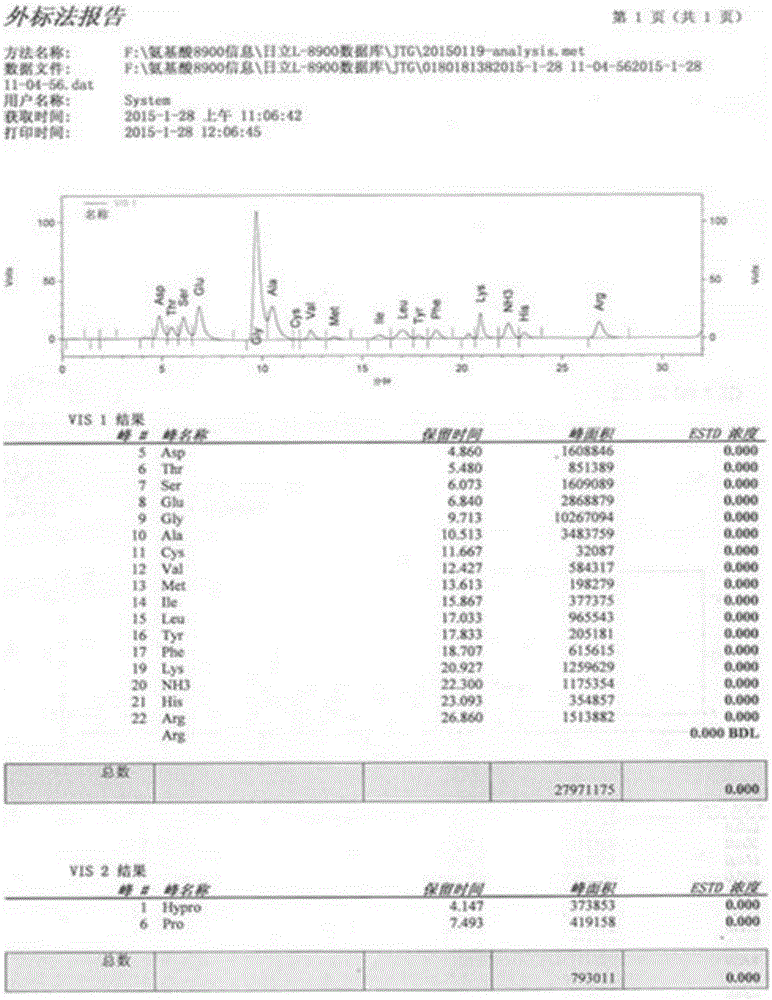
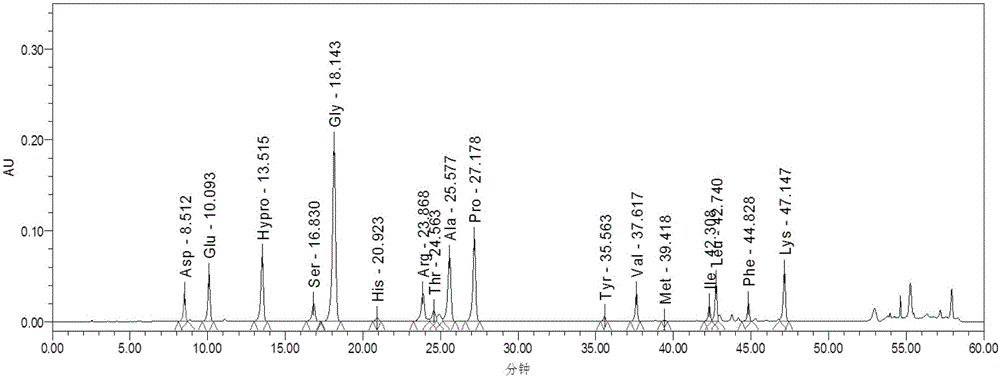
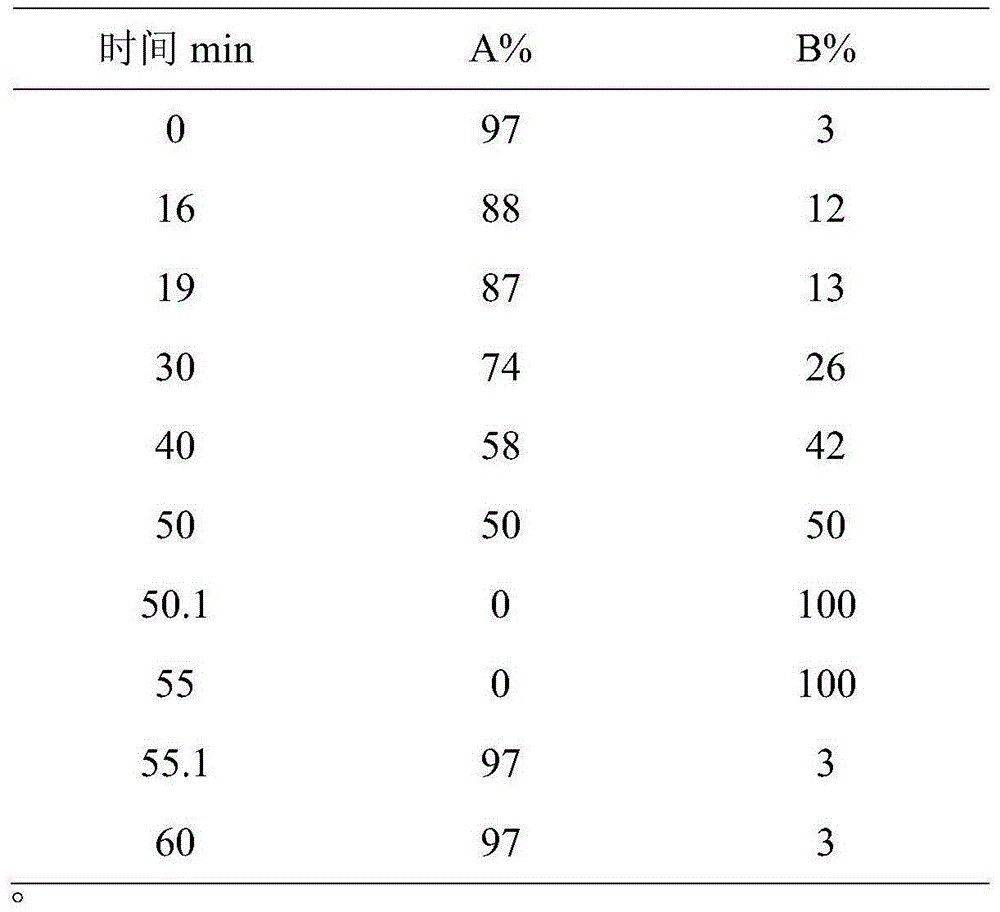
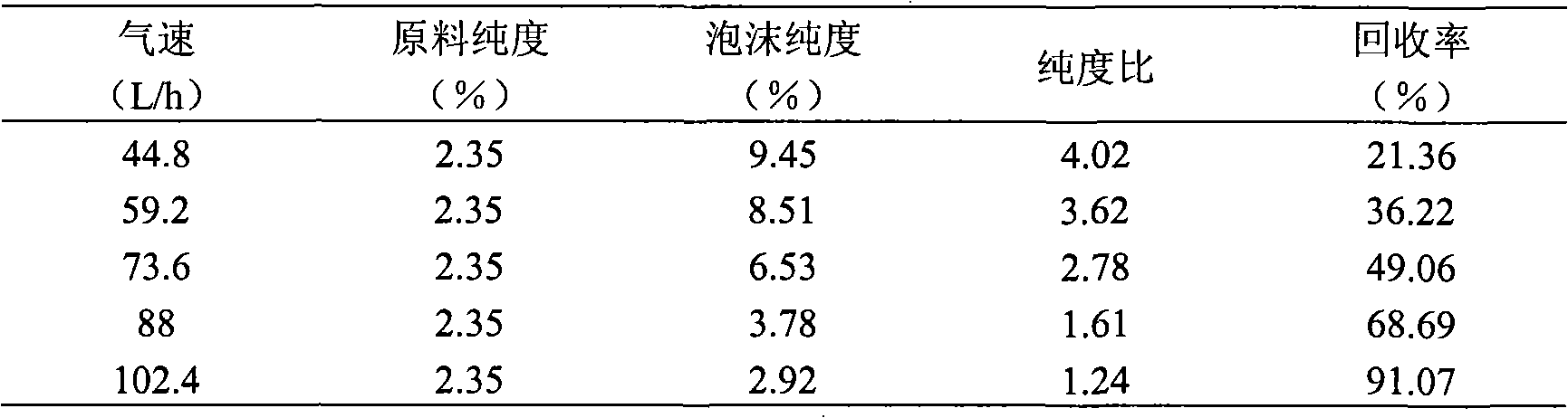
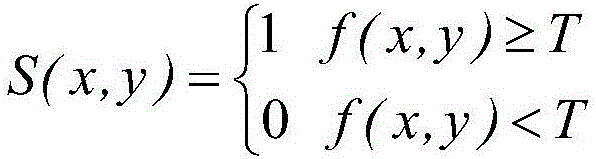Patents
Literature
320 results about "Drug quality" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The Office of New Drug Quality Assessment (ONDQA) assesses the critical quality attributes and manufacturing processes of new drugs, establishes quality standards to assure safety and efficacy ...
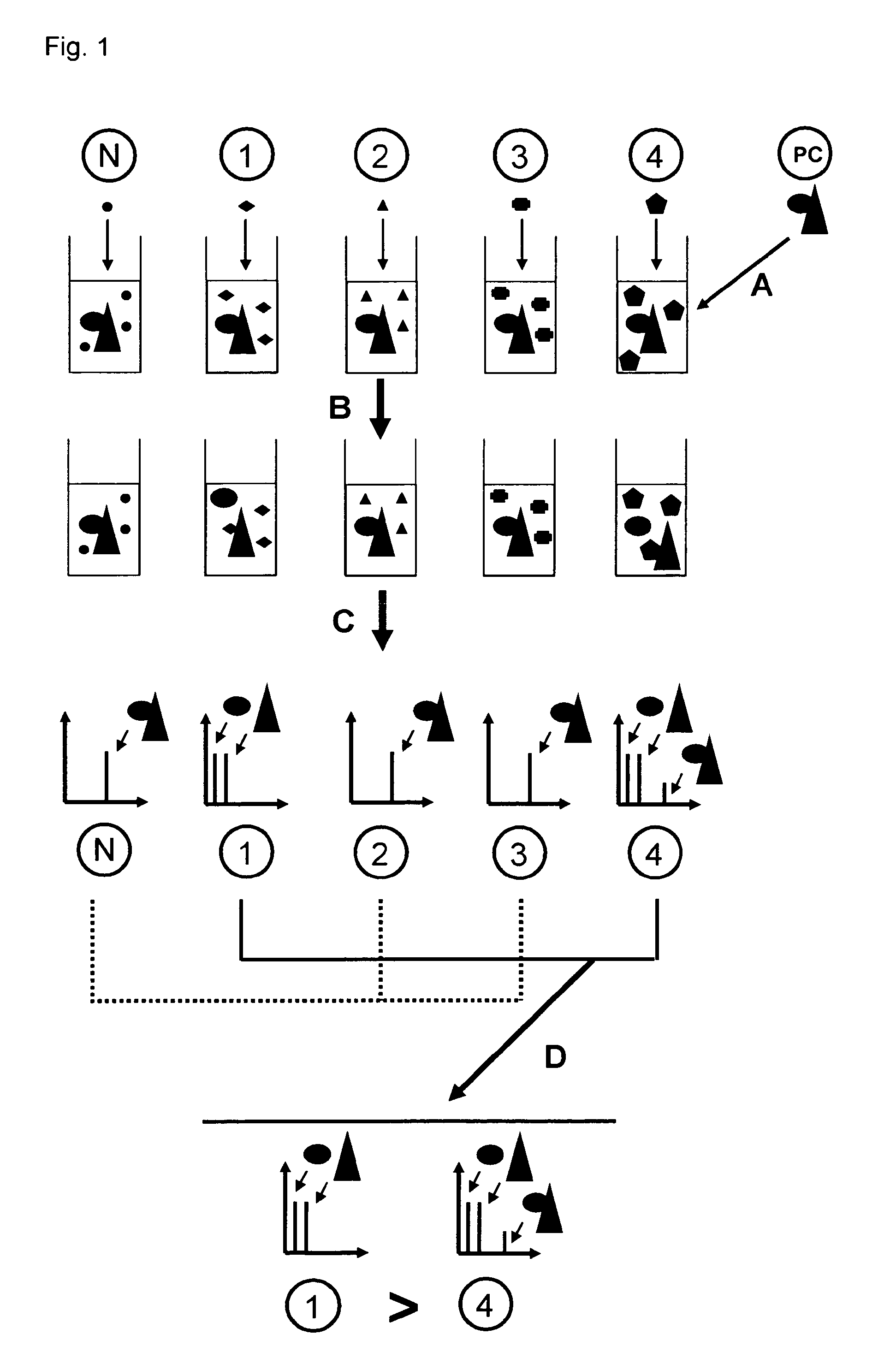
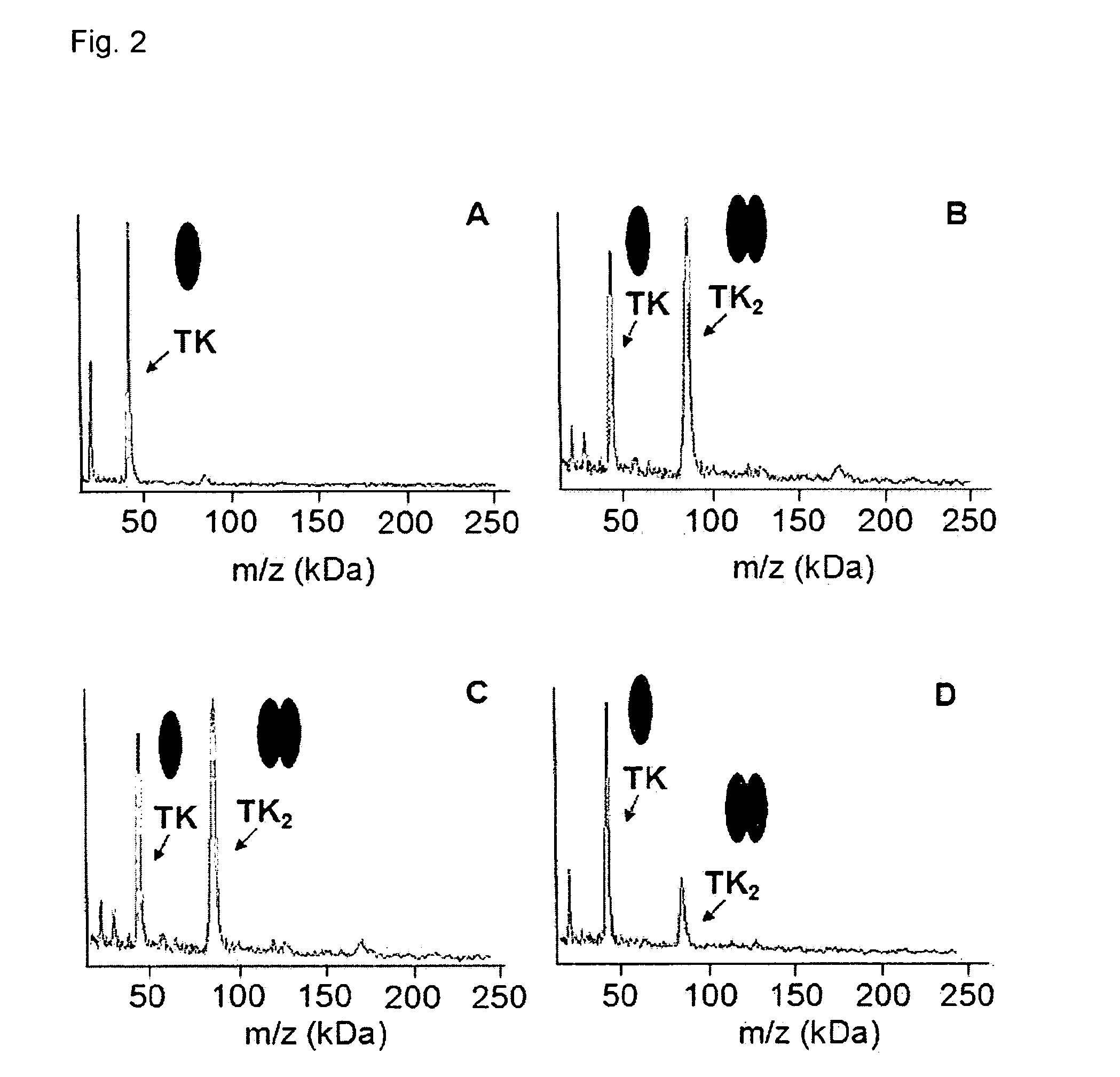
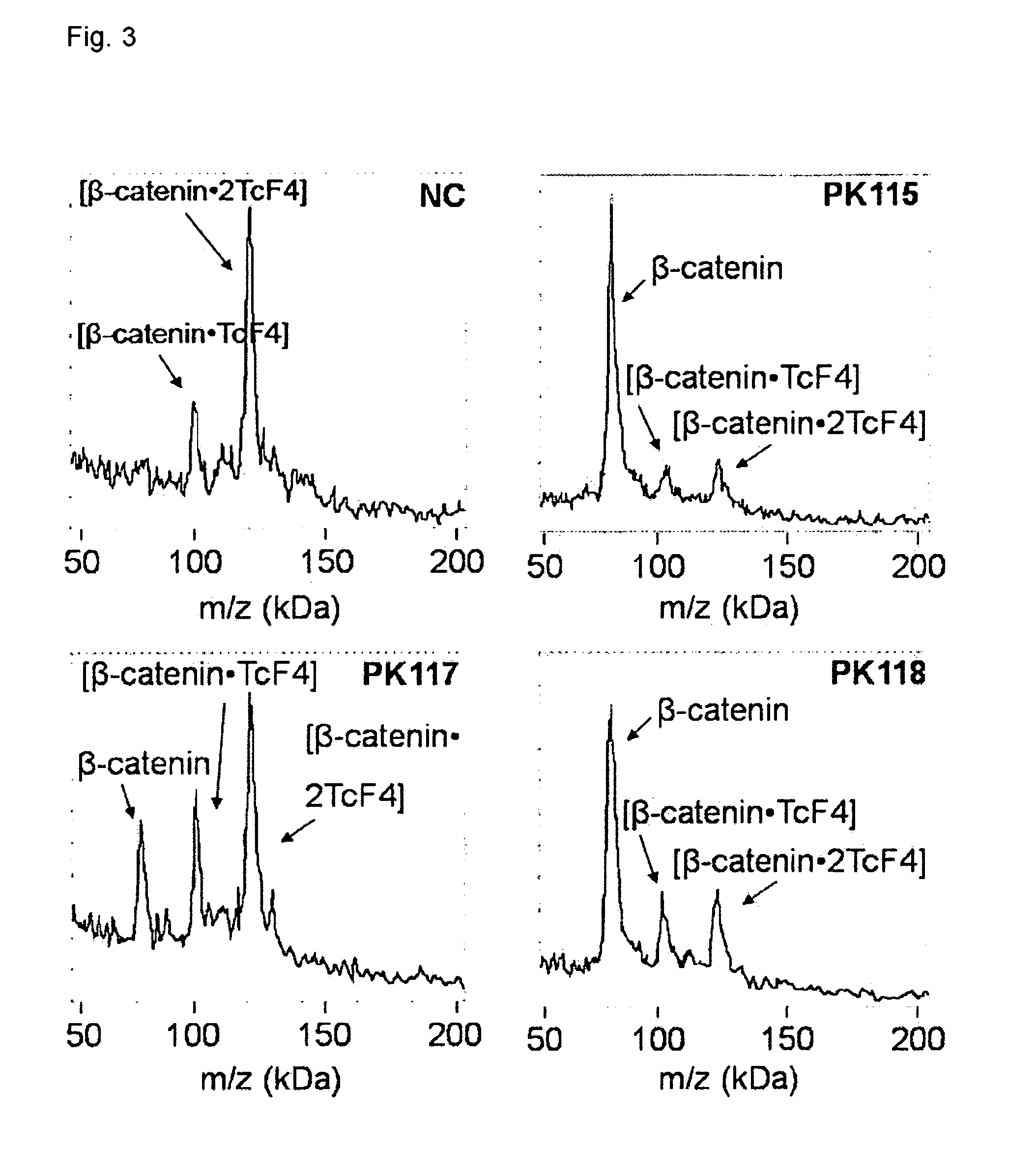
Direct mass spectrometric analysis of drug candidates targeting protein complexes
ActiveUS20100099200A1Improve throughputEasy to detectBioreactor/fermenter combinationsBiological substance pretreatmentsProtein targetProtein-protein complex
The invention relates to a method of using high mass matrix assisted laser desorption-ionization (MALDI) mass spectrometry for the qualitative and quantitative analysis of the effect of drug candidates on protein complexes such as protein-protein interactions in purified samples or complex biological matrices, as well as to the use of this method for lead compound optimization, drug characterization, drug manufacturing processes, and drug quality control processes, including automated high throughput applications.
Owner:COVALX
Slow/controlled release pellet composition containing ginkgo leaf extracts and preparation method thereof
InactiveCN101375869ASmall toxicityStable blood concentrationGranular deliveryGinkgophyta medical ingredientsSustained release pelletsHard Capsule
The invention belongs to the field sustained / controlled-release preparations, in particular to an oral sustained / controlled-release pellet combination containing ginkgo biloba extract and a preparation method. The oral sustained / controlled-release pellet combination is composed of (A) a core containing a pill; (B) an insulating coating layer; (C) a sustained-release coating layer; (D) and an enteric-coated coating layer. The invention is the traditional Chinese medicine multi-component sustained-release pellet combination which is taken once by 24 hours and the multi-unit sustained-release pellet combined preparation with the different drug release systems, the core containing the pill is prepared by adopting the extrusion pill rolling method, a novel sustained-release multi-layer coating technology and a fluidized bed are utilized for coating the sustained-release pellet, the rapid-release part and the sustained-release part of the coated pellet are mixedly filled into a hard capsule or pressed into a pellet tablet. The sustained-release pellet has stable coating process and good reproducibility, thereby being applicable to the industrial mass production; and the drug quality of the preparation is stable through the long-term storage. The in vitro release test shows that the multiple components of the traditional Chinese medicine can achieve the sustained-release role, the sustained-release preparation can significantly increase the transmembrane absorption and the stability of various effective active ingredients by oral drug administration, the curve of plasma drug concentration in vivo is smooth, and the design purpose of 24-hour sustained-release is achieved.
Owner:CHINA PHARM UNIV
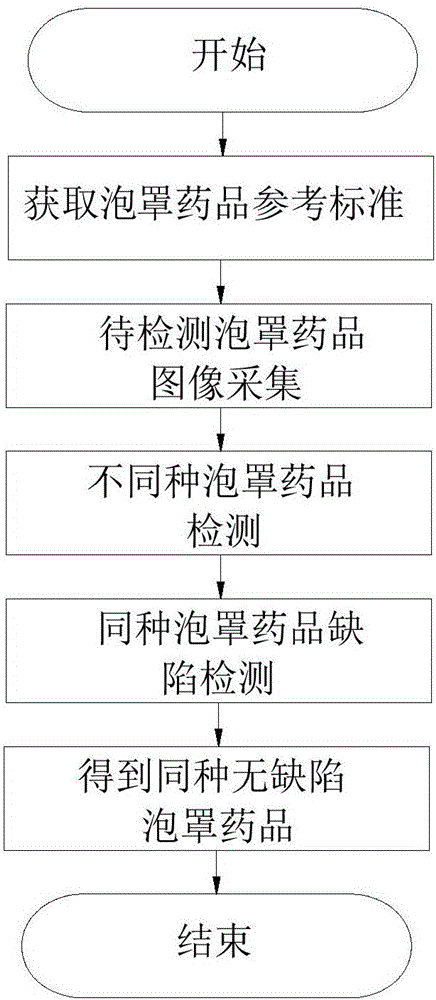
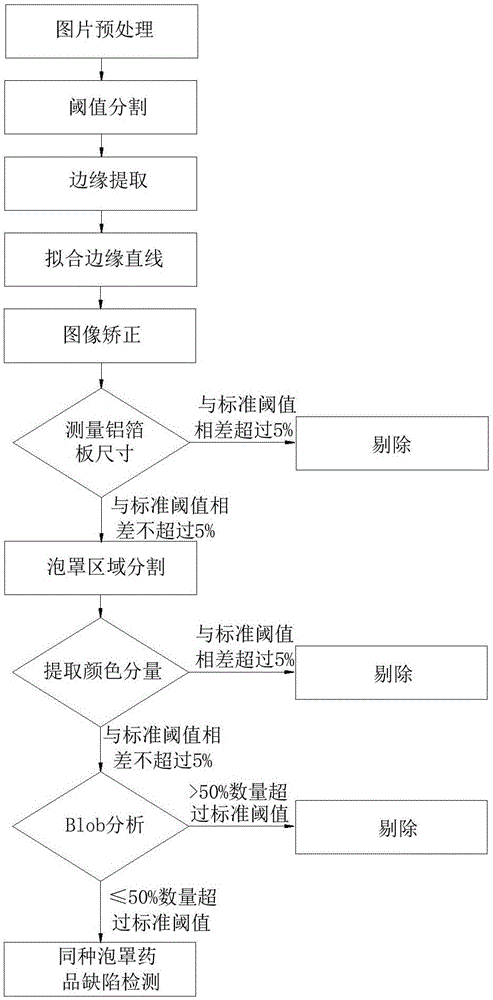
Method for classification and online defect visual detection of blister drugs on production line
InactiveCN107525808AReduce labor intensityNot affected by subjective factorsImage enhancementImage analysisProduction linePattern recognition
