Preparation method of candesartan cilexetil
A technology of candesartan cilexetil and sartan medoxomil, applied in the field of chemistry, can solve problems such as excessive dosage and types, high cost and environmental protection, increase solvent residue, etc., and achieves less dosage, low production cost, and product yield. and excellent product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
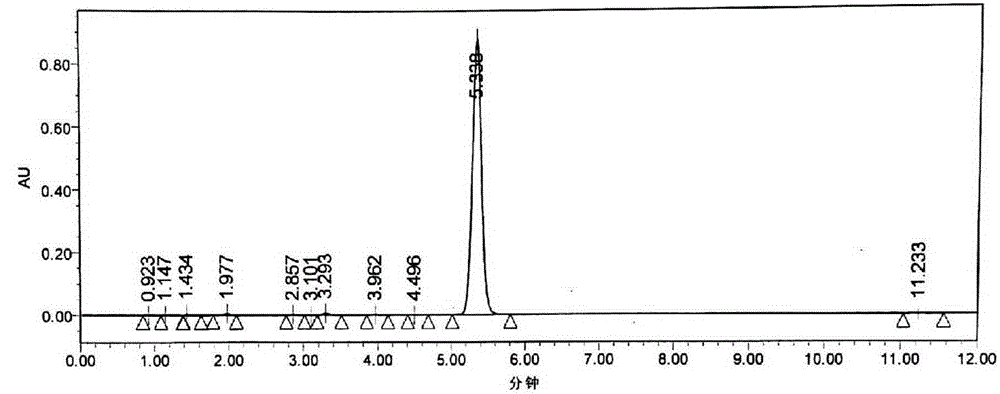
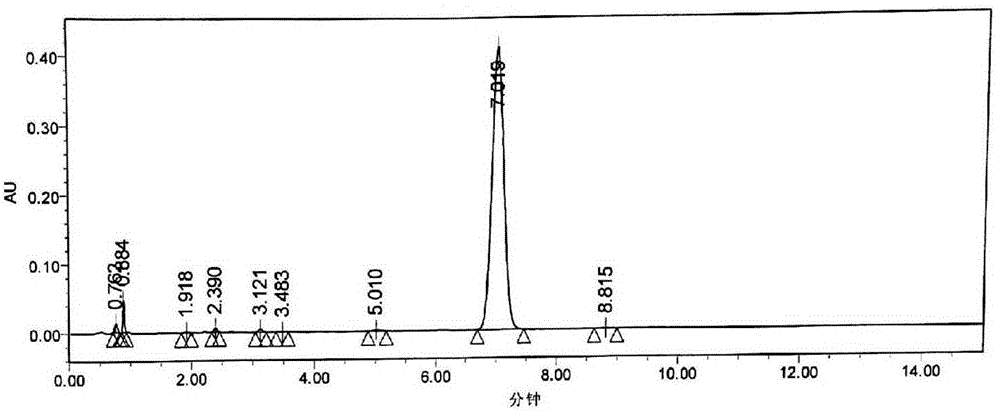
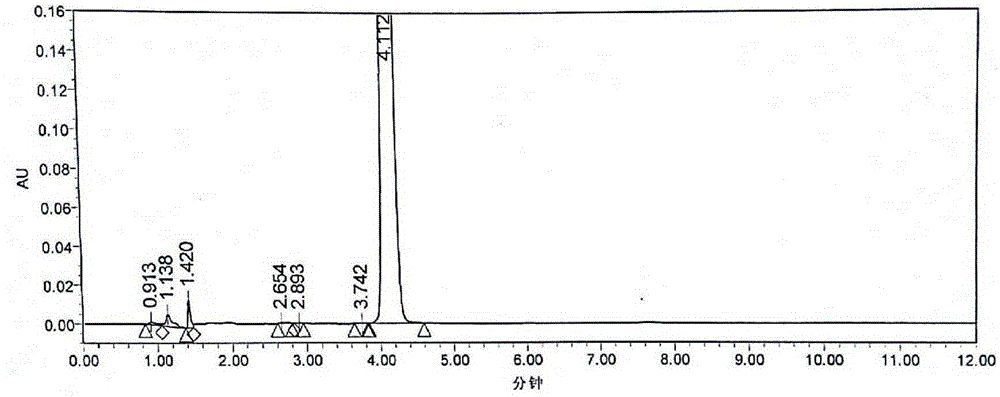
Image
Examples
Embodiment 1
[0054] Example 1 A kind of preparation method of candesartan cilexetil
[0055] 1. Candesartan (10kg) and dichloromethane (100kg) are added in the reactor, cooled to 15°C, slowly drip triethylamine (4.5kg); after the dropwise addition, the temperature of the reaction system rises to 23°C, Add triphenylchloromethane (7kg) in batches; After addition, keep the temperature of the reaction system at 23° C., and react for 3.5 hours; TLC monitoring (developing agent dichloromethane:methanol=10:1 (V / V), R f =0.78) After the reaction is complete, add 0.1mol / L HCl30L at one time (system pH=5.4), then slowly add 5L9mol / LHCl to pH=2.2; let it stand, separate the water layer and the organic layer, and use 60L saturated saline for the organic layer After washing, move it into a vacuum kettle, and recover dichloromethane under reduced pressure; add 65L of ethanol to the residue in the kettle, raise the temperature to 45°C and stir for 3 hours until a large amount of white solid precipitate...
Embodiment 2
[0075] Example 2 A kind of preparation method of candesartan cilexetil
[0076] 1. Candesartan (10kg) and dichloromethane (100kg) are added in the reactor, cooled to 10°C, slowly drip triethylamine (4.5kg); after the dropwise addition, the temperature of the reaction system rises to 21°C, Add triphenylchloromethane (7kg) in batches; Add, keep reaction system temperature 21 ℃, react for 4 hours; TLC monitoring (developing agent dichloromethane:methanol=10:1 (V / V), R f =0.78) After the reaction is complete, add 0.1mol / L HCl at one time (system pH=5.2), then slowly add 9mol / L HCl dropwise to adjust to pH=2.2; let stand, separate the water layer and the organic layer, and use 60L saturated salt for the organic layer After washing with water, move it into a vacuum kettle, and recover dichloromethane under reduced pressure; add 65L of ethanol to the residue in the kettle, raise the temperature to 40°C and stir for 3 hours until a large amount of white solids are precipitated, stop...
Embodiment 3
[0089] Example 3 A kind of preparation method of candesartan cilexetil
[0090] 1. Candesartan (10kg) and dichloromethane (100kg) are added in the reactor, cooled to 13°C, slowly drip triethylamine (4.5kg); after the dropwise addition, the temperature of the reaction system rises to 25°C, Triphenylchloromethane (7kg) was added in batches; the addition was completed, and the temperature of the reaction system was kept at 25°C for 4 hours of reaction; TLC monitoring (developing agent dichloromethane:methanol=10:1 (V / V)) after the reaction was complete, Add 0.1mol / L HCl at one time to make the system pH = 6, then slowly add 9mol / L HCl dropwise to adjust to pH = 2.6; let it stand, separate the water layer and the organic layer, wash the organic layer with 60L saturated saline, and then transfer it to a vacuum kettle. Recover dichloromethane under reduced pressure; add 65L of ethanol to the residue in the kettle, raise the temperature to 50°C and stir for 3 hours until a large am...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com