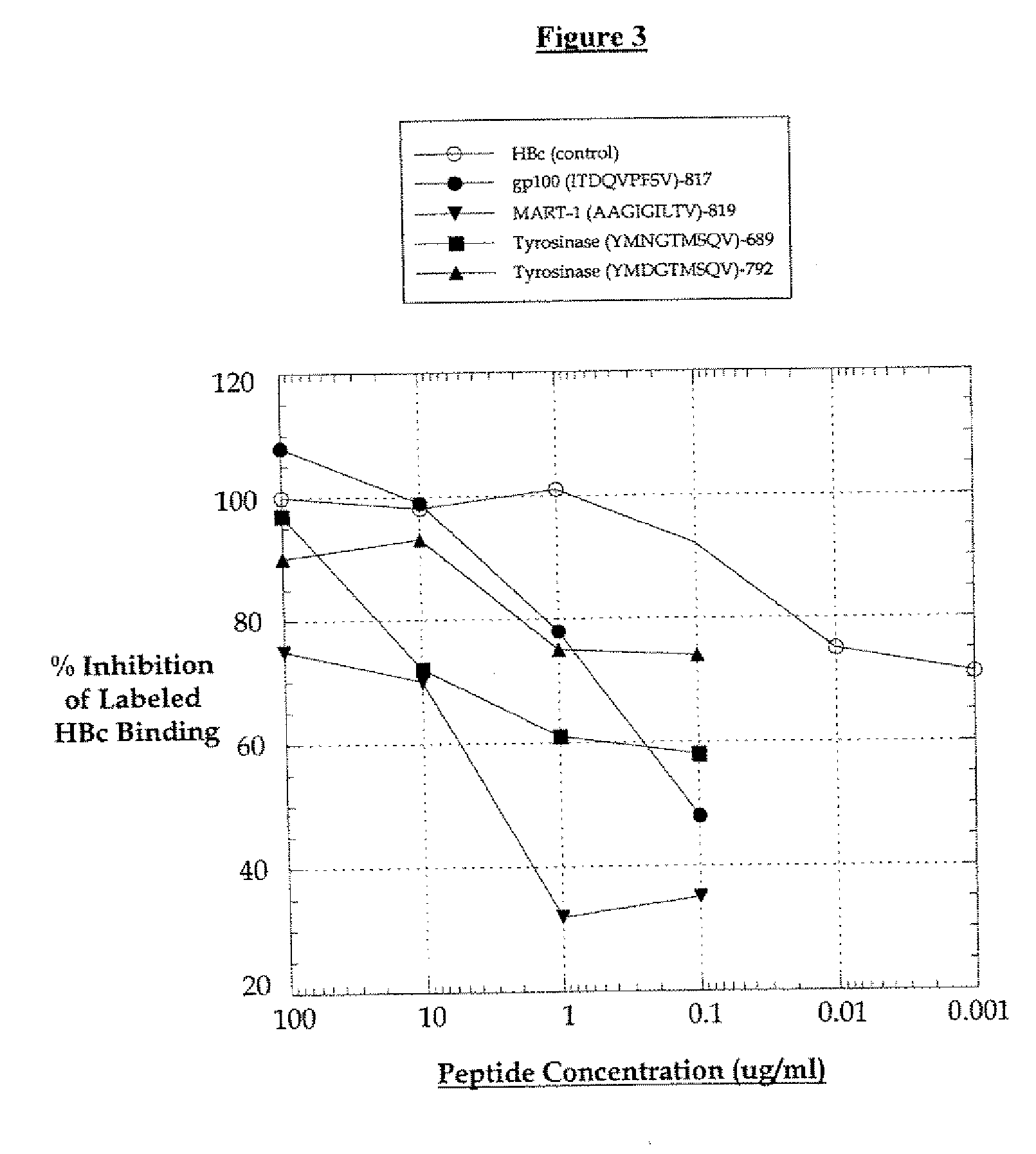
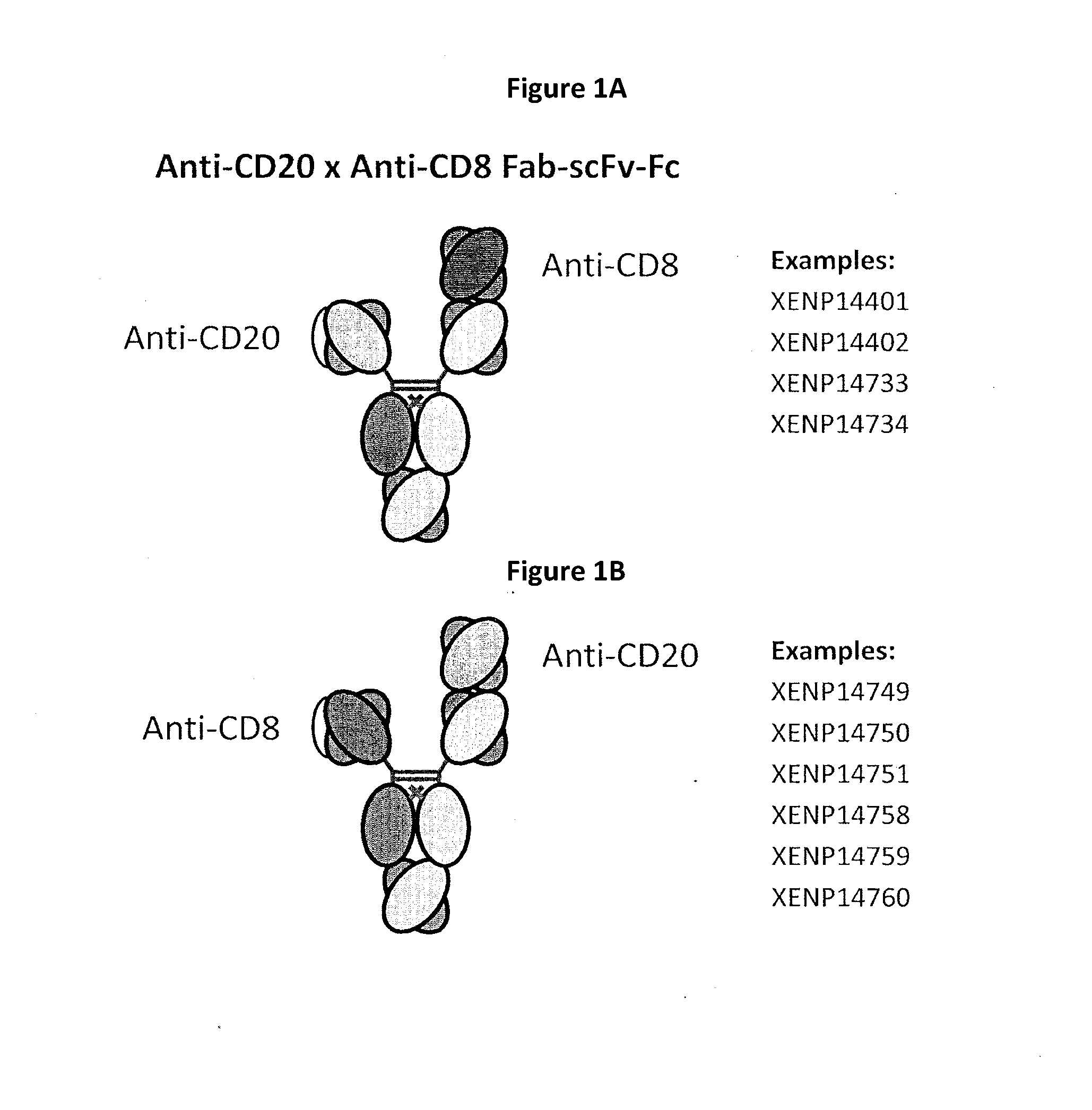
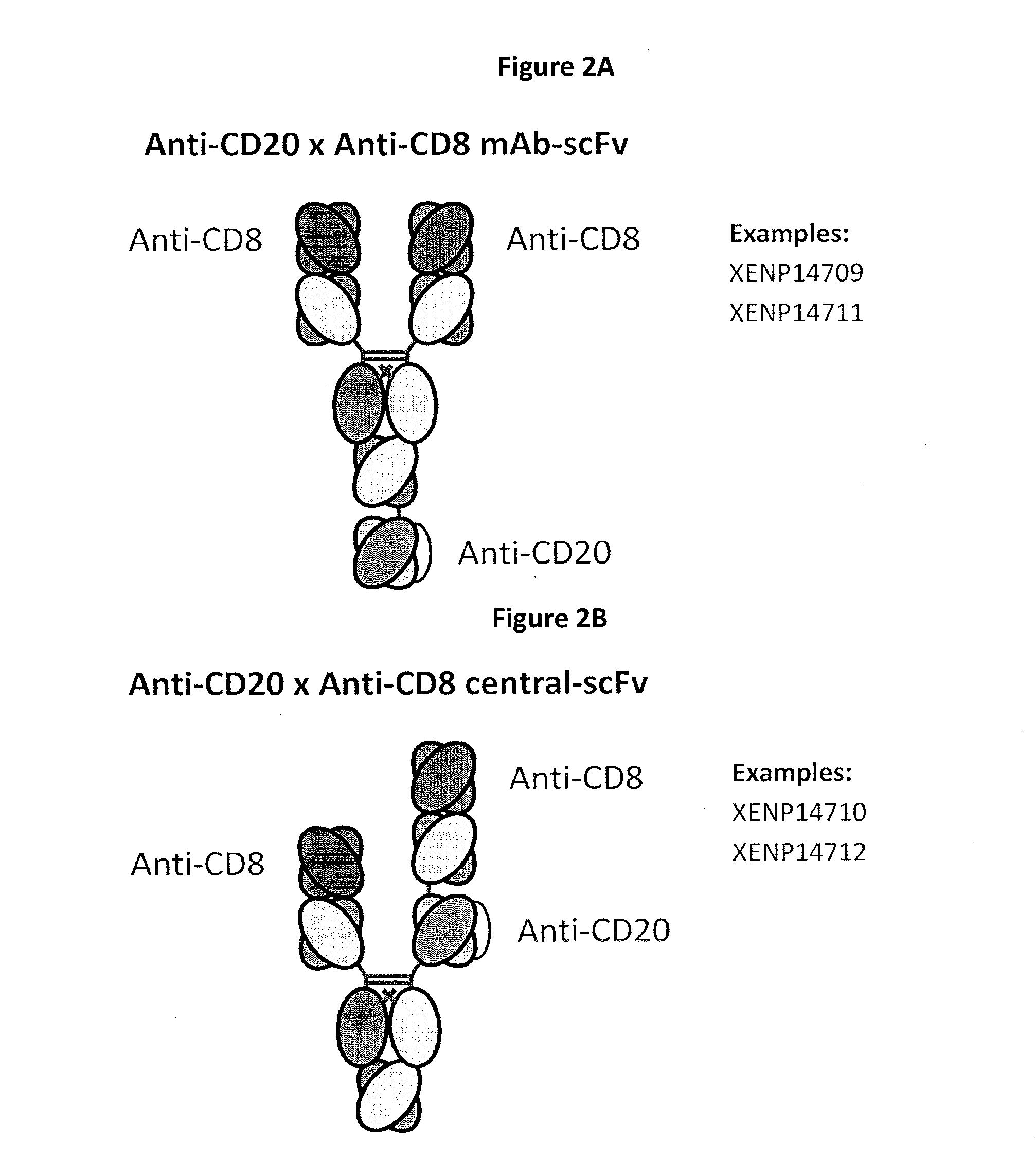
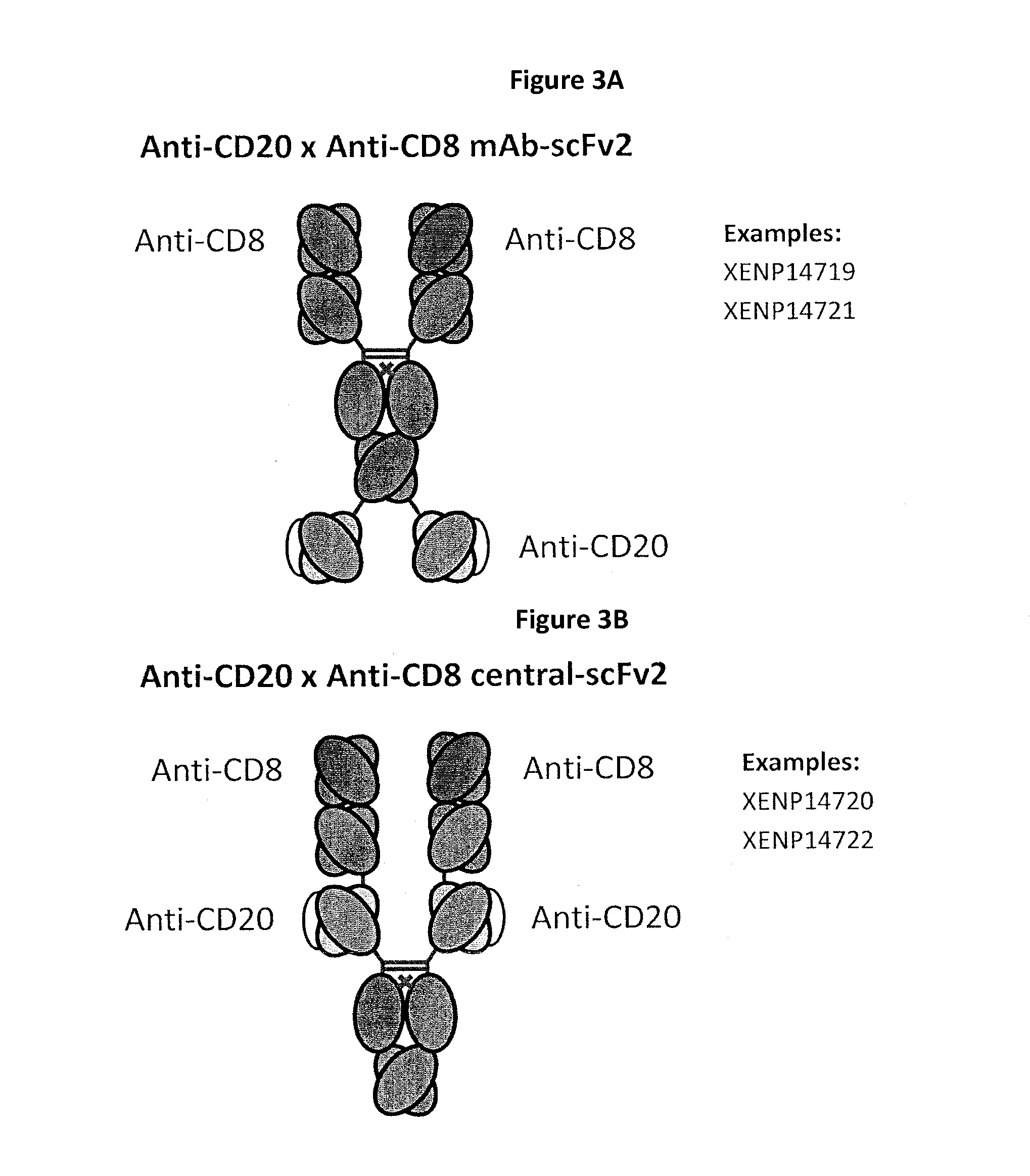
Patents
Literature
988 results about "CD8" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
CD8 (cluster of differentiation 8) is a transmembrane glycoprotein that serves as a co-receptor for the T cell receptor (TCR). Like the TCR, CD8 binds to a major histocompatibility complex (MHC) molecule, but is specific for the class I MHC protein. There are two isoforms of the protein, alpha and beta, each encoded by a different gene. In humans, both genes are located on chromosome 2 in position 2p12.
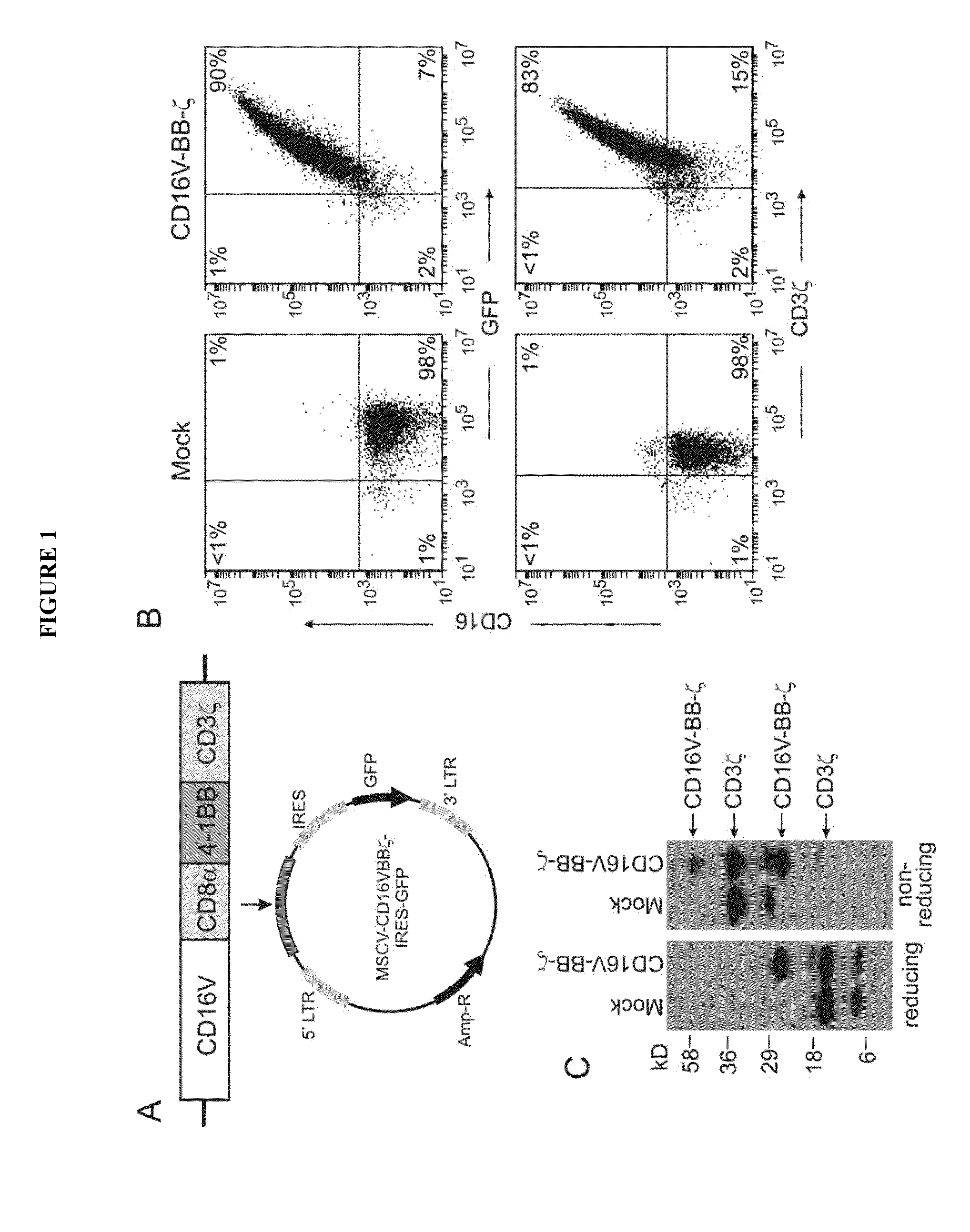
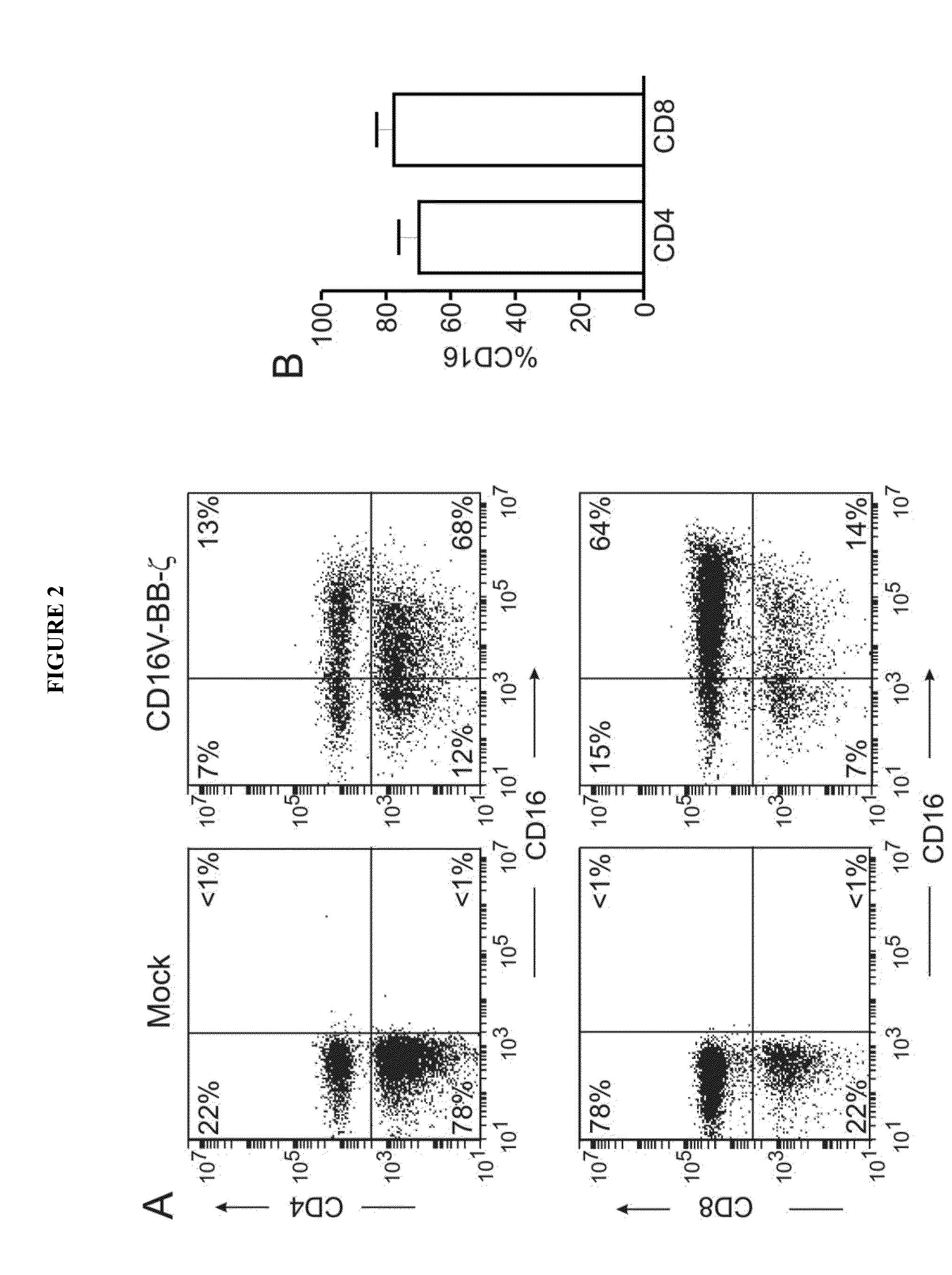
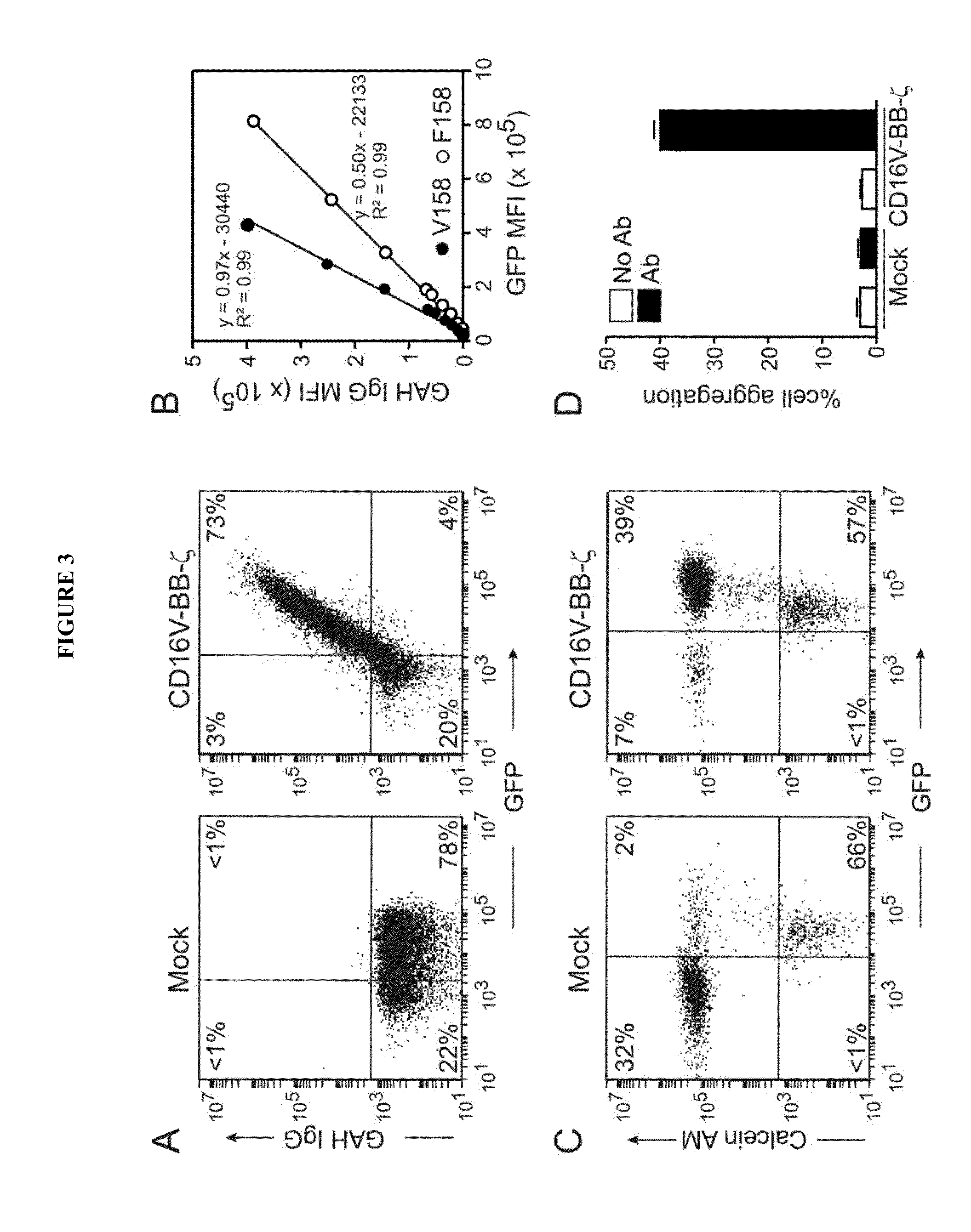
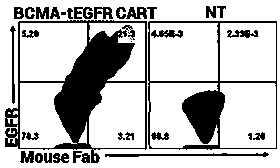
Chimeric receptors and uses thereof in immune therapy
ActiveUS20150139943A1Good curative effectEnhanced ADCC activityVirusesPeptide/protein ingredientsCytotoxicityCD8
Owner:COGENT BIOSCIENCES INC +2
Methods of producing enriched populations of tumor-reactive t cells from tumor
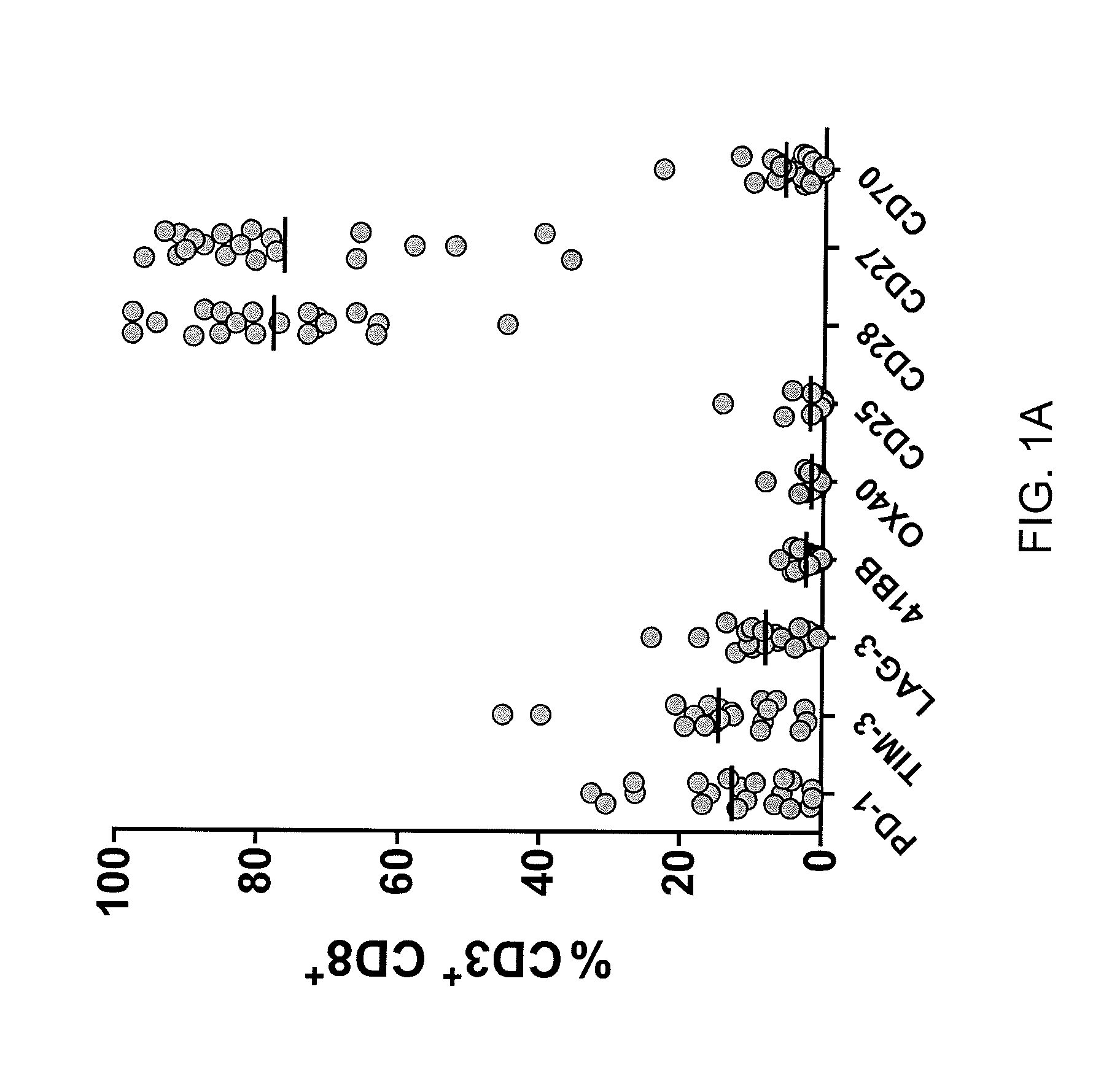
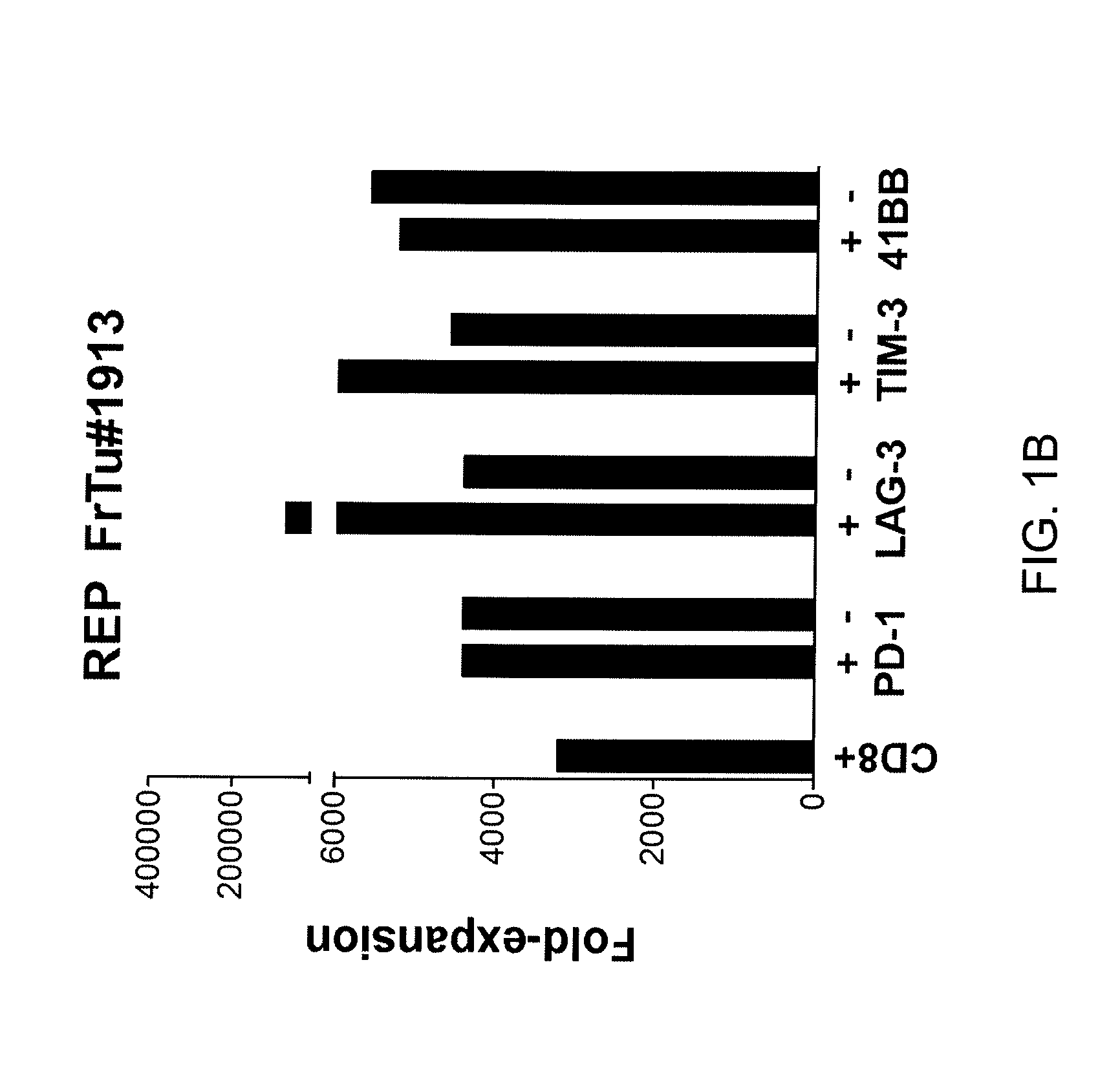
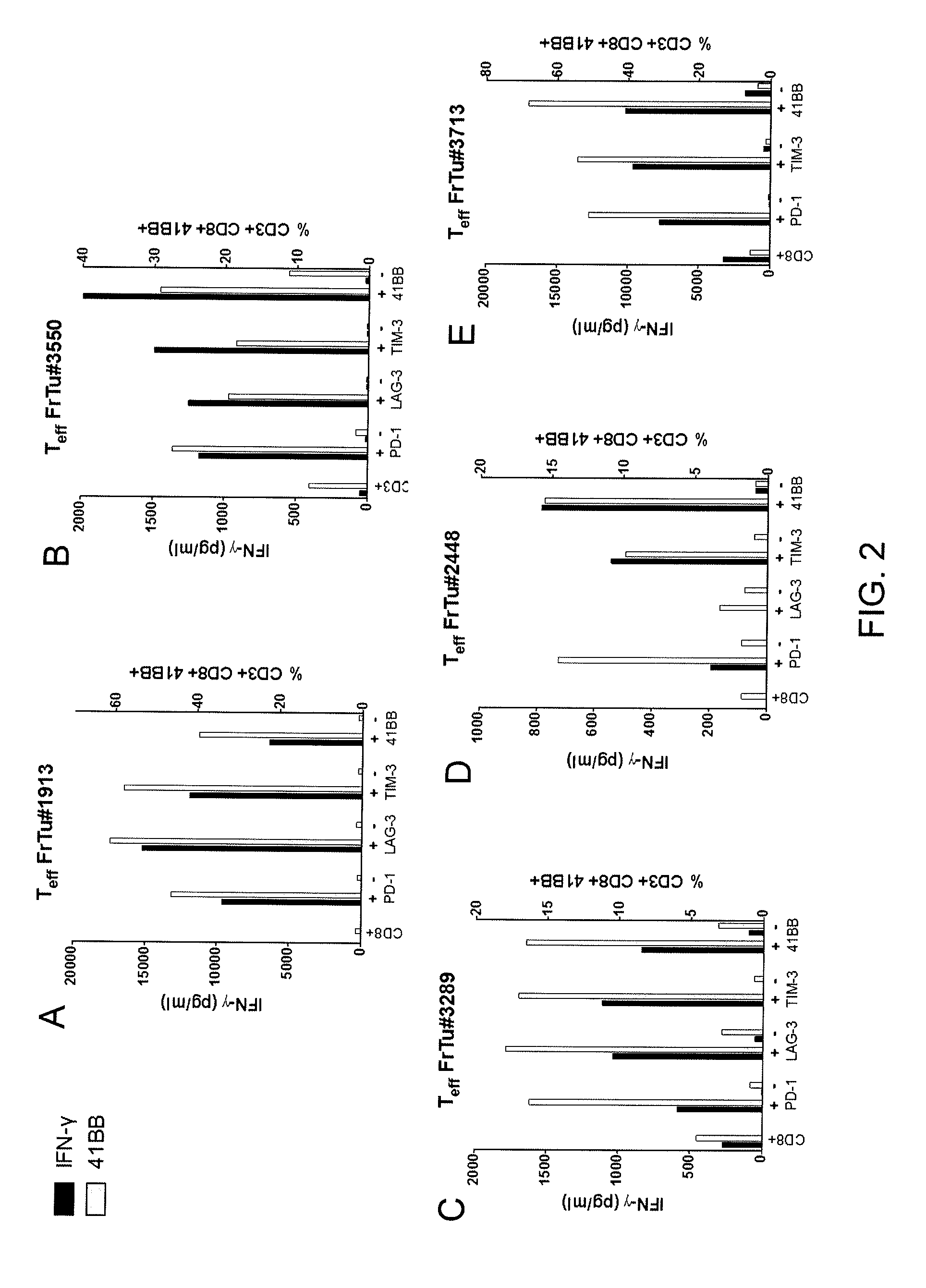
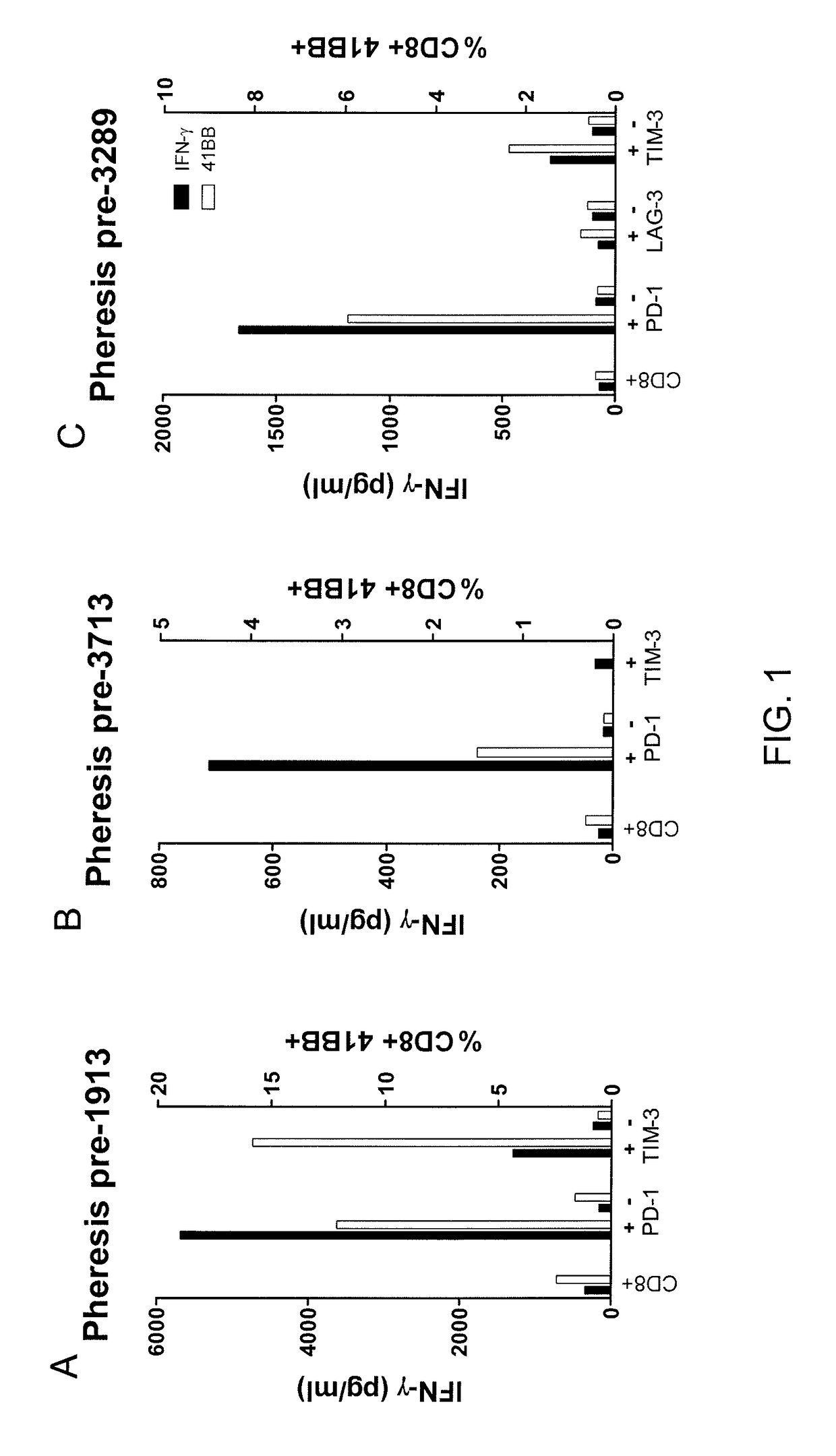
Methods of obtaining a cell population enriched for tumor-reactive T cells, the method comprising: (a) obtaining a bulk population of T cells from a tumor sample; (b) specifically selecting CD8+ T cells that express any one or more of TIM-3, LAG-3, 4-1BB, and PD-1 from the bulk population; and (c) separating the cells selected in (b) from unselected cells to obtain a cell population enriched for tumor-reactive T cells are disclosed. Related methods of administering a cell population enriched for tumor-reactive T cells to a mammal, methods of obtaining a pharmaceutical composition comprising a cell population enriched for tumor-reactive T cells, and isolated or purified cell populations are also disclosed.
Owner:UNITED STATES OF AMERICA
Use of synthetic glycolipids as universal adjuvants for vaccines against cancer and infectious diseases
InactiveUS20050192248A1Enhancement and extension of durationBiocideOrganic active ingredientsDiseaseAdjuvant
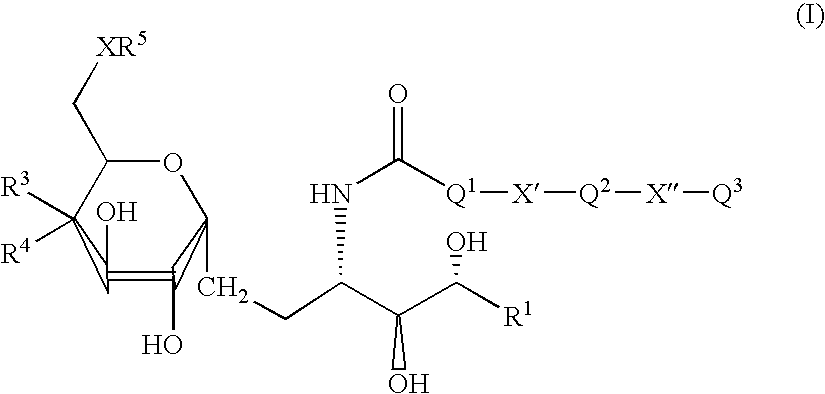
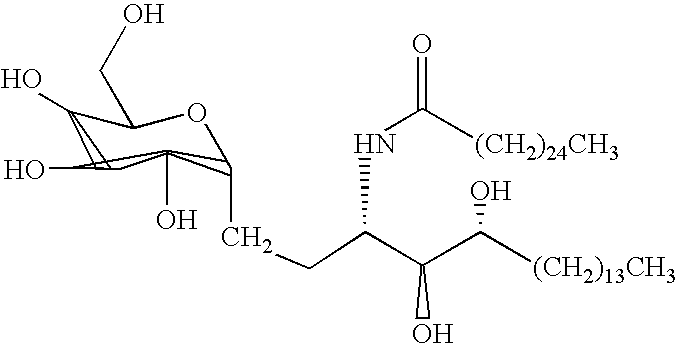
The present invention relates to methods and compositions for augmenting an immunogenicity of an antigen in a mammal, comprising administering said antigen together with an adjuvant composition that includes a synthetic glycolipid compound of Formula I, as described herein. According to the present invention, the use of a compound of Formula I as an adjuvant is attributed at least in part to the enhancement and / or extension of antigen-specific Th1-type responses, in particular, CD8+ T cell responses. The methods and compositions of the present invention can be useful for prophylaxis and treatment of various infectious and neoplastic diseases.
Owner:NEW YORK UNIV +1
Methods of producing enriched populations of tumor reactive T cells from peripheral blood
ActiveUS9844569B2Mammal material medical ingredientsBlood/immune system cellsAbnormal tissue growthPeripheral blood mononuclear cell
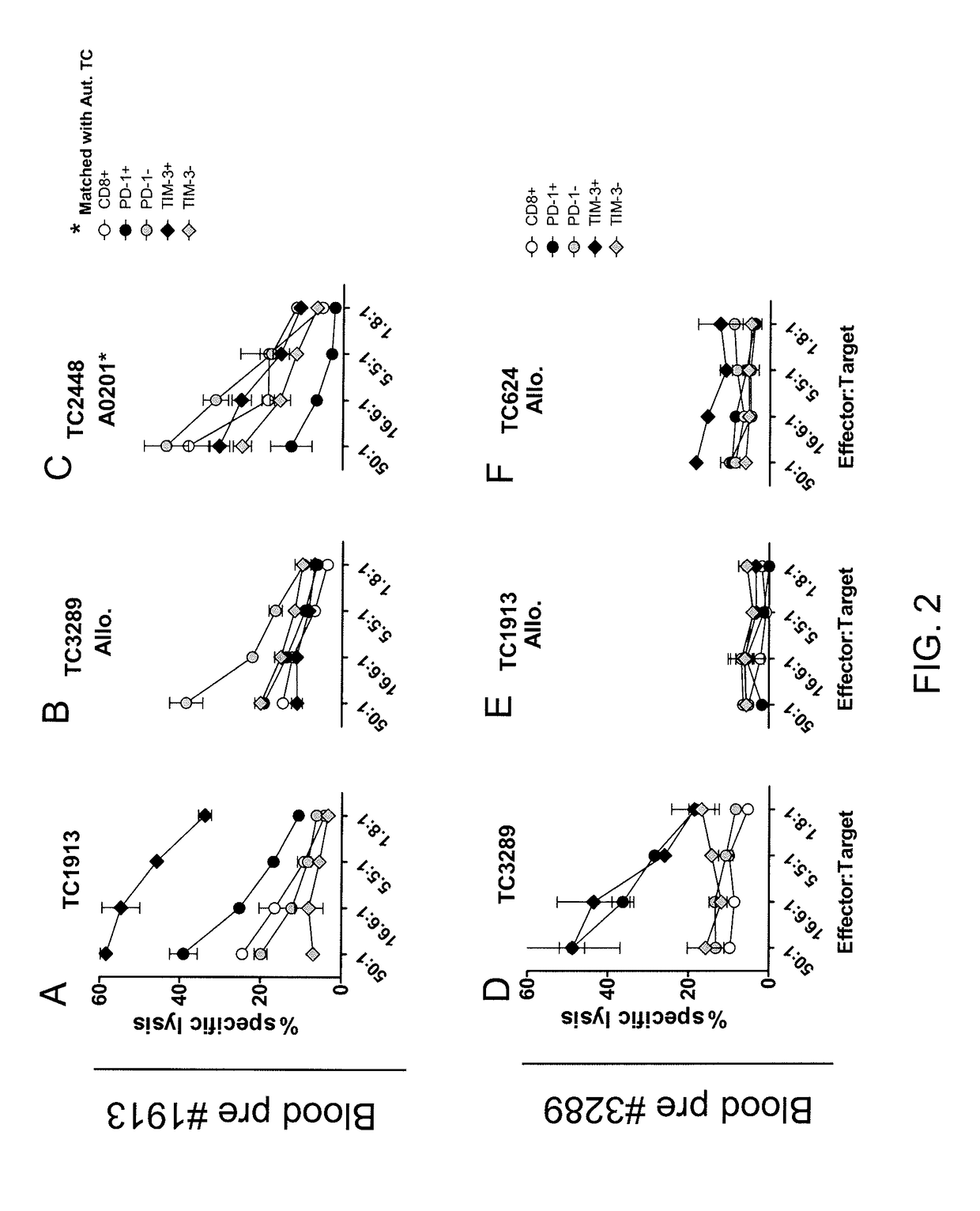