The invention relates to a method for classification and online defect visual detection of blister drugs on a production line. The method comprises the following steps: S1, acquiring and storing the reference standards of all characteristics of the blister drugs; S2, acquiring images of the blister drugs to be detected; S3, carrying out detection of the different types of blister drugs on the blister drugs to be detected according to the standard threshold values of all the characteristics of the blister drugs; S4, carrying out defect detection of the same type of blister drugs on the blister drugs to be detected according to the standard threshold values of all the characteristics of the blister drugs; S5, obtaining the same type of blister drugs without defects. The method replaces the traditional artificial blister drug quality inspection with a machine vision inspection technology, and has the advantages of being low in labor intensity, not susceptible to subjective factors, high in reliability and detection efficiency, low in loss detecting rate and false detecting rate, and the like.
Owner:FOSHAN NANHAI GUANGDONG TECH UNIV CNC EQUIP COOP INNOVATION INST +1
Electronic tag shopping system of printing article
InactiveCN1694122ASave shopping timeReduce queuingOther printing matterSensing record carriersEngineeringPurchasing
This invention relates to a system for putting electric label on printing article belongs to the domain of net purchasing and electric label technology. Presently, print production is propagandized by different commodity price paper, poster, and magazines. There're product picture, price, favourable agio, from one to dozens of them, attracts the customer to purchase. The electric label provides the introduction of production, the function of guards against falsely. It was obtained by the customers themselves that it wasters time and money e.g. shortcoming. This invention of drug quality detecting system sets electric label to print production. It reads label by mobile phone reader through different net connection of server background, to finish the purchasing of products.
Owner:SHANGHAI ZHONGCE IND & TRADING +1
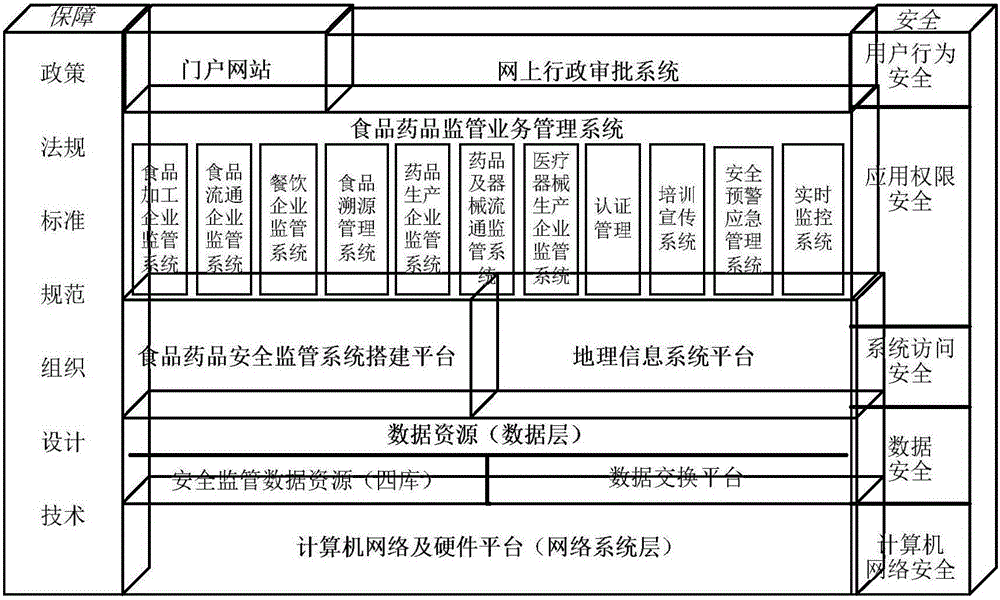
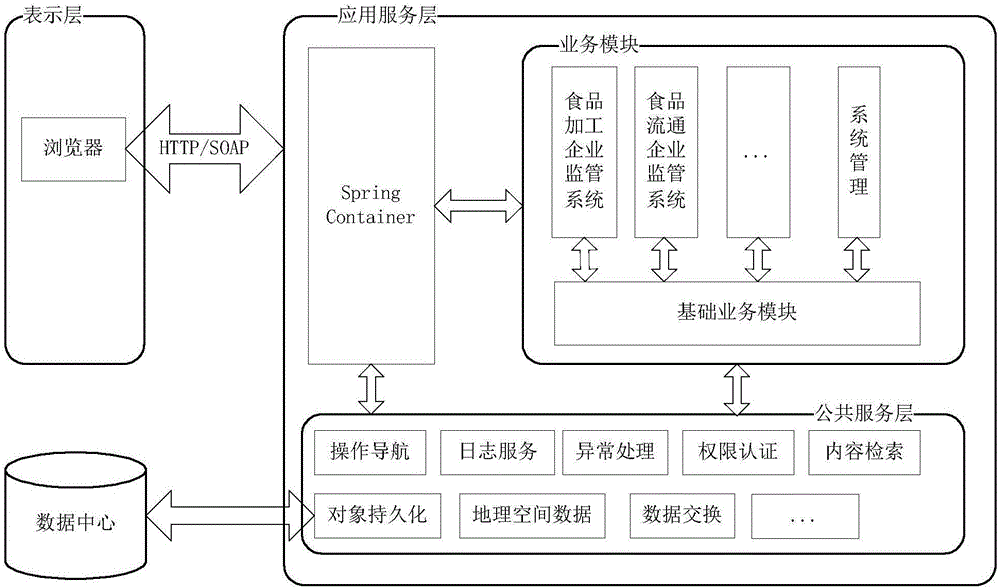
Food and drug safety supervisory system
InactiveCN106096973AGuarantee food safetyRealize chain managementCommerceInformatizationBusiness management
The invention discloses a food and drug safety supervisory system, which comprises a computer network and hardware platform, a data layer, a system building platform, a business management system and a web portal, wherein the data layer is used for establishing an organization, management, maintenance and update system of a business database according to a database management technology; the system building platform is the supporting environment of an information system and comprises a system permission management and message platform mechanism and a log management, unified certification and Gis building platform; the business management system is used for realizing the informatization of food and drug safety supervisory business under a network environment; and the web portal is used for finishing the communication and the management of information associated with the public through the web portal under the support of each business system. The invention provides the food safety supervisory system which is suitable for the situation of China and emphasizes the prevention, the whole process supervision of safe production and circulation of key food and drugs is realized, a food and drug quality safety situation is guaranteed to be constantly stable and good, a food and drug safety level is obviously improved, and the diet safety of urban and rural residents is exactly guaranteed.
