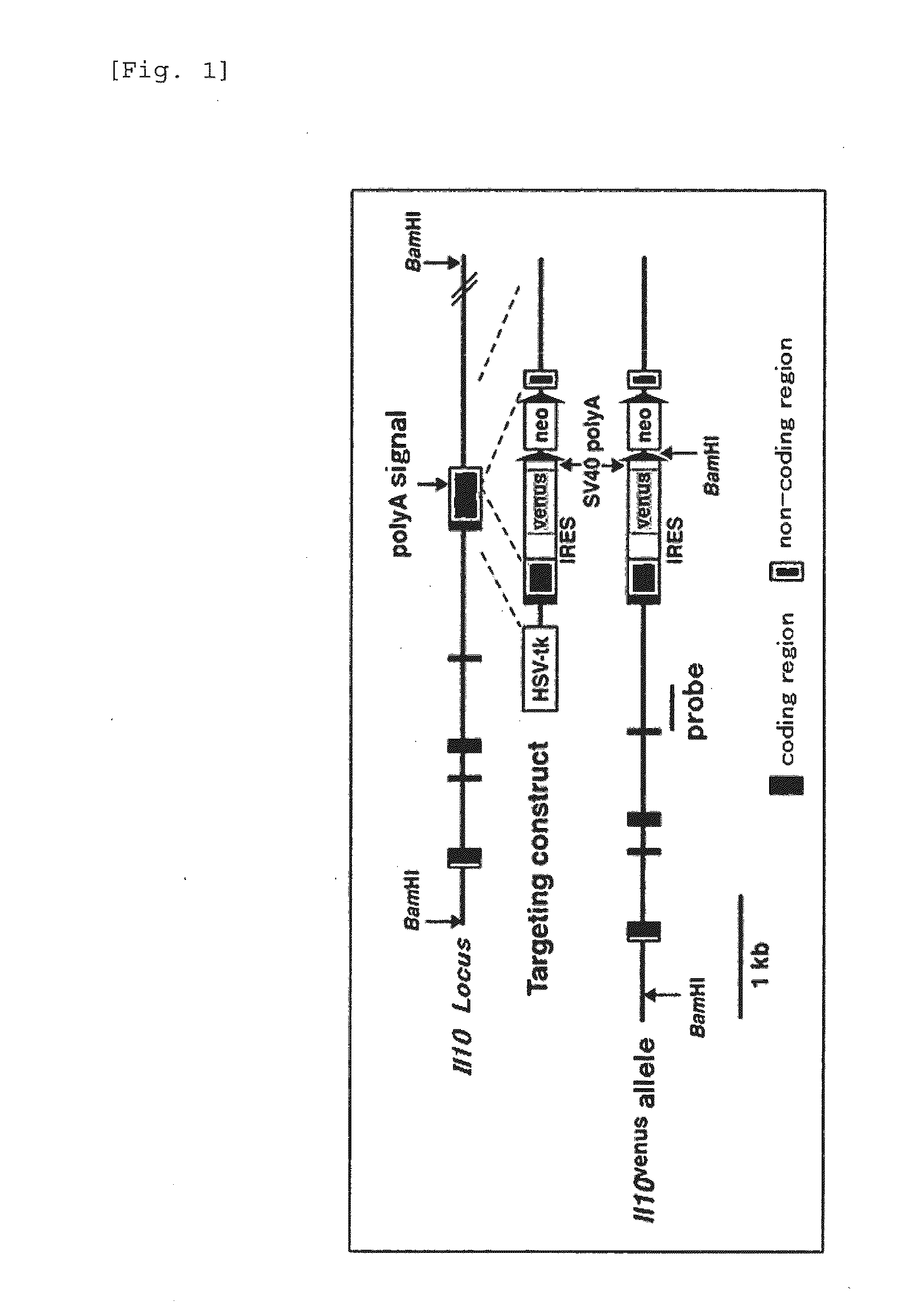
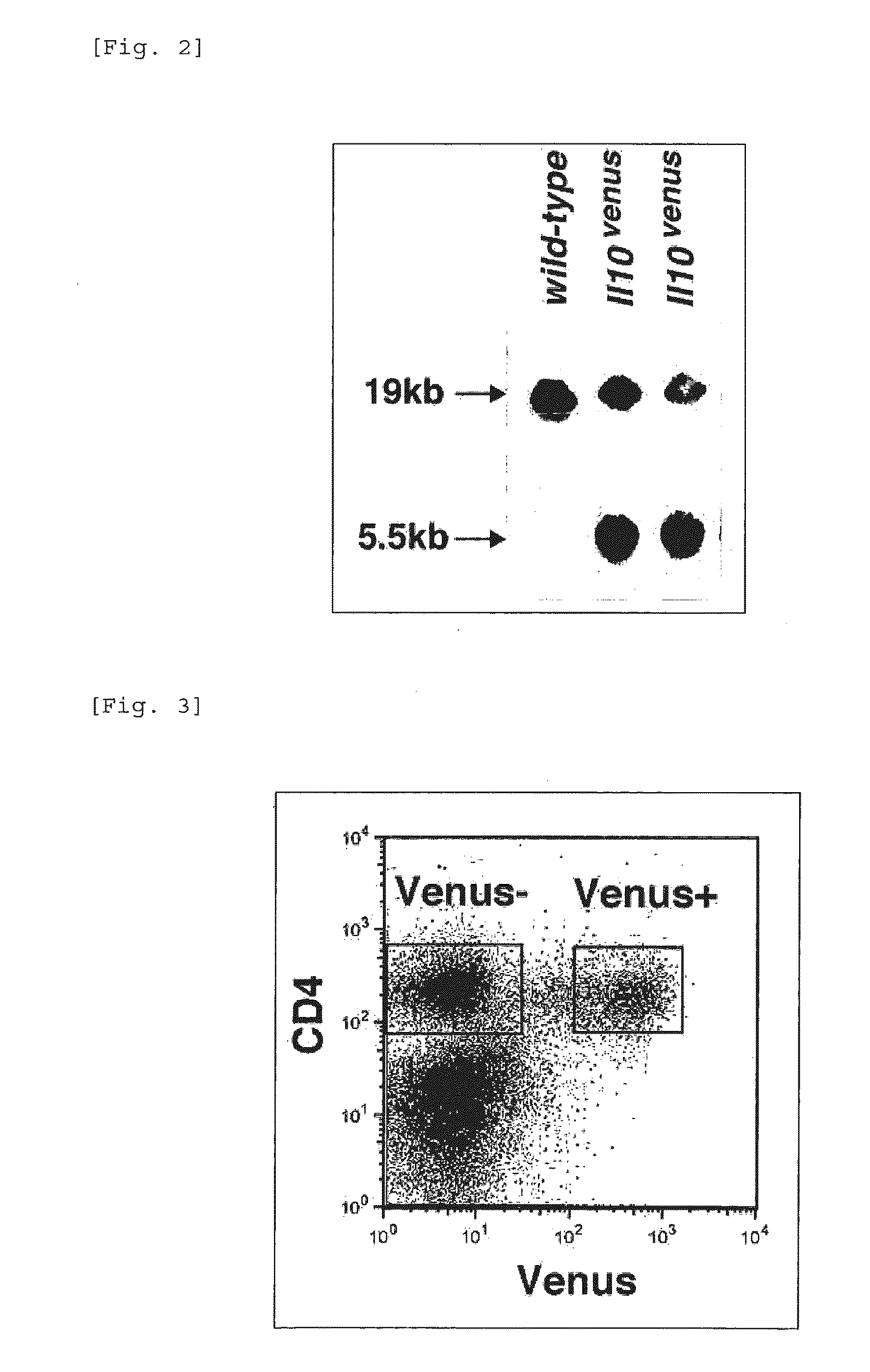
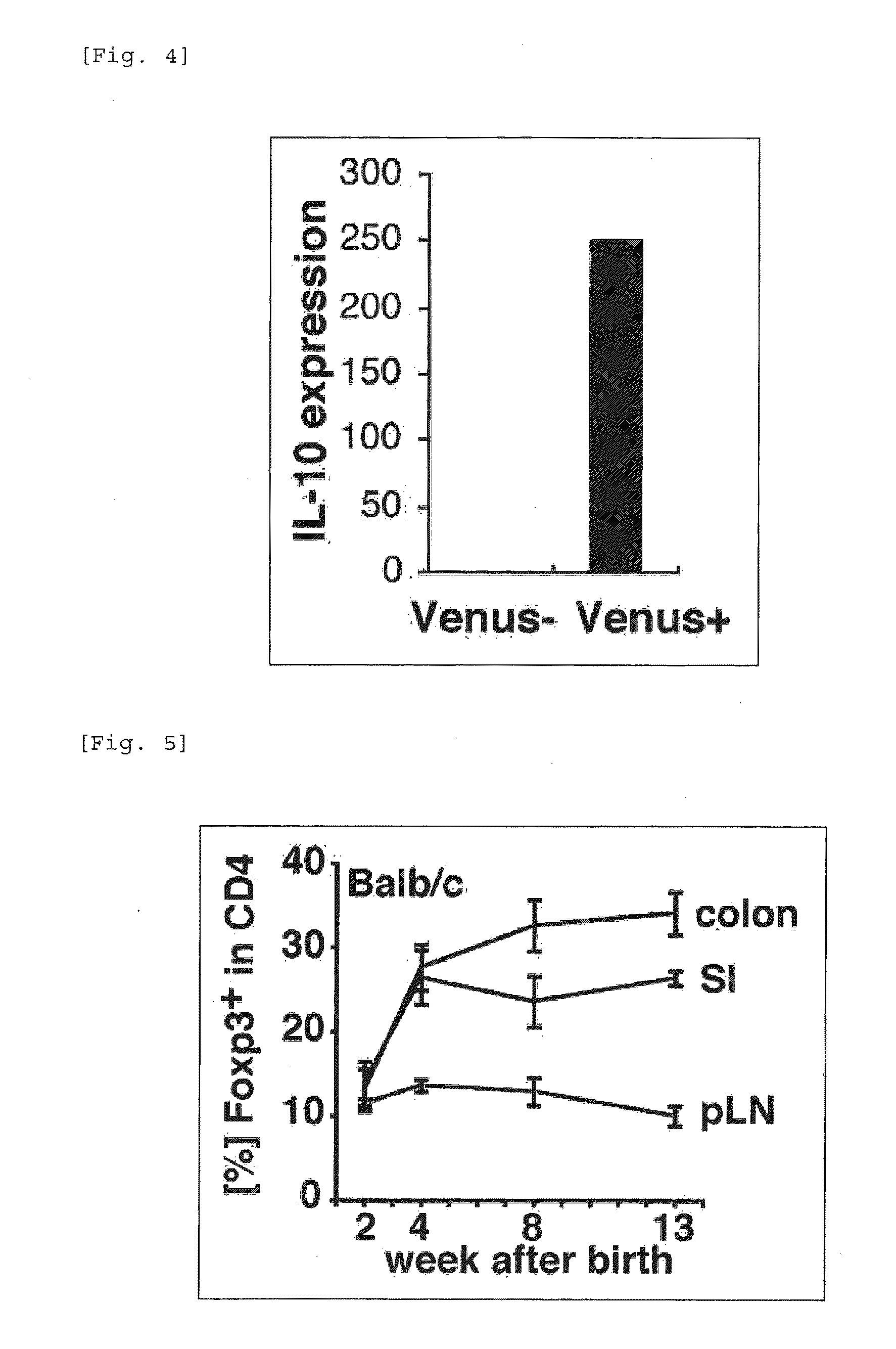
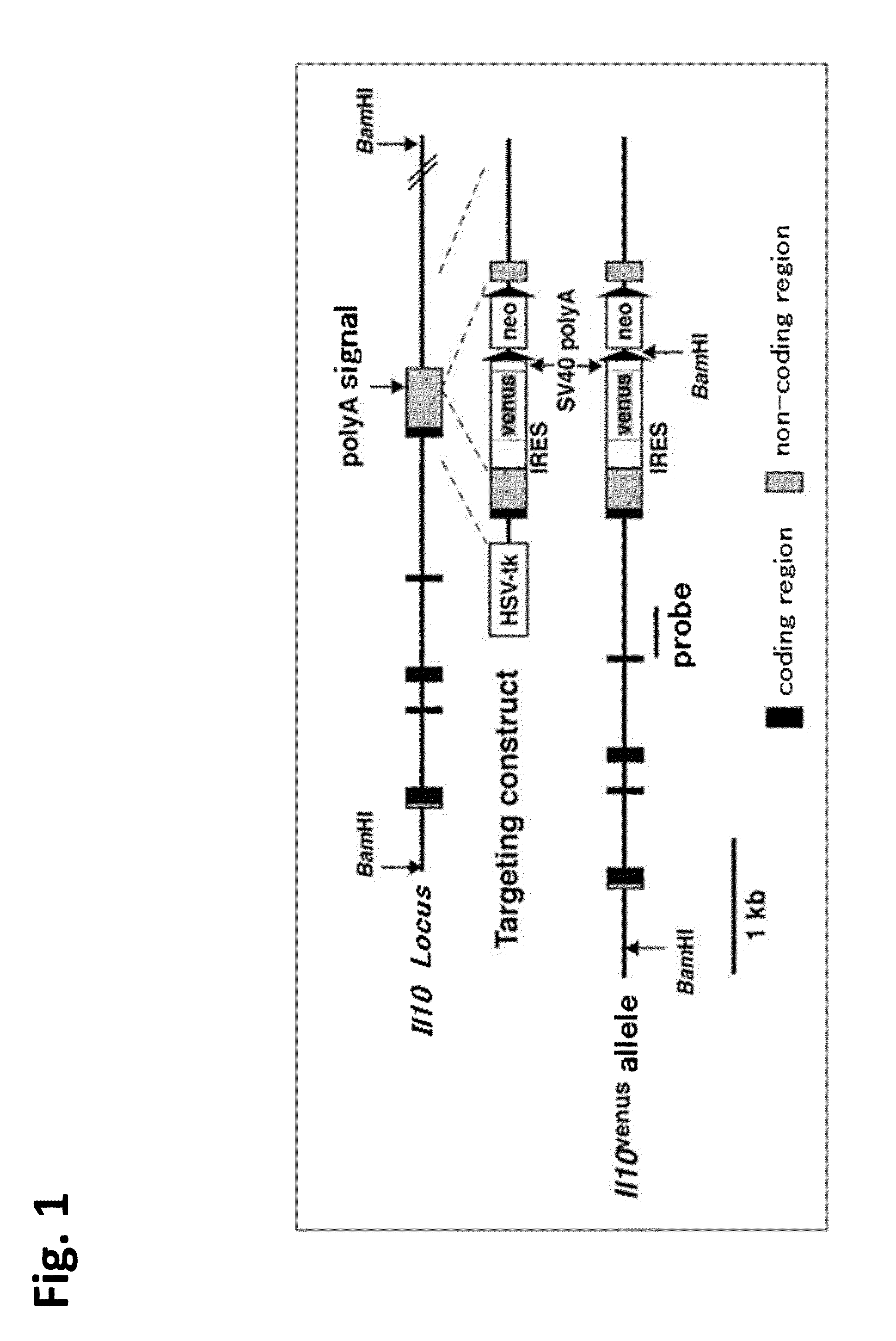
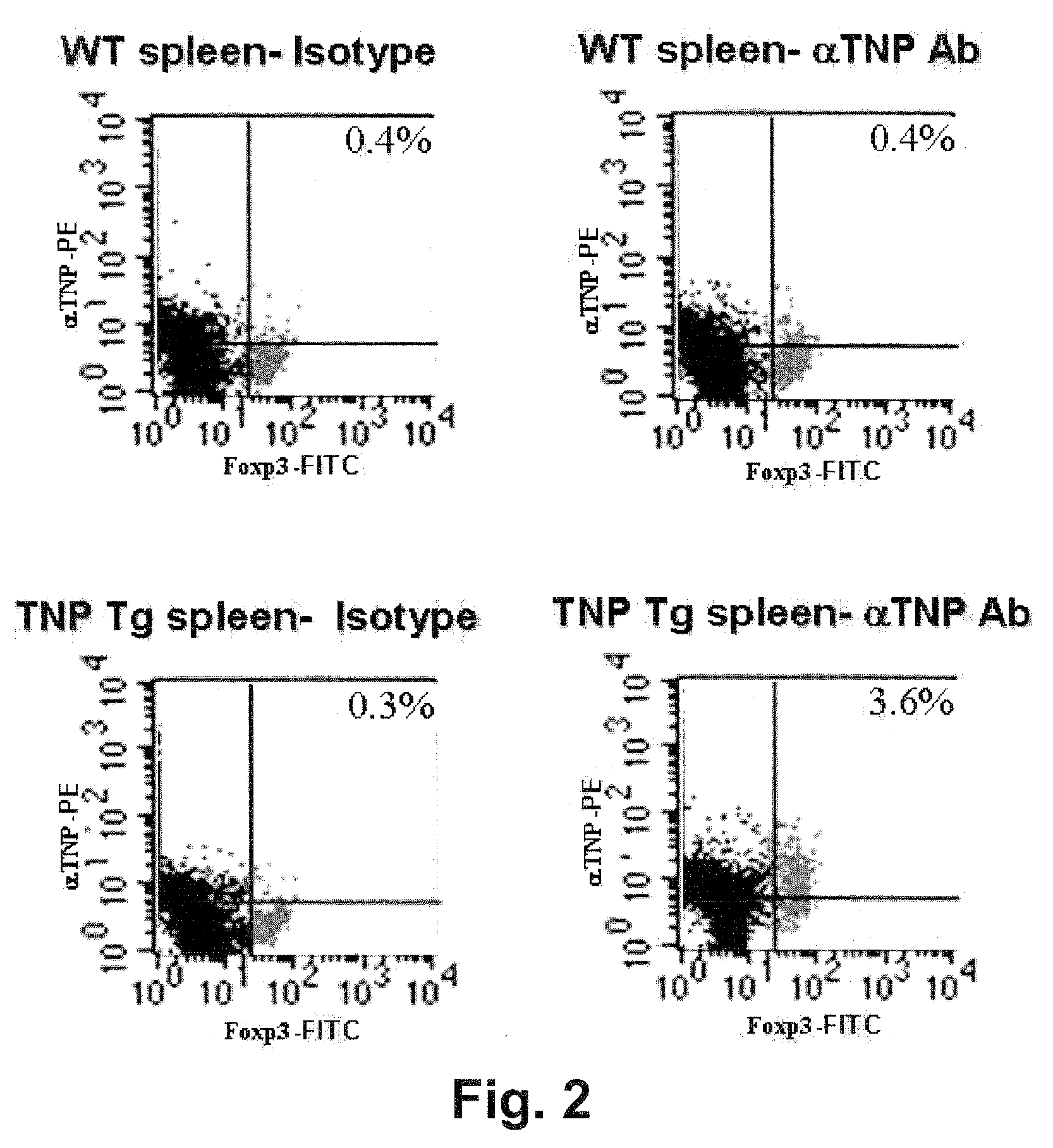
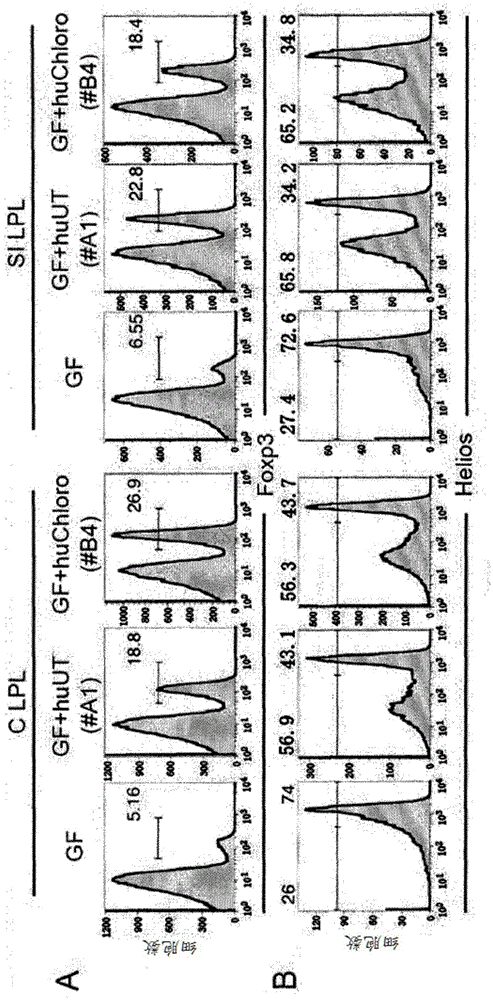
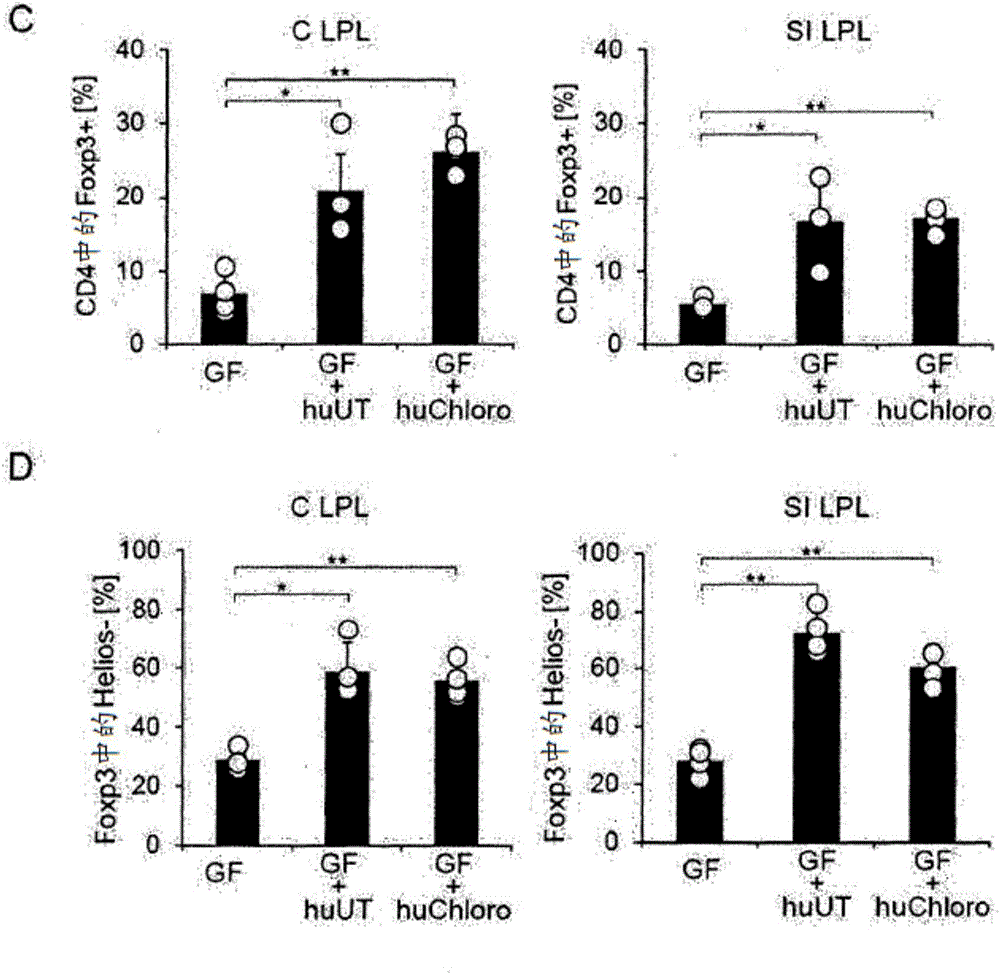
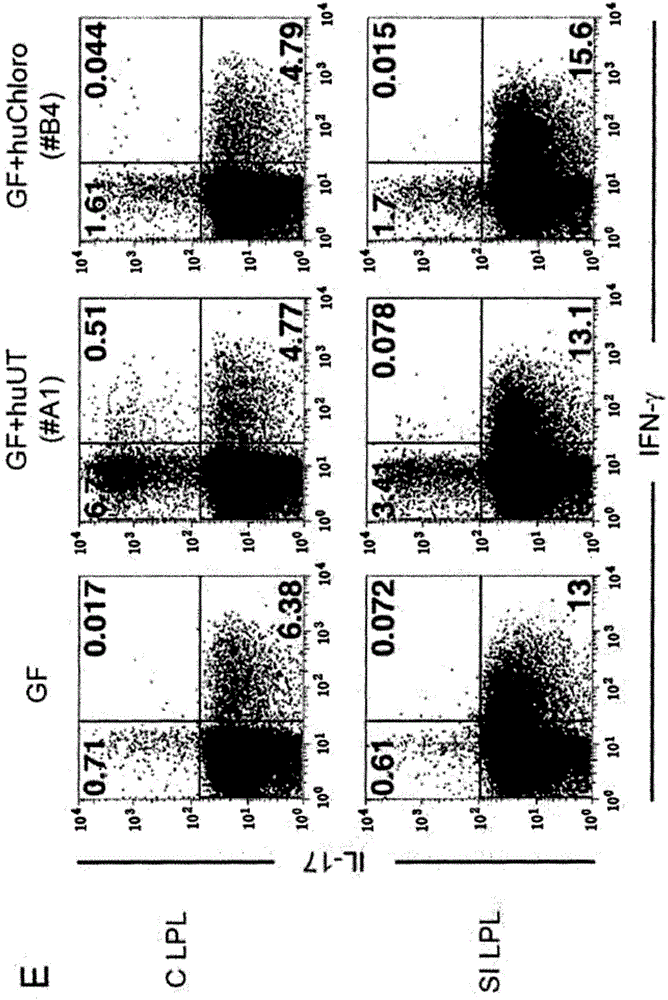
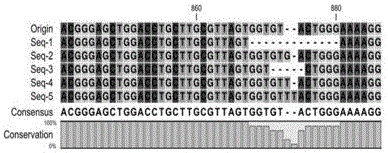
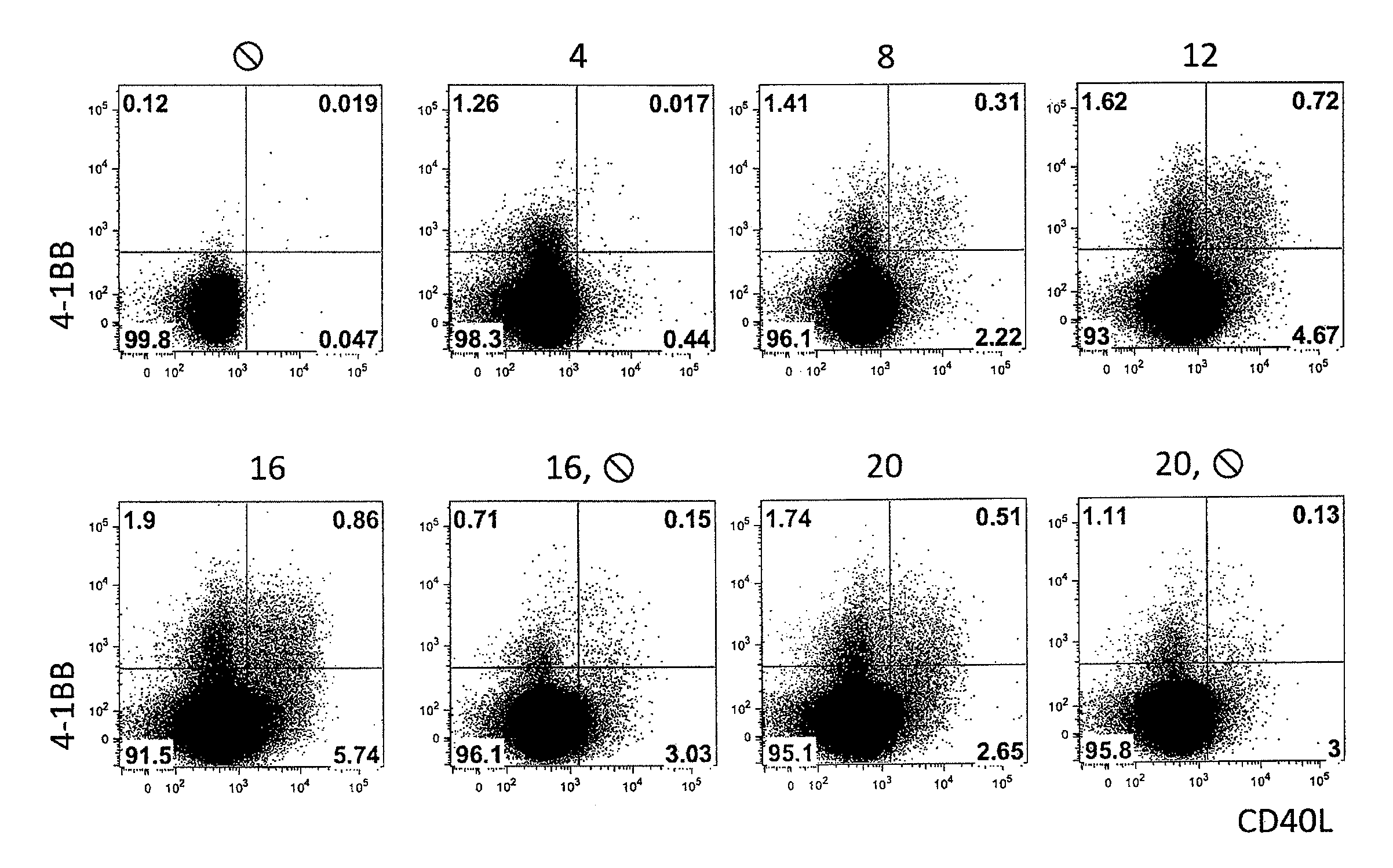
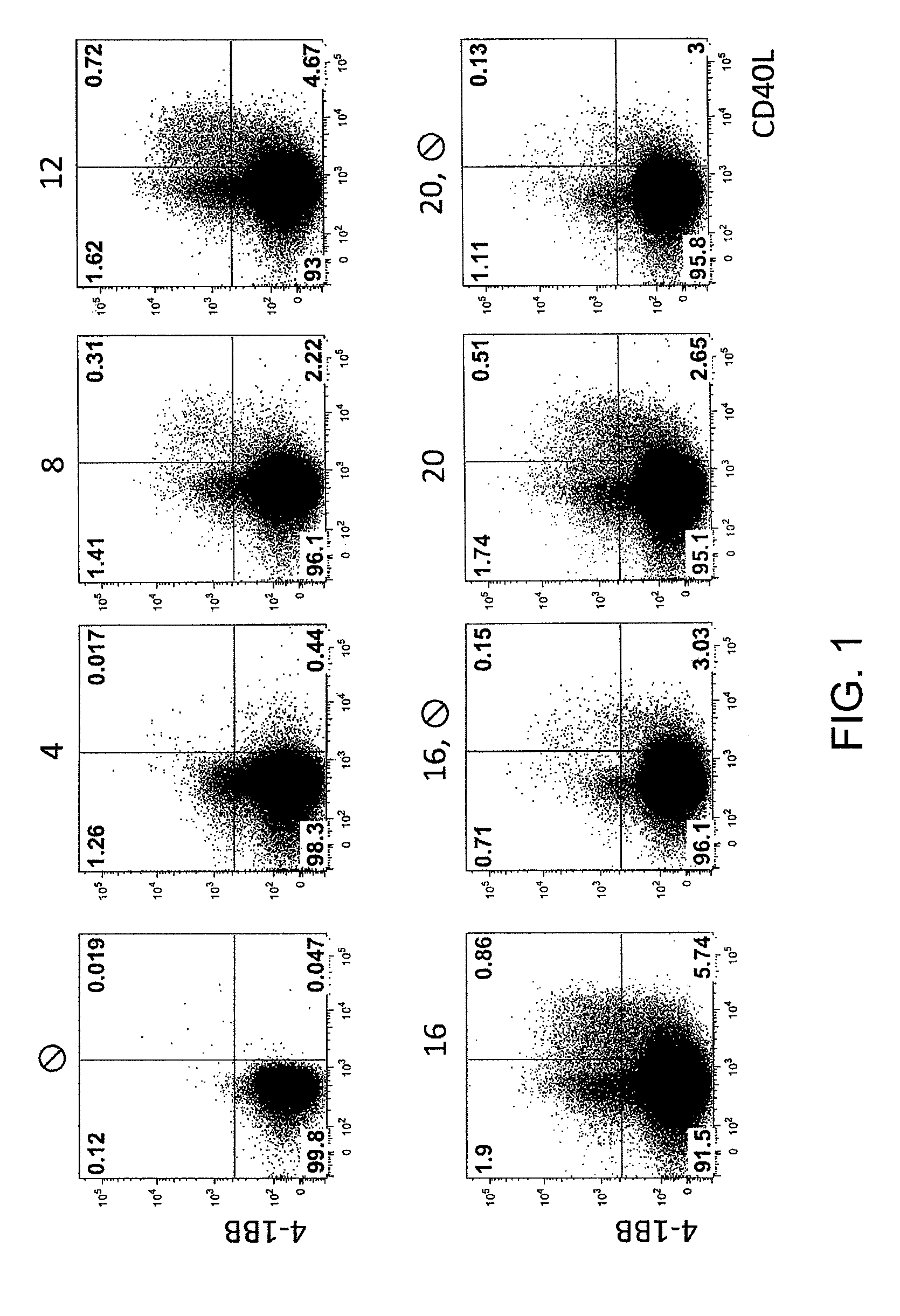
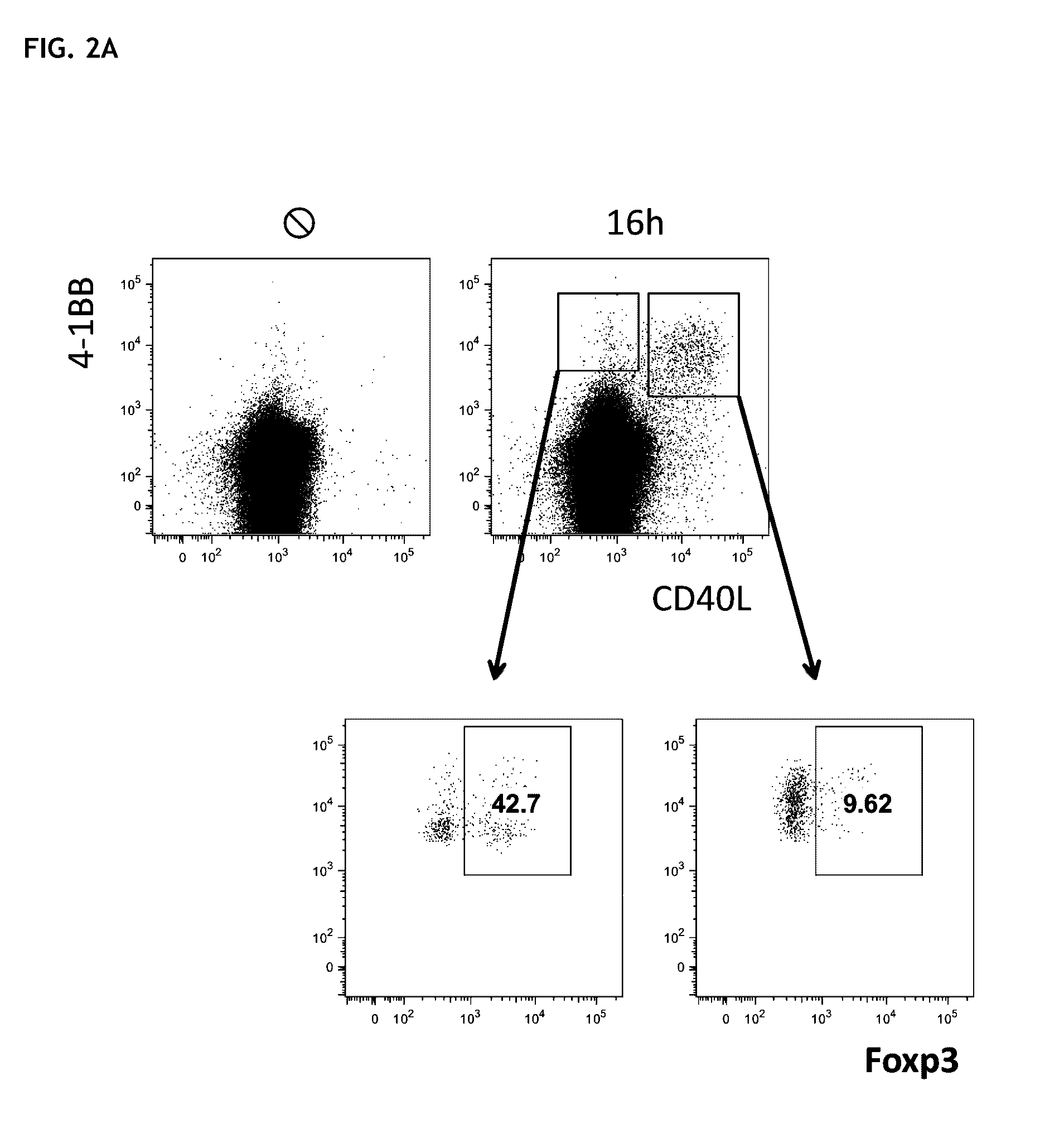
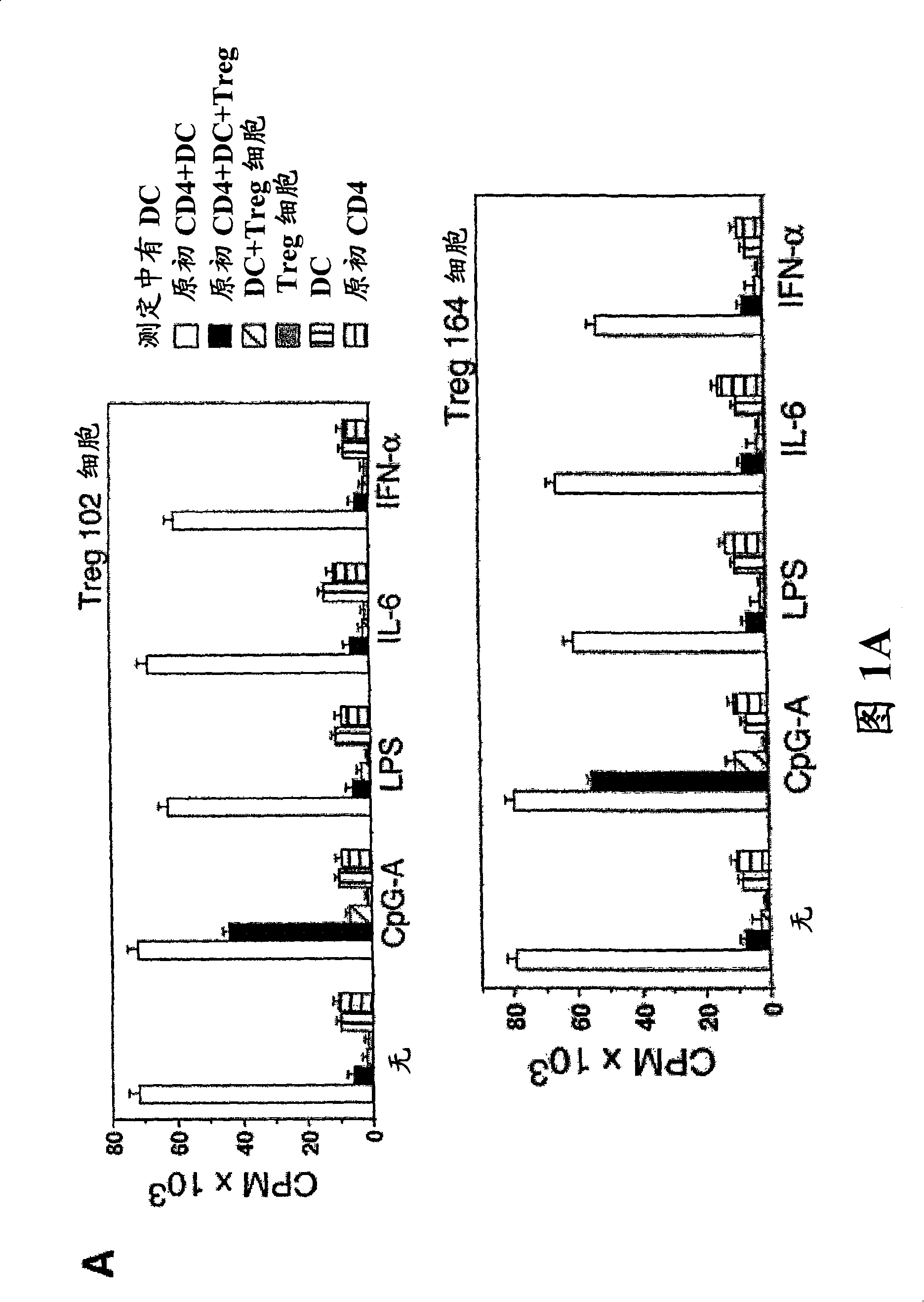
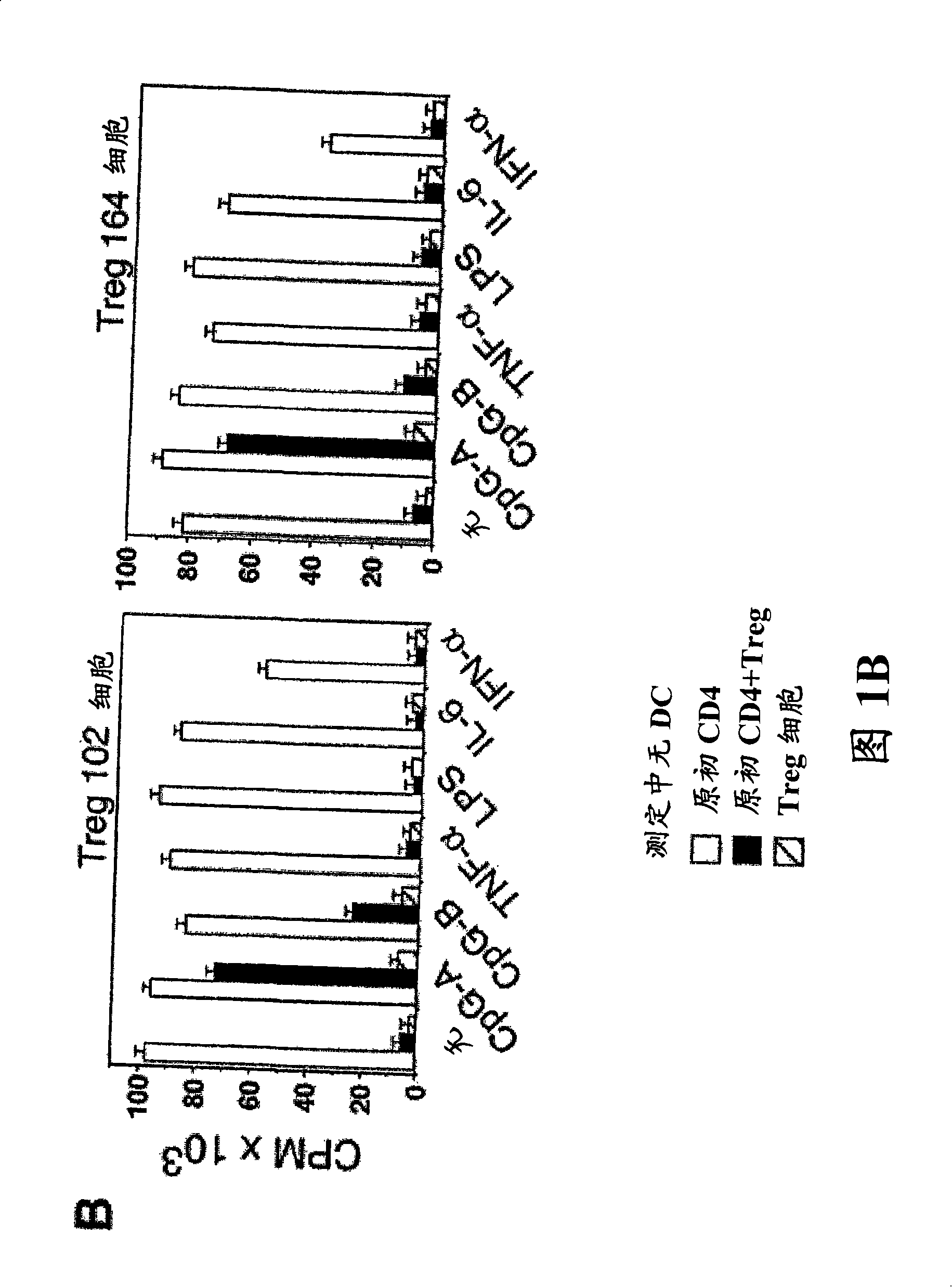
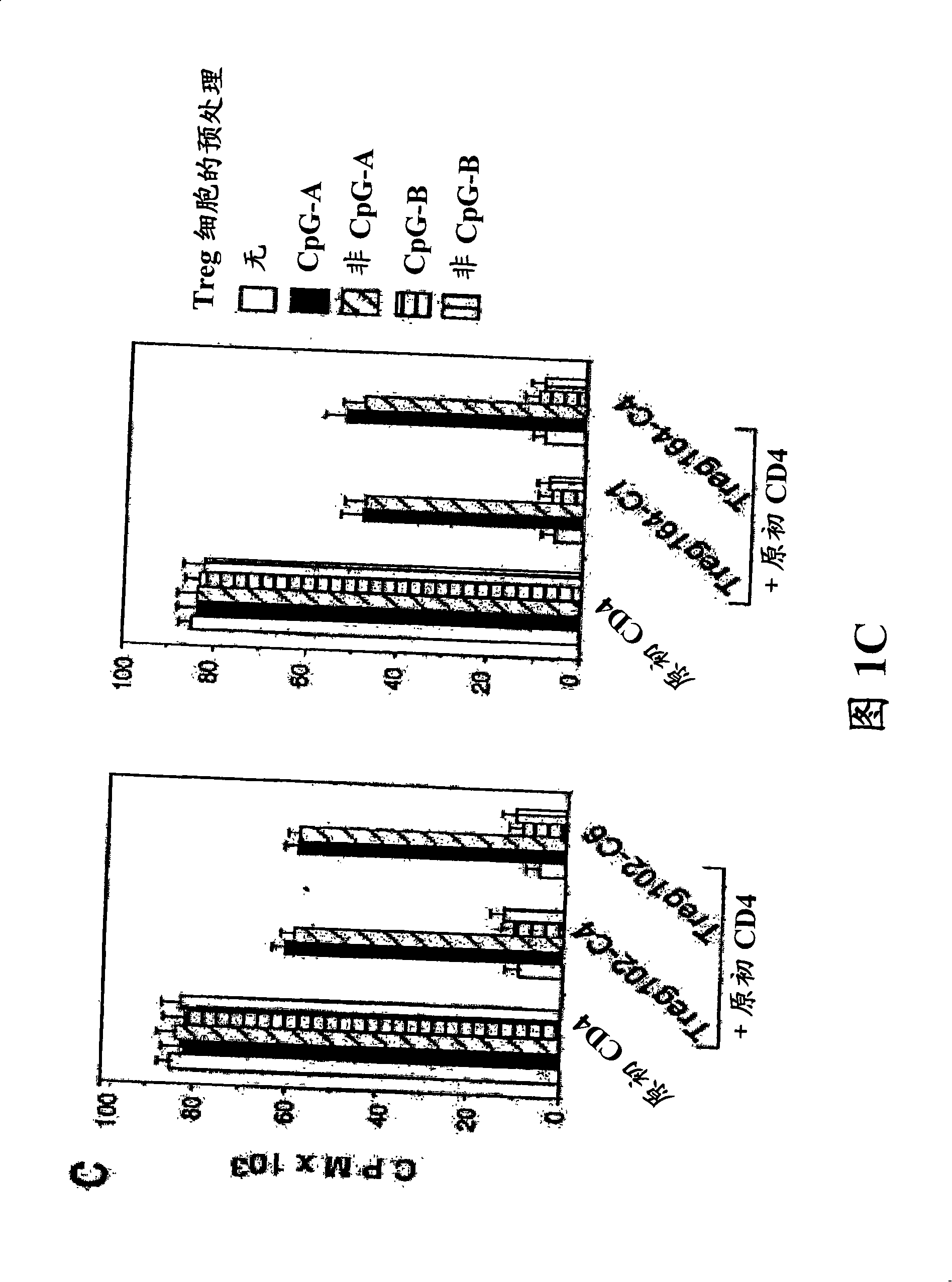
Patents
Literature
212 results about "Treg cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Definitions - treg cells. Treg Cells (n.) 1.(MeSH)CD4-positive T cells that inhibit immunopathology or autoimmune disease in vivo. They inhibit the immune response by influencing the activity of other cell types.
Composition for inducing proliferation or accumulation of regulatory t cells
InactiveUS20130149339A1Induced proliferationInduce accumulationAntibacterial agentsCompound screeningRegulatory T cellT cell
It was found that bacteria belonging to the genus Clostridium induce accumulation of regulatory T cells (Treg cells) in the colon. Moreover, the present inventors found that regulatory T cells (Treg cells) induced by from these bacteria suppressed proliferation of effector T-cells. From these findings, the present inventors found that the use of bacteria belonging to the genus Clostridium or a physiologically active substance derived therefrom made it possible to induce proliferation or accumulation of regulatory T cells (Treg cells), and further to suppress immune functions.
Owner:THE UNIV OF TOKYO
Composition for inducing proliferation or accumulation of regulatory t cells
ActiveUS20150143557A1Induce proliferation and accumulationImmunity in a living organism can be suppressedAntibacterial agentsCompound screeningRegulatory T cellT cell
It was found that bacteria belonging to the genus Clostridium induce accumulation of regulatory T cells (Treg cells) in the colon. Moreover, the present inventors found that regulatory T cells (Treg cells) induced by from these bacteria suppressed proliferation of effector T-cells. From these findings, the present inventors found that the use of bacteria belonging to the genus Clostridium or a physiologically active substance derived therefrom made it possible to induce proliferation or accumulation of regulatory T cells (Treg cells), and further to suppress immune functions.
