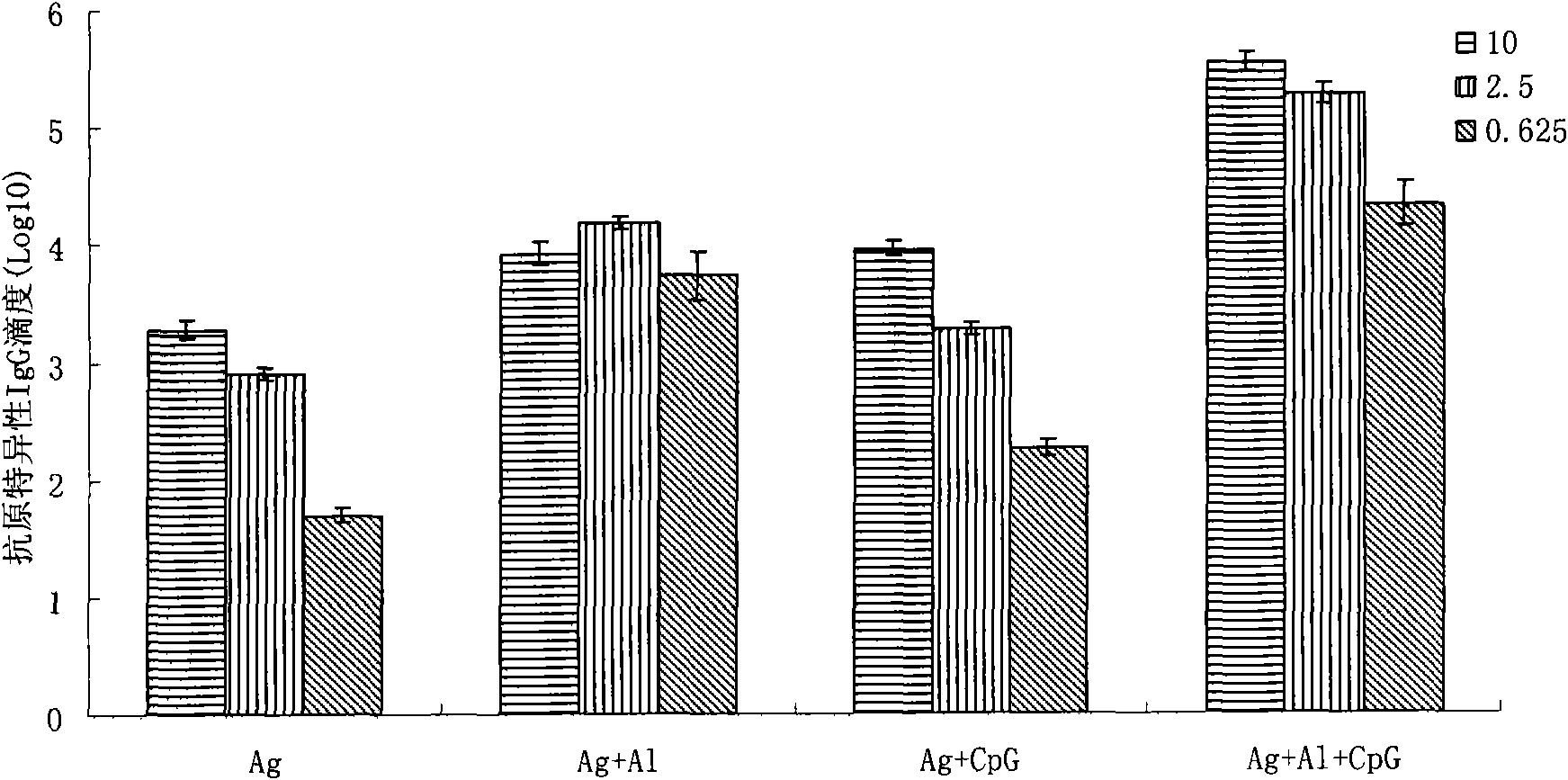
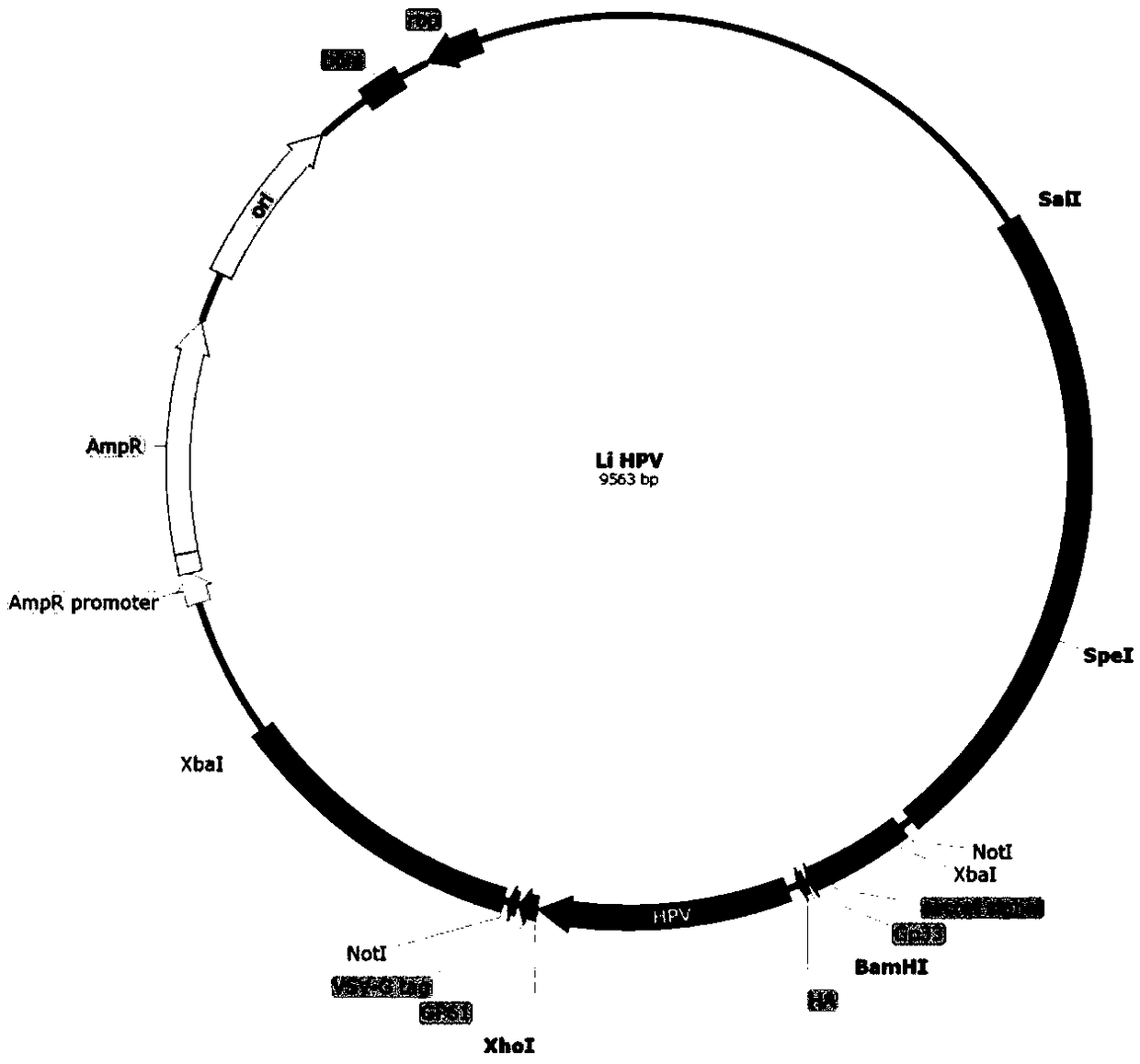
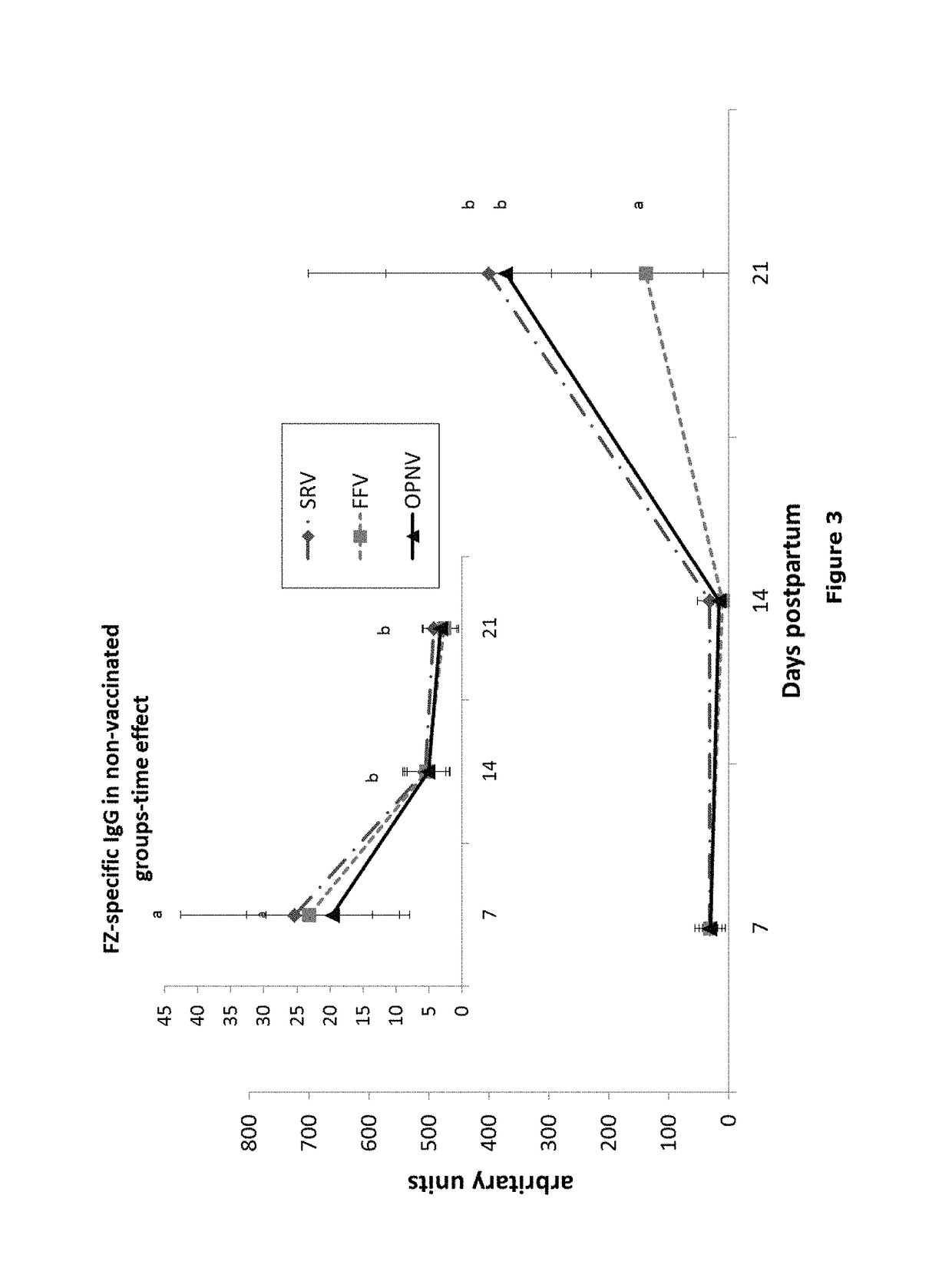
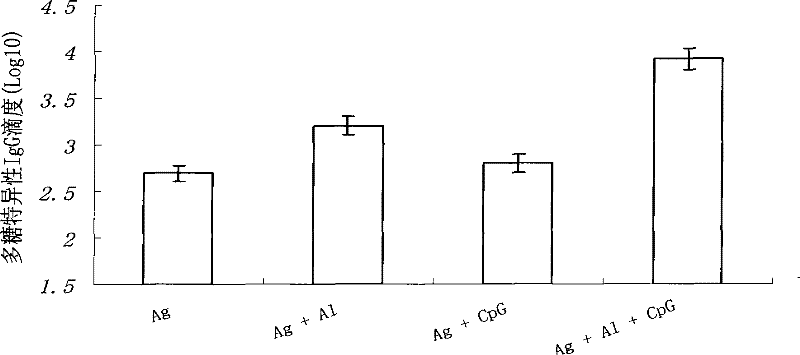
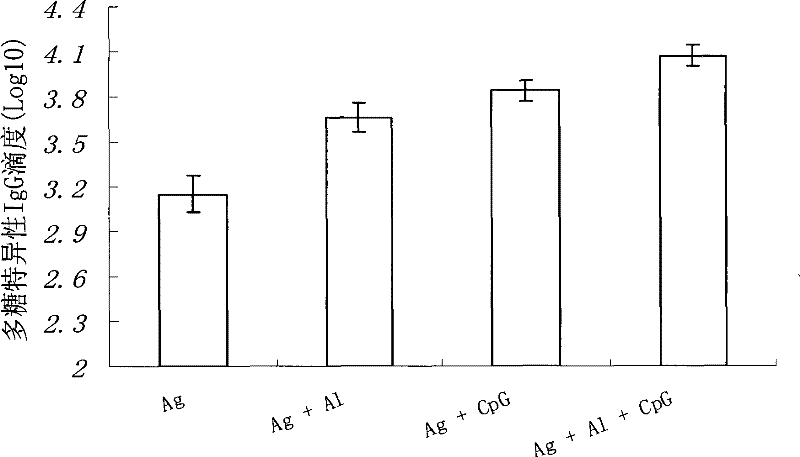
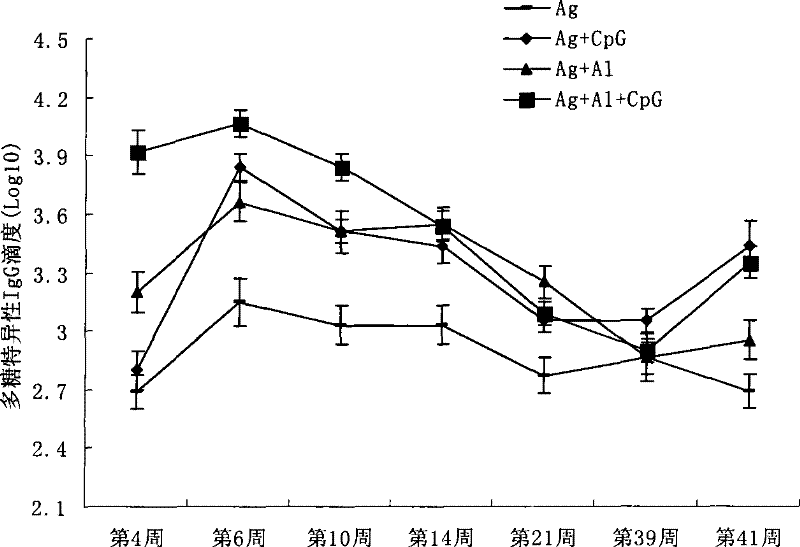
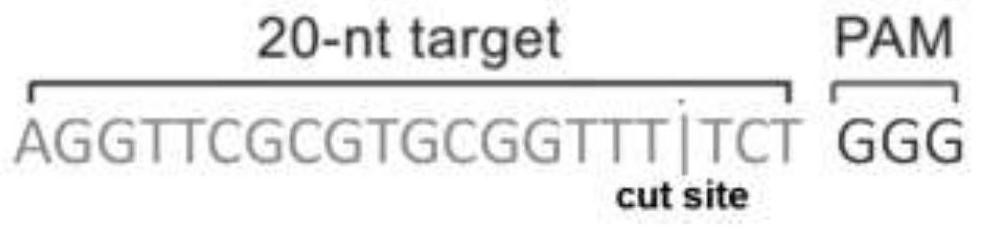
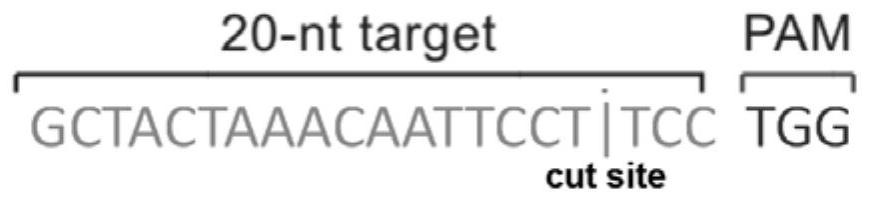
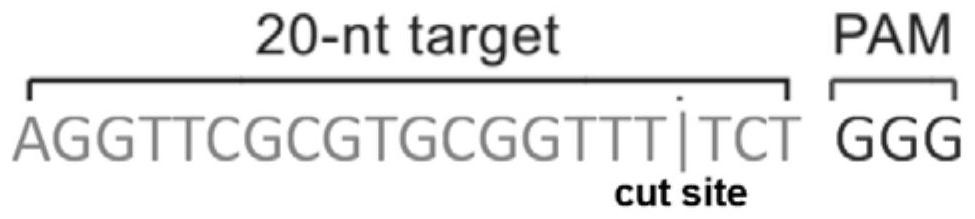
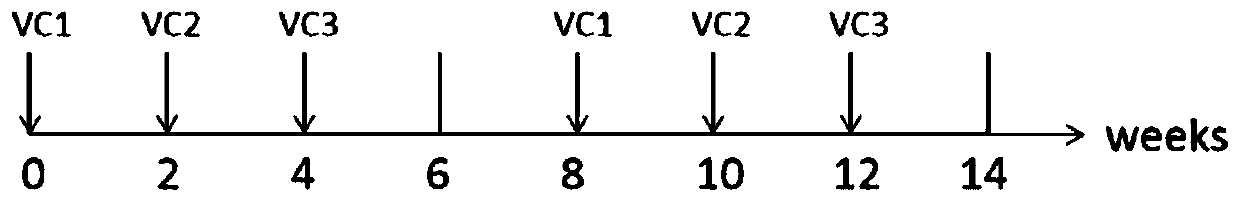
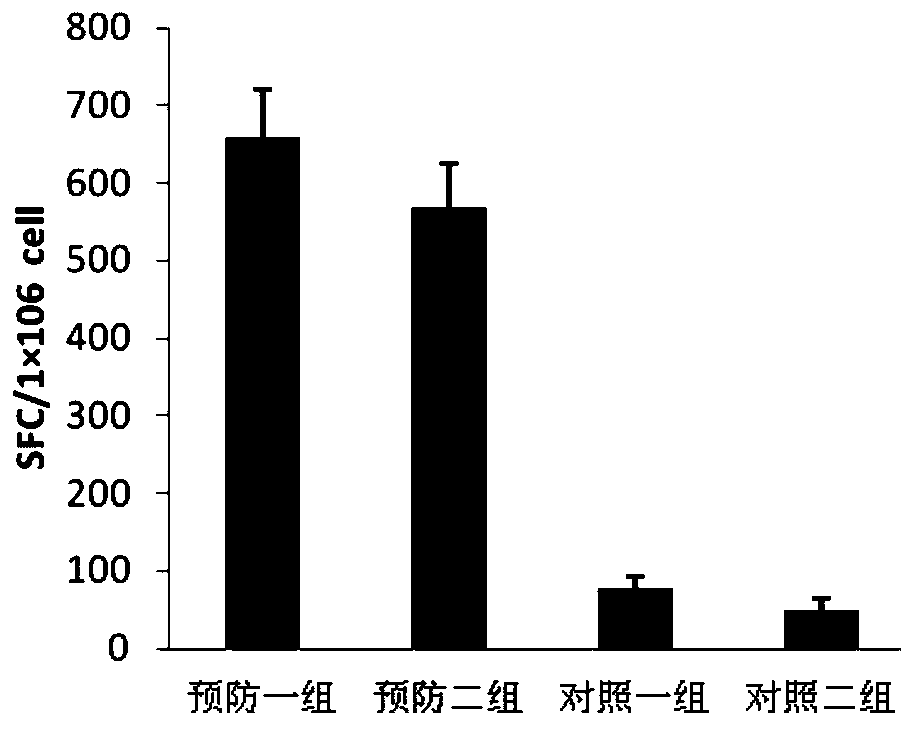
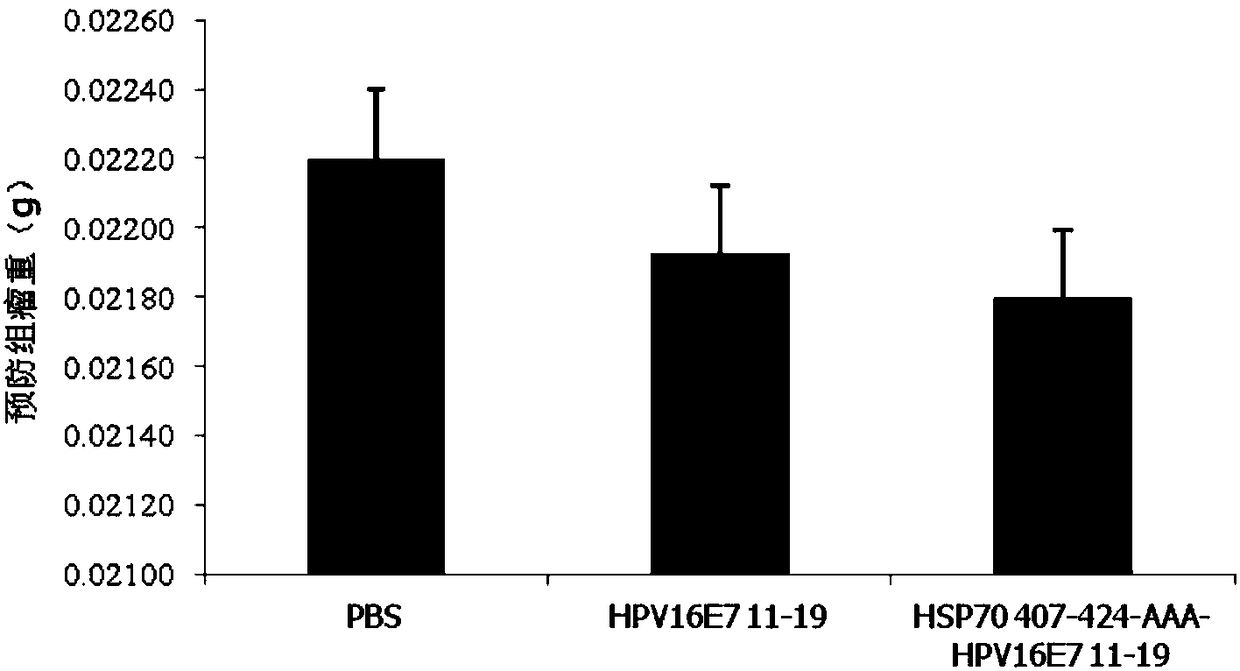
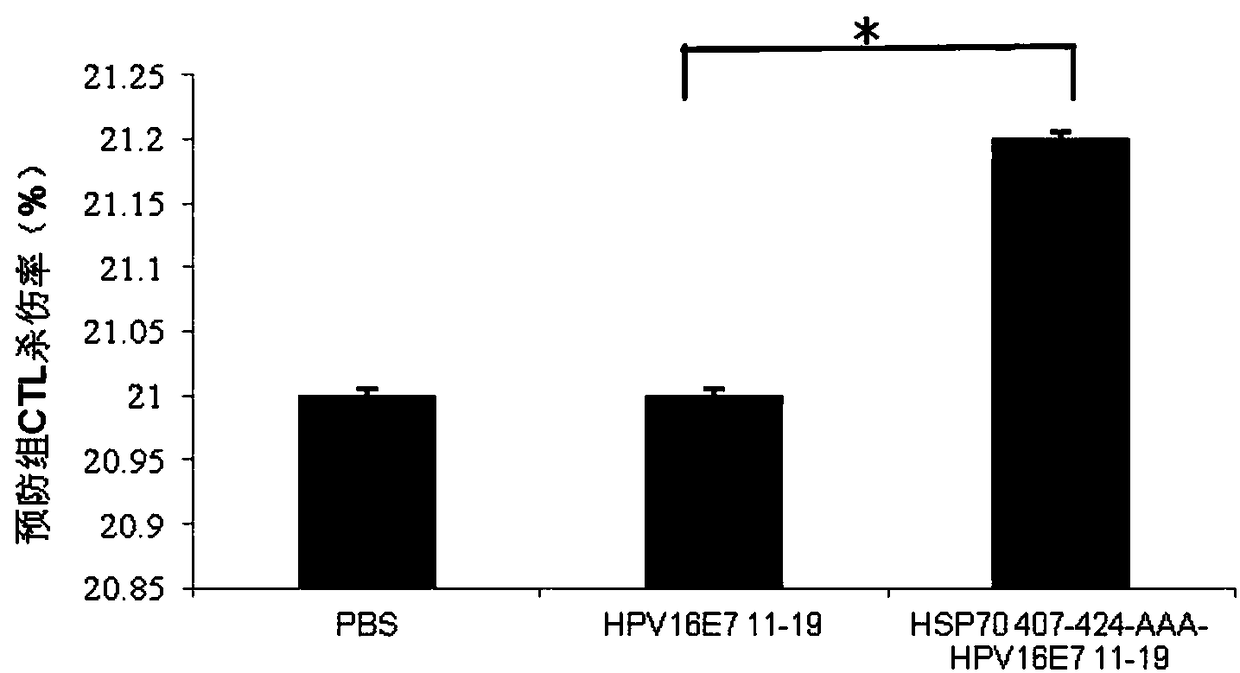
Patents
Literature
49results about How to "Enhance specific immune response" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Drug and tumor whole-cell vaccine for treating or preventing tumor, and preparation methods and applications of drug and whole-cell vaccine
InactiveCN102343086AImprove immunityAmplify specific immune responseAntibody medical ingredientsAntineoplastic agentsAntineoplastic ImmunotherapeuticWhite blood cell
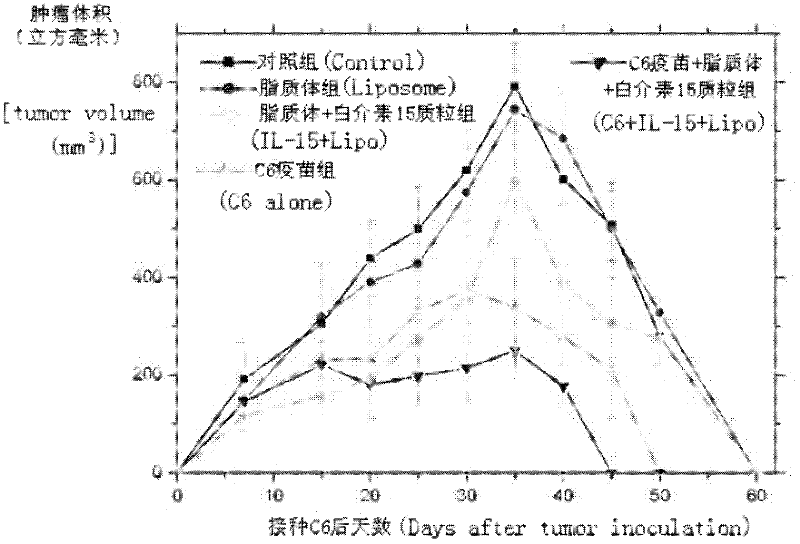
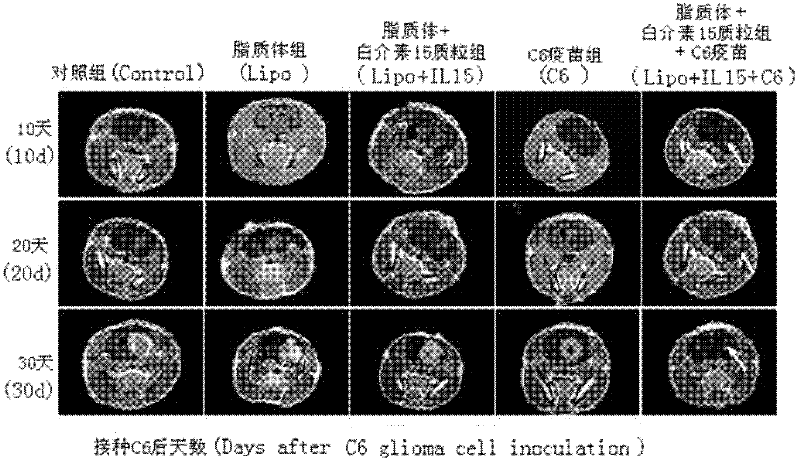
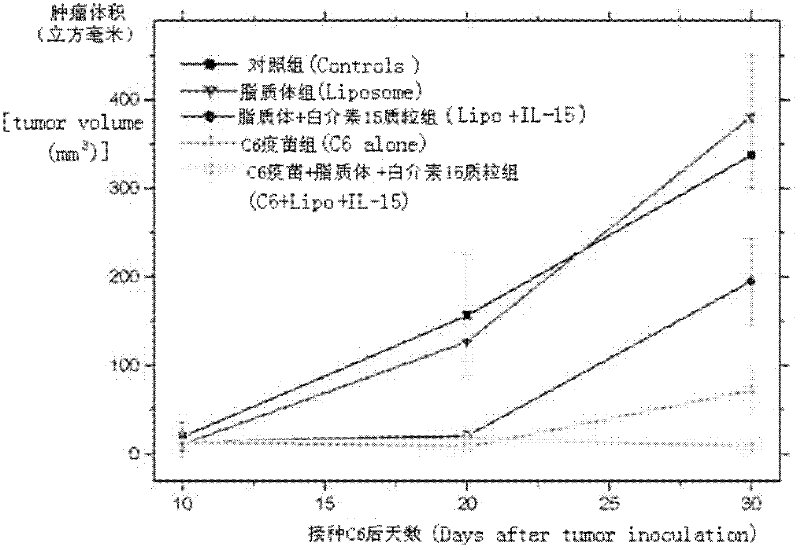
The invention relates to a drug and a tumor whole-cell vaccine for treating or preventing tumor, and preparation methods and applications of the drug and the whole-cell vaccine, and belongs to the fields of bioengineering and biological immunology. The invention provides a drug with good effect for treating or preventing tumor and a tumor whole-cell vaccine. The drug comprises the tumor whole-cell vaccine, a cation liposome, and recombinant plasmid for encoding interleukin-15 gene. The drug has the dual actions of tumor prevention immunization and anti-tumor immune treatment, and has durable action and obvious tumor prevention or treatment effect.
Owner:SICHUAN UNIV
Oral vaccine delivery system and application thereof
InactiveCN106215181AGood biocompatibilityNo side effectsAntibacterial agentsBacterial antigen ingredientsDiseaseAdjuvant
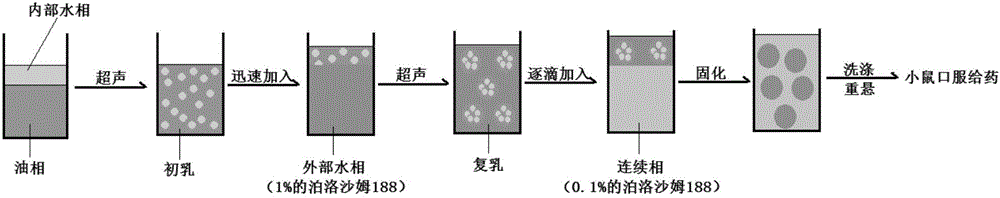
The invention discloses an oral vaccine delivery system. W1 of W1 / O / W2 type nanoparticles is a vaccine medicine phase, an organic phase O is selected from PLGA and HP55, an external water phase W2 and a continuous water phase are selected from poloxamer 188, and a vaccine medicine is a double epitope-double adjuvant helicobacter pylori vaccine. The method adopts a polymer material having good biological safety and good compatibility to prepare the nanoparticles, a preparation process and a formula are optimized, the oral vaccine delivery system is non-toxic, free of any side effects, easy to manufacture and low in cost. In the field of biological medicines, the nanoparticles can be used for oral taking of a helicobacter pylori subunit vaccine, accordingly helicobacter pylori infection related diseases are prevented and treated, and huge economic benefits and social benefits are brought.
Owner:CHINA PHARM UNIV
Carbohydrate specific cellular immunity inducing microorganisms and fractions thereof
InactiveCN101600454AEnhance specific immune responseEffective immune responseBacterial antigen ingredientsBacteriaMolecular biologyAbnormal tissue growth
The present invention relates to the field of prevention and treatment of disorders associated with the occurrence of certain carbohydrate epitopes. More particularly, the present invention relates to the prevention and treatment of carbohydrate epitope positive tumors. It relates to formulations and methods for the induction of an effective carbohydrate specific cellular immune response.
