Patents
Literature
46results about How to "Enhance humoral immune response" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Adjuvant compositions and methods for enhancing immune responses to polynucleotide-based vaccines
InactiveUS7105574B1Enhance immune responseEnhance humoral immune responseAntibacterial agentsBiocideLipid formationVaccination
The invention provides adjuvants, immunogenic compositions, and methods useful for polynucleotide-based vaccination and immune response. In particular, the invention provides an adjuvant of cytofectin:co-lipid mixture wherein cytofectin is GAP-DMORIE.
Owner:VICAL INC
Human interleukin-2 (IL-2)/Fc fusion protein
ActiveCN102174111AEnhance humoral immune responseImprove immunityPeptide/protein ingredientsDigestive systemRegulatory T cellHalf-life
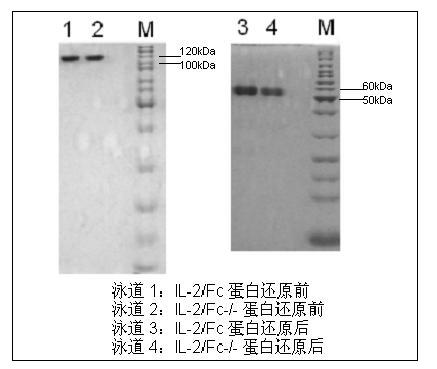
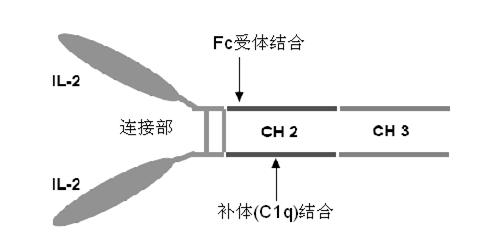
The invention provides human interleukin interleukin-2 (IL-2) / Fc fusion protein. The human IL-2 of the fusion protein comprises all sequences of a human IL-2 extracellular region; the Fc fragments comprise a hinge region, a CH2 region and a CH3 region; the human IL-2 / Fc sequences are fused directly or through a connection sequence; and the Fc fragments are human or animal IgG, IgM, IgD and IgA orsubtypes thereof. The ADCC and CDC effective factor action can be eliminated, and in addition, the human IL-2 / Fc fusion protein has the compatibility with a recombinant IL-2 receptor so that the half-life period is obviously prolonged and also has all the biological activity of the IL-2 receptor. The IL-2 / Fc obviously improves the humoral immune response stimulated by the hepatitis B vaccine and the immunity of the CD8+T cells targeted to the hepatitis B vaccine. Moreover, the balance immune (suppression) of the effective T cells and the regulatory t cells can be adjusted under the action of the cyclosporine A so that the pancreatic islet transplantation immune tolerance is induced.
Owner:上海百英生物科技股份有限公司
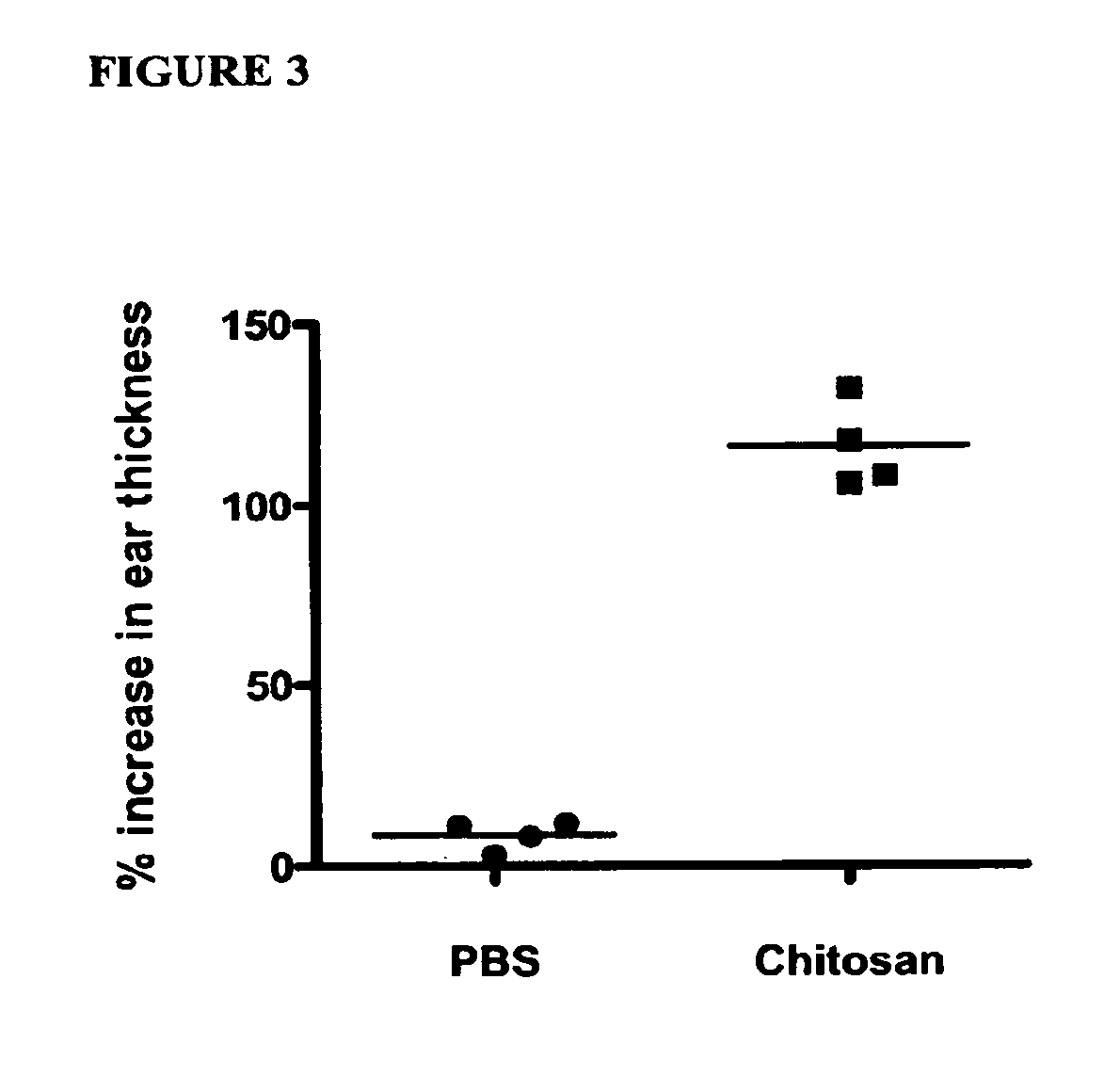
Compositions and methods for chitosan enhanced immune response
InactiveUS20100150960A1Increasing cell mediated immune responseEnhance immune responseSsRNA viruses negative-sensePeptide/protein ingredientsAntigenDisease
Owner:UNITED STATES OF AMERICA
Ginseng total saponin or monomer saponin RbI vaccine immunological adjuvant application
InactiveCN1367022ALong validity periodShort validity periodAntibody medical ingredientsSide effectAluminium hydroxide
The present invention discloses an application of ginseng total saponin or monomer saponin Rb1 as vaccine immunologic adjuvant. The ginseng total saponin or monomer saponin Rb1 and aluminium hydroxide are mixed and used as vaccine immunologic adjuvant, and as compared with traditional aluminium hydroxide adjuvant said invention possesses the following advantages: (1) its side effect is less; (2) said vaccine can be stored under the condition of frozen low-temp. and it can prolong effective period of vaccine; (3) its method is simple and convenient, its quality is easy to be controlled; and (4) it can induce body to produce higher humoral immunity response.