Owner:THE UNIV OF TOKYO
Redirected, genetically-engineered t regulatory cells and their use in suppression of autoimmune and inflammatory disease
InactiveUS20100135974A1Effective quantityOvercome scarcityBiocideAntipyreticIntracellular signallingInflammatory Bowel Diseases
A redirected Treg cell is endowed with specificity toward a selected target antigen or ligand. The cell contains a chimeric receptor polypeptide that is expressed in a single, continuous chain, with an extracellular recognition region displayed on the surface of the cell, a transmembrane region and an intracellular signaling region. The extracellular recognition region is specific for the selected target antigen or ligand. The intracellular signaling region includes a combination of T-cell signaling polypeptide moieties, which combination, upon binding of the extracellular recognition region to the selected target antigen or ligand, triggers activation of the redirected Treg cells to cause suppression of T-cell mediated immunity. Such redirected Treg cells may be used to suppress undesired activity of T effector cells thereby mediating an immune or inflammatory response. They are particularly useful in treating T effector cell-mediated diseases, such as inflammatory bowel disease, transplant rejection and GVH disease.
Owner:YEDA RES & DEV CO LTD
Human-derived bacteria that induce proliferation or accumulation of regulatory t cells
InactiveCN104160014APromote proliferationPromote accumulationBacteriaAntipyreticBacteroidesRegulatory T cell
Species of human-derived bacteria belonging to the Clostridia class have been shown to induce accumulation of regulatory T cells (Treg cells) in the colon and suppress immune functions. Pharmaceutical compositions containing these bacteria can be used to prevent and treat immune-mediated diseases such as autoimmune diseases.
Owner:THE UNIV OF TOKYO +1
Methods for Generating Improved Immune Reponse
InactiveUS20080220000A1Enhance immune responseViral antigen ingredientsAntibody ingredientsImmunologyTreg cell
This application relates to a method for generating an improved immune response in a host. The method involves the step of administering a vectored vaccine in the presence of an agent that impairs Treg cell function.
Owner:ISIS INNOVATION LTD
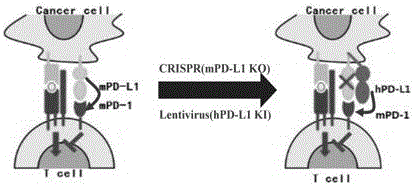
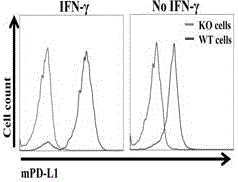
Humanized PD-L1 tumor cell line, animal model with same and application of humanized PD-L1 tumor cell line and animal model
ActiveCN105950560ASpeed up the processLethalCompounds screening/testingCell receptors/surface-antigens/surface-determinantsPD-L1Wilms' tumor
The invention provides a humanized PD-L1 tumor cell line MC-38-hPD-L1, a builtanimal tumor model with the same and a method for constructing the humanized PD-L1 tumor cell line. The method particularly includes knocking out animal-origin PD-L1 by the aid of CRISPR-CAS9; carrying out amplification and cultivation to obtain knocked-out cell banks; extracting DNA (deoxyribonucleic acid) and carrying out PCR (polymerase chain reaction) amplification; recycling and cloning amplification products; carrying out over-expression on human-origin PD-L1 in MC-38 cell lines of mPD-L1 KO by the aid of lentivirus systems; packaging lentivirus and screening Puromycin to obtain the humanized MC-38 cell line of PD-L1. The humanized PD-L1 tumor cell line, the animal tumor model and the method have the advantages that as shown by results, high killing efficiency and multiplication capacity are obviously presented by tumor infiltration CD8 T lymphocytes after antibody treatment is carried out, tumor infiltration Treg cells can be obviously inhibited after antibody treatment is carried out, and accordingly the method is proved to be effective and feasible from the aspect of molecular mechanisms.
Owner:SUZHOU INST OF SYST MEDICINE
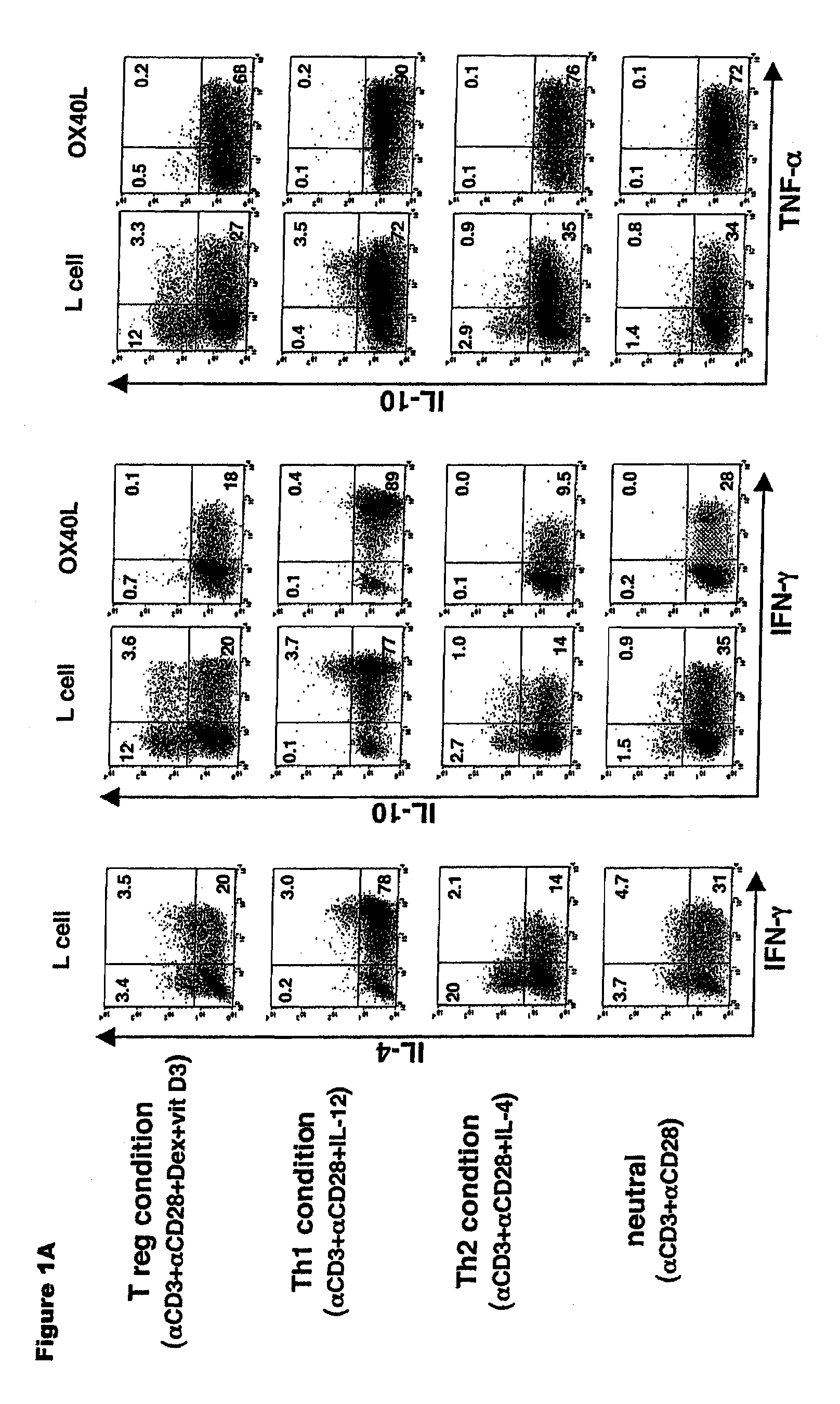
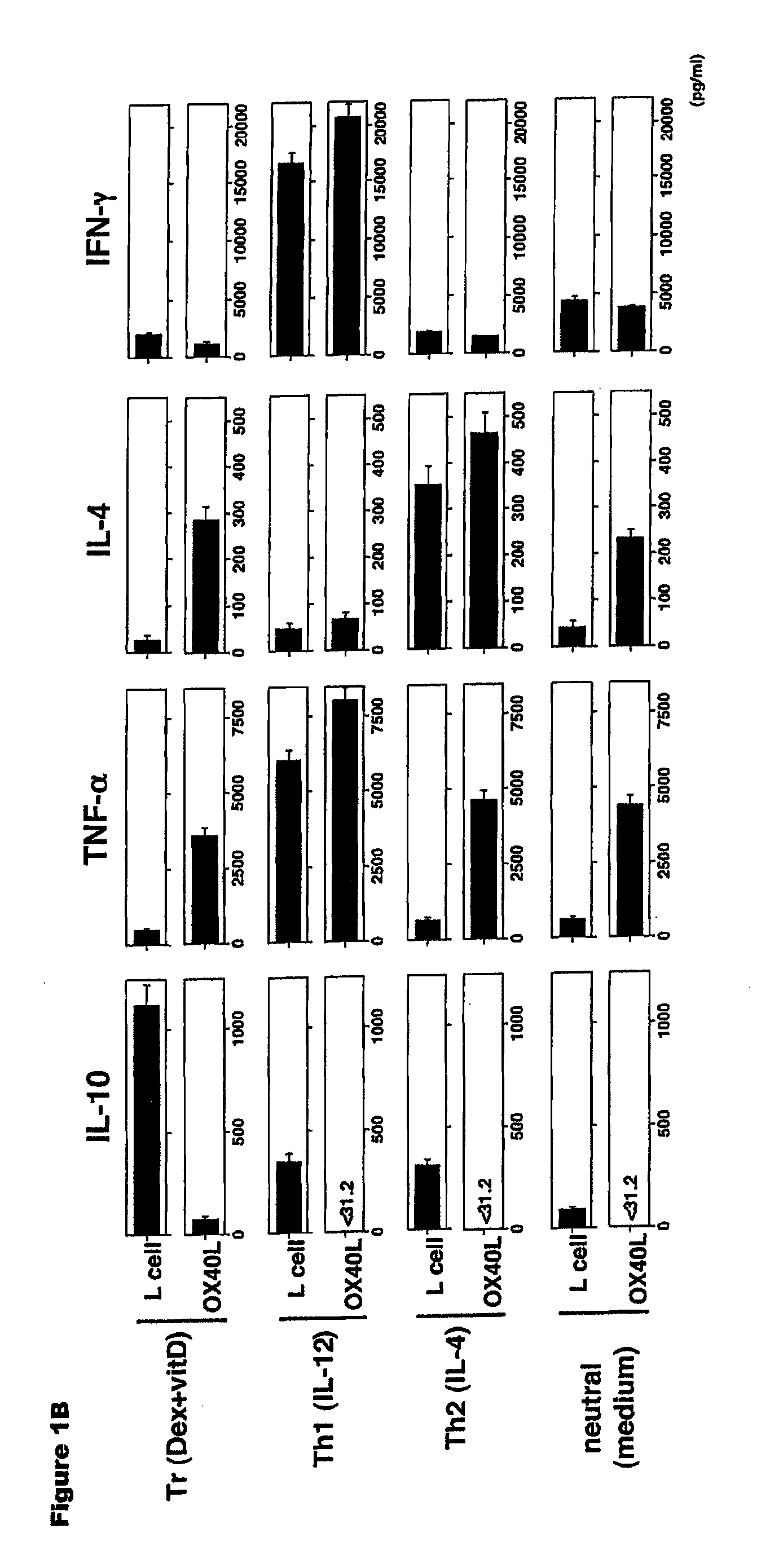
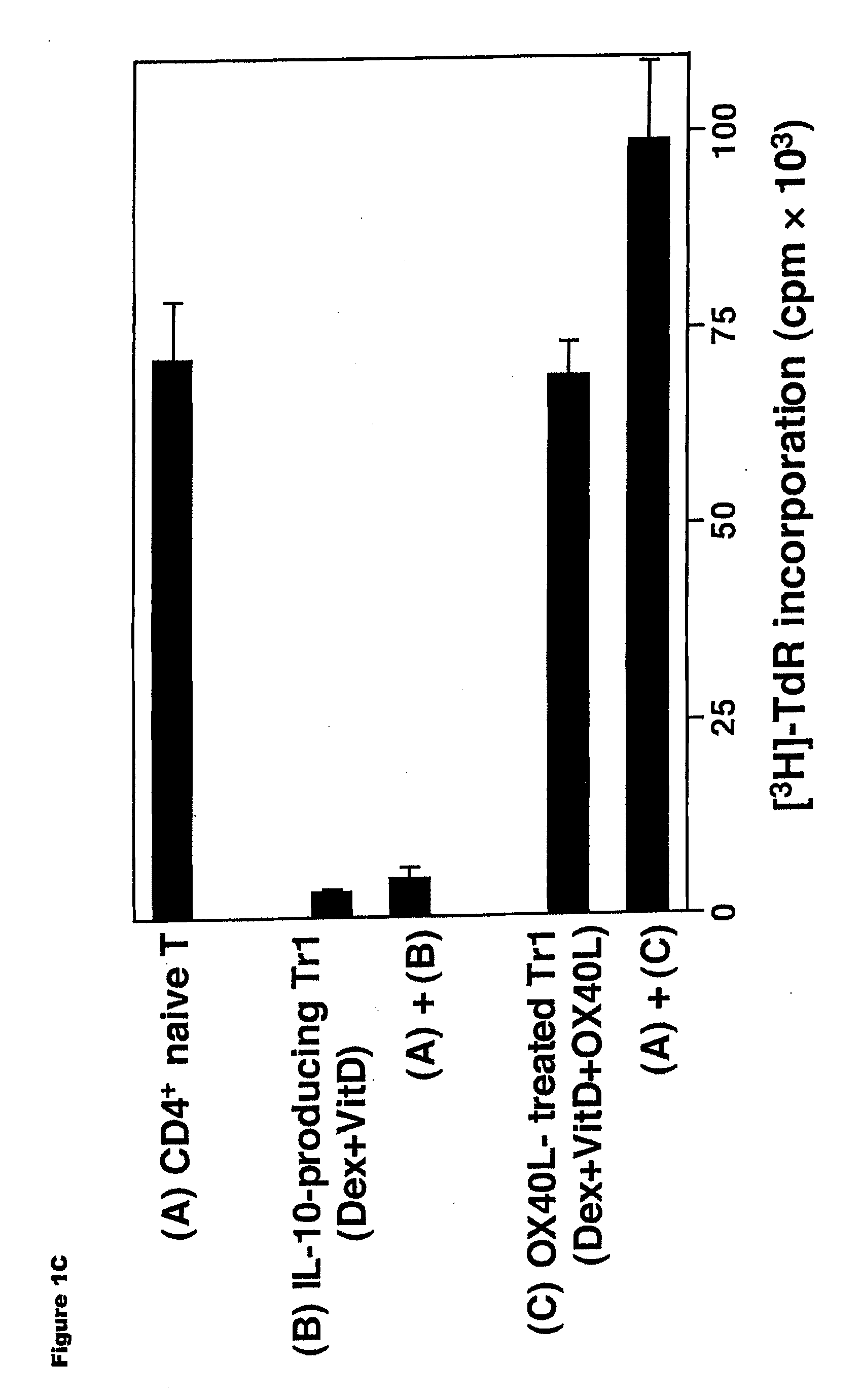
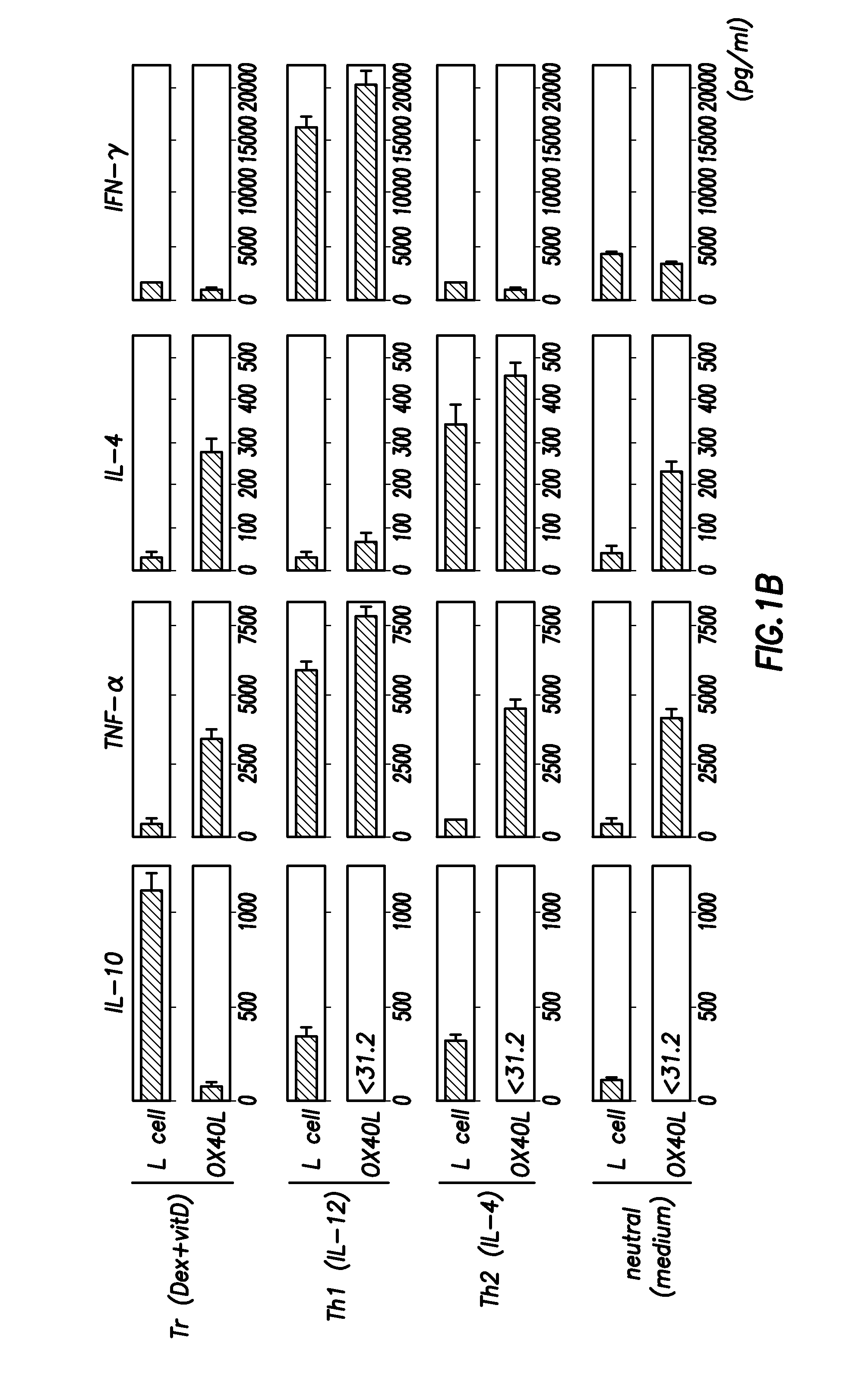
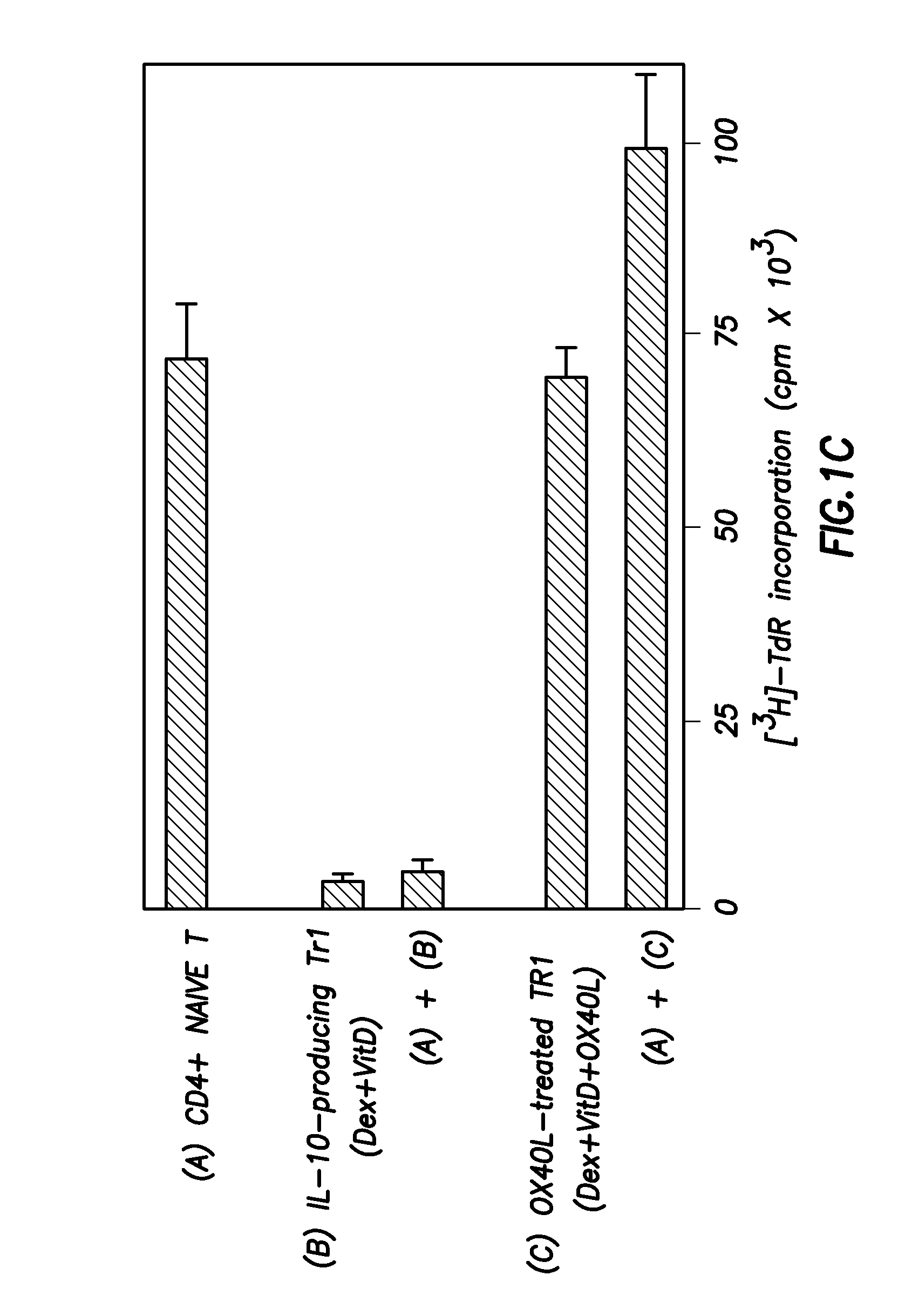
Methods of modulating the ox40 receptor to treat cancer
Numerous disease states, such as human allergic, autoimmune, and autoimmune diseases, and cancer, may be treated by targeting OX40 / OX4OL. OX4OL inhibits the generation of Tr1 cells from naïve and memory CD4+ T cells. This unique function of OX4OL is not shared by two other costimulatory TNF-family members, GITR-ligand and 4-1BB-ligand. It has been shown that signaling the OX40-receptor on human T cells by antibodies, small molecules, or the OX4OL modulates the generation and function of IL-10 producing Foxp3+ Treg immunosuppressive T cells and blocks Foxp3+ Treg function. Further, provided are high throughput methods for identifying compounds that can inhibit the immunosuppressive function of IL-10 producing Tr1 cells.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Amplifying, freezing and storing and recovering method of activated lymphocyte with CD3+CD8+as major
InactiveCN102839153ASolve the problem of multiple blood collectionHigh purityDead animal preservationBlood/immune system cellsPatient needT lymphocyte
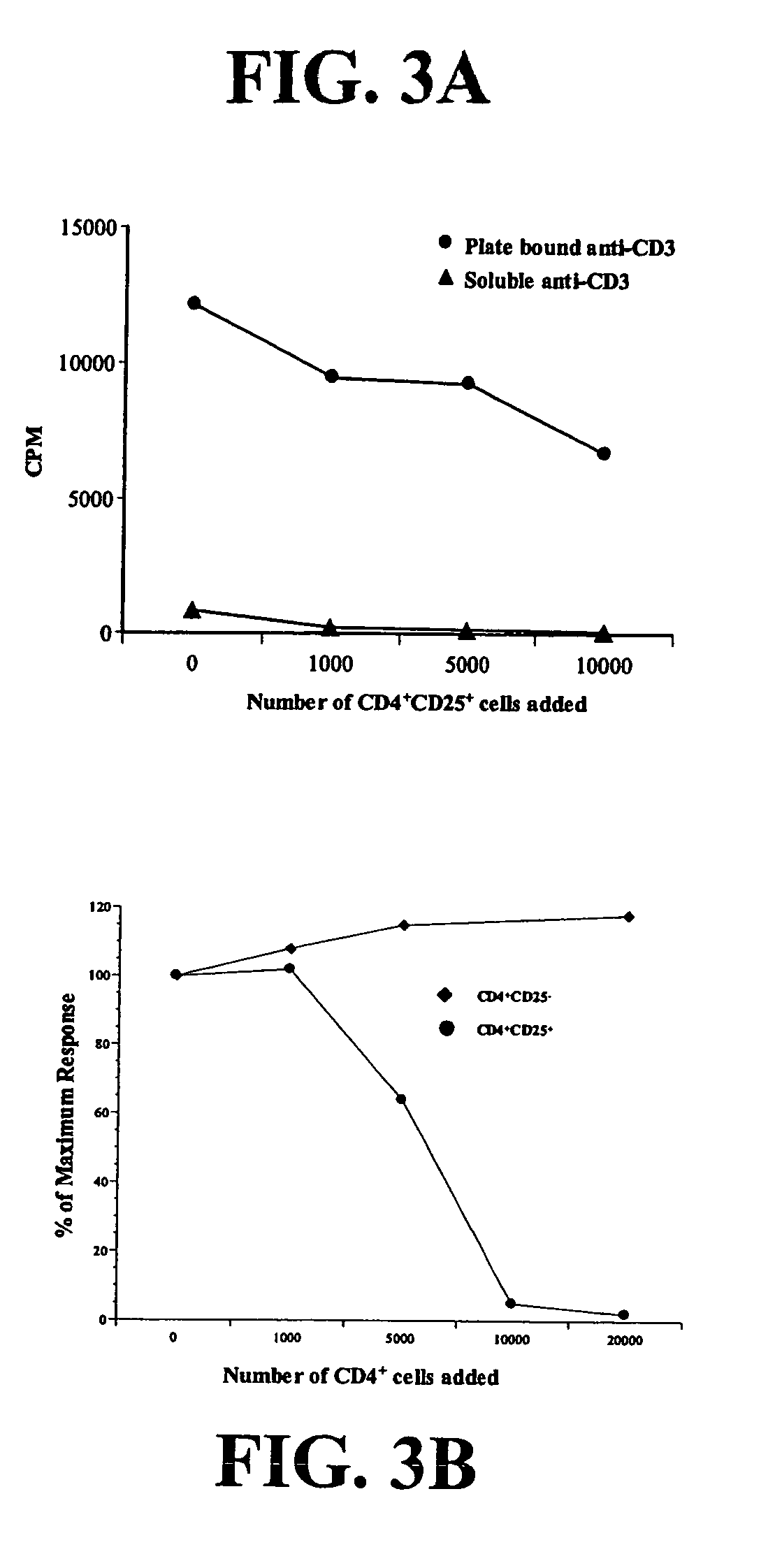
The invention discloses culturing, freezing and recovering methods of an activated lymphocyte with CD3+CD8+as major, which can solve problems that a patient needs carrying out blood sampling for many times caused by continuously utilizing the activated lymphocyte with CD3+CD8+as major. The method comprises the following steps of: (1) contacting the extracted lymphocyte of the peripheral blood with IL-2, IL-15, an anti-CD3 antibody and an anti-CD28 antibody, so as to amplify the activated lymphocyte with CD3+CD8+as major; (2) freezing and storing the activated lymphocyte; and (3) recovering the activated lymphocyte. The activated lymphocyte cultured via the method disclosed by the invention has clear components, and comprises few CD4+CD25+Treg cells and more CD8+T lymphocyte; feedback time and frequency of the activated lymphocyte can be adjusted according to other treatments for a patient, such as a radiotherapy or a chemotherapy, so that diseases, such as tumor, infectious diseases and immunodeficiency can be treated well.
