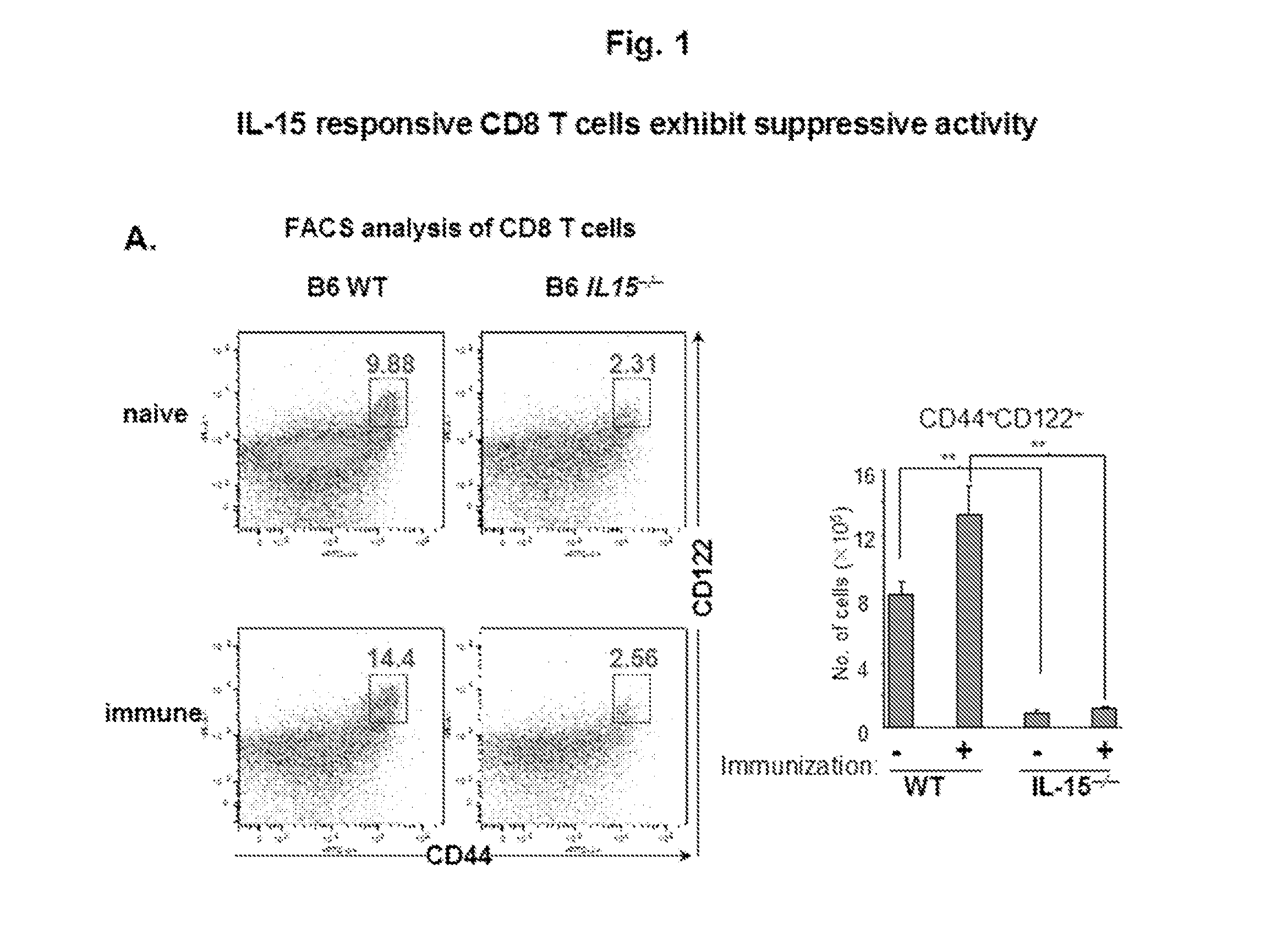
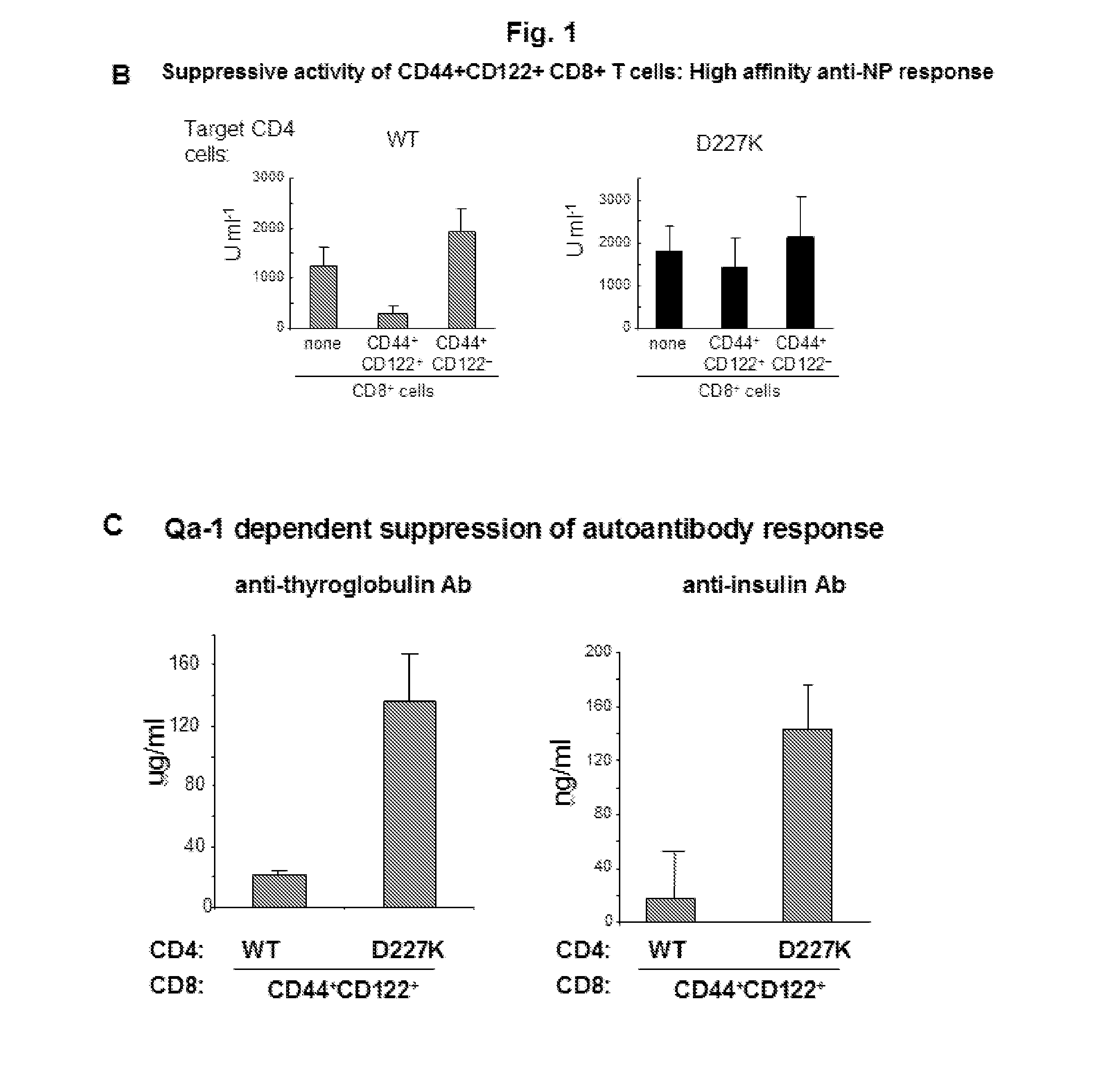
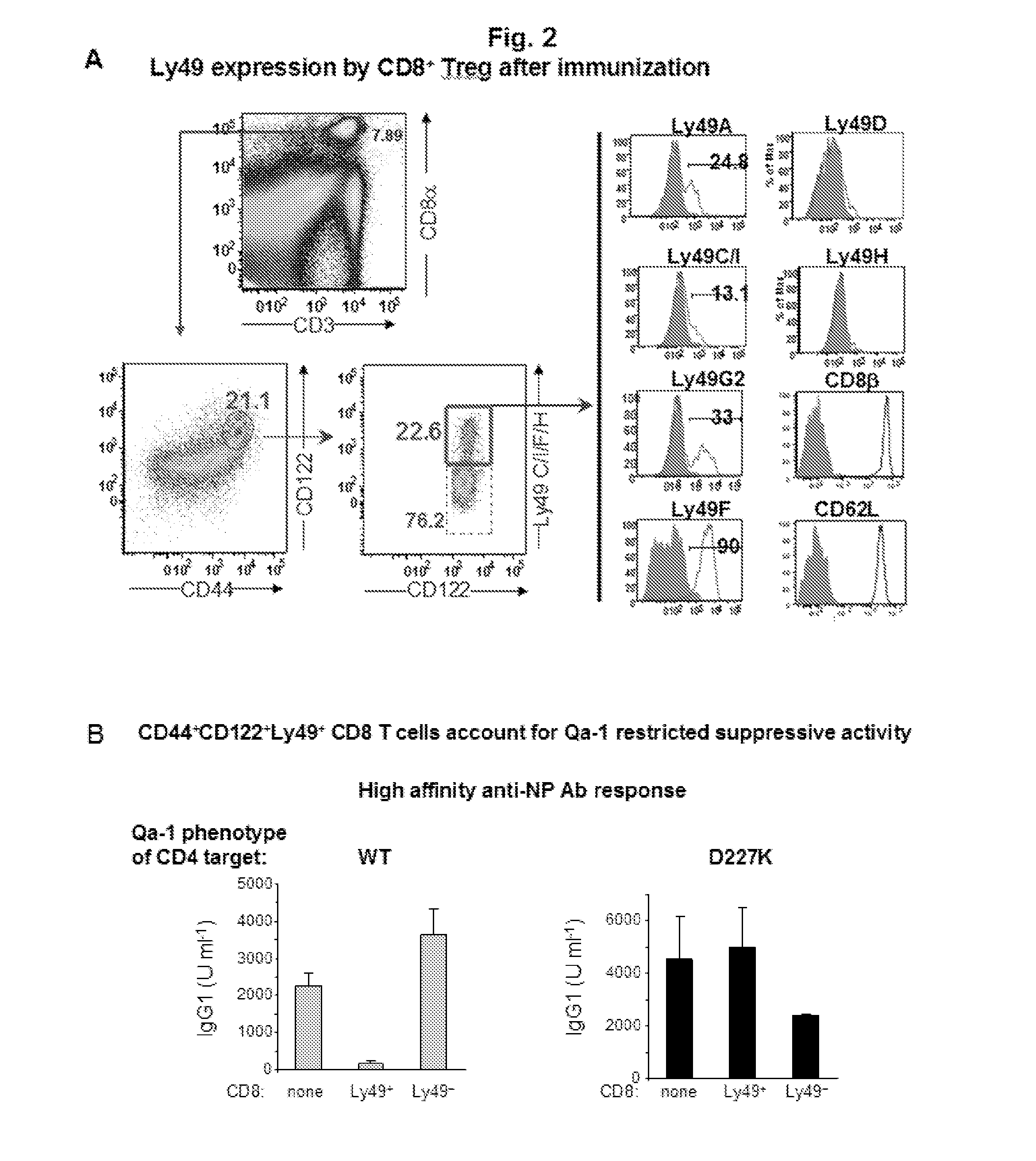
Discovery of regulatory t cells programmed to suppress an immune response
a technology immune responses, applied in the field of discovery of regulatory t cells programmed to suppress an immune response, can solve the problems of insufficient cell-intrinsic mechanisms to prevent the development of autoimmune disorders, incomplete process, and inability to define autoimmune diseases, so as to maintain self-acceptance and improve the symptom of autoimmune diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
