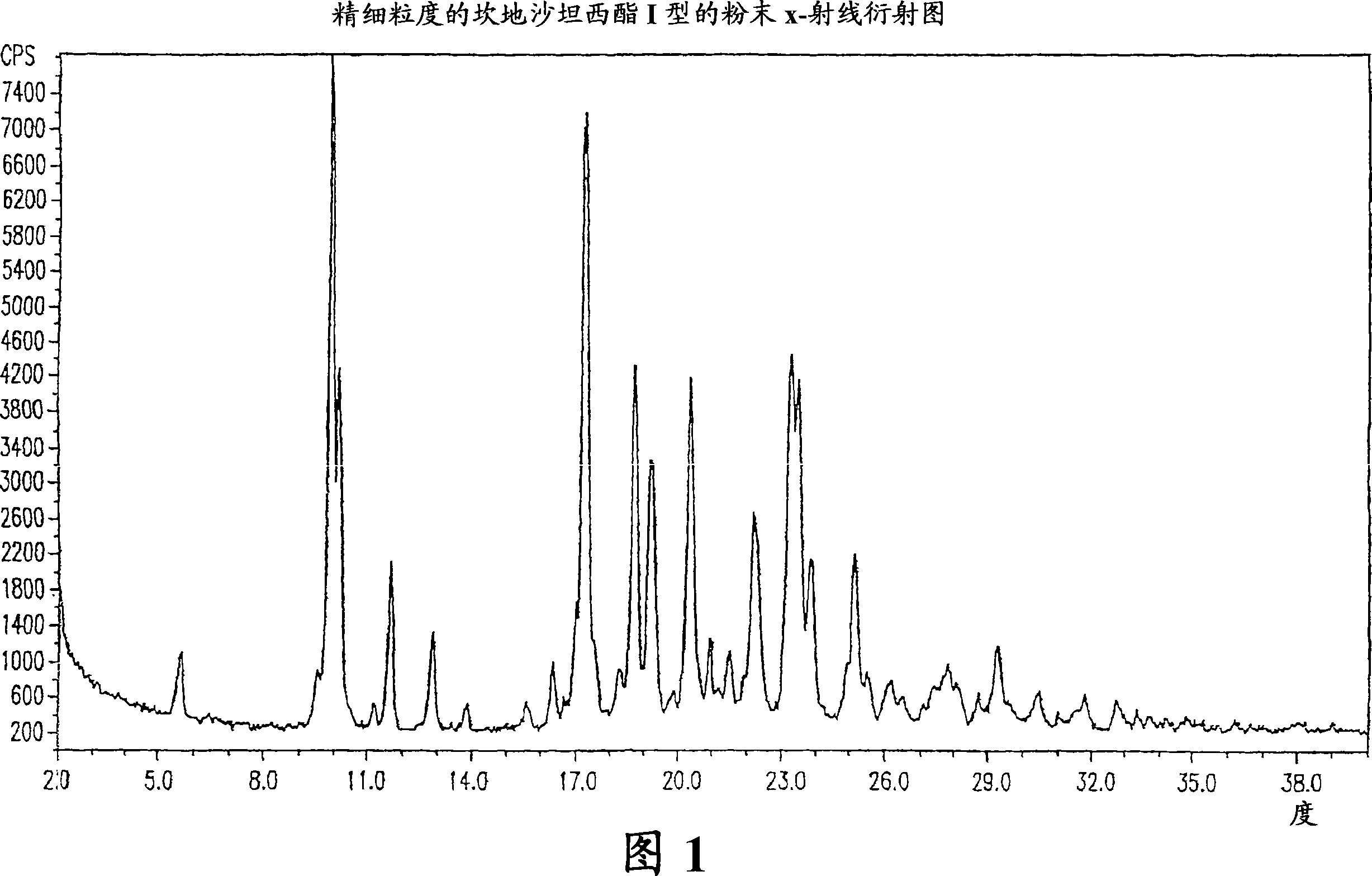
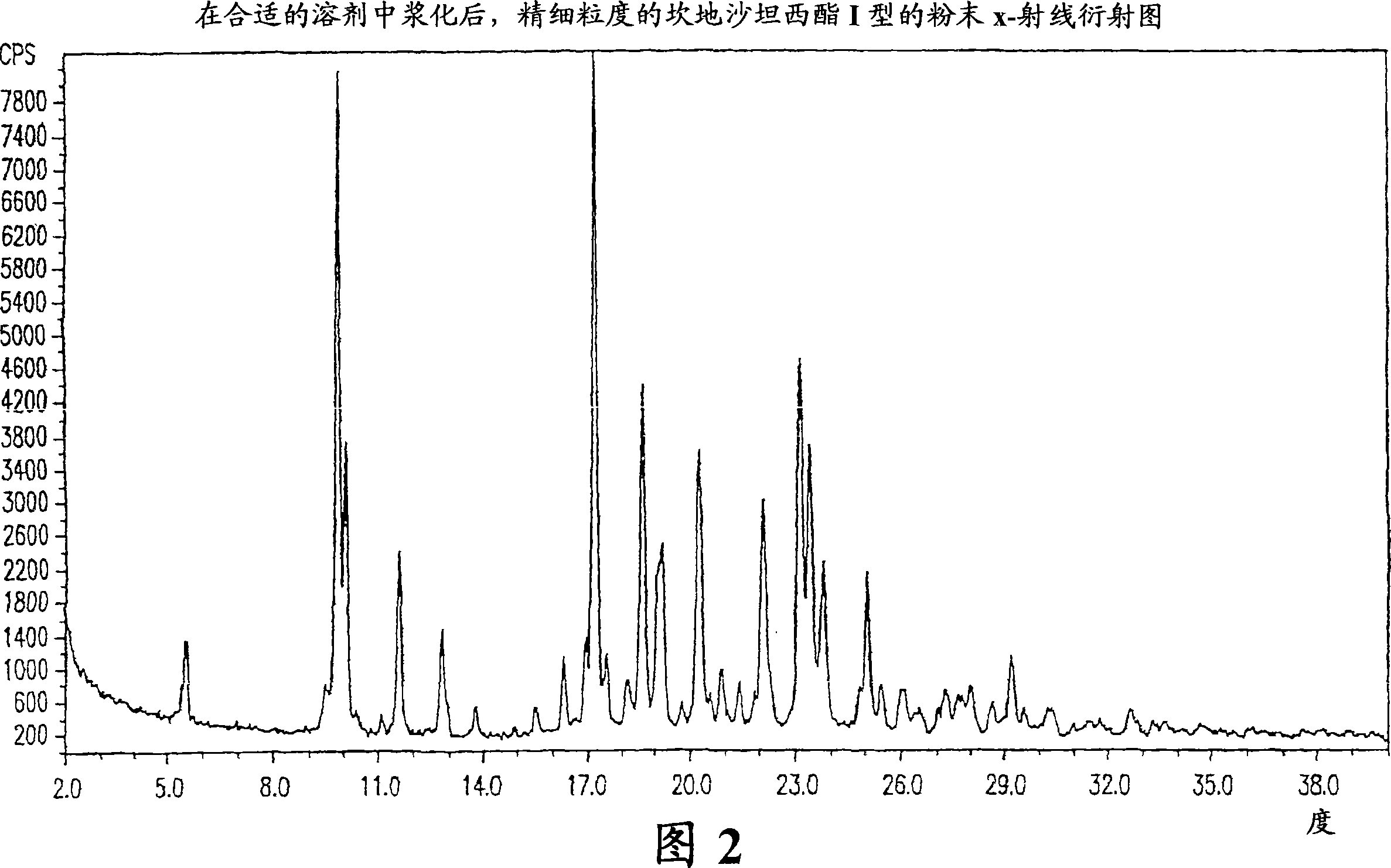
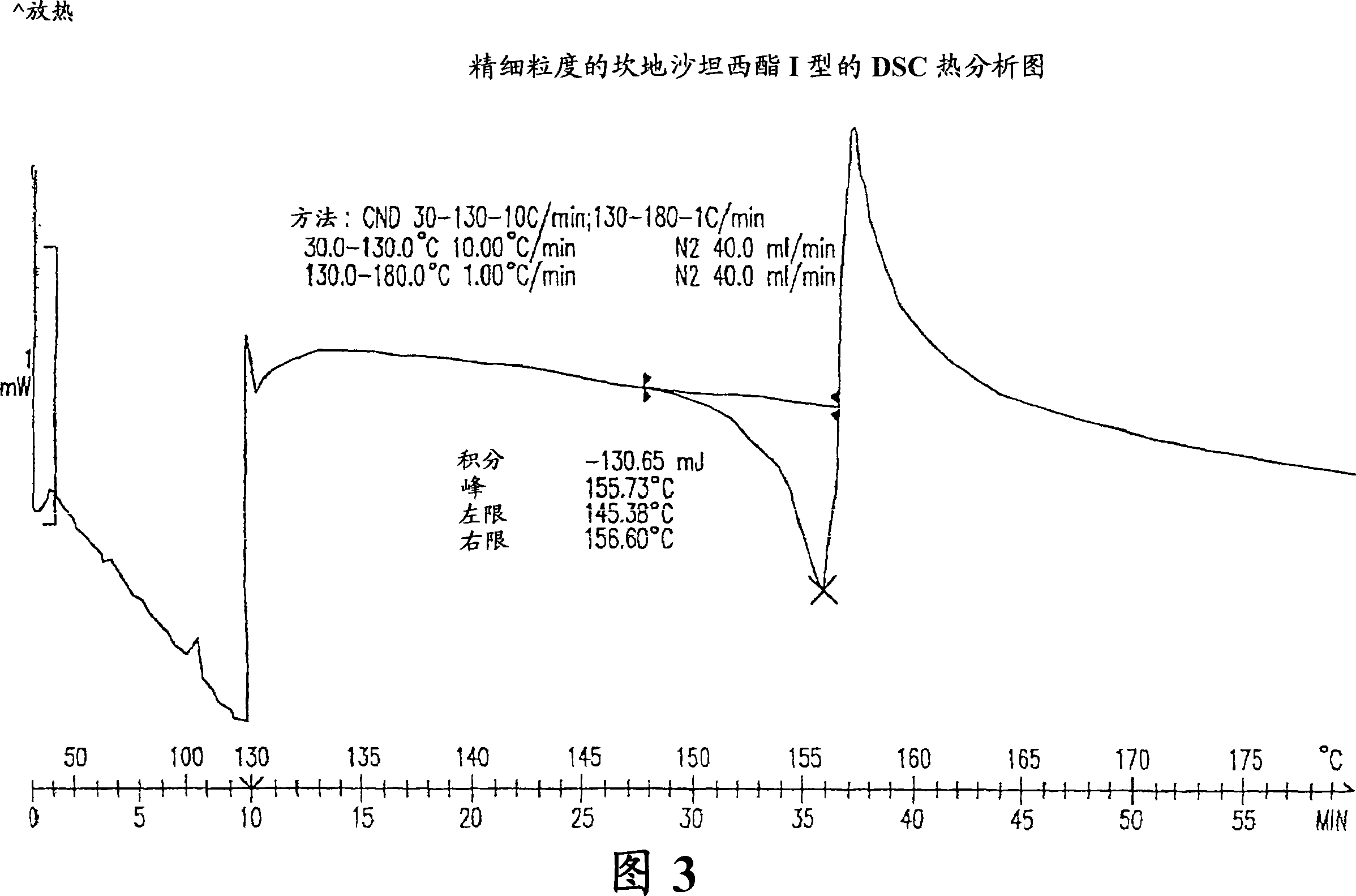
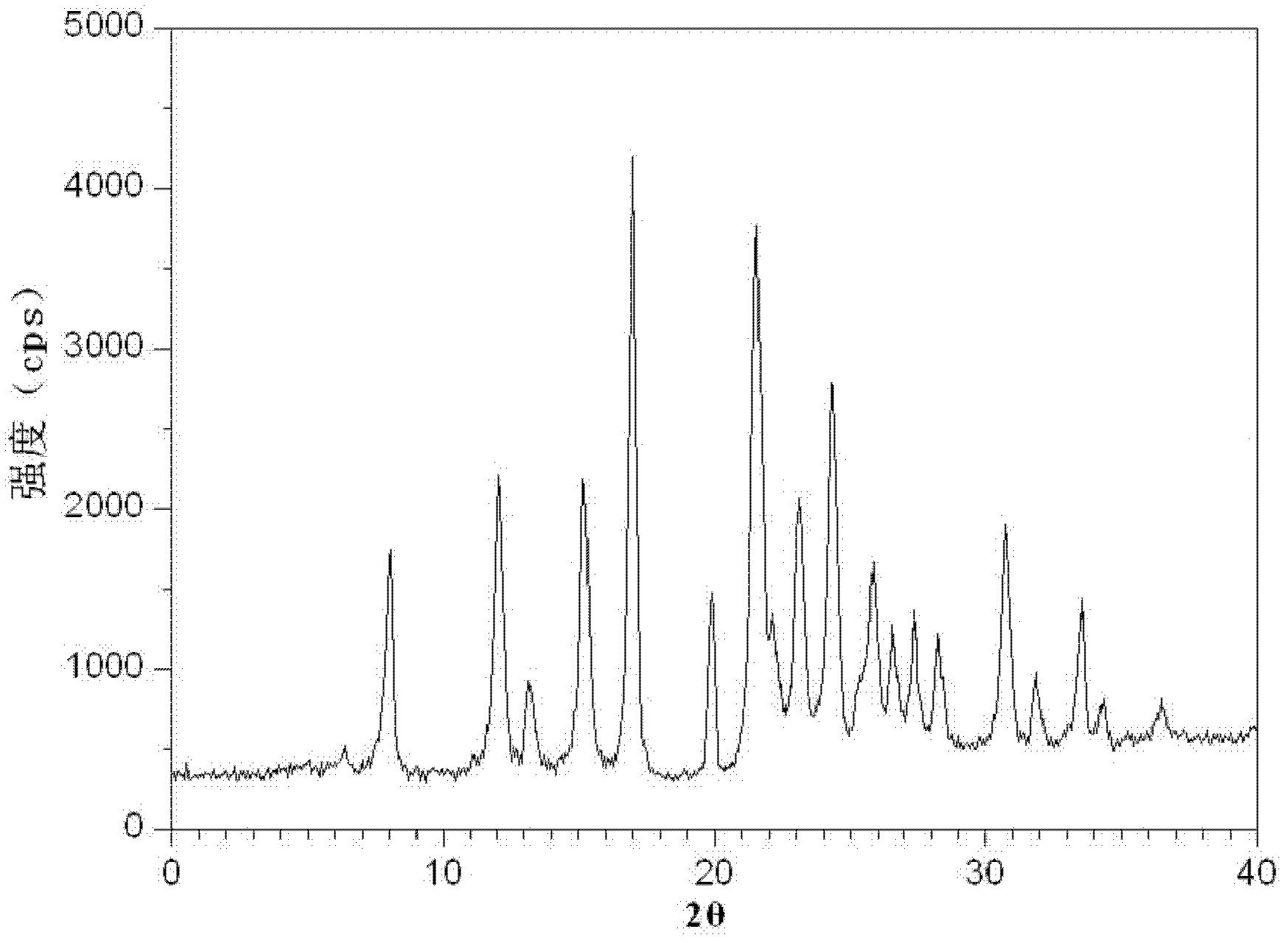
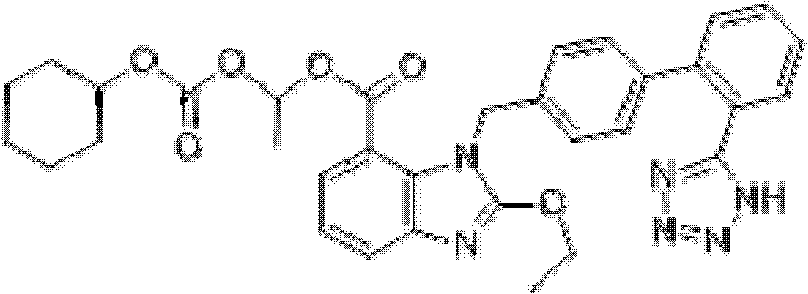
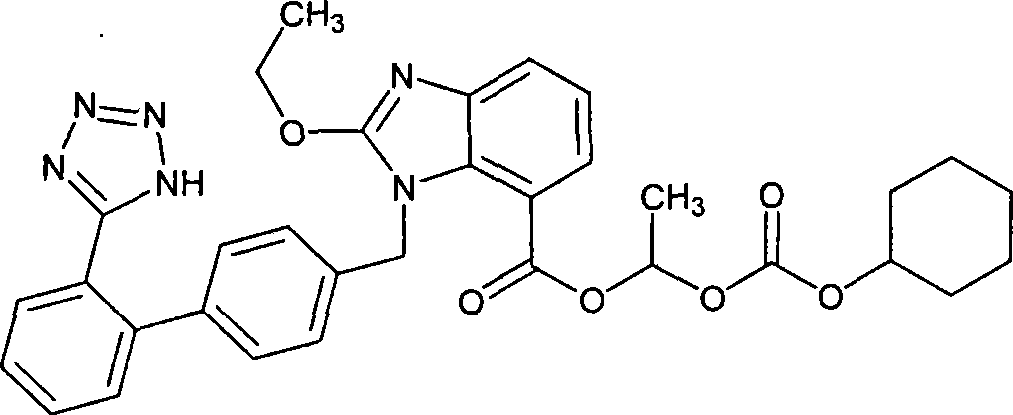
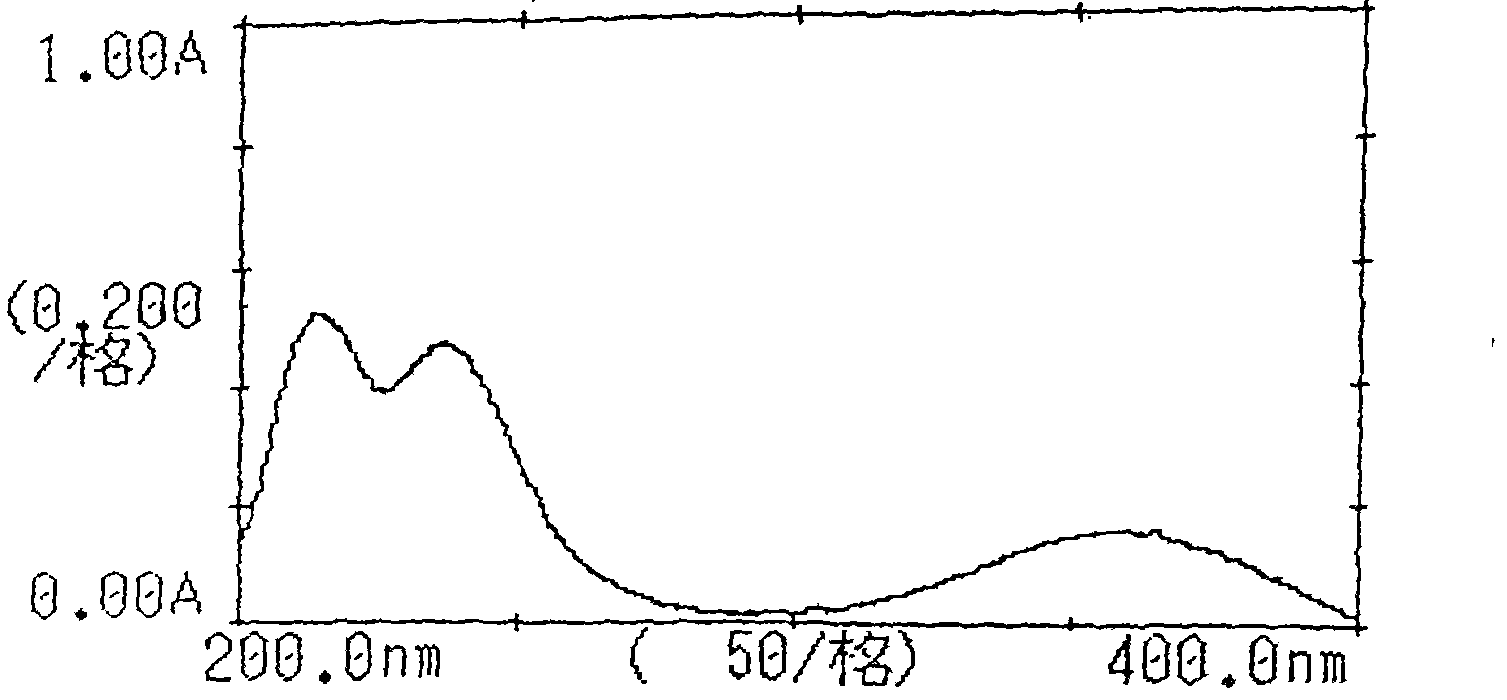
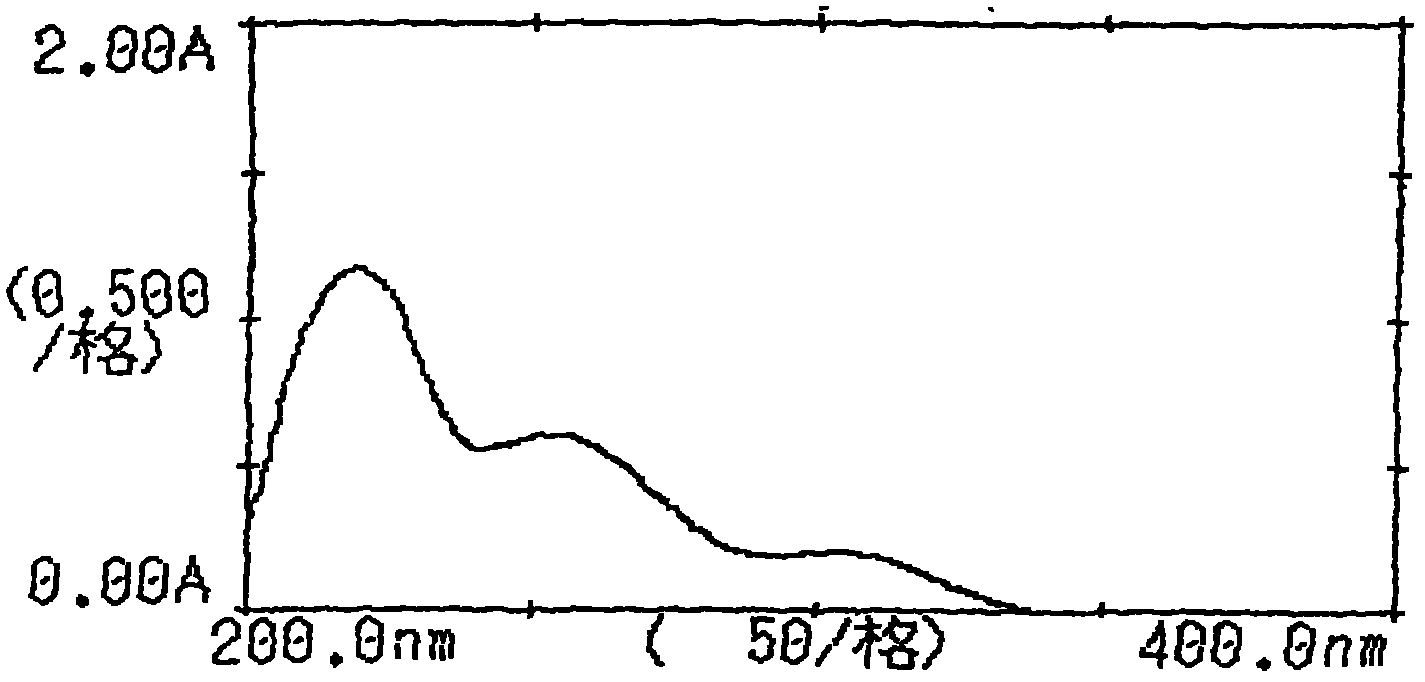
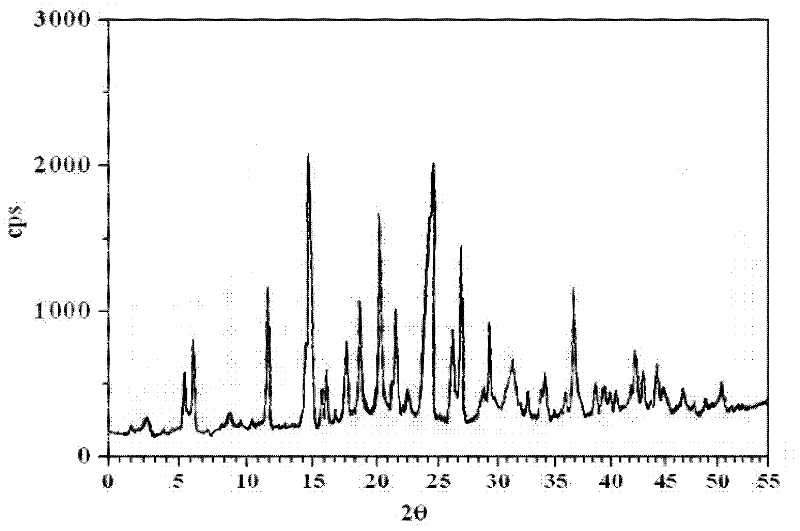
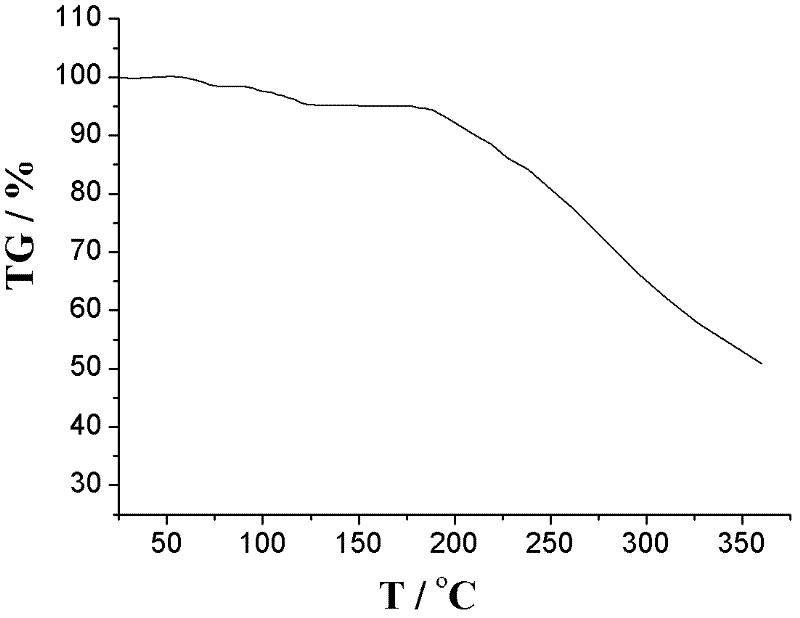
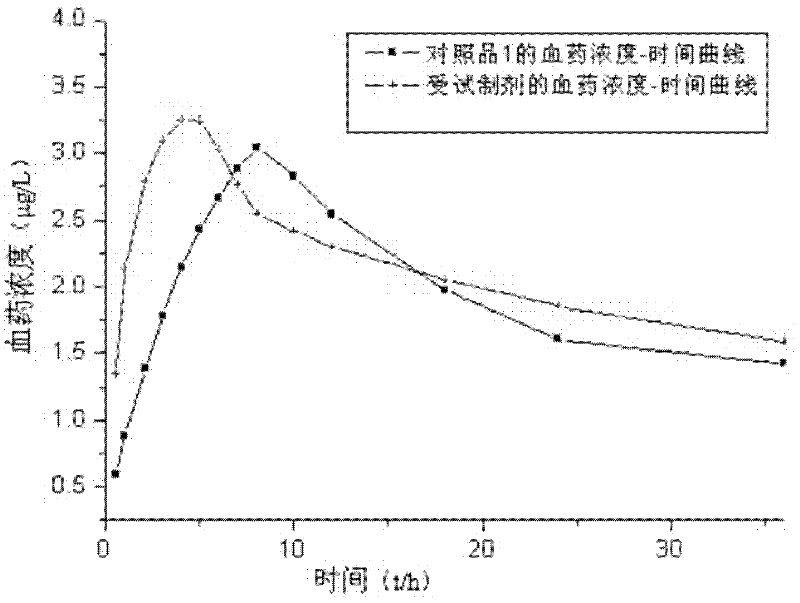
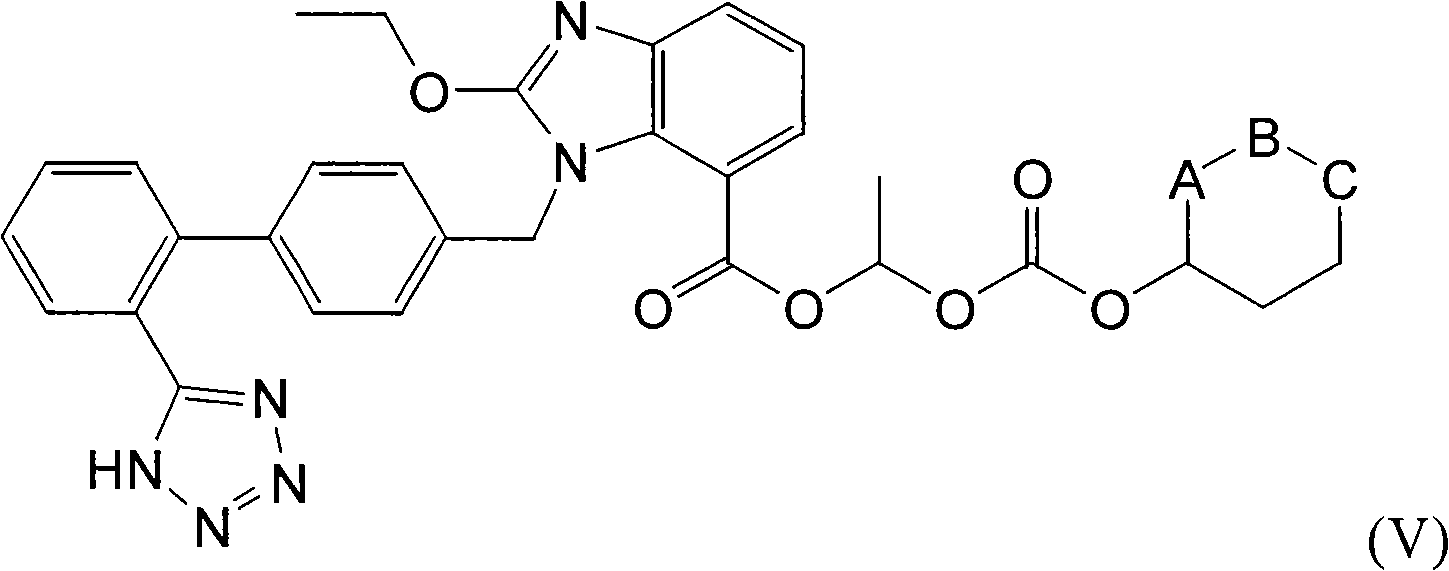
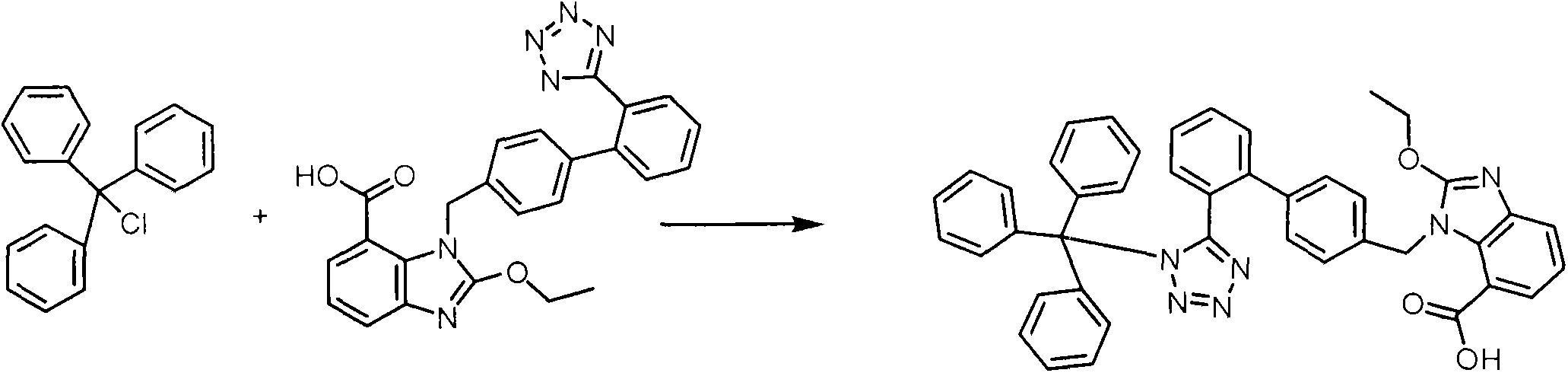
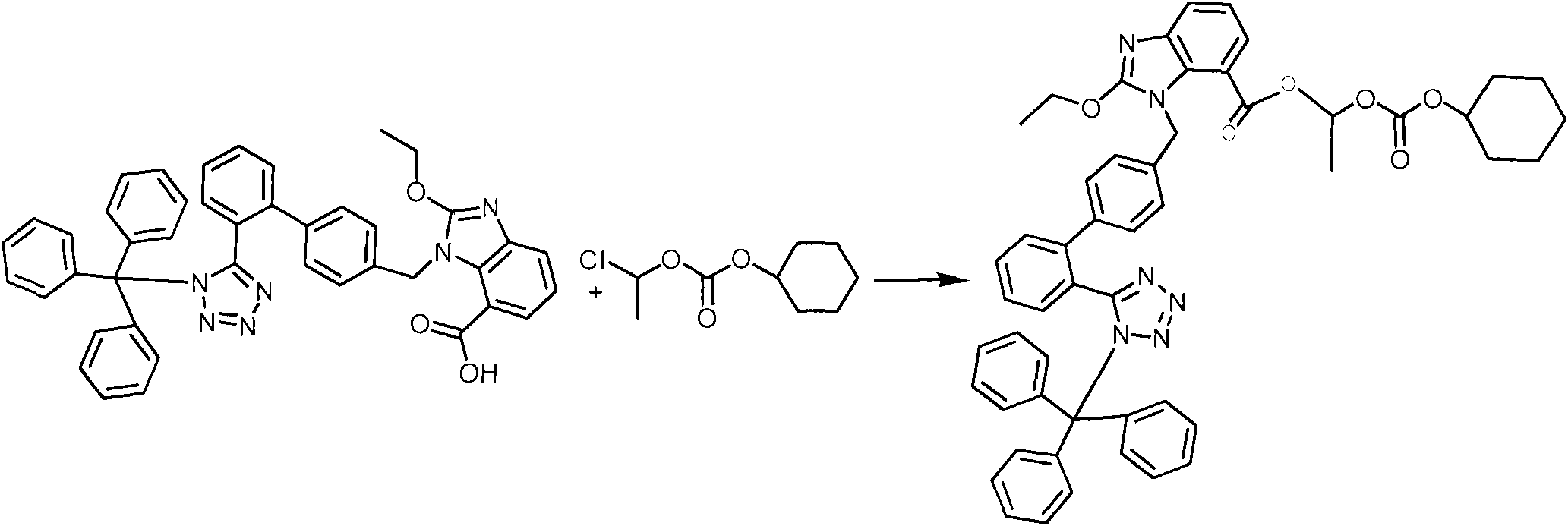
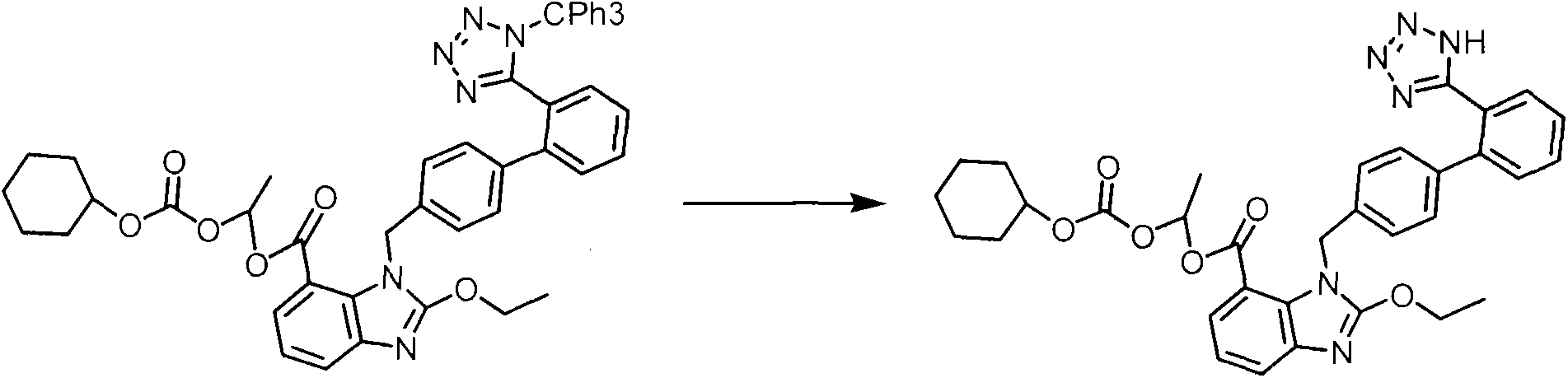
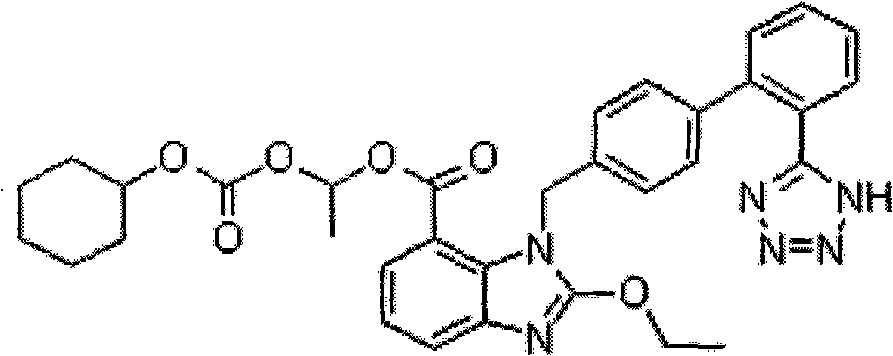
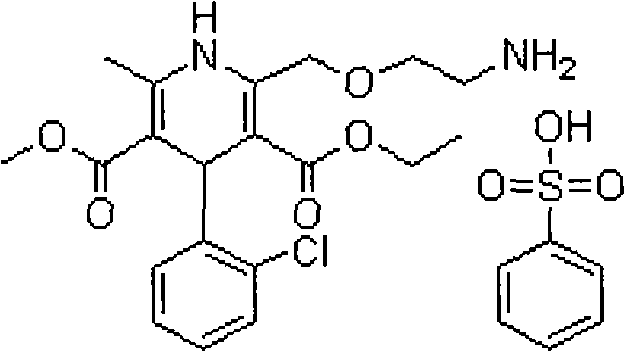
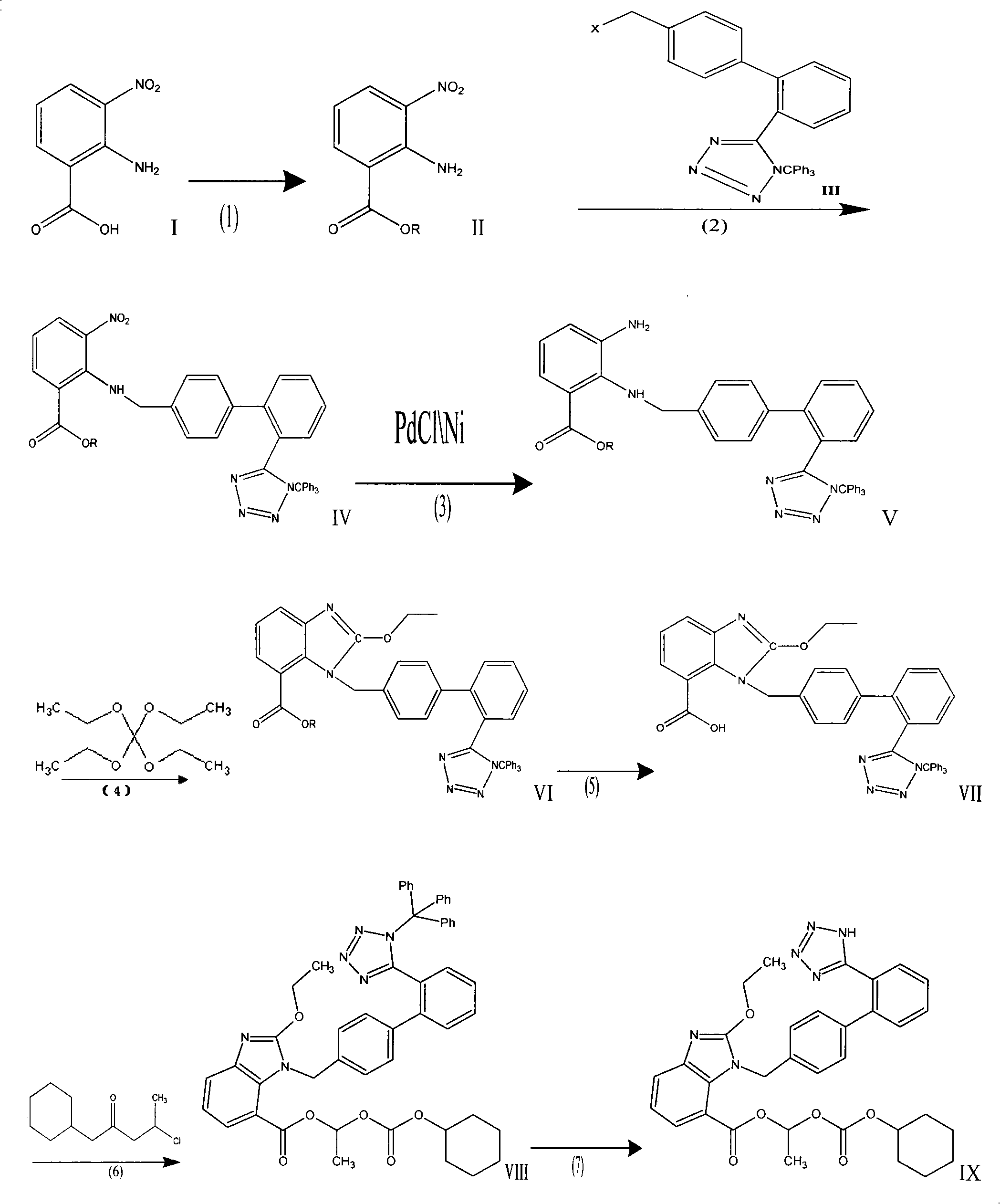
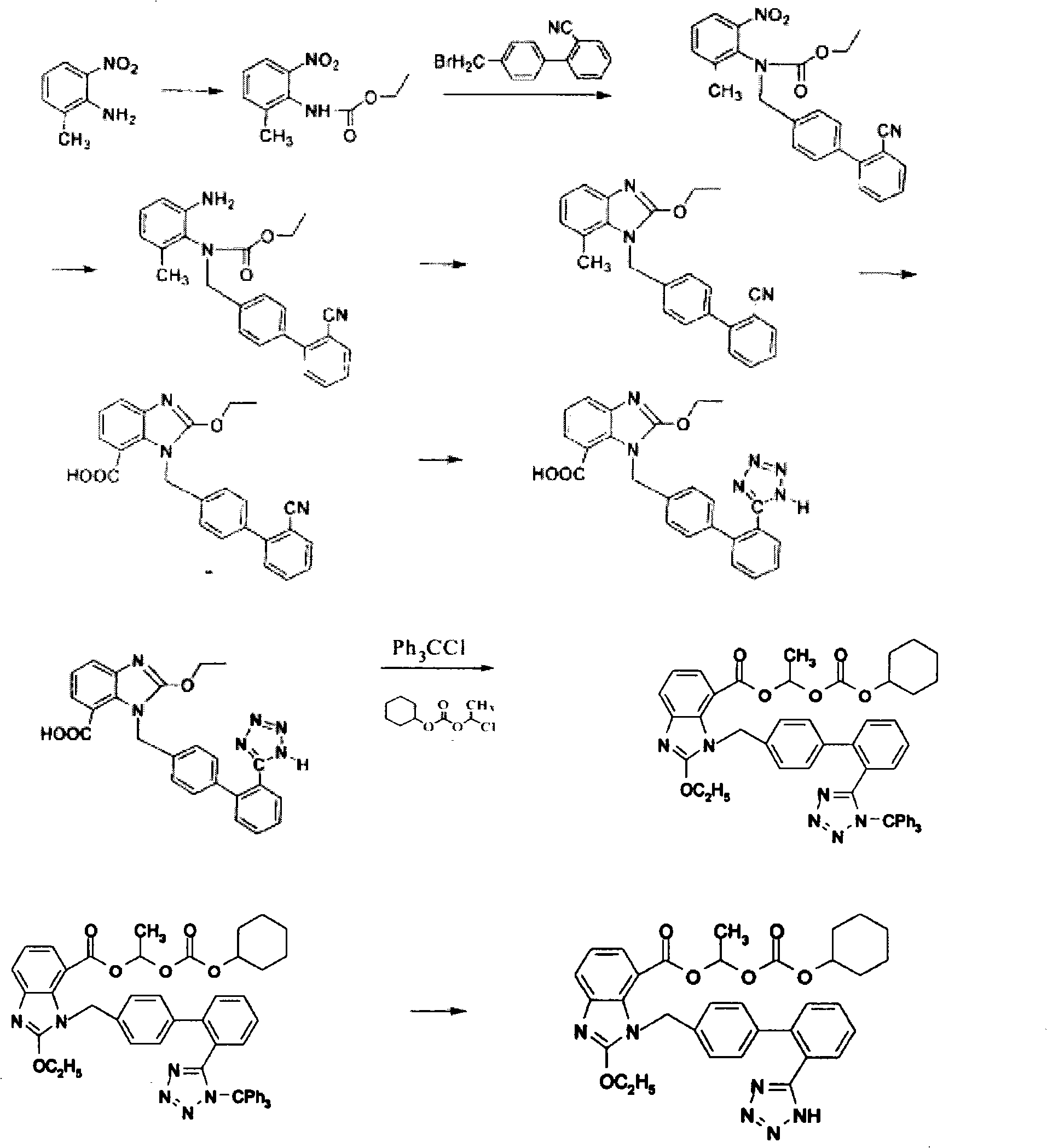
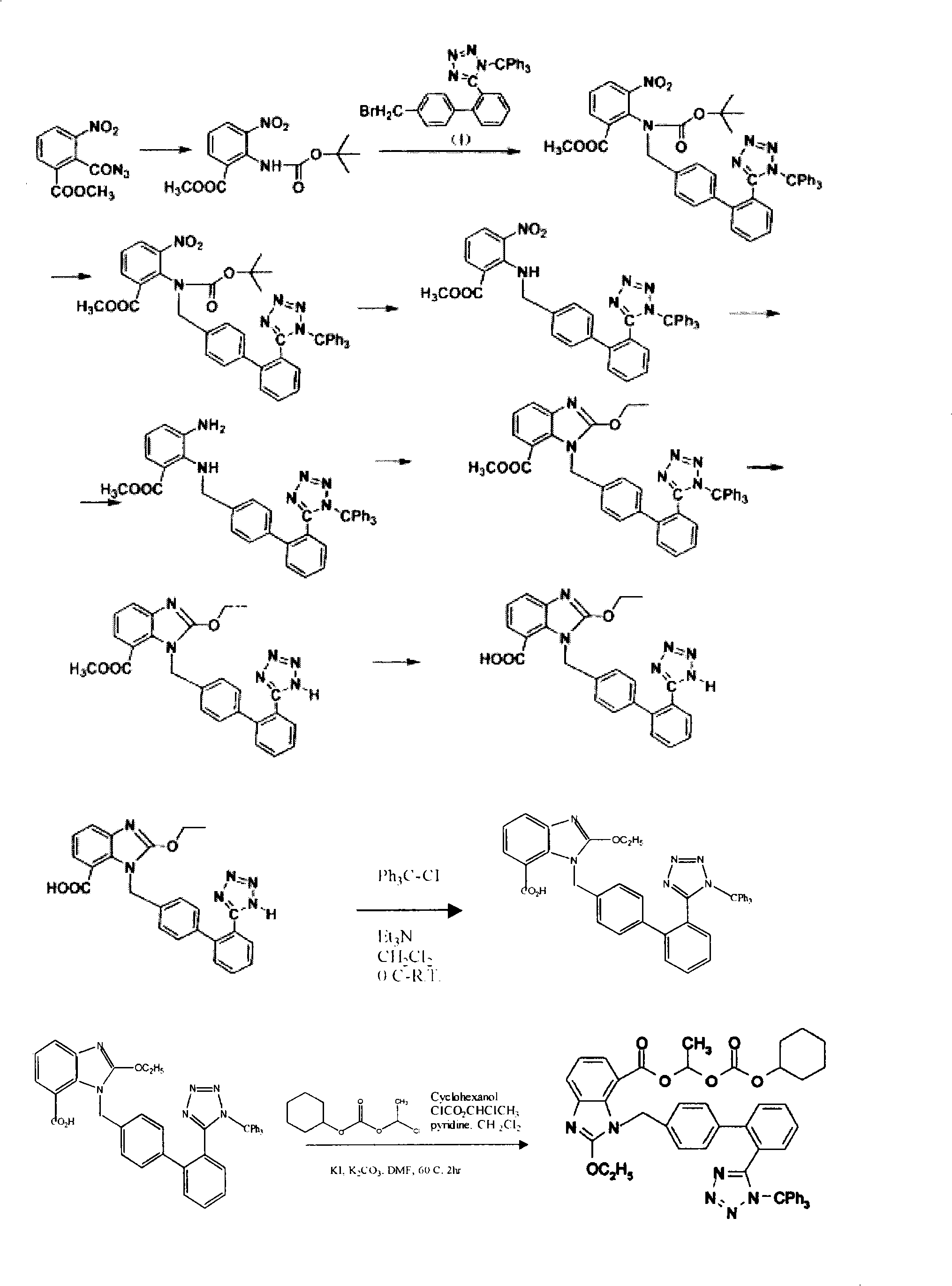
