Patents
Literature
4482 results about "Constipation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Infrequent, irregular or difficult evacuation of the bowels.
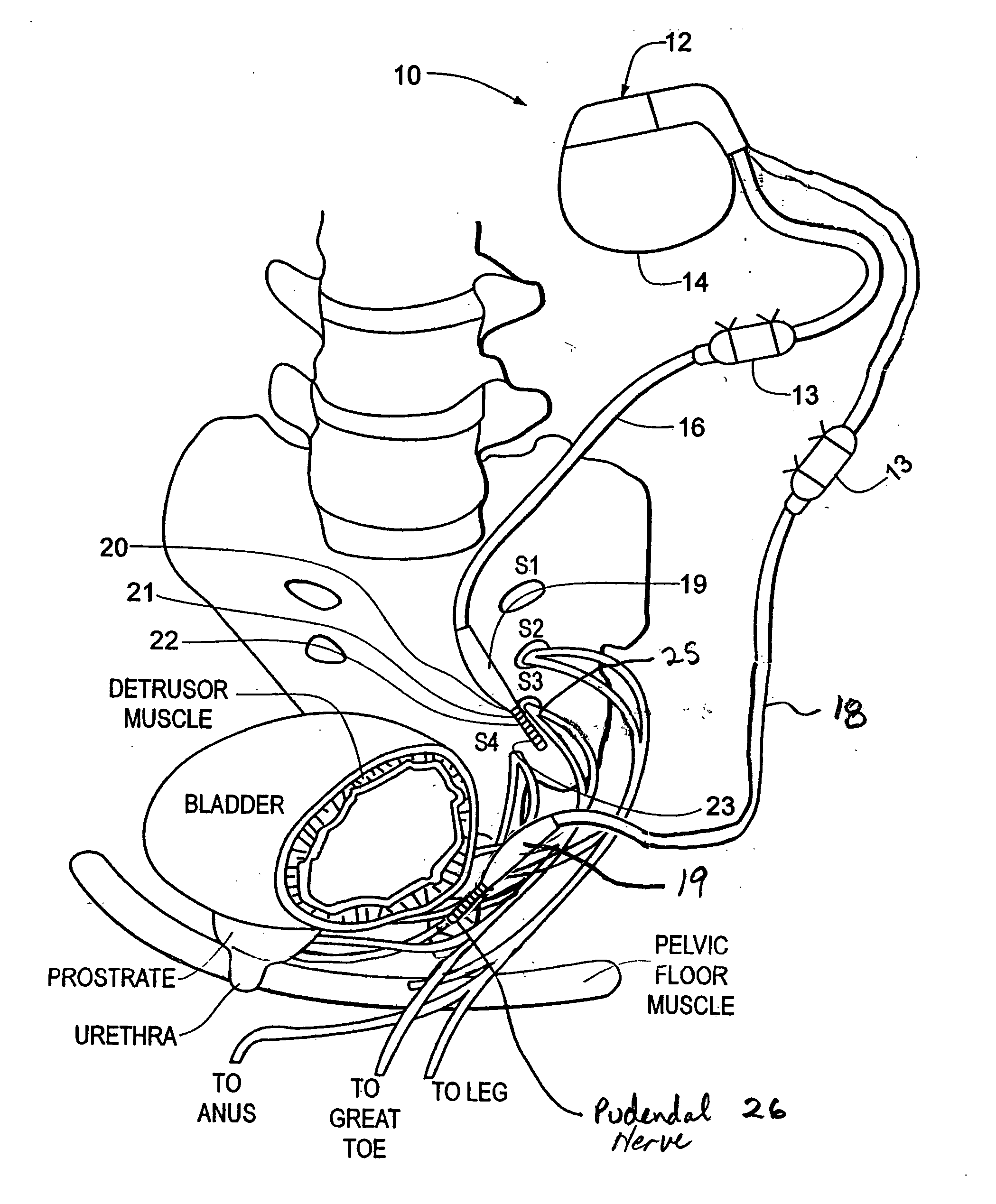
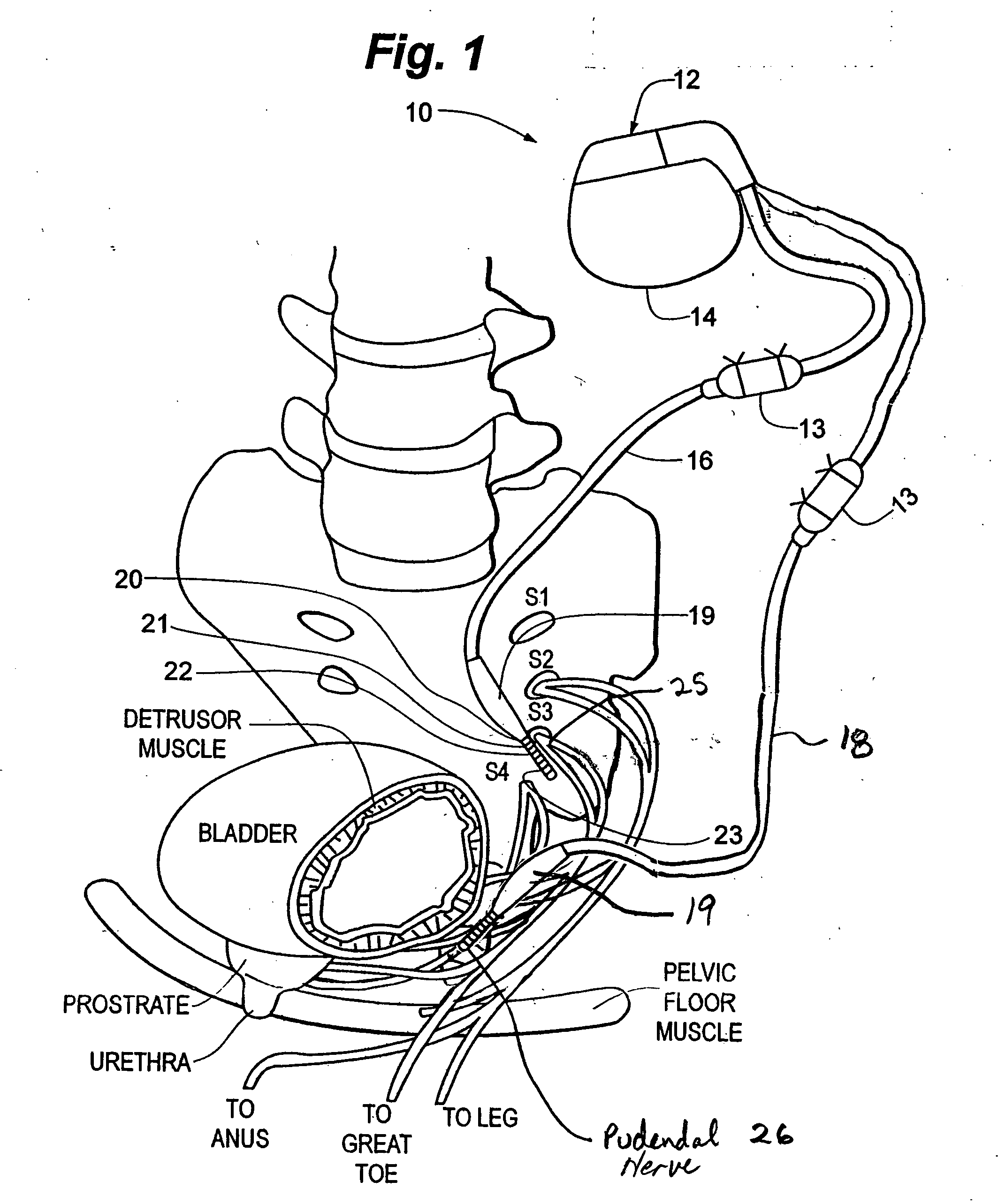
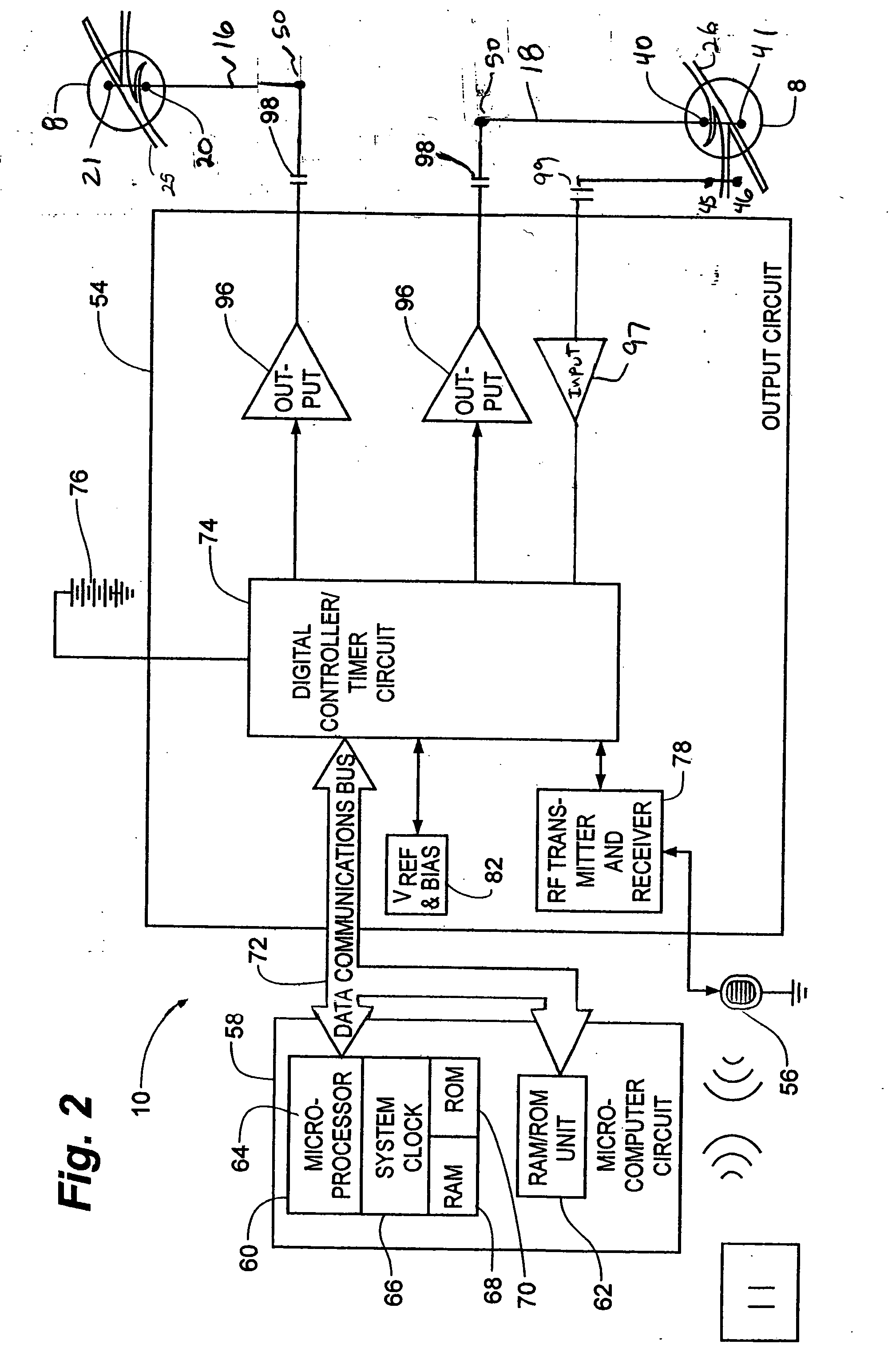
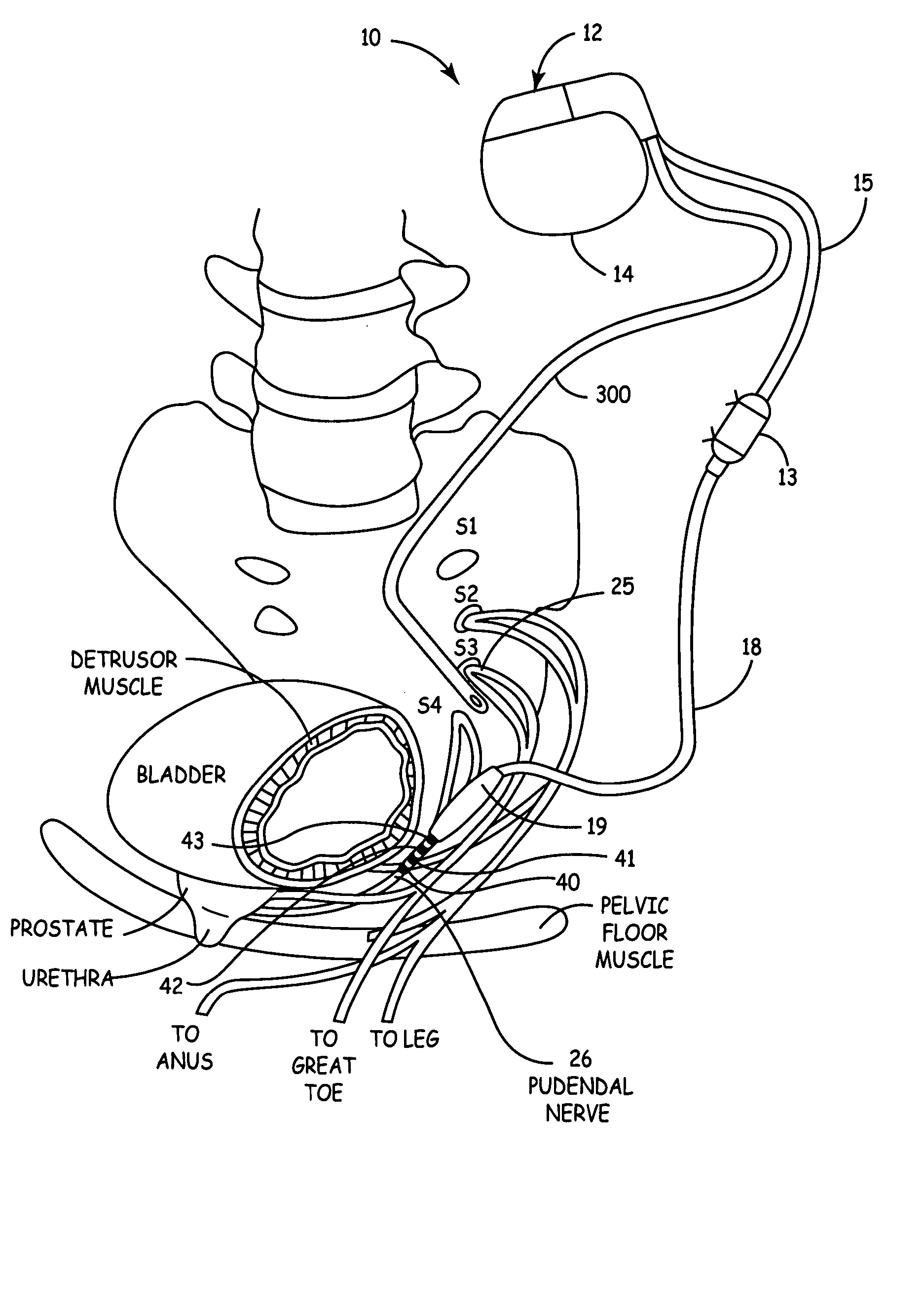
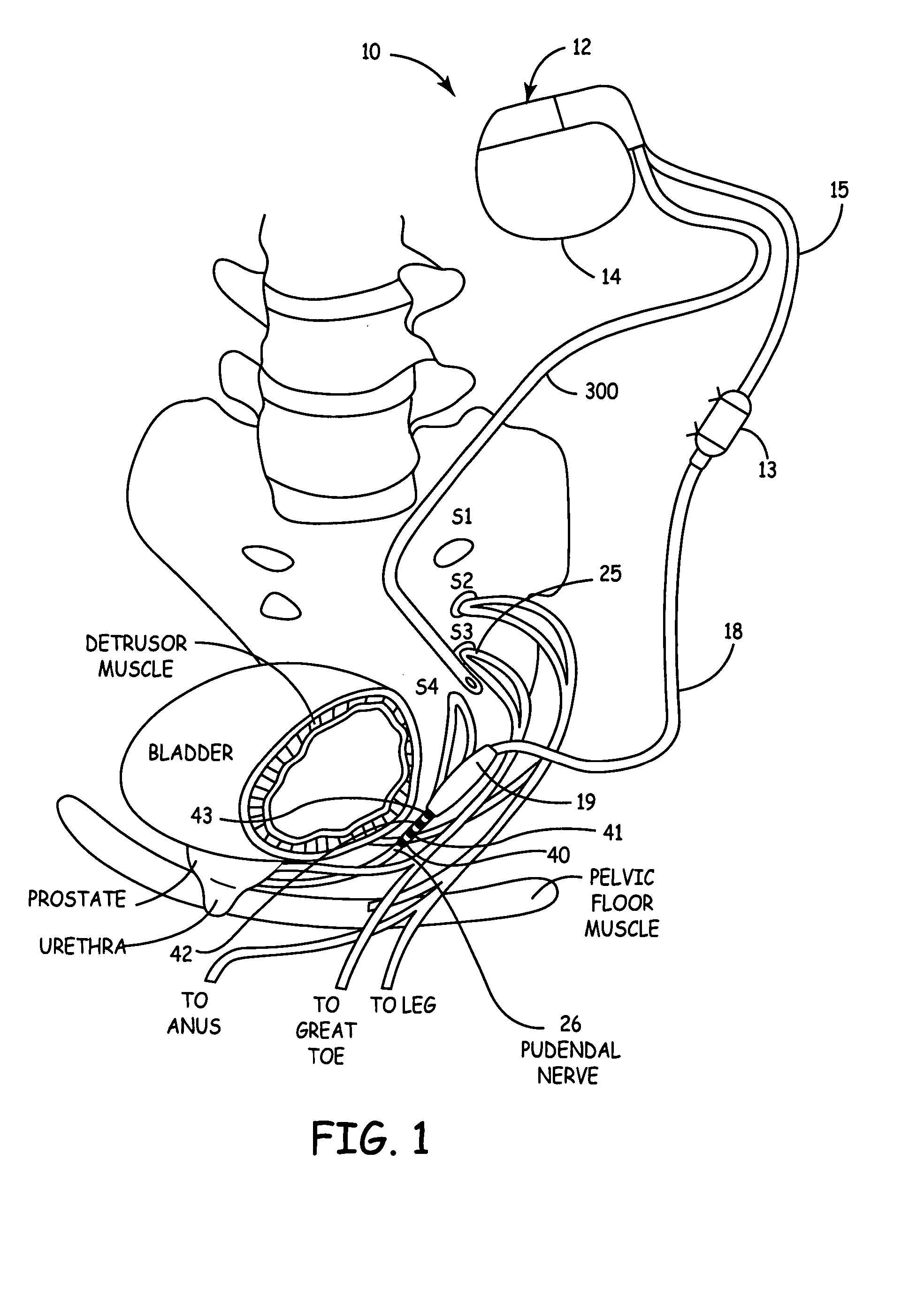
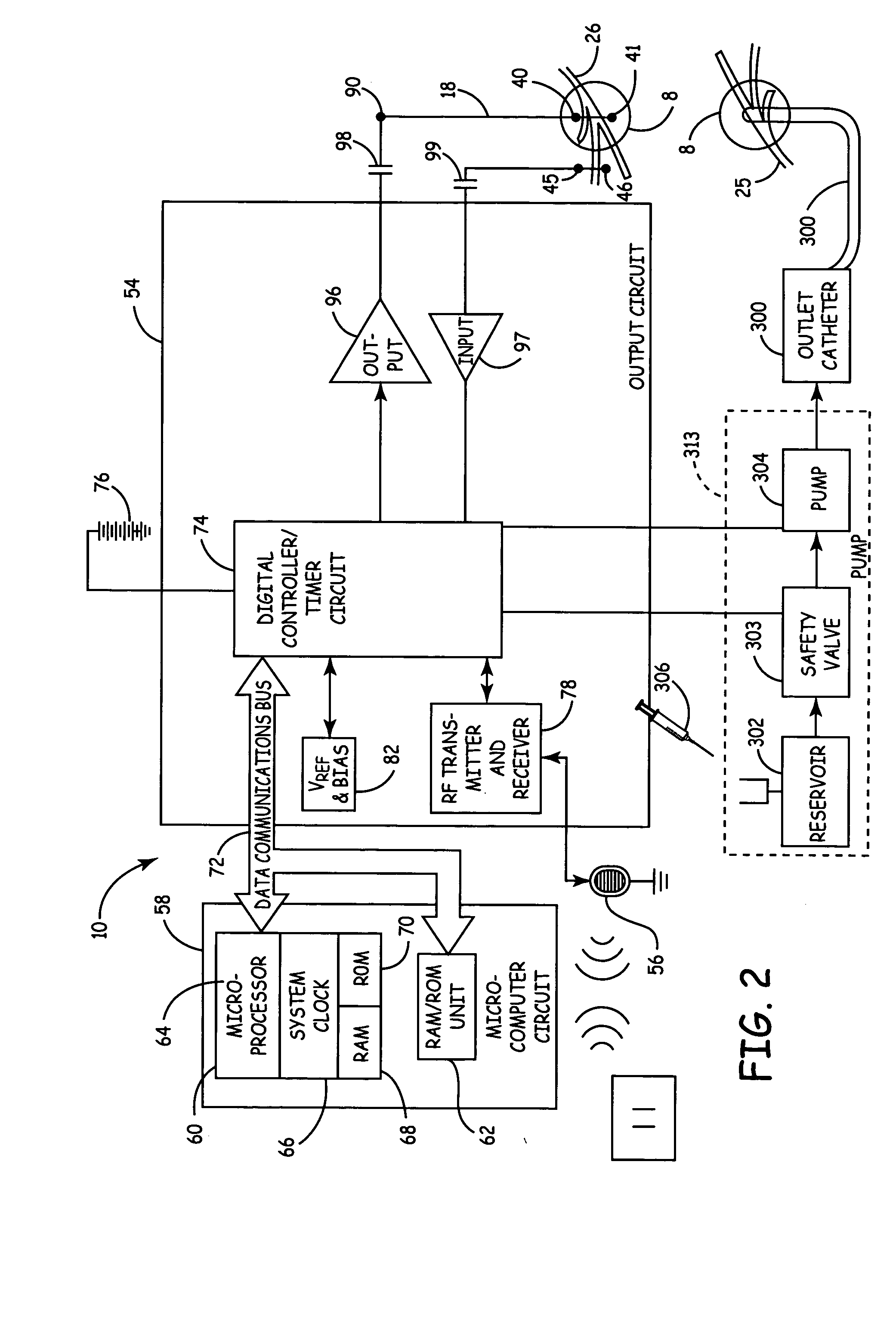
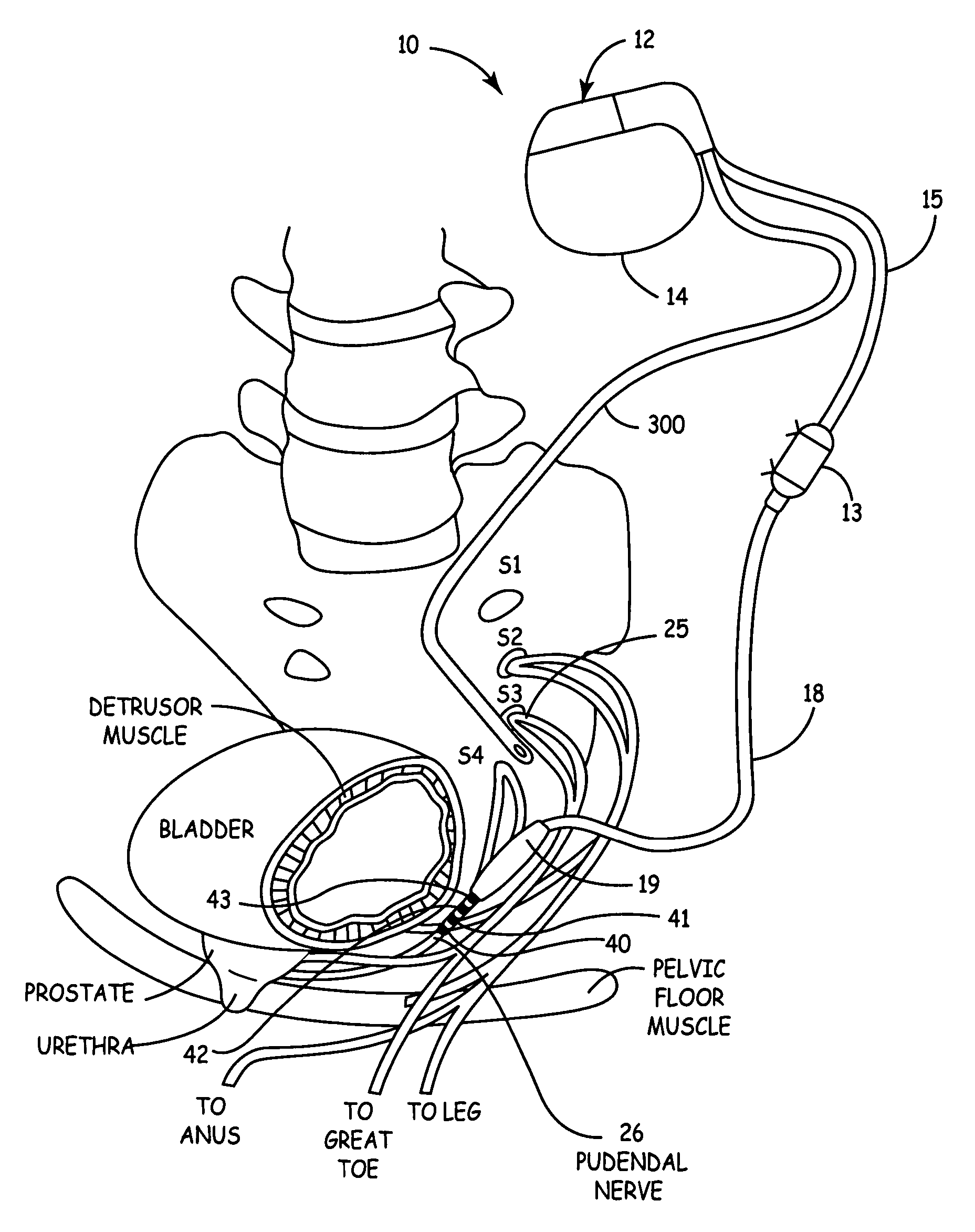
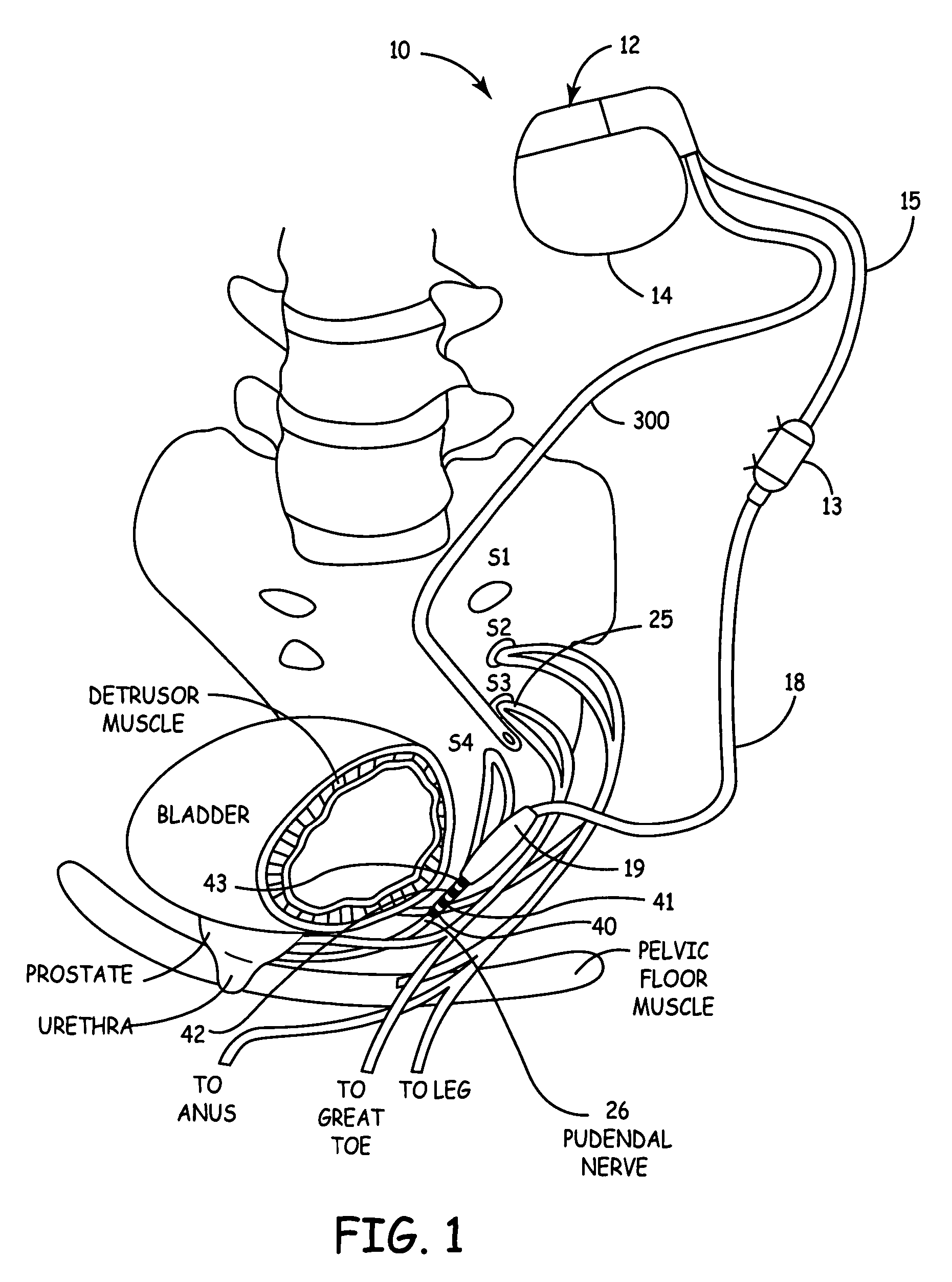
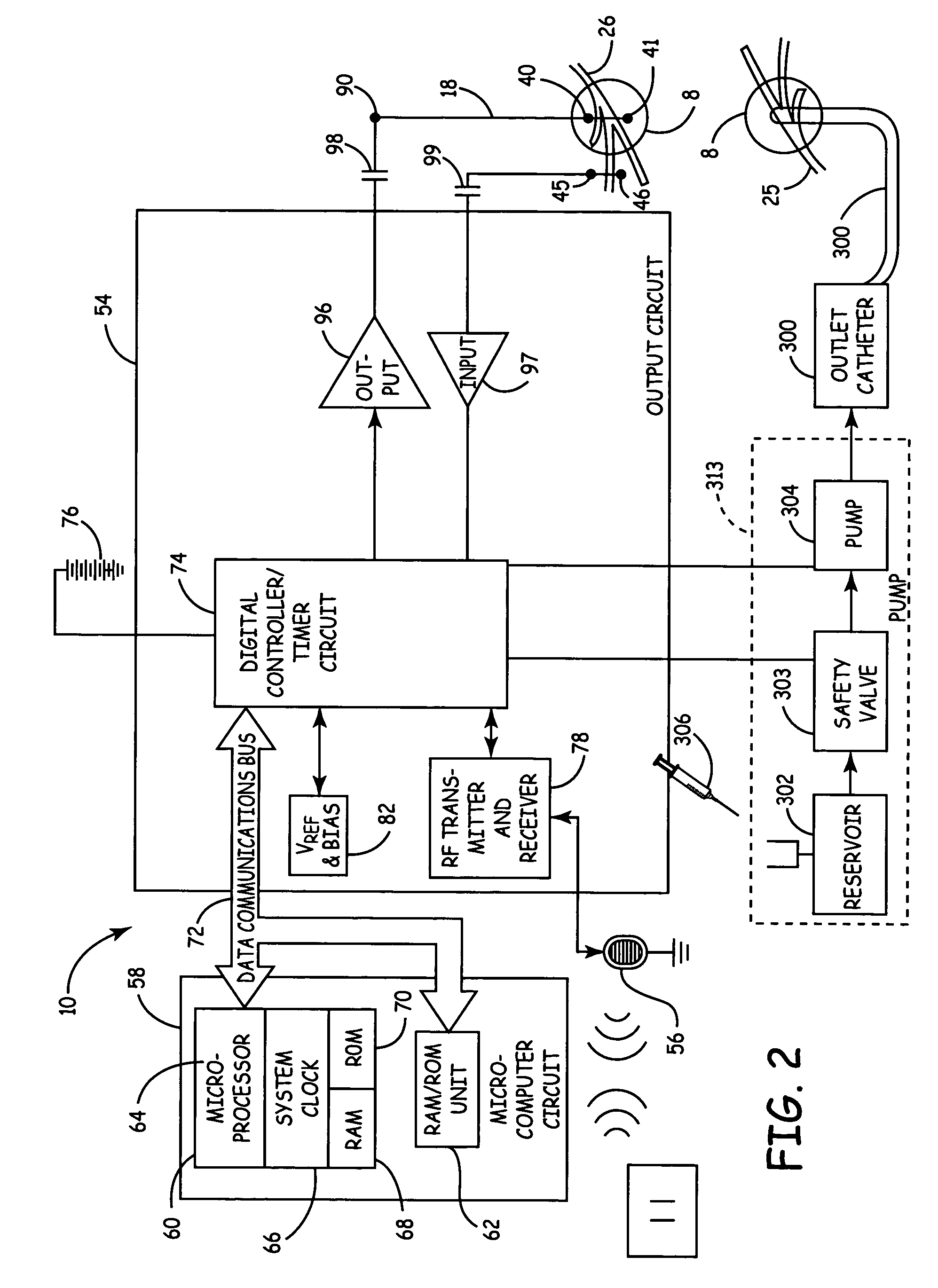
Method, system and device for treating various disorders of the pelvic floor by electrical stimulation of the pudendal nerves and the sacral nerves at different sites
Described are implantable devices and methods for treating various disorders of the pelvic floor by means of electrical stimulation of the pudendal and sacral nerves, or portions thereof, and optional means for delivering drugs in association therewith. Two or more electrical stimulation regimes are applied on a continuous, alternating, intermittent or other basis to the sacral and pudendal nerves, and optionally one or more drugs are infused, injected or otherwise administered, to appropriate portions of a patient's pelvic floor and pudendal nerve and / or sacral nerve, or portions thereof, in an amount and manner effective to treat a number of disorders, including, but not limited to, urinary and / or fecal voiding dysfunctions such as constipation, incontinence disorders such as urge frequency and urinary retention disorders, sexual dysfunctions such as orgasmic and erectile dysfunction, pelvic pain, prostatitis, prostatalgia and prostatodynia.
Owner:MEDTRONIC INC
Composite probiotics micro-ecological formulation and preparation method
InactiveCN101496822AEnsure balancePromotes the detoxification processBacteria material medical ingredientsDigestive systemDiseaseChronic diarrhea
The invention relates to a composite probiotic bacterium microecological preparation and a preparation method thereof. The preparation is prepared by mixing 1.0 to 20 percent of composite microbial bacterium powder, 5.0 to 50 percent of composite oligosaccharide, 30 to 80 percent of auxiliary protective carrier and 0.1 to 0.2 percent of edible essence. The composite probiotic bacterium microecological preparation is prepared from a beneficial microbial preparation and a microbial growth-promoting factor, namely the oligosaccharide, through special combination and technological recombination. The composite probiotic bacterium microecological preparation has the advantages that beneficial active bacteria and the oligosaccharide promote each other, so