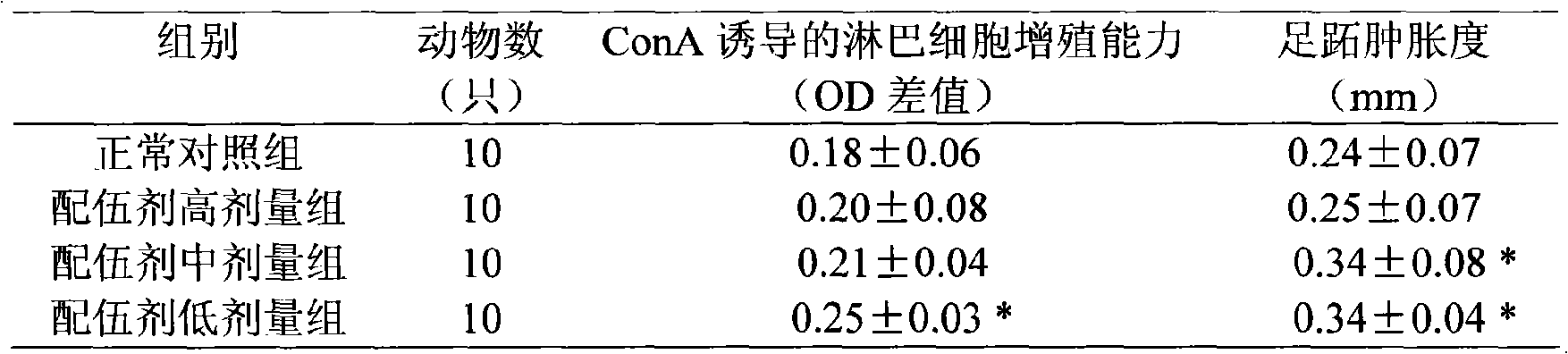
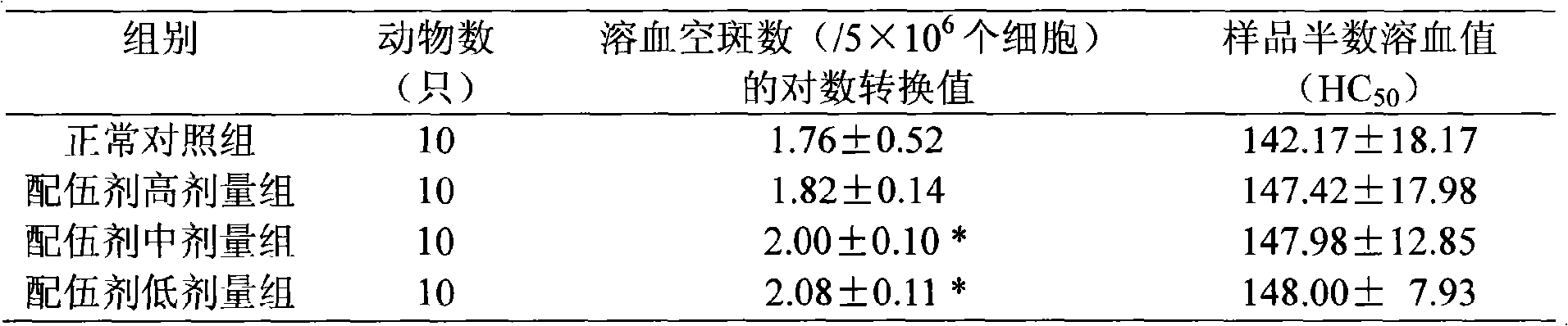
Patents
Literature
69 results about "Lymphopoiesis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lymphopoiesis (lĭm'fō-poi-ē'sĭs) (or lymphocytopoiesis) is the generation of lymphocytes, one of the five types of white blood cell (WBC). It is more formally known as lymphoid hematopoiesis. Pathosis in lymphopoiesis leads to any of various lymphoproliferative disorders, such as the lymphomas and lymphoid leukemias.
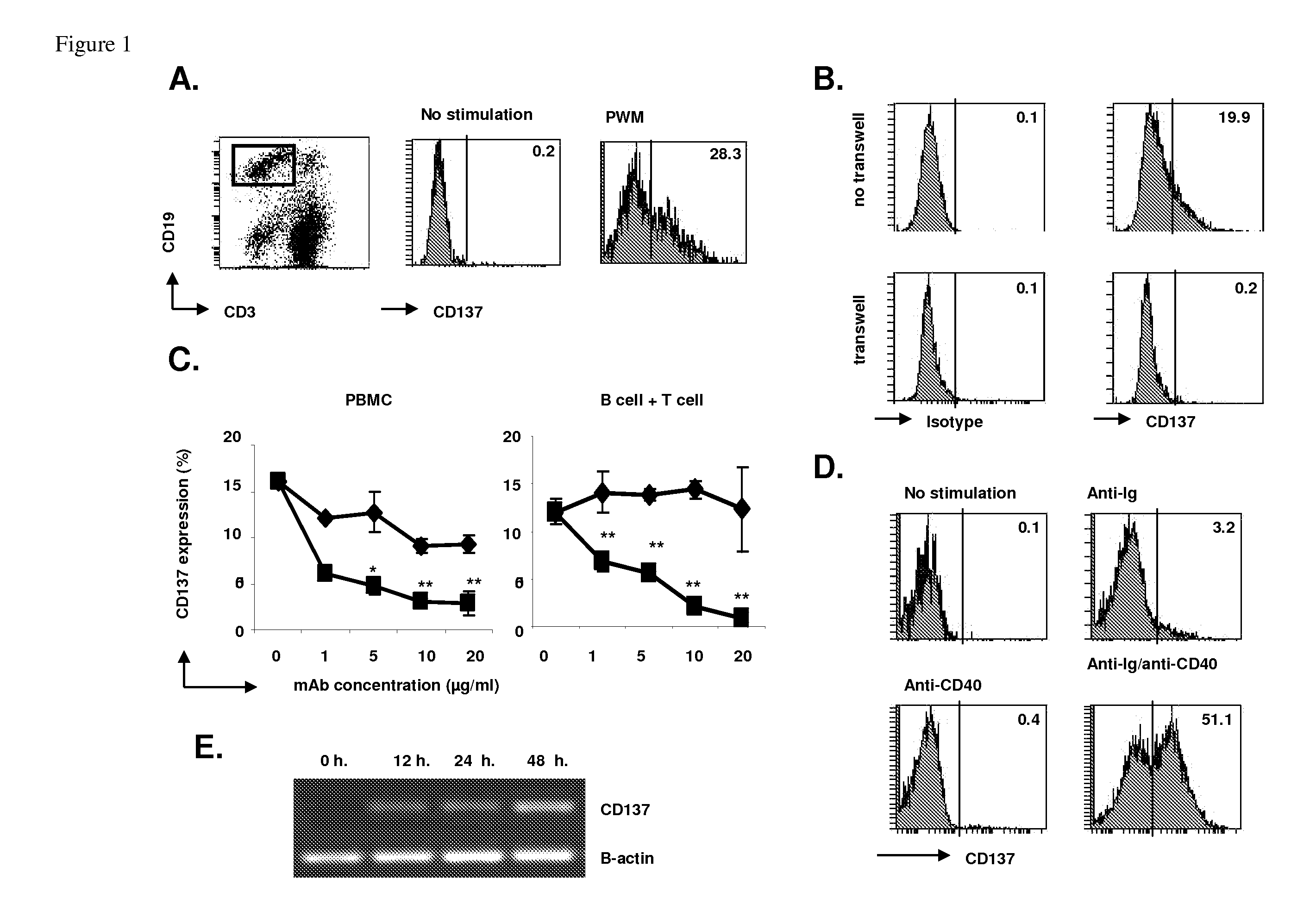
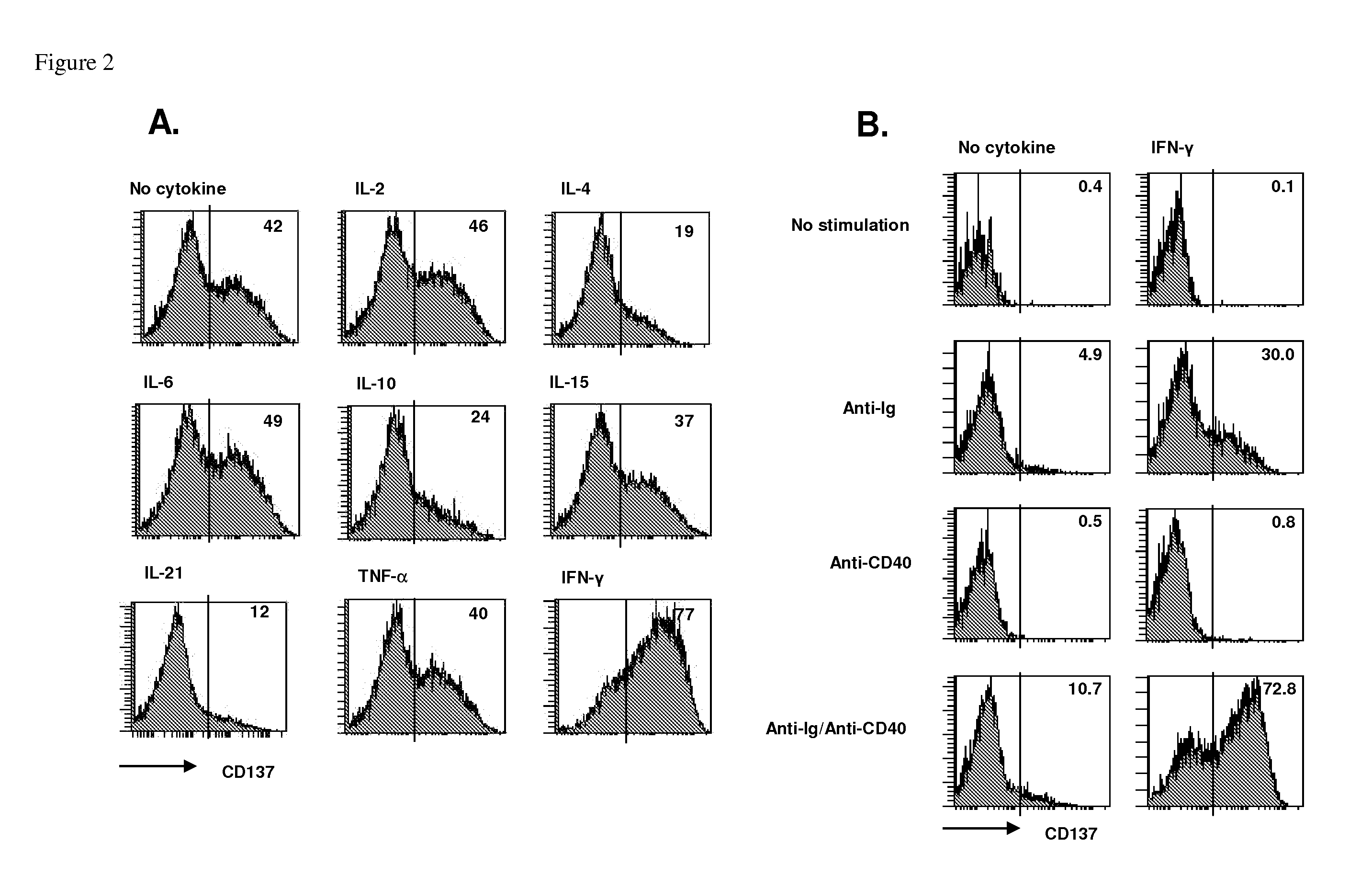
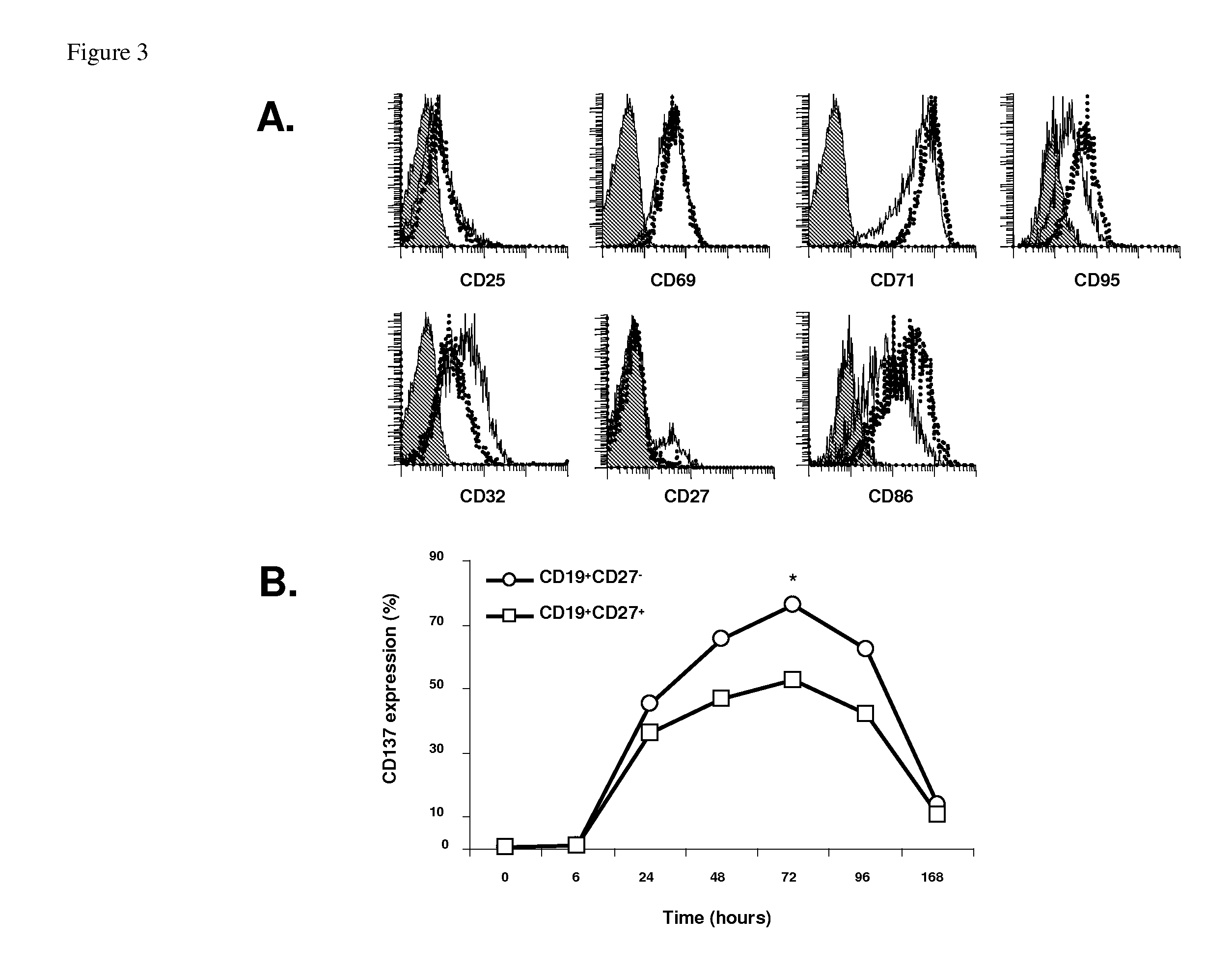
Methods for treating cancers and diseases associated with 4-1bb (CD137) expression
InactiveUS20120076722A1Conducive to survivalPrevent proliferationPeptide/protein ingredientsSkeletal disorderDiseaseCD137
The present invention relates to the role of 4-1BB (CD137) ligand and anti-4-1BB (CD137) antibody in the treatment of cancers and diseases associated with 4-1BB (CD137) expression. More particularly, the present invention relates to the use of (i) 4-1BB (CD137) ligand for inducing proliferation and activation and promoting survival of B lymphocytes and (2) anti-4-1BB (anti-CD137) antibody for inhibiting proliferation and activation and inducing death of B lymphocytes.