Methods of obtaining a cell population enriched for tumor-reactive T cells, the method comprising: (a) obtaining a bulk population of peripheral blood mononuclear cells (PBMCs) from a sample of peripheral blood; (b) specifically selecting CD8+T cells that also express PD-1 and / or TIM-3 from the bulk population; and (c) separating the cells selected in (b) from unselected cells to obtain a cell population enriched for tumor-reactive T cells are disclosed. Related methods of administering a cell population enriched for tumor-reactive T cells to a mammal, methods of obtaining a pharmaceutical composition comprising a cell population enriched for tumor-reactive T cells, and isolated or purified cell populations are also disclosed.
Owner:UNITED STATES OF AMERICA
Full-humanized anti-PD-1 monoclonal antibody and preparation method and application thereof
InactiveCN103242448AHigh affinityImproving immunogenicityAntiviralsImmunoglobulins against cell receptors/antigens/surface-determinantsAutoimmune diseaseFactor ii
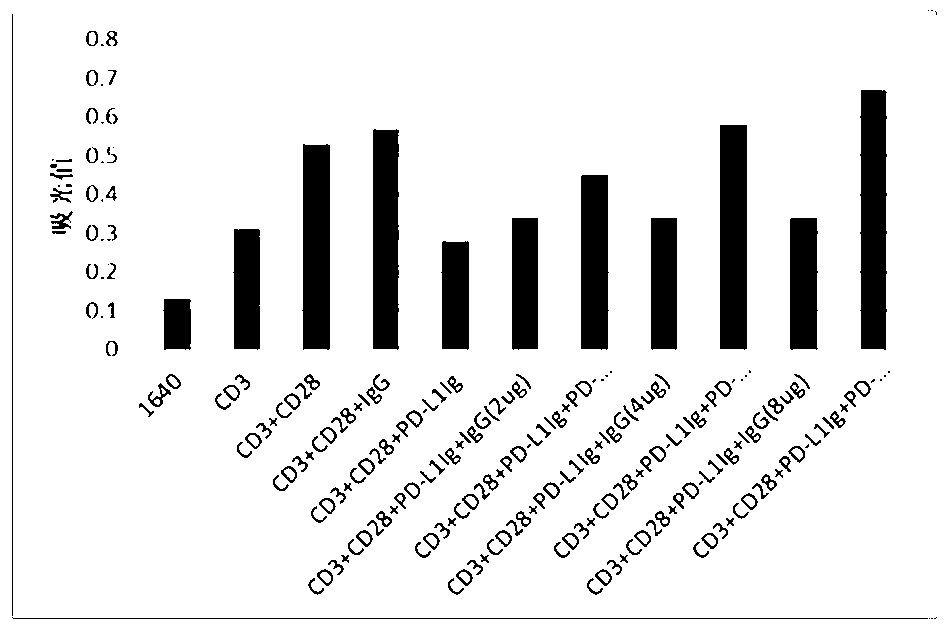
The invention discloses a full-humanized anti-PD-1 monoclonal antibody having a heavy-chain amino acid sequence shown in a sequence 7 in a sequence table and a light-chain amino acid sequence shown in a sequence 8 in the sequence table. The full-humanized anti-PD-1 monoclonal antibody has the advantages that the antibody has high appetency and low immunogenicity on PD-1, is efficiently expressed in animal cells, and can be used for industrial production. An experiment proves that the full-humanized anti-PD-1 monoclonal antibody specifically blocks a PD-1 / PD-L inhibiting signal, so that a disabled effector cell in an organism restores a biological function; activation proliferation of a tumor and a virus specificity CD8+T cell and secretion of a cell factor are facilitated; the killability of lymphocyte on tumor antigen, an exotic invasive virus and the like is enhanced; the immunity of the organism is improved; and the tumor cell and the virus are timely cleared. Therefore, the antibody disclosed by the invention has a wide application prospect on treatment of tumors, infectious diseases and autoimmune diseases.
Owner:ZHENGZHOU UNIV
A cell therapy method for the treatment of tumors
T cell responses are often diminished in humans with a compromised immune system. We have developed a method to isolate, stimulate and expand naïve cytotoxic T lymphocyte precursors (CTLp) to antigen-specific effectors, capable of lysing tumor cells in vivo. This ex vivo protocol produces fully functional effectors. Artificial antigen presenting cells (AAPCs; Drosophila melanogaster) transfected with human HLA class I and defined accessory molecules, are used to stimulate CD8+ T cells from both normal donors and cancer patients. The class I molecules expressed to a high density on the surface of the Drosophila cells are empty, allowing for efficient loading of multiple peptides that results in the generation of polyclonal responses recognizing tumor cells endogenously expressing the specific peptides. The responses generated are robust, antigen-specific and reproducible if the peptide epitope is a defined immunogen. This artificial antigen expression system can be adapted to treat most cancers in a significant majority of the population.
Owner:JANSSEN PHARMA INC
Heterodimeric antibodies including binding to cd8
The invention provides bispecific antibodies that co-engage CD8 (preferably bivalently) and a target tumor antigen.
