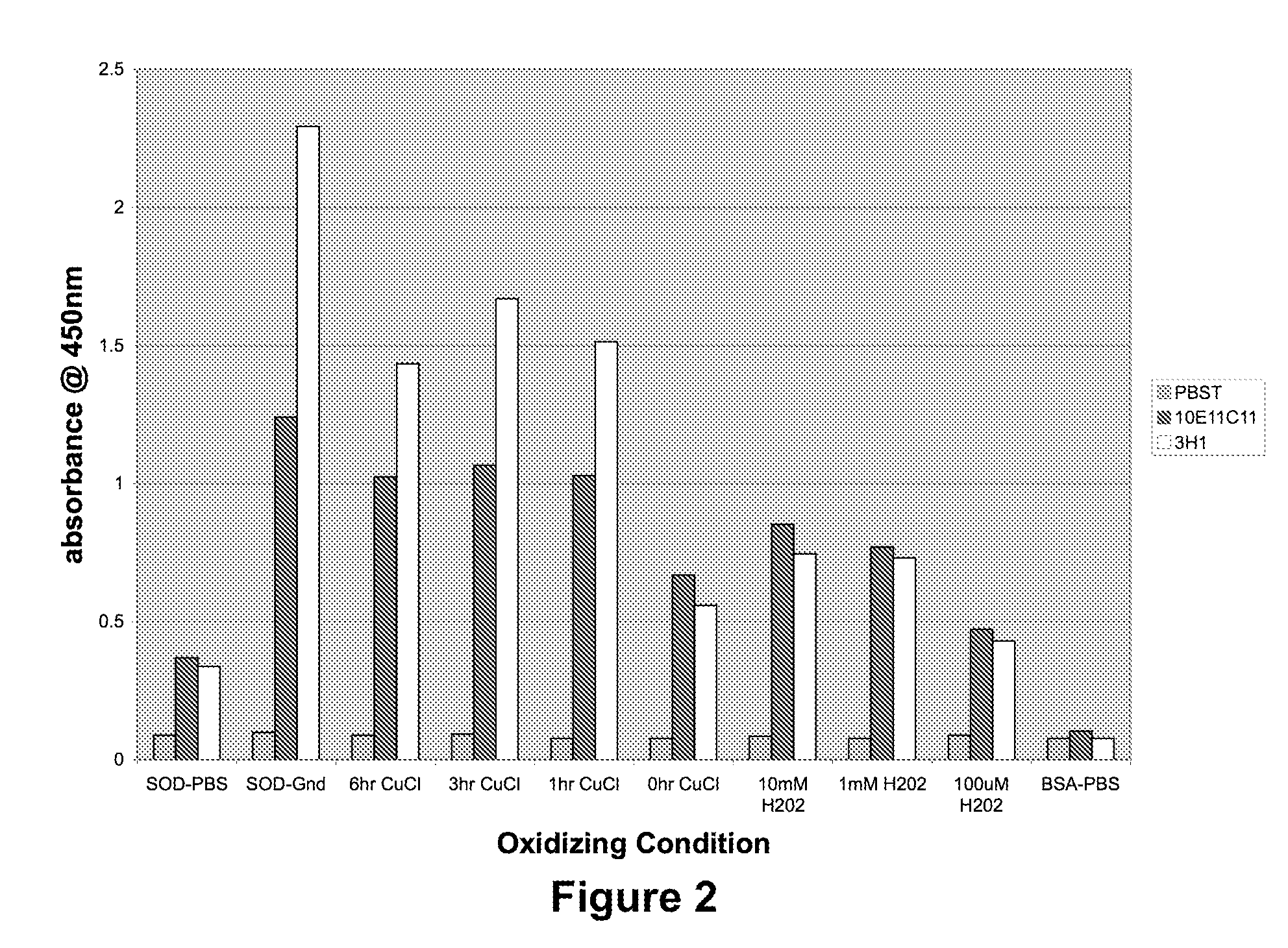
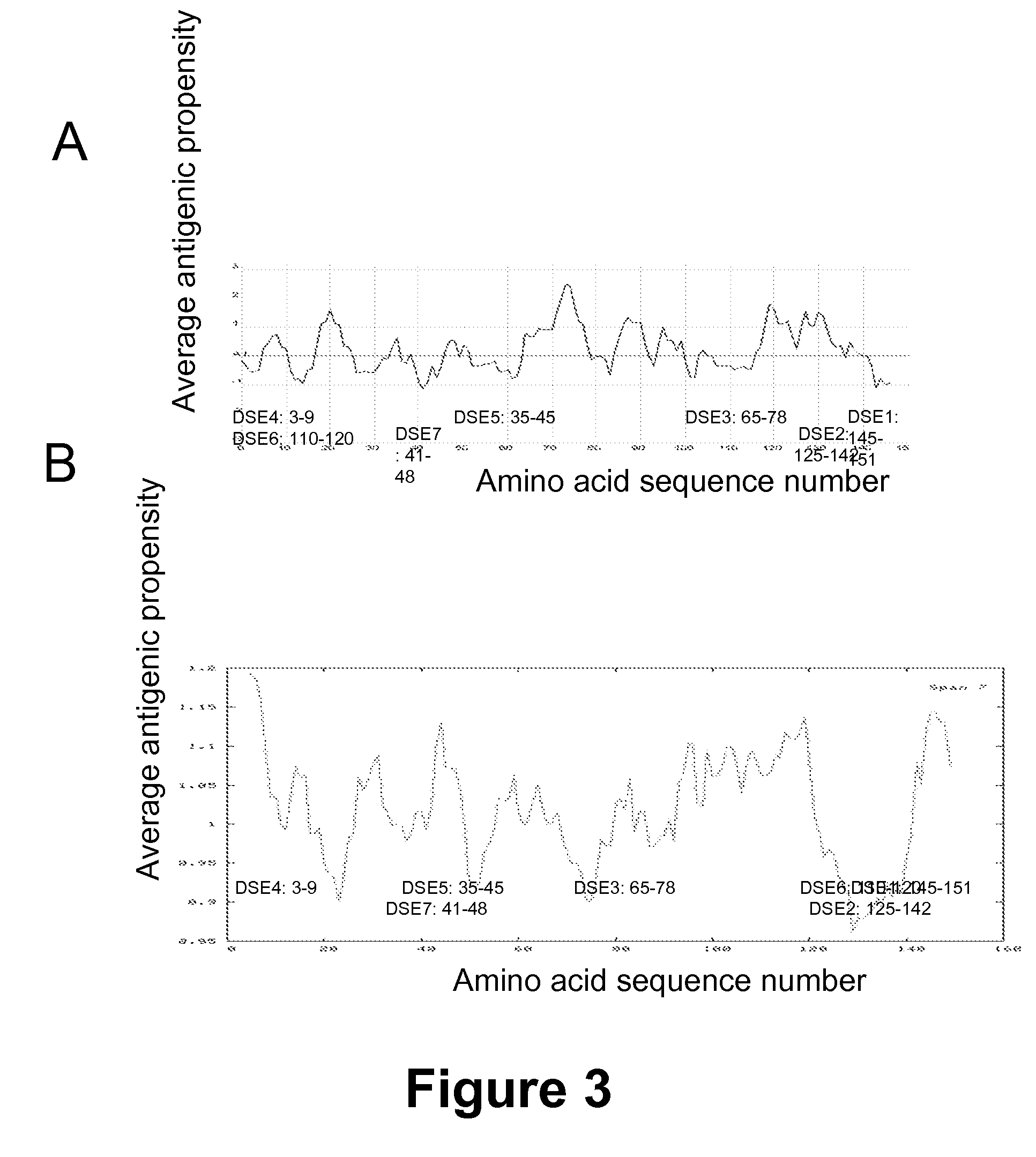
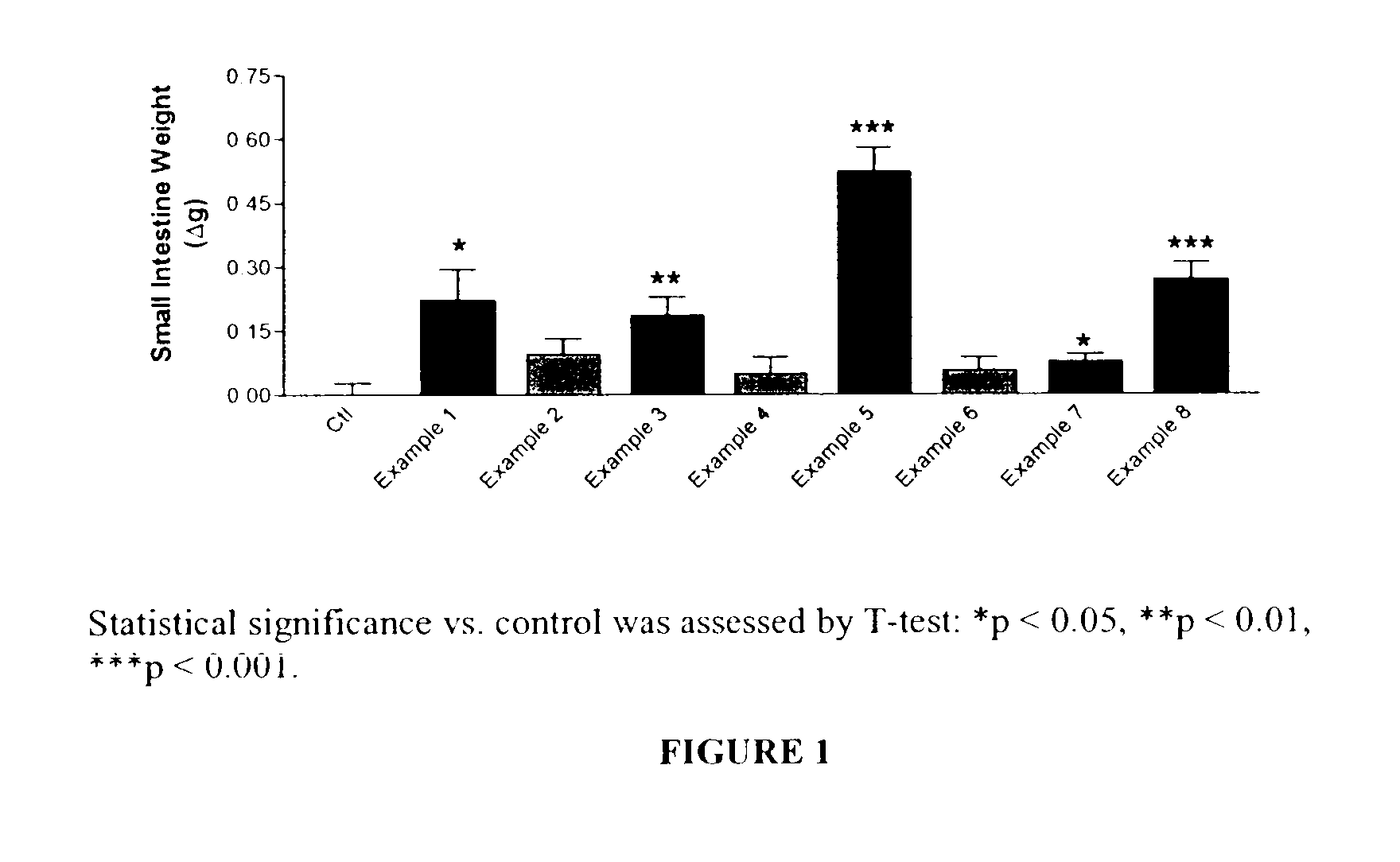
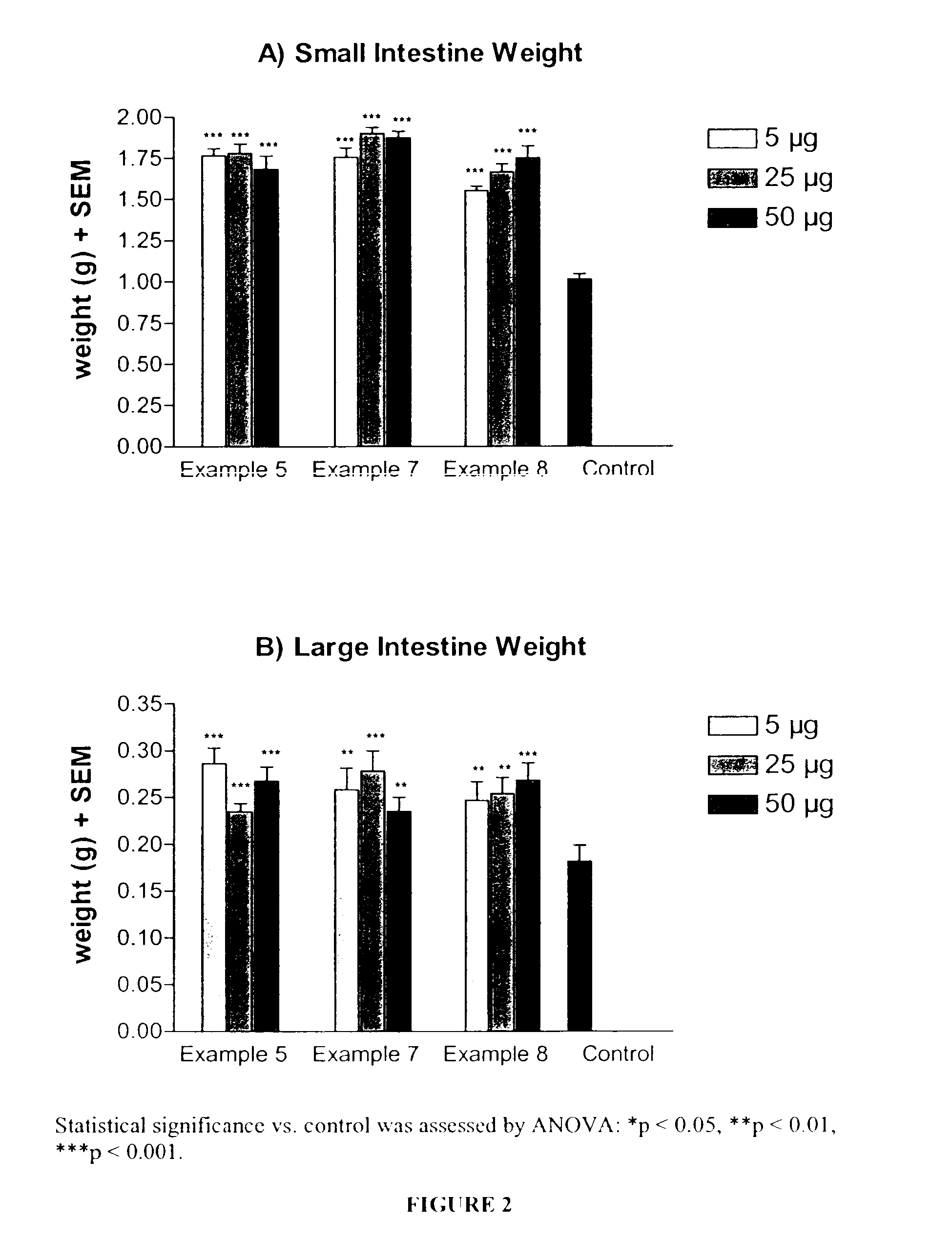
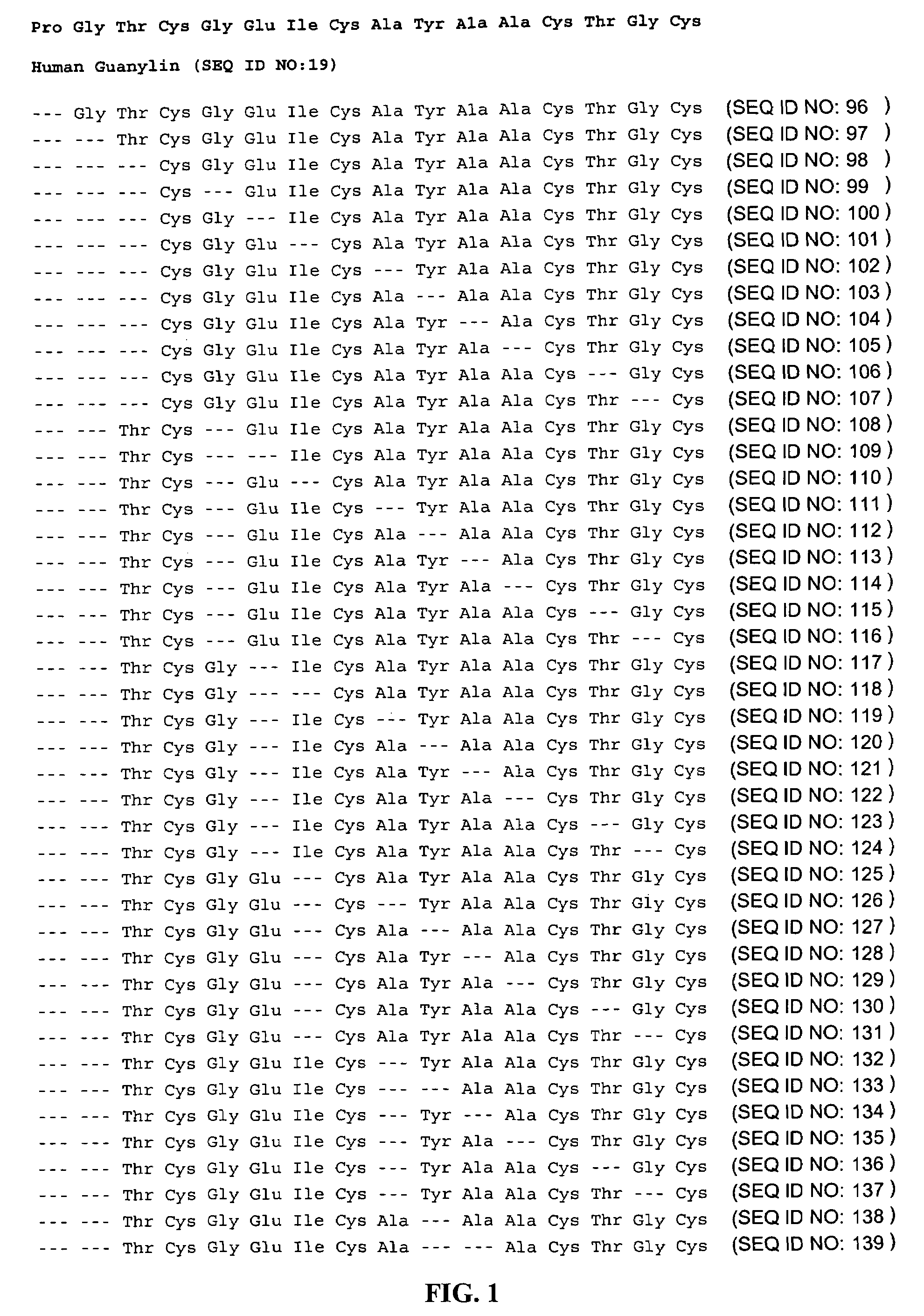
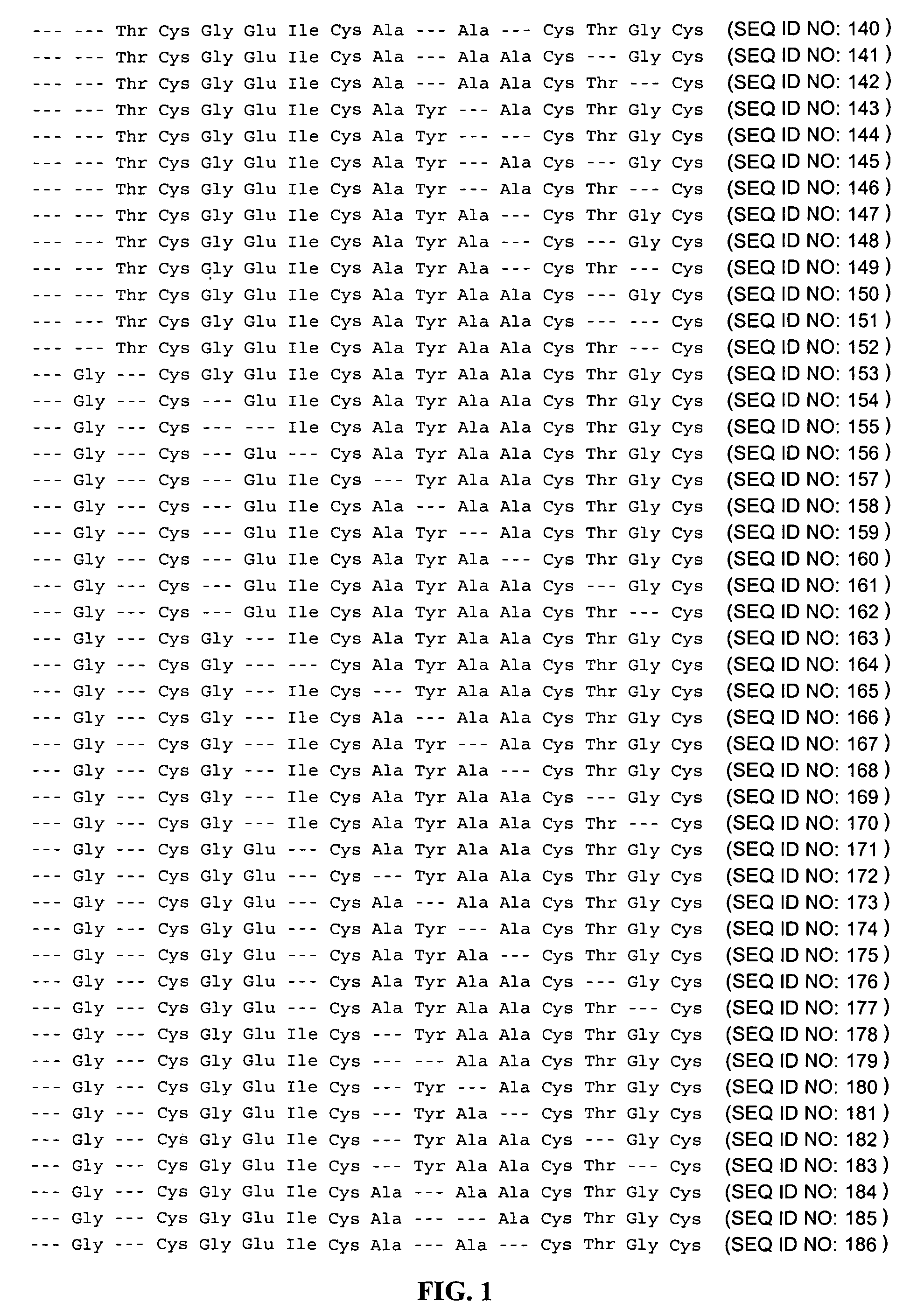
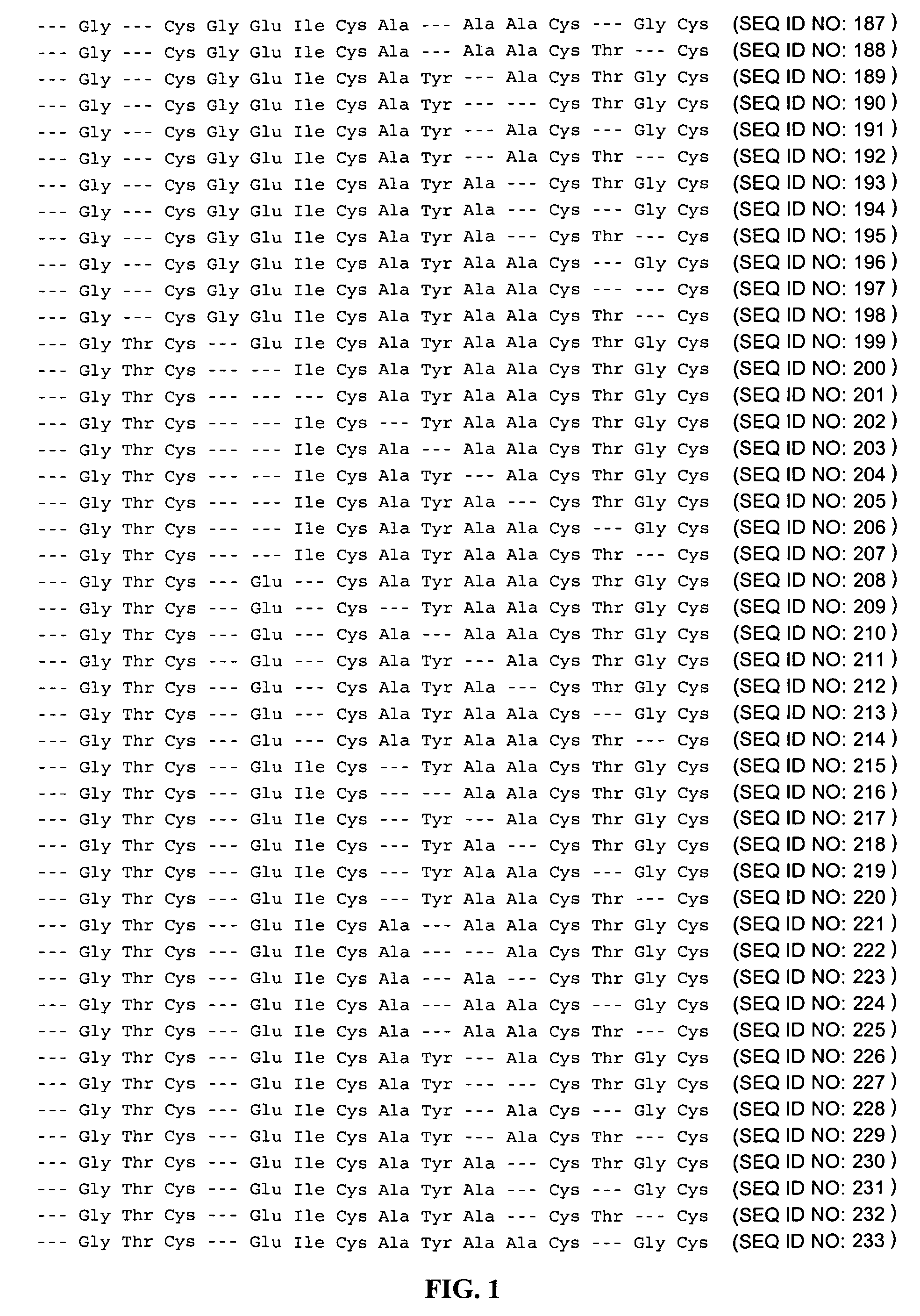
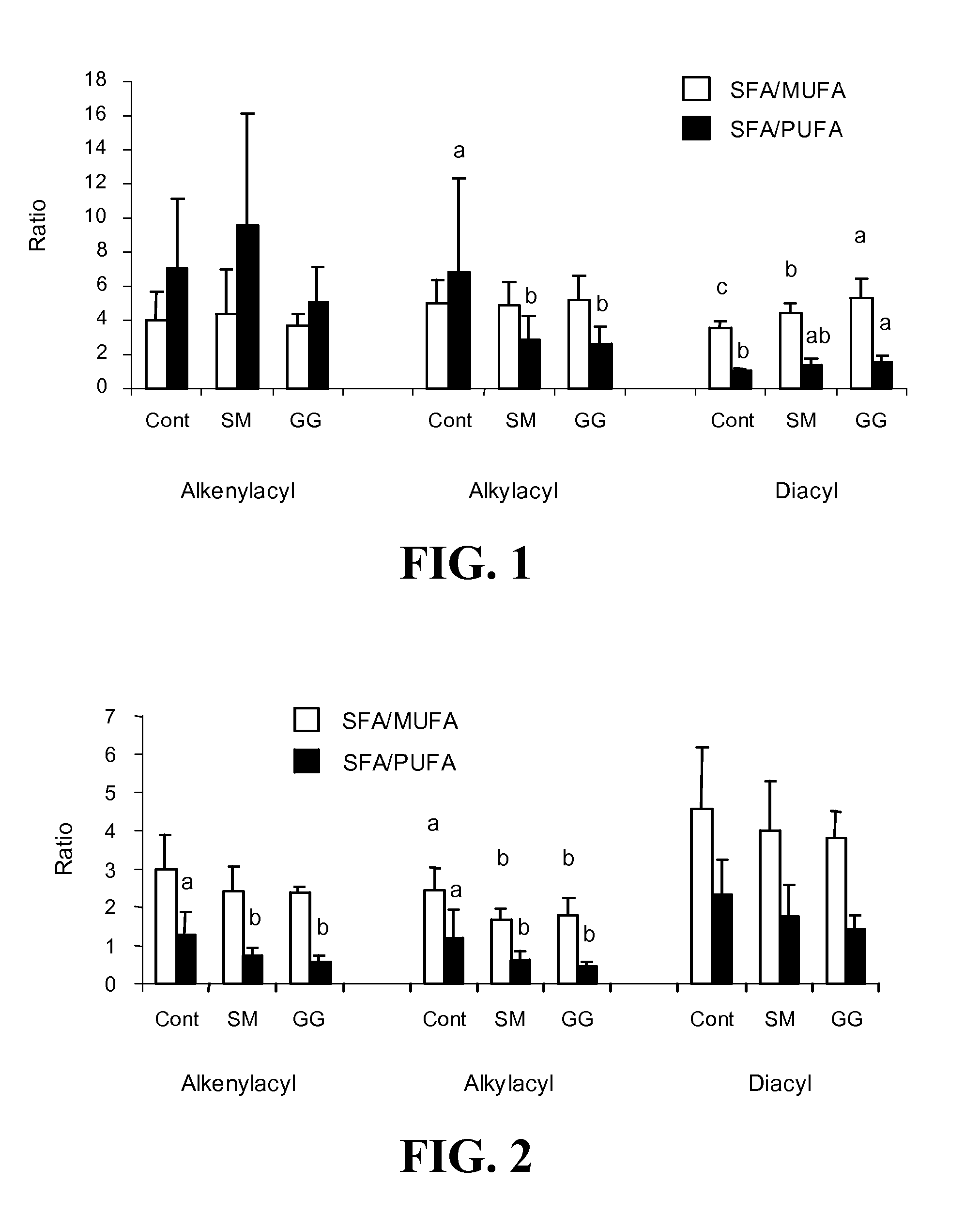
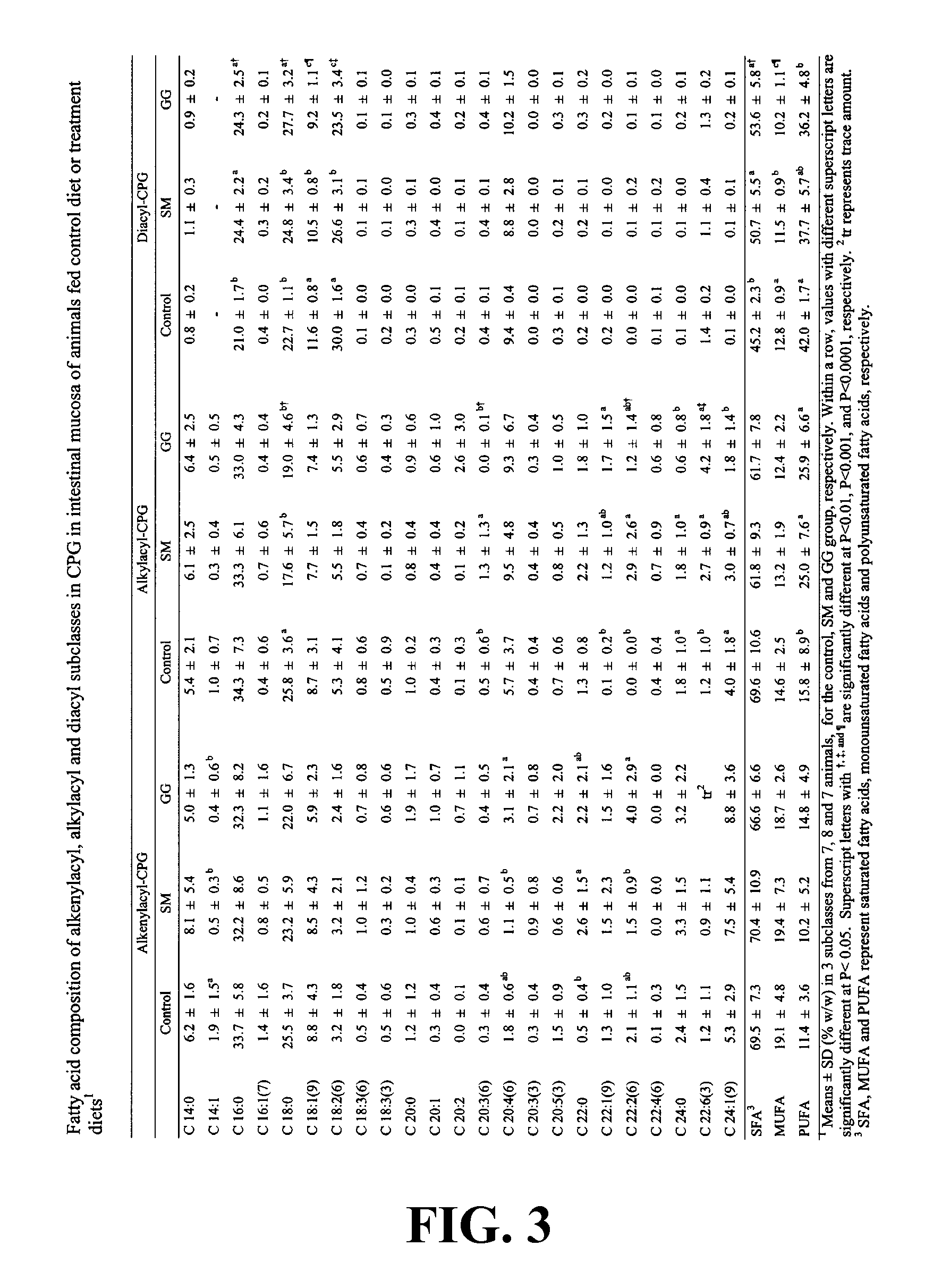
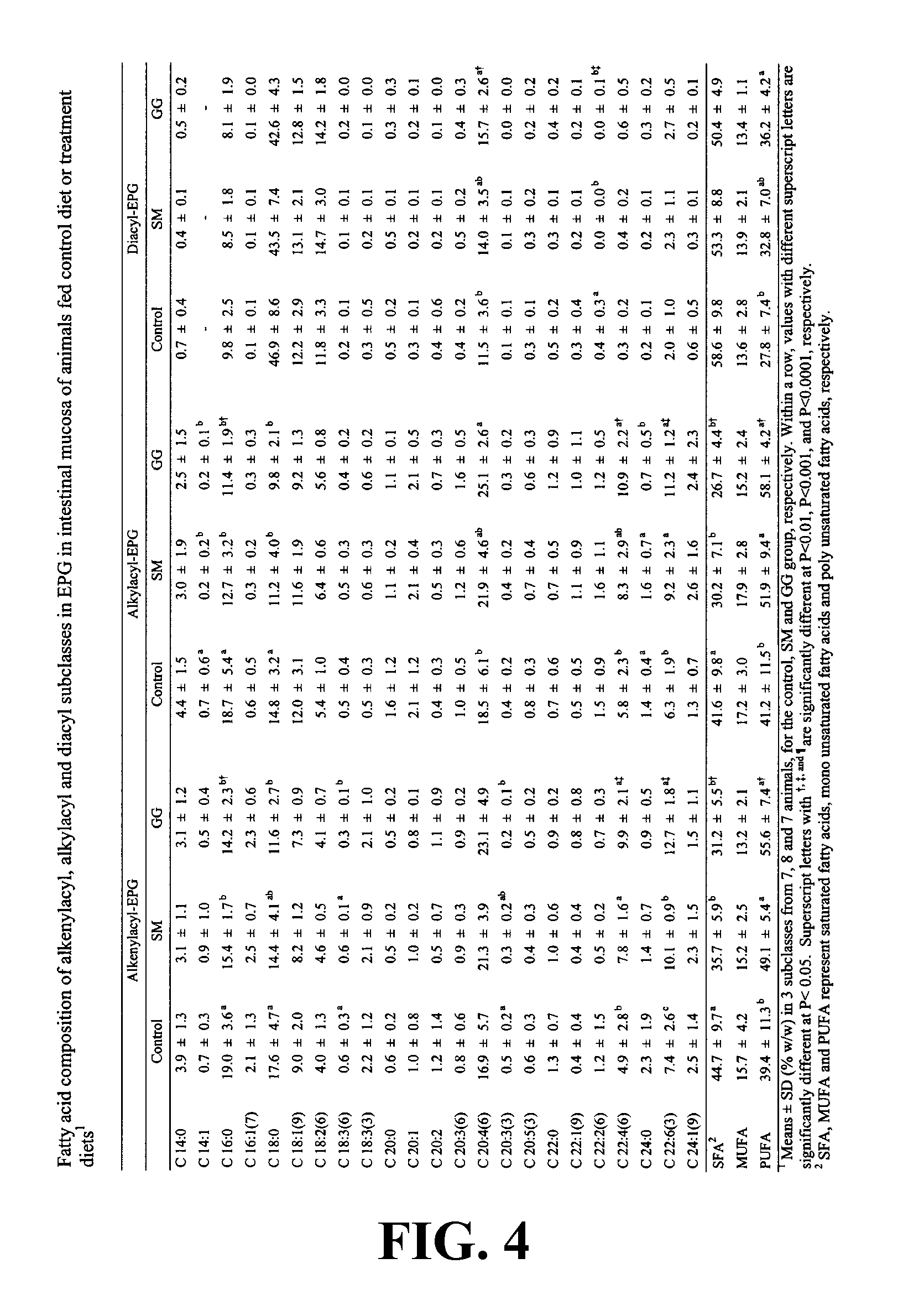
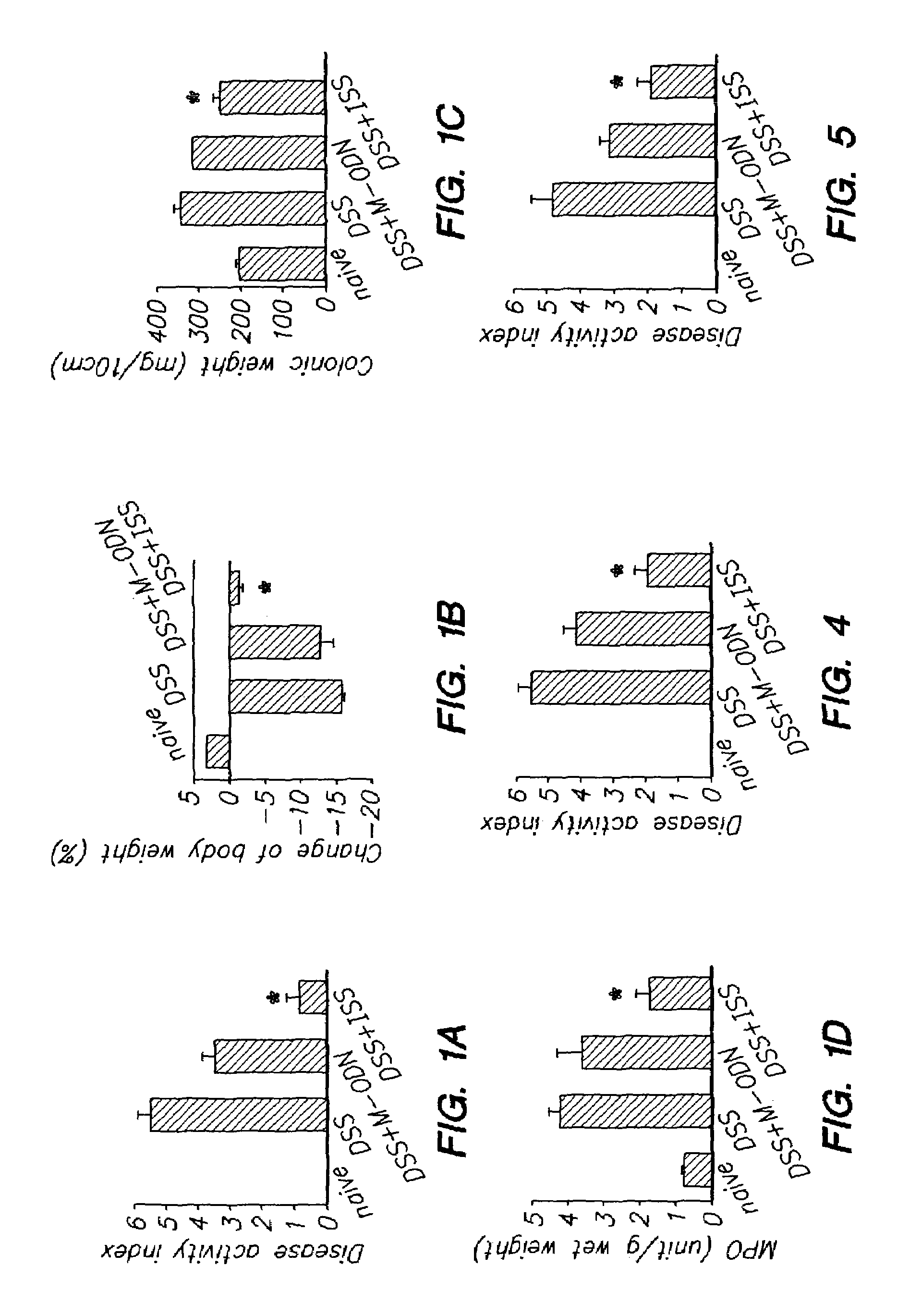
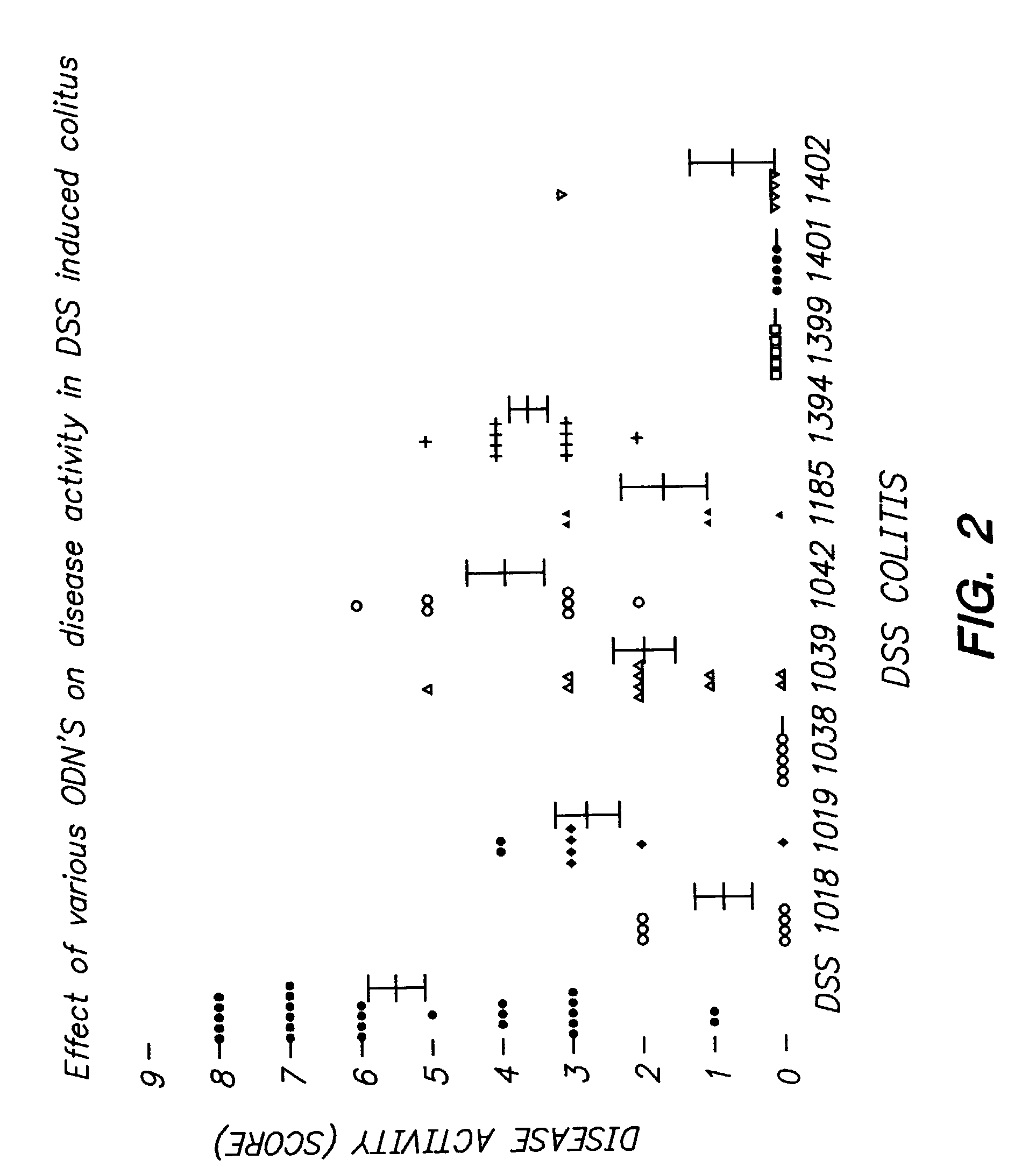
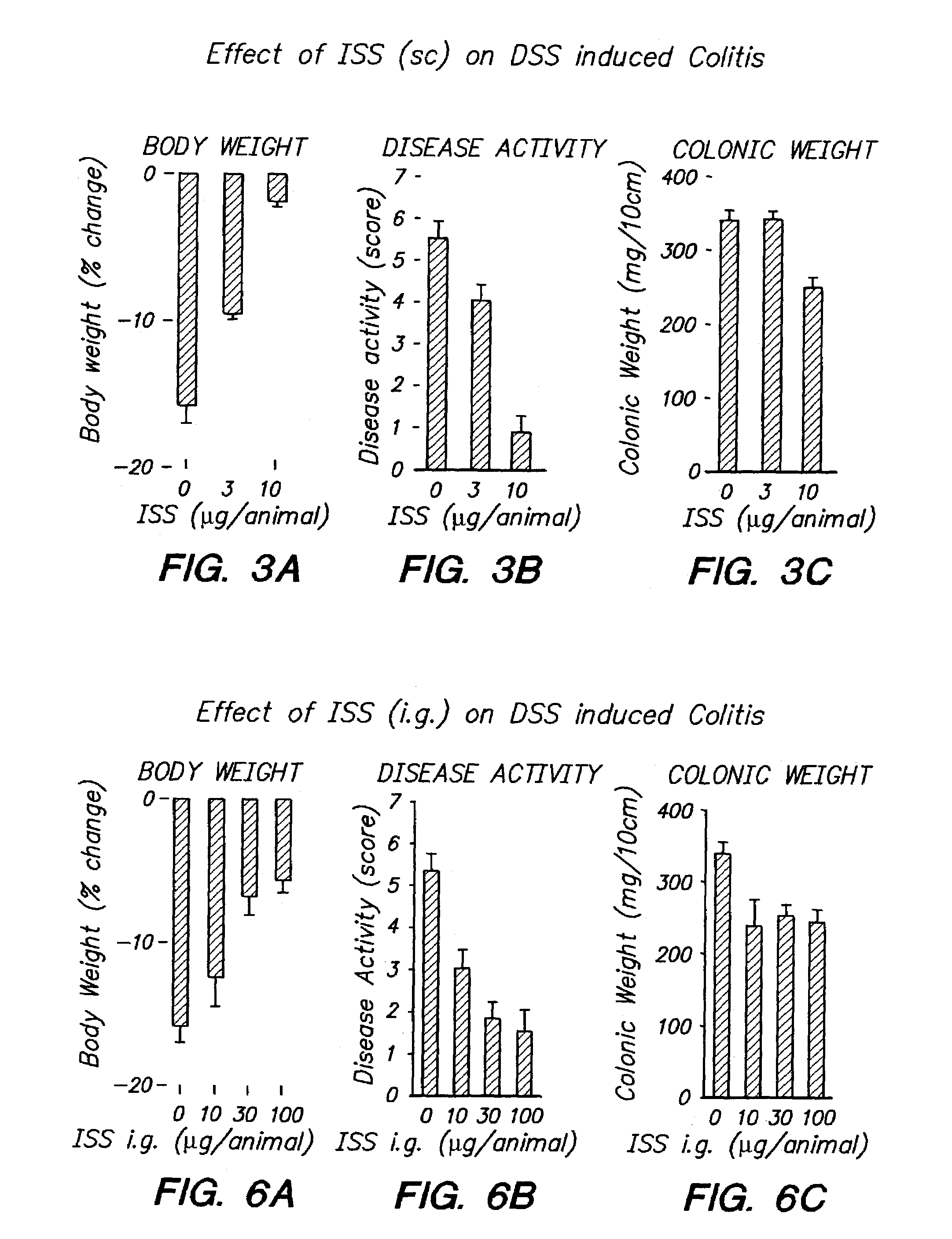
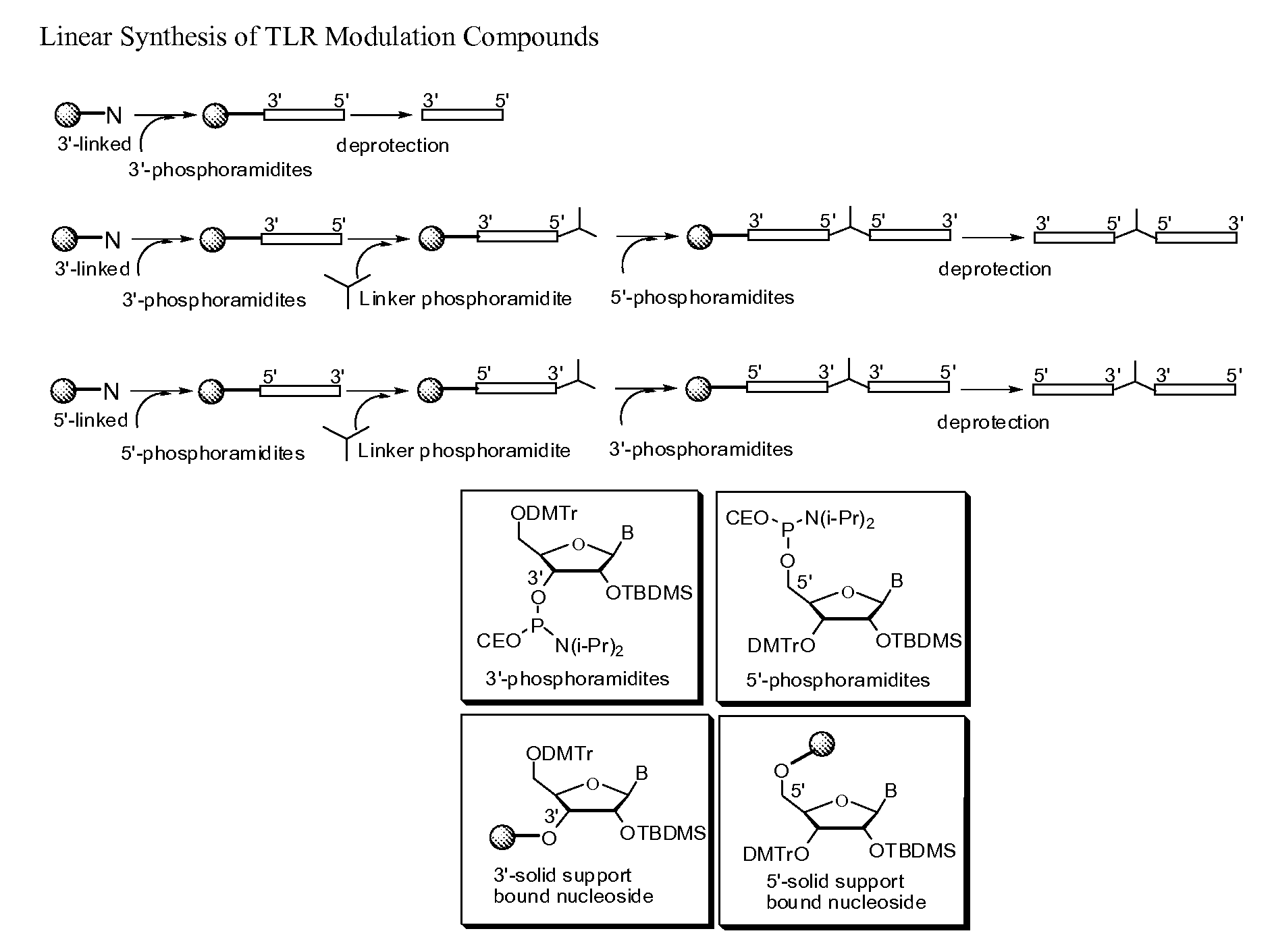
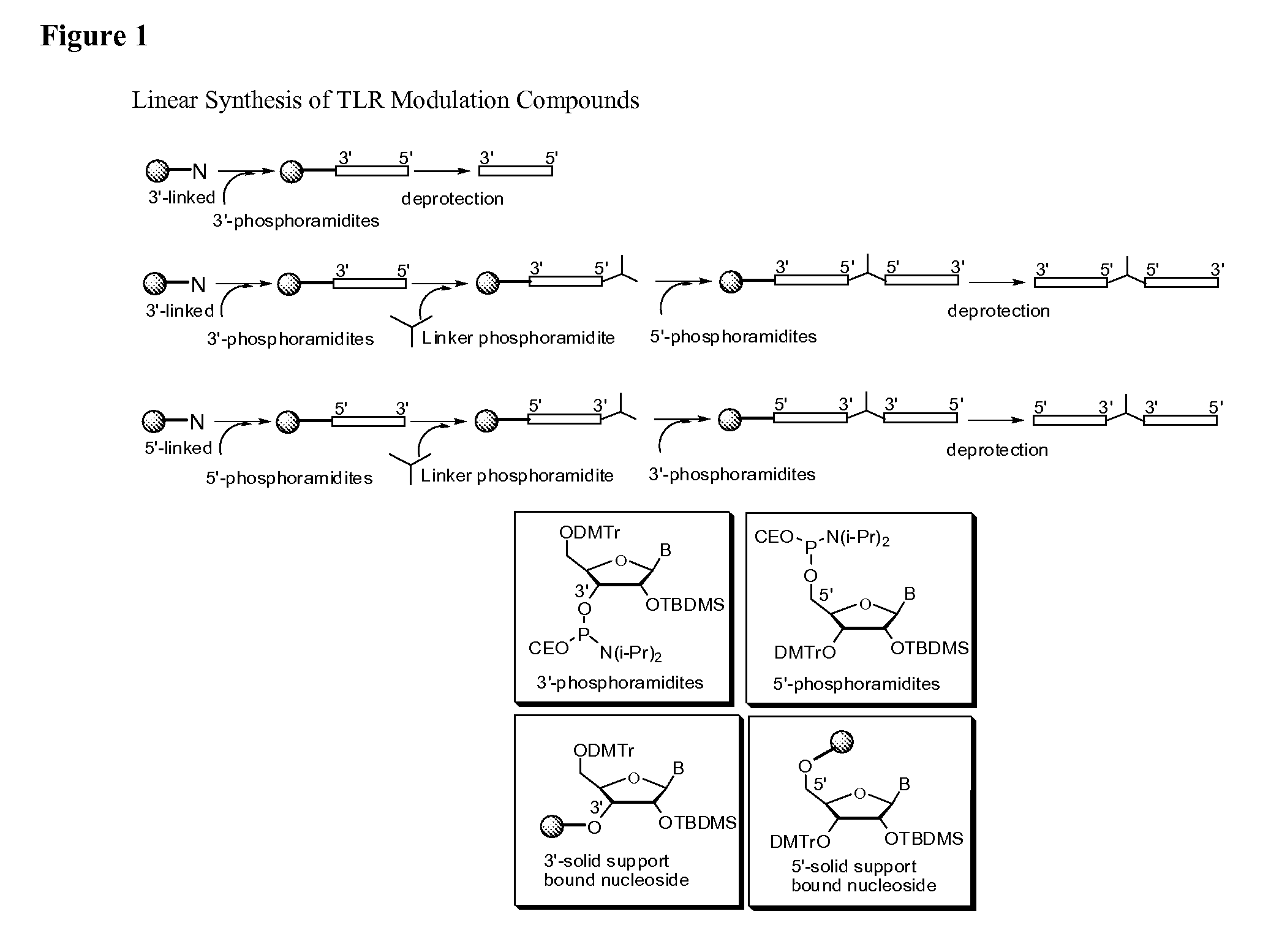
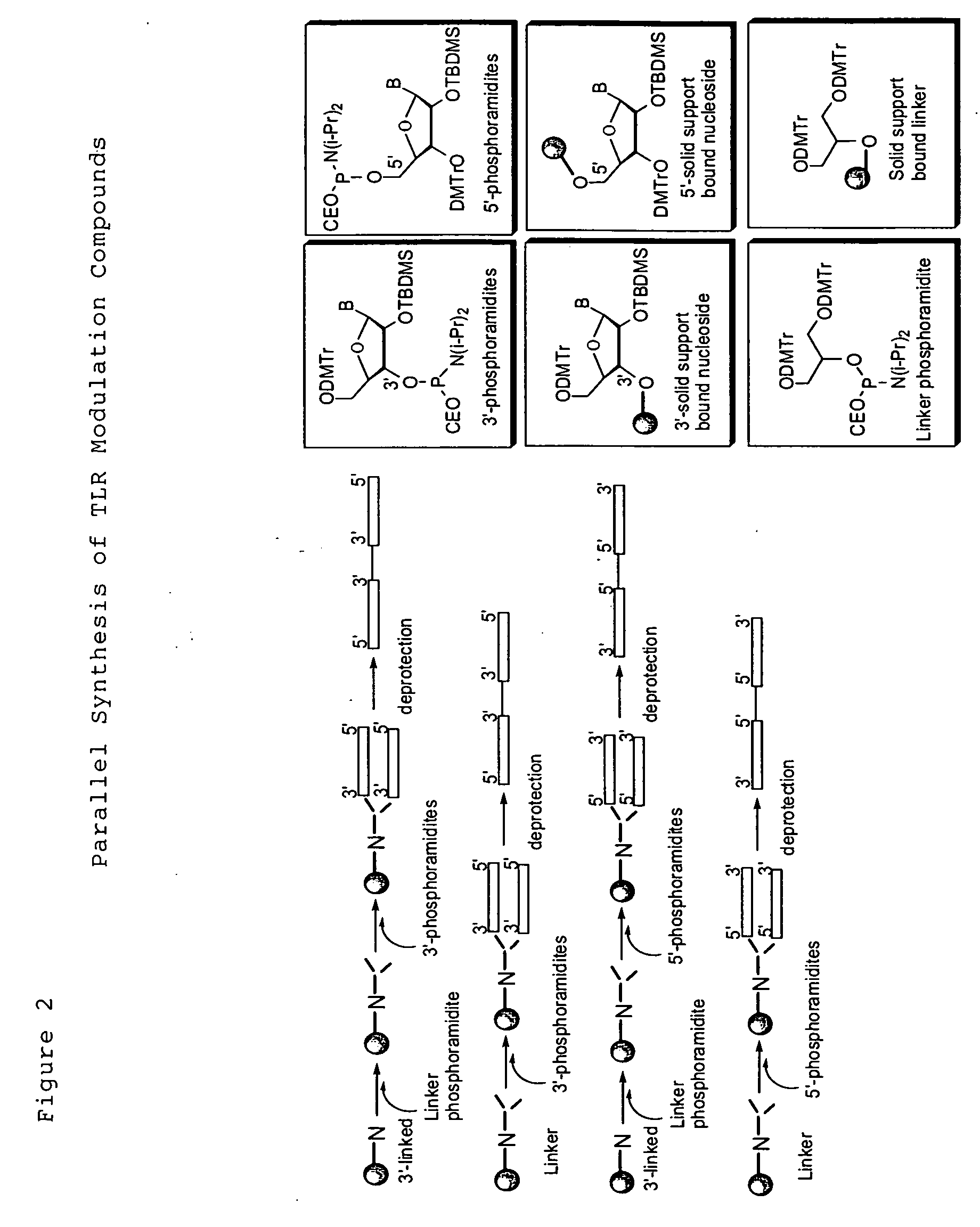
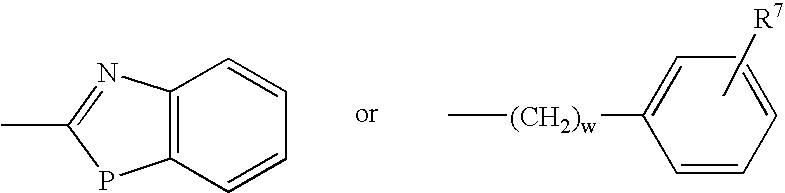Patents
Literature
215 results about "Inflammatory Bowel Diseases" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Inflammatory Bowel Diseases is a monthly peer-reviewed medical journal covering all aspects of inflammatory bowel disease. It was established in 1995 and is published by Lippincott Williams & Wilkins. It is the official journal of the Crohn's & Colitis Foundation. The editors-in-chief are Bret A. Lashner (Cleveland Clinic) and Fabio Cominelli (Case Western Reserve University). According to the Journal Citation Reports, the journal has a 2016 impact factor of 4.525, ranking it 15th out of 79 journals in the category "Gastroenterology & Hepatology".
