Preparation method of proscar tablet, and prepared proscar tablet
A technology of finasteride and androsteride tablets, which is applied in the directions of non-active ingredient medical preparations, medical preparations containing active ingredients, pharmaceutical formulas, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 5mg finasteride tablets
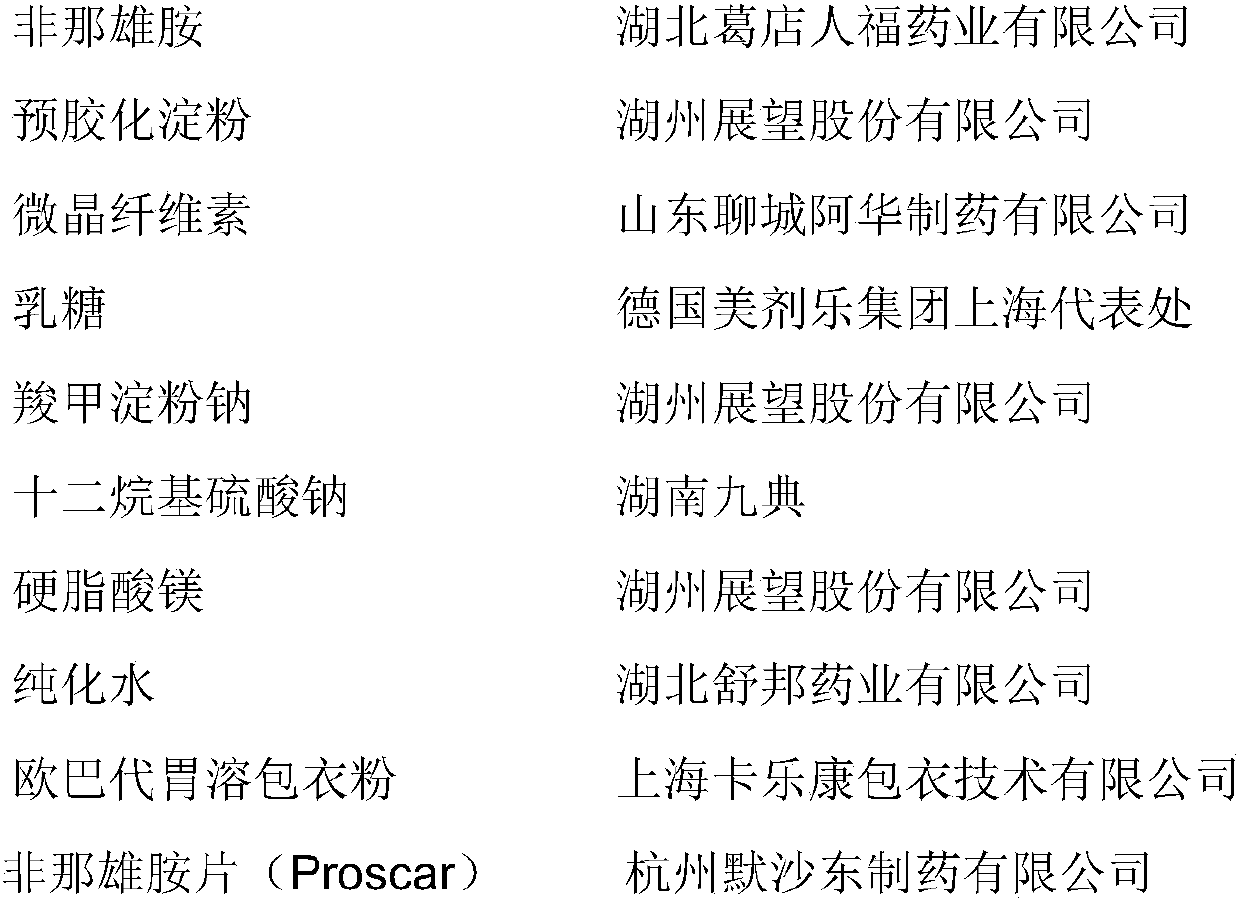
[0043] (1) Plain tablets
[0044]
[0045] (2) Coating
[0046] Stomach Dissolved Opadry: 8%
[0047] Purified water: 92%
[0048] Preparation process: After mixing the finasteride raw material and lactose, pregelatinized starch, microcrystalline cellulose, sodium starch glycolate, stir for 4 minutes to make the mixture uniform, add sodium lauryl sulfate aqueous solution for stirring, shearing and granulation 1min, 24 mesh sieve wet granules, after drying at 60-65°C until the moisture content is less than 4.0%, 24 mesh sieve, mixed with magnesium stearate, and compressed according to the theoretical tablet weight. If the uniformity and friability of the plain tablets are tested, they can be coated.
