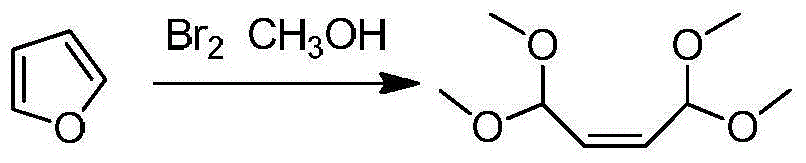
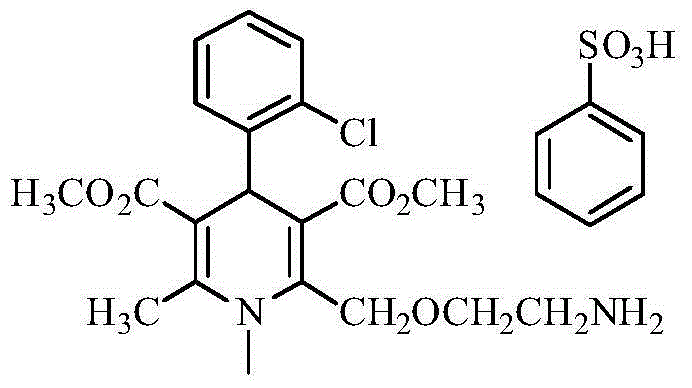
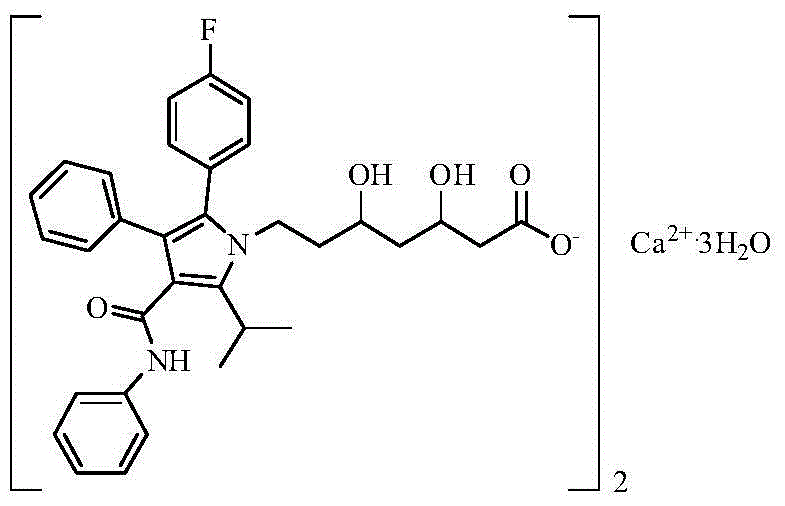
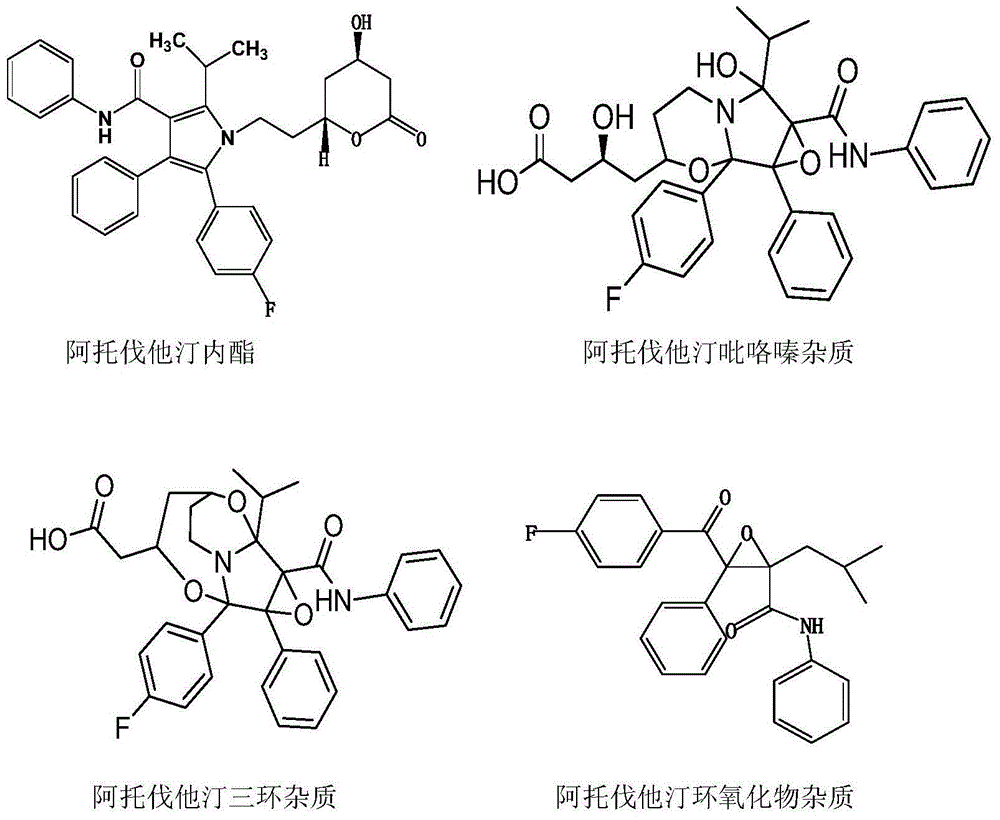
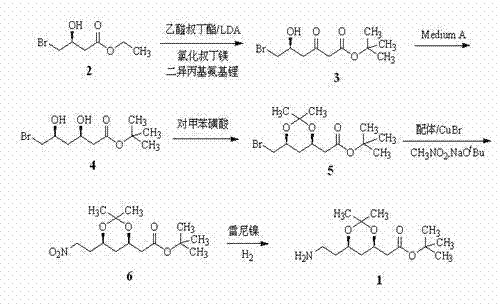
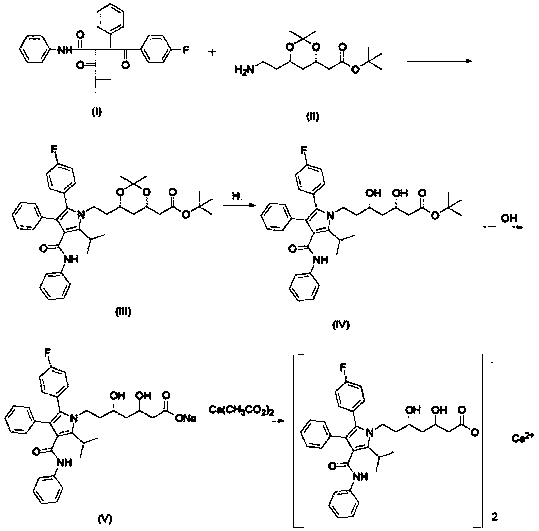
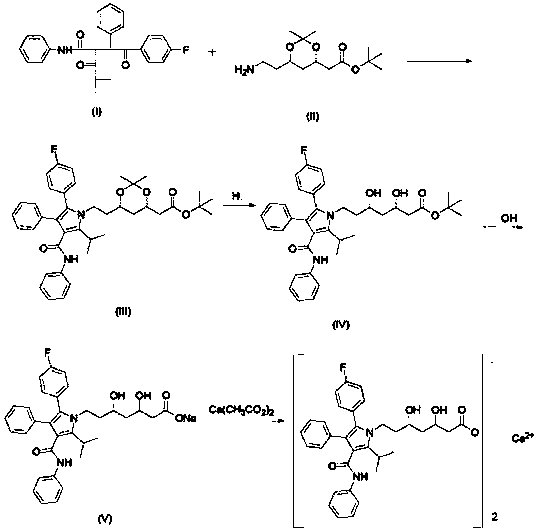
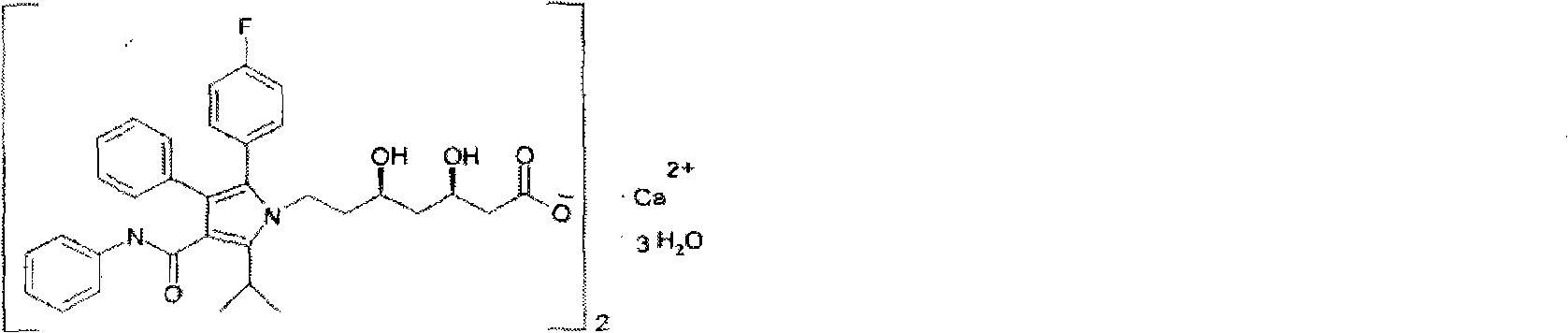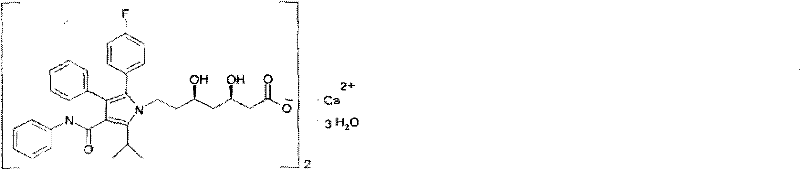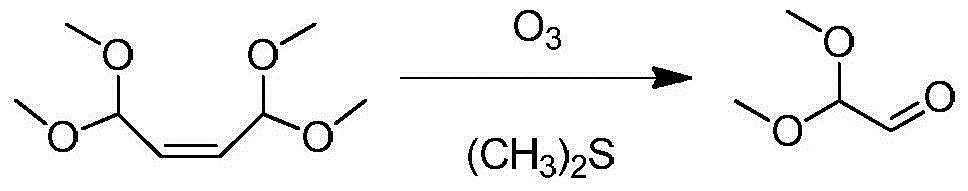Patents
Literature
282 results about "Atorvastatin calcium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Stablized pharmaceutical composition comprising an amorphous active substance
The invention relates to the pharmaceutical composition comprising the amorphous active substance which is atorvastatin calcium. The process of stabilization of the pharmaceutical composition comprising the pharmaceutical formulation with amorphous atorvastatin calcium, the process of stabilization of the pharmaceutical formulation comprising amorphous atorvastatin calcium and the process of stabilization of atorvastatin calcium in an amorphous form is described.
Owner:LEK PHARMA D D
Quick-disintegration tablets of calcium atovastatine, and its prepn. method
ActiveCN1911209AIncreased or improved disintegrationEnhanced or improved dissolutionMetabolism disorderPill deliveryLubricantChemistry
Owner:CSPC OUYI PHARM CO LTD
Atorvastatin calcium tablet and preparation method thereof
ActiveCN102920675AHigh dissolution rateImprove bioavailabilityMetabolism disorderPharmaceutical non-active ingredientsFiller ExcipientHardness
The invention discloses an atorvastatin calcium tablet and a preparation method thereof. The tablet consists of the following components in parts by mass: 7.22 parts of main medicine atorvastatin calcium, 84.55 parts of filler, 6 parts of disintegrating agent croscarmellose sodium, 1.33 parts of adhesive hydroxy propyl cellulose, 800.4 parts of cosolvent polysorbate and 0.5 part of lubricating agent magnesium stearate, wherein the filler comprises the following raw materials in parts by mass: 22.01 parts of calcium carbonate, 21.87 parts of milk sugar and 40.67 parts of microcrystalline cellulose. The atorvastatin calcium tablet has the characteristics of short disintegrating time, fast dissolving-out speed, high bioavailability and small particle diameter, and is convenient to take. Furthermore, the hardness of the tablet can reach 60-70N, so that the tablet is hardly broken, and therefore, the packing and transporting costs are reduced, and the industrialized popularization of the tablet is easily realized.