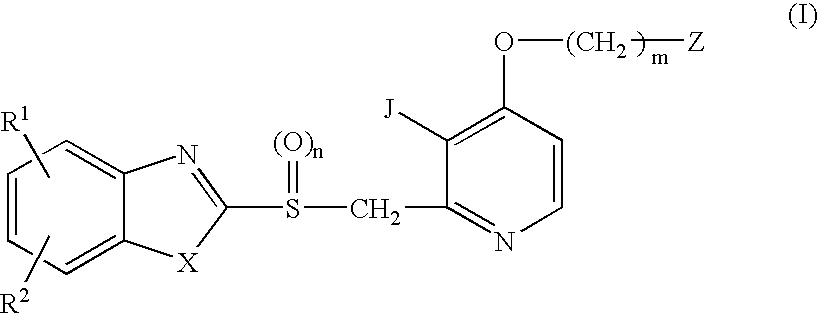
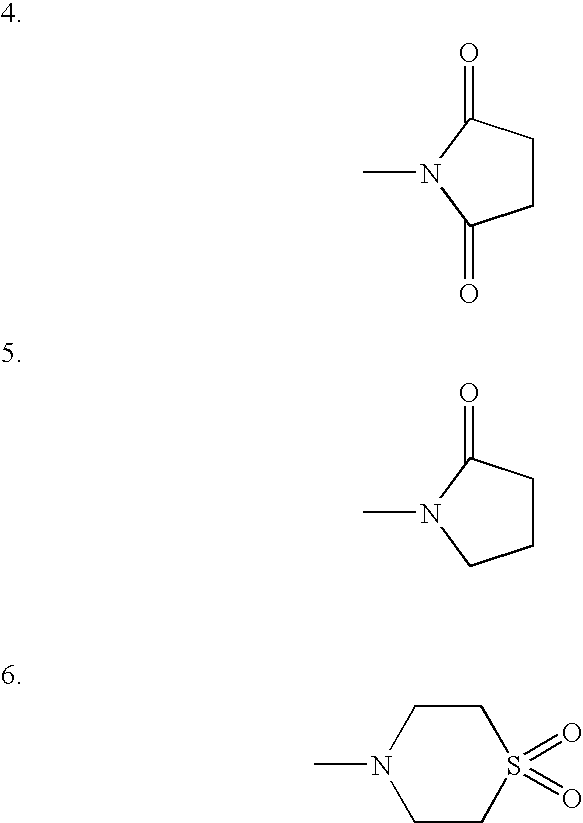
Organic Compounds
InactiveUS20090186022A1Reducing required dosagingReduce potential side effectsSugar derivativesImmunoglobulins against cytokines/lymphokines/interferonsInflammatory Bowel DiseasesAtopic dermatitis
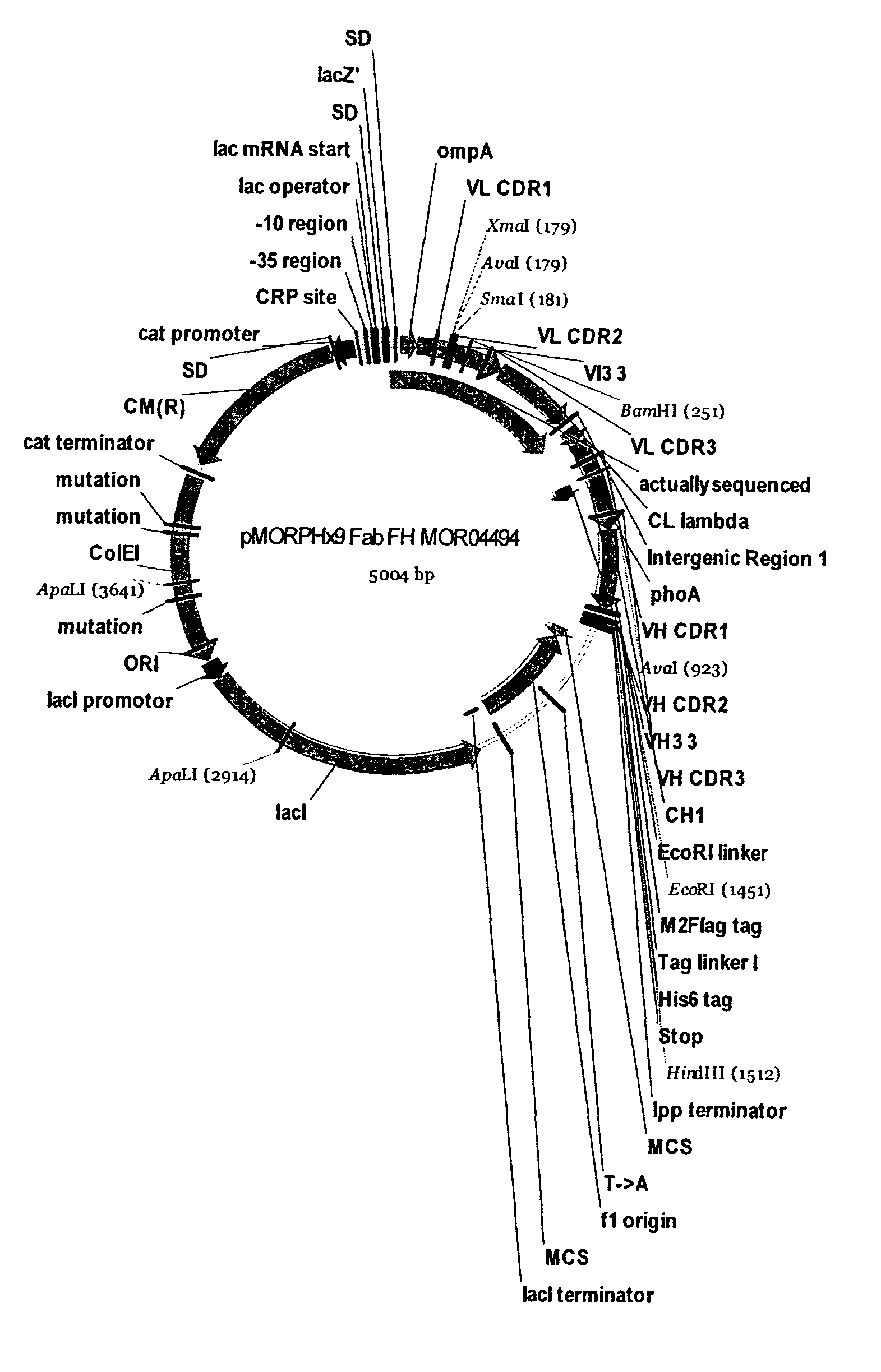
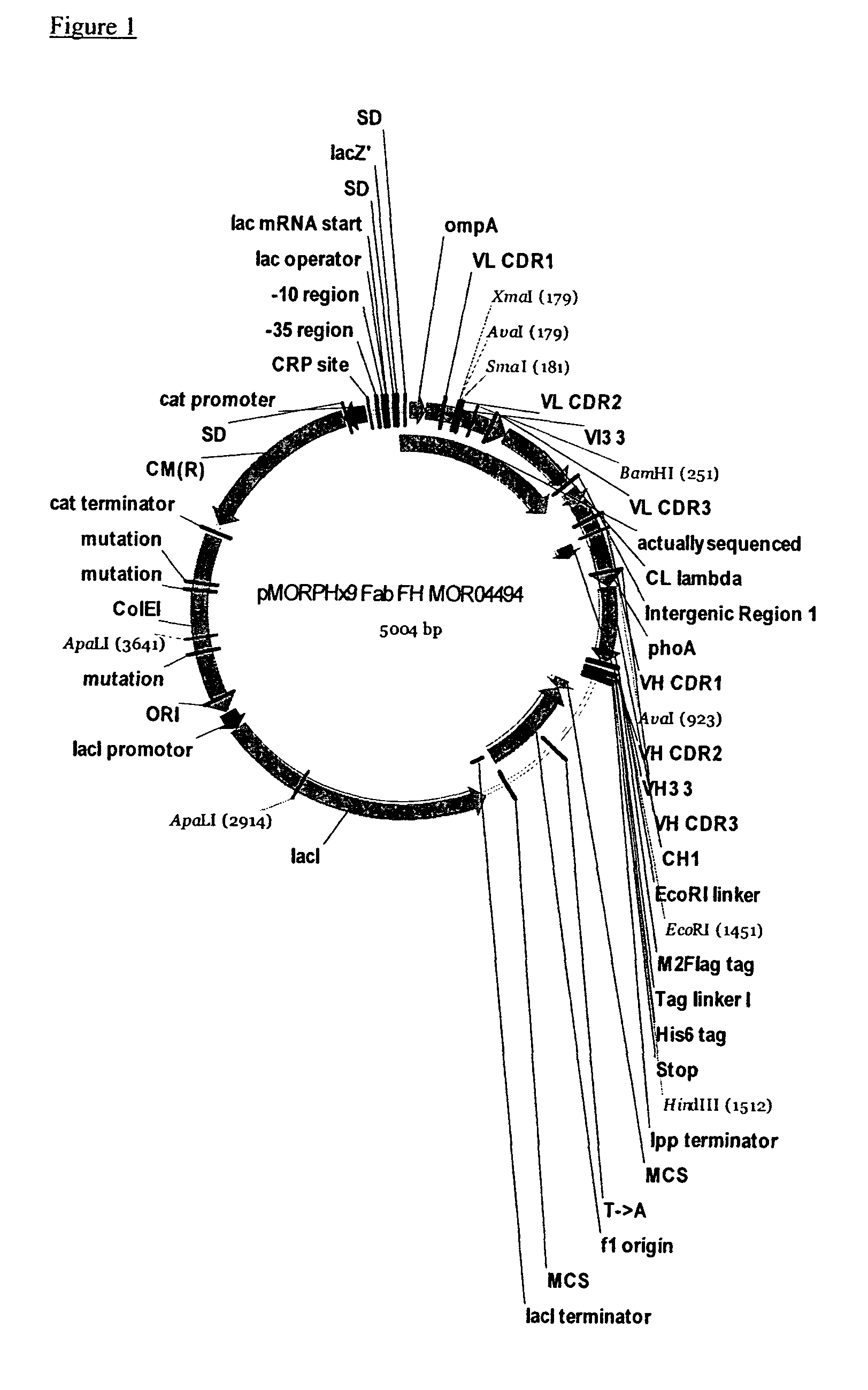
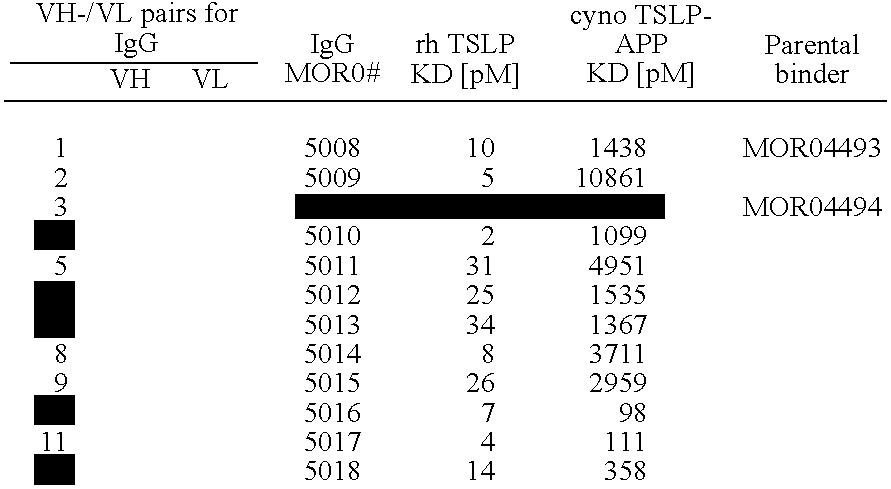
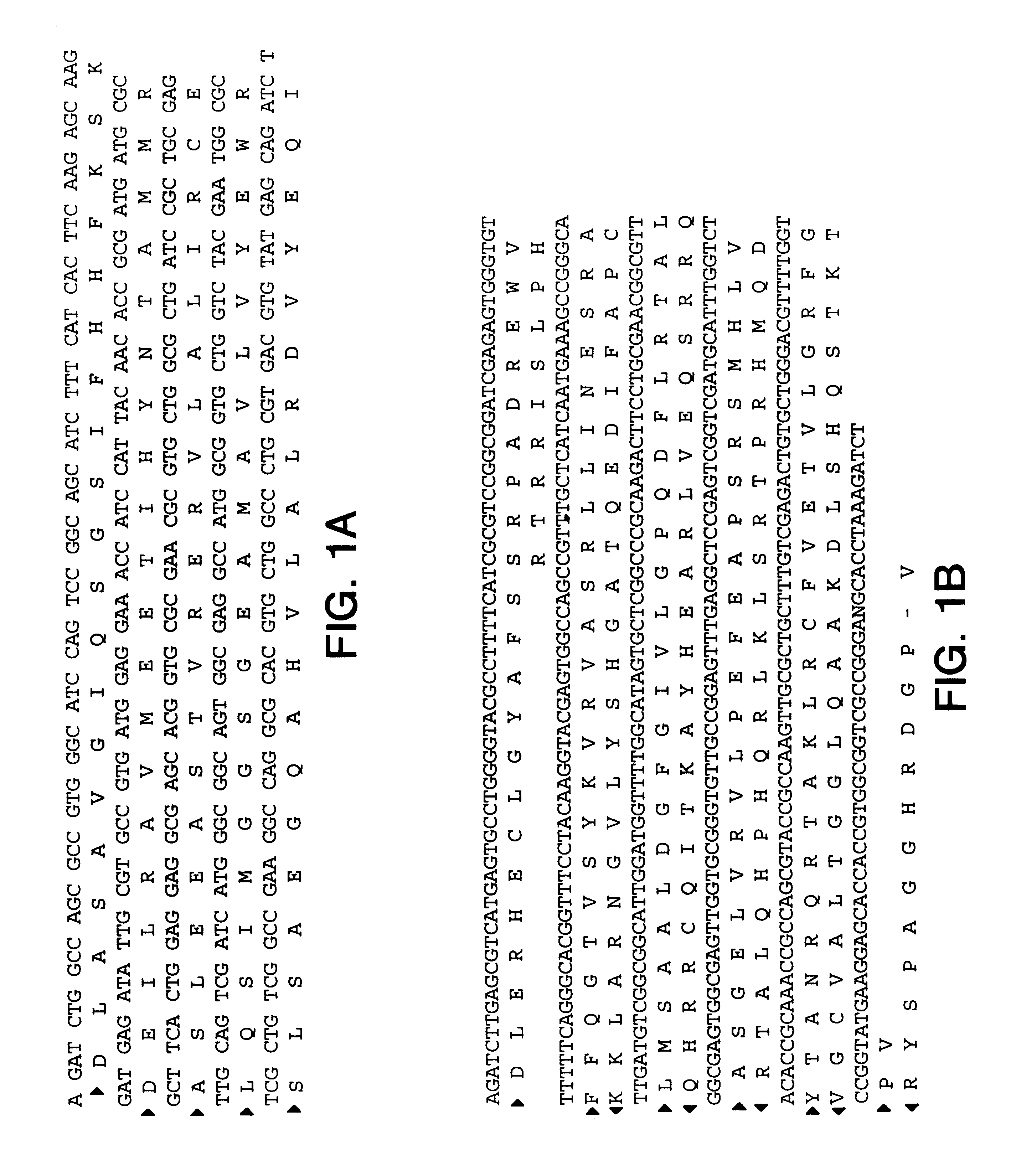
The present invention relates to human thymic stromal lymphopoietin (hTSLP) antibodies and especially those which neutralize hTSLP activity. It further relates to methods for using anti-hTSLP antibody molecules in diagnosis or treatment of hTSLP related disorders, such as asthma, atopic dermatitis, allergic rhinitis, fibrosis inflammatory bowel disease, and Hodgkin's lymphoma.
Owner:NOVARTIS AG
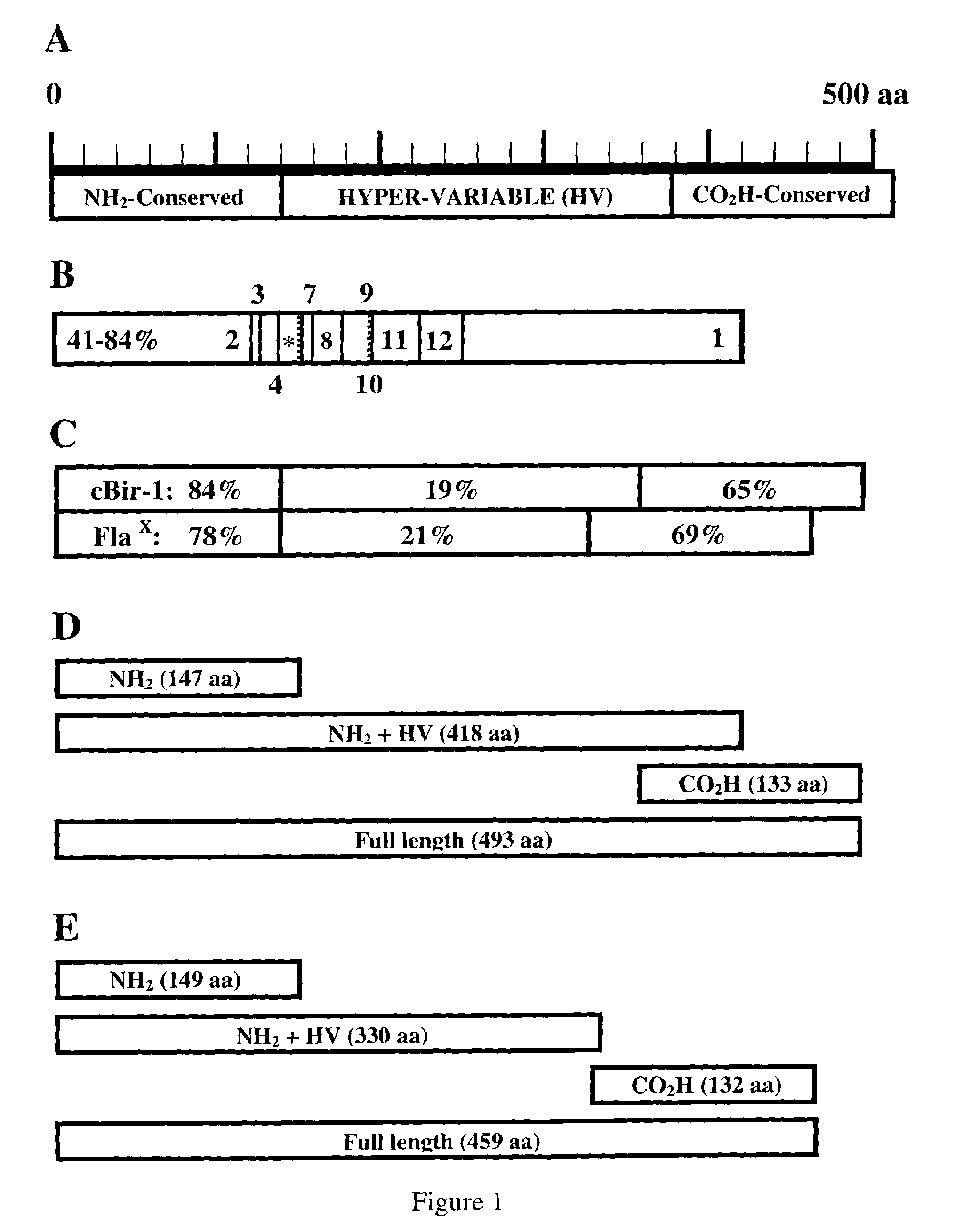
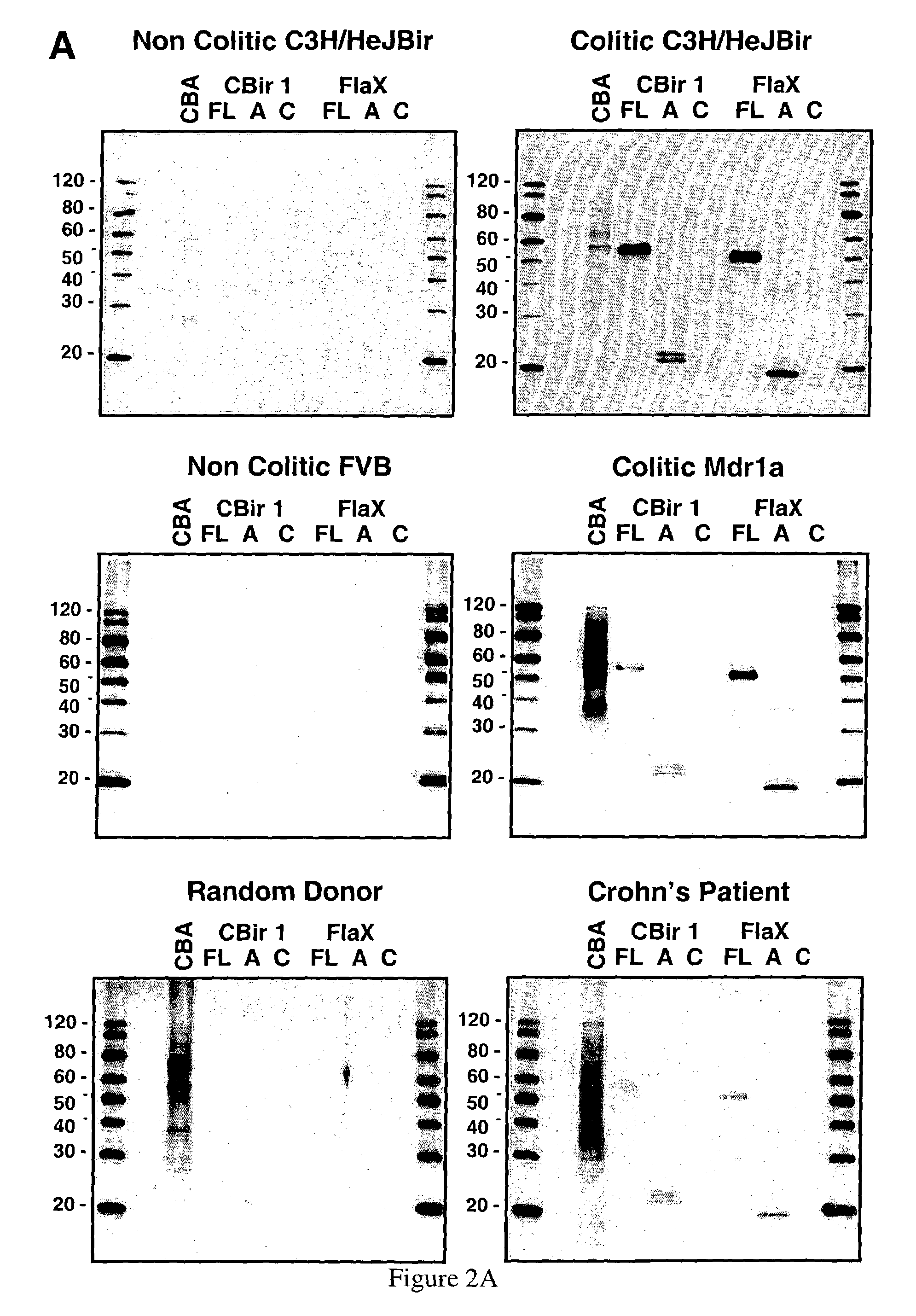
IBD-associated microbial antigens and methods of using same
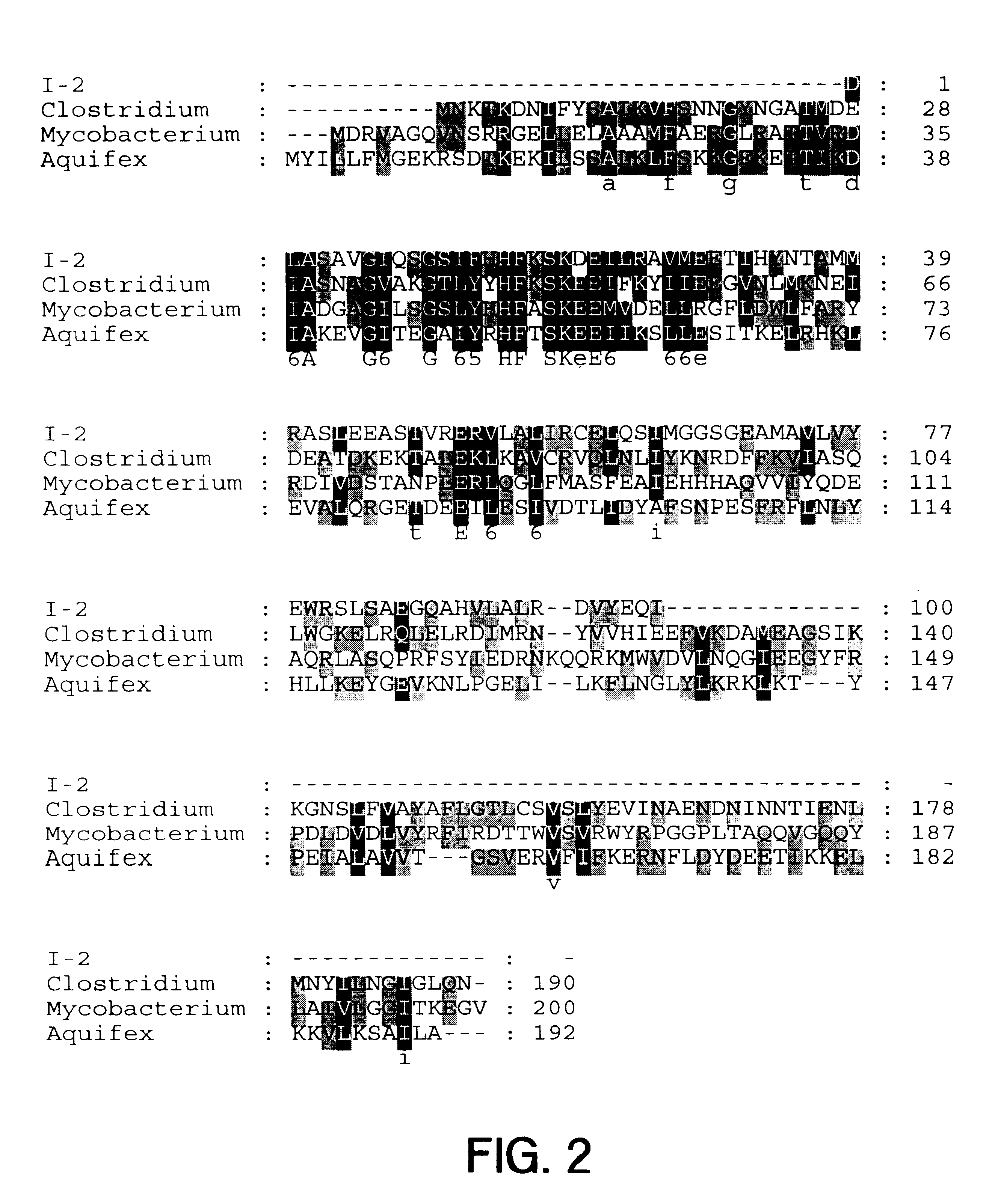
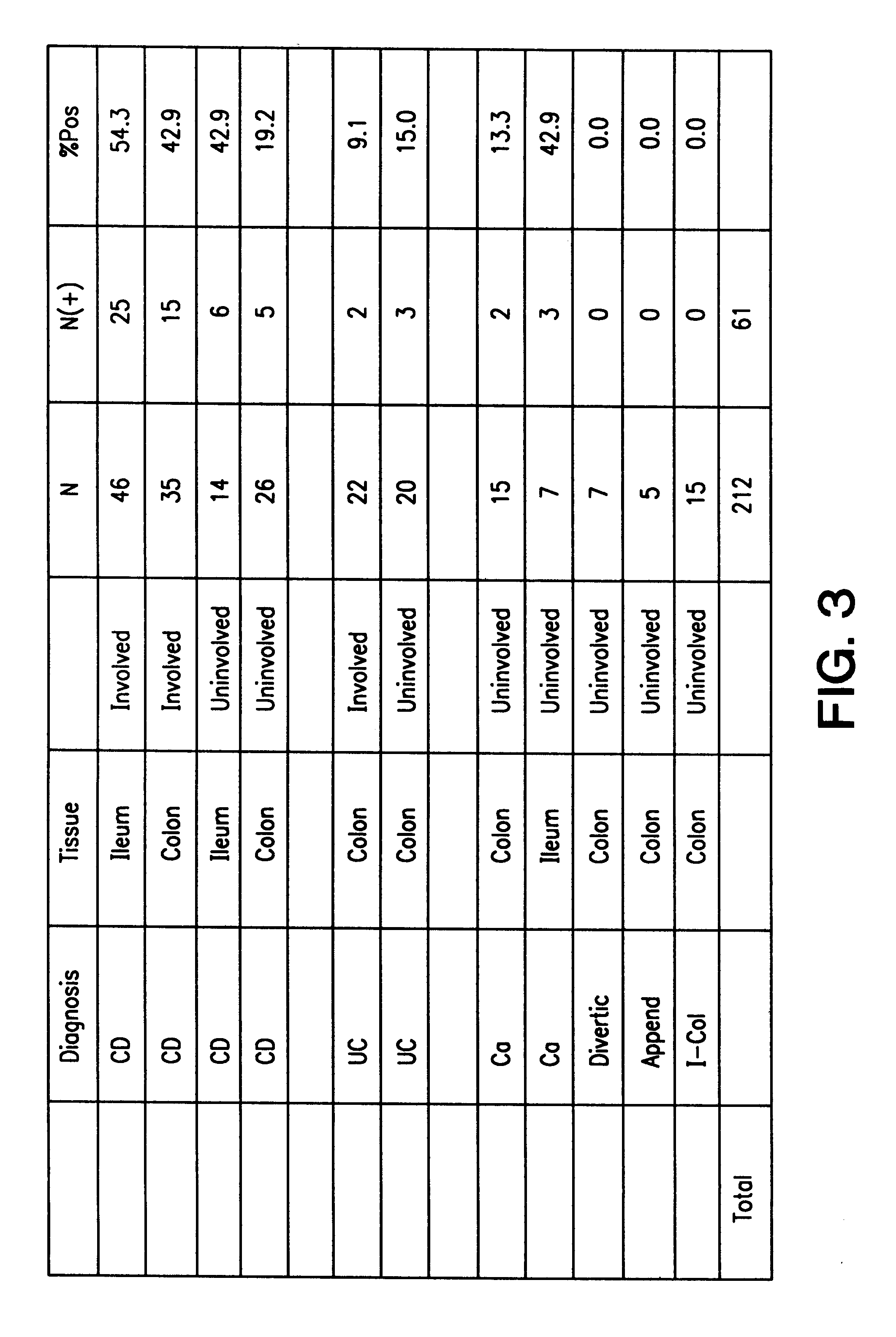
The present invention provides nucleic acid and amino acid sequence of the novel I-1 and I-2 polypeptides, which are associated with human inflammatory bowel disease (IBD). Methods of diagnosing and treating inflammatory bowel disease using the IBD-associated I-1 and I-2 antigens also are provided.