Embodiment 2
[0050] 5mg finasteride tablets
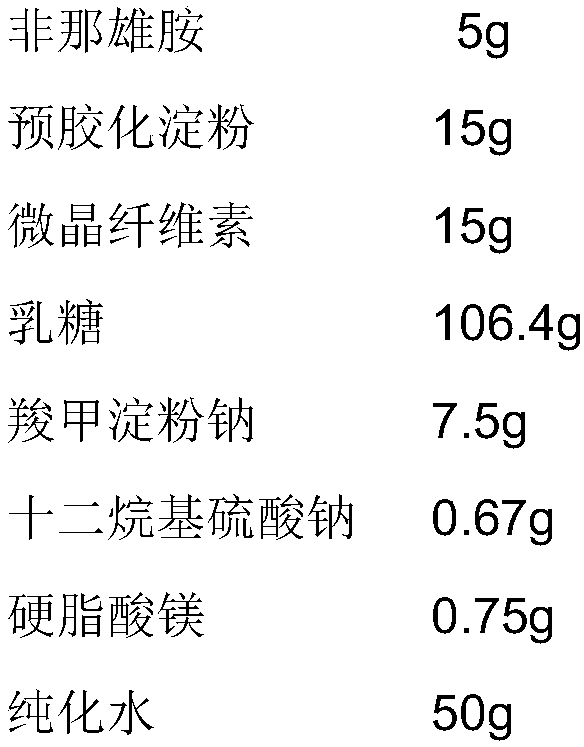
[0051] (1) Plain tablets
[0052]
[0053] (2) Coating
[0054] Stomach dissolving Opadry: 10%
[0055] Purified water: 90%
[0056] Preparation process: After mixing the raw material of finasteride with lactose, pregelatinized starch, microcrystalline cellulose and sodium starch glycolate, stir for 5 minutes to make the mixture uniform, add sodium lauryl sulfate aqueous solution for stirring, shearing and granulation 1min, 24 mesh sieve wet granules, dried at 60-65°C until the moisture content is less than 4.0%, 24 mesh sieve, mixed with lubricant, and compressed according to theoretical tablet weight. If the uniformity and friability of the plain tablets are tested, they can be coated.
Embodiment 3
[0058] 5mg finasteride tablets
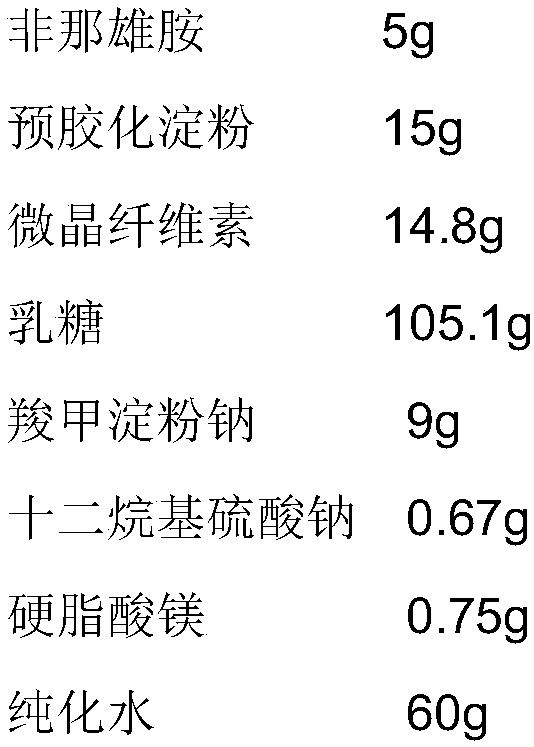
[0059] (1) Plain tablets
[0060]
[0061]
[0062] (2) Coating
[0063] Stomach Dissolved Opadry: 8%
[0064] Purified water: 92%
[0065] Preparation process: After mixing finasteride raw material with lactose, pregelatinized starch, microcrystalline cellulose, sodium starch glycolate, stir for 5 minutes to make the mixture uniform, add sodium lauryl sulfate aqueous solution for stirring, shearing and granulation 1min, 24 mesh sieve wet granules, dried at 60-65°C until the moisture content is less than 4.0%, 24 mesh sieve, mixed with lubricant, and compressed according to theoretical tablet weight. If the uniformity and friability of the plain tablets are tested, they can be coated.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com