Owner:HENAN RUNHONG PHARMA
Atorvastatin calcium form vi or hydrates thereof
InactiveUS20060122403A1High purityImprove solubilityOrganic chemistryMetabolism disorderX-rayPowder diffraction
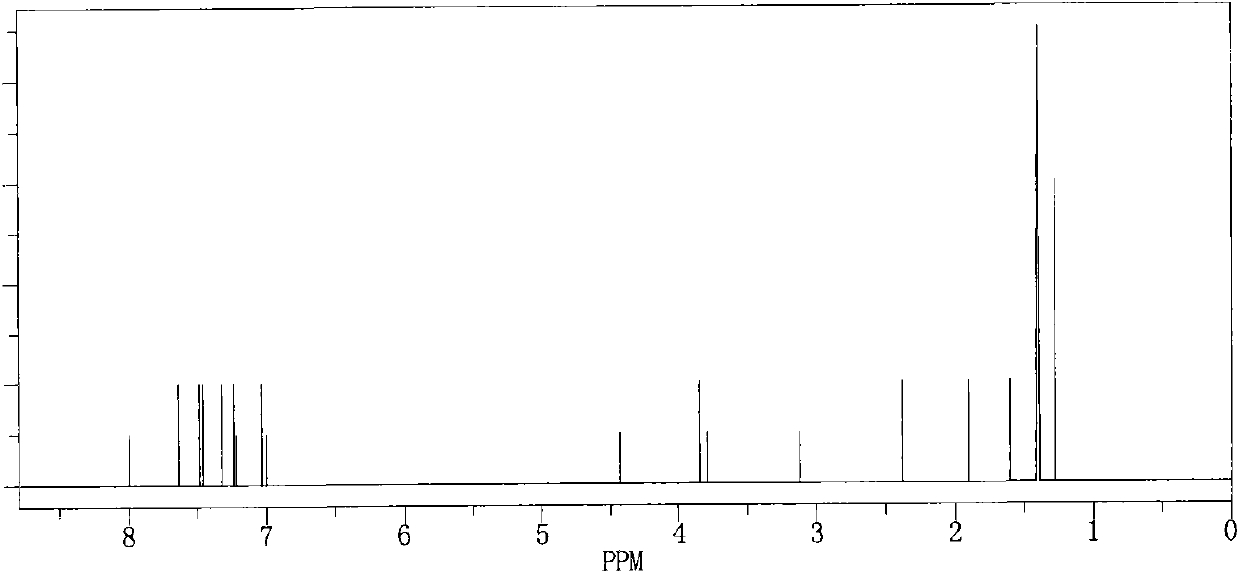
Atorvastatin calcium Form VI Or hydrates thereof, characterized by its X-ray powder diffraction and / or solid state NMR is described, as well as methods for the preparation of the same.
Owner:SURI SANJAY +3
Process for the preparation of amorphous atorvastatin calcium
InactiveUS6646133B1Suitable for preparationShort timeOrganic chemistryOrganic solventSecondary hyperlipidemia