Owner:UNIV OF MARYLAND BALTIMORE
Method for preparing immunocompetent soybean peptide by enzymolysis and membrane separation
ActiveCN101974589AImprove the quality of immune activityPeptide preparation methodsFermentationImmunocompetenceHydrolysis
The invention relates to a method for preparing an immunocompetent soybean peptide by enzymolysis and membrane separation, belonging to the technical field of soybean protein deep processing. The method comprises the following steps of: by taking defatted soybean protein powder as a raw material, firstly, carrying out acid cleaning on the defatted soybean protein powder to obtain acid washed soybean protein; then carrying out enzyme hydrolysis by using an Alcalase protease under certain conditions to obtain coarse soybean peptide liquid; clarifying the prepared coarse soybean peptide liquid by adopting microfiltration; then carrying out enrichment and desalination treatment by adopting ultrafiltration and nanofiltration; and finally, drying to obtain the powdery immunocompetent soybean peptide. The activity of the soybean peptide is determined by adopting a neutral red phagocytosis test and a lymphopoiesis method, which proves that the prepared soybean peptide has high immunocompetence.
Owner:安徽盛美诺生物技术有限公司
Method for enhancing proliferation or differentiation of a cell using ob protein
InactiveUS7074397B1Easy to implantFacilitates mobilizationPeptide/protein ingredientsDepsipeptidesMyelopoiesisIn vivo
Uses for WSX ligands in hematopoiesis are disclosed. In particular, in vitro and in vivo methods for stimulating hematopoiesis (e.g., myelopoiesis, erythropoiesis and especially, lymphopoiesis) using a WSX ligand (e.g., anti-WSX receptor agonist antibodies or OB protein), and optionally another cytokine, are described.
Owner:GENENTECH INC
Ganoderma lucidum polysaccharide and preparation method thereof
InactiveCN101935358AEasy to prepareSuitable for mass productionDigestive systemAntinoxious agentsImmunocompetenceNitric oxide
The invention provides ganoderma lucidum polysaccharide and a preparation method thereof. The ganoderma lucidum polysaccharide is obtained through carrying out water extraction and ultrafiltration on a ganoderma lucidum fruiting body, decoloring and then freeze drying. The decolored ganoderma lucidum polysaccharide has remarkably improved immunocompetence and can stimulate macrophage to generate nitric oxide and stimulate lymphopoiesis.