Owner:GLYCOTOPE GMBH
Recombinant pasteurella multocida toxin protein and application thereof
ActiveCN104059134AImprove immunityImprove efficiencyAntibacterial agentsAntibody mimetics/scaffoldsPasteurella multocida toxinEpitope
The present invention relates to a Pasteurella multocida toxin recombinant protein and use thereof, the recombinant protein comprising at least one epitope of Pasteurella multocida toxin recombinant protein, and when the number of the epitopes is a plurality, the epitopes can be connected by a connector therebetween. The recombinant protein can further comprise the amino acid sequence of at least one unit of a complement cleavage C3d, and the epitopes and the amino acid sequence of the complement cleavage C3d can be connected by a connector. The present invention also relates to a nucleotide sequence encoding the above-mentioned Pasteurella multocida toxin recombinant protein, and a porcine atrophic rhinitis immunogenic composition comprising the above-mentioned Pasteurella multocida toxin recombinant protein.
Owner:维克香港贸易有限公司
Feed additive containing yeast cell polysaccharides
InactiveCN104621361AIncrease profitImprove immunityAnimal feeding stuffBiotechnologyBacillus licheniformis
The invention provides a feed additive containing yeast cell polysaccharides. The feed additive is prepared from 90-95 percent of yeast cell polysaccharides, 2-6 percent of saccharomycetes, 1-3 percent of bacillus subtilis and 1-3 percent of bacillus licheniformis. The saccharomycetes, bacillus subtilis, bacillus licheniformis and yeast cell polysaccharides are compounded and matched with one another, so that the intestinal health level of piglets is improved, the body immunity is enhanced, and the antibody valence of piglet inject vaccines is improved.
Owner:广东雅琪生物科技股份有限公司
Meningococcus vaccine
InactiveCN101559225AImprove immunityLow costAntibacterial agentsCarrier-bound antigen/hapten ingredientsSpecific immunityCarrier protein
The invention relates to a meningococcus vaccine comprising a chemical conjugate of meningococcus capsular polysaccharide and carrier protein, and a first adjuvant, wherein the first adjuvant is oligodeoxynucleotide comprising at least one non-methylation CpG dinucleotide, and the length of the oligodeoxynucleotide is 15-35 ribonucleotides. The adjuvant used for increasing the immunization effect of the meningococcus vaccine is a novel human vaccine adjuvant with favorable potential application prospect, and the chemical essence of the adjuvant is the oligodeoxynucleotide which is synthesized manually and contains the CpG dinucleotide. By a receptor TLR9 activated immune system on immune cells, the adjuvant reinforces specific immune response aiming at vaccine antigens. The immunization effect of a vaccine can be increased by adding the vaccine adjuvant into the vaccine, so that the dosage of the vaccine antigen can be reduced, the immunization injection times can be reduced and the vaccine cost can be reduced.
Owner:NAT VACCINE & SERUM INST
Controlled live tumor cell vaccine and preparation method and application thereof
InactiveCN101559220ASubmit fullyHigh potencyAntibody medical ingredientsFermentationAbnormal tissue growthSpecific immunity
