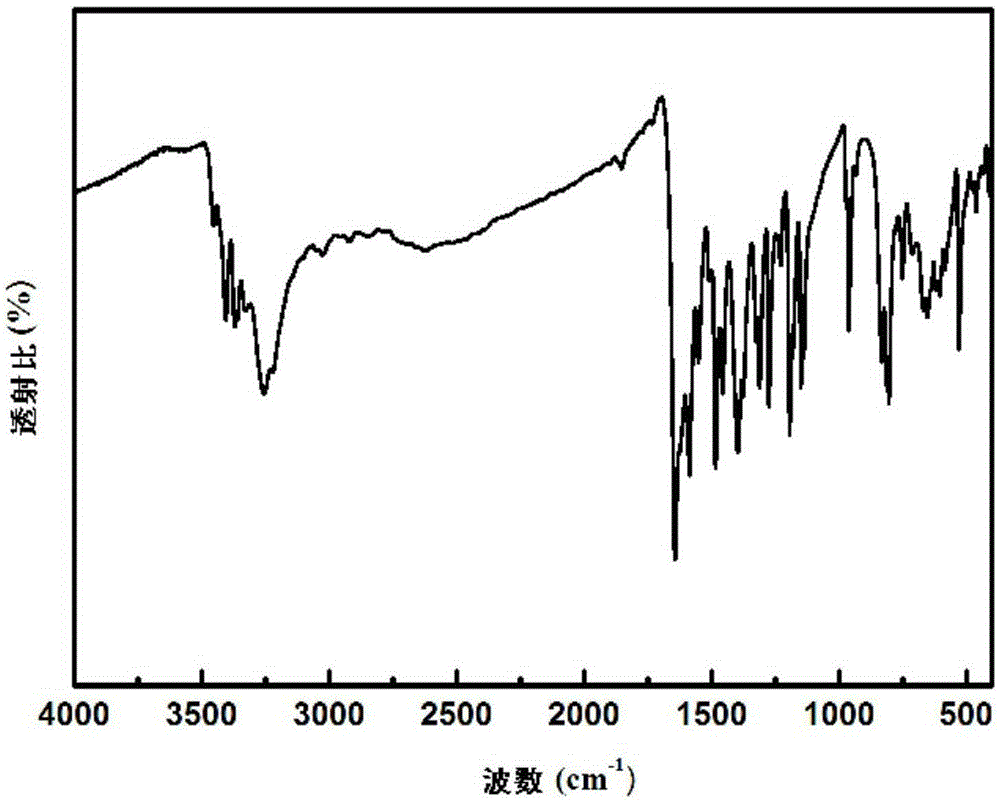
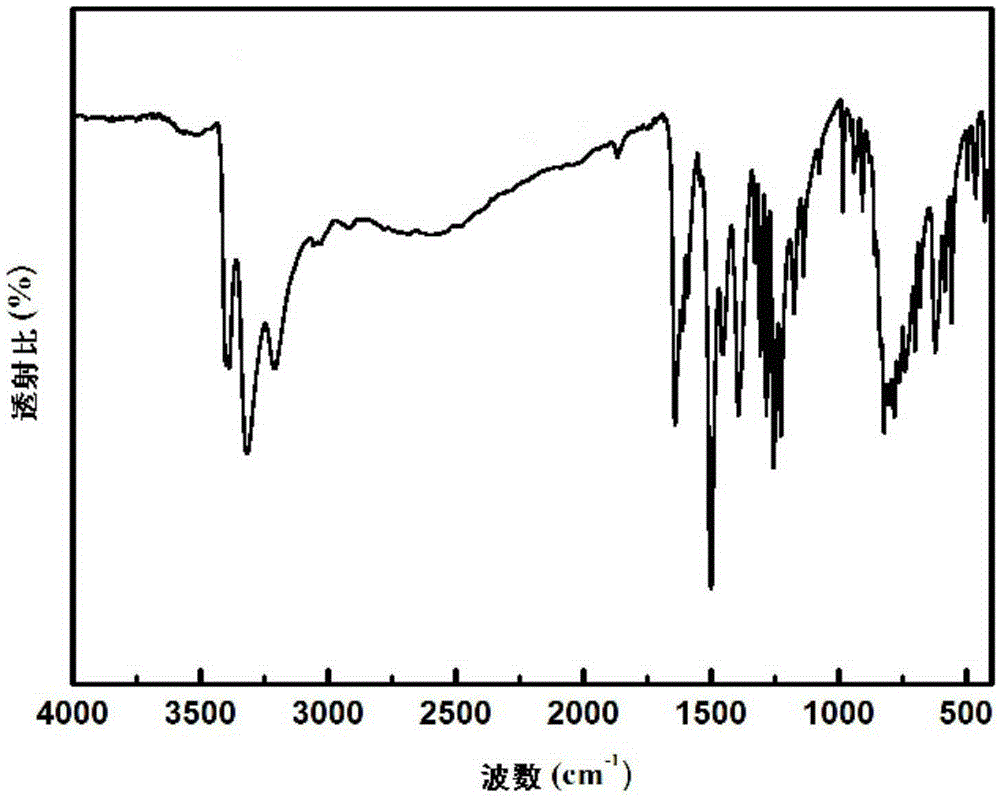
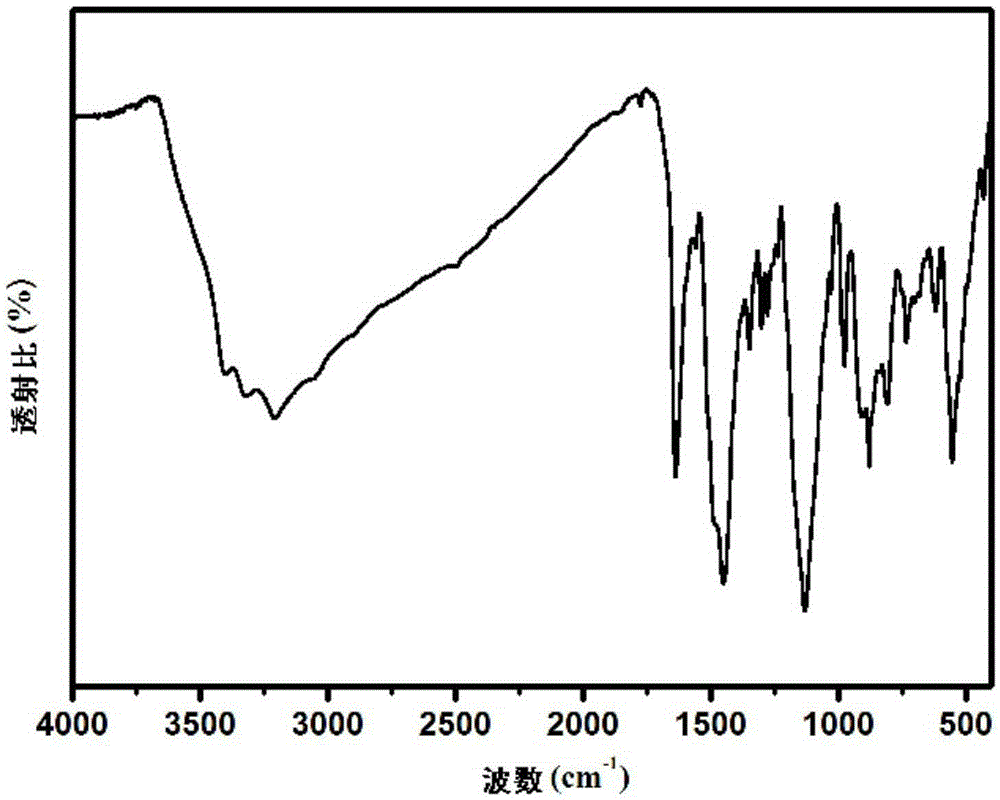
Hydroxyl-containing benzimidazole diamines and preparation method thereof
A kind of technology of hydroxybenzimidazole diamine and triaminobenzene, which is applied in the field of synthesis of organic compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] The present invention also provides a kind of preparation method of hydroxy-containing benzimidazole diamine, comprising the following steps:
[0046] A1) Reaction of 1,2,4-triaminobenzene hydrochloride with a salicylic acid derivative having a structure shown in formula (IV) to obtain a hydroxy-containing benzimidazole diamine having a structure shown in formula (I) ;
[0047]
[0048] or
[0049] A2) Reaction of 1,2,4-triaminobenzene hydrochloride with a salicylic acid derivative having a structure shown in formula (V) to obtain a hydroxy-containing benzimidazole diamine having a structure shown in formula (II) ;
[0050]
[0051] or
[0052] A3) Reaction of 3,3',4,4'-tetraaminobiphenyl with a salicylic acid derivative having a structure shown in formula (IV) to obtain a hydroxy-containing benzimidazole having a structure shown in formula (III) Diamine;
[0053]
[0054] In the present invention, the step A1) preferably specifically includes the following ...
Embodiment 1
[0126] (1) Under the condition of nitrogen protection, 58.82g (0.3mol) of 1,2,4-triaminobenzene hydrochloride, 600gP 2 o 5 Content is 80% polyphosphoric acid, 1.35g (0.006mol) SnCl 2 · 2 h 2 O, stirred at 80°C for 8h to obtain the first mixed solution of 1,2,4-triaminobenzene;
[0127] (2) Add 60g (0.42mol) P to the above first mixed solution 2 o 5 , 46.84g (0.33mol) 4-aminosalicylic acid, reacted at 210°C for 8h to obtain a reaction mixture;
