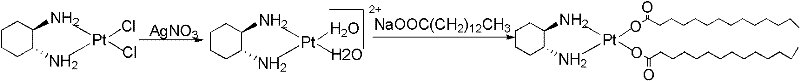Patents
Literature
230 results about "Drug production" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
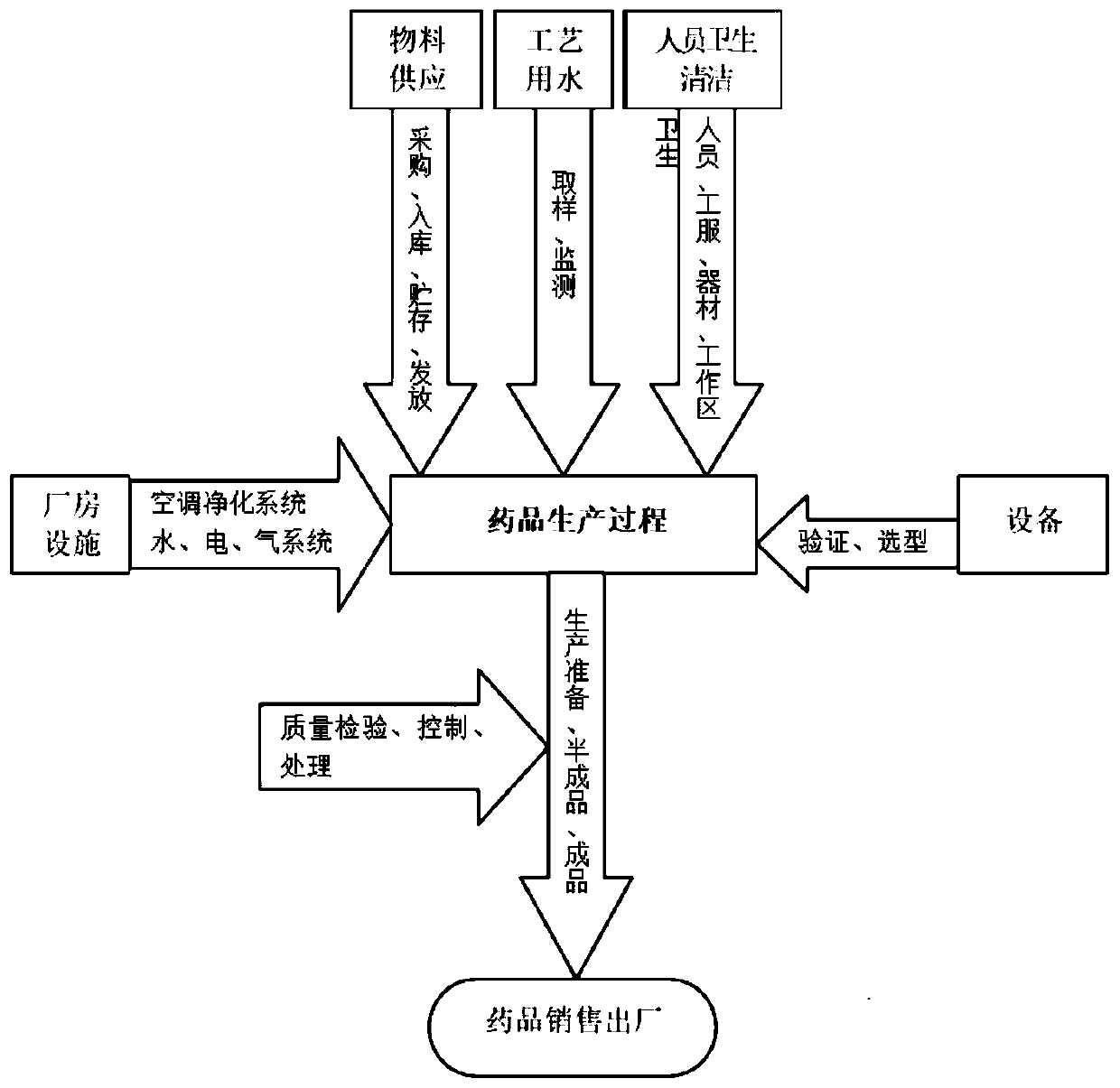
Drug "manufacturing," in a criminal law setting, occurs when an individual is involved in any step of the illicit drug production process. Those who sell certain precursor chemicals, specialized equipment, or simply offer to help produce drugs also may be charged with the crime.
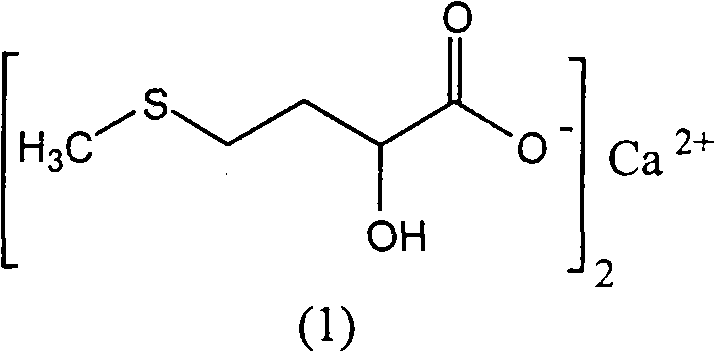
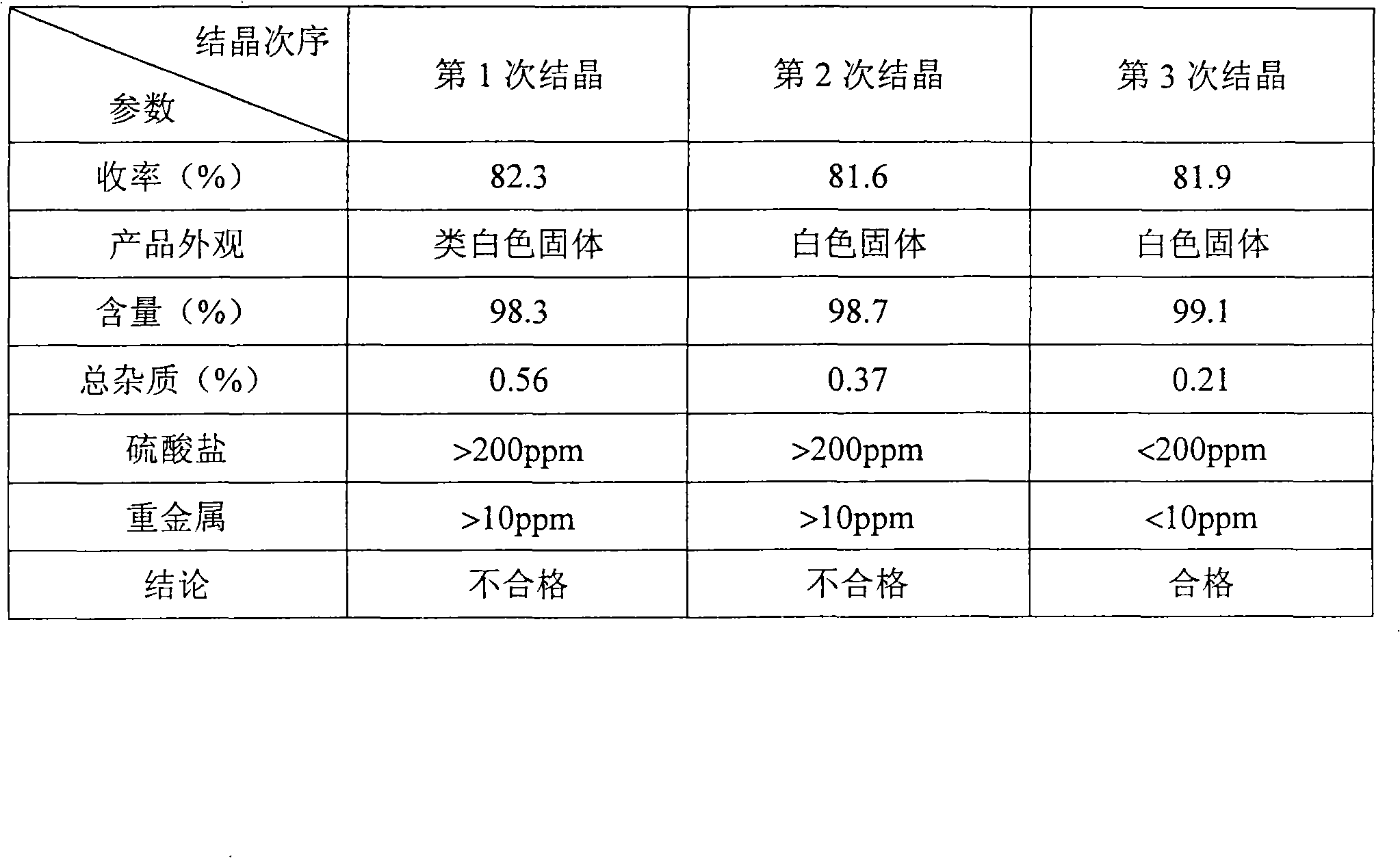
Preparation of medicinal D,L-2-hydroxy-4-methylthio calcium butyrate
The invention discloses a method for preparing a D, L-2-hydroxyl-4-methylthio butanoic calcium salt for medicinal purpose. The method comprises following steps of: a. using a D,L-2-hydroxyl-4-methylthio butanoic acid and an alcohol with a general formula of ROH as the raw materials to carry out esterification to obtain a D, L-2-hydroxyl-4-methylthio butyrate, and; b. hydrolyzing the D, L-2-hydroxyl-4-methylthio butyrate in step a and calcium oxide in a solvent to produce the D, L-2-hydroxyl-4-methylthio butanoic calcium salt. The method provided by the invention for the production of the D, L-2-hydroxyl-4-methylthio butanoic calcium salt has the advantages of short course, readily available the raw materials, low cost, easy control over the quality of the product, and more importantly, the method can prepare high-purity D, L-2-hydroxyl-4-methylthio butanoic calcium salt for medicinal purpose, thereby satisfying the requirements of State Food and Drug Administration Bureau and Good Manufacturing Practice (GMP) for drug production, and facilitating the preparation of pharmaceutical preparations.
Owner:NANJING LIFENERGY R & D +1
Green extraction process for artemisinin
The invention discloses a green extraction process for artemisinin, and relates to the field of drug production. The process comprises the following steps: (1) drying of raw materials, that is, putting the raw materials into drying equipment for drying; (2) primary processing of artemisinin, that is, putting the dried raw materials into an extraction tank, adding petroleum ether, heating the extraction tank, carrying out countercurrent extraction, allowing the extract to flow out from the extraction tank, standing the extract to obtain a supernatant, allowing the supernatant to pass through asilica gel column, eluting the silica gel column with petroleum ether, collecting artemisinin fractions, putting the artemisinin fractions into a concentrating tank for concentration until crystals are precipitated, putting the obtained concentrate into a crystallizing tank for crystallization, carrying out filtering to obtain crystals, and drying the crystals so as to obtain crude products of artemisinin; (3) refining of artemisinin, that is, dissolving the crude products of artemisinin in an alcohol precipitating tank with ethanol, wherein the ethanol with a concentration of 93 to 95% is 65to 75 times the amount of the crude products, standing the solution, taking the supernatant of the solution for secondary filter, concentrating the filtrate in the concentrating tank, standing the concentrate for crystallization, carrying out filtering to obtain crystals, and drying the crystals under vacuum so as to obtain refined products of artemisinin.
Owner:GUANGXI XIANCAOTANG PHARMA
Short bifidobacteria with functions of anti-gastrointestinal tract pathogen, oxidation resistance and blood pressure reduction
ActiveCN101314763AStrong resistanceImprove antioxidant capacityAntibacterial agentsBacteriaBiotechnologyFeces
The invention discloses a novel Bifidobacterium breve with excellent probiotic function. The Bifidobacterium breve strains are obtained by screening the feces of the long-lived elders in Hetian, XinJiang, and the obtained strains have outstanding resistance to acid and bile acid salts, adhesion characteristics to intestinal tract epidermic cells, excellent antibacterial performance and good oxidization resistance; and the strains have an effect on reducing the blood pressure as well as the edible safety, therefore, the Bifidobacterium breve can be widely applied in the fields such as functional additives, dairy products, beverage, functional food, drug production, etc.
Owner:PRESIDENT ENTERPRISES (CHINA) INVESTMENT CO LTD +1
System and methods for the production of personalized drug products
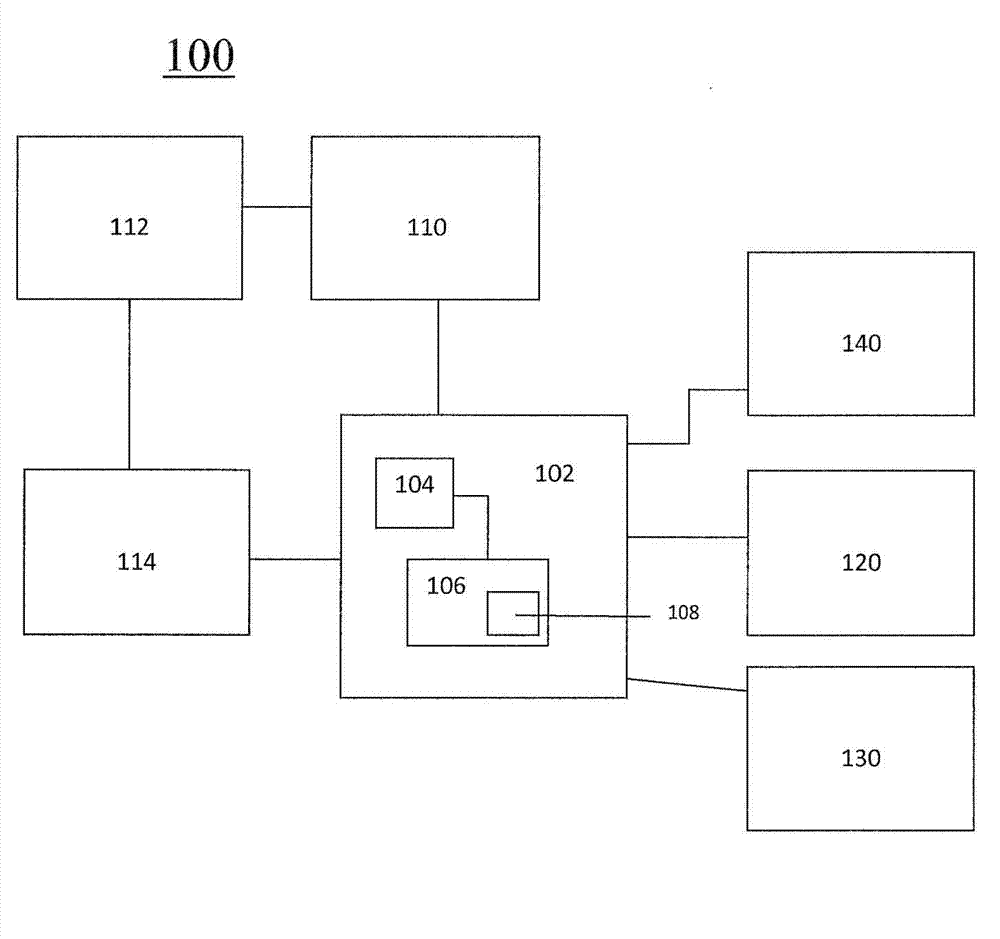
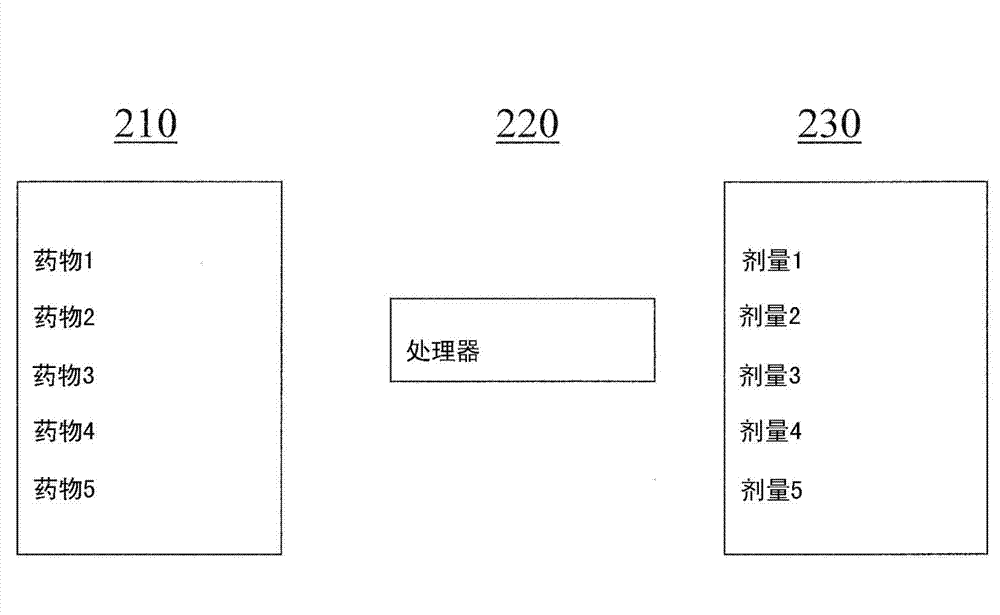
InactiveCN103250176AData processing applicationsDrug and medicationsTransdermal patchDrug dispensing
A system and method for determining an optimal combination drug product for a particular patient includes a processor that receives patient information and determines an optimal combination drug product based on the received information. A system which can provide information regarding predicted events or pathologies based on received patient information and guidance on subsequent steps to ameliorate, treat or intervent. A drug production device includes a plurality of drug containers, each of which are coupled to a drug dispensing channel. A controller controls the dispensing of drug through each channel, and a combination drug product is produced from the dispensed drugs. A combination drug product includes a plurality of discrete units of a first drug, and a plurality of discrete units of a second drug.; A transdermal patch includes a plurality of drug compartments, each containing a quantity of drug product, and a controller for controlling the release of drugs from each compartment. Feedback loop elements can enable iterations to optimized personalized doses.