that the composite probiotic bacterium microecological preparation has the advantages of obviously improving the microecological environment of the whole intestine and contributing to the digestion, preventing and treating intestine diseases, treating diarrhea, chronic diarrhea, diarrhea which can not be cured by antibiotic, constipation, dyspepsia and abdominal distension which are caused by intestine dysbacteriosis, recovering the intestine flora balance, protecting the liver, reinforcing the functions of detoxication and toxin expulsion of the liver, reinforcing the disease prevention and resistance such as the resistance of human body, and the like.
Owner:上海谱莱生物技术有限公司
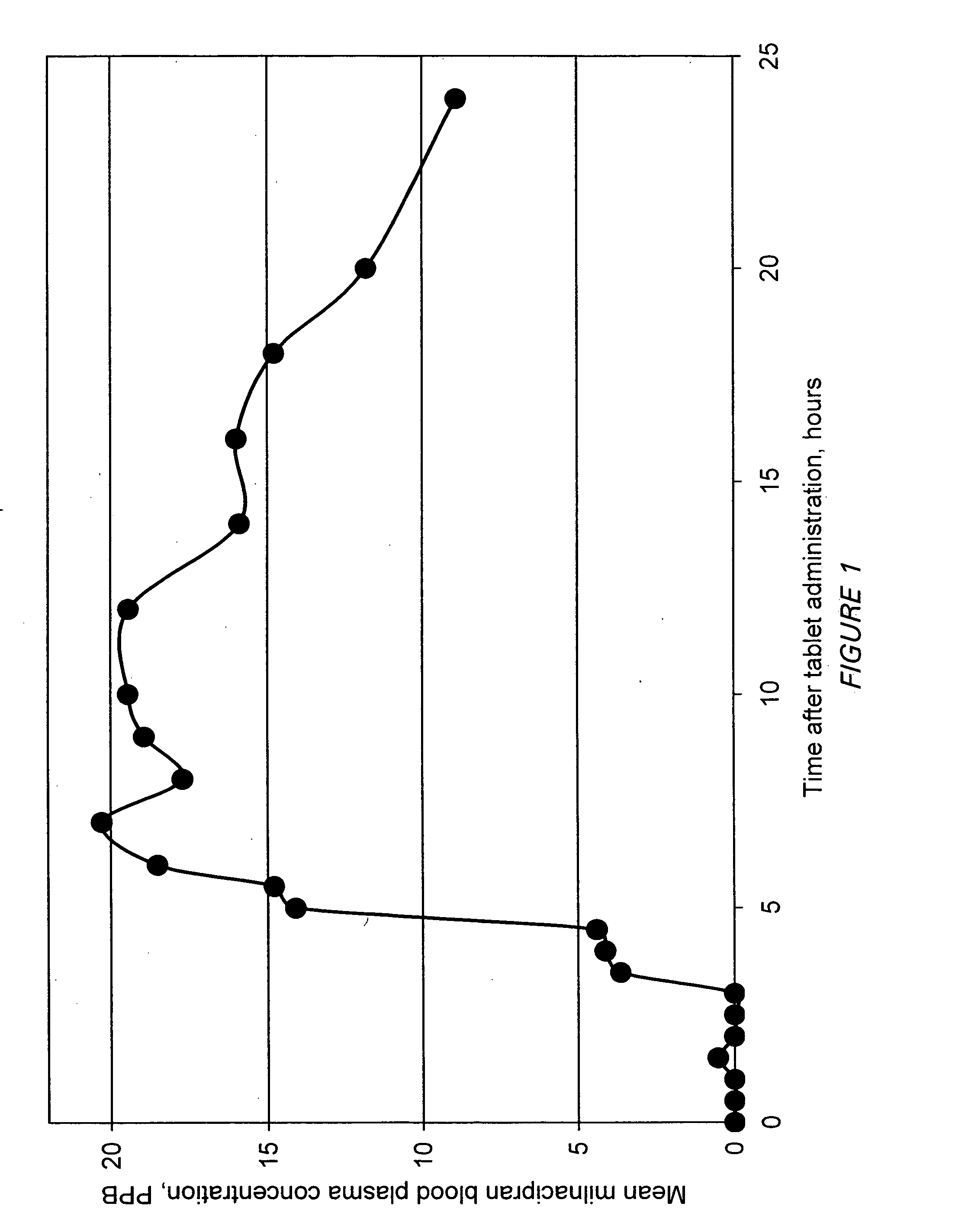
Modified release compositions of milnacipran
A once-a-day oral milnacipran modified release formulation has been developed. The formulation comprises an extended release dosage unit (optionally containing the immediate release portion) coated with delayed release coating. The milnacipran composition, when administered orally, first passes through the stomach releasing from zero to less than 10% of the total milnacipran dose and then enters the intestines where drug is released slowly over an extended period of time. The release profile is characterized by a 0.05-4 hours lag time period during which less than 10% of the total milnacipran dose is released followed by a slow or extended release of the remaining drug over a defined period of time. The composition provides in vivo drug plasma levels characterized by Tmax at 4-10 hours and an approximately linear drop-off thereafter and Cmax below 3000 ng / ml, preferably below 2000 ng / ml, and most preferably below 1000 ng / ml. The composition allows milnacipran to be delivered over approximately 24 hours, when administered to a patient in need, resulting in diminished incidence or decreased intensity of common milnacipran side effects such as sleep disturbance, nausea, vomiting, headache, tremulousness, anxiety, panic attacks, palpitations, urinary retention, orthostatic hypotension, diaphoresis, chest pain, rash, weight gain, back pain, constipation, vertigo, increased sweating, agitation, hot flushes, tremors, fatigue, somnolence, dyspepsia, dysoria, nervousness, dry mouth, abdominal pain, irritability, and insomnia.
Owner:COLLEGIUM PHARMA INC
Method, system and device for treating disorders of the pelvic floor by delivering drugs to various nerves or tissues
InactiveUS20050010259A1Strong specificityLower medical costsElectrotherapyMedical devicesDiseaseLigament structure