The invention relates to a process for the preparation of amorphous atorvastatin calcium by recrystallization of crude atorvastatin from an organic solvent which comprises dissolving crude amorphous atorvastatin calcium in a lower alkanol containing 2-4 carbon atoms or a mixture of such alkanols under heating and isolating the amorphous atorvastatin calcium precipitated after cooling. The atorvastatin calcium obtained is a known valuable agent useful in treating hyperlipidemia and hypercholestrolemia.
Owner:EGIS GYOGYSZERGYAR NYILVANOSAN MUKODO RESZVENY TARSASAG
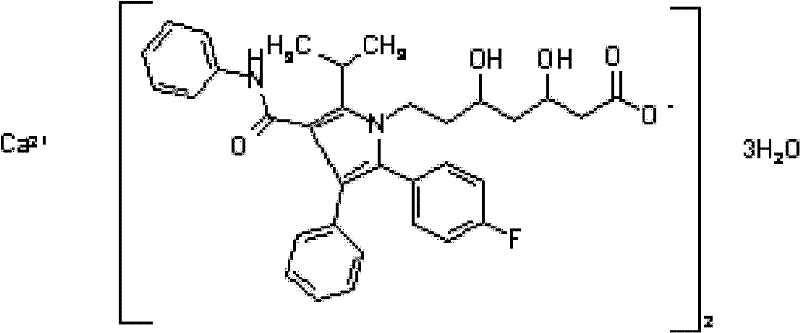
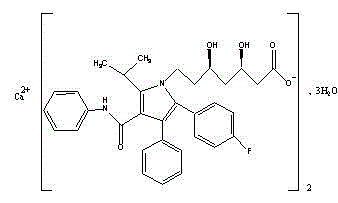
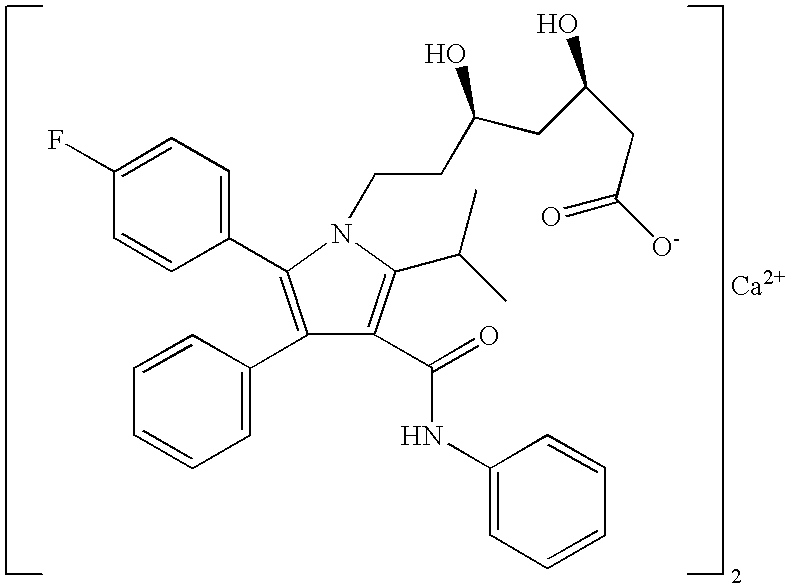
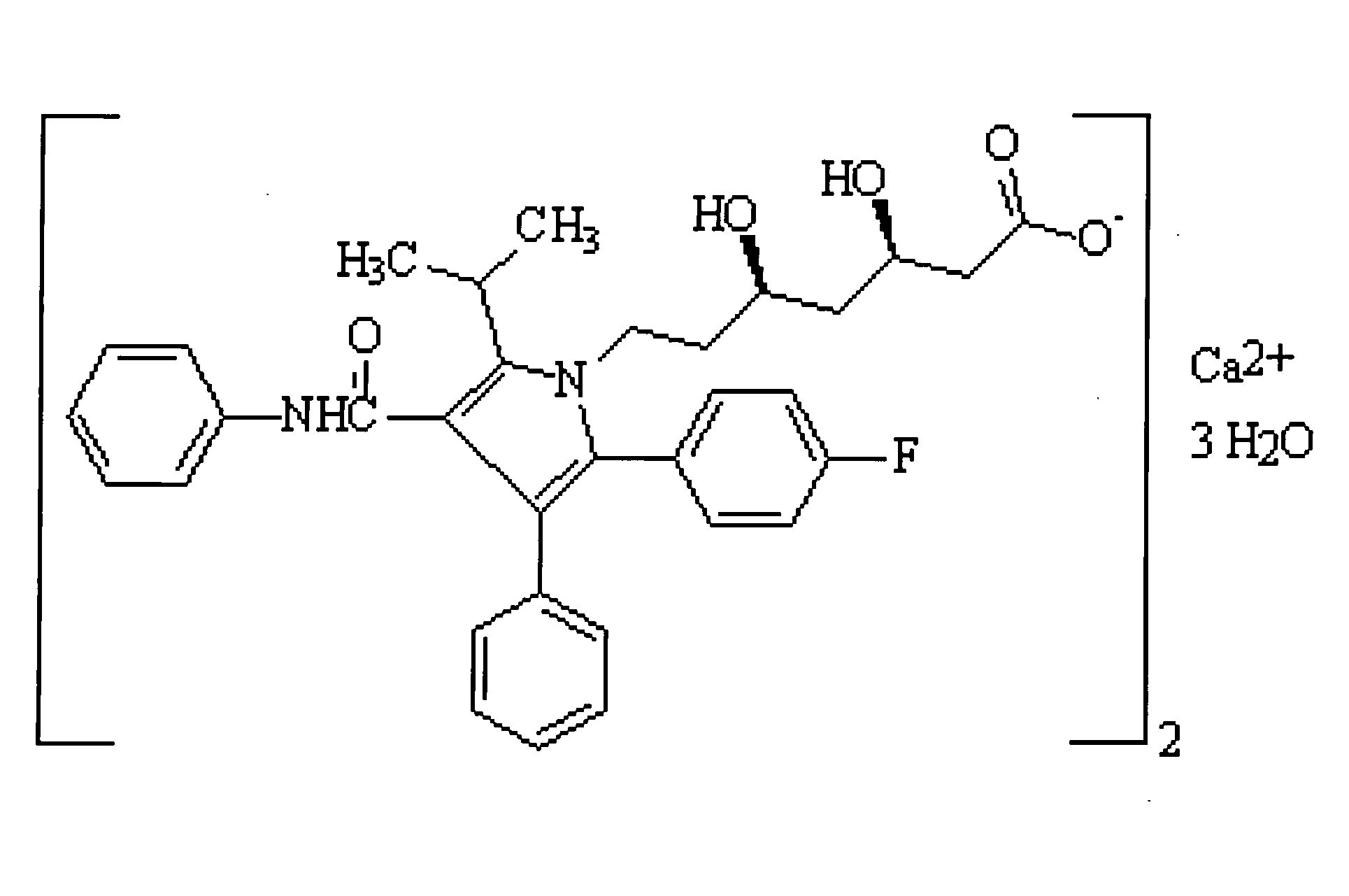
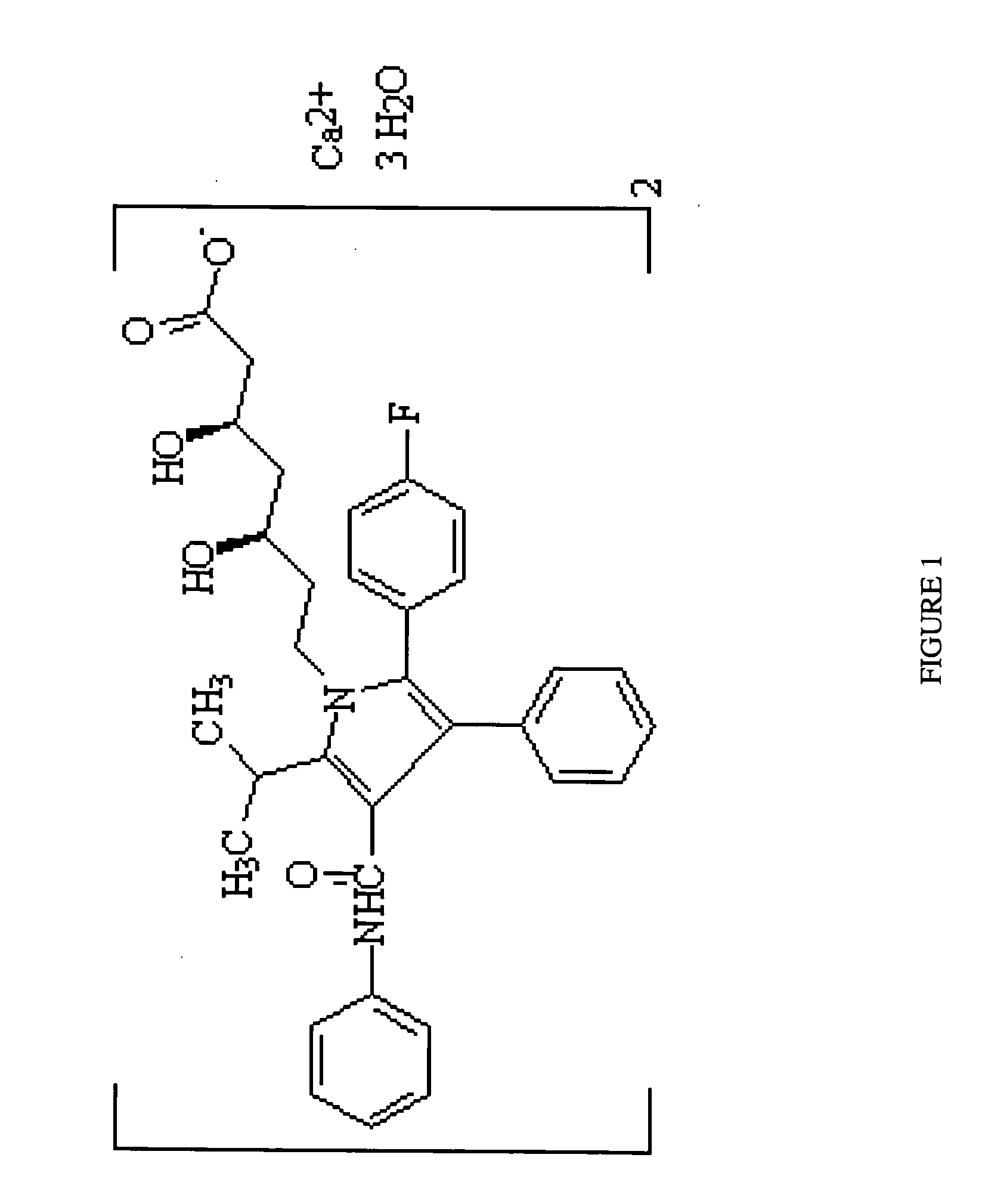