Owner:INTELLIMEDICINE +1
Lingzhiol A and application of lingzhiol A in drug production and foods
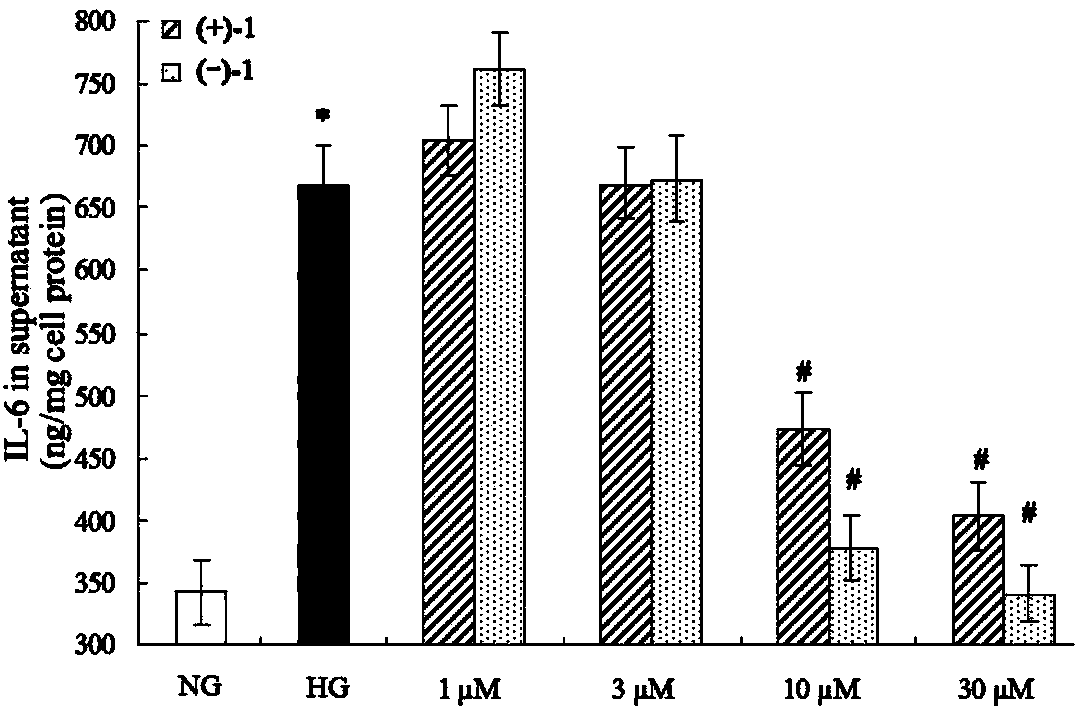
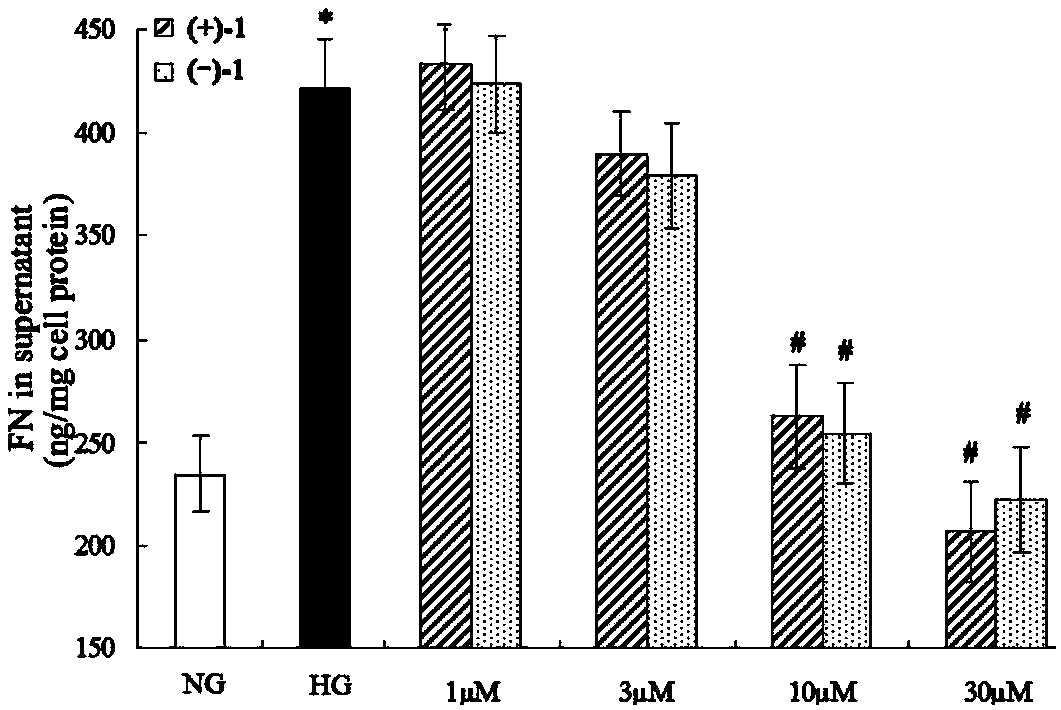
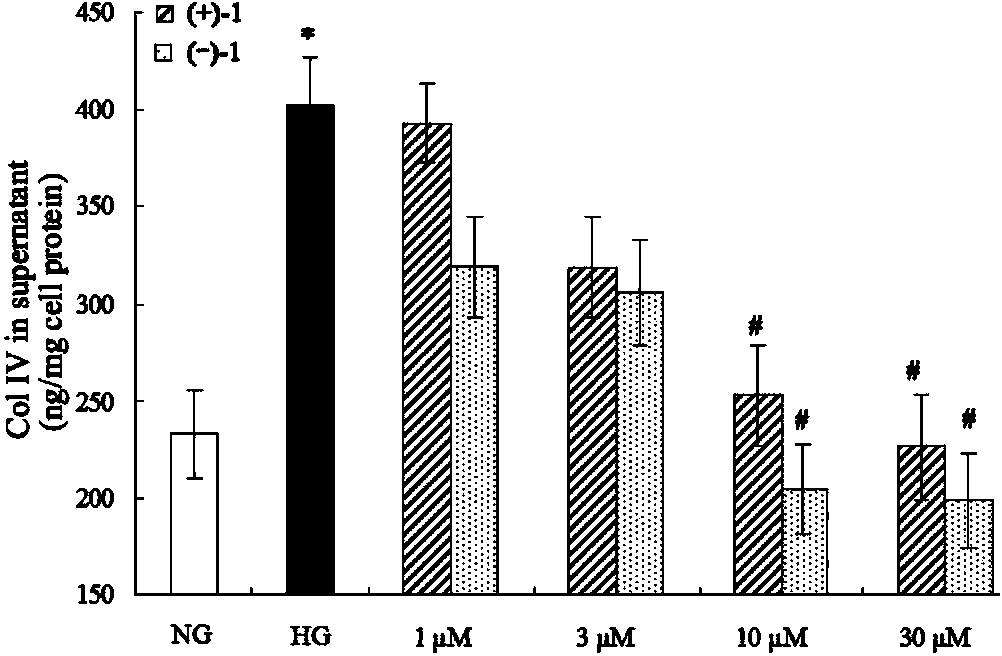
Glossy ganoderma as a traditional Chinese medicine is called immortal grass since ancient times and known as the ability to treat various diseases; a pair of lingzhiol A optical enantiomers is purified from ganoderma lucidum, and the lingzhiol A optical enantiomers have obvious effects on inhibiting rat renal mesangial cell strains induced by high glucose to generate reactive oxide species, IL-6, fibronectin and IV type collagen, and also can obviously inhibit the phosphorylation of the renal tubular epithelial cell Smad3 induced by TGF-beta1, so that the application prospect of the compound in preparation of medicines for treating diabetic nephropathy and chronic nephropathy is shown.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI
Block chain-based drug traceability method and device and medium
PendingCN111008844AIncreased sense of securityGuaranteed credibilityDatabase distribution/replicationDigital data protectionDrug productComputer science
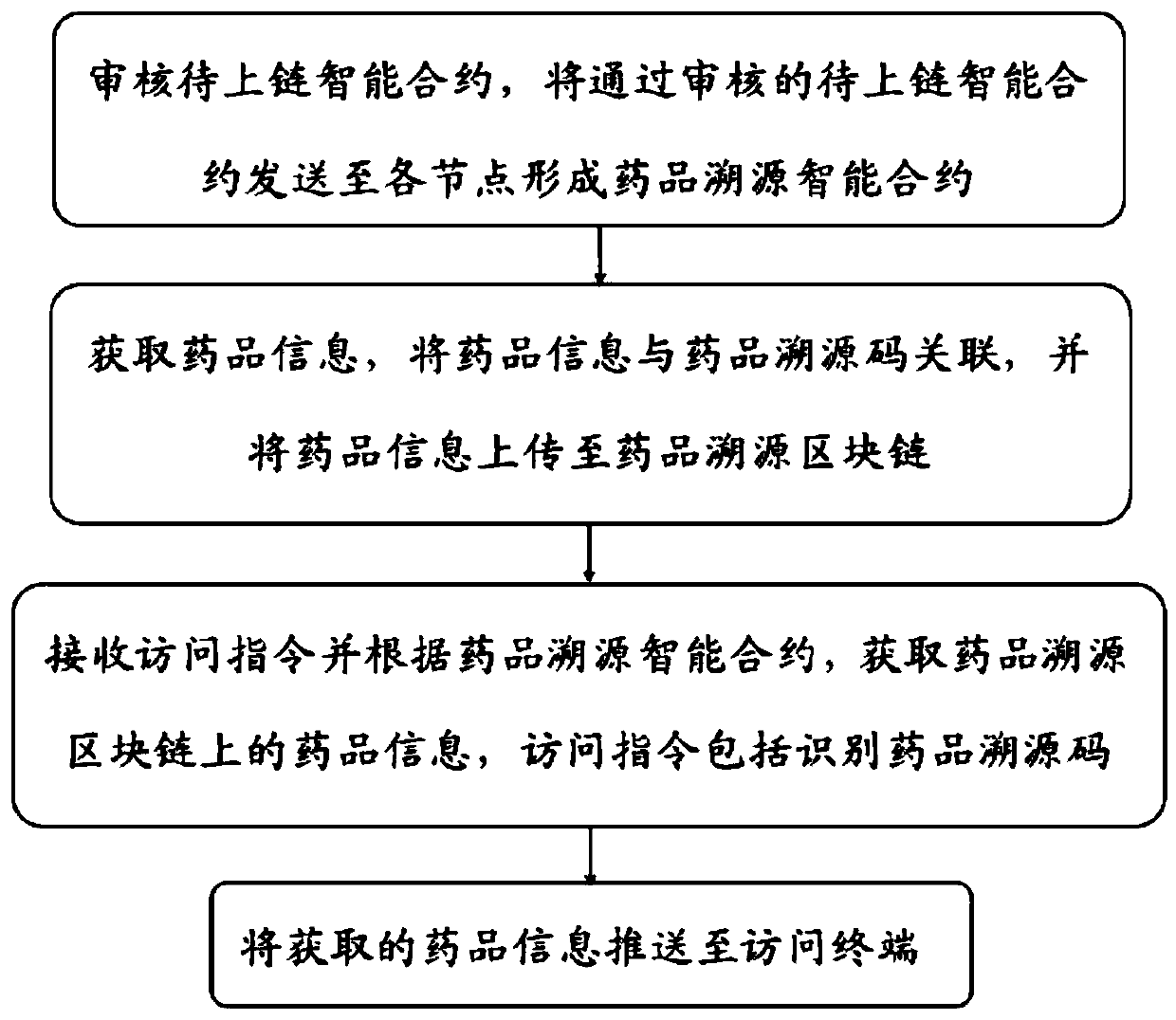
The invention discloses a block chain-based drug traceability method and device and a medium. The block chain-based drug traceability method comprises the steps: auditing a to-be-uploaded intelligentcontract, and sending the to-be-uploaded intelligent contract passing the auditing to each node to form a drug traceability intelligent contract; obtaining drug information, associating the drug information with the drug traceability code, and uploading the drug information to the drug traceability block chain; receiving an access instruction and obtaining the drug information on the drug traceability block chain according to the drug traceability intelligent contract, wherein the access instruction comprises identifying the drug traceability code; and pushing the acquired drug information toan access terminal. The drug traceability block chain related to the drug traceability method is is tamper-proof and can retain the uniqueness of the drug information and the detection certificates and can accurately and quickly provide data such as materials in different states in the drug production, circulation and detection process to consumers in real time so as to realize the tracing of thewhole drug production process.
Owner:山东浪潮质量链科技有限公司
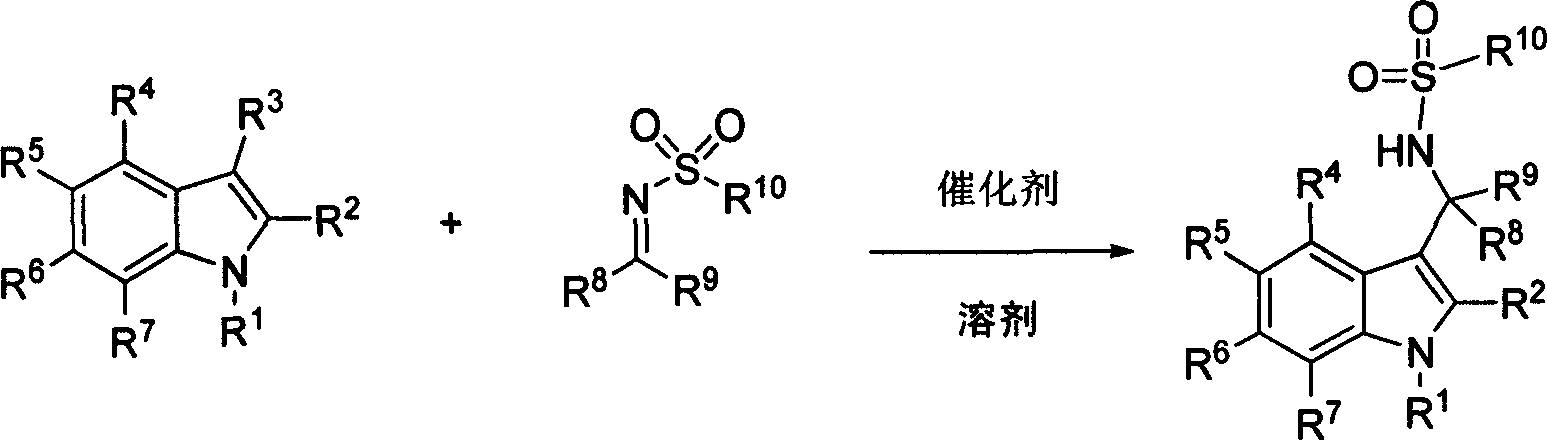
Process of synthesizing 3-methyl amino indole compound
InactiveCN100999490AImprove efficiencyHigh enantioselectivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsImidePhosphate
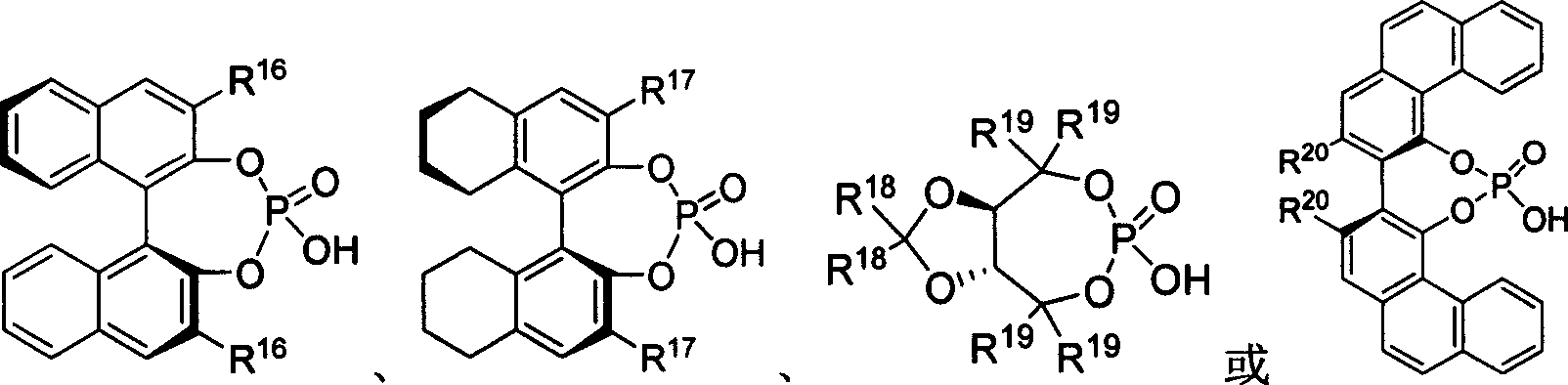
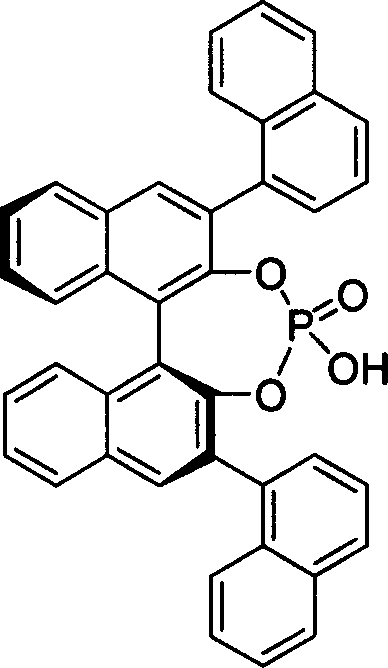
The present invention takes chiral phosphate as catalyst, uses sulfonyl imide and indole compounds synthesize 3-methylamino-indole compound of high-efficiency and high enantioselectivity. Compared with the existing method, it can be applied to many different types of indole and sulfonyl imide compounds, mild reaction conditions, simple operation. In addition, the reaction without joining any metal salts compounds, so useful for drug production and processing. And the reaction yields are better, high enantioselectivity.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
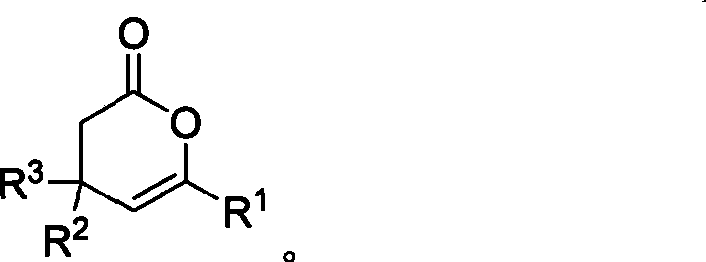
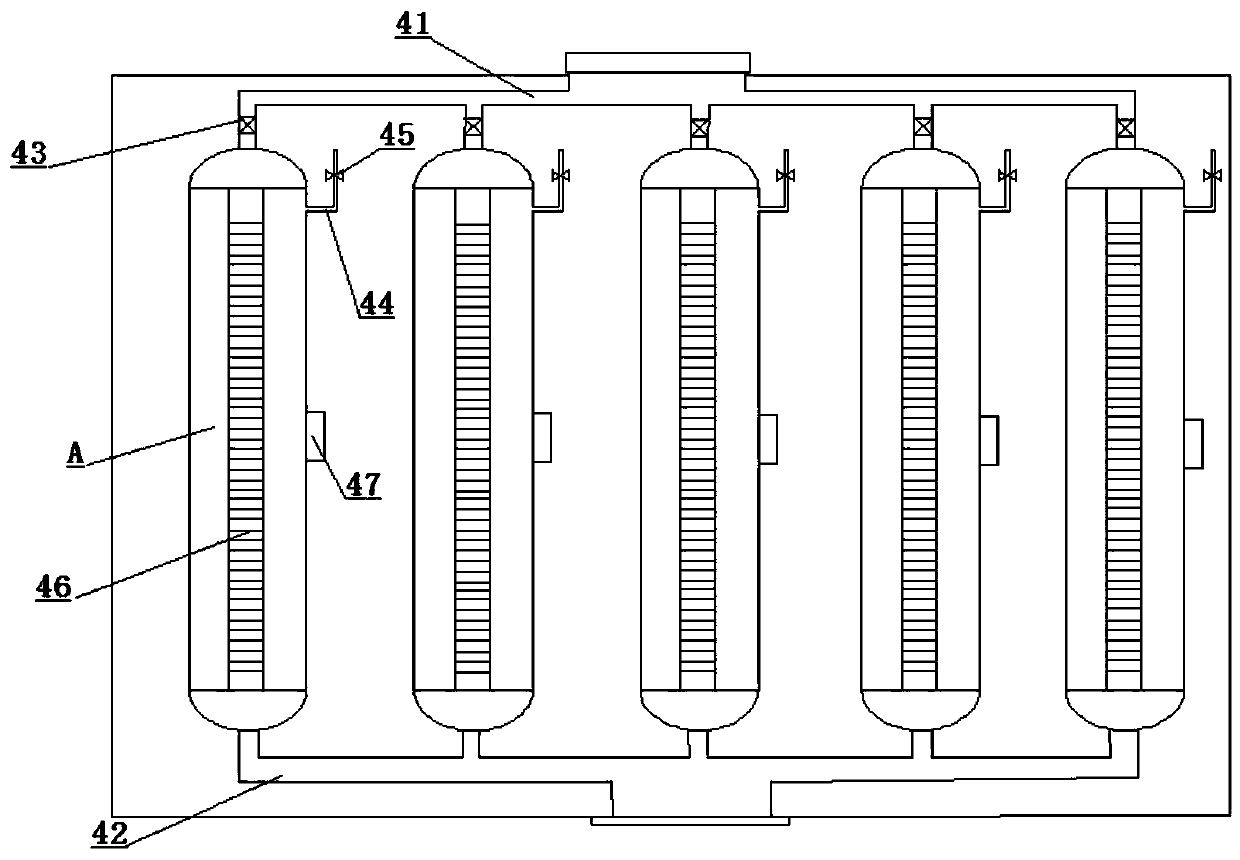
Method for synthesizing 4,6-substituted 3,4- dihydro-pyran-2-ketone derivative
InactiveCN101481369AIncrease production capacityEasy to handleOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsKetoneCarbene
The invention provides a method for effectively synthesizing a 4,6-substituted 3,-4-dihydro-pyran-2one derivative by efficiently synthesizing aldehyde-substituted cyclopropane compound with N-heterocyclic carbene as a catalyst. Compared with the existing method, the method has the advantages of wide suitable substrate range, convenient catalyst acquisition, mild reaction condition, simple operation, and high reaction efficiency. The method is performed without any metallic salt compounds, which is good for drug production and treatment.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Verification method of low pH incubation virus inactivation
InactiveCN108342368AImprove scalabilitySimple purification processSsRNA viruses negative-senseMicrobiological testing/measurementVirus inactivationValidation methods
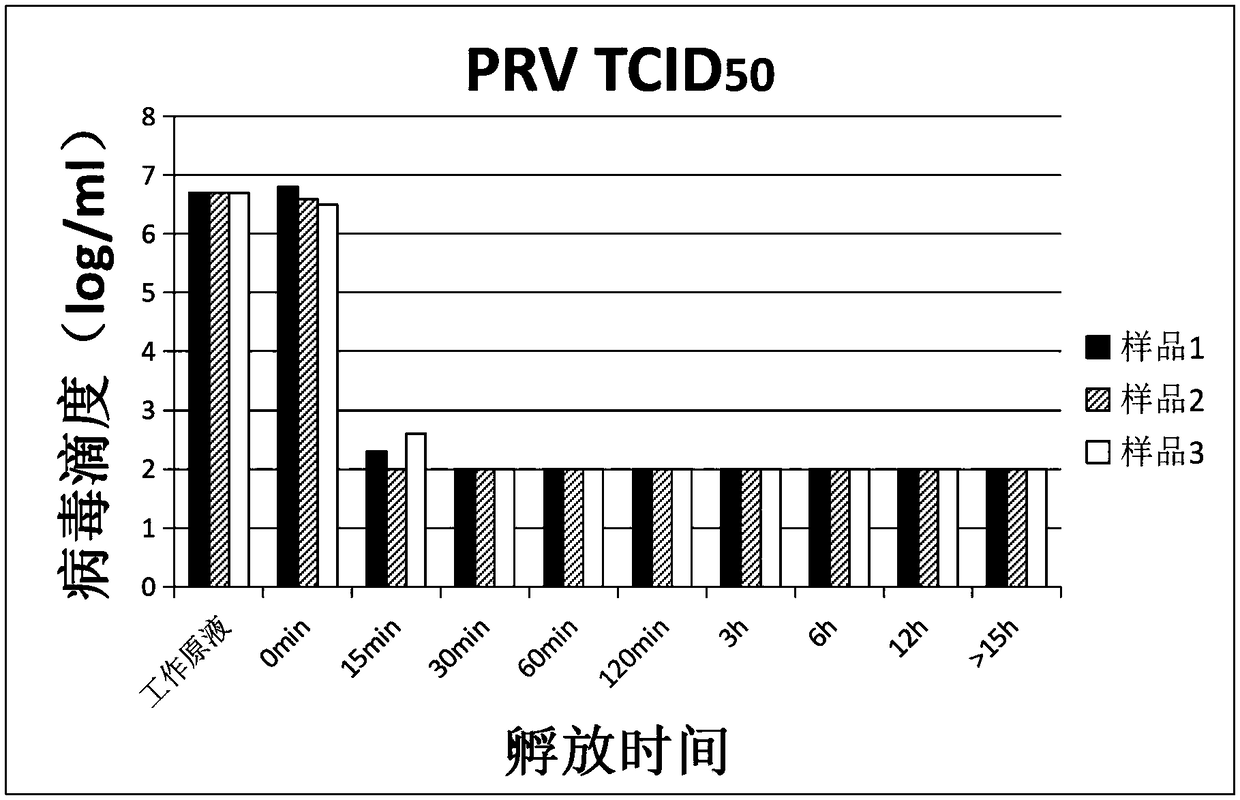
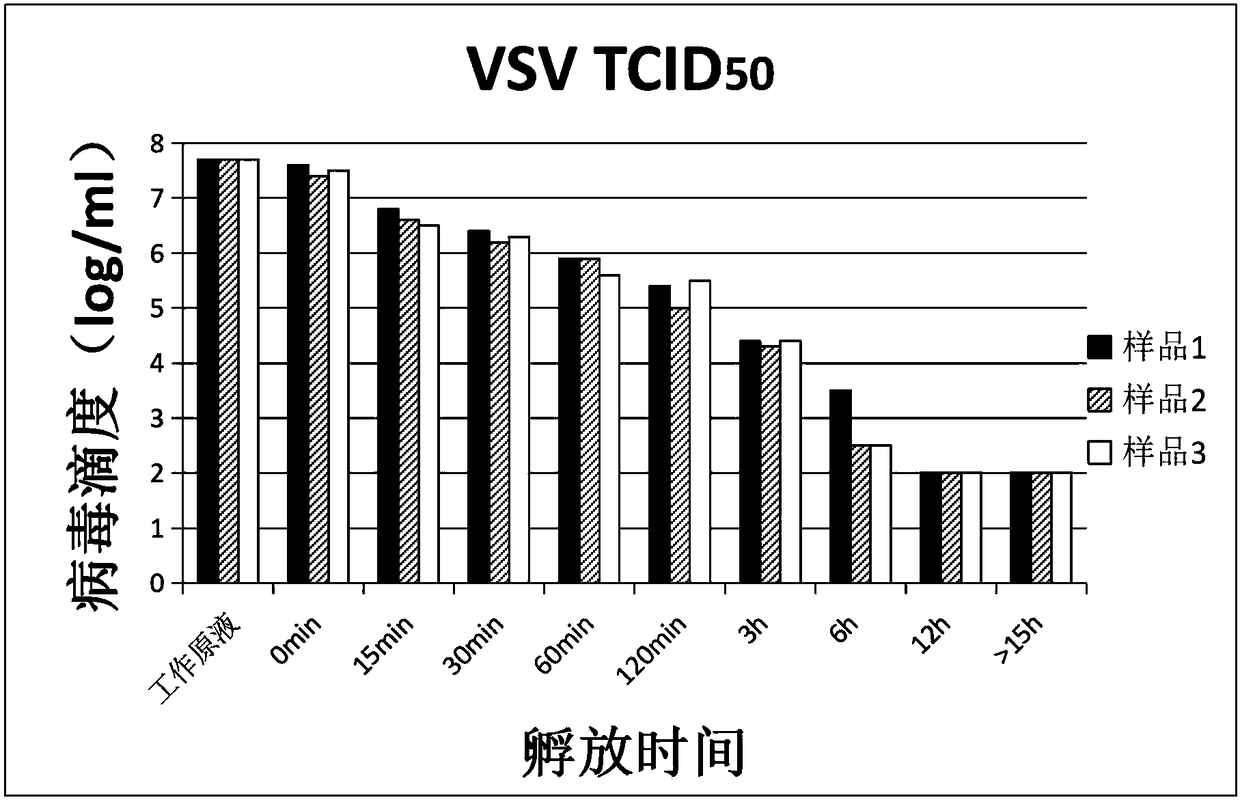
The invention relates to a verification method of low pH incubation virus inactivation. The method comprises the steps of selecting indicator viruses and corresponding host cells; amplifying and purifying the indicator viruses; titering virus working primary liquid and samples; optimizing the pH value of virus inactivation verification experiments; estimating the low pH incubation virus inactivation effect. By means of the method, the full process of genetically engineered drug production technology virus inactivation can be simulated in a laboratory, and the amplifying and purifying technology of the indicator viruses is optimized, so that the titer of the working primary liquid of the indicator viruses is increased, the pH value is unified to 7.0, the quality is stable, and subsequent operation is facilitated; meanwhile, the specific technological details like the determination of the optimal incubation pH value and the optimization of the pH adjusting mode of low pH incubation are optimized, and the efficiency and the effectiveness of the inactivation of common DNA viruses, RNA viruses, enveloped viruses and non-enveloped viruses are improved.
