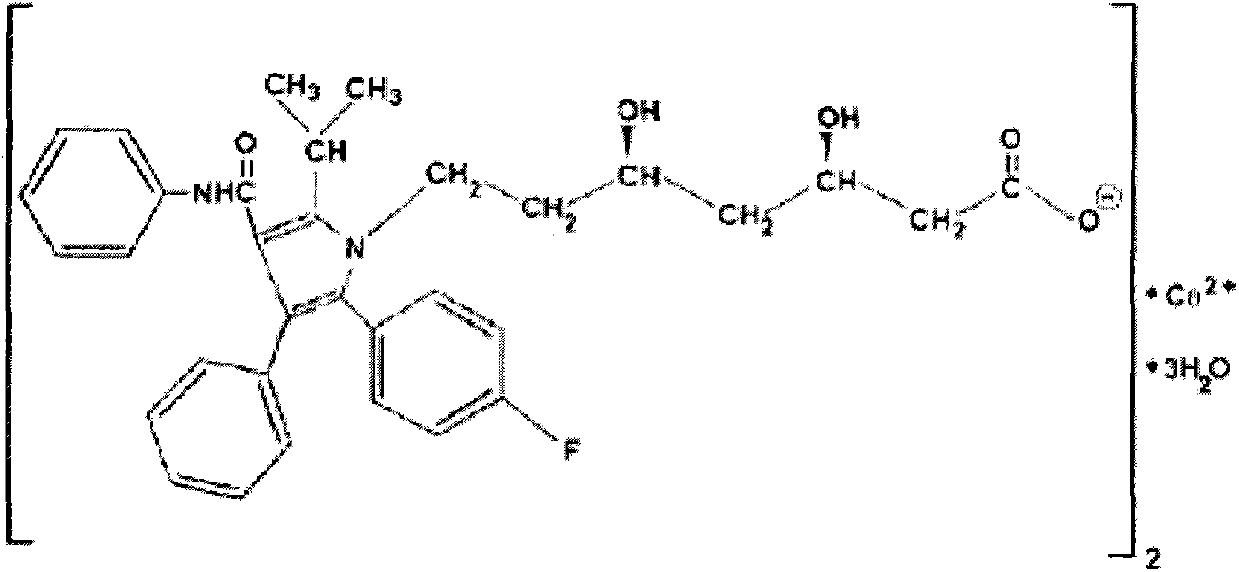
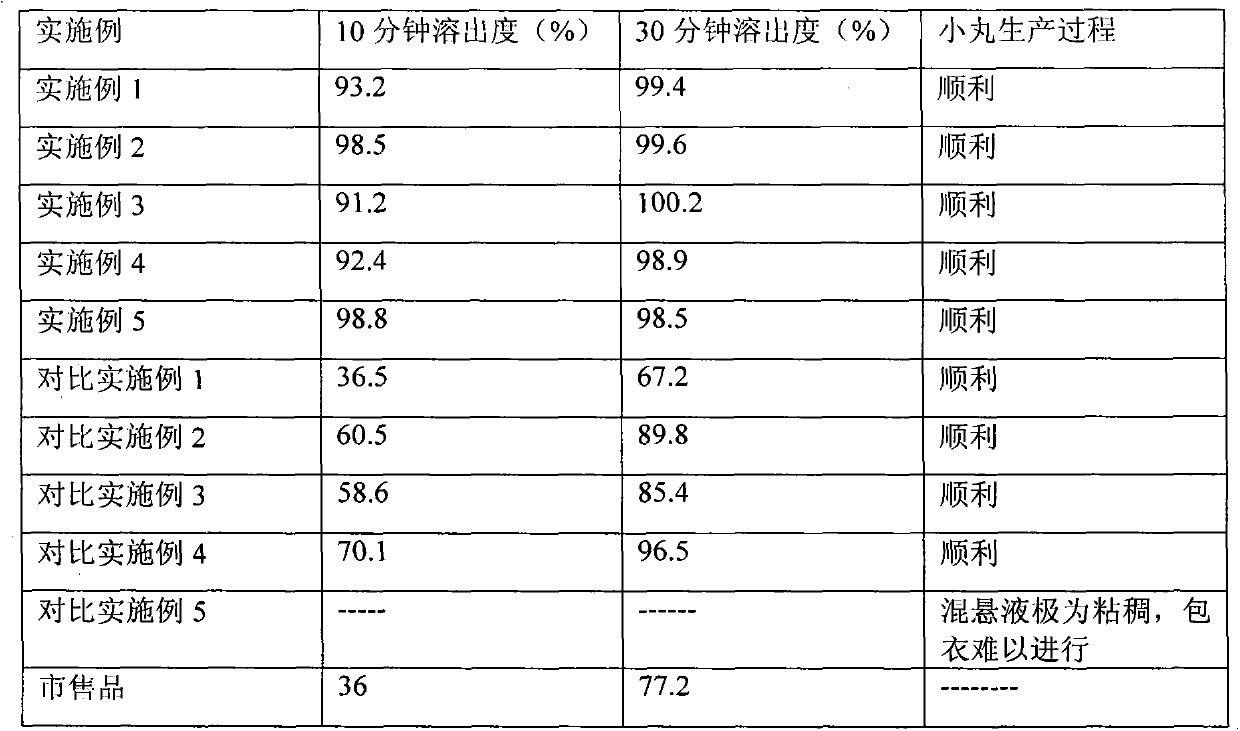
Quickly-dissolved atorvastatin calcium tablet and preparation method thereof
An atorvastatin calcium and rapid technology, applied in the direction of pill delivery, metabolic diseases, active ingredients of heterocyclic compounds, etc., can solve the problems of reduced pharmacological activity, poor stability, complicated process, etc., to increase drug safety and reduce viscosity Increase, the effect of simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) atorvastatin calcium 1 part
[0031] Methanol 10 parts
[0032] 0.3 parts of crospovidone
[0033] (2) 20 servings of lactose pellets
[0034] (3) 50 parts of microcrystalline cellulose
[0035] Crospovidone 0.5 parts
[0036] 1 part magnesium stearate
[0037] Preparation Process:
[0038] The raw material of atorvastatin calcium is dissolved in methanol, crospovidone is added, and ground by a ball mill to control the particle size of the suspension d90<20 μm. Then coating is carried out in a fluidized bed, the suspension is coated on the outer layer of lactose pellets, after the coating is completed, it is evenly mixed with microcrystalline cellulose, crospovidone and magnesium stearate, and compressed into tablets to obtain the product.
Embodiment 2
[0040] (1) atorvastatin calcium 1 part
[0041] Methanol 10 parts
[0042] 3 parts crospovidone