The invention relates to a controlled live tumor cell vaccine and a preparation method and application thereof. The preparation method is characterized in that a suicide gene thymidine kinase TK and an immunoregulatory gene are allowed to transfect tumor cells by slow virus infection and liposome mediation to perform transgene, cells stably expressing the target gene are screened for cloning culture and expansion to obtain a double-gene modified tumor cell vaccine. The controlled live tumor cell vaccine can help activate lymphopoiesis of an immune system, generate a specific CTL killing efficacy and / or a non-specific NK killing efficacy and generate significantly enhanced anti-tumor immune response in a host. The controlled live tumor cell vaccine has higher titer, more complete tumor antigen presentation, and increased and stable expression of exogenous immunoregulatory genes, and better transgene efficacy, thus enhancing specific immune reaction induced by the tumor cell vaccine and increasing efficacy of the tumor cell vaccine.
Owner:GUANGZHOU GENERAL HOSPITAL OF GUANGZHOU MILITARY COMMAND
Poliomyelitis vaccine
InactiveCN101559224AImprove immunityLow costViral antigen ingredientsAntiviralsSpecific immunityRibonucleotide synthesis
The invention relates to a poliomyelitis vaccine comprising an inactivated poliomyelitis virus used as an antigen, and a first adjuvant, wherein the first adjuvant is oligodeoxynucleotide comprising at least one non-methylation CpG dinucleotide, and the length of the oligodeoxynucleotide is 15-35 ribonucleotides. The adjuvant used for increasing the immunization effect of the inactivated poliomyelitis vaccine is a novel human vaccine adjuvant with favorable potential application prospect, and the chemical essence of the adjuvant is the oligodeoxynucleotide which is synthesized manually and contains the CpG dinucleotide. By a receptor TLR9 activated immune system on immune cells, the adjuvant reinforces specific immune response aiming at vaccine antigens. The immunization effect of a vaccine can be increased by adding the vaccine adjuvant into the vaccine, so that the dosage of the vaccine antigen can be reduced and the vaccine cost can be reduced finally.
Owner:NAT VACCINE & SERUM INST
Cervical cancer therapeutic vaccine based on recombinant attenuating sheep listeria ivanovii
ActiveCN108939064ABreak immune toleranceMake up for the disadvantage of lower immunogenicityBacteriaViral antigen ingredientsAbnormal tissue growthListeria floridensis
The invention relates to a cervical cancer therapeutic vaccine based on recombinant attenuating sheep listeria ivanovii. By taking the attenuating sheep listeria ivanovii as a vector, specific immuneresponse in a tumor microenvironment is improved effectively and immune tolerance of a body is broken by means of a unique characteristic that the listeria ivanovii can grow in a main phagocyte and isa natural T cell immune activation adjuvant, so that continuous virus infection of the body is eliminated and the good therapeutic effect of eliminating the focus and inhibiting tumor development isachieved. The vaccine has the advantages that the defect that nucleic acid and protein polypeptide vaccines are relatively low in immunogenicity is made up, and the attenuating sheep listeria ivanoviialso has high safety while retaining the characteristic that an original strain can grow in cells to achieve antigen presentation.