Owner:CANVEST WUHAN BIOTECH
Process for preparing mefenamic acid
ActiveCN101475505AImprove productivityLow yieldOrganic chemistryOrganic compound preparationDimethylaniline N-oxideAnti-inflammatory analgesics
The invention belongs to the technical field of anti-inflammatory drug production, and relates to a method for preparing mefenamic acid. The method comprises: adding o-chlorobenzoic acid and 2,3-dimethylaniline into a system which is formed by a non-protonic polar solvent and a dehydrant, performing condensation reaction in the presence of an acid-binding agent, a catalyst and a phase-transfer catalyst, and obtaining mefenamic sodium; acidifying the mefenamic sodium and obtaining coarse mefenamic acid products; and refining the coarse mefenamic acid products in an organic solvent and water and obtaining finished mefenamic acid products. The method improves the production efficiency of the mefenamic acid and reduces the production cost of the mefenamic acid.
Owner:BAOJI TIANXIN PHARM CO LTD
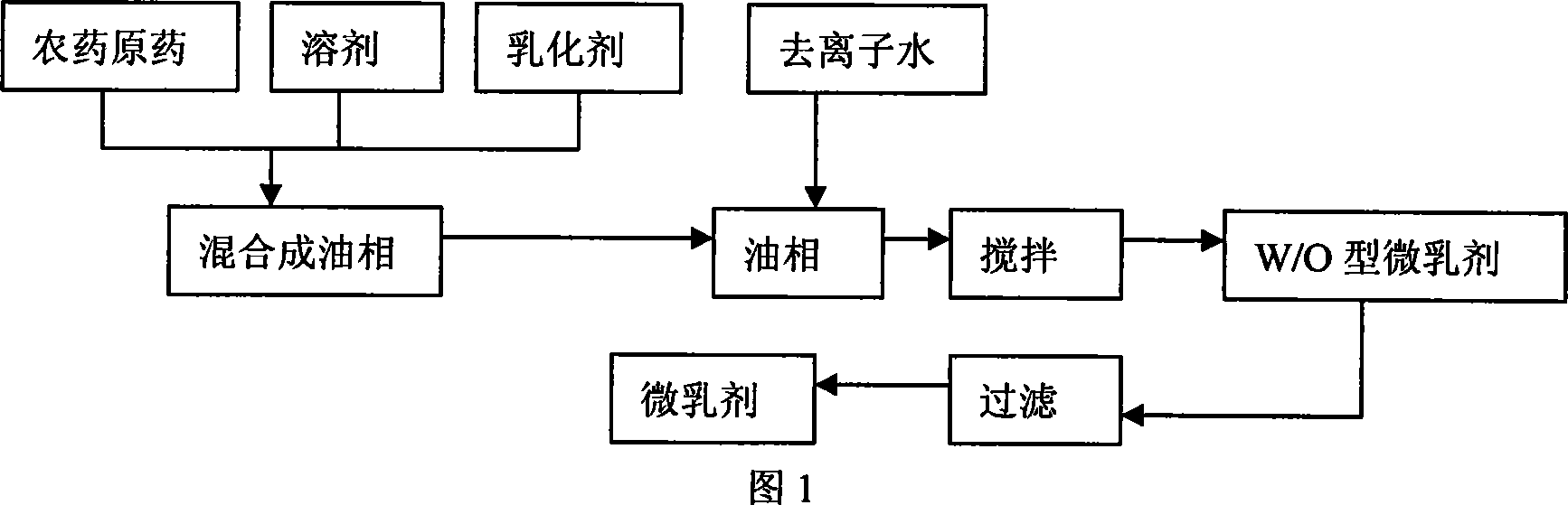
Ginkgo snail-killing micro emulsion and preparation method thereof
The invention discloses a gingko snail killing microemulsion agent and a preparing method, which relates to the technical field of plant source agricultural drug production. The invention takes the seed shell extract of the gingko containing a ginkgoic acid as the source drug, and the invention is made by configuring auxiliary surface active agents, solution agent and antifreeze agent as well as water. The producing method comprises the following operating steps: the gingko external seed shell extract, the microemulsion agent and the solution agent are blended to be an even and transparent oil phase; the steamed water or the deionized water are added slowly to form an oil enclosed water type emulsion during the agitating process; through agitating and heating, the emulsion is rapidly converted into a water enclosed oil type; a steady O or W type emulsion after being cooled to reach the constant indoor temperature is obtained. The invention has fine environmental compatibility of the emulsion conformation, also completely realizes the advantages of the gingko snail killing agent with high efficiency to the snail and safety to people and animals. The microemulsion agent takes water as the main solvent with slight influence to environment. In addition, the invention has remarkable synergism; the LC 50 and LC 90 of the 24h respectively are 0.56mg per liter and 3.5mg per liter, which are obviously better than that of the 24h snail killing effect (LC50 is equal to 1.35mg per liter and the LC90 is equal to 3.85mg per liter) of the external seed shell extract of the original drug.
Owner:JIANGSU UNIV
Quality control method for rhizoma gastrodiae capsule
ActiveCN104306745AToxicEasy to emulsifySenses disorderNervous disorderMedicineThin layer chromatographic
The invention relates to a quality control method for a rhizoma gastrodiae capsule and belongs to the technical field of biomedicine. The quality control method comprises the steps of raw material control, preparation method control, an identification method and a content measurement method. Modern drug production management is integrated in quality control. The quality of drugs and solvents used in production is examined from the source. Product quality control in the production process is established, perfect product quality standards are established, the thin-layer chromatographic detection ratio is higher than national requirements, the gastrodin content is greatly increased, the increase degree reaches 28.3%, and the quality and the therapeutic effect of the drug product are ensured.
Owner:YUNNAN YONGZITANG PHARMA
Novel biopharmacy tablet grinding forming device
ActiveCN109604019ASolve the phenomenon that it is easy to stamp and cause fragmentationSolve the phenomenon of fragmentationPharmaceutical product form changeGrain treatmentsEngineeringDrug powder
The invention relates to medical instruments, in particular to a novel biopharmacy tablet grinding forming device. The novel biopharmacy tablet grinding forming device comprises a grinding box base and a forming box. The problem that during biopharmacy, tablet stamping is the common method of drug production, before tablets are stamped, grinding, mixing and transporting are carried out, then, stamping is carried out, steps are tedious and much, and most steps are manually finished can be solved, and the problem that an existing stamping device is supported to carry out single-time stamping forming, the stamping of the stamped stables is prone to be hollow, and fragmentation is generated, and through one-time stamping molding, drug powder in the mold is prone to being extruded out of the mold can be solved.
