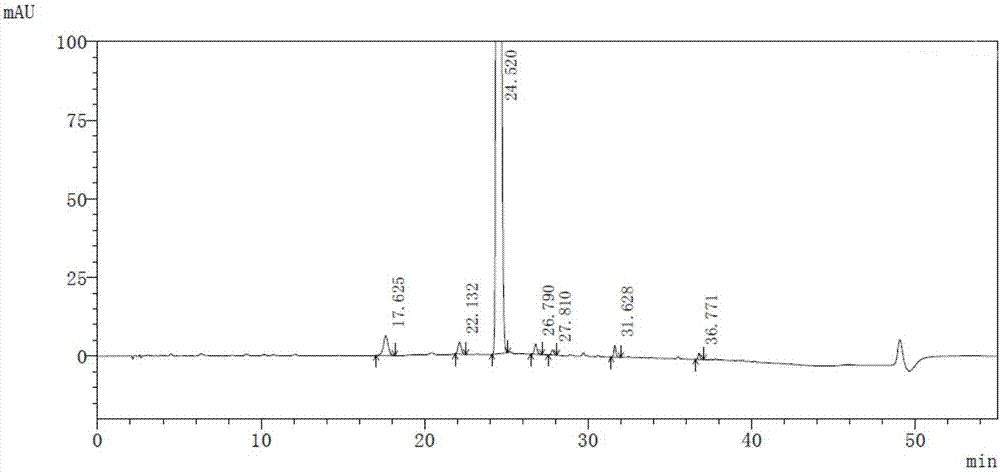
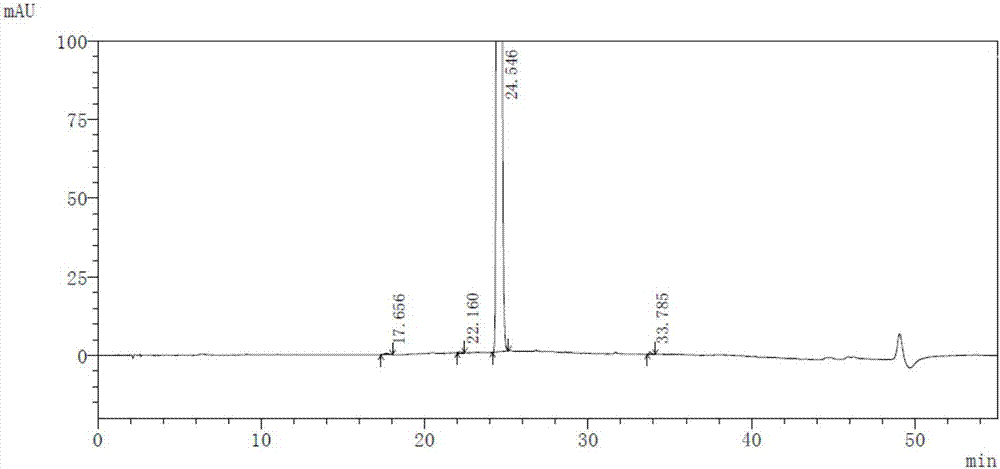
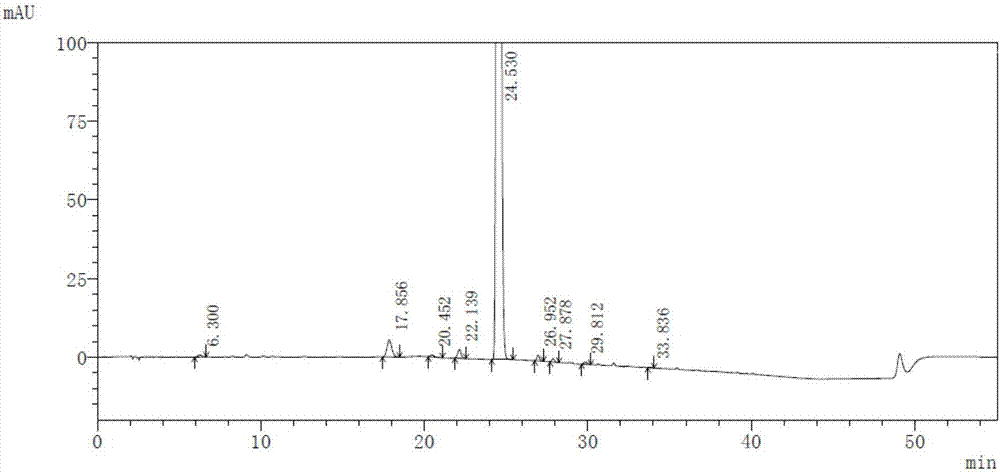
High-purity selexipag
A high-purity technology of Selexipah, which is applied in the field of high-efficiency preparation of prostacyclin receptor agonist Selexipah, achieves the effect of low cost, high purity and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Add 5-bromo-2,3-diphenylpyrazine (100g, 0.32mol), 4-isopropylamino-1-butanol (127g, 0.96mol) and potassium iodide (15.9g, 0.096mol) in the reaction flask , heated to 150°C for 16 hours. After the system was cooled to room temperature, ethyl acetate (800 mL) was added, washed with water (800 mL×2), dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated under reduced pressure, and the residue was washed with dichloromethane / heptane (1.2 L, v / v =1:5) recrystallized to obtain 4-((5,6-diphenylpyrazin-2-yl)-(isopropyl)amino)-1-butanol (75.5 g, yield: 65.3%) .