Owner:南京颂悦生物科技有限公司
Pollen product capable of adjusting human body immunity
InactiveCN103933146AAnti-atherosclerosisWith lipid-lowering effectUnknown materialsImmunological disordersZiziphus jujubaImmune Regulators
The invention relates to a pollen product capable of adjusting human body immunity, and the pollen product is prepared by using of traditional immune regulator-wall-broken pine pollen as a main raw material and adding of blood-nourishing and enriching donkey hide gelatin, jujube, oligo isomaltose, crystalline fructose, lemon tree and the like. The pollen product formulation is rich in polysaccharides and physiological active substances, can obviously promote human immune organ growth, improves the nonspecific cellular immune function and specific humoral immune ability of human body so as to play the role of regulating and improving the human body immunity, eliminating body fatigue and comprehensive aging resistance.
Owner:CHENGGU QIANCAITANG BEE IND
Medicinal and edible Chinese herbal medicine fermented beverage for improving female immunity, and preparation method
PendingCN112515069AIncrease contentFunction increaseUnknown materialsFungi medical ingredientsPolygonum odoratumAngelica Sinensis Root
The invention discloses a medicinal and edible Chinese herbal medicine fermented beverage for improving the female immunity, and a preparation method. The medicinal and edible Chinese herbal medicinefermented beverage comprises the following Chinese herbal medicine components in parts by weight: 10-30 parts of Chinese dates, 1-10 parts of spina date seeds, 1-10 parts of raspberries, 10-30 parts of mulberries, 1-10 parts of Chinese wolfberry, 2-20 parts of longans, 1-10 parts of polygonatum odoratum, 0-2 parts of polygala tenuifolia, 0-5 parts of pleurotus citrinopileatus, 0-2 parts of Angelica sinensis, 10-20 parts of acalypha australis and 0-10 parts of honey. The medicinal and edible Chinese herbal medicine fermented beverage is prepared by deep fermentation of probiotics, and has common food properties. The medicinal and edible Chinese herbal medicine fermented beverage has the effects of nourishing yin and blood, tranquilizing mind by nourishing the heart, nourishing the heart andspleen, strengthening the brain and benefiting intelligence, tonifying qi and reducing weight, removing dampness and food retention, enhancing immunity, inhibiting bacteria, regulating intestinal health, beautifying the features and hairdressing and the like, is particularly suitable for women to drink, has a wide consumer market, and has relatively good economic and social benefits.
Owner:BAISE UNIV
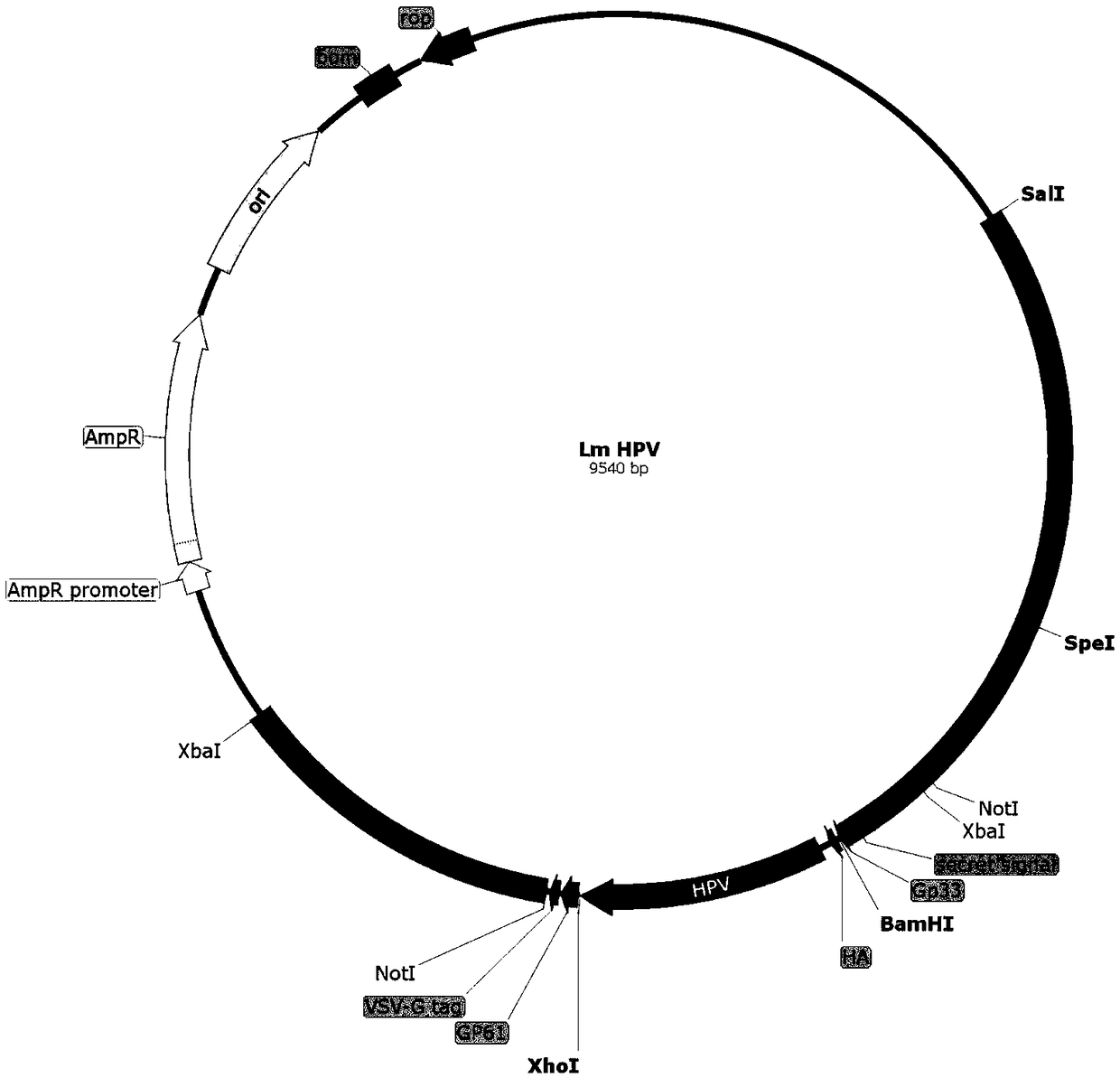
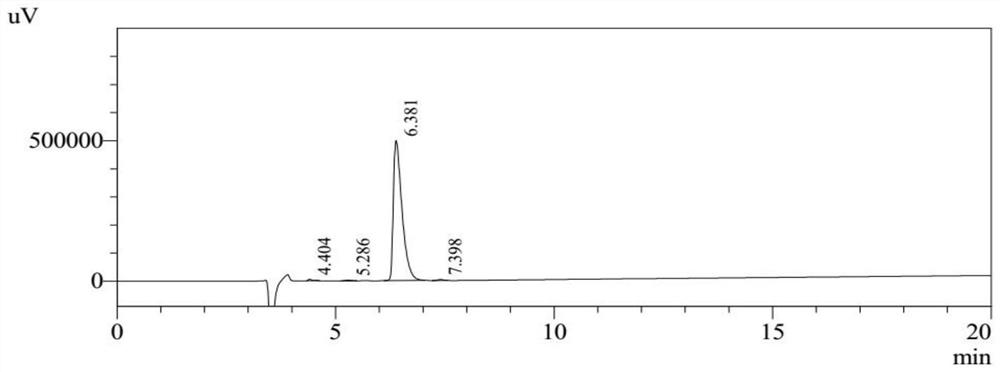
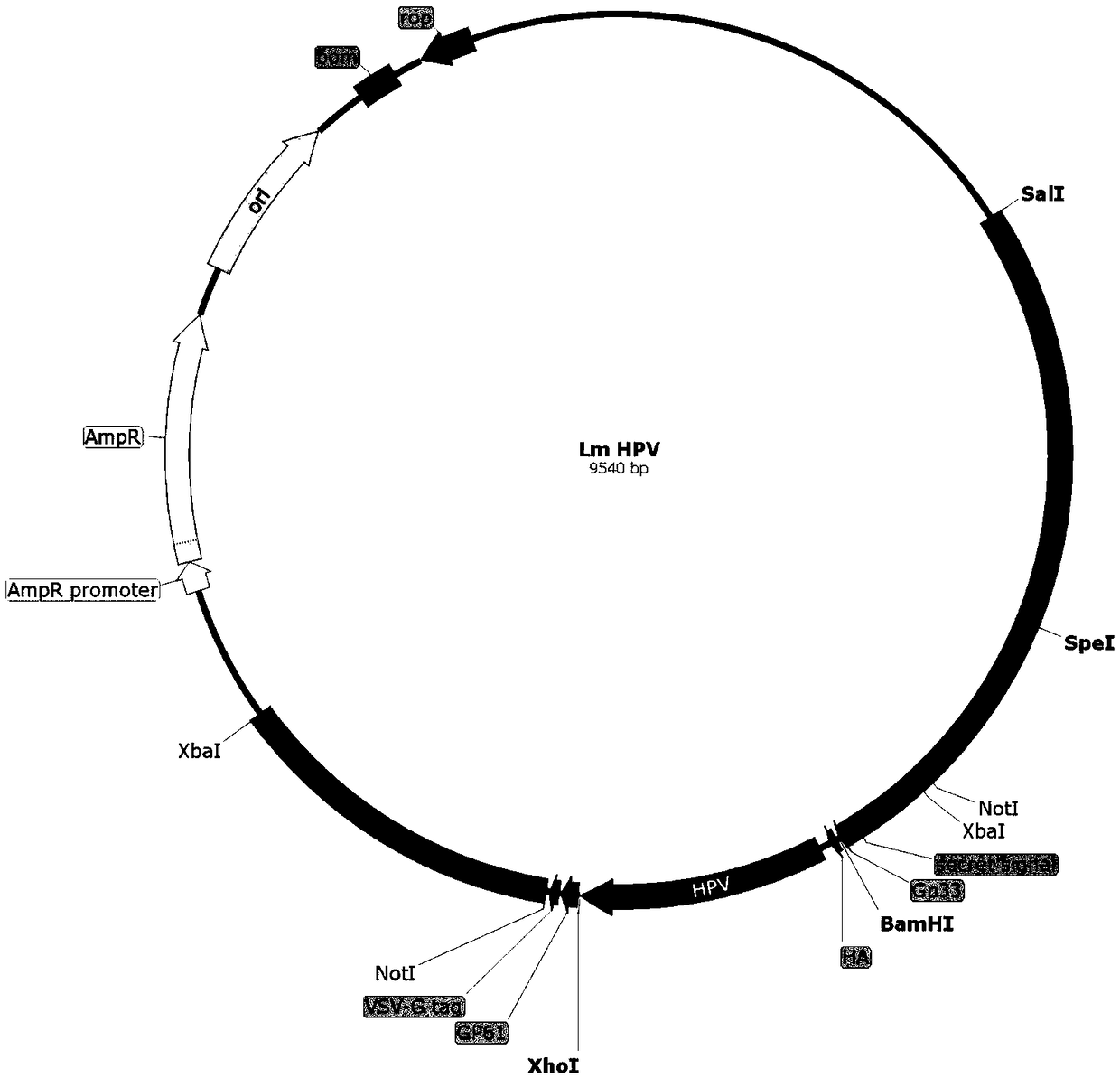
Cervical cancer therapeutic vaccine based on recombinant attenuated Listeria monocytogenes
ActiveCN108992665ABreak immune toleranceMake up for the disadvantage of lower immunogenicityBacteriaViral antigen ingredientsAdjuvantSpecific immunity
The invention relates to a cervical cancer therapeutic vaccine based on recombinant attenuated Listeria monocytogenes. Attenuated Listeria monocytogenes (hereinafter referred to as Lm) is used as a vector, and the unique ability of Listeria to grow in a host phagocytic cell is the characteristic of a natural T cell immune activation adjuvant, and is used to effectively improve the specific immuneresponse in a tumor microenvironment and break the immune tolerance of bodies, so persistent viral infection of the bodies is removed, and good therapeutic effects of clearing focus and restraining tumor development are achieved. The cervical cancer therapeutic vaccine makes up for the shortcoming of low immunogenicity of nucleic acid and protein peptide vaccines, and the attenuated Listeria reserves the intracellular growth and antigen presentation completion characteristics of an original strain, and has a high safety.
Owner:南京颂悦生物科技有限公司
Haemonchus contortus nano-material subunit vaccine and application thereof
ActiveCN111558034AEnhance specific immune responseProlong or enhance specific immune responseProtozoa antigen ingredientsPeptidesDendritic cellAdult worm
The invention discloses a Haemonchus contortus nano-material subunit vaccine and applications thereof. The Haemonchus contortus nano-material subunit vaccine is a subunit vaccine prepared by wrappinghaemonchus contortus recombinant protein HCA59 with nano-material PLGA, the recombinant HCA59 protein has an amino acid sequence as shown in SEQ ID NO. 1. The subunit vaccine can be used for preventing haemonchus contortus infection of sheep. The particle size of the nano subunit vaccine is 75 to 402nm; the Haemonchus contortus nano-material subunit vaccine has good biocompatibility and unique physicochemical properties, has the advantages of targeting property, slow release property, safety, high efficiency and the like, and can be decomposed and metabolized in an animal body; the vaccine hasan irritant antigen effect of stimulating dendritic cells, can promote the capability of converting mononuclear cells into the dendritic cells, has relatively strong immunoprotection capability, andcan remarkably reduce the ovum discharge rate and adult reduction rate of goats infected with haemonchus contortus clinically.
