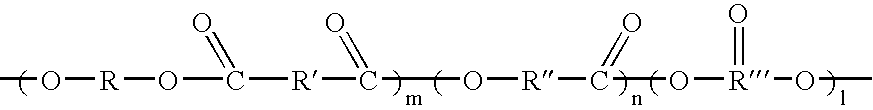
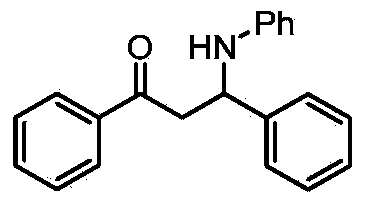
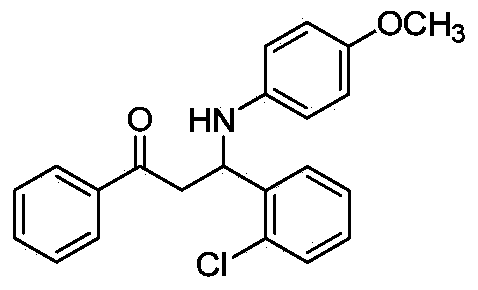
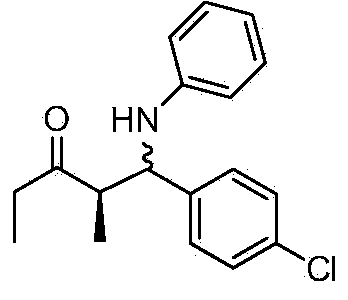
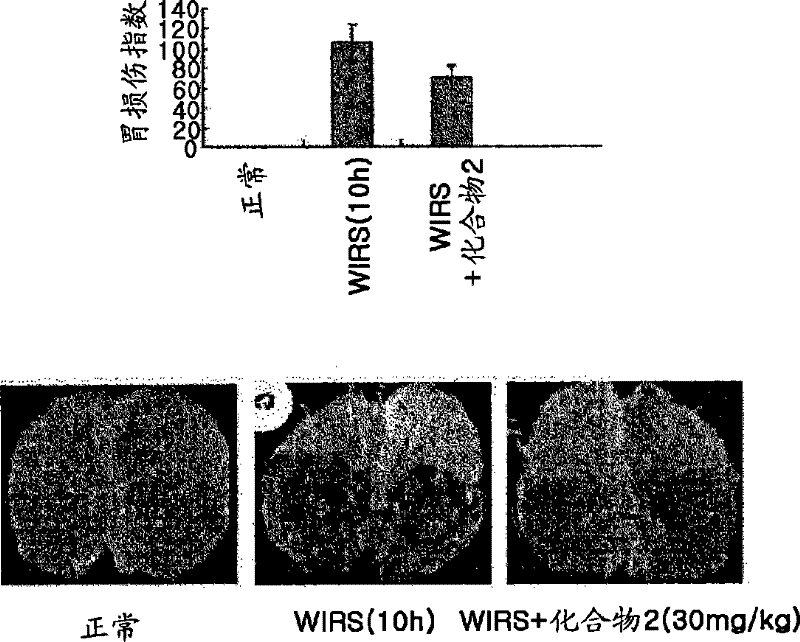
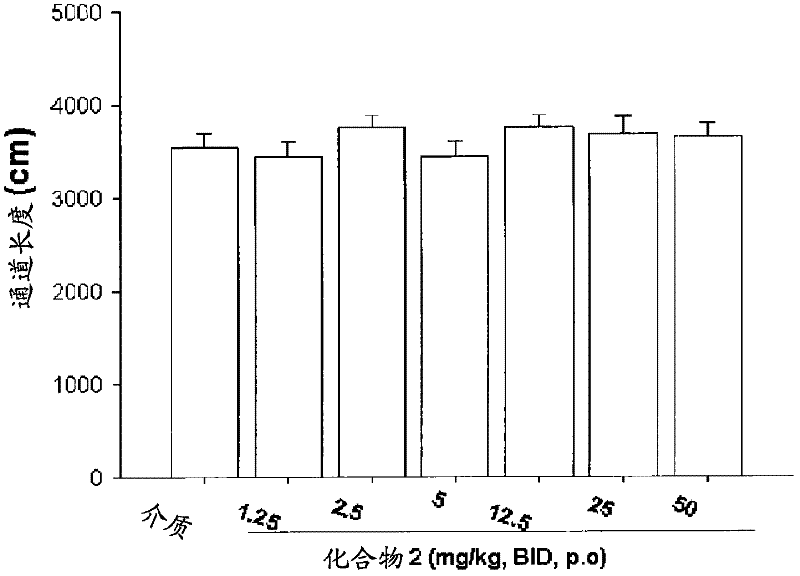
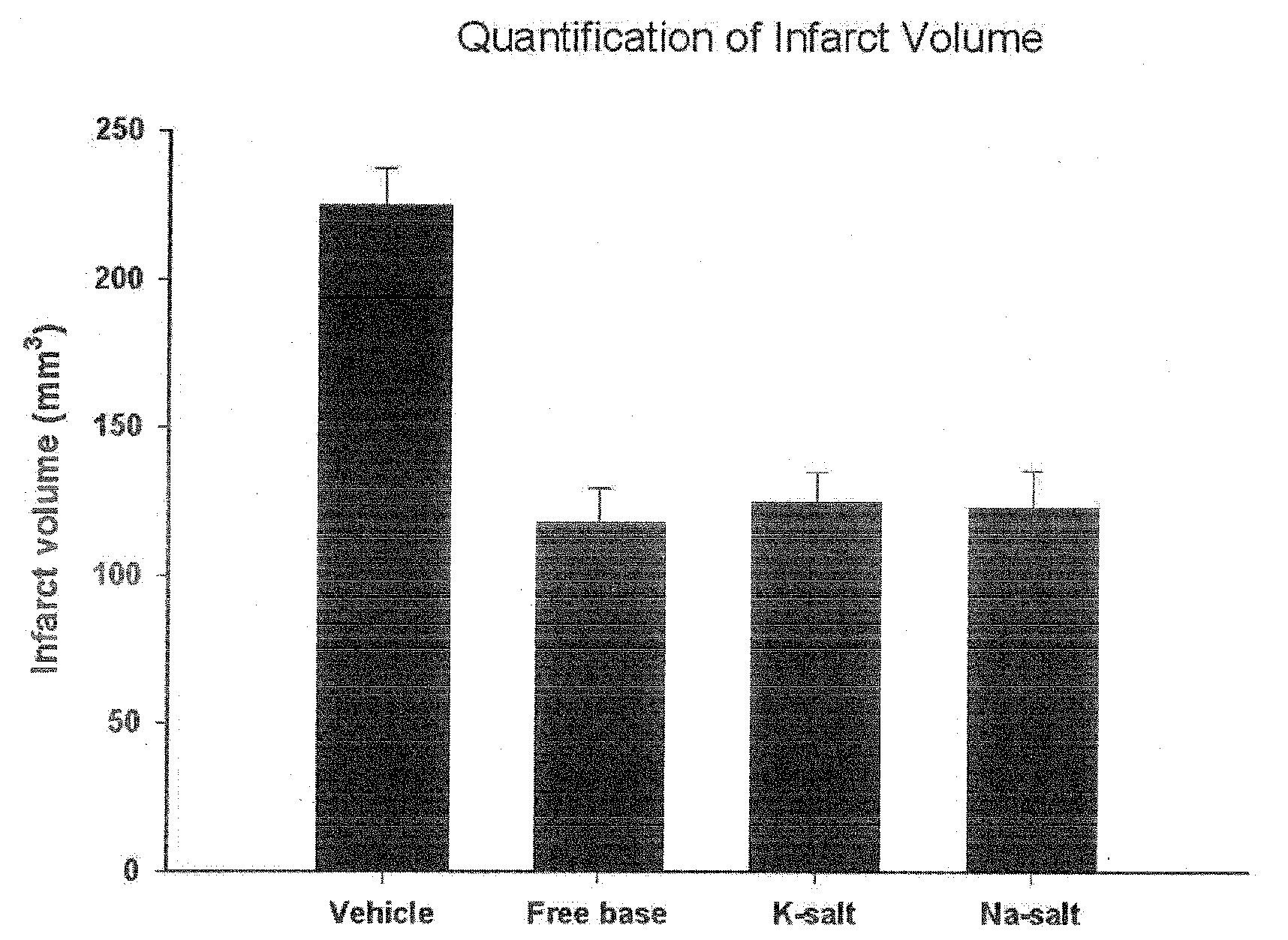
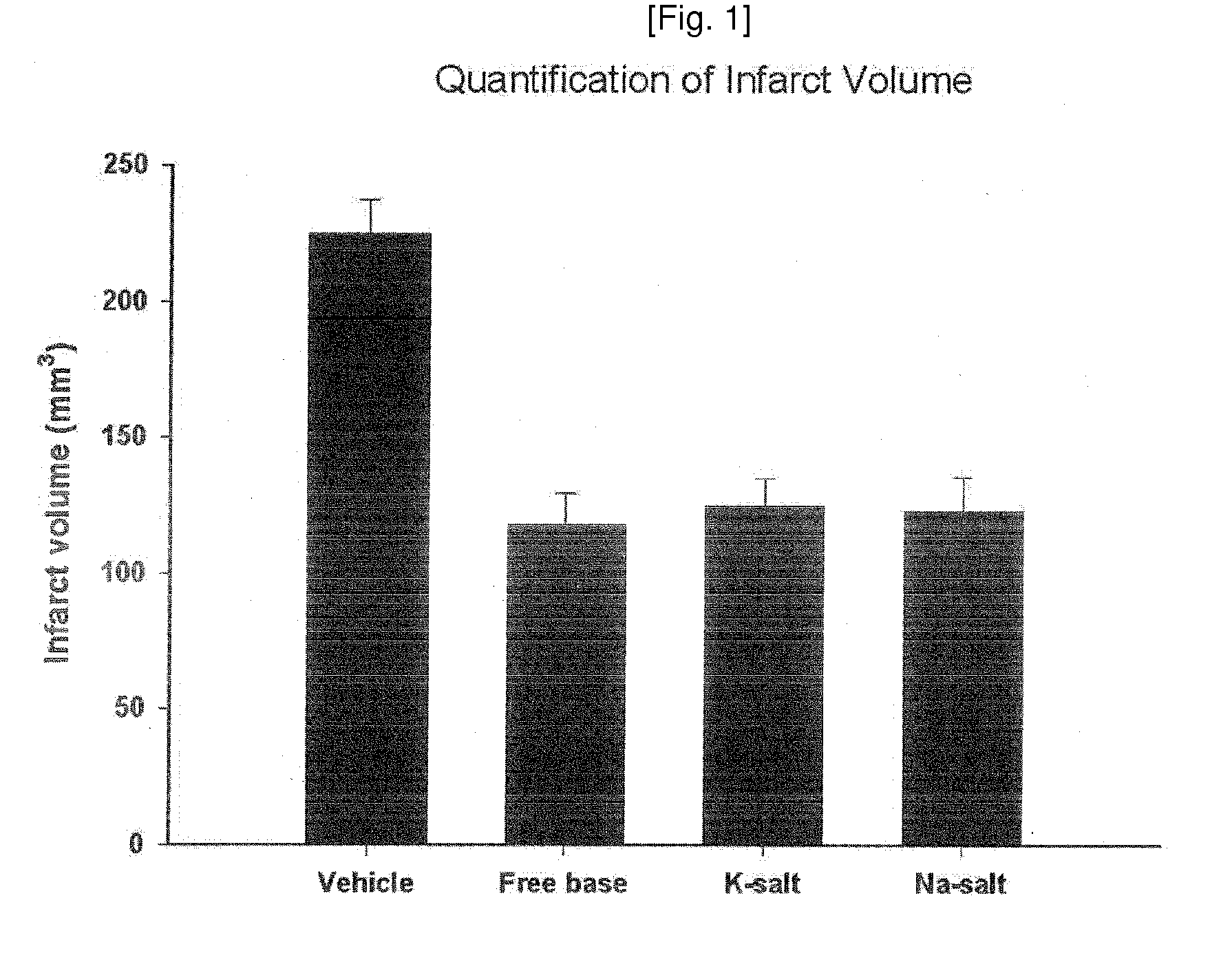
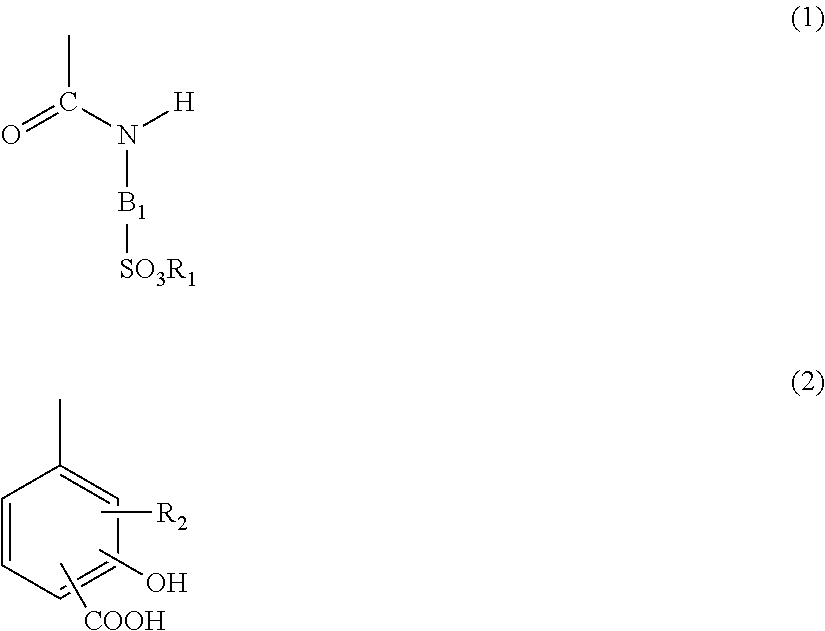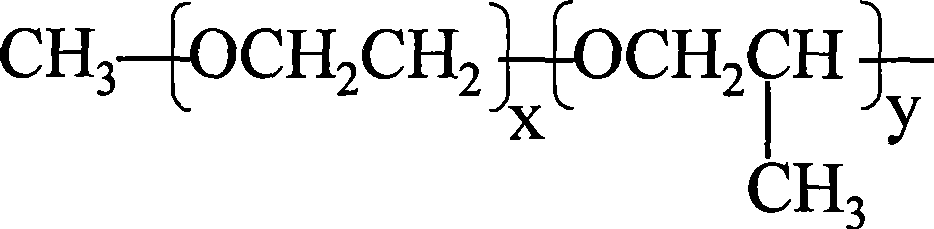Patents
Literature
151 results about "Salicylic acid derivatives" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
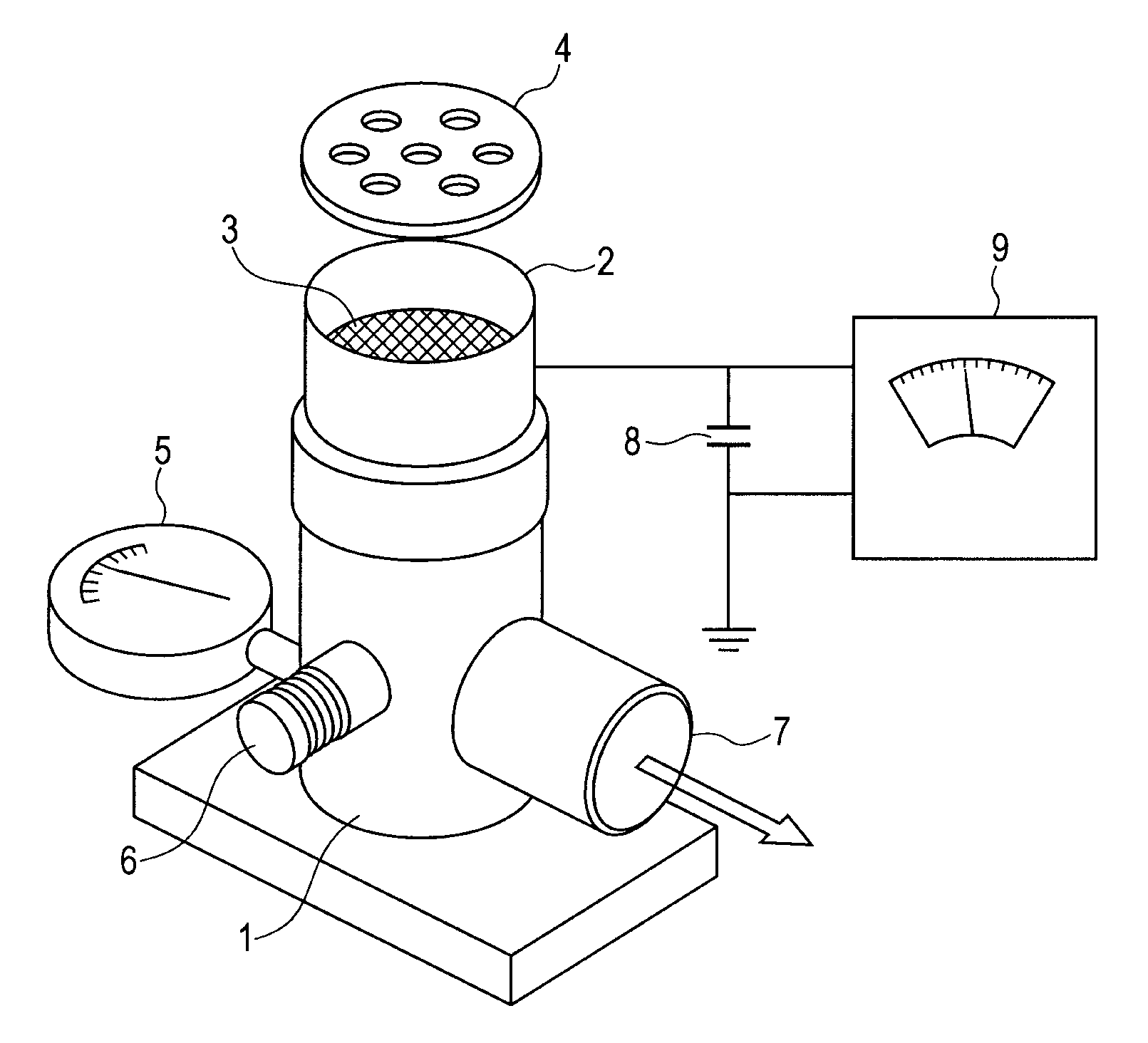
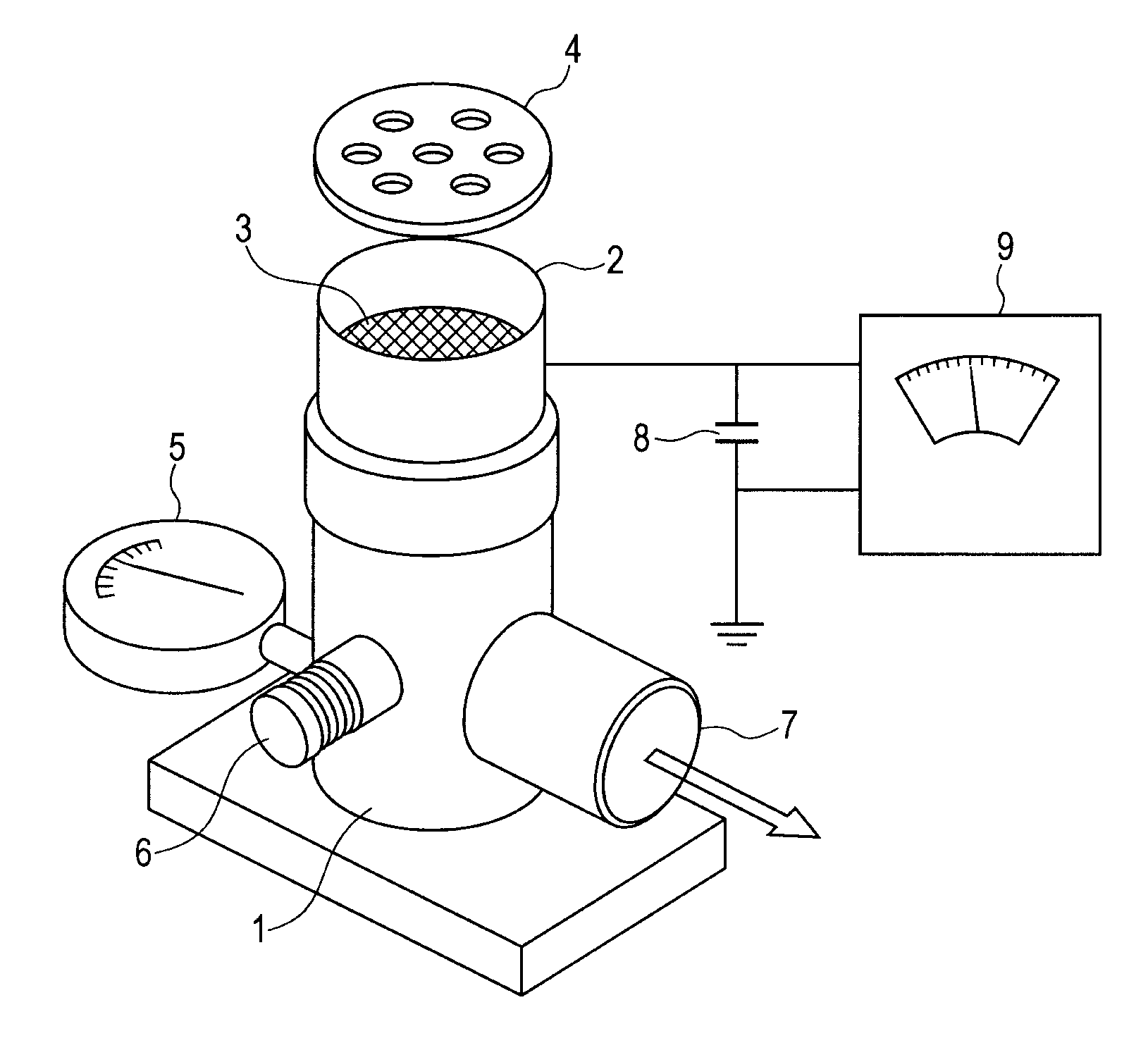
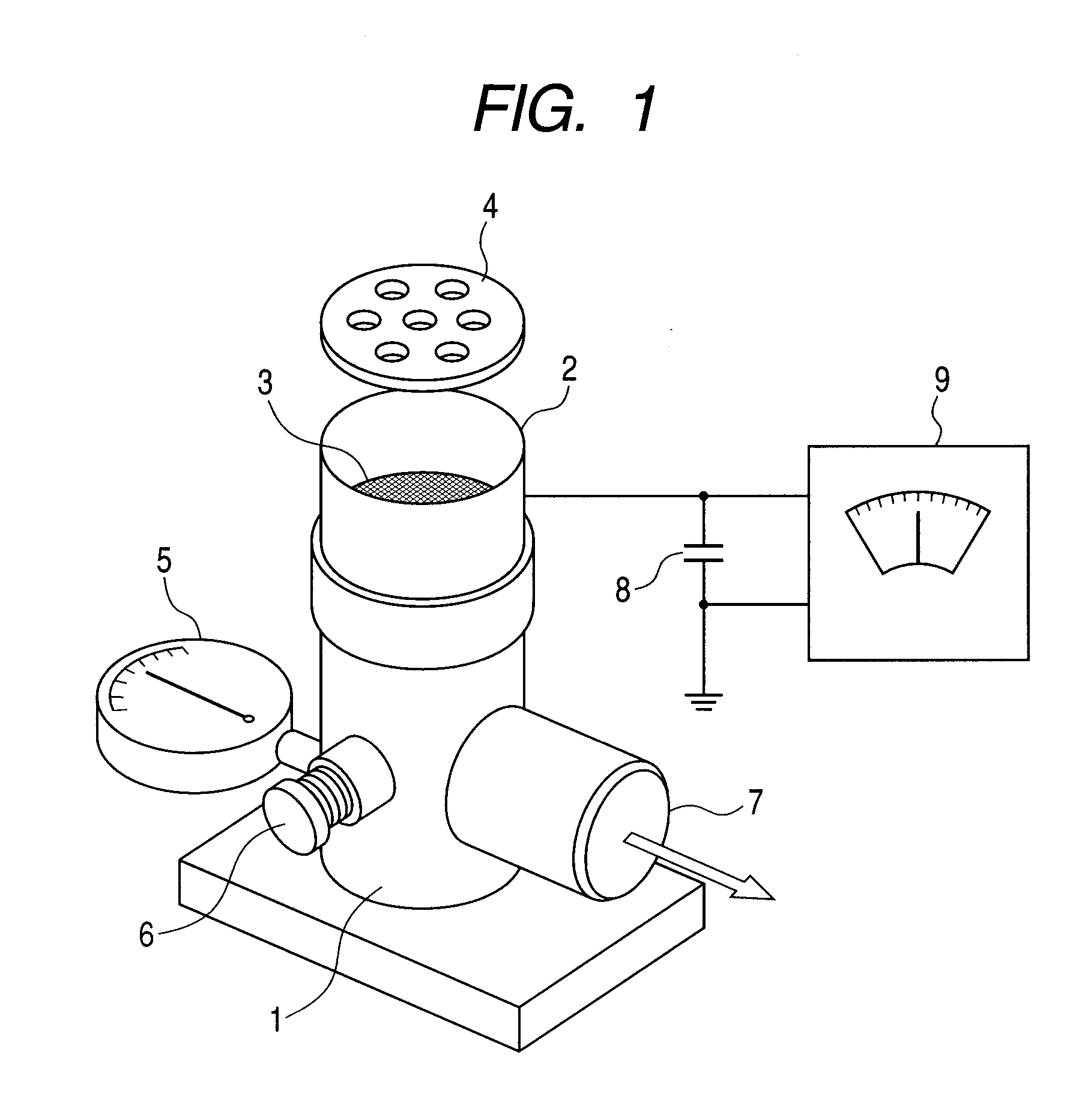
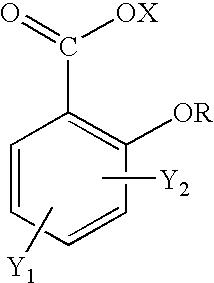
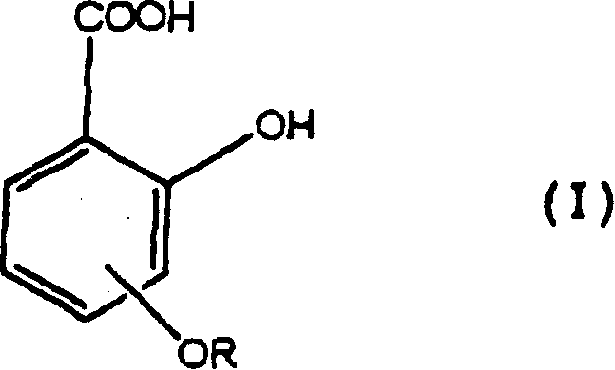
Salicylic acid is the 2-hydroxy derivative of benzoic acid having the following structure: This formulation has been shown to provide gradual and prolonged release of the active ingredient into the skin.
Therapy for constipation
Compositions comprising colchicine and at least one amino-salicylic acid derivative, preferably olsalazine is used for treatment of prophylaxis of constipation.
Owner:BORODY THOMAS JULIUS
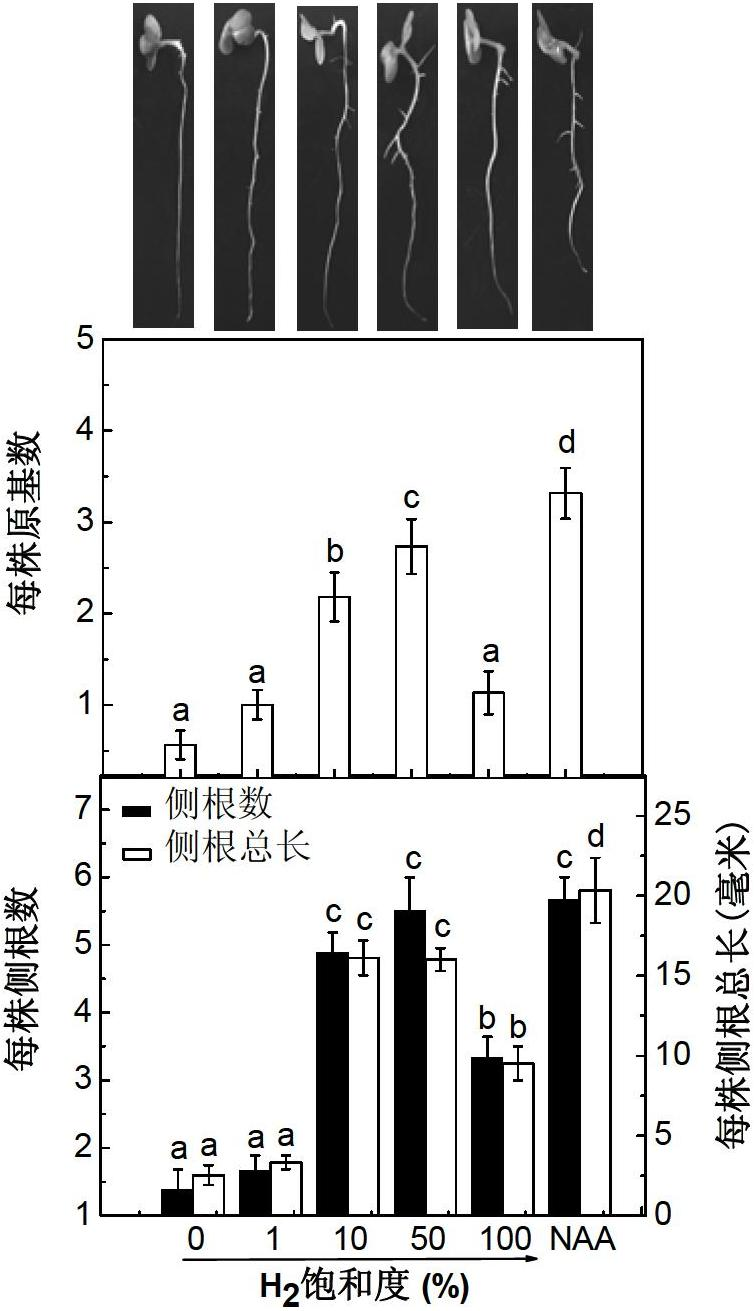
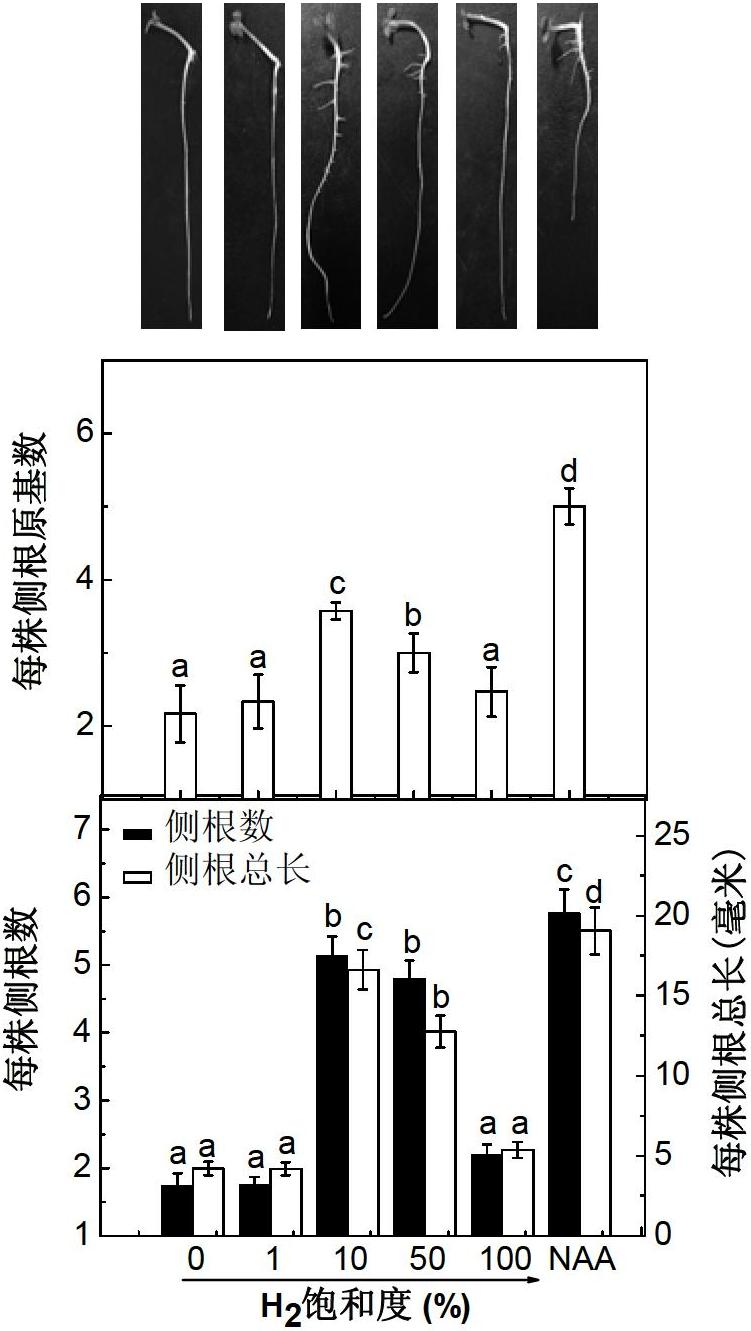
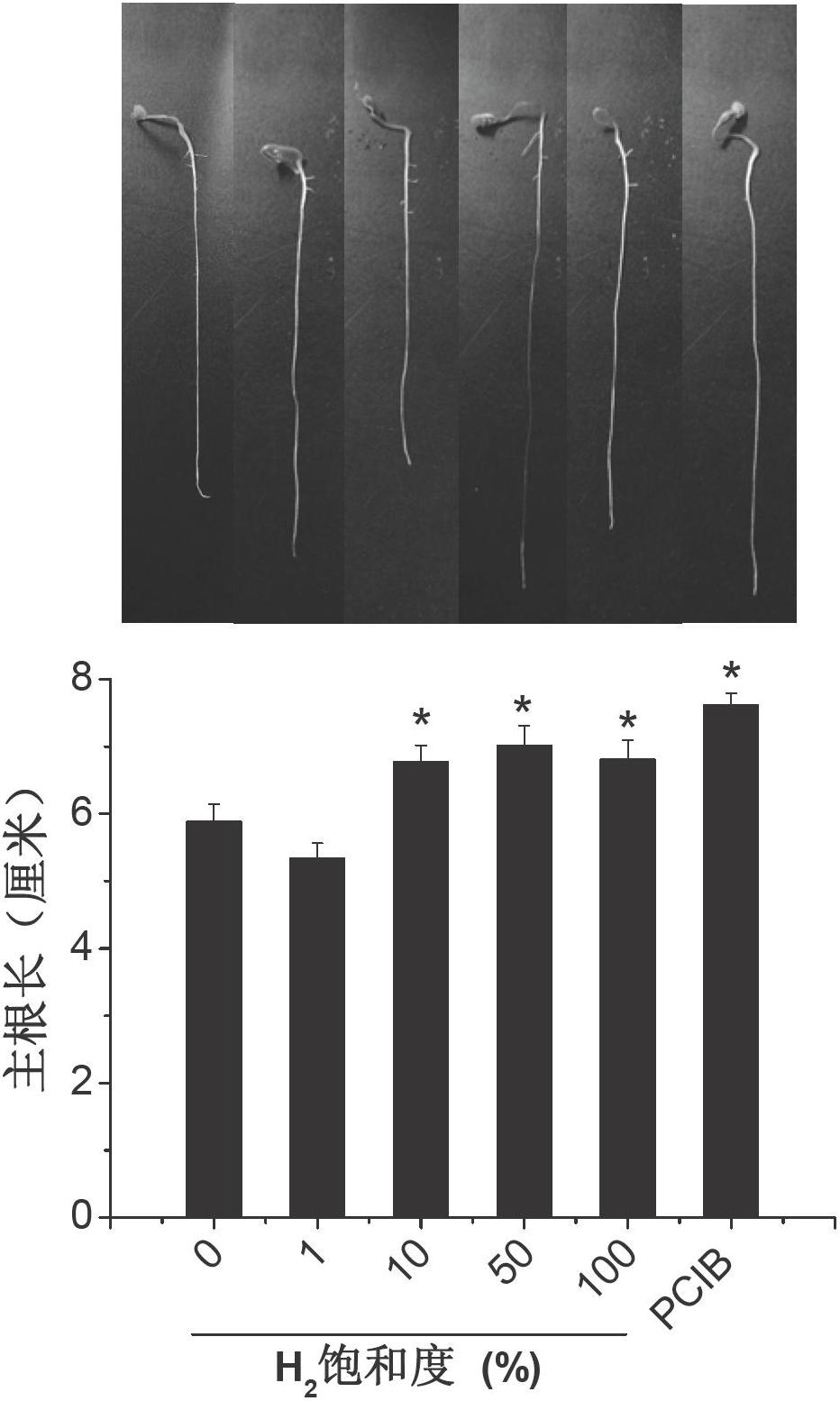
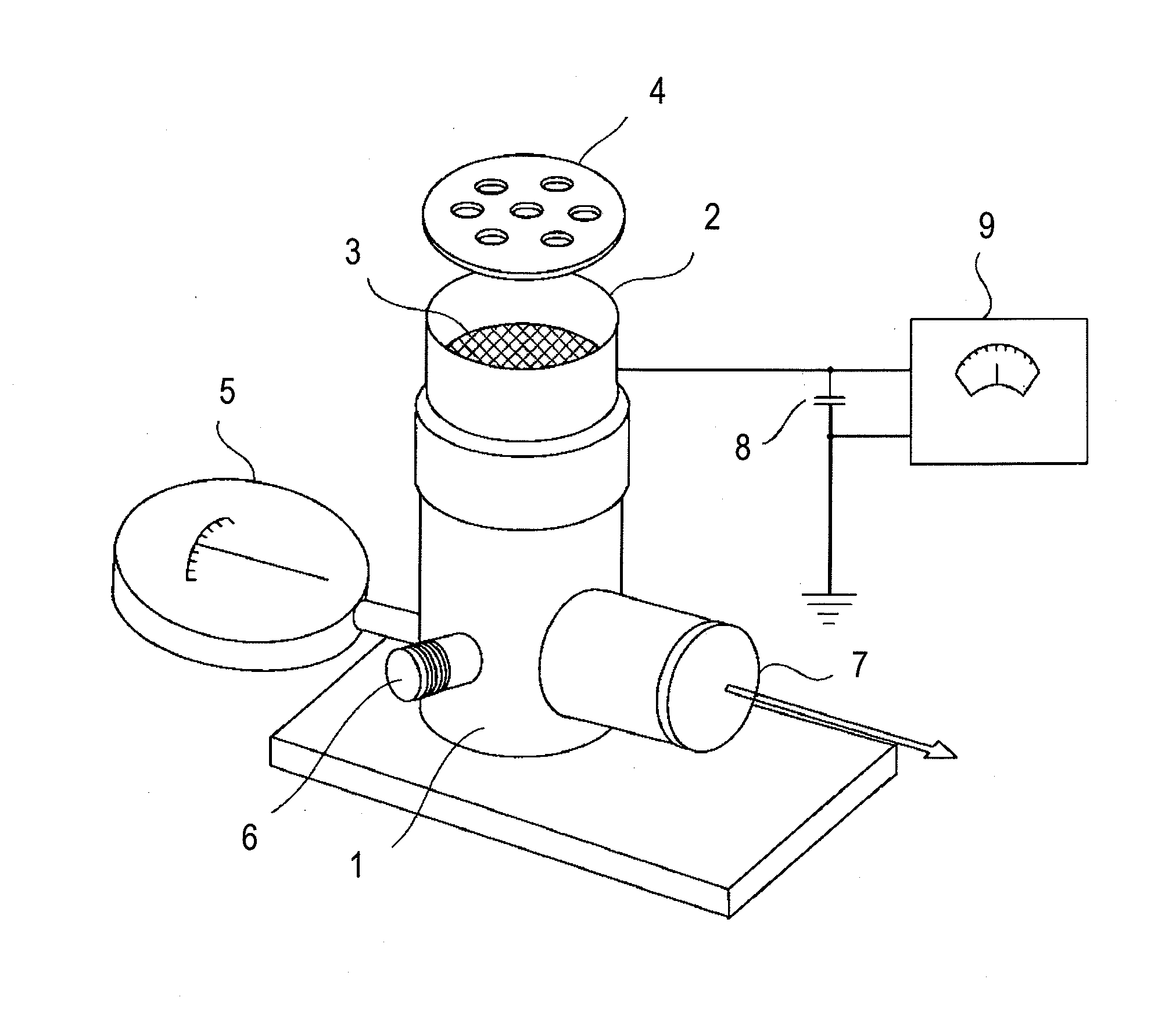
Hydrogen-rich liquid plant growth regulator, and preparation method and application thereof
ActiveCN102657221AWide variety of sourcesLow costBiocidePlant growth regulatorsCut flowersNutrient solution
The invention belongs to the fields of development and utilization of plant growth regulating substances, and discloses a hydrogen-rich liquid plant growth regulator. The saturation of hydrogen in a solvent is 0.1 to 100 percent, and the solvent is water, a 25 to 100 percent Hoagland nutrient solution, a