Owner:XENCOR INC
Prostate stem cell antigen vaccines and uses thereof
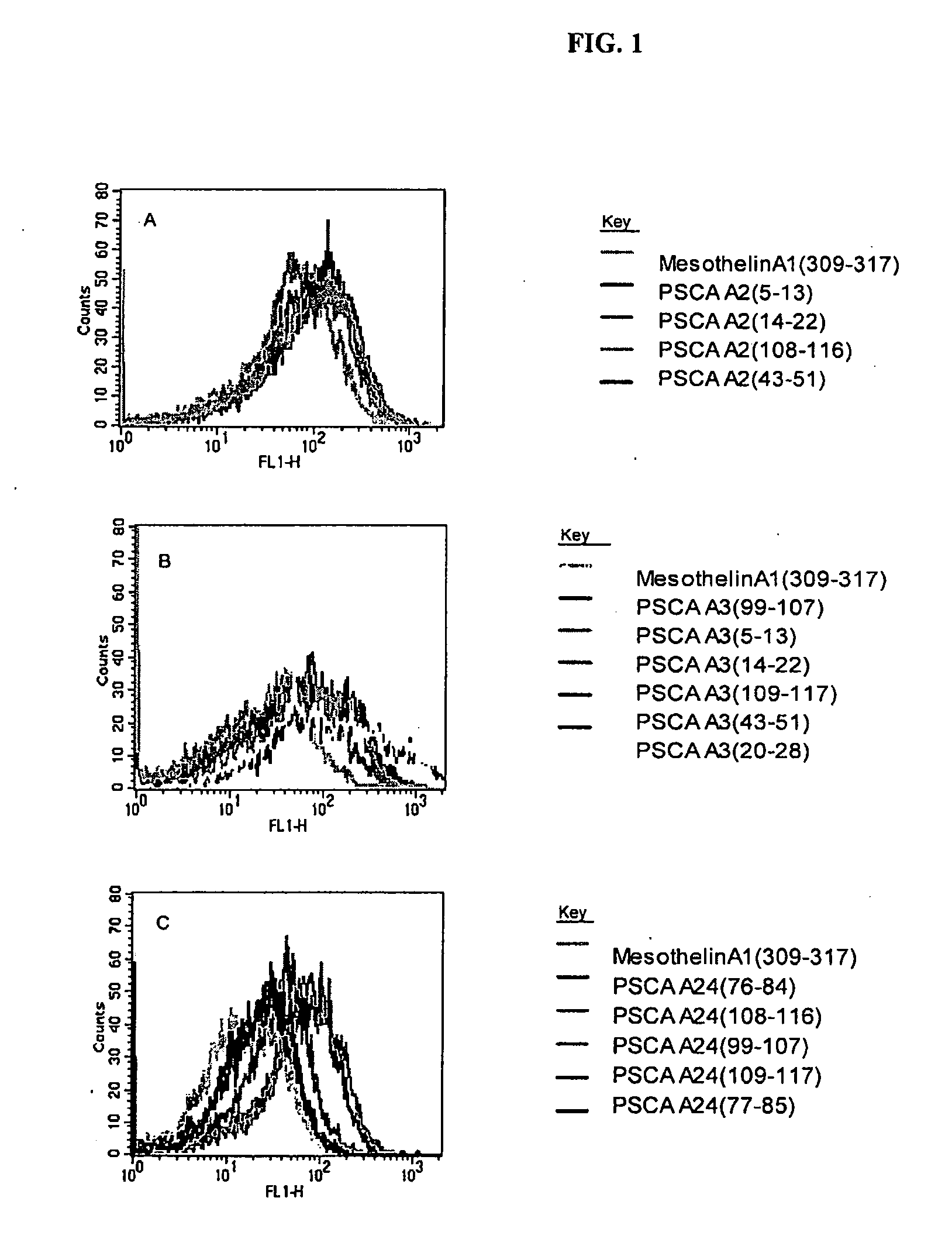
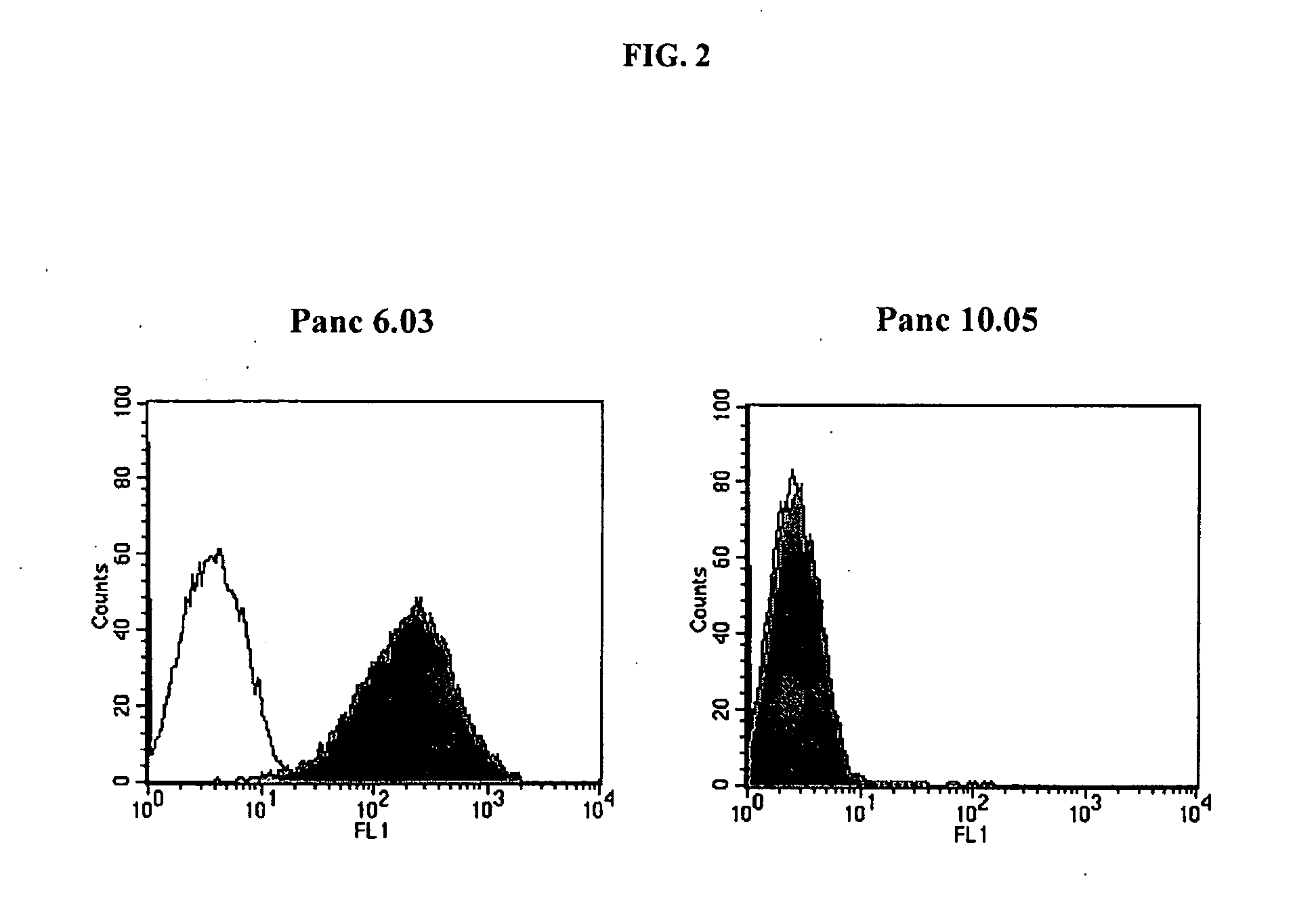
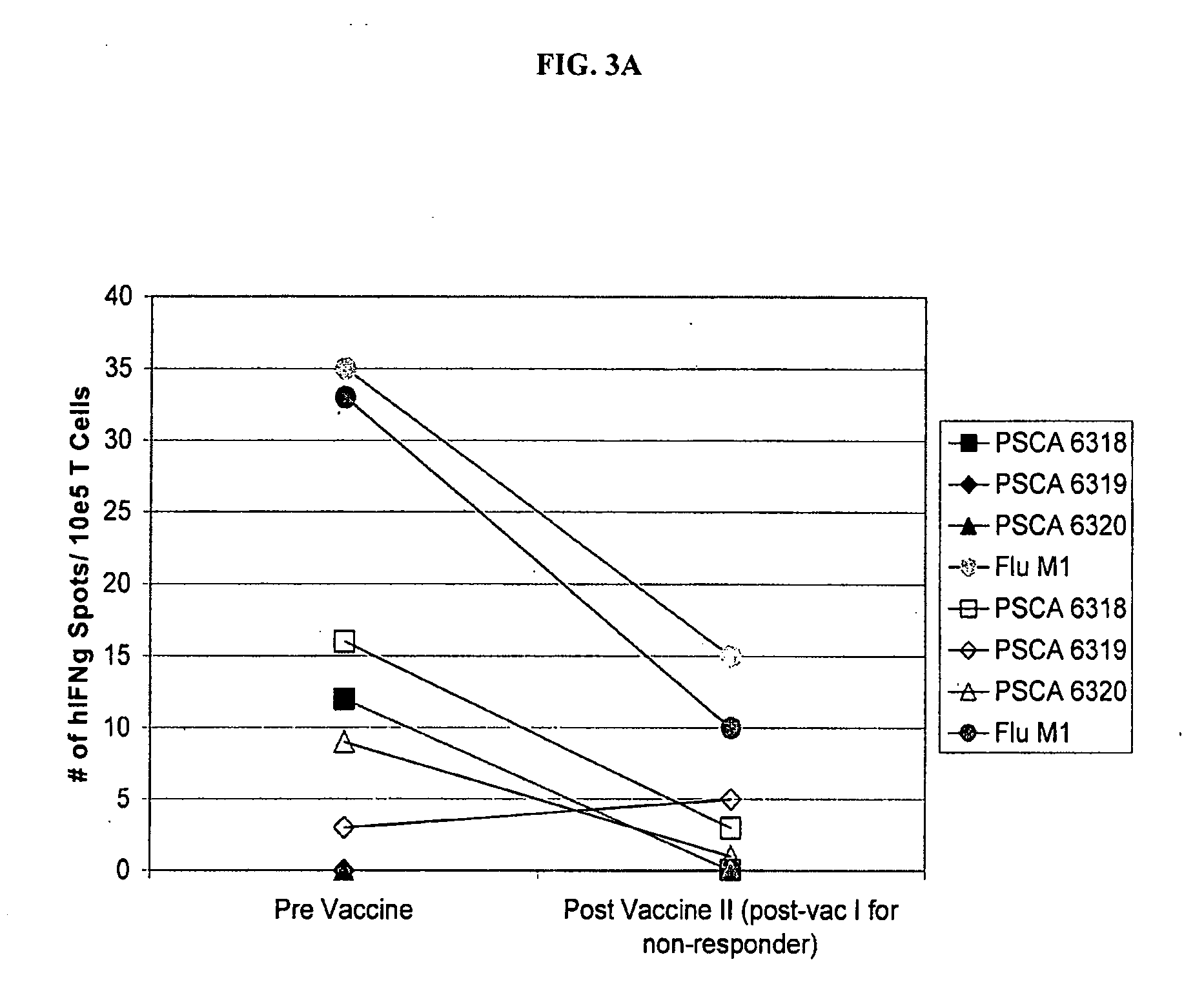
This invention relates to the identification of prostate stem cell antigen (PSCA) as a target of clinically relevant antitumor immune responses. The invention provides vaccines comprising PSCA, or fragments thereof, which are useful in inducing antitumor immune responses, including PSCA specific CD8+ T cell responses. Methods of using the compositions to treat cancer are also provided. The invention further provides methods of identifying compounds useful in antitumor vaccines and methods of assessing the responses of patients to cancer immunotherapy.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
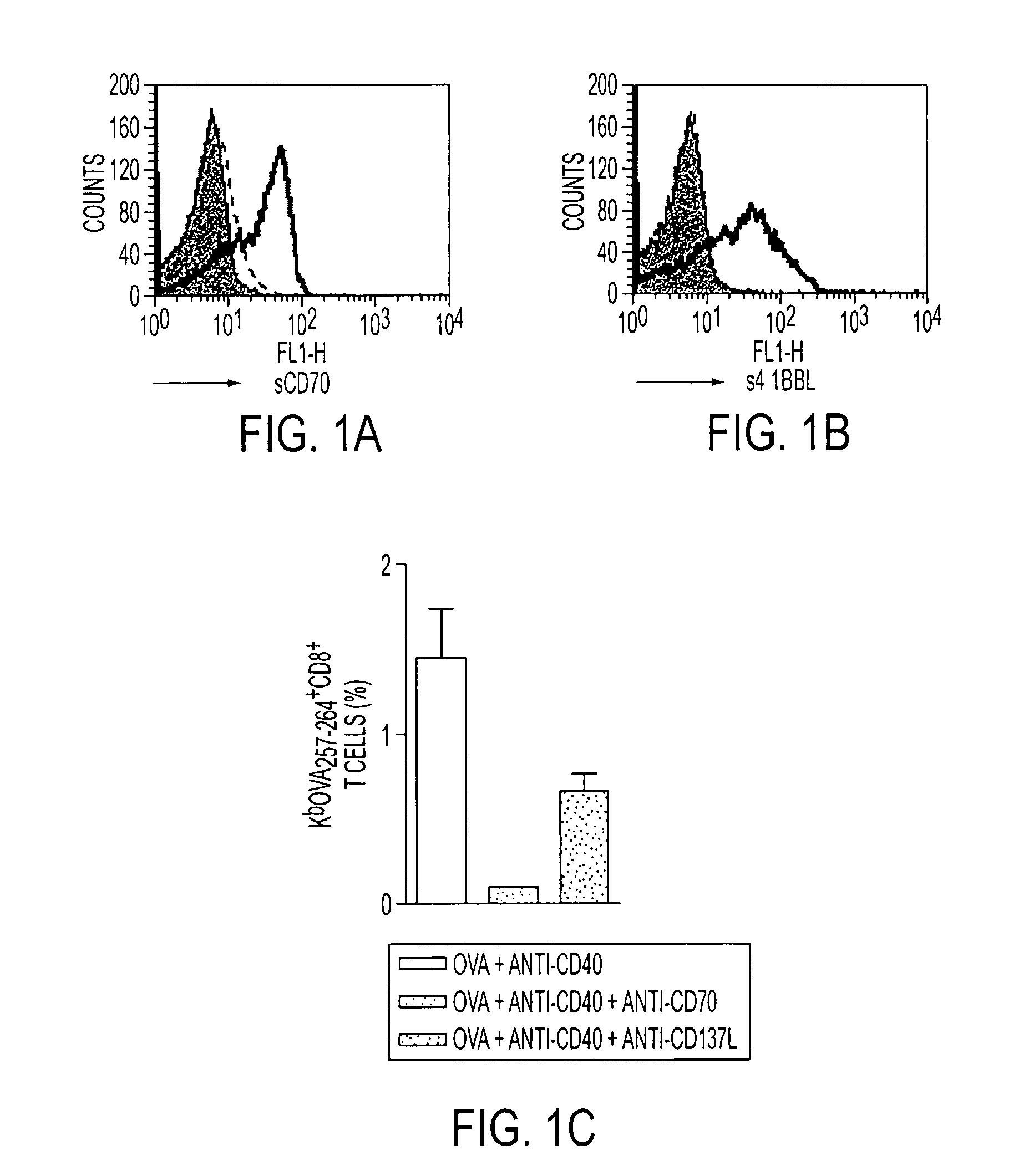
Human immune therapies using a CD27 agonist alone or in combination with other immune modulators
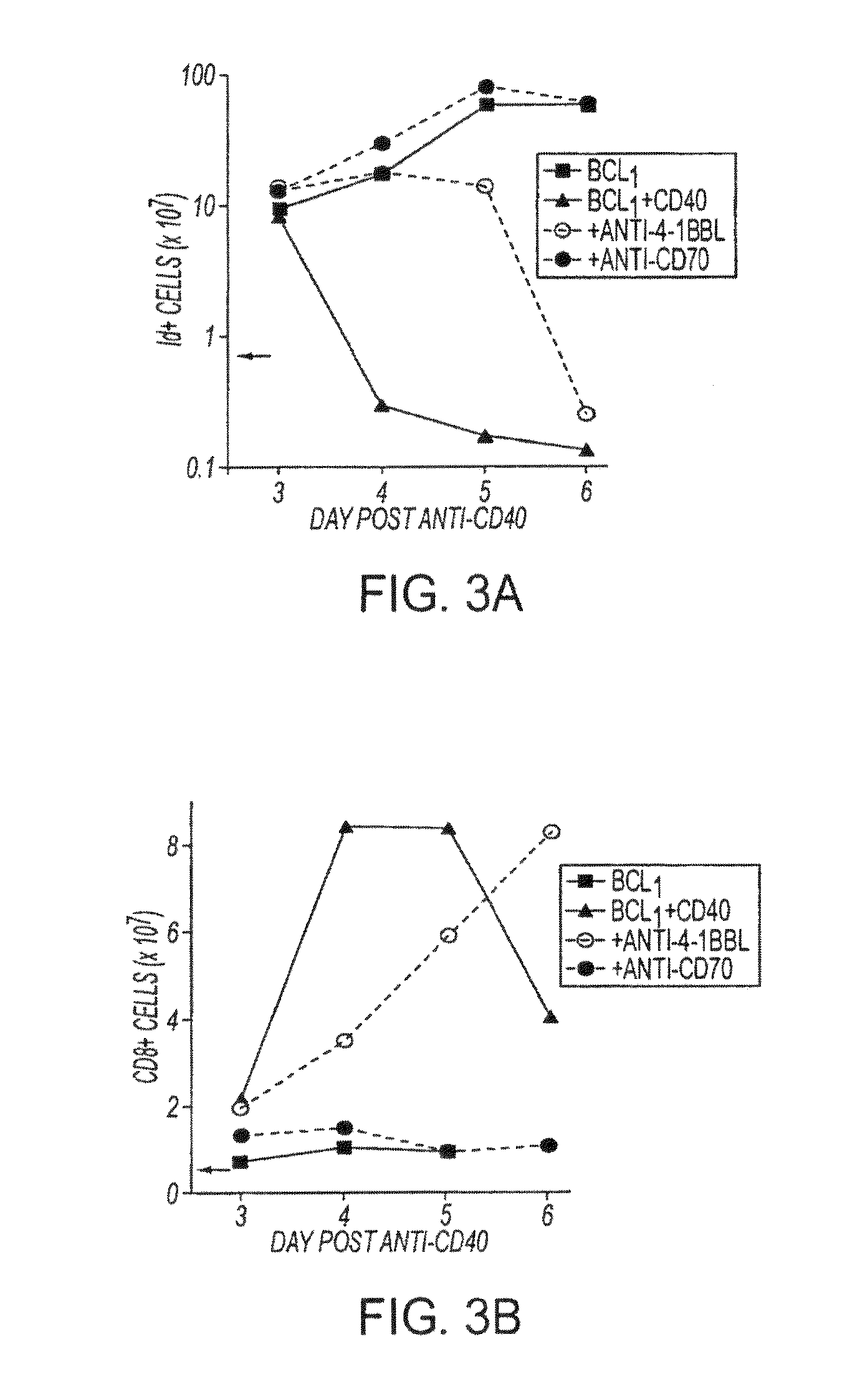
ActiveUS8481029B2Promotes strong expression of 4-1BBImprove responseAntibacterial agentsNervous disorderAutoimmune responsesImmune modulator
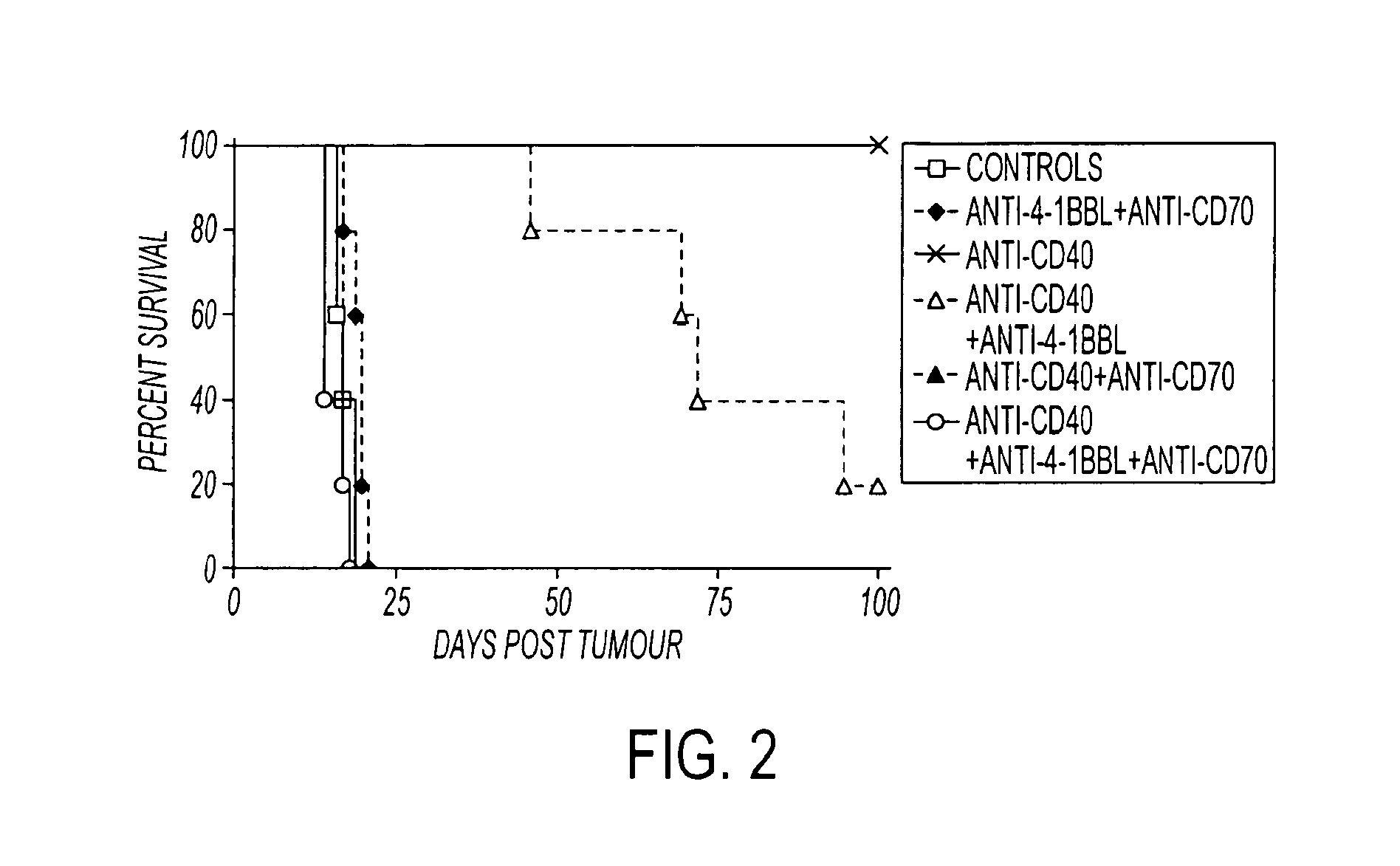
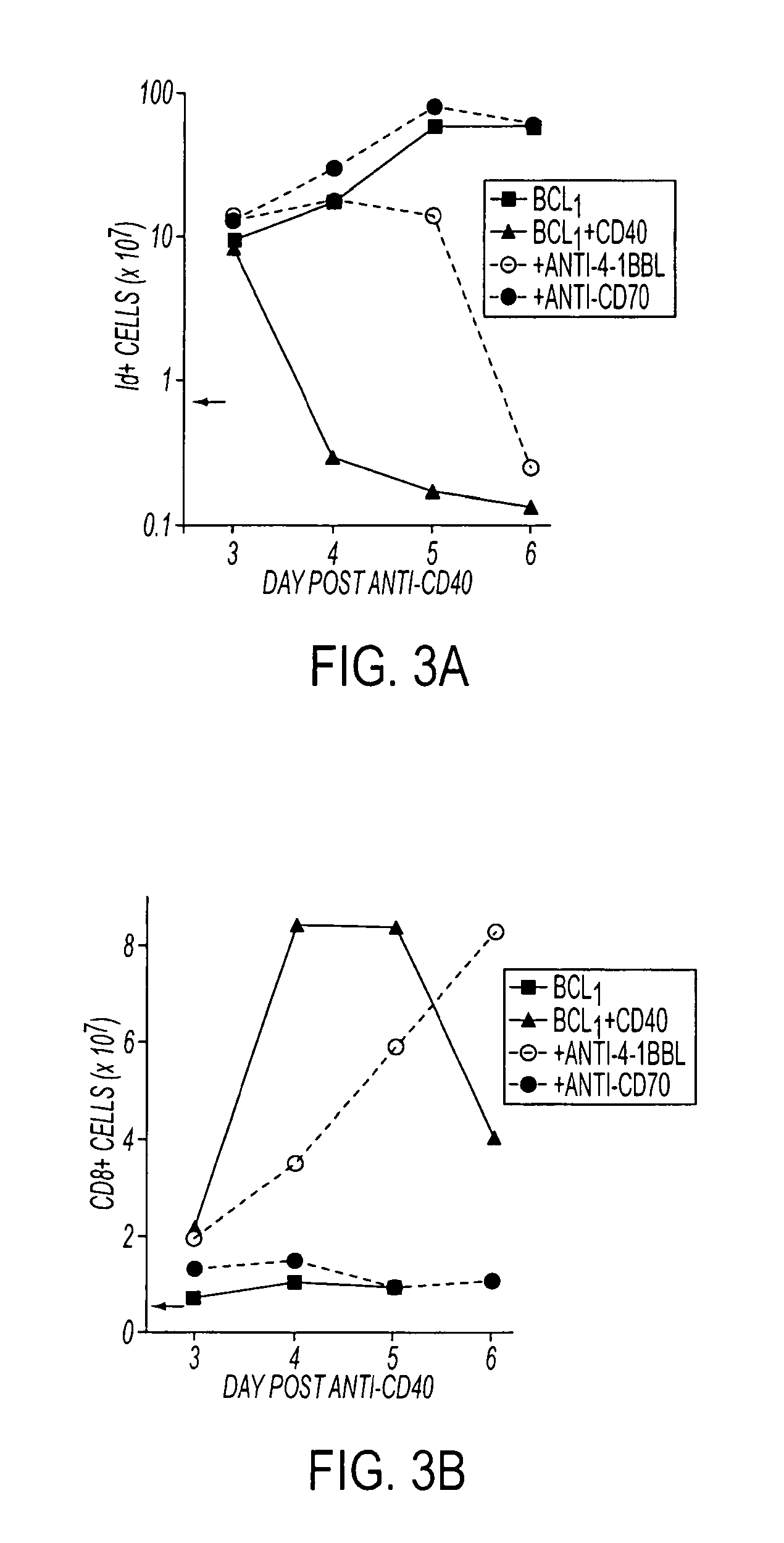
Methods of inducing T cell proliferation and expansion in vivo for treating conditions wherein antigen-specific T cell immune response are therapeutically desirable such as cancer, infection, inflammation, allergy and autoimmunity and for enhancing the efficacy of vaccines are provided. These methods comprise the administration of at least one CD27 agonist, preferably an agonistic CD27 antibody, alone or in association with another moiety such as immune stimulant or immune modulator such as an anti-CD40, OX-40, 4-IBB, or CTLA-4 antibody or an agent that depletes regulatory cells, or a cytokine. These mono and combination therapies may also optionally include the administration of a desired antigen such as a tumor antigen, an allergen, an autoantigen, or an antigen specific to an infectious agent or pathogen against which a T cell response (often CD8+) is desirably elicited.
