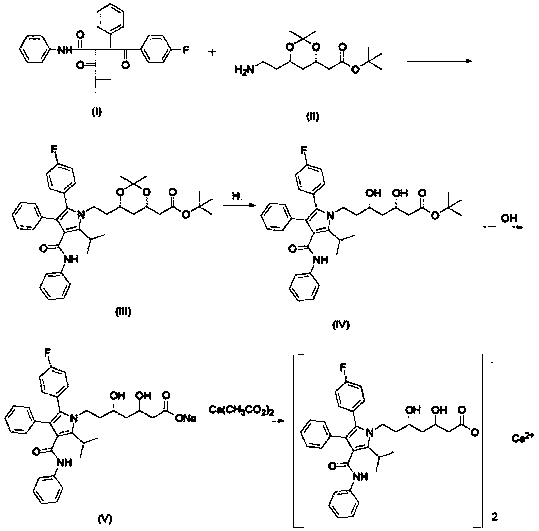
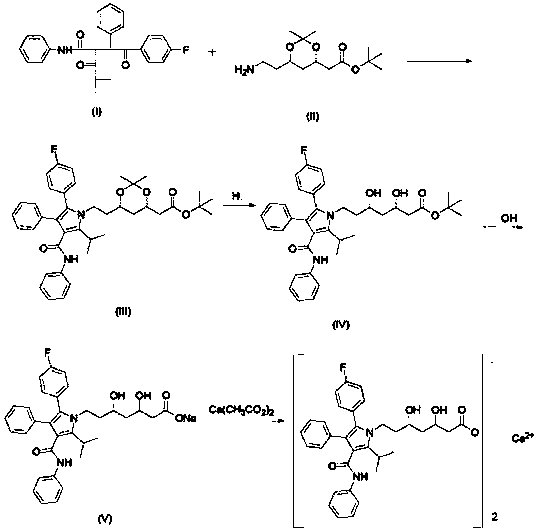
Preparing method of high-purity atorvastatin calcium
A high-purity technology of atorvastatin calcium, applied in the field of preparation of high-purity atorvastatin calcium, can solve the problem of poor stability of amorphous atorvastatin calcium, poor bioavailability, high energy consumption, etc. problems, to achieve the effect of high product purity, improved purity, and high economic added value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (a) (4R-cis)-6-[2-[2-(4-fluorophenyl)-5-(1-isopropyl)-3-phenyl-4-[(aniline)hydroxyl]-1H Preparation of -pyrrol-1-yl]ethyl]-2,2-dimethyl-1,3-dioxolane-4-acetic acid tert-butyl ester
[0035] Put 1kg (4R-cis) 6-aminoethyl-2,2-dimethyl-1,3-dioxane-4-tert-butyl acetate into a 10L dry and clean reactor, 6L toluene, 0.41kg Pivalic acid, stirred at room temperature for 1 hour; add 1.1kg of 2-[2-(4-fluorophenyl)-2-oxo-1-phenylethyl]-4-methyl-3- Oxo-N-phenylpentanamide, heated to reflux (105°C-110°C) until the end of the reaction (TLC detection and tracking, developing solvent: ethyl acetate:petroleum ether=1:2). Stop heating, cool down to 25°C-30°C, add 5L of saturated sodium bicarbonate solution to wash once, and then wash the organic phase with 2*5L purified water twice, then transfer the organic phase to a rotary evaporator, at 60°C Concentrate to dryness under reduced pressure to give 4R-cis)-6-[2-[2-(4-fluorophenyl)-5-(1-isopropyl)-3-phenyl-4-[(aniline) Hydroxy]-1H-pyrr...
Embodiment 2
[0045] (a) (4R-cis)-6-[2-[2-(4-fluorophenyl)-5-(1-isopropyl)-3-phenyl-4-[(aniline)hydroxyl]-1H Preparation of -pyrrol-1-yl]ethyl]-2,2-dimethyl-1,3-dioxolane-4-acetic acid tert-butyl ester
[0046] Put 1kg (4R-cis) 6-aminoethyl-2,2-dimethyl-1,3-dioxane-4-tert-butyl acetate into a 10L dry and clean reactor, 6L toluene, 0.41kg Pivalic acid, stirred at room temperature for 1 hour; add 1.1kg of 2-[2-(4-fluorophenyl)-2-oxo-1-phenylethyl]-4-methyl-3- Oxo-N-phenylpentanamide, heated to reflux (105°C-110°C) until the end of the reaction (TLC detection and tracking, developing solvent: ethyl acetate:petroleum ether=1:2). Stop heating, cool down to 25°C-30°C, add 5L of saturated sodium bicarbonate solution to wash once, and then wash the organic phase with 2*5L purified water twice, then transfer the organic phase to a rotary evaporator, at 60°C Concentrate to dryness under reduced pressure to give 4R-cis)-6-[2-[2-(4-fluorophenyl)-5-(1-isopropyl)-3-phenyl-4-[(aniline) Hydroxy]-1H-pyrr...
Embodiment 3
[0056] (a) (4R-cis)-6-[2-[2-(4-fluorophenyl)-5-(1-isopropyl)-3-phenyl-4-[(aniline)hydroxyl]-1H Preparation of -pyrrol-1-yl]ethyl]-2,2-dimethyl-1,3-dioxolane-4-acetic acid tert-butyl ester
[0057] Put 1kg (4R-cis) 6-aminoethyl-2,2-dimethyl-1,3-dioxane-4-tert-butyl acetate into a 10L dry and clean reactor, 6L toluene, 0.41kg Pivalic acid, stirred at room temperature for 1 hour; add 1.1kg of 2-[2-(4-fluorophenyl)-2-oxo-1-phenylethyl]-4-methyl-3- Oxo-N-phenylpentanamide, heated to reflux (105°C-110°C) until the end of the reaction (TLC detection and tracking, developing solvent: ethyl acetate:petroleum ether=1:2). Stop heating, cool down to 25°C-30°C, add 5L of saturated sodium bicarbonate solution to wash once, and then wash the organic phase with 2*5L purified water twice, then transfer the organic phase to a rotary evaporator, at 60°C Concentrate to dryness under reduced pressure to give 4R-cis)-6-[2-[2-(4-fluorophenyl)-5-(1-isopropyl)-3-phenyl-4-[(aniline) Hydroxy]-1H-pyrr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com