[0128] (3) Cool the above reaction mixture to 120°C, then pour it into ice water and stir for 2 hours, then filter and wash with distilled water, then take out the filter cake, add a small amount of water, wash with saturated NaHCO 3 The pH value of the solution was adjusted to 8, then filtered, washed with distilled water, and finally vacuum-dried at 120°C for 24 hours to obtain a crude product of hydroxybenzimidazole-containing diamine;
[0129] (4) Using dimethyl sulfoxide as a solvent, recrystallize the crude diamine containi...
Embodiment 2
[0135] (1) Under the condition of nitrogen protection, 58.82g (0.3mol) of 1,2,4-triaminobenzene hydrochloride, 650gP 2 o 5 Polyphosphoric acid with a content of 80%, 0.36g (0.003mol) tin powder, stirred at 100°C for 6h to obtain the first mixed solution of 1,2,4-triaminobenzene;
[0136] (2) Add 42.57g (0.3mol) P 2 o 5 , 45.34g (0.3mol) of 5-aminosalicylic acid, reacted at 200°C for 6h to obtain a reaction mixture;
[0137] (3) Cool the above reaction mixture to 120°C, then pour it into ice water and stir for 2 hours, then filter and wash with distilled water, then take out the filter cake, add a small amount of water, wash with saturated NaHCO 3 The pH value of the solution was adjusted to 8, then filtered, washed with distilled water, and finally vacuum-dried at 120°C for 24 hours to obtain a crude product of hydroxybenzimidazole-containing diamine;
[0138] (4) The crude product of hydroxy-containing benzimidazole diamine was sublimed at 330° C. to obtain hydroxy-contai...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Breaking strength | aaaaa | aaaaa |
| Modulus | aaaaa | aaaaa |
| Breaking strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com