Owner:RGT UNIV OF CALIFORNIA
Compounds as rearranged during transfection (RET) inhibitors
This invention relates to novel compounds which are inhibitors of the Rearranged during Transfection (RET) kinase, to pharmaceutical compositions containing them, to processes for their preparation, and to their use in therapy, alone or in combination, for the normalization of gastrointestinal sensitivity, motility and / or secretion and / or abdominal disorders or diseases and / or treatment related to diseases related to RET dysfunction or where modulation of RET activity may have therapeutic benefit including but not limited to all classifications of irritable bowel syndrome (IBS) including diarrhea-predominant, constipation-predominant or alternating stool pattern, functional bloating, functional constipation, functional diarrhea, unspecified functional bowel disorder, functional abdominal pain syndrome, chronic idiopathic constipation, functional esophageal disorders, functional gastroduodenal disorders, functional anorectal pain, inflammatory bowel disease, proliferative diseases such as non-small cell lung cancer, hepatocellular carcinoma, colorectal cancer, medullary thyroid cancer, follicular thyroid cancer, anaplastic thyroid cancer, papillary thyroid cancer, brain tumors, peritoneal cavity cancer, solid tumors, other lung cancer, head and neck cancer, gliomas, neuroblastomas, Von Hippel-Lindau Syndrome and kidney tumors, breast cancer, fallopian tube cancer, ovarian cancer, transitional cell cancer, prostate cancer, cancer of the esophagus and gastroesophageal junction, biliary cancer, adenocarcinoma, and any malignancy with increased RET kinase activity.
Owner:GLAXOSMITHKLINE INTPROP DEV LTD
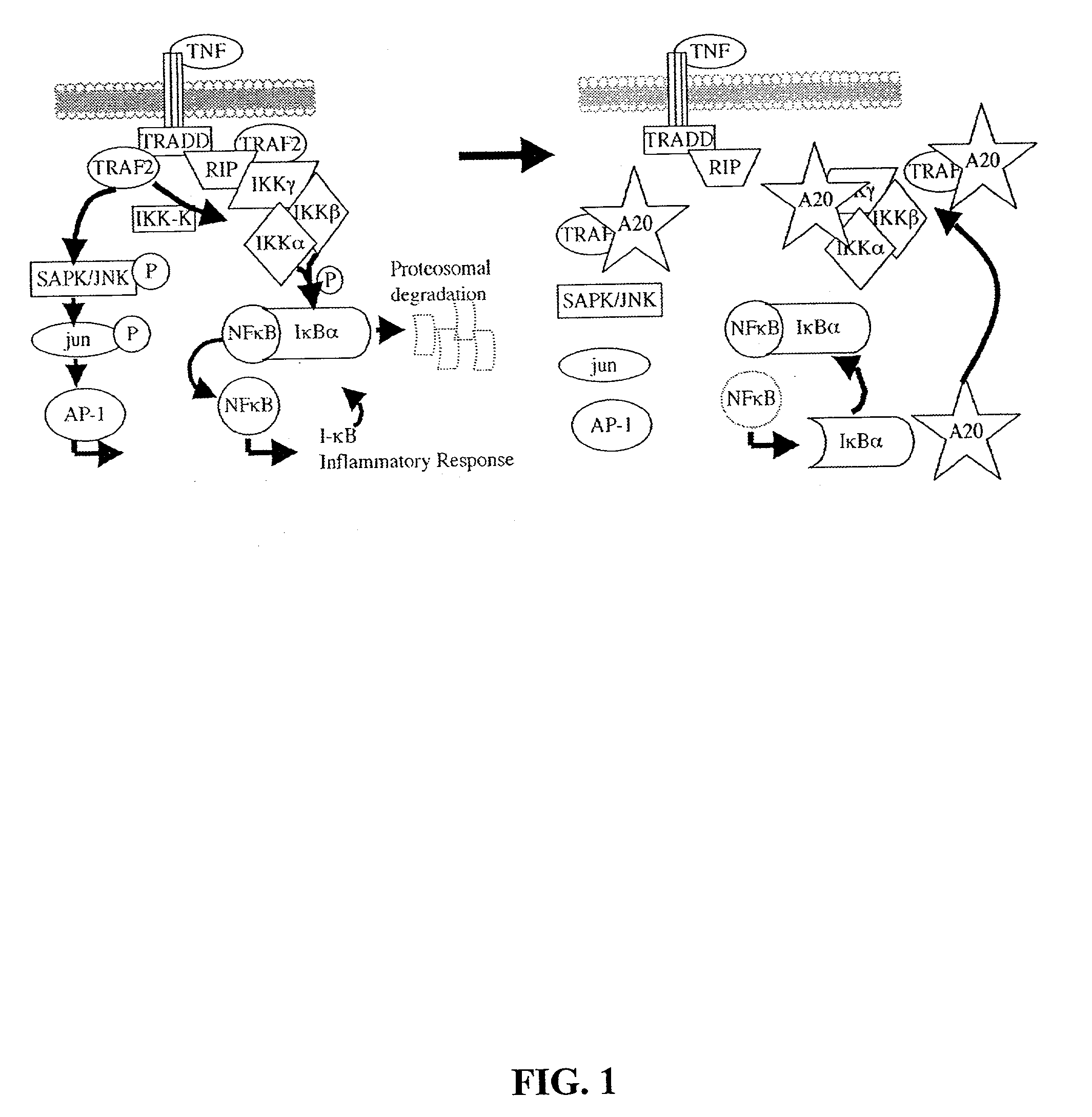
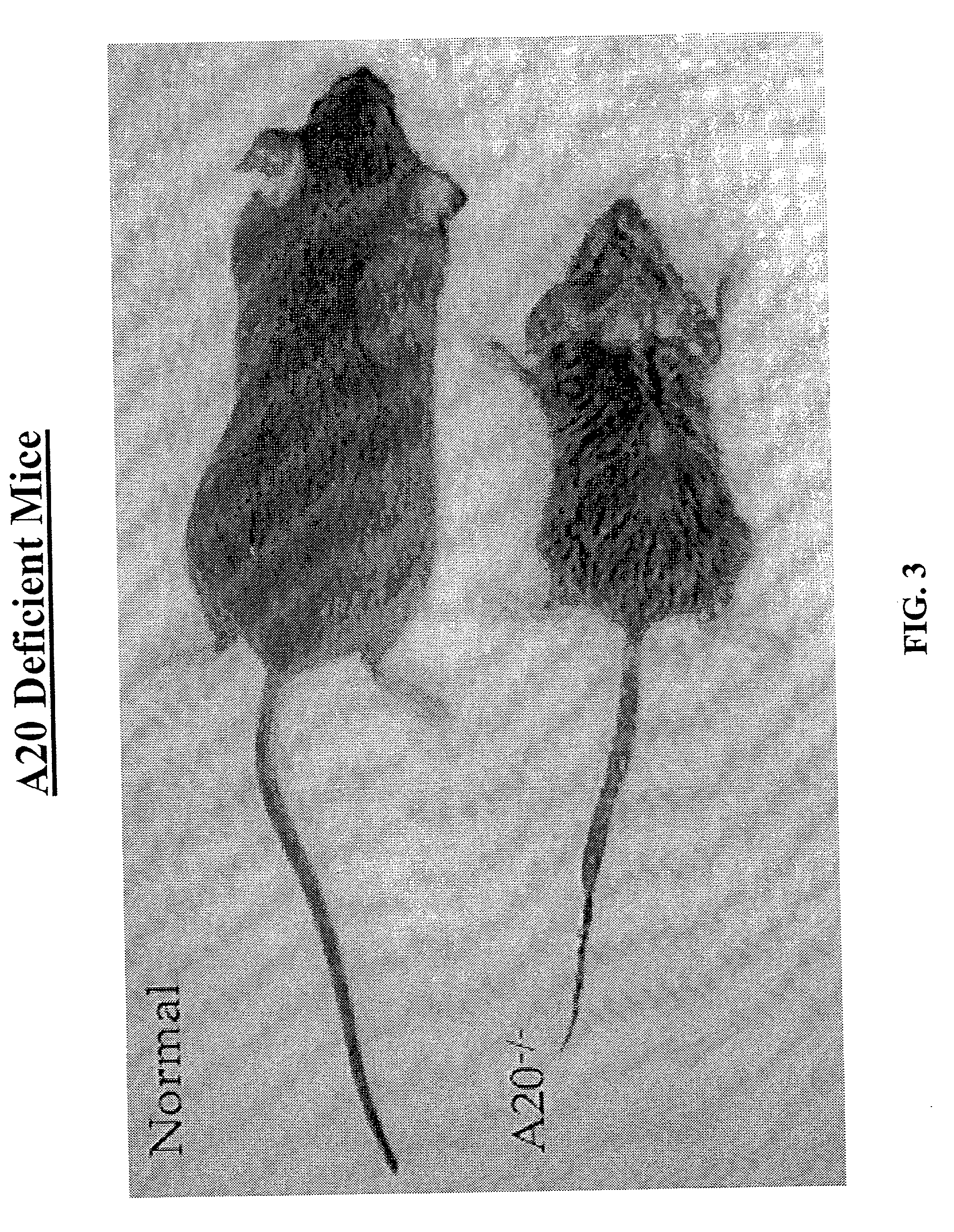
Methods and compositions relating to modulation of A20
The invention provides compositions and methods for treating diseases characterized by aberrant programmed cell death and / or inflammation, comprising mediating A20 function in the subject. Such diseases include Crohn's disease, inflammatory bowel disease, a disease associated with ischemic injury, a toxin-induced liver disease and cancer. The invention further provides methods and compositions for assays for modulators of A20.
Owner:UNIVERSITY OF CHICAGO
Immunostimulatory nucleic acid for treatment of non-allergic inflammatory diseases
InactiveUS20050250726A1Reduces and prevents non-allergic inflammationOrganic active ingredientsGenetic material ingredientsInflammatory Bowel DiseasesNon allergic
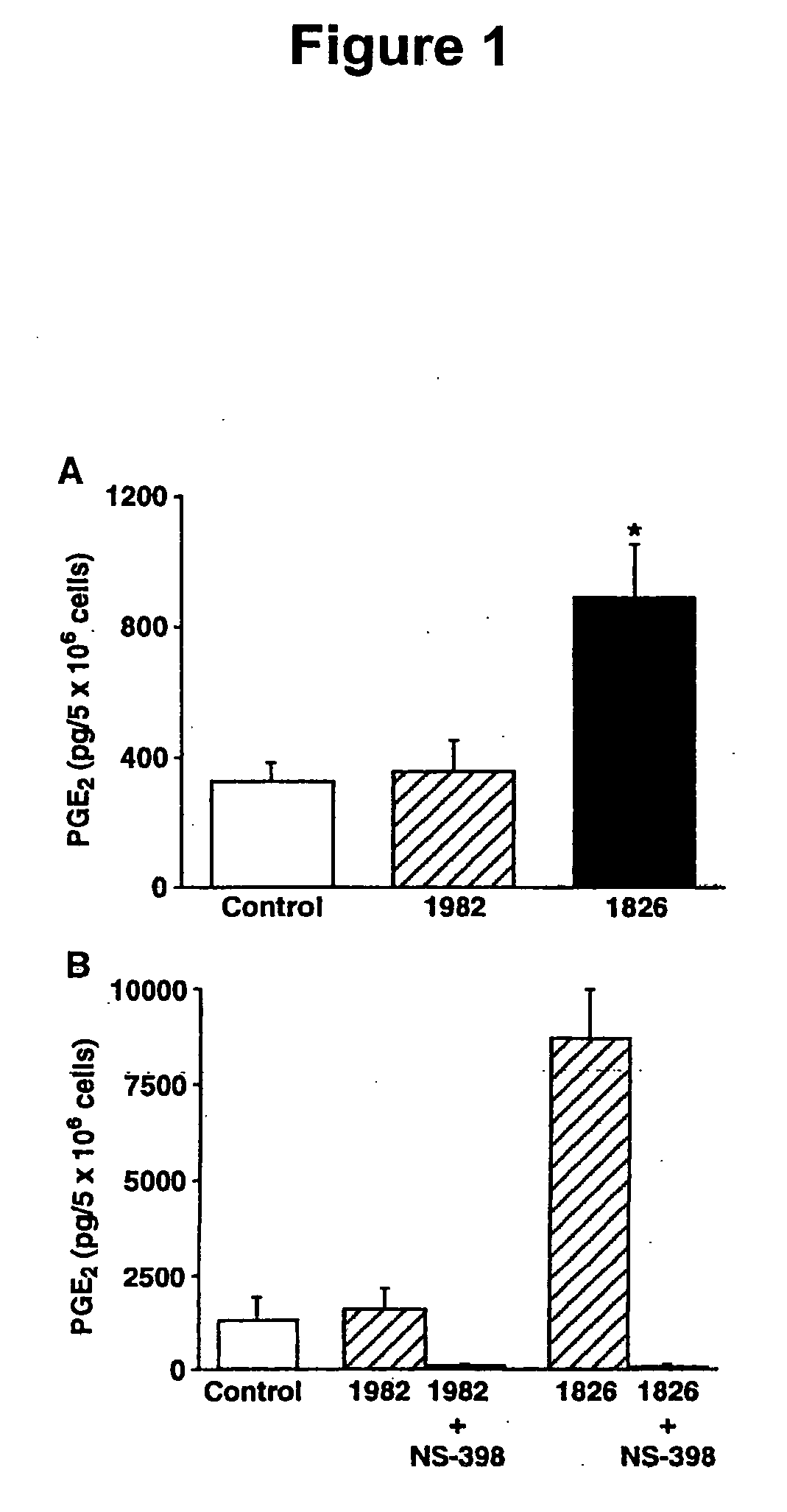
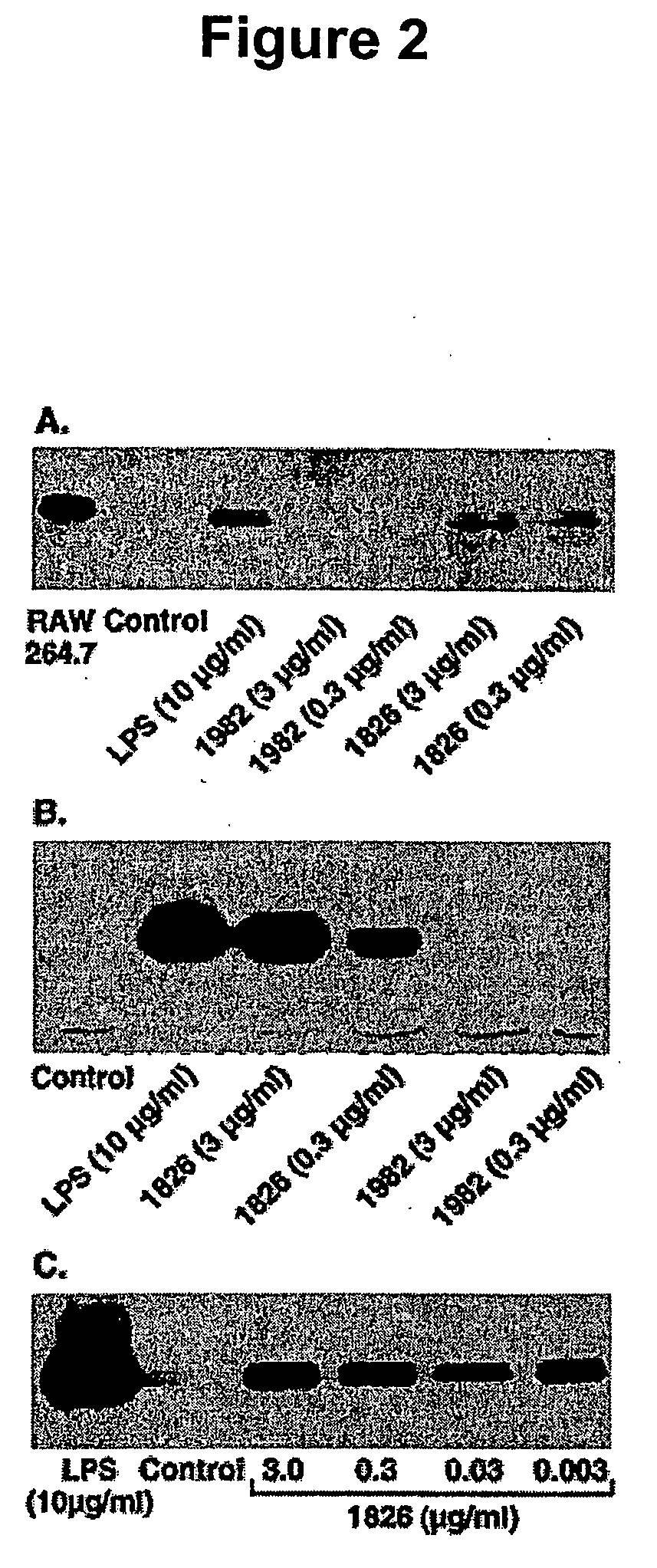
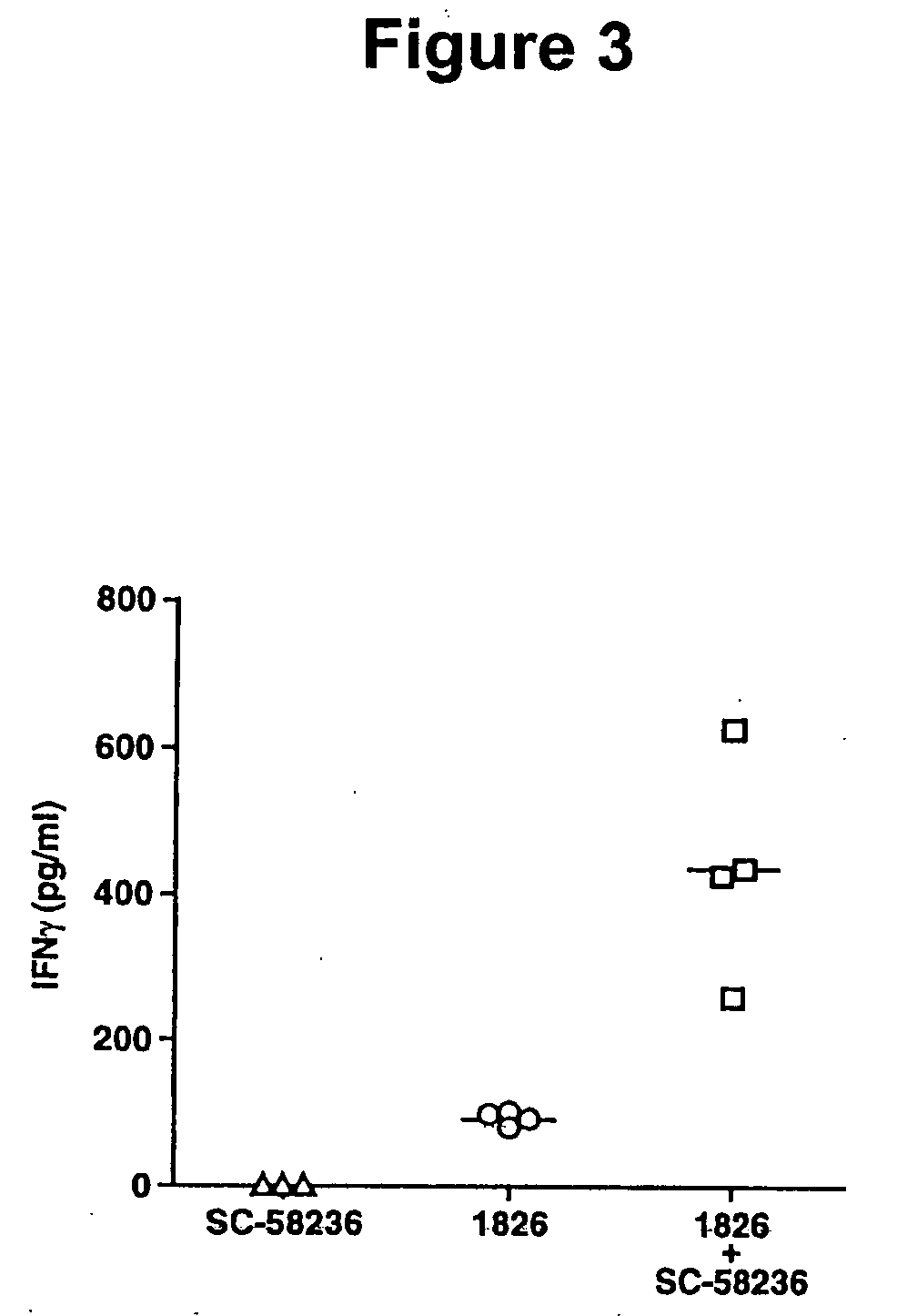
The invention provides methods and compositions for using immunostimulatory nucleic acids to treat non-allergic inflammatory diseases. Non-allergic inflammatory diseases that may be treated according to the methods and products of the invention include psoriasis and inflammatory bowel disease. The invention further provides methods for augmenting a Th1 response to immunostimulatory nucleic acid involving inhibition of prostaglandin-mediated counter-regulatory response.
Owner:UNIV OF IOWA RES FOUND
Method and compositions for the treatment of gastrointestinal disorders
ActiveUS20060094658A1Reduce accumulationIncrease gastrointestinal motilityPeptide/protein ingredientsMetabolism disorderGastroparesisInflammatory Bowel Diseases
Compositions and related methods for treating IBS and other gastrointestinal disorders and conditions (e.g., gastrointestinal motility disorders, functional gastrointestinal disorders, gastroesophageal reflux disease (GERD), duodenogastric reflux, Crohn's disease, ulcerative colitis, inflammatory bowel disease, functional heartburn, dyspepsia (including functional dyspepsia or nonulcer dyspepsia), gastroparesis, chronic intestinal pseudo-obstruction (or colonic pseudoobstruction), and disorders and conditions associated with constipation, e.g., constipation associated with use of opiate pain killers, post-surgical constipation, and constipation associated with neuropathic disorders as well as other conditions and disorders are described. The compositions feature peptides that activate the guanylate cyclase C (GC-C) receptor.
