Oral proliposome containing atorvastatin calcium and preparation method of oral proliposome
A technology of atorvastatin calcium and proliposome, which is applied in the direction of liposome delivery, medical preparations of non-active ingredients, pharmaceutical formulas, etc., can solve the problem that the pharmacological activity of atorvastatin cannot be effectively exerted and the limitations Problems such as clinical application of drugs and poor bioavailability, to meet the needs of clinical drug administration, improve bioavailability, and high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The distribution ratio of the components is: atorvastatin calcium 10mg, soybean lecithin 180mg, poloxamer 50mg, sodium cholate 8mg, absolute ethanol 1ml.
[0026] Preparation process: according to the formula, add the required amount of atorvastatin calcium, soybean lecithin, poloxamer and sodium cholate into the required amount of absolute ethanol solvent, ultrasonically dissolve the mixture to dissolve into a clear anhydrous solution, Obtained atorvastatin calcium proliposome preparation.
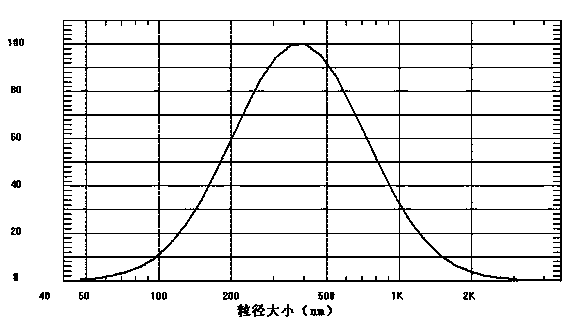
[0027] Adding the prepared atorvastatin calcium proliposomes to 50 times the amount of distilled water can quickly form an atorvastatin calcium liposome solution with an average particle size of 470.1 nm and an encapsulation efficiency of >90%.
[0028] The physical and chemical characteristics of the finished product:
[0029] Finished product traits: It is light yellow clear and transparent solution.
[0030] Hydration dispersibility: After adding distilled water and shaking to...
Embodiment 2
[0035] The distribution ratio of the components is: atorvastatin calcium 17mg, soybean lecithin 240mg, poloxamer 58mg, sodium deoxycholate 10mg, absolute ethanol 1.5ml.
[0036] The preparation process is the same as in Example 1.
[0037] Adding the prepared atorvastatin calcium proliposomes to 50 times the amount of distilled water can quickly form an atorvastatin calcium liposome solution with an average particle size of 80%.
Embodiment 3
[0039]The distribution ratio of the components is: atorvastatin calcium 20mg, soybean lecithin 270mg, poloxamer 60mg, Tween 80 16mg, absolute ethanol 1.8ml.
[0040] The preparation process is the same as in Example 1.
[0041] Adding the prepared atorvastatin calcium proliposomes to 50 times the amount of distilled water can quickly form an atorvastatin calcium liposome solution with an average particle size of 85%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com