Owner:浙江豆豆宝中药研究有限公司
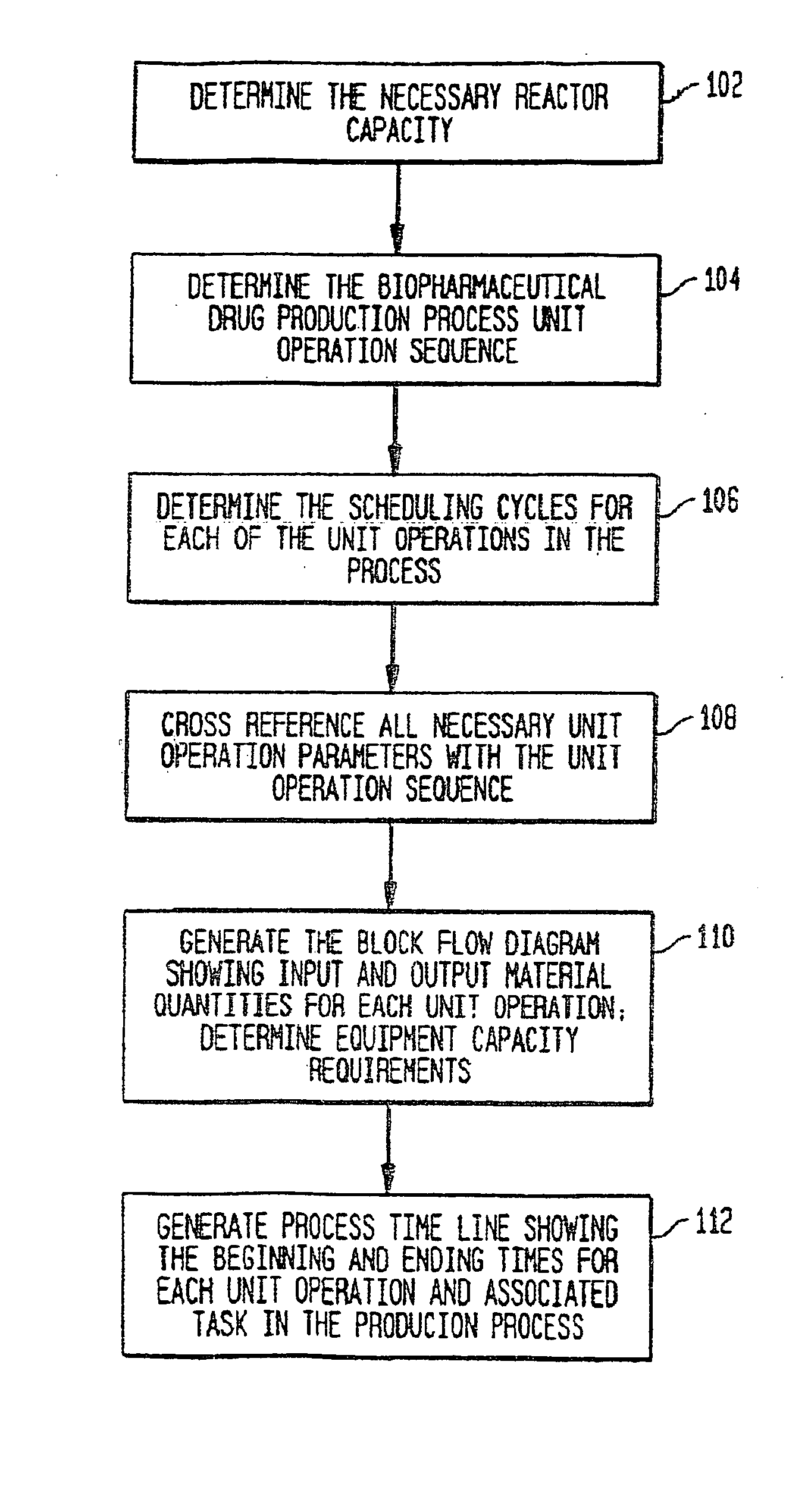
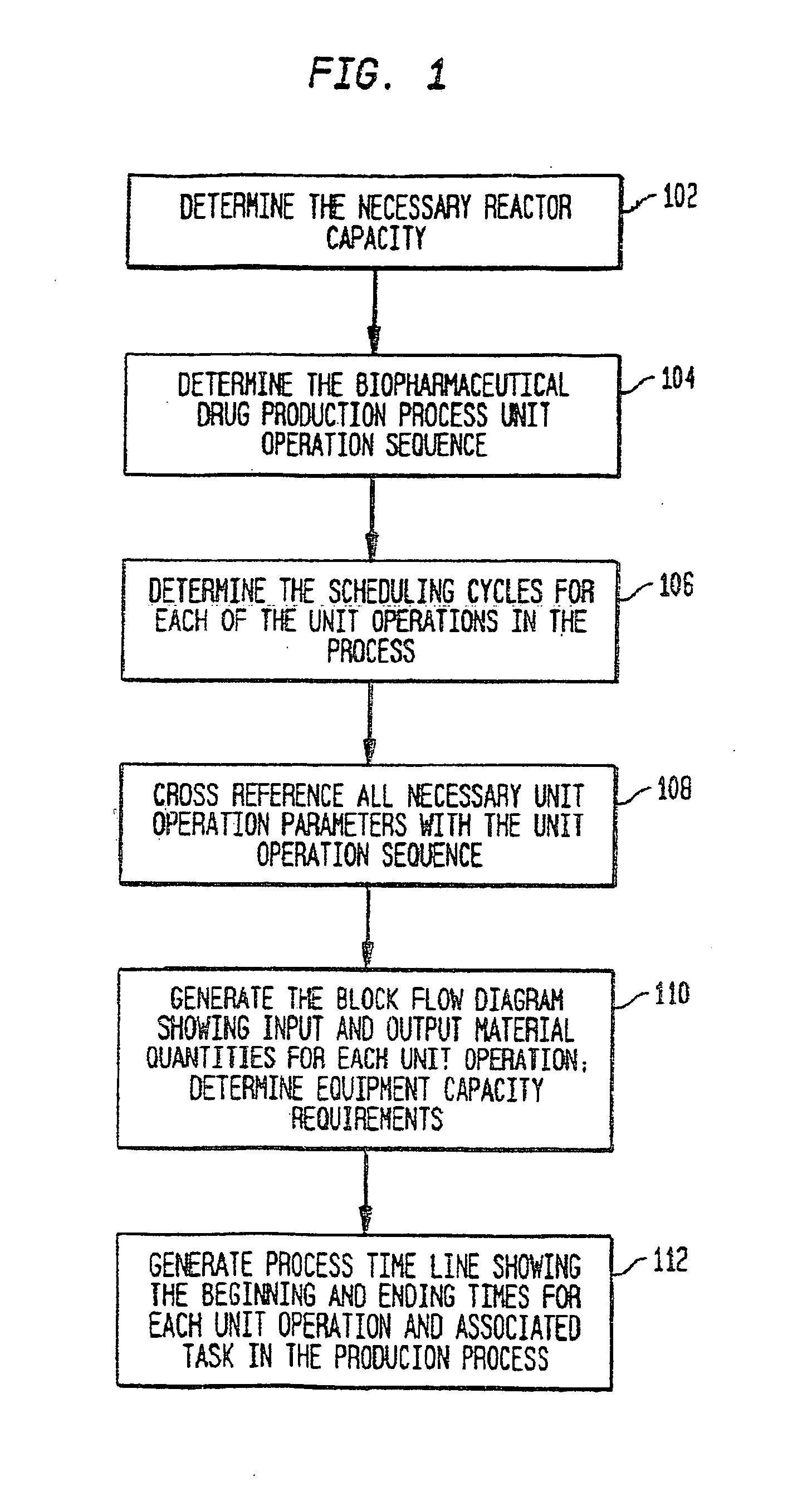
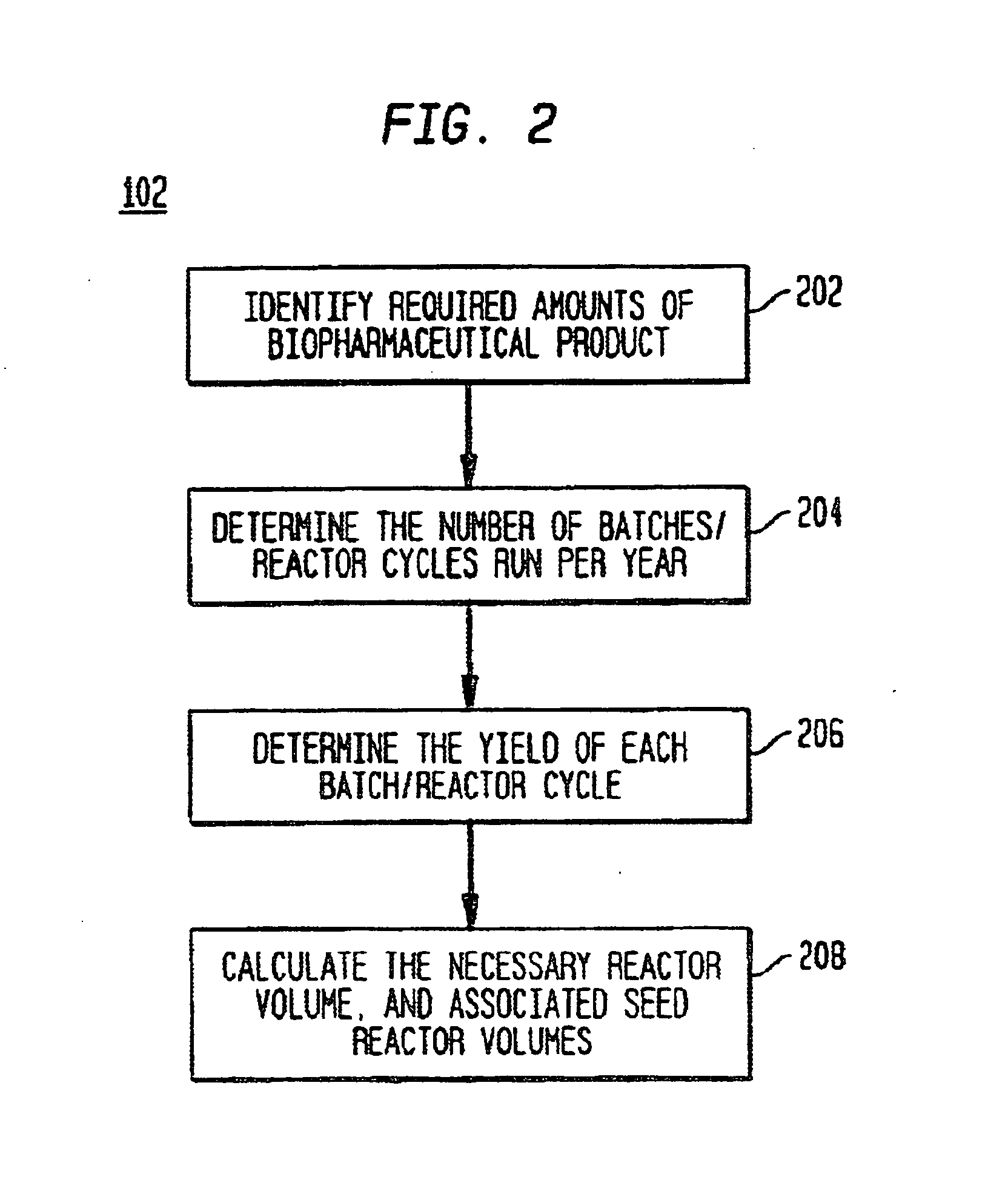
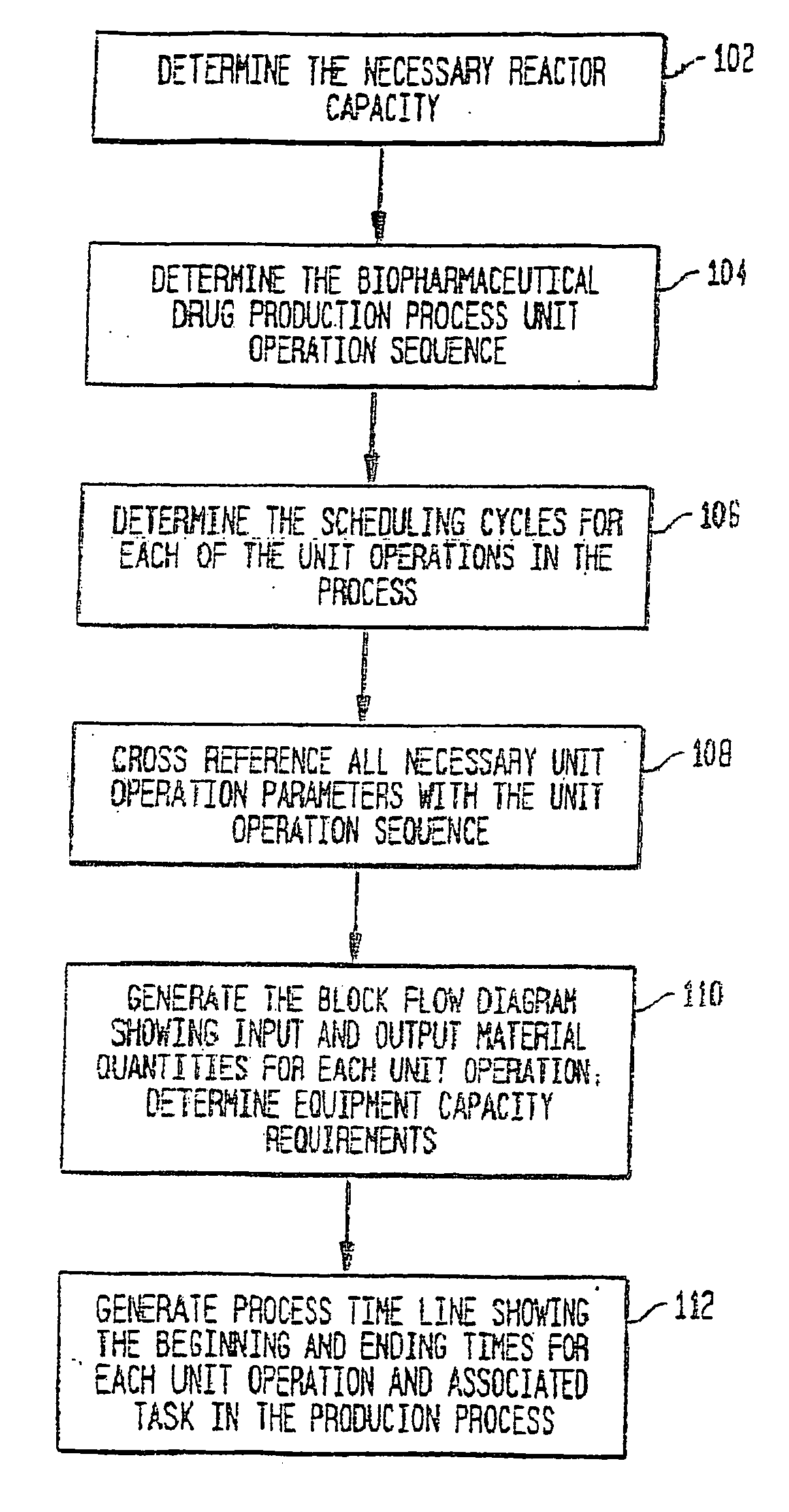
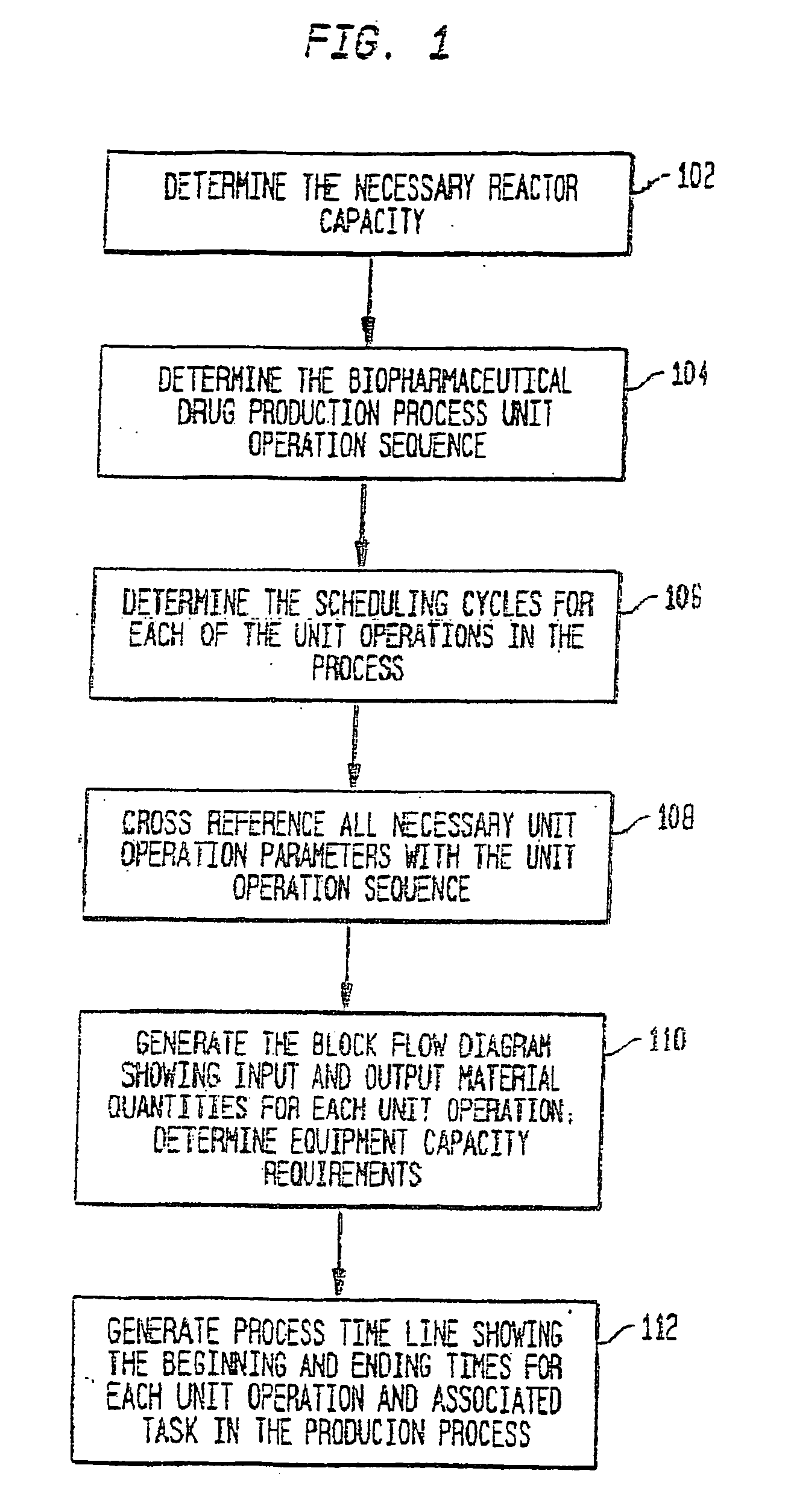
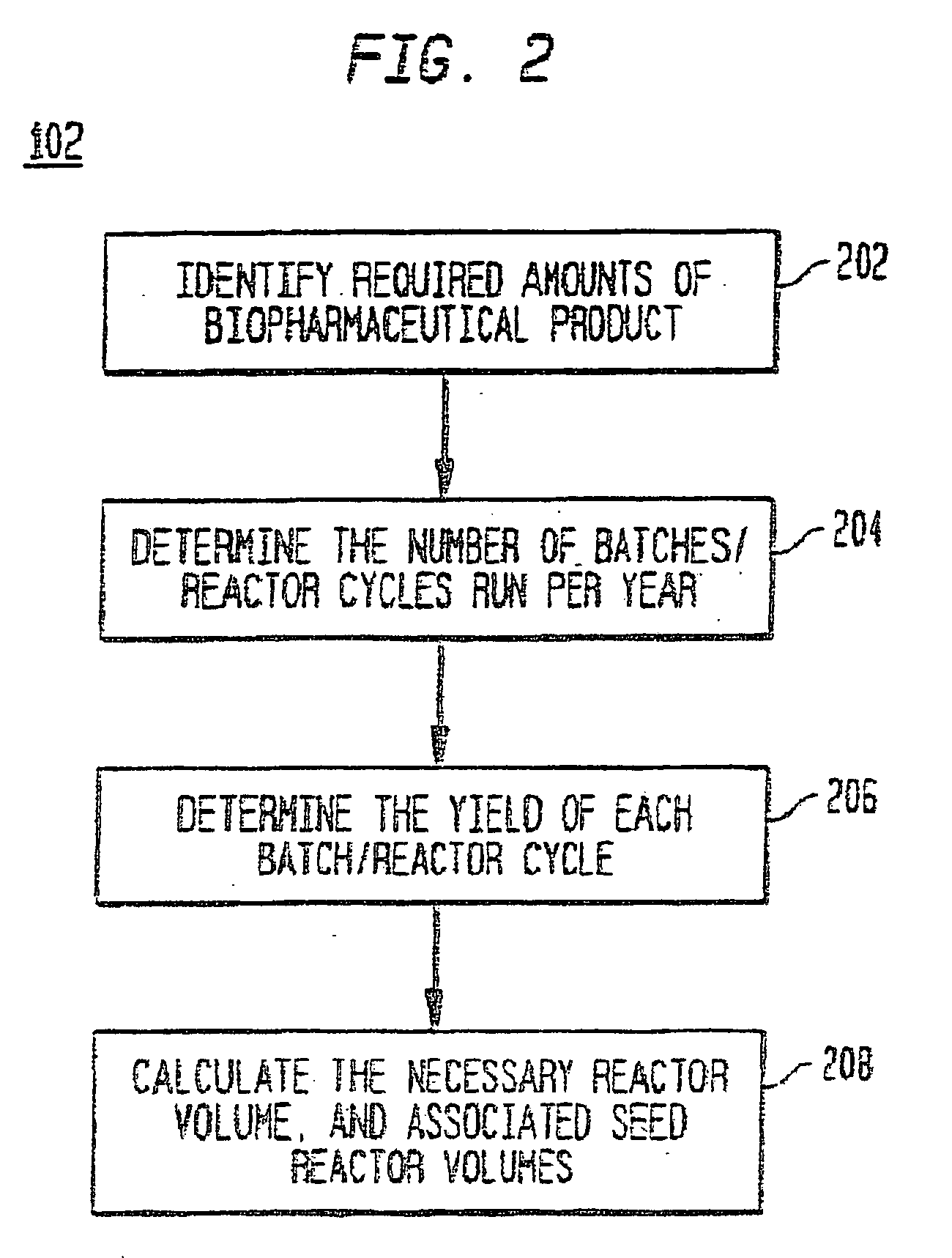
Use of sub (partial) cycles, nested cluster cycles, and lot cycles for determining equipment capacities in a batch manufacturing facility
A system and method for the simulation and modeling of biopharmaceutical batch process manufacturing facilities using process time lines is described herein. The system employs an eleven-field delimited string code which specifies the unit identifier code and the iteration value for each of the ten levels of nested scheduling cycles of the biopharmaceutical drug production process being modeled. The method includes generating a process time line using operational parameters, a block flow diagram, and a set of scheduling cycles for each of a sequence of unit operations. The process time line is used as a tool for batch processing and facility design.