Owner:JINAN TAISHENG BIOLOGICAL TECH CO LTD
Methods for generating antigen-specific effector T cells
InactiveCN101415827AImmunoglobulin superfamilyMicroinjection basedAutoimmune conditionAutoimmune disease
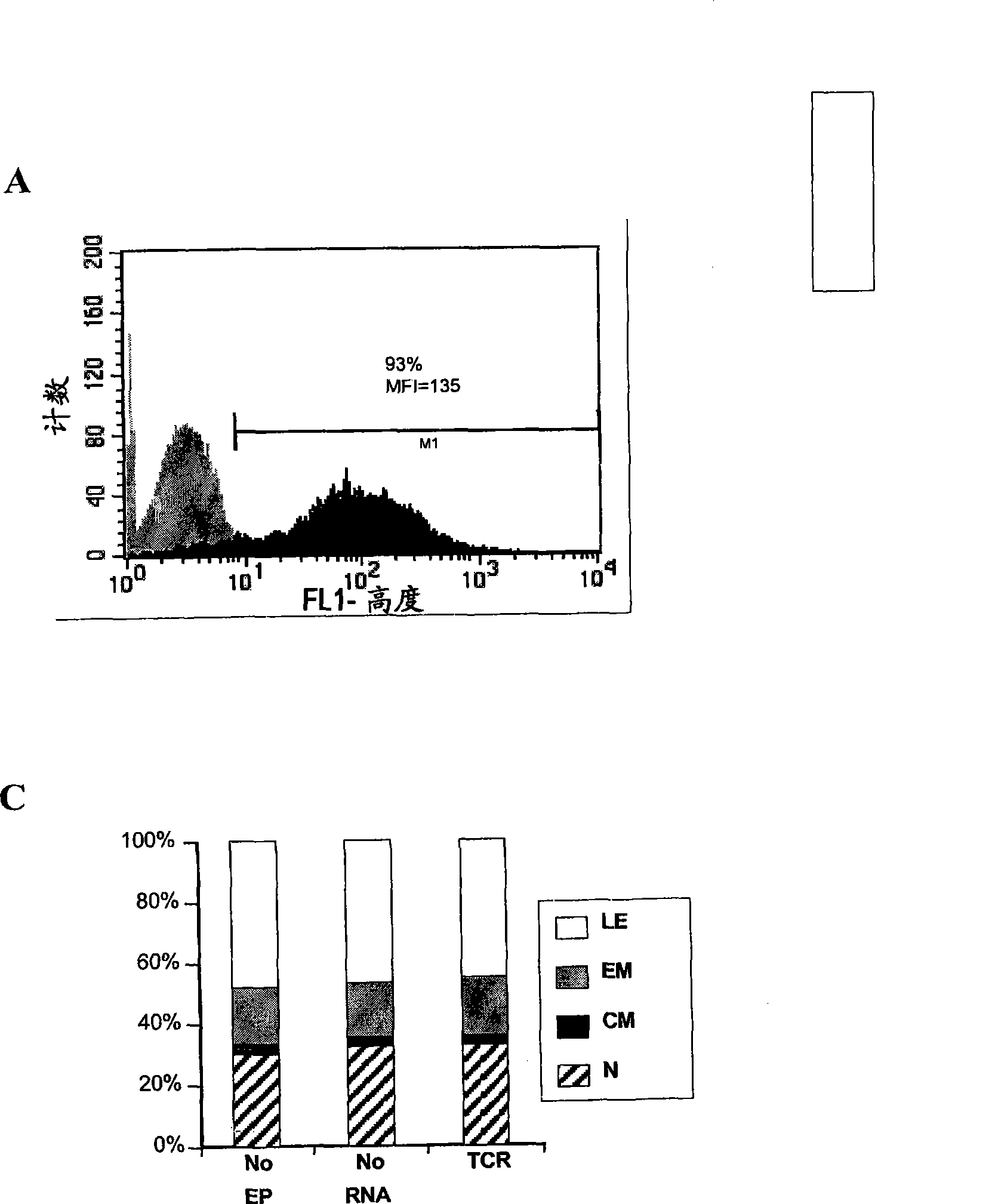
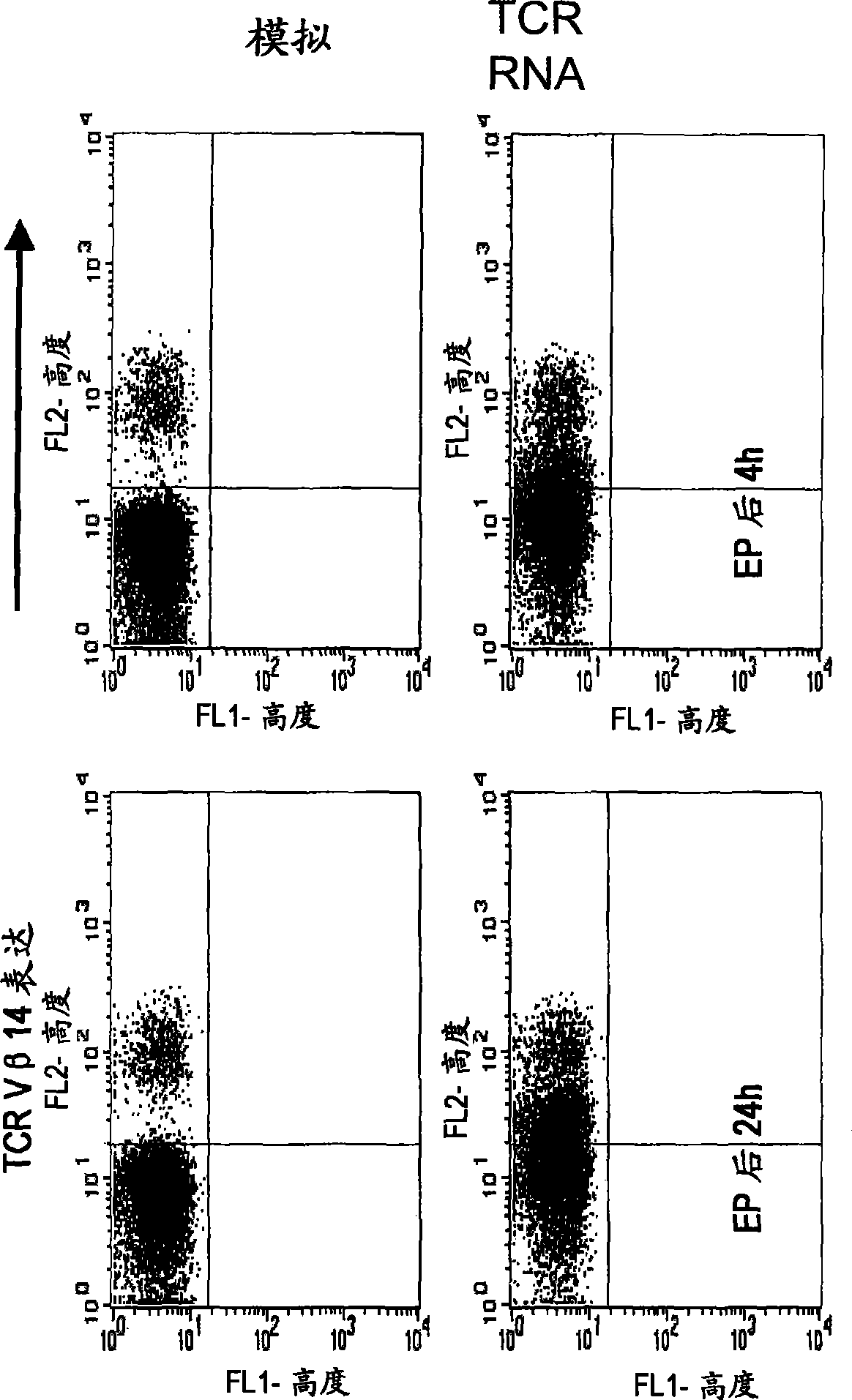
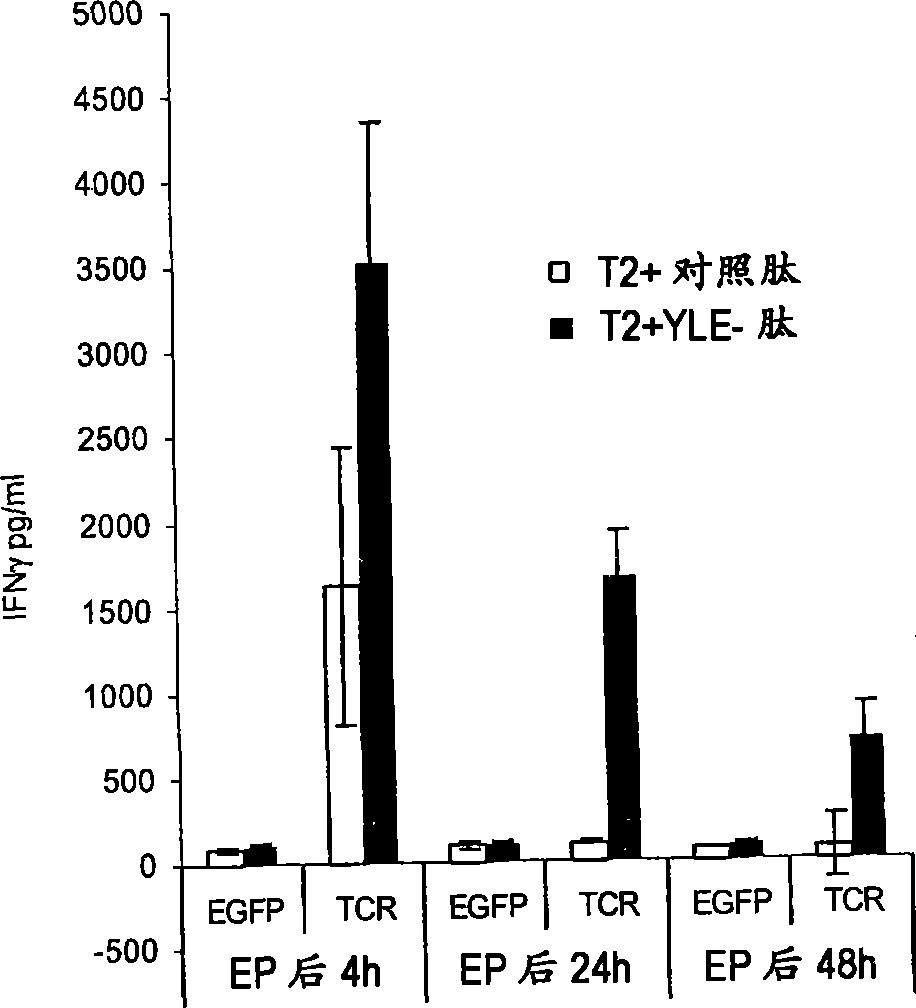
The invention relates to T cells transiently transfected with RNA, especially RNA encoding a T cell receptor and / or FoxP3, and methods of transfecting T cells with RNA by electroporation. Compositions of the invention include an effector T cell transiently transfected with RNA encoding a T cell receptor (TCR) specific for an antigen, wherein the T cell demonstrates effector function specific for cells presenting the antigen in complex with an MHC molecule. Treg cells comprising an exogenous RNA encoding FoxP3 are also provided. The transfected T cells are useful for immunotherapy, particularly in the treatment of tumors, pathogen infection, autoimmune disease, transplant rejection and graft versus host disease.
Owner:ARGOS THERAPEUTICS INC
Preparation method of high-purity, high-multiplication capacity and high-cytotoxin activity CIK (cytokine induced kill) cell
ActiveCN102154206AIncrease the number ofHigh activityMammal material medical ingredientsBlood/immune system cellsPhytohemagglutininsPeripheral blood mononuclear cell
The invention belongs to the technical field of cell culture in vitro, and particularly relates to a preparation method of high-purity, high-multiplication capacity and high-cytotoxin activity CIK (cytokine induced kill) cell. The method comprises the following steps: collecting and separating peripheral blood mononuclear cell of a patient, eliminating CD4+CD25+Treg cell by means of Mini MACS (magnetic active cell sorting) method, and sorting to obtain CD3+, CD4+ and CD8+T cells; and putting the obtained cells into culture solution containing phytohemagglutinin (PHA), so that the PHA concentration in the suspension liquid is 100ng / ml, hatching for 24h under the culture condition of 5% CO2 at 37 DEG C, transferring the hatched suspension liquid into a cell culture bottle coated by CD3 monoclonal antibody (1mug / ml), adding IFN (interferon)-gamma (1000U / ml), adding IL (interleukin)-2(500U / ml) and IL (interleukin)-21(1000U / ml) after 48h, compensating sodium selenite-containing (0.005mg / L)cell culture after four days, and continuously culturing for 7-14 days to obtain the high-purity, high-multiplication capacity and high-cytotoxin activity CIK (cytokine induced kill) cell. The quantity, the activity and the purity of the CIK cell which is prepared by the method and amplified in vitro are improved, so that the antineoplastic function of the CIK is enhanced.
Owner:郑骏年
Methods of modulating the ox40 receptor to treat cancer
Numerous disease states, such as human allergic, autoimmune, and autoimmune diseases, and cancer, may be treated by targeting OX40 / OX40L. OX40L inhibits the generation of Tr1 cells from naïve and memory CD4+ T cells. This unique function of OX40L is not shared by two other costimulatory TNF-family members, GITR-ligand and 4-1BB-ligand. It has been shown that signaling the OX40-receptor on human T cells by antibodies, small molecules, or the OX40L modulates the generation and function of IL-10 producing Foxp3+ Treg immunosuppressive T cells and blocks Foxp3+ Treg function. Further, provided are high throughput methods for identifying compounds that can inhibit the immunosuppressive function of IL-10 producing Tr1 cells.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
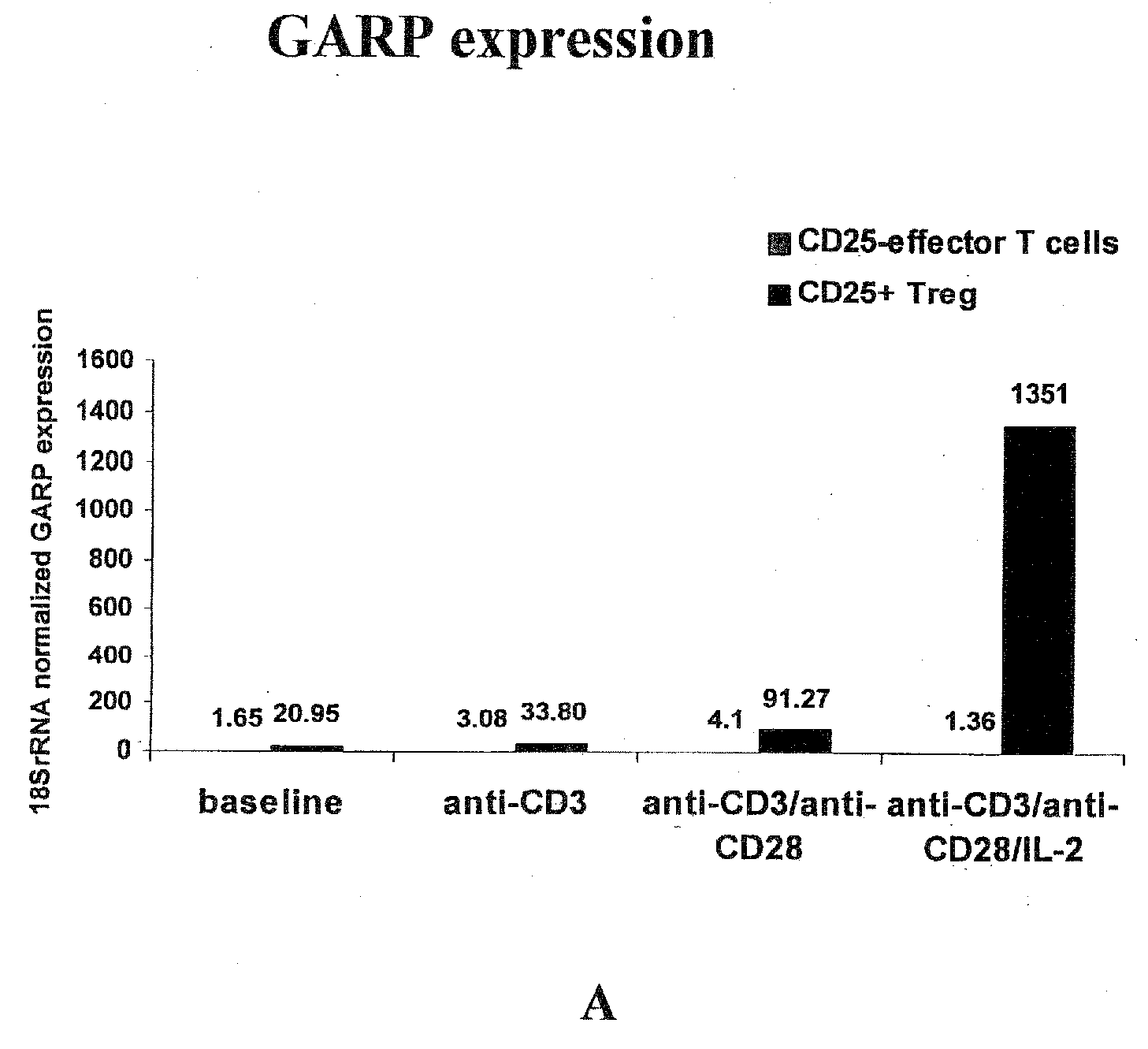
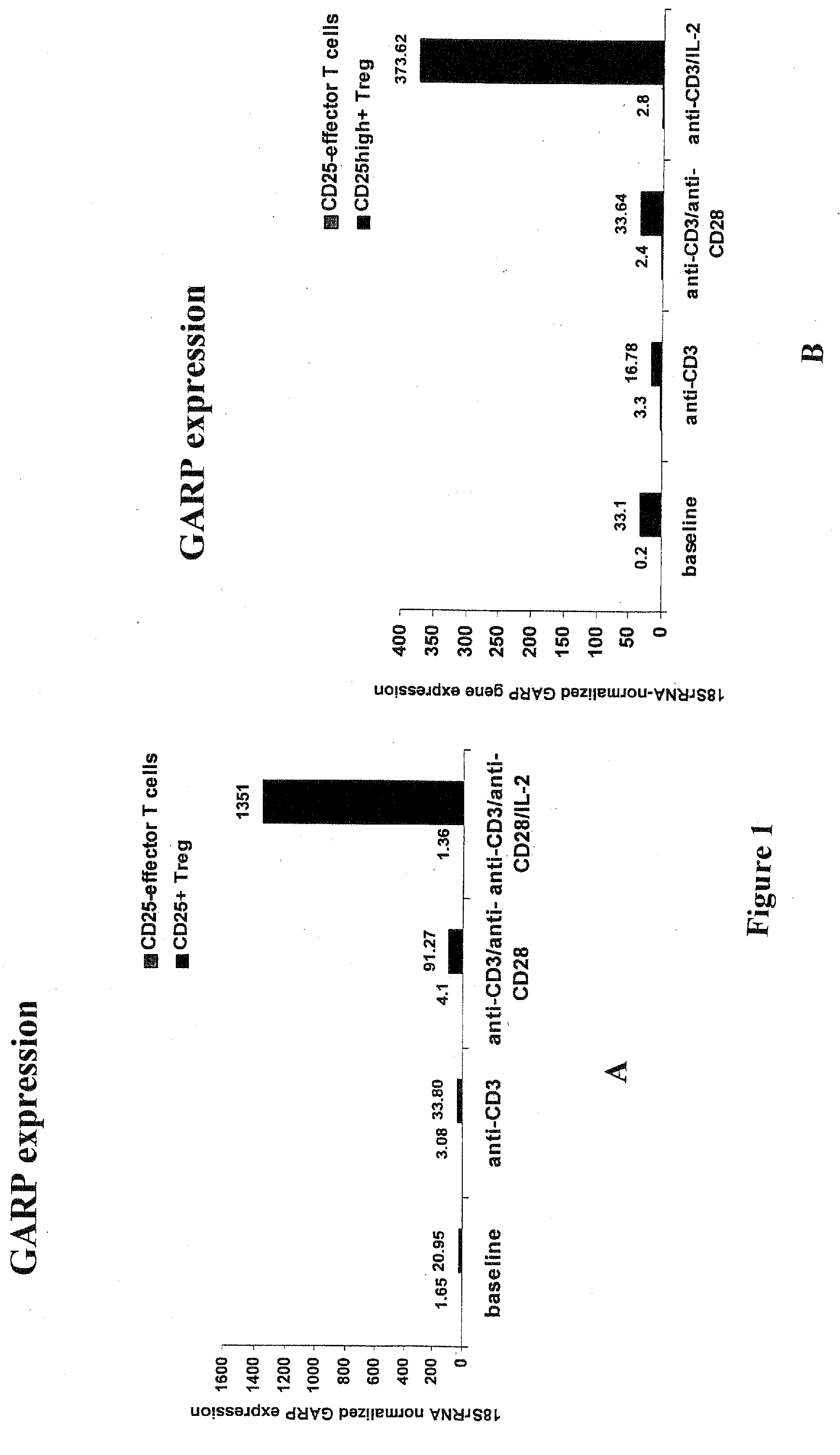
Methods and Reagents for Identifying/Isolating T Regulatory (Treg) Cells and for Treating Individuals
An affinity ligand is reactive to the GARP protein may be capable of binding to an extracellular domain of GARP protein expressed on regulatory T (Treg) cells. The affinity ligand may be an antibody and may be used to identify Treg cells. A method comprises providing a blood sample from a subject and determining the amount of Treg cells in that sample. A composition containing Treg cells may be administered to an individual to suppress effector T cell activity in the individual. A composition containing an affinity ligand capable of binding to a GARP domain may be administered to an individual to suppress Treg cell activity and increase effector T cell activity in the individual. A kit for detecting Treg cells may include an affinity ligand reactive with mammalian GARP protein.