Crystalline 1-(cyclohexyloxycarbonyloxy) ethyl 1-((2'-cyanobiphenyl-4-yl)methyl)-2-ethoxy-1h-benzo[d]imidazole-7-carboxylate and a process for its preparation
InactiveCN101679300ACost-effective manufacturingStable crystal formOrganic active ingredientsOrganic chemistryCandesartanCarboxylic acid
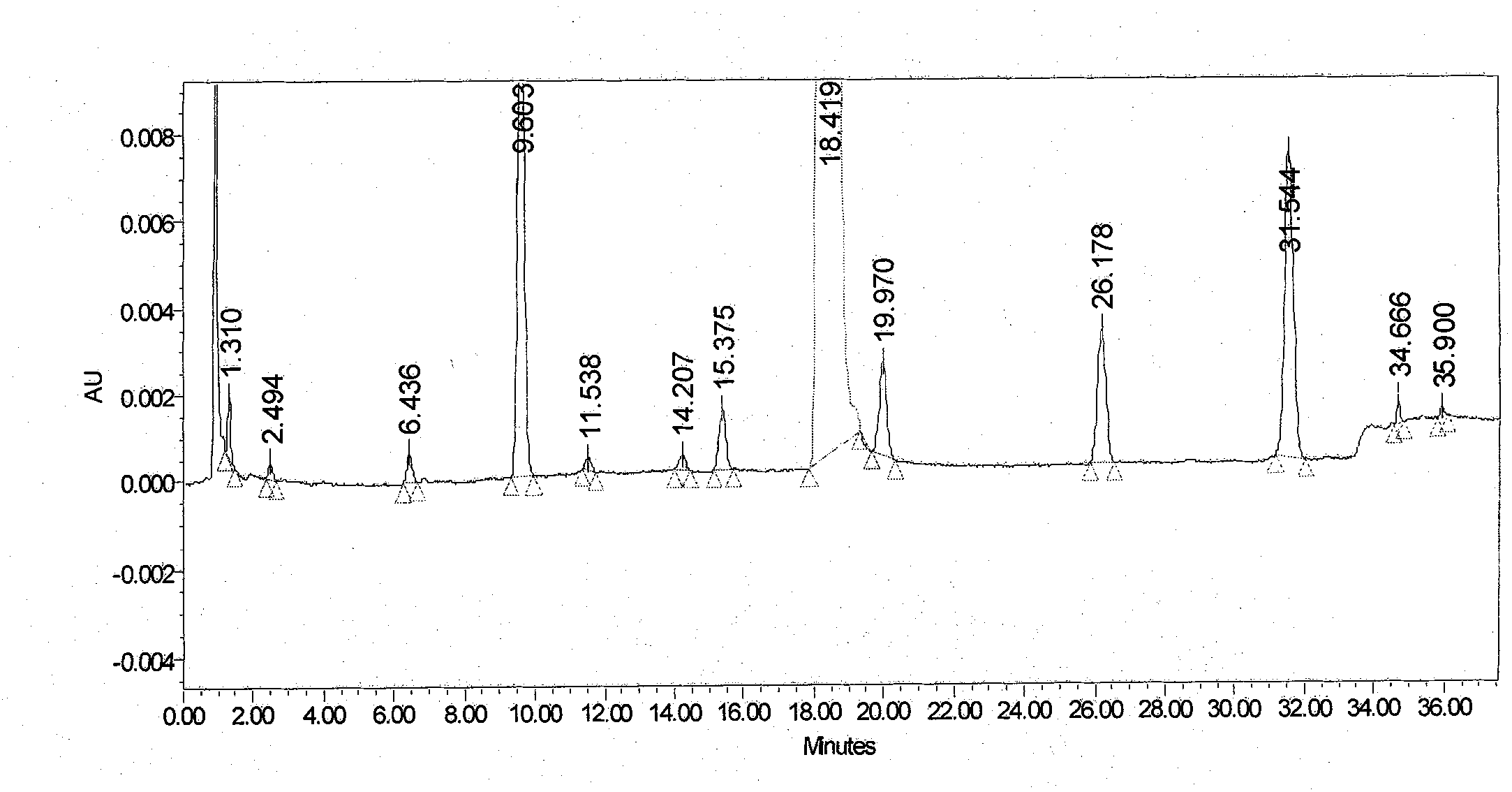
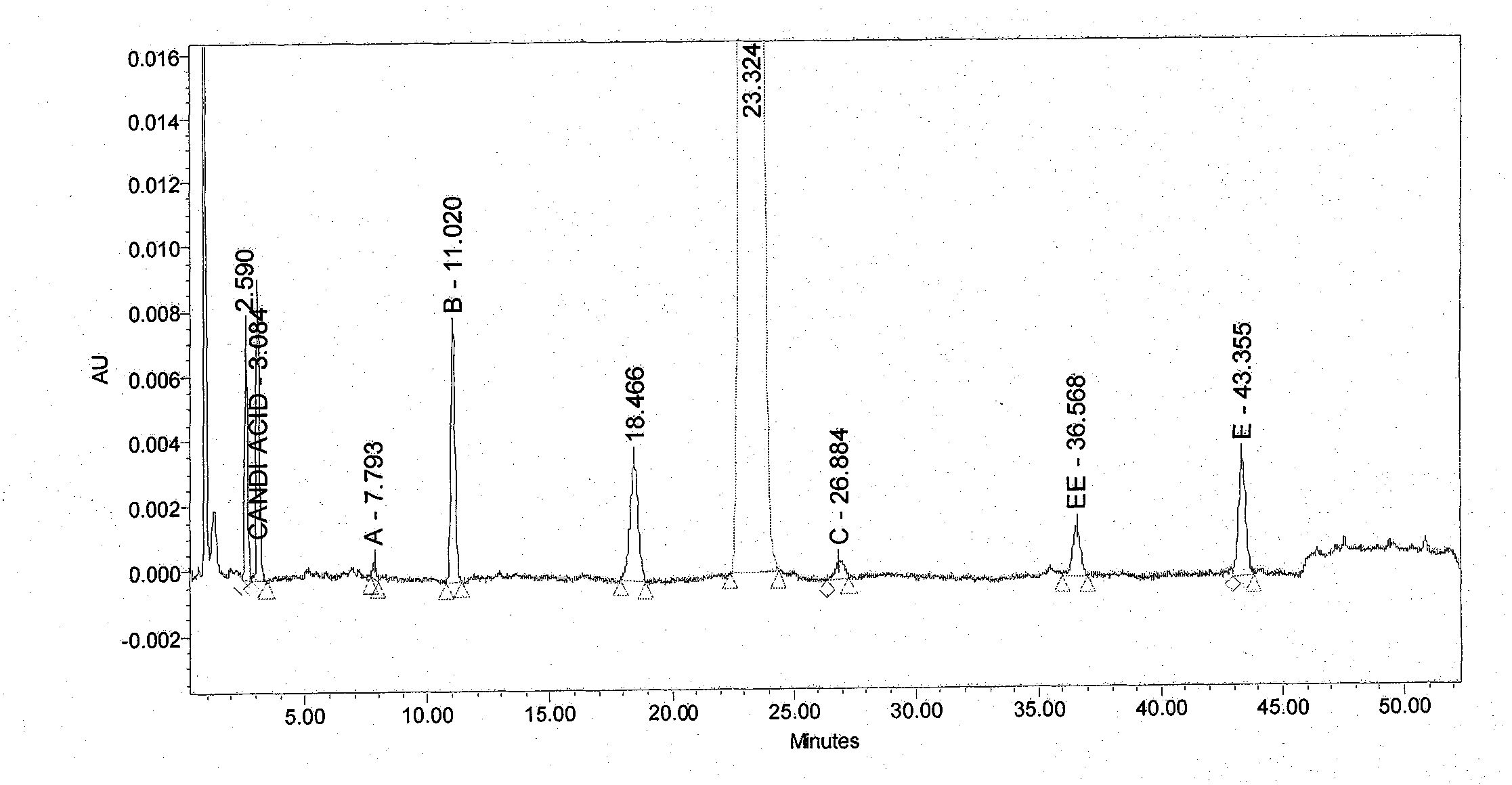
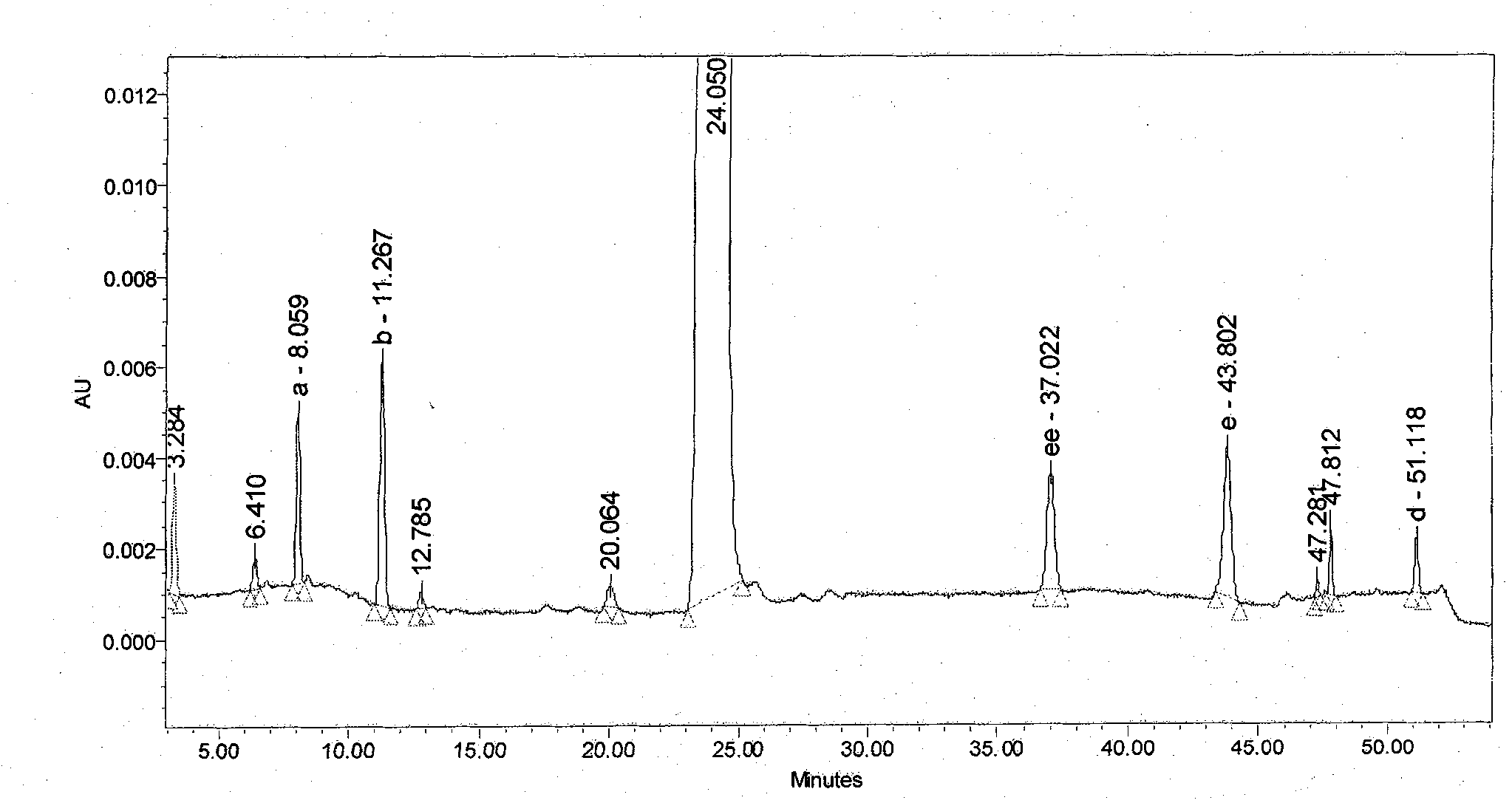
The present invention relates to 1-(cyclohexyloxycarbonyloxy)ethyl 1-((2'-cyanobiphenyl-4-yl)methyl)-2-ethoxy-1H-benzo[d]imidazole-7-carboxylate in crystalline form and a process for its preparation,which is useful intermediate in the preparation of candesartan cilexetil. The present invention also relates to the preparation of candesartan cilexetil and pharmaceutical composition comprising candesartan cilexetil.
Owner:KRKA D D NOVO MESTO
Preparation method of candesartan cilexetil