Owner:NANJING AGRICULTURAL UNIVERSITY
Mammalian milk osteopontin for enhancing immune responsiveness
ActiveUS9956283B2Enhance specific immune responseEnhance immune responsePeptide/protein ingredientsImmunological disordersVaccinationInfectious Disorder
Owner:ARLA FOODS AMBA
Meningococcus vaccine
InactiveCN101559225BImprove immunityLow costAntibacterial agentsCarrier-bound antigen/hapten ingredientsSpecific immunityCarrier protein
The invention relates to a meningococcus vaccine comprising a chemical conjugate of meningococcus capsular polysaccharide and carrier protein, and a first adjuvant, wherein the first adjuvant is oligodeoxynucleotide comprising at least one non-methylation CpG dinucleotide, and the length of the oligodeoxynucleotide is 15-35 ribonucleotides. The adjuvant used for increasing the immunization effectof the meningococcus vaccine is a novel human vaccine adjuvant with favorable potential application prospect, and the chemical essence of the adjuvant is the oligodeoxynucleotide which is synthesizedmanually and contains the CpG dinucleotide. By a receptor TLR9 activated immune system on immune cells, the adjuvant reinforces specific immune response aiming at vaccine antigens. The immunization effect of a vaccine can be increased by adding the vaccine adjuvant into the vaccine, so that the dosage of the vaccine antigen can be reduced, the immunization injection times can be reduced and the vaccine cost can be reduced.
Owner:NAT VACCINE & SERUM INST
Recombinant adenovirus vaccine for African swine fever and construction method of recombinant adenovirus vaccine
PendingCN113897394AKnockout avoidanceLow immunogenicityHydrolasesViral antigen ingredientsClassical swine fever virus CSFVAntigen
The invention discloses an African swine fever virus vaccine. The vaccine is obtained by constructing a recombinant adenovirus vector co-expressed by four antigen genes of an African swine fever virus and packaging the recombinant adenovirus vector through 293TD37 cells. Wherein the four antigen genes of the African swine fever virus are P72, B602L, P30 and P54 respectively. The construction of the recombinant adenovirus vector co-expressed by the four antigen genes of the African swine fever virus mainly comprises the following steps: knocking out E1, E3, E2a and E4 genes of an adenovirus vector through CRISPR / cas9 to construct shuttle plasmids in E1 and E4 regions, and respectively expressing the P72, B602L, P30 and P54 genes by using the shuttle plasmids, thereby obtaining a brand-new adenovirus vector. Compared with a first-generation adenovirus vector, the vector has the advantages that the vector capacity is increased by about 3kb, and the recombinant adenovirus with relatively high titer is obtained by carrying out packaging through a 293TD37 cell line and is used for preparing a recombinant adenovirus vaccine for an African swine fever. According to the African swine fever virus vaccine, the capacity of the adenovirus vector vaccine can be greatly increased, and the specific immune response to the African swine fever virus is enhanced by simultaneously expressing four independent antigens of the African swine fever on one adenovirus vector.
Owner:JIAXING ANYU BIOTECH CO LTD
Recombinant adenovirus vaccine for African swine fever and construction method of recombinant adenovirus vaccine
PendingCN113897390AOptimal knockout siteKnockout avoidanceHydrolasesViral antigen ingredientsClassical swine fever virus CSFVAntigen
The invention discloses an African swine fever virus vaccine. The vaccine is obtained by constructing a recombinant adenovirus vector co-expressed by four antigen genes of African swine fever virus and packaging the recombinant adenovirus vector through 293TD37 cells. Wherein the four antigen genes of the African swine fever virus are L8Lubiquitin, I215L, I73Rhbsag and E146L respectively. The construction of the recombinant adenovirus vector co-expressed by the four antigen genes of the African swine fever virus mainly comprises the following steps: knocking out E1, E3, E2a and E4 genes of an adenovirus vector through CRISPR / cas9 to construct shuttle plasmids in E1 and E4 regions, and respectively expressing the L8Lubiquitin, I215L, I73Rhbsag and E146L genes by using the shuttle plasmids, thereby obtaining a brand-new adenovirus vector. Compared with a first-generation adenovirus vector, the vector has the advantages that the vector capacity is increased by about 3kb, and the recombinant adenovirus with relatively high titer is obtained by carrying out packaging through a 293TD37 cell line and is used for preparing a recombinant adenovirus vaccine for an African swine fever. According to the African swine fever virus vaccine, the capacity of the adenovirus vector vaccine can be greatly increased, and the specific immune response to the African swine fever virus is enhanced by simultaneously expressing four independent antigens of the African swine fever on one adenovirus vector.
Owner:JIAXING ANYU BIOTECH CO LTD
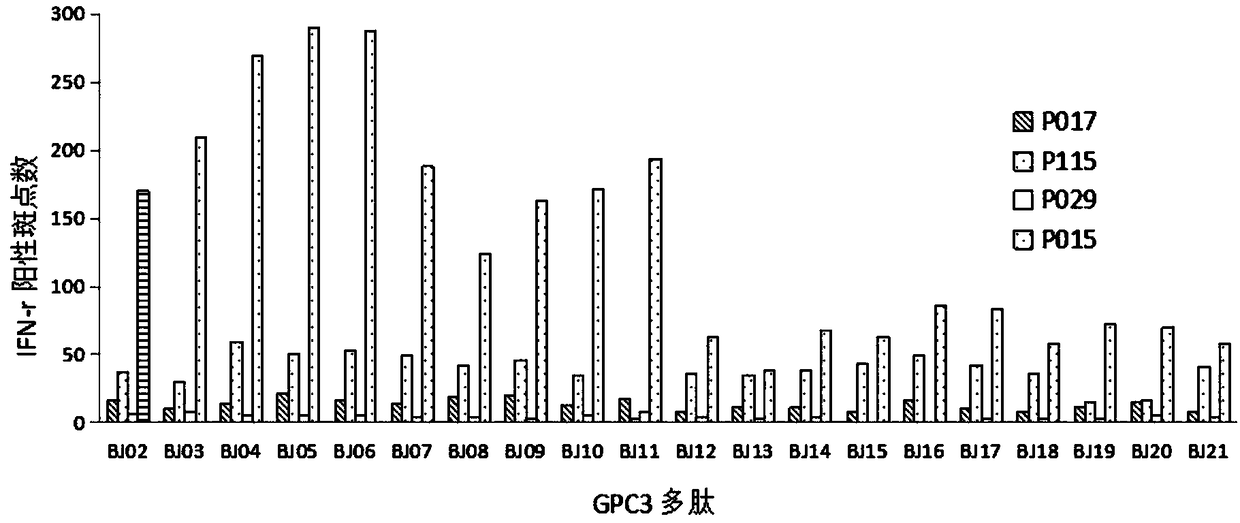
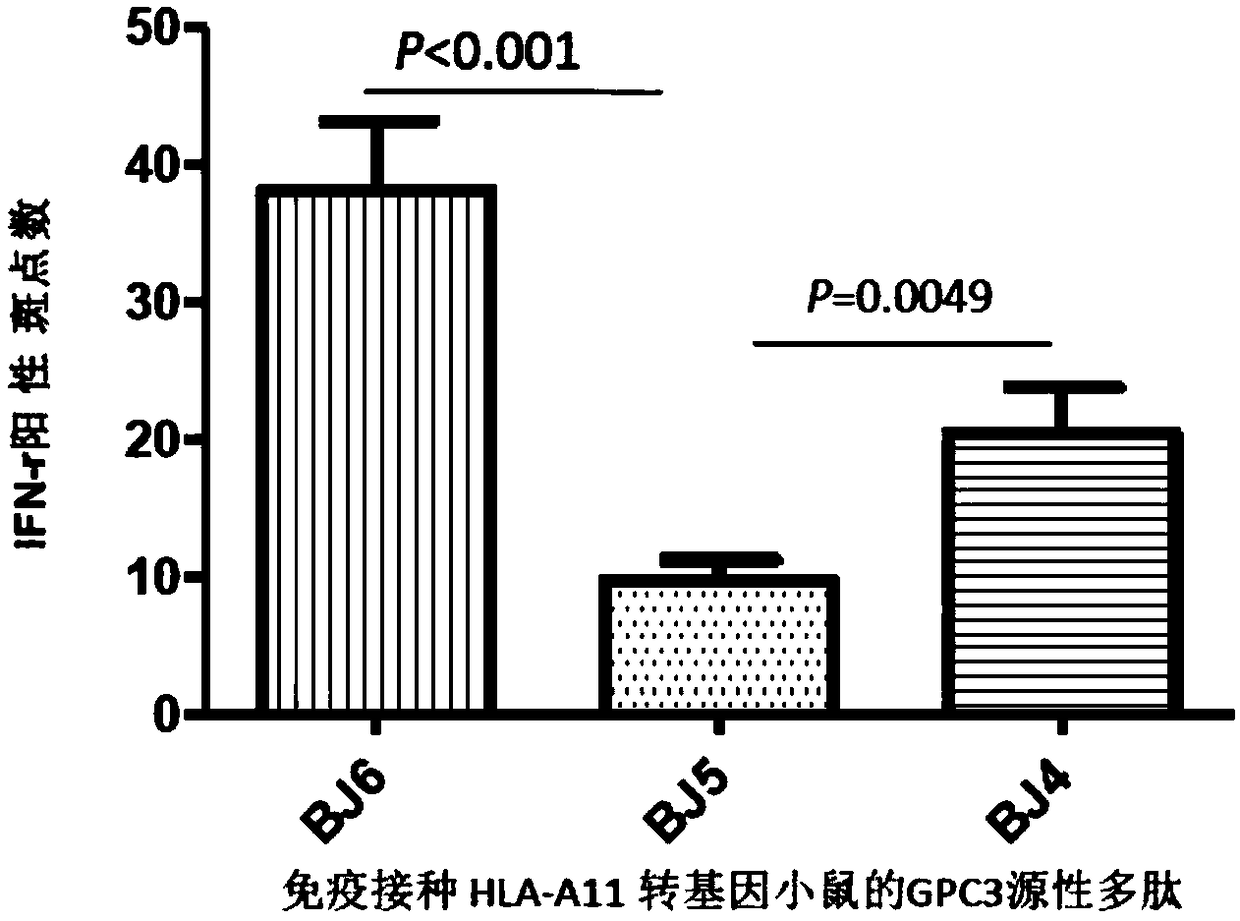
HLA-A11 restricted GPC3-derived polypeptide and vaccine containing polypeptide
InactiveCN108250266AEnhance specific immune responseEnhance immune induction therapyGenetically modified cellsSkeletal/connective tissue cellsAntigenSpecific immunity
The invention discloses an HLA-A11 restricted GPC3-derived polypeptide and a vaccine containing the polypeptide, and belongs to the technical field of biological pharmacy. The HLA-A11 restricted GPC3-derived polypeptide is composed of an amino acid sequence of SEQ ID NO:1 or SEQ ID NO:2. The invention also discloses the vaccine containing the above polypeptide, an application of the above polypeptide in preparing an immune-inducing irritant of a GPC3-positive tumor or cancer, a preparation capable of inducing tumor-reactive T cells, a pharmaceutical composition used for treating and / or preventing the GPC3-positive tumor or cancer, an antibody, and an antigen presenting cell. The polypeptide provided by the invention can induce and produce a specific immune response, and can be used for immunoprophylaxis and immunotherapy.