Owner:库尔勒市食品药品监督管理局
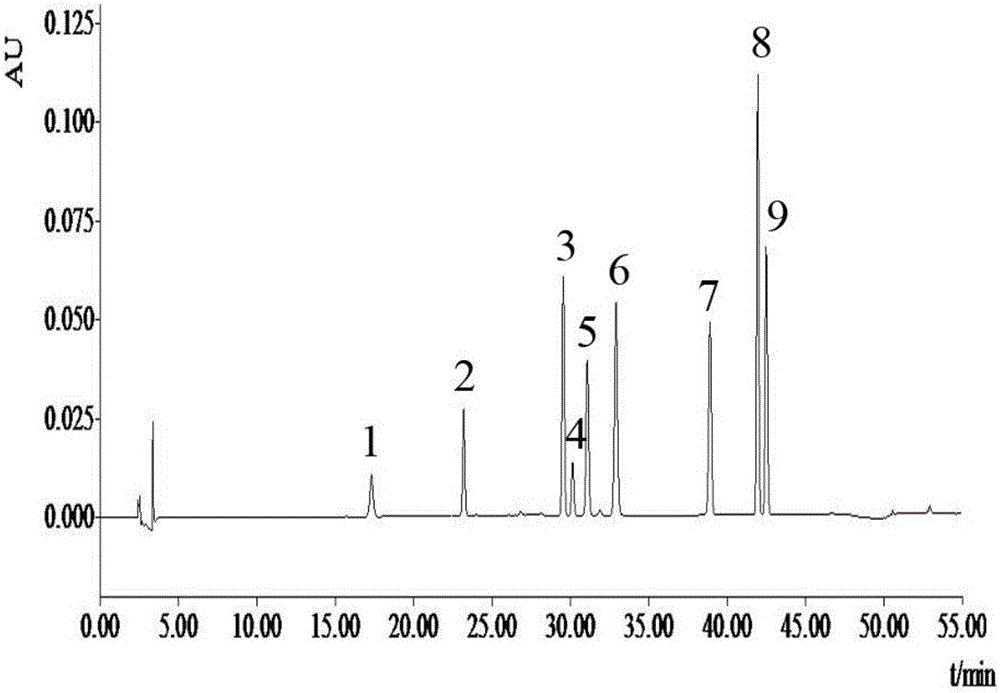
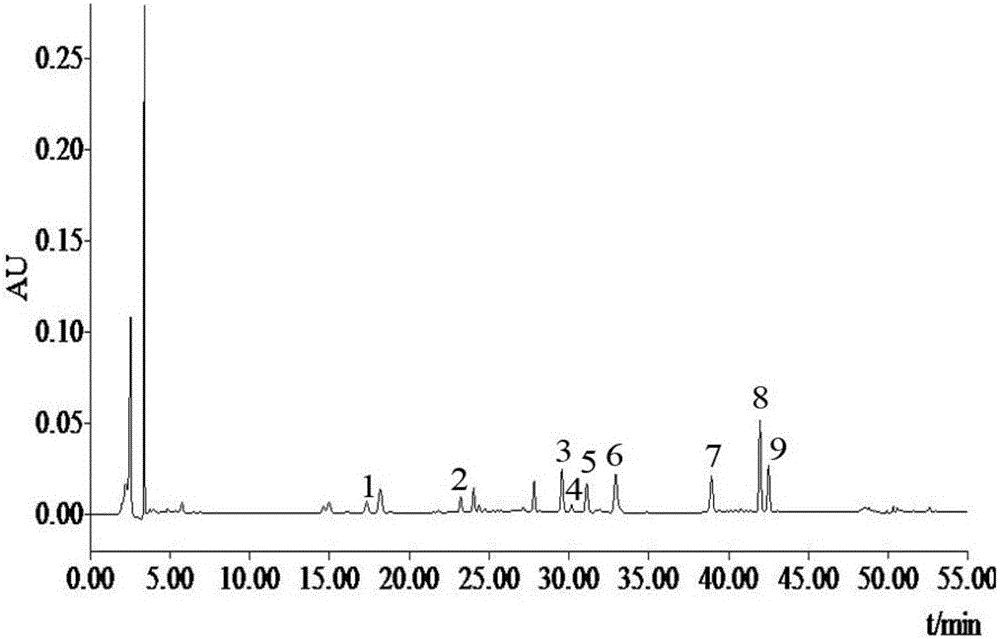
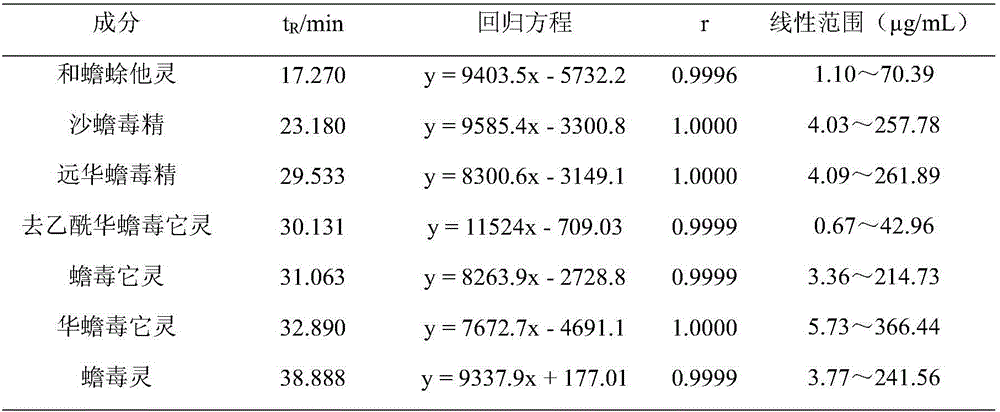
Method for detecting bufadienolide components in Liushen pill
InactiveCN106706812AQuality improvementImprove detection efficiencyComponent separationReference sampleColumn temperature
The invention discloses a method for detecting bufadienolide components in a Liushen pill. The method comprises the following steps: preparing a reference sample solution; preparing a test sample solution; and calculating the content through an HPLC assay determination method and a standard curve method. According to the invention, optimal chromatographic conditions such as mobile phase composition, a chromatographic column, an elution program, detection wavelength and column temperature are screened out through a large number of experiments, and the chromatographic conditions are optimized to obtain the optimal detection conditions. The detection method provided by the invention can realize quick, accurate and reliable determination, has high specificity and favorable reproducibility, can simultaneously detect 9 bufadienolide components in the Liushen pill, and can be used for quality control on the Liushen pill, thereby having important significance for drug quality control.
Owner:雷允上药业集团有限公司
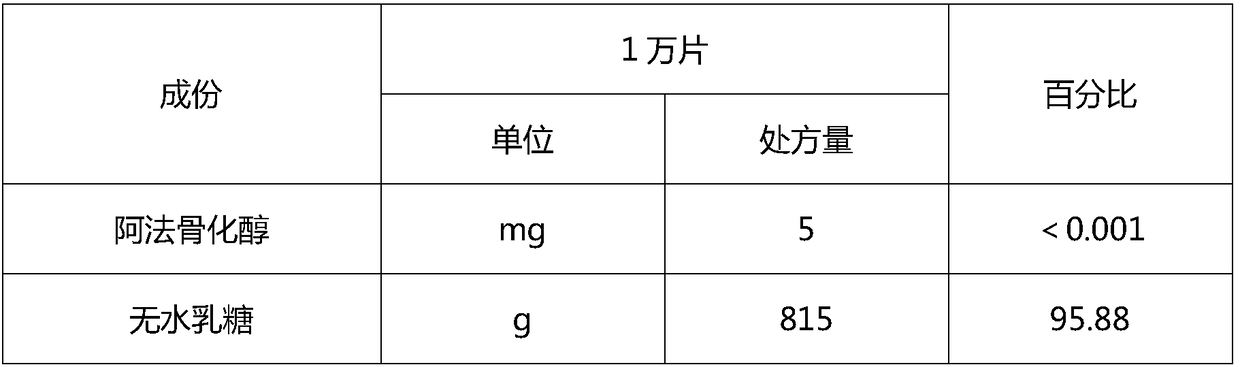
Vitamin D analog preparation and preparation method thereof
ActiveCN108420797AContent uniformity is not highHighly uniform dispersionOrganic active ingredientsSkeletal disorderEldecalcitolDrug product
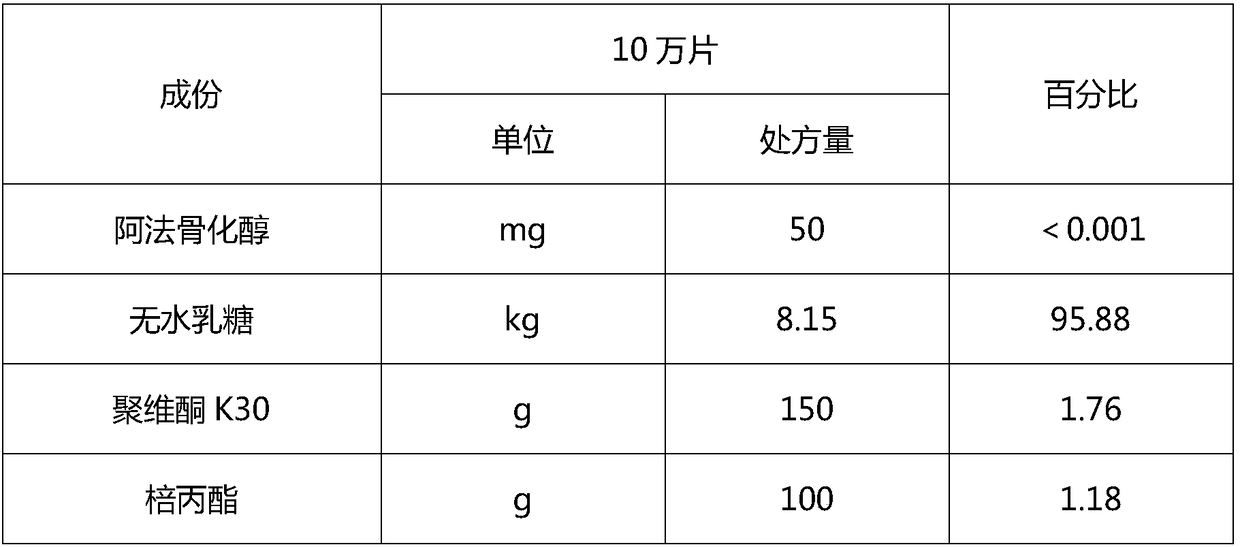
The invention discloses a vitamin D analog preparation and a preparation method thereof. The invention aims to solve the problems that a conventional preparation method of vitamin D analog has difficulty in meeting the uniformity requirement and during the granulation process, drugs are converted and degraded easily. For the first time, a double screw extrusion technology is used to prepare a solid preparation of vitamin D analog. Various vitamin D analogs are taken as model drugs, on the basis that a preparation technology of solid preparations is deeply researched, alfacalcidol, eldecalcitol, or calcitriol is chosen to prepare the solid preparation by using a double screw extruding machine; the uniformity meets the pharmacopeia requirements; the drug stability is obviously enhanced, andthe drug quality is greatly improved.