The invention provides a preparation method of candesartan cilexetil. The preparation method comprises steps I-IV. The step I concretely comprises the following substeps of adding candesartan and dichloromethane in a reaction container; slowly and dropwise adding triethylamine at the temperature of 10-15 DEG C; raising the temperature of a reaction system to 21-25 DEG C after dropwise adding of the triethylamine is fnished; adding triphenylchloromethane in batches; reacting for 3-4 hours; adding 0.1 mol / L HCl at one step after reaction is complete and adjusting pH (potential of hydrogen) to be 5-6; then slowly and dropwise adding 9 mol / L HCl and adjusting pH to be 2-3; leaving standstill; separating a water layer and an organic layer; adding saturated salt water in the organic layer to wash the organic layer; leaving standstill to achieve a layering effect; separating out the organic layer; performing decompress concentration on the organic layer to remove the dichloromethane; adding absolute ethyl alcohol in residual viscous substances; raising temperature to 45-50 DEG C; stirring for 3 hours; stopping heating after a large amount of white solids are separated out; reducing to room temperature; performing suction filtration; washing filter cakes with ethyl alcohol; and drying to obtain trityl candesartan.
Owner:山西皇城相府药业股份有限公司
Method for preparing candesartan ring compound
The invention relates to a method for preparing a candesartan ring compound, which comprises the following steps: 1) preparing 3-animo-2-ethyl ethoxy formacyl amino benzoate; 2) preparing 2-ethyoxylbenzimidazole-4-ethyl indole carboxylate; and 3) preparing candesartan ring compound, namely 2-ethyoxyl-1-1[[(2-cyanobiphenyl-4-yl) mthyl] benzimidazole]-7-carboxylate. In the invention, a benzimidazole ring