Kimura B nutrient solution, a TAP nutrient solution or an MS culture solution. Calcium salt, salicylic acid, salicylate, salicylic acid derivative, humid acid or humic acid salt at the final concentration of 0-1,000mu mol / L is added into the solvent. Plants, plant tissues and seeds are irrigated, sprayed or soaked, the plant growth and morphogenesis can be effectively promoted, seed germination is quickened, heavy metal accumulation is reduced, and the oxidation resistance and stress resistance / tolerance are improved. The method has the characteristics of no pollution, environmental protection, low cost, and wide application range, and is applied to the fields such as farmland chemical regulation, provenance agriculture, environment and plant nutrition, plant tissue culture and cut flower preservation time prolonging.
Owner:NANJING AGRICULTURAL UNIVERSITY
Toner
InactiveUS20130065174A1Not easy to influenceGood dispersibilityDevelopersEngineeringSalicylic acid derivatives
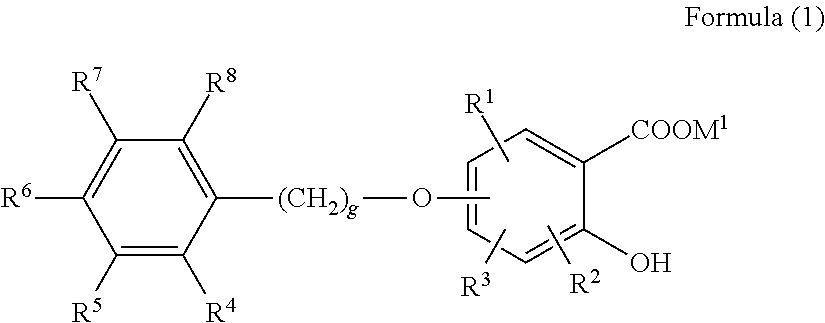
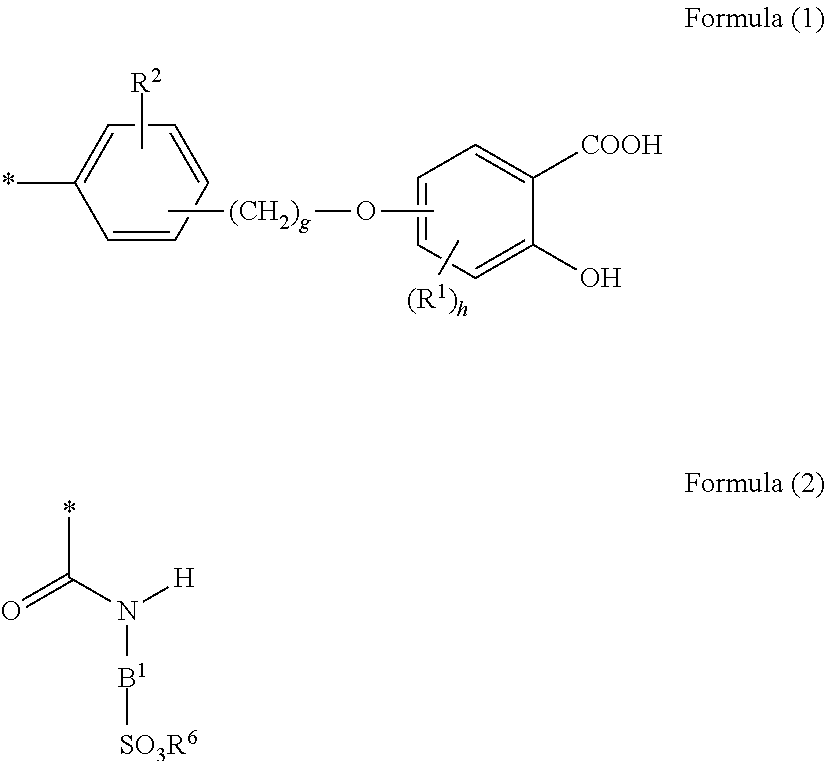
An object of the present invention is to provide a toner in which a charging amount and rise of the charging amount are hardly influenced by change in a temperature and humidity environment, and pigment dispersibility is high in order to provide an image output having high transparency, color mixing properties, and color nuance stability. The present invention is a toner comprising toner particles each comprising a metallic compound and a colorant, wherein the metallic compound is a compound having a structure bonded to a metal in a site derived from —COOM1 and / or —OH of a salicylic acid structural moiety or a salicylic acid derivative structural moiety of an aromatic compound represented by formula (1) below:
Owner:CANON KK