Owner:NANJING HERON PHARM CO LTD
Frame-removing manipulator for freezing-dry line
ActiveCN102189547AAutomatic and efficient pick and placeAutomatic and efficient transmissionProgramme-controlled manipulatorGripping headsFreeze-dryingWorking environment
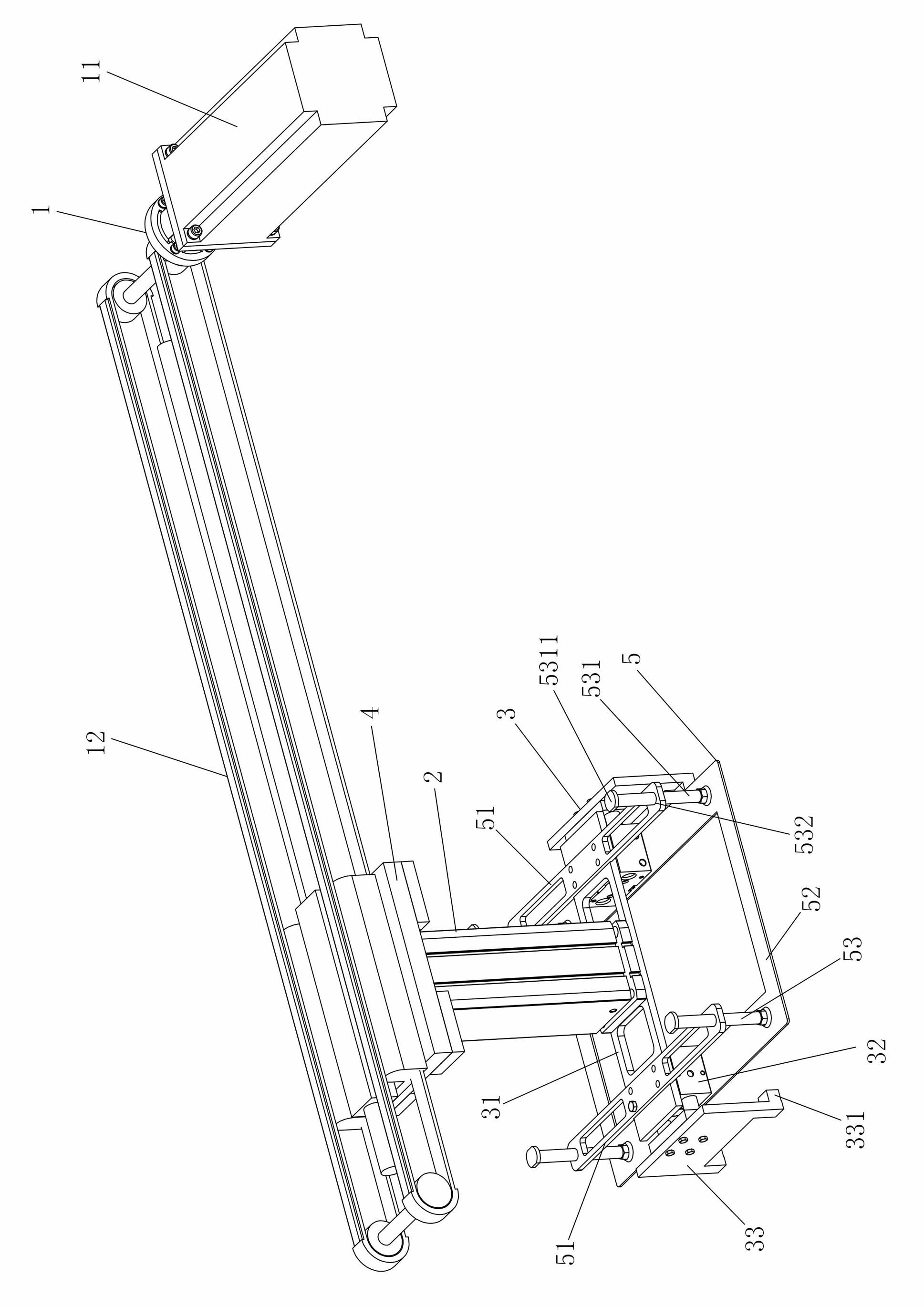
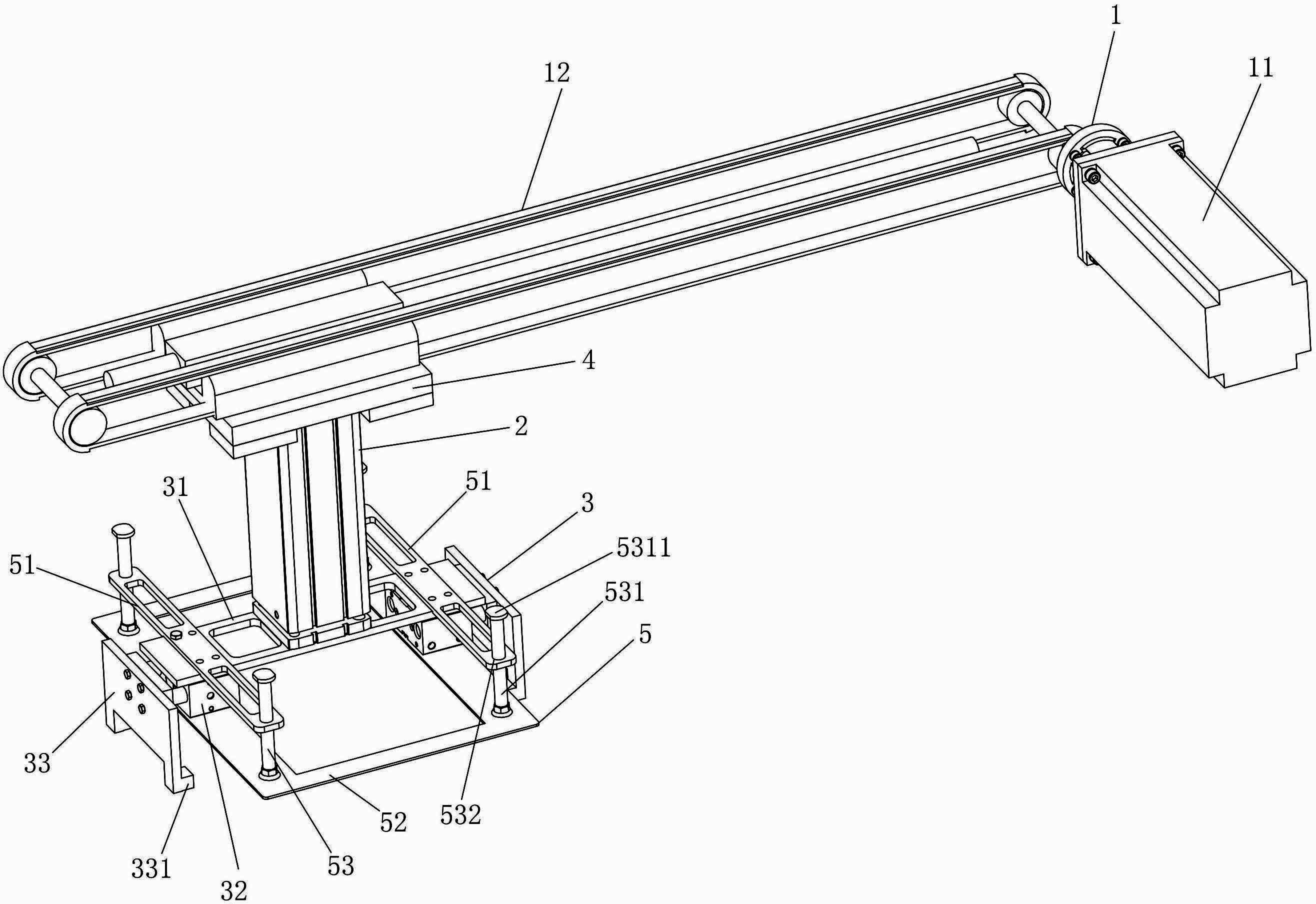
The invention discloses a frame-removing manipulator for a freezing-dry line, which comprises a horizontal drive mechanism, a lifting mechanism and a clamping assembly used for clamping a bottle-collecting frame, wherein one end of the lifting mechanism is connected with the drive end of the horizontal drive mechanism, the other end of the lifting mechanism is connected with the clamping assembly, and a pressing assembly used for pressing bottle bodies in the bottle-collecting frame during frame-removing is arranged on the clamping assembly. The frame-removing manipulator disclosed by the invention has the advantages of simple structure, high frame-removing precision and efficiency, greatly improved sterile work environment of equipment, increased drug quality, capability of automatic frame removal and the like.
Owner:TRUKING TECH LTD
Bone-strengthening drug quality detection method
ActiveCN105021729AImprove quality control standardsHigh level of quality standardsComponent separationColor/spectral properties measurementsMicrowaveMedicine
The invention discloses a bone-strengthening drug quality detection method, and belongs to the drug quality detection technical field. The adopted technical scheme comprises that the bone-strengthening drug is made into capsules by an artificial tiger bone powder and auxiliary materials, and the quality detection method includes the following determining steps: 1) after the bone-strengthening drug is hydrolyzed, determining the content of total amino acids in the samples, wherein each capsule contains not less than 65 mg of the total amino acids; and 2) determining the content of total calcium in the bone-strengthening drug by adopting atomic absorption spectrophotometry, wherein each capsule contains not less than 55 mg of calcium. The determination method for determining the content of the total amino acids in the preparation by adopting microwave hydrolysis and automatic online derivation, using a full-automatic amino acid analyzer or adopting traditional hydrolysis and PITC derivation method and using high performance liquid chromatography is established, the determination method for determining the content of calcium in the samples by adopting the atomic absorption spectrophotometry is increased, the accuracy and advancement of the quality detection method is ensured, and the product quality of Jintiange capsules can be effectively controlled.
Owner:XIAN DAQING PHARMA FACTORY JINHUA ENTERPRISE GROUP CORP
Method for preparing amorphous atorvastatin calcium
The invention discloses a method for preparing amorphous Atorvastatin calcium. The current method uses more solvent, so the cost is over high; and the residual quantity of the solvent in a product is large, thereby causing big influence on the quality of the product and causing serious environmental pollution. In the method, alcohol solvent and water are combined into mixed alcohol-water solvent which dissolves Atorvastatin calcium containing one or more crystal forms completely; proper temperature is kept for ensuring that the Atorvastatin calcium is not precipitated; and the amorphous Atorvastatin calcium is precipitated by a spray drying method. The method uses the mixed alcohol-water solvent which is removed from the product easily, has little organic residue and causes less influence on drug quality; the usage amount of the solvent is reduced greatly; the concentration of the Atorvastatin calcium in the solution is high; and the purity of the prepared product of the amorphous Atorvastatin calcium is high.
Owner:ZHEJIANG JINGXIN PHARMA
Natural indigo prepared by froth flotation method and its preparation method and device
ActiveCN101306026AQuality improvementImprove qualityFlotationBlood disorderIndigo redFroth flotation
The invention provides an indigo prepared by adopting the froth-flotation method, which comprises the components by weight percentages as follows: 2.63%-5.51% of indigo blue and 0.13%-0.61% of indigo red. The invention also provides a preparation method and a device for the indigo. The preparation method for the indigo of the invention has the advantages that the operation and the management are convenient, the separation is not limited by environment or climate, and the running is stable and reliable. The preparation method is a novel technique for indigo separation; by adopting the novel technology for machining, the large scale production can be realized on the basis of ensuring the drug quality, in addition, the indigo product quality is increased; the preparation method has simple and convenient operation and low technical difficulty. The quality of the indigo which is prepared by the preparation method is higher than the pharmacopoeia standards obviously.
Owner:雅安迅康药业有限公司
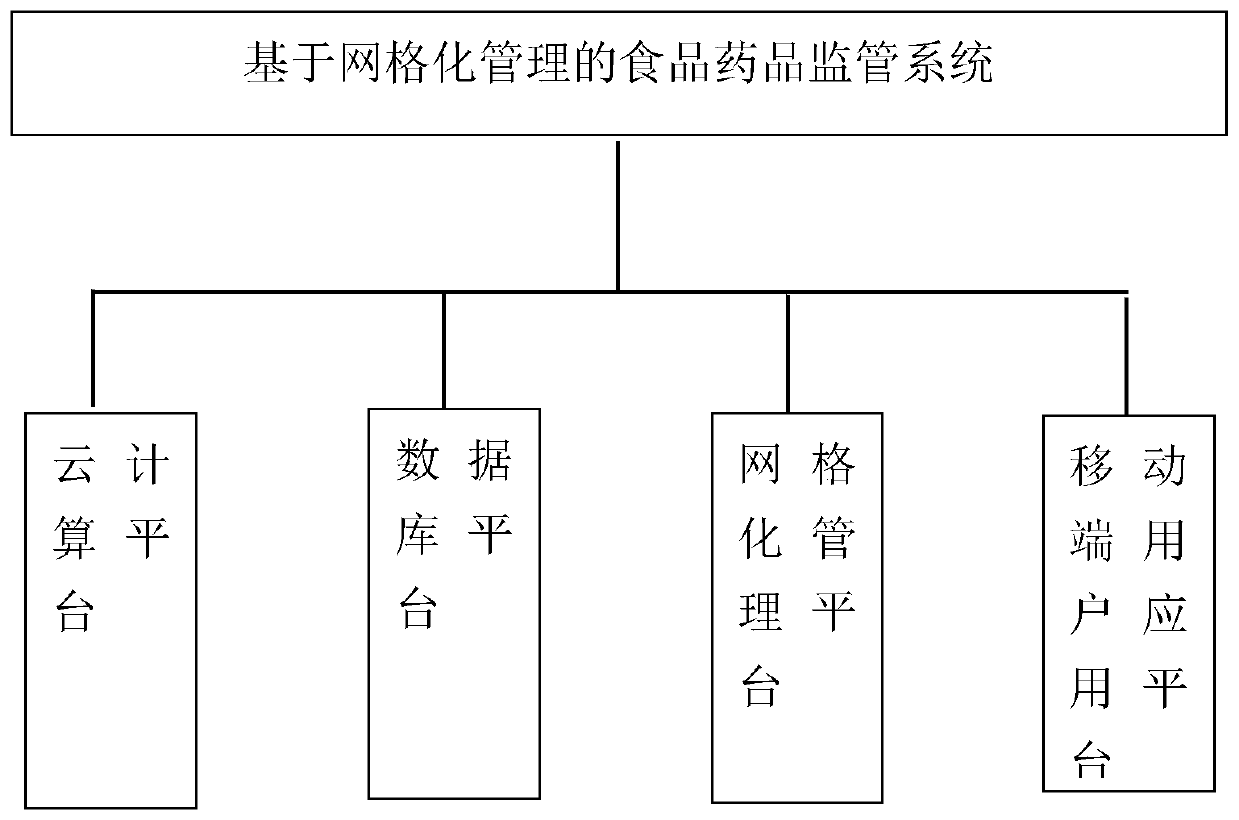
Food and drug supervision system and method based on gridding management
InactiveCN109801004ARealize intelligent serviceRealize refined managementResourcesFood safetyDrug product
The invention discloses a food and drug supervision system and method based on gridding management. The supervision system comprises a cloud computing platform, a database platform, a gridding management platform and a mobile terminal user application platform, the gridding management platform is used for dividing the supervision range into grids, and food and drug production and circulation information of all supervision enterprises in the grids is marked on a geographic map information module (GIS); The supervision method comprises the steps of administrative supervision, technical supervision and inspection case handling, and inspection case handling comprises the steps of grid division, information collection, task providing, task dispatching, task processing, processing feedback, casechecking and checking and comprehensive evaluation. According to the invention, a food safety supervision system based on a GIS professional map for prevention is established, food and drug safety production and circulation process supervision are realized, and food and drug quality safety is guaranteed.
Owner:郭承湘 +2
Novel processing method for manyflower solomonseal rhizome
The invention provides a novel processing process method for manyflower solomonseal rhizome. According to the method, process indexes of an existing nine-steaming and nine-airing process are adjusted, and a braising process is further added, so that the processing of manyflower solomonseal rhizome is optimized, the drug quality is beneficially improved, the content of polygahatous polysaccharides is maximum, and the pesticide effect of manyflower solomonseal rhizome is further improved.
Owner:安徽华善堂中药饮片有限公司
Method for detecting quality of blood-activating analgesic plaster
ActiveCN101745091AEffective Quality MonitoringThe method is simpleHydroxy compound active ingredientsAntipyreticMedicineGas phase
The invention relates to the drug quality detection field, in particular to a method for detecting the quality of a blood-activating analgesic plaster, which comprises the following steps: angelica sinensis, hesperidin, atropine sulfate, chrysophanol and cortex periplocae in the blood-activating analgesic plaster are identified by a thin layer chromatography, and then component contents in the blood-activating analgesic plaster are detected by a gas chromatograph. The method for detecting the quality of the blood-activating analgesic plaster combines the thin layer chromatography and the gas chromatograph for detecting the effective components and contents of the blood-activating analgesic plaster, the detected result is accurate and reliable, the quality of the blood-activating analgesicplaster can be effectively monitored, and the method is simple and convenient and is easy to operate.