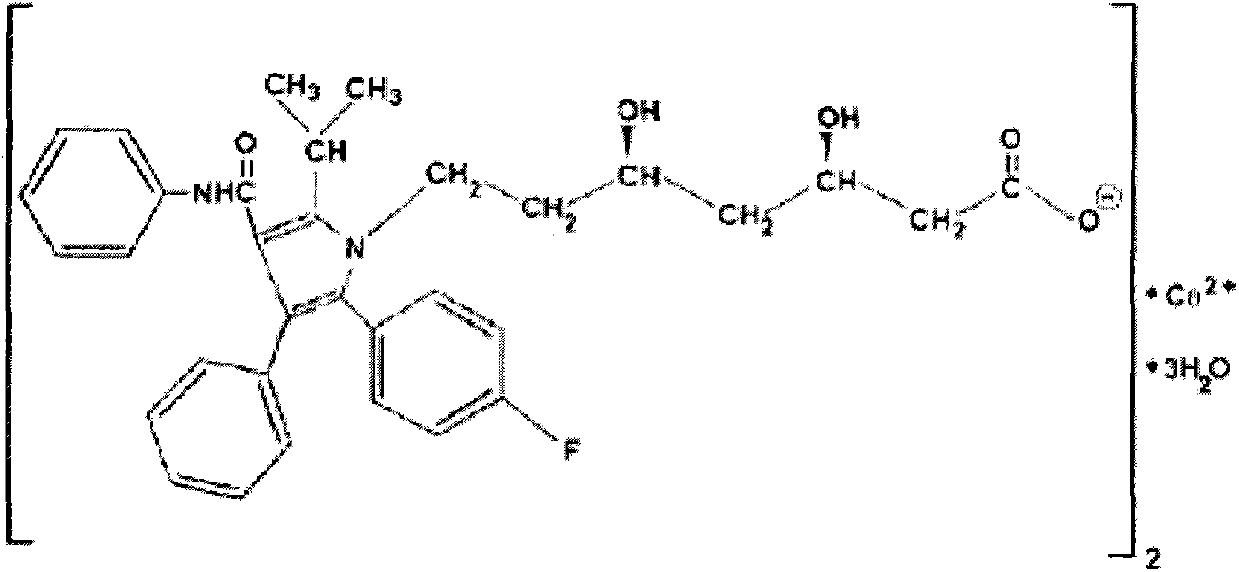
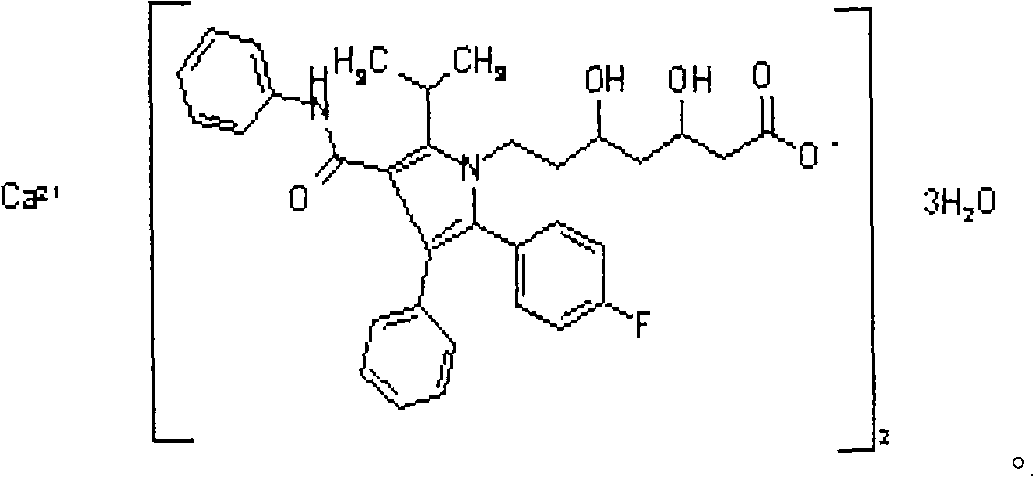
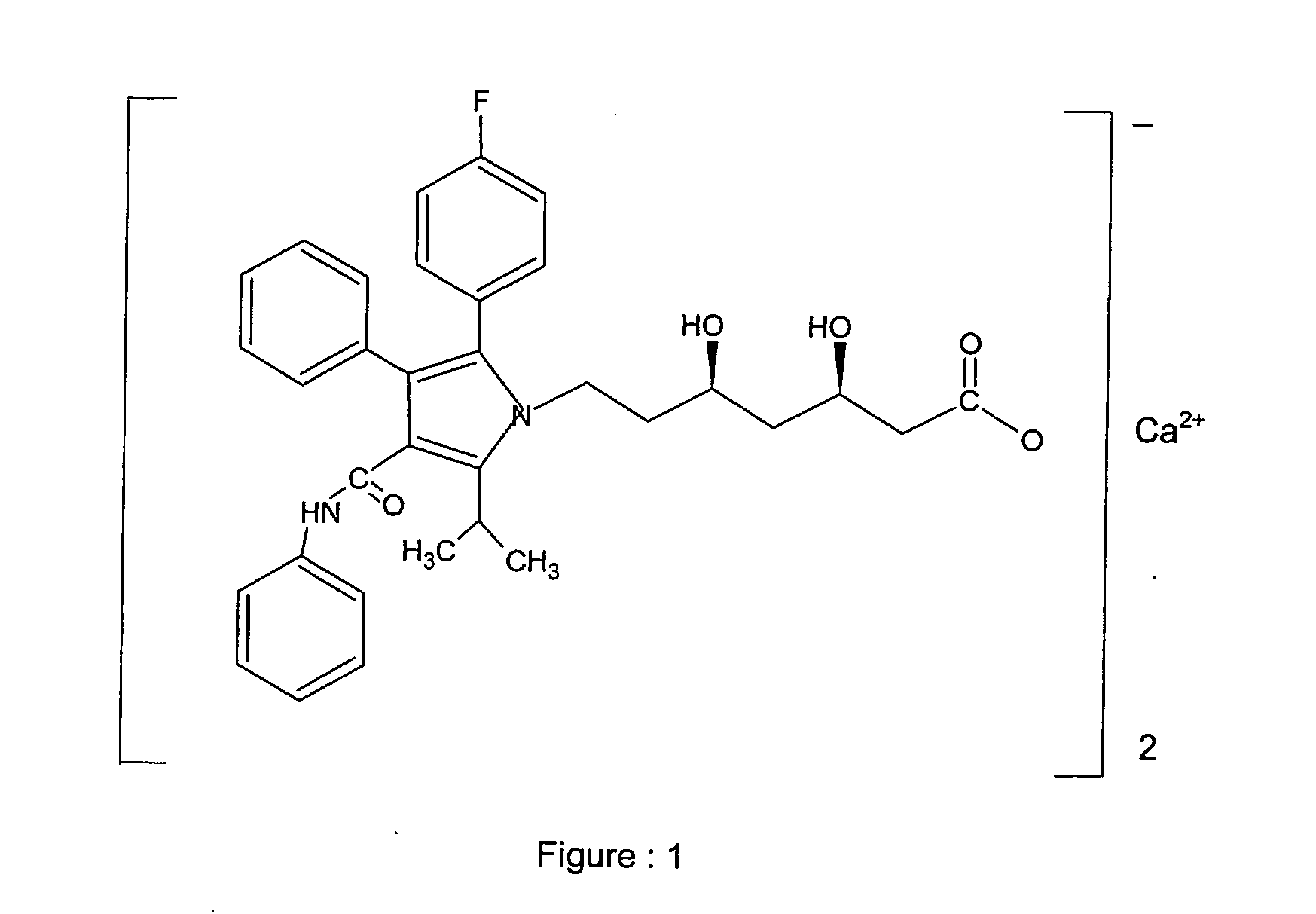
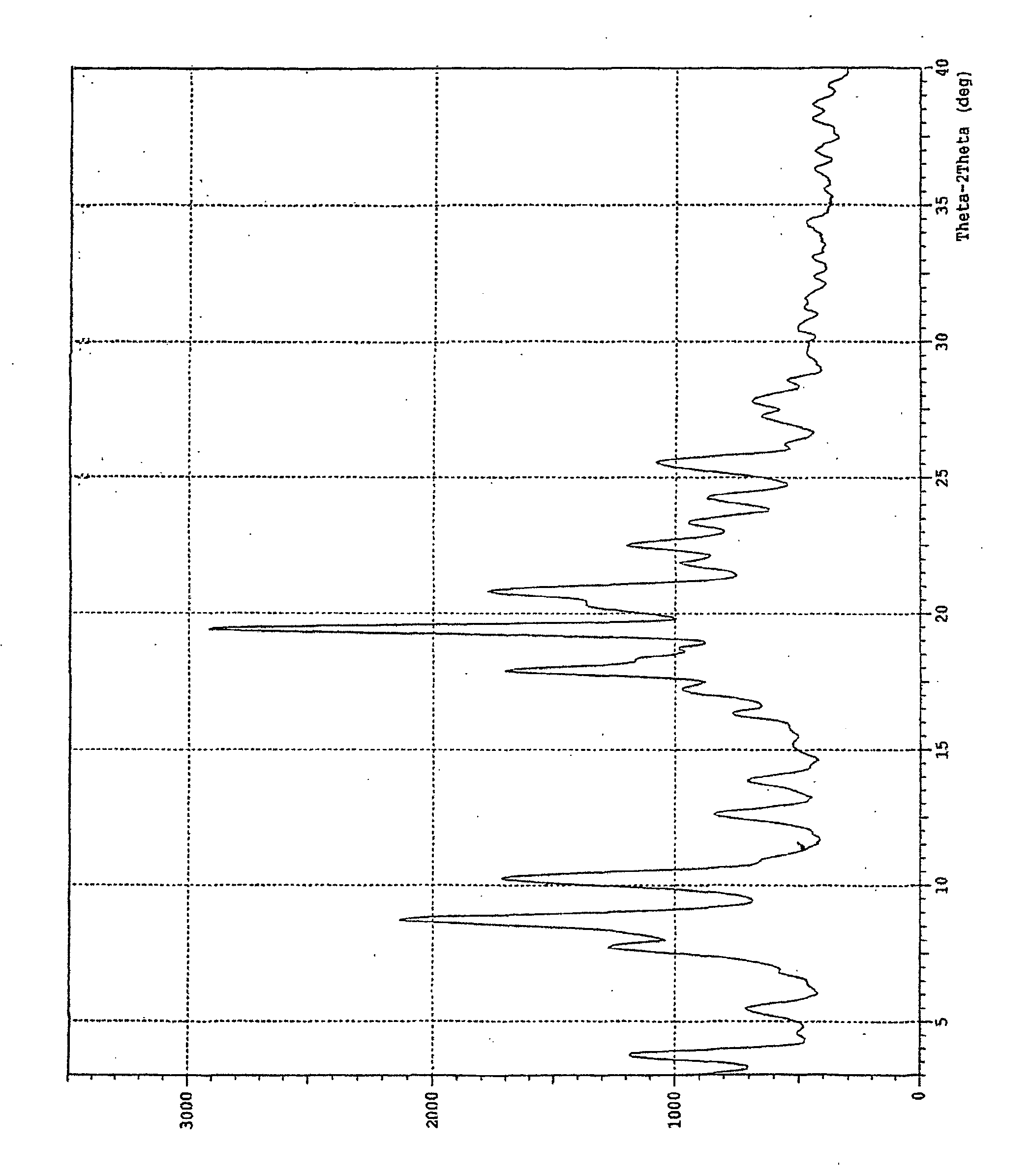
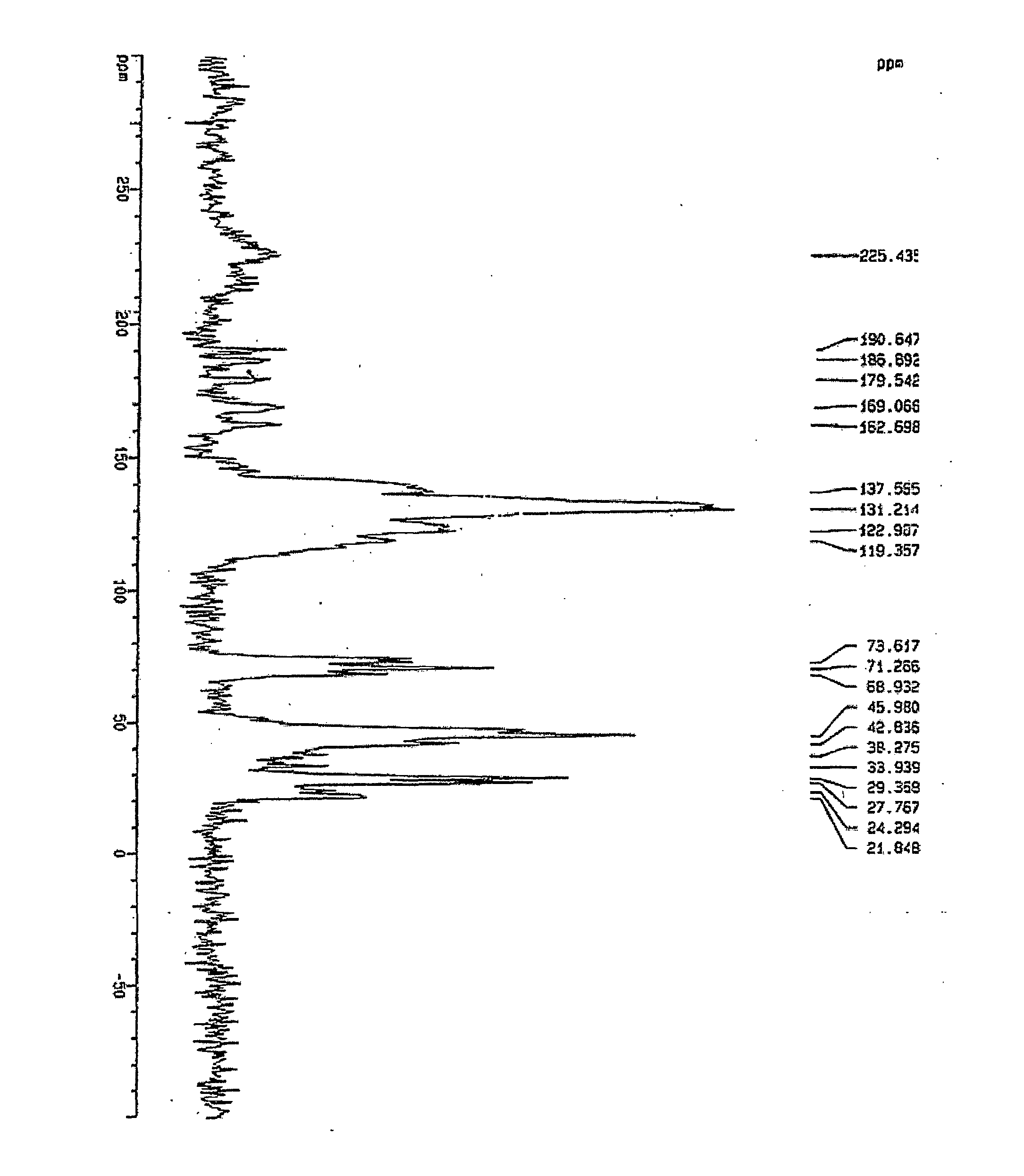
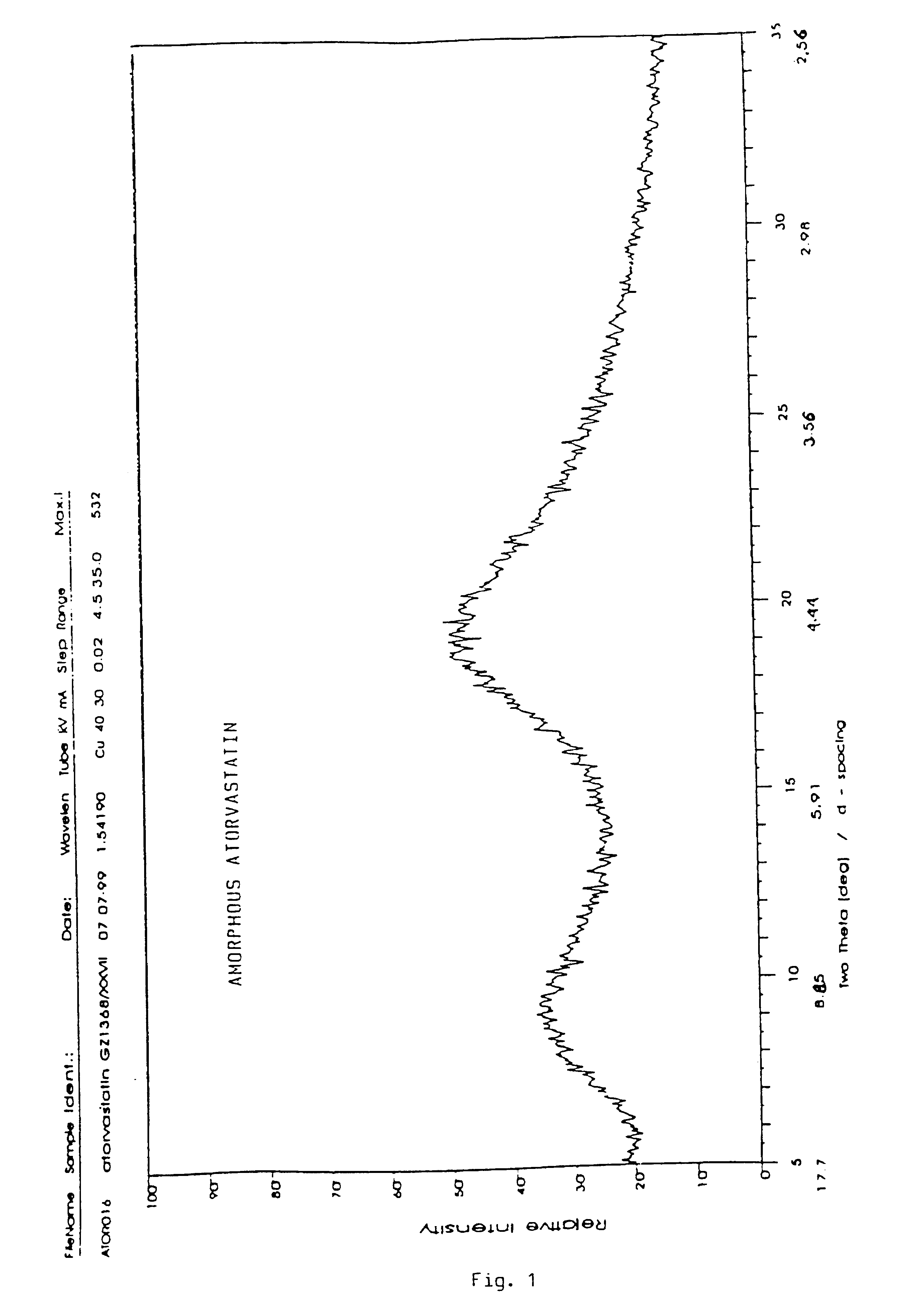
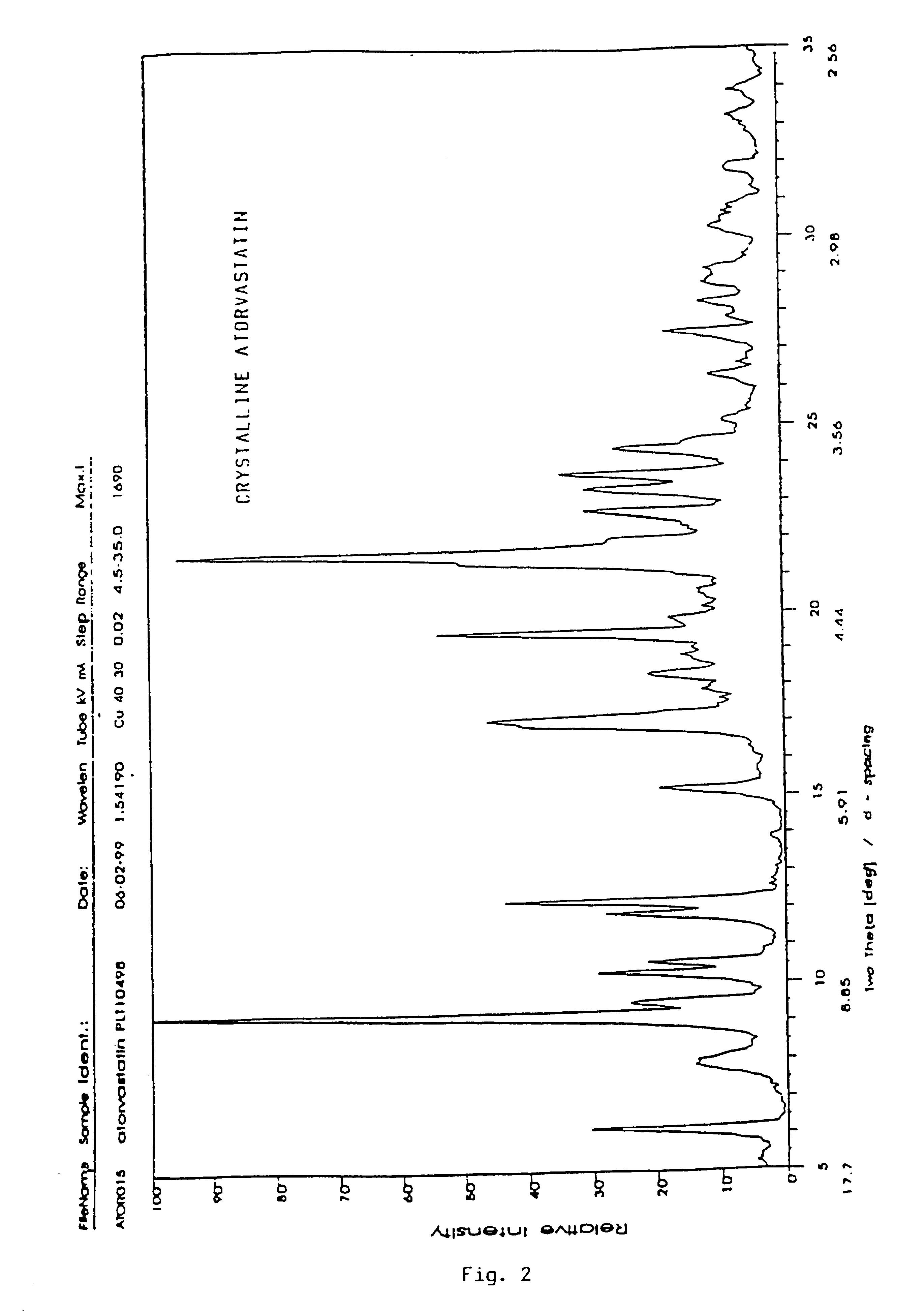
Crystalline [R-(R*,R*)]-2-(4-fluorophenyI)-beta,delta-dihydroxy-5-(1-methylethyl)-3-phenyl- 4-[(phenylamino)carbonyl]-1H-pyrrole-heptanoic acid calcium salt (2:1)
Novel crystalline forms of atorvastatin calcium designated Fa and Je which are prepared by crystallization from non-aqueous, non-polar solvents at a temperature above 90° C. The crystalline forms are useful as agents for treating hyperlipidemia and hypercholesterolemia.