Toner, and developer, image forming method, image forming apparatus and process cartridge using the toner
ActiveUS20050003289A1Stable controlKeeping charge quantityDevelopersElectrographic processes using charge patternParticulatesSilicon dioxide
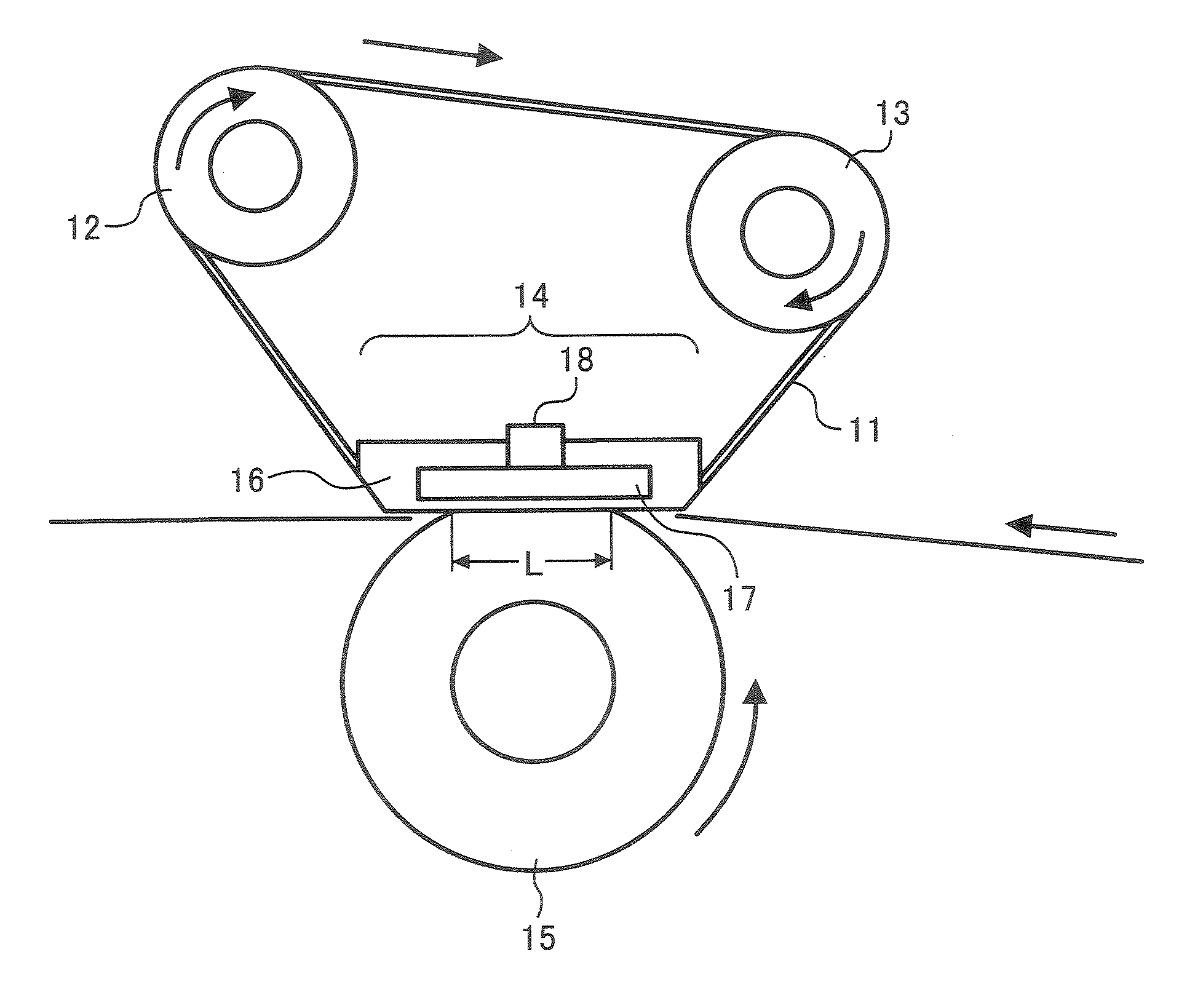
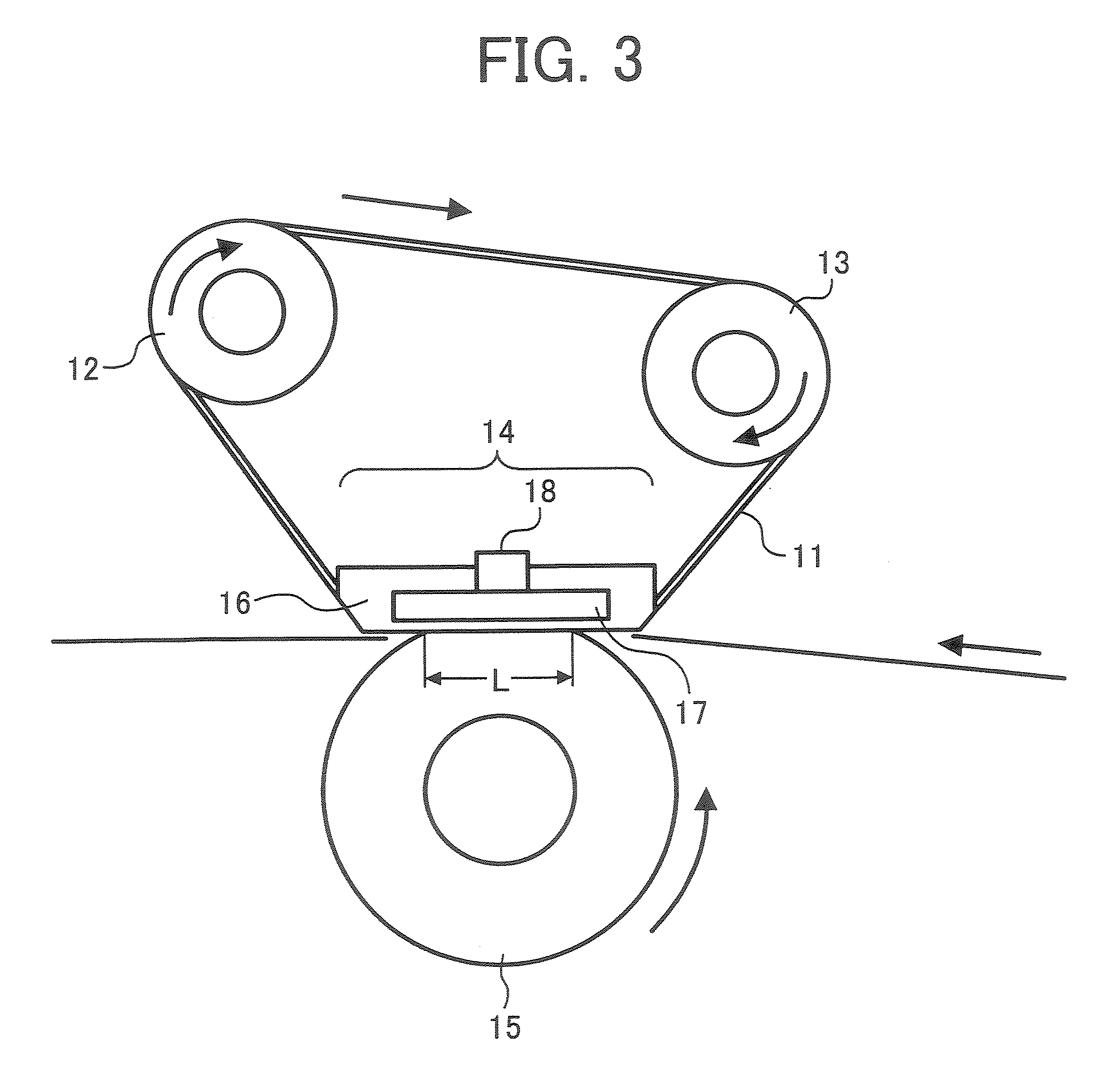
A toner is provided that contains: (A) a binder resin; (B) a colorant; (C) a charge controlling agent; and (D) an additive; wherein the binder resin (A) contains: a polyester resin containing no tetrahydrofuran-insoluble component; and having a molecular weight distribution wherein components having a molecular weight not greater than 5×102 are present in an amount of 4% or less by weight, and wherein a main peak is present in a molecular weight range of from 3×103 to 9×103 when the molecular weight distribution is determined by gel permeation chromatography; wherein the charge controlling agent (C) contains: a metal salt of salicylic acid or salicylic acid derivative; and wherein the additive (D) contains: a hydrophobized silica having a primary particle diameter of from 0.01 to 0.03 μm; and a hydrophobized titanium oxide having a primary particle diameter of from 0.01 to 0.03 μm and a specific surface area of from 60 to 140 m2 / g, wherein the hydrophobized titanium oxide is prepared by surface-treating a particulate titanium oxide prepared by a wet process, wherein the hydrophobized titanium dioxide has one or more water-soluble components in an amount of 0.2% or more by weight, and has a transmittance not less than 35% for light having a wavelength of 300 nm and a transmittance not less than 80% for light having a wavelength of 600 nm; and its use in a developer, an image forming method, image forming apparatus and a process cartridge containing the toner.