Owner:ANHUI ANKE YULIANGQING PHARMA
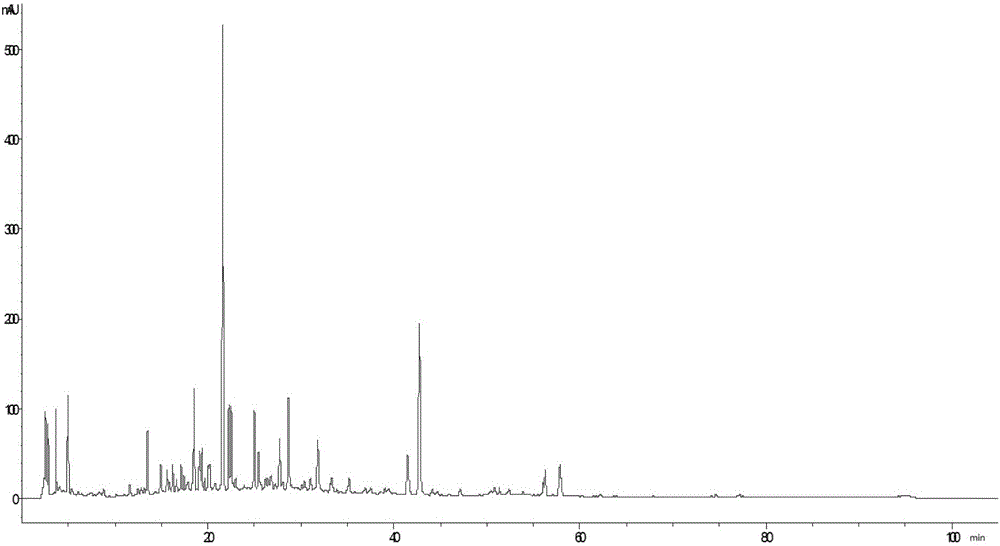
Establishing method of fingerprint spectrum of honeysuckle-fructus forsythiae heat-clearing tablets and fingerprint spectrum
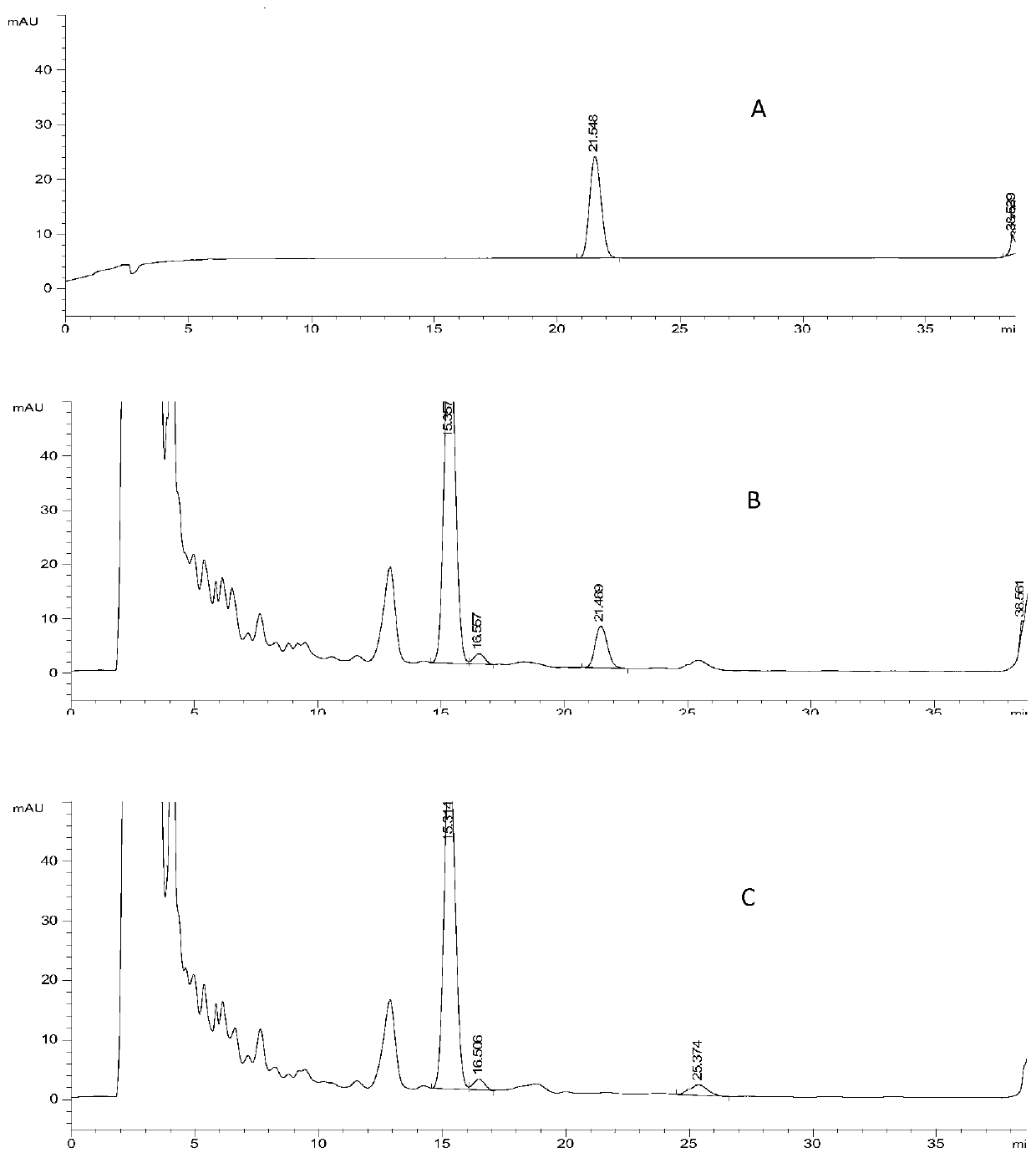
The invention discloses an establishing method of a fingerprint spectrum of honeysuckle-fructus forsythiae heat-clearing tablets and the fingerprint spectrum. The establishing method of the fingerprint spectrum of the honeysuckle-fructus forsythiae heat-clearing tablets includes the following steps: step 1, preparing a test sample solution of the honeysuckle-fructus forsythiae heat-clearing tablets; step 2, respectively precisely sucking the test sample solution and injecting into liquid chromatographs, and recording a chromatogram; step 3, exporting the honeysuckle-fructus forsythiae heat-clearing tablet fingerprint spectrum obtained in the step 2 from the instrument, and importing into a traditional Chinese medicine chromatographic fingerprint spectrum similarity evaluation system; selecting chromatographic peaks existing in chromatograms of different batches of the honeysuckle-fructus forsythiae heat-clearing tablets as common peaks; generating a reference fingerprint spectrum of the honeysuckle-fructus forsythiae heat-clearing tablets by an average value calculation method; and calculating the relative retention time and the relative peak area of each common peak. The honeysuckle-fructus forsythiae heat-clearing tablet fingerprint spectrum established by the method provided by the invention can effectively characterize the quality of the honeysuckle-fructus forsythiae heat-clearing tablets, and is conducive to comprehensive supervisory control of the drug quality. The method has the advantages of being simple, convenient, stable, high in precision, good in reproducibility and the like.
Owner:JIANGSU KANION PHARMA CO LTD
Novel T cell immune modulator bioactivity detection method
ActiveCN107916276ASuitable for quality controlSuitability for detectionMicrobiological testing/measurementNucleic acid vectorCellular componentImmune modulator
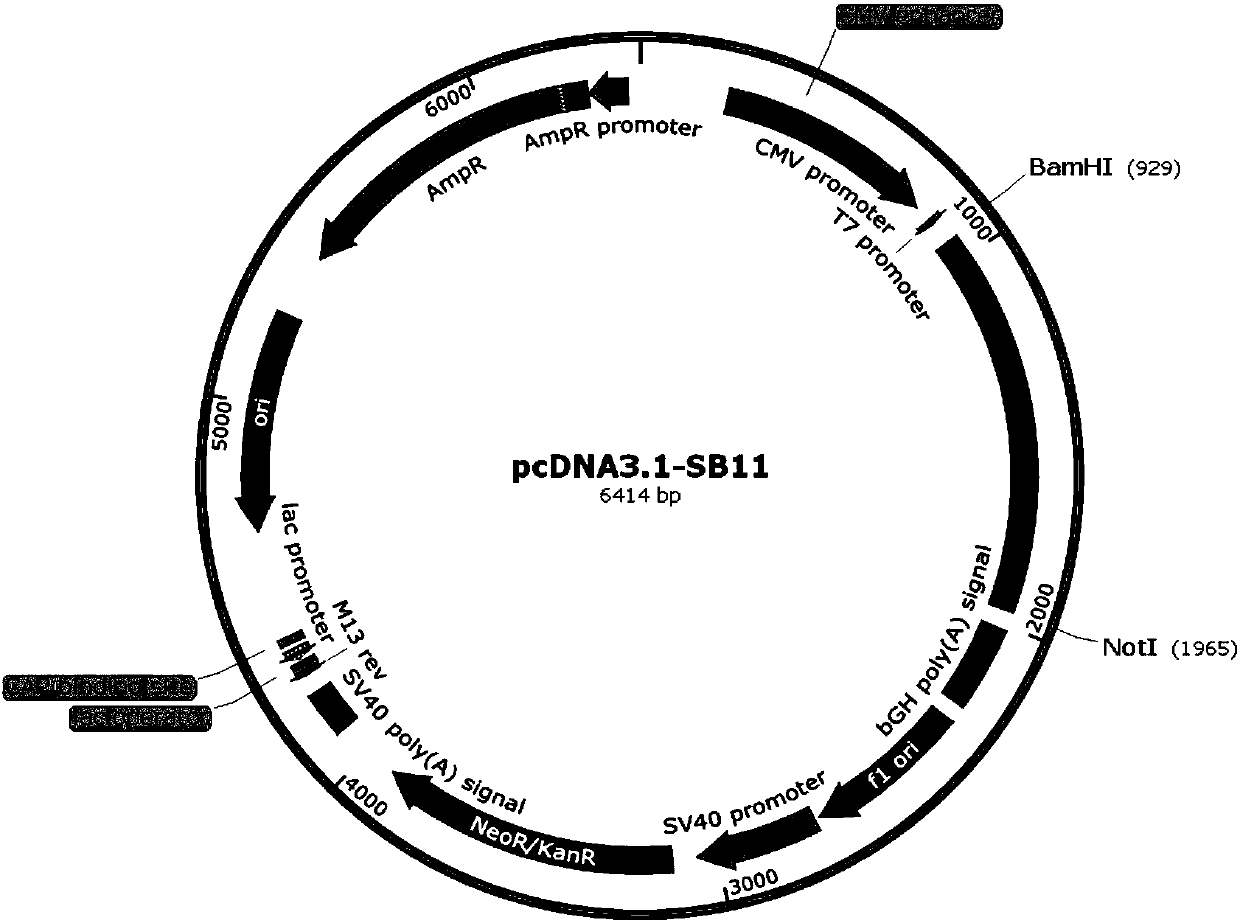
The invention relates to the technical field of biological medicines, and particularly discloses a novel T cell immune modulator bioactivity detection method. Eukaryotic expression plasmids and co-expression resistance genes of sleeping beauty transposase and reporter genes driven by different response elements are expressed, eukaryotic expression plasmids of transposon inverted repetitive sequences are inserted into two ends of the reporter genes and the co-expression resistance genes and jointly transferred into host Jurkat T cells, pressurized screening and monoclonal separation are performed to obtain stable monoclonal effect cells, target cells and T cell activating modulators are added, and bioactivity of T cell immune modulator is measured by measuring activity of the reporter genes. The method can be applied to a bioactivity reporter gene detection system for cell immune modulators such as PD-1 / PD-L1 monoclonal antibodies and CTAL4-Fc fusion proteins. Any human blood-derived cell components or other components are omitted, testing results are stable, operation is simple and convenient, testing time is short, and the method is quite suitable for later drug quality control and batch release detection and can be widely applied.
Owner:BEIJING DONGFANG BIOTECH
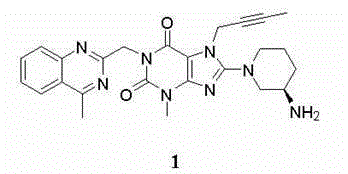
Dipeptidyl peptidase IV inhibitor pharmaceutical composition, use and preparation method thereof
ActiveCN105456270AReduce typesLow costMetabolism disorderPharmaceutical non-active ingredientsStarch cornMagnesium stearate
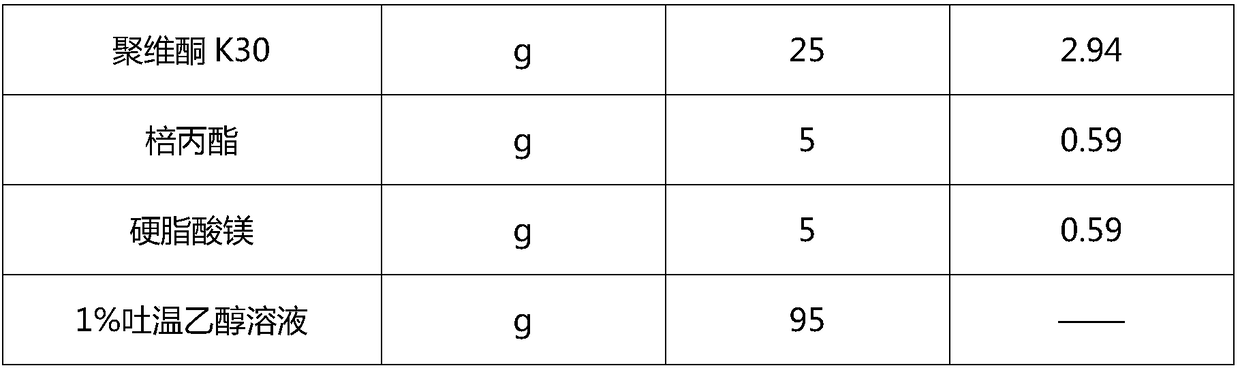
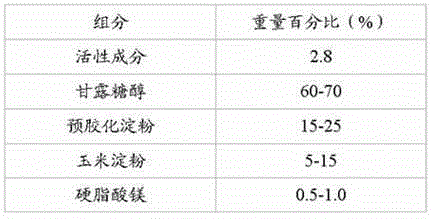
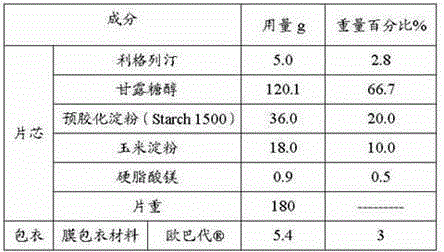
The invention discloses a pharmaceutical composition containing a dipeptidyl peptidase IV inhibitor linagliptin, use and preparation method thereof. The linagliptin containing pharmaceutical composition provided by the invention consists of linagliptin or a salt thereof serving as the active ingredient, and pharmaceutical excipients mannitol, pregelatinized starch, corn starch and magnesium stearate. The linagliptin containing pharmaceutical composition provided by the invention reduces the types of excipients, increases the stability of the preparation, reduces the cost of raw materials, and solves the hardness and friability problems of linagliptin tablets by controlling the particle size of the key excipient mannitol. The obtained table has all indicators especially the dissolution rate in line with the drug quality standards, and the process is simple, thus being more suitable for large scale production.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
A clean production method of medium molecular weight hydroxyethyl starch
The invention provides a clean production method of medium molecular weight hydroxyethyl starch, in particular to a clean production method in the refining process of chemical raw materials hydroxyethyl starch 200 / 0.5 and hydroxyethyl starch 130 / 04 used for plasma expanders. Bacterial endotoxin is the key toxic substance leading to clinical infusion pyrogenic reaction. The bacterial endotoxin in the raw material drug of hydroxyethyl starch exceeds the limit, which can easily lead to the limit of bacterial endotoxin in the final product of the preparation, hydroxyethyl starch injection, and the unqualified rate increases. The method of the invention is controlled by necessary process conditions such as clean air spray drying under clean conditions, microbial control under high temperature conditions, and on-line cleaning and disinfection of equipment, so that the quality of the bacterial endotoxin index of the obtained final product is obviously better than the current national drug quality standards. At the same time, it provides the necessary conditions for the direct preparation method of raw materials without microfiltration, which is helpful for the qualification of the insoluble particle index of the preparation, shortens the process flow of the final preparation, and reduces the probability of pollution.