Owner:IVAX PHARMA
Atorvastatin calcium tablet
ActiveCN102309462AQuality improvementImprove stabilityMetabolism disorderPill deliveryWestern medicinePharmacology
The invention which relates to an atorvastatin calcium tablet for treating hyperlipidemia belongs to the technical field of western medicine preparations. In the tablet, the weight ratio of atorvastatin calcium: a filler: a disintegrant: a lubricant is 1:8-15:0.5-1.5:0.1-0.3. The tablet of the present invention has the advantages of stable quality, rapid disintegration and leaching, moderate tablet hardness, and simple preparation technology.
Owner:SUZHOU CHUNGHWA CHEM & PHARMA IND
Atorvastatin calcium tablets and preparation method thereof
ActiveCN102138910AGood dispersionRapid dissolutionMetabolism disorderPill deliveryDissolutionLactose
The invention relates to atorvastatin calcium tablets and a preparation method thereof. The tablets comprise the following components in parts by weight: 1 part of atorvastatin calcium (in terms of atorvastatin), 1 part of croscarmellose sodium, 15 parts of lactose (80M), and 0.2 part of magnesium stearate. The preparation method comprises the following steps: screening the atorvastatin calcium and the croscarmellose sodium based on the amounts of the formula through a 120-meshed sieve respectively, and mixing uniformly; uniformly mixing the mixture with the lactose based on the amount of the formula according to a uniform progressive increasing method; and uniformly mixing the magnesium stearate based on the amount of the formula, and tabletting. The tablets have the advantages of stable quality, controllable related substances, good dispersibility and high dissolution speed.
Owner:LUNAN PHARMA GROUP CORPORATION
Atorvastatin calcium oral disintegrating tablet and preparation method thereof
InactiveCN101791297AEasy to takeHas hardnessMetabolism disorderPill deliveryMicroparticleMedical prescription
The invention provides an atorvastatin calcium oral disintegrating tablet prescription and a preparation method thereof. The atorvastatin calcium oral disintegrating tablet prescription comprises atorvastatin calcium, a bitter covering agent, a filling agent, a disintegrant, a lubricant, and the like. The invention is technically characterized by providing a particle preparing technology which not only can effectively cover the bitter of the atorvastatin calcium, but also can lead the atorvastatin calcium oral disintegrating tablet to be disintegrated in the mouth. The atorvastatin calcium oral disintegrating tablet with pressure of above 4kg and disintegrating time within 30 seconds can be prepared by adding normal supplementary materials of filling agents, disintegrants, lubricants, and the like of oral disintegrating tablets and is easy to realize industrial scale production.
Owner:CHINA PHARM UNIV
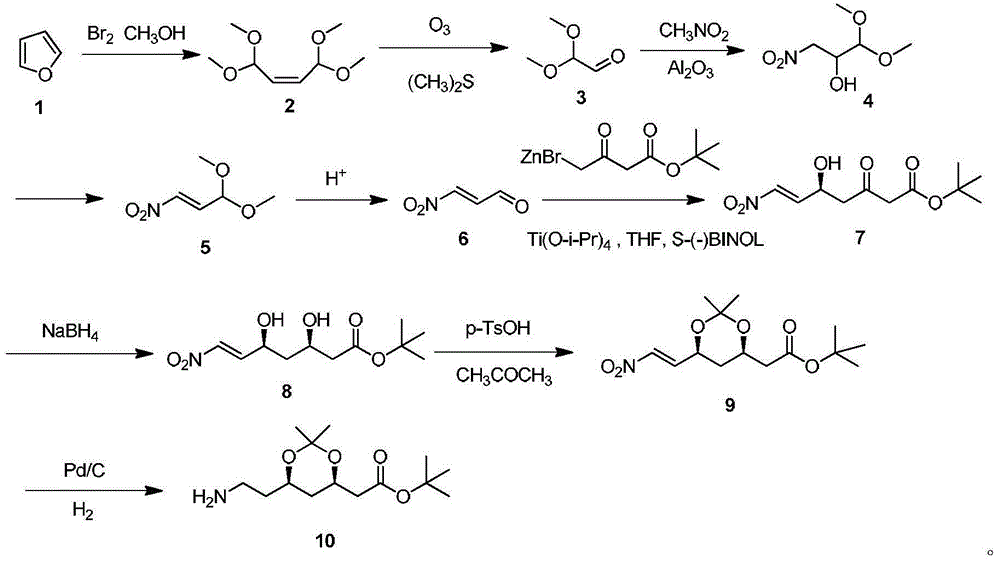
Method for preparing chiral intermediate of atorvastatin
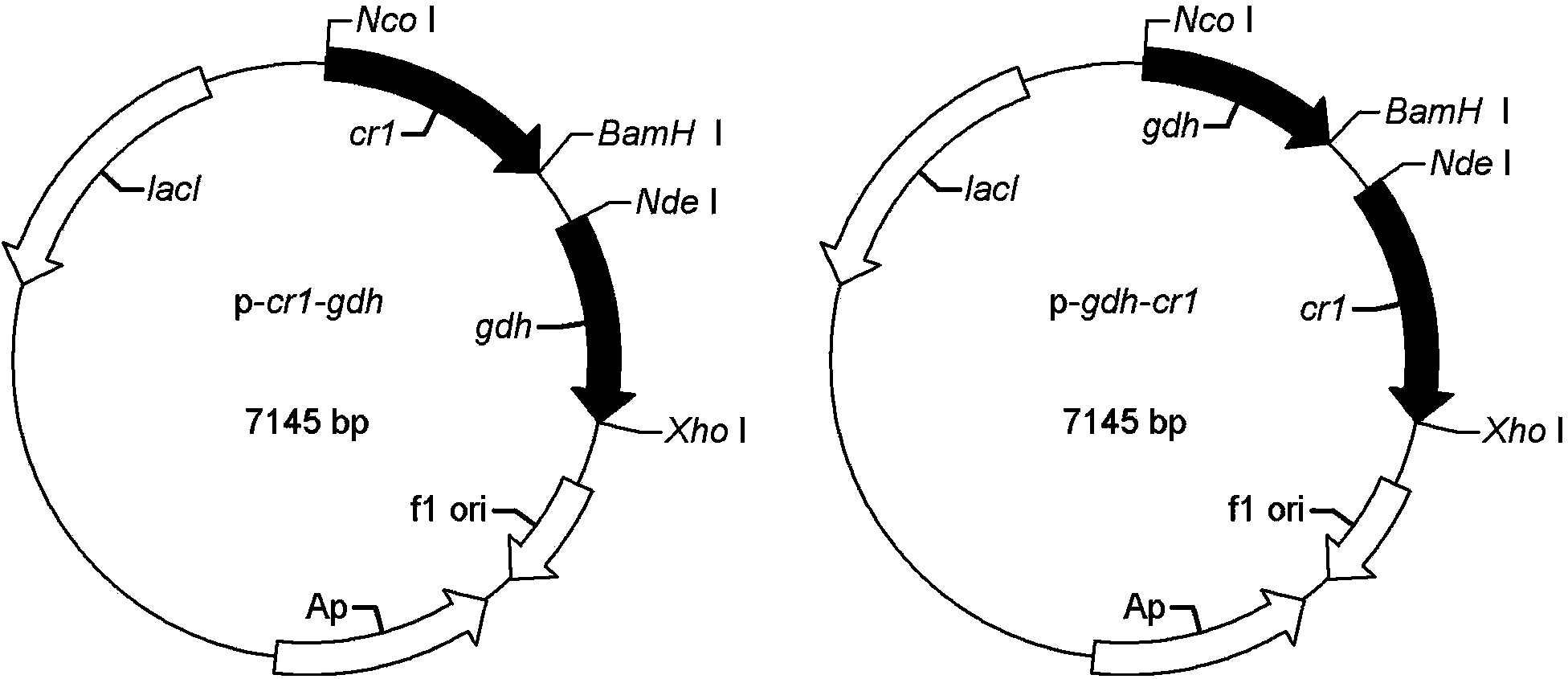
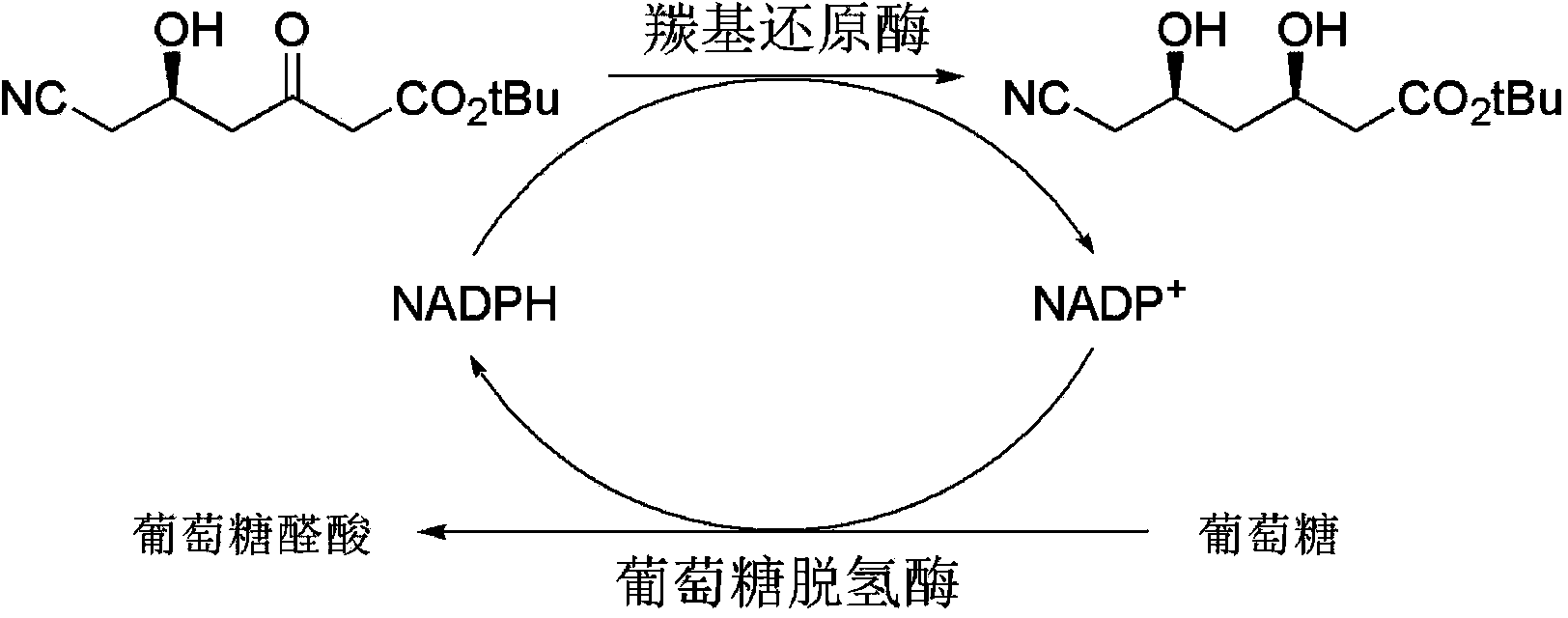
InactiveCN103911403AEfficient coenzyme cycleReduce processing costsBacteriaMicroorganism based processesSide chainGenetically engineered
The invention discloses a method for preparing 6-cyano-(3R, 5R)-dihydroxyl tert-butyl caproate by using genetically engineered bacteria as a whole-cell biocatalyst. The method specifically comprises the following steps of designing and optimizing a tandem co-expression policy of a carbonyl reductase and a glucose dehydrogenase according to the expression characteristics of the carbonyl reductase and the glucose dehydrogenase, establishing a brand-new biological catalysis system in which cyclic regeneration of coenzymes is matched with the reduction of the 6-cyano-(3R, 5R)-dihydroxyl tert-butyl caproate, thus realizing in-situ biological synthesis of the 6-cyano-(3R, 5R)-dihydroxyl tert-butyl caproate, namely a chiral side chain synthesis precursor of atorvastatin. By optimizing expression conditions and reaction conditions and under the conditions that a cosolvent is 5% dimethyl sulfoxide, a reaction solution pH is 7.0, the temperature is 20 DEG C, and a ratio of glucose to a substrate is 1.2: 1, the concentration of the substrate for the in-situ biological synthesis of the 6-cyano-(3R, 5R)-dihydroxyl tert-butyl caproate can be 35g / L, and meanwhile, the addition of an exogenous coenzyme is completely avoided, and therefore, the method has wide application prospect.