Described are implantable devices and methods for treating various disorders of the pelvic floor by delivering one or more drugs to a patient's hypogastric nerve or branches or portions thereof, prostatic plexus nerve or branches or portions thereof, sacral splanchnic nerve or branches or portions thereof, pelvic splanchnic nerve or branches or portions thereof, prostate or branches or portions thereof, pelvic floor, colon or branches or portions thereof, bladder or portions thereof, vagina or portions thereof, anus or portions thereof, external anal sphincter or portions thereof, urethra or portions thereof, penile dorsal nerve or portions thereof, inferior rectal nerves or branches or portions thereof, perineal nerves or branches or portions thereof, scrotal nerves or branches or portions thereof, scrotum or portions thereof, Alcock's Canal or branches or portions thereof, sacro-tuberous ligament or branches or portions thereof, ischial tuberosity or branches or portions thereof, greater sciatic foramen or branches or portions thereof, or lesser sciatic foramen or branches or portions thereof. One, two or more drug delivery regimes are provided on a continuous, alternating, intermittent or other basis to one or more selected nerves, nerve portions or tissues in an amount and manner effective to treat a number of disorders, including, but not limited to, urinary and / or fecal voiding dysfunctions such as constipation, incontinence disorders such as urge frequency and urinary retention disorders, sexual dysfunctions such as orgasmic and erectile dysfunction, pelvic pain, prostatitis, prostatalgia and prostatodynia.
Owner:MEDTRONIC INC
Method and compositions for the treatment of gastrointestinal disorders
ActiveUS20060094658A1Reduce accumulationIncrease gastrointestinal motilityPeptide/protein ingredientsMetabolism disorderGastroparesisInflammatory Bowel Diseases
Compositions and related methods for treating IBS and other gastrointestinal disorders and conditions (e.g., gastrointestinal motility disorders, functional gastrointestinal disorders, gastroesophageal reflux disease (GERD), duodenogastric reflux, Crohn's disease, ulcerative colitis, inflammatory bowel disease, functional heartburn, dyspepsia (including functional dyspepsia or nonulcer dyspepsia), gastroparesis, chronic intestinal pseudo-obstruction (or colonic pseudoobstruction), and disorders and conditions associated with constipation, e.g., constipation associated with use of opiate pain killers, post-surgical constipation, and constipation associated with neuropathic disorders as well as other conditions and disorders are described. The compositions feature peptides that activate the guanylate cyclase C (GC-C) receptor.