Owner:ZHEJIANG UNIV
Tuberculosis subunit vaccine containing unite adjuvant
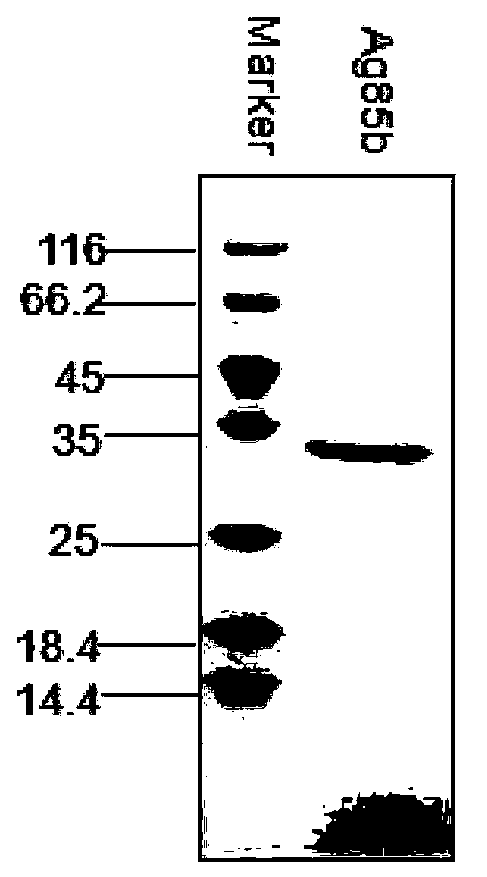
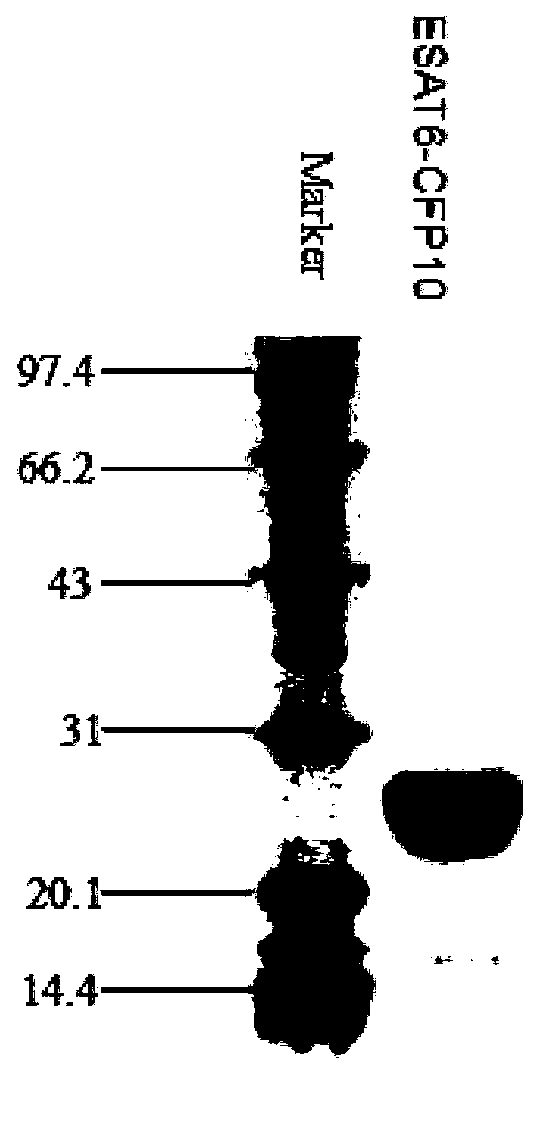
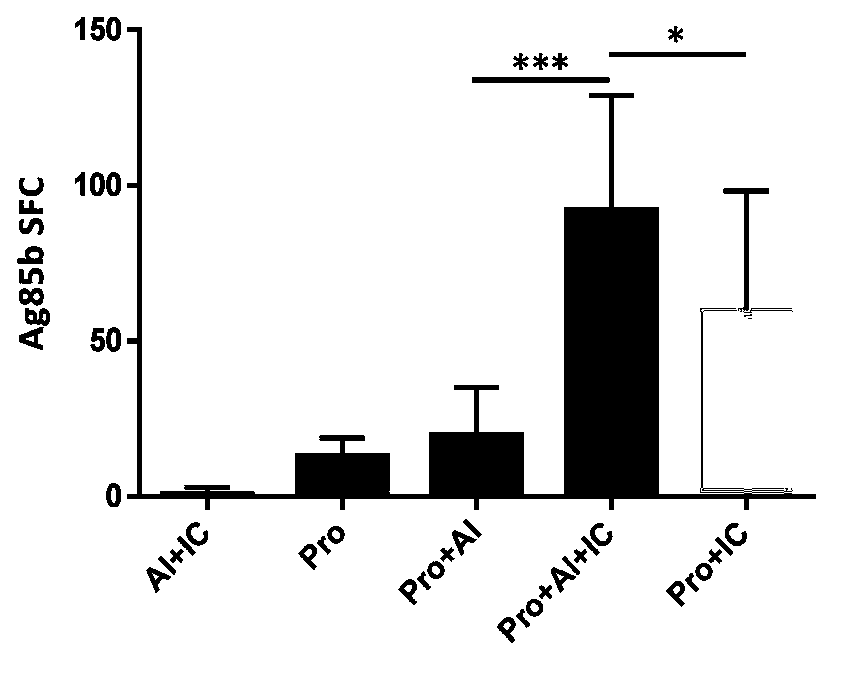
ActiveCN103386128AEnhance cellular immunityEnhance humoral immune responseAntibacterial agentsBacterial antigen ingredientsTuberculomaAdjuvant
The invention provides a novel tuberculosis mycobacterium subunit vaccine containing unite adjuvant, which takes Ag85b protein, ESAT 6-CFP10 fusion protein as antigen component, and aluminum and PolyIC as a composite adjuvant. The adjuvant provided by the invention can effectively improve cellular immunity response of body to the tuberculosis subunit vaccine; at the same time, the adjuvant combines with the tuberculosis mycobacterium antigen Ag85b protein, ESAT6-CFP10 fusion protein, therefore the immunization effect is better than the compatibility effect of using other single adjuvant component and Ag85b protein and ESAT6-CFP10 fusion protein.
Owner:NAT INST FOR FOOD & DRUG CONTROL
Carbohydrate specific cellular immunity inducing microorganisms and fractions thereof
InactiveCN101600454AEnhance specific immune responseEffective immune responseBacterial antigen ingredientsBacteriaMolecular biologyAbnormal tissue growth
The present invention relates to the field of prevention and treatment of disorders associated with the occurrence of certain carbohydrate epitopes. More particularly, the present invention relates to the prevention and treatment of carbohydrate epitope positive tumors. It relates to formulations and methods for the induction of an effective carbohydrate specific cellular immune response.