Owner:UNIV OF SOUTHAMPTON
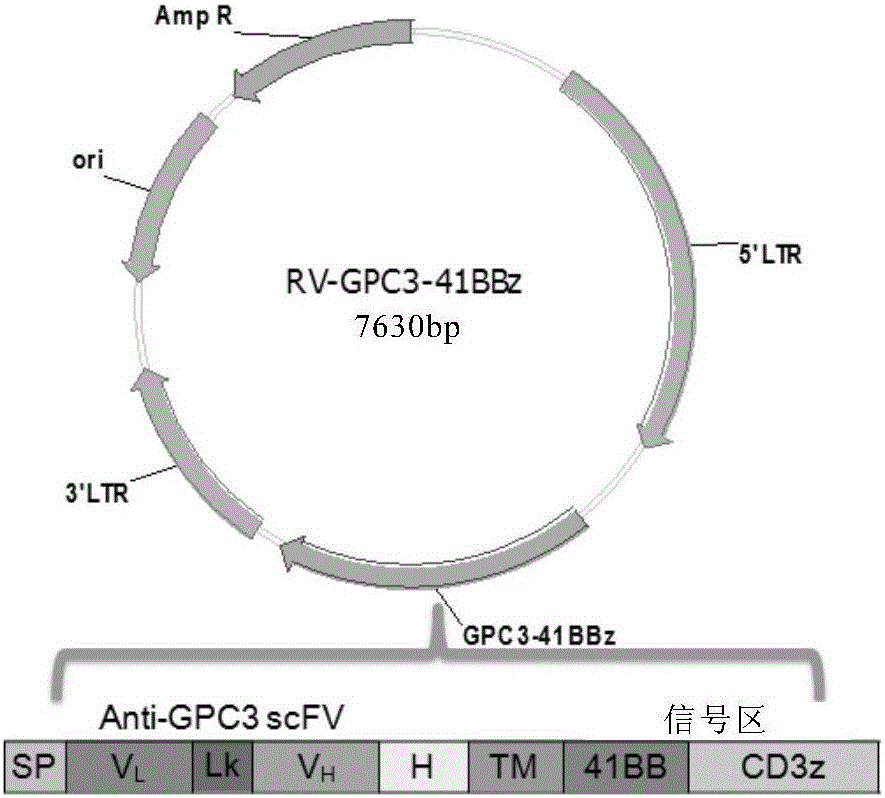
Chimeric antigen receptor of targeted GPC3 (Glypican 3) and application thereof
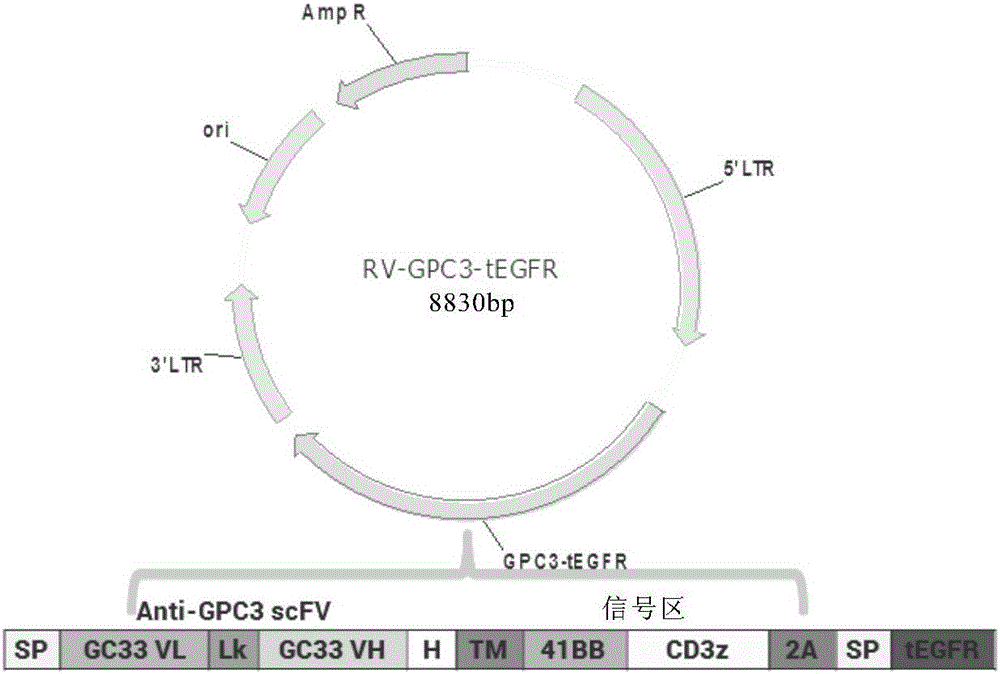
The invention relates to a chimeric antigen receptor of a targeted GPC3 (Glypican 3) and an application thereof. Specifically, the invention provides a polynucleotide sequence, wherein the polynucleotide sequence is selected from: polynucleotide sequences (1) containing a coding sequence of a CD8 antigen leading peptide, a coding sequence of an anti-GPC3 single-chain antibody, a coding sequence of a human CD8 alpha hinge region, a coding sequence of a human CD8 transmembrane region, a coding sequence of a human 41BB intracellular region, and a coding sequence of a human CD3 zeta intracellular region, and a coding sequence of an optional fragment of an EGFR (Epidermal Growth Factor Receptor) sequence containing an extracellular domain III and an extracellular domain IV, wherein the coding sequences are sequentially connected; and complementary sequences (2) of the polynucleotide sequences (1). The invention provides a CAR (chimeric antigen receptor) coded by the polynucleotide sequences and a T cell expressing the CAR. The prepared CAR-T cell has a function of strongly killing a specific tumor cell, and the kill effectiveness is more than 90% under the condition that the effector-target ratio is 20:1.
Owner:HRAIN BIOTECHNOLOGY CO LTD
Tolerogenic synthetic nanocarrier compositions with transplantable graft antigens and methods of use
InactiveUS20120276156A1Eliminate side effectsReduce generationAntipyreticAnalgesicsNanocarriersPlant Antigens
Disclosed are synthetic nanocarrier compositions, and related methods, comprising APC presentable transplant antigens and immunosuppressants that provide tolerogenic immune responses (e.g., a reduction in CD8+ T cell proliferation and / or activity) specific to the APC presentable transplant antigens.
Owner:SELECTA BIOSCI
Methods and reagents for vaccination which generate a CD8 T cell immune response
InactiveUS6663871B1Increase boost effectGood effectVirusesPeptide/protein ingredientsAntigenVaccination
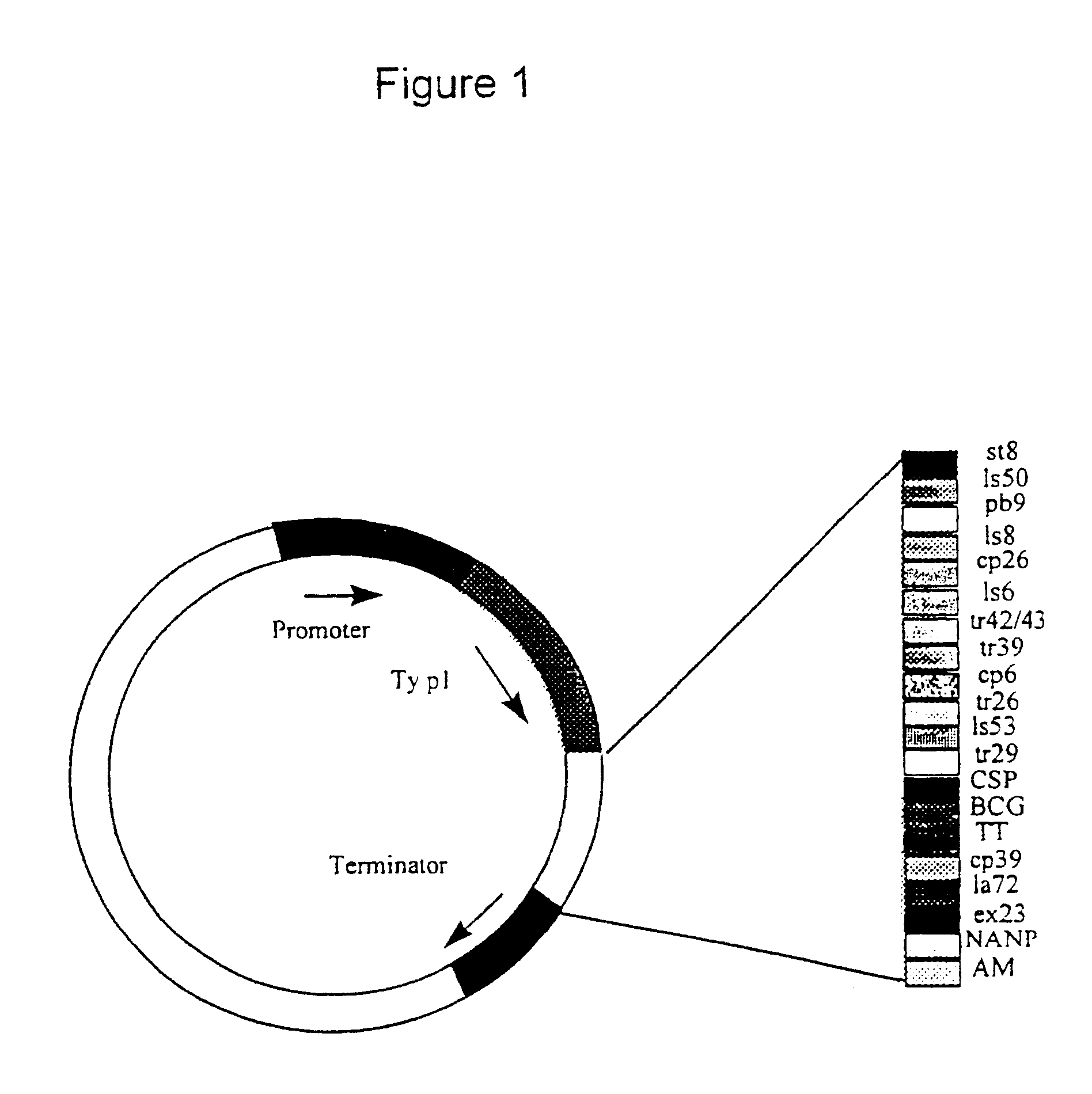
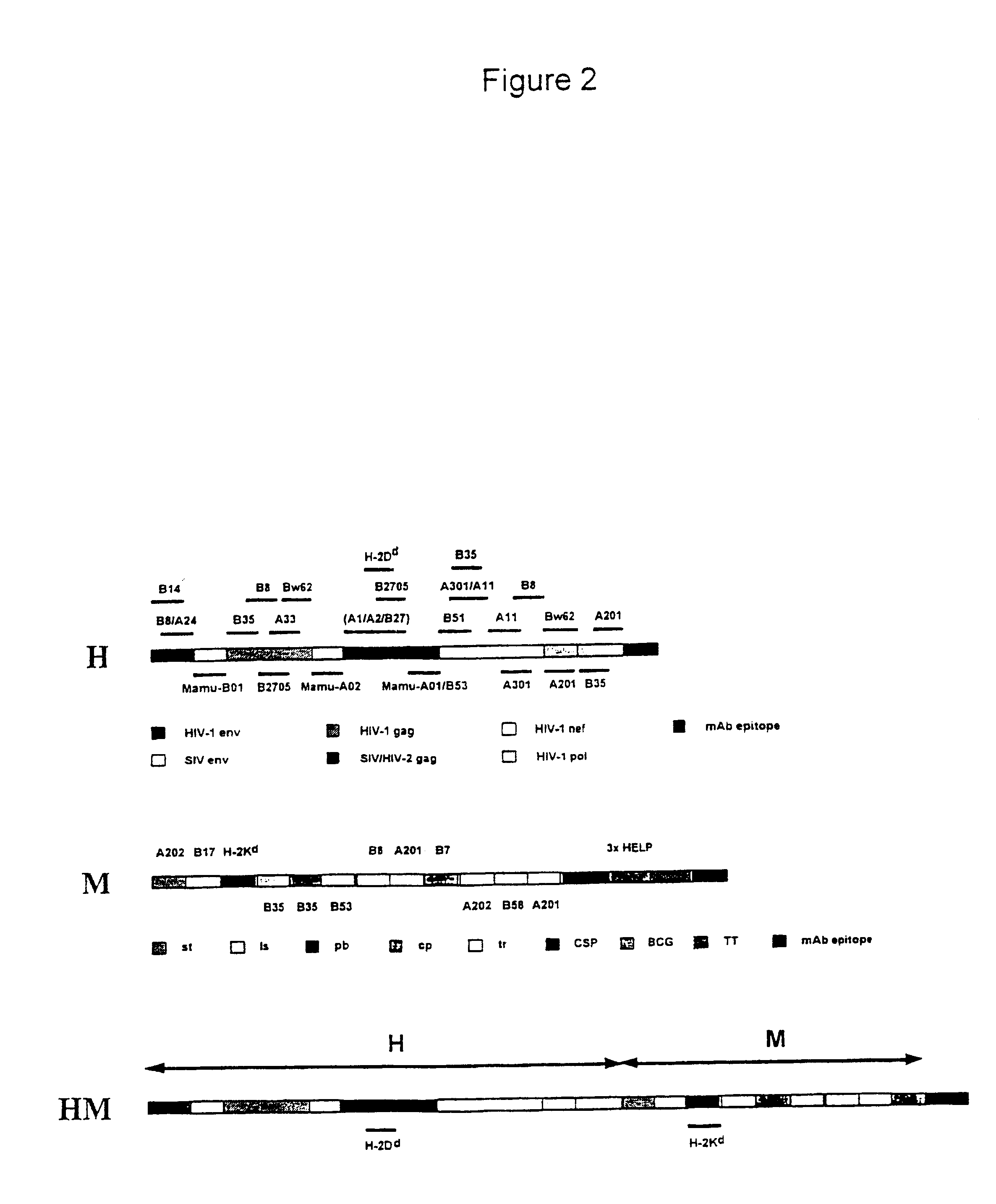
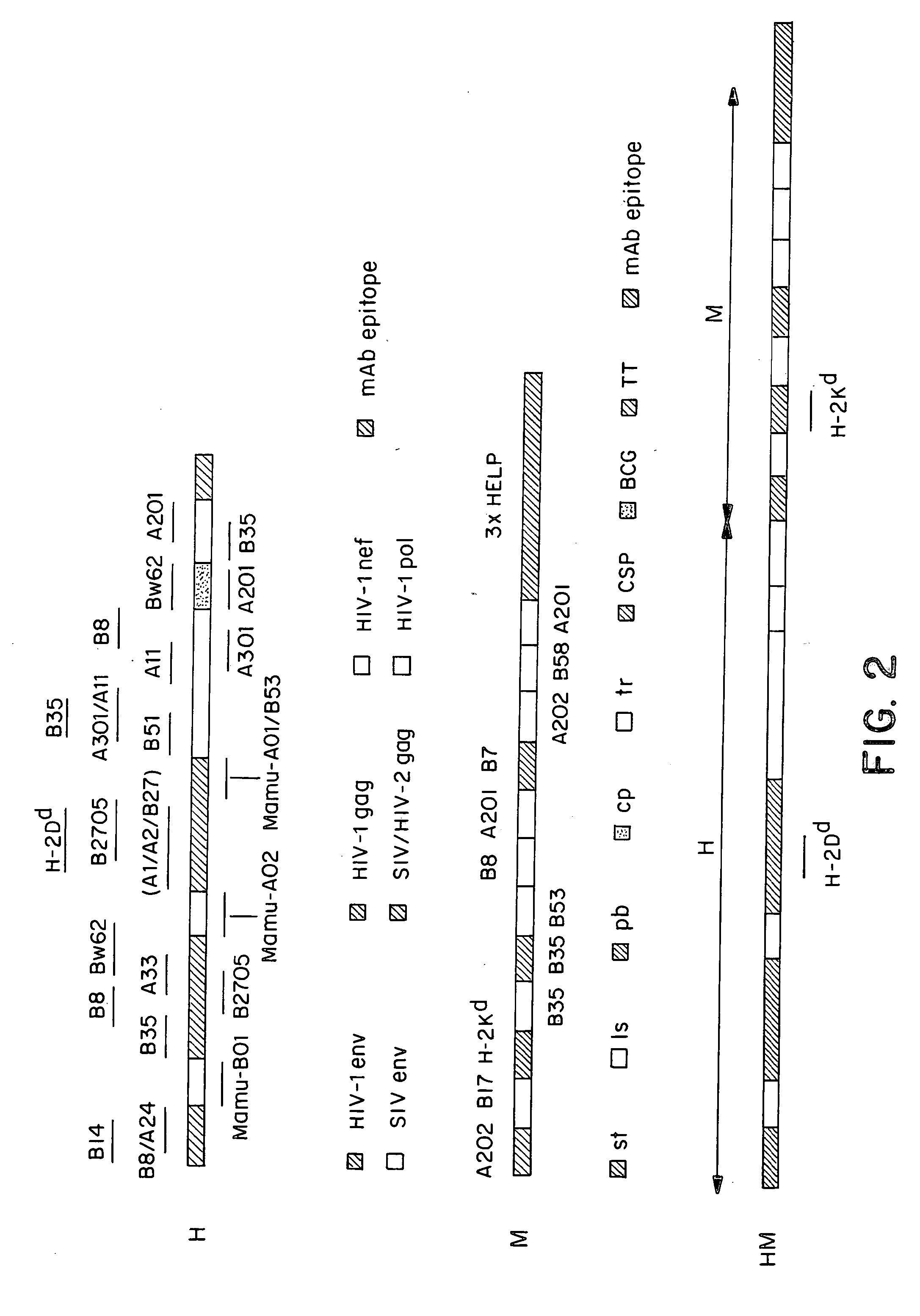
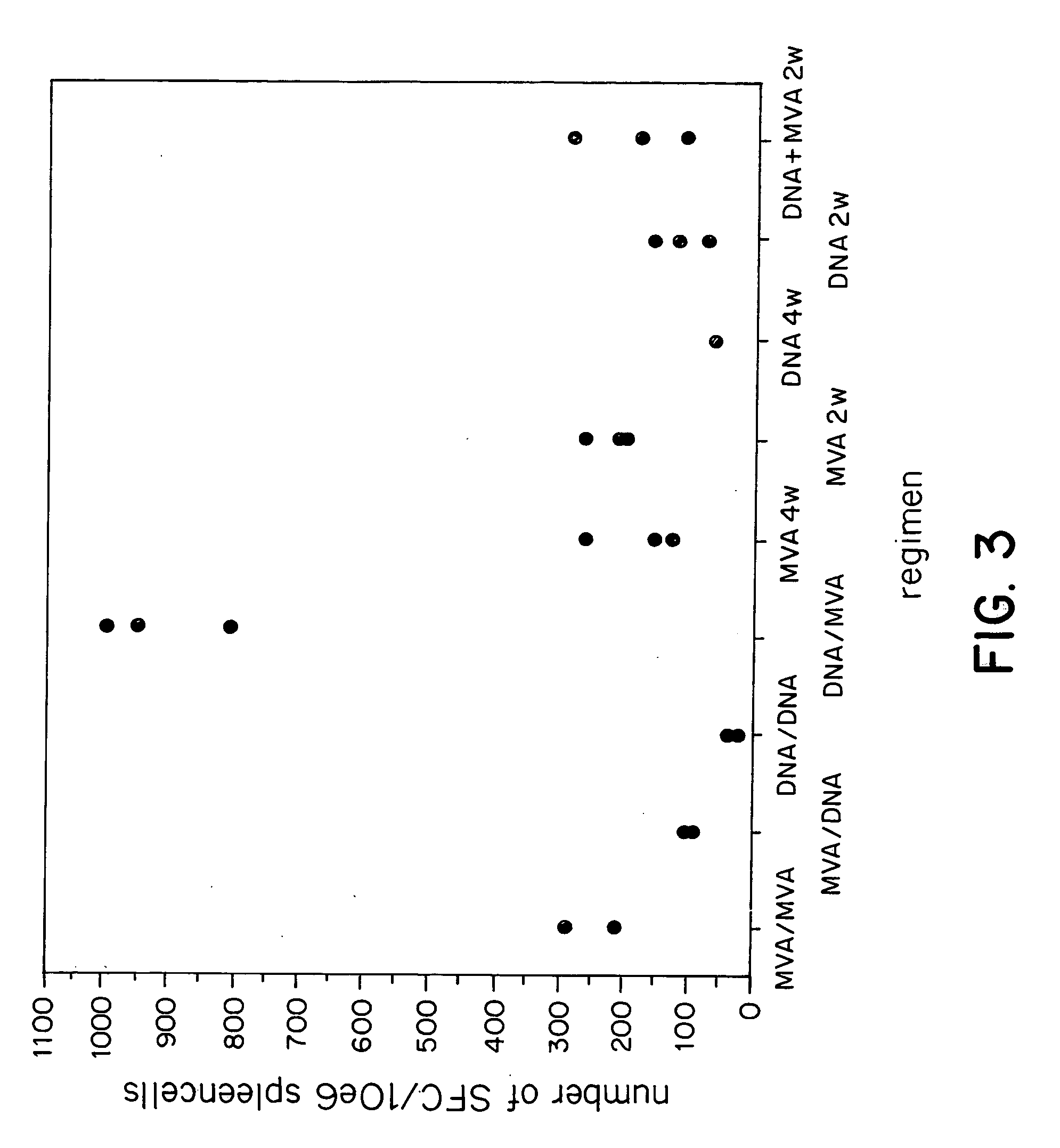
New methods and reagents for vaccination are described which generate a CD8 T cell immune response against malarial and other antigens such as viral and tumour antigens. Novel vaccination regimes are described which employ a priming composition and a boosting composition, the boosting composition comprising a non-replicating or replication-impaired pox virus vector carrying at least one CD8 T cell epitope which is also present in the priming composition.