[0043] (2) 20 servings of lactose pellets
[0044] (3) 50 parts of microcrystalline cellulose
[0045] 3.5 parts of crospovidone
[0046] 1 part magnesium stearate
[0047] Preparation Process:
[0048] The raw material of atorvastatin calcium is dissolved in methanol, crospovidone is added, and ground by a ball mill to control the particle size of the suspension d90<20 μm. Then coating is carried out in a fluidized bed, the suspension is coated on the outer layer of lactose pellets, after the coating is completed, it is evenly mixed with microcrystalline cellulose, crospovidone and magnesium stearate, and compressed into tablets to obtain the product.
Embodiment 3
[0050] (1) atorvastatin calcium 1 part
[0051] Methanol 10 parts
[0052] Sodium carboxymethyl starch 0.3 parts
[0053] (2) Mannitol pellets 20 parts
[0054] (3) 50 parts of microcrystalline cellulose
[0055] Crospovidone 0.5 parts
[0056] 1 part magnesium stearate
[0057] Preparation Process:
[0058] The raw material of atorvastatin calcium is dissolved in methanol, crospovidone is added, and ground by a ball mill to control the particle size of the suspension d90<20 μm. Then coat in a fluidized bed, wrap the suspension on the outer layer of mannitol pellets, mix evenly with microcrystalline cellulose, crospovidone, and magnesium stearate after coating, and press into tablets to obtain .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com