Owner:GLYCOTOPE GMBH
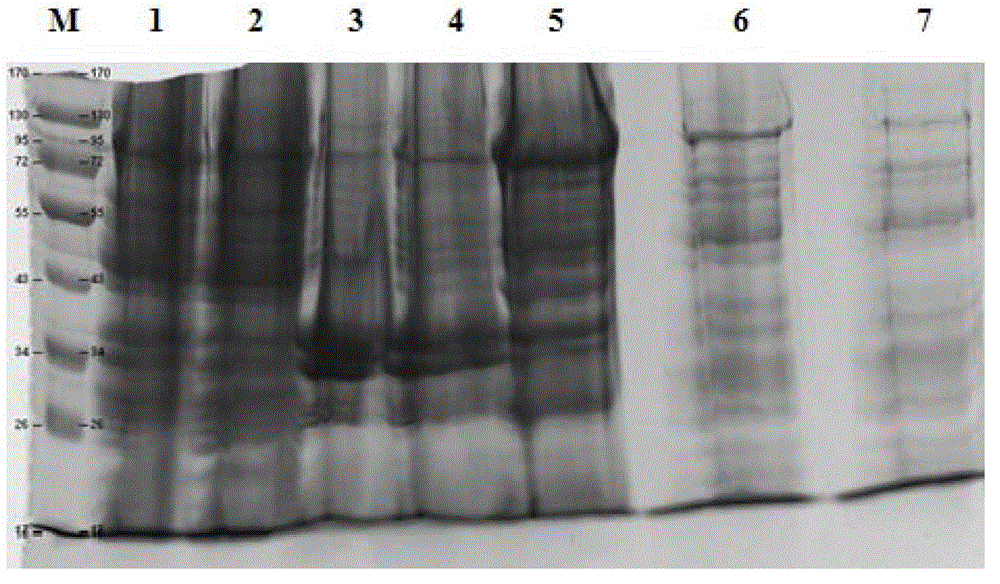
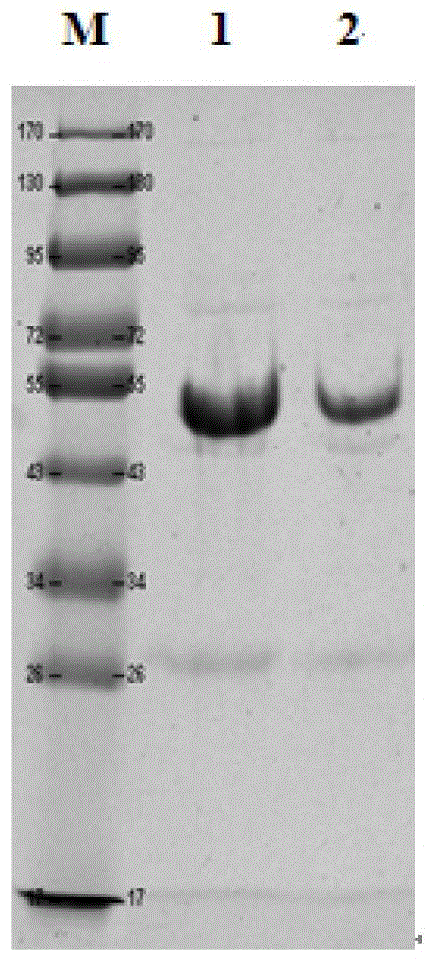
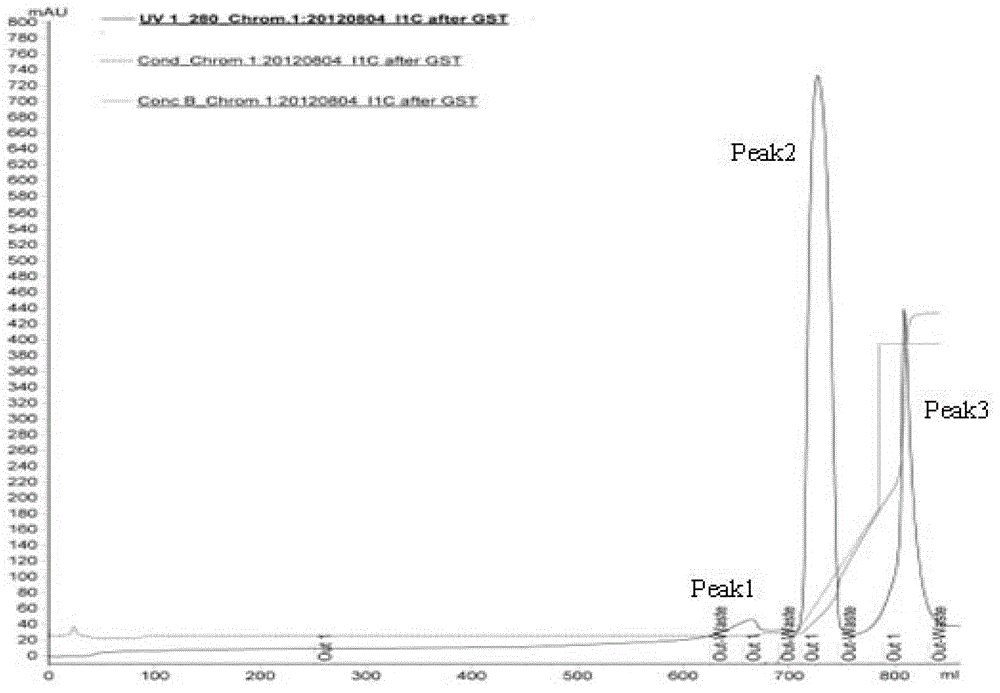
Purifying method of methicillin-resistant staphylococcus aureus MRSA recombinant protein vaccine I1C
InactiveCN103333254AHigh puritySimple processPeptide preparation methodsHybrid peptidesEscherichia coliAntigen
The invention discloses a purifying method of a methicillin-resistant staphylococcus aureus MRSA recombinant protein vaccine I1C, wherein the I1C protein is obtained by recombining and fusing active functional fragments of two antigen molecules of IsdB and ClfA of methicillin-resistant staphylococcus aureus and expressing escherichia coli genetically engineered bacteria. The high purity MRSA recombinant dual subunit genetic engineering protein I1C is obtained by technologies on the genetically engineered bacteria such as high pressure breaking, salting out, GST (Glutathione S Transferase) affinity chromatography, PP (Prescission Protease) digestion, adhere chromatography, gel filtration chromatography and the like. The method disclosed by the invention is simple and rapid in purifying process, easiness in application and good in repeatability. The target protein obtained is high in purity. Animal tests verify that the vaccine can effectively stimulate the organism to generate higher humoral immune response and good immunoprotection.
Owner:CHONGQING YUANLUN BIOTECH +1
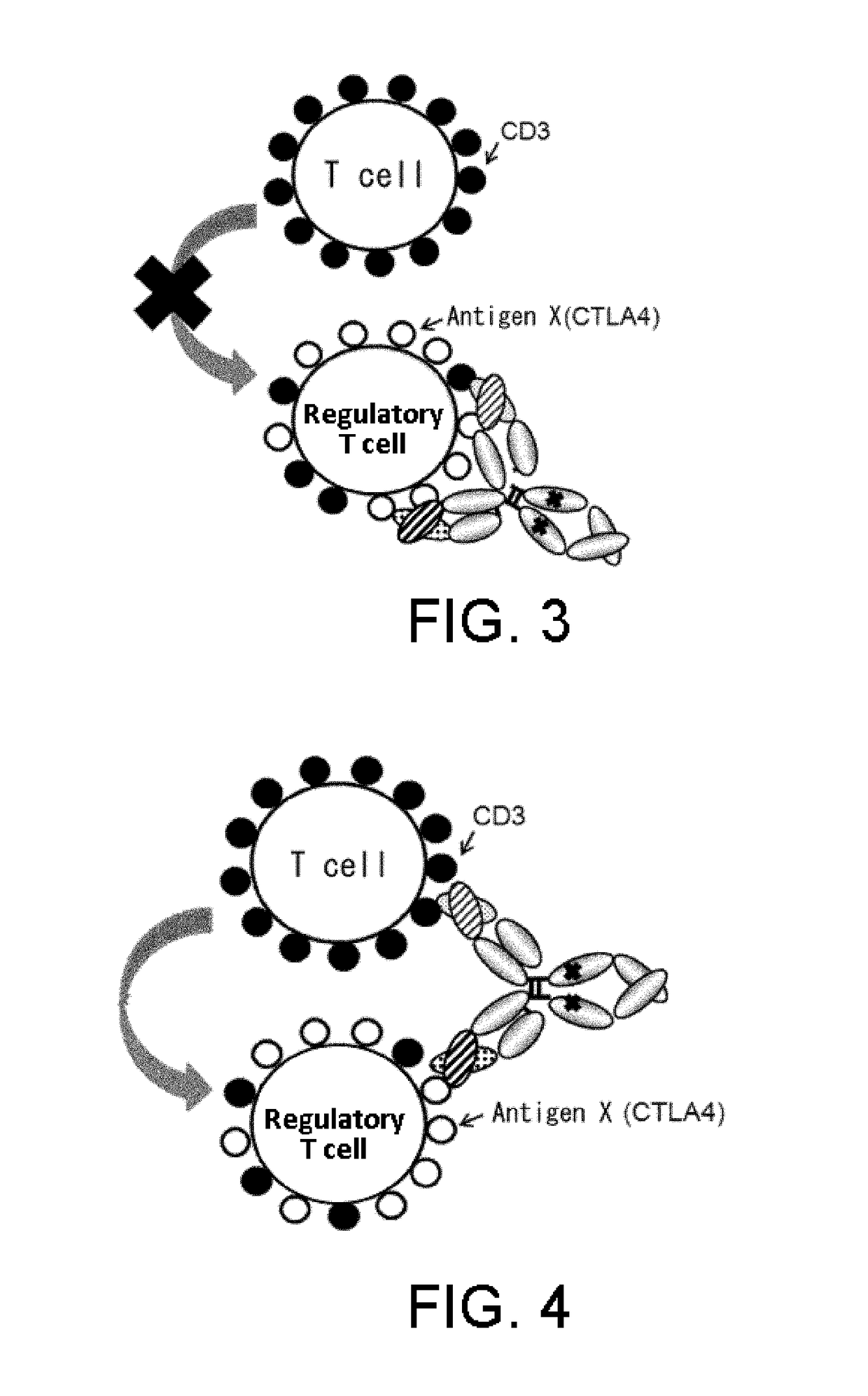
Method for enhancing humoral immune response
ActiveUS20190077872A1Enhance humoral immune responseEffective treatmentSerum immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsDrugPeptide
The present invention provides methods for producing antibodies against peptides, proteins, or such to which immune tolerance is easily established, by using antigen-binding molecules comprising a domain that binds to a molecule expressed on the surface of a cell having an immune response-suppressing function and a T cell receptor (TCR) complex-binding domain. The present invention also provides pharmaceutical compositions for use in combination with therapeutic vaccines and agents for enhancing a humoral immune response, each comprising the antigen-binding molecules as active ingredients.