Owner:RICOH KK
Pharmaceutically acceptable salts containing local anesthetic and anti-inflammatory activities and methods for preparing the same
The present invention provides pharmaceutically acceptable salts having local anesthetic and anti-inflammatory activities. The preferred pharmaceutically acceptable salt is a diclofenac salt of lidocaine. Diclofenac is a non-steroidal anti-inflammatory drug (“NSAID”). Lidocaine is a local anesthetic. Other NSAID (except the salicylic acid derivatives of NSAID) can be used to replace diclofenac and / or other local anesthetics can be used to replace lidocaine. The pharmaceutically acceptable salts are crystalline compounds, which are distinctively different from either the NSAID alone or the local anesthetic alone, as indicated by differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), High Performance Liquid Chromatography (HPLC) and Fourier-Transformed Infrared Spectroscopy (FTIR) analyses. These pharmaceutically acceptable salts are suitable for use in topical treatment or parenteral injection to treat patients with localized pain, including muscle pain, joint pain, pain associated with herpes infection, and wound pain (such as surgical wound, burn wound etc.).
Owner:YUNG SHIN PHARMACEUTICALS INDUSTRIAL CO LTD
Toner
Provided is a toner having high charging rapidity to reach a sufficient charging amount in a short time, high stability of charging from the initial stage to a time when a large amount of sheets is printed out, and high stability of charging under a high temperature and high humidity. In a toner including toner particles, each of which contains at least a binder resin, a colorant, and a charge controlling resin, the charge controlling resin is a copolymer of a structure A having at least a specific salicylic acid derivative structure and a structure B having sulfonic acid or sulfonic acid ester as a substituent.
Owner:CANON KK
Resin for toners, and toner
To obtain a resin for toners, or a toner, which is superior in the quickness in its rise of charging to a sufficient charge quantity in a short time, in the stability of charging that extends from the initial stage up to the printing on a large number of sheets, and in the stability of charging that comes in a high-temperature and high-humidity environment. As a charge control resin, it is characterized by containing a specific unit having sulfonic acid or a sulfonate as a substituent and a specific unit having a salicylic acid derivative.
Owner:CANON KK
Cosmetic composition with a heating effect
Cosmetic composition containing, in an anhydrous medium, at least one exothermic metal salt, at least one carboxylic acid and at least one salicylic acid compound, chosen from salicylic acid and salicylic acid derivatives. The composition may be used in particular for cleansing and / or removing makeup from and / or scrubbing the skin.
Owner:LOREAL SA
Topical use of halosalicylic acid derivatives
InactiveUS20050136015A1Accelerated sebumAccelerated acne controlAntibacterial agentsBiocideDiseaseSkin callus
Halosalicylic acid compounds of the present invention, having at least one halogen substituent on the aromatic ring, are employed to produce desquamation of skin, treat nail disorders or dandruff, remove calluses, control acne and excess sebum production, reduce skin pore size or control blackheads. Topical compositions containing the halosalicylic acid compound and a cosmetically acceptable vehicle are disclosed.
Owner:AVON PROD INC
Overall-efficiency oil-controlling acne-removing pockmark-eliminating skin-beautifying essence
ActiveCN105395441AReduced activitySuppress generationCosmetic preparationsToilet preparationsBetaineMedicine
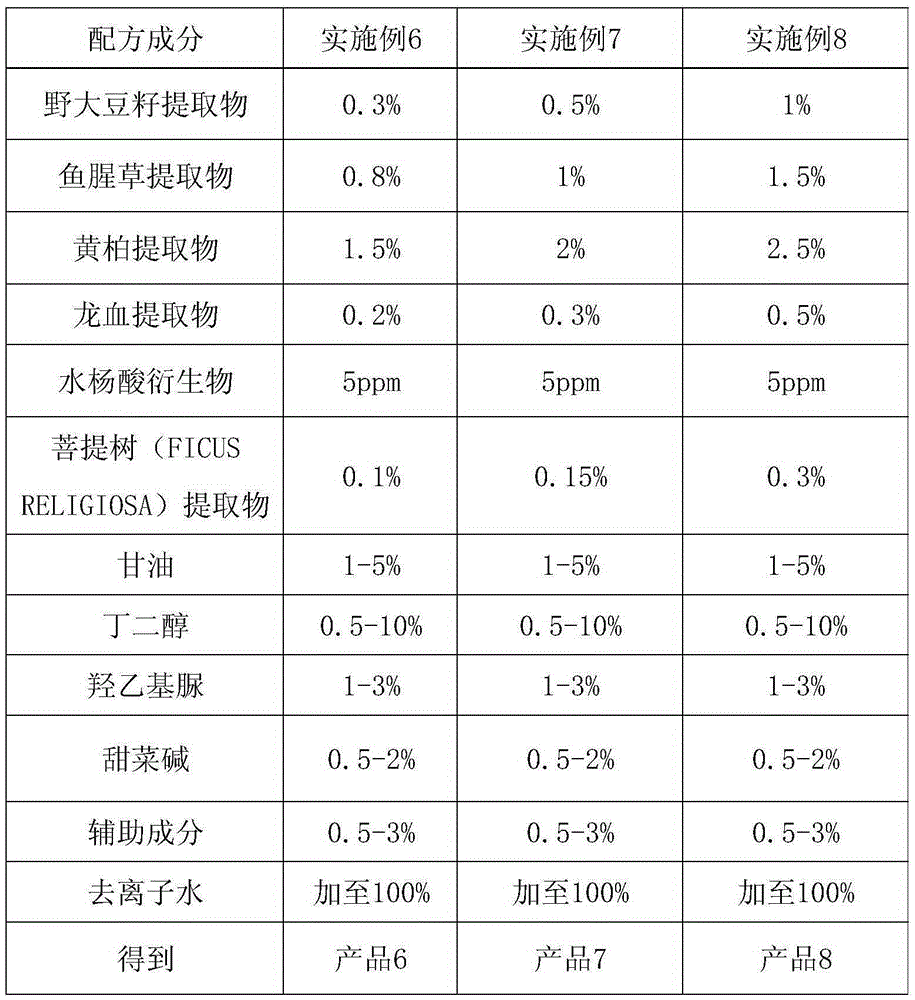
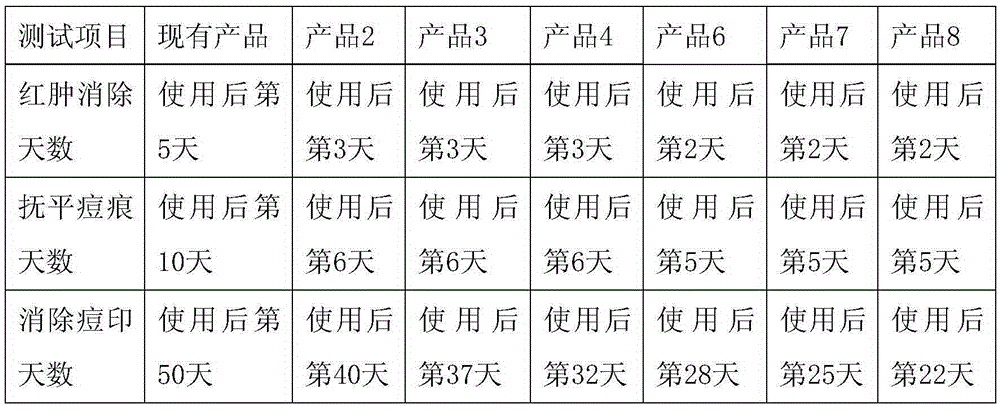
The invention discloses a overall-efficiency oil-controlling acne-removing pockmark-eliminating skin-beautifying essence. The essence comprises, by weight, 0.1 to 2% of extract of wild soybean seeds, 0.5 to 3% of extract of cordate houttuynia, 1 to 5% of extract of cortex phellodendron, 0.1 to 1% of extract of dragon's blood, 5 to 20 ppm of a salicylic acid derivative, 1 to 5% of glycerin, 0.5 to 10% of butylene glycol, 1 to 3% of hydroxyethylcarbamide, 0.5 to 2% of betaine and 0.5 to 3% of auxiliary components, with the balance being deionized water. According to the invention, through perfect combination of the extract of wild soybean seeds, the extract of cordate houttuynia, the extract of cortex phellodendron, the extract of dragon's blood and the salicylic acid derivative, the overall-efficiency oil-controlling acne-removing pockmark-eliminating skin-beautifying essence has overall effects of aiding the skin in continuous oil control, unblocking and astringing pores, removing acne, eliminating pockmarks, smoothing the skin, etc.
Owner:广州蜜妆生物科技有限公司
Carbonic diester, aromatic polycarbonate, production apparatus, and process for production
A process for producing through melt polymerization an aromatic polycarbonate reduced in coloring and improved in thermal stability, hue, etc. by keeping the pressure inside a starting-material dissolution vessel and the linear velocity of a gas in a transport piping in respective given ranges, keeping the concentration of a basic nitrogen compound in a specific range, recycling a by-product containing impurities in a specific amount, reducing the contents of nitrogen compounds, metallic elements, and salicylic acid derivatives in a carbonic diester to respective given values, using a reactor made of a specific material, and / or using a reactor having an oxide layer formed on the inner wall surface thereof.
Owner:TEIJIN LTD
Method for the preparation of a polyester block copolymer, a polyester block copolymer composition and method for the preparation thereof
The present invention I relates to a method for the preparation of a polyester block copolymer (P1) characterized in that in the method for the preparation of 100% by weight of the polyester block copolymer (P1) by allowing to react A% by weight of a crystalline aromatic polyester with B% by weight of lactones (A+B=100), (B+0.5)% by weight of lactones are added into A% by weight of a crystalline aromatic polyester, and not less than 0.5% by weight of unreacted lactones are remained with respect to 100% by weight of the polyester block copolymer (P1) after reaction. The present invention II relates to a method for the preparation of a polyester block copolymer (P'1) having a high molecular weight characterized in that the polyester block copolymer (P1) is allowed to further react in a solid phase. The present invention III relates to a polyester block copolymer composition (R) obtained by thermally-processing a polyester block copolymer composition (Q) obtained by melt-mixing 100 parts by weight of a polyester block copolymer (P) with 0.1-5 parts by weight of an epoxy compound (C) having one or more epoxy groups under an inert gas atmosphere and not less than 120° C. in a solid phase, and further, at a temperature lower than a melting point of the polyester block copolymer composition (R). The present invention IV relates to a polyester block copolymer composition which comprises thermally-kneading 0.5-5.0 parts by weight of a mono or more functional epoxy compound (C) and 0.01-3.0 parts by weight of a complex-formable agent for a metal (G) such as an oxalic acid derivative and a salicylic acid derivative or a hydrazide derivative with 100 parts by weight of a polyester block copolymer (P1) obtained by a reaction of a crystalline aromatic polyester (A1) with lactones (B). The present invention V relates to a polyester block copolymer composition which comprises, in obtaining the polyester block copolymer composition by allowing to react the crystalline aromatic polyester (A1) with lactones (B), adding and thermally-kneading 0.5-5.0 parts by weight of an epoxy compound (C) having one or more pieces of epoxy groups (including at least 0.2 part by weight of two or more functional epoxy compound) and, optionally, 0-2.0 parts by weight of a carbodiimide compound (E) to 100 parts by weight of a polyester block copolymer (P3) obtained by allowing to react 0.1-100% by mol of at least one of a multifunctional compound (D) having at least three pieces of at least one kind of carboxylic group (i), hydroxyl group (ii), and / or an ester-formable group therefrom (iii) with 100% by mol of a crystalline aromatic polyester (A1). The present invention VI relates to a polyester block copolymer composition which comprises, in obtaining said polyester block copolymer composition by allowing to react the crystalline aromatic polyester (A1) with lactones (B), adding and thermally-kneading 0.1-5.0 parts by weight of an epoxy compound (C) having one or more pieces of epoxy groups and 0-2.0 parts by weight of a carbodiimide compound (E) to 100 parts by weight of a polyester block copolymer (P3) obtained by allowing to react 0.1-200% by mol of at least one of an aliphatic or aromatic multifunctional compound (D) having at least three pieces of carboxylic group hydroxyl group (ii), and / or an ester-formable group therefrom (iii) with 100% by mol of a crystalline aromatic polyester (A1). The present invention VII relates to a polyester block copolymer composition (R) which comprises, in obtaining the polyester block copolymer composition by allowing to react the crystalline aromatic polyester (A1) with lactones (B), heating a polyester block copolymer composition (Q) at a solid phase, and the composition (Q) is obtained by adding and thermally-kneading 0.1-5.0 parts by weight of at least one kind of an epoxy compound (C) having one or more pieces of epoxy groups to 100 parts by weight of a polyester block copolymer (P) obtained by allowing to react 0.1-200% by mol of at least one of a multifunctional compound (D)
Owner:WATANABE JUN
Method for preparing beta-amino-carbonyl compound
InactiveCN103467321AGreat application potentialAtom economy is highOrganic chemistryOrganic compound preparationRoom temperatureKetone
The invention provides a method for preparing a beta-amino-carbonyl compound. Aldehydes, ketones and amines are directly mixed under the condition of synergetic catalysis of titanocene dichloride and a ligand, and react at room temperature to obtain the beta-amino-carbonyl compound, wherein the ligand is salicylic acid or salicylic acid derivatives or structural analogues thereof. According to the method, the catalyst used in the method is low in cost, nontoxic and stable and effective to air and water, the reaction condition is mild, a solvent is not needed, the operation is simple, and the atom economy is high.
Owner:SHAANXI NORMAL UNIV +1
Toner, and developer, image forming method, image forming apparatus and process cartridge using the toner
InactiveUS20070264035A1Stable controlKeep it steadyDevelopersElectrographic process apparatusSilicon oxideSilicon dioxide
A toner is provided that contains: (A) a binder resin; (B) a colorant; (C) a charge controlling agent; and (D) an additive; wherein the binder resin (A) contains: a polyester resin containing no tetrahydrofuran-insoluble component; and having a molecular weight distribution wherein components having a molecular weight not greater than 5×102 are present in an amount of 4% or less by weight, and wherein a main peak is present in a molecular weight range of from 3×103 to 9×103 when the molecular weight distribution is determined by gel permeation chromatography; wherein the charge controlling agent (C) contains: a metal salt of salicylic acid or salicylic acid derivative; and wherein the additive (D) contains: a hydrophobized silica having a primary particle diameter of from 0.01 to 0.03 μm; and a hydrophobized titanium oxide having a primary particle diameter of from 0.01 to 0.03 μm and a specific surface area of from 60 to 140 m2 / g, wherein the hydrophobized titanium oxide is prepared by surface-treating a particulate titanium oxide prepared by a wet process, wherein the hydrophobized titanium dioxide has one or more water-soluble components in an amount of 0.2% or more by weight, and has a transmittance not less than 35% for light having a wavelength of 300 nm and a transmittance not less than 80% for light having a wavelength of 600 nm; and its use in a developer, an image forming method, image forming apparatus and a process cartridge containing the toner.