Owner:SHANGHAI ACAD OF AGRI SCI
Enterobacter cloacae, selenium-rich polysaccharide thereof and use of selenium-rich polysaccharide
InactiveCN101475916AEasy to produceIncrease productionOrganic active ingredientsBacteriaMicroorganismMouse Lymphocyte
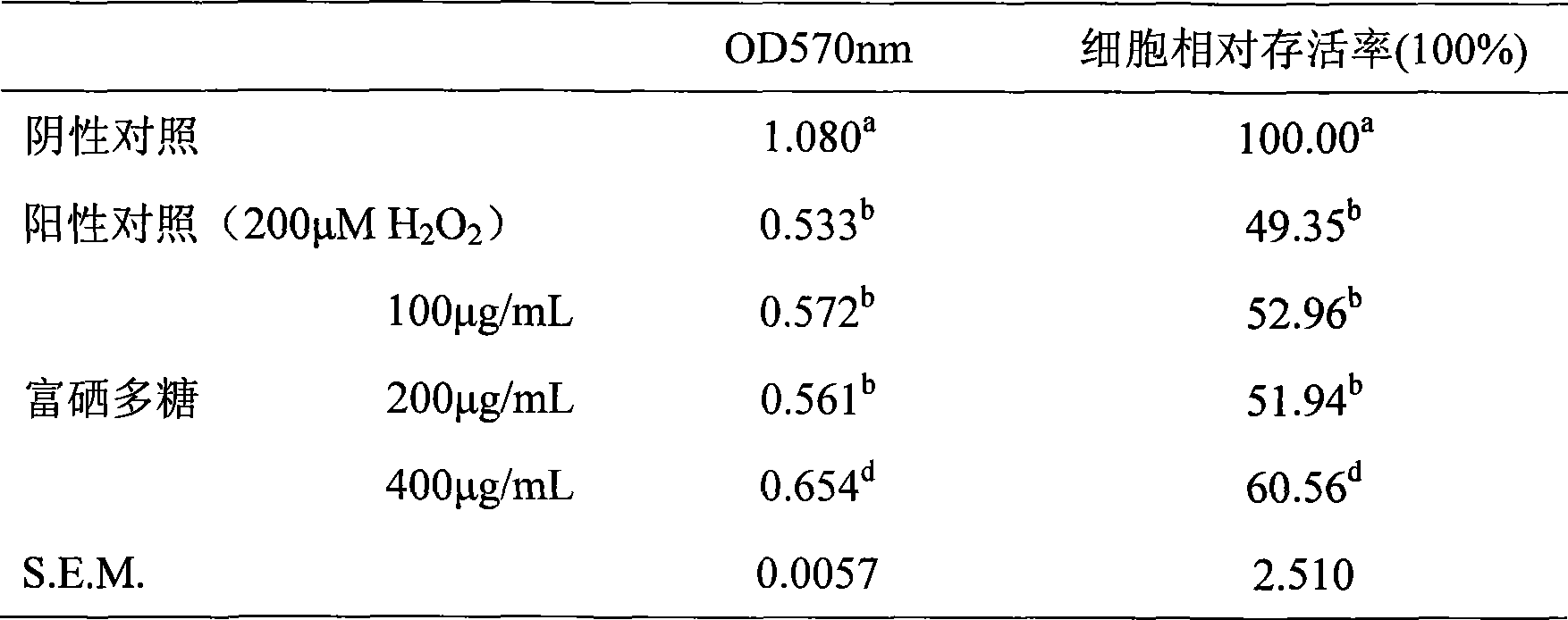
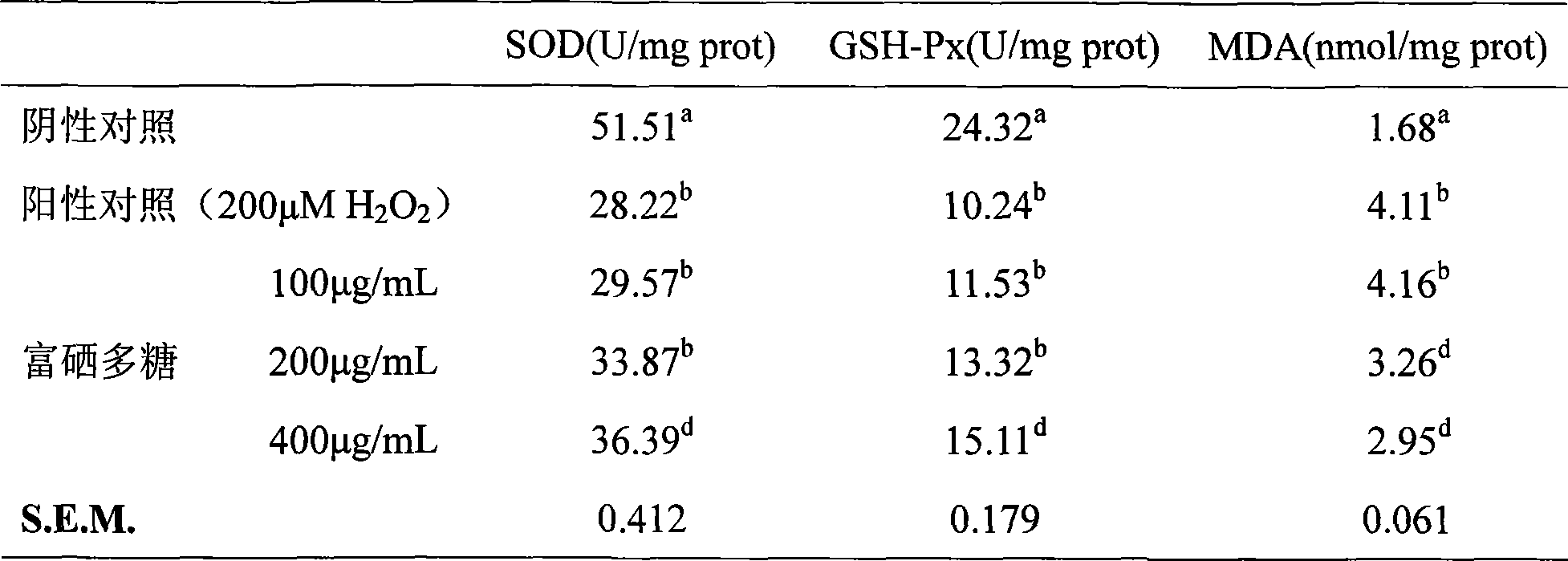
The invention discloses an Enterobacter cloacae Z0206, which has been stored in 'China microorganism strain preservation management committee common micro organism center' at December 3rd, 2007 and whose storage number is CGMCC NO 2279; also discloses is an Enterobacter cloacae Selenium-rich polysaccharide preparation method and applications of Selenium-rich polysaccharide. The invention has advantages that: the invention has simple production process of extracting the Selenium-rich polysaccharide, high product yield and higher purity, higher Selenium-rich levels; the selenium-enriched polysaccharide prepared by the method can significantly improve the lymphopoiesis capability as well as cytokine level of mice with low immune capacity, and has significant role in the regulation of the immune function of mice with low immune capacity; the selenium-enriched polysaccharide prepared by the method can significantly improve the activity of anti-oxidase, to enhance the function of the antioxidant defense system, has a clear protective effect to the oxidative damage of RAW264.7 cells induced by H2O2, which is an effective antioxidant.