Owner:CHUGAI PHARMA CO LTD
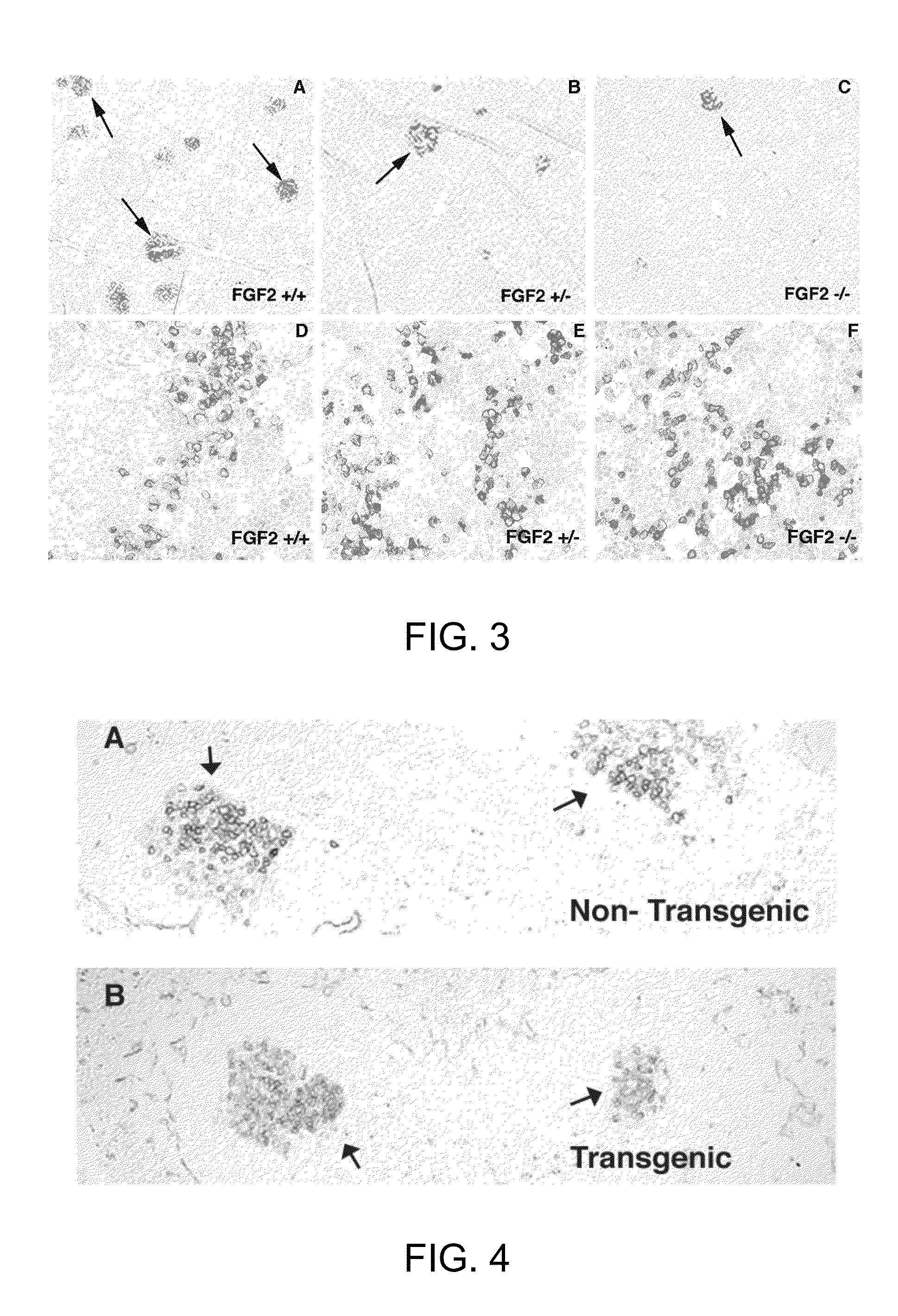
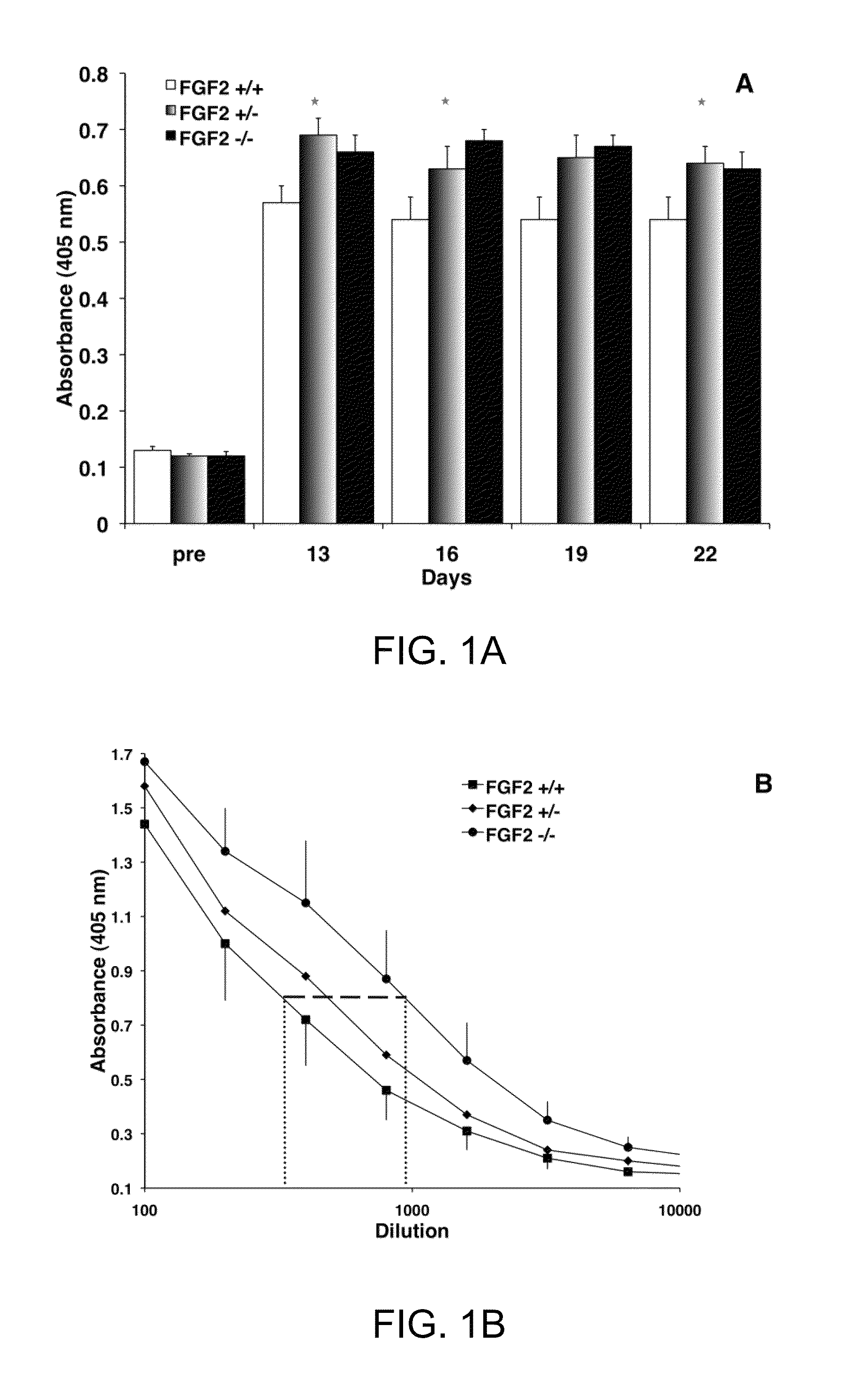
FGF modulation of in vivo antibody production and humoral immunity
ActiveUS8435525B1Enhance humoral immune responseInhibitory activitySnake antigen ingredientsImmunoglobulins against growth factorsDecreased Antibody ProductionIn vivo
The invention provides methods for increasing or decreasing antibody production in vivo by inhibiting or promoting the activity of fibroblast growth factor-2 (FGF2) respectively.