is constructed, tetraethyl orthocarbonate is avoided, and intramolecular dehydration construction is realized; and finally alkylation is accomplished, canobromobiphenyl is introduced; and thus, the minimum consumption of canobromobiphenyl is realized and the cost of the candesartan ring compound is minimized.
Owner:ZHANGJIAGANG XINYI CHEM
Process for preparing candesartan cilexetil C-form crystal
Type-C candesartan cilexetil crystal is prepared through dissolving corresponding non-type C crystal material organic solvent capable of consoluting with water except C1-C3 lower alcohol and acetone, adding water to the solution while stirring to separate out crystal, and collecting the separated crystal as the type-C candesartan cilexetil. The said process is simple, low in organic solvent consumption, type-C candesartan cilexetil yield near the theoretical value, high crystal purity, high crystal quality and environment friendly.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD
Stable candesartan cilexetil amlodipine pharmaceutical composition and its preparation method
ActiveCN102743381AShorten production timeSave labor hoursOrganic active ingredientsPharmaceutical non-active ingredientsCandesartanPolyethylene glycol
The invention relates to a stable candesartan cilexetil and amlodipine pharmaceutical-containing composition and its preparation method. The invention is characterized in that a) each tablet contains 4mg-8mg of candesartan cilexetil, b) each tablet contains 2-10mg of amlodipine or its pharmaceutically acceptable salt, c) each tablet contains polyethylene glycol, wherein the weight of polyethylene glycol accounts for 2-10% of the tablet, and d) the tablet is prepared by a compressed tablet technology by using a dry method.