Owner:CASE WESTERN RESERVE UNIV
Materials and methods for modulating immune responses
The present invention provides nanoparticle-coupled tolerogenic Treg cell therapy for treatment of immune and / or autoimmune disorders. In certain specific embodiments, the present invention can be used in the prevention and / or treatment of autoimmune diseases including, but not limited to, type 1 diabetes, lupus erythematosus (SLE), multiple sclerosis (MS), inflammatory bowel disease (IBD), rheumatoid arthritis, oophoritis, and autoimmune pathology associated with Graft versus Host Disease (GvHD) following hematopoietic stem cell transplantation.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Method for obtaining treg-cells
InactiveUS20110123502A1Preventing transplant rejectionBiocideMetabolism disorderSurface markerRegulatory T cell
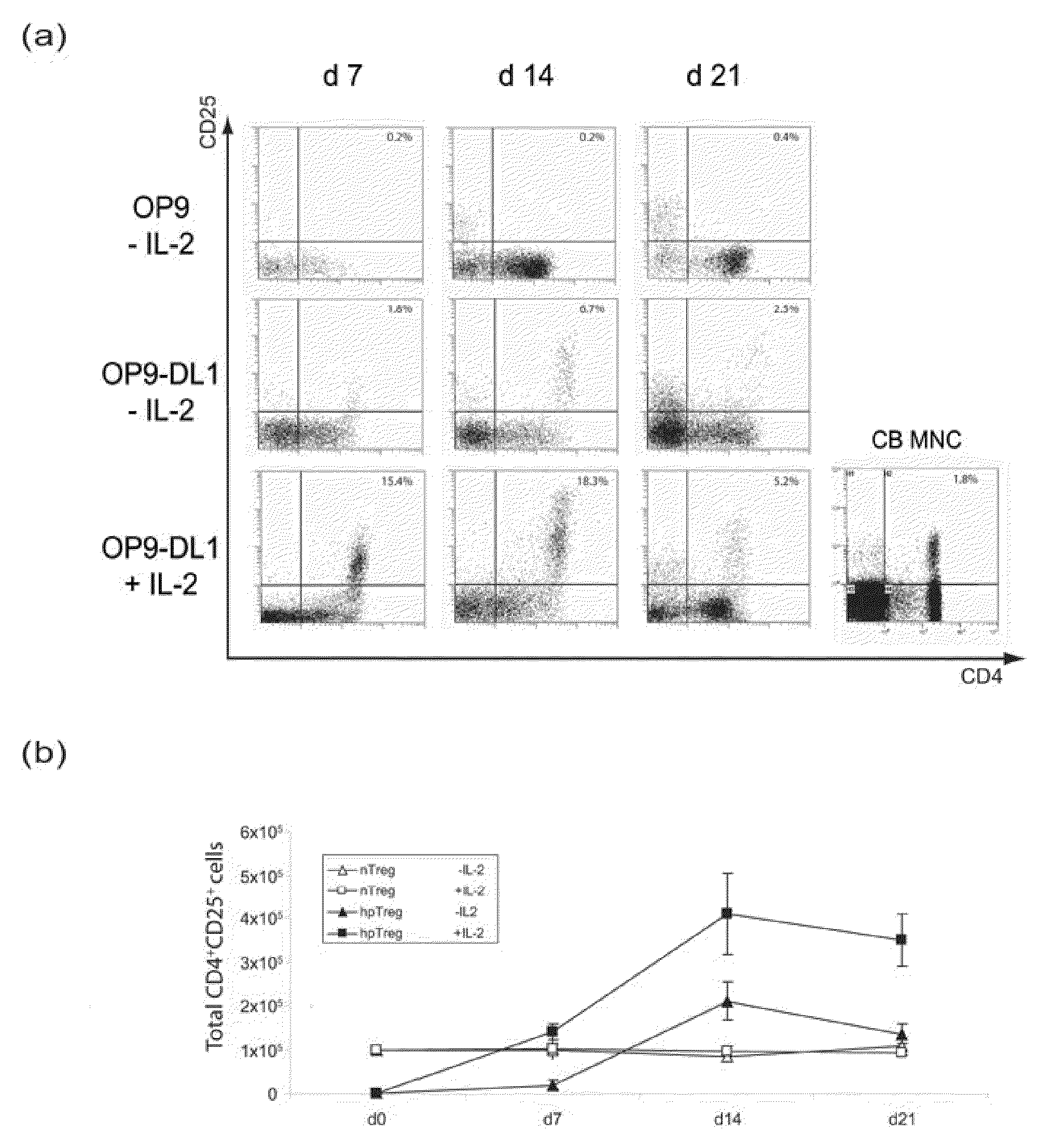
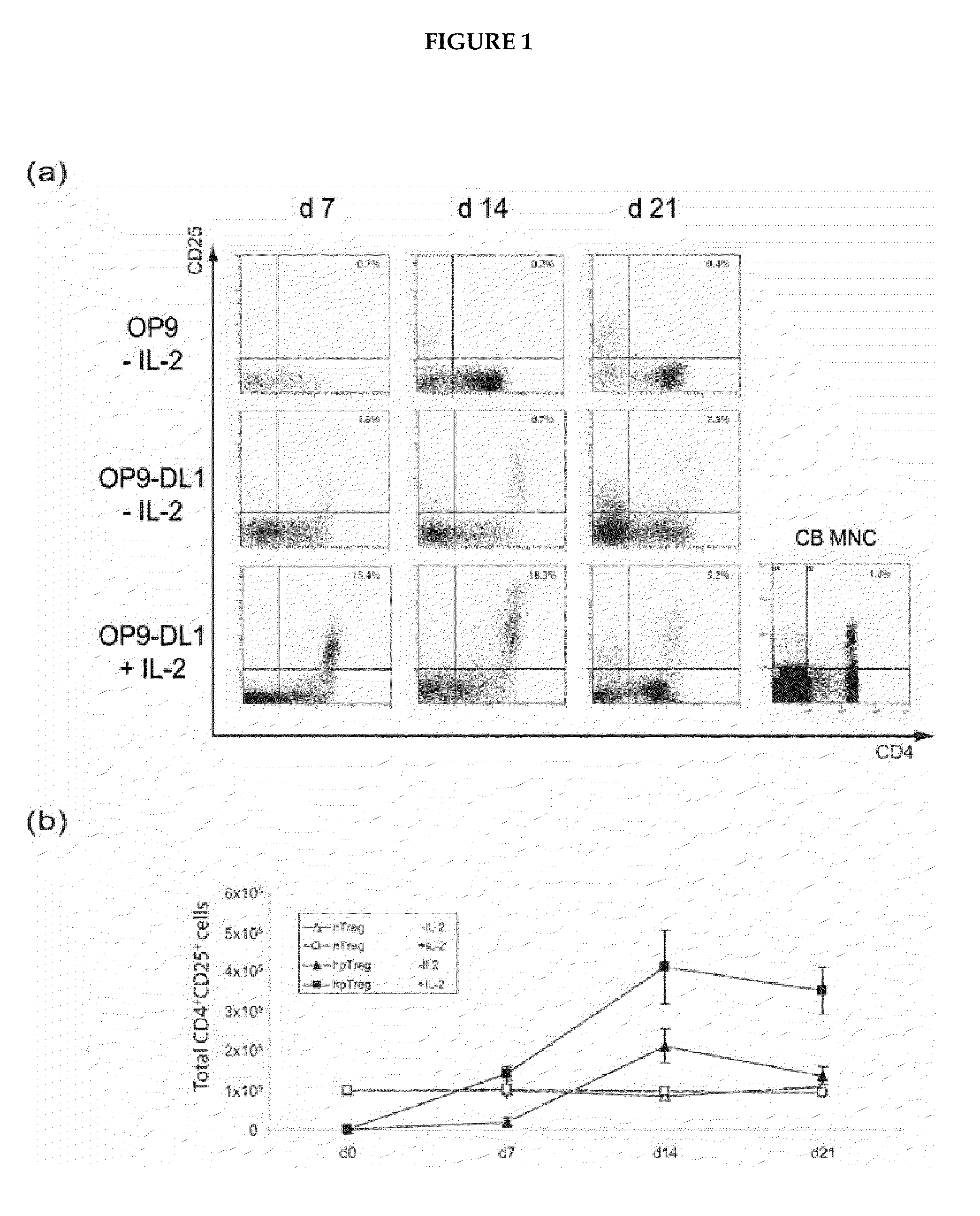
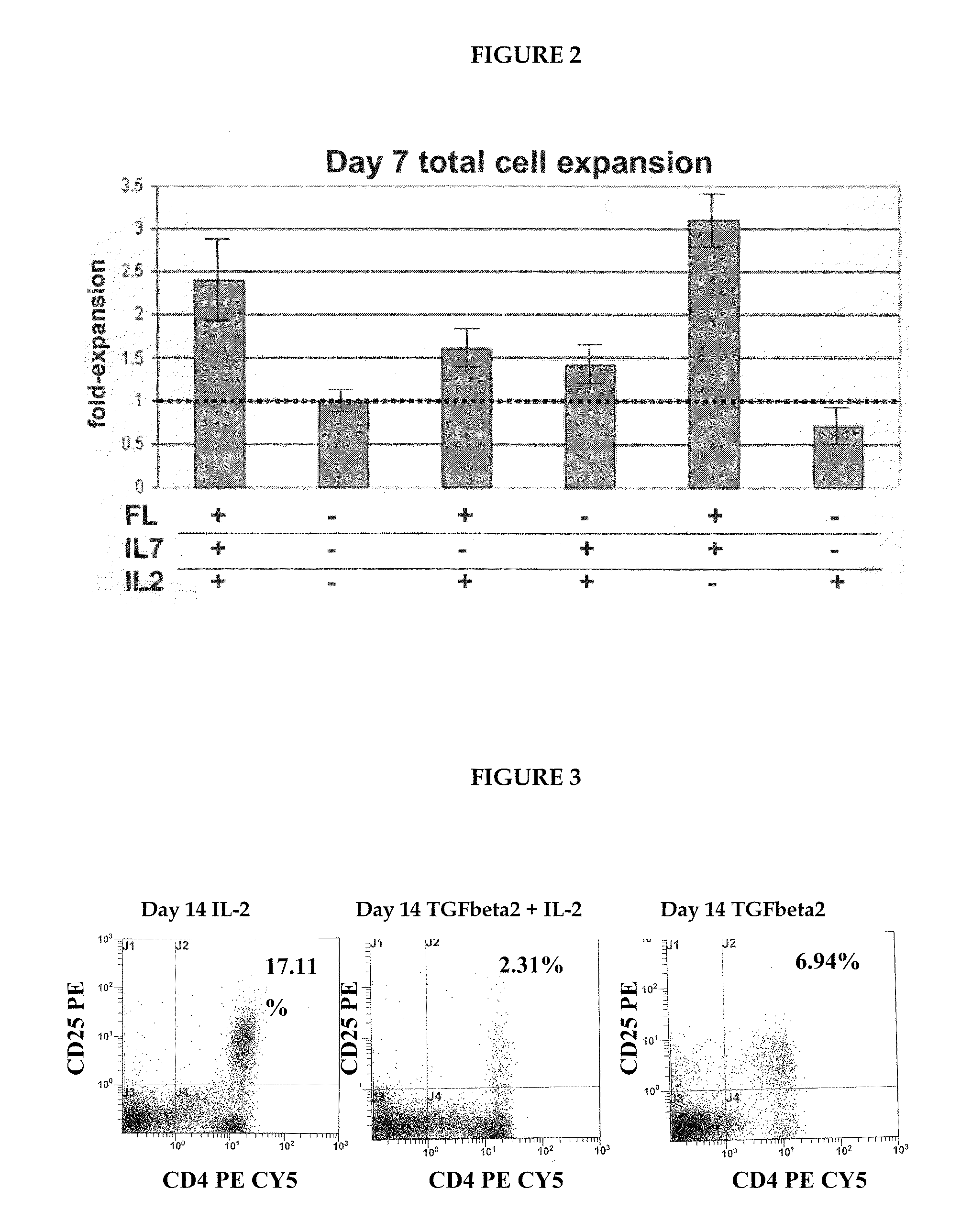
A method for generating a population of functional regulatory T cells (TREG-cells), which are a subset of the T cell lineage having the ability to actively suppress immune activation and maintain peripheral immune tolerance, is described. The method comprises the steps of first culturing haemopoietic stem cells (HSC) and / or haemopoietic progenitor cells in the presence of a Notch ligand that supports T cell differentiation, and then isolating T cells having a TREG-cell surface marker phenotype. A suitable source of HSC is cord blood (CB) and a suitable culture medium is OP9 cells engineered to express the Notch Ligand Delta-Like 1 (DL1) (OP9-DL1 cell line). Examples of TREG-cell surface marker phenotypes are CD4+CD25+, CD45RO+, CD45RA+, CD127LOW−, LAG-3 and / or CD39+.
Owner:ADELAIDE RES & INNOVATION PTY LTD +1
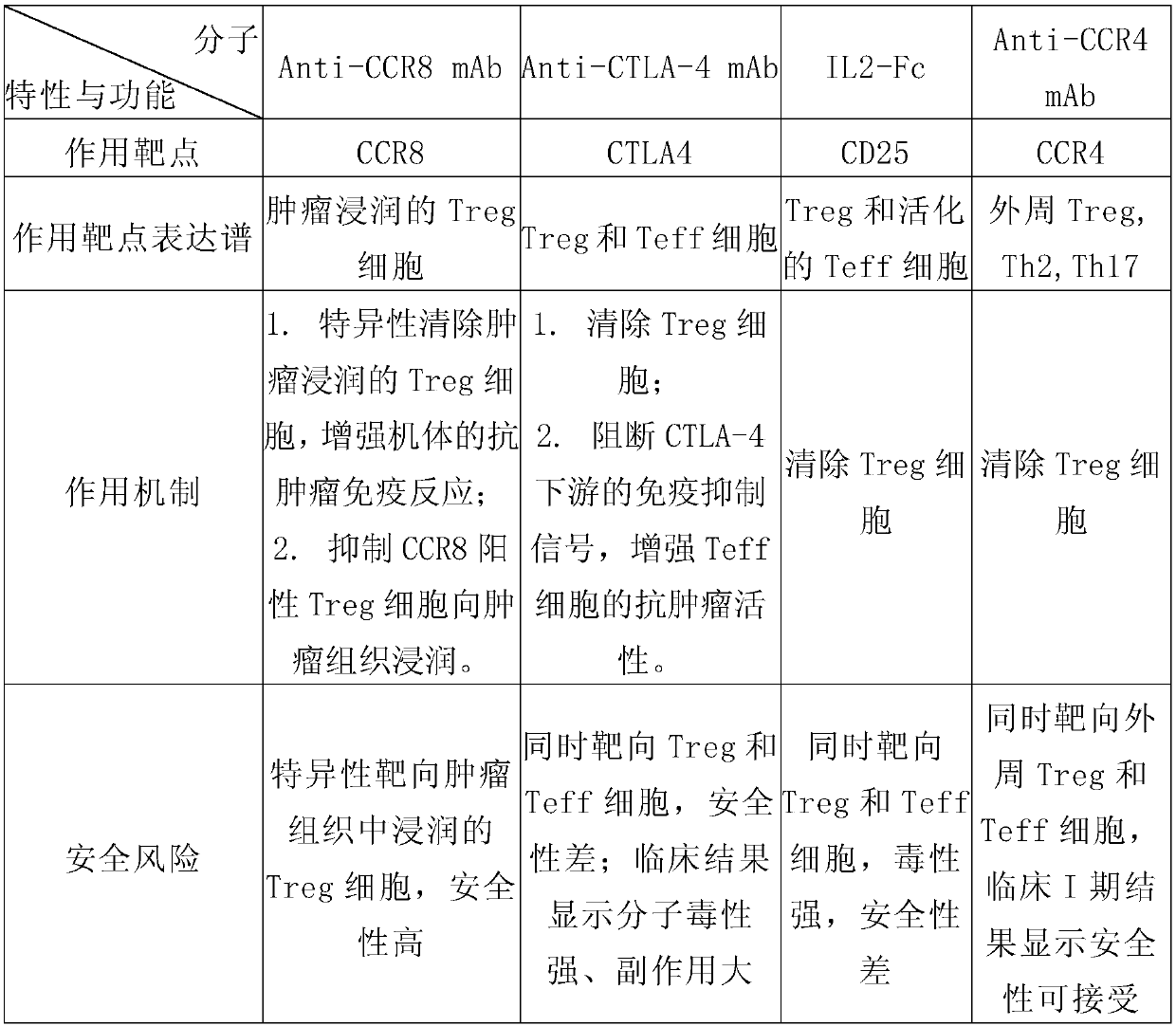
Anti-CCR8 monoclonal antibody and application thereof
InactiveCN110835371AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsTherapeutic antibodyAntiendomysial antibodies
The invention relates to an anti-CCR8 monoclonal antibody and an application thereof. The invention discloses the anti-CCR8 monoclonal antibody. The antibody has a function of blocking binding betweenCCR8 and a ligand thereof, and the monoclonal antibody specifically targets Treg cells infiltrated in tumor tissues and having immunosuppressive activity, but does not target inflammatory the Treg cells and non-Treg type T cells. Compared with other targeting broad-spectrum Treg cell antibodies, the therapeutic antibody targeting CCR8 has better safety and effectiveness, so that the antibody is particularly suitable for immunotherapy of tumors.
Owner:普米斯生物技术(苏州)有限公司
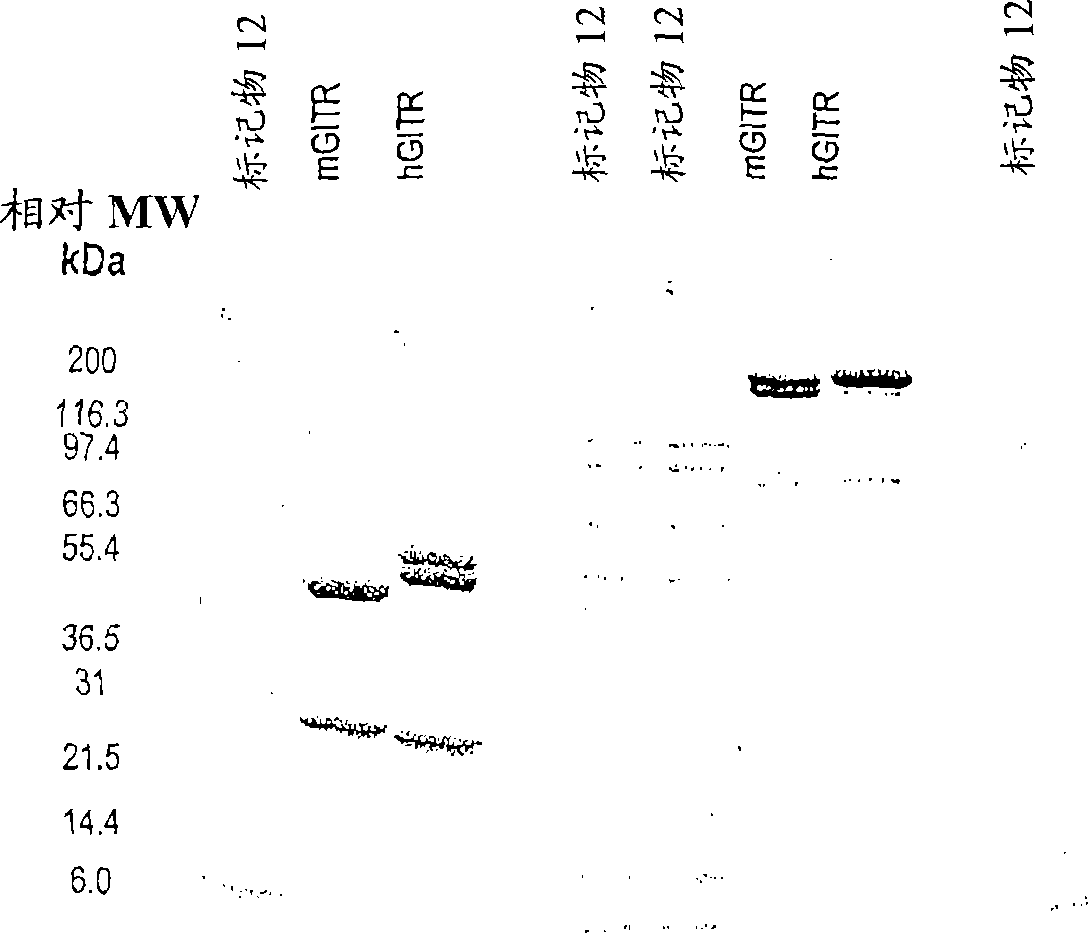
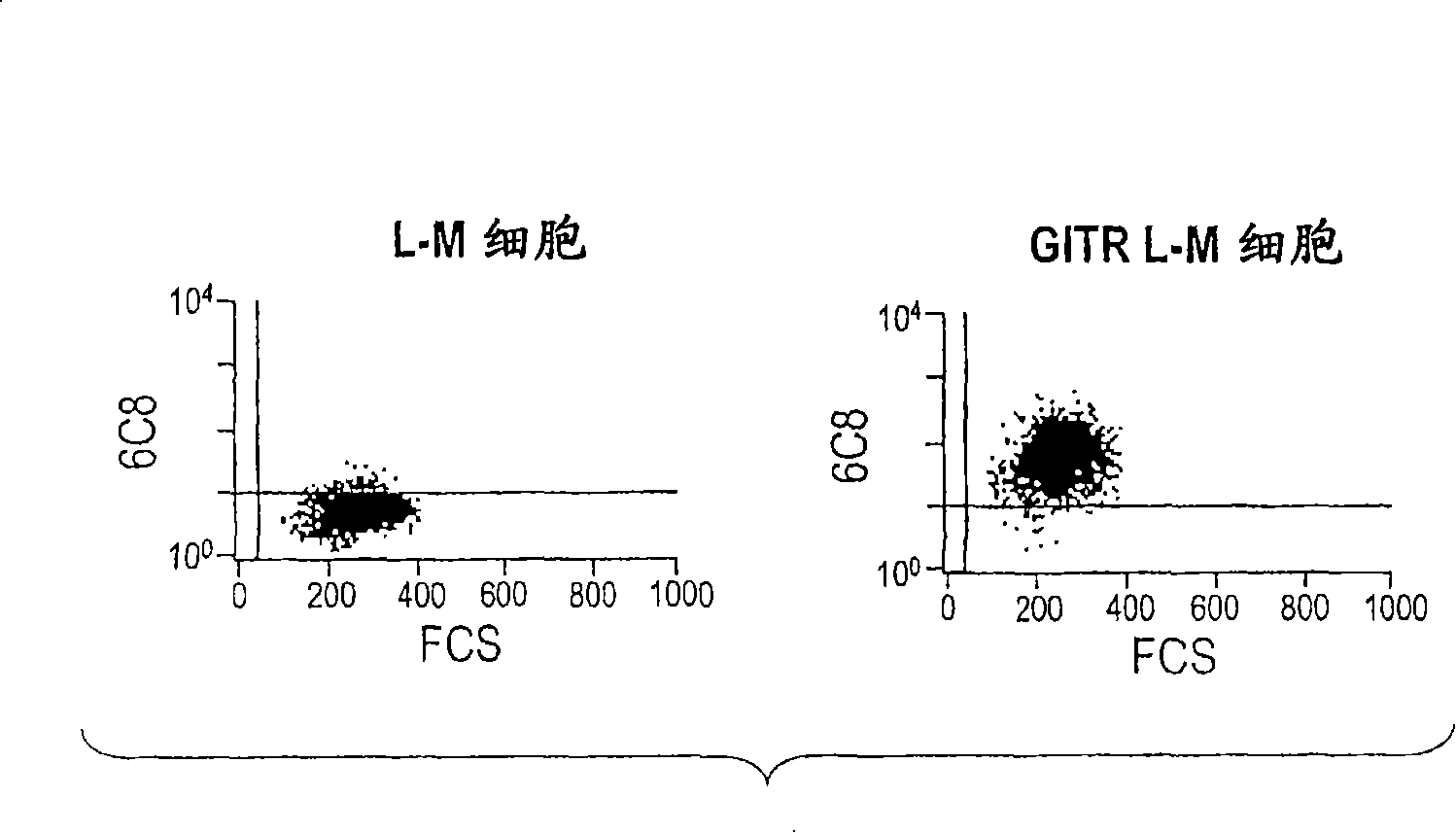
GITR binding molecules and uses therefor
InactiveCN101218257AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDendritic cellIn vivo
The present invention provides binding molecules that specifically bind to GITR, e.g., human GITR (hGITR), on T cells and dendritic cells. Binding molecules of the invention are characterized by binding to hGITR with high affinity, in the presence of a stimulating agent, e.g., CD3, are agonistic, and abrogate the suppression of Teff cells by Treg cells. Various aspects of the invention relate to binding molecules, and pharmaceutical compositions thereof, as well as nucleic acids, recombinant expression vectors and host cells for making such binding molecules. Methods of using a binding molecule of the invention to detect human GITR or to modulate human GITR activity, either in vitro or in vivo, are also encompassed by the invention.
Owner:GITR
Preparation method of CIK (Cytokine Induced Killer) cells with high proliferation capacity, high cytotoxic activity and high survival rate, associated CIK cells and application
InactiveCN102827809AImprove proliferative abilityHighly toxic activityMammal material medical ingredientsBlood/immune system cellsPeripheral blood mononuclear cellClinical efficacy
The invention relates to a preparation method of CIK (Cytokine Induced Killer) cells with high proliferation capacity, high cytotoxic activity and high survival rate. The preparation method comprises the steps of: (1) sorting and removing CD4<+>CD25<+>Treg cells of peripheral blood mononuclear cell to obtain CIK pre-cells; (2) cultivating the CIK pre-cells in a cell culture fluid containing 100ng / ml of PHA, 100ng / ml of IL-6 and 10ng / ml of PGE2 for 24h; (3) transferring the CIK pre-cells to a cell culture bottle coated with 1microgramme / ml of CD3 monoclonal antibody, and adding 1000U / ml of IFN-gamma for cultivating for 48h; (4) adding 1000U / ml of IL-2 and 100ng / ml of IL-1alpha for cultivating for 4 days; and (5) adding 1microgramme / ml of insulin to continuously cultivate for 7-14 days. The invention further provides associated CIK cells, a method for inhibiting the peripheral blood mononuclear cell to be differentiated to the CD4<+>CD25<+>Treg cells and a method for promoting the proliferation of the CIK cells. The preparation method of the CIK cells provided by the invention is skillful in design, and the tumor killing cells-CIK cells prepared have stronger proliferation capacity, higher cytotoxic activity and better tumor killing efficiency, so that the clinical efficacy is improved, and the preparation method is appropriate for wide application in a large scale.