Owner:BUSH ANDREW B
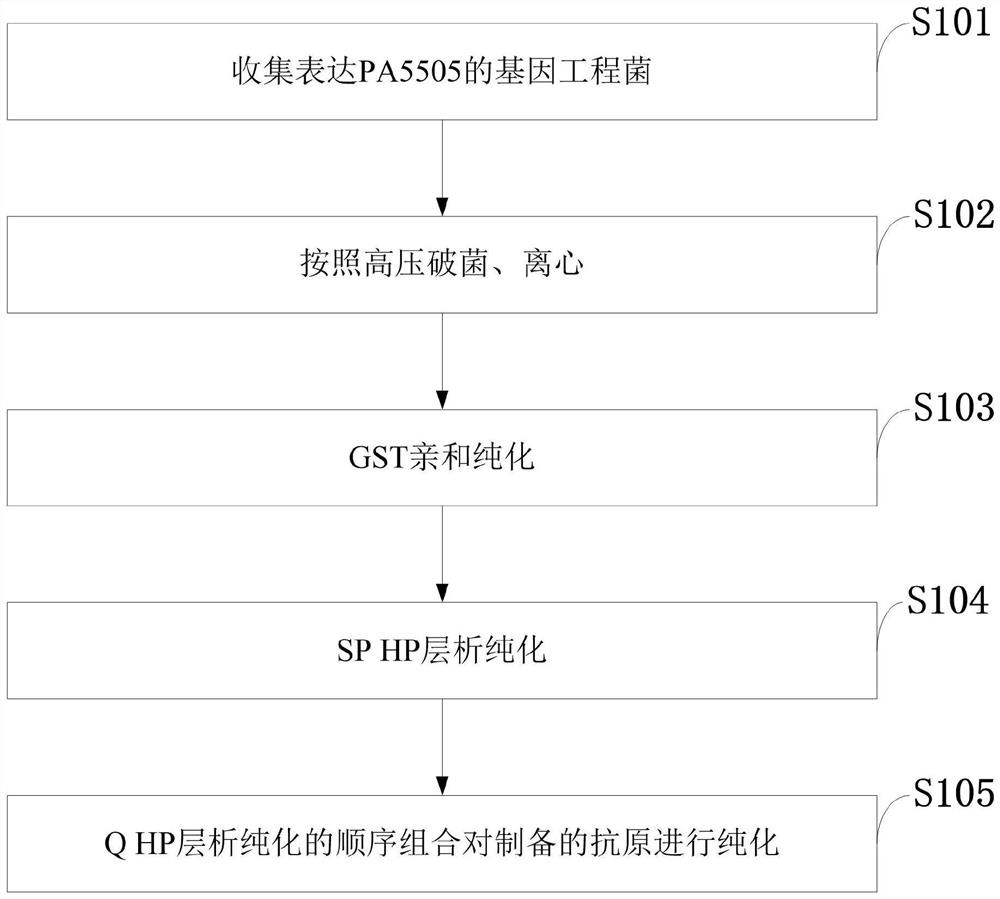
Pseudomonas aeruginosa gene engineering vaccine candidate antigen PA5505 purification method
ActiveCN109486736AHigh purityEasy diagnosisBacteriaMicroorganism based processesEscherichia coliAntigen
The invention belongs to the technical field of biological pharmacy, and discloses a pseudomonas aeruginosa gene engineering vaccine candidate antigen PA5505 purification method. A DNA (deoxyribonucleic acid) sequence for encoding PA5505 protein activity function fragment is cloned to a pGEX-6p-2 vector by a gene engineering technology, escherichia coli recombinant bacteria pGEX-6p-2-PA5505 / XL-1 blue are built, and PA5505 proteins are acquired by inducible expression. Gene engineering bacteria expressing PA5505 are subjected to technologies such as high-pressure bacteria breaking, GST affinitychromatography, PP enzyme digestion, SP HP chromatography and Q HP chromatography to obtain high-purity vaccine candidate antigen PA5505. The purification method is simple in purification process, easy to amplify and good in repeatability, the acquired target proteins are high in purity, and animal experiments prove that a body can be effectively stimulated to generate high humoral immune response and good immune protection functions.
Owner:重庆艾力彼生物科技有限公司
Respiratory syncytial virus vaccine, and preparation method and application thereof
ActiveCN105983095AEnhance humoral immune responseSuppression of cellular immune responseOrganic active ingredientsAntiviralsViral antibodyCell immune response
The invention discloses a respiratory syncytial virus vaccine, and a preparation method and an application thereof. The active components of the respiratory syncytial virus vaccine comprise a respiratory syncytial virus G protein and an immunosuppressant; the amino acid sequence of the respiratory syncytial virus G protein is represented by sequence 2 in a sequence table; and the immunosuppressant is cyclosporins A. Test proves that the respiratory syncytial virus vaccine can enhance the humoral immunoreaction in the virus neutralizing antibody level of immune animals, can inhibit too strong cellular immunity reactions, effectively and specifically inhibits inflammation related pathological reactions, and has the advantages of manure technology, low cost, no side effects and easy promotion.
Owner:ADVACCINE SUZHOU BIOPHARMACEUTICALS CO LTD +1
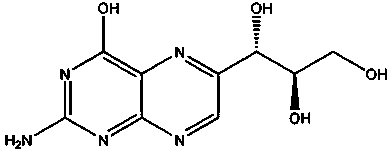
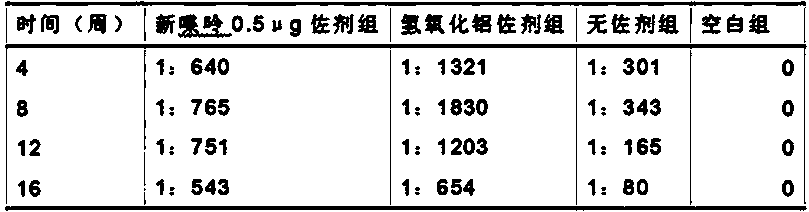
Neopterin adjuvant and vaccine containing neopterin adjuvant
ActiveCN104056266ASmall side effectsRaw materials are easy to getImmunological disordersAntibody medical ingredientsSide effectEngineered genetic
The invention provides a neopterin adjuvant and a vaccine containing the neopterin adjuvant, and belongs to the technical field of immunology. According to the vaccine containing the neopterin adjuvant, each single vaccine contains 0.5-1 microgram of the neopterin adjuvant. The neopterin adjuvant belongs to an endogenous substance in the human body, is small in toxic and side effects, is safe and reliable when used in an immunizing dose range, and can effectively induce antigenic specificity humoral immune responses, and the induced humoral immune response effect is better than that of a group without adjuvants and similar to that of a group with an aluminum adjuvant. Raw materials of the neopterin adjuvant are easy to obtain, the neopterin adjuvant is a commercially available product and low in cost and can be added to multiple traditional vaccines and genetic engineering vaccines to be used as a vaccine adjuvant.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Vaccine for prevention and/or treatment of respiratory syncytial virus infection
ActiveCN103239734ASuppress immune responseEnhance humoral immune responseSsRNA viruses negative-senseViral antigen ingredientsDiseaseRSV Infections
The invention discloses a co-immune vaccine for prevention and / or treatment of diseases caused by respiratory syncytial virus infection. The vaccine is composed of a DNA vaccine and a subunit vaccine. The DNA vaccine is a recombinant expression vector containing an encoding gene of a protein as shown in a sequence 2 in a sequence table. The subunit vaccine is a protein as shown in the sequence 2 in the sequence table. Experimental results show that the co-immune vaccine provided by the present invention can enhance the humoral immune response of immune animals, also inhibit too strong cellular immune response, effectively inhibit inflammation, protect lung tissues of mammalians and prevent function from destruction of RSV infection.