Owner:IRONWOOD PHARMA
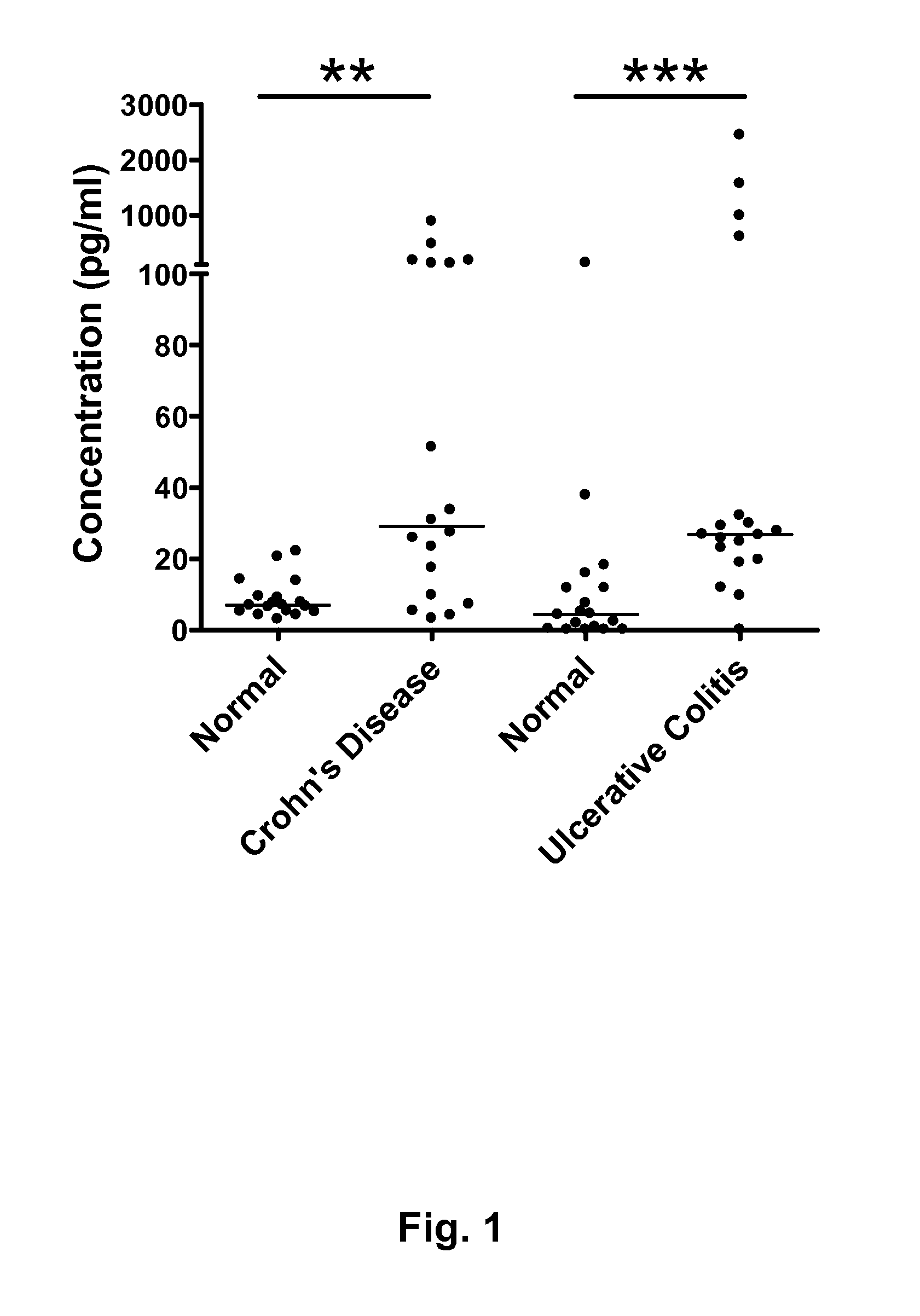
Inflammatory bowel disease biomarkers and related methods of treatment
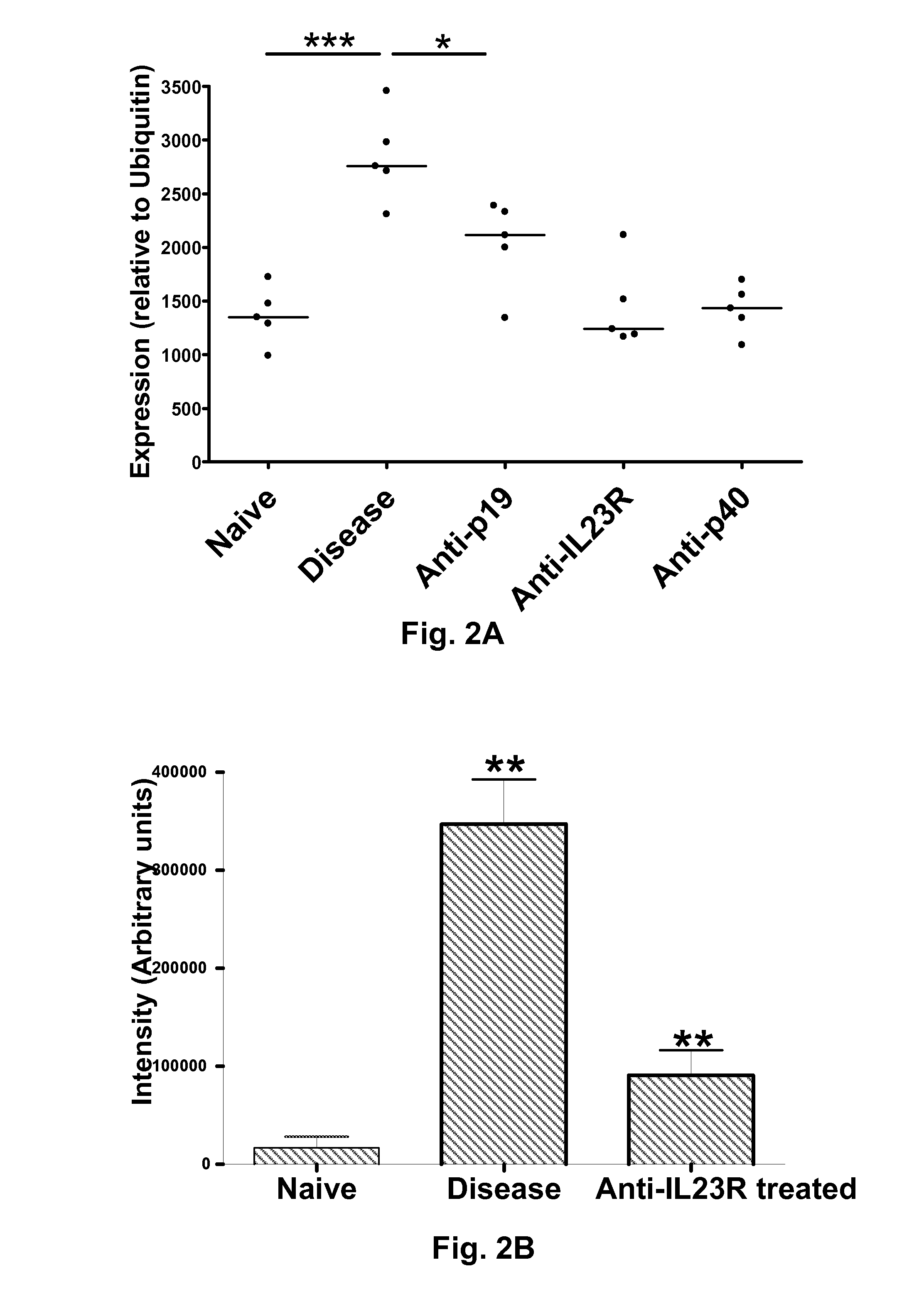
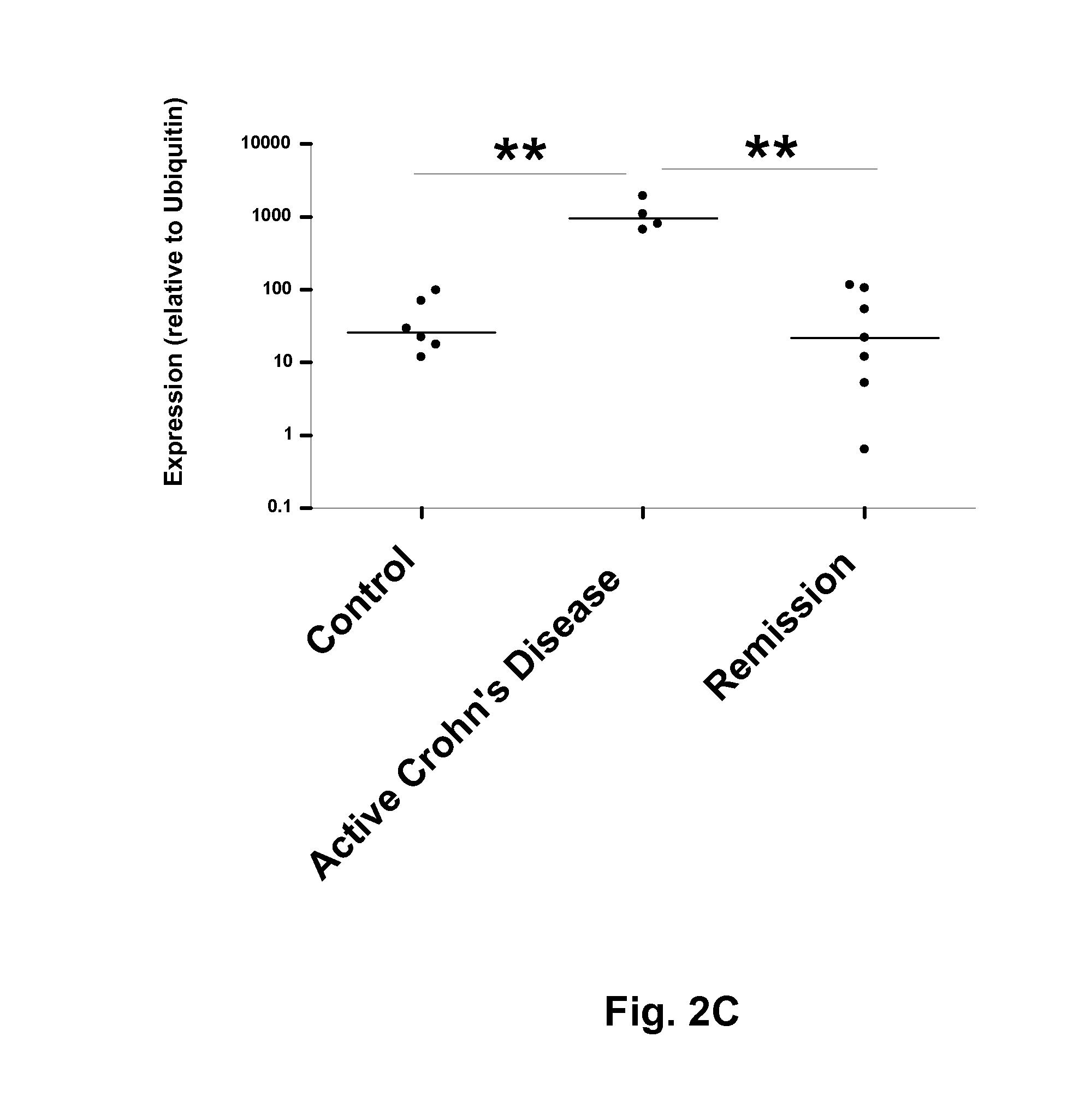
Biomarkers associated with inflammatory bowel disease (IBD) are provided, as well as methods of using such biomarkers for diagnosing, assessing and monitoring disease progression. The biomarkers may be measured at the protein level or the gene expression level Biomarkers may be tracked individually or in groups of two or more. The disclosed biomarkers may find particular utility in monitoring a course of therapy, such as treatment with an IL-23 antagonist. Changes in biomarker levels can also be used to confirm target engagement and therapeutic efficacy. Changes in biomarkers can also be used inform modification of a therapeutic regimen, for example to increase or decrease dosing of a therapeutic agent, such as an anti-IL-23 or anti-IL-23R anti-body.
Owner:MERCK SHARP & DOHME CORP
Compositions and methods for the therapy and diagnosis of inflammatory bowel disease
Compositions and methods for the therapy and diagnosis of Inflammatory Bowel Disease (IBD), including Crohn's Disease and Ulcerative Colitis, are disclosed. Illustrative compositions comprise one or more bacterial polypeptides, immunogenic portions thereof, polynucleotides that encode such polypeptides, antigen presenting cell that expresses such polypeptides, and T cells that are specific for cells expressing such polypeptides. The disclosed compositions are useful, for example, in the diagnosis, prevention and / or treatment of IBD.
Owner:CORIXA CORP
Method of treating mast cell activation-induced diseases with a proteoglycan
InactiveUS6689748B1Decrease in urinaryBiocidePeptide/protein ingredientsInflammatory Bowel DiseasesInterstitial cystitis
The invention provides a method for preventing and treating the harmful biological effects of biochemicals secreted from activated mast cells in the organism of warm blooded animals and more especially human beings, said effects being associated with allergy (including but not limited to allergic conjunctivitis, allergic rhinitis, allergic otitis, asthma, allergic uticaria, food allergy and atopic dermatitis), hyperproliferative diseases such as leukemia and systemic mastocytosis, interstitial cystitis, inflammatory bowel disease, irritable bowel syndrome, osteoporosis and scleroderma. The method consists in administering to said animals and especially to human beings an effective amount of a proteoglycan such as chondroitin sulfate with mast cell secretion inhibitory activity, alone or in combination with one or more synergistic adjuvants such those belonging to the class of flavonoids or compounds with histamine-1 receptor antagonist activity.
Owner:THETA BIOMEDICAL CONSULTING & DEVMENT
Methods and compositions for the treatment of gastrointestinal disorders
InactiveUS20070010450A1Increase gastrointestinal motilityReduce inflammationPeptide/protein ingredientsMetabolism disorderIntestinal tract diseasesGastroparesis
Compositions and related methods for treating IBS and other gastrointestinal disorders and conditions (e.g., gastrointestinal motility disorders, functional gastrointestinal disorders, gastroesophageal reflux disease (GERD), duodenogastric reflux, Crohn's disease, ulcerative colitis, inflammatory bowel disease, functional heartburn, dyspepsia (including functional dyspepsia or nonulcer dyspepsia), gastroparesis, chronic intestinal pseudo-obstruction (or colonic pseudoobstruction), and disorders and conditions associated with constipation, e.g., constipation associated with use of opiate pain killers, post-surgical constipation, and constipation associated with neuropathic disorders as well as other conditions and disorders are described. The compositions feature peptides that activate the guanylate cyclase C (GC-C) receptor.
Owner:IRONWOOD PHARMA
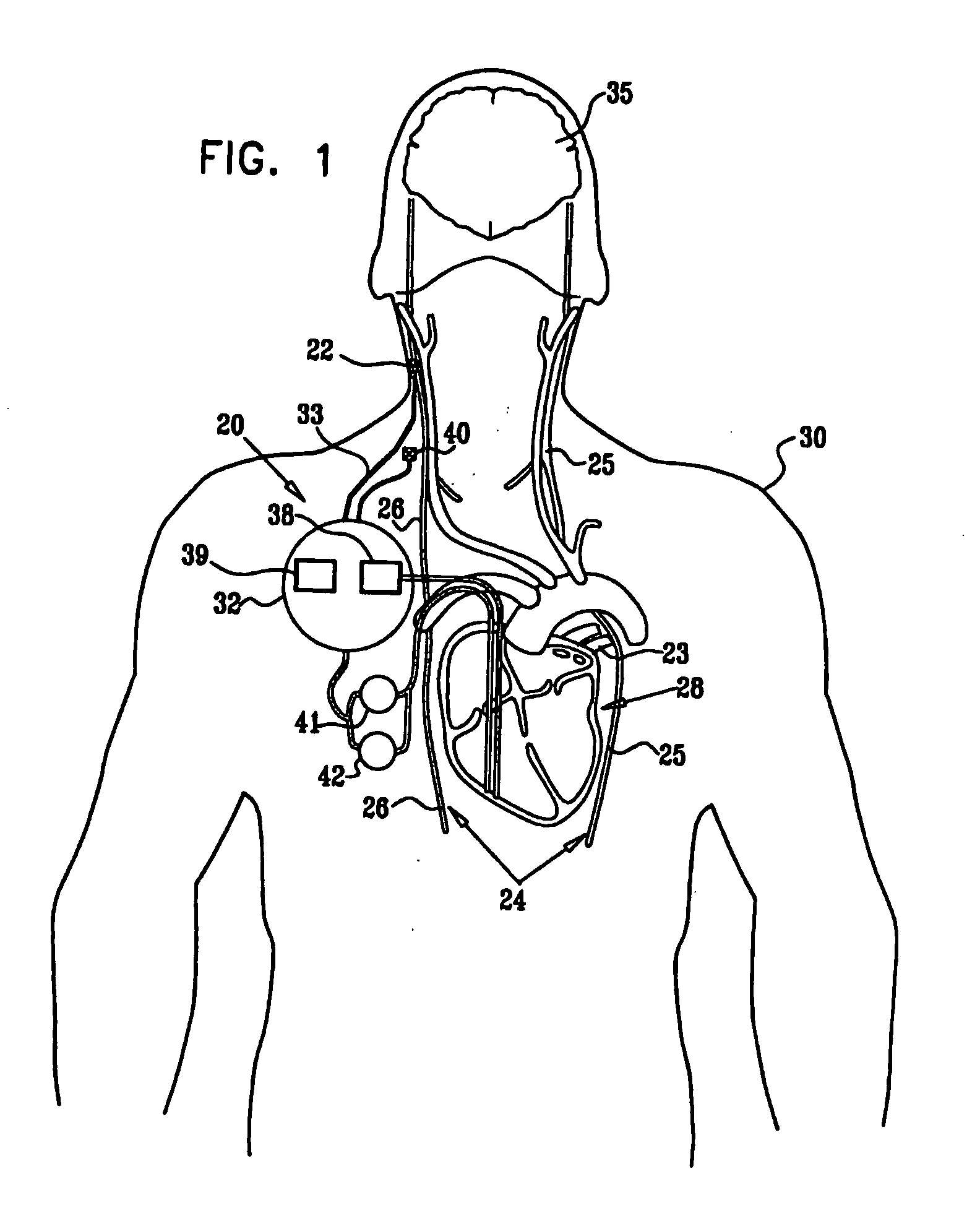
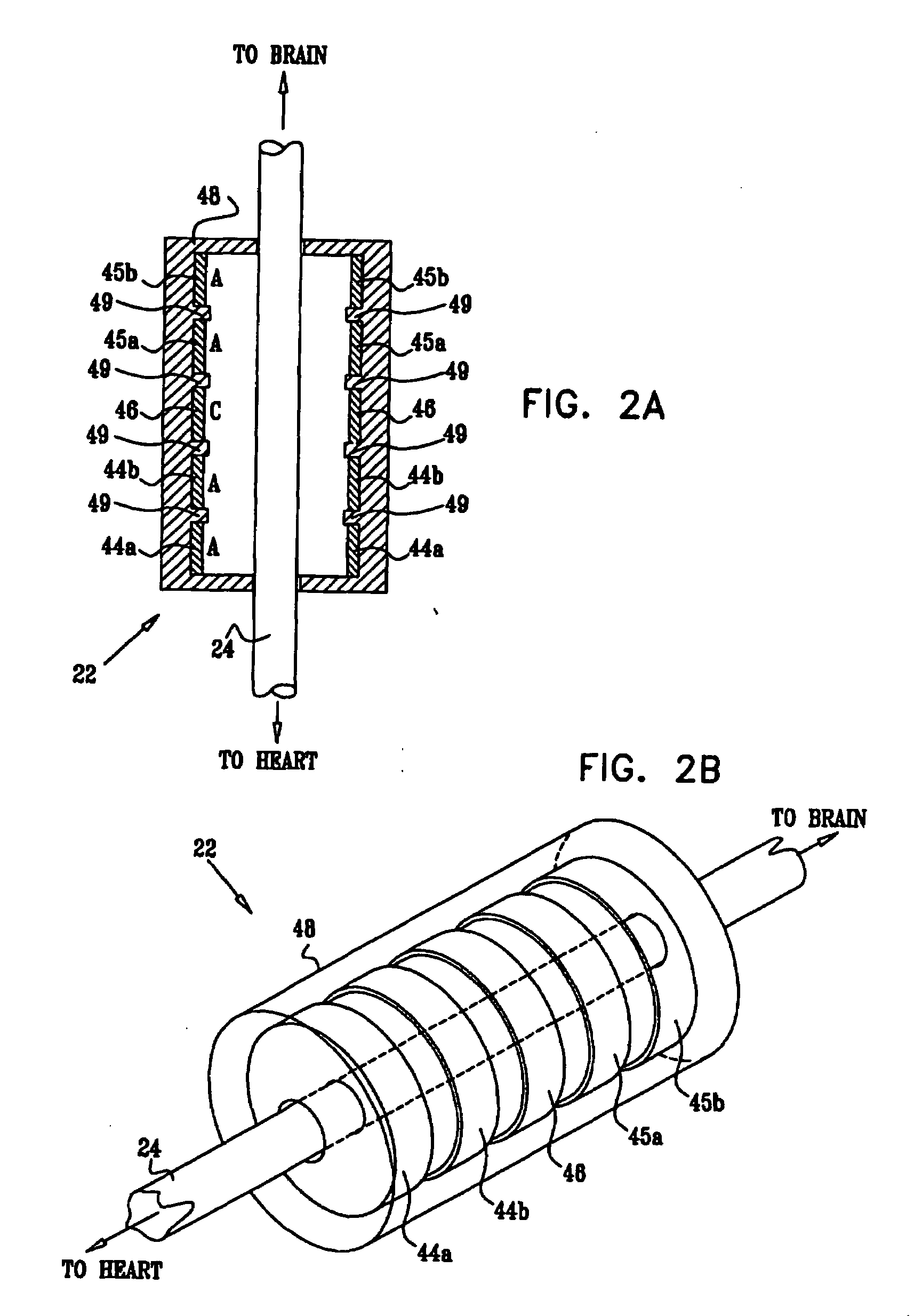
Combined parasympathetic stimulation and drug therapy
InactiveUS20080125843A1Enhancing and sustaining efficacyImprove efficiencyHeart defibrillatorsMedical devicesNervous systemMyelitis
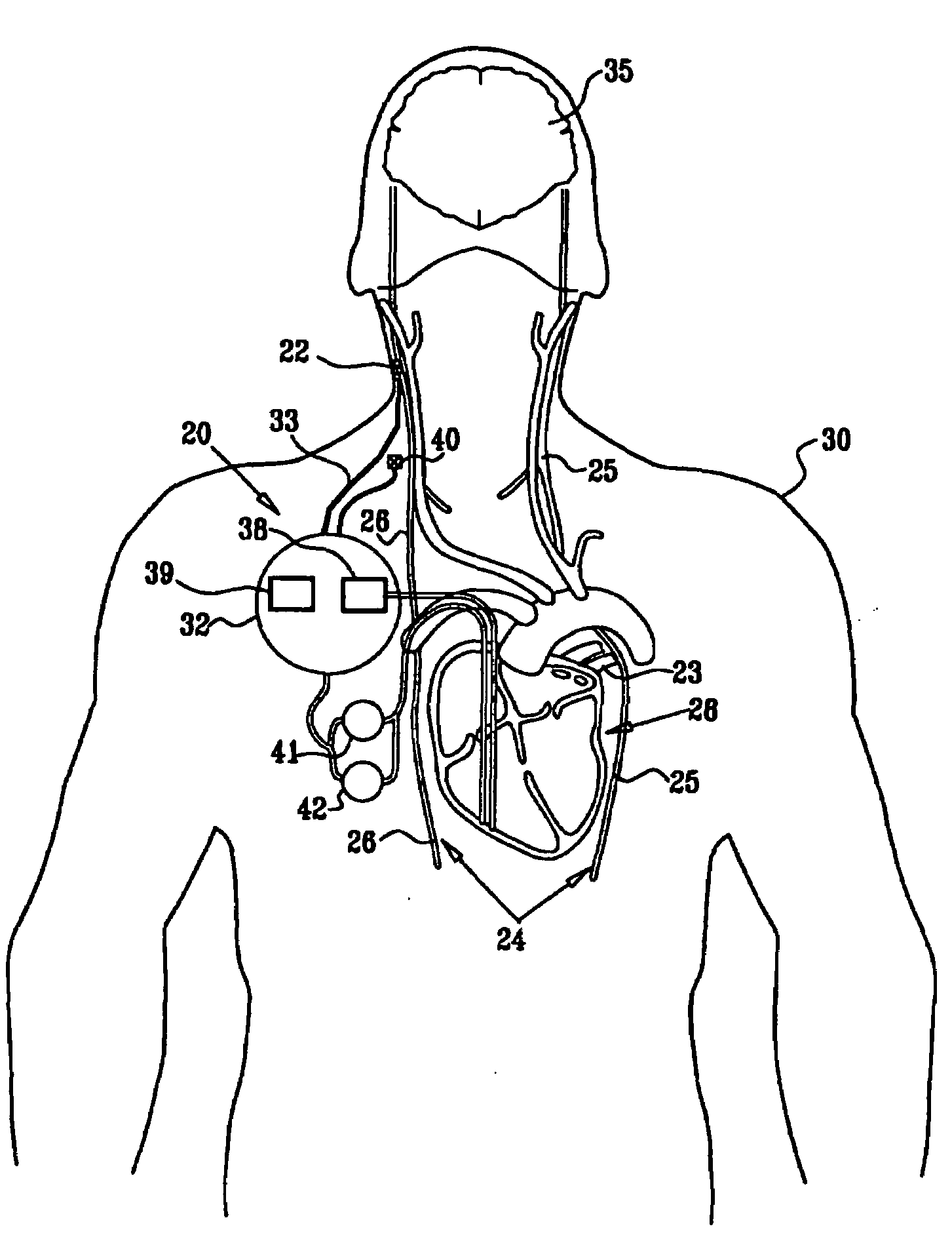
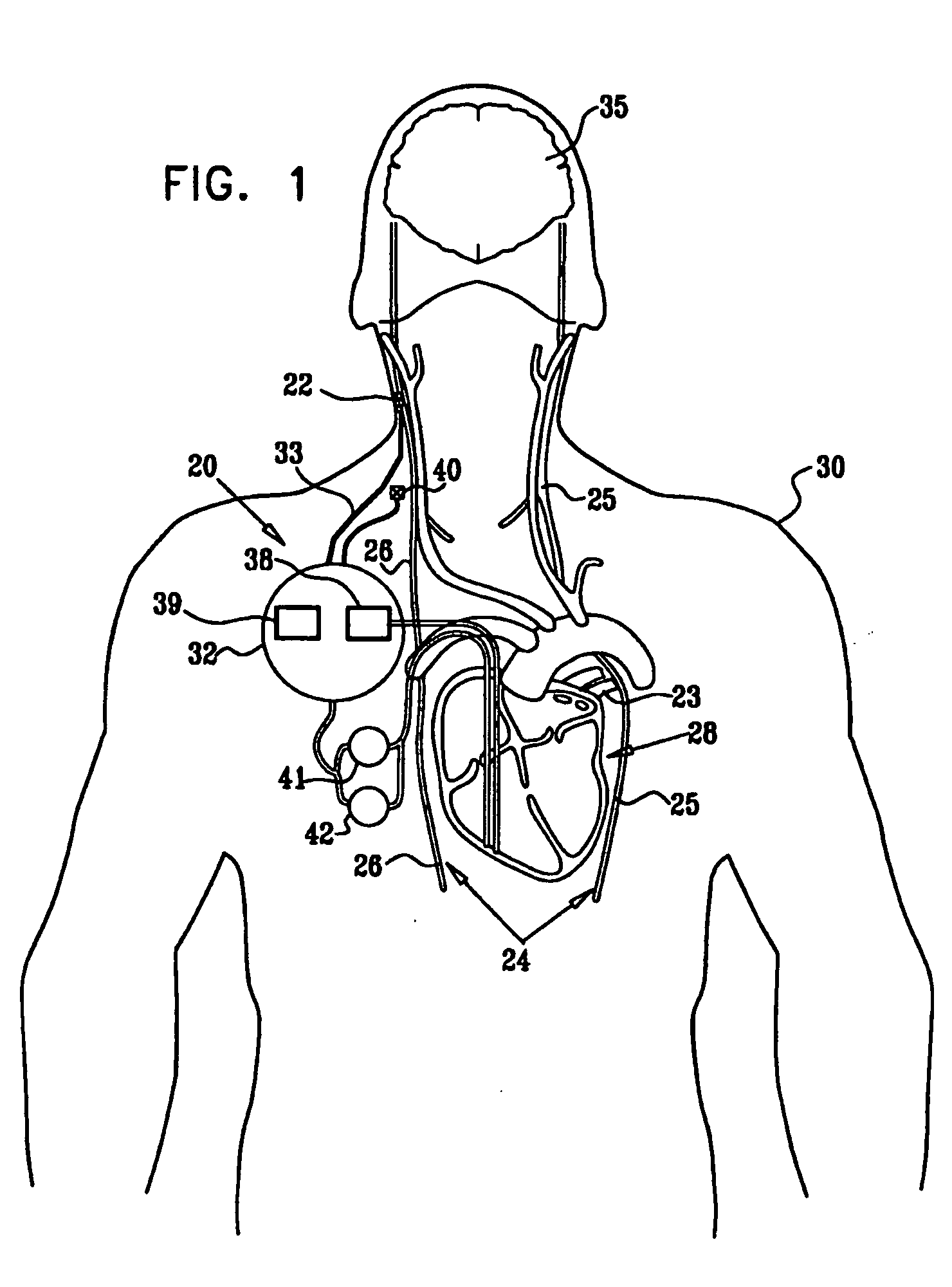
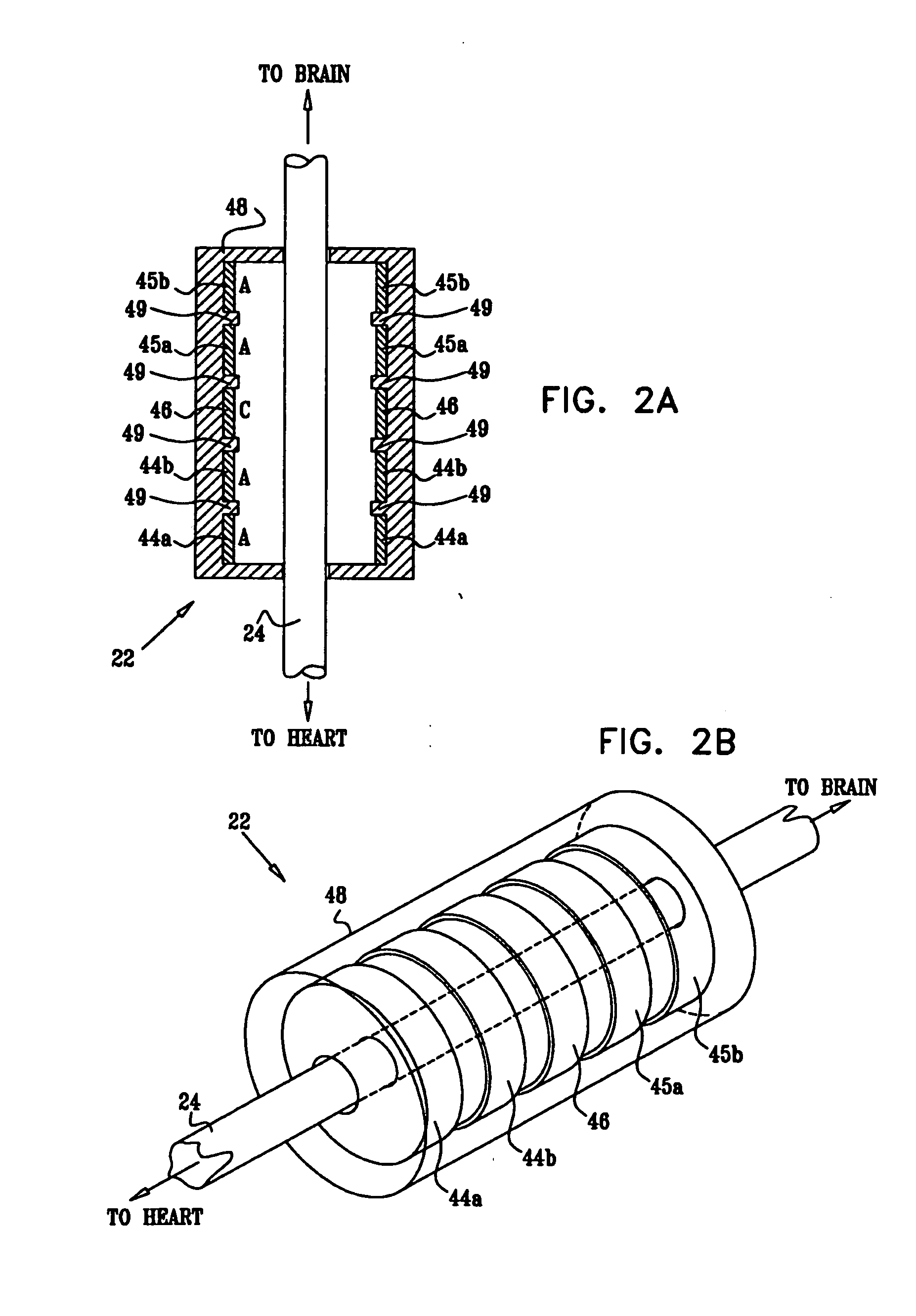
A method is provided for treating a subject, including applying a current to a site of the subject selected from the list consisting of: a vagus nerve of the subject, an epicardial fat pad of the subject, a pulmonary vein of the subject, a carotid artery of the subject, a carotid sinus of the subject, a vena cava vein of the subject, and an internal jugular vein of the subject. The method also includes configuring the current so as to treat a condition of the subject selected from the list consisting of: an autoimmune disease, an autoimmune inflammatory disease, multiple sclerosis, encephalitis, myelitis, immune-mediated neuropathy, myositis, dermatomyositis, polymyositis, inclusion body myositis, inflammatory demyelinating polyradiculoneuropathy, Guillain Barre syndrome, myasthenia gravis, inflammation of the nervous system, inflammatory bowel disease, Crohn's disease, ulcerative colitis, SLE (systemic lupus erythematosus), rheumatoid arthritis, vasculitis, polyarteritis nodosa, Sjogren syndrome, mixed connective tissue disease, glomerulonephritis, thyroid autoimmune disease, sepsis, meningitis, a bacterial infection, a viral infection, a fungal infection, sarcoidosis, hepatitis, and portal vein hypertension.