Owner:FUSHIMI HIROYUKI +1
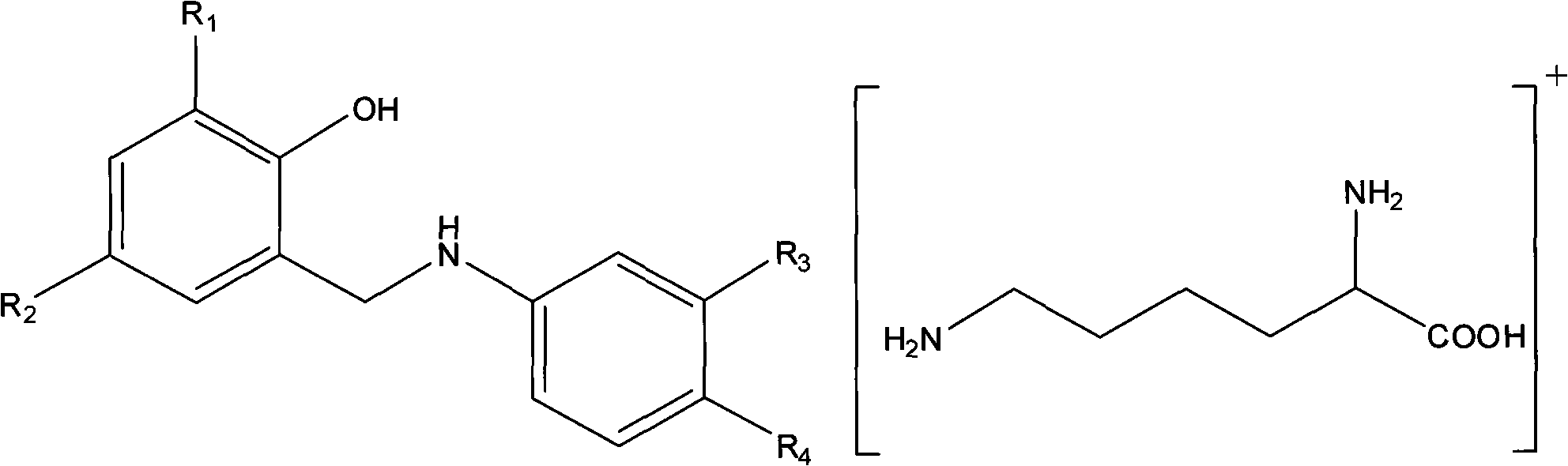
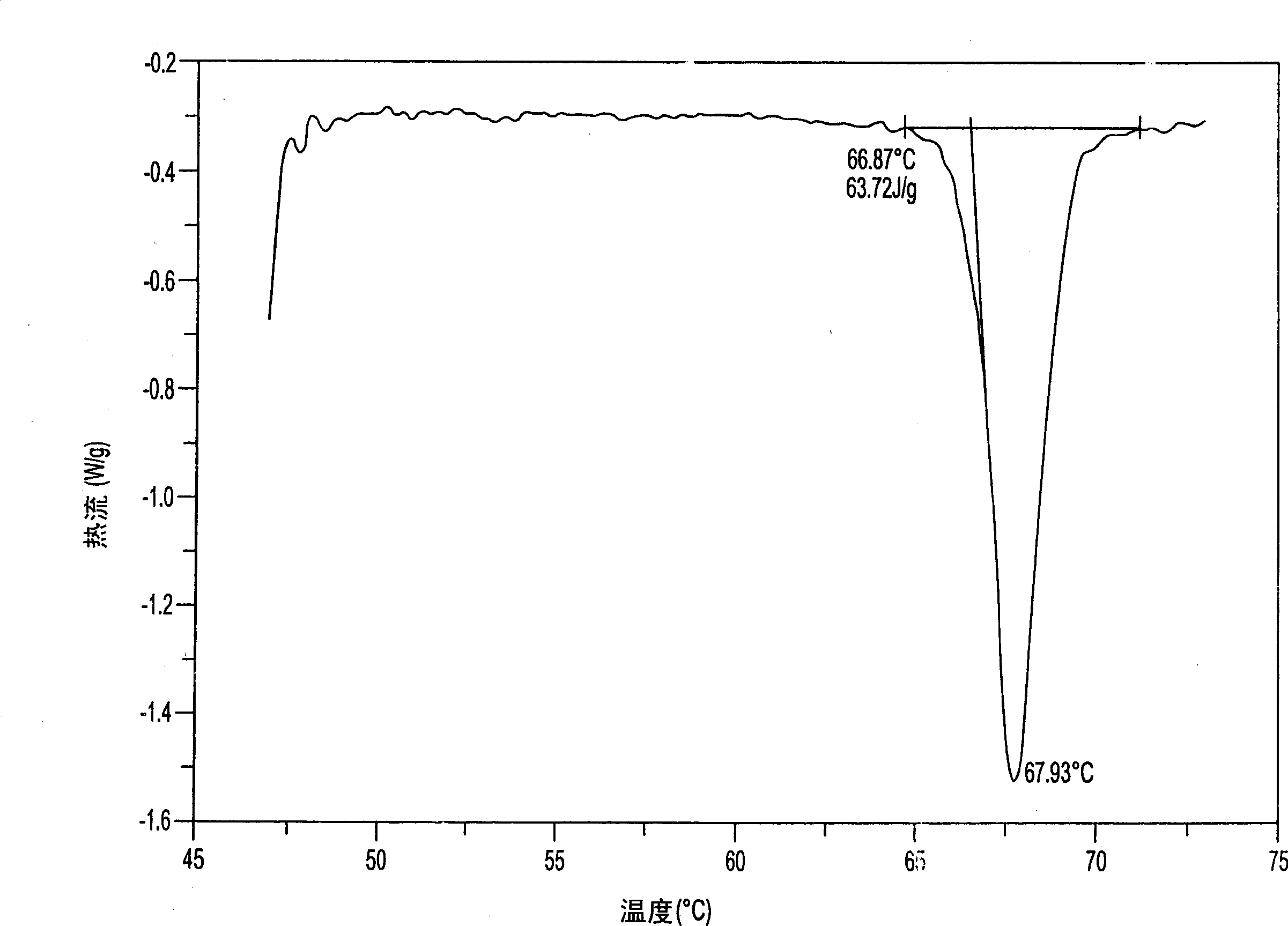
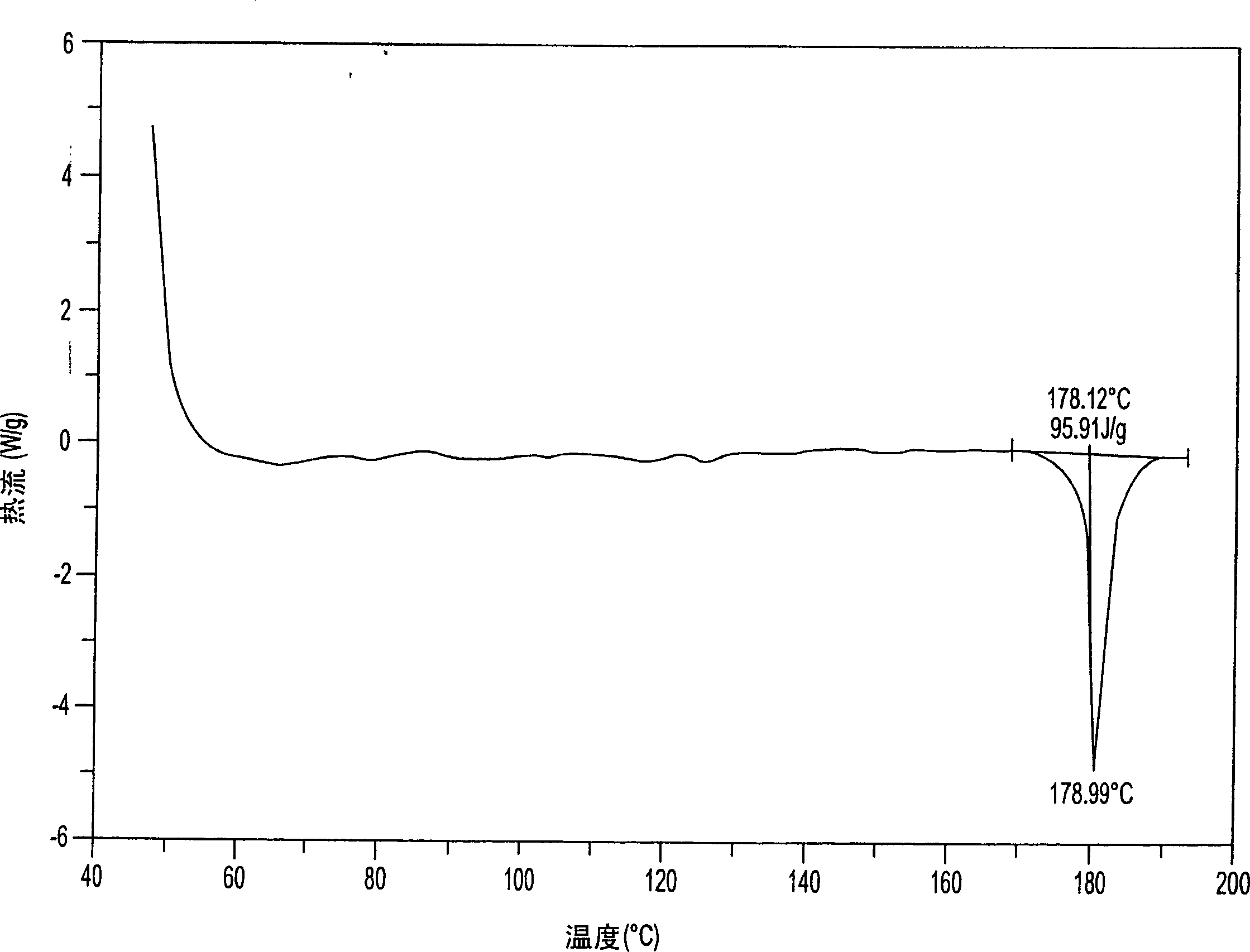
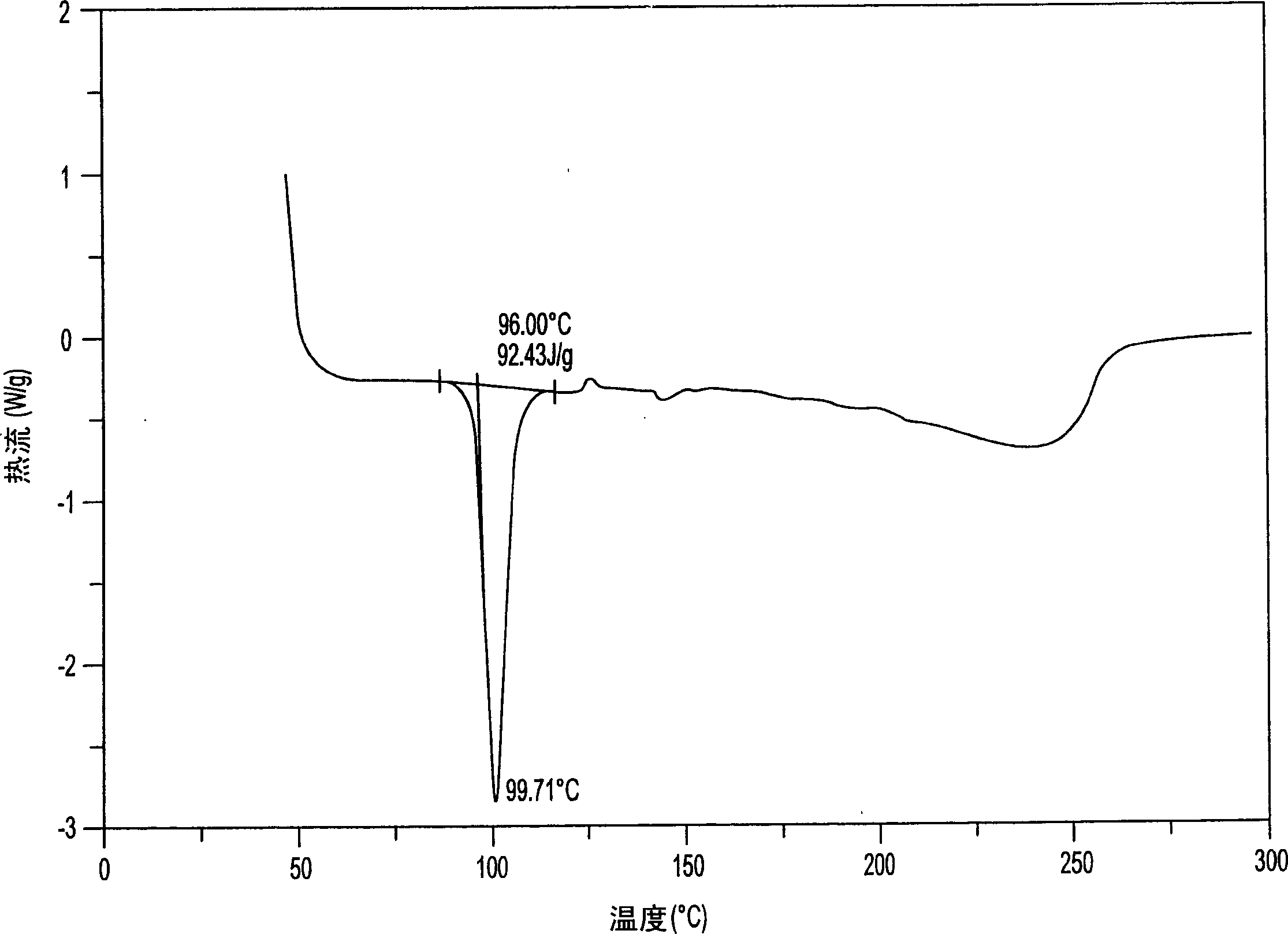
Aminosalicylic acid derivative lysine complex salt, preparation method and application thereof
InactiveCN101792399AGood water solubilityOrganic active ingredientsNervous disorderNeuropathic painSalicylic acid derivatives
The invention relates to aminosalicylic acid derivative lysine complex salt, a preparation method and application thereof. The structure of the aminosalicylic acid derivative lysine complex salt conforms to the following general formula (I), R1 stands for -H, -Cl or -Br; R2 stands for -F, -Cl or -Br; one of R3 and R4 stands for COO-, and the other one stands for -OH or -OCOCH3. A target compound of the aminosalicylic acid derivative lysine complex salt has the pharmacological action of treating neuropathic pain.
Owner:NANJING MEDICAL UNIV
Medicinal salt with local narcosis and antiphlogosis activity and its prepn process
InactiveCN1486690AOrganic active ingredientsOrganic chemistryNon steroid anti inflammatory drugTopical treatment
The present invention provides with local narcosis and antiphlogosis activity, and the optimal salt is diclofenac salt of lidocaine. Diclofenac acid is one kind of non-steroid anti-inflammatory drug (NSAID), and lidocaine is one kind of local anaesthetic, with diclofenac acid and / or lidocaine being replaceable with other NSAID and / or other local anaesthetic separately. The medicinal salt is crysalline compound dissimilar to NSAID and lidocaine obviously, as DSC, TGA, HPLC and FTIR show. The medicinal salt is especially suitable for local treatment and injection outside gastrointestinal tract to treat local pain including muscle pain, joint pain, pain related to herpes, wound pain, etc.
Owner:YUNG SHIN PHARMACEUTICALS INDUSTRIAL CO LTD
Hydroxyl-containing benzimidazole diamines and preparation method thereof
ActiveCN105348201AHigh strengthHigh modulusOrganic chemistryMonocomponent synthetic polymer artificial filamentSalicylic acid derivativesDiamine
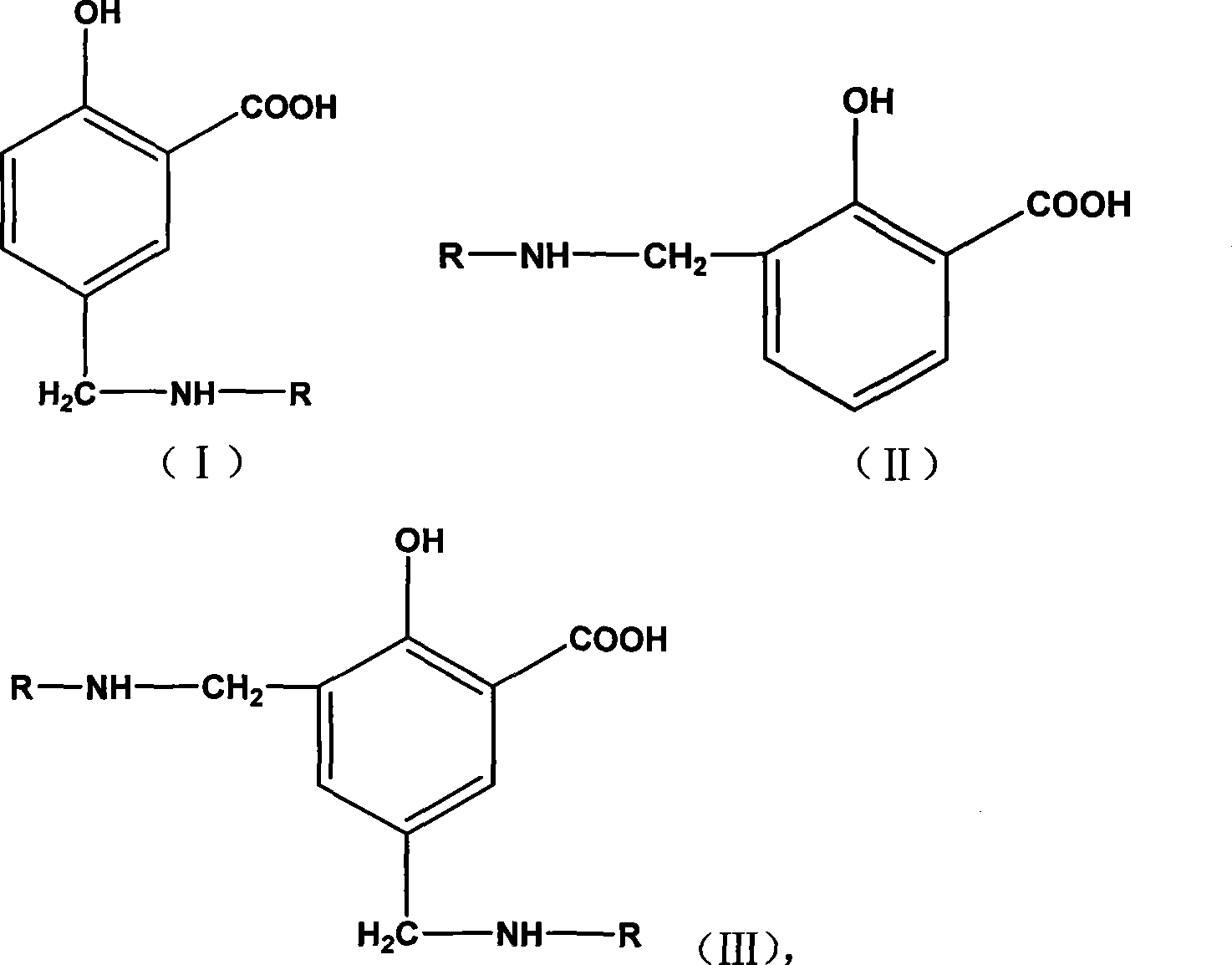
The invention provides hydroxyl-containing benzimidazole diamines and a preparation method thereof. The hydroxyl-containing benzimidazole diamines possess the structures shown as a formula (I), a formula (II) or a formula (III). The preparation method comprises the following steps: A1) reacting 1,2,4-benzenetriamine dihydrochloride with a salicylic acid derivative with the structure shown as a formula (IV), so as to obtain a hydroxyl-containing benzimidazole diamine with the structure shown as a formula (I); or A2) reacting 1,2,4-benzenetriamine dihydrochloride with a salicylic acid derivative with the structure shown as a formula (V), so as to obtain a hydroxyl-containing benzimidazole diamine with the structure shown as a formula (II); or A3) reacting 3,3'-diaminobenzidine with a salicylic acid derivative with the structure shown as a formula (IV), so as to obtain a hydroxyl-containing benzimidazole diamine with the structure shown as a formula (III). Compared with the prior art, polyimides prepared from the provided hydroxyl-containing benzimidazole diamines are high in strength and large in modulus.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
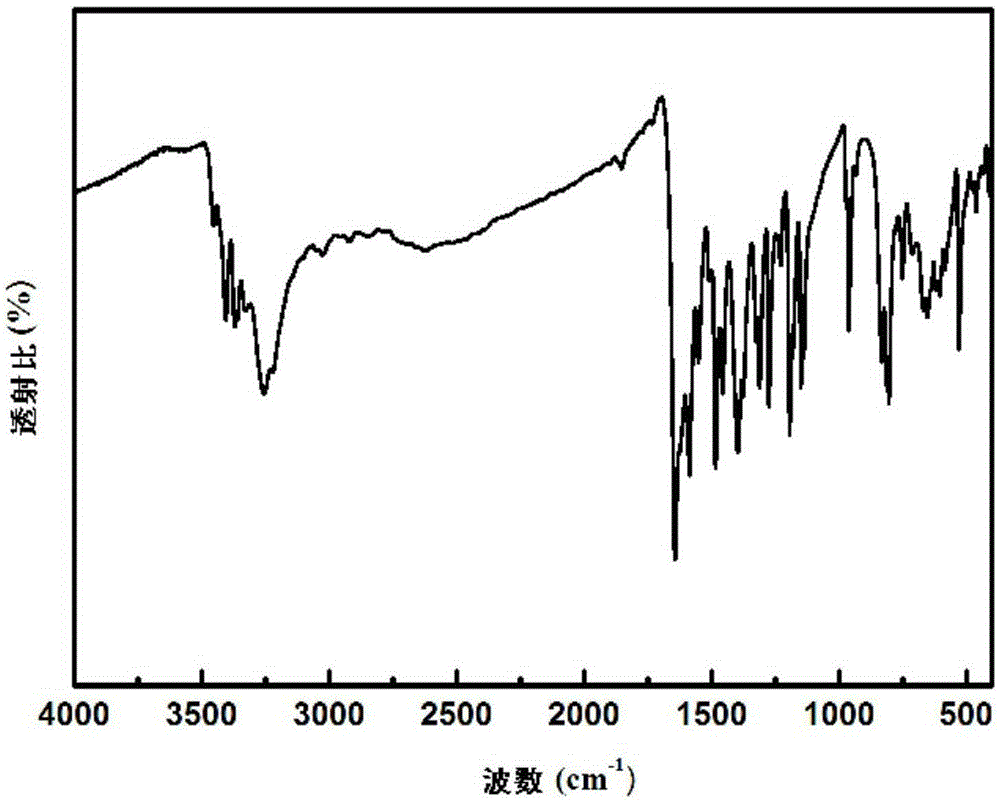
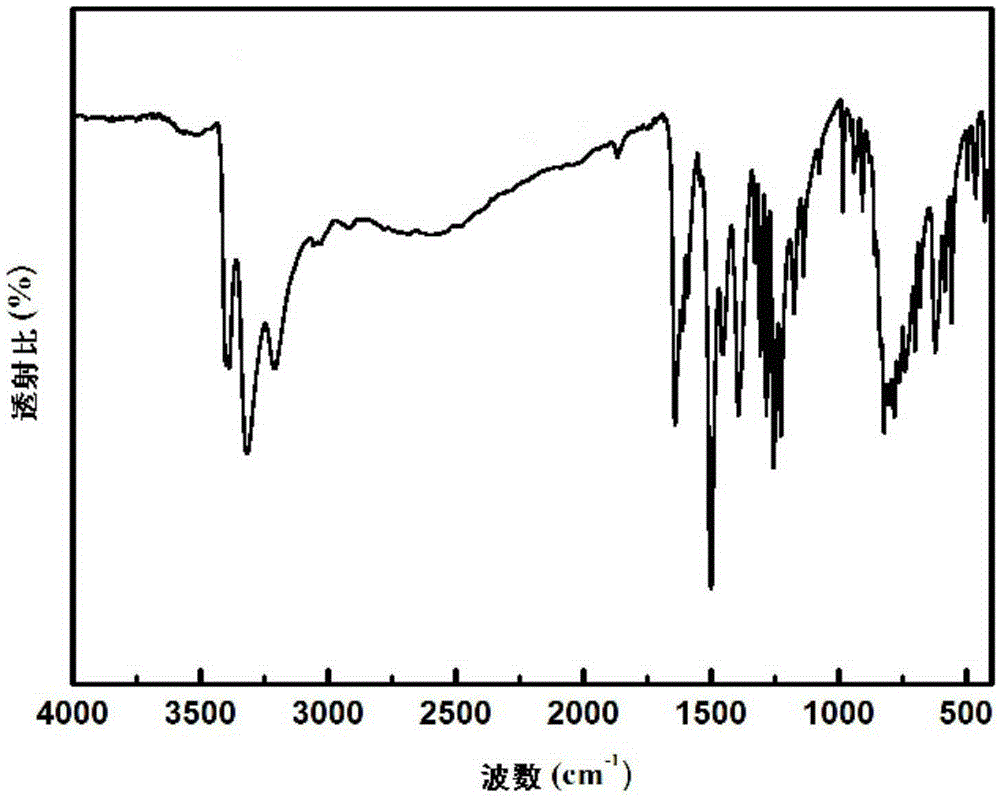
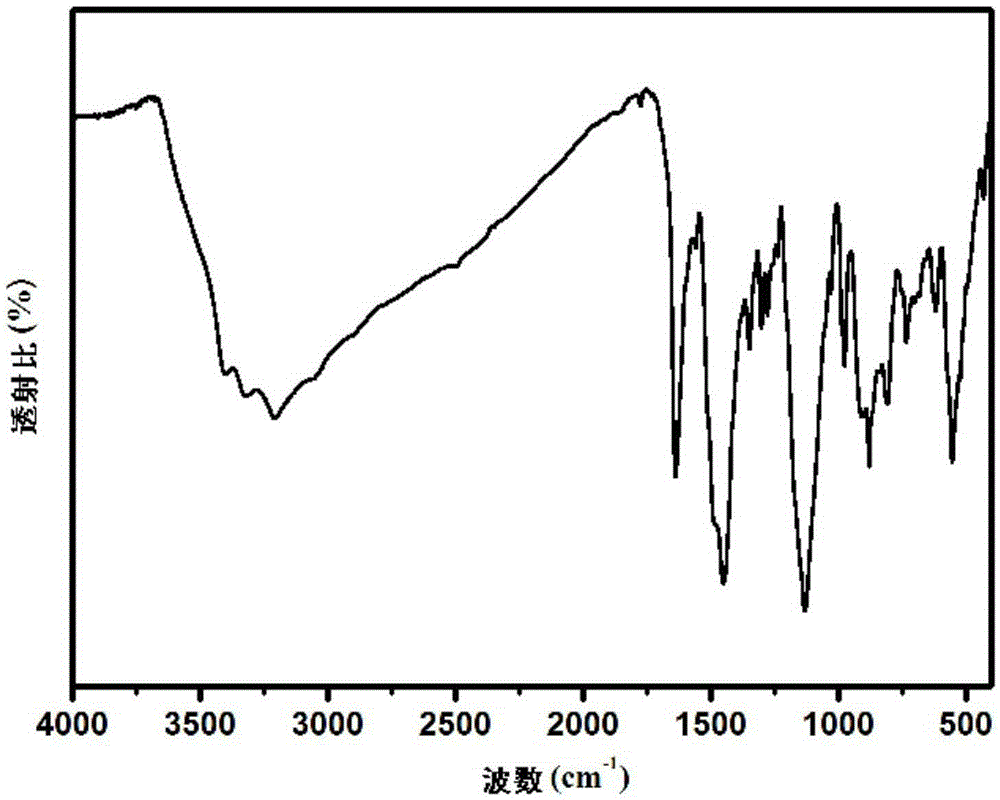
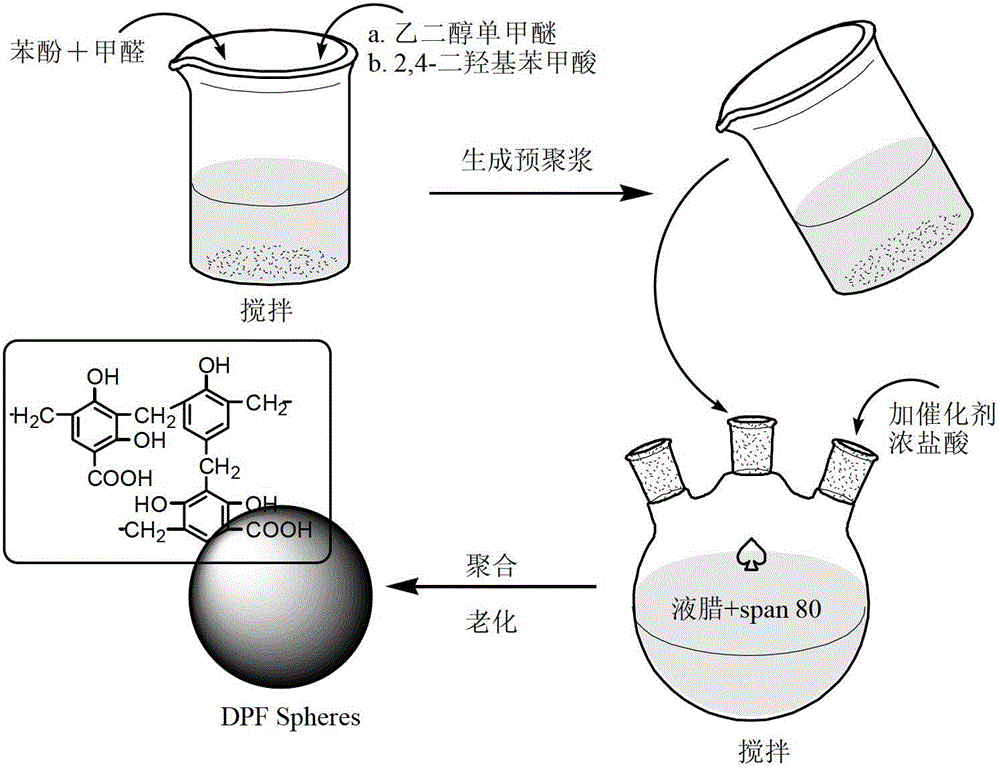
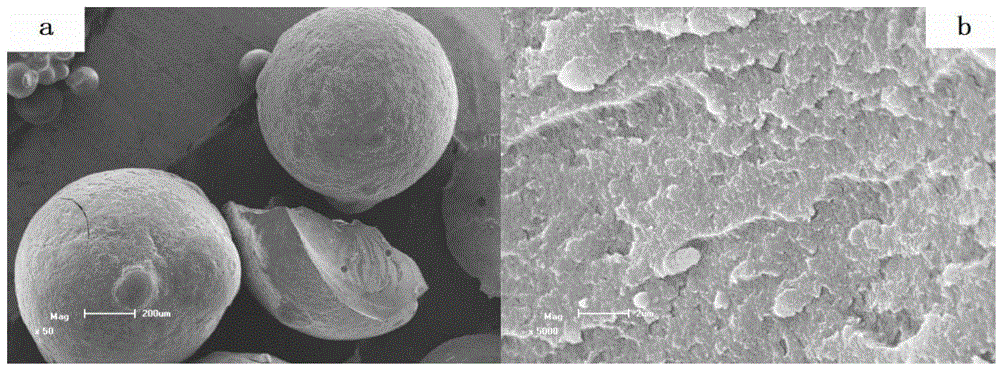
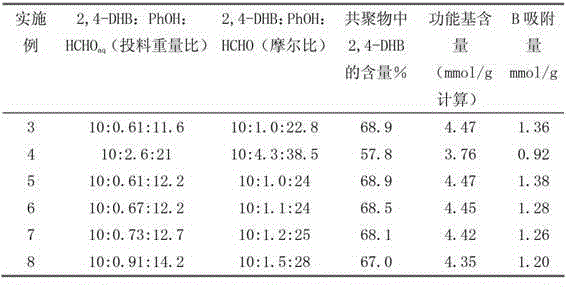
Preparation method of condensation type beaded salicylic acid derivative boron-specific chelating resin
ActiveCN103145936AImprove adsorption capacitySimple processIon-exchange process apparatusIon-exchanger regenerationBenzoic acidCross-link