Owner:ZHEJIANG UNIV
Recombinant staphylococcus aureus enterotoxin M and its prepn and application
InactiveCN101066993ATypical superantigen activityGrowth inhibitionBacteriaPeptide/protein ingredientsAntigenStaphylococcus cohnii
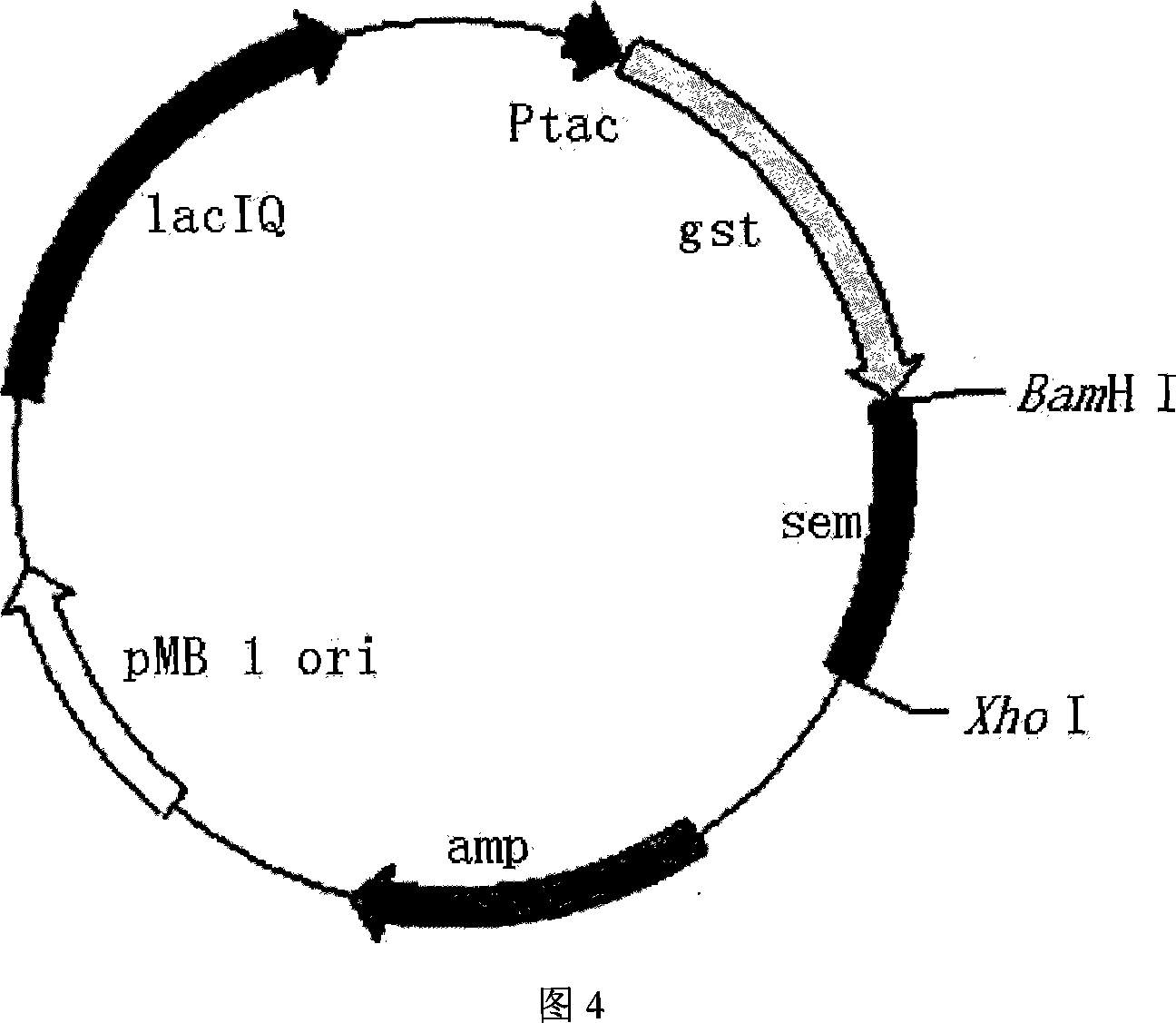
The recombinant staphylococcus aureus enterotoxin M as one kind of superantigen with the amino acid sequence as shown in SEQ ID No. 1 is obtained through recombining gene originated from staphylococcus aureus and coding SEM with one kind of plasmid vector, transforming to proper host for expressing, and affiliation purifying. It is proved through extracorporeal mouse spleen lymphopoiesis experiment and tumor cell inhibiting experiment that the recombinant protein has typical superantigen activity stronger than that of SEC and may be applied in preparing medicine for stimulating lymphopoiesis and inhibiting tumor cell growth. The present invention is suitable for preparing high purity enterotoxin with superantigen activity and developing superantigen preparation. The present invention has reasonable design, efficient expression on recombinant SEM in the expression strength of about 15-25 % of staphylococcus aureus protein, and other advantages.