Owner:ADVACCINE SUZHOU BIOPHARMACEUTICALS CO LTD
Metal aluminum nano adjuvant, preparation method thereof, vaccine composition, preparation method of vaccine composition and application of metal aluminum nano particle
PendingCN112704734AEnhance humoral immune responseEnhance immune responsePowder deliveryCancer antigen ingredientsImmunologic disordersAutoimmune condition
The invention discloses a metal aluminum nano adjuvant, a preparation method thereof, a vaccine composition of the vaccine composition and an application of a metal aluminum nano particle. The vaccine adjuvant comprises the metal aluminum nano particle, and can be used as a candidate adjuvant of preventive vaccines and therapeutic vaccines for various diseases such as infection, autoimmune diseases and tumors. The vaccine adjuvant provided by the invention is combined with an antigen for use, so that humoral immune response and cellular immune response of the vaccine can be effectively enhanced, and the enhancement effect of the vaccine adjuvant is remarkably superior to that of a commercially available aluminum hydroxide adjuvant.
Owner:JILIN UNIV
FGF modulation of in vivo antibody production and humoral immunity
InactiveUS9107908B2Enhance humoral immune responseInhibitory activityAntibody ingredientsFibroblast growth factorDecreased Antibody ProductionIn vivo
The invention provides methods for increasing or decreasing antibody production in vivo by inhibiting or promoting the activity of fibroblast growth factor-2 (FGF2) respectively.
Owner:BUSH ANDREW B
Ginseng total saponin or monomer saponin RbI vaccine immunological adjuvant application
InactiveCN1141143CLittle side effectsLong validity periodAntibody medical ingredientsSide effectAluminium hydroxide
The present invention discloses an application of ginseng total saponin or monomer saponin Rb1 as vaccine immunologic adjuvant. The ginseng total saponin or monomer saponin Rb1 and aluminium hydroxide are mixed and used as vaccine immunologic adjuvant, and as compared with traditional aluminium hydroxide adjuvant said invention possesses the following advantages: (1) its side effect is less; (2) said vaccine can be stored under the condition of frozen low-temp. and it can prolong effective period of vaccine; (3) its method is simple and convenient, its quality is easy to be controlled; and (4) it can induce body to produce higher humoral immunity response.
Owner:ZHEJIANG UNIV
Micromolecular water cluster composition and preparation method thereof
InactiveCN111789773AStrengthens the skin barrierImproves antimicrobial humoral responseAntibacterial agentsCosmetic preparationsAntimicrobial humoral responseImmunity response
The invention relates to the technical field of biological medicines, in particular to a micromolecular water cluster composition and a preparation method thereof. According to the invention, the micromolecular water cluster composition is prepared from micromolecular water and biological enzyme, and the selected specific biological enzyme can stabilize the micromolecular water for a long time. Meanwhile, the micromolecular water cluster composition prepared by the preparation method disclosed by the invention can be used for promoting keratinocyte end differentiation and up-regulation expression of transcriptional levels of intercellular connexin and keratinocyte envelope protein so as to achieve an effect of enhancing skin barrier, can remarkably improve anti-microbial humoral response,humoral immune response, antibacterial peptide-mediated anti-microbial humoral immune response, bacterial response, bacterial defense response and defense response related genes for other organisms, and has an immune regulation effect.
Owner:GUANGZHOU HENGGUANG COMPOSITE MATERIAL CO LTD
Ebola virus disease vaccine taking human replication deficient adenovirus as vector
ActiveUS10172932B2Induce robust cellular and humoral immune responseShort timeSsRNA viruses negative-senseViral antigen ingredientsProtective antigenD'Aguilar virus
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
A kind of composite adjuvant, the vaccine containing the composite adjuvant and the preparation method of the vaccine
ActiveCN106474468BRaw materials are easy to getSimple ingredientsSsRNA viruses positive-senseViral antigen ingredientsAdjuvantSide effect
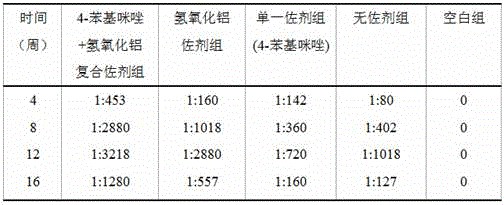
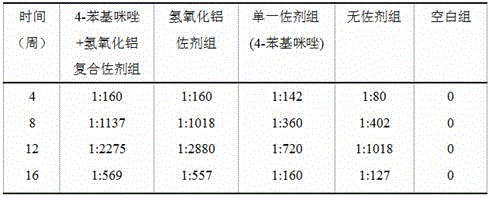
The invention relates to a composite adjuvant, a vaccine comprising the same and a preparation method of the vaccine. The composite adjuvant comprises a noncompetitive inhibitor of indoleamine-2,3 dioxygenase and aluminum salts, the noncompetitive inhibitor of the indoleamine-2,3 dioxygenase is 4-benzimidazole, the content of the 4-benzimidazole is 0.5-1.5 mg, the aluminum salts are aluminium hydroxide, and the content of the aluminium hydroxide is 300 mu g. The invention further relates to the vaccine comprising the composite adjuvant and a preparation method of vaccine. The raw materials of the composite adjuvant are easy to obtain, a preparation process of the vaccine is simple, the cost is low, the performance is stable, toxic and side effects are small, immune response is promoted jointly from the positive aspect and the negative aspect of organism immunoregulation, the composite adjuvant is safe and reliable when used in a range of immunizing dose, humoral immune response of antigenic specificity can be induced effectively, humoral immune responses of the antigenic specificity are enhanced remarkably, and the effect of the composite adjuvant is higher than the effects of a group without adjuvant, a group with a single adjuvant and a group with an aluminum adjuvant.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Ebola Virus Disease Vaccine Taking Human Replication Deficient Adenovirus As Vector
ActiveUS20180264100A1Short timeSimple preparation processSsRNA viruses negative-senseViral antigen ingredientsProtective antigenEbola virus
Provided are an Ebola virus envelope glycoprotein (that is GP protein) codon optimized nucleotide sequence, a human replication deficient recombinant adenovirus capable of expressing the nucleotide sequence, and applications in preparing a vaccine for preventing Ebola virus diseases. The nucleotide sequence takes a replication deficient 5 type adenovirus that is lack of E1 and E3 in a combined mode as a vector. HEK293 cells that integrate adenovirus E1 genes serve as a packaging cell line, and carried protective antigenic genes are codon optimized Zaire type Ebola virus Makona strain envelope glycoprotein genes. After the envelope glycoprotein genes are optimized by codon, the expression level in transfection cells is obviously improved.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Staphylococcus aureus mntc recombinant protein and its preparation method and application
ActiveCN103694323BHigh expressionEasy to separate and purifyAntibacterial agentsBacteriaStaphylococcusAnimal testing
The invention belongs to the field of biotechnology, and relates to a MntC recombinant protein of staphylococcus aureus (SA), a carrier comprising the recombinant protein, a host, a composition or a kit, application, preparation, fermentation and purification method of the protein. The MntC recombinant protein prepared by the method has strong immunogenicity, is safe and non-toxic, and is proved by animal tests to be able to effectively stimulate an organism to generate high efficient humoral immune response and good immune protection.
Owner:CHENGDU OLYMVAX BIOPHARM +1
A kind of respiratory syncytial virus vaccine and its preparation method and application
ActiveCN105983095BSuppress immune responseInhibition of pathological reactionsOrganic active ingredientsAntiviralsSide effectViral antibody
The invention discloses a respiratory syncytial virus vaccine, and a preparation method and an application thereof. The active components of the respiratory syncytial virus vaccine comprise a respiratory syncytial virus G protein and an immunosuppressant; the amino acid sequence of the respiratory syncytial virus G protein is represented by sequence 2 in a sequence table; and the immunosuppressant is cyclosporins A. Test proves that the respiratory syncytial virus vaccine can enhance the humoral immunoreaction in the virus neutralizing antibody level of immune animals, can inhibit too strong cellular immunity reactions, effectively and specifically inhibits inflammation related pathological reactions, and has the advantages of manure technology, low cost, no side effects and easy promotion.