The invention discloses a preparation method of condensation type beaded salicylic acid derivative boron-specific chelating resin. The boron-specific chelating resin has two hydroxyl groups on a benzene ring and is a vinyl acetate monomer (DPFSphere) prepared by taking 2,4-dihydroxy-benzoic acid as a functional monomer, taking phenol as a cross-linking agent, taking concentrated hydrochloric acid (AR) as a catalyst and taking glycol or ethylene glycol monomethyl ether as a pore-foaming agent through a reversed phase suspension polycondensation method. The preparation method has the advantages that the particle size of the boron-specific chelating resin is 0.1-1.0mm; boron is absorbed from a boron-contained water solution by using the beaded resin, and the maximum absorption capacity of the boron-specific chelating resin in the boron-contained water solution is 1.2-1.4mmol / g; the boron-contained water solution is adjusted to a neutral or alkaline solution with a pH value of 8-10, which is beneficial to the absorption of boron; the absorption of boron cannot be influenced when the concentrations of alkali metal and alkali metal ions are 0.01-0.2mmol / L; and the preparation method is simple in process and easy to implement and is beneficial to large-scale popularization and application.
Owner:NANKAI UNIV
Cool and refreshing toilet water capable of relieving itching and expelling mosquito
ActiveCN101780020AReduce penetration rateLow toxicityCosmetic preparationsAntipyreticIrritationPeppermints
The invention discloses a cool and refreshing toilet water capable of relieving itching and expelling mosquito, comprising the following components in percentage by weight: 40-75% of edible alcohol, 0.1-2% of peppermint derivative, 0.1-2% of salicylic acid derivative, 1-20% of humectant, 1-15% of plant mosquito repellent, 1-10% of synthesized mosquito repellent, and water as the rest. The toilet water has the effect of slowly releasing refrigerant and is capable of keeping cool for a long time and diminishing inflammation without stimulating skin by reducing the permeation rate of salicylic acid, thereby avoiding irritant effect for skin and improving the comfort when in use; and the artificially synthesized mosquito repellent is complexly used with natural mosquito repellent, thereby improving the mosquito repelling effect and prolonging the mosquito repelling time and decreasing the toxicity of synthesized mosquito repellent with relatively higher safety.
Owner:广东名臣日化有限公司
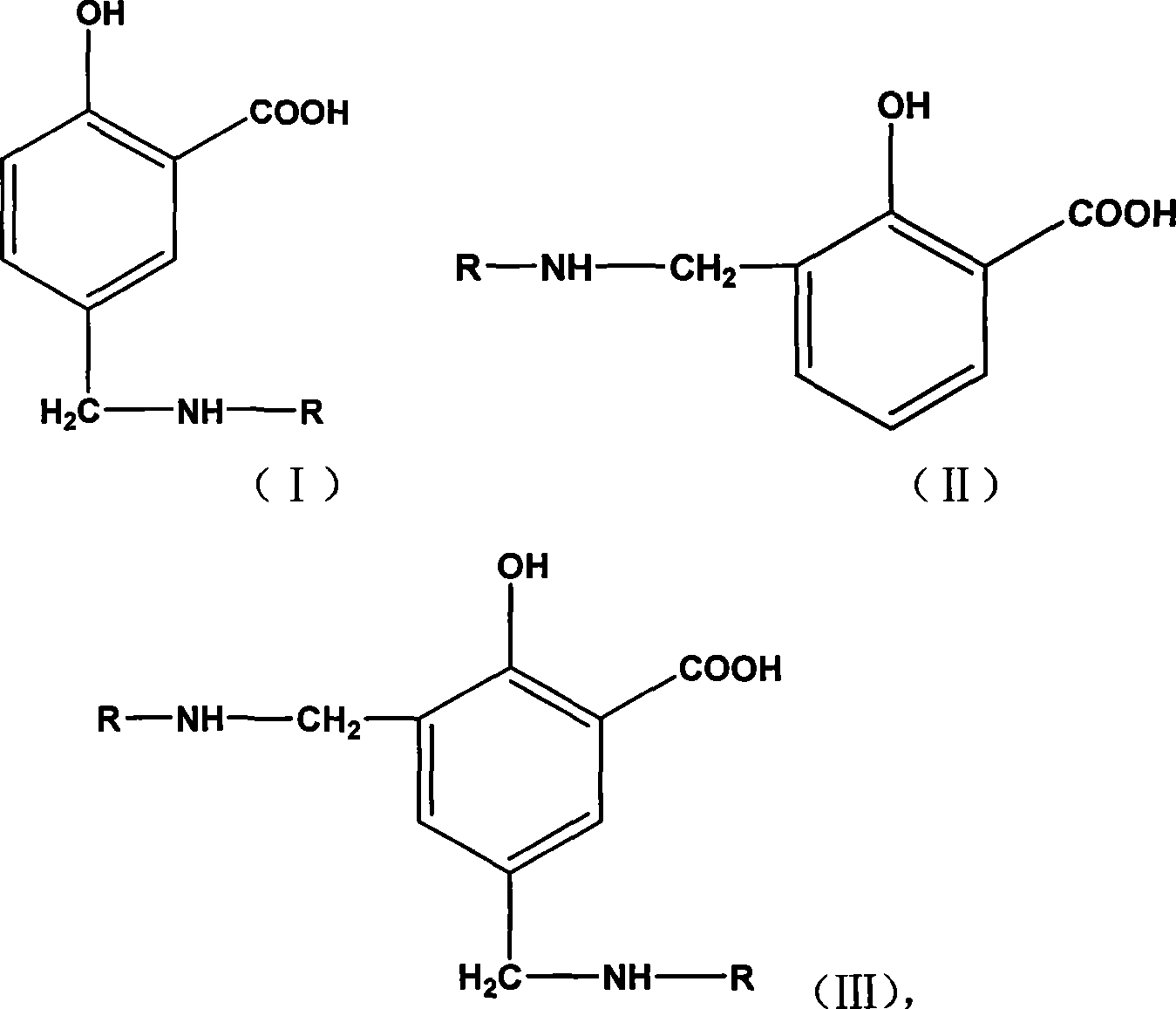
Dispersant for salicylic acid derivatives pigments and preparation method and use thereof
InactiveCN101380555AHigh tinting strengthHigh strengthTransportation and packagingInksBenzoic acidWater based
The invention relates to a dispersant used for salicylic acid derivative pigments, a preparation method and the application thereof; the dispersant has the structures of formula (I), (II) and (III) and is liquid; wherein, the matrix is salicylic acid (ortho-hydroxy benzoic acid), linking group is amine methyl-CH2NH-, and solvated chain R is alkylene oxide polymer; the preparation method comprises the following steps: polyether primary amine and the salicylic acid are heated, stirred fully and evenly, and dipped with formaldehyde solution slowly, and the amine methylating product of the salicylic acid is prepared by Mannich reaction; the application thereof lies in improving the dispersibility of lake or disazo pigments used for solvent or water-based printing ink. The dispersant of the invention is colourless and improves the colour intensity of the pigments by virtue of synergistic action of the dispersant and the pigments; the printing ink produced by adding the pigments of the dispersant has higher colour intensity, transparency and low viscosity, and is applied widely.