Owner:IRONWOOD PHARMA
Compound vegetable healthcare product and method for preparing same
InactiveCN102228242ATo promote metabolismPromote peristalsisFood preparationBiotechnologyFood additive
The invention relates to a nutrient healthcare product and a method for preparing the same, in particular to a compound vegetable healthcare product and a method for preparing the same. The compound vegetable nutrient powder of the invention consists of the following components in part by weight: 0.1 to 100 parts of dried vegetable powder, 0 to 10 parts of alga-type plant regulating powder, 0 to 96 parts of auxiliary component, 0 to 5 parts of natural flavoring agent and 0 to 5 parts of food additive. The preparation method adopts a simple production process and is suitable for industrialized production, and the product prepared by the method is rich in nutrition and stable in quality, meets the demands of people on various nutrients, regulates the intestines and stomach, improves enterogastric peristalsis, enhances in vivo metabolism of sulfur-contained substances, avoids the adverse effects of human body caused by malnutrition and unbalanced nutrition and prevents and helps to treat diseases such as ulcerative stomatitis, diabetes and constipation. The product has also the advantages of convenience for carrying and long storage period.
Owner:肖天存
Therapy for constipation
Compositions comprising colchicine and at least one amino-salicylic acid derivative, preferably olsalazine is used for treatment of prophylaxis of constipation.
Owner:BORODY THOMAS JULIUS
Infant formula with improved protein content
InactiveUS6863918B2Promote absorptionPrevent and reduce regurgitationVitamin food ingredientsHydrolasesHydrolysateConstipation
An improved infant formula resulting in reduced constipation, abdominal discomfort and gastrointestinal problems, comprises at least one protein component having a phosphorus content of less than 0.75 g P / 100 g protein, and at least one lipid component that can be easily digested by an infant. Preferably, it further comprises at least one prebiotic component, and at least one viscosity-improving component. The protein fraction of the formula is preferably a hydrolysate prepared by hydrolysing a protein starting material, especially a whey protein with a combination of at least one endo- and at least one exoproteinase.
Owner:NUTRICIA
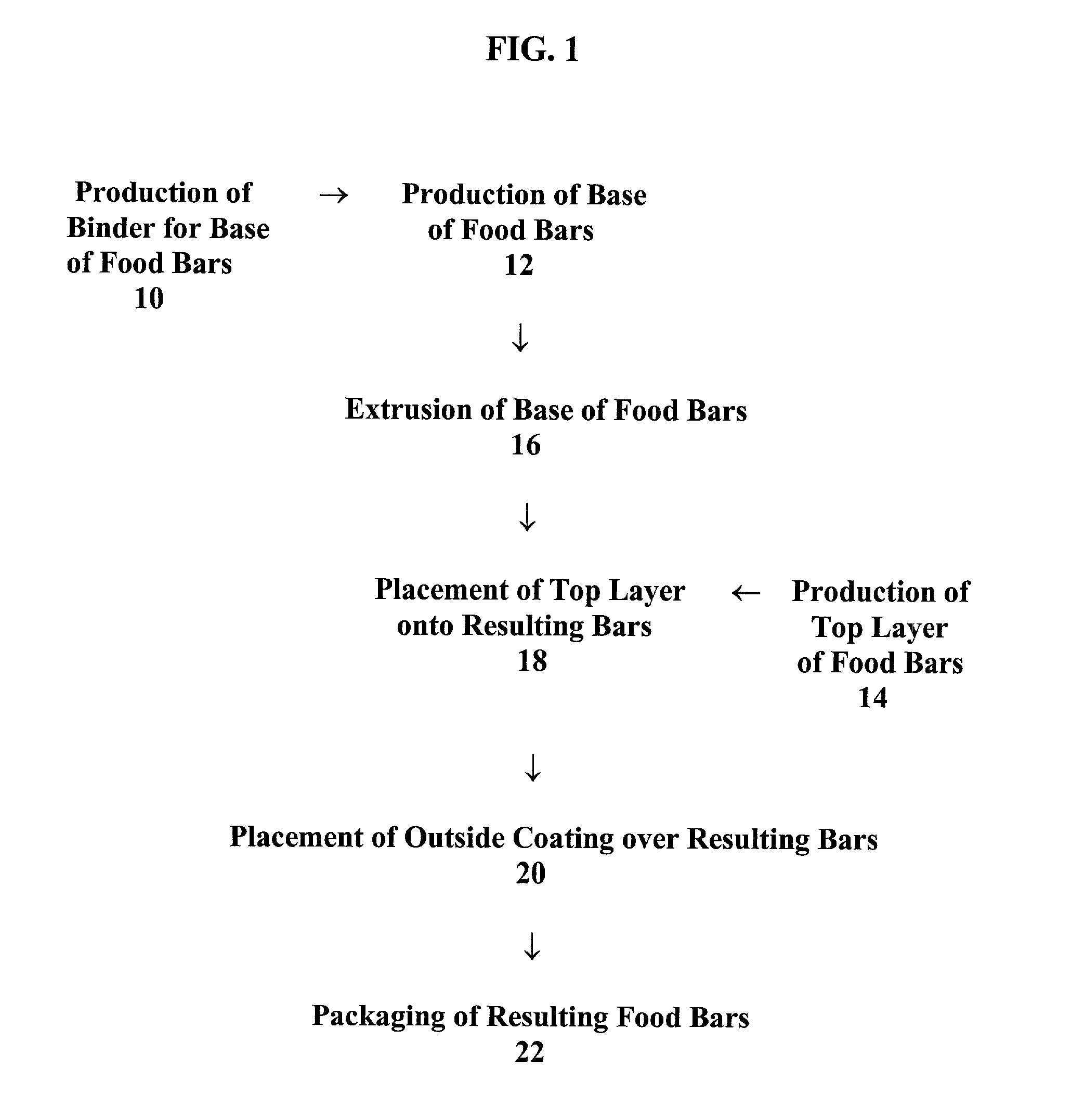
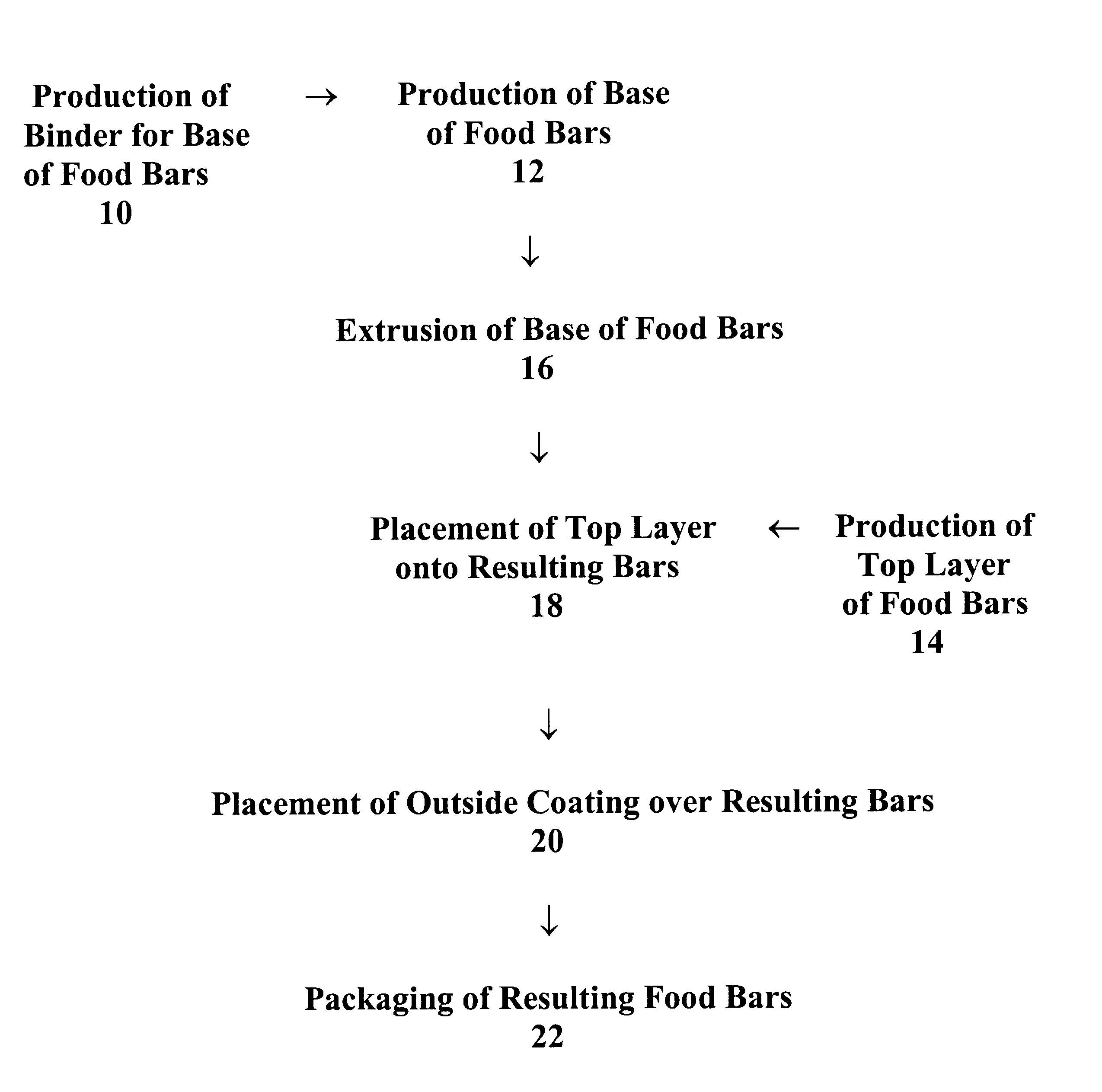
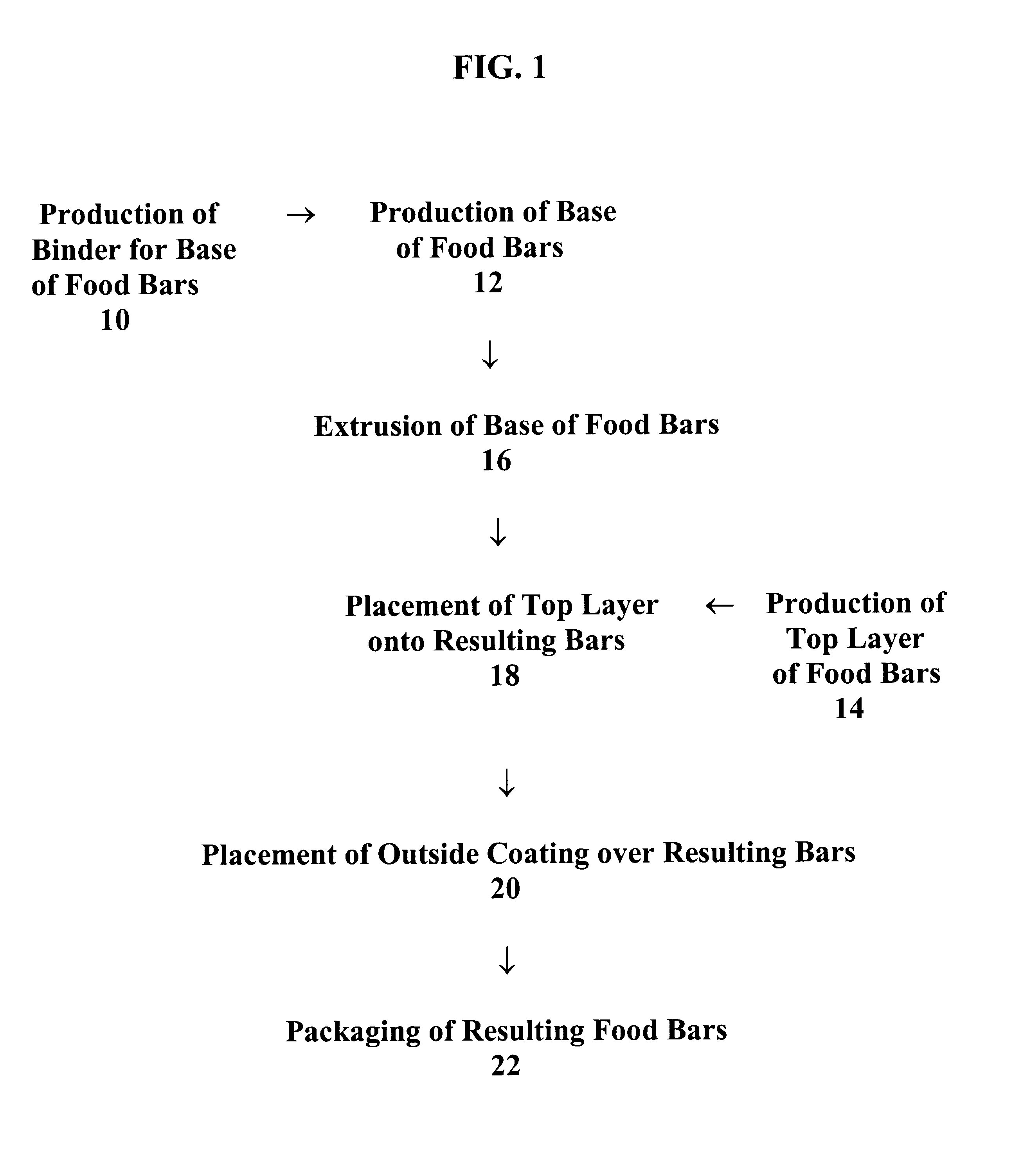
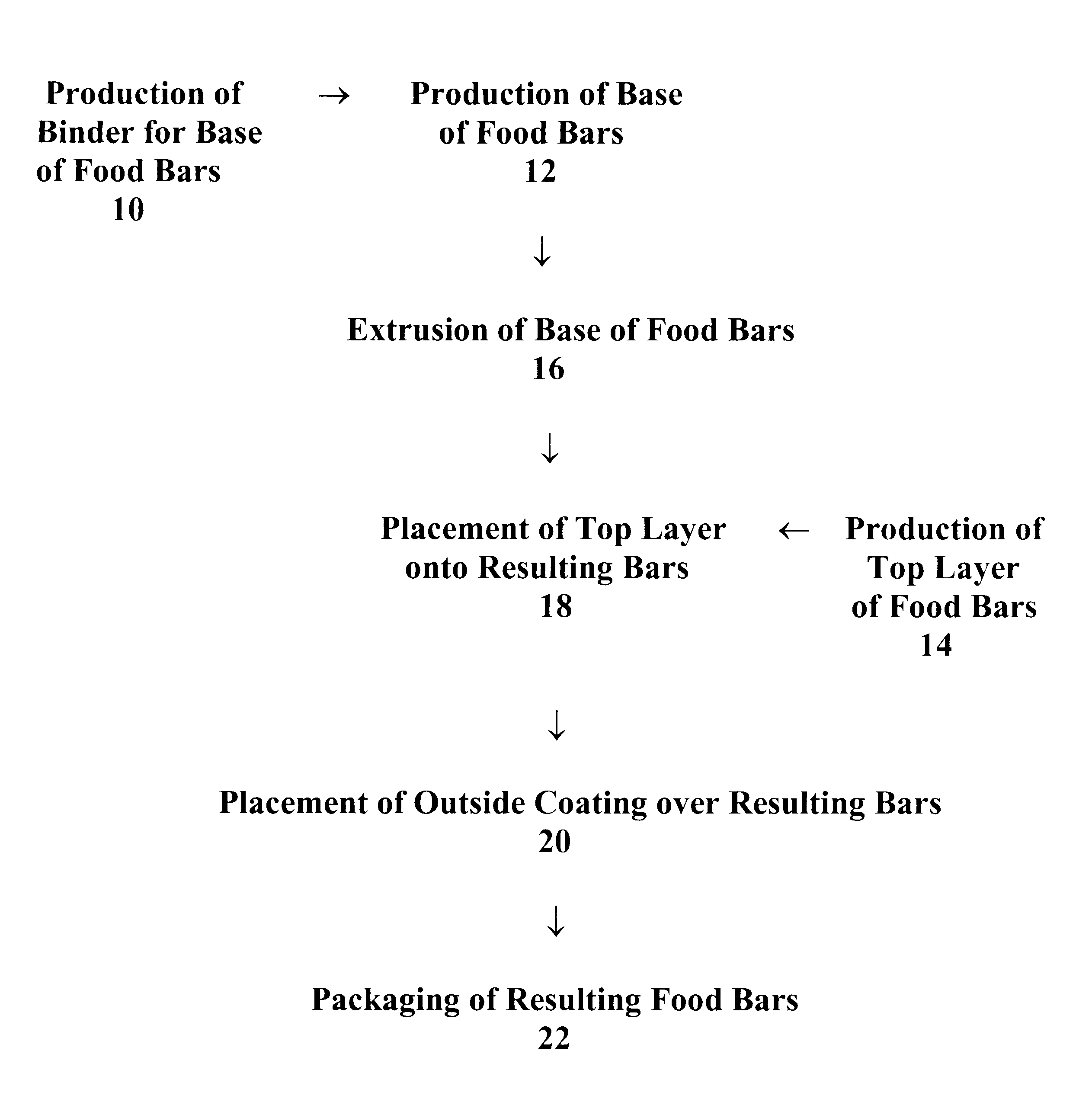
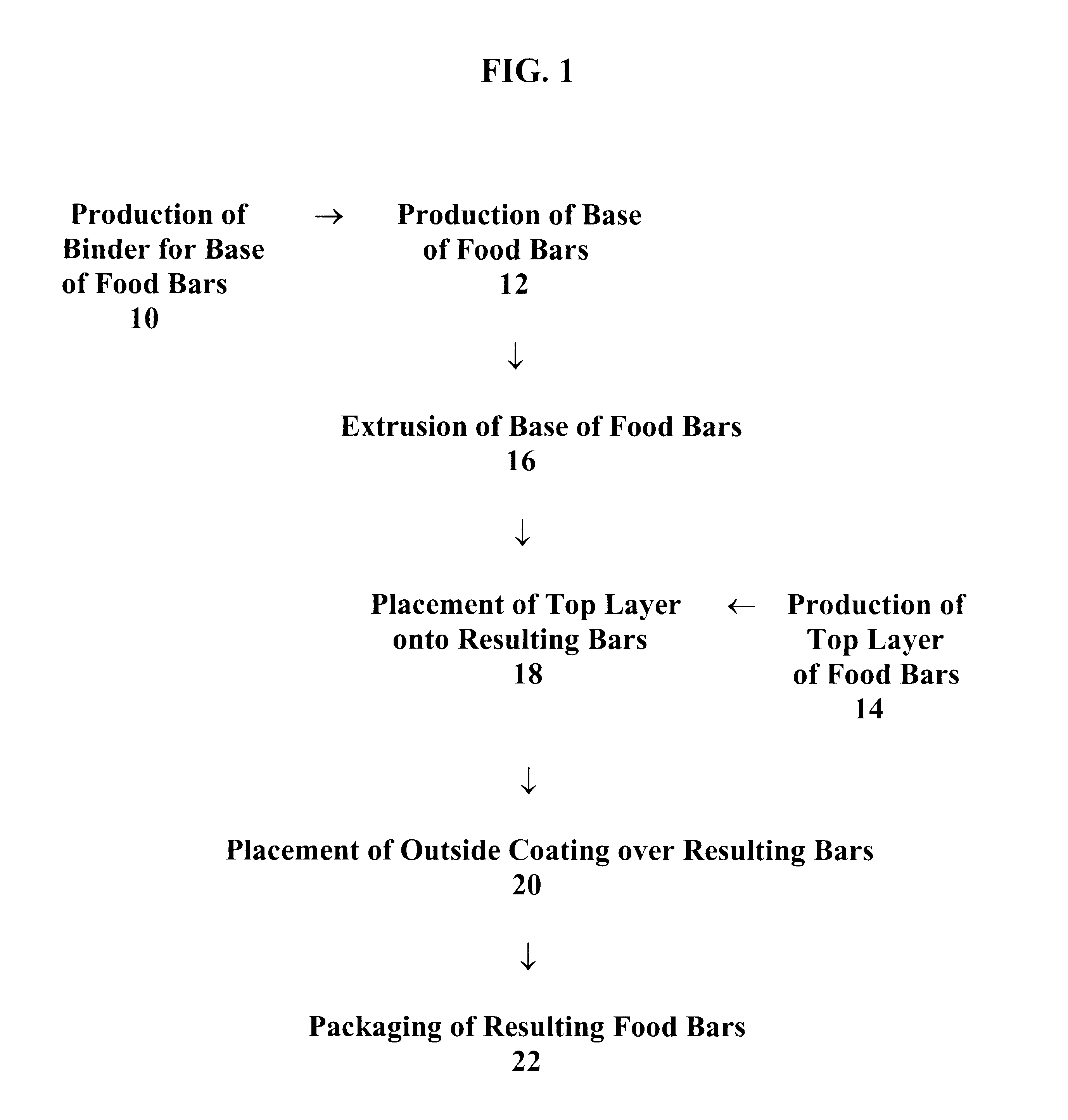
Food bars containing nutritional supplements and Anti-constipation and regularity -maintaining agents
The present invention provides food bars for consumption by pregnant women, lactating women or women of childbearing potential that are attempting to become pregnant containing one or more vitamins and / or minerals, and one or more anti-constipation and regularity-maintaining agents, methods for preparing these food bars, and methods for supplementing the dietary requirements of pregnant women, lactating women or women of childbearing potential that are attempting to become pregnant. The food bars of the invention