Owner:OXXON THERAPEUTICS LTD
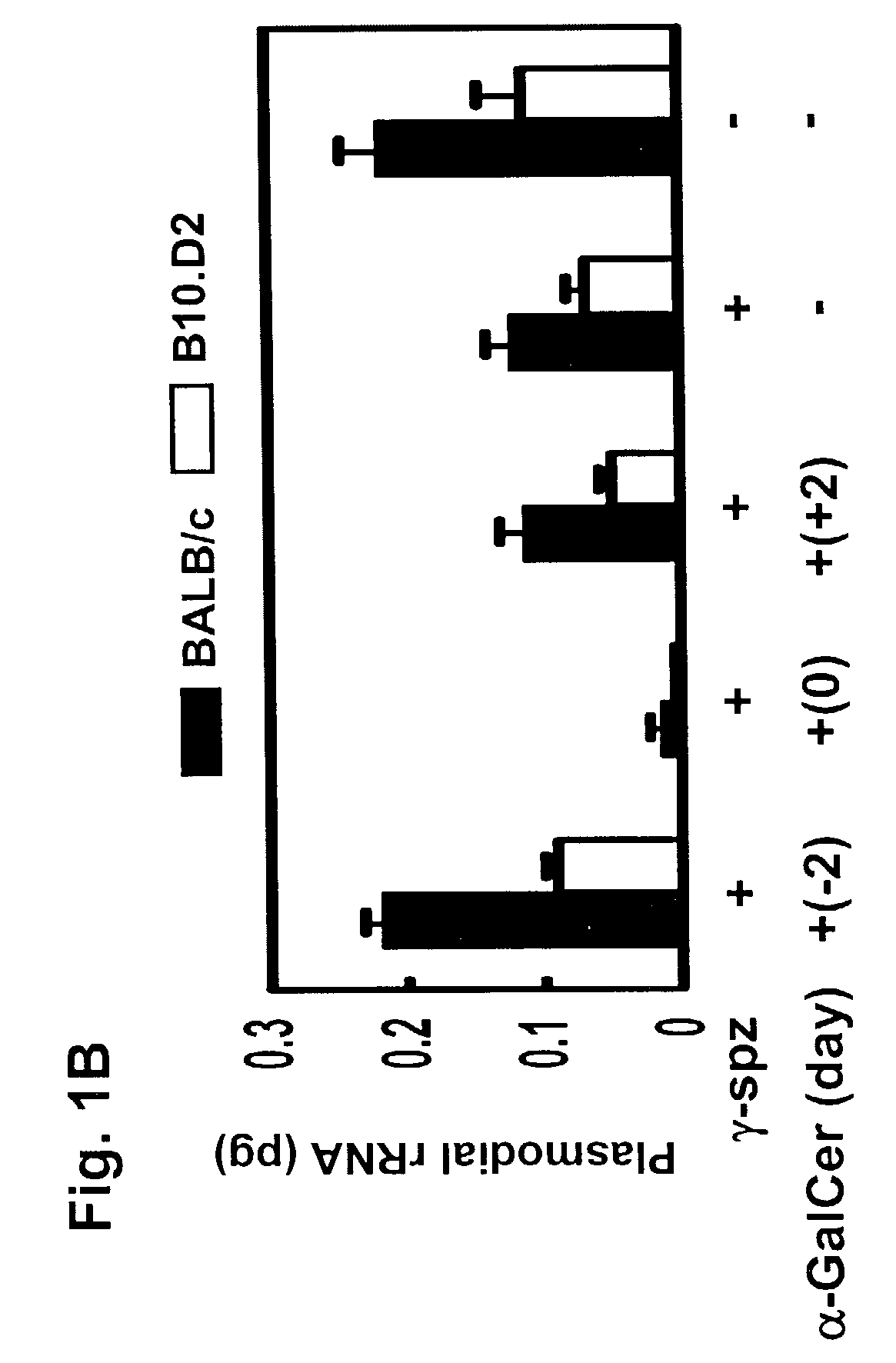
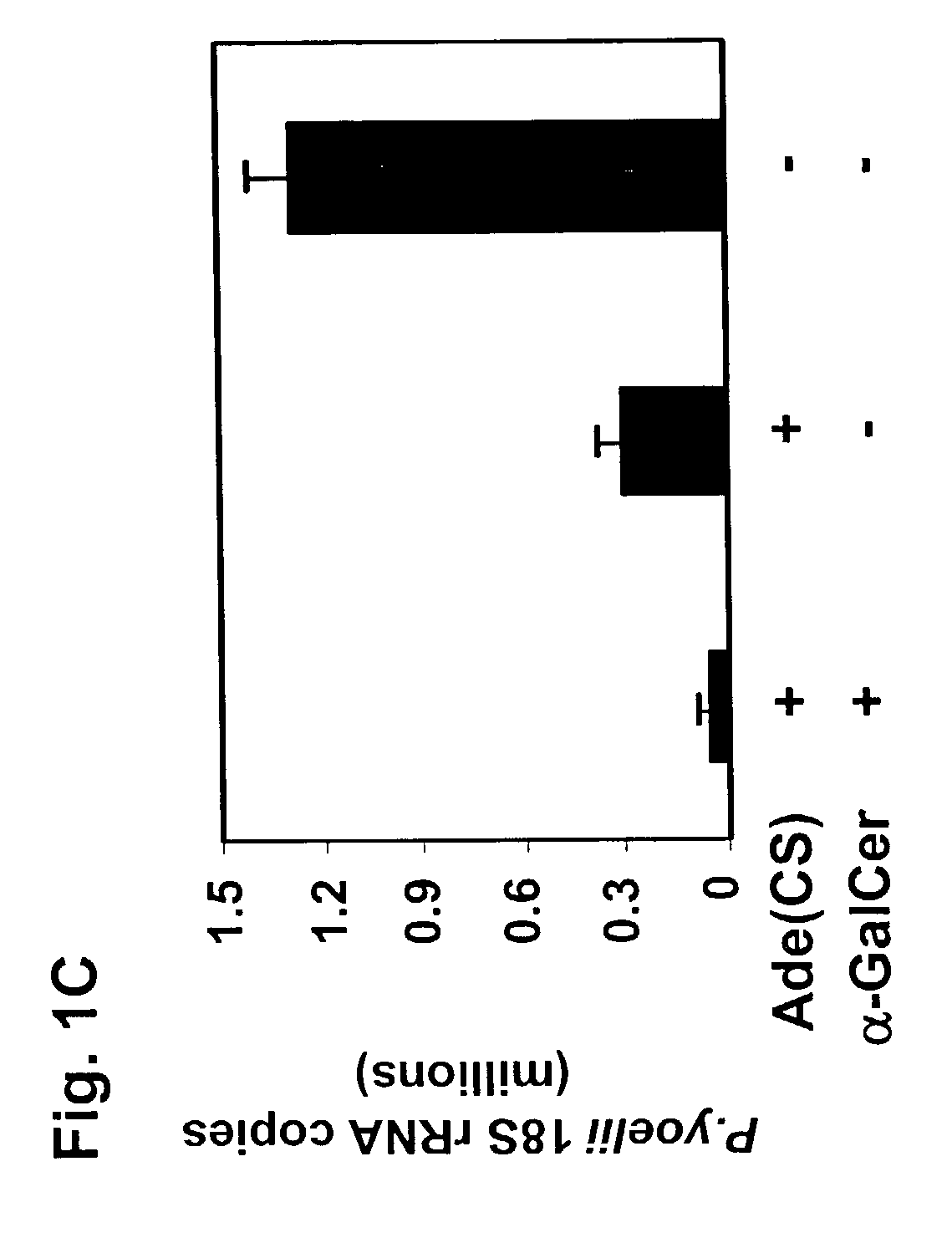
Use of glycosylceramides as adjuvants for vaccines against infections and cancer
InactiveUS7488491B2Enhancement and extension of durationAntibacterial agentsOrganic active ingredientsAdjuvantMammal
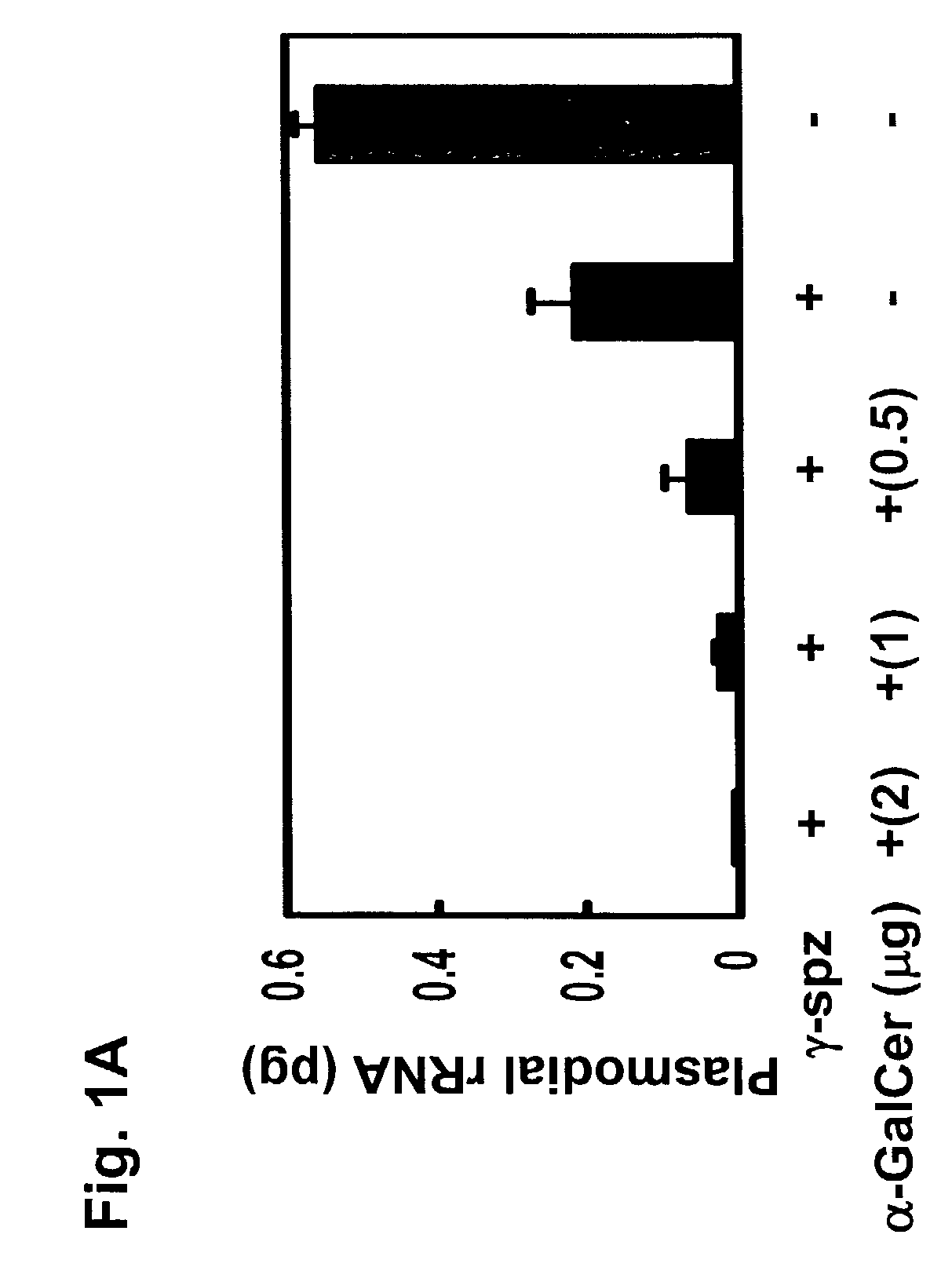
The present invention relates to methods and compositions for augmenting an immunogenicity of an antigen in a mammal, comprising administering said antigen together with an adjuvant composition that includes glycosylceramide, preferably α-galactosylceramide (α-GalCer). According to the present invention, the use of glycosylceramide as an adjuvant is attributed at least in part to the enhancement and / or extension of antigen-specific Th1-type responses, in particular, CD8+ T cell responses. The methods and compositions of the present invention can be useful for prophylaxis and treatment of various infectious and neoplastic diseases.
Owner:NEW YORK UNIVERSITY
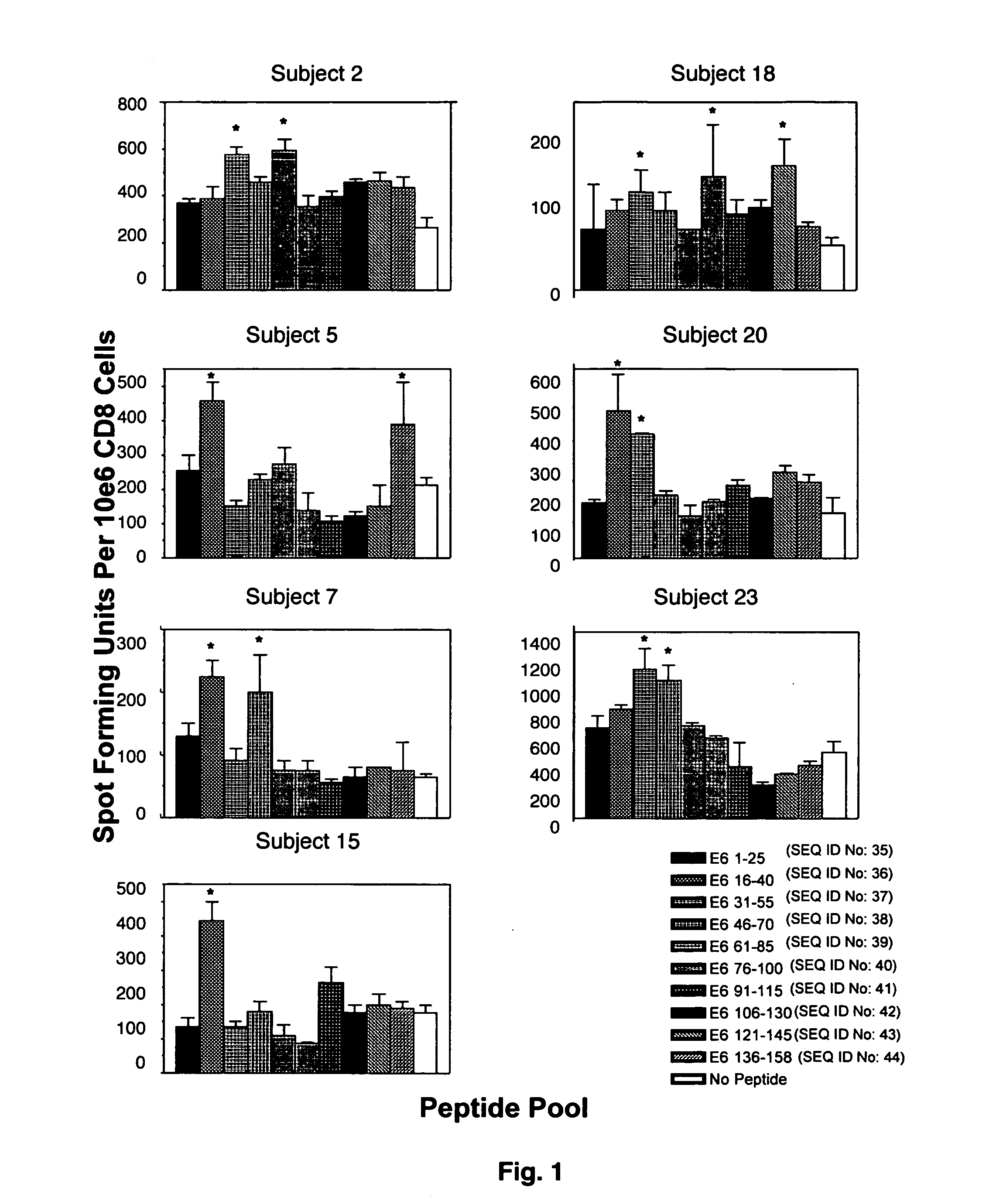
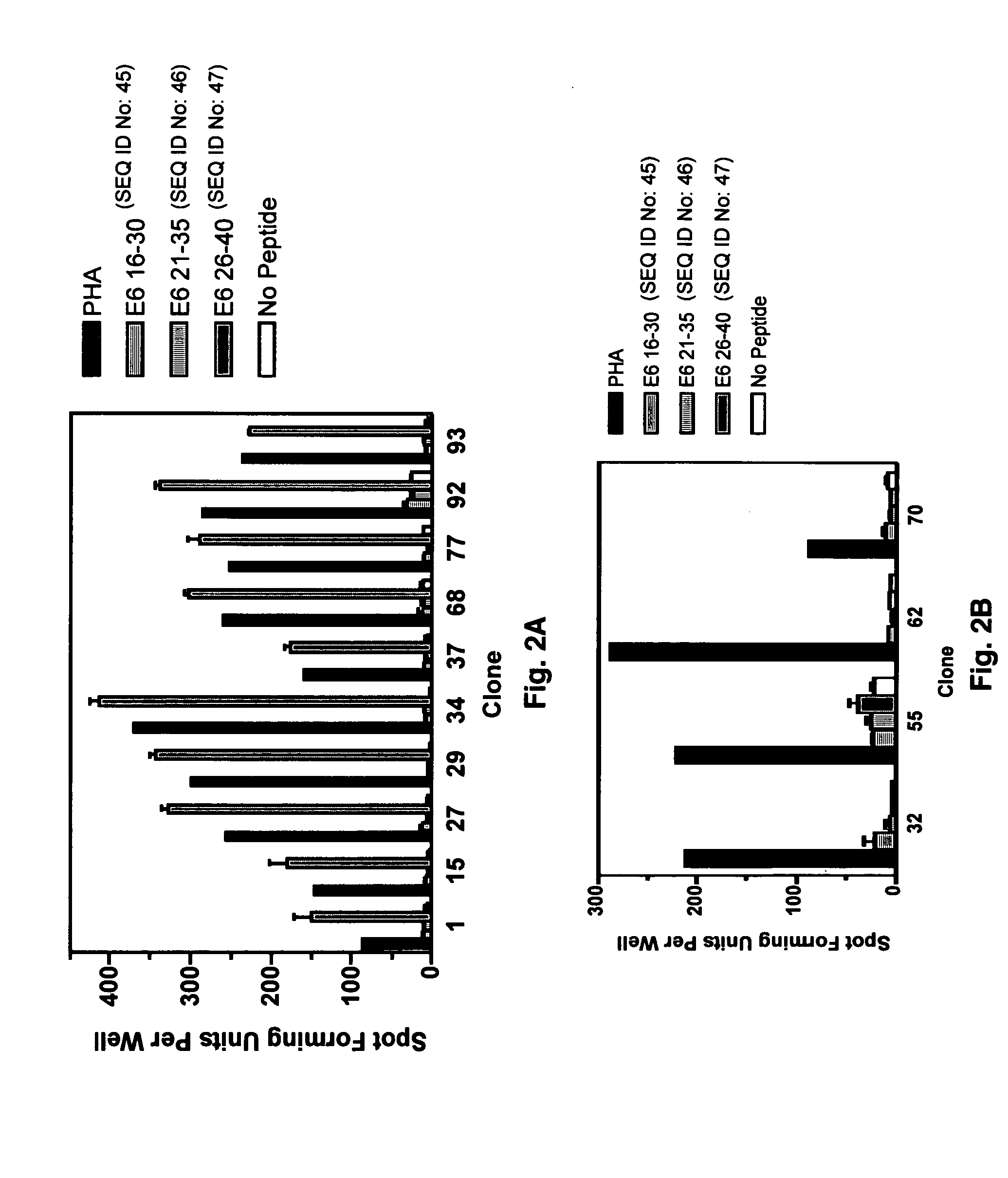
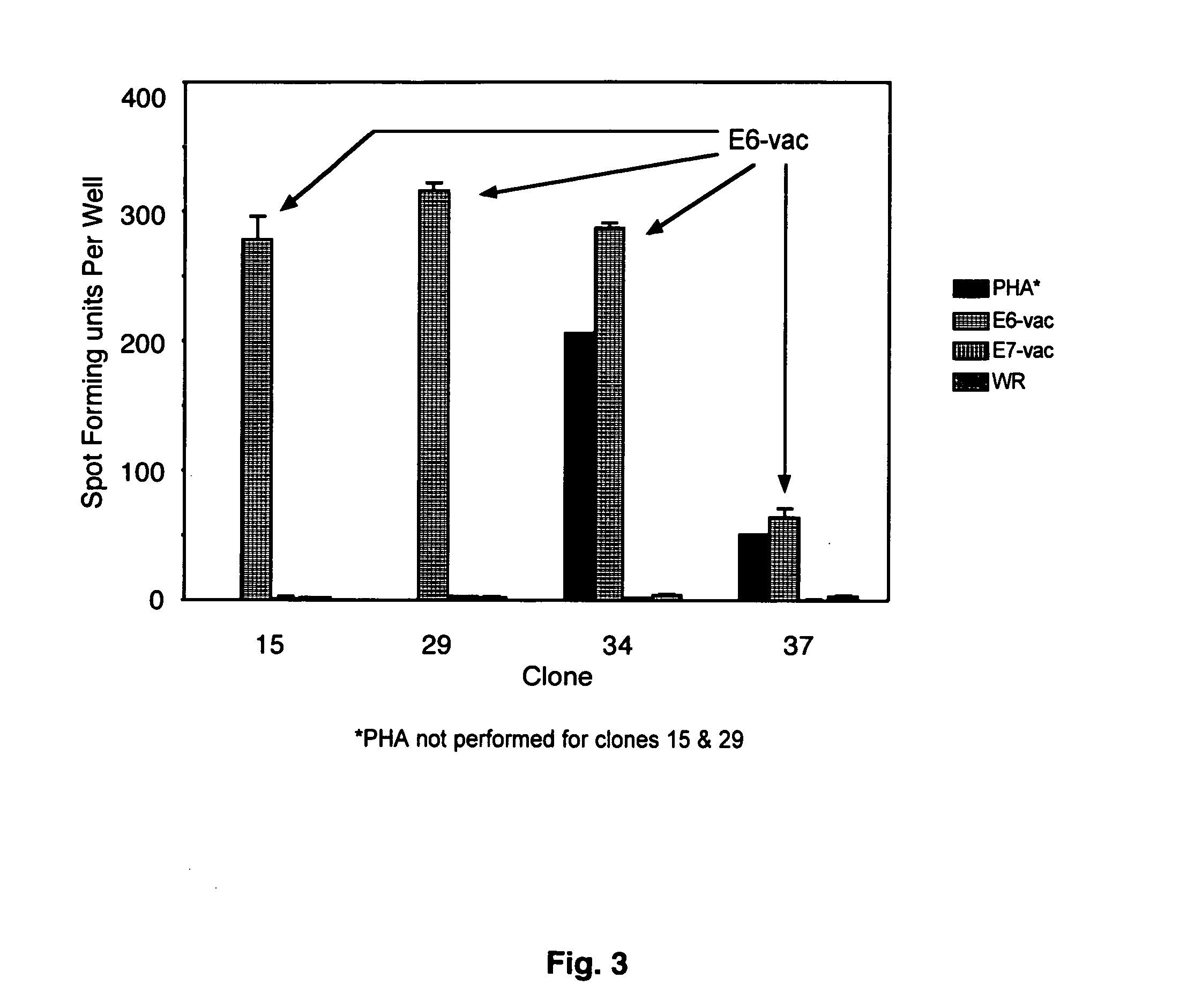
CD8 T cell epitopes in HPV 16 E6 and E7 proteins and uses thereof
InactiveUS20060182763A1Peptide/protein ingredientsViral antigen ingredientsHuman papillomavirusDendritic cell
The present invention is directed to the examination of the pattern of immunodominant CD8 T cell epitopes in the E6 and E7 protein of Human Papillomavirus (HPV) and its further characterization in terms of its amino acid sequence and HLA restriction. These epitopes are identified based on their ability to induce strong CD8 T cell response and therefore, are important as sources of antigens for dendritic cell immunotherapy to treat cervical cancer. The present invention contemplates identifying a number of similar epitopes restricted by a wide variety of HLA types so that they can be used in concert to develop a preventative vaccine, which can be used for general population.
Owner:BIOVENTURES LLC
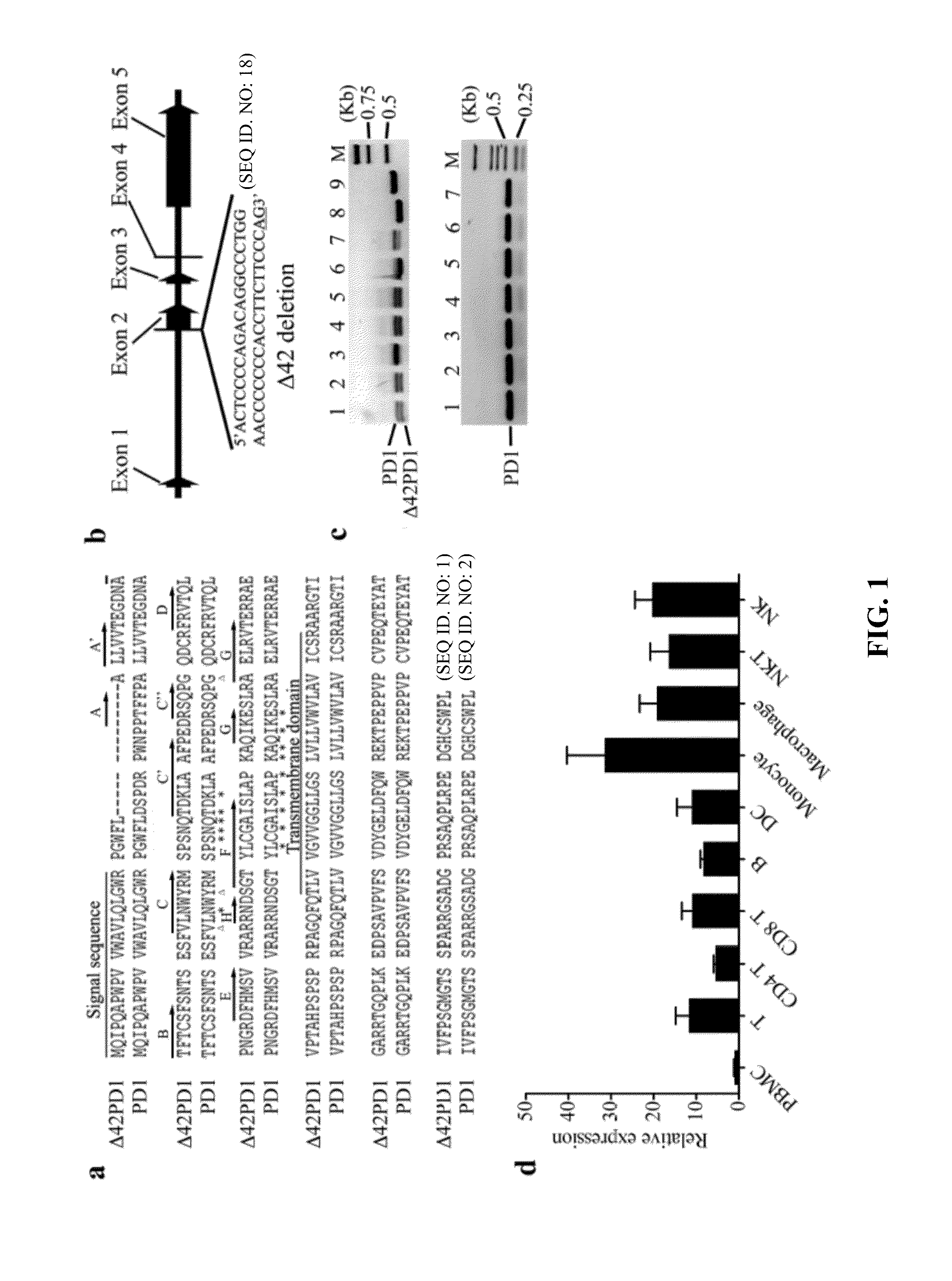
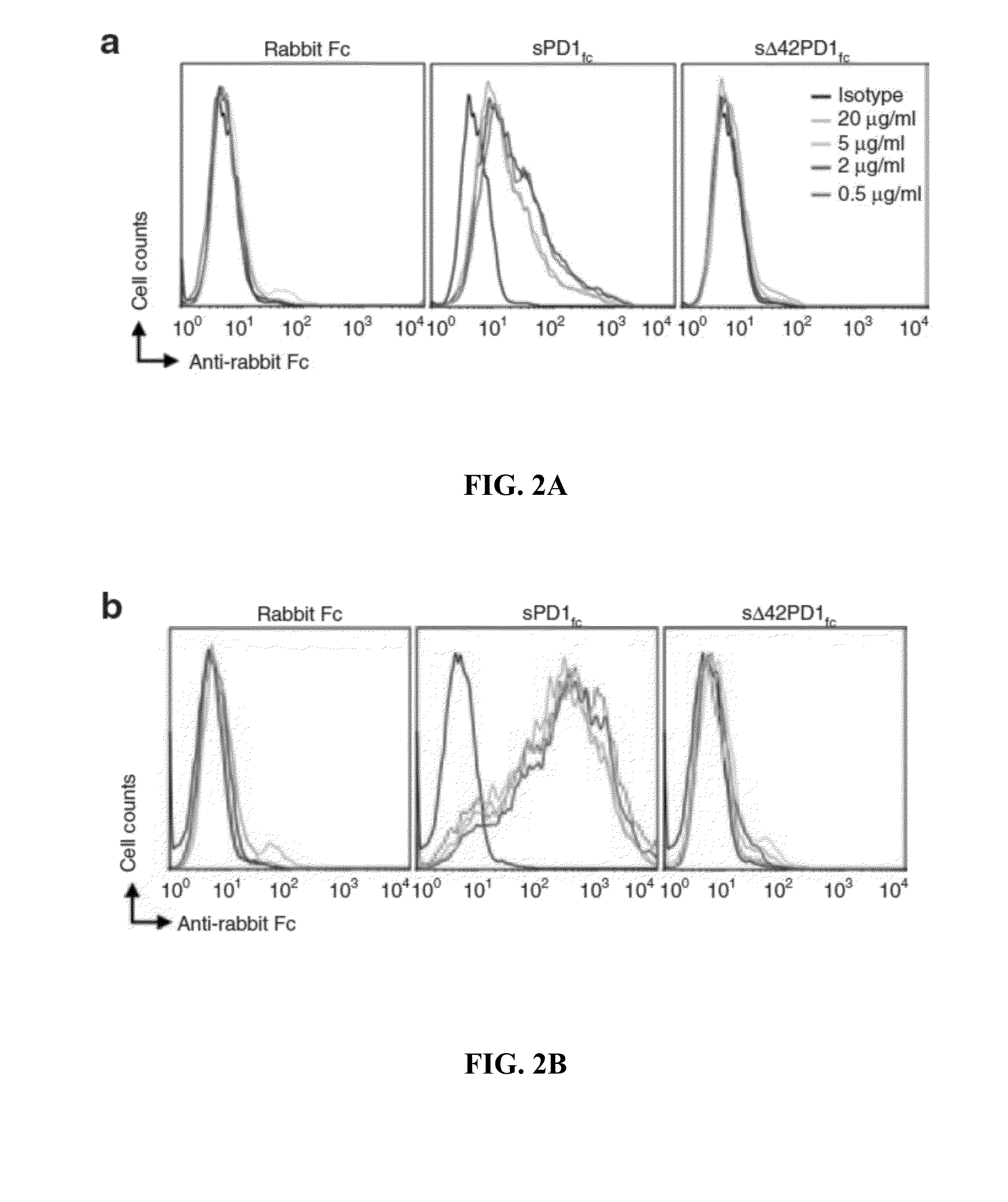
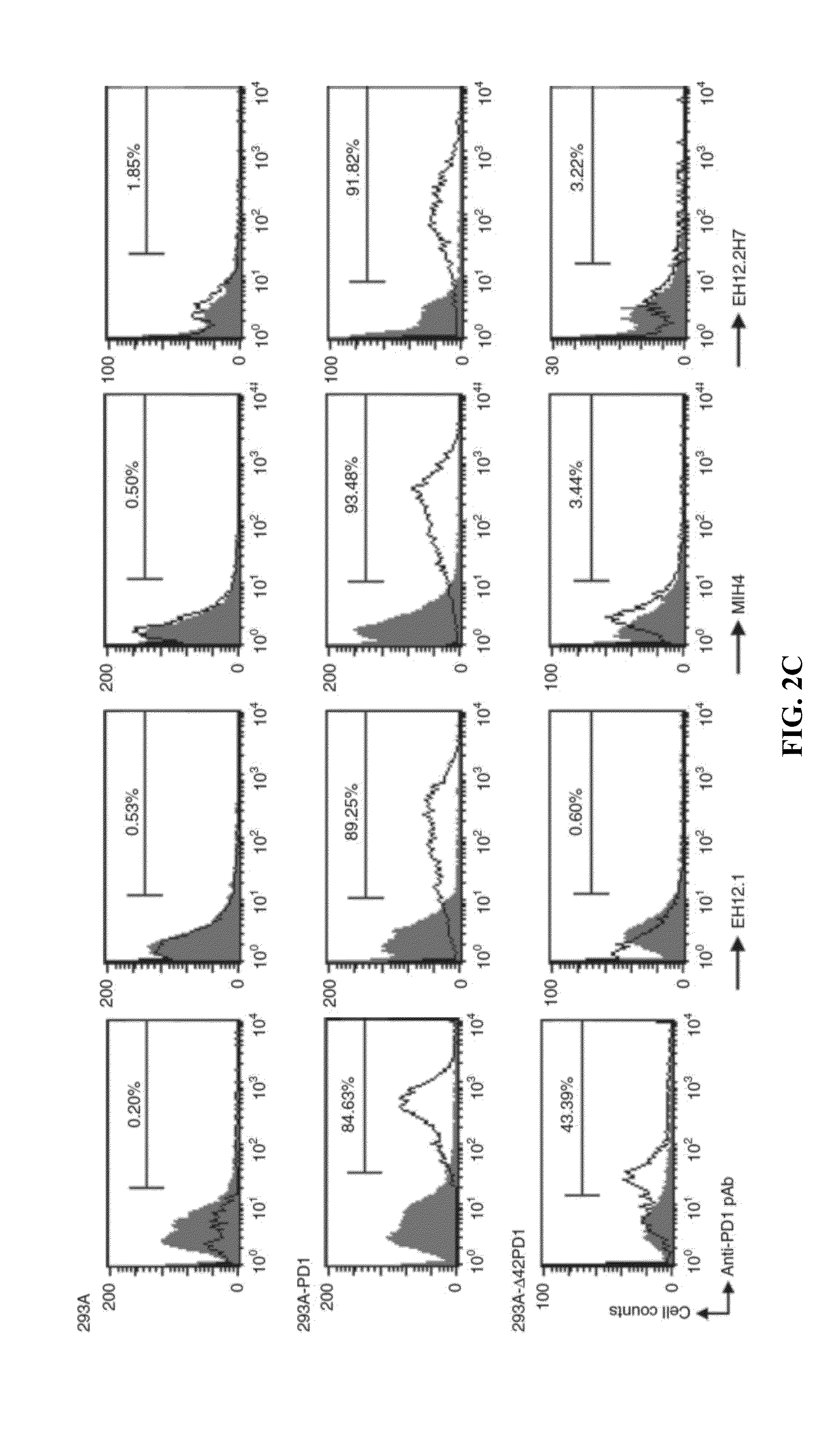
Novel pd1 isoforms, and uses thereof for potentiating immune responses
ActiveUS20140302070A1Prevention treatment ameliorationPeptide/protein ingredientsGenetic material ingredientsAutoimmune conditionAdjuvant
In one embodiment, the present invention provides a new isoform of human PD1 (Δ42PD1) that contains a 42-nucleotide in-frame deletion located at exon 2 domain. Δ42PD1 does not engage PD-L1 / PD-L2, and can induce the production of pro-inflammatory cytokines In one embodiment, Δ42PD1 can be used as an intramolecular adjuvant to develop a fusion DNA vaccine for enhancing antigen-specific CD8+ T cell immunity and for prevention of pathogenic infection and / or cancer. In one embodiment, soluble Δ42PD1 protein could be a therapeutic target for autoimmune diseases. In other embodiments, proteins or peptides or nucleic acids encoding proteins or peptides containing Δ42PD1 could be used as immunogens for developing antibodies binding specifically to Δ42PD1. In yet another embodiment, neutralizing antibodies could block sΔ42PD1 function and accordingly could be used as treatment for autoimmune disorders.
