Carbohydrate specific cellular immunity inducing microorganisms and fractions thereof
A technology of carbohydrates and cellular immunity, applied in the direction of microorganisms, microorganism-based methods, biological tests, etc., can solve the problems of non-CH-specificity, ineffective immune response, lack of specificity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0789] Example 1 Anaerobic culture technology and culture medium
[0790] 568 The anaerobic technology for bacterial culture is based on the previously described method summarized by Breznak and Costilow. Disperse the medium prepared with cysteine hydrochloride (cysteine·HCl) as a reducing agent in an anaerobic culture tube (Ochs, Bovenden, Germany) or a glass serum bottle, leaving about half to one third of the total container volume as The gas headspace is sealed with a butyl rubber plug. The solution prepared without using a reducing agent (such as PBS-a) is boiled before dispersion. Before autoclaving, use N 2 / CO 2 (80 / 20, v / v) instead of gas phase. In order to achieve this, a needle was pierced through a butyl rubber stoppered bottle, and the bottle was evacuated by means of a vacuum pump (Vacuubrand, Wertheim, Germany). After evacuating, use N to shake the bottle repeatedly throughout the process 2 / CO 2 (80 / 20, v / v) inflation. The evacuation and inflation steps are perfo...
Embodiment 2
[0793] Example 2 Affinity enrichment of core-1 positive microorganisms
[0794] 571 2.1 TF1 and TF2 coated Preparation
[0795] The respective volume of 100μl (M-450 Rat Anti-Mouse IgM, Dynal Biotech ASA, Oslo, Norway) placed in a 2ml Safe-Lock Eppendorf tube (Eppendorf, Hamburg, Germany), using Dyna Magnetic Particle -S( -S, Dynal Biotech, Oslo, Norway), with 2ml of phosphate buffered saline a (PBS-a: 8.1g l -1 NaCl, 0.16g l -1 NaH 2 PO 4 ·H 2 O, 0.98g l -1 Na 2 HPO 4 ·2H 2 O, 1g l -1 BSA, pH 7.4) washed twice, and suspended in 25μl PBS-a. The lyophilized TF1 or TF2 cell culture supernatant was dissolved in 1 ml milli-Q synthetic grade water (Millipore, Billerica, MA, USA). Add the dissolved TF1 or TF2 cell culture supernatant (1ml) to the And incubate on a test tube rotator (model 34528, Snijders Scientific, Netherlands) at 4C for 30 min. Tube placed -S, and let it stand for 3 minutes before removing the liquid with a pipette. Make Resuspend in 2ml PBS-a and place -S, an...
Embodiment 4
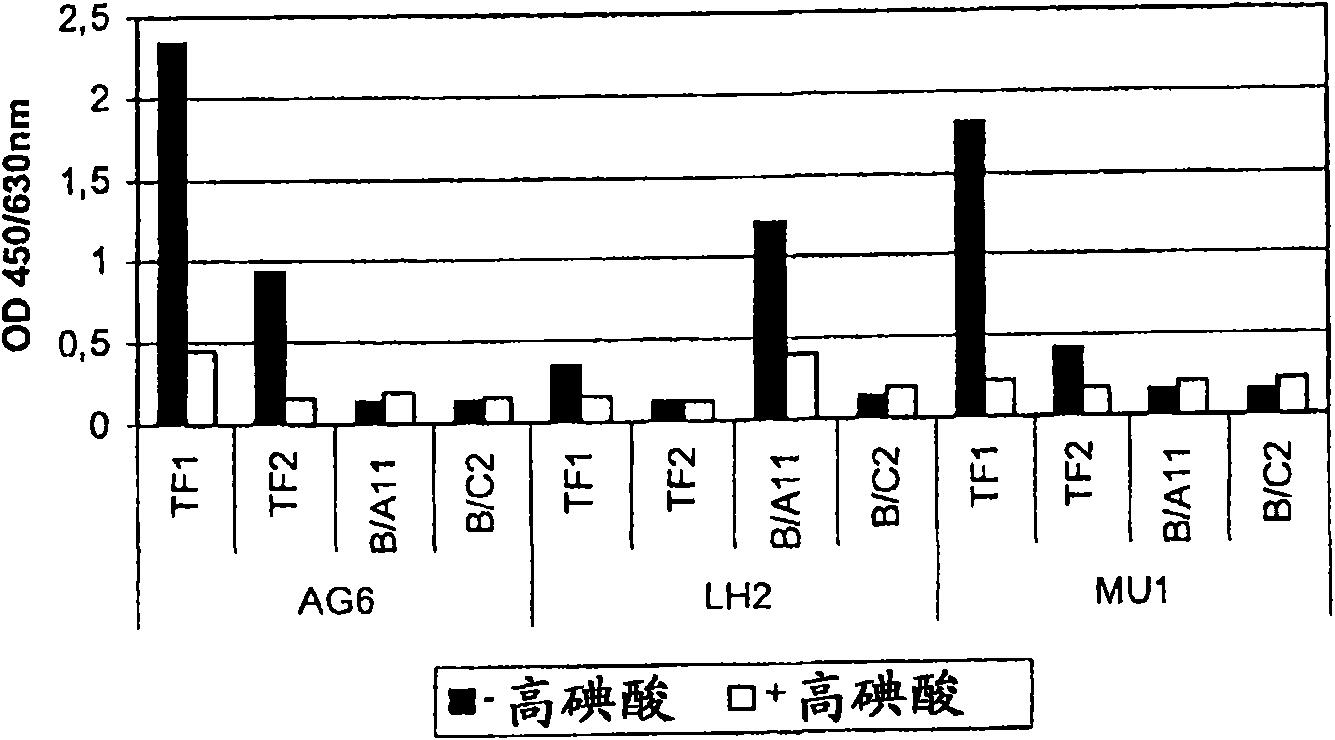
[0825] Example 4 Growth and immobilization of bacteria based on ELISA screening
[0826]590 well-separated clones were randomly selected from selective and non-selective agar plates, and re-streaked three times on non-selective medium. Pick a single clone and depending on which one gives the best growth, inoculate it into ST (agar omitted), WC or MRS broth, and grow overnight at 37°C. These cultures were inoculated (1%) into 300 ml of fresh ST, WC or MRS broth and grown overnight at 37°C. The cells were pelleted (8000×g, 15min, 4C) and resuspended in 10ml PBS-c (8g l -1 NaCl, 0.2g l -1 KCl, 1.44g l -1 Na 2 HPO 4 , 0.24g l -1 KH 2 PO 4 )(12). The suspension was fixed at 4C for 3 to 4 hours by adding 30 ml of 4% paraformaldehyde (PFA) solution in PBS-c (prepared according to (8)). Next, the sample was washed with 40ml PBS-c (8000×g, 15min, 4C), and the pellet was suspended in 15ml PBS-c, and then an equal volume of 96% ice-cold ethanol was added. The samples are stored at -20C until...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com