generally comprise one or more vitamins and minerals recommended for consumption by pregnant women, lactating women or women of childbearing potential that are attempting to become pregnant in an amount that is effective for enhancing the nutrition of pregnant women, lactating women or women of childbearing potential that are attempting to become pregnant, and that is not harmful to developing fetuses or breast-feeding babies, one or more anti-constipation and regularity maintaining agents in an amount that is effective for reducing or eliminating constipation, and that is not harmful to developing fetuses or breast-feeding babies, from about 0 to about 99 weight percent of carbohydrates, from about 0 to about 80 weight percent of proteins, and from about 0 to about 60 weight percent of fats.
Owner:PBM PHARMA
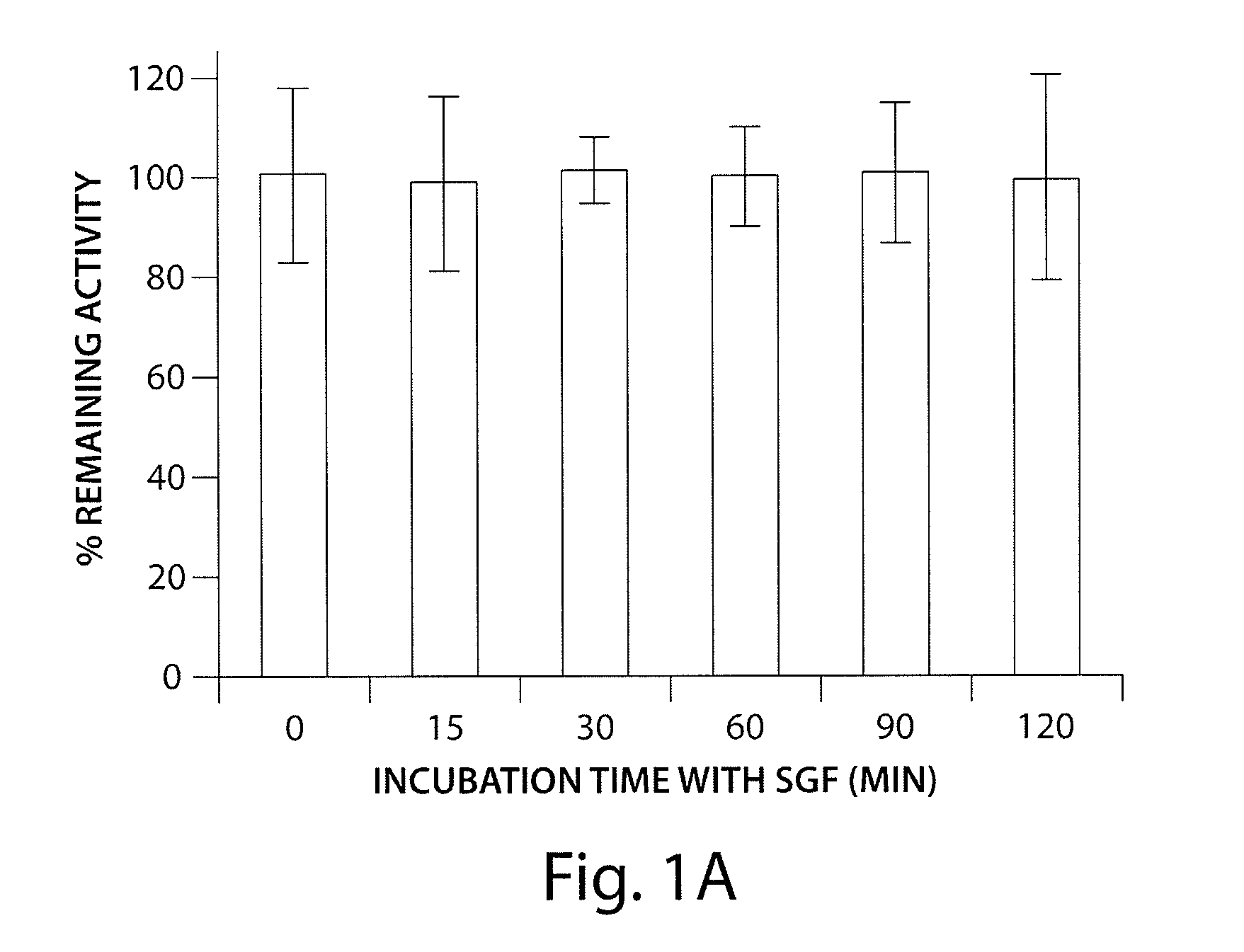
Agonists of guanylate cyclase useful for the treatment of gastrointestinal disorders, inflammation, cancer and other disorders
ActiveUS20090048175A1Maintain good propertiesIncrease resistanceOrganic active ingredientsSenses disorderPhosphodiesteraseGastrointestinal cancer
The invention provides novel guanylate cyclase-C agonist peptides and their use in the treatment of human diseases including gastrointestinal disorders, inflammation or cancer (e.g., a gastrointestinal cancer). The peptides can be administered either alone or in combination with an inhibitor of cGMP-dependent phosphodiesterase. The gastrointestinal disorder may be classified as either irritable bowel syndrome, constipation, or excessive acidity etc. The gastrointestinal disease may be classified as either inflammatory bowel disease or other GI condition, including Crohn's disease and ulcerative colitis, and cancer.
Owner:BAUSCH HEALTH IRELAND LTD
Pharmaceutical composition and method for the transdermal delivery of magnesium
InactiveUS20050196434A1Reduce disadvantagesBiocideAerosol deliveryAutonomic bladder dysfunctionMagnesium salt
The present invention relates to a method and transdermal pharmaceutical composition for preventing magnesium deficiency or imbalances associated with magnesium deficiency including diabetes, hypertension, high cholesterol, cardiac arrhythmias, acute myocardial infarction, arteriosclerosis, atherosclerosis, preeclampsia, dysautonomia, mitral valve prolapse, asthma, constipation, irritable bowel syndrome, migraines, muscle spasms and cramping, premenstrual syndrome, osteoporosis, kidney stones, chronic fatigue syndrome, and fibromyalgia. The transdermal pharmaceutical composition includes a therapeutically effective amount of a pharmaceutically acceptable salt of magnesium and a pharmaceutically acceptable carrier. A therapeutically effective amount of a pharmaceutically acceptable salt of zinc a vitamin such as B-complex vitamin, a carotenoid, a mineral, or a combination thereof may also be included in the transdermal pharmaceutical composition. A therapeutically effective amount of progesterone may also be included in the transdermal pharmaceutical composition. The transdermal pharmaceutical composition may be topically administered to prevent magnesium deficiency or imbalances caused by magnesium deficiency.