Owner:DONGHUA UNIV
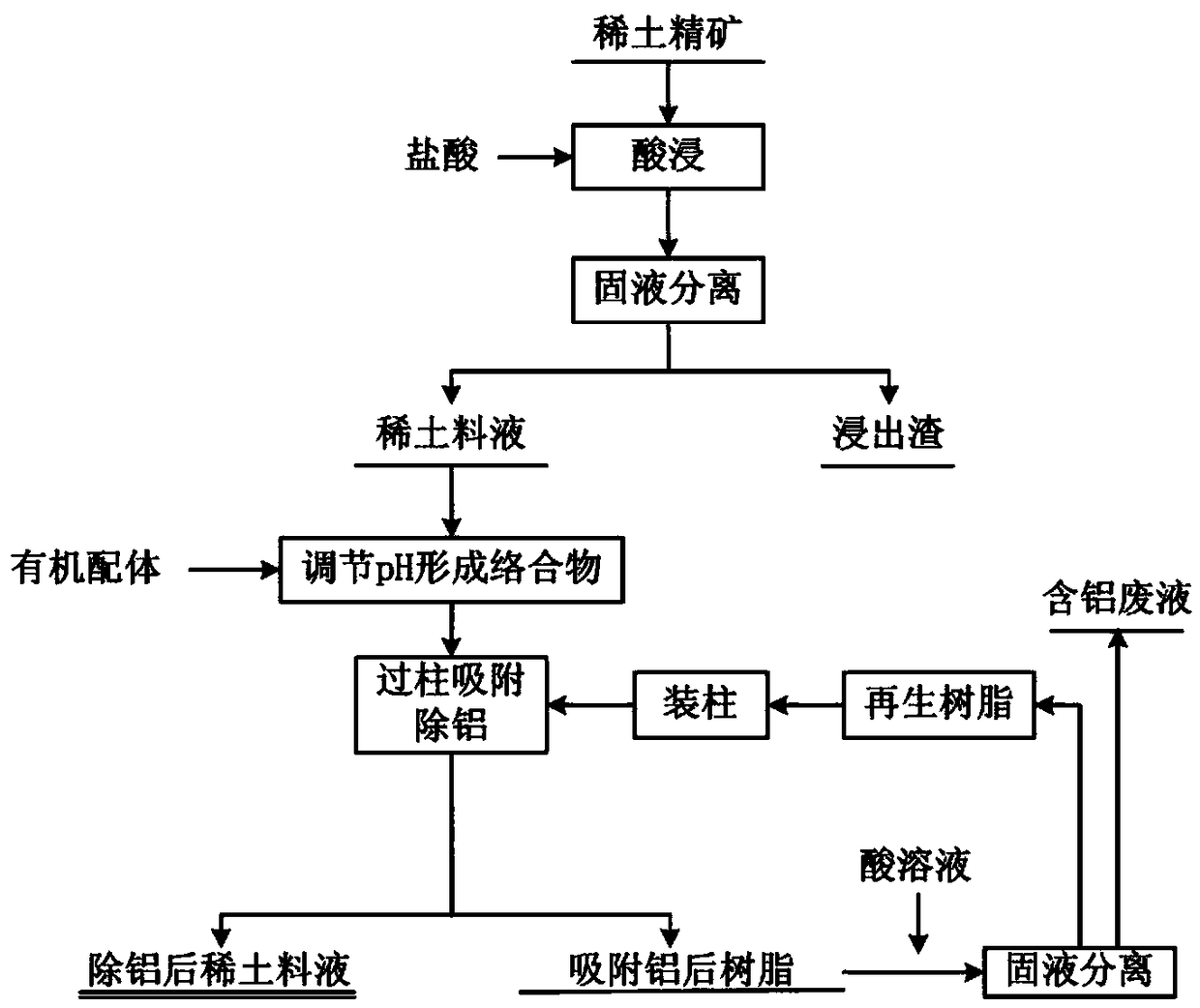
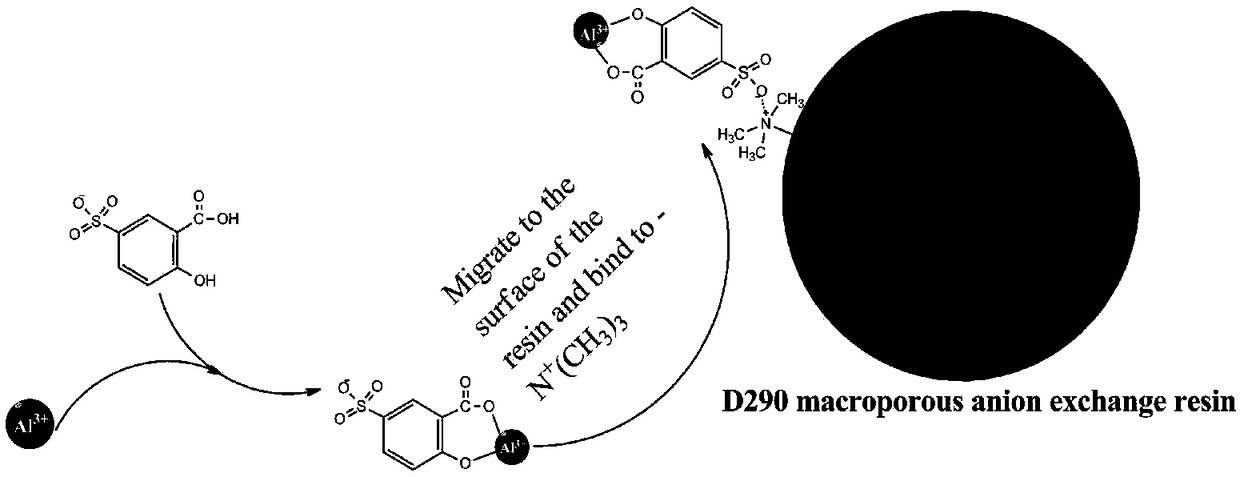
Method for adsorbing and removing aluminum from rare earth material liquor through complexing-ion exchange synergistic effect
ActiveCN108866358AReduce concentrationAchieve removalProcess efficiency improvementAluminum IonRare earth
The invention discloses a method for adsorbing and removing aluminum from rare earth material liquor through a complexing-ion exchange synergistic effect. Salicylic acid derivative is utilized as an organic ligand to treat a rare earth solution, then D290 type anion exchange resin is utilized to adsorb complexing anion generated by reaction between aluminum ions and the organic ligand, and aluminum ions can be removed from the material liquor. By means of controlling the organic ligand use amount, a reaction temperature, a solution pH value, the rare earth material liquor and a resin column flow velocity, a removal rate of aluminum ions in the rare earth material liquor can reach 70% or more, and rare earth loss does not exceed 5%. Compared with the prior art, the method for adsorbing andremoving aluminum from rare earth material liquor through the complexing-ion exchange synergistic effect has a low equipment requirement and simpleness in operation, and a lot of factory areas required by multilevel extraction of an extraction method are avoided; meanwhile, the problems that aluminum hydroxide flocculent precipitate is difficult to filter and has seriousness in entrainment; furthermore, the used D290 type anion exchange resin can be recycled, and production cost is reduced.
Owner:JIANGXI UNIV OF SCI & TECH
Medical use of 5-benzylaminosalicylic acid derivatives or salts thereof
The present invention relates to a pharmaceutical composition useful for the treatment or prevention of stress-related disorders, depression, and anxiety disorders, containing 5-benzylaminosalicylic acid derivatives and / or pharmaceutically acceptable salts thereof as active ingredients. In addition, the present invention relates to a method for treating or preventing stress-related disorders, depression, and anxiety disorders, wherein the method comprises a step for administrating 5-benzylaminosalicylic acid derivatives and / or pharmaceutically acceptable salts thereof to a subject required for treatment.
Owner:NEUROTECH PHARMA
Method and composition for treating hairy hoof warts
InactiveUS6863898B2Prevent hairy hoof wartsHeavy metal active ingredientsBiocideAspirinSodium Bentonite
Hairy hoof warts in animals are treated by topically administering an effective amount of a composition of aspirin or other compound or derivative of salicylic acid that preferably contains at least 50% by weight and more preferably between about 75% and 100% by weight aspirin or other compound or derivative of salicylic acid. A preferred composition includes aspirin, hydrated lime, sodium bentonite, and chalk or iron oxide.
Owner:CLAWSON MICHAEL D
Topical dermal anaesthetic
InactiveUS20020094343A1Increase painRelief the painBiocideOrganic active ingredientsSide effectIrritation
A liquid composition applied transdermally for relief of pain comprising alcohol in an amount by weight of about 57 to about 91 percent; glycerin in an amount by weight of about 1 to about 12 percent; an analgesic agent in an amount by weight of about 2 to about 28 percent, the analgesic agent comprising a derivative of salicylic acid; methylsulfonylmethane in an amount by weight of about 0.02 to 5 percent; and emu oil in an amount by weight of about 0.01 to 3 percent, the liquid composition permeating skin to relieve pain. The composition further comprising, as an additional feature, aloe vera in an amount by weight of at least about 0.05 percent and having an amount by weight of about 0.05 to 4 percent. The composition features transdermal pain relief such that a patient can apply the analgesic agent directly to an area of pain without such side effects as stomach irritation which is normally associated with aspirin. The composition may be sprayed or rolled directly onto the painful area. Because of the unique formula, the composition is safe to vital internal organs, requires no mixing before use, and is shelf stable for marketing purposes.