Owner:ZHEJIANG UNIV
Staphylococcus aureus enterotoxin 1 and preparation and use
InactiveCN1900119ATypical superantigen activityGrowth inhibitionAntibody medical ingredientsHybrid peptidesAntigenNucleotide
The present invention provides a kind of recombinant staphylococcus aureus enterotoxin I prepared through genetic engineering process, and the protein is one kind of super antigen with the amino acid sequence of SEQ ID No. 1. The recombinant plasmid is reconstructed with pGEX-4T-1 and the nucleotide sequence of SEQ ID No. 2. Extracorporeal experiment shows that the prepared high purity recombinant SEI protein can excite the splenic lymphopoiesis of mouse effectively in dosage depending relationship and the excited splenic lymphocyte of the mouse has obvious tumor cell killing effect. Compared with available staphylococcus aureus enterotoxin C as the main effective component in the staphylococcus aureus filtrate preparation, the recombinant SEI protein has even high super antigen activity and may be prepared into super antigen preparation for inhibiting tumor growth effectively.
Owner:HANGZHOU MINSHENG PHARM CO LTD
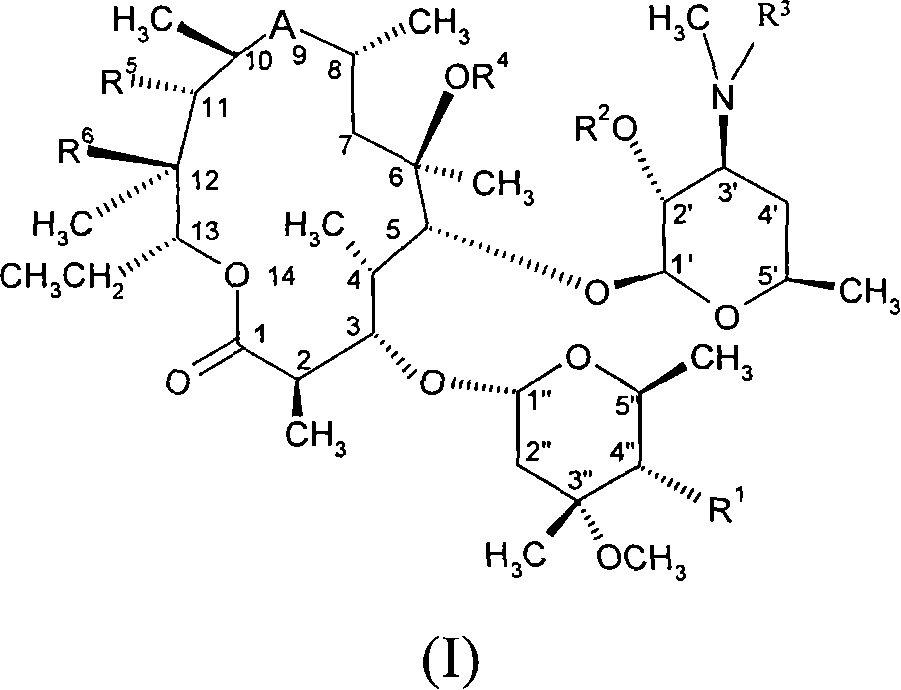
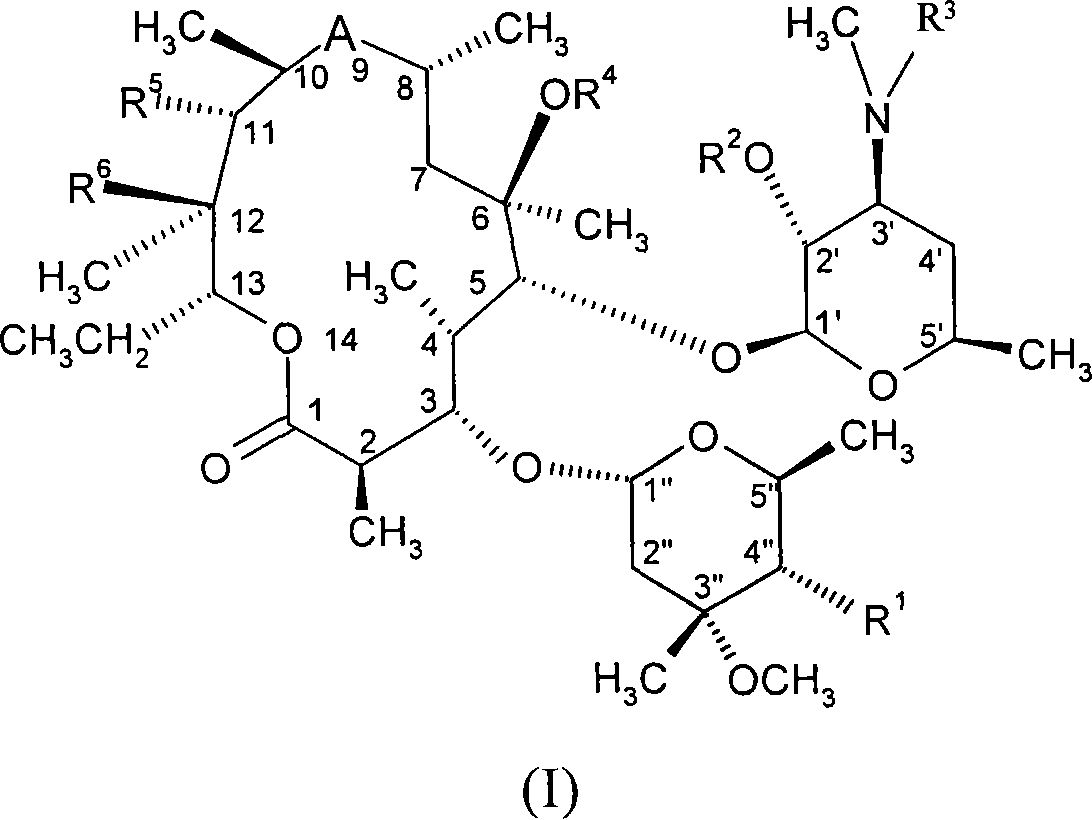
Macrolides with anti-inflammatory activity
The present invention relates to novel semisynthetic macrolides with anti-inflammatory activity. More specifically, the present invention relates to 14- and 15-membered macrolides substituted at the 4" position, their pharmaceutically acceptable derivatives, their preparation methods and intermediates, pharmaceutical compositions containing them and Their activity and use in the treatment of inflammatory diseases and conditions in humans and animals, especially those associated with excessive secretion of TNF-alpha, IL-1, IL-8, IL-2 or IL-5; and / or for use as Activity and uses of inhibitors of lymphocyte hyperproliferation and / or granulocyte degranulation.