Owner:MEDTRONIC INC
Agonists of Guanylate Cyclase UseFul For The Treatment of Gastrointestinal Disorders, Inflammation, Cancer and Other Disorders
ActiveUS20100093635A1Reduce inflammationPositive therapeutic effectAntipyreticAnalgesicsPhosphodiesteraseGastrointestinal cancer
The invention provides novel guanylate cyclase-C agonist peptides and their use in the treatment of human diseases including gastrointestinal disorders, inflammation or cancer (e.g., a gastrointestinal cancer). The peptides can be administered either alone or in combination with an inhibitor of cGMP-dependent phosphodiesterase. The gastrointestinal disorder may be classified as either irritable bowel syndrome, constipation, or excessive acidity etc. The gastrointestinal disease may be classified as either inflammatory bowel disease or other GI condition including Crohn's disease and ulcerative colitis, and cancer.
Owner:BAUSCH HEALTH IRELAND LTD
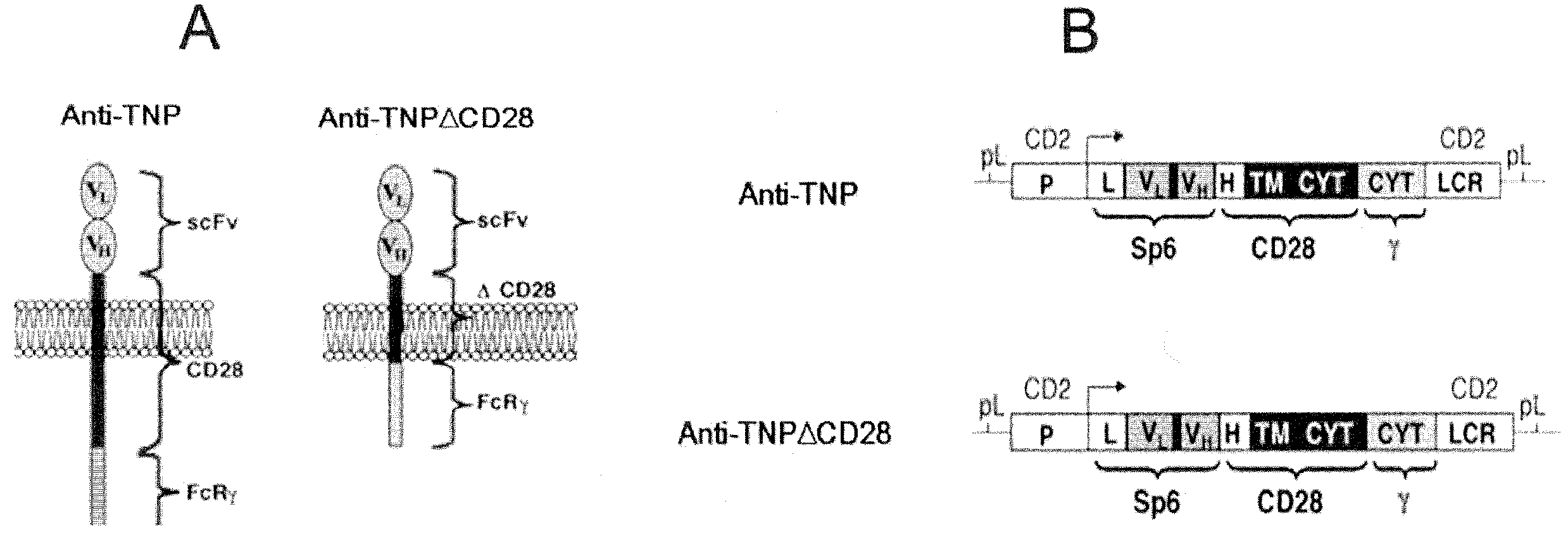
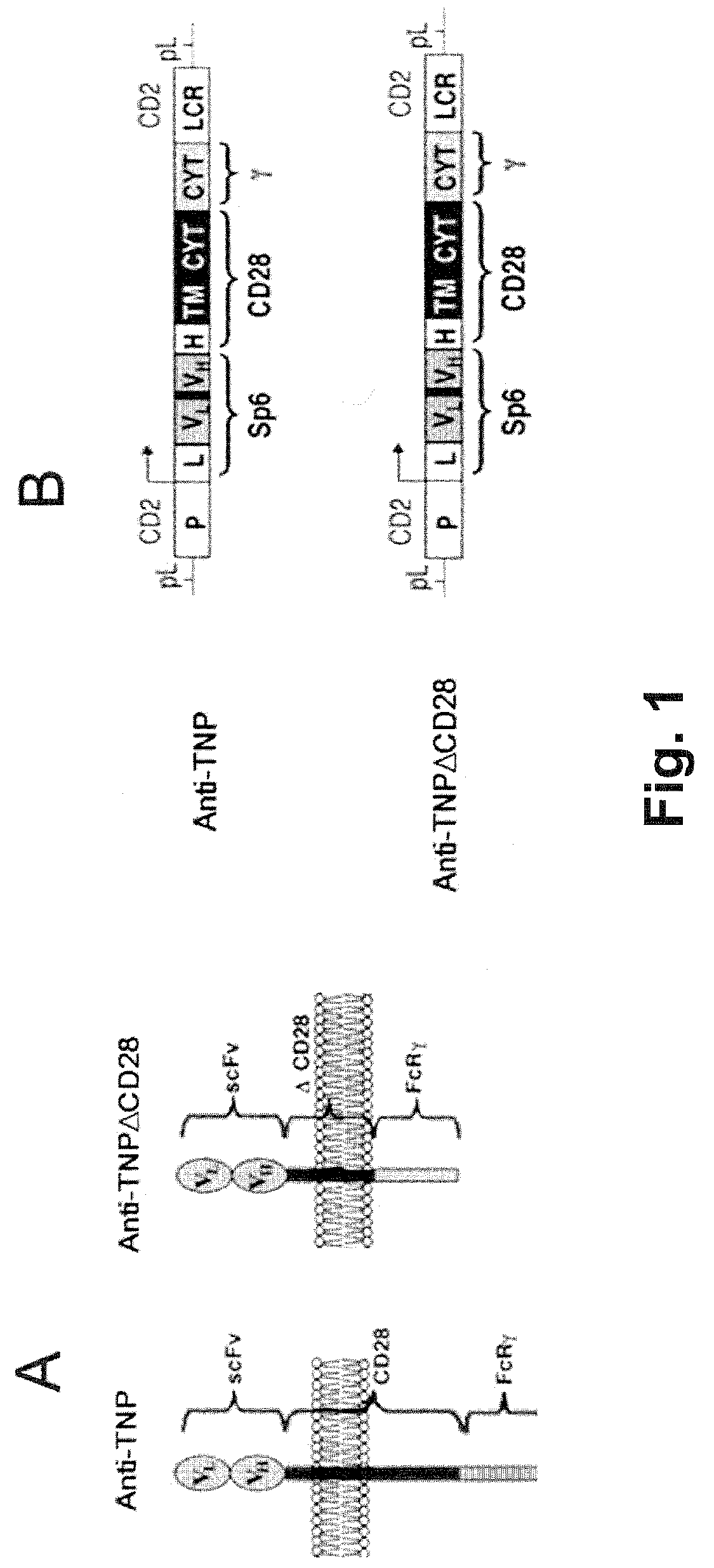
Redirected, genetically-engineered t regulatory cells and their use in suppression of autoimmune and inflammatory disease
InactiveUS20100135974A1Effective quantityOvercome scarcityBiocideAntipyreticIntracellular signallingInflammatory Bowel Diseases
A redirected Treg cell is endowed with specificity toward a selected target antigen or ligand. The cell contains a chimeric receptor polypeptide that is expressed in a single, continuous chain, with an extracellular recognition region displayed on the surface of the cell, a transmembrane region and an intracellular signaling region. The extracellular recognition region is specific for the selected target antigen or ligand. The intracellular signaling region includes a combination of T-cell signaling polypeptide moieties, which combination, upon binding of the extracellular recognition region to the selected target antigen or ligand, triggers activation of the redirected Treg cells to cause suppression of T-cell mediated immunity. Such redirected Treg cells may be used to suppress undesired activity of T effector cells thereby mediating an immune or inflammatory response. They are particularly useful in treating T effector cell-mediated diseases, such as inflammatory bowel disease, transplant rejection and GVH disease.
Owner:YEDA RES & DEV CO LTD
Compositions and Methods for Assessing and Treating Inflammatory Diseases and Disorders
InactiveUS20140377278A1BiocideBacteria material medical ingredientsAltered microbiotaInflammatory Bowel Diseases
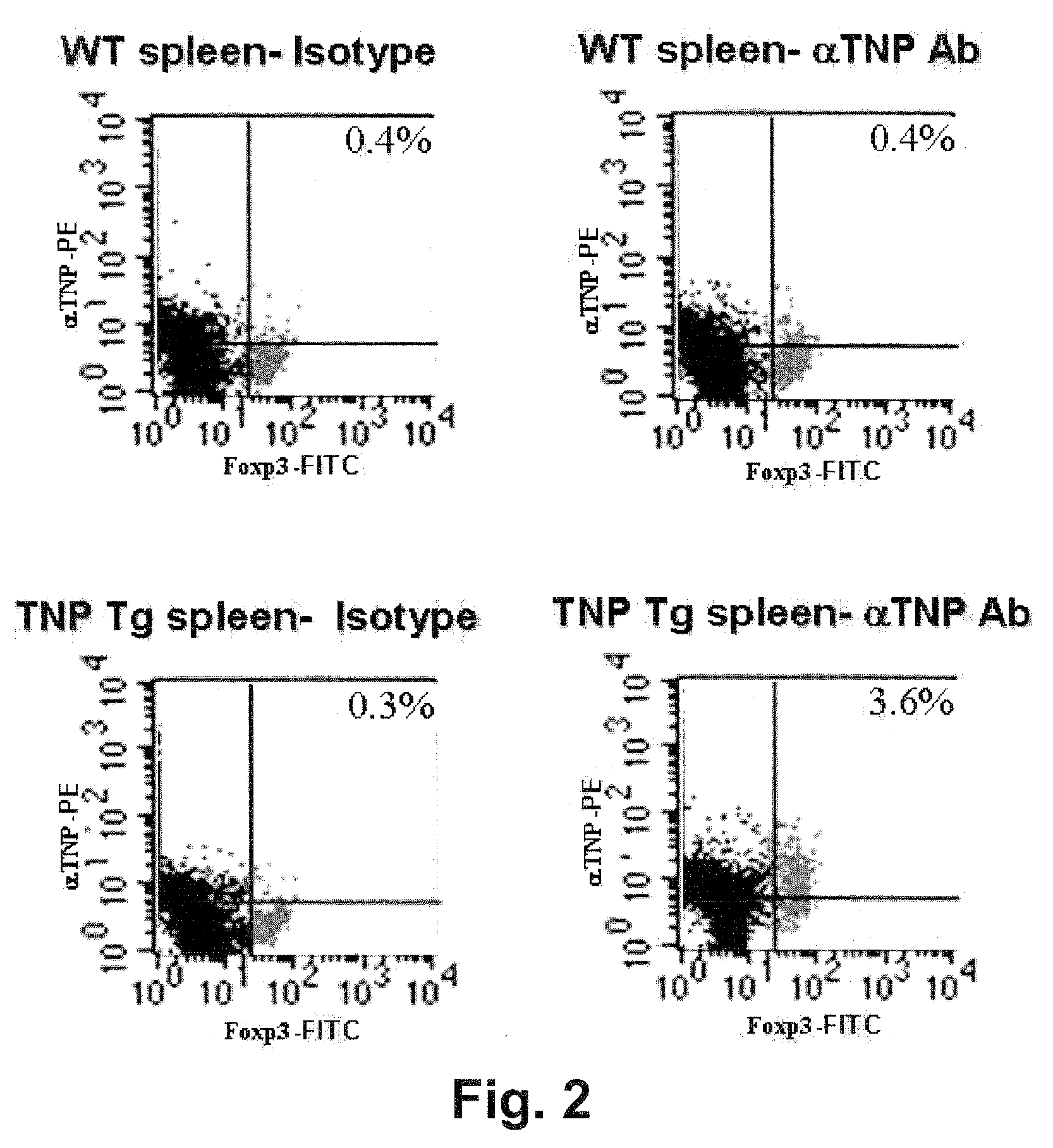
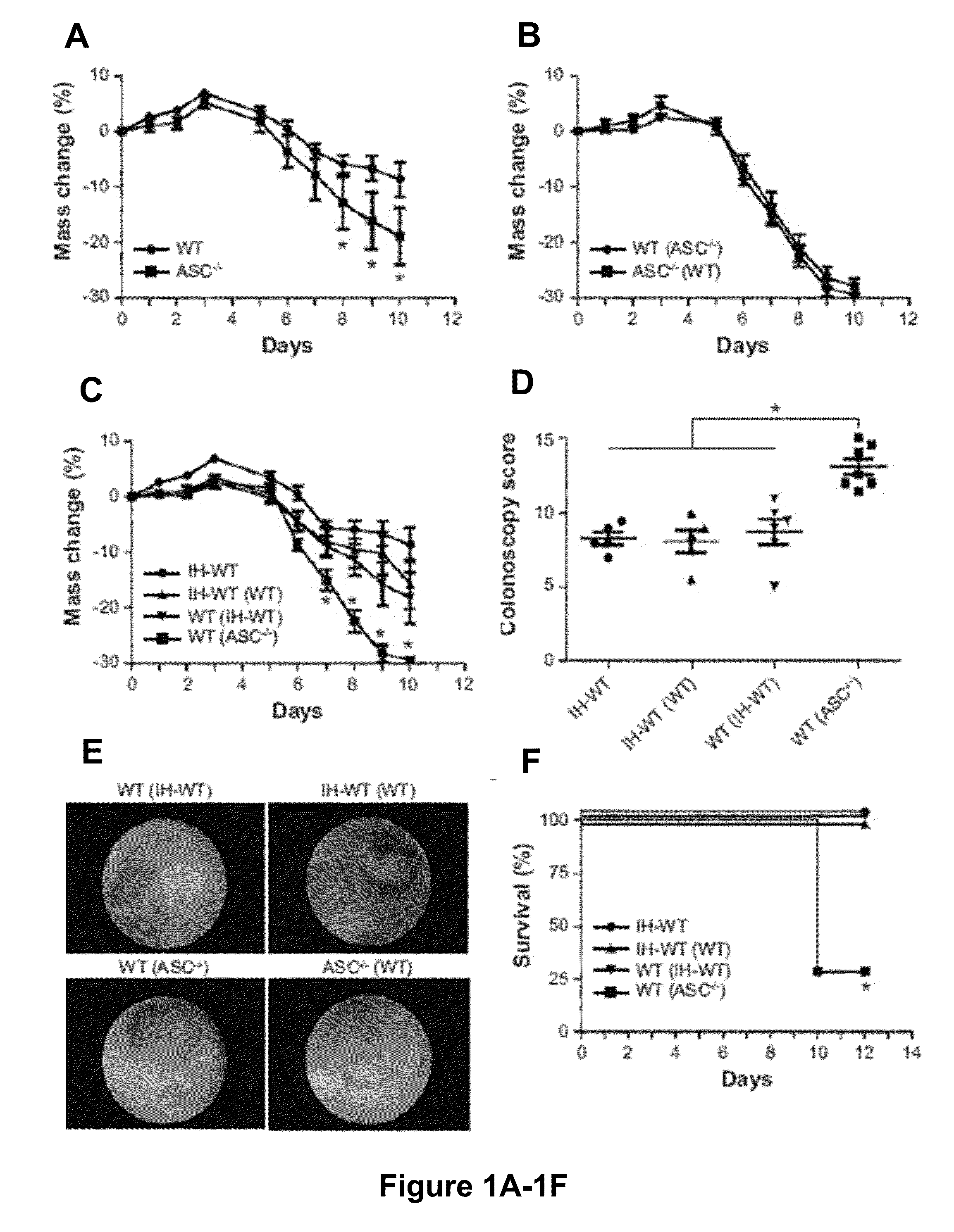
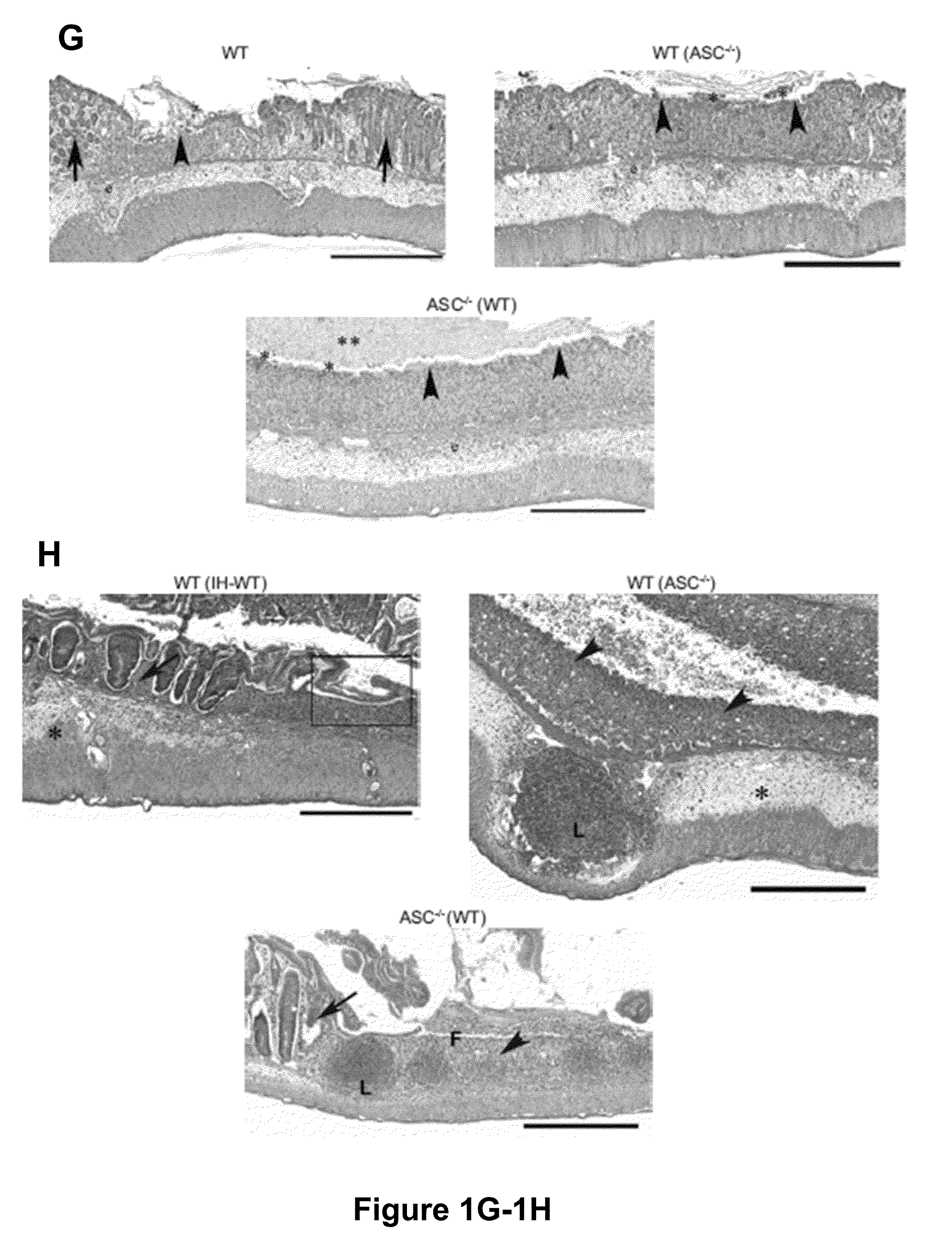
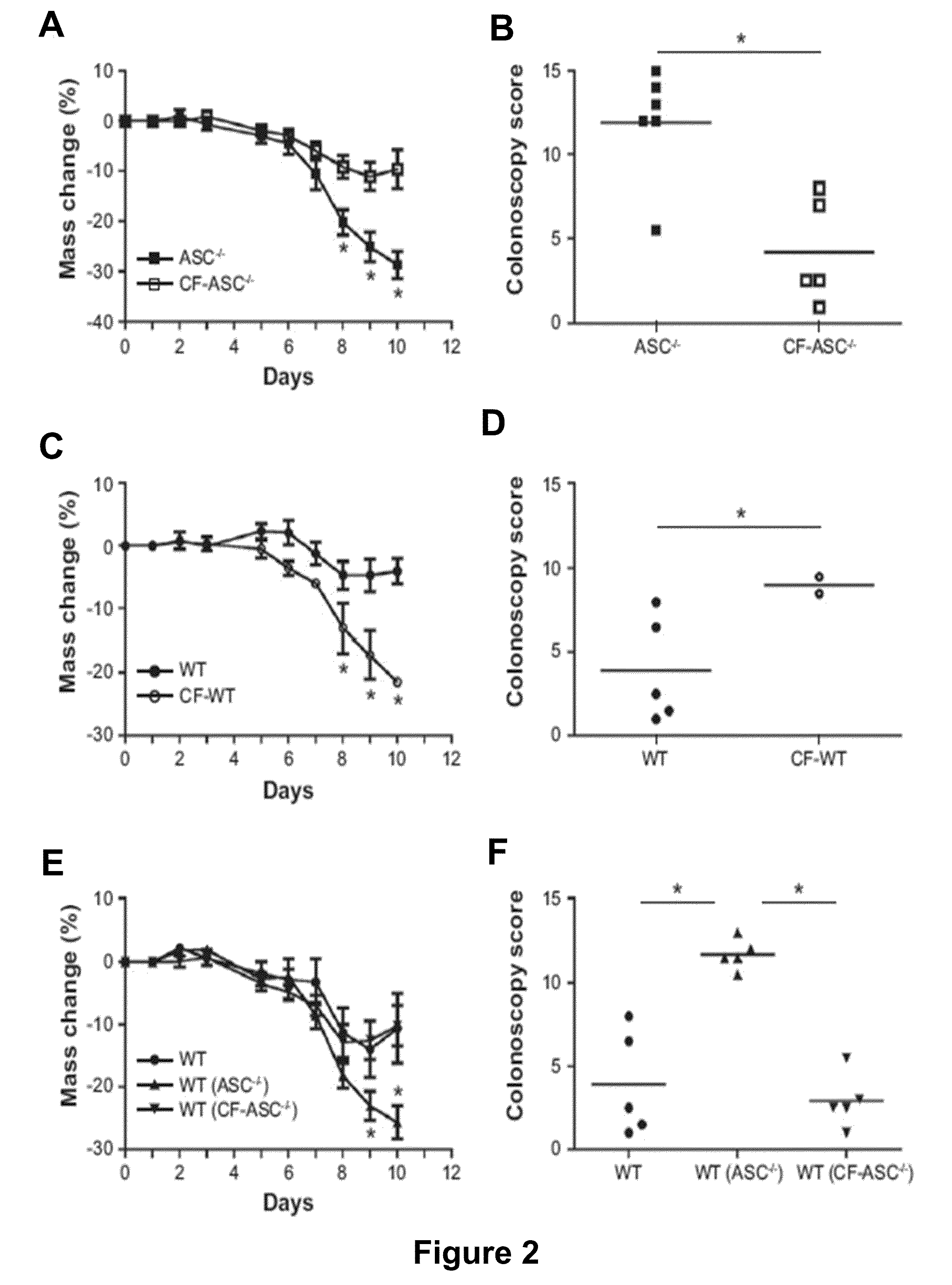
The present invention relates to the discovery that the disruption of inflammasome function leads to an altered microbiota that affects the development and progression of inflammatory diseases and disorders. Thus, the invention relates to compositions and methods for detecting and determining the relative proportions of the constituents of a subject's microbiota, methods of modifying an altered microbiota population in a subject, and compositions and methods for treating inflammatory diseases and disorders in a subject in need thereof.