Owner:VERSITECH LTD
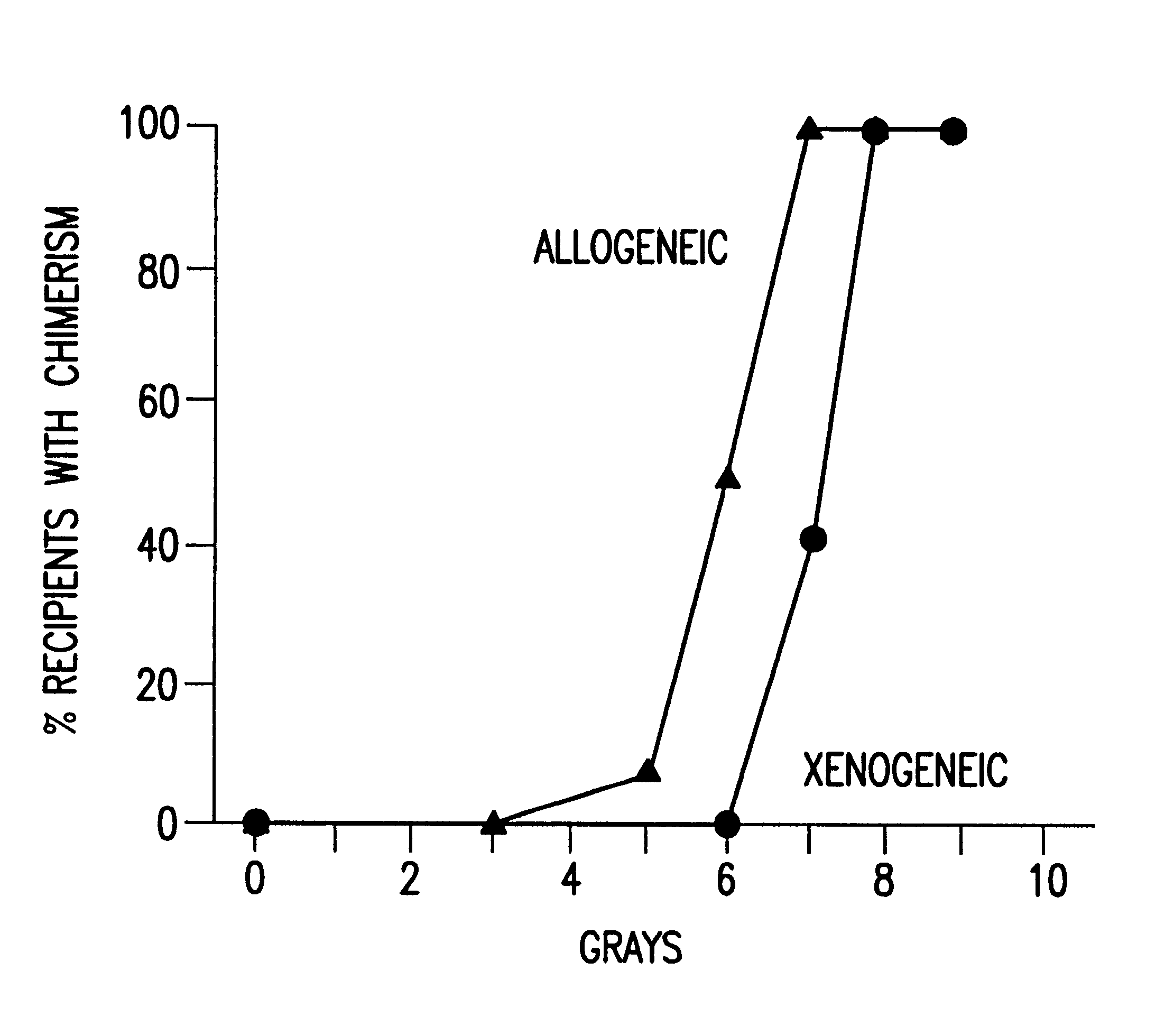
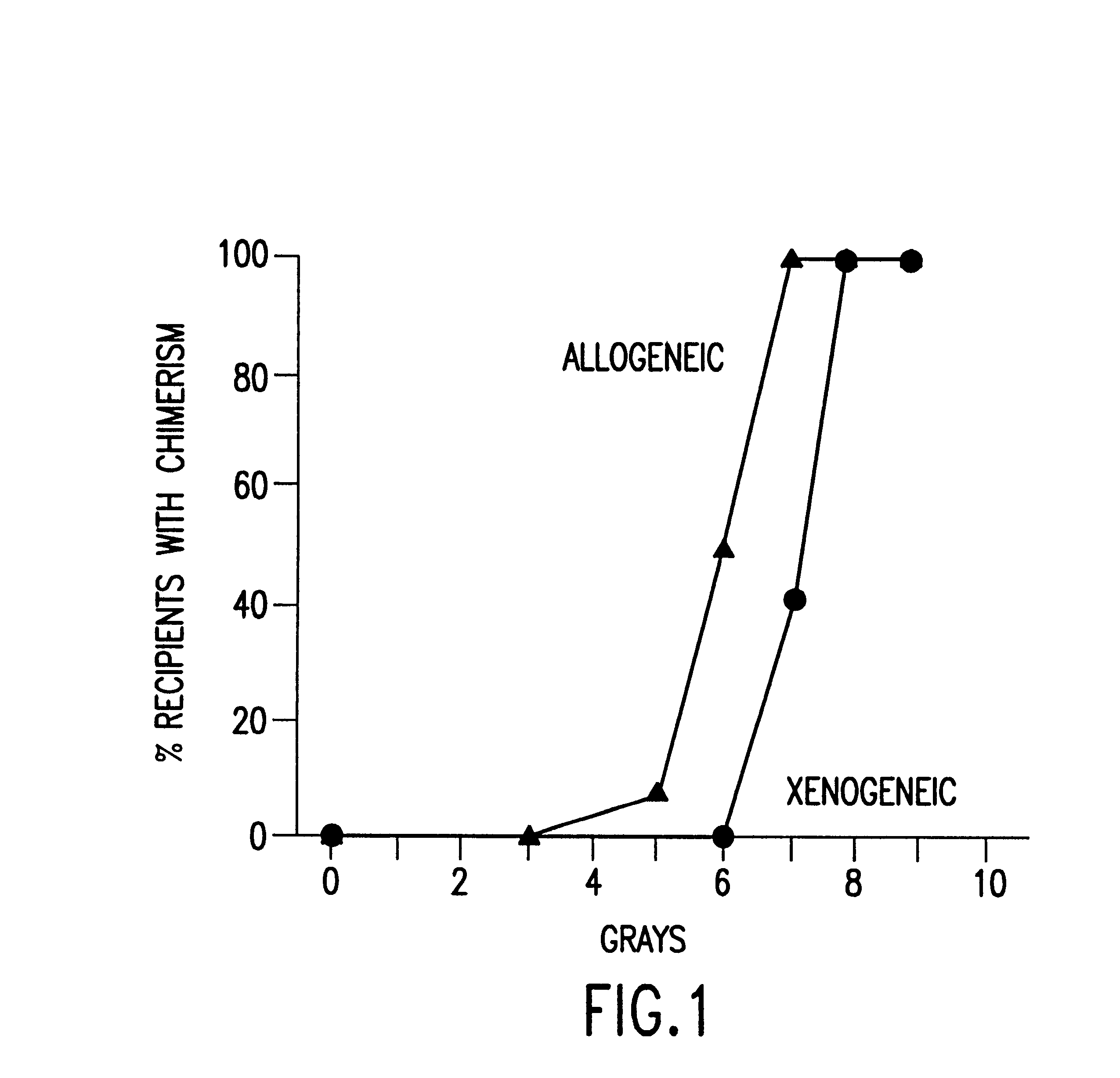
Non-lethal methods for conditioning a recipient for bone marrow transplantation
InactiveUS6217867B1Reduce proliferationDiminished cytotoxic activityBiocideOrganic active ingredientsGackstroemiaAcquired immunodeficiency
The present invention relates to non-lethal methods of conditioning a recipient for bone marrow transplantation. In particular, it relates to the use of nonlethal doses of total body irradiation, total lymphoid irradiation, cell type-specific or cell marker-specific antibodies, especially antibodies directed to bone marrow stromal cell markers, NK cells, or the CD8 cell marker, cytotoxic drugs, or a combination thereof. The methods of the invention have a wide range of applications, including, but not limited to, the conditioning of an individual for hematopoietic reconstitution by bone marrow transplantation for the treatment of hematologic malignancies, hematologic disorders, autoimmunity, infectious diseases such as acquired immunodeficiency syndrome, and the engraftment of bone marrow cells to induce tolerance for solid organ, tissue and cellular transplantation.
Owner:PITTSBURGH UNIV OF
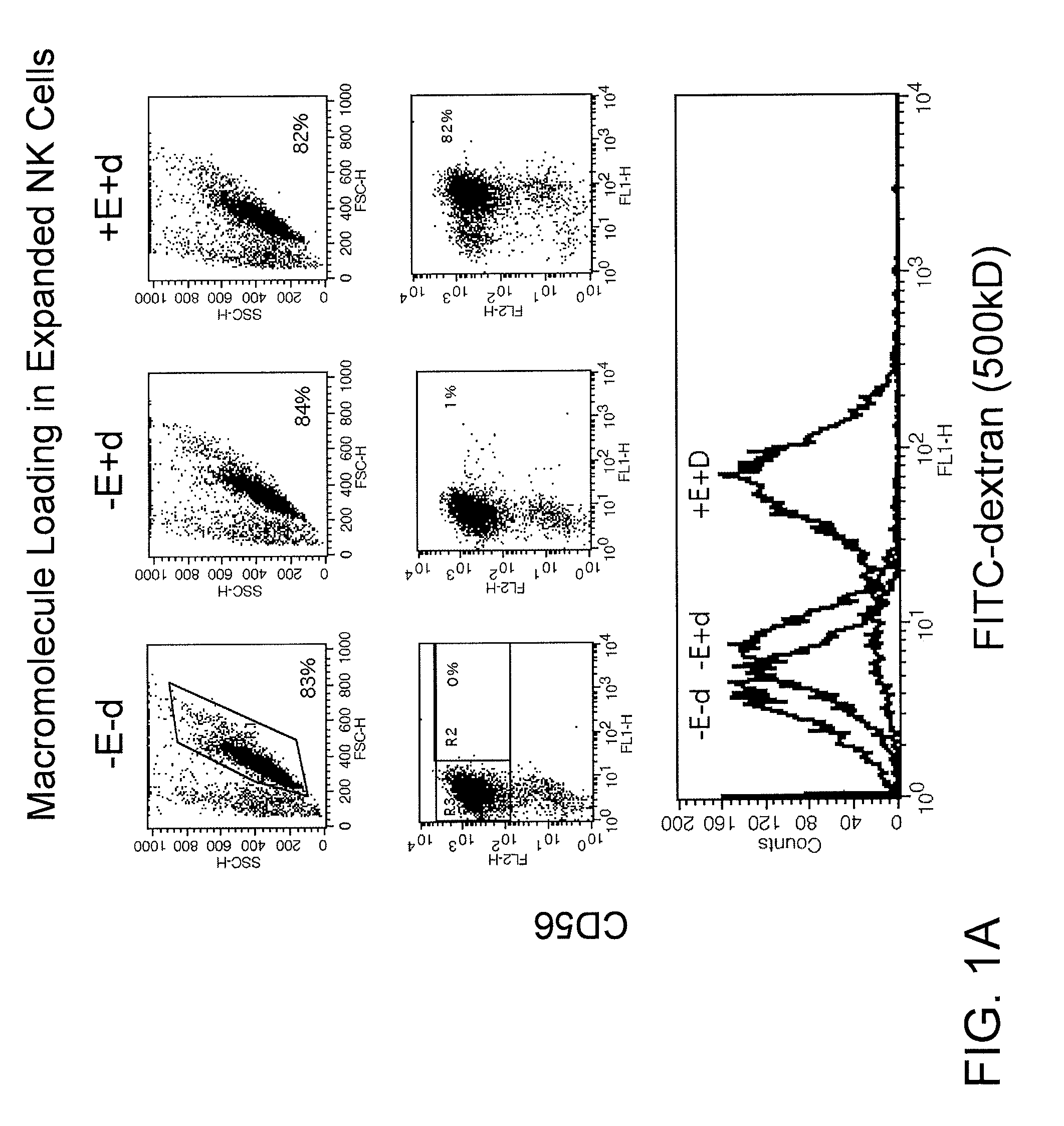
Engineering and delivery of therapeutic compositions of freshly isolated cells
ActiveUS8450112B2Simple procedureGood treatment effectGenetic material ingredientsFermentationNatural Killer Cell Inhibitory ReceptorsCD8
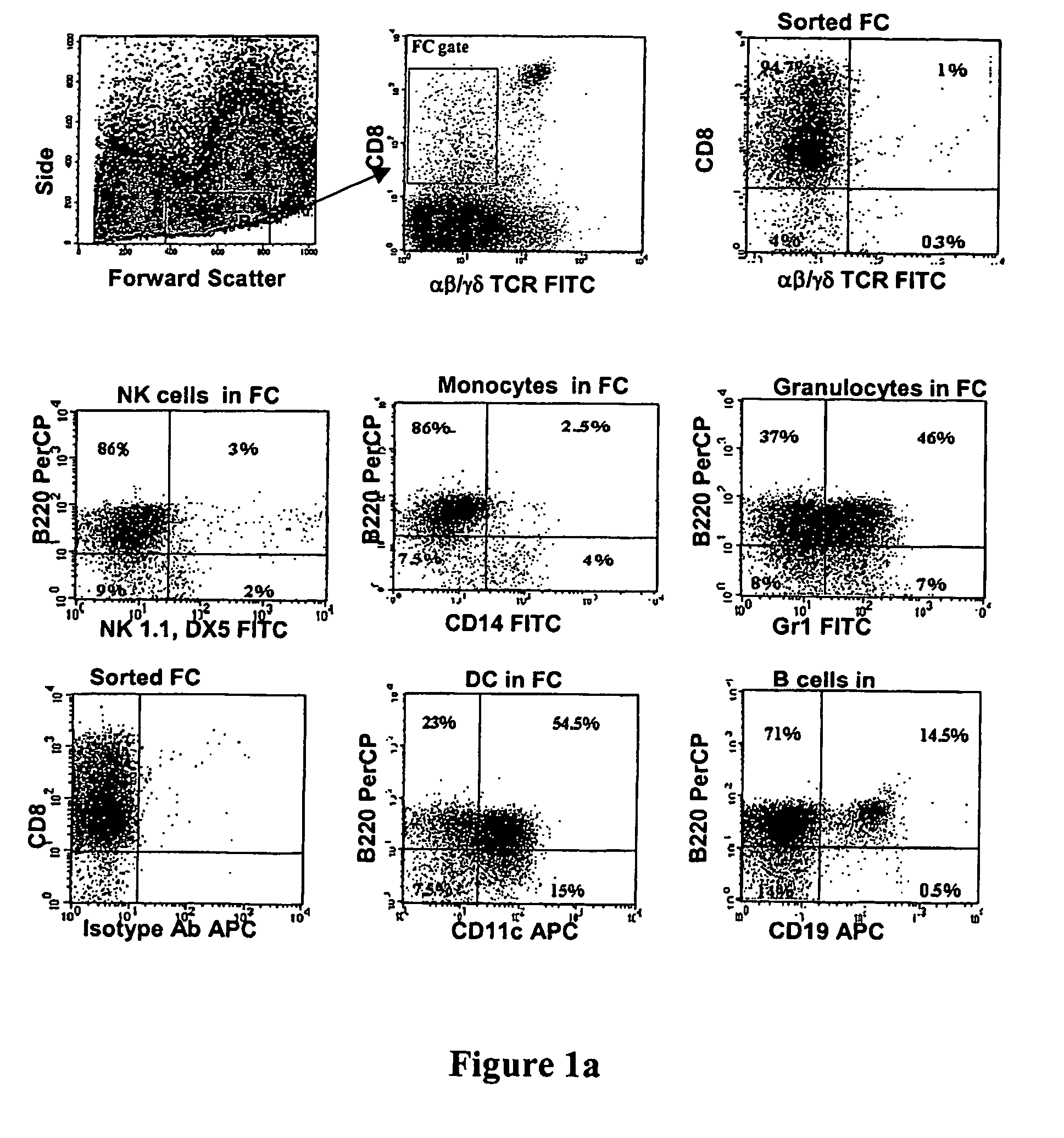
The present invention relates to the transient modification of cells. In particular embodiments, the cells are immune systems, such as PBMC, PBL, T (CD3+ and / or CD8+) and Natural Killer (NK) cells. The modified cells provide a population of cells that express a genetically engineered chimeric receptor which can be administered to a patient therapeutically. The present invention further relates to methods that deliver mRNA coding for the chimeric receptor to unstimulated resting PBMC, PBL, T (CD3+ and / or CD8+) and NK cells and which delivers the mRNA efficiently to the transfected cells and promotes significant target cell killing.
Owner:MAXCYTE
Human immune therapies using a cd27 agonist alone or in combination with other immune modulators
InactiveUS20130336976A1Promotes strong expression of 4-1BBImprove responseAntibacterial agentsAntimycoticsIMMUNE STIMULANTSCD8
Methods of inducing T cell proliferation and expansion in vivo for treating conditions wherein antigen-specific T cell immune response are therapeutically desirable such as cancer, infection, inflammation, allergy and autoimmunity and for enhancing the efficacy of vaccines are provided. These methods comprise the administration of at least one CD27 agonist, preferably an agonistic CD27 antibody, alone or in association with another moiety such as immune stimulant or immune modulator such as an anti-CD40, OX-40, 4-1BB, or CTLA-4 antibody or an agent that depletes regulatory cells, or a cytokine. These mono and combination therapies may also optionally include the administration of a desired antigen such as a tumor antigen, an allergen, an autoantigen, or an antigen specific to an infectious agent or pathogen against which a T cell response (often CD8+) is desirably elicited.
Owner:UNIV OF SOUTHAMPTON
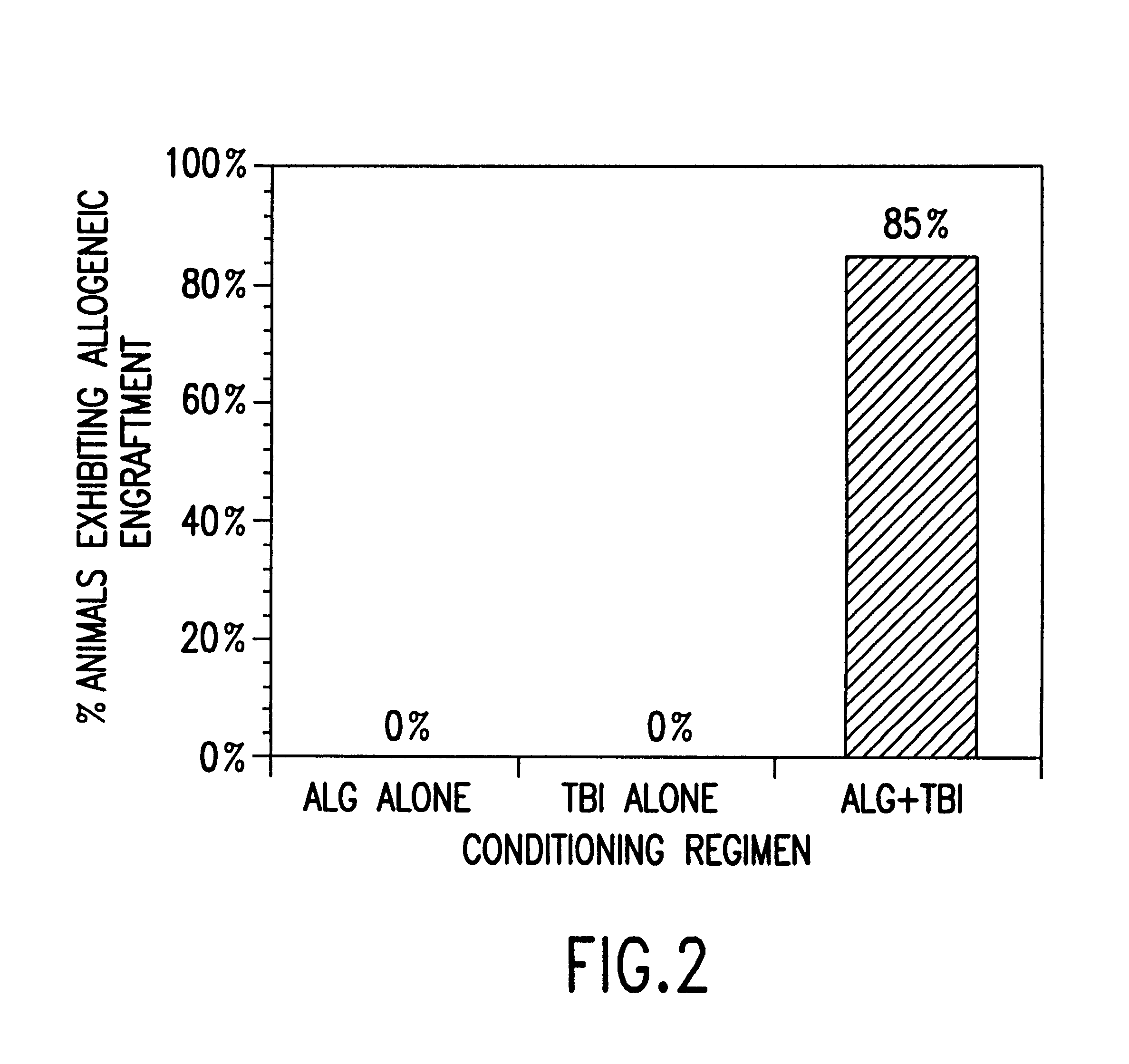
Methods for enhancing engraftment of purified hematopoietic stem cells in allogenic recipients
InactiveUS20070098693A1Facilitate HSC engraftmentEnhance engraftmentBiocidePeptide/protein ingredientsDendritic cellGraft versus host disease induction
CD8+ / TCR− bone marrow cells facilitate engraftment of hemapoietic stem cells (HSQ in allogeneic recipients without causing graft versus host disease. The present invention identifies the main subpopulation (55-65%) of CD8+ / TCR− facilitating cells (FQ as plasmacytoid precursor dendritic cells (p-preDC). The present invention notably demonstrates that FC and p-preDC share many phenotypic, morphological, functional features, including IFN-α production, activation and survival after stimulation, and expansion and maturation after FIO-Ligand (FL) treatment. FL mobilized FC, the majority of which express a pre-DC phenotype, facilitate HSC engraftment. Although p-preDC significantly enhance HSC engraftment, they do so with less efficiency than FC. The present invention for the first time defines a direct functional role for p-preDC in HSC engraftment and will have a significant impact on strategies to design effective facilitating cell-based therapies for transplantation.
Owner:UNIV OF LOUISVILLE RES FOUND INC
Methods and reagents for vaccination which generate a CD8 T cell immune response
New methods and reagents for vaccination are described which generate a CD8 T cell immune response against malarial and other antigens such as viral and tumour antigens. Novel vaccination regimes are described which employ a priming composition and a boosting composition, the boosting composition comprising a non-replicating or replication-impaired pox virus vector carrying at least one CD8 T cell epitope which is also present in the priming composition.