Owner:CHINA PHARM UNIV
Oral proliposome containing atorvastatin calcium and preparation method of oral proliposome
InactiveCN103690485AHighlight substantive featuresSignificant progressMetabolism disorderPharmaceutical non-active ingredientsOral medicationPhospholipid
The invention relates to an oral proliposome containing atorvastatin calcium and a preparation method of the oral proliposome. The oral proliposome comprises the following components: 0.5%-10% of the atorvastatin calcium, 10%-30% of phospholipid, 2%-10% of poloxamer, 50%-87.3% of absolute ethanol and 0.2%-3% of a stabilizer. The preparation method comprises the following steps: adding the atorvastatin calcium, the phospholipid, the poloxamer and the stabilizer into the absolute ethanol solvent, mixing in the absence of water, and dissolving the mixture into a clear anhydrous solution so as to obtain an atorvastatin calcium proliposome preparation. The proliposome can be quickly self-assembled into the medicated proliposome preparation with a proper particle size and high encapsulation efficiency after being diluted by a proper hydration medium before use and is used for oral administration, the operation is easy and convenient, and the oral administration mode can be easily accepted by patients.
Owner:RUNZE PHARMACEUTICAL (SUZHOU) CO LTD
Method for preparing amorphous atorvastatin calcium
The invention discloses a method for preparing amorphous Atorvastatin calcium. The current method uses more solvent, so the cost is over high; and the residual quantity of the solvent in a product is large, thereby causing big influence on the quality of the product and causing serious environmental pollution. In the method, alcohol solvent and water are combined into mixed alcohol-water solvent which dissolves Atorvastatin calcium containing one or more crystal forms completely; proper temperature is kept for ensuring that the Atorvastatin calcium is not precipitated; and the amorphous Atorvastatin calcium is precipitated by a spray drying method. The method uses the mixed alcohol-water solvent which is removed from the product easily, has little organic residue and causes less influence on drug quality; the usage amount of the solvent is reduced greatly; the concentration of the Atorvastatin calcium in the solution is high; and the purity of the prepared product of the amorphous Atorvastatin calcium is high.
Owner:ZHEJIANG JINGXIN PHARMA
Method for preparing amorphous atorvastatin calcium
The invention provides a method for preparing amorphous atorvastatin calcium. The amorphous atorvastatin calcium is prepared by undergoing a four-step reaction. The method comprises the following steps of: continually performing acidolysis deprotection and an alkali hydrolysis reaction in the same boiler under the condition that methanol and tetrahydrofuran are taken as solvents; particularly extracting a generated calcium salt with propyl acetate or butyl acetate or ethyl acetate; adding acetone for dissolving; concentrating a part; and directly drying under reduced pressure to obtain amorphous atorvastatin calcium, wherein the total yield is more than or equal to 75 percent. Due to the adoption of the method, the reaction period is greatly shortened, the preparing period can be shortened by more than 30 hours, the production cost is lowered, environmental pollution is reduced, the product quality is improved, the process operating steps are simplified, industrial production is easy, operation is convenient, the synthesis yield is high, the raw material cost is low, the product quality is good, and the obtained atorvastatin calcium is amorphous.
Owner:JIANGXI FUSHINE PHARMA CO LTD
Preparation method of atorvastatin calcium preparation
ActiveCN104688708AWell mixedMetabolism disorderPharmaceutical delivery mechanismAlkaline earth metalAdhesive
The invention relates to a preparation method of an atorvastatin calcium preparation. According to the method, atorvastatin calcium is mixed with a hydrophilic diluent and an alkali diluent in an equal increment way, and then mixed with a disintegrating agent and aerosil; an adhesive is added into the mixture; ethanol solution wet granulation is carried out; and then microcrystalline cellulose is added; and tabletting and film coating are carried out to obtain the preparation. The method can well solve the problem of influence of alkaline earth metal salt on bioavailability of atorvastatin calcium preparation, so as to realize good stability, bioavailability and dissolution rate of atorvastatin calcium preparation.
Owner:BEIJING WINSUNNY PHARMA CO LTD
Atorvastatin calcium oral disintegrating tablet and preparation method thereof
InactiveCN101791297BEasy to takeHas hardnessMetabolism disorderPill deliveryMicroparticleMedical prescription
Owner:CHINA PHARM UNIV
Synthesis method for chiral intermediate of atorvastatin calcium
ActiveCN105153110AEasy to operateGood repeatabilityOrganic chemistry methodsChemical synthesisCyanide
The invention discloses a synthesis method for a chiral intermediate of atorvastatin calcium, and belongs to the technical field of medical intermediate synthesis. The synthesis method is characterized in that according to the process route, not only are dangerous, highly toxic and expensive chemicals such as butyl lithium, editpotassium cyanide and periodic acid in chemical synthesis prevented from being used, but also an ee value of the chiral intermediate is effectively improved due to usage of a mixed chiral catalysts of titanium iso-propylate and S-xenol. According to the synthesis method, the raw materials are low in cost and easy to obtain, the route operation is easy, the repeatability is good, the yield is very high, and the synthesis method is suitable for industrial production.
Owner:北京华素制药股份有限公司
Preparation method of high-stability amlodipine atorvastatin calcium tablet
InactiveCN104644633ASimple controlMetabolism disorderPharmaceutical delivery mechanismAmlodipine besilateLubricant
The invention relates to a preparation method of a high-stability amlodipine atorvastatin calcium tablet. The preparation method of the high-stability amlodipine atorvastatin calcium tablet is characterized in that the amount of peroxide in microcrystalline cellulose is controlled. The preparation method of the high-stability amlodipine atorvastatin calcium tablet comprises the following steps: A, granulating of atorvastatin calcium, concretely comprising the steps of firstly dissolving a surfactant in water, adding a binding agent, stirring and dissolving, secondly mixing atorvastatin calcium, calcium carbonate, other diluents and a disintegrant, thirdly, granulating, and fourthly, drying wet granules obtained in the step three, so that dry atorvastatin calcium granules are prepared; and B, preparing of finished granules, concretely comprising the steps of firstly adding amlodipine besylate, a disintegrant and a flow aid into the dry atorvastatin calcium granules, secondly, uniformly mixing powder obtained in the step one in a mixing machine, thirdly, adding a lubricating agent, and uniformly mixing, and fourthly, pressing the powder into tablets.
Owner:CHINA RESOURCES SAIKE PHARMA
Synthetic method of atorvastatin calcium intermediate
InactiveCN103614430AFew stepsLow costOrganic chemistryMicroorganism based processesEthyl groupNitromethane
The invention discloses a synthetic method of an atorvastatin calcium intermediate. The synthetic method comprises the following steps: preparing [(4R, 6S)-6- brooethyl-2,2-diemthyl-1,3-dioxane-4-group] tert-butyl acetate (5) by (3S)-4-bromine-3-hydroxyl ethyl butyrate through condensation, asymmetric biological catalytic reduction and hydroxyl protection; and preparing an atorvastatin calcium intermediate [(4R, 6S)-6-(2-aminoethyl)-2,2-dimethyl-1,3-dioxane-4-group] tert-butyl acetate through catalytic hydrogenation reduction of raney nickel after 5 is condensed with nitromethane under action of cuprous bromide and a nitrogen-containing compound ligand. According to the synthetic method disclosed by the invention, steps are shortened, cost is lowered, and therefore, the synthetic method is more suitable for large-scale preparation.
Owner:SUZHOU HEALTH COLLEGE
Preparing method of high-purity atorvastatin calcium
The invention discloses a preparing method of high-purity atorvastatin calcium, and belongs to the technical field of organic synthesis. The method includes the steps of making a compound V and calcium acetate react in a water and alcohol mixed solvent system, and conducting cooling crystallization and filtration after the reaction is completed to obtain an atorvastatin calcium crude product, wherein the reaction temperature is 40-70 DEG C, the volume ratio of alcohols to water in the reaction system is 1:(2-8), and the mass percentage concentration of the compound V in a mixed solvent is 5-10%; dissolving the atorvastatin calcium crude product in a recrystallization solvent A, adding I-type atorvastatin calcium crystals at 45-85 DEG C for crystal transformation, and conducting cooling crystallization, filtration, washing and drying after crystal transformation is completed to obtain a fine product, wherein the mass percentage concentration of the atorvastatin calcium crude product inthe recrystallization solvent A is 5-10%. The method has the advantages of being high in product purity, easy and safe to operate, high in yield and the like and is suitable for large-scale industrialproduction.
Owner:HUBEI GUANGJI PHARMA
Stable pharmaceutical formulation comprising atorvastatin calcium
InactiveUS20080038332A1Inhibit degradationsInhibit formationBiocidePowder deliveryAntioxidantWater insoluble
The invention relates to a stable pharmaceutical formulation comprising an intimate admixture or admixture of crystalline or amorphous atorvastatin calcium, and a stabilizing-effective amount of a water-insoluble alkaline excipient or a combination of one or more water-insoluble alkaline excipients thereof, a stabilizing-effective amount of an antioxidant or a combination of one or more antioxidants thereof, and at least one or more additional pharmaceutically acceptable inert excipients or carriers, and a method for the preparation of the said formulation by wet and dry granulation. The invention further relates to a stabilized intimate admixture of atorvastatin calcium, a water-insoluble alkaline excipient and an antioxidant and a method for the preparation of the said intimate admixture by co-precipitation and co-milling.