Owner:BROWN OWEN (I) (US)
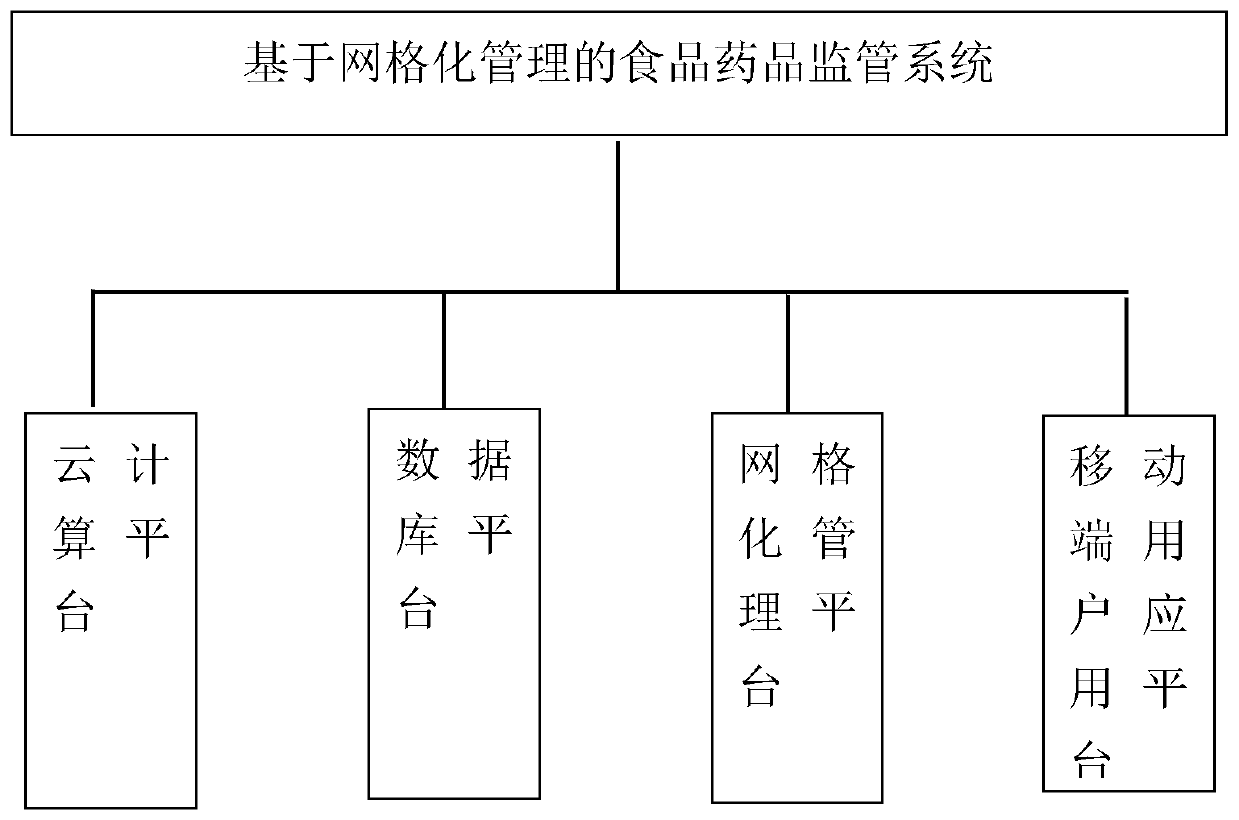
Food and drug supervision system and method based on gridding management
InactiveCN109801004ARealize intelligent serviceRealize refined managementResourcesFood safetyDrug product
The invention discloses a food and drug supervision system and method based on gridding management. The supervision system comprises a cloud computing platform, a database platform, a gridding management platform and a mobile terminal user application platform, the gridding management platform is used for dividing the supervision range into grids, and food and drug production and circulation information of all supervision enterprises in the grids is marked on a geographic map information module (GIS); The supervision method comprises the steps of administrative supervision, technical supervision and inspection case handling, and inspection case handling comprises the steps of grid division, information collection, task providing, task dispatching, task processing, processing feedback, casechecking and checking and comprehensive evaluation. According to the invention, a food safety supervision system based on a GIS professional map for prevention is established, food and drug safety production and circulation process supervision are realized, and food and drug quality safety is guaranteed.
Owner:郭承湘 +2
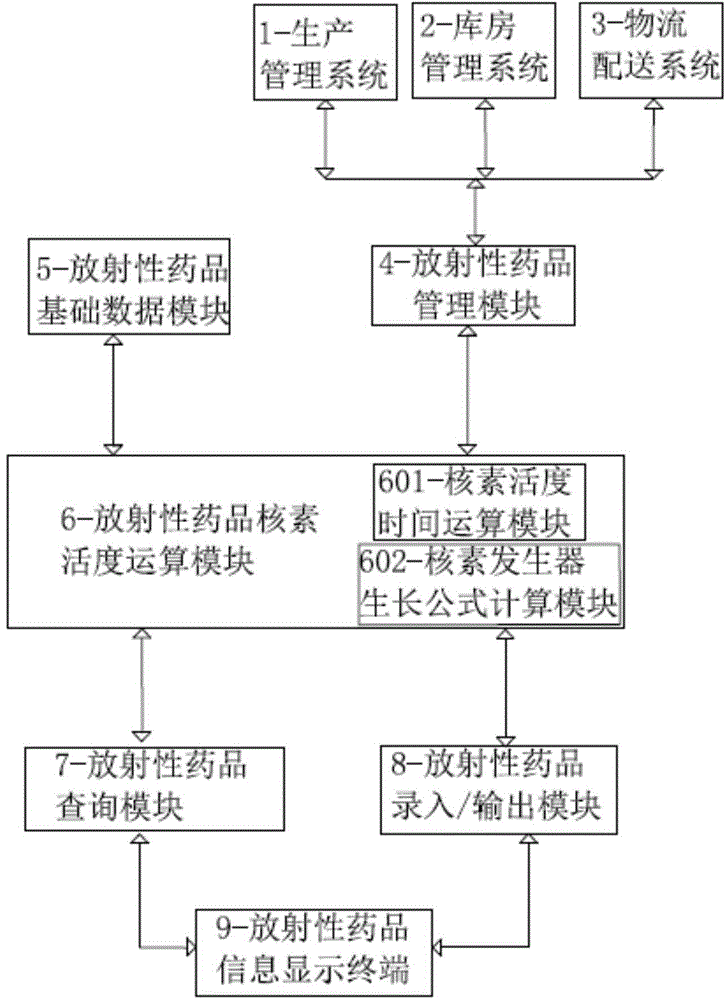
Dynamic calibration system for radiopharmaceutical radionuclide activity
ActiveCN104484793ASolve the accuracy problemFix error-prone problemsOffice automationManufacturing computing systemsEngineeringLogistic distribution
The invention relates to the technical field of radiopharmaceutical production equipment, in particular to a dynamic calibration system for radiopharmaceutical radionuclide activity. The invention provides the dynamic calibration system for the radiopharmaceutical radionuclide activity. The dynamic calibration system comprises a radiopharmaceutical basic data module, a production management system, a warehouse management system and a logistics distribution system, wherein the production management system, the warehouse management system and the logistics distribution system respectively carry out two-way signal transmission with a radiopharmaceutical management module, the system also comprises a radiopharmaceutical radionuclide activity operation module which respectively carries out data transmission with the radiopharmaceutical management module and the radiopharmaceutical basic data module, and the radiopharmaceutical radionuclide activity operation module is respectively connected with a radiopharmaceutical inquiry module and a radiopharmaceutical medicine input / output module. The dynamic calibration system has the advantages that in a nuclear drug production management system, a radionuclide activity automatic calibration module is added, a radionuclide database is built, production materials and products of the radiopharmaceutical are realized, and the activity value at any moment can be inquired through the real-time calculation from initial parameters by inputting a product name or a chemical formula.
Owner:BEIJING ZHIBO BIO MEDICAL TECH +1
Drug production process tracing system based on block chain technology
InactiveCN107767142AReal-time temperature monitoringHumidity real-time monitoringCommerceEngineeringProduction control
The invention relates to a drug production process tracing system based on the block chain technology, which includes a data acquisition unit, an inspection unit, an infrared monitoring unit, an operation monitoring unit, an environment monitoring unit and a data analysis unit. The functions of the units are clear. The materials in different states and other data in the production process can be offered to the drug production management personnel in real time, accurately and quickly. The whole drug production process can be traced.
Owner:SHANGHAI WEILIAN INFORMATION TECH CO LTD
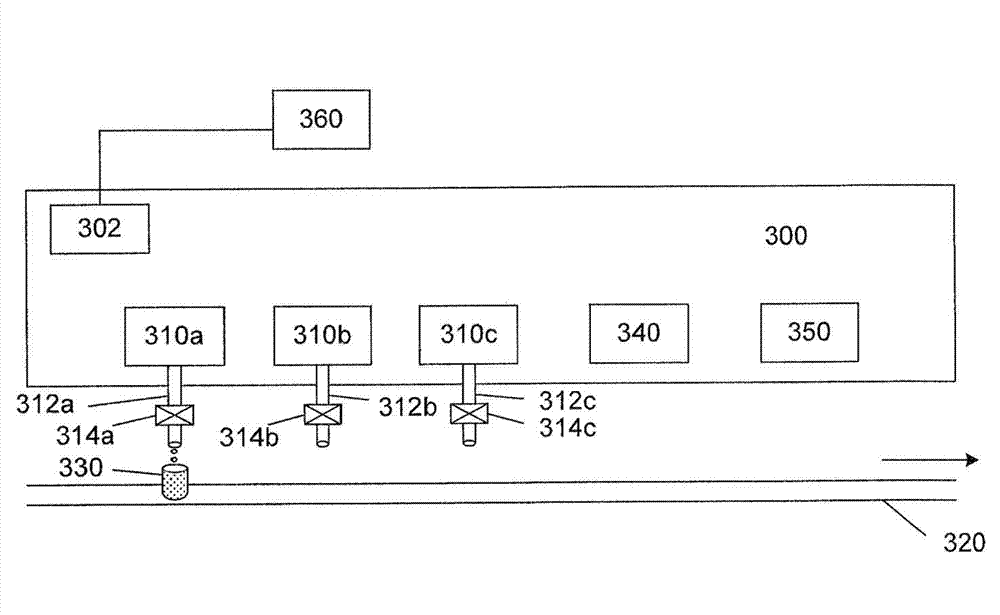
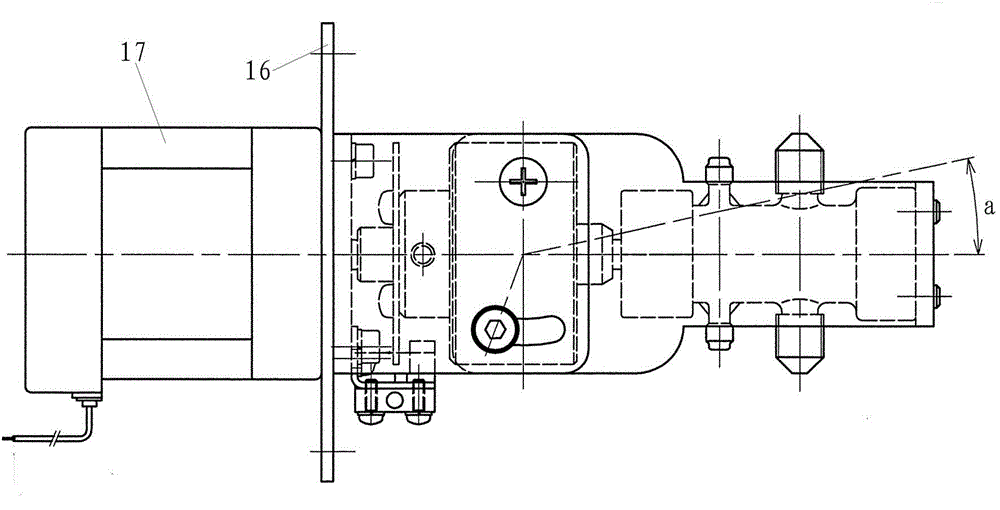
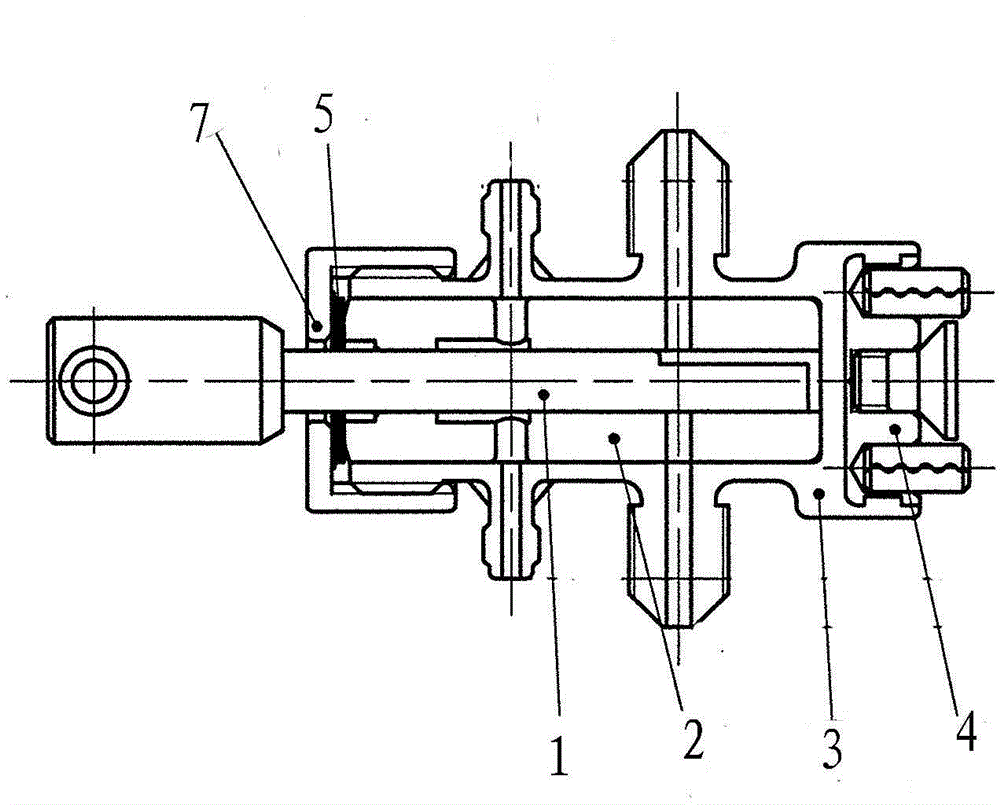
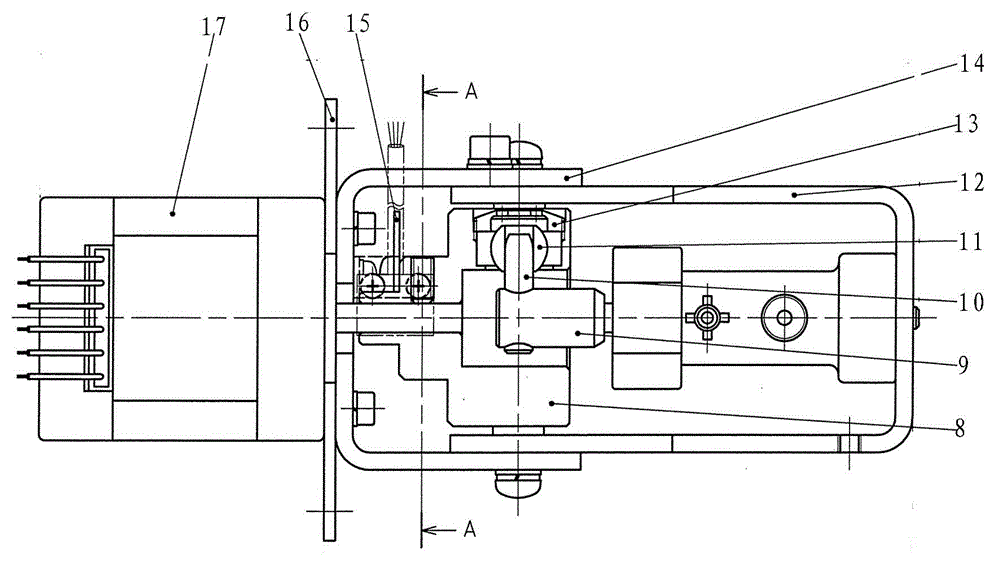
Micro liquid metering ceramic pump for drug production
InactiveCN103557133AEliminates air locksEliminate cloggingPumpsPositive-displacement liquid enginesEngineeringMetering pump
The invention discloses a micro liquid metering ceramic pump for drug production, and belongs to the technical field of metering pumps. The ceramic pump comprises a driving section and a pump head, wherein the center of the driving section is connected with an output shaft of a stepping motor; a self-aligning roller bearing is arranged on an inner side wall of a cavity of the driving section; a cylinder body is arranged in the pump head in a sleeving manner; a liquid inlet and a liquid outlet which are corresponding mutually are formed in the cylinder body; a plunger capable of rotating and sliding relatively is arranged in the cylinder body in a matching manner; an opening is formed at the inner end of the plunger; the outer end of the plunger extends out of the cylinder body; a plunger sleeve is arranged at the outer end of the plunger, and connected with a plunger pin perpendicular to the plunger sleeve; the plunger pin is connected with the self-aligning roller bearing from the inner side of the cavity of the driving section, so that the plunger can be obliquely arranged relative to the driving section; and an included angle a between a central line of the plunger and a central line of the driving section is 0-12 degrees. According to the micro liquid metering ceramic pump for the drug production, a valveless structure effectively solves the common problems of a general metering pump, such as blocking and seizure.