Owner:上海优立赛尔生物医药科技有限公司
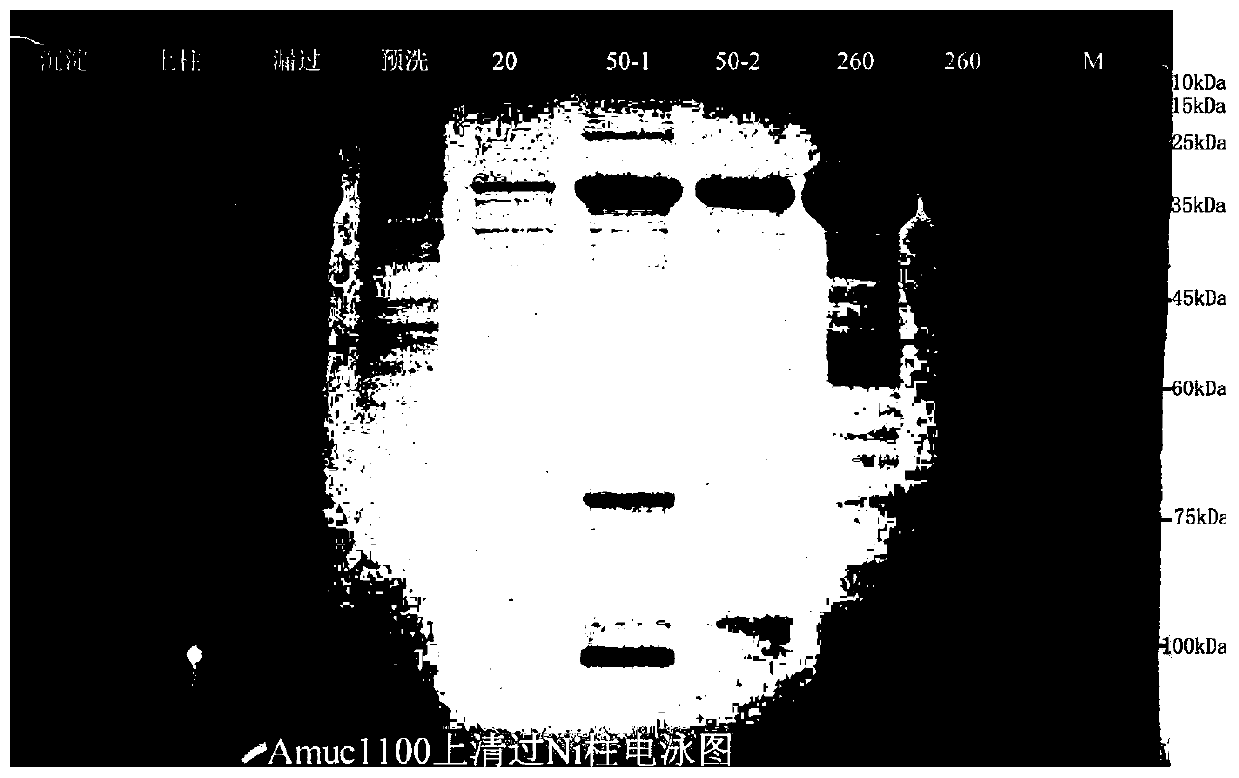
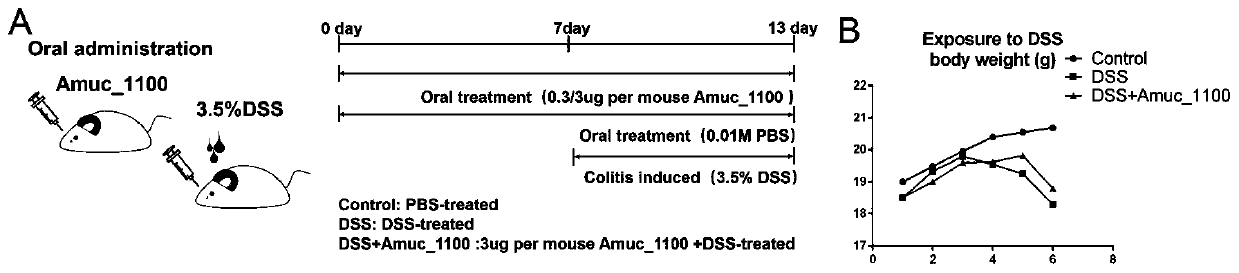
Recombinantly-expressed Akkermansia membrane protein Amuc_1100 and application thereof
ActiveCN110698547ARegulate immune responseStimulate immune responseBacteriaPeptide/protein ingredientsLamina propriaColonic lamina propria
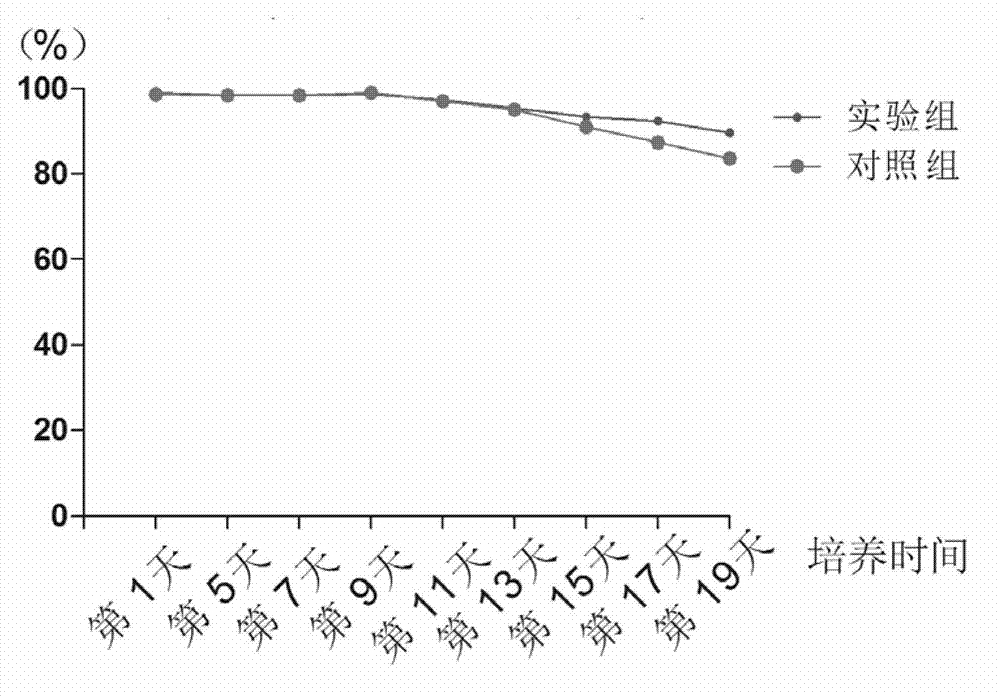
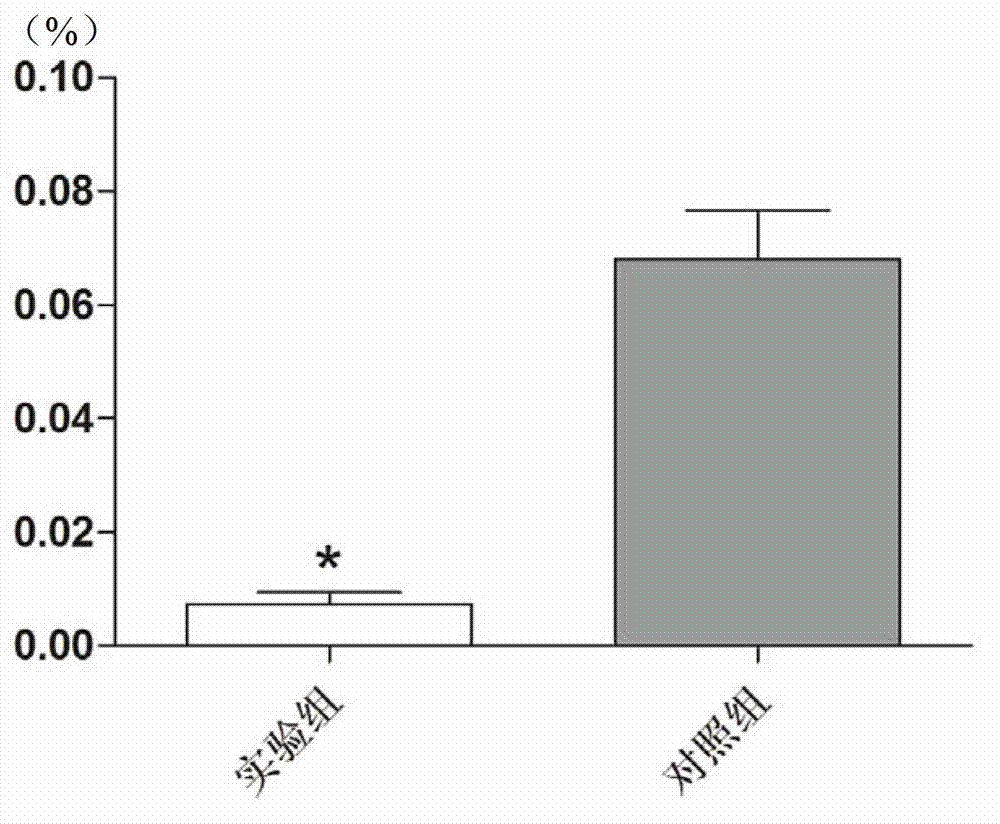
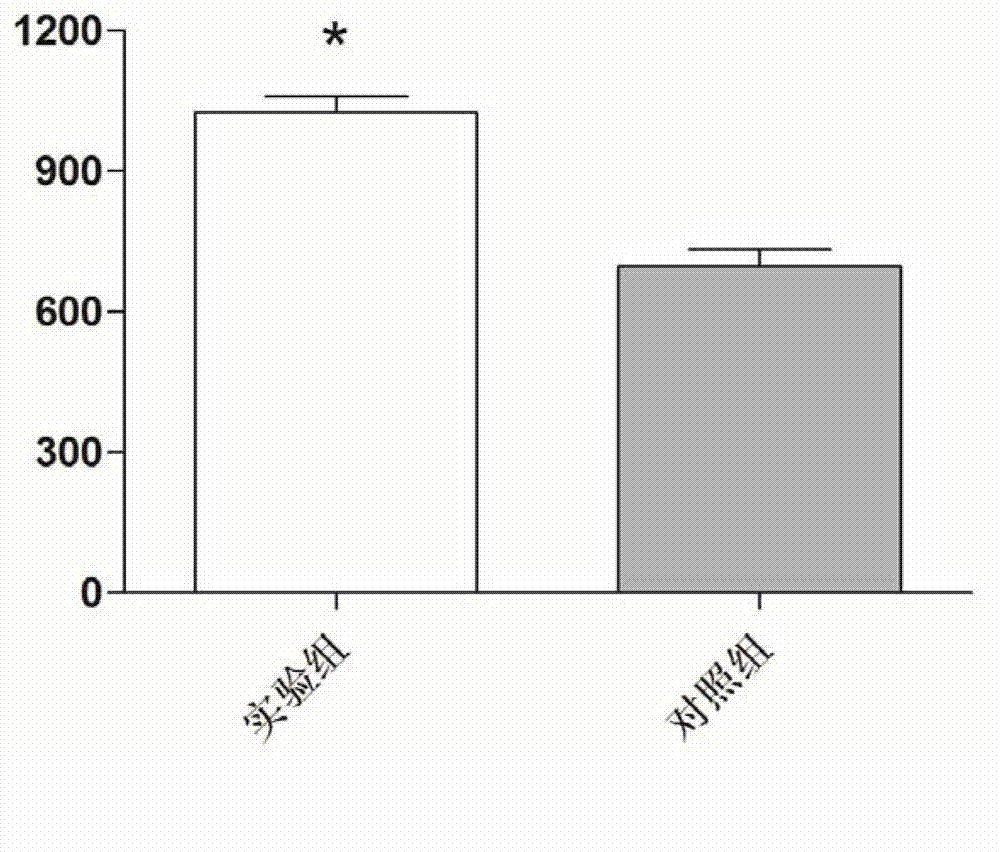
The invention discloses recombinantly-expressed Akkermansia membrane protein Amuc_1100 and an application thereof. The invention recombinantly expresses the Akkermansia membrane protein Amuc_1100, through animal experiments, we find that oral administration of the Akkermansia membrane protein Amuc_1100 can significantly improve the body weight of mice, increase colon length and crypt depth, and activate proliferation of intestinal stem cells to protect mice from colitis damage. Isolation of lymphocytes from the lamina propria of the colon shows that the proportion of Treg cells is significantly up-regulated and that of Th17 cells is significantly down-regulated, proving that Amuc_1100 can restore colon damage by adjusting the proportion of immune cells Treg / Th17. Therefore, the protein isexpected to be developed into a protein product for preventing or treating colitis.
Owner:NANJING AGRICULTURAL UNIVERSITY
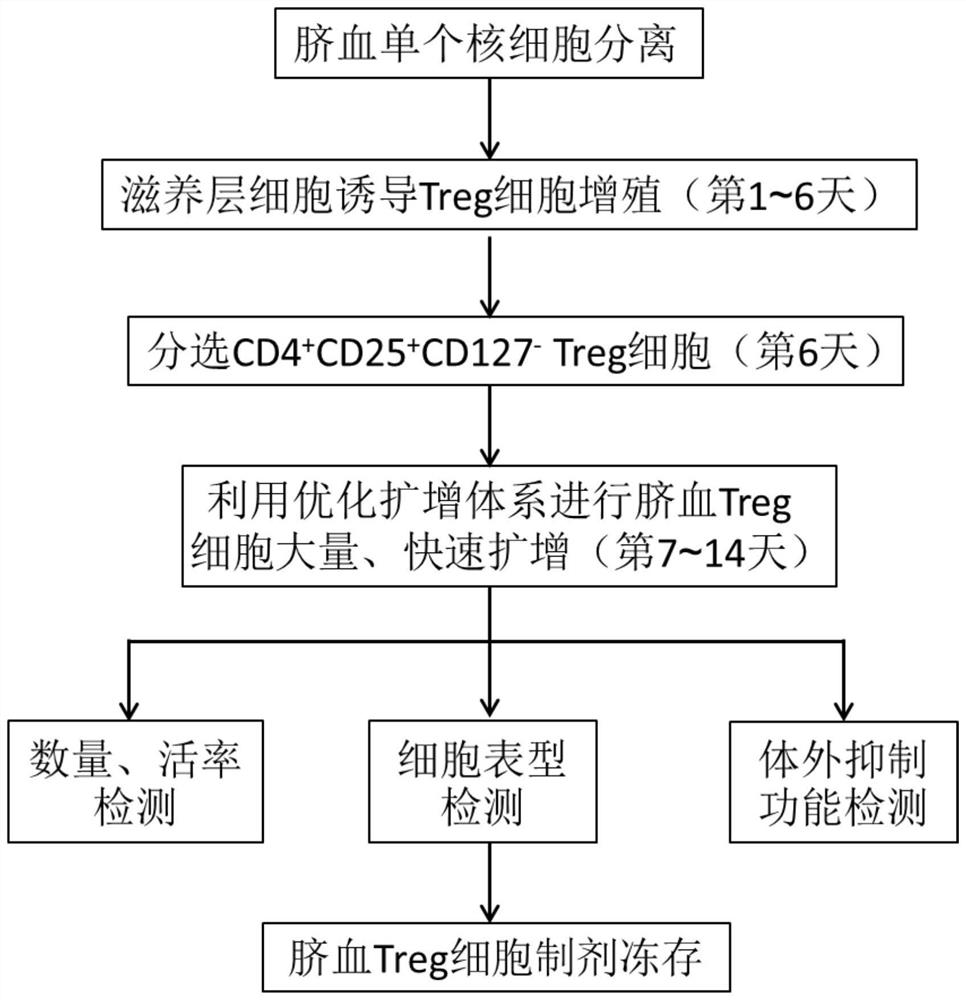
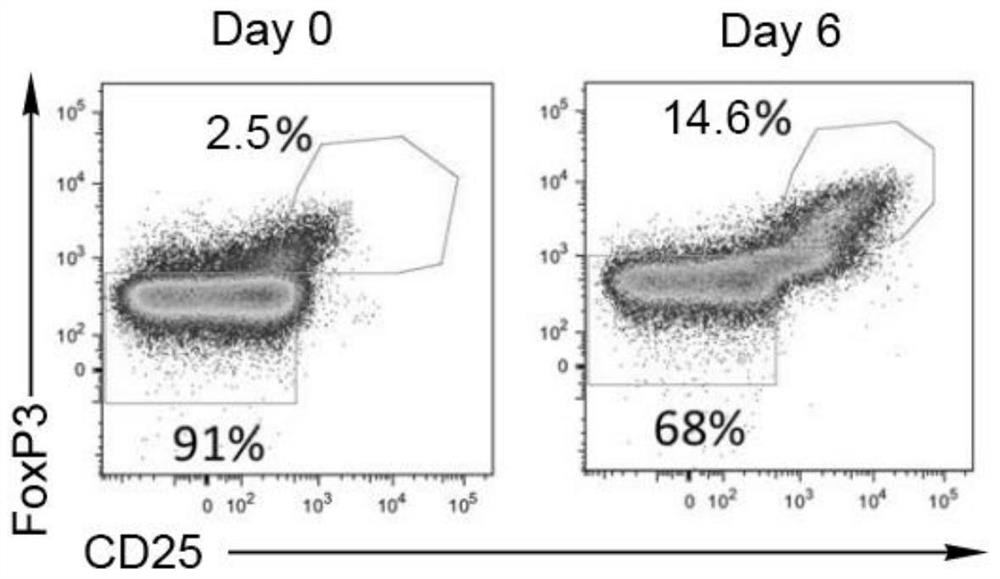
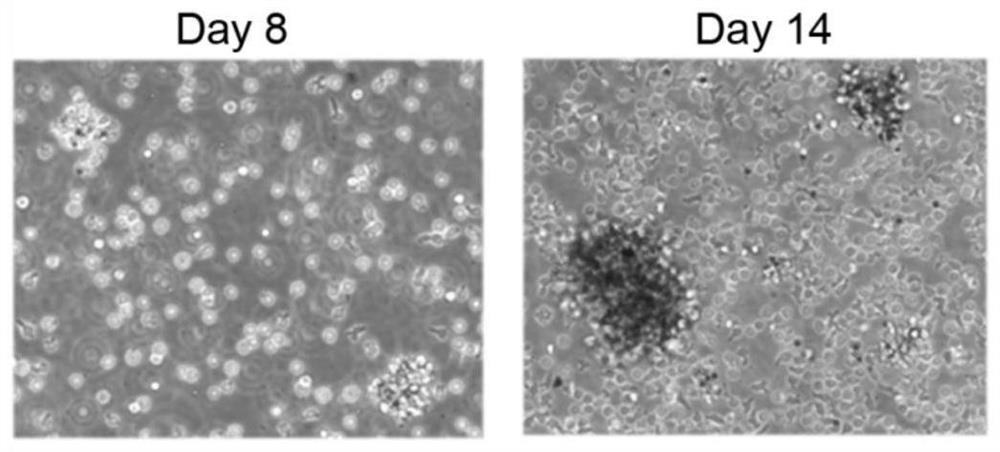
Umbilical cord blood Treg cell in-vitro amplification method based on trophoblastic cells and application
ActiveCN112458053APromote amplificationRaise the ratioAntipyreticDigestive systemAutoimmune diseaseTrophoblast
The invention discloses an umbilical cord blood Treg cell in-vitro amplification method based on trophoblastic cells and application. The specific technical method comprises the steps that firstly, umbilical cord Wharton's jelly mesenchymal stem cells are adopted as the trophoblastic cells to induce preliminary proliferation of Treg cells in umbilical cord blood mononuclear cells; then, pure Tregcells are obtained through magnetic bead sorting; and finally, the Treg cells are stimulated to be rapidly amplified by using optimized amplification factors. According to the amplification method, human AB plasma, IL-2, rapamycin, an RARA agonist and a DNA methyltransferase inhibitor are used as the optimized amplification factors, and a large number of umbilical cord blood Treg cells with high purity and high activity can be prepared within two weeks. Umbilical cord blood is used as a raw material for Treg cell amplification, batch preparation can be achieved, and Treg cell quality fluctuation caused by individual differences of samples can be reduced. The umbilical cord blood Treg cells have low immunogenicity and can be used as universal cells for clinical research, such as autoimmunediseases, graft-versus-host diseases and the like.