Owner:WUHAN HUST LIFE SCI & TECH
HPLC analytic method for Nicorandil-related substances
The invention belongs to the technical field of pharmaceutical analysis, and particularly relates to an HPLC analytic method for Nicorandil-related substances. The HPLC analytic method is characterized in that a stationary phase is a reversed-phase column of carbon octadecyl silane, and a moving phase is a mixed solution of a buffer solution, methyl alcohol and tetrahydrofuran. The HPLC analytic method is quick, simple and accurate, is good in repeatability and suitable for control and stability study of the related substances, lays a foundation for formulating quality standards of the related substances, and makes drug quality control and medication safety possible. Through scaling down organic solvents and volatile salt, which have high toxicity, the acquisition time is shortened, the economic cost is lowered, the HPLC analytic method is suitable for large-scale production, and meanwhile, harms to experimenters and environments are reduced.
Owner:XIAN KAIBEI NAITE INTELLIGENT ENG
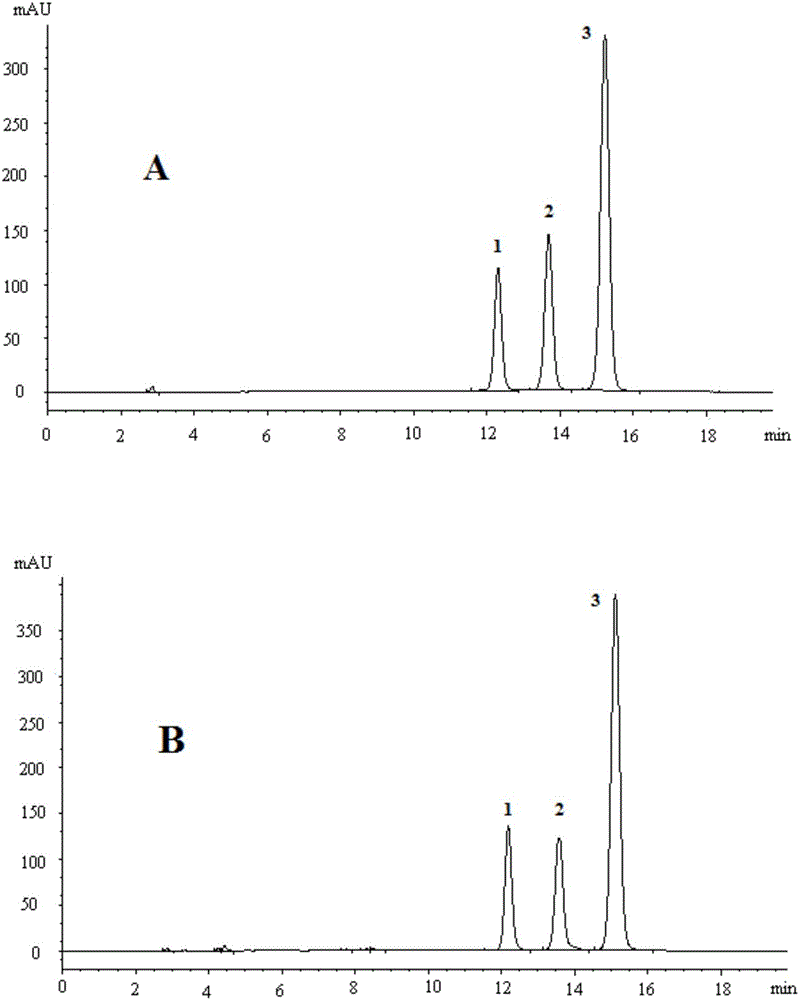
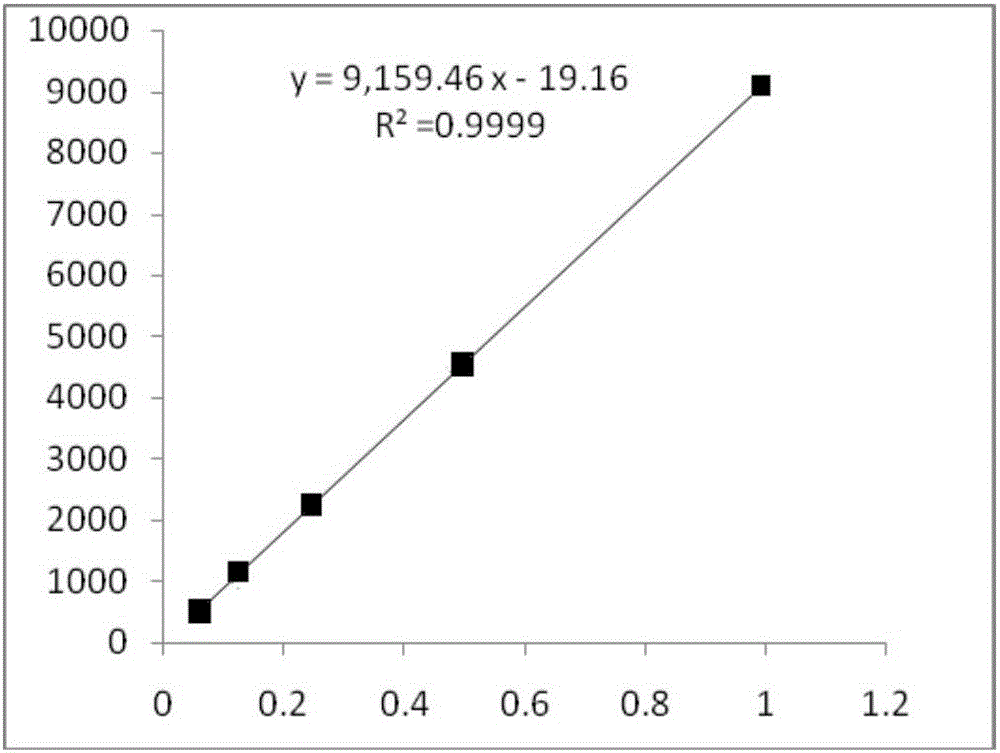
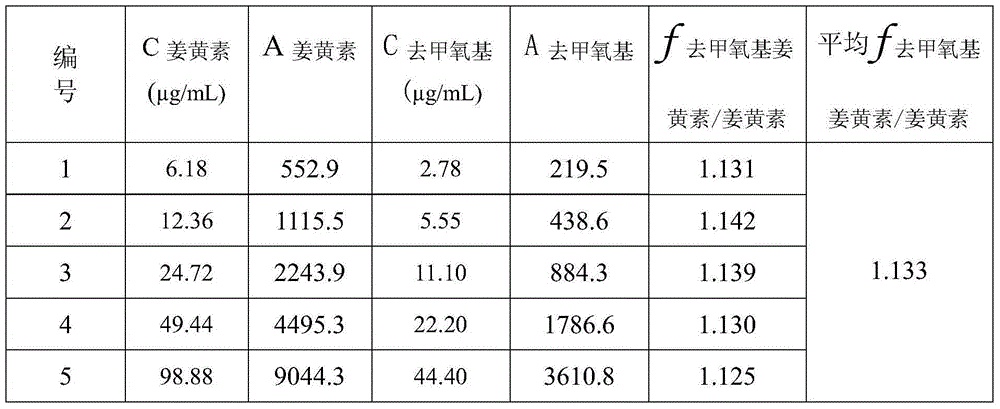
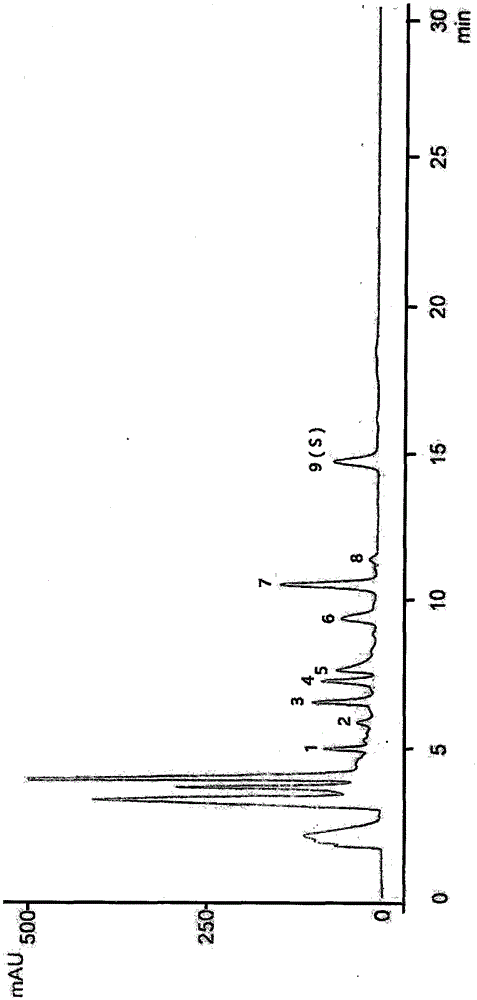
Method for calculating contents of three effective components in curcuma longa product through relative correction factor
InactiveCN105116061AReduce the number of weighings with the balanceReduce wasteComponent separationMedicineBisdemethoxycurcumin
The invention relates to a method for calculating the contents of three effective components in a curcuma longa product through a relative correction factor, and belongs to the technical field of drug quality determination. The method comprises: (1) preparing a reference substance stock solution; (2) preparing a reference substance solution; (3) preparing a sample solution; (4) determining through high performance liquid chromatography; (5) determining the relative correction factor value; (6) calculating the curcumin content; and (7) calculating the demethoxycurcumin content and the bisdemethoxycurcumin content. According to the present invention, the relative correction factor is used to detect the contents of the three effective components in the curcuma longa product, such that the demethoxycurcumin reference substance preparation and the bisdemethoxycurcumin reference substance preparation in each experiment are eliminated, the detection cost is reduced, and the detection efficiency is improved; the relative correction factor f is verified under different detection equipment, different chromatographic columns, different detection wavelengths, different column temperatures, different flow rates and different flow relative ratios; and the method has characteristics of rapidness, efficiency, high precision, low cost and the like, and is the feasible and effective detection method.
Owner:SICHUAN NEO GREEN PHARMA TECH DEV
Preparation method of clindamycin phosphate injection
ActiveCN102144966AImprove stabilityGood curative effectAntibacterial agentsOrganic active ingredientsMedicineClindamycin Phosphate
The invention discloses a chemical injection, and particularly relates to a preparation method of a clindamycin phosphate injection. The clindamycin phosphate injection is prepared from the following raw materials in grams: clindamycin phosphate 300-400 g and sodium hydroxide 24.3-25.5 g. The preparation method comprises the following steps: dissolving 24.3-25.5g of sodium hydroxide in 1500-1900 g of water for injection, dissolving 300-400 g of clindamycin phosphate in the sodium hydroxide solution, stirring uniformly, adding water for injection to a constant volume of 2,000 mL, performing coarse filtering with a 0.65 mu m microporous filter membrane, performing fine filtering with a 0.22 mu m microporous filter membrane for sterilizing, and filling to obtain the clindamycin phosphate injection. The preparation method provided by the invention has the characteristics of applicability to mass production, high product yield, stable drug quality, and good therapeutic effect of drugs.
Owner:辽宁格林生物药业集团股份有限公司
Fingerprint spectrum control method of low-sugar strong loquat syrup
ActiveCN105372350AOvercoming the difficulty of reflecting the real feeding situation of the productQuantitative authenticityComponent separationAdditive ingredientSugar
The invention provides a fingerprint spectrum control method of low-sugar strong loquat syrup and relates to the technical field of drug quality control. The method includes the following steps that 1, an ingredient standard feature spectrum in the low-sugar strong loquat syrup is established; 2, a low-sugar strong loquat syrup sample feature spectrum to be tested is tested with the same mentioned method; 3, the low-sugar strong loquat syrup sample feature spectrum to be tested is compared with a standard low-sugar strong loquat syrup sample feature spectrum, and the quality and authenticity of the low-sugar strong loquat syrup to be tested are judged. The fingerprint spectrum control method has the advantages that integrity, the macroscopic property and fuzziness are achieved, and the defect that it is difficult to reflect the real feeding situation of products through individual ingredient tests is overcome. By means of the method, a novel method and means are provided for completely and accurately evaluating the quality of the strong loquat syrup; similarities and differences of spectrums are distinguished visually with vision, and the authenticity and merits of a sample are quantized through a semi-quantitative indicator.
Owner:HEFEI JINYUE PHARMA
Qingpeng ointment and quality control method of Qingpeng ointment preparation
The invention discloses a quality control method of a pharmaceutical composition, and specially, relates to Qingpeng ointment and a quality control method of a Qingpeng ointment preparation. Compared with a quality control method of a traditional Qingpeng ointment, the quality control method of the Qingpeng ointment preparation provided by the invention is added with thin layer determination processes on Chinese rhubarb, benzoin and gallic acid and a content determination process on anthraquinones in Chinese rhubarb. The quality control method is simple and fast, has strong specificity, enables technicians of the field to fast and accurately determine authenticity of products in the market, and effectively controls preparation quality and stability. Through an improved quality standard, product quality can be effectively controlled so that drug safety, drug effectiveness and drug quality controllability are realized really.