Owner:SICHUAN EMEISHAN PHARMA
High-purity selexipag
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST
Preparation method for high purity L-calcium levofolinate
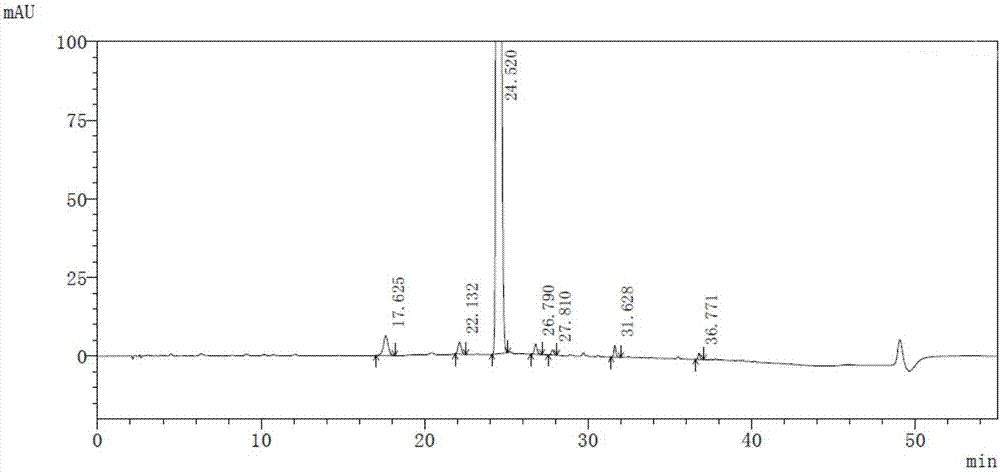
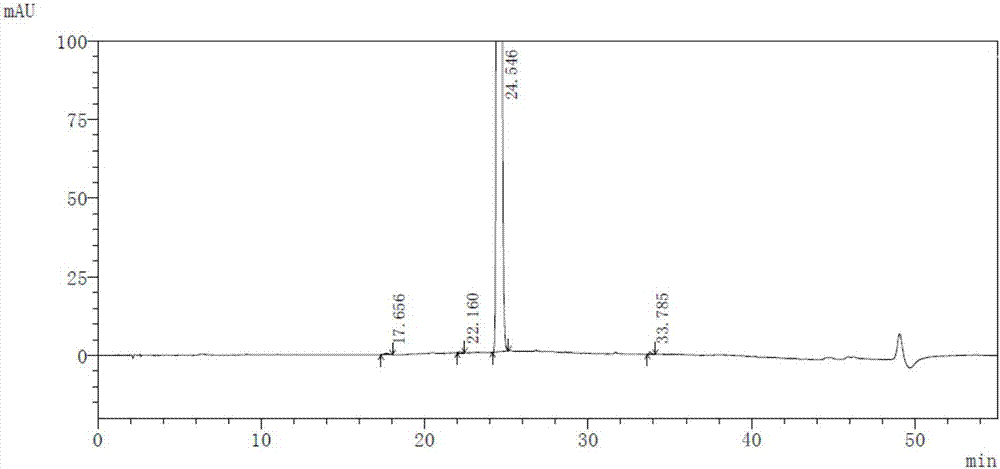
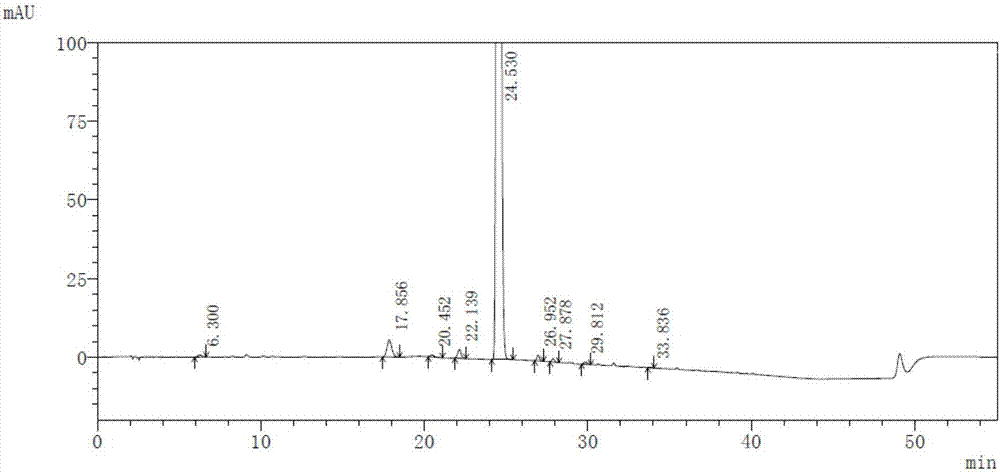
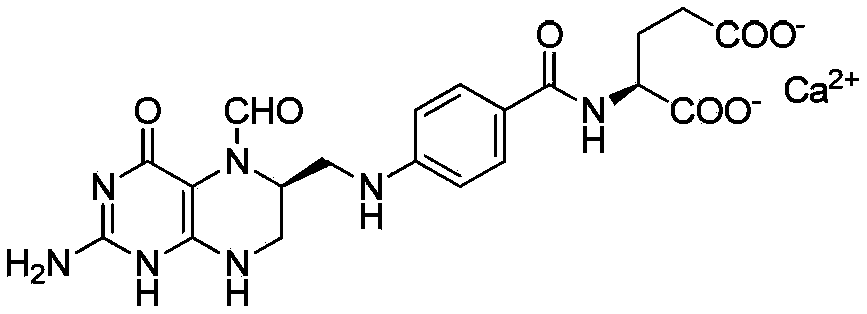
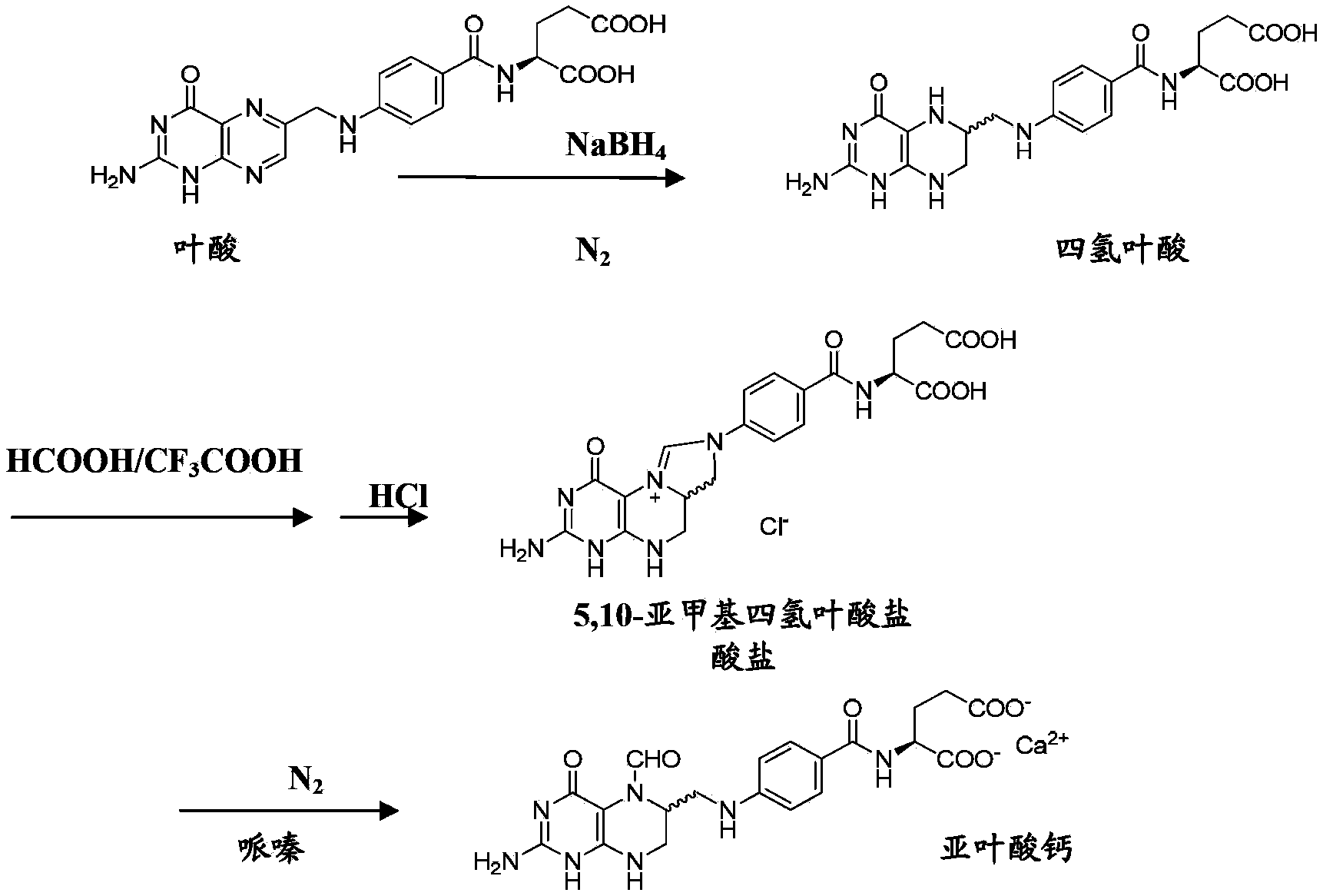
InactiveCN104045640AAvoid distillationLower requirementOrganic chemistryAntipyreticCalcium levofolinateActive ingredient
The invention provides a preparation method for high purity L-calcium levofolinate. With folic acid as a starting raw material, the method relates to reduction, formylation, intermediate recrystallization splitting, hydrolysis ring opening, recrystallization refining and other steps. The L-calcium levofolinate prepared by the invention has a color ranging from white to light yellow, optical purity of not less than 98.5%, in related substance inspection, the total impurities are not greater than 1% and a single impurity is not greater than 0.3%. The preparation method for high purity L-calcium levofolinate provided by the invention has the advantages of simple operation, short process period and high product quality, and can be used for industrialized production of related bulk drugs by drug production enterprises.
Owner:天津康鸿医药科技发展有限公司
Fosaprepitant dimeglumine freeze-dried powder and preparation method thereof
InactiveCN103565760AShorten freeze-drying timeReduce manufacturing costPowder deliveryOrganic active ingredientsFreeze-dryingFosaprepitant dimeglumine
The invention discloses fosaprepitant dimeglumine freeze-dried powder and a preparation method thereof, and relates to the technical field of drugs and drug production. The freeze-dried powder injection contains fosaprepitant dimeglumine, lactose, polysorbate 80 and ethanol water solution. The freeze-dried powder adopts the ethanol water solution as freeze-dried preparation solvent, so that the freeze-dried time is largely shortened, the energy consumption is decreased, and the production cost is reduced.
Owner:NANJING CORE TECH CO LTD
Vegetable insecticide
InactiveCN105941498ASimple processEasy to operateBiocideDead animal preservationMedicinal herbsContinuous use
The invention discloses a vegetable insecticide and relates to the field of agricultural drug production. The vegetable insecticide comprises, by weight, 8-12 parts of sophora flavescens, 15-25 parts of pepper leaves, 10-25 parts of tobacco leaves, 20-35 parts of argy wormwood, 15-30 parts of oleander leaves, 15-20 parts of pericarpium citri reticulatae, 15-25 parts of radix glycyrrhizae and 5-8 parts of spreader. The vegetable insecticide is simple in process, convenient to operate, capable of realizing broad-spectrum insect killing, strong in contact killing, safe and reliable to use, nonhazardous to human and livestock, free of residue, free of environmental pollution and reliable in efficacy, Chinese medicinal herbs resources are fully excavated and utilized, production cost is lowered greatly, insects do not produce antibodies, and continuous use can be realized.
Owner:HENAN HDF CHEM CO LTD
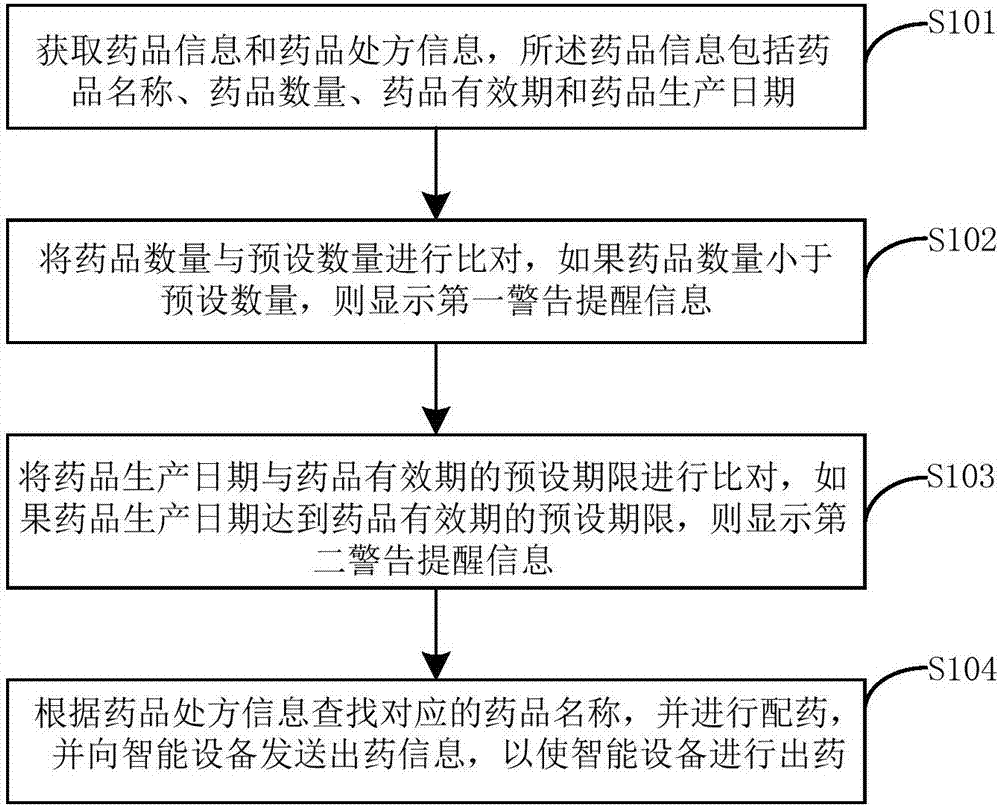
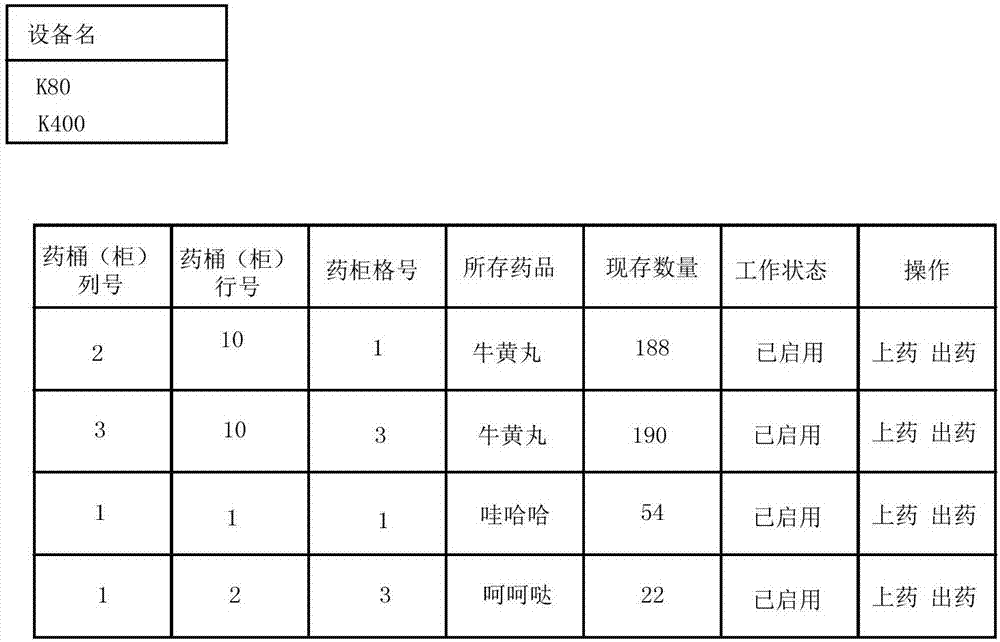
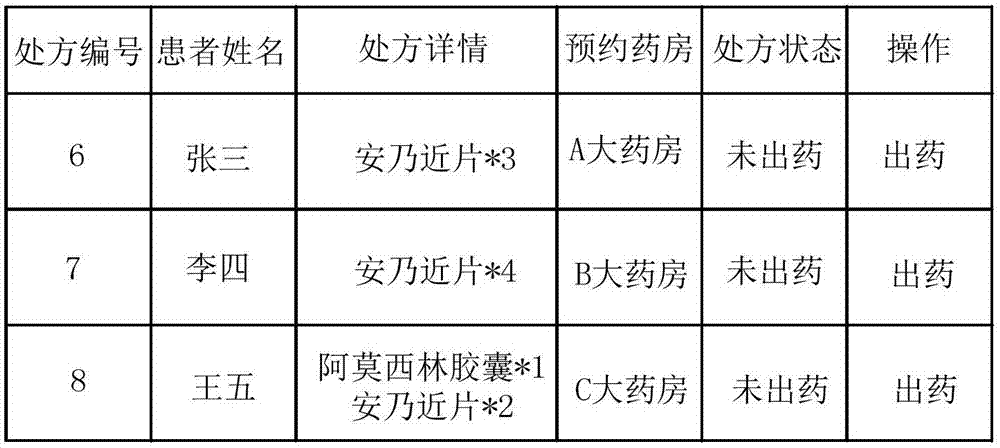
Intelligent drugstore management method and system
InactiveCN107358044AEffective monitoringEfficient managementAlarmsSpecial data processing applicationsDrug productDrugs prescriptions
The invention provides an intelligent drugstore management method and system. The method comprises the steps of obtaining drug information and drug prescription information, wherein the drug information comprises a drug name, a drug quantity, a drug validity date and a drug production date; comparing the drug quantity with a preset quantity, and if the drug quantity is smaller than the preset quantity, displaying first warning prompt information; comparing the drug production date with a preset deadline of the drug validity date, and if the drug production date reaches the preset deadline of the drug validity date, displaying second warning prompt information; and searching for the corresponding drug name according to the drug prescription information, performing drug dispensation, and sending drug output information to an intelligent device, thereby enabling the intelligent device to perform drug output. Therefore, drugs can be effectively monitored and managed.