Owner:福安药业集团庆余堂制药有限公司
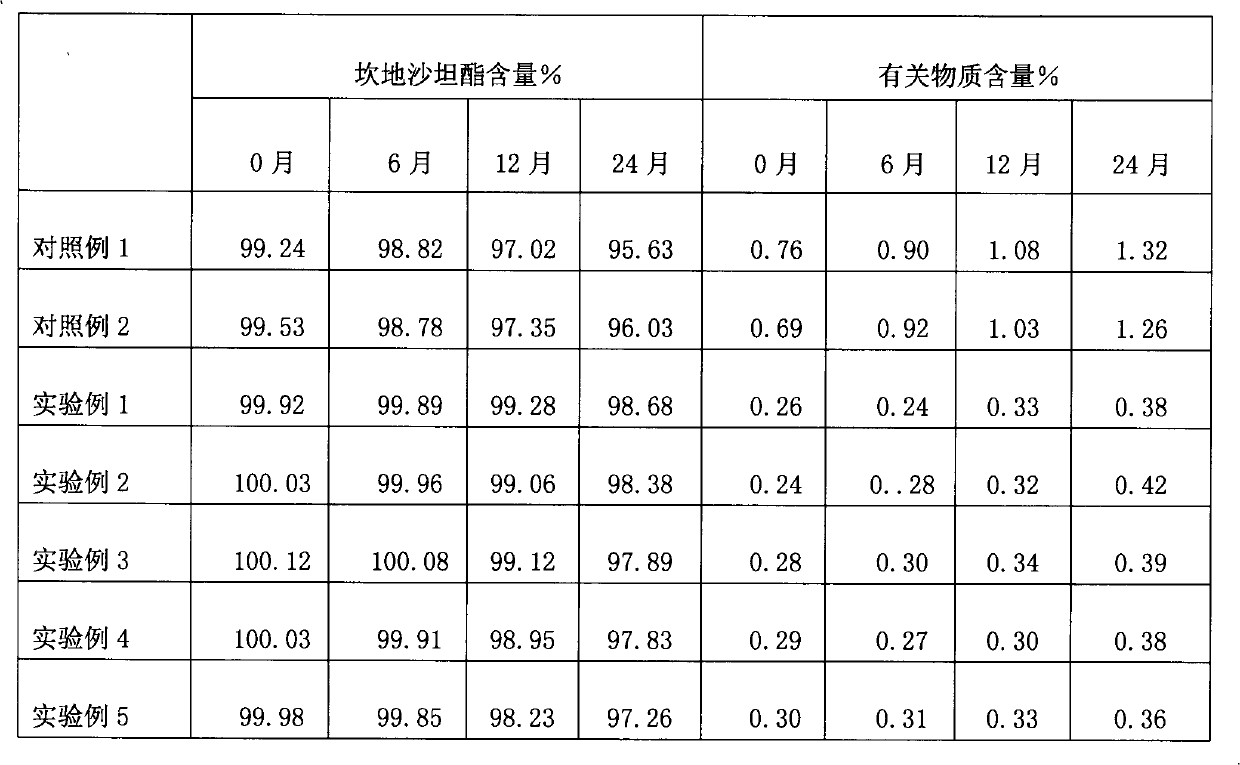
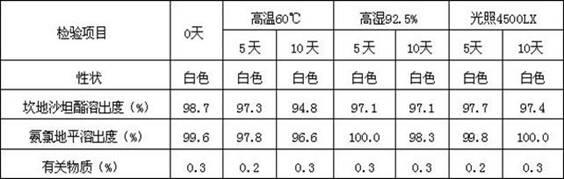
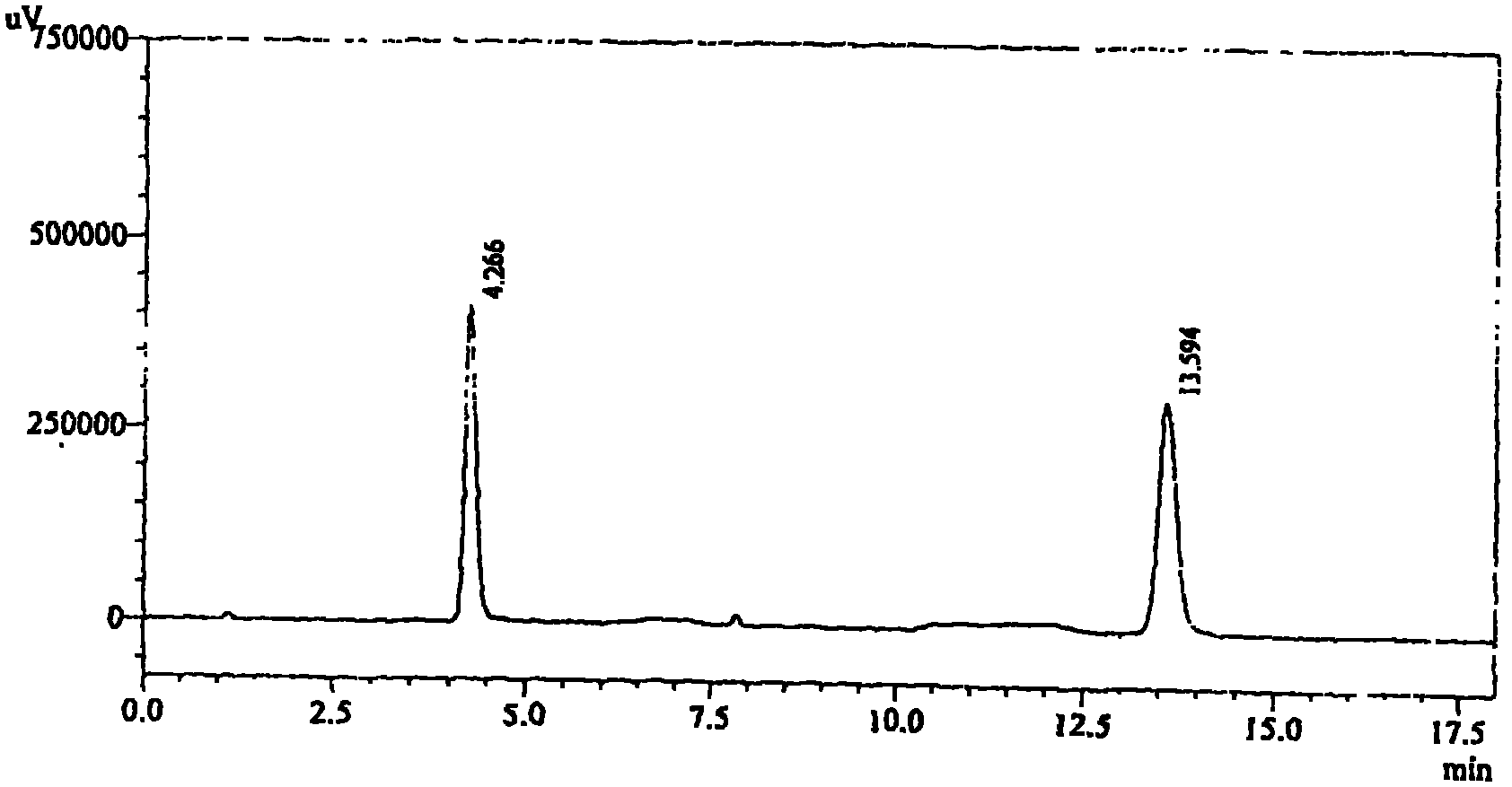
High performance liquid chromatography for analyzing candesartan cilexetil
The invention relates to high performance liquid chromatography for analyzing related substances of candesartan cilexetil. In the method, gradient elution is performed by adopting a reversed phase chromatographic column and taking a low-pH buffer salt of acetonitrile as a mobile phase. By the method, all known impurities of the candesartan cilexetil can be simultaneously analyzed, the problems that unknown impurities interfere with the candesartan cilexetil in the prior art and the degradation product of the candesartan cilexetil cannot be controlled by a conventional liquid phase analytic method are solved, and the quality of raw materials or preparation of the candesartan cilexetil can be more comprehensively and effectively controlled. The method has the advantages of simple operation,high repeatability and higher specificity.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Candesartan cilexetil/hydrochlorothiazide capsule and preparation method thereof
The invention provides a candesartan / hydrochlorothiazide capsule and a preparation method thereof. The invention has the obvious advantages as follows: on the one hand, the invention can obviously reduce the blood pressure of a hypertensive patient, effectively protect the heart, the brain and the kidney organ and reduce the incidence of complications and has safety, effectiveness, high tolerance,and the like; on the other hand, the invention also has the advantages of less tablet process link, short production period, less quality control points of production process, and the like besides keeping the advantages of accurate dosage, convenient application, stable quality, convenience for carrying, transport and storage, high degree of production mechanization and automatization, large yield, and the like of the prior tablet / dispersible tablet and can simplify the industrial production, thereby being beneficial to the reduction of drug production and management costs and the selection of doctors and patients for cheap and good drugs.
Owner:北京瑞伊人科技发展有限公司 +1
Process for manufacturing a pharmaceutical dosage form comprising nifedipine and candesartan cilexetil
InactiveUS20130309302A1Low variabilityReduce production lossBiocidePretreated surfacesNifedipineCandesartan
The present invention relates to manufacturing processes for the preparation of a pharmaceutical dosage form comprising nifedipine and candesartan cilexetil and optionally at least one diuretic characterized in that nifedipine is released in the body in a controlled (modified) manner and the candesartan cilexetil is released rapidly (immediate release (IR)) and optionally the diuretic is released rapidly (immediate release (IR)) and the pharmaceutical dosage forms obtainable by these processes.
Owner:BAYER PHARMA AG
Novel oral solid medicinal composition and preparation method thereof
ActiveCN102342942AReduce dosageSolving Quality Control IssuesOrganic active ingredientsOrganic chemistryCandesartanLevamlodipine
The invention discloses a novel oral solid medicinal composition. The novel oral solid medicinal composition is an oral preparation prepared from hydrochlorothiazide, levamlodipine besylate, candesartan cilexetil and pharmaceutically acceptable auxiliary materials. The novel oral solid medicinal composition can be processed into tablets, capsules and the like. Specifically, the novel oral solid medicinal composition comprises: be weight, 5 to 25 parts of hydrochlorothiazide, 2.5 to 5 parts of levamlodipine besylate, 4 to 20 parts of candesartan cilexetil, 30 to 60 parts of microcrystalline cellulose, 30 to 60 parts of compressible starch, 30 to 50 parts of crosslinked polyvinylpyrrolidone, 1 to 2 parts of silica and 0.5 to 2 parts of magnesium stearate. The novel oral solid medicinal composition has a scientific and reasonable formula, low auxiliary material content and high bioavailability. Therefore, the novel oral solid medicinal composition is a drug of first choice for the treatment of hypertension.
Owner:HAINAN JINRUI PHARMA
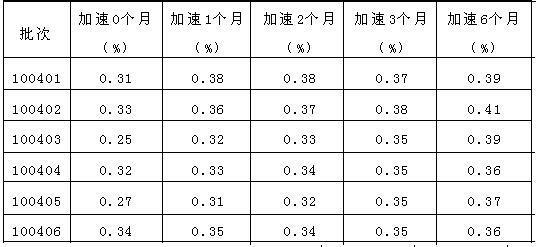
Stable micronized candesartan cilexetil and methods for preparing thereof
The invention encompasses sable candesartan cilexetil of fine particle size, wherein desethyl-candesartan (desethyl-CNS) within the stable candesartan cilexetil does not increase to more than about 0.1%w / w by HPLC relative to the initial amount of candesartan cilexetil, when the stable candesartan cilexetil is maintained at a temperature of about 550 DEG C for at least 2 weeks, methods of making the same and pharmaceutical compositions thereof.
Owner:TEVA PHARMA IND LTD
Brand new drug composition containing levamlodipine besylate and candesartan cilexetil and preparation method thereof
ActiveCN102349902AImprove solubilityRapid dissolutionOrganic active ingredientsPill deliverySolubilityCandesartan
The invention relates to a brand new drug composition containing levamlodipine besylate and candesartan cilexetil and a preparation method thereof. The composition comprises the active ingredients such as levamlodipine besylate and candesartan cilexetil and pharmaceutically acceptable auxiliaries, wherein levamlodipine besylate is levamlodipine besylate crystals; and the characteristic peak in the X-ray powder diffraction pattern obtained after measuring the crystals with a Cu-Kalpha ray is displayed at 2theta, namely 8.0 degrees, 12.1 degrees, 15.4 degrees, 17.0 degrees, 19.8 degrees, 21.6 degrees, 23.0 degrees, 24.3 degrees, 25.7 degrees, 27.4 degrees, 30.7 degrees and 33.5 degrees. The drug composition and the preparation method have the following advantages: the crystals can improve the solubility of levamlodipine besylate to some extent, thus amlodipine besylate in the drug composition is dissolved out more quickly and the drug composition has a better curative effect and good stability; and the method adopts a direct powder compression technology, has the advantages of simple technology, short production period and low production cost and is easy to realize industrial production.
Owner:HAINAN JINRUI PHARMA
Stable candesartan cilexetil tablet combination
ActiveCN103127010AAvoid crystal transformationAvoid elevationOrganic active ingredientsPharmaceutical non-active ingredientsCandesartanMagnesium stearate
The invention relates to a candesartan cilexetil tablet combination and a preparation method. The candesartan cilexetil tablet combination is characterized in that a combination of a unit dose comprises a candesartan cilexetil C type crystal of 8 milligrams, hydroxypropylcellulose of 12 milligrams, lactose of 55 milligrams, starch of 15 milligrams, microcrystalline cellulose of 30 milligrams, sodium carboxymethylcellulose of 0.7 milligram and magnesium stearate of 1.2 milligrams. The hardness of a tablet is in a range of 2.20-2.60 kilograms. The preparation method is characterized in that the candesartan cilexetil C type crystal does not participate in granulating, and the tablet manufacturing pressure is 10-35 kilonewtons.