Owner:JINHE TIBETAN MEDICINE
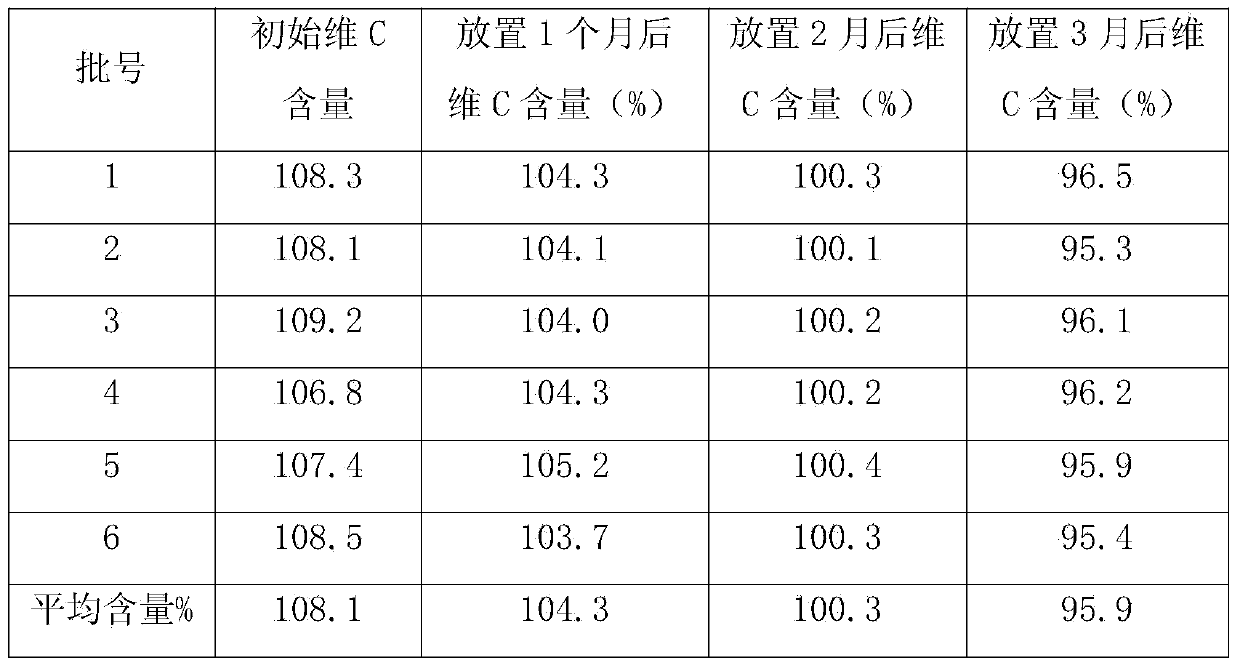
Vitamin C Yinqiao tablets and preparation method thereof
InactiveCN104189588AIncrease polarityReduce adverse reactionsOrganic active ingredientsAntipyreticVitamin CGLYCYRRHIZA EXTRACT
The invention discloses vitamin C Yinqiao tablets. The vitamin C Yinqiao tablets are prepared in the following steps: adding 60-80 parts of folium isatidis, 70-90 parts of radix bupleuri, 70-90 parts of platycodon grandiflorus, 50-70 parts of astragalus, 40-60 parts of Cyrtomium fortunei, 30-60 parts of chrysanthemum and 40-60 parts of isatis root into a formula of the vitamin C Yinqiao tablets, adding a proper amount of auxiliary materials to be prepared into 1000 tablets, coating, thereby obtaining the vitamin C Yinqiao tablets. The invention also discloses a process for preparing the vitamin C Yinqiao tablets. According to the vitamin C Yinqiao tablets disclosed by the invention, the active ingredients of medicinal materials such as the radix bupleuri, liquorice, isatis root and folium isatidis are added into the prescription under the condition that the curative effect of the medicine is basically stable, and the adverse reaction of chlorpheniramine maleate, acetaminophen and vitamin C taken by patients can be relieved. Meanwhile, the stability, drug quality and drug effect of the vitamin C Yinqiao tablets in the production process are improved, the validity of the vitamin C Yinqiao tablets is prolonged, and the content of vitamin C is still larger than 95 percent after the vitamin C Yinqiao tablets are stored within 3 months at room temperature.
Owner:JIANGXI JIUHUA PHARMA
Fingerprint checking method of gadol injection
InactiveCN101361781AEnsure drug qualityEnsure curative effectBiological testingCardiovascular disorderAdditive ingredientCurative effect
The invention relates to a quality inspection method for traditional Chinese medicine preparations, in particular to a fingerprint inspection method of integripetal rhodiola herb injection. The method comprises the steps of: (1) establishing the standard fingerprint of the integripetal rhodiola herb injection; (2) establishing the fingerprints of integripetal rhodiola herb injection samples; and (3) making a comparison between the fingerprints of the integripetal rhodiola herb injection samples and the standard fingerprint of the integripetal rhodiola herb injection and selecting a sample that meets the requirements of the standard fingerprint of the integripetal rhodiola herb injection. The method can qualitatively determine the major ingredients of the integripetal rhodiola herb injection and establish the proportion relationship between active ingredients and inactive ingredients in the injection so as to ensure the drug quality and the therapeutic effect of the integripetal rhodiola herb injection, effectively and reliably represent the product quality of the integripetal rhodiola herb injection and provide a new comparing standard for completely and correctly evaluating the quality of the integripetal rhodiola herb injection.
Owner:TONGHUA YUSHENG PHARMA
Portable rapid food and drug quality detection device and method
InactiveCN104697932AReduce adverse effectsGuaranteed excitation wavelengthMaterial analysis by optical meansGas phasePulsed laser
The invention discloses a portable rapid food and drug quality detection device and method. The device comprises a sample pool, a laser radiating unit, an ultrasonic detector, a signal processing unit, a control display unit and the like. By virtue of an opto-acoustic non-destructive testing technology, the detection method, compared with a liquid / gas phase chromatographic method, can be used for directly detecting a tested sample without performing complex preprocessing operation on the sample, and irreversible destructiveness on the nature of the tested sample can be avoided; and compared with non-destructive detection methods such as near infrared spectroscopy, the detection method can relatively well avoid adverse impact of scattered light from components inside the sample on the transmission or reflection spectrum measurement of the tested sample, so that the detection accuracy is greatly improved. As an embedded system and a control display unit touch screen adopted by a large pulsed laser and a data processing unit are replaced by virtue of a laser diode rotating device, the detection device disclosed by the invention is relatively high in integration degree, and is relatively low in cost when multiple excitation wavelengths of a light source is guaranteed.
Owner:JIANGXI SCI & TECH NORMAL UNIV
Automatic control device of powder height
InactiveCN103605382AEfficient detectionRealize real-time controlLevel controlCapacitanceAutomatic control
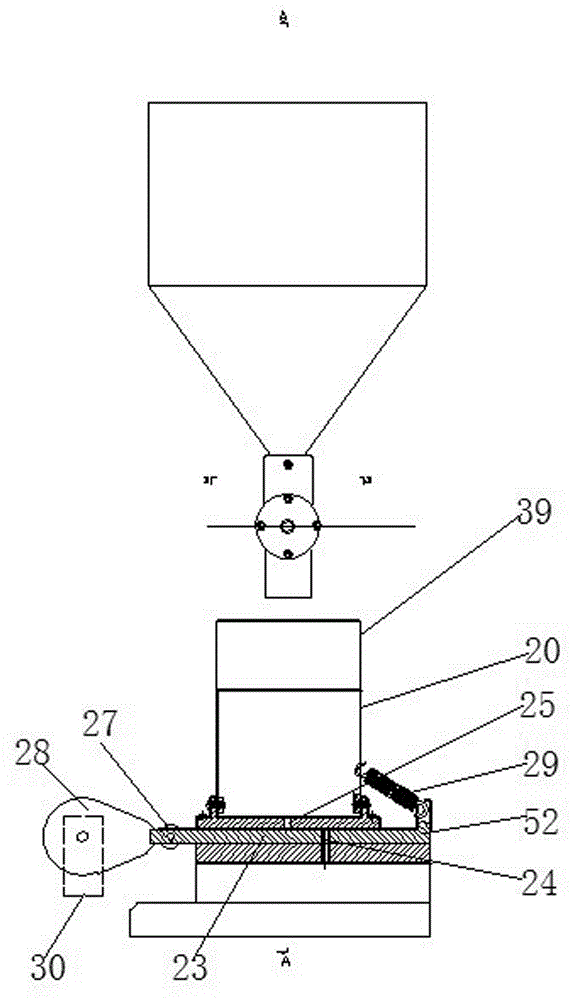
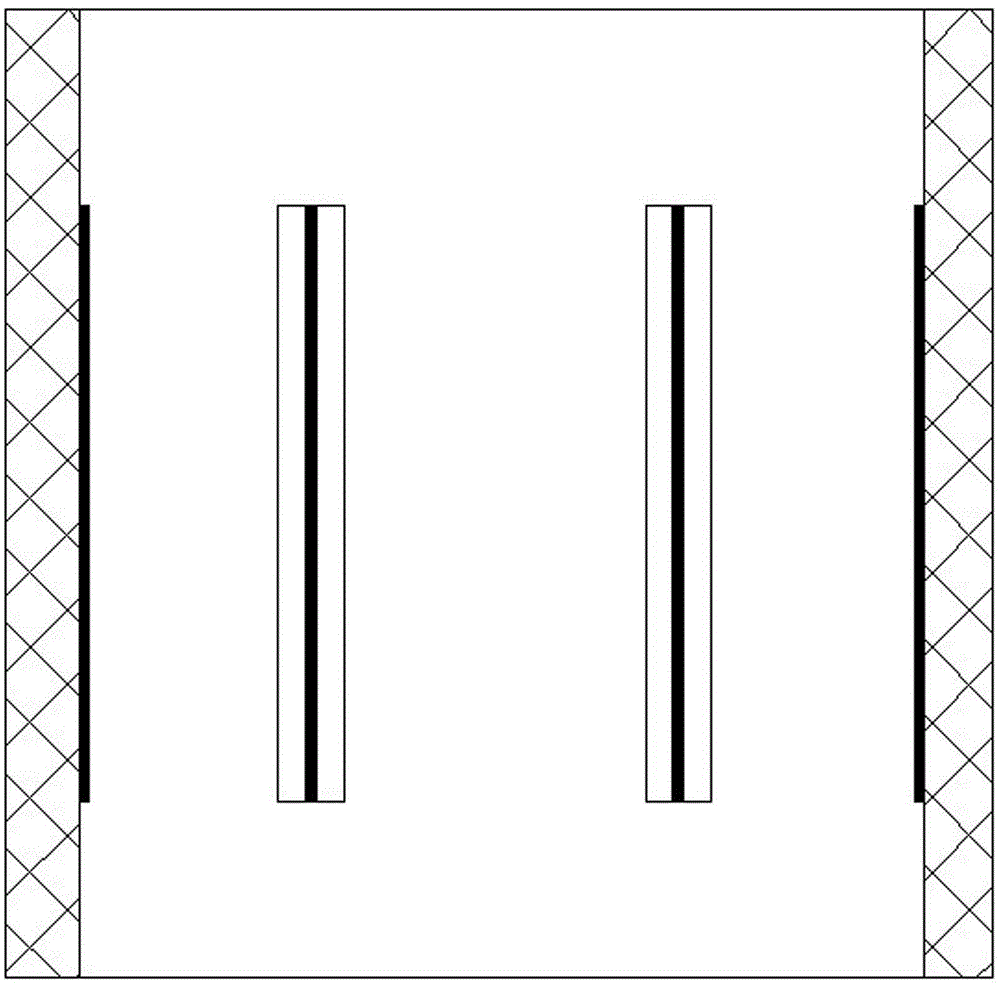
An automatic control device of a powder height comprises a feeding mechanism and a material powder height detection machine. The material powder height detection machine comprises a square charging barrel, a supporting plate, a support, a quantitative discharging supporting plate, a quantitative discharging pulling plate and a material powder height detector. The charging barrel is fixed on the supporting plate. The quantitative discharging pulling plate is arranged in a pulling plate chute on an upper end surface of the support. The supporting plate is fixed on the upper end surface of the support. During a blanking state, a discharging hole on the supporting plate forms butt joint with a discharging hole on the quantitative discharging pulling plate. The quantitative discharging pulling plate is driven by a cam driving mechanism. The material powder height detection machine is formed by four capacitance pole plates. The two capacitance pole plates are sticked on inner walls of left and right sidewalls of the charging barrel respectively, the other two capacitance pole plates are arranged in a central section of the charging barrel and two ends are fixedly connected with a front wall and a rear wall of the charging barrel respectively. By using the device of the invention, the powder height can be effectively detected; real-time control of the powder height is realized and finished drug quality is increased.