Owner:成都云测医学生物技术有限公司
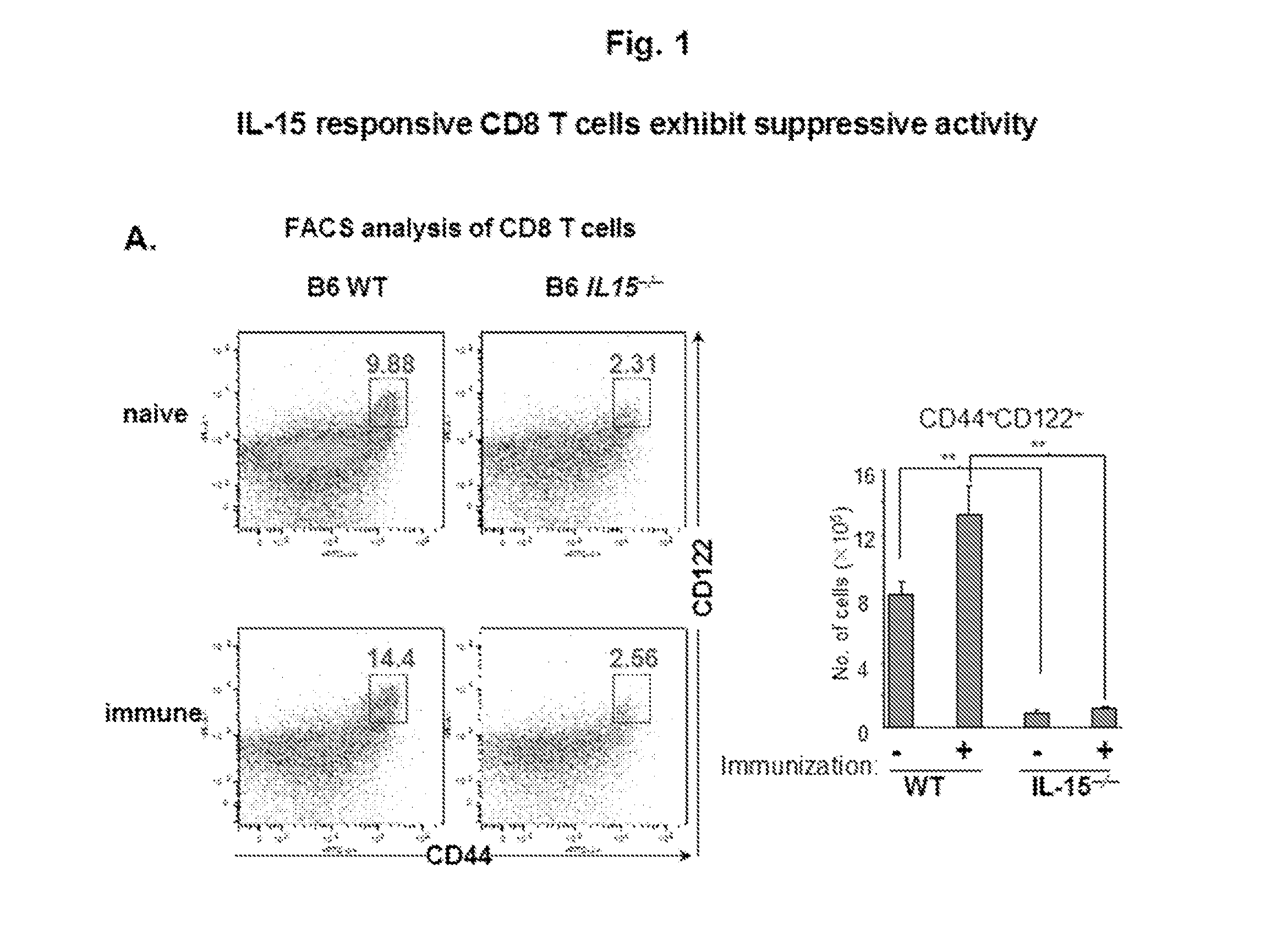
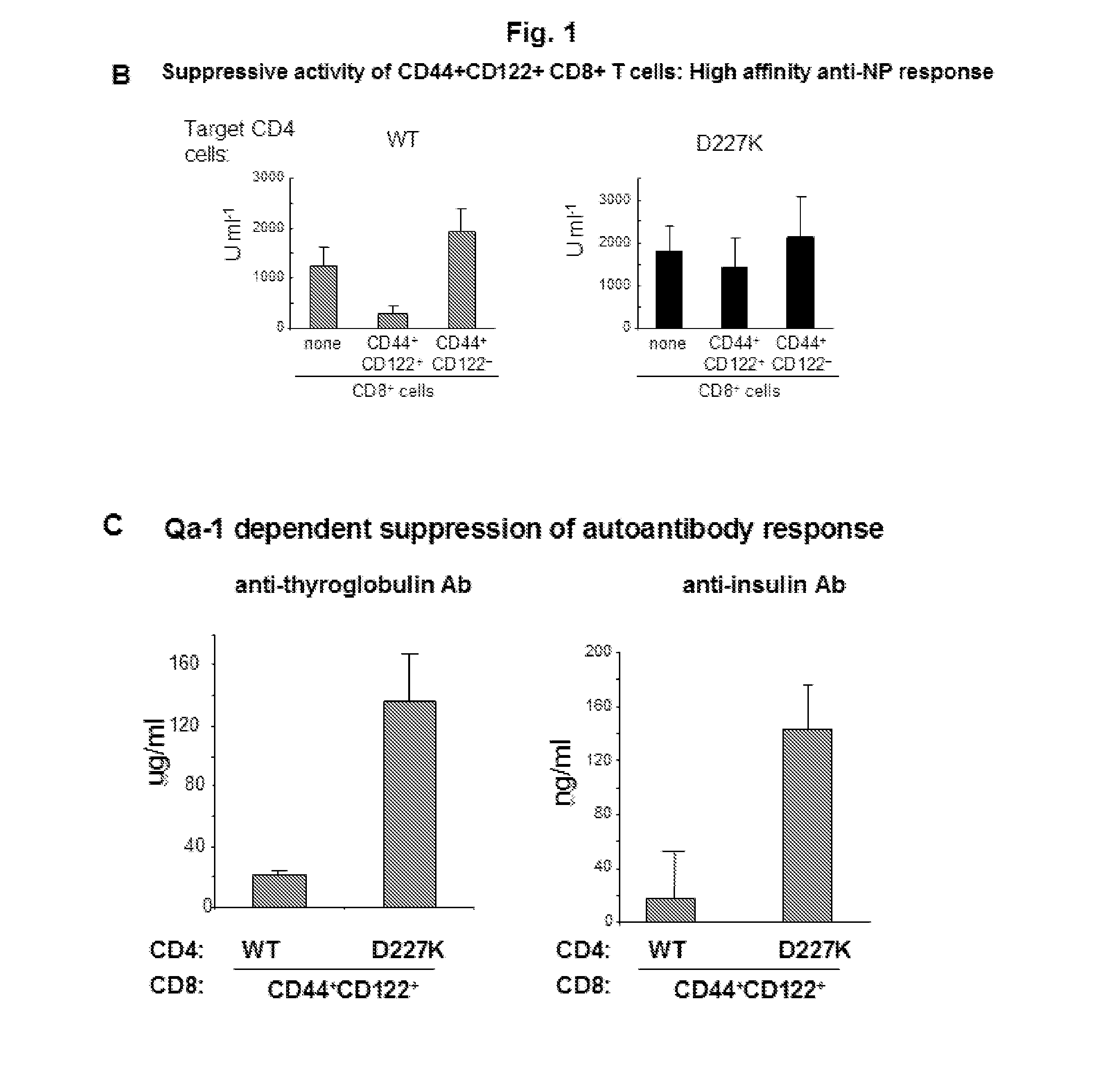
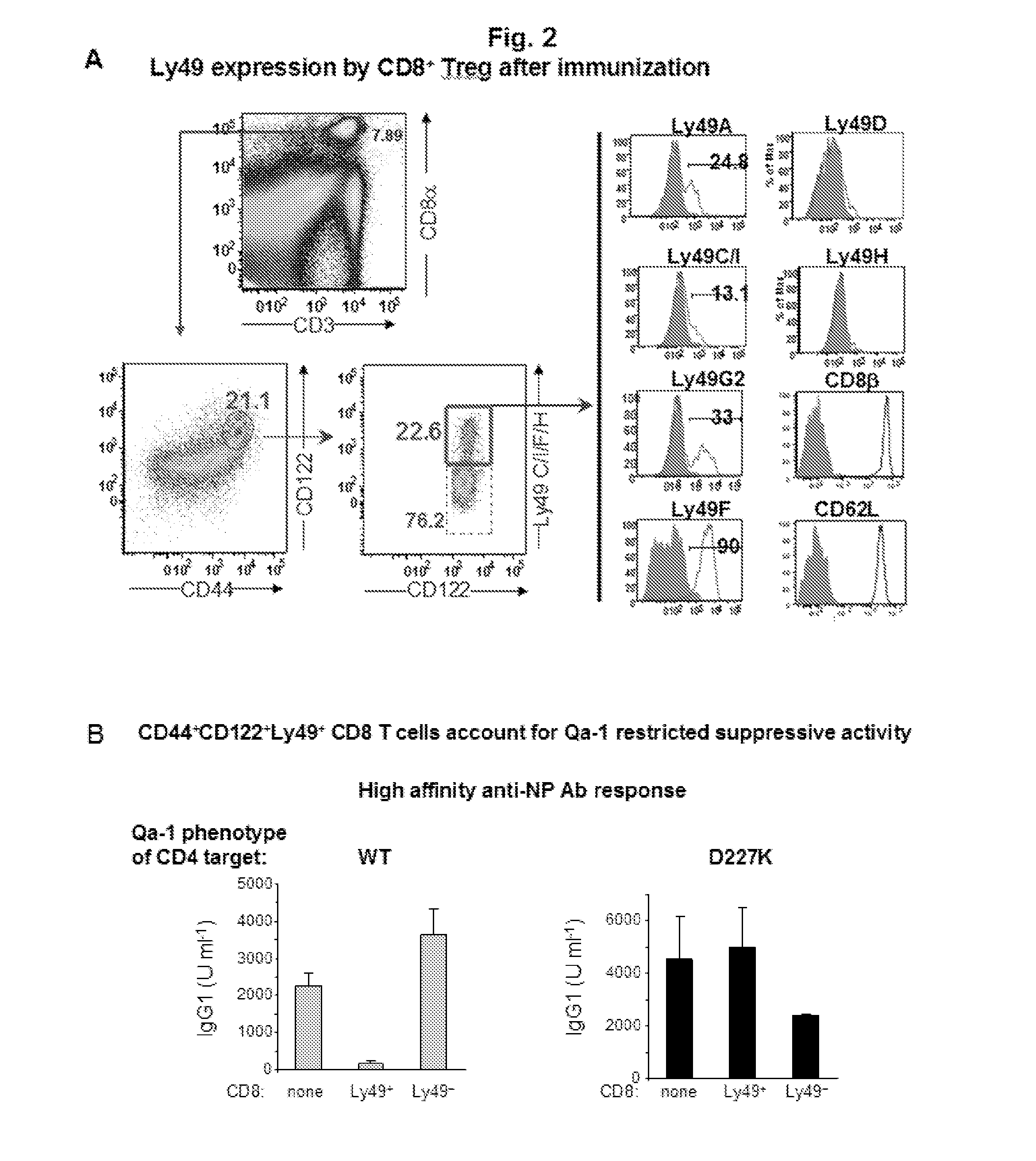
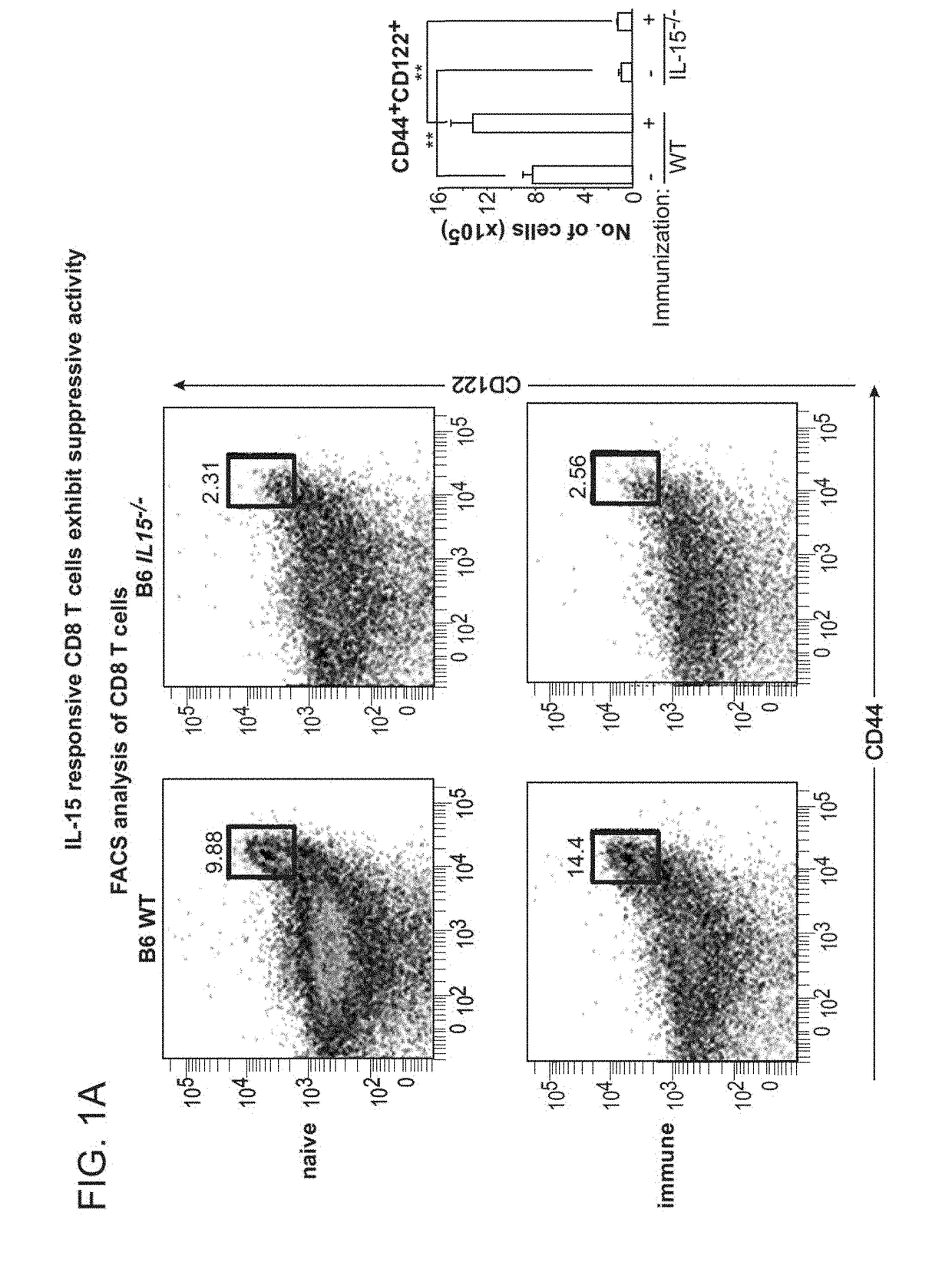
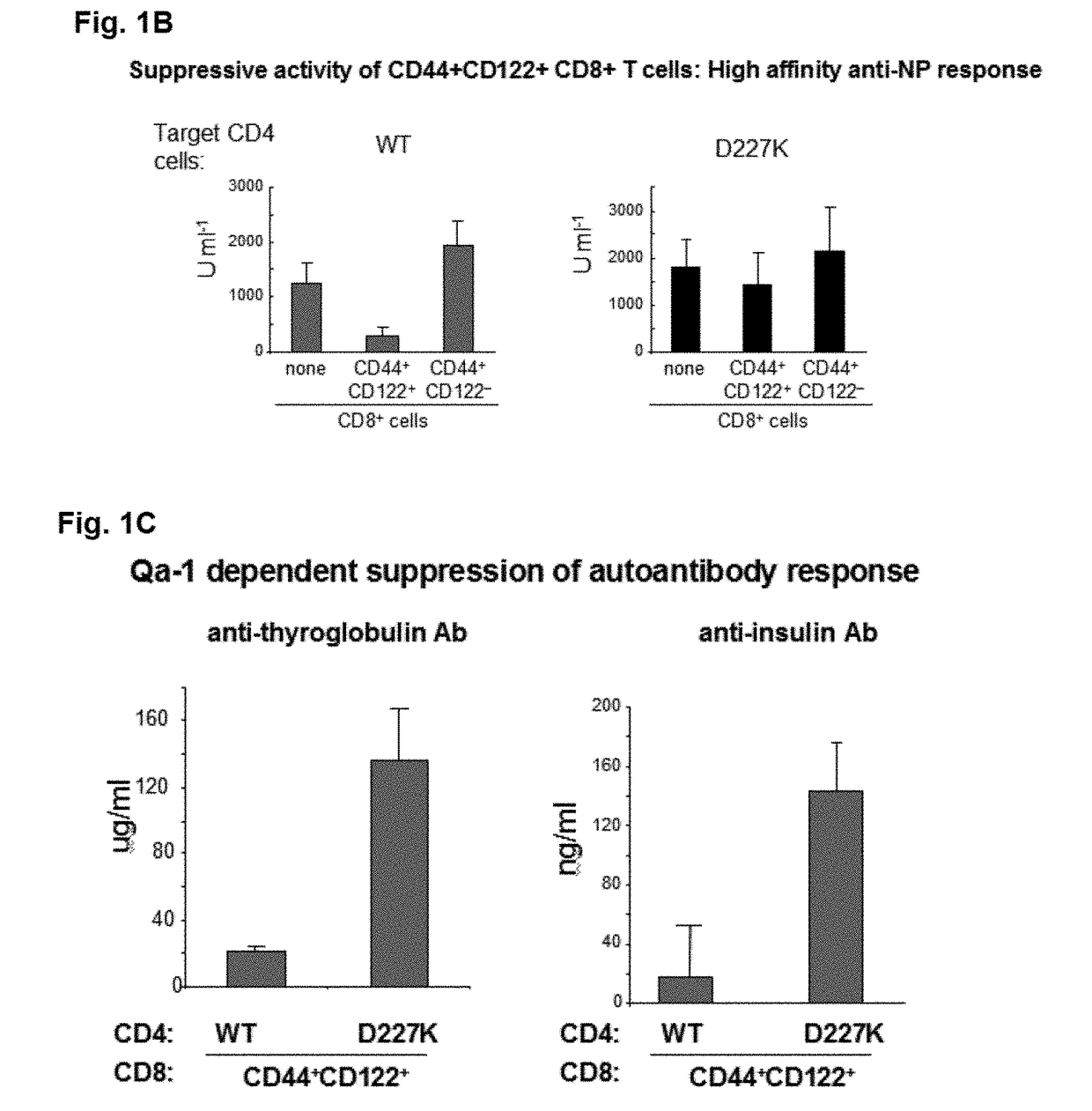
Discovery of regulatory t cells programmed to suppress an immune response
InactiveUS20130302276A1Maintenance of toleranceEffective to ameliorateBiocideNervous disorderAntigenDisease
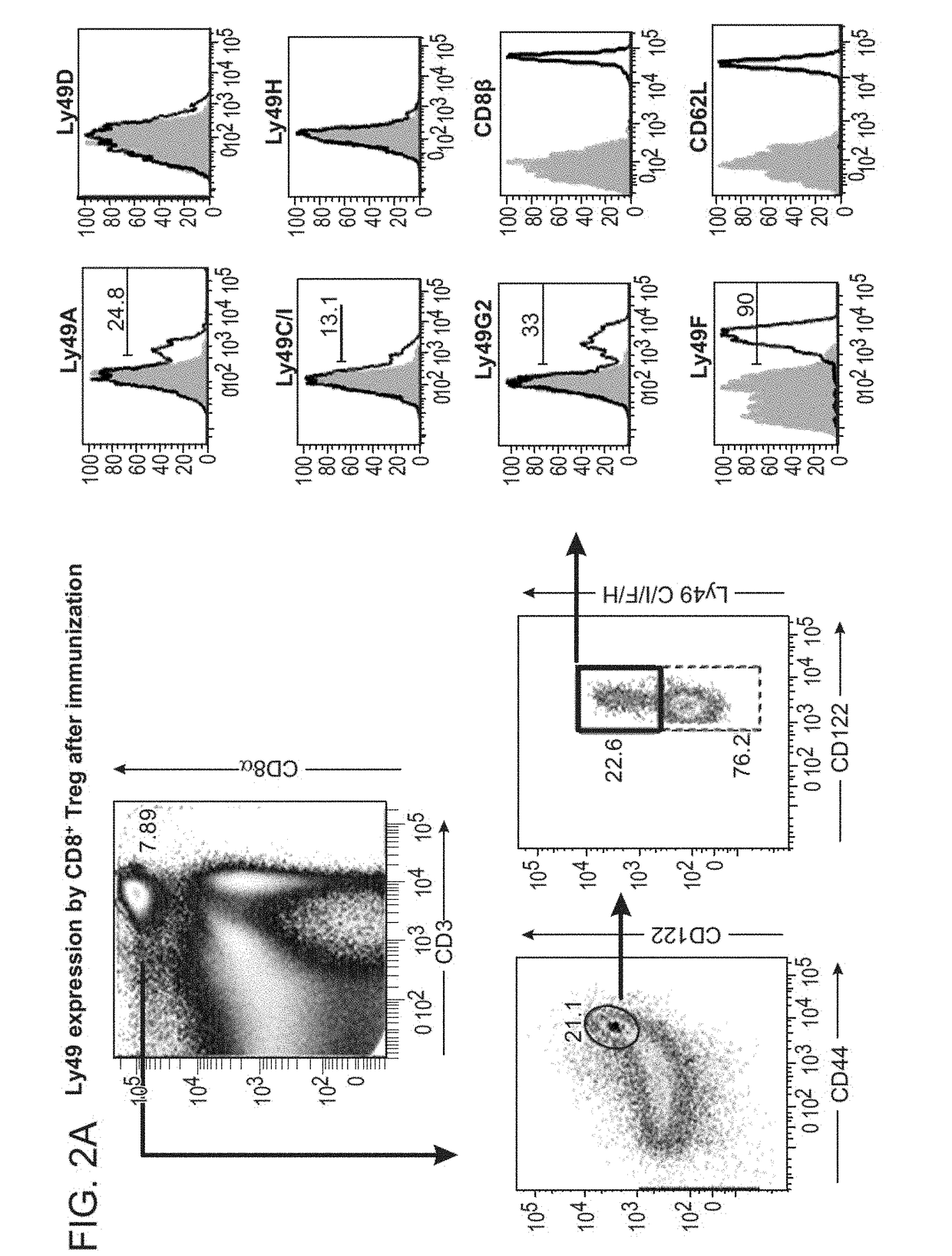
A method to treat an autoimmune disease is provided. The method involves administration of interleukin-15 receptor (IL-15R) agonists in an amount effective to ameliorate a symptom of the autoimmune disease. The invention also involves a method to treat an autoimmune disease by ex-vivo expansion of CD44+CD122+Kir+ CD8+ Treg cells and administration of the CD44+CD122+Kir+ CD8+ Treg cells. Compositions comprising CD44+CD122+Kir+ CD8+ Treg cells are also provided. Methods for stimulating an immune response to an antigen are also provided.
Owner:DANA FARBER CANCER INST INC
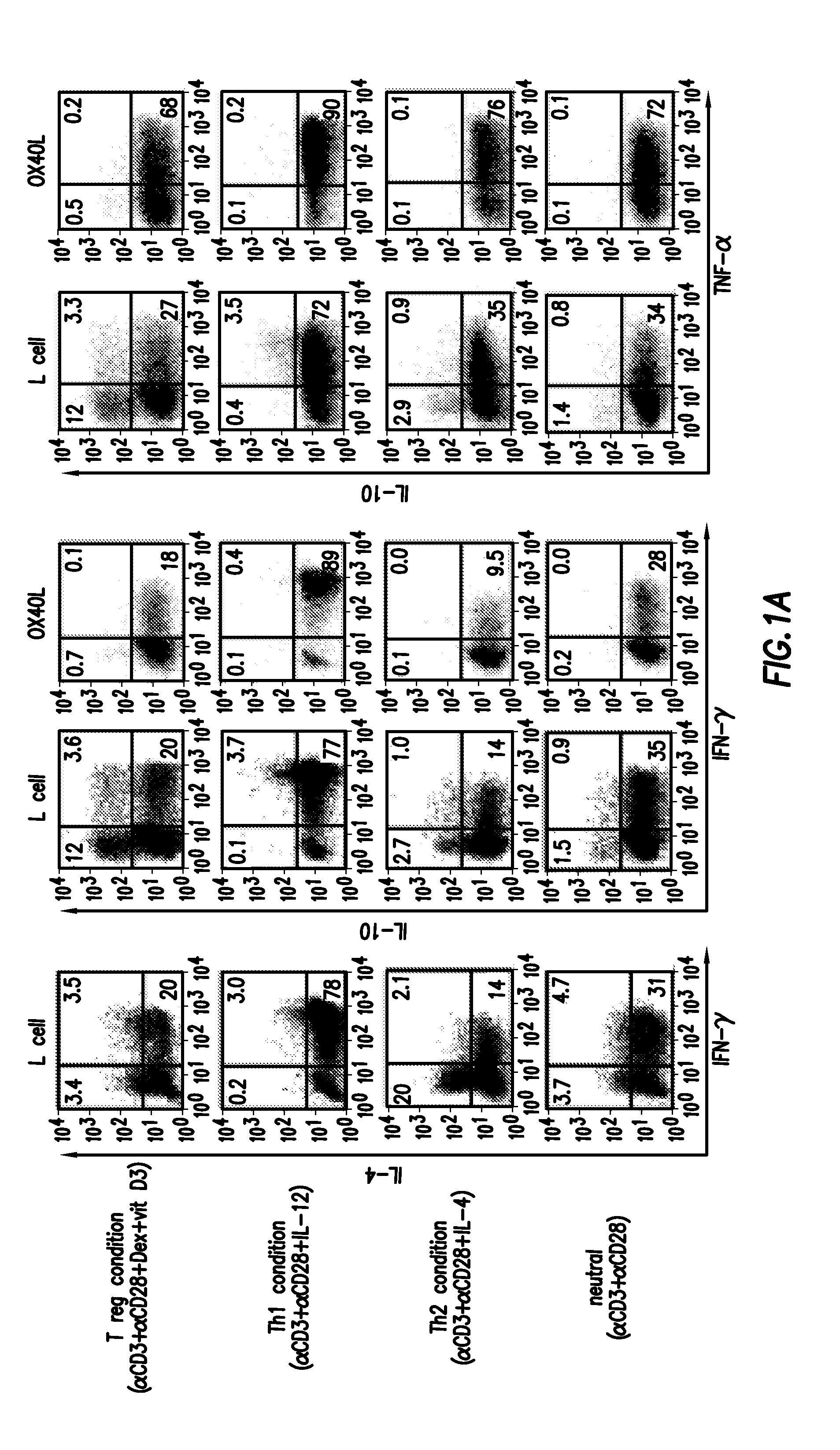
Regulatory T cells and their use in immunotherapy and suppression of autoimmune responses
ActiveUS7651855B2Improve abilitiesIncreased activationBiocideMammal material medical ingredientsAutoimmune ReactionsRegulatory T cell
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Lactobacillus casei with immunomodulatory, anti-inflammatory and anti-cervical cancer effects and application
ActiveCN111560330AIncrease the number ofImprove colitis symptomsMilk preparationBacteriaFatty acidThelial cell
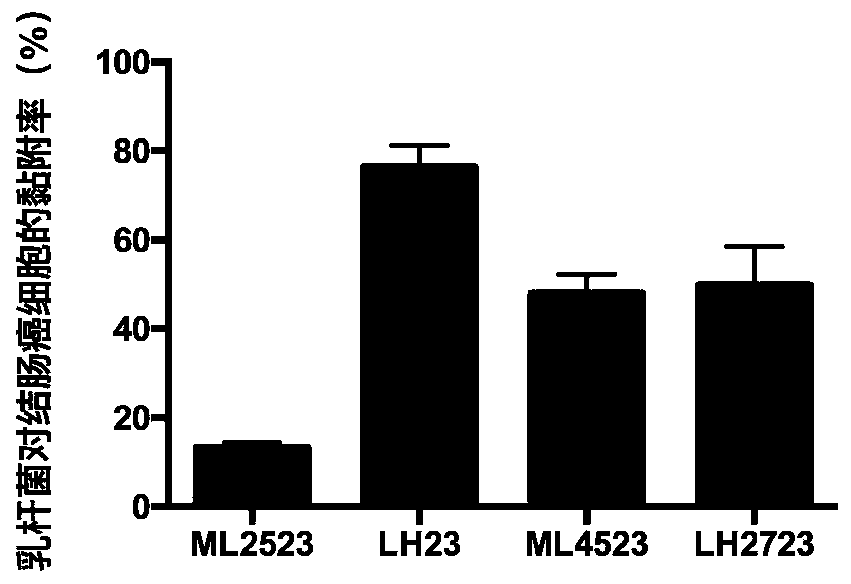
The invention relates to lactobacillus casei with immunoregulation, anti-inflammatory and anti-cervical cancer effects. The lactobacillus casei strain is named as LH23, the preservation number is CGMCC No.16656, and the strain can be used for regulating immunity, regulating M1 / M2 macrophage balance, increasing the number of Treg cells, improving the symptoms of mouse colitis, shortening the colonlength and apoptosis of intestinal epithelial cells, inhibiting excessive secretion of proinflammatory factors (TNF-alpha, IL-1beta and IL-6) and recovering the concentration of short-chain fatty acid(SCFAs) in the intestinal tract, can resist cervical cancer, inhibit proliferation of cervical cancer HeLa cells and acetylation modification of histone, cause apoptosis of the HeLa cells and inhibitexpression of human papilloma virus HPVE6 and E7 proteins. The strain is used for preparing fermented food for inhibiting inflammatory bowel diseases and cervical cancer, and has a very wide application prospect.