Owner:YALE UNIV
Parasympathetic stimulation for heart conditions
InactiveUS20080125819A1Enhancing and sustaining efficacyImprove efficiencyHeart defibrillatorsHeart stimulatorsMyelitisNervous system
A method is provided for treating a subject, including applying a current to a site of the subject selected from the list consisting of: a vagus nerve of the subject, an epicardial fat pad of the subject, a pulmonary vein of the subject, a carotid artery of the subject, a carotid sinus of the subject, a vena cava vein of the subject, and an internal jugular vein of the subject. The method also includes configuring the current so as to treat a condition of the subject selected from the list consisting of: an autoimmune disease, an autoimmune inflammatory disease, multiple sclerosis, encephalitis, myelitis, immune-mediated neuropathy, myositis, dermatomyositis, polymyositis, inclusion body myositis, inflammatory demyelinating polyradiculoneuropathy, Guillain Barre syndrome, myasthenia gravis, inflammation of the nervous system, inflammatory bowel disease, Crohn's disease, ulcerative colitis, SLE (systemic lupus erythematosus), rheumatoid arthritis, vasculitis, polyarteritis nodosa, Sjogren syndrome, mixed connective tissue disease, glomerulonephritis, thyroid autoimmune disease, sepsis, meningitis, a bacterial infection, a viral infection, a fungal infection, sarcoidosis, hepatitis, and portal vein hypertension.
Owner:MEDTRONIC INC
Methods and Compositions to Treat and Detect Misfolded-SOD1 Mediated Diseases
ActiveUS20080206251A1Reduce and inhibit participationInhibit and neutralize toxic effectOrganic active ingredientsSenses disorderInflammatory Bowel DiseasesCerebral infarction
The invention provides a method for treating a medical condition, disease, or disorder mediated by a misfolded form of superoxide dismutase (SOD) in a subject in need of treatment. The method optionally comprises administering to the subject a composition comprising a pharmaceutically acceptable vehicle and an agent selected from (1) an exogenous antibody or fragment thereof that binds selectively to the misfolded form of SOD, and / or (2) an immunogen that elicits production of an endogenous antibody that binds selectively to the misfolded form of SOD, and / or (3) a nucleic acid sequence encoding (1) or (2). In certain embodiments, the invention provides methods of treating diseases such as Alzheimer's Disease, Parkinson's Disease or amyotrophic lateral sclerosis and macular degeneration, glaucoma, ischemia, cerebral infarction, myocardial infarction, atherosclerosis, multiple sclerosis, inflammatory bowel disease, ulcerative colitis, Crohn's disease or necrotizing enterocolitis using disease-specific epitopes, and compositions including these epitopes. The invention also provides antibodies that bind to monomeric or misfolded SOD1, and not on the molecular surface of native homodimeric SOD1. In addition, the invention includes methods of diagnosing Alzheimer's Disease, Parkinson's Disease or amyotrophic lateral sclerosis in a subject. Also, the invention provides methods of identifying substances for the treatment or prevention of Alzheimer's Disease, Parkinson's Disease or amyotrophic lateral sclerosis and kits using the binding proteins of the invention.
Owner:PROMIS NEUROSCI
Antagonizing interleukin-21 receptor activity
InactiveUS20080241098A1Reduced activityImprove inflammation symptomsCompounds screening/testingPeptide/protein ingredientsWhite blood cellInflammatory Bowel Diseases
Methods and compositions for inhibiting interleukin-21 (IL-21) / IL-21 receptor (MU-1) activity using antagonists of IL-21 or IL-21 receptor (“IL-21R” or “MU-1”), are disclosed. IL-21 / IL-21R antagonists can be used to induce immune suppression in vivo, e.g., for treating, ameliorating or preventing autoimmune or inflammatory disorders, including, e.g., inflammatory bowel disease (IBD), rheumatoid arthritis (RA), transplant / graft rejection, psoriasis, asthma, fibrosis, and systemic lupus erythematosus (SLE).
Owner:WYETH LLC
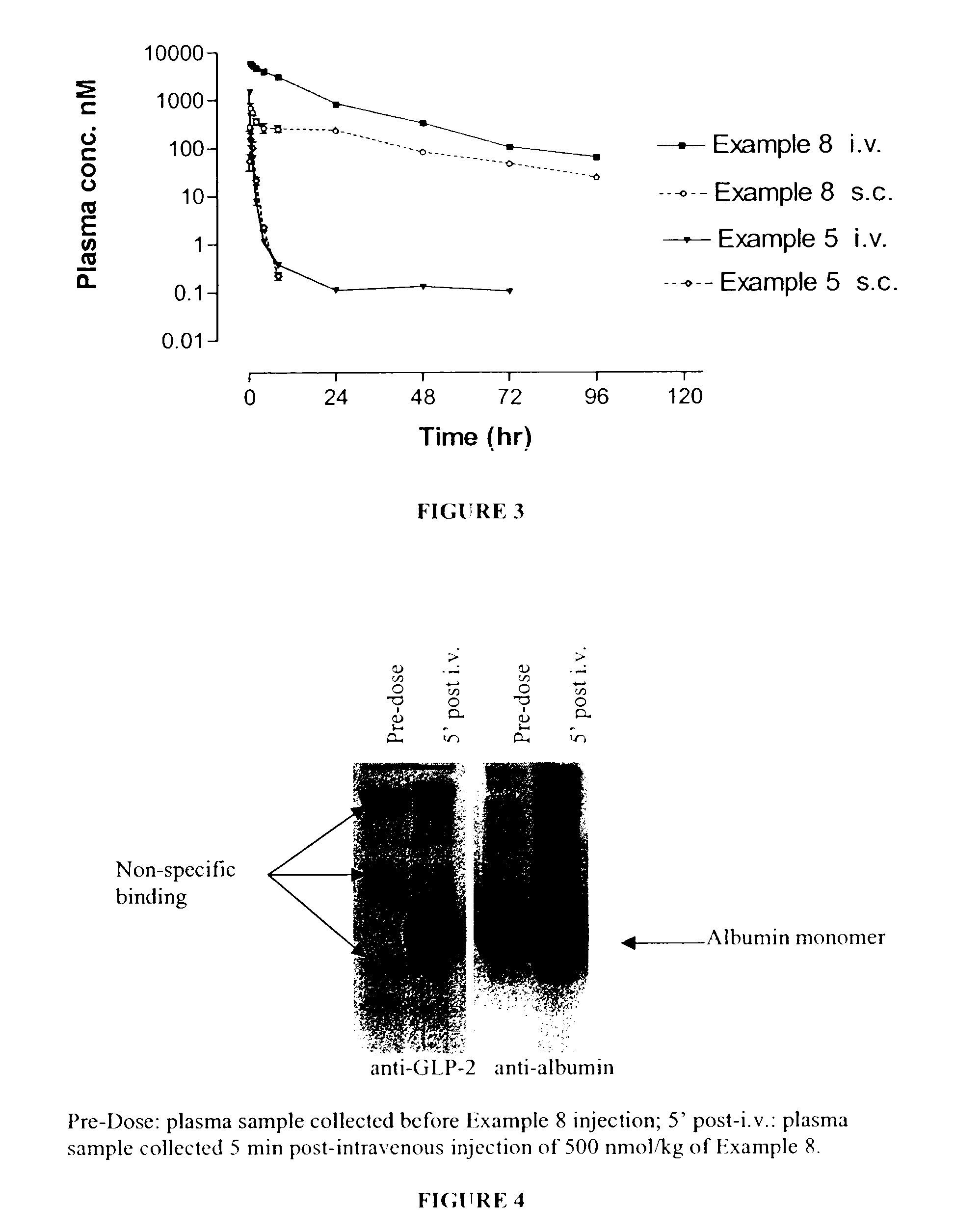
Long lasting glucagon-like peptide 2 (glp-2) for the treatment of gastrointestinal diseases and disorders
InactiveUS7112567B2Prevents undesirable cleavageHigh activityPeptide/protein ingredientsMetabolism disorderInflammatory Bowel DiseasesHalf-life
This invention relates to glucagon-like peptide 2 (GLP-2) derivatives. In particular this invention relates to GLP-2 peptide derivatives having an extended in vivo half-life, for the treatment or prevention of gastrointestinal disorders or diseases such as inflammatory bowel disease and other gastrointestinal functions, from any segment of the gastrointestinal tract, from the oesophagus to the anus.
Owner:CONJUCHEM
Novel compounds as rearranged during transfection (RET) inhibitors
This invention relates to novel compounds which are inhibitors of the Rearranged during Transfection (RET) kinase, to pharmaceutical compositions containing them, to processes for their preparation, and to their use in therapy, alone or in combination, for the normalization of gastrointestinal sensitivity, motility and / or secretion and / or abdominal disorders or diseases and / or treatment related to diseases related to RET dysfunction or where modulation of RET activity may have therapeutic benefit including but not limited to all classifications of irritable bowel syndrome (IBS) including diarrhea-predominant, constipation-predominant or alternating stool pattern, functional bloating, functional constipation, functional diarrhea, unspecified functional bowel disorder, functional abdominal pain syndrome, chronic idiopathic constipation, functional esophageal disorders, functional gastroduodenal disorders, functional anorectal pain, inflammatory bowel disease, proliferative diseases such as non-small cell lung cancer, hepatocellular carcinoma, colorectal cancer, medullary thyroid cancer, follicular thyroid cancer, anaplastic thyroid cancer, papillary thyroid cancer, brain tumors, peritoneal cavity cancer, solid tumors, other lung cancer, head and neck cancer, gliomas, neuroblastomas, Von Hippel-Lindau Syndrome and kidney tumors, breast cancer, fallopian tube cancer, ovarian cancer, transitional cell cancer, prostate cancer, cancer of the esophagus and gastroesophageal junction, biliary cancer, adenocarcinoma, and any malignancy with increased RET kinase activity.
Owner:GLAXOSMITHKLINE INTPROP DEV LTD
Method for treating congestive heart failure and other disorders
ActiveUS7494979B2Increase gastrointestinal motilityReduce complicationsPeptide/protein ingredientsMetabolism disorderGastroparesisInflammatory Bowel Diseases
Compositions and related methods for treating IBS and other gastrointestinal disorders and conditions (e.g., gastrointestinal motility disorders, functional gastrointestinal disorders, gastroesophageal reflux disease (GERD), duodenogastric reflux, Crohn's disease, ulcerative colitis, inflammatory bowel disease, functional heartburn, dyspepsia (including functional dyspepsia or nonulcer dyspepsia), gastroparesis, chronic intestinal pseudo-obstruction (or colonic pseudoobstruction), and disorders and conditions associated with constipation, e.g., constipation associated with use of opiate pain killers, post-surgical constipation, and constipation associated with neuropathic disorders as well as other conditions and disorders are described. The compositions feature peptides that activate the guanylate cyclase C (GC-C) receptor.
Owner:IRONWOOD PHARMA
Formulations for mediating inflammatory bowel disorders
InactiveUS20070173480A1Reduce cholesterol absorptionReduce absorptionBiocideSugar derivativesInflammatory Bowel DiseasesLower blood cholesterol
The invention provides formulations and methods for mediating inflammation, in particular an inflammatory bowel disorder such as necrotizing enterocolitis, and for. Further, the formulations are effective in lowering blood cholesterol and decreasing blood cholesterol absorption. The formulations comprise at least one ganglioside, which may be selected from the group consisting of: GD3, GM1, GM2, GM3, and GD1b. The invention provides a method of treating or preventing inflammatory diseases, such as necrotizing enterocolitis by delivery of at least one ganglioside to a subject in need thereof. Supplementation of foods or liquids with gangliosides, fore example infant formula or infant foods, can be employed according to the invention.
Owner:MTI META TECH
Method for treating inflammatory bowel disease and other forms of gastrointestinal inflammation
InactiveUS7485627B2Improve colitisSugar derivativesEnergy modified materialsGastrointestinal inflammationInflammatory Bowel Diseases
The invention provides a method for ameliorating gastrointestinal inflammation, particularly chronic gastrointestinal inflammation such as inflammatory bowel disease (IBD), in a subject. In one embodiment, the method comprises administering an immunomodulatory nucleic acid to a subject suffering from or susceptible to gastrointestinal inflammation.
Owner:RGT UNIV OF CALIFORNIA +1
Toll like receptor modulators
ActiveUS20090053148A1Prevent diseaseLower immune responseSenses disorderNervous disorderInflammatory Bowel DiseasesAutoimmune responses
The invention relates to TLR9 antagonist compounds and their therapeutic or prophylactic use. The invention provides novel immune regulatory oligonucleotides and immunomers as antagonist of TLRs and methods of use thereof. These immune regulatory oligonucleotides have unique sequences that suppress, without completely ablating, TLR-mediated signaling in response to a TLR ligand or TLR signaling agonist. The methods may have use in the prevention and treatment of autoimmunity, inflammation, inflammatory bowel disease, lupus, allergy, asthma, infection, sepsis, cancer and immunodeficiency.
Owner:IDERA PHARMA INC
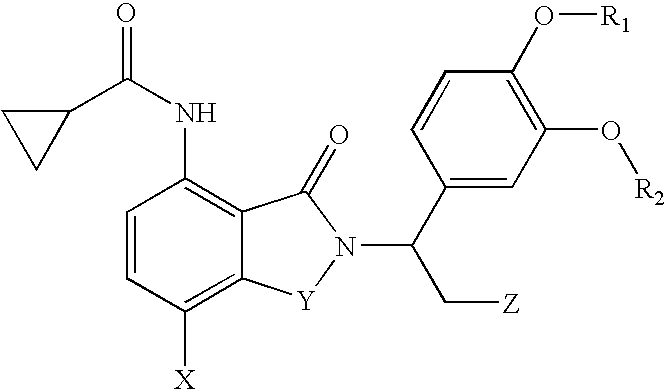
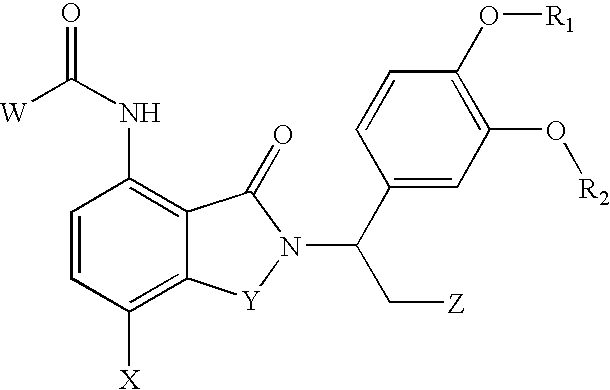
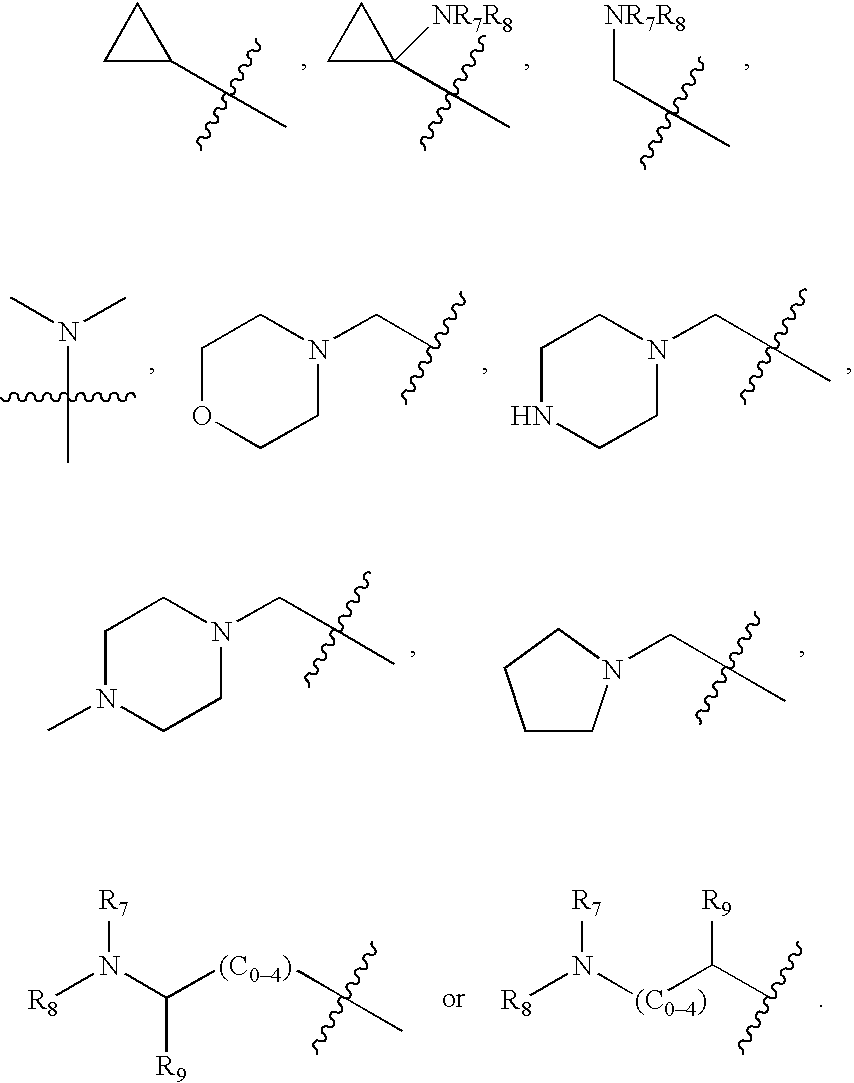
7-Amido-isoindolyl compounds and their pharmaceutical uses
The invention encompasses 7-amido-isoindolyl compounds and methods of using these compounds and compositions in mammals for treatment, prevention or management of various diseases and disorders. Examples inlcude, but are not limited to, cancer, inflammatory bowel disease and myelodysplastic syndrome.
Owner:CELGENE CORP
T-cell antigens, and their use in diagnosis and treatment of T-cell mediated conditions
InactiveUS6566082B1Eliminating other undesired immune responsesOptimizationPeptide/protein ingredientsAntipyreticAutoimmune ReactionsAntibody conjugate
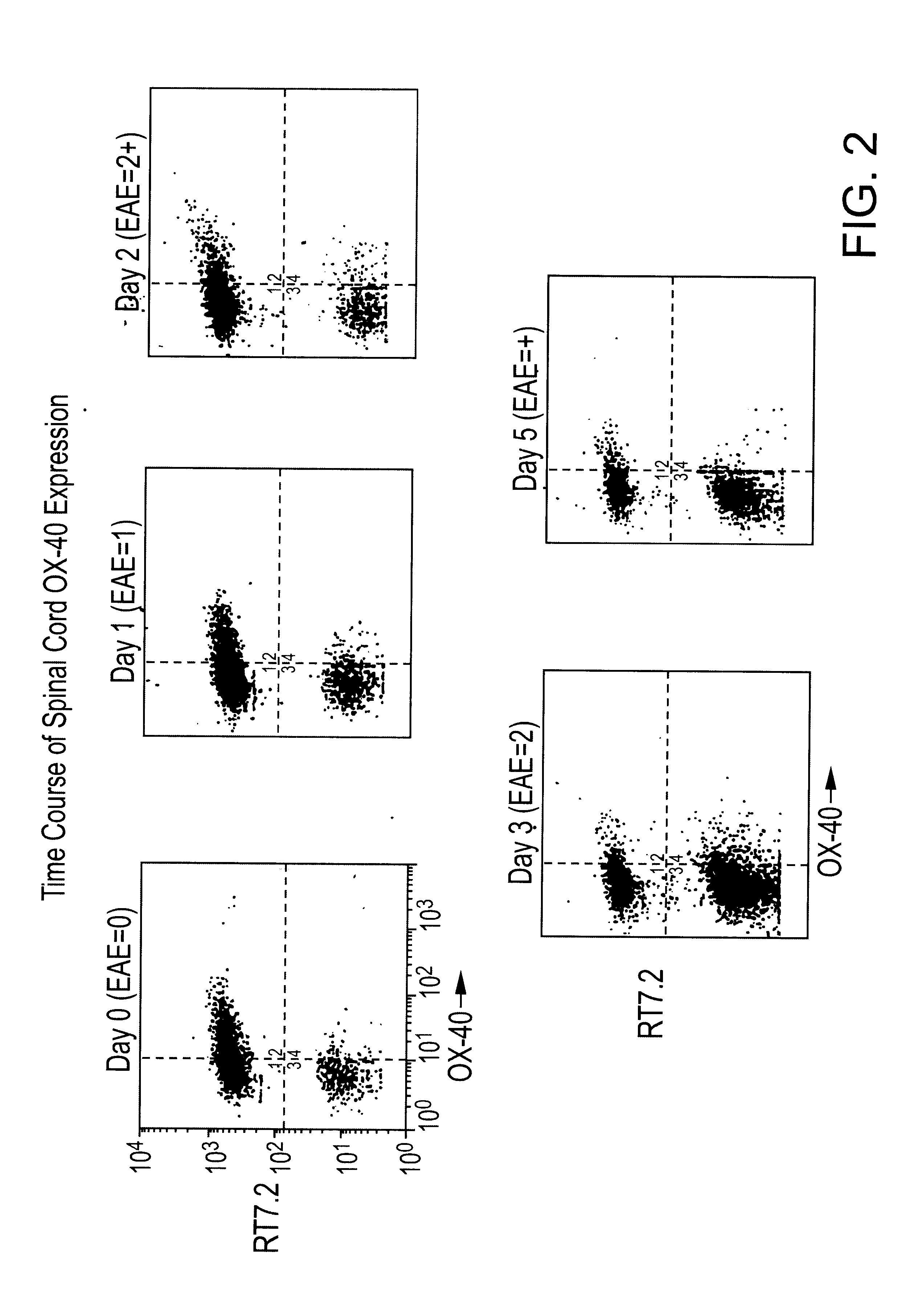
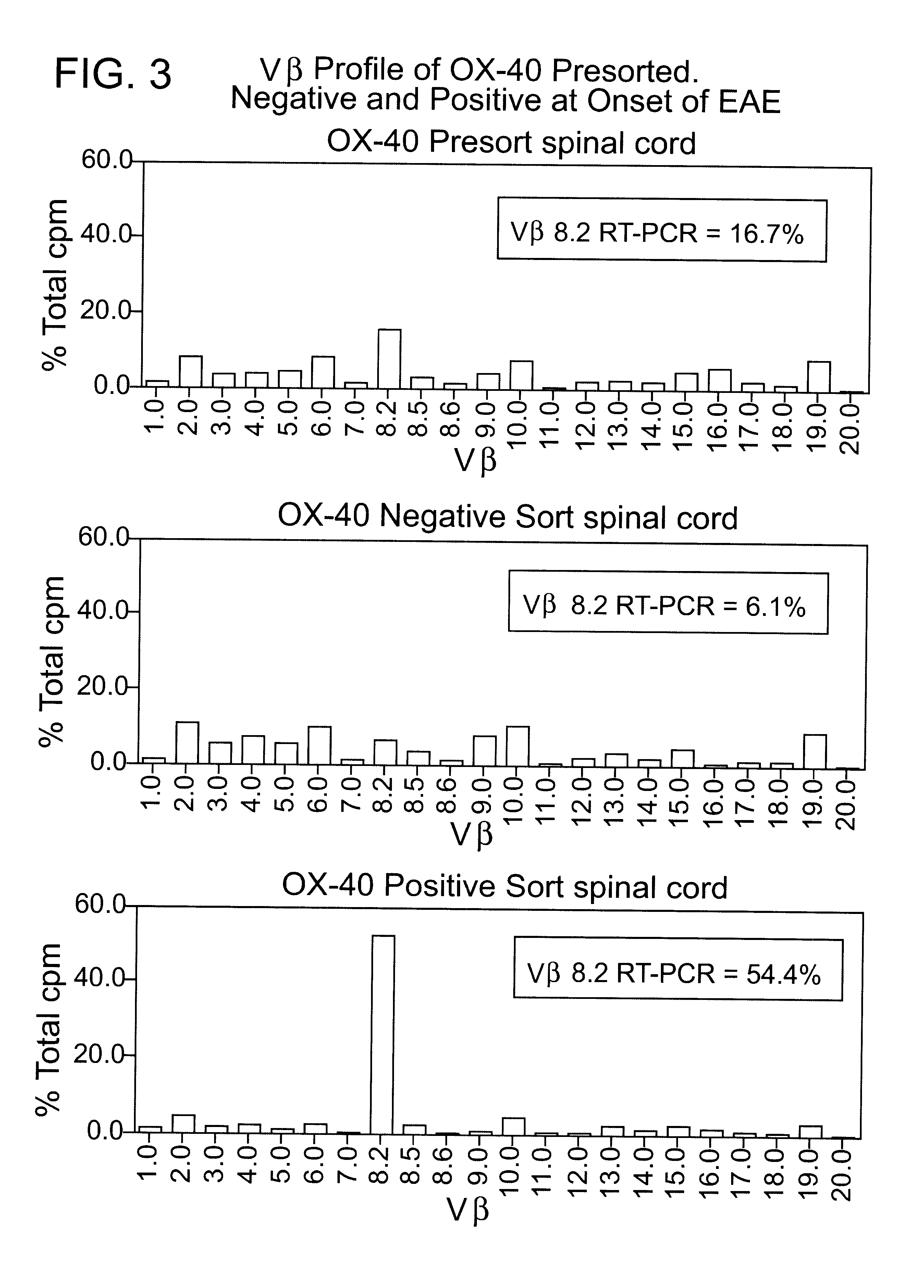
The OX-40 antigen is characterized and claimed together with variants and derivatives thereof. Also described are binding agents for the antigen and the use of these in diagnosis and therapy. Examples of such use include a method for the selective depletion of activated CD4+ T-cells in vivo by using immunotoxins comprising an OX-40 antibody conjugated to a toxic molecule (such as Ricin-A chain). The administration of these specific immunotoxins is used therapeutically to deplete autoimmune reactive CD4+ T-cells which have been implicated in diseases including Multiple Sclerosis, Rheumatoid Arthritis, Sarcoidosis, and Autoimmune Uveitis as well as inflammatory bowel disease and graft-versus-host disease. This type of therapy is also beneficial for eradicating CD4+ T-cell lymphomas and alloreactive CD4+ T-cells involved with a transplantation reaction. The use of the human form of the OX-40 antibody will also help in the early diagnosis of all the diseases mentioned above.
Owner:INNOCOMM TECH
EphA2, hypoproliferative cell disorders and epithelial and endothelial reconstitution
InactiveUS20050049176A1Convenient treatmentReduces and avoids unwanted effectPeptide/protein ingredientsAntipyreticCell layerInflammatory Bowel Diseases
The present invention relates to methods and compositions designed for the treatment, management, or prevention of a hypoproliferative cell disorder, especially those disorders relating to the destruction, shedding, or inadequate proliferation of epithelial and / or endothelial cells, particularly interstitial cystitis (IC) and lesions associated with inflammatory bowel disease (IBD). The methods of the invention comprise the administration of an effective amount of one or more agents that are antagonists of EphA2. In certain embodiments, the EphA2 antagonistic agent of the invention decreases EphA2-endogenous ligand binding, upregulates EphA2 gene expression and / or translation, increases EphA2 protein stability or protein accumulation, decreases EphA2 cytoplasmic tail phosphorylation, promotes EphA2 kinase activity (other than autophosphorylation or ligand-mediated EphA2 signaling), increases proliferation of EphA2 expressing cells, increases survival of EphA2 expressing cells, and / or maintains / reconstitutes epithelial and / or endothelial cell layer integrity. The invention also provides pharmaceutical compositions comprising one or more EphA2 antagonistic agents of the invention either alone or in combination with one or more other agents useful for therapy for a hypoproliferative cell disorder. Diagnostic methods and methods for screening for therapeutically useful agents are also provided.