Owner:GLAXO GROUP LTD
Composition having effect of enhancing immunity function and usage thereof
InactiveCN101559214AEnhance immune functionImprove proliferative abilityOrganic active ingredientsPeptide/protein ingredientsBiotechnologyLymphocyte
The invention discloses a composition having effect of enhancing immunity function, which is prepared by mixing marine protein peptide and nucleotide according to the weight ratio of 45:8 and can be used for preparing medicine, health-care food or food that can enhance immunity function. The obtained preparation has the characteristics of easy and rapid absorption, low viscosity, good water solubility, etc. The preparation can remarkably enhance the mouse delayed-type hypersensitivity (DTH) capability and T lymphopoiesis stimulated by ConA, and obviously increases the quantity of antibody-producing cells. The result proves that the compatibility of the marine protein peptide and the nucleotide can enhance the enhancing immunity function.
Owner:珍奥集团股份有限公司
Rebuild golden staphylococcus enterotoxin N and preparation and application thereof
InactiveCN101037478ATypical superantigen activityGrowth inhibitionPeptide/protein ingredientsDepsipeptidesAntigenStaphylococcus cohnii
A recombinant Methicillin-resistant Staphylococcus aureus enterotoxin N belongs to a super antigen with SEQ ID NO.1 amino acid sequence, which is expressed by a recombination of a gene coding SEN from the Staphylococcus aureus and a plasmid vector, and a transformation to the proper host and is obtained a recombinant a highly purified SEN protein by affinity purification. The invention proves the recombinant protein has a typical super antigen activity better than SEC and