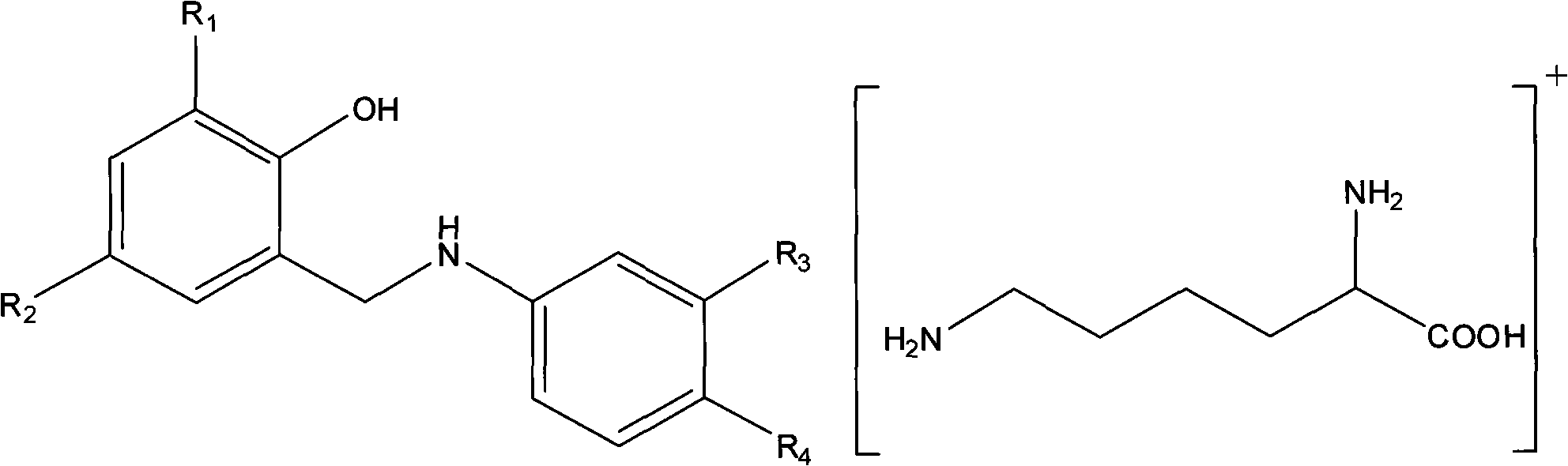
Aminosalicylic acid derivative lysine complex salt, preparation method and application thereof
A technology of aminosalicylic acid and lysine, which is applied to the preparation of cyanide reaction, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of poor solubility and achieve good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1 4-(5-fluoro-2-hydroxybenzylamino)-2-hydroxybenzoic acid lysine salt
[0016] Under room temperature and electromagnetic stirring, 1.46g (0.01mol) lysine 10mL was added dropwise to 2.77g (0.01mol) 4-(5-fluoro-2-hydroxybenzylamino)-2-hydroxybenzoic acid in 10mL ethanol solution The aqueous solution, then cooled to 5-15 ° C, reacted for 2-5 hours, and precipitated solid. Filter and dry in vacuo to obtain white crystals.
[0017] 1 H NMR (DMSO-d 6 )δ: 1.42(m, 2H); 1.56(m, 2H); 1.70(m, 2H); 2.77(t, J=7.5Hz, J=7.5Hz, 2H); 3.30(t, J=6Hz, J =6Hz, 1H); 4.21(s, 2H); 5.92-7.46(m, 6H); 9.60(s, 2H); 11.44(s, 1H);
Embodiment 2
[0018] Example 2 4-(5-bromo-2-hydroxybenzylamino)-2-hydroxybenzoic acid lysine salt
[0019] At room temperature and under electromagnetic stirring, add 1.46 g (0.01 mol) lysine 10 mL dropwise to 3.38 g (0.01 mol) of 4-(5-bromo-2-hydroxybenzylamino)-2-hydroxybenzoic acid in 10 mL of ethanol The aqueous solution, then cooled to 5-15 ° C, reacted for 2-5 hours, and precipitated solid. Filter and dry in vacuo to obtain white crystals.
[0020] 1 H NMR (DMSO-d 6 )δ: 1.42(m, 2H); 1.56(m, 2H); 1.70(m, 2H); 2.77(t, J=7.5Hz, J=7.5Hz, 2H); 3.30(t, J=6Hz, J =6Hz, 1H); 4.24(s, 2H); 6.72-7.78(m, 6H); 9.96(s, 2H); 11.44(s, 1H);
Embodiment 3
[0021] Example 3 4-(3,5-dichloro 2-hydroxybenzylamino) 2-hydroxybenzoic acid lysine salt (compound 1)
[0022] At room temperature and under electromagnetic stirring, 1.46 g (0.01 mol) of lysine was added dropwise to a solution of 3.28 g (0.01 mol) of 4-(3,5-dichloro-2-hydroxybenzylamino)-2-hydroxybenzoic acid in 10 mL of ethanol. Amino acid 10mL aqueous solution, then lower the temperature to 5-15°C, react for 2-5 hours, and a solid is precipitated. Filter and dry in vacuo to obtain white crystals.
[0023] 1 HNMR (DMSO-d 6 )δ: 1.42(m, 2H); 1.56(m, 2H); 1.70(m, 2H); 2.77(t, J=7.5Hz, J=7.5Hz, 2H); 3.30(t, J=6Hz, J =6Hz, 1H); 4.24(s, 2H); 5.74-7.39(m, 5H, ph); 8.41(s, 6H);
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com