Owner:BEIJING YOUAN HOSPITAL CAPITAL MEDICAL UNIV
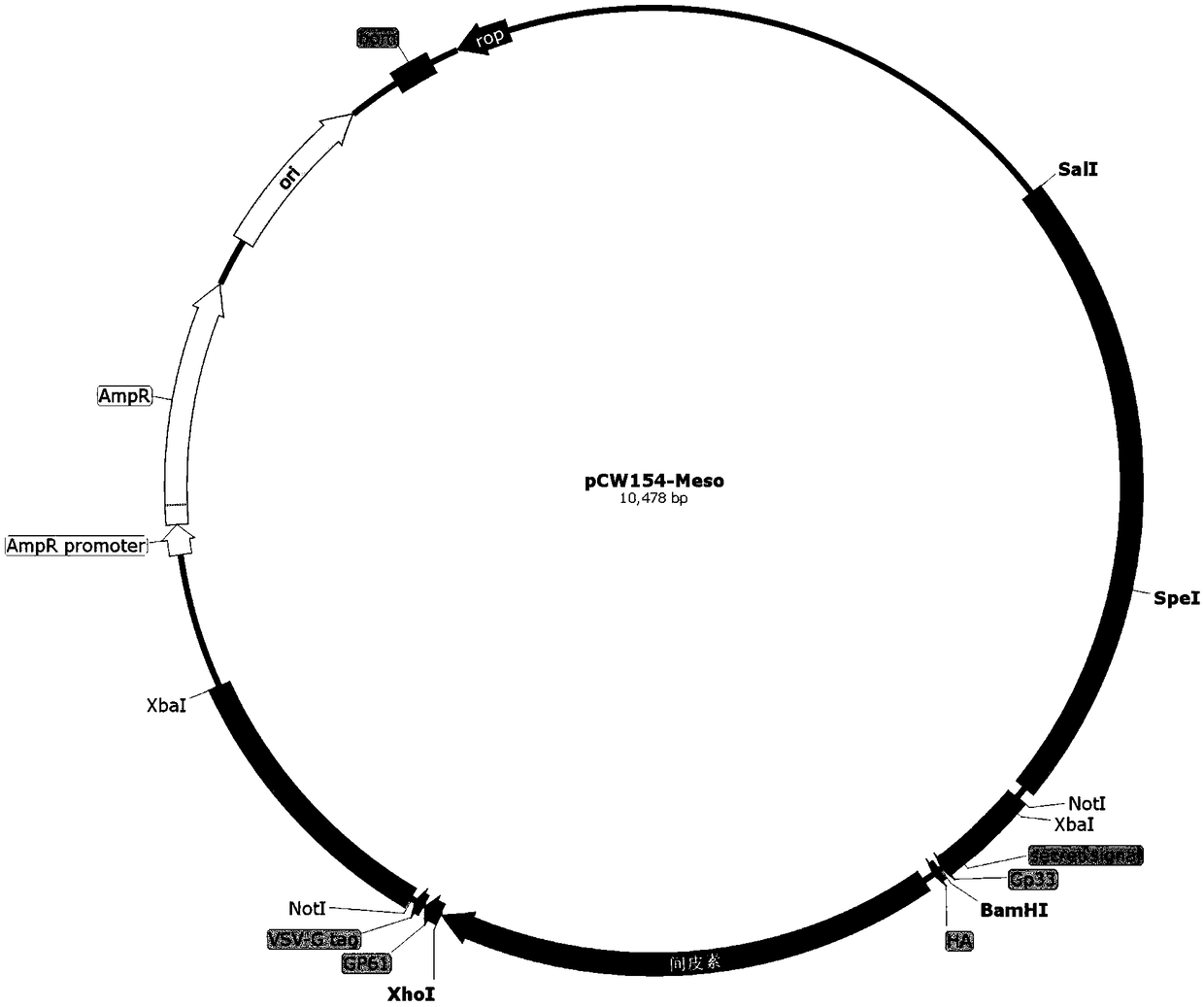
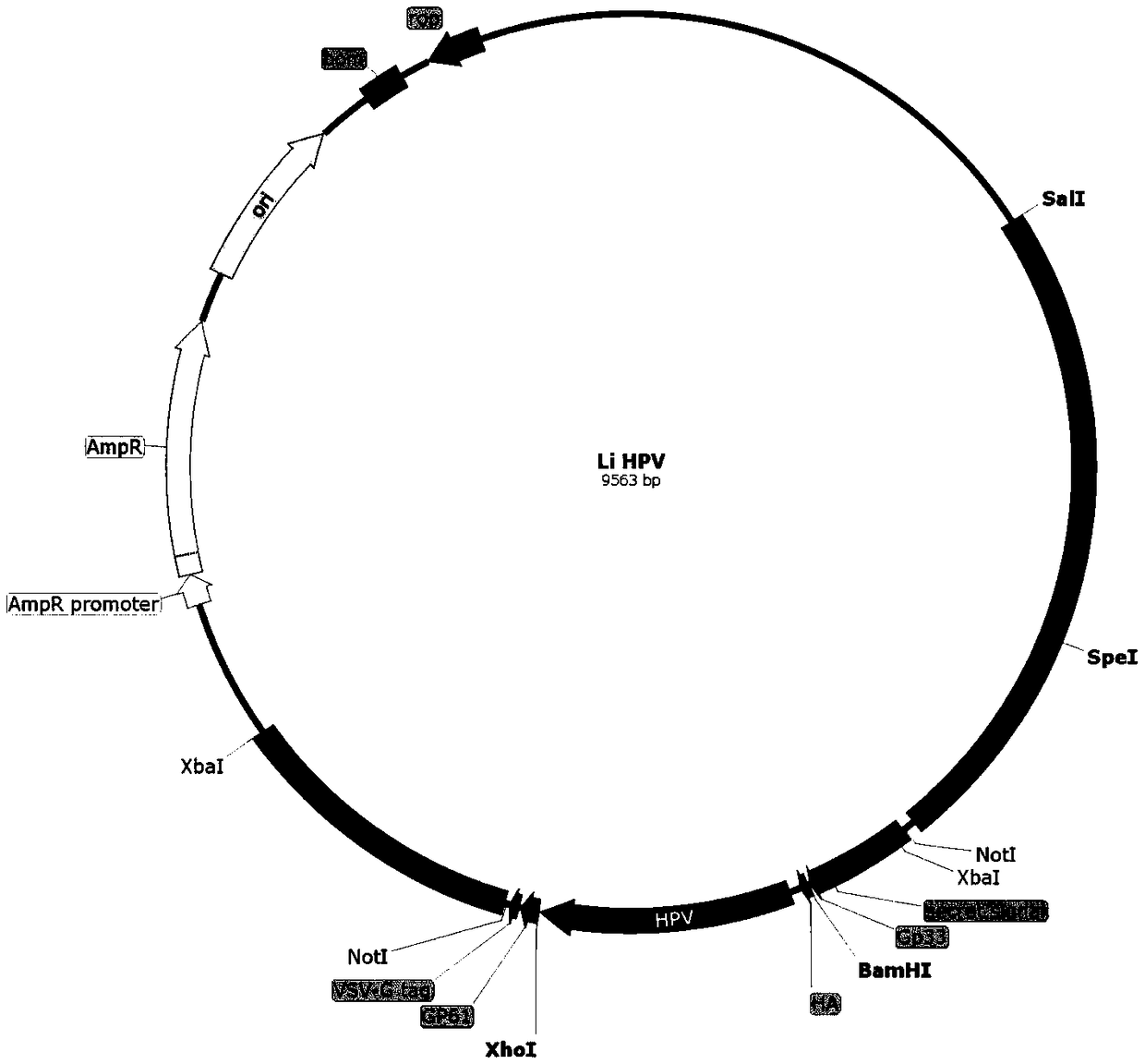
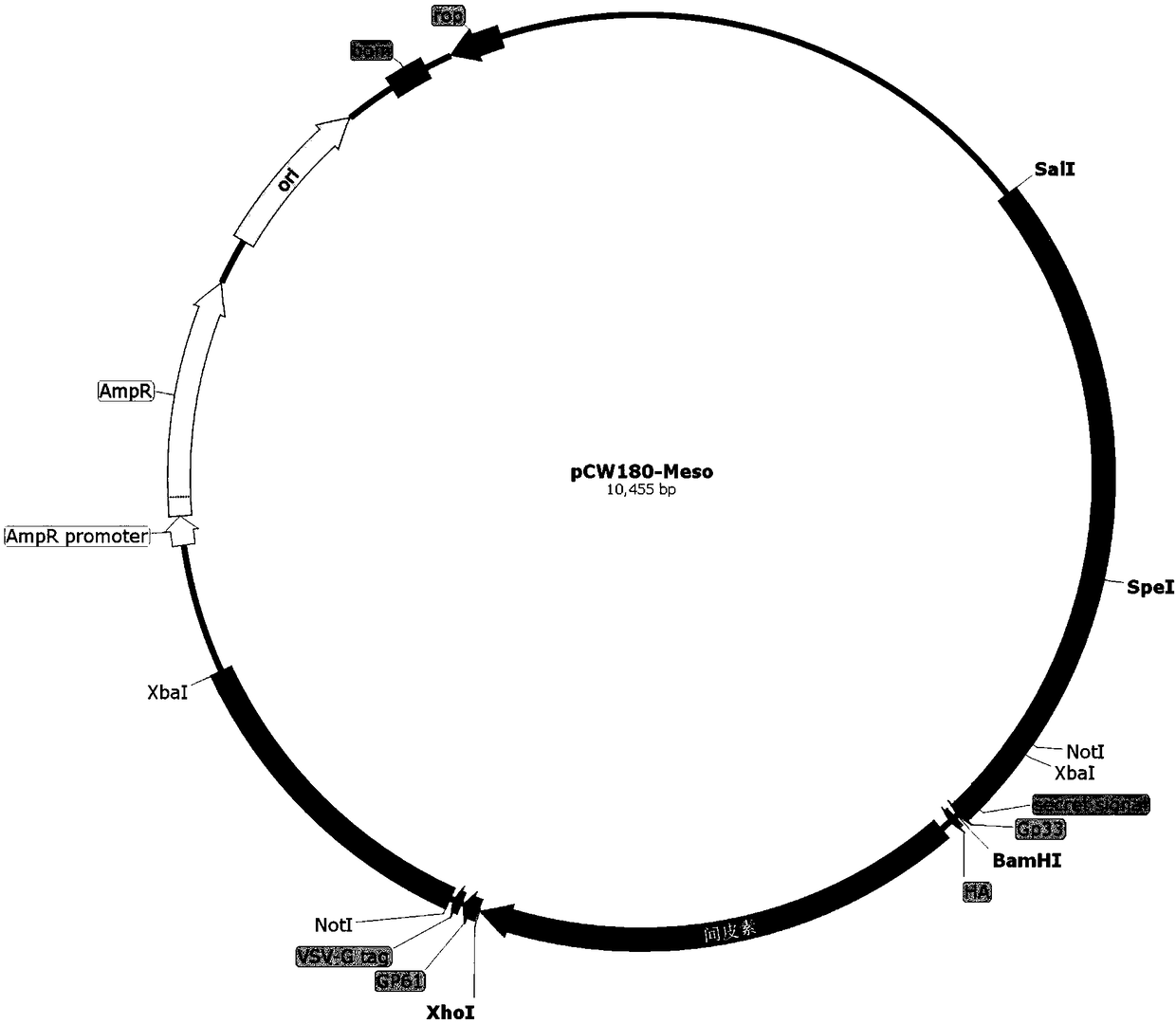
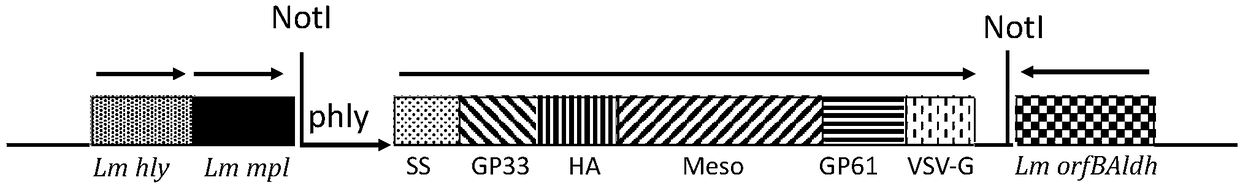
Application of recombinant attenuated listeria in preparation of mesothelin high-expression cancer therapeutic vaccine
ActiveCN108714210ABreak immune toleranceMake up for the disadvantage of lower immunogenicityBacteriaMicroorganism based processesAbnormal tissue growthPhagocyte
The invention relates to application of recombinant attenuated listeria in the preparation of a mesothelin high-expression cancer therapeutic vaccine. By taking attenuated Listeria monocytogenes (Lm for short hereinafter) and attenuated Listeria ivanovii (Li for short hereinafter) as carriers, and special listeria can grow in phagocyte of a host and is a natural T-cell immune activation adjuvant,so that the specific immune response in a tumor microenvironment is effectively improved, the immune tolerance of a body is broken through, the continuous virus infection of the body is eliminated, and good treatment effects on the elimination of an infection focus and the containment of tumor development are achieved. The application has the advantages that the disadvantages that nucleic acid type vaccines and protein polypeptide type vaccines are relatively low in immunogenicity are remedied, the attenuated listeria preserves the properties that an original strain can grow in the cells and finish antigen presentation, and meanwhile, the attenuated listeria has relatively high safety.