Owner:BRIERRE BARBARA T
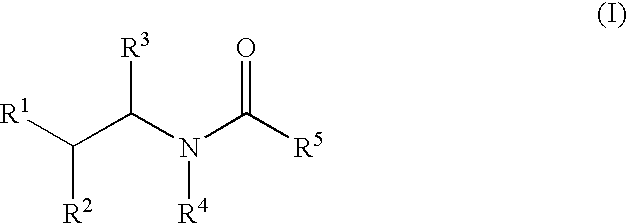
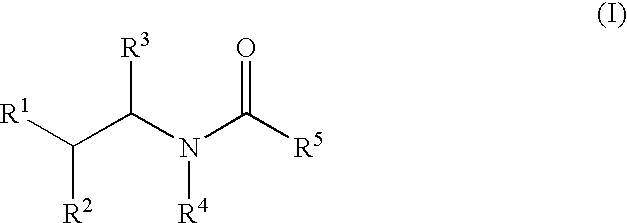
Substituted aryl amides
Novel compounds of structural formula (I) are antagonists and / or inverse agonists of the Cannabinoid-1 (CB1) receptor and are useful in the treatment, prevention and suppression of diseases mediated by the CB1 receptor. The compounds of the present invention are useful as psychotropic drugs in the treatment of psychosis, memory deficits, cognitive disorders, migraine, neuropathy, neuro-inflammatory disorders including multiple sclerosis and Guillain-Barre syndrome and the inflammatory sequelae of viral encephalitis, cerebral vascular accidents, and head trauma, anxiety disorders, stress, epilepsy, Parkinson's disease, movement disorders, and schizophrenia. The compounds are also useful for the treatment of substance abuse disorders, the treatment of obesity or eating disorders, as well as, the treatment of asthma, constipation, chronic intestinal pseudo-obstruction, and cirrhosis of the liver.
Owner:MERCK & CO INC
Method, system and device for treating disorders of the pelvic floor by delivering drugs to various nerves or tissues
InactiveUS7427280B2EfficaciousMore delivery of drugElectrotherapyMedical devicesDiseaseLigament structure
Described are implantable devices and methods for treating various disorders of the pelvic floor by delivering one or more drugs to a patient's hypogastric nerve or branches or portions thereof, prostatic plexus nerve or branches or portions thereof, sacral splanchnic nerve or branches or portions thereof, pelvic splanchnic nerve or branches or portions thereof, prostate or branches or portions thereof, pelvic floor, colon or branches or portions thereof, bladder or portions thereof, vagina or portions thereof, anus or portions thereof, external anal sphincter or portions thereof, urethra or portions thereof, penile dorsal nerve or portions thereof, inferior rectal nerves or branches or portions thereof, perineal nerves or branches or portions thereof, scrotal nerves or branches or portions thereof, scrotum or portions thereof, Alcock's Canal or branches or portions thereof, sacro-tuberous ligament or branches or portions thereof, ischial tuberosity or branches or portions thereof, greater sciatic foramen or branches or portions thereof, or lesser sciatic foramen or branches or portions thereof. One, two or more drug delivery regimes are provided on a continuous, alternating, intermittent or other basis to one or more selected nerves, nerve portions or tissues in an amount and manner effective to treat a number of disorders, including, but not limited to, urinary and / or fecal voiding dysfunctions such as constipation, incontinence disorders such as urge frequency and urinary retention disorders, sexual dysfunctions such as orgasmic and erectile dysfunction, pelvic pain, prostatitis, prostatalgia and prostatodynia.
Owner:MEDTRONIC INC
Food bars containing nutritional supplements
Owner:PBM PHARMA
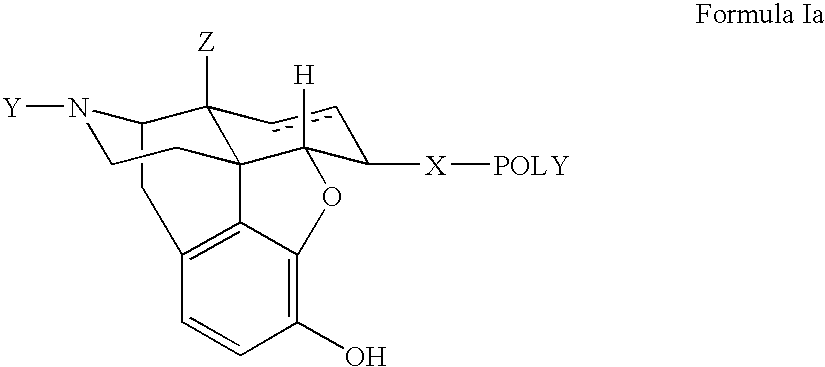
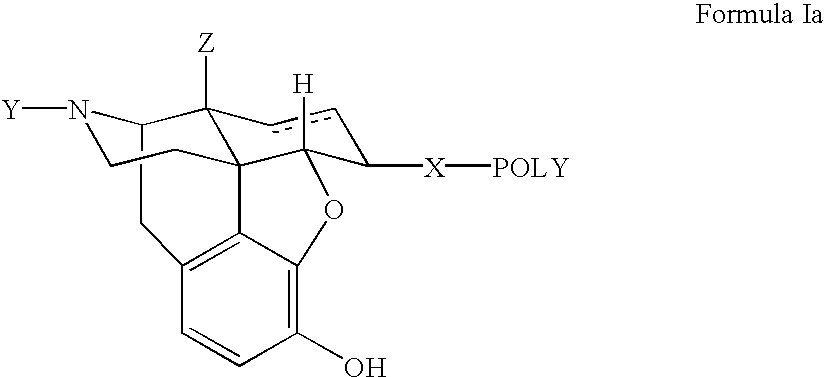
Polymer conjugates of opioid antagonists
The invention provides polymer conjugates of opioid antagonists comprising a polymer, such as poly(ethylene glycol), covalently attached to an opioid antagonist. The linkage between the polymer and the opioid antagonist is preferably hydrolytically stable. The invention also includes a method of treating one or more side effects associated with the use of opioid analgesics, such as constipation, nausea, or pruritus, by administering a polymer conjugate of the invention.
Owner:NEKTAR THERAPEUTICS INC
Lady beer and production technology thereof
The invention relates to a beer produced from materials of 1-7 degree beerwort produced with a common beer production method, fresh fruit and vegetable juice, healthcare plant juice, traditional Chinese medicine extract, vitamins, a microelement mineral preparation, a foam stabilizer, and ethanol, which are blended according to a certain weight ratio. The lady beer provided by the invention has characteristics of digestion and absorbance promoting, beautifying, toxin expelling, freckle reducing, weight reducing, aging resisting, fatigue resisting, bowel loosening, constipation relieving, metabolism balancing, endocrine regulating, blood sugar reducing, and blood fat reducing. The contents of beerwort and ethanol in the beer are low. With the lady beer, original flavors of beer are preserved, abundant nutrients are provided, beer belly is not caused with long-term drinking, obesity is not caused, and a good healthcare effect is provided. The application scope of the lady beer is wide, and the lady beer is especially suitable for ladies.
Owner:张华
Methods and compositions for the treatment of gastrointestinal disorders
InactiveUS20070010450A1Increase gastrointestinal motilityReduce inflammationPeptide/protein ingredientsMetabolism disorderIntestinal tract diseasesGastroparesis
Compositions and related methods for treating IBS and other gastrointestinal disorders and conditions (e.g., gastrointestinal motility disorders, functional gastrointestinal disorders, gastroesophageal reflux disease (GERD), duodenogastric reflux, Crohn's disease, ulcerative colitis, inflammatory bowel disease, functional heartburn, dyspepsia (including functional dyspepsia or nonulcer dyspepsia), gastroparesis, chronic intestinal pseudo-obstruction (or colonic pseudoobstruction), and disorders and conditions associated with constipation, e.g., constipation associated with use of opiate pain killers, post-surgical constipation, and constipation associated with neuropathic disorders as well as other conditions and disorders are described. The compositions feature peptides that activate the guanylate cyclase C (GC-C) receptor.
Owner:IRONWOOD PHARMA
Substituted amides
Novel compounds of the structural formula (I) are antagonists and / or inverse agonists of the Cannabinoid-1 (CB1) receptor and are useful in the treatment, prevention and suppression of diseases mediated by the CB1 receptor. The compounds of the present invention are useful as centrally acting drugs in the treatment of psychosis, memory deficits, cognitive disorders, migraine, neuropathy, neuro-inflammatory disorders including multiple sclerosis and Guillain-Barre syndrome and the inflammatory sequelae of viral encephalitis, cerebral vascular accidents, and head trauma, anxiety disorders, stress, epilepsy, Parkinson's disease, movement disorders, and schizophrenia. The compounds are also useful for the treatment of substance abuse disorders, the treatment of obesity or eating disorders, as well as the treatment of asthma, constipation, chronic intestinal pseudo-obstruction, and cirrhosis of the liver.
Owner:MERCK SHARP & DOHME LLC
Therapy for the treatment of disease
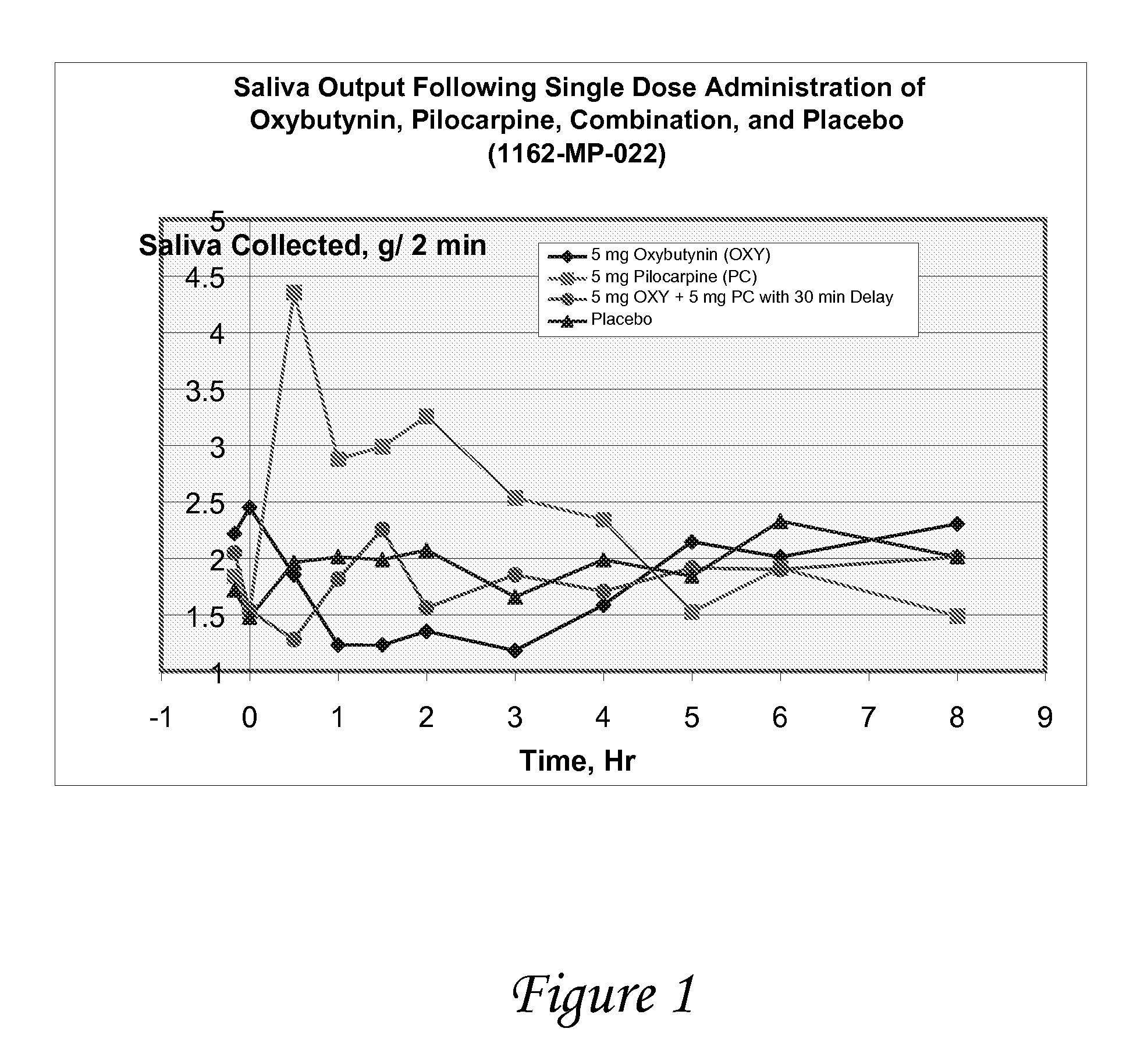
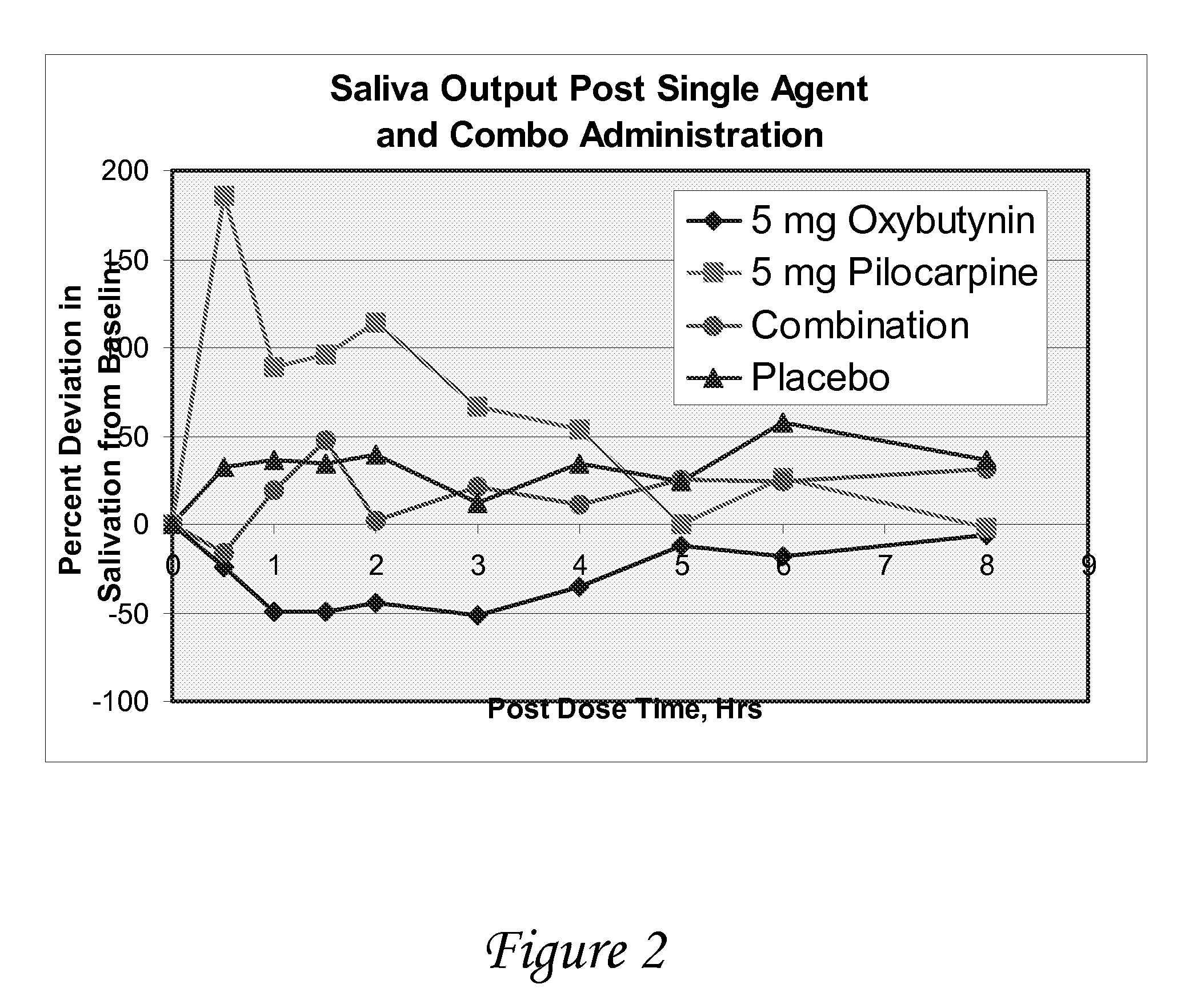
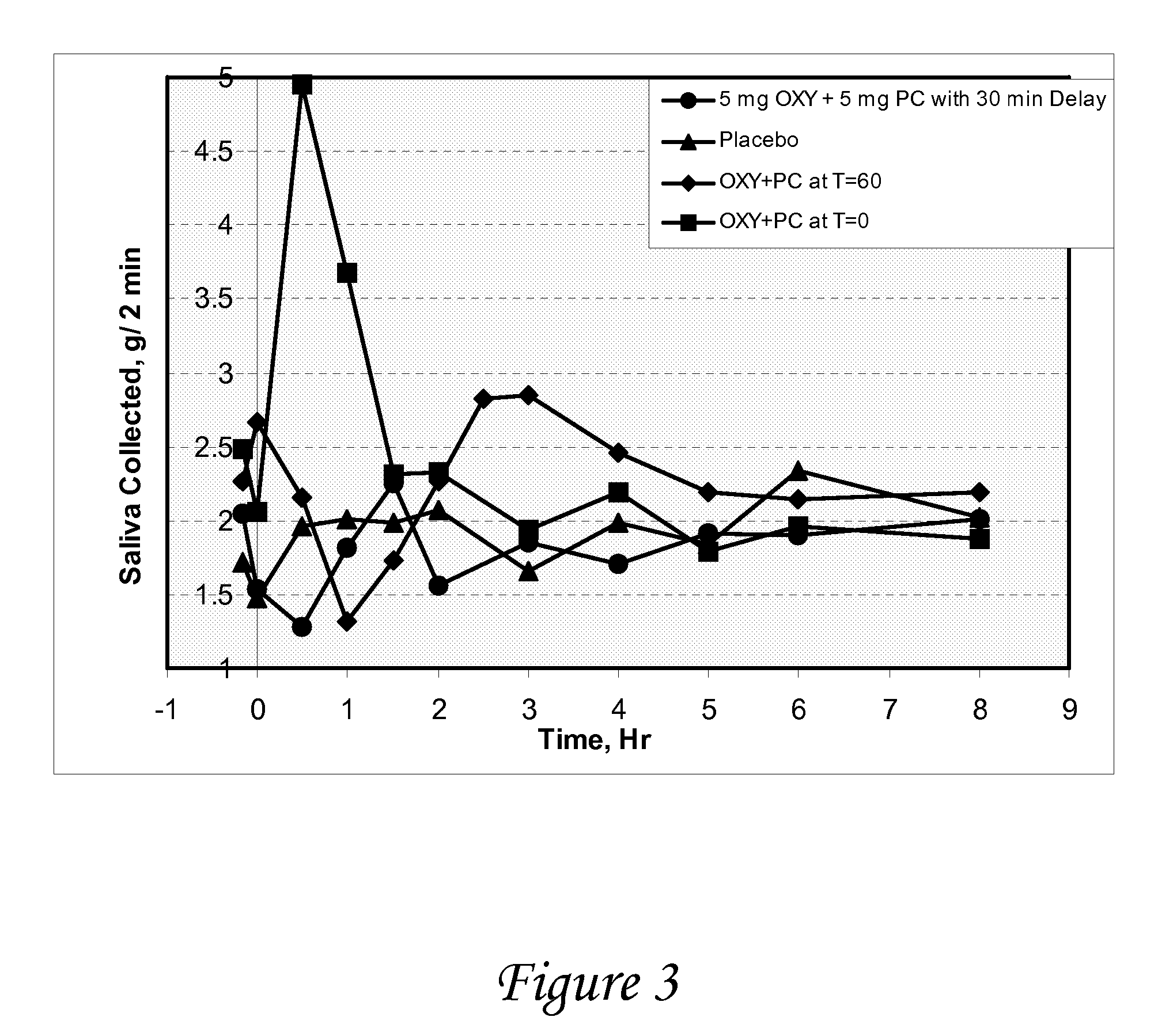
