Stable candesartan cilexetil tablet combination
A technology of sartan cilexetil tablet and candesartan cilexetil, which is applied in the field of candesartan cilexetil C-type crystal tablet composition, can solve the problems of difficult process operation and long time consumption, and meet the friability requirements , The preparation process is simple and easy, and the effect of good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
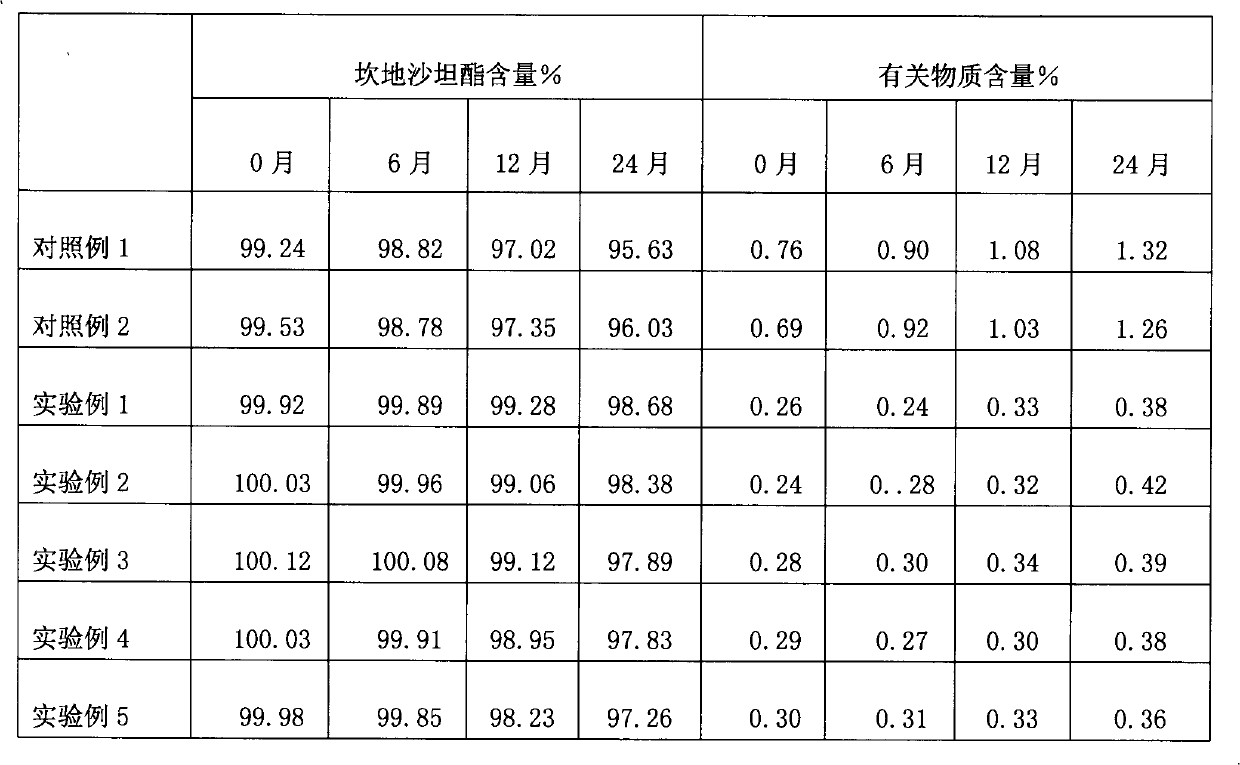
Image
Examples
Embodiment 1
[0015] Example 1 Candesartan cilexetil C crystal 8.000g, hydroxypropyl cellulose 12.000g, lactose 55.000g, starch 15.000g, microcrystalline cellulose 30.000g, carboxymethylcellulose sodium 0.700g, magnesium stearate 1.200g g, make 1000 pieces according to the following method.
[0016] The first step is to weigh the auxiliary materials according to the prescription and pass through a 120-mesh sieve.
[0017] In the second step, add the prescription amount of sodium carboxymethylcellulose into an appropriate amount of 95% ethanol, stir until it is completely dissolved, then add an appropriate amount of purified water, stir evenly, and prepare a 50% ethanol solution containing 1% of sodium carboxymethylcellulose (w / w) as a binder, set aside.
[0018] The third step is to add the prescribed amount of hydroxypropyl cellulose, lactose, starch, and microcrystalline cellulose to the wet granulator in order for dry mixing, and stir and mix for 5 minutes. Then slowly add the prescrib...
Embodiment 2
[0023] Example 2 Candesartan cilexetil C-type crystal 8.000g, hydroxypropyl cellulose 12.000g, lactose 55.000g, starch 15.000g, microcrystalline cellulose 30.000g, carboxymethylcellulose sodium 0.700g, magnesium stearate 1.200g g, made into 1000 pieces. Prepared according to the method of Example 1, the difference is that the tabletting pressure is 35 kN.
[0024] According to the method prescribed in the Pharmacopoeia of the People's Republic of China, the hardness of the tablet was measured to be 2.60 kg.
Embodiment 3
[0025] Example 3 Candesartan cilexetil C crystal 8.000g, hydroxypropyl cellulose 12.000g, lactose 55.000g, starch 15.000g, microcrystalline cellulose 30.000g, carmellose sodium 0.700g, magnesium stearate 1.200g g, made into 1000 pieces. Prepared according to the method of Example 1, the difference is that the tabletting pressure is 30 kN.
[0026] According to the method prescribed in the Pharmacopoeia of the People's Republic of China, the hardness of the tablet was measured to be 2.52 kg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com