Owner:OXXON THERAPEUTICS LTD
Immuno-reactive peptide CTL epitopes of human cytomegalovirus
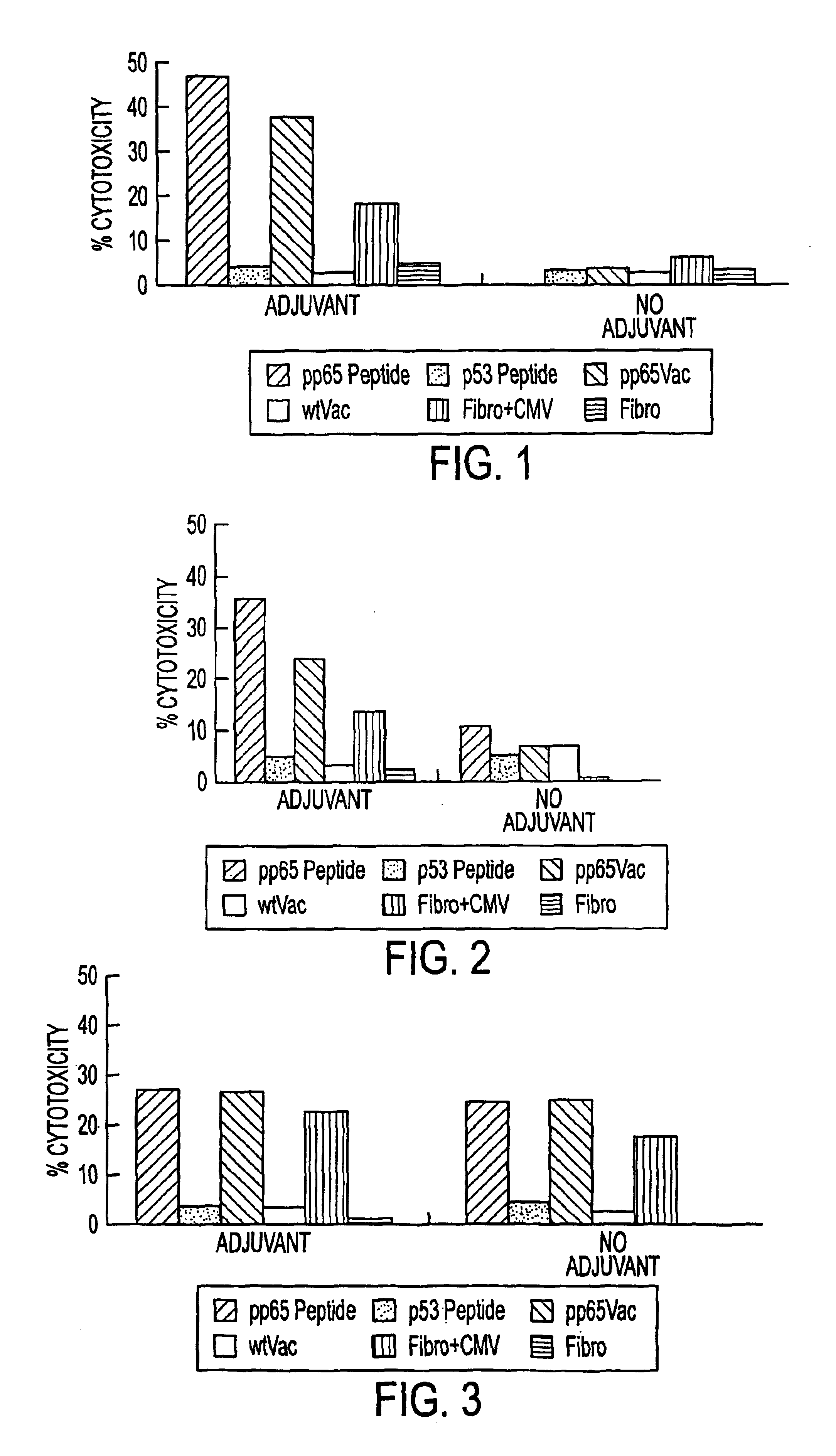
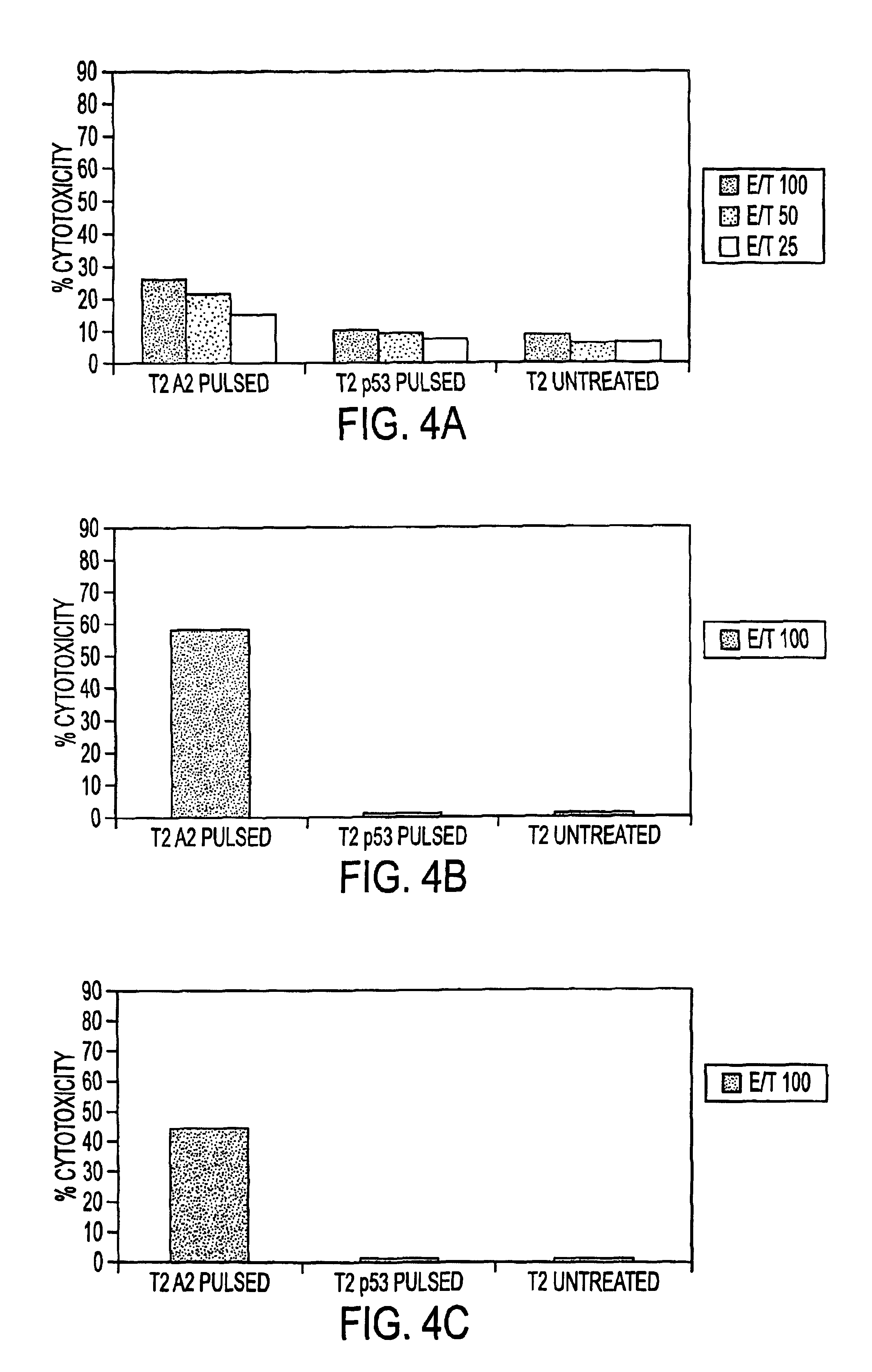
The invention provides a plurality of peptides (and immunologically functional variants thereof) which are immunogenic epitopes recognized by CD8<+> class I MHC restricted cytotoxic T-lymphocytes of patients harboring latent human cytomegalovirus (HCMV) infection. The peptides are capable of activating CTLs and CTLps in the absence of active viral replication, and thus are useful for eliciting a cellular immune response against HCMV by normal and immunodeficient subjects. Peptide and lipopeptide vaccines, with and without adjuvants, also are disclosed. Cellular vaccines comprising the peptides form a further embodiment of this invention.
Owner:CITY OF HOPE
Suppression of transplant rejection
InactiveUS20070166307A1Vertebrate cellsImmunoglobulins against cell receptors/antigens/surface-determinantsAutoimmune conditionRegulatory T cell
The present invention relates to a transplant rejection in an animal suppressed by administration of an antibody directed at a cell surface antigen selected from the group consisting of CD4, CD8, CD154, LFA-1, CD80, CD86 and ICAM-1, preferably an anti-CD4 antibody, together with a non-cellular protein antigen to generate in the animal a population of regulatory T-lymphocytes; reactivating said population of regulatory T-lymphocytes by further administration to the animal of the non-cellular protein antigen; and transplanting said organ or tissue whilst said population of regulatory T-lymphocytes is activated. Regulatory T cells can be generated ex vivo by culturing T cells with an antibody directed at a cell surface antigen selected from the group consisting of CD4, CD8, CD154, LFA-1, CD80, CD86 and ICAM-1, in the presence of cells that present either alloantigen or a non-cellular protein antigen. Ex vivo generated T-lymphocytes can be used as an alternative method of overcoming transplant rejection or in combination with the in vivo method. A similar approach can be adopted for the treatment of autoimmune conditions.
Owner:ISIS INNOVATION LTD
Immunotherapy of cancer through combination of local and systemic immune stimulation
Provided herein are compositions and methods for immunotherapy of cancers, said methods combining systemic vaccination with a recombinant expression vector encoding a viral antigen and / or a cancer specific tumor antigen(s) to induce activated CD8 T-cells, with a local (e.g., intratumoral) immune stimulation using standard or modified application of a TLR agonist, to induce local inflammatory and innate immune responses which recruit T-cells to the tumor.
Owner:IMMUNE DESIGN CORP
CIK cell, as well as preparation method and cell preparation thereof
InactiveCN101519646AIncrease the number of proliferationPromote cell proliferationMammal material medical ingredientsTissue cultureAbnormal tissue growthPhytohemagglutinins
The invention discloses a CIK cell, as well as a preparation method and a cell preparation thereof. The method for preparing the CIK cell comprises the following steps: placing a separated mononuclear cell in a culture fluid containing phytohemagglutinin; transplanting the mononuclear cell into a culture flask enveloped by antiCD3 monoclonal antibody and antiCD28 monoclonal antibody after the cell is cultured for 24 to 72 hours; and adding a culture fluid containing IL-1alpha and IL-2 into the culture flask to keep on culturing for 5 to 15 days, and separating the cells into different flasks to be cultured every 2 to 3 days. The CIK cell prepared by the method has the characteristics of obvious improved cell proliferation, great increase of CD8 cell proportion, wide antineoplastic spectrum and strengthened antineoplastic activity. The CIK cell and the cell preparation can effectively prevent the metastasis and the recrudescence for of postoperative patients with tumor, can be combined with chemicotherapy to effectively reduce toxic and side effects of the chemicotherapy, strengthens the survivability tolerance of the patients to improve healing efficacy, and can obviously prolong lifecycle to of end-stage patients so as to improve the life living quality.
Owner:上海德嘉生物科技有限公司 +1
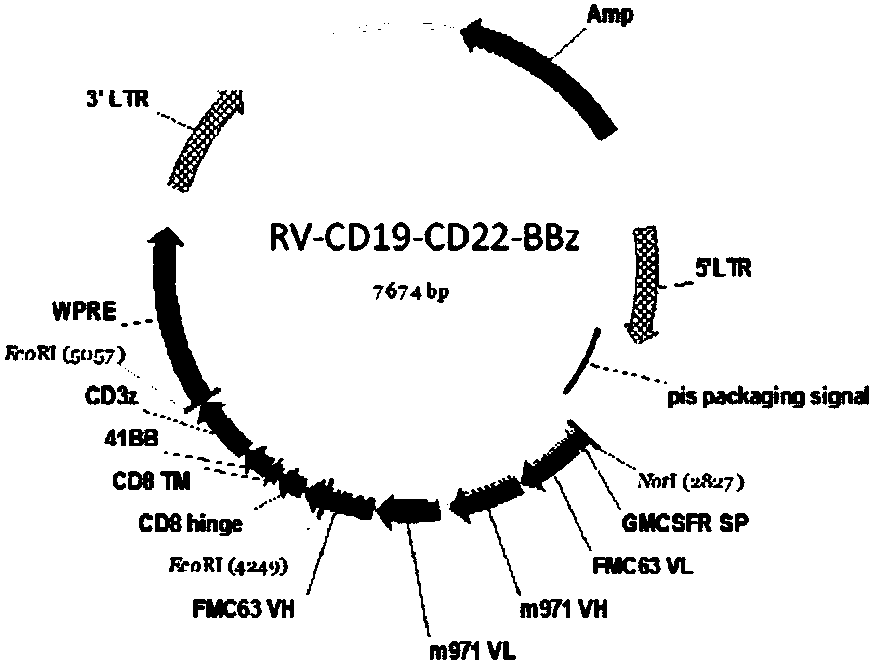
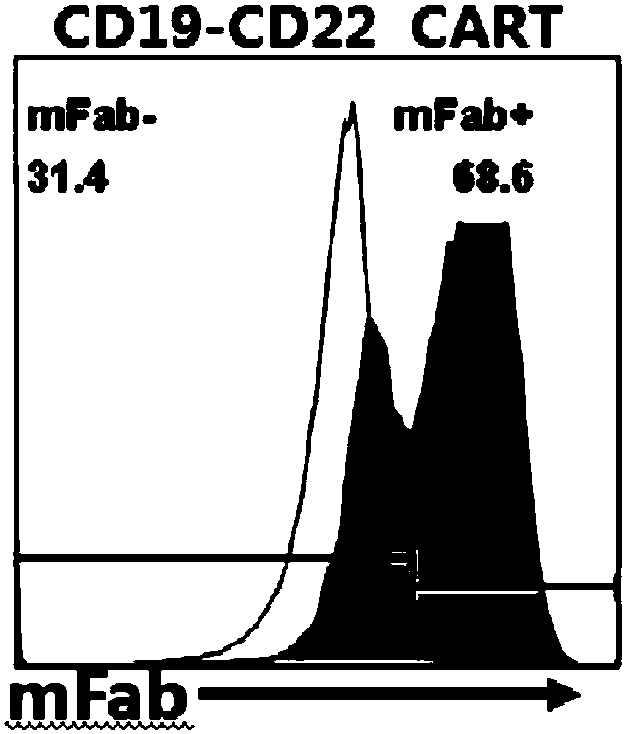
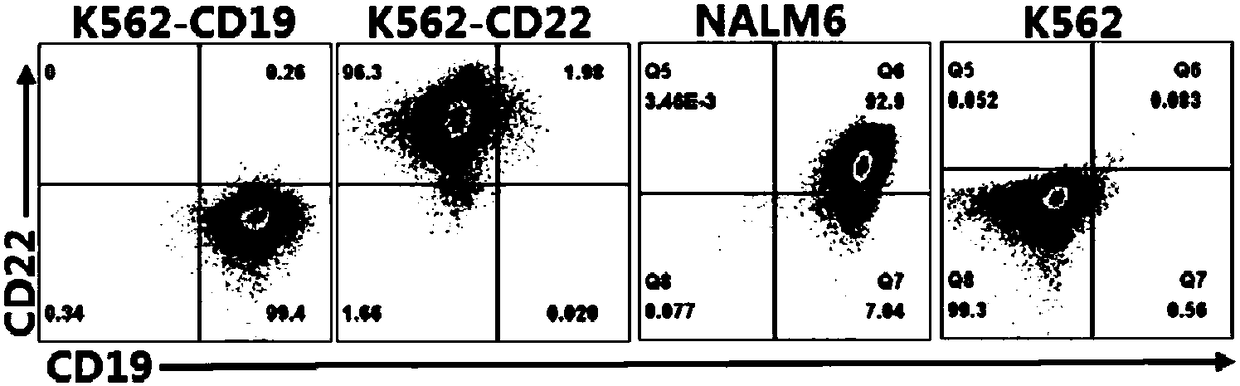
Target CD19 and CD22 chimeric antigen receptor and application thereof
PendingCN108504668AShorten the timeStable expressionMammal material medical ingredientsImmunoglobulinsSingle-Chain AntibodiesAntigen receptors
The invention relates to a double-target CD19 and CD22 chimeric antigen receptor and application thereof, in particular to a polynucleotide sequence which is selected from (1) a coding sequence comprising anti-CD22 and anti-CD19 single-chain antibodies which are connected sequentially, a coding sequence of a human CD8 hinge region, a coding sequence of human CD8 transmembrane region, a coding sequence of a human 41BB intracellular region, a coding sequence of a human CD3 zeta intracellular region and a polynucleotide sequence of a coding sequence of a fragment, comprising an extracellular domain III and a extracellular domain IV, of an optional EGFR; and (2) a complementary sequence of the (1) polynucleotide sequence. The invention further provides a relevant fusion protein, a carrier comprising the coding sequence and application of the fusion protein, the coding sequence and the carrier. The prepared CD19-CD22-BBzCAR-T cell has a strong killing function to the specific tumor cell, CD107a expression and IFN gamma secretion are high, and the killing efficiency to the target cell can reach about 80% under the situation that the effector-target ratio is 10:1.
Owner:HRAIN BIOTECHNOLOGY CO LTD
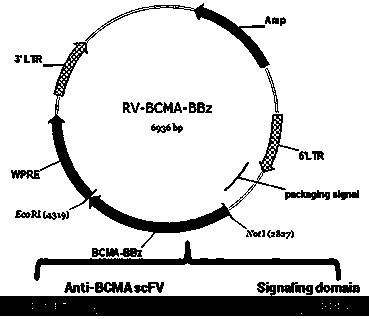
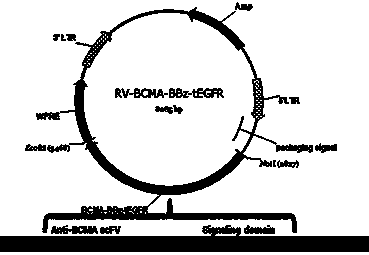
Chimeric antigen receptor targeting BCMA and use thereof
ActiveCN108018299AMammal material medical ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenSingle-Chain Antibodies
The present invention relates to a chimeric antigen receptor targeting BCMA and use thereof. In particular, the present invention provides a polynucleotide sequence selected from the group consistingof (1) a polynucleotide sequence containing sequentially linked an anti-BCMA single chain antibody coding sequence, a human CD8 alpha hinge region coding sequence, a human CD8 transmembrane domain coding sequence, a human 41 BB intracellular region coding sequence, a human CD 3 zeta intracellular region coding sequence and an optional extracellular domain III or extracellular domain IV fragment-containing coding sequence of EGFR; and (2) a complementary sequence of the polynucleotide sequence. The present invention also provides related fusion proteins, vectors containing the coding sequences,and uses of the fusion proteins, the coding sequences, and the vectors.
Owner:HRAIN BIOTECHNOLOGY CO LTD
Multi-epitopic vaccine
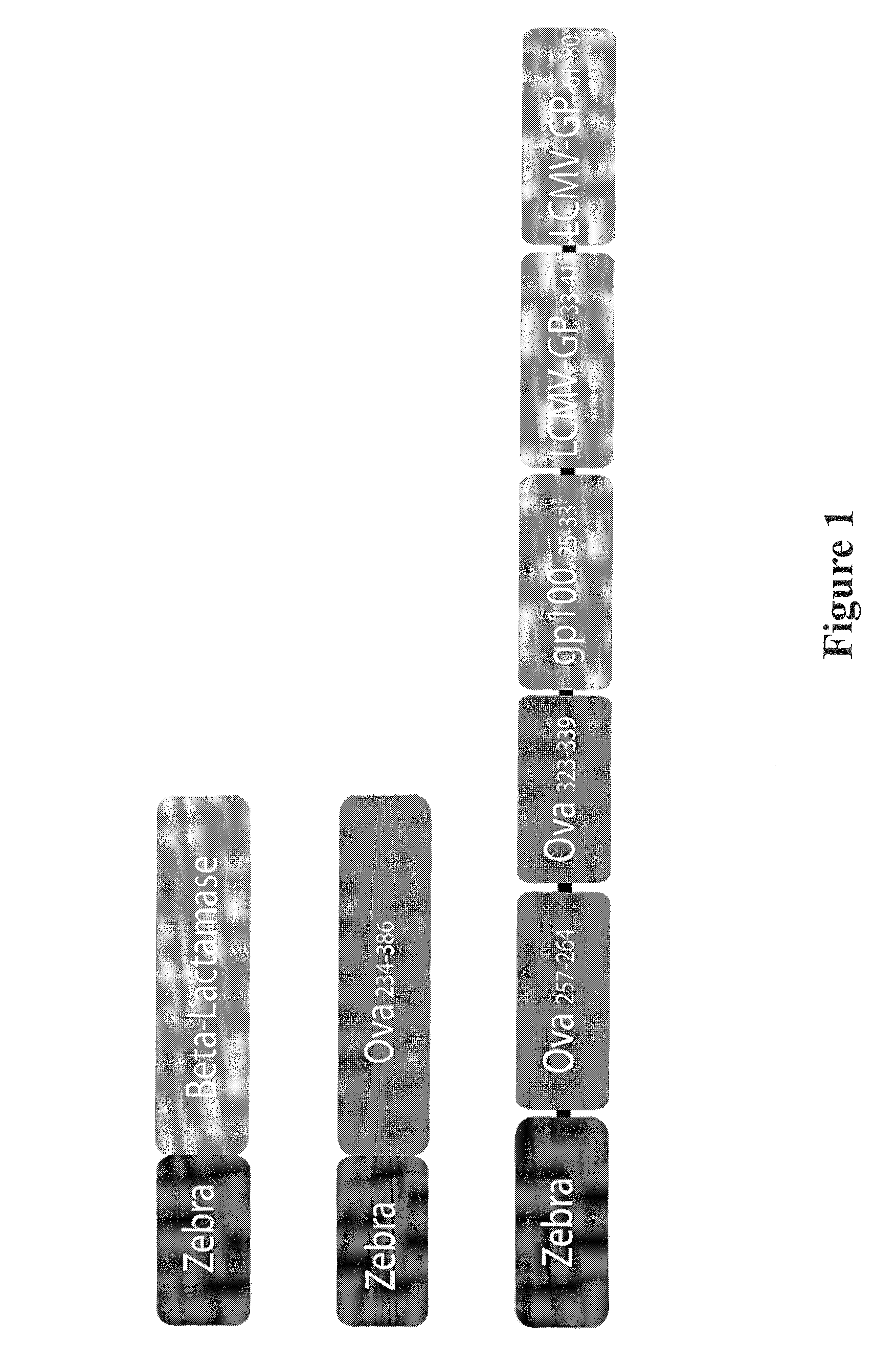
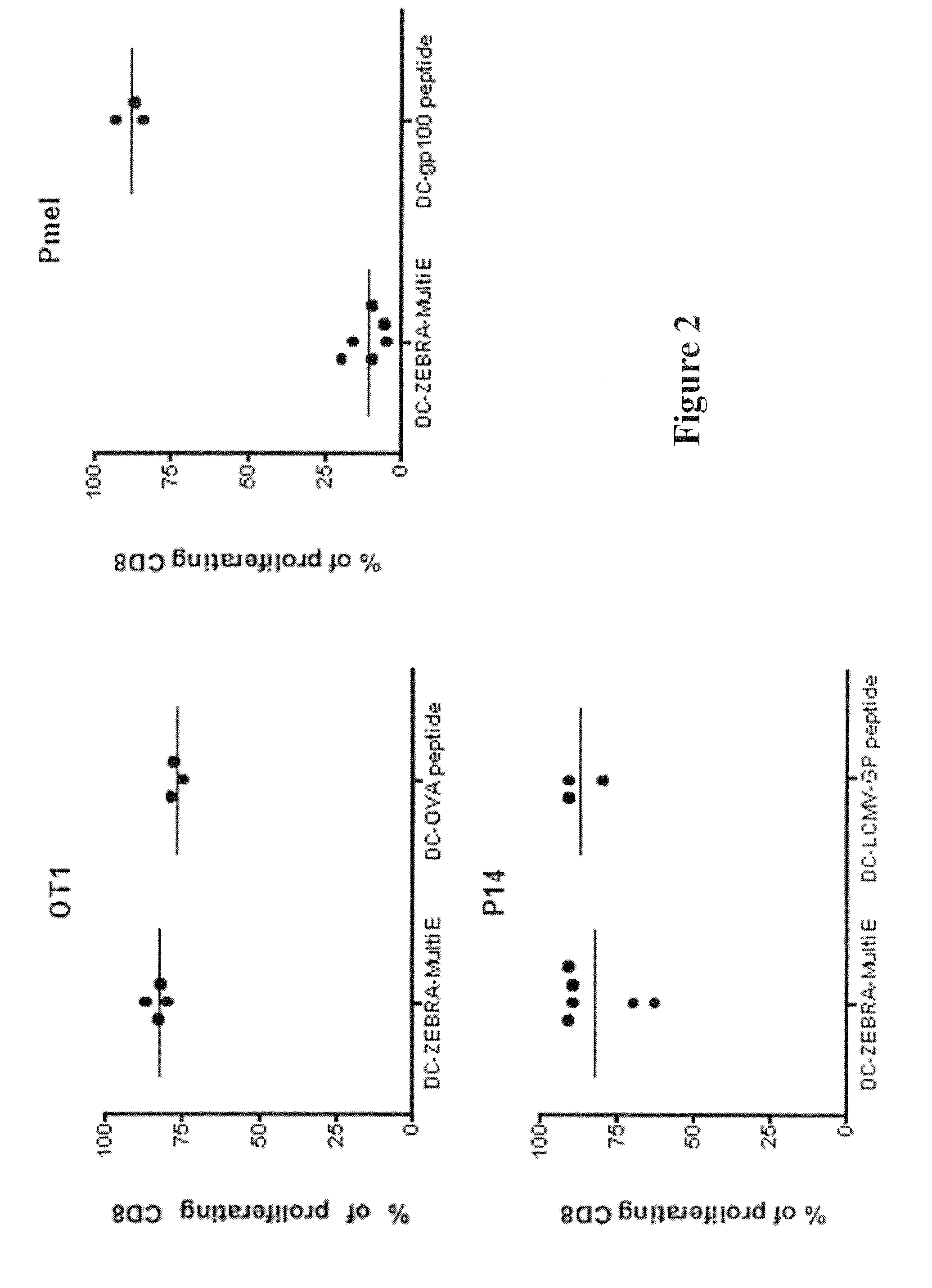
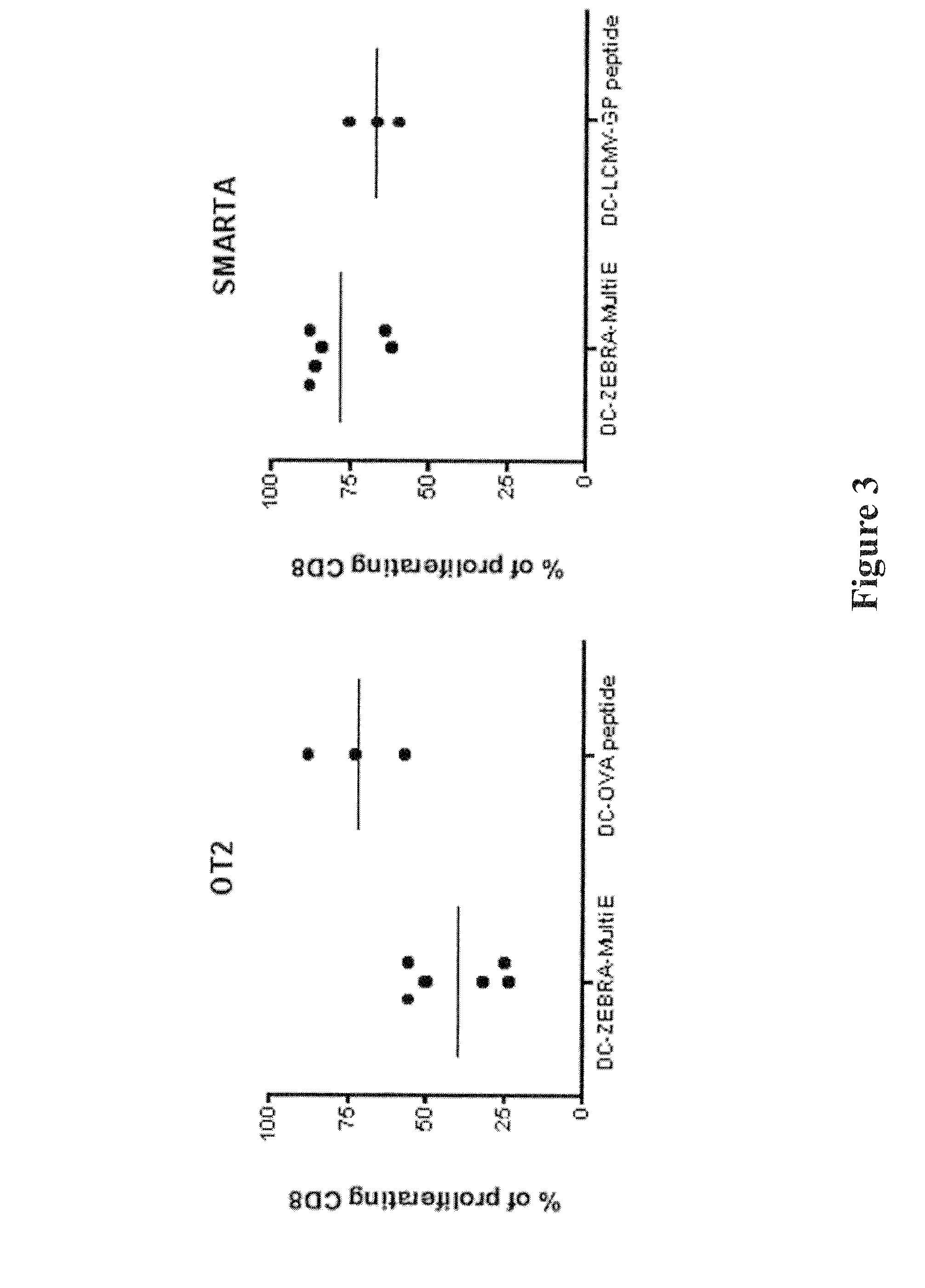
The present invention relates to isolated polypeptides comprising: (i) a protein transduction domain consisting of ZEBRA or a fragment thereof that retains the capacity of internalization, (ii) at least one CD4+ epitope; and (iii) at least one CD8+ epitope. It also relates to antigen presenting cells loaded with said polypeptides, and the use thereof in immunotherapy including prevention and / or treatment of cancers or infectious diseases.