Owner:苏州圣苏新药开发有限公司 +1
Polypeptide for promoting pig body to generate broad-spectrum immune response and application thereof
ActiveCN113929746APromotes Broad Spectrum Immune ResponsePromote proliferationViral antigen ingredientsAntiviralsAntigenSpecific immunity
The invention provides a polypeptide for promoting a pig body to generate broad-spectrum immune response and application thereof, and belongs to the technical field of biomedicine. The polypeptide comprises one or more of a first polypeptide, a second polypeptide, a third polypeptide, a fourth polypeptide and a fifth polypeptide; the amino acid sequences of the first polypeptide, the second polypeptide, the third polypeptide, the fourth polypeptide and the fifth polypeptide are respectively shown as SEQ ID NO. 1 to SEQ ID NO. 5. The polypeptide provided by the invention can promote the pig body to generate broad-spectrum immune response by simultaneously promoting the proliferation of lymphocytes and mononuclear macrophages in the pig body; moreover, the polypeptide provided by the invention can promote the specific immune response of ASFV antigen in the pig body, so that the polypeptide can be used as an effective component of an ASFV subunit vaccine to enhance the immune response of pigs.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Application of recombinant attenuated Listeria in preparation of therapeutic vaccine for cervical cancer
ActiveCN109010819ABreak immune toleranceMake up for the disadvantage of lower immunogenicityAntibody mimetics/scaffoldsViral antigen ingredientsAdjuvantSpecific immunity
The invention relates to application of recombinant attenuated Listeria in preparation of a therapeutic vaccine for cervical cancer. Attenuated Listeria monocytogenes (hereinafter referred to as Lm) and attenuated Listeria ivanovii (hereinafter referred to as Li) are used as vectors, by use of the unique characteristic that the Listeria can grow in phagocytose cells in a host, and is a natural T cell immune activation adjuvant, specific immune response in tumor microenvironment can be effectively enhanced, body's immune tolerance can be broken, body's persistent viral infection can be eliminated, and good treatment results of clearing focuses and curbing tumor development can be achieved. The Listeria has the advantages that shortcomings of low immunogenicity of nucleic acid and protein peptide vaccines can be made up, the attenuated Listeria retains the characteristic that an attenuated original strain can grow intracellularly to complete antigen presentation, and meanwhile the attenuated Listeria has higher safety.
Owner:南京颂悦生物科技有限公司
GFP-2 nano oligopeptide collagen and a preparing method and application thereof
ActiveCN109022522AImprove immune efficiencyHigh antibody titerMicroorganism based processesPeptide preparation methodsUltrafiltrationChemistry
The invention relates to GFP-2 nano oligopeptide collagen and a preparing method and application thereof. The GFP-2 nano oligopeptide collagen is prepared by (1) preparing a brown sugar medium and performing sterilization; (2) inoculating GFP-2 bacteria, a bacterial glycerol stock and an LB medium and performing shake culture; (3) transferring a product to the brown sugar medium, performing shakeculture, and then performing centrifugation, with supernatant liquid being collected and bacterial cells being abandoned; (4) salting out to precipitate protein and nano oligopeptide collagen, then performing centrifugation again, removing supernatant liquid and collecting crude protein; (5) resuspending the crude protein with tris-HCl, allowing the resuspended crude protein to pass through a membrane and performing dialysis with tris-HCl; and (6) performing ultrafiltration with an ultrafiltration tube, collecting nano oligopeptide collagen the molecular weight of which is less than 10 KD, cooling the nano oligopeptide collagen and freeze-drying the nano oligopeptide collagen to obtain the GFP-2 nano oligopeptide collagen. The prepared GFP-2 nano oligopeptide collagen can be used as an adjuvant and can greatly boost immunity.
Owner:杭州洪晟生物技术股份有限公司
Recombinant adenovirus vaccine for African swine fever and construction method of recombinant adenovirus vaccine
ActiveCN113897395AOptimal knockout siteKnockout avoidanceHydrolasesViral antigen ingredientsClassical swine fever virus CSFVAntigen
The invention discloses an African swine fever virus vaccine. The vaccine is obtained by constructing a recombinant adenovirus vector co-expressed by four antigen genes of an African swine fever virus and packaging the recombinant adenovirus vector through 293TD37 cells. Wherein the four antigen genes of the African swine fever virus are EP402R, EP153R, I177L and K205Rubiqutin respectively. The construction of the recombinant adenovirus vector co-expressed by the four antigen genes of the African swine fever virus mainly comprises the following steps: knocking out E1, E3, E2a and E4 genes of an adenovirus vector through CRISPR / cas9 to construct shuttle plasmids in E1 and E4 regions, and respectively expressing the EP402R, EP153R, I177L and K205Rubiqutin genes by using the shuttle plasmids, thereby obtaining a brand-new adenovirus vector. Compared with a first-generation adenovirus vector, the vector has the advantages that the vector capacity is increased by about 3kb, and the recombinant adenovirus with relatively high titer is obtained by carrying out packaging through a 293TD37 cell line and is used for preparing a recombinant adenovirus vaccine for an African swine fever. According to the African swine fever virus vaccine, the capacity of the adenovirus vector vaccine can be greatly increased, and the specific immune response to the African swine fever virus is enhanced by simultaneously expressing four independent antigens of the African swine fever on one adenovirus vector.
Owner:JIAXING ANYU BIOTECH CO LTD
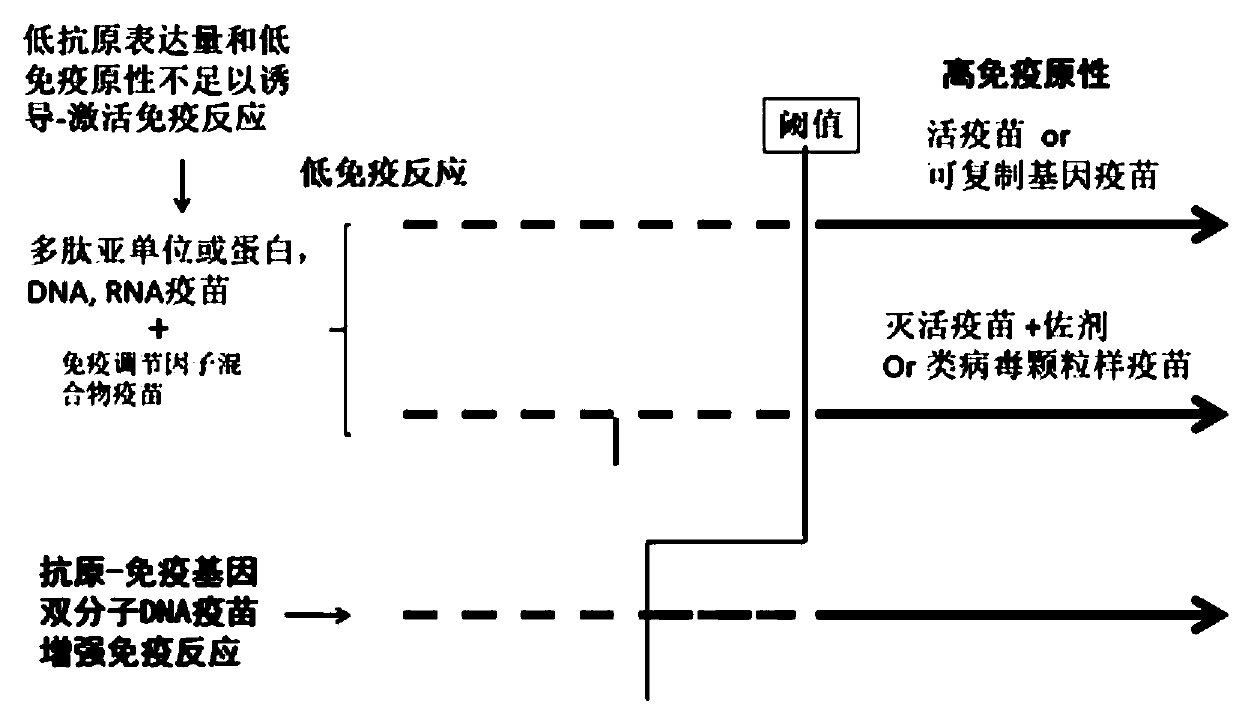
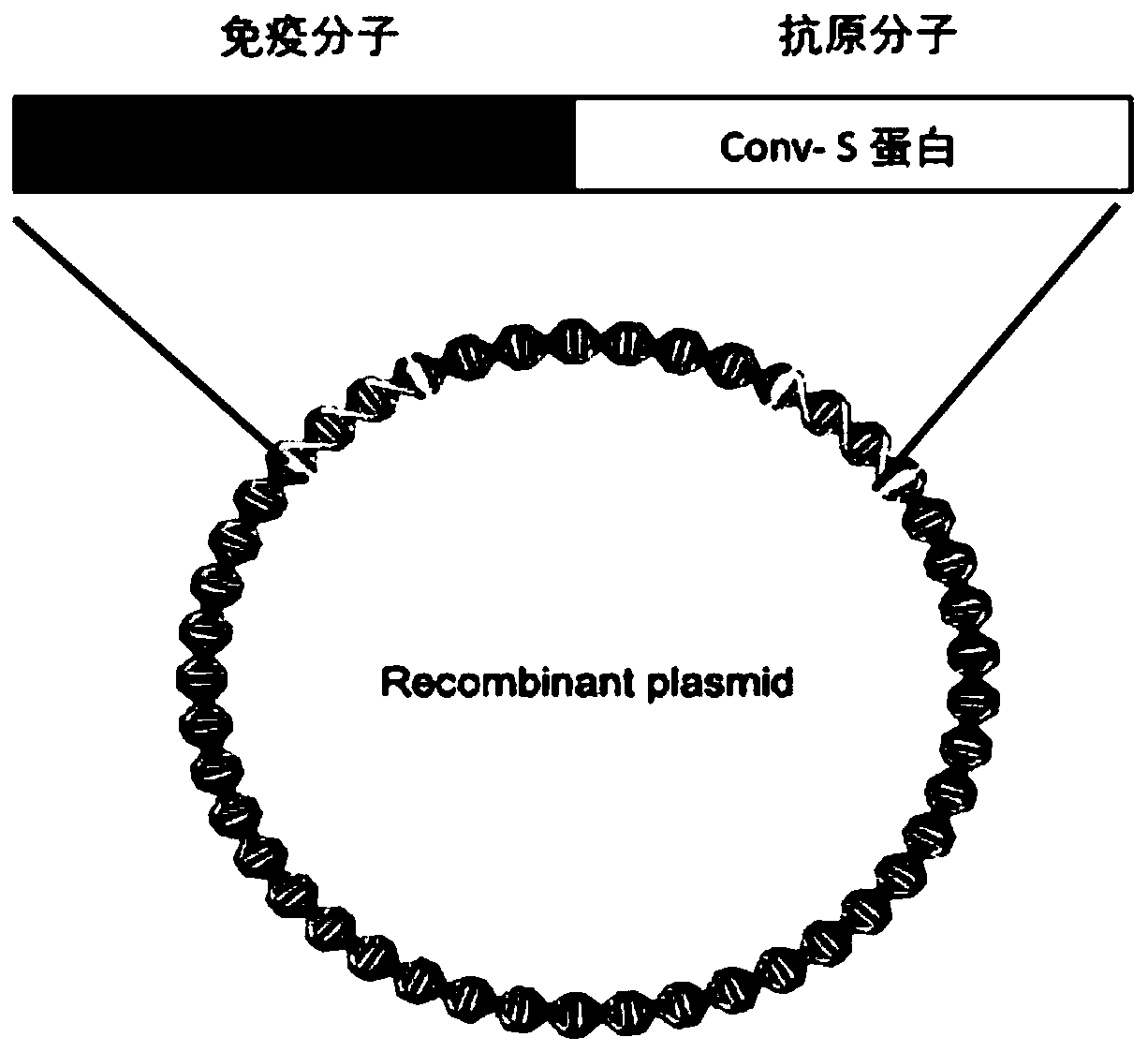
Bimolecular DNA vaccine of virus antigen-immune coactivator
PendingCN111588843AEnhance specific immune responseSsRNA viruses positive-senseViral antigen ingredientsDiseaseImmunocompetence
The invention discloses a bimolecular DNA vaccine of a virus antigen-immune coactivator, and relates to the technical field of biological medicines. An expression vector is a DNA plasmid; a virus antigen is any virus immunogenicity (antigen) molecule; and an immunocompetence factor is a T cell co-activation factor. According to the vaccine disclosed by the invention, an exogenous gene segment forexpressing a T cell co-activation factor and a virus gene segment of a virus antigen molecule are jointly constructed in the DNA plasmid and are simultaneously expressed in the same cell, and the double-molecule activates systematic immune response of T cells on virus. The bimolecular DNA vaccine is also a novel vaccine technology platform, and is suitable for developing preventive vaccines for various infectious diseases. Aiming at global COVID-19 prevalence, the bimolecular DNA vaccine of the new coronavirus S antigen fragment-immune co-activation factor can be constructed through the technical platform, and achieves immunoprophylaxis and control.
Owner:SICHUAN CHENGYU BIOLOGICAL PROD INC
Therapeutic DC composite vaccine against herpes simplex viruses and preparation method thereof
ActiveCN109701008ATreatment Prevention and TreatmentBreak immune toleranceAntiviralsAntibody medical ingredientsSequence signalImmune effects
The present invention discloses a therapeutic DC composite vaccine against herpes simplex viruses and a preparation method thereof. The therapeutic DC composite vaccine comprises dendritic cells modified with three loaded HSV-2 virus-specific immune antigen gene fragments. The three loaded HSV-2 virus-specific immune antigen gene fragments are gC+US10, DC-UL47 and gD+ICP4, respectively. The therapeutic DC composite vaccine uses granulocyte-macrophage cluster factor receptor signal peptides to guide expressions of antigens, can improve expression efficiency of the antigens, enhances immune effects, and generates a stronger anti-HSV-specific immune response.