Owner:VELTRAN LP
Process of Preparation of Substituted Tetrafluorobenzylaniline Compound and Its Pharmaceutically Approved Salts
InactiveUS20080167492A1Inhibition formationLess toxicityNervous disorderOrganic compound preparationSalicylic acidMedicinal chemistry
This invention relates to a method for preparing tetrafluorobenzyl-5-aminosalicylic acid derivative and its pharmaceutically acceptable salt compounds. In particular, this invention relates to a method for preparing tetrafluorobenzyl-5-aminosalicylic acid derivative and its pharmaceutically acceptable salts by using tetrafluorobenzilidine-5-aminosalicylic acid derivative as an intermediate.
Owner:JW PHARMA CORP
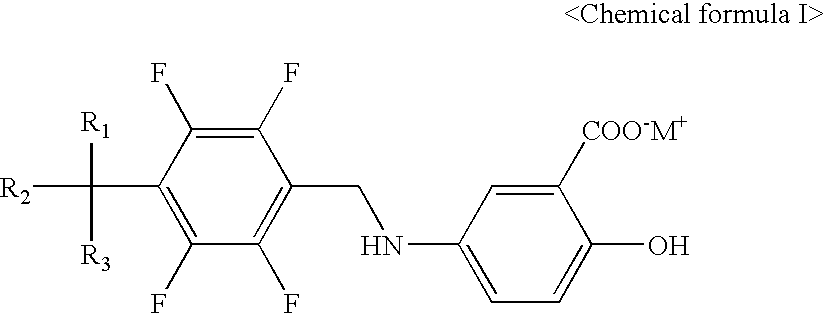
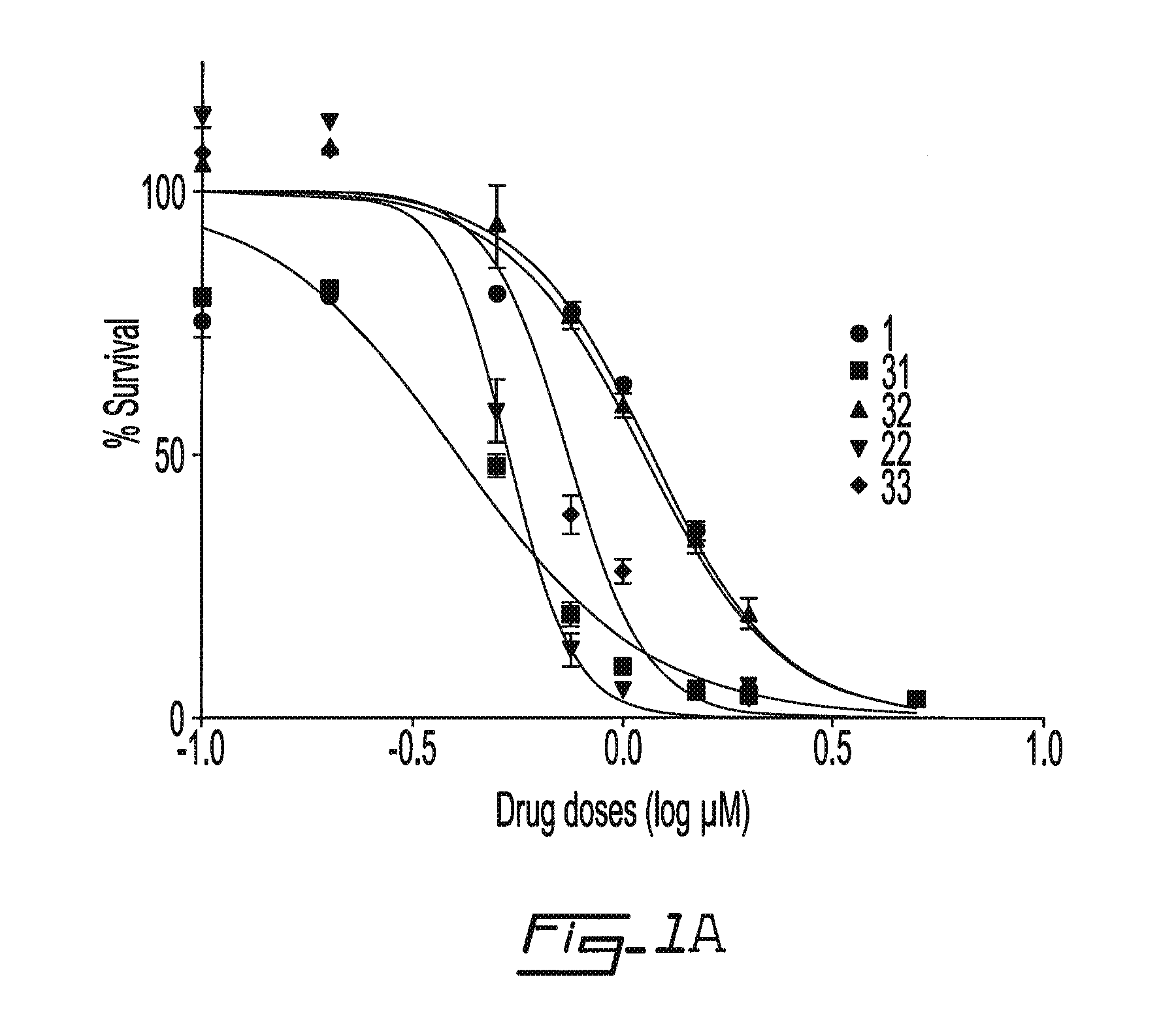
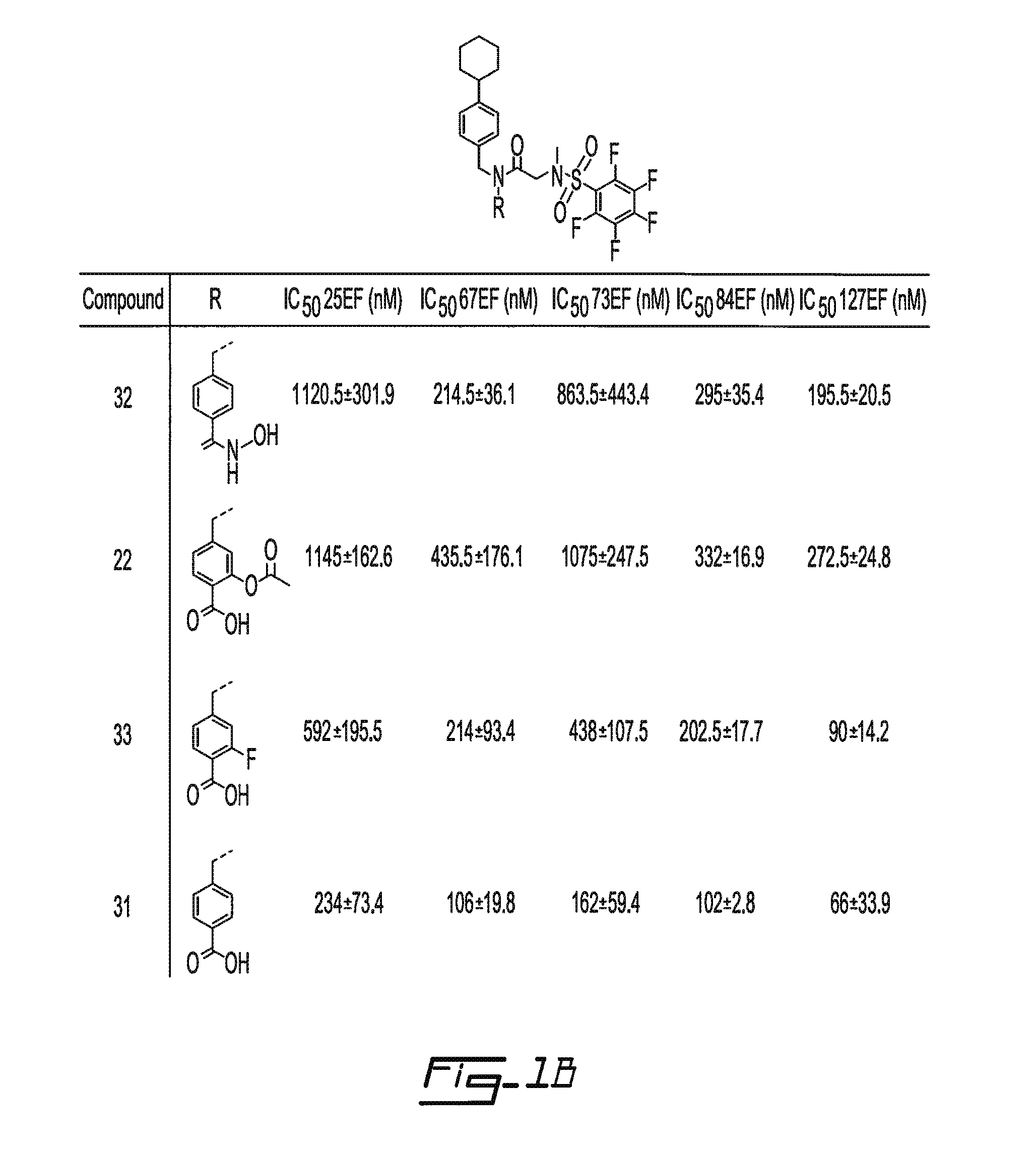
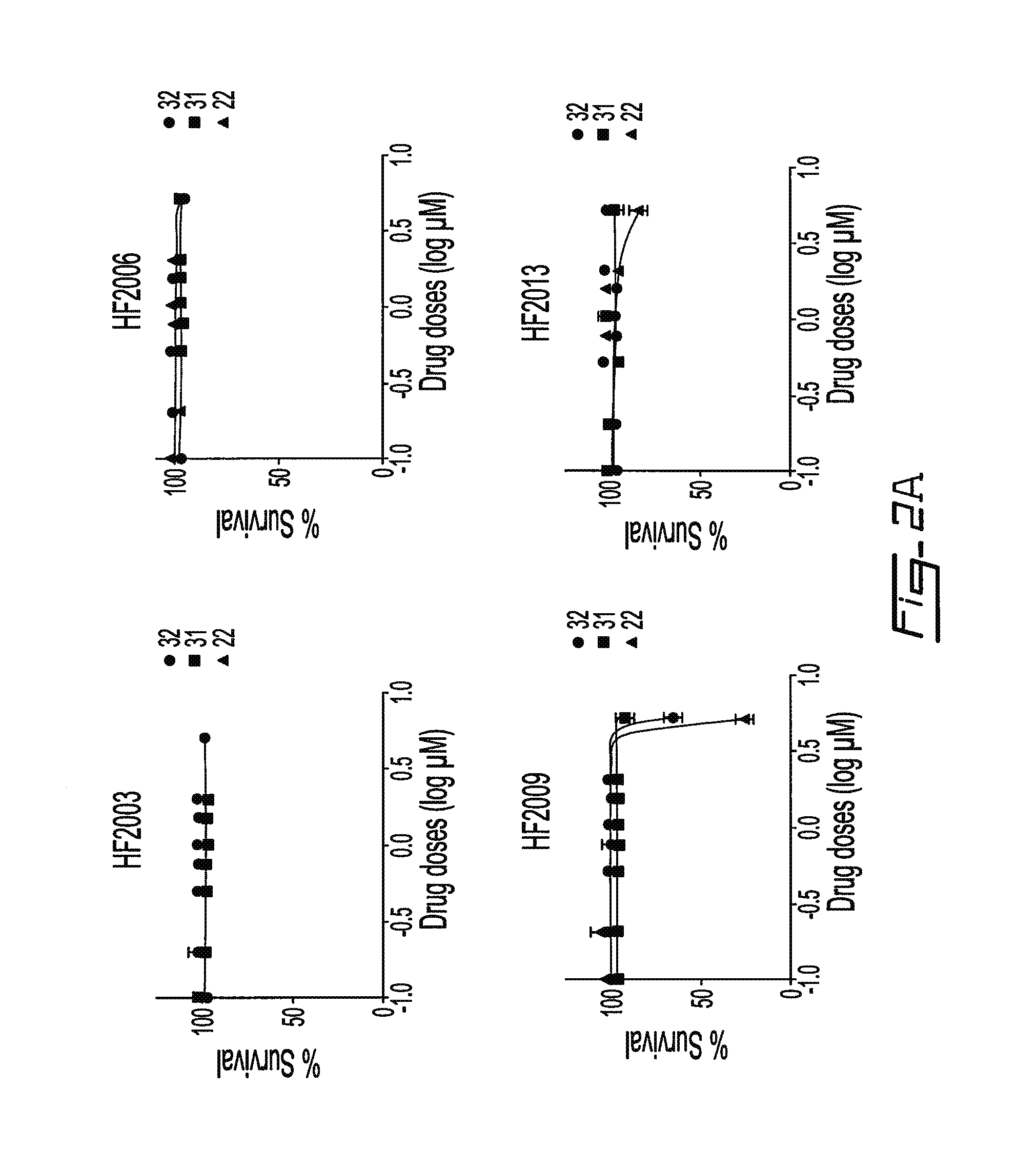
Salicyclic acid derivatives, pharmaceutically acceptable salt thereof, composition thereof and method of use thereof
Owner:UTI LLP +2
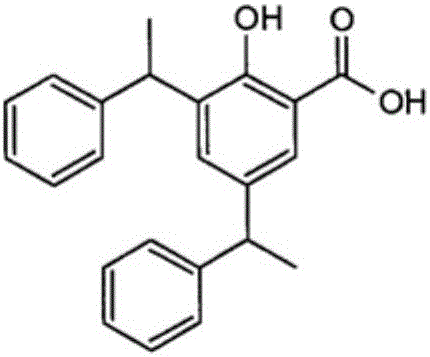
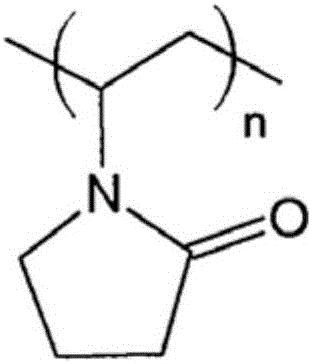
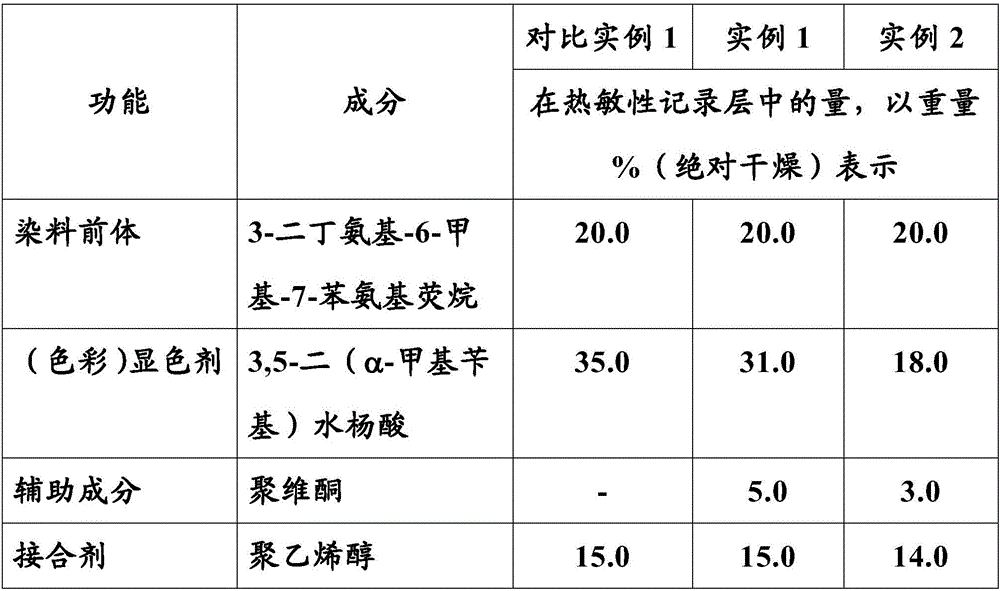
Heat-sensitive recording material with salicylic acid derivative as (color) developer reactive with a dye precursor
A heat-sensitive recording material is proposed, comprising a web substrate having a facing side and a reverse side opposite it, at least one heat-sensitive recording layer on at least one of the two sides of the web substrate, the heat-sensitive recording layer comprising at least one dye precursor and at least one salicylic acid derivative as (color) developer reactive with this at least one dye precursor, characterized in that: the at least one salicylic acid derivative is 3,5-di(alpha-methylbenzyl)salicylic acid, the heat-sensitive recording layer further comprises polyvinylpyrrolidone as an auxiliary component assisting the (color) developer.
Owner:MITSUBISHI HITEC PAPER EURO
Water-in-oil emulsion composition
ActiveCN1636550ANo damage to performance and functionGood stability over timeCosmetic preparationsToilet preparationsMethoxylaricinolic acidPolyethylene glycol
The present invention provides a water-in-oil type that does not impair the function of the component in a system containing a salicylic acid derivative (alkoxysalicylic acid, etc.), has good emulsification stability over time, and is excellent in usability emulsified composition. The water-in-oil emulsified composition contains (a) a salicylic acid derivative (such as methoxysalicylate, ethoxysalicylate, etc.) and (b) in an aqueous phase (inner phase). ) salts, and (c) polyethylene glycol with a molecular weight of 1,000 to 200,000, and if necessary, the oil phase (external phase) contains 30% by mass or more of the oil phase (external phase) that is liquid at normal temperature silicone oil.
Owner:SHISEIDO CO LTD
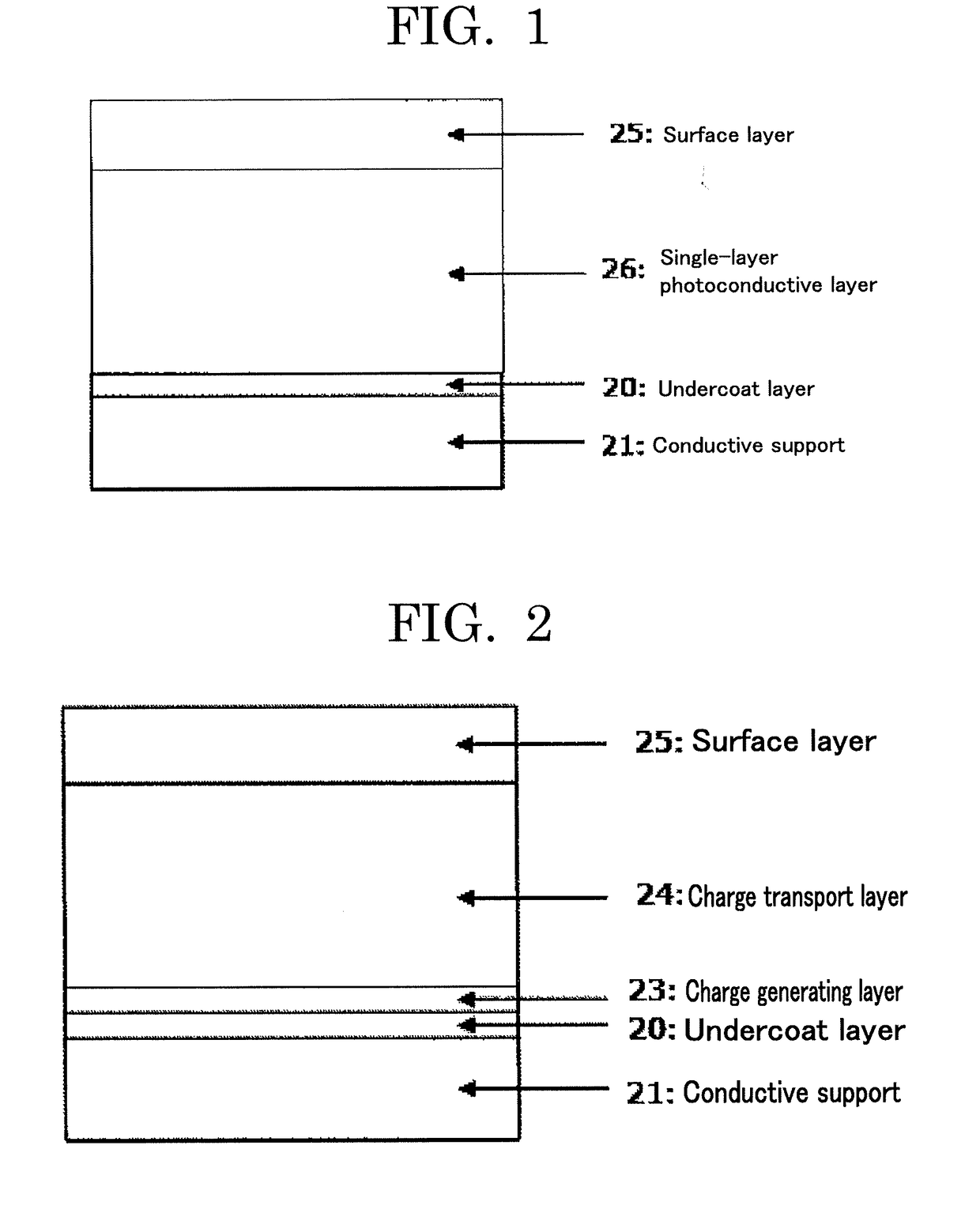
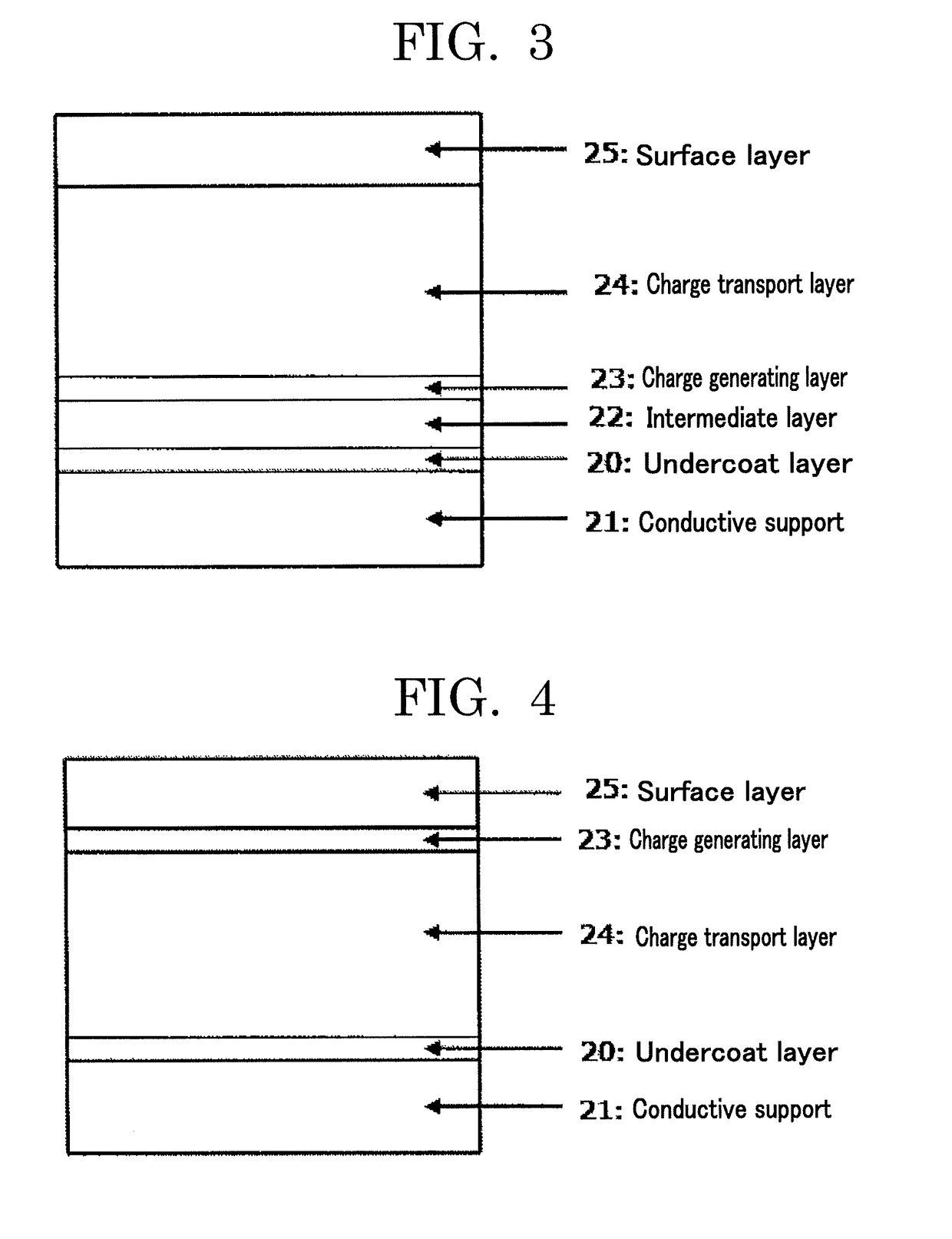
Electrophotographic photoconductor, image forming apparatus, and process cartridge
ActiveUS20190011845A1Solve problemsElectrography/magnetographyCoatingsElectrical conductorMicrometer
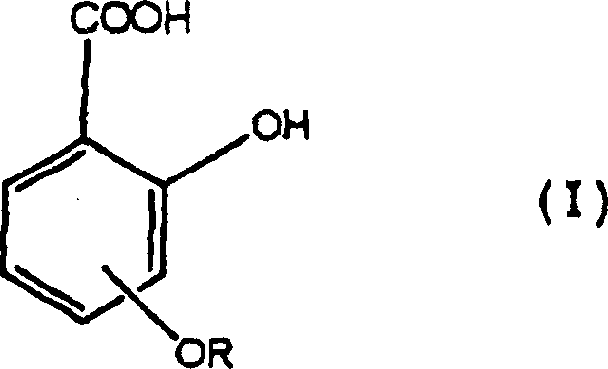
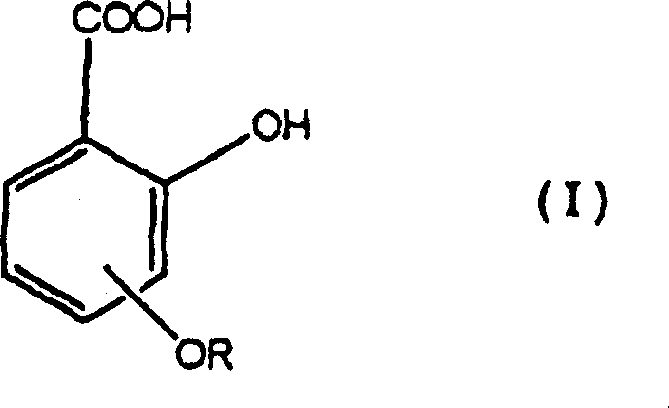
Provided is an electrophotographic photoconductor including: a conductive support; an undercoat layer; and a photoconductive layer, wherein the undercoat layer and the photoconductive layer are provided over the conductive support in the order of reciting, wherein the undercoat layer contains at least metal oxide particles, a binder, and a salicylic acid derivative such as 4-methylsalicylic acid, wherein the metal oxide particles have a volume resistivity of 1×105 Ω·cm or higher but 1×108 Ω·cm or lower, wherein the undercoat layer has a volume resistivity of 0.001×106 Ω·cm or higher but 0.02×106 Ω·cm or lower, and wherein the undercoat layer has a thickness of 2 micrometers or greater but 20 micrometers or less.
Owner:RICOH KK
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com