Owner:MAI DE
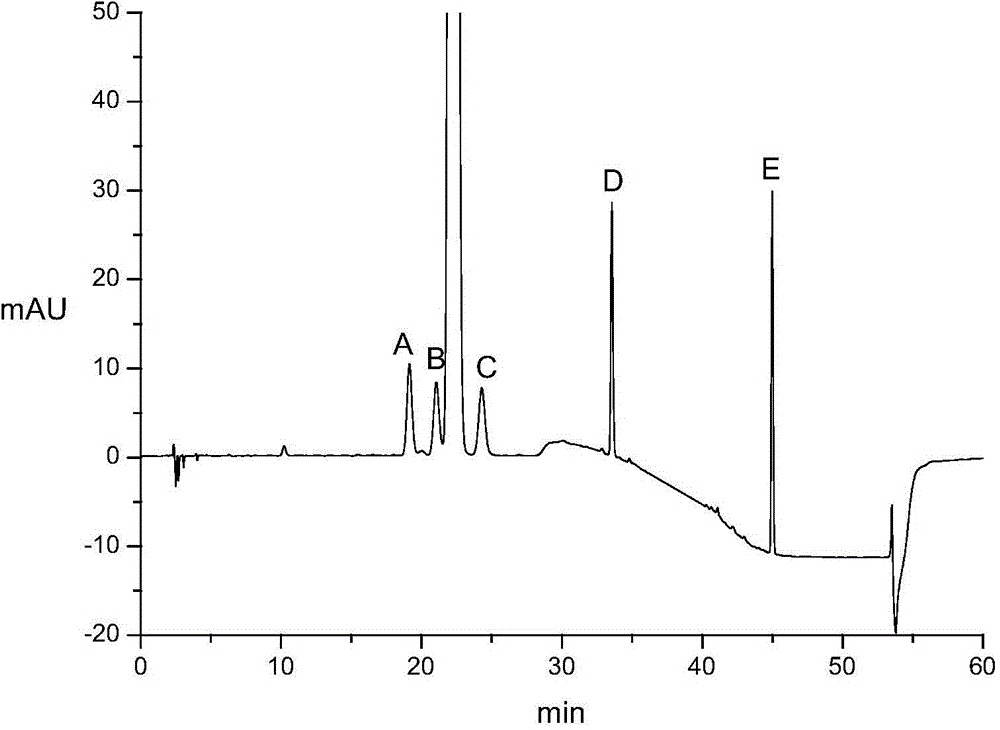
Determining method of atorvastatin calcium related substance
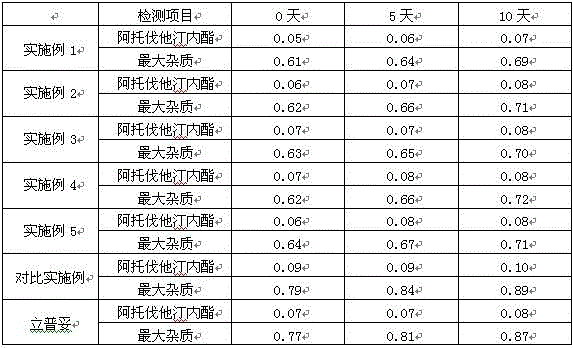
InactiveCN104931599AEasy to separateImprove separation rateComponent separationGradient elutionSolvent
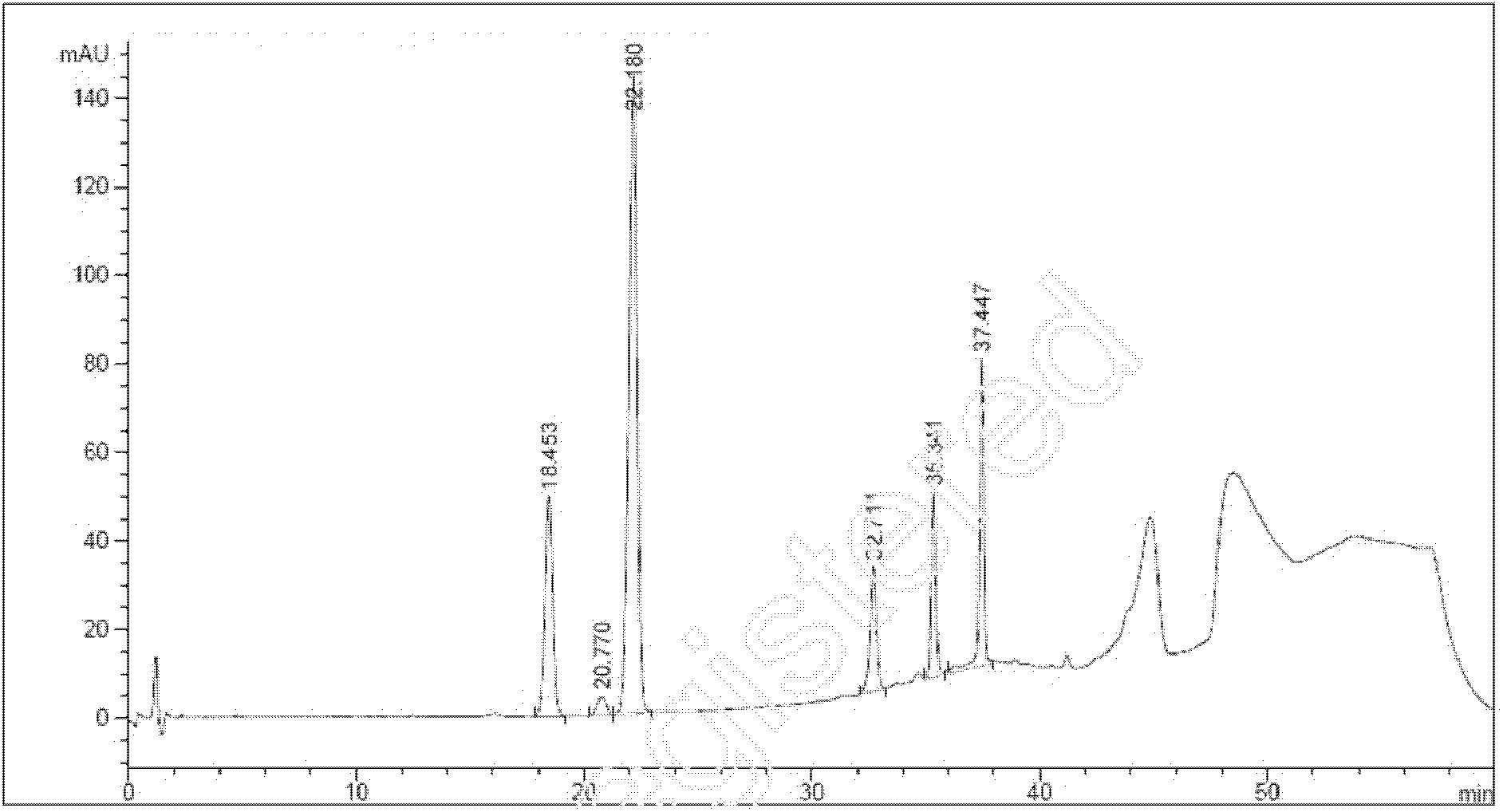
The invention relates to a determining method of an atorvastatin calcium related substance. The method comprises the following steps of 1, preparing a test solution, i.e. taking a proper amount of atorvastatin calcium, adding a solvent to dissolve and quantitatively dilute the atorvastatin calcium until about 1mg of atorvastatin calcium is contained in 1ml of solution, and taking the solution as the test solution; 2, preparing a mixed reference substance solution, i.e. weighing a proper amount of reference substances of an impurity A, an impurity B, an impurity C, an impurity D and an impurity E, and a proper amount of reference substance of the atorvastatin calcium, adding the solvent to dissolve and quantitatively dilute the reference substances until about 3 micrograms of impurity A, 2 micrograms of impurity B, 2 micrograms of impurity C, 2 micrograms of impurity D, 2 micrograms of impurity E and 10 micrograms of atorvastatin calcium solution are contained in 1ml of solution, and taking the solution as the mixed reference substance solution; 3, performing HPLC (High Performance Liquid Chromatography) analysis, i.e. taking 20 microliters of test solution and 20 microliters of mixed reference substance soulution, respectively filling the test solution and the mixed reference substance into a liquid chromatograph, recording a chromatogram until a gradient elution program is finished, and according to the peak area of the chromatogram, calculating the content of each component.
Owner:BEIJING JIALIN PHARM INC
Stabilized pharmaceutical compositions comprising an HMG-CoA reductase inhibitor
InactiveUS20090247603A1Improve bioavailabilityImprove stabilityBiocideMetabolism disorderHMG-CoA reductaseSolubility
The present invention is a new stable drug composition particularly suitable for use as an antihypercholesterolaemic or antihyperlipidaemic agent. The present invention is specifically a drug composition comprising a pharmaceutical, a complexing agent and a surfactant, and a method for manufacturing same. When applied to unstable drugs with low solubility and poor bioavailability, like HMG-CoA reductase inhibitors and especially atorvastatin calcium amorphous form, the resulting drug composition is more stable and is characterized by an improved dissolution profile.
Owner:ORBUS PHARMA INC
Preparation method of atorvastatin calcium intermediate
ActiveCN102127060AIncrease reaction rateHigh reaction yieldOrganic chemistryWater insolubleBoiling point
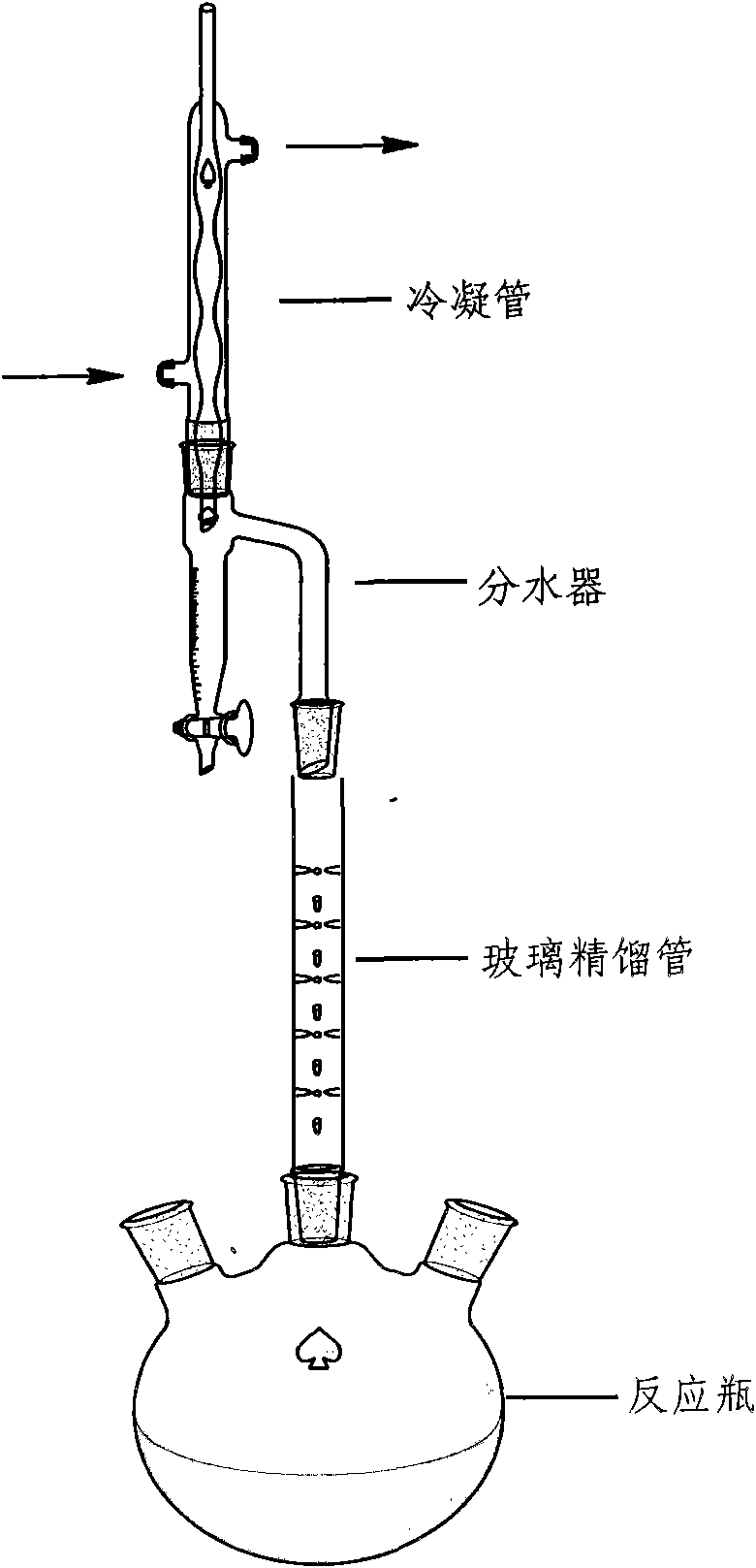
The invention relates to a preparation method of a compound shown as a formula (I) of an atorvastatin calcium intermediate. The structure of the compound is shown in the specifications. In the preparation method of the compound shown as the formula (I), a water-insoluble mixed solvent with an appropriate boiling point and a constant-temperature reflux water diversion mode are adopted, so that the reaction time is greatly shortened, and the yield is high.