Owner:DISHA PHARMA GRP
Candesartan cilexetil composition
ActiveCN104758252AOrganic active ingredientsPharmaceutical non-active ingredientsCandesartanAdhesive
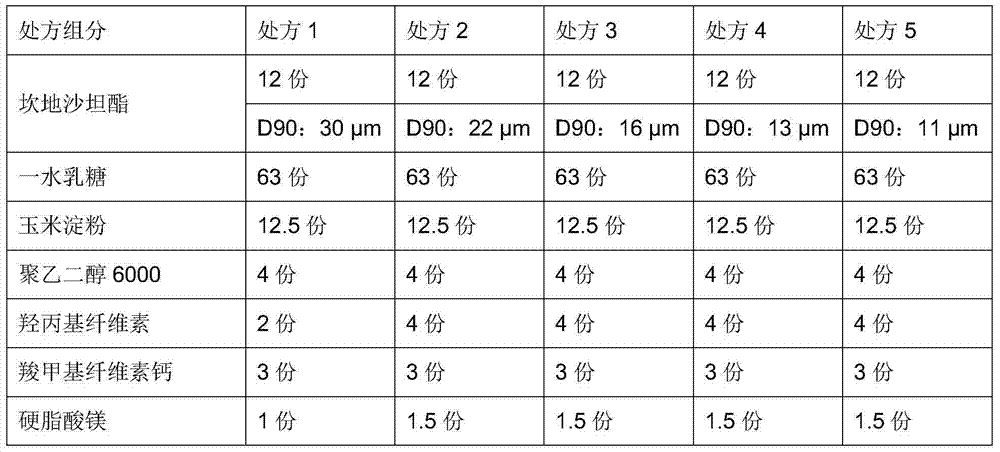
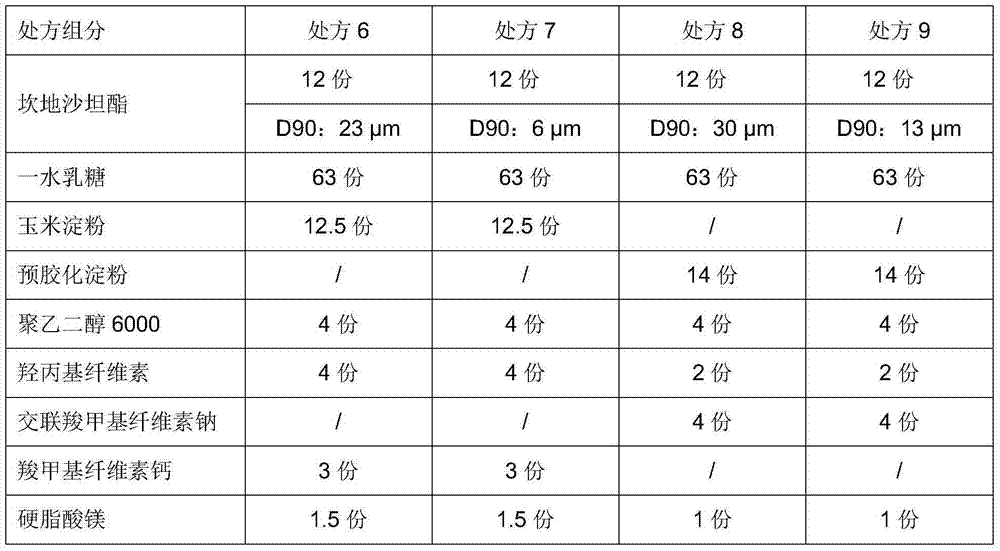
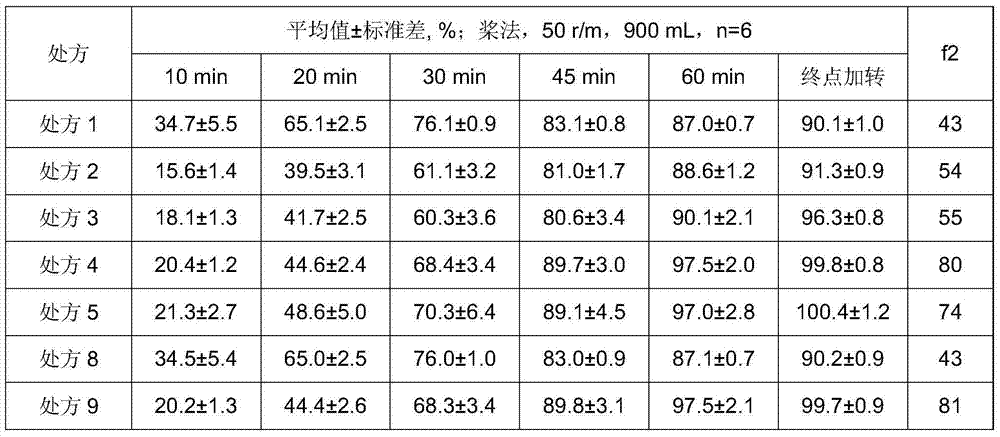
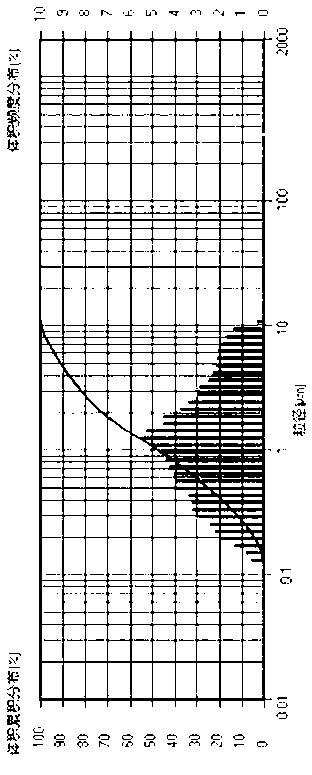
The invention provides a candesartan cilexetil granule with a specific particle size range, and a preparation method and a composition thereof, belonging to the technical field of pharmacy. When the cumulative particle size distribution number of the candesartan cilexetil granule reaches 90%, a corresponding particle size is less than 16 [mu]m and higher than 11[mu]m; and the candesartan cilexetil composition including candesartan cilexetil in such a particle size range comprises 1 to 20 parts of the candesartan cilexetil granule, 65 to 80 parts of a filler, 1 to 20 parts of an adhesive, 1 to 10 parts of a disintegrating agent and 1 to 5 parts of a lubricant. The composition has good dissolution performance.
Owner:SUNSHINE LAKE PHARM CO LTD
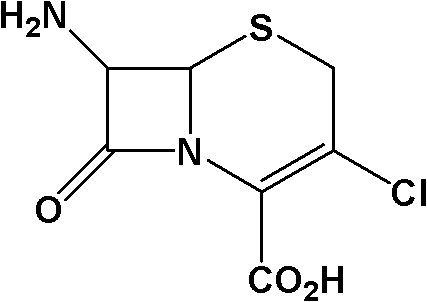
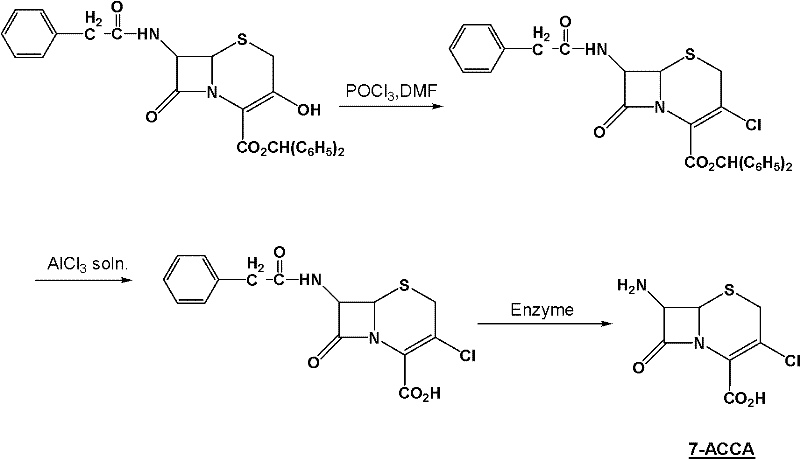
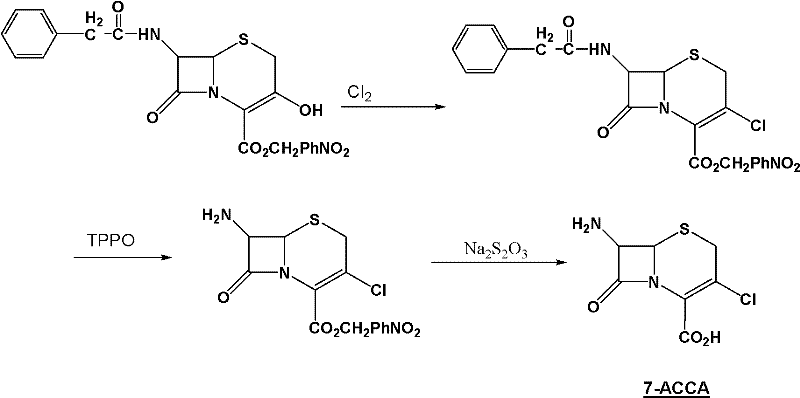
Method for preparing 7-ACCA
The invention relates to an important intermediate 7-ACCA of cephalosporin cefaclor, comprising the following steps of: 1) preparation of 3-amino-2-ethoxyformyl anesthesin; 2) preparation of 2-ethoxybenzimidazole-4-carboxylic acid ethyl ester; 3) preparation of candesartan cyclic compound. According to the invention, the benzimidazole ring is constructed, tetraehtyl orthocarbonate is avoided, andintramolecular dehydration is accomplished; and alkylation is accomplished in the final stage and cyanobromobiphenyl is introduced to minimize the consumption of cyanobromobiphenyl as well as the cost of the candesartan cyclic compound.
Owner:ZHANGJIAGANG XINYI CHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Crystalline 1-(cyclohexyloxycarbonyloxy) ethyl 1-((2'-cyanobiphenyl-4-yl)methyl)-2-ethoxy-1h-benzo[d]imidazole-7-carboxylate and a process for its preparation Crystalline 1-(cyclohexyloxycarbonyloxy) ethyl 1-((2'-cyanobiphenyl-4-yl)methyl)-2-ethoxy-1h-benzo[d]imidazole-7-carboxylate and a process for its preparation](https://images-eureka.patsnap.com/patent_img/002ad7ec-c106-4989-9608-5d408eb3f096/A2008800129400033H1.PNG)
![Crystalline 1-(cyclohexyloxycarbonyloxy) ethyl 1-((2'-cyanobiphenyl-4-yl)methyl)-2-ethoxy-1h-benzo[d]imidazole-7-carboxylate and a process for its preparation Crystalline 1-(cyclohexyloxycarbonyloxy) ethyl 1-((2'-cyanobiphenyl-4-yl)methyl)-2-ethoxy-1h-benzo[d]imidazole-7-carboxylate and a process for its preparation](https://images-eureka.patsnap.com/patent_img/002ad7ec-c106-4989-9608-5d408eb3f096/A20088001294000061.PNG)
![Crystalline 1-(cyclohexyloxycarbonyloxy) ethyl 1-((2'-cyanobiphenyl-4-yl)methyl)-2-ethoxy-1h-benzo[d]imidazole-7-carboxylate and a process for its preparation Crystalline 1-(cyclohexyloxycarbonyloxy) ethyl 1-((2'-cyanobiphenyl-4-yl)methyl)-2-ethoxy-1h-benzo[d]imidazole-7-carboxylate and a process for its preparation](https://images-eureka.patsnap.com/patent_img/002ad7ec-c106-4989-9608-5d408eb3f096/A20088001294000071.PNG)