ActiveUS20070053995A1Relieve constipationBiocideNervous disorderAnticholinergic agentsCompound (substance)
Disclosed herein are pharmaceutical compositions comprising various combinations of an antimuscarinic or an anticholinergic agent, a compound that causes stimulation of salivary glands, and a compound that relieves constipation. Also disclosed are methods of treating a patient suffering from overactive bladder comprising administering to the patient the above pharmaceutical composition.
Owner:THERAVIDA INC
Agonists of Guanylate Cyclase UseFul For The Treatment of Gastrointestinal Disorders, Inflammation, Cancer and Other Disorders
ActiveUS20100093635A1Reduce inflammationPositive therapeutic effectAntipyreticAnalgesicsPhosphodiesteraseGastrointestinal cancer
The invention provides novel guanylate cyclase-C agonist peptides and their use in the treatment of human diseases including gastrointestinal disorders, inflammation or cancer (e.g., a gastrointestinal cancer). The peptides can be administered either alone or in combination with an inhibitor of cGMP-dependent phosphodiesterase. The gastrointestinal disorder may be classified as either irritable bowel syndrome, constipation, or excessive acidity etc. The gastrointestinal disease may be classified as either inflammatory bowel disease or other GI condition including Crohn's disease and ulcerative colitis, and cancer.
Owner:BAUSCH HEALTH IRELAND LTD
Agonists of Guanylate Cyclase Useful for the Treatment of Gastrointestinal Disorders, Inflammation, Cancer and Other Disorders
ActiveUS20100069306A1Maintain good propertiesIncrease resistancePeptide/protein ingredientsAntipyreticPhosphodiesteraseGastrointestinal cancer
The invention provides novel guanylate cyclase-C agonist peptides and their use in the treatment of human diseases including gastrointestinal disorders, inflammation or cancer (e.g., a gastrointestinal cancer). The peptides can be administered either alone or in combination with an inhibitor of cGMP-dependent phosphodiesterase. The gastrointestinal disorder may be classified as either irritable bowel syndrome, constipation, or excessive acidity etc. The gastrointestinal disease may be classified as either inflammatory bowel disease or other GI condition including Crohn's disease and ulcerative colitis, and cancer.
Owner:BAUSCH HEALTH IRELAND LTD
Food bars containing nutritional supplements and anti-constipation and regularity maintaining-agents
The present invention provides food bars for consumption by pregnant women, lactating women or women of childbearing potential that are attempting to become pregnant containing one or more vitamins and / or minerals, and one or more anti-constipation and regularity-maintaining agents, methods for preparing these food bars, and methods for supplementing the dietary requirements of pregnant women, lactating women or women of childbearing potential that are attempting to become pregnant. The food bars of the invention generally comprise one or more vitamins and minerals recommended for consumption by pregnant women, lactating women or women of childbearing potential that are attempting to become pregnant in an amount that is effective for enhancing the nutrition of pregnant women, lactating women or women of childbearing potential that are attempting to become pregnant, and that is not harmful to developing fetuses or breast-feeding babies, one or more anti-constipation and regularity-maintaining agents in an amount that is effective for reducing or eliminating constipation, and that is not harmful to developing fetuses or breast-feeding babies, from about 0 to about 99 weight percent of carbohydrates, from about 0 to about 80 weight percent of proteins, and from about 0 to about 60 weight percent of fats.
Owner:PBM PHARMA
Compound probiotics fermented Chinese herbal medicine active health care liquid and preparation method thereof
ActiveCN103027231AAntioxidantHigh food and medicinal valuePre-extraction tea treatmentFood preparationMetaboliteCoronary heart disease
The invention provides compound probiotics fermented Chinese herbal medicine active health care liquid and a preparation method thereof. The health care liquid is prepared by the following raw materials: folium ginkgo, gingko pollen, fructus lycii, tea leaves, bacillus natto, saccharomyces cerevisiae, lactobacilli, acetobacter xylinum, bifidobactirium, white granulated sugar, brown sugar, defatted soy flour, honey, oligosaccharide, sodium chloride, deionized water and the like. The compound probiotics fermented Chinese herbal medicine active health care liquid integrates disease treatment and health care functions of several single products, contains Chinese herbal medicine active ingredients with the functions of preventing and treating hyperlipidemia, hyperglycemia and hypertension, preventing tumors and cancers, enhancing memory, protecting livers, improving immunity, preventing and treating coronary heart diseases, and also contains probiotics groups and metabolic products of the probiotics groups which can regulate balance in the stomach and intestines, resist aging, prolong the life, facilitate digestion, expel toxin for beauty, and prevent and treat thrombi, constipation and diarrhea. The health care liquid integrates health care and treatment, is very wide in market prospect, and conforms to the existing medical viewpoint of people that the prevention, health care, treatment and recovery are combined.
Owner:湖南现代资源生物科技有限公司
Agonists of guanylate cyclase useful for the treatment of gastrointestinal disorders, inflammation, cancer and other disorders
ActiveUS7879802B2Excellent propertyIncrease resistanceOrganic active ingredientsSenses disorderPhosphodiesteraseGastrointestinal cancer
Owner:BAUSCH HEALTH IRELAND LTD
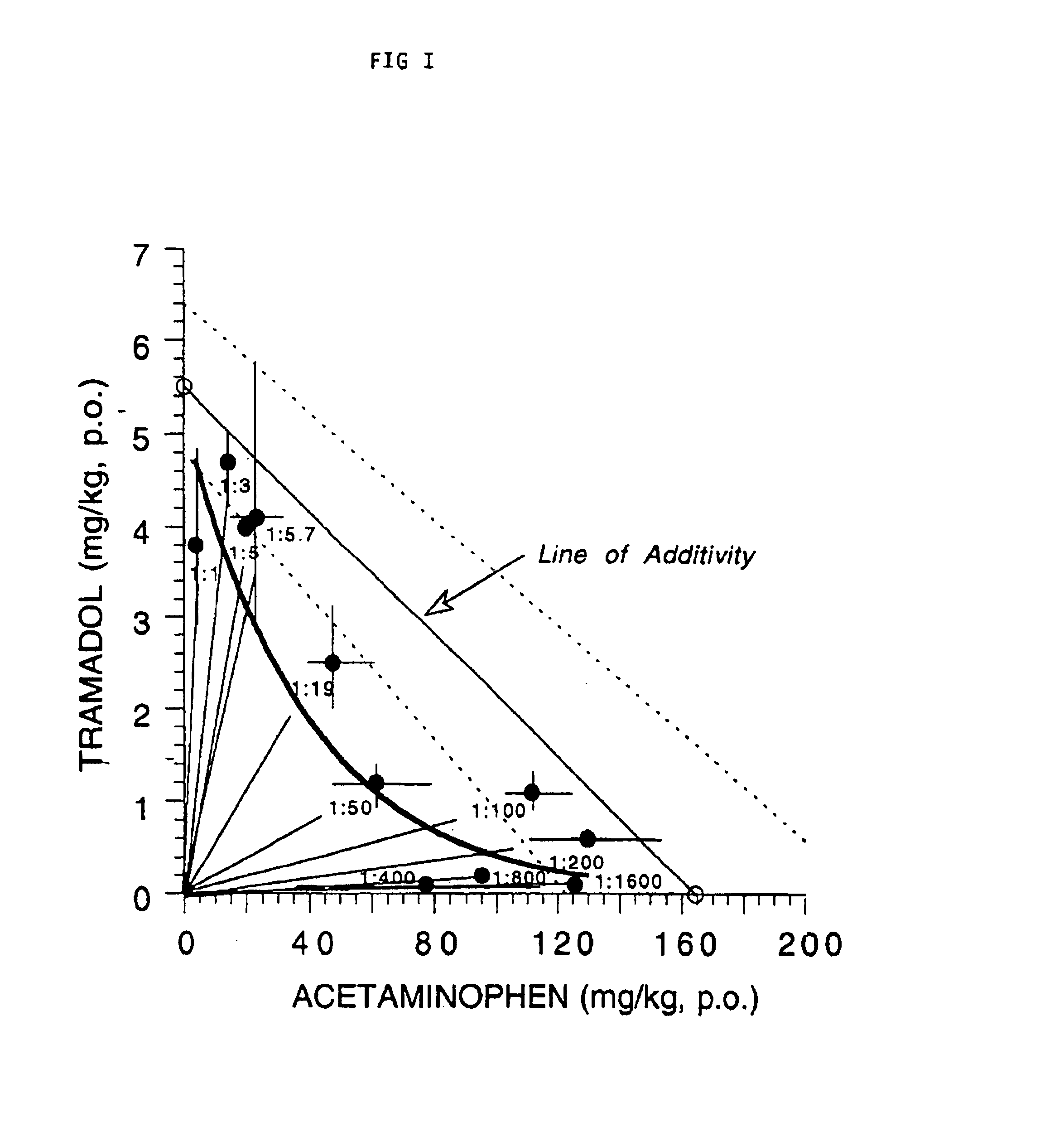
Composition comprising a tramadol material and acetaminophen and its use
This invention relates to a composition comprising a tramadol material and acetaminophen, and its use. As used herein tramadol refers to various forms of tramadol. The compositions are pharmacologically useful in treating pain and tussive conditions. The compositions are also subject to less opioid side-effects such as abuse liability, tolerance, constipation and respiratory depression. Furthermore, where the components of the compositions are within certain ratios the pharmacological effects of the compositions are superadditive (synergistic).
Owner:ORTHO MCNEIL PHARM INC
Polymer conjugates of opioid antagonists
The invention provides polymer conjugates of opioid antagonists comprising a polymer, such as poly(ethylene glycol), covalently attached to an opioid antagonist. The linkage between the polymer and the opioid antagonist is preferably hydrolytically stable. The invention also includes a method of treating one or more side effects associated with the use of opioid analgesics, such as constipation, nausea, or pruritus, by administering a polymer conjugate of the invention.