Owner:BENGBU BBCA MEDICINE SCI DEV
Determination method of related substances in atorvastatin calcium capsules
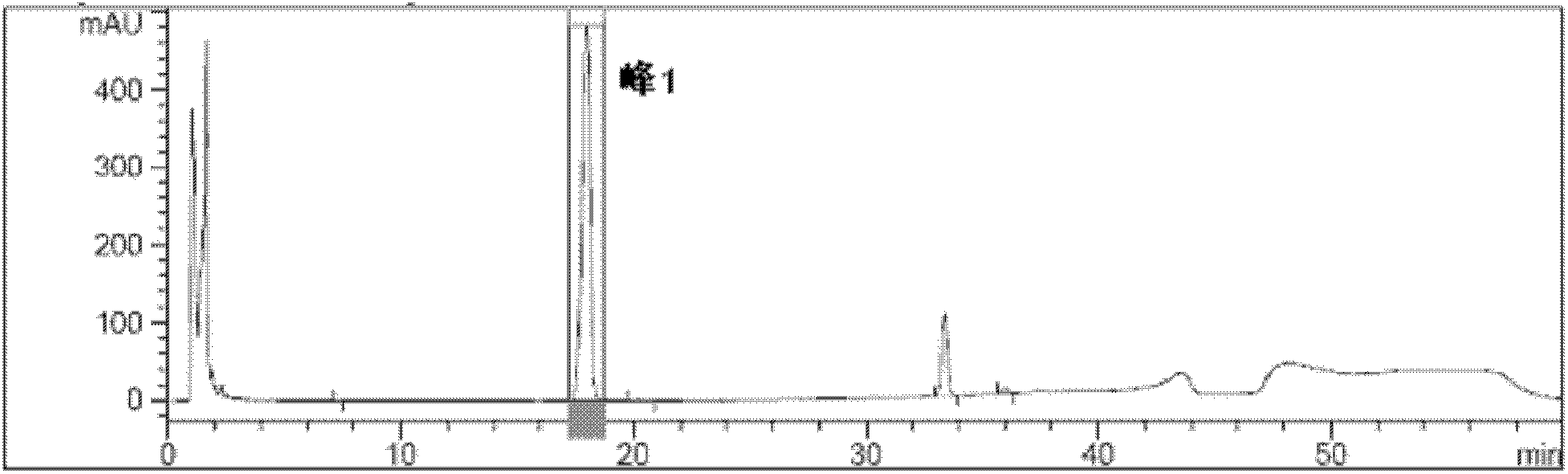
A detection method of atorvastatin calcium capsule related substances of the invention relates to a method which tests or analyzes a drug by separating the drug into various components by adsorption, and the method can detect a single component of related substances in an atorvastatin calcium capsule, and facilitates the improvement of quality standard of atorvastatin calcium capsules. According to the invention, a sample solution to be tested, a self-control solution, and a blank solution are weighed; the solutions are injected into a liquid chromatography, wherein the chromatographic conditions are as follows: a chromatographic column with octadecyl silane bonded silica as a filler is selected; acetonitrile-tetrahydrofuran is used as a mobile phase A; 0.56% ammonium biphosphate solution-mobile phase A is used as a mobile phase B; 0.56% ammonium biphosphate solution- mobile phase A-methanol is used as a mobile phase C; gradient elution is performed; the detection wavelength is 240-250 nm, and the column temperature is 20 DEG C-30 DEG C, wherein in the 0.56% ammonium biphosphate solution, 0.56% is a mass-volume ratio; and a chromatogram is recorded.
Owner:北京汉典中西药研究开发中心
Quickly-dissolved atorvastatin calcium tablet and preparation method thereof
InactiveCN103006602AHigh specific surface areaImprove securityMetabolism disorderPill deliveryTreatment effectSurface-active agents
The invention provides a quickly-dissolved atorvastatin calcium tablet and a preparation method thereof. The atorvastatin calcium tablet provided by the invention does not contain surface active agents and alkaline materials, is simple in process, and can be quickly dissolved in the stomach, thereby enhancing the compliance and treatment effect of the administration of patients.
Owner:QINGDAO UNIV
Composition containing ezetimibe and atorvastatin calcium and preparation method of composition
InactiveCN104013617AImprove stabilityReduce contentMetabolism disorderPharmaceutical delivery mechanismDiseasePharmaceutical drug
The invention relates to a tablet composition containing ezetimibe and atorvastatin calcium and a preparation method of the composition. According to the method, the stability of atorvastatin calcium in a pharmaceutical composition can be effectively improved, and the in-vivo bioavailability of the medicine is improved. Moreover, the method has the advantages that the process is simple and feasible to operate, the requirement on preparation equipment is low, and the production cost is reduced. The solid oral preparation is used for treating or preventing cardiovascular and cerebrovascular diseases.
Owner:AVENTIS PHARMA HAINAN
Process for producing important synthesis midbody of high purity atorvastatin
ActiveCN101429195AEasy to recycleImprove conversion rateOrganic chemistryFiltrationTertiary butyl acetate
The invention relates to a method for preparing high-purity (4R, 6R)-6-{2-[5-isopropyl-3-phenyl-2(4-fluorophenyl)-4-(phenylcarbamoyl)-yrrol-1-yl]- ethyl}-2, 2-dimethyl-[1, 3]-dioxane-4-yl-tert-butyl acetate, which comprises the following steps: step one, [5-methyl-4-isopropyl-2-phenyl-1(4-fluorophenyl)-3-(phenylcarbamoy1)-1,4-hexanedione](II) and [(4R,6R)-2,2-dimethyl-6-(2-aminoethyl)-[1,3]-dioxane-4-yl-tert-butyl acetate](III) with a mol ratio of between 0.71 and 1.12 to 1 are weighed, an acid catalyst which is 1.05 to 1.15 times of mol number of the formula (II) is weighed, the mixture is dissolved in a non-hydroxy solvent which is 3.0 to 4.2 times of the weight of the formula (II) under the protection of nitrogen and the stirring, and heating reflux and azeotropic water entrainment are performed until an HPLC shows that the reaction is finished; and second two, the solvent is removed under vacuum, then a water-isopropanol mixed solvent with a volume ratio of 2 to 5 is used to recrystallize the mixture, and a key intermediate of synthetic atorvastatin calcium which has an HPLC purity not less than 99.0 percent and is expressed by the formula (I) is obtained after the pump filtration and drying. The method has the advantages of simple process, low equipment requirement, low cost, convenient and quick recovery of the solvent, less environmental pollution, and high product purity.
Owner:安徽美诺华药物化学有限公司
Preparation process of atorvastatin calcium
The invention provides a preparation process of atorvastatin calcium, which is characterized by being divided into two steps when preparing a condensation compound (4R-cis)-1,1-dimethyl ethyl-6-[2-[2-(4-fluorophenyl)-5-(1-methyl ethyl)-3-phenyl-4-[(phenyl amino)-carbonyl]-1H-pyrrole-1-yl]ethyl]-2,2-dimethyl-1,3-dioxane-4-acetate: 1), adding (4R-cis)-6-aminoethyl-2,2-dimethyl-1,3-dioxane-4-tertbutyl acetate (called ATS-9 for short) into a mixed solvent of n-heptane, tetrahydrofuran and toluene to react with pivalic acid; and 2), adding 4-fluorine-alpha-[2-methyl-1-oxygen-propyl]-gamma-oxo-N,beta-diphenyl phenyl butyramide (called M-4 for short) and rising the temperature for reaction. The invention has the advantage of capability of greatly improving the yield of the intermediate condensation compound and is more in favor of industrialized production.
Owner:SHANDONG XINHUA PHARMA CO LTD
Atorvastatin calcium dried suspension and preparation method thereof
InactiveCN101637452AMask bitternessGreat tastePowder deliveryMetabolism disorderDispersityFiller Excipient
The invention relates to an atorvastatin calcium dried suspension and a preparation method thereof. The atorvastatin calcium dried suspension comprises the following components in portion by weight: 1to 10 portions of atorvastatin calcium, 10 to 95 portions of filling agent, 1 to 30 portions of suspension and / or suspending agent, 2 to 40 portions of flavoring agent, and 0.2 to 2.0 portions of lubricating agent. The atorvastatin calcium dried suspension has the advantages of high dispersity, even distribution, quick absorption, high bioavailability, good adaptability, good mouthfeel, stabilityand the like. The atorvastatin calcium dried suspension can be applied to regulation of blood fat.
Owner:FOSHAN DAYI TECH LTD
Ezetimibe and atorvastatin calcium tablet and preparation method thereof
ActiveCN105832723AImprove distribution uniformityImprove hydrophilicityMetabolism disorderDrageesFiller ExcipientWater insoluble
The invention discloses an ezetimibe and atorvastatin calcium tablet and a preparation method thereof. The tablet is prepared from ezetimibe particles and atorvastatin calcium particles through double-layer tabletting, wherein the ezetimibe particles comprise the following components: ezetimibe, a binder, a surfactant, a water-soluble filling agent, a water-insoluble filling agent, a disintegrating agent and a lubricant; during pelletizing of the ezetimibe particles, the ezetimibe and the surfactant are dissolved or dispersed into a binder solution; the water-soluble filling agent is added to uniformly mix; and other auxiliary materials are added for pelletizing. During the preparation of the ezetimibe layer, the auxiliary materials are added in specific sequence, so that the in-vitro release problem of the ezetimibe is effectively solved.
Owner:ZHEJIANG JUTAI PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Crystalline [R-(R*,R*)]-2-(4-fluorophenyI)-beta,delta-dihydroxy-5-(1-methylethyl)-3-phenyl- 4-[(phenylamino)carbonyl]-1H-pyrrole-heptanoic acid calcium salt (2:1) Crystalline [R-(R*,R*)]-2-(4-fluorophenyI)-beta,delta-dihydroxy-5-(1-methylethyl)-3-phenyl- 4-[(phenylamino)carbonyl]-1H-pyrrole-heptanoic acid calcium salt (2:1)](https://images-eureka.patsnap.com/patent_img/c53f8800-eff8-4a45-9d70-27d2e69fbc35/US20050209306A1-20050922-D00001.png)
![Crystalline [R-(R*,R*)]-2-(4-fluorophenyI)-beta,delta-dihydroxy-5-(1-methylethyl)-3-phenyl- 4-[(phenylamino)carbonyl]-1H-pyrrole-heptanoic acid calcium salt (2:1) Crystalline [R-(R*,R*)]-2-(4-fluorophenyI)-beta,delta-dihydroxy-5-(1-methylethyl)-3-phenyl- 4-[(phenylamino)carbonyl]-1H-pyrrole-heptanoic acid calcium salt (2:1)](https://images-eureka.patsnap.com/patent_img/c53f8800-eff8-4a45-9d70-27d2e69fbc35/US20050209306A1-20050922-D00002.png)
![Crystalline [R-(R*,R*)]-2-(4-fluorophenyI)-beta,delta-dihydroxy-5-(1-methylethyl)-3-phenyl- 4-[(phenylamino)carbonyl]-1H-pyrrole-heptanoic acid calcium salt (2:1) Crystalline [R-(R*,R*)]-2-(4-fluorophenyI)-beta,delta-dihydroxy-5-(1-methylethyl)-3-phenyl- 4-[(phenylamino)carbonyl]-1H-pyrrole-heptanoic acid calcium salt (2:1)](https://images-eureka.patsnap.com/patent_img/c53f8800-eff8-4a45-9d70-27d2e69fbc35/US20050209306A1-20050922-D00003.png)