Owner:ADVACCINE SUZHOU BIOPHARMACEUTICALS CO LTD +1
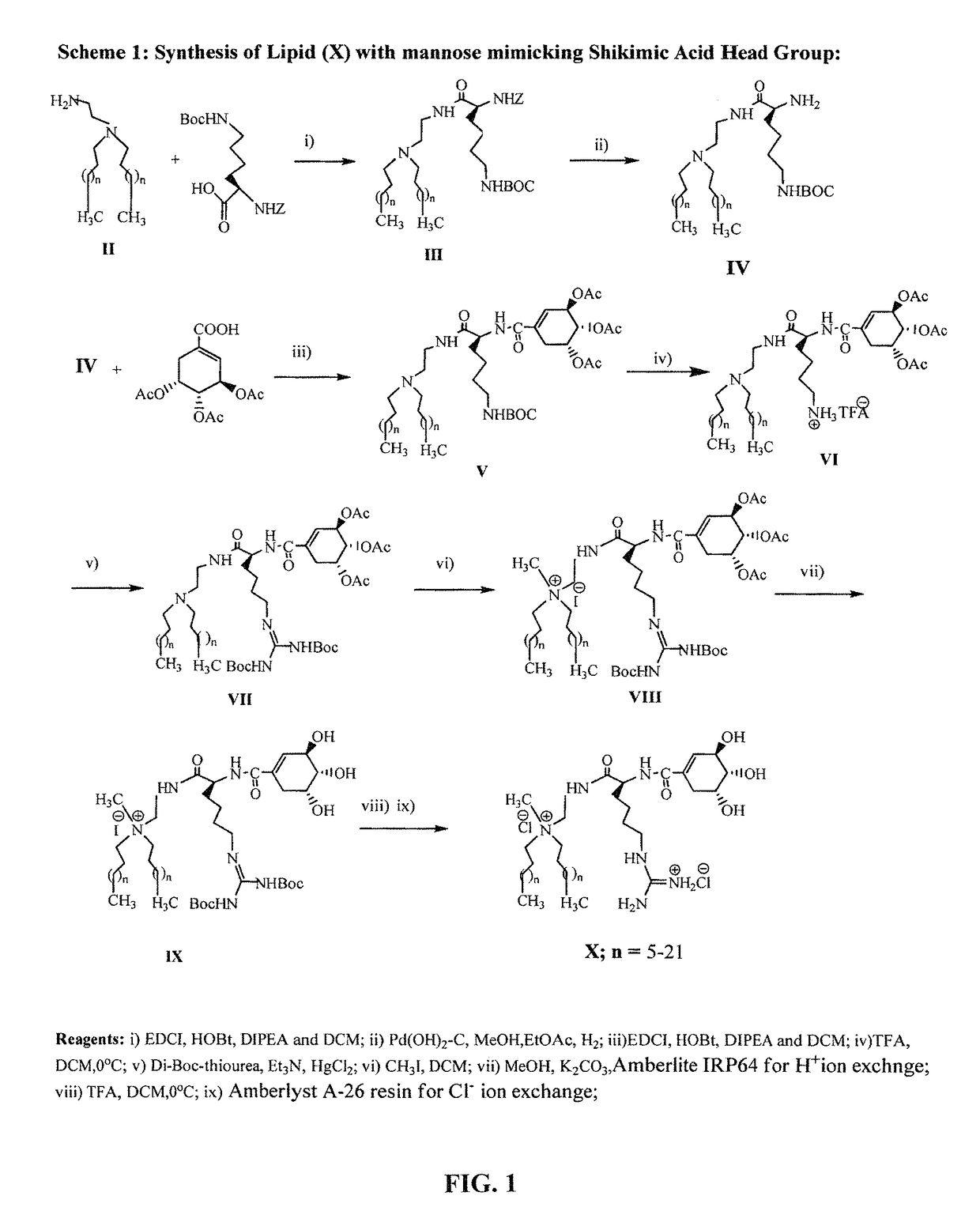
Mannose-receptor selective lysinylated cationic amphiphiles and a process for preparation thereof
ActiveUS9840530B2Efficient deliveryEnhance humoral immune responseEsterified saccharide compoundsOrganic active ingredientsDendritic cellTyrosinase
The present invention relates to the mannose-receptor selective lysinylated cationic amphiphile and a process for preparation thereof. The compounds of the present invention can target DNA vaccines to antigen presenting cells (APCs) such as macrophages and dendritic cells (DCs), via mannose receptors expressed on the cell surface of APCs. The cationic amphiphiles disclosed herein show enhanced cellular and humoral immune response compared to their mannosyl counterparts in genetic immunization in mice. The present invention discloses that immunization with electrostatic complexes (lipoplexes) of DNA vaccines encoding melanoma antigens (gp100 and tyrosinase) and liposome of the presently described novel lysinylated cationic amphiphiles with mannose-mimicking shikimoyl head-groups provides long-lasting (100 days post melanoma tumor challenge) protective immunity in all immunized mice. Cationic amphiphiles with mannose-mimicking shikimoyl head-groups described in the present invention are likely to find future applications in the field of genetic immunization.
Owner:COUNCIL OF SCI & IND RES
Purification method of candidate antigen pa5505 of Pseudomonas aeruginosa genetic engineering vaccine
ActiveCN109486736BHigh purityEasy diagnosisBacteriaMicroorganism based processesEscherichia coliEnzyme digestion
The invention belongs to the technical field of biopharmaceuticals, and discloses a method for purifying Pseudomonas aeruginosa genetic engineering vaccine candidate antigen PA5505, in which the DNA sequence encoding the active functional fragment of the PA5505 protein is cloned onto the pGEX‑6p‑2 carrier through genetic engineering technology , construct the Escherichia coli recombinant engineering strain pGEX‑6p‑2‑PA5505 / XL‑1 blue, and obtain the PA5505 protein by inducing expression. In the present invention, high-purity vaccine candidate antigen PA5505 is obtained by performing high-pressure bacteriostasis, GST affinity chromatography, PP enzyme digestion, SP HP chromatography, Q HP chromatography and other technologies on genetically engineered bacteria expressing PA5505. The purification process of the invention is simple, easy to scale up, and has good repeatability, and the obtained target protein has high purity. Animal experiments have proved that it can effectively stimulate the body to produce a higher humoral immune response and good immune protection.