Owner:TIANJIN UNIV OF SCI & TECH
Method for identifying antigen-specific regulatory t cells
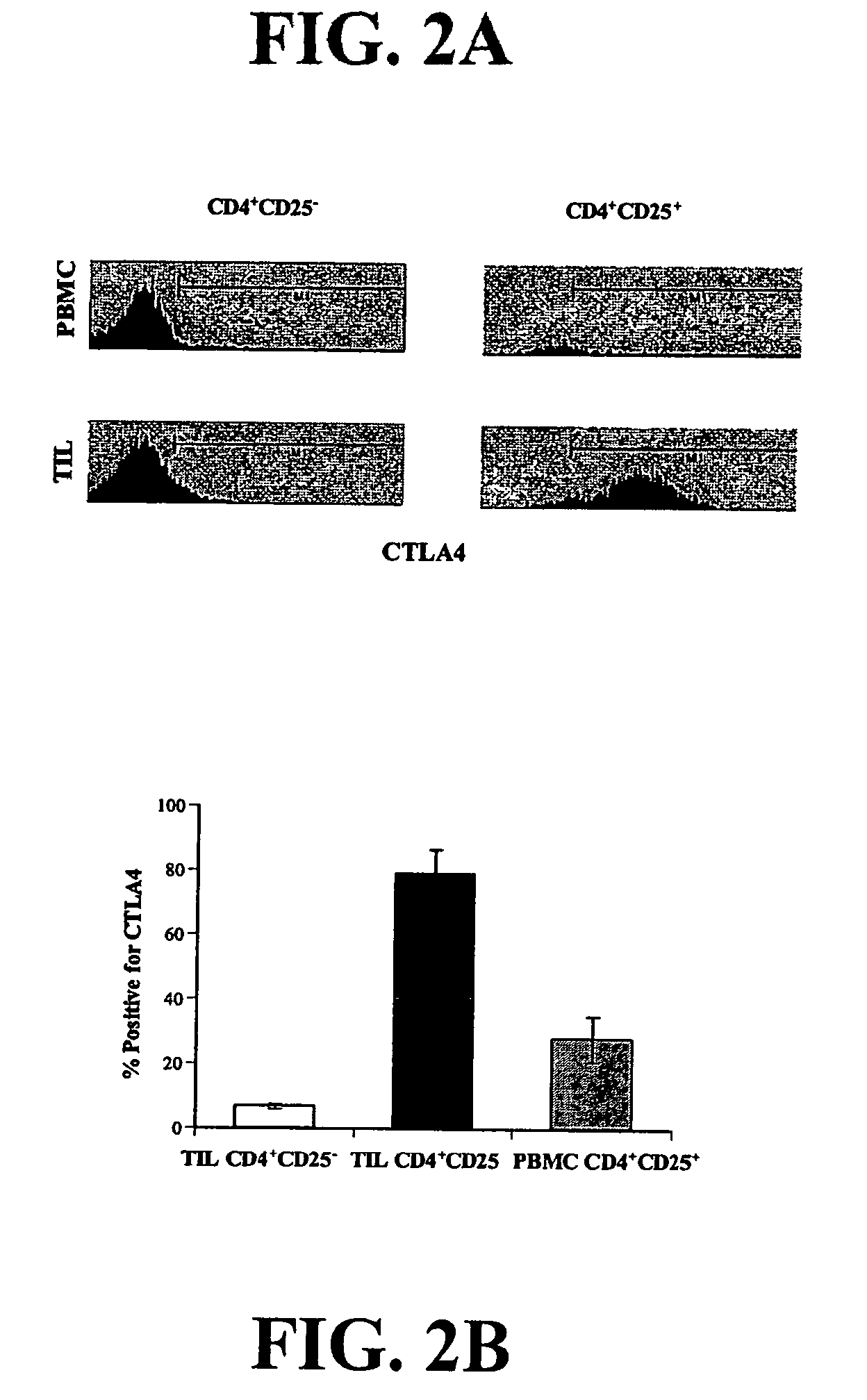
A method of identifying an antigen-specific regulatory T cell (Treg) from a subject is discussed wherein the method comprises quantitatively or qualitatively detecting co-expression of each of cell markers CD4, CD25 and CD134, or alternatively, N each of cell markers CD8, CD25 and CD137, as well as one or more cell markers selected from the group of Treg cell markers consisting of CD39, CD73, CD127, CTLA-4 and Foxp3 on a cell in a suitable lymphocyte-containing sample from the subject in response to exposure to a target antigen. Also discussed are methods of isolating and expanding the identified antigen-specific Treg population, which may permit antigen-specific Treg cell therapy.
Owner:ST VINCENTS HOSPITAL SYDNEY
Method for the identification and separation of non-regulatory T-cells from a mixture of regulatory T-cells
The present invention relates to a method of identifying and separating non-regulatory T-cells (conventional T-cells) from a mixture comprising regulatory T-cells by using of the CD154 molecule (CD40 ligand) through depletion of CD154+ T-cells from the mixture or in combination with additional positive selection of Treg using markers that are specific for regulatory T-cells, such as for example, CD25, GITR, CTLA4 or markers which are specific for activated regulatory T-cells, such as, for example, CD137, “latent TGF-beta (LAP)”, GARP (LRRC32), CD121a / b, thereby generating a cell composition of activated Treg cells. The invention relates also to a kit comprising an antibody for detecting CD154 and at least one additional antibody for detecting markers for activated or non-activated regulatory T-cells. The antibodies can be coupled to a fluorescent dye or magnetic microparticles.
Owner:MILTENYI BIOTEC B V & CO KG
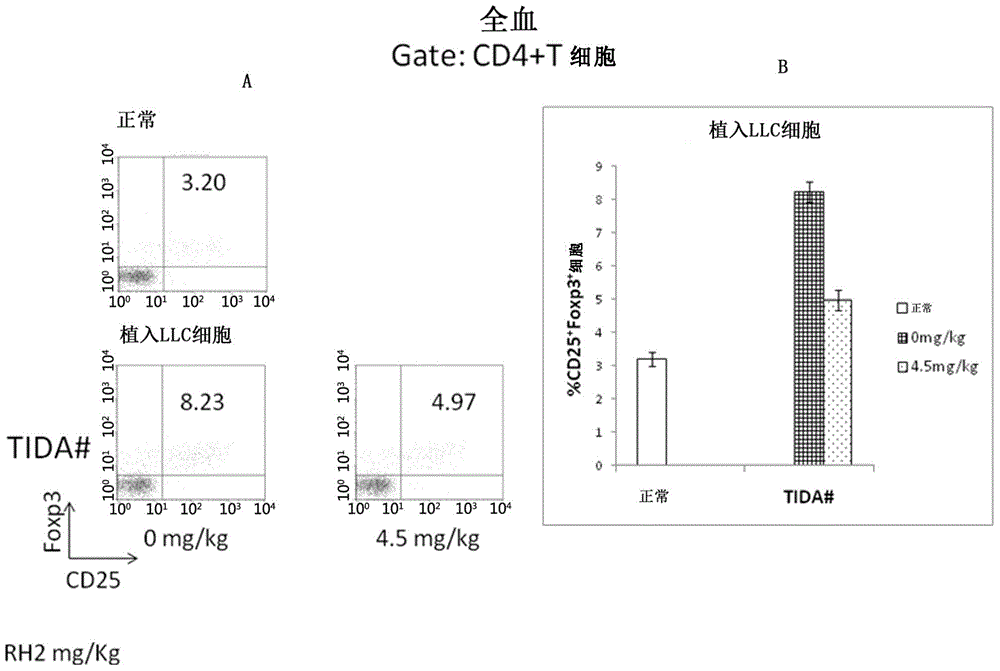
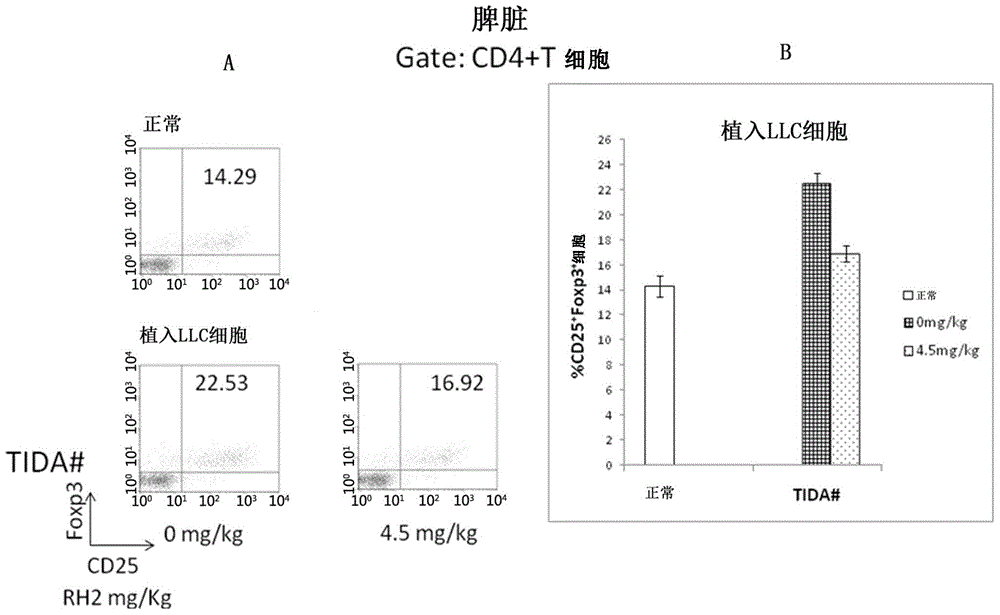
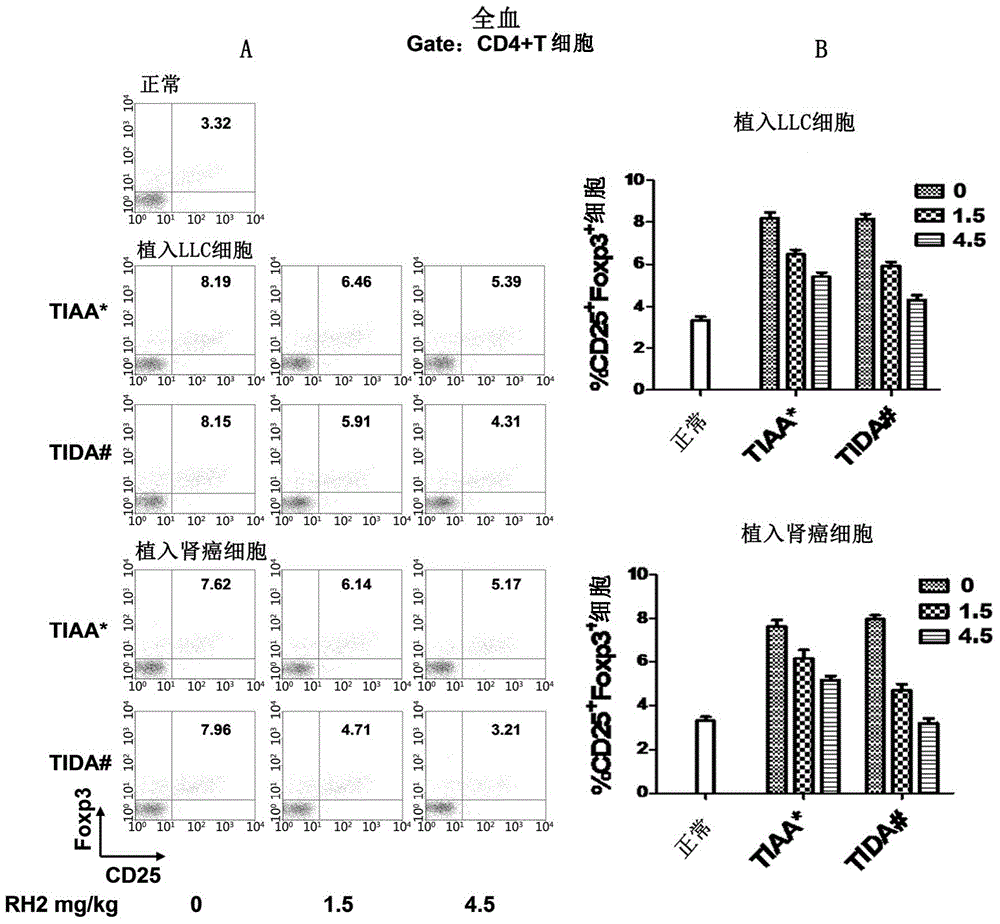
Application of ginsenoside Rh2 to inhibition of Treg cells
InactiveCN105287611AGrowth inhibitionImprove the completion rate of radiotherapy and chemotherapyOrganic active ingredientsAntineoplastic agentsAutoimmune responsesGinsenoside Rh2
The invention discloses application of ginsenoside Rh2 and particularly verifies that ginsenoside can be applied to preparation of inhibitors for inhibiting Treg cells or preparation of compositions for preventing and / or treating tumors with Treg cells increased. The ginsenoside Rh2 is capable of improving balance of T cells in human bodies by inhibition of the Treg cells, autoimmunity can be further enhanced, and accordingly an anti-tumor effect or an immunodeficiency disease improvement effect can be achieved.
Owner:JIANGSU MICROMEDMARK BIOTECH +1
Methods and compositions for expanding T regulatory cells
InactiveCN101378783AHigh affinityEfficient signal transductionCell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsDendritic cellAutoimmune disease
The present invention provides methods and compositions for expanding Treg cells ex vivo or in vivo using one or more conjugates comprising a costimulatory moiety that stimulates at least one of three signals involved in Treg cell development and / or using dendritic cells pulsed with antigens and modified to display TGF-beta, or hematopoetic stem cells or bone marrow cells modified to display TGF-beta. The methods and compositions are useful, for example, in the treatment and prevention of autoimmune disease, including Type 1 diabetes and in preventing foreign graft rejection, as well as to establish mixed chimerism, induce tolerance to autoantigens, alloantigens or xenoantigens, beta cell regeneration, prevention of foreign graft rejection, and treatment of a genetically inherited hematopoietic disorder.
Owner:UNIV OF LOUISVILLE RES FOUND INC
Methods and compositions for expanding T regulatory cells
InactiveUS7745215B2Peptide/protein ingredientsAntibody mimetics/scaffoldsAutoimmune conditionDendritic cell
The present invention provides methods and compositions for expanding Treg cells ex vivo or in vivo using one or more conjugates comprising a costimulatory moiety that stimulates at least one of three signals involved in Treg cell development and / or using dendritic cells pulsed with antigens and modified to display TGF-β, or hematopoetic stem cells or bone marrow cells modified to display TGF-β. The methods and compositions are useful, for example, in the treatment and prevention of autoimmune disease, including Type 1 diabetes and in preventing foreign graft rejection, as well as to establish mixed chimerism, induce tolerance to autoantigens, alloantigens or xenoantigens, beta cell regeneration, prevention of foreign graft rejection, and treatment of a genetically inherited hematopoietic disorder.
Owner:UNIV OF LOUISVILLE RES FOUND INC
Discovery of regulatory t cells programmed to suppress an immune response
A method to treat an autoimmune disease is provided. The method involves administration of interleukin-15 receptor (IL-15R) agonists in an amount effective to ameliorate a symptom of the autoimmune disease. The invention also involves a method to treat an autoimmune disease by ex-vivo expansion of CD44+CD122+Kir+ CD8+ Treg cells and administration of the CD44+CD122+Kir+ CD8+ Treg cells. Compositions comprising CD44+CD122+Kir+ CD8+ Treg cells are also provided. Methods for stimulating an immune response to an antigen are also provided.
Owner:DANA FARBER CANCER INST INC
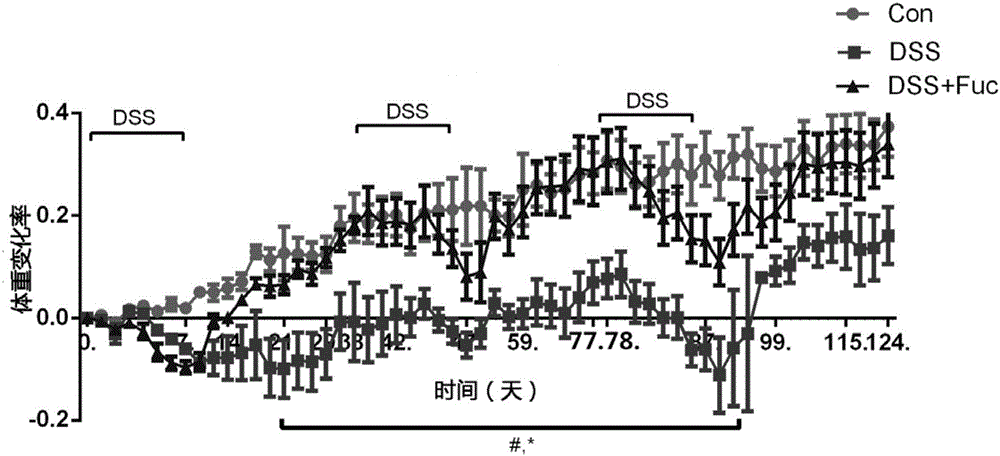
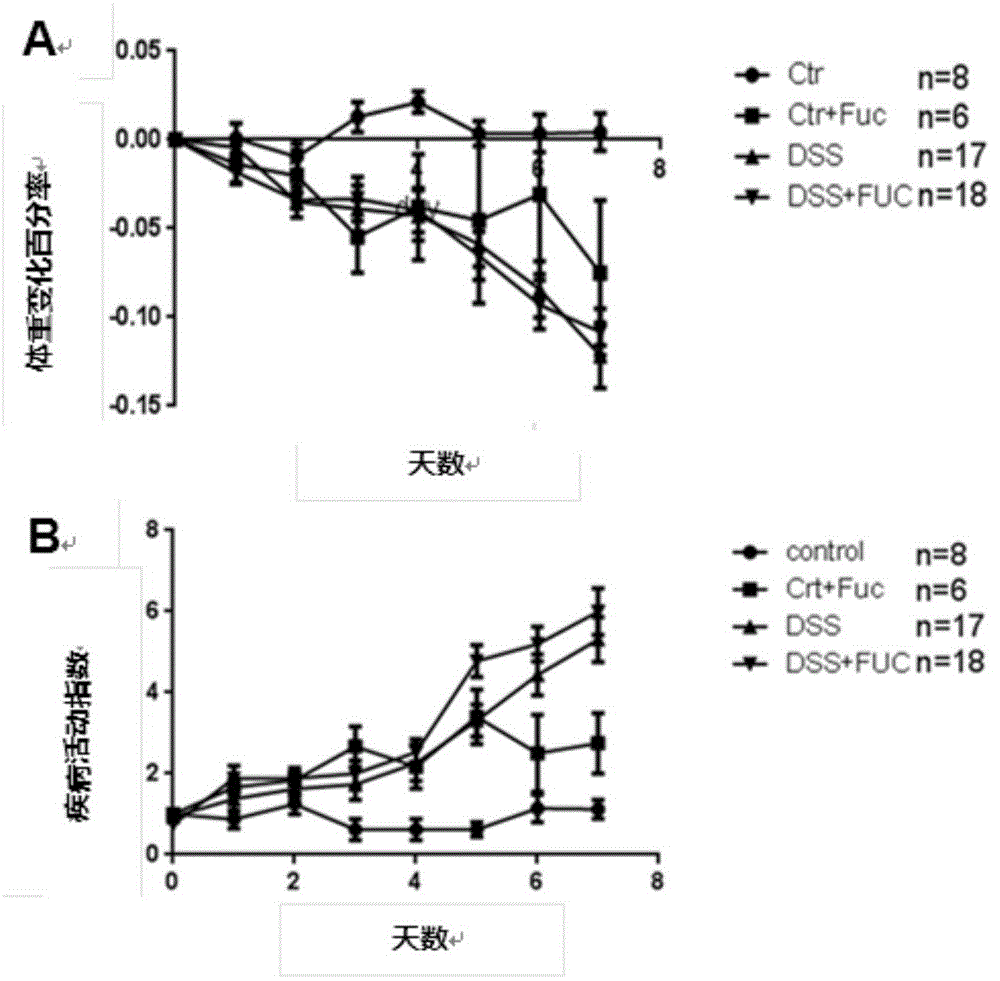
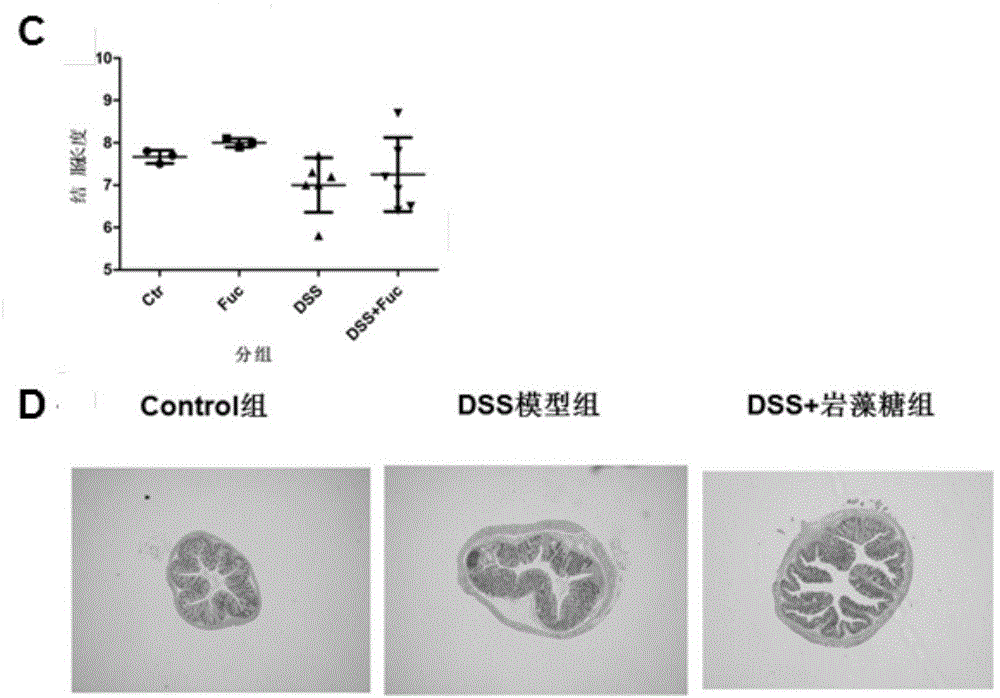
Application of L-fucose in medicines and health-care products for treating gastrointestinal lesions
InactiveCN106668034AReduce incidenceReduce inflammatory damageOrganic active ingredientsDigestive systemTolerabilityPoor compliance
The invention discloses application of L-fucose in medicines and health-care products for treating gastrointestinal lesions. The L-fucose is used for treating the gastrointestinal lesions, such as inflammatory bowel disease, functional gastrointestinal disorder and irritable bowel syndrome. The medicine is a carbohydrate medicine, since the adverse effect is less, and the tolerance of a patient is good, so that the medicine can be widely applied into clinic. The medicine has the advantages that the L-fucose is used in an early period of the gastrointestinal lesions, the activation of mesenteric lymph node Treg cells is regulated by DC cells, the differentiation potential of colons Th1 and Th17 is inhibited, the generation of bile acid is inhibited by regulating the intestinal flora, the function of inhibiting the contraction and spasm of intestinal muscles is realized by an nNOS path, the intestinal inflammation and weight-losing conditions are improved, and the patient can obtain an obvious long-term prognosis benefit; because of limitations of related existing therapy medicines, namely multiple medicine types, large side effect and poor compliance, a new therapy strategy for treating the patients with the gastrointestinal lesions, such as the inflammatory bowel disease, the functional gastrointestinal disorder and the irritable bowel syndrome, is provided.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
Direct reversal of the suppressive function of cd4+ regulatory t cells via toll-like receptor 8 signaling
InactiveCN101184852AEasy to optimizeSimple designSugar derivativesMicrobiological testing/measurementTLR8Disease
CD4<+> regulatory T (Treg) cells profoundly suppress host immune responses and thus protect against autoimmune disease while restricting desired immune responses such as antitumor immunity. Synthetic phosphorothioate-protected, guanosine-containing oligonucleotides can directly reverse the suppressive activity of Treg cells without involving dendritic cells. This effect appears to be transduced by signaling through Toll-like receptor (TLR) 8 and engagement of the MyD88 and IRAK4 molecules in Treg cells. Stimulation of Treg cells with natural ligands for human TLR8 recapitulated the effect of the synthetic guanosine-containing oligonucleotides .
Owner:BAYLOR COLLEGE OF MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com