Owner:NEKTAR THERAPEUTICS INC
Yacon ice cream and manufacturing method thereof
The invention discloses yacon ice cream and a manufacturing method thereof, wherein, the yacon ice cream comprises whole milk powders accounting for 12 to 15 percent, unsweetened evaporated milk accounting for 6 to 9 percent, cream accounting for 7 to 9 percent, white granulated sugar accounting for 3 to 4 percent, starch syrup accounting for 1 to 1.5 percent, sodium alginate accounting for 0.15 to 0.2 percent, monoglyceride accounting for 0.15 to 0.2 percent, yacon granules accounting for 20 to 25 percent and appropriate drinking water. The manufacturing method of the yacon ice cream comprises the steps of yacon granule preparation, raw material mixing preparation, sterilization, homogenizing, cooling, ageing, congealing, filling, rigidification and the like; the yacon ice cream contains yacon granules, achieves cool and delicious taste, achieves double functions of nourishment and health care, and further achieves the nourishment and health care effects of reducing temperature, relieving summer-heat, clearing intestines and stomach, resolving liver toxicity, reducing blood fat, reducing blood pressure, helping digestion, preventing oxidation, preventing constipation and the like compared with the traditional ice cream.
Owner:武杰
Agonists of guanylate cyclase useful for the treatment of gastrointestinal disorders, inflammation, cancer and other disorders
ActiveUS8034782B2Excellent propertyIncrease resistanceAntipyreticAnalgesicsPhosphodiesteraseGastrointestinal cancer
The invention provides novel guanylate cyclase-C agonist peptides and their use in the treatment of human diseases including gastrointestinal disorders, inflammation or cancer (e.g., a gastrointestinal cancer). The peptides can be administered either alone or in combination with an inhibitor of cGMP-dependent phosphodiesterase. The gastrointestinal disorder may be classified as either irritable bowel syndrome, constipation, or excessive acidity etc. The gastrointestinal disease may be classified as either inflammatory bowel disease or other GI condition including Crohn's disease and ulcerative colitis, and cancer.
Owner:BAUSCH HEALTH IRELAND LTD
Health-care tea
InactiveCN101040644AImprove drinking tasteHigh nutritional valuePre-extraction tea treatmentMetabolism disorderDiseaseSide effect
The invention relates to a health-care tea and relative technique, which uses hemp seed and sweet tea as basic materials, assisted with other natural Chinese medicine drug, to be used on various diseases. The inventive health-care tea combines the characters of food, health-care product and drug, with good taste, and treatment on hypertension, hyperlipemia, high cholesterol and constipation, without side effect.
Owner:广西巴马常春藤生命科技发展有限公司
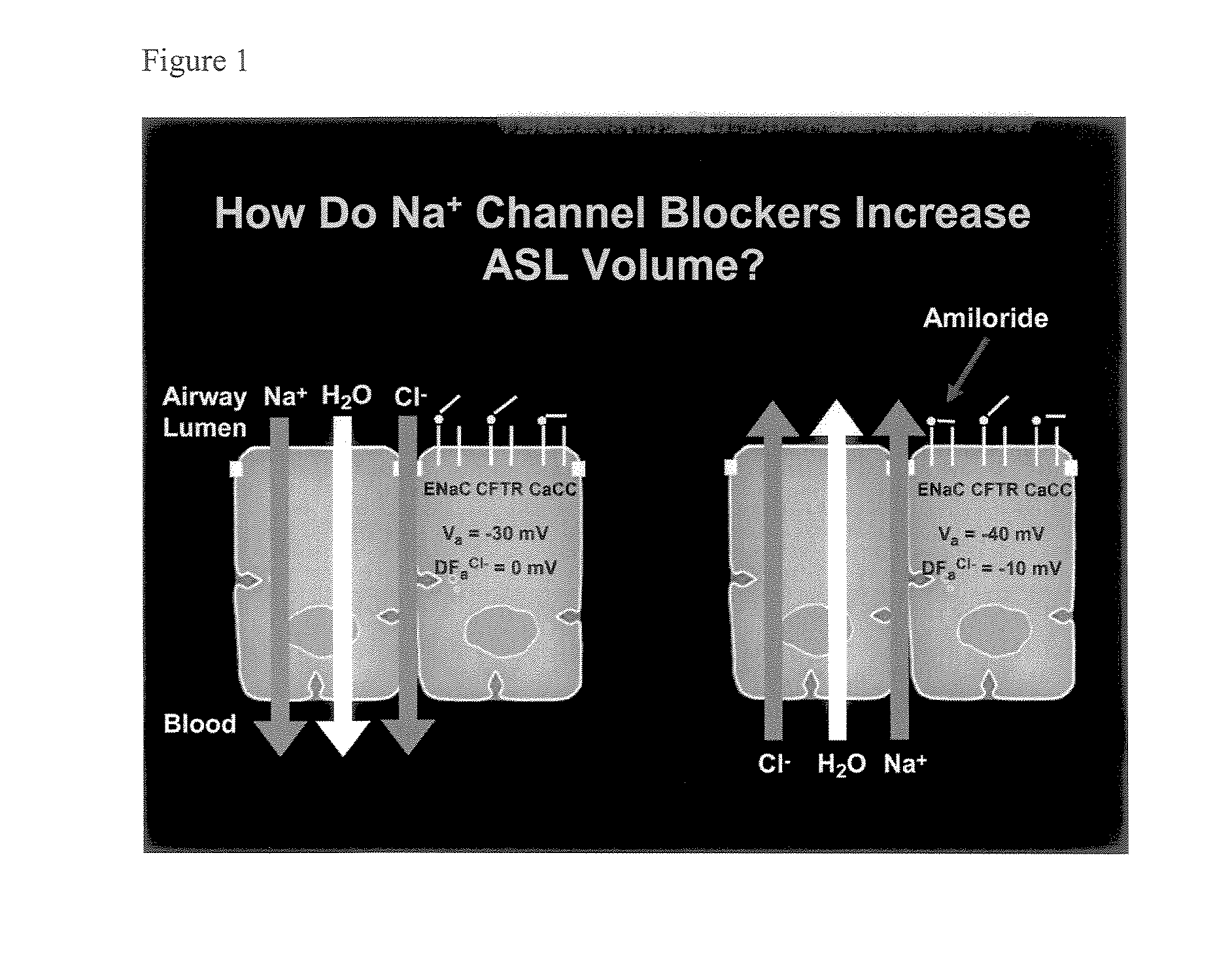
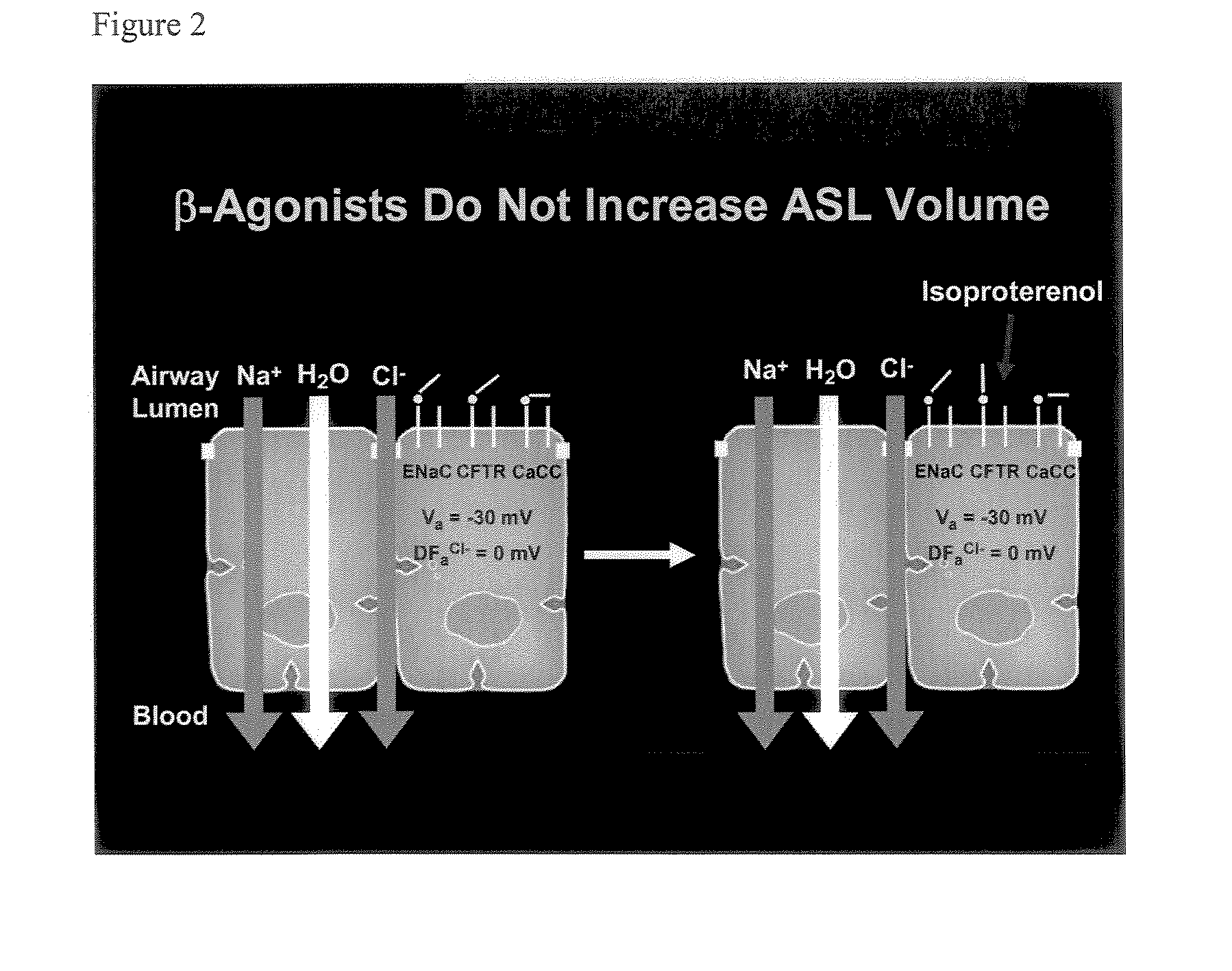
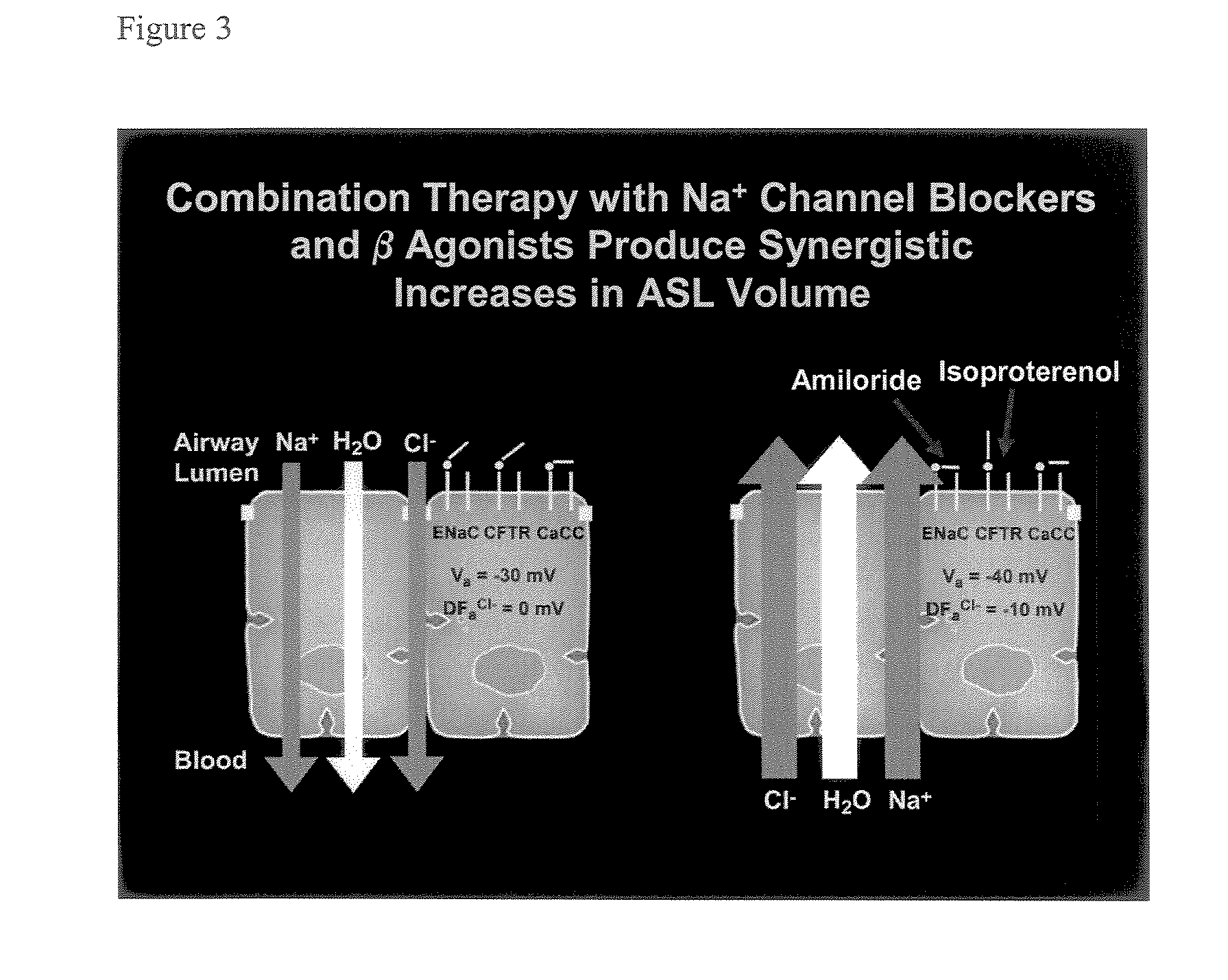
Aliphatic pyrazinoylguanidine sodium channel blockers with beta agonist activity
ActiveUS8324218B2More potent and/or absorbed less rapidlyLess reversibleOrganic active ingredientsSenses disorderDiseaseSinusitis
The present application provides sodium channel blockers exemplified by the following structure:The compounds of the invention useful for treating chronic bronchitis, cystic fibrosis, sinusitis, vaginal dryness, dry eye, Sjogren's disease, distal intestinal obstruction syndrome, dry skin, esophagitis, dry mouth (xerostomia), nasal dehydration, ventilator-induced pneumonia, asthma, primary ciliary dyskinesia, otitis media, chronic obstructive pulmonary disease, emphysema, pneumonia, constipation, and chronic diverticulitis, for example.
Owner:PARION SCI DURHAM NC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com