[0053] 1 H NMR (400MHz, CDCl 3 )δ8.01 (s, 1H), 7.45 (d, J = 5.8Hz, 2H), 7.35 (d, J = 6.8Hz, 2H), 7.28–7.21 (m, 6H), 4.79 (hept, J = 6.6 Hz,1H),3.68(t,J=6.3Hz,2H),3.47–3.38(m,2H),1.86(s,1H),1.80–1.72(m,2H),1.69–1.58(m,2H) ,1.27(d,J=6.7Hz,6H).
[0054] 13 C NMR (100MHz, CDCl 3 )δ151.6, 149.0, 139.6, 139.5, 139.0, 129.8, 129.3, 128.1, 128.0, 127.9, 127.1, 126.9, 62.4, 46.2, 42.2, 30.0, 25.7, ...
Embodiment 2
[0056] Add 4-((5,6-diphenylpyrazin-2-yl)-isopropylamino)-1-butanol (72.3g, 0.20mol), toluene (200mL), tetrabutylsulfuric acid in the reaction flask Ammonium hydrogen (33.9g, 0.10mol) and 40% potassium hydroxide solution (150mL, w%) were added dropwise with tert-butyl bromoacetate (78.0g, 0.40mol) under an ice-water bath, and the reaction was continued for 20 hours after dropping . Add concentrated hydrochloric acid dropwise to adjust the pH of the system to 5-6, extract with ethyl acetate (500mL), wash with water (500mL×2), and concentrate under reduced pressure to obtain 2-(4-((5,6-diphenylpyrazinyl ) (isopropyl) amino) butoxy) tert-butyl acetate crude product, directly carry out next step reaction.
Embodiment 3
[0058] Tetrahydrofuran (300 mL) and 10% sodium hydroxide solution (300 mL, w%) were added to the crude product obtained in Example 2 above, heated to reflux, and the reaction was complete as detected by TLC. Concentrate under reduced pressure to remove tetrahydrofuran, extract the aqueous phase with methyl tert-butyl ether (300mL×2), then adjust the pH to 2-3 with 1N hydrochloric acid, extract with ethyl acetate (800mL), concentrate under reduced pressure, and wash the residue with acetic acid Ethyl ester (500 mL) was recrystallized to give 2-(4-((5,6-diphenylpyrazin-2-yl)(isopropyl)amino)butoxy)acetic acid (S-I) (55.8 g, two steps Yield: 66.5%).
[0059] 1 H NMR (400MHz, CDCl 3 )δ8.10(s,1H),7.42(dd,J=7.6,1.8Hz,2H),7.32(dd,J=7.4,2.1Hz,2H),7.27–7.21(m,6H),4.85(hept ,J=6.6Hz,1H),4.07(s,2H),3.61(t,J=6.0Hz,2H),3.48–3.39(m,2H),1.81–1.70(m,4H),1.26(d, J=6.7Hz,6H).
[0060] 13 C NMR (400MHz, CDCl 3 )δ173.3, 152.0, 149.6, 139.4, 138.8, 138.4, 129.9, 129.5, 128.1, 128.0, 127.9, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com