Owner:重庆艾力彼生物科技有限公司
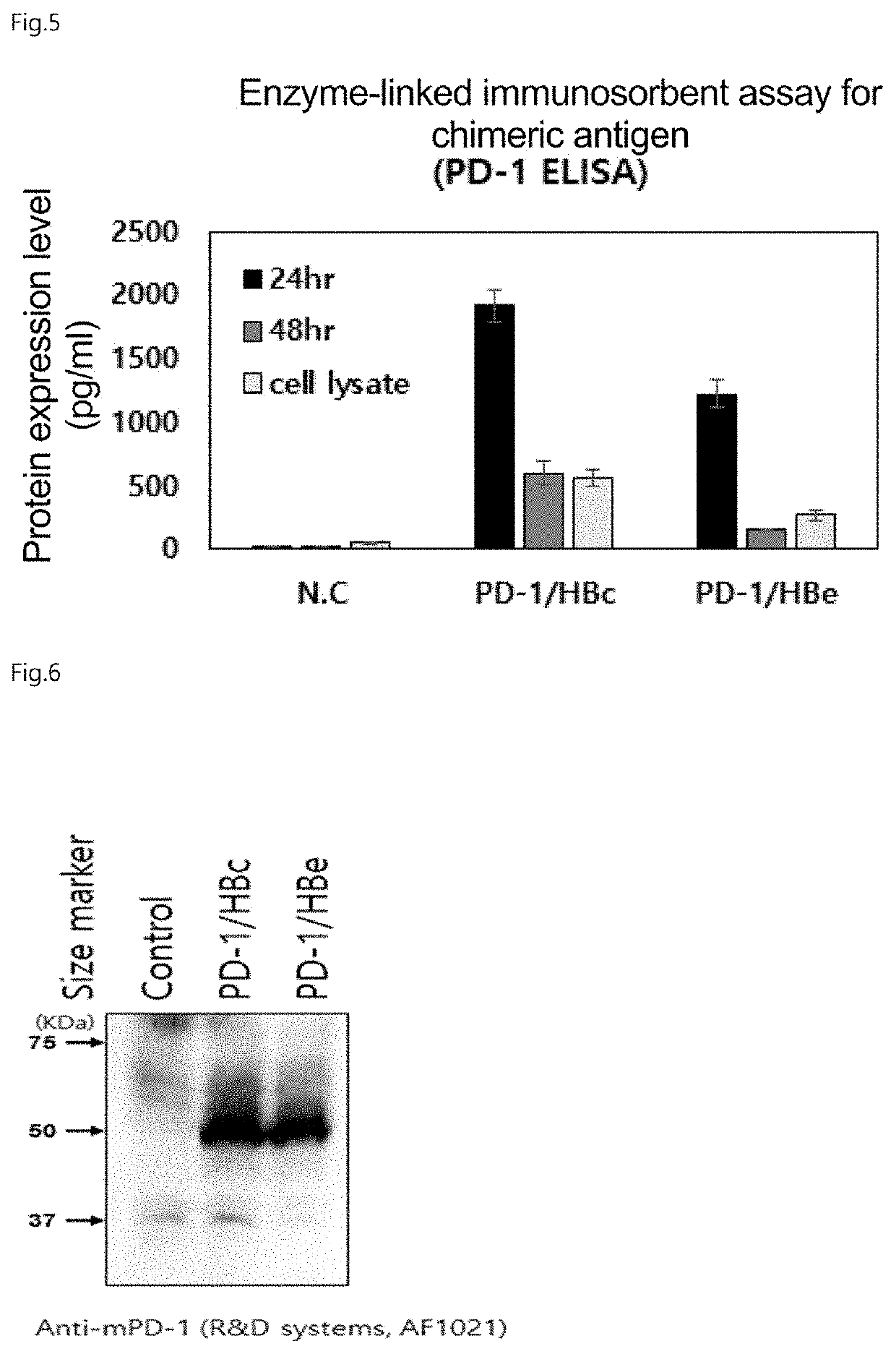
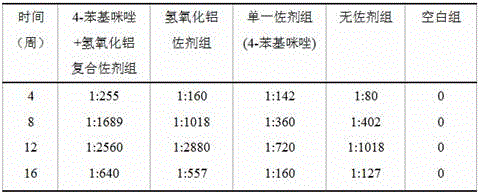
Chimeric antigen with enhanced multi-immune function through specific binding to target cell, and use thereof
PendingUS20210355168A1Enhances multiple immune functionEnhancing multiple immune functionCell receptors/surface-antigens/surface-determinantsViral antigen ingredientsImmunopotencyMedicine
The present invention relates to a chimeric antigen, which binds specifically to target cells and enhances multiple immune functions, and the use thereof. Specifically, the present invention relates to: a chimeric antigen for inducing multiple immune functions wherein an immune response-inducing domain and a domain for inducing target cell-specific binding are fused to each other; a pharmaceutical composition for preventing or treating cancer, containing, as an active ingredient, the chimeric antigen for enhancing multiple immune functions; a pharmaceutical composition for preventing or treating infectious disease; a composition for enhancing immunity; and a vaccine composition.
Owner:RNAGENE INC
Composite adjuvant, vaccine comprising the same and preparation method of vaccine
ActiveCN106474468ARaw materials are easy to getSimple ingredientsSsRNA viruses positive-senseViral antigen ingredientsSide effectAdjuvant
The invention relates to a composite adjuvant, a vaccine comprising the same and a preparation method of the vaccine. The composite adjuvant comprises a noncompetitive inhibitor of indoleamine-2,3 dioxygenase and aluminum salts, the noncompetitive inhibitor of the indoleamine-2,3 dioxygenase is 4-benzimidazole, the content of the 4-benzimidazole is 0.5-1.5 mg, the aluminum salts are aluminium hydroxide, and the content of the aluminium hydroxide is 300 mu g. The invention further relates to the vaccine comprising the composite adjuvant and a preparation method of vaccine. The raw materials of the composite adjuvant are easy to obtain, a preparation process of the vaccine is simple, the cost is low, the performance is stable, toxic and side effects are small, immune response is promoted jointly from the positive aspect and the negative aspect of organism immunoregulation, the composite adjuvant is safe and reliable when used in a range of immunizing dose, humoral immune response of antigenic specificity can be induced effectively, humoral immune responses of the antigenic specificity are enhanced remarkably, and the effect of the composite adjuvant is higher than the effects of a group without adjuvant, a group with a single adjuvant and a group with an aluminum adjuvant.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Preparation method and application of chitosan-Pickering emulsion interleukin 12 adjuvant system
ActiveCN113940994AHigh immune inductionEnhance humoral immune responseAntibacterial agentsChlamydiaceae ingredientsAdjuvantWhite blood cell
The invention relates to the field of immunology, in particular to a preparation method and application of a chitosan-Pickering emulsion interleukin 12 adjuvant system. Mixing chitosan with water to obtain a water phase; and mixing the water phase with oil phase liquid paraffin, and carrying out ultrasonic treatment to obtain the chitosan-Pickering emulsion. Combining a chitosan-Pickering emulsion with IL-12 to form an adjuvant, and then forming a vaccine with the adjuvant and pORF5. The invention provides a vaccine preparation prepared from a chitosan-Pickering emulsion / IL-12 adjuvant system and chlamydia trachomatis recombinant protein. Experiments prove that the chitosan-Pickering emulsion / IL-12 adjuvant system enhances the humoral immunity and cellular immunity level of an antigen better than those of an antigen without an adjuvant or containing a single adjuvant.
Owner:NANHUA UNIV
Newcastle disease live vaccine adjuvant and preparation method thereof
PendingCN111494621AEnhance humoral immune responseThe preparation process is stableSsRNA viruses negative-senseViral antigen ingredientsCelluloseAntiendomysial antibodies
The invention provides a newcastle disease live vaccine adjuvant. The newcastle disease live vaccine adjuvant comprises the following raw materials in percentage by weight: 60-75% of a buffer solution, 5%-10% of squalane, 5%-10% of squalene, 3%-5% of pluronic, 1%-3% of an emulsifier, 2%-4% of hydroxymethyl cellulose, 2%-4% of dimethyl dioctadecyl ammonium bromide and 1%-3% of polycaprolactone. Theadjuvant composition can remarkably improve humoral immune response and cellular immune response of the newcastle disease vaccine, not only can generate a relatively high antibody level in a short time, but also can stimulate an organism to generate efficient protective force for a long time, when the adjuvant composition is used for preparing vaccines, large emulsification equipment is not needed, the vaccine preparation process is stable, and the side reaction after vaccine immunization is small.
Owner:JIANGSU ACAD OF AGRI SCI
Immunoadjuvant Compositions and uses Thereof
InactiveUS20160193328A1Accelerate and prolong and enhance qualityEnhance immune responseAntibody medical ingredientsOil/fats/waxes non-active ingredientsAdjuvantAgonist
The present invention relates to an immunoadjuvant composition comprising at least adjuvant and at least one MyD88-dependent pathway agonist.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
Altered avian virus for in-ovo inoculation and methods of use thereof
ActiveUS10196616B2Reduce productionImprove survival rateSsRNA viruses negative-senseViral antigen ingredientsHeterologousHeterologous Antigens
An altered avian NDV that contains the coding sequence of avian interleukin-4 (IL-4), or a portion thereof, in the reverse orientation suppresses in-ovo IL-4 production via RNAi when administered to embryonic birds. An immunogenic composition containing this altered NDV is included in this invention. The altered avian NDV can, optionally contain a polynucleotide encoding a heterologous antigen from a heterologous avian pathogen and can produce said heterologous antigen in-ovo.
Owner:US SEC AGRI +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com