Materials and Methods
Mice.
[0056]C57BL / 6J (B6), Rag2− / −, IL-15− / − and B6.Qa-1(D227K) mice (backcrossed for 11 generations) were housed in pathogen-free conditions. All experiments were performed in compliance with federal laws and institutional guidelines as approved by Dana Farber Cancer Institute's Animal Care and Use Committee.
Reagents and Flow Cytometry.
[0057]Single-cell suspensions were prepared and maintained in the dark at 4° C. for immunofluorescent analysis, washed in ice-cold FACS buffer (2% fetal calf serum, 0.1% NaN3 in PBS) and incubated with each antibody for 30 min and washed with FACS buffer before analyzing. Anti-CD4, anti-CD8, anti-B220, anti-CD44, anti-CD62L, anti-Fas, anti-ICOS, anti-IgM, anti-CD200 (BD Bioscience) or anti-CD8β, anti-Ly49C / I / F / H, anti-Ly49A, Ly-49G2, Ly49C / I, Ly49D, Ly49F, Ly49H (eBioscience) were used followed by analysis of cells using a FACSCanto (BD Biosciences) and FlowJo software (TriStar).
Cell Purification and Adoptive Transfer.
[0058]Naïve ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| soluble | aaaaa | aaaaa |
| affinity | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com