A kind of GFP-2 small peptide and its preparation method and application
A GFP-2, culture medium technology, applied in the preparation method of peptides, biochemical equipment and methods, chemical instruments and methods, etc., can solve the problems of inability to achieve immune body efficacy, low immunogenicity, etc., and improve immune efficacy. , Improve antibody titer, strengthen the effect of specific immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] First of all, the present invention provides a preparation method of GFP-2 small peptide, the preparation method comprising the following steps:
[0026] (1) Prepare brown sugar medium and sterilize it;
[0027] (2) Inoculate GFP-2 bacteria, glycerol bacteria and LB medium, and culture on a shaking table;
[0028] (3) Transfer to brown sugar culture medium, carry out shaking table culture, then carry out centrifugation, collect supernatant, and discard bacteria;
[0029] (4) Salt out to precipitate proteins and small peptides, centrifuge again after salting out, remove supernatant, and collect crude protein;
[0030] (5) Resuspend the crude protein with tris-HCl, pass the resuspended crude protein through the membrane, and dialyze with tris-HCl;
[0031] (6) Perform ultrafiltration with an ultrafiltration tube to collect small peptides with a molecular weight of less than 10KD, cool the obtained small peptides, and freeze-dry to obtain GFP-2 small peptides.
[0032] ...
Embodiment 1
[0045] Preparation of GFP-2 Small Peptide
[0046] (1) Prepare 1 L of brown sugar medium and sterilize at 110°C for 15 minutes;
[0047] (2) Inoculate GFP-2 bacteria, 5mL LB medium per tube, and inoculate glycerol bacteria and medium at a ratio of 1:100. A total of 2 tubes of GFP-2 bacterial solution were connected and cultured on a shaking table for 12 hours;
[0048] (3) Transfer to 1L brown sugar medium, culture on a shaker for 12 hours, centrifuge at 4000 rpm for 1 hour, collect the supernatant, and discard the bacteria;
[0049] (4) Prepare saturated ammonium sulfate. Salt out the supernatant and saturated ammonium sulfate at a ratio of 4:1 to precipitate proteins and small peptides. After salting out, centrifuge again at 12,000 rpm for 20 minutes, remove the supernatant, and collect crude protein.
[0050] (5) Resuspend the crude protein with tris-HCL, pass the resuspended crude protein through a 0.45mm filter membrane, dialyze with tris-HCL for 5 hours, and change the...
Embodiment 2
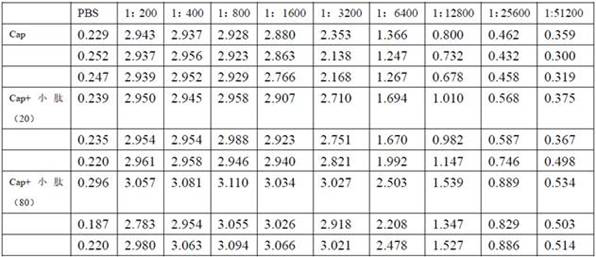
[0053] Study of GFP-2 Small Peptide on Improving Antibody Titer
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com