Owner:SHENYANG LIGONG UNIV
Detection method for traditional Chinese medicine composition, and applications thereof
ActiveCN109709240AMonitor qualityMonitor Quality PerformanceComponent separationBeta-asaroneHplc method
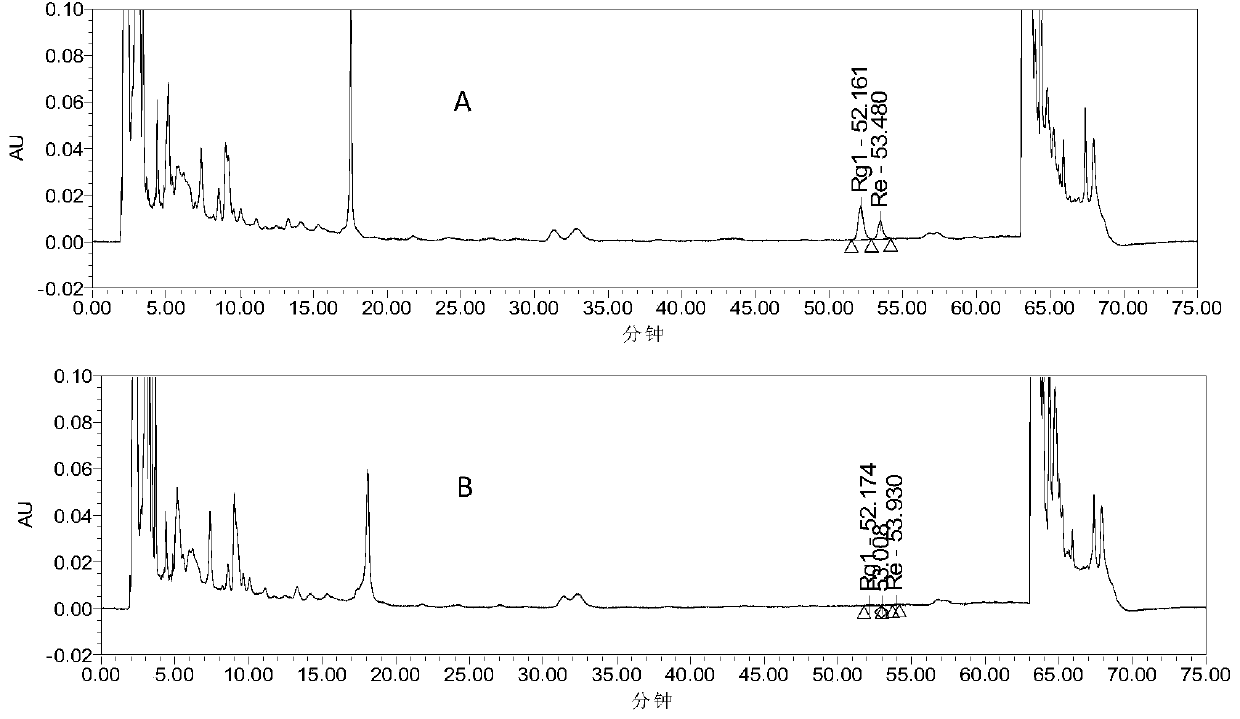
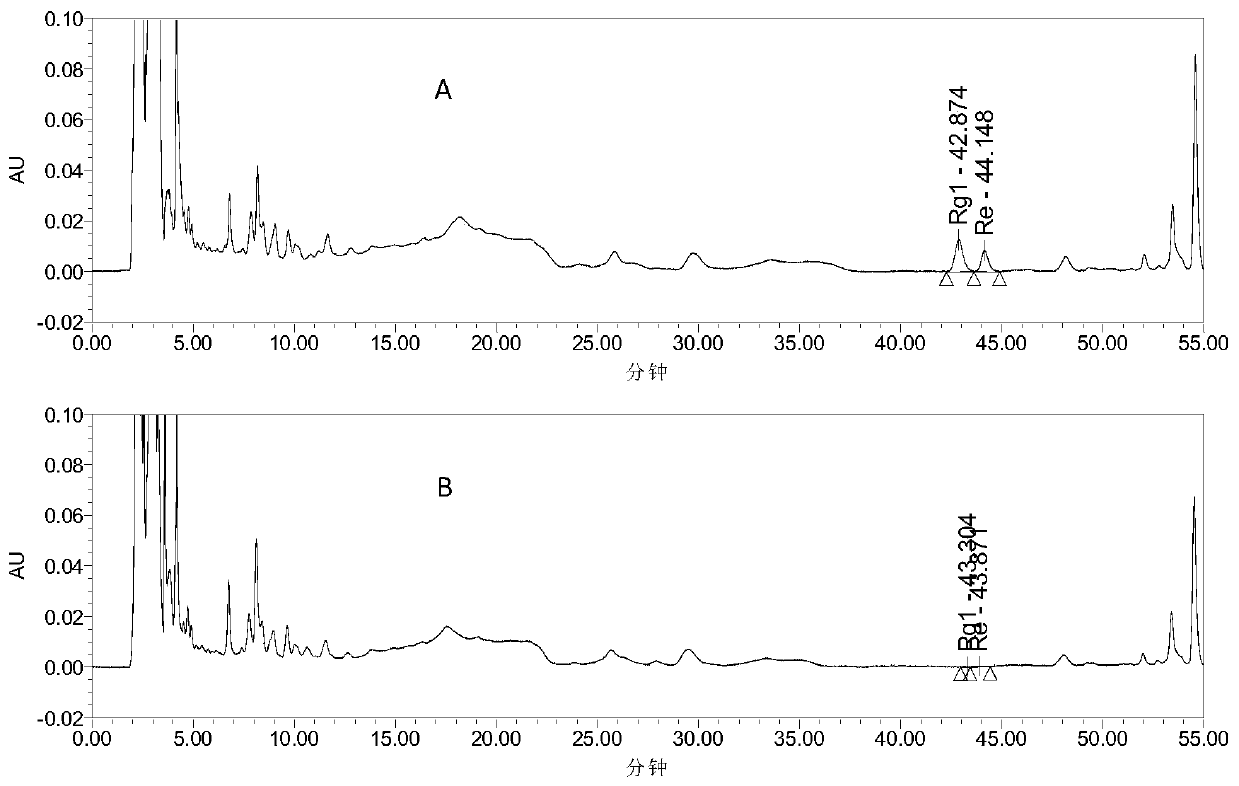
The invention provides a detection method for a traditional Chinese medicine composition. The traditional Chinese medicine composition includes, by weight, 4-9 parts of ginseng, 4-9 parts of fried spine date seed, 4-9 parts of honey-fried herba epimedii, 5-12 parts of prepared rehmannia root, 3-8 parts of largehead atractylodes rhizome stir-fried with bran, 3-8 parts of processed radix polygalae,4-9 parts of grassleaf sweetflag rhizome, 5-12 parts of Chinese angelica, and 1-5 parts of honey-fried liquorice root. The detection method includes taking octadecylsilane bonded silica gel as a filling agent, taking pure water as a mobile phase A, taking acetonitrile as a mobile phase B, and performing gradient elution, a detection wavelength being 198-208 nm; determining ginsenoside Rg1 and ginsenoside Re content; utilizing an HPLC method to determine effective component beta-asarone icariin content in the composition, and identifying the Chinese angelica; and utilizing a TLC method to realize qualitative identification on the prepared rehmannia root and the honey-fried liquorice root in a prescription. The method is high in accuracy and sensitivity and wide in detection scope, so that the effective controlling of drug quality can be realized; and the method can be taken as the quality monitoring method for traditional Chinese medicine compositions and preparations.
Owner:BEIJING ZHONGYAN TONGRENTANG CHINESE MEDICINE R & D +1
Method for establishing HPLC (High Performance Liquid Chromatography) fingerprints of stiff silkworms
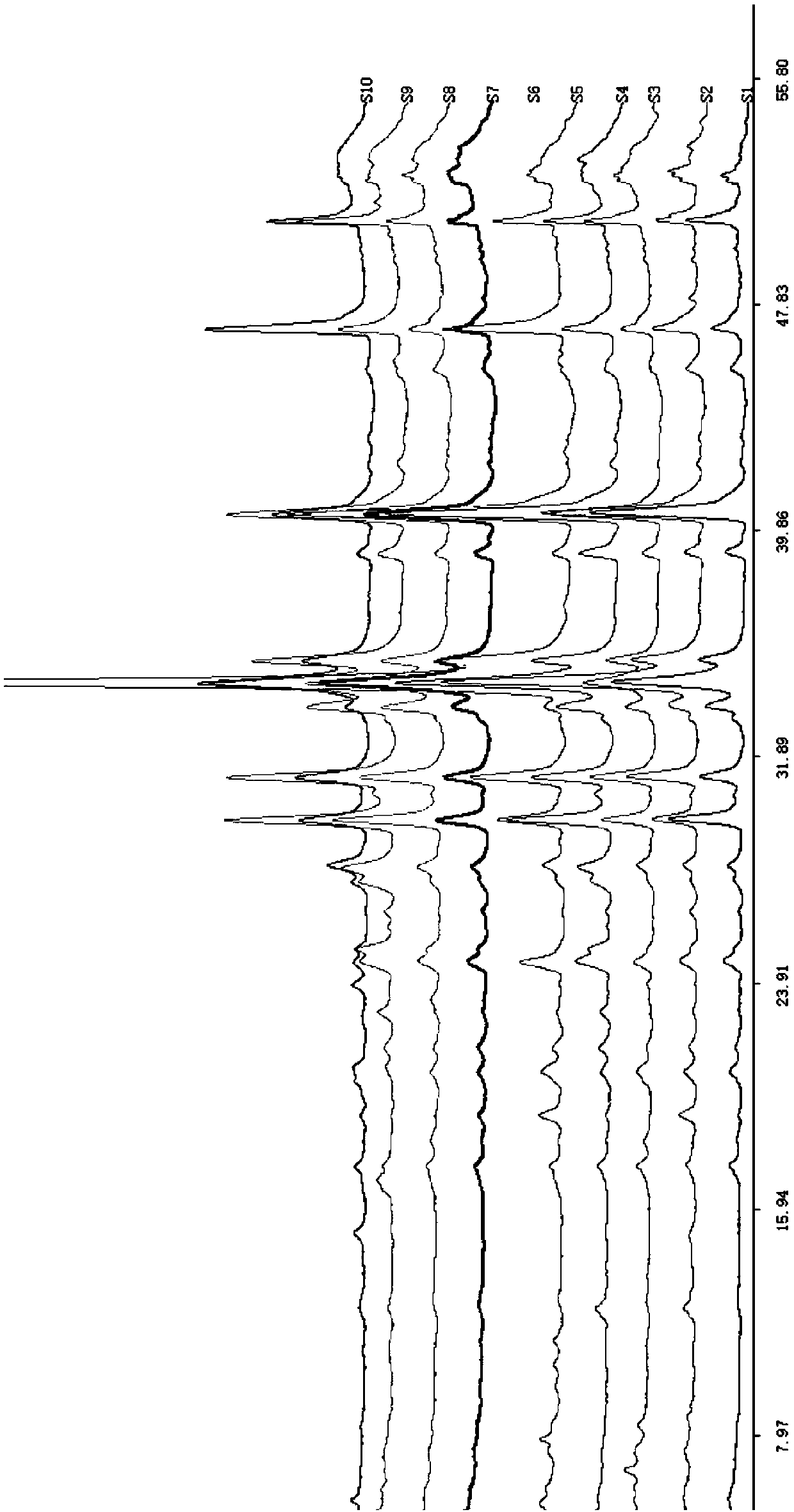
InactiveCN109521132AGuaranteed stabilityGuaranteed validityComponent separationHplc fingerprintHplc mass spectrometry
The invention discloses a method for establishing HPLC (High Performance Liquid Chromatography) fingerprints of stiff silkworms. The method comprises the following steps: weighing stiff silkworm powder, performing ultrasonic extraction with an 80% (V / V) ethanol solution according to a solid-liquid ratio of 1:20, filtering, evaporating to dryness, fixing the volume with methanol, and filtering by amicrofiltration membrane so as to obtain a test solution; preparing a reference solution; sucking 10uL of the test solution or mixed reference solution, injecting into a high performance liquid chromatograph to detect, and recording the fingerprints within 60 minutes; determining HPLC fingerprints of multiple batches of stiff silkworm medicinal materials, and analyzing by a similarity evaluationsystem, thereby obtaining the HPLC characteristic fingerprints of the traditional Chinese medicinal material stiff silkworms. The HPLC reference fingerprints of the stiff silkworms established by highperformance liquid chromatography have excellent precision, reproducibility and stability, and can achieve effects of comprehensively reflecting the types and quantities of the chemical components contained in the stiff silkworms and performing overall description and evaluation on the drug quality.
Owner:SERICULTURE & AGRI FOOD RES INST GUANGDONG ACAD OF AGRI SCI +1
Indapamide sustained-release drug composite and preparation method thereof
InactiveCN103142529AAvoid sudden releaseRelease stabilityOrganic active ingredientsPharmaceutical delivery mechanismDrug release rateAdhesive
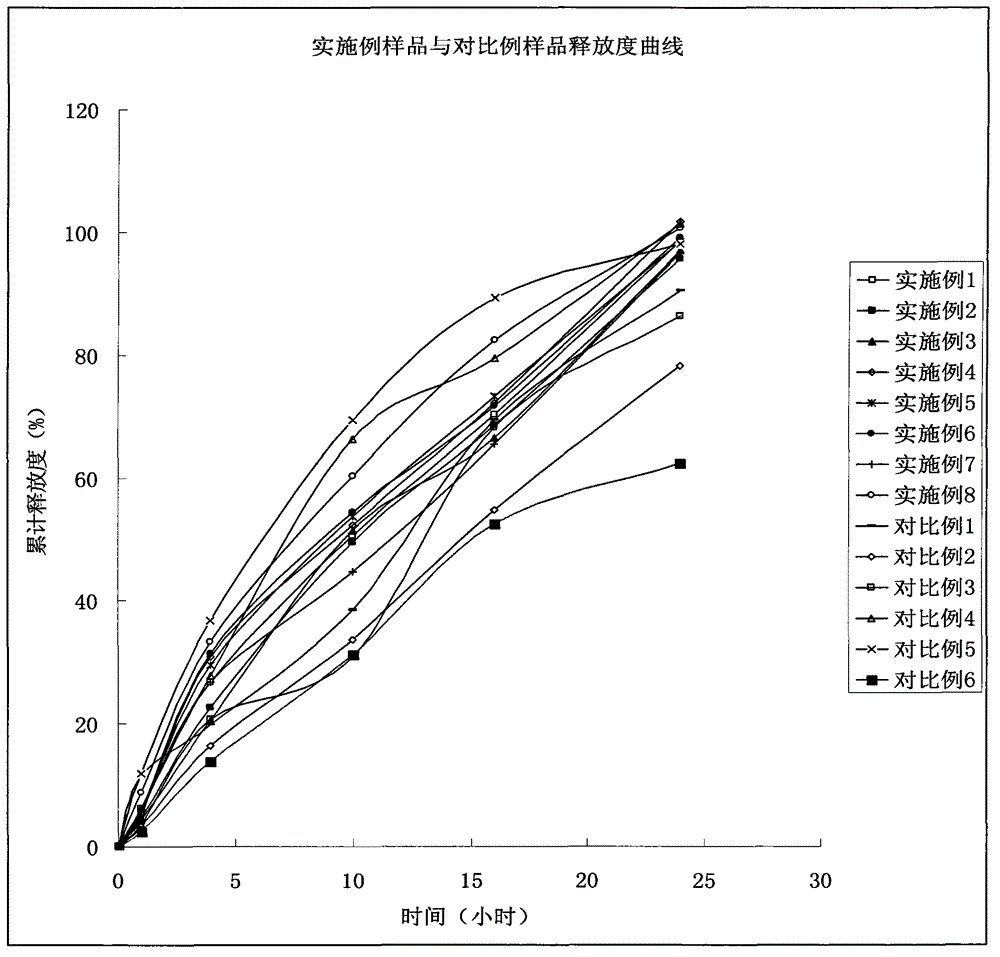
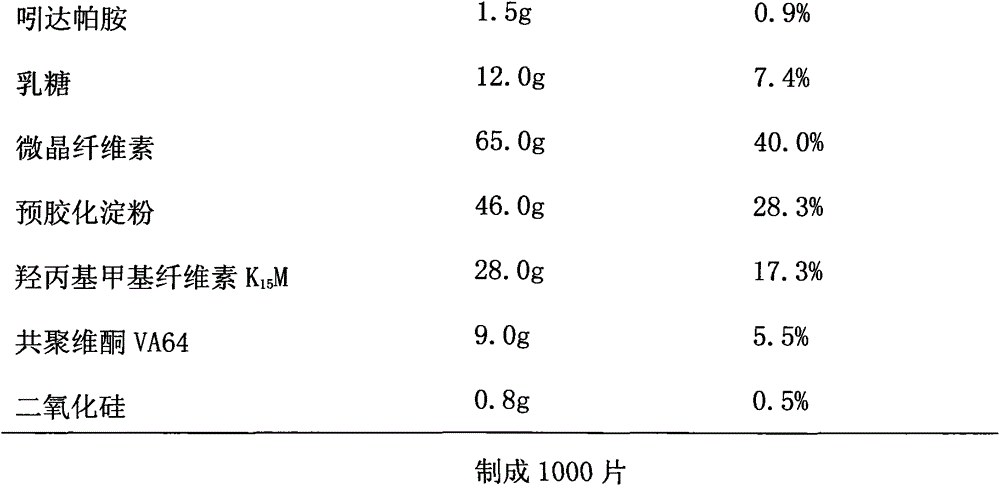
The invention discloses an indapamide sustained-release drug composite and a preparation method thereof. The indapamide sustained-release drug composite comprises the following ingredients: indapamide serving as an active ingredient and a proper amount of filler, framework material, lubricant and copovidone (VA64). The indapamide sustained-release tablet disclosed by the invention is used for treating primary hypertension and is characterized in that the VA64 is added in the prescription so that the burst release of the drug is prevented, the stable release of the drug is guaranteed and the hypokalemia caused by overhigh blood concentration is avoided; in the process, the indapamide is micronized to below 50 micrometers so that the release of the drug is improved and the delayed release of the drug is avoided; and the direct powder compression process is adopted so that the problems that the framework material coheres to form sticky balls due to adhesive and the homogeneity of drug releasing rate is influenced are avoided. The prepared indapamide sustained-release tablet has the advantages that the drug releasing rate is stable; the homogeneity of the releasing rate is good; the drug bioavailability is increased; and the drug quality is guaranteed.
Owner:KANGYA OF NINGXIA PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com