can be applied in the preparation of activating the lymphocytes multiplication and inhibiting the tumor cell proliferation. The product is suitable for preparing the highly purified enterotoxin with the super antigen activity and exploiting super antigen agent. The invention has a wise design, purifies the target protein with affinity chromatography, has a simple process and a high purification speed.
Owner:ZHEJIANG UNIV
Prepn and application of recombinant staphylococcus aureus enterotoxin O
ActiveCN101045924ATypical superantigen activityGrowth inhibitionBacteriaDepsipeptidesAntigenNucleotide
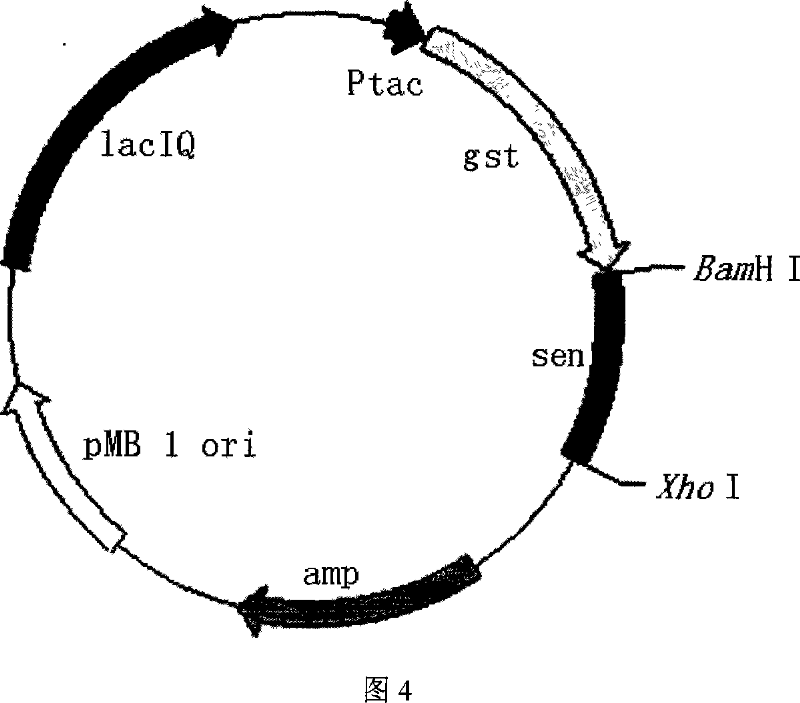
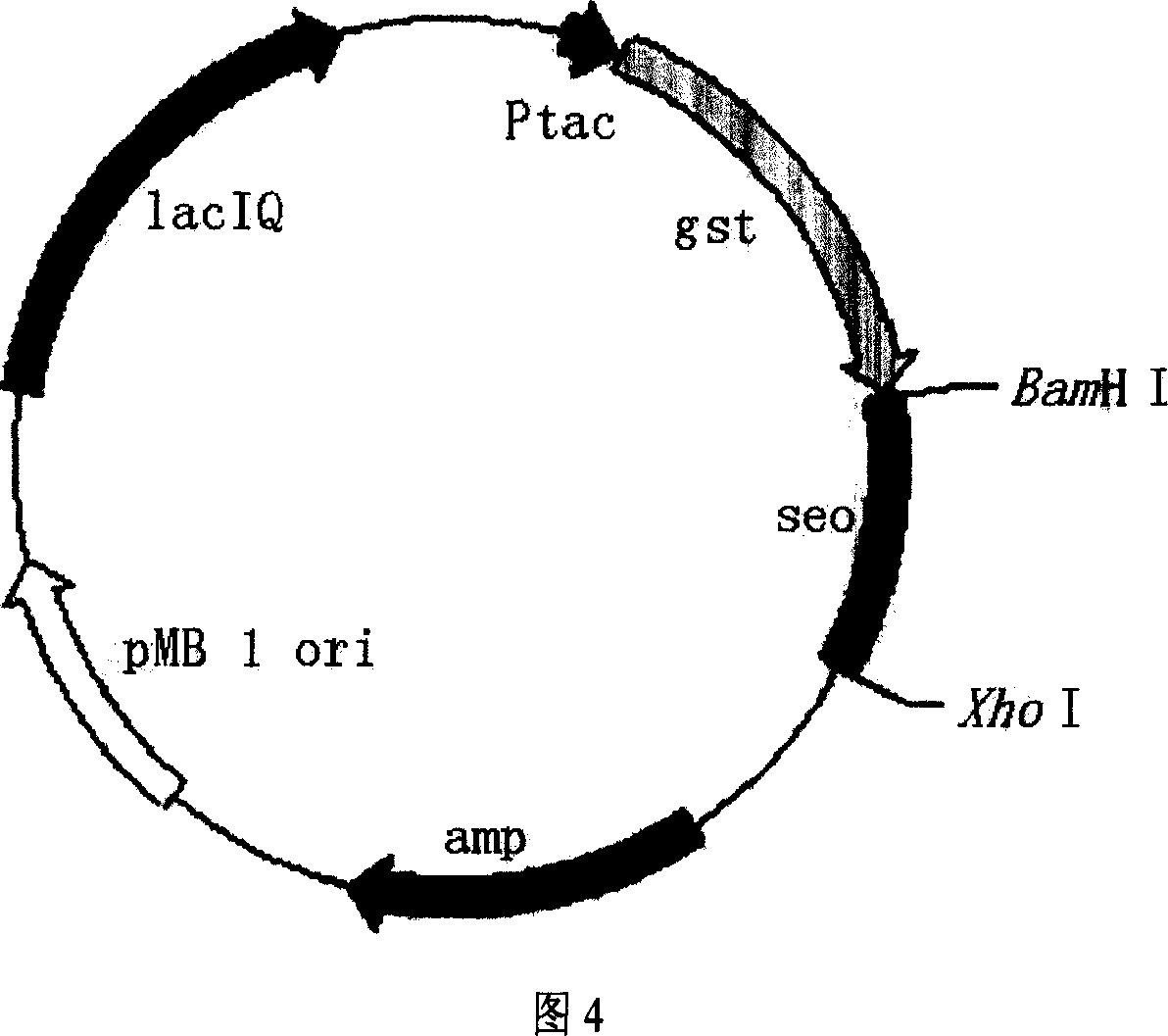
The present invention provides process of preparing recombinant staphylococcus aureus enterotoxin O, as one kind of superantigen with the amino acid sequence as shown in SEQ ID No. 1. The present invention constructs one kind of recombinant plasmid for expression staphylococcus aureus enterotoxin O via recombining pGEX-4T-1 and the polynucleotide sequence of SEQ ID No. 1, and obtains initial fusion GST-SEO protein accounting for 15-25 % of total bacterial protein content. The present invention utilizes the recombinant staphylococcus aureus enterotoxin O with superantigen activity in promoting extracorporeal spleen lymphopoiesis and inhibiting extracorporeal tumor cell growth. It is proved that the recombinant staphylococcus aureus enterotoxin O has superantigen activity similar to that of SEC. The present invention is suitable for use in preparing high purity enterotoxin with superantigen activity and developing superantigen preparation.
Owner:HANGZHOU ENSHI GENE TECH DEV
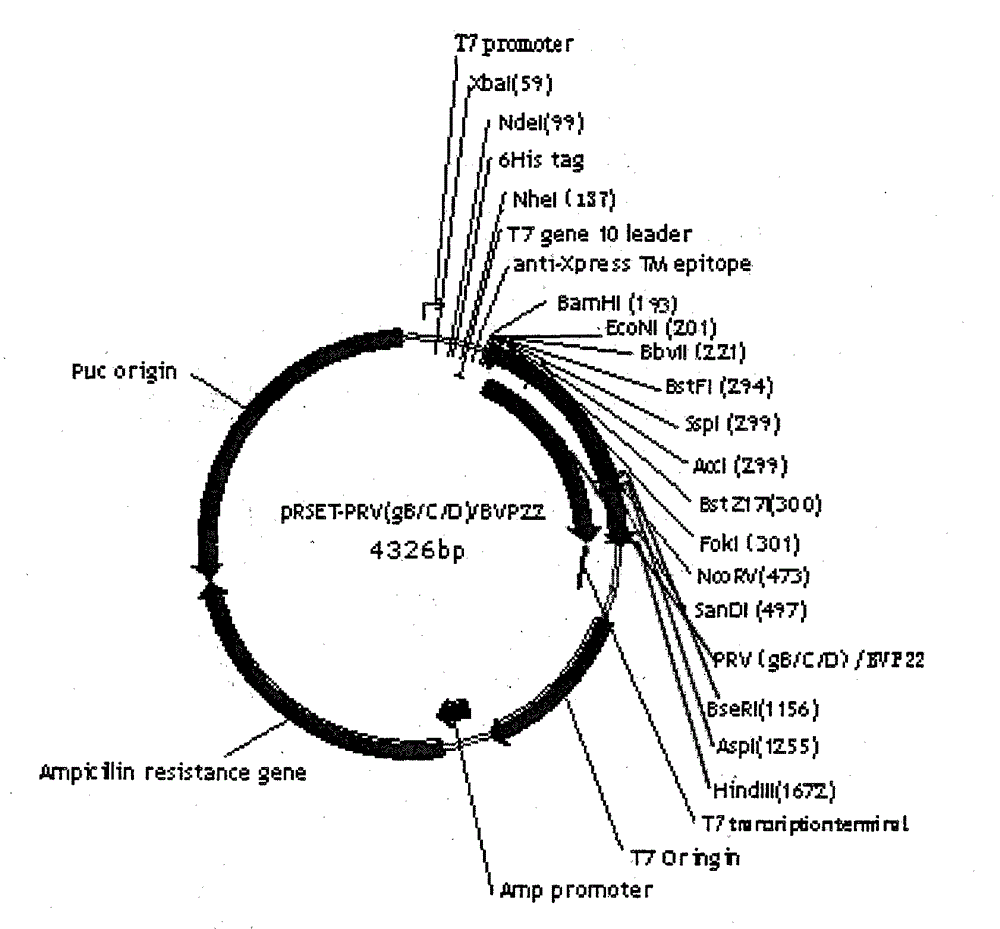