Owner:UNIVERSITY OF GENEVA +1
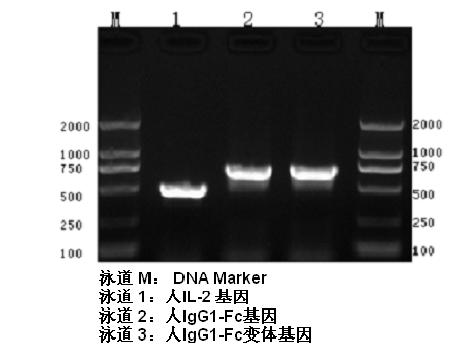
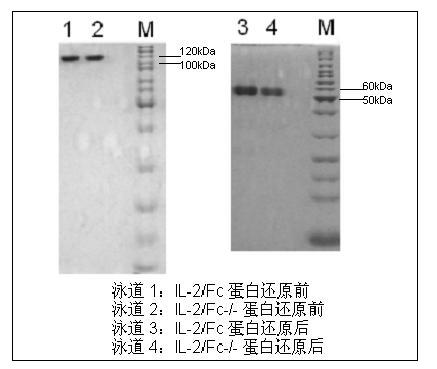
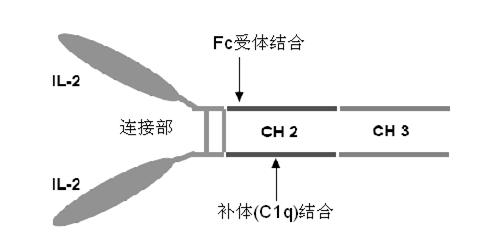
Human interleukin-2 (IL-2)/Fc fusion protein
ActiveCN102174111AEnhance humoral immune responseImprove immunityPeptide/protein ingredientsDigestive systemRegulatory T cellHalf-life
The invention provides human interleukin interleukin-2 (IL-2) / Fc fusion protein. The human IL-2 of the fusion protein comprises all sequences of a human IL-2 extracellular region; the Fc fragments comprise a hinge region, a CH2 region and a CH3 region; the human IL-2 / Fc sequences are fused directly or through a connection sequence; and the Fc fragments are human or animal IgG, IgM, IgD and IgA orsubtypes thereof. The ADCC and CDC effective factor action can be eliminated, and in addition, the human IL-2 / Fc fusion protein has the compatibility with a recombinant IL-2 receptor so that the half-life period is obviously prolonged and also has all the biological activity of the IL-2 receptor. The IL-2 / Fc obviously improves the humoral immune response stimulated by the hepatitis B vaccine and the immunity of the CD8+T cells targeted to the hepatitis B vaccine. Moreover, the balance immune (suppression) of the effective T cells and the regulatory t cells can be adjusted under the action of the cyclosporine A so that the pancreatic islet transplantation immune tolerance is induced.
Owner:上海百英生物科技股份有限公司
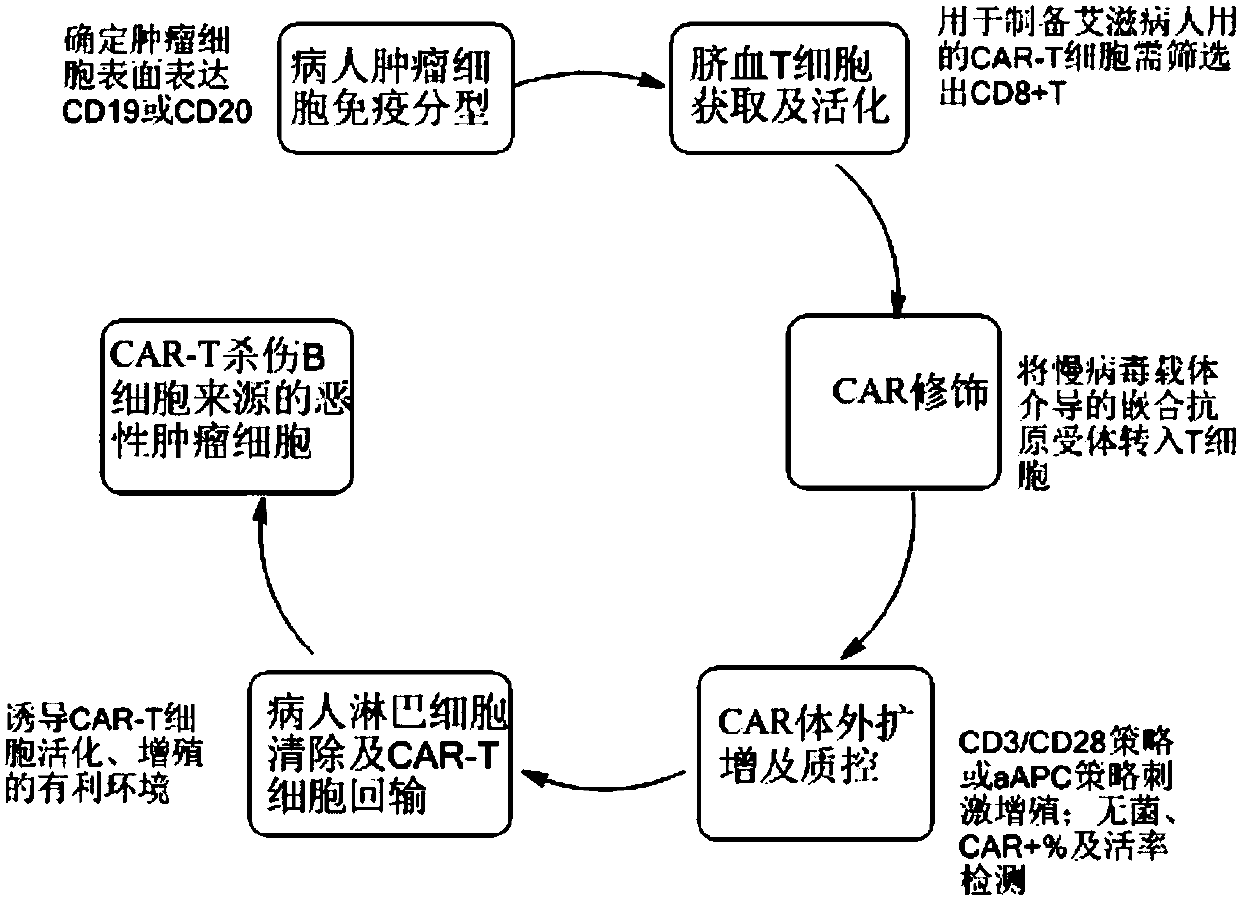
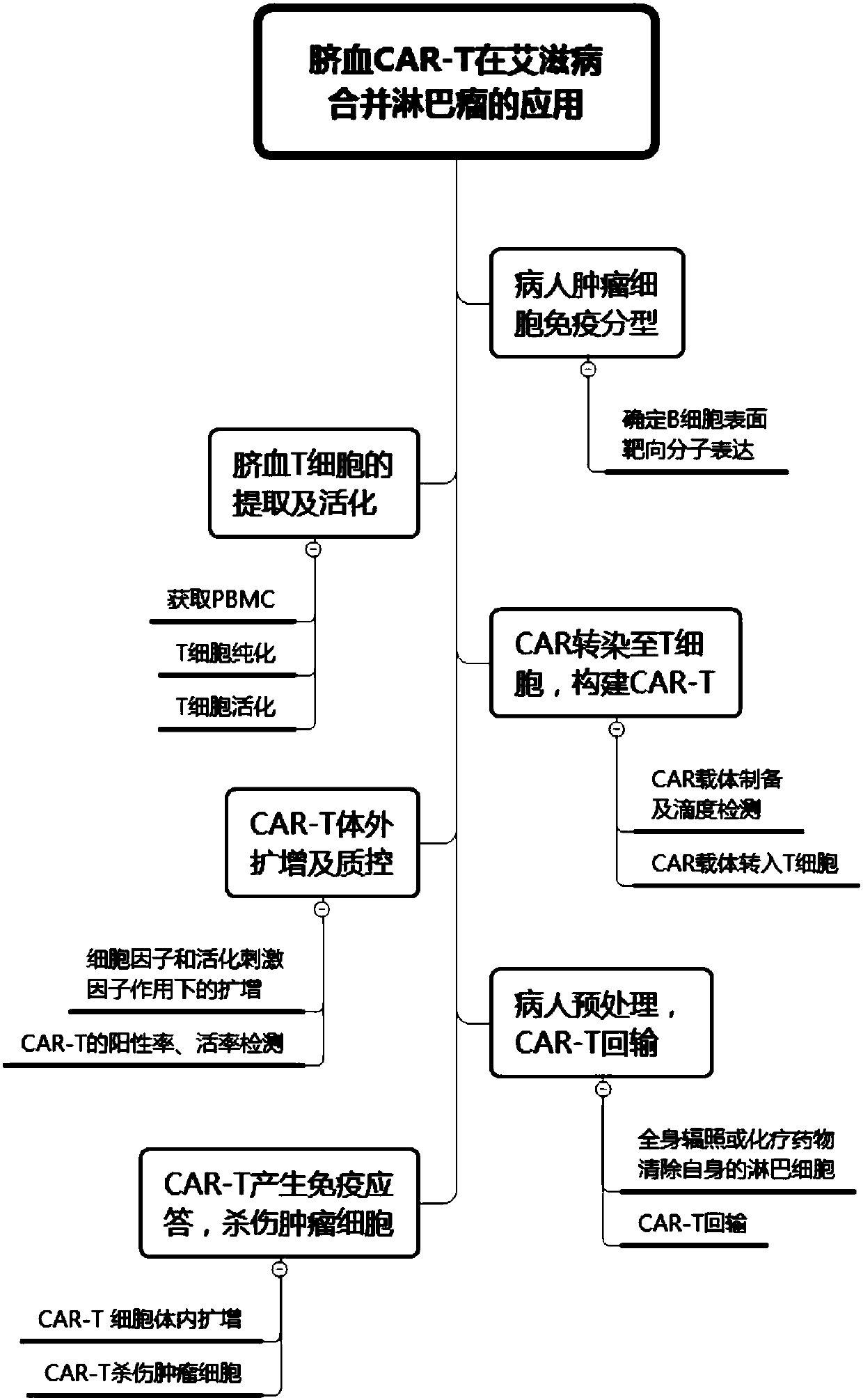
CAR-T cell for treating AIDS-associated lymphoma, and preparation method and application thereof
PendingCN107904259ALow storm riskSmall storm riskGenetically modified cellsAntiviralsAIDS-related lymphomaCord blood stem cell
The invention discloses a preparation method of a CAR-T cell for treating AIDS-related lymphoma. A CD8+ T cell is used to produce the CAR-T cell, and the CD8-T cell derives from a cord blood T cell. The method includes the following steps: preparing cord blood mononuclear cells from cord blood, removing tumors and other cells by using a human T cell purification kit to obtain T cells, and carryingout separation by using magnetic beads to obtain the CD8+ T cell; activating the CD8+ T cell by using an appropriate medium and appropriate stimulation conditions; transferring CAR to the CD8+ T cellby using lentivirus to prepare the CAR-T cell; and amplifying the CAR-T cell in vitro by using cytokines and activating stimulators to achieve the desired effective dose. The invention also relates to the CAR-T cell prepared by the method, and an application thereof. The CAR-T cell prepared in the invention has a good cell activity and a high proliferation speed, and reduces the attack risk of GVHD; and only the CD8+T cell of the umbilical blood T cells of AIDS patients is used to prepare the CAR-T cell for, so the risk of input CAR-T infected with in-vivo HIV is reduced.
Owner:SHANGHAI LONGYAO BIOTECH CO LTD
Method of promoting b-cell proliferation and activation with CD40 ligand and cyclosporin
InactiveUS6465251B1Efficient inductionModulate immune responseDead animal preservationArtificial cell constructsAntigenCyclosporins
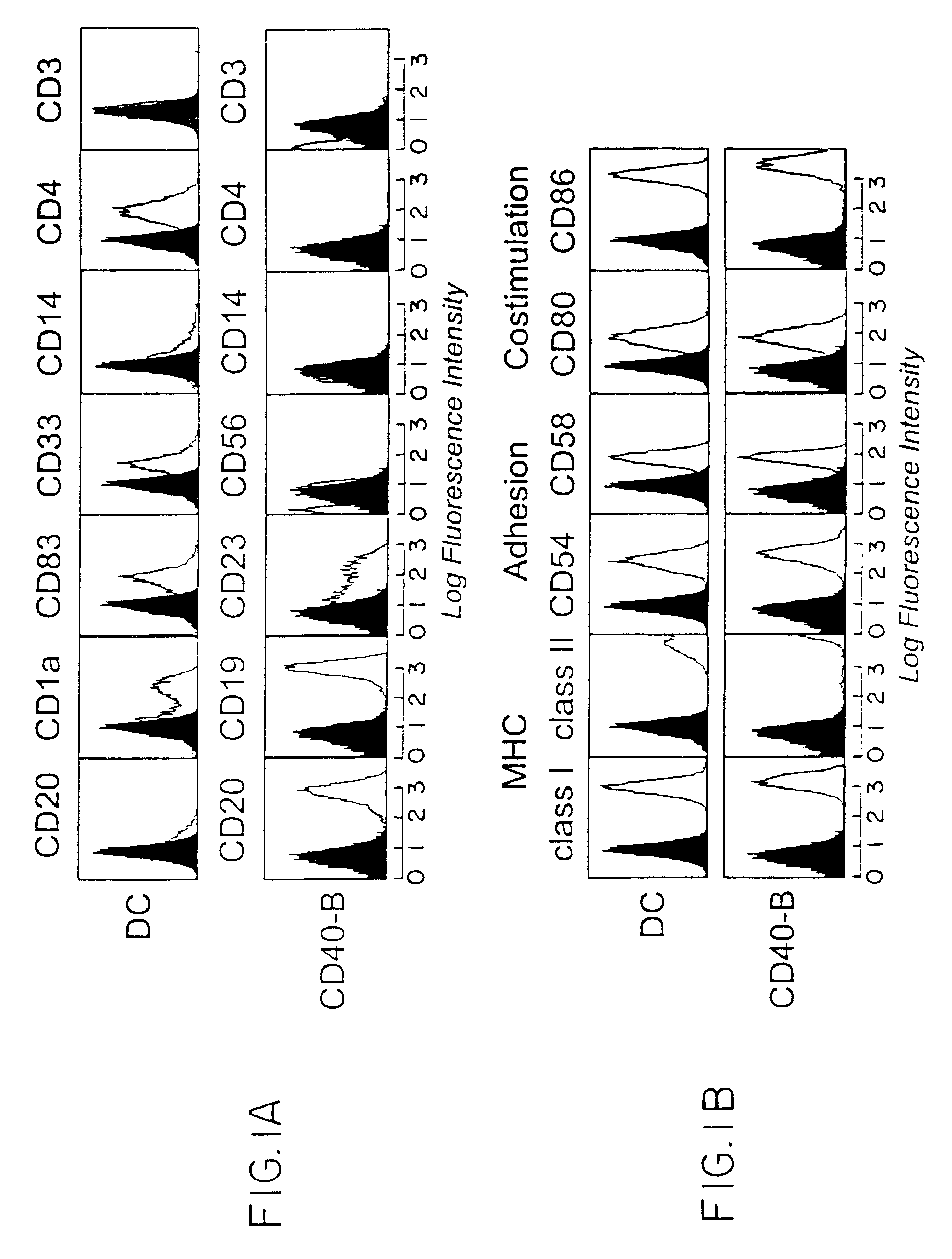
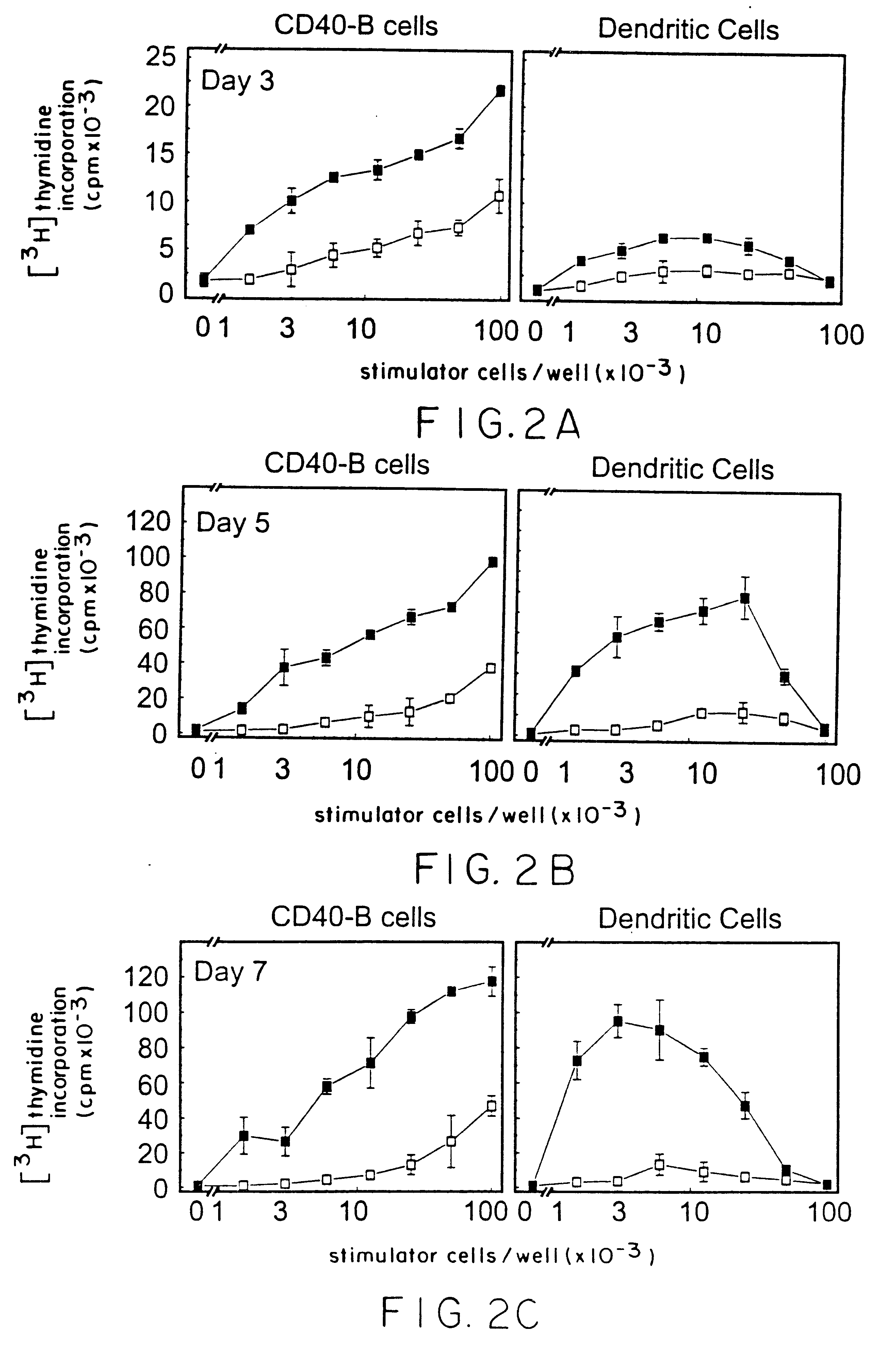
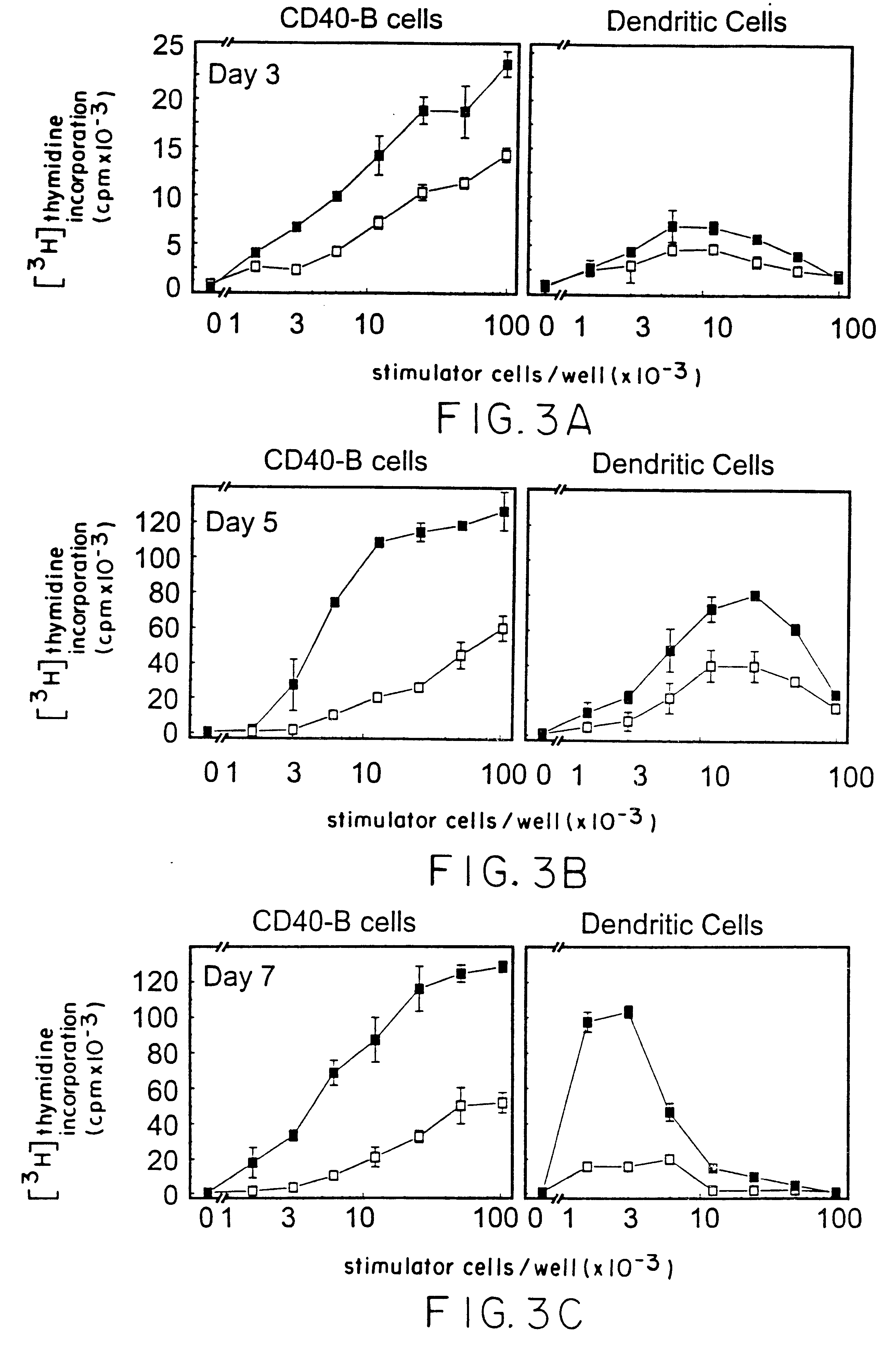
We teach a strategy to obtain large quantities of desired APCs, activated B cells, which are superior in their capacity to present tumor protein antigen in a multiadministration protocol. Human B cells can be obtained from peripheral blood in large numbers. These cells can be activated in vitro by coculture with CD40L (CD40-B cells) and an immunosuppressive agent such as cyclosporin A. They can expanded up to 1x103 to 1x104 fold in 2 weeks or 1x105 to 1x106 fold in 2 months. We demonstrate these cells are most efficient APCs comparable to DCs in stimulating allogeneic CD4+ CD45RA+, CD4+ CD45RO+, and CD8+ T cells. In contrast to DCs, CD40-B cells are fully functional even in the presence of immunosuppressive cytokines such as IL-10 and TGFbeta.
Owner:DANA FARBER CANCER INST INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com