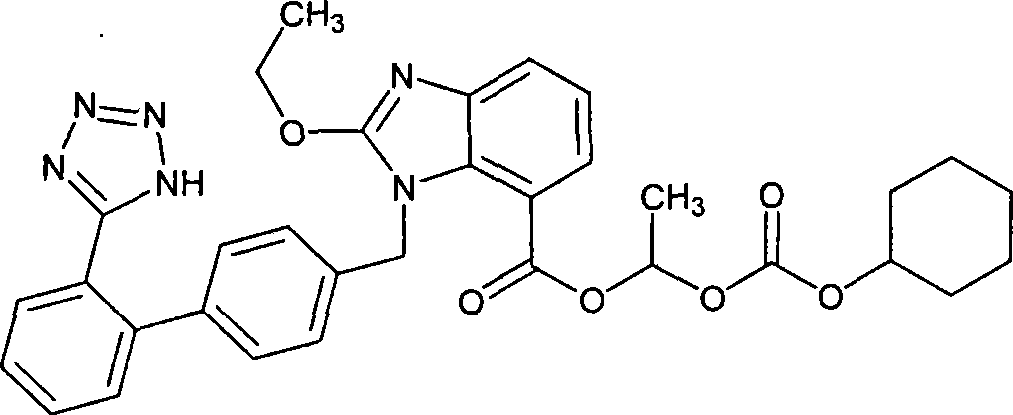
Calldesartan ci1exetil medicine compounds
A technology of candesartan cilexetil and composition, which is applied in the field of candesartan cilexetil composition, can solve the problems of poor stability of candesartan cilexetil, easy hydrolysis, prolonging the validity period of candesartan cilexetil preparations, etc. Effective period, long-term stable effect of inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The preparation of embodiment 1 candesartan cilexetil liposome (high pressure emulsifier)
[0018] Candesartan cilexetil 8g, soybean lecithin 20g, dehydrated alcohol 20ml, fully dispersed and emulsified in a high-pressure emulsifier, removed dehydrated alcohol under 50Pa to obtain the candesartan cilexetil liposome composition.
Embodiment 2
[0019] The preparation (ultrasonic wave) of embodiment 2 candesartan cilexetil liposomes
[0020] Candesartan cilexetil 4g, egg yolk lecithin 8g, absolute ethanol 20ml, ultrasonic wave is fully dispersed and emulsified, and dehydrated alcohol is removed under 50Pa to obtain candesartan cilexetil liposome composition.
Embodiment 3
[0021] The preparation of embodiment 3 candesartan cilexetil liposomes (high pressure emulsifier)
[0022] Candesartan cilexetil 8g, soybean lecithin 4g, dehydrated alcohol 20ml, fully dispersed and emulsified in a high-pressure emulsifier, removed dehydrated alcohol under 50Pa to obtain the candesartan cilexetil liposome composition.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com