Owner:MEDIMMUNE LLC
Compositions and methods for diagnosing colon disorders
InactiveUS20090197249A1Increases the diagnostic certaintyEasy to monitorMicrobiological testing/measurementBiostatisticsInflammatory Bowel DiseasesActive disease
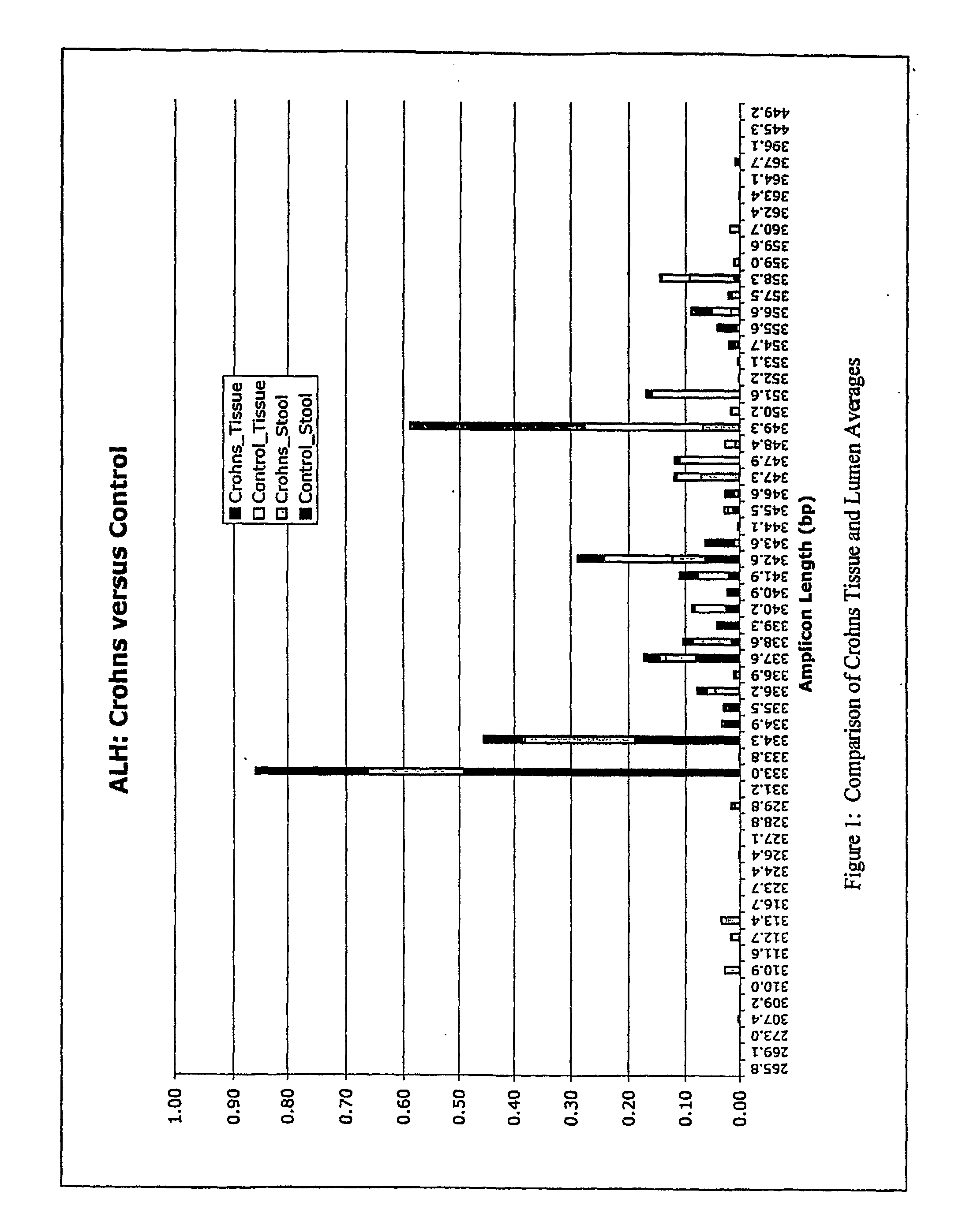
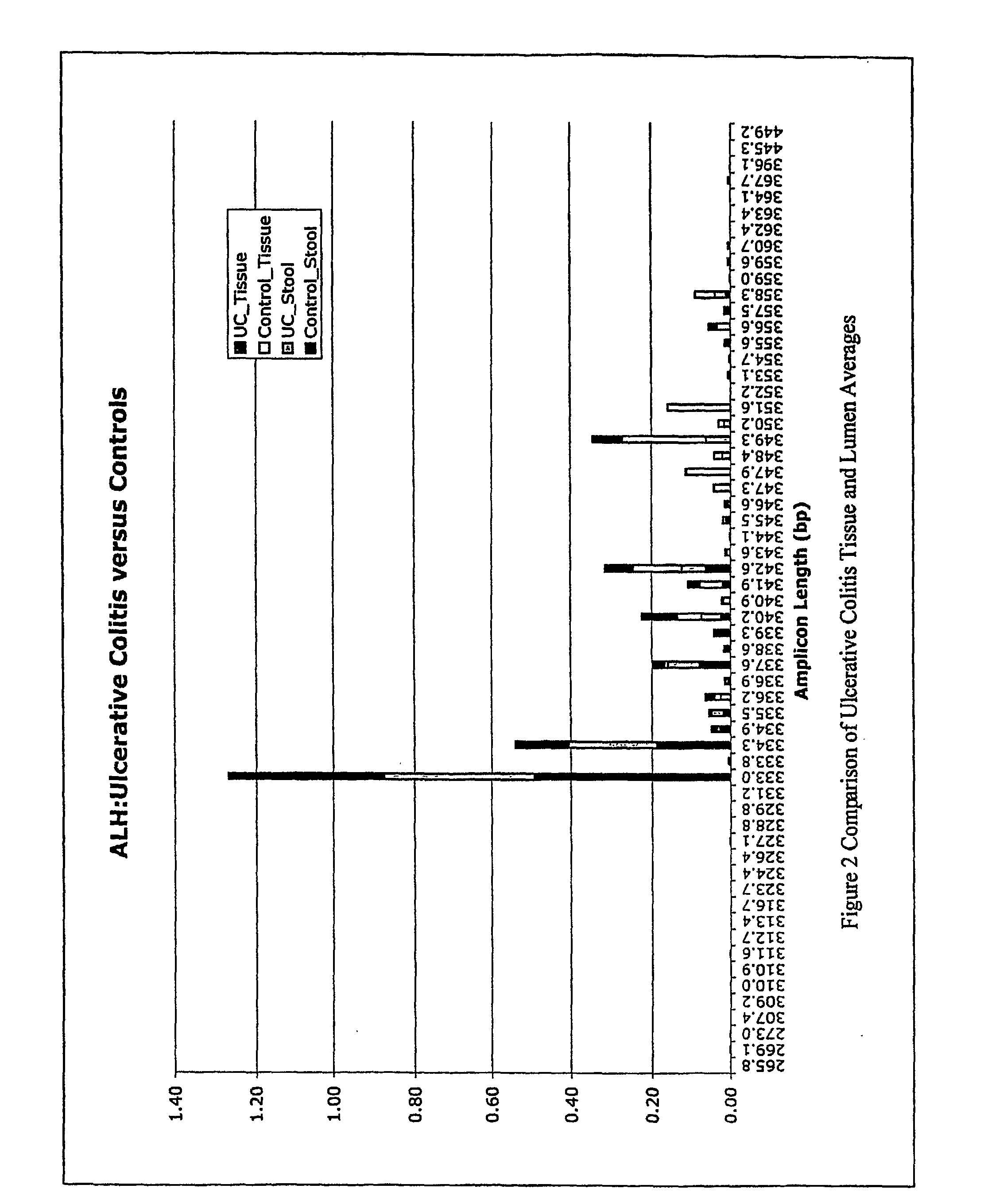
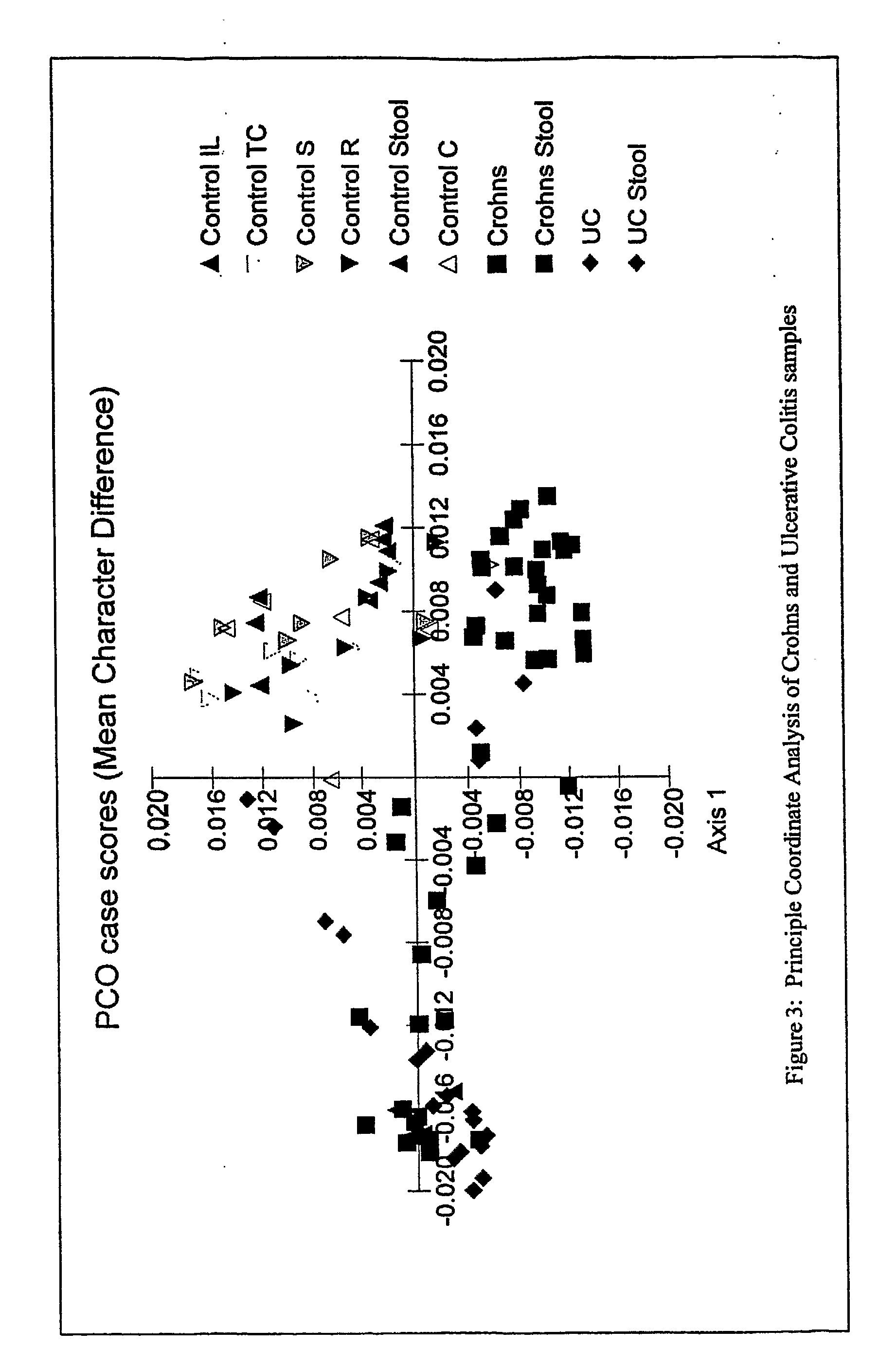
The present invention relates to methods and compositions for diagnosing, monitoring, prognosticating, analyzing, etc., polymicrobial diseases. The present invention also relates to the microbial community present in the digestive tract and lumen in normal subjects, and subjects with digestive tract diseases, especially diseases of the colon, such as inflammatory bowel disease, including ulcerative colitis, Crohn's syndrome, and pouchitis. The present invention especially relates to compositions and methods for diagnosing and prognosticating the mentioned diseases and conditions, e.g., to determine the presence of the disease in a subject, to determine a therapeutic regimen, to determine a therapeutic regimen, to determine the onset of active disease, to determine the predisposition to the disease, etc.
Owner:GEORGE MASON INTPROP INC +1
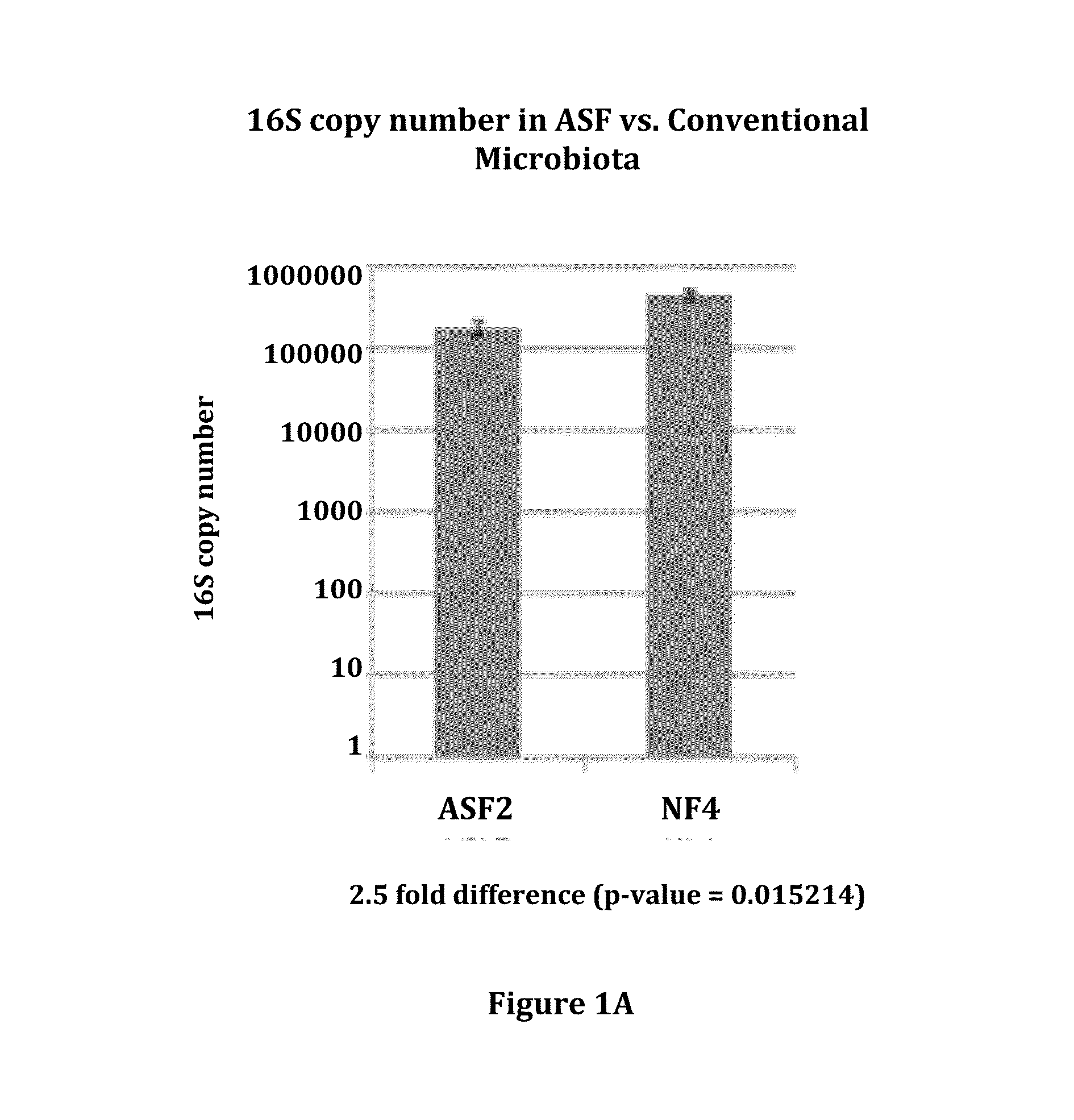
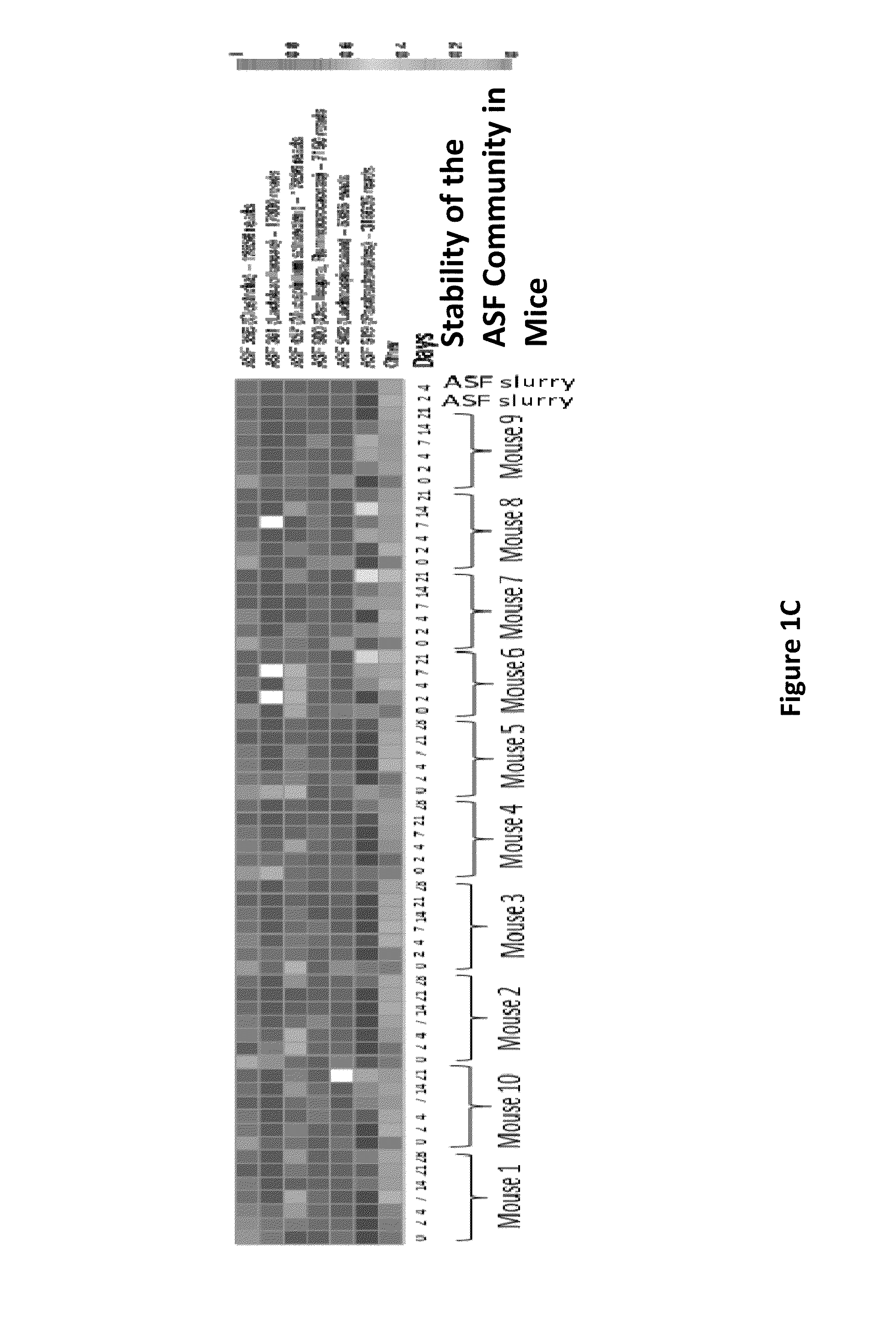
Compositions and methods comprising a defined microbiome and methods of use thereof
ActiveUS20160243175A1Minimal urease activityReduce bacteria countBacteriaBacteria material medical ingredientsMicroorganismInflammatory Bowel Diseases
The invention features the use of a defined microbial consortia for the replacement of a gut microbiome associated with disease. In particular, the invention provides for the treatment of hyperammonemia, Clostridium difficile colitis, hepatic encephalopathy associated with cirrhosis, and inflammatory bowel disease.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Methods using proton pump inhibitors
The invention provides methods of treating and preventing asthma, laryngitis, symptomatic gastroesophageal reflux disease, pregnancy-induced gastroesophageal reflux disease, noncardiac chest pains, coughing, apnea, dyspepsia, inflammatory bowel disease, irritable bowel syndrome, gastritis, stress ulcers, bleeding peptic ulcers, acute gastrointestinal bleeding, infectious enteritis, collagenous colitis, lymphocytic colitis, chronic diarrhea in immunocompromised patients, esophageal ulcers in immunocompromised patients, idiopathic gastric acid hypersecretion, gastroparesis, gastrointestinal motility disorders, Zollinger-Ellison syndrome, short bowel syndrome, emesis, regurgitation, early satiety, chronic sore throat, abdominal pain, abdominal bloating, nausea, sour stomach, diarrhea, constipation, bacterial infections, refractory ulcers, gastrointestinal disorders induced by NSAIDs, Barrett's esophagus, gastrointestinal disorders caused by steroids, gastrointestinal disorders induced by cholinergic compounds, and fungal or viral-induced ulcers in the gastrointestinal tract by administering a therapeutically effective amount of at least one proton pump to a patient in need thereof. The invention also provides on demand relief of symptoms associated with gastroesophageal reflux disease (GERD), and provides relief from symptoms caused by the consumption of excessive amounts of food and / or alcohol by administering a therapeutically effective amount of at least one proton pump inhibitor to a patient in need thereof. The invention also provides methods for treating parasitic infections, such as malaria, by administering a therapeutically effective amount of at least one proton pump inhibitor to a patient in need thereof.
Owner:EISAI CO LTD
Chromone derivatives as matrix metalloproteinase inhibitors
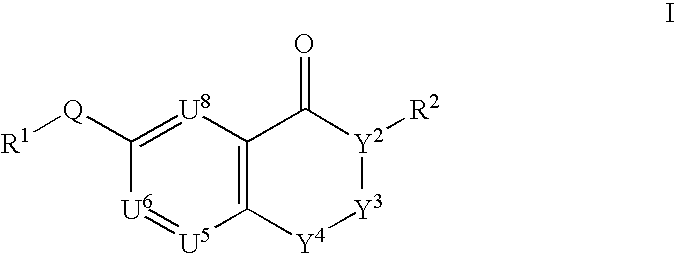
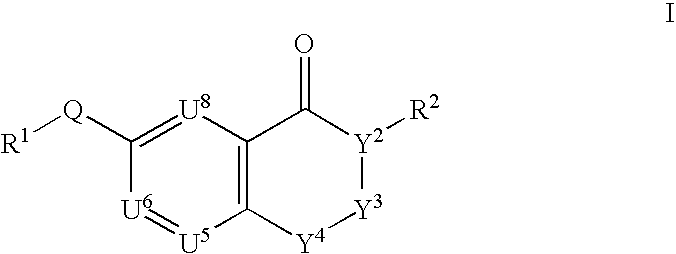
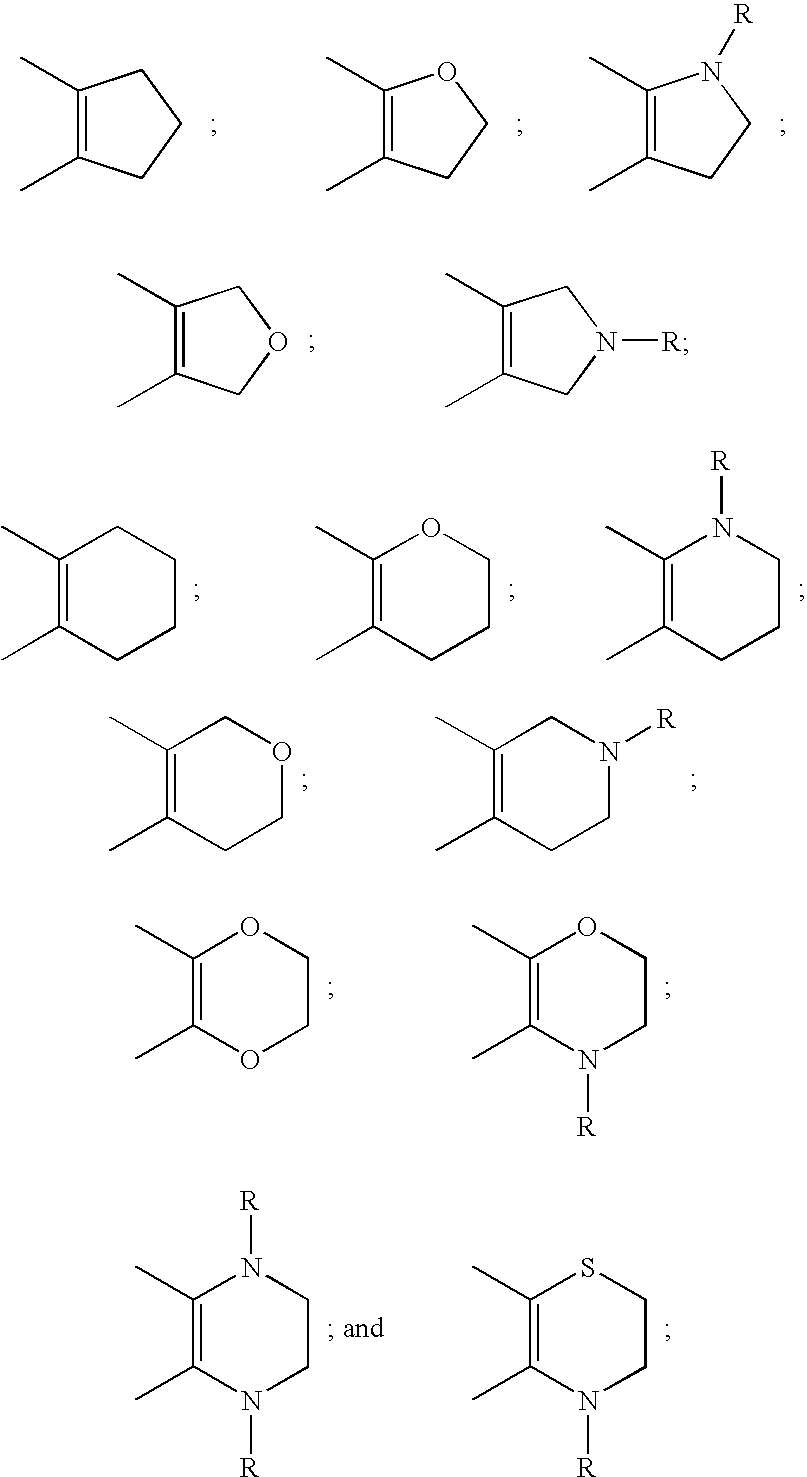
This invention provides compounds defined by Formula I or a pharmaceutically acceptable salt thereof,wherein R1, Q, Y2, Y3, Y4, U5, U6, U8, and R2 are as defined in the specification. The invention also provides pharmaceutical compositions comprising a compound of Formula I, or a pharmaceutically acceptable salt thereof, as defined in the specification, together with a pharmaceutically acceptable carrier, diluent, or excipient. The invention also provides methods of inhibiting an MMP-13 enzyme in an animal, comprising administering to the animal a compound of Formula I, or a pharmaceutically acceptable salt thereof. The invention also provides methods of treating a disease mediated by an MMP-13 enzyme in a patient, comprising administering to the patient a compound of Formula I, or a pharmaceutically acceptable salt thereof, either alone or in a pharmaceutical composition. The invention also provides methods of treating diseases such as heart disease, multiple sclerosis, osteo- and rheumatoid arthritis, arthritis other than osteo- or rheumatoid arthritis, cardiac insufficiency, inflammatory bowel disease, heart failure, age-related macular degeneration, chronic obstructive pulmonary disease, asthma, periodontal diseases, psoriasis, atherosclerosis, and osteoporosis in a patient, comprising administering to the patient a compound of Formula I, or a pharmaceutically acceptable salt thereof, either alone or in a pharmaceutical composition. The invention also provides combinations, comprising a compound of Formula I, or a pharmaceutically acceptable salt thereof, together with another pharmaceutically active component as described in the specification.
Owner:WARNER-LAMBERT CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com