Owner:上海兴瑞一达生物科技有限公司
Multi-epitope combined peptide used for treating and preventing human papillomavirus infection and related diseases
InactiveCN108409866AImprove bindingImprove survivabilityViral antigen ingredientsAntibody mimetics/scaffoldsCtl epitopeDisease
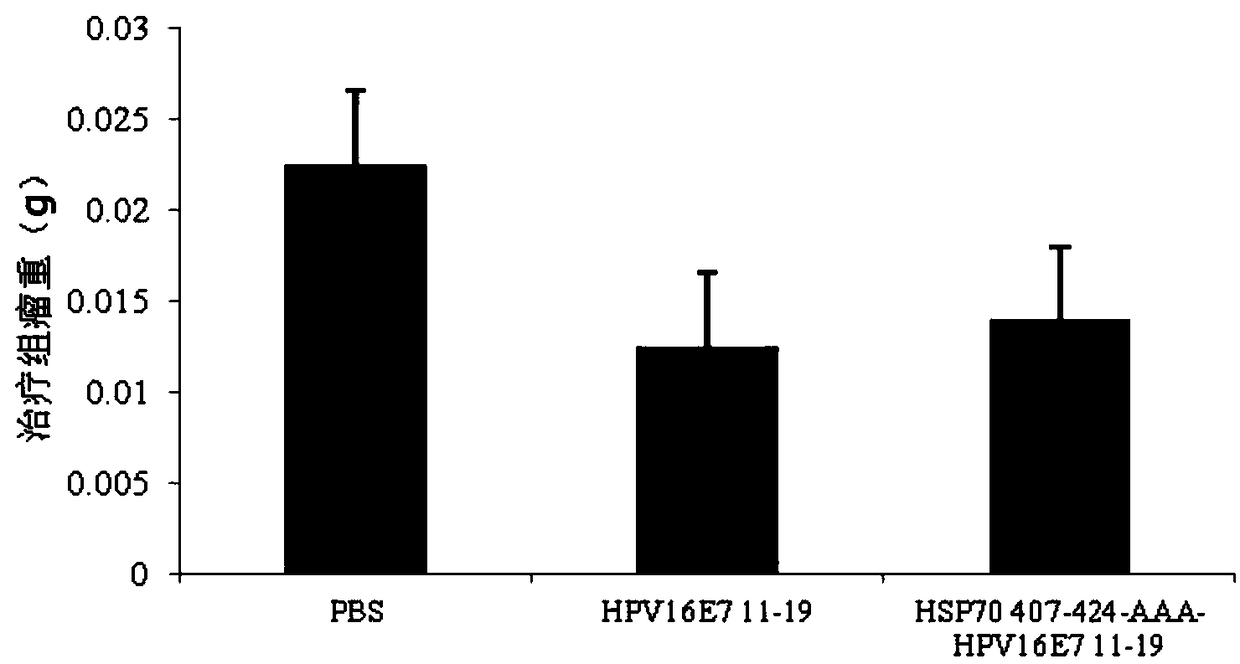
The invention relates to a multi-epitope combined peptide used for treating and preventing human papillomavirus (HPV) infection and related diseases. The multi-epitope combined peptide consists of a binding domain and a hinge domain of carboxyl-terminated peptide of mycobacterium tuberculosis heat shock protein 70 (HSP70), and antigen epitope peptides of T cells of HPV E6 and E7, and the multi-epitope combined peptide is in linear arrangement through the arrangement mode of HSP stimulated epitope peptide-hinge area-HPV CTL epitope. The multi-epitope combined peptide provided by the invention can be used as a vaccine to induce the immune response mediated by specific T lymphocytes through injection of intradermal, hypodermic, focus or mucosal tissues, and can induce effective antiviral effect in tissues and local tissues for treating and preventing HPV infection and related diseases. The multi-epitope combined peptide provided by the invention has the advantages of low use dose and no need of adding artificial excipients.
Owner:HEFEI RUICHENGSHENG BIOTECH CO LTD
Mesothelin high-expression cancer treatment vaccine based on recombinant attenuated listeria monocytogenes
InactiveCN108704126ABreak immune toleranceMake up for the disadvantage of lower immunogenicityBacteriaBacteria peptidesTreatment effectAdjuvant
The invention relates to a mesothelin high-expression cancer treatment vaccine based on recombinant attenuated listeria monocytogenes. The attenuated listeria monocytogenes (Lm) is used as a carier, the characteristics that the Lm can specifically grow in a host phagocyte and is a natural T cell immune activated reagent are utilized, specific immune response in a tumor microenvironment can be effectively improved, the immune tolerance of a body can be broken, persistent viral infection of the body can be cleaned, and an excellent treatment effect for cleaning the focus and inhibiting tumor development can be achieved. The mesothelin high-expression cancer treatment vaccine has the advantages of overcoming the defect of relatively low immunogenicity of nucleotide and protein polypeptide vaccines, the attenuated listeria remained with original strains can grow in cells, so that higher safety is achieved while the characteristic of antigen presentation can be completed.
Owner:南京颂悦生物科技有限公司
An oral drug delivery system using composite nanomaterials as a carrier
ActiveCN107823185BGood biocompatibilityReduce degradation damageAntibacterial agentsBacterial antigen ingredientsDrugs solutionDisease
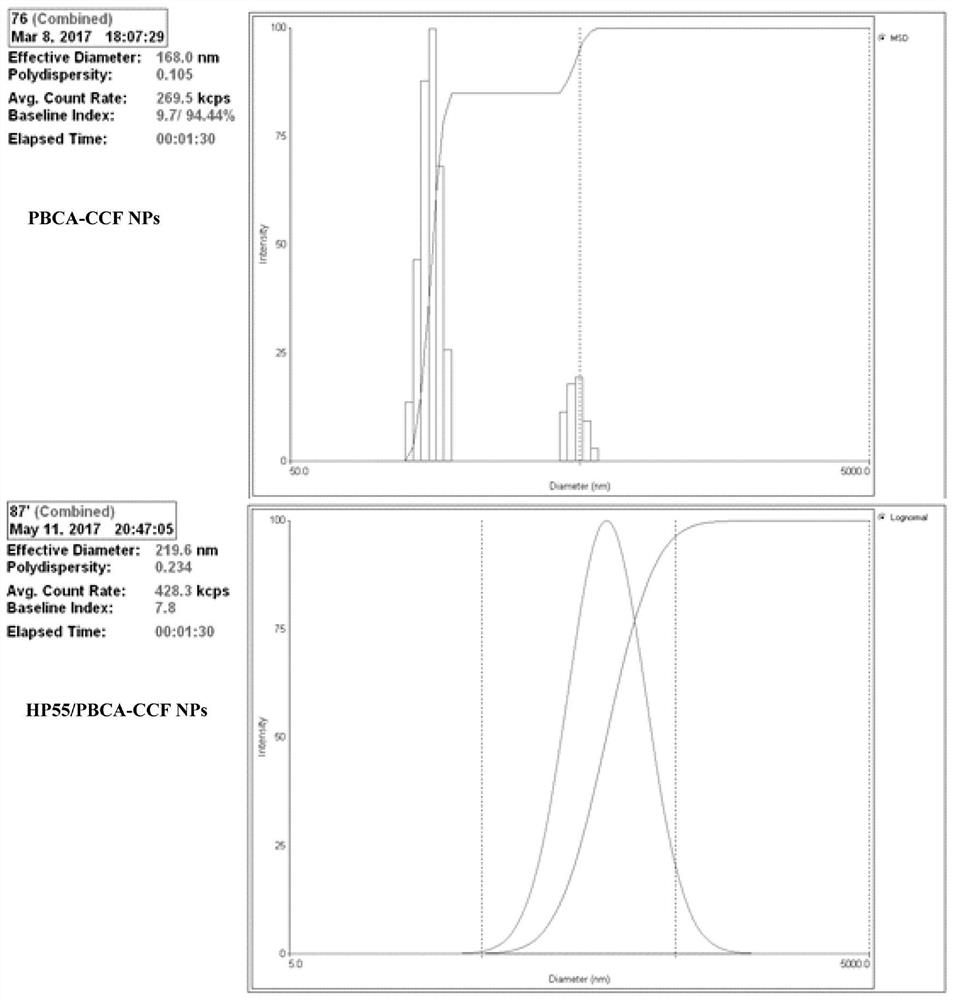
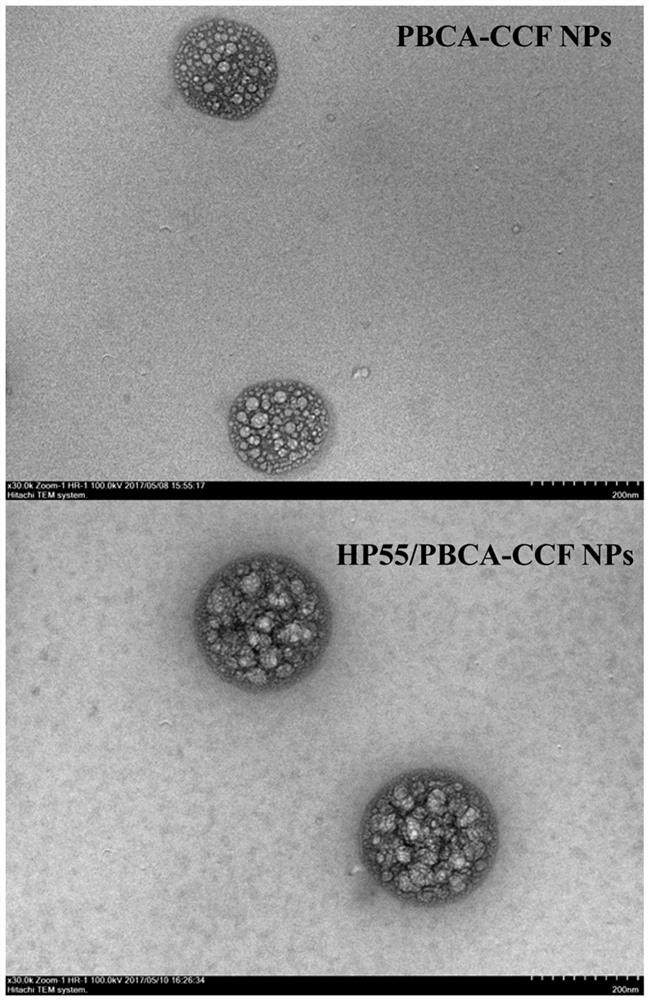
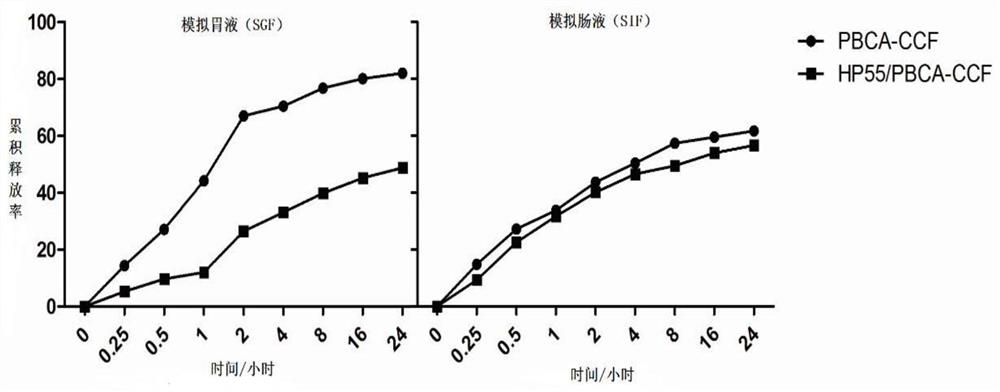
The invention discloses an oral drug delivery system using composite nanomaterials as carriers. The composite nanomaterials HP55 and PBCA are used as shells to coat drug solutions to form nanoparticles. The method adopts the biodegradable and good biocompatibility composite nanometer material as the carrier of the oral medicine, which can improve the bioavailability of the oral medicine. In the field of biomedicine, the nanoparticle can be used for oral delivery of Helicobacter pylori subunit vaccines, which can play a huge role in preventing and treating diseases related to Helicobacter pylori infection.
Owner:CHINA PHARM UNIV
A kind of GFP-2 small peptide and its preparation method and application
ActiveCN109022522BImprove immune efficiencyHigh antibody titerMicroorganism based processesDepsipeptidesAdjuvantSmall peptide
Owner:杭州洪晟生物技术股份有限公司
Human sperm specific transfer factor oral preparation and preparation process thereof
InactiveCN102626422AImprove immunityRich sourcesUnknown materialsAntibody medical ingredientsTransfer cellBULK ACTIVE INGREDIENT
The invention discloses a human sperm specific transfer factor oral preparation and a preparation process thereof. The process mainly comprises the following steps of: preparing an immunogen; immunizing in an animal; and preparing a human sperm specific transfer factor, and the like. The oral preparation has the functions of transferring cell immune information, mediating a cell immune reaction, and specifically inhibiting and killing human sperms, and has the function of enhancing non-specific immunity; the oral preparation does not cause anaphylaxis, is practical and safe, has a remarkable effect, and does not cause medicament resistance after use; and the product is taken orally without being limited by conditions, is convenient, does not influence the biological activities of active ingredients, is a safe and effective preparation, and has potential social value and market application prospect.
Owner:周建伟
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com