Owner:SHANGHAI VIEW VALLEY TECH
Method and system for simulating and modeling a batch manufacturing facility
A system and method for the simulation and modeling of biopharmaceutical batch process manufacturing facilities using process time lines is described herein. The system employs an eleven-field delimited string code which specifies the unit identifier code and the iteration value for each of the ten levels of nested scheduling cycles of the biopharmaceutical drug production process being modeled. The method includes generating a process time line using operational parameters, a block flow diagram, and a set of scheduling cycles for each of a sequence of unit operations. The process time line is used as a tool for batch processing and facility design.
Owner:APPLIED PROCESS TECH
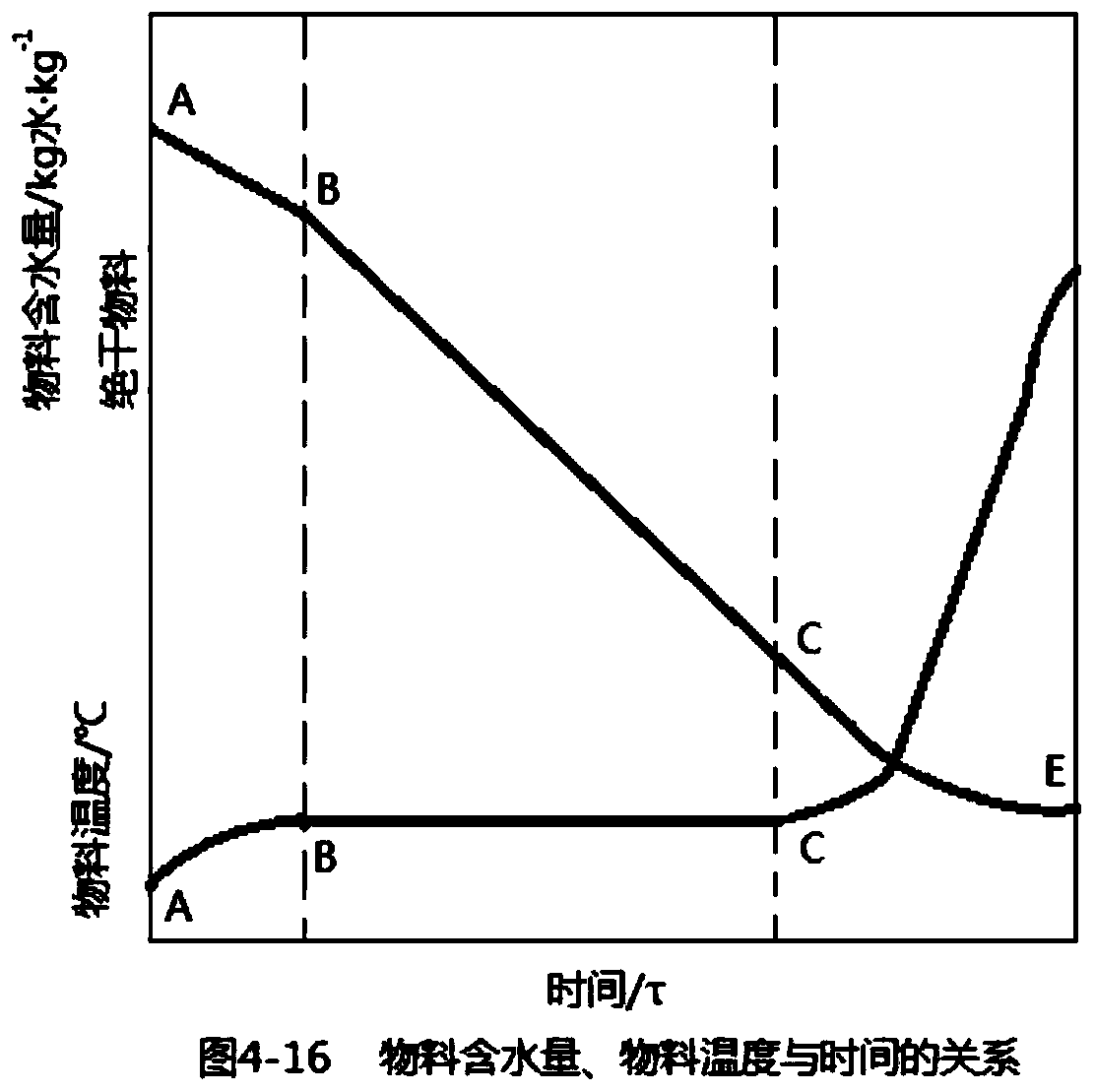
Granulating equipment for preparing hydrotalcite chewable tablets and production process of hydrotalcite chewable tablets
ActiveCN110131965ALow costAccurate detectionDrying solid materials with heatMaterial analysis by observing effect on chemical indicatorModern medicineBiochemical engineering
The invention discloses an ebullated dryer for preparing hydrotalcite chewable tablets and a production process of hydrotalcite chewable tablets. The production process of the hydrotalcite chewable tablets comprises the following steps of weighing, mixing, wet granulation, drying and tabletting. The production process of the hydrotalcite chewable tablets is simple and convenient, and has simple operation. The ebullated dryer for preparing the hydrotalcite chewable tablets has the function of automatically controlling a drying endpoint, is simple in device, low in cost, accurate in detection and high in automation degree, and meets the requirement of modern drug production on automation.
Owner:TEYI PHARMACEUTICAL GROUP CO LTD
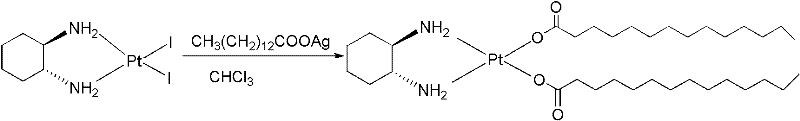
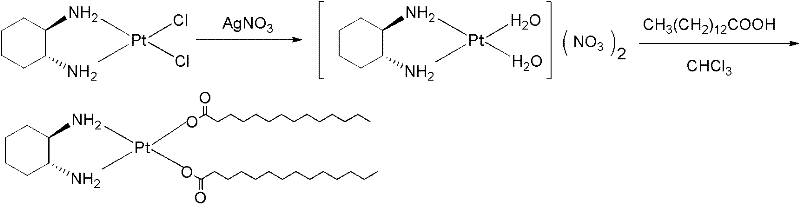
Method for purifying platinum
The invention relates to a method for purifying platinum of an antitumor drug, wherein the process comprises: platinum is dissolved in a mixed solution of n butanol and anhydrous ethyl alcohol, an insoluble substance is filtered, and a proper amount of deionized water is added in the solution for obtaining a platinum elaboration. When the purification method of the present invention is used for purifying platinum crude products with the content of 80 - 95%, the purity of platinum crude products can be raised more than 98%. The purified platinum products can be directly used for the corresponding drugs production.
Owner:KUNMING GUIYAN PHARMA
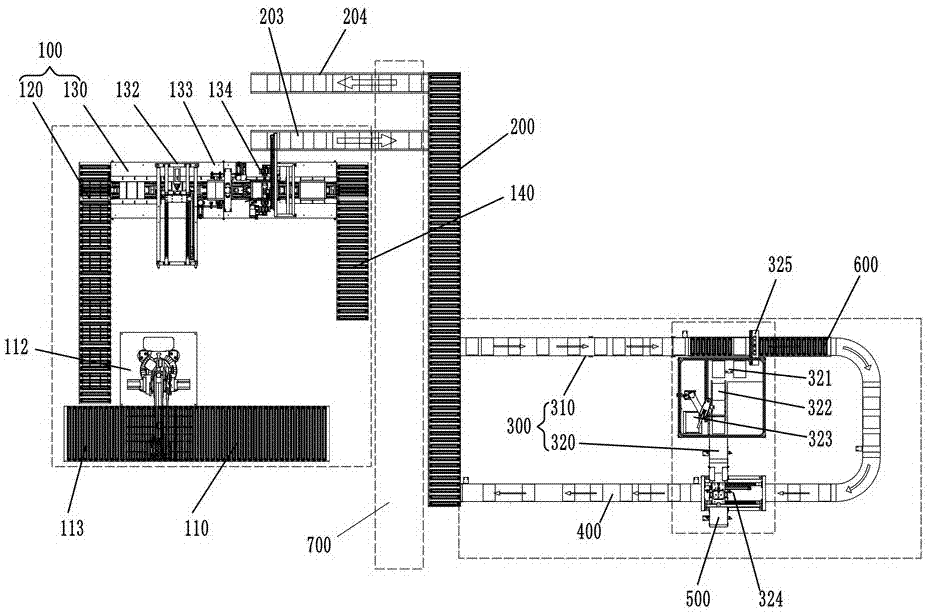
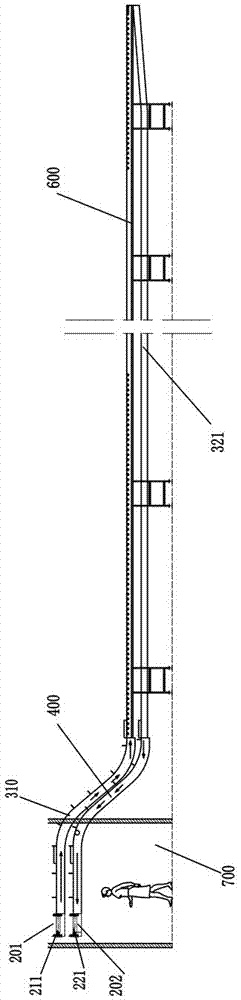
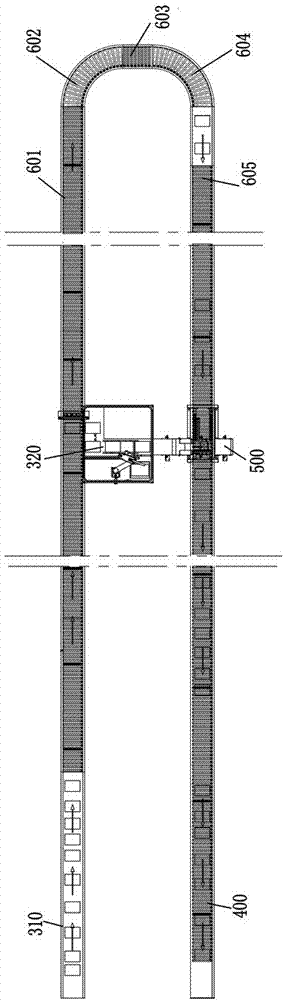
Logistics conveying system
ActiveCN107284968AImprove space utilizationImprove automationMechanical conveyorsLogistics managementButt joint
The invention discloses a logistics conveying system. The logistics conveying system comprises a first conveying rail, a transition conveying rail, a second conveying rail and a packing material recycling rail; the first conveying rail is arranged on one side of the transition conveying rail, and the second conveying rail and the packing material recycling rail are arranged on the other side of the transition conveying rail; the transition conveying rail comprises an upper-layer conveying rail and a lower-layer conveying rail which are vertically arranged in parallel, the first conveying rail is connected with the upper-layer conveying rail in a butt-joint manner, the second conveying rail is connected with the upper-layer conveying rail in a butt-joint manner, and the packing material recycling rail is connected with the lower-layer conveying rail in a butt-joint manner; the first conveying rail is provided with unboxing stations, the second conveying rail is provided with unpacking stations, and the packing material recycling rail is used for receiving packing materials unpacked on the unpacking stations. The logistics conveying system has the advantages that layout is reasonable, the structure is compact, the space utilization rate of a plant area is increased, and automation of a drug production line can be achieved.
Owner:TRUKING TECH LTD
Informationized intelligent control and management type drug production system
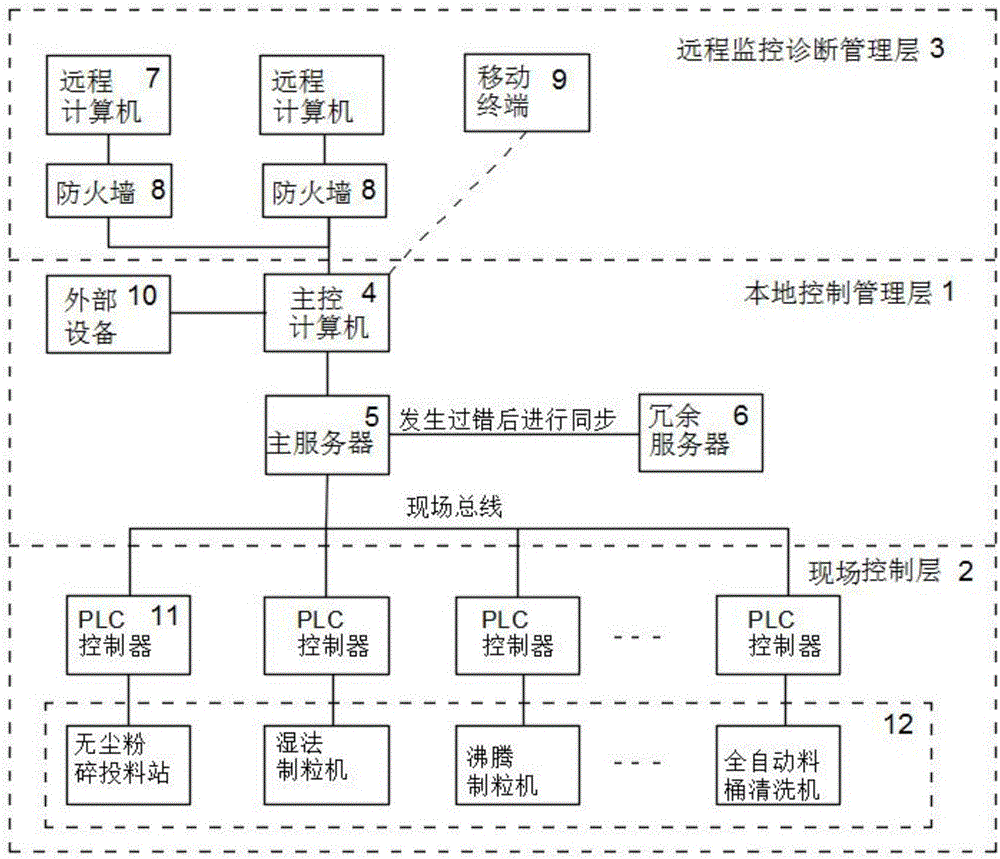
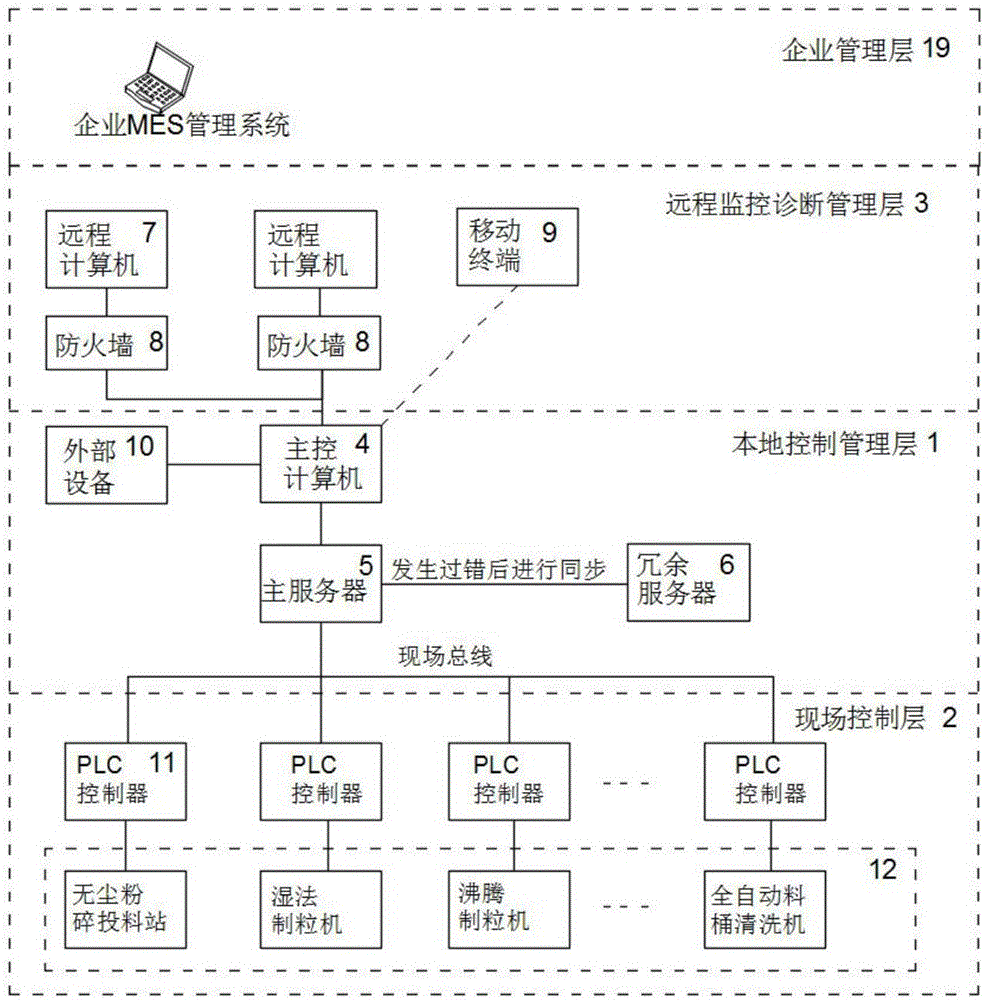
InactiveCN106527377AAvoid wastingOperation coordinationTotal factory controlProgramme total factory controlInformatizationControl layer
The invention discloses an informationized intelligent control and management type drug production system, wherein the in-place, local and / or remote automatic monitoring and management are realized. The system comprises a field production device and a corresponding management control system. The management control system comprises a local control and management layer corresponding to the field production device, a field control layer and a remote monitoring management layer, wherein the field control layer and the remote monitoring management layer are respectively connected with the local control and management layer. The system is characterized in that the overall management and control system of the system adopts a Browser / Server structure. The part selection is conducted by a Wincc Web Navigator. In this way, the monitored operation of the field production device and / or the corresponding production process is realized through applying the WWW browsing technology and inputting a corresponding IP address in an IE browser based on a variety of scripting languages and active techniques of the browser.
Owner:YICHUN WANSHEN PHARMA MACHINERY
Preparation method of porous carbon-based composite material
ActiveCN109879266AImprove performanceImprove electrochemical performanceHybrid capacitor electrodesCell electrodesComposite electrodePorous carbon
The invention discloses a preparation method of a porous carbon-based composite material, and belongs to the field of waste recycling and regeneration. Waste mushroom dregs are recycled, the finally prepared product has excellent performances when used as an electrode material, and can realize industrial application, and activation and compounding are integrally carried out to prepare the uniformcomposite porous carbon-based electrode material with excellent electrochemical performances; an alkaline solution is adopted to activate and dissolve a mushroom dreg suspension into a homogeneous solution, an electrochemically active material or its precursor is added, a compounding process is carried out, and curing and heat treatment processes are carried out to finally construct the electrochemically-active porous carbon-based composite electrode material; and the preparation method has the advantages of realization of harmlessness and recycling hazardous wastes in the drug production process, simple process flow, and low cost, and the obtained composite material has the advantages of good conductivity, high capacity and excellent cycle performances when used as an electrode material for energy storage devices, and is suitable for large-scale production.
Owner:CENT SOUTH UNIV
High-efficiency wet straightening granulator
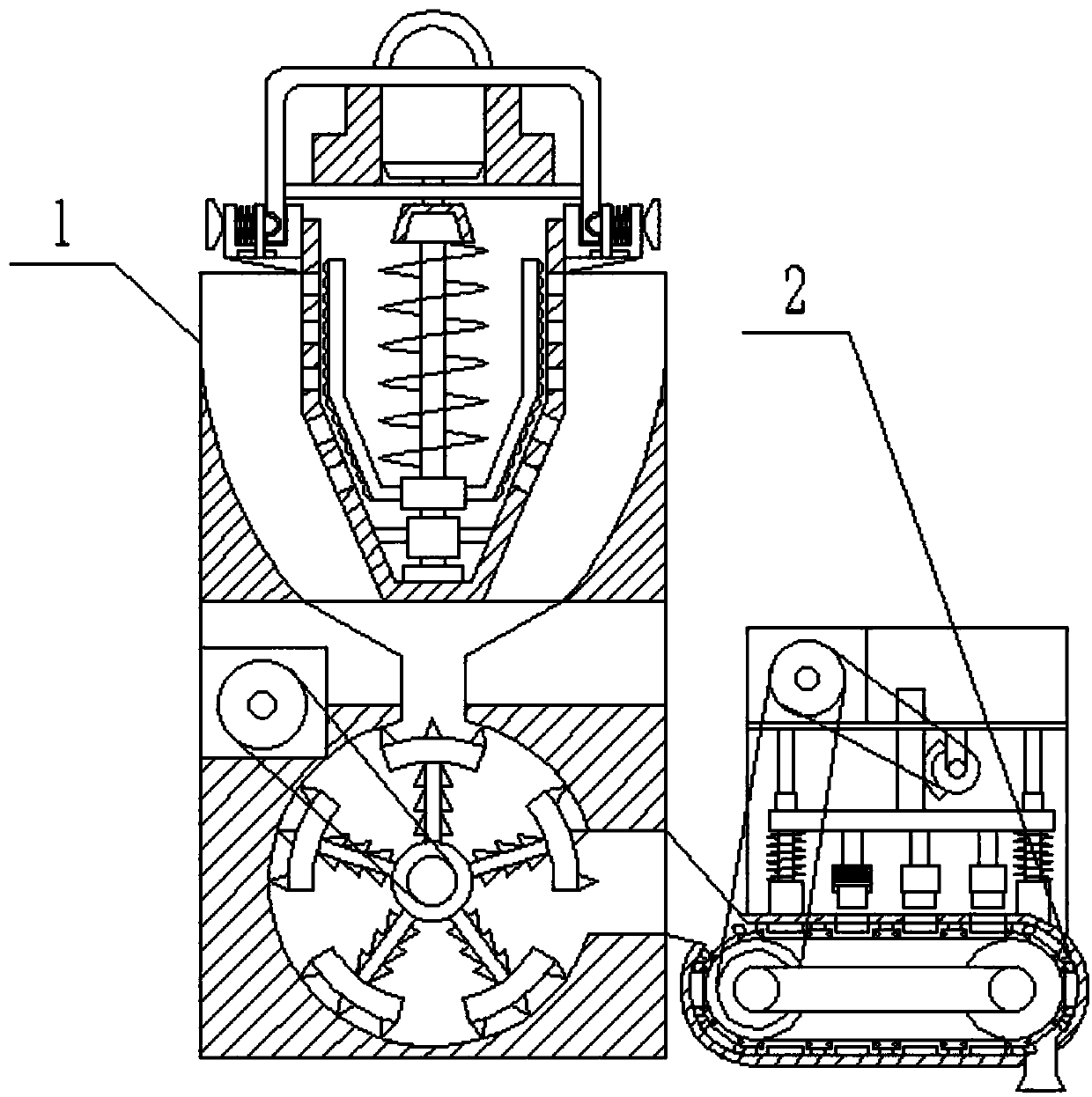
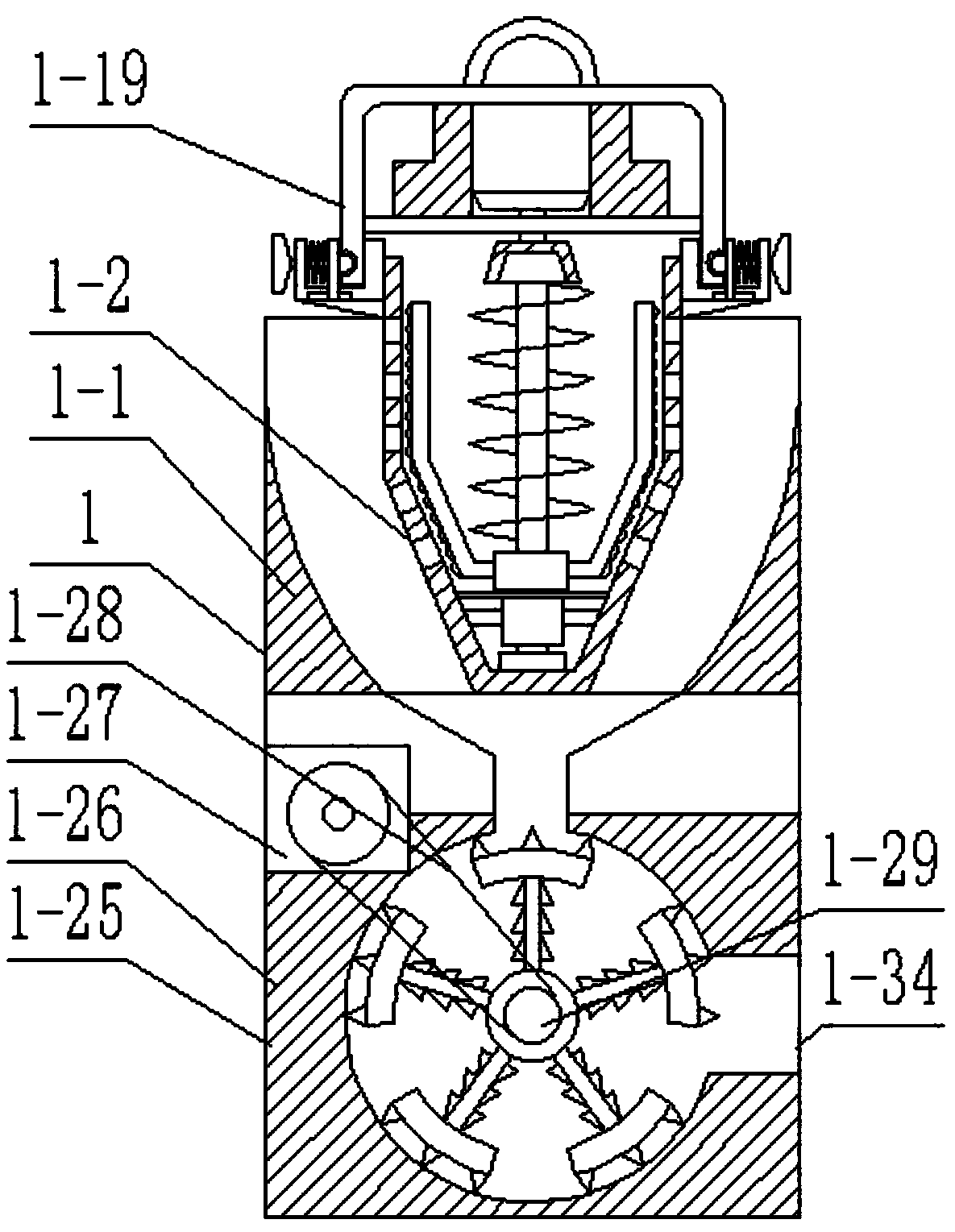
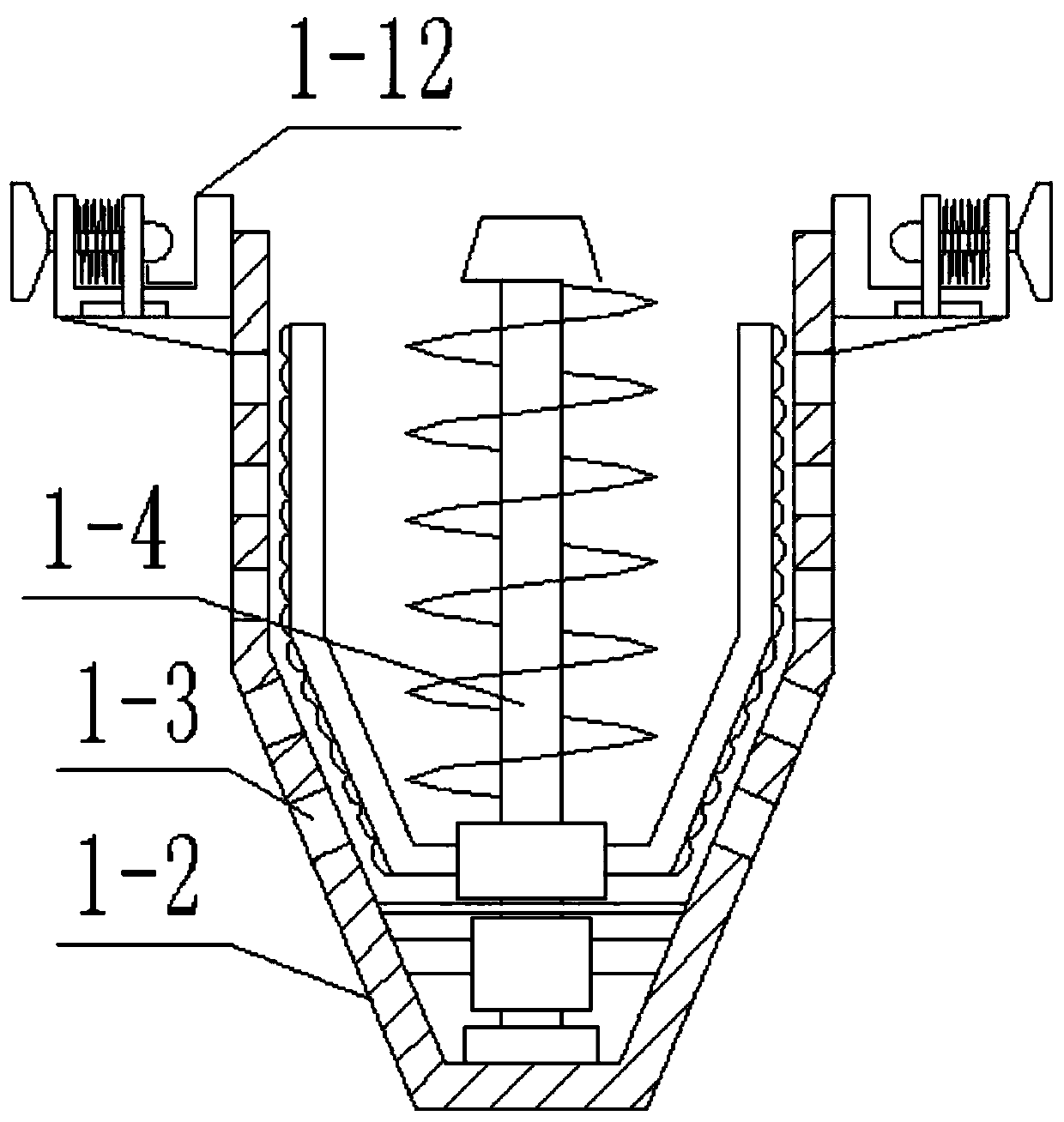
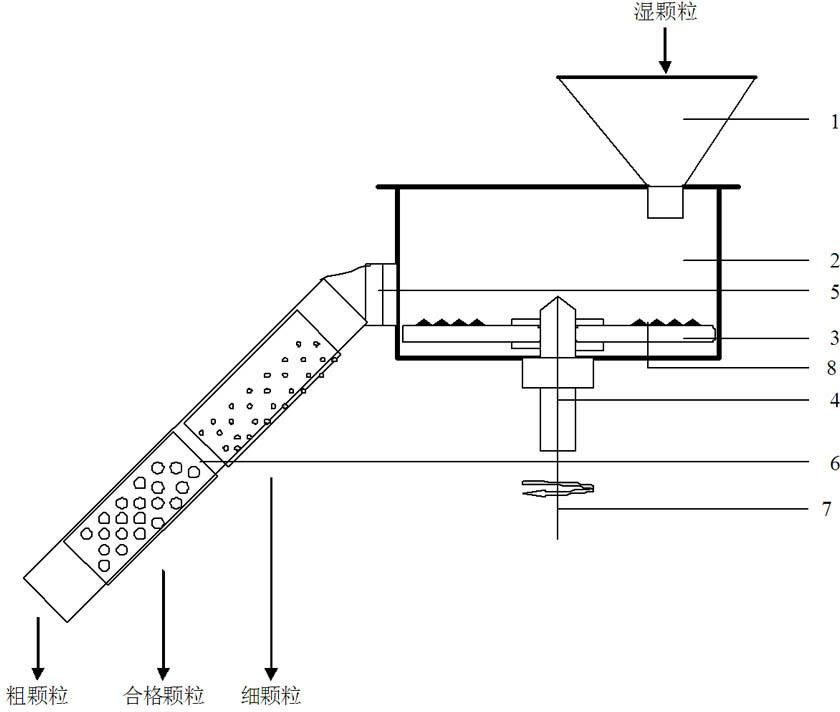
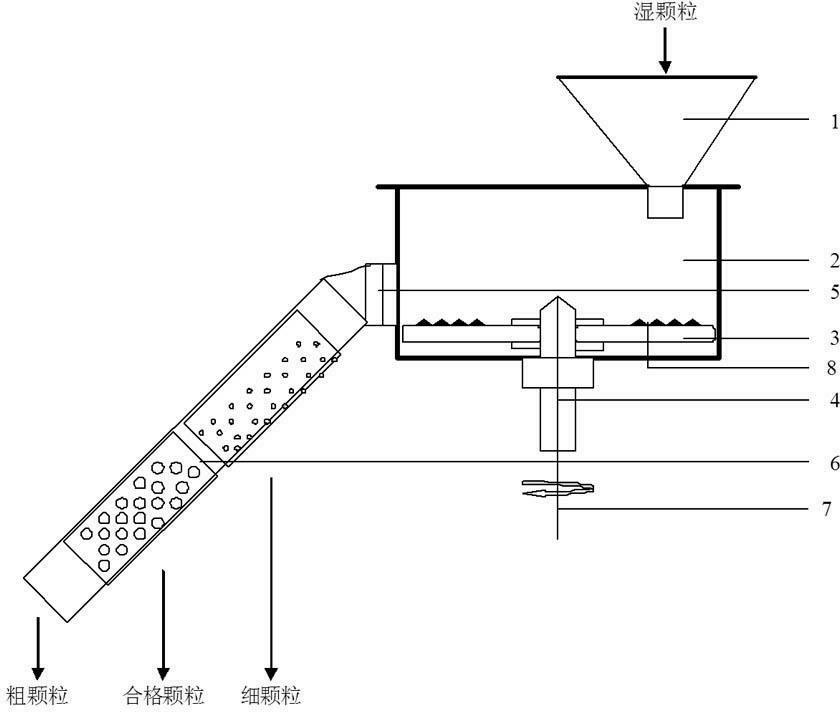
InactiveCN102688795AReduce contact surfaceAvoid sticking to each otherSievingScreeningMotor driveEngineering
The invention provides a high-efficiency wet straightening granulator, and relates to drug production equipment, in particular to a wet straightening granulator for producing granular medicines. The high-efficiency wet straightening granulator consists of a hopper, a cylinder body, a saw tooth turntable, a rotating shaft, a discharge valve, a screening pipeline and a motor, wherein the hopper is arranged at one side of the upper part of the cylinder body, the bottom end of the other side of the cylinder body is connected with the discharge valve, the discharge valve is connected with the screening pipeline, screening holes on the screening pipeline are sequentially arranged from small to large, one end with the small screening holes is arranged on the top end of the screening pipeline, the saw tooth turntable is arranged at the bottom in the cylinder body, the rotating shaft is connected with the motor, the motor drives the rotating shaft to rotate, and the rotating shaft drives the saw tooth turntable to rotate. The high-efficiency wet straightening granulator provided by the invention is very short in time for straightening granules, i.e. one granule straightening step can be completed within 3-5min generally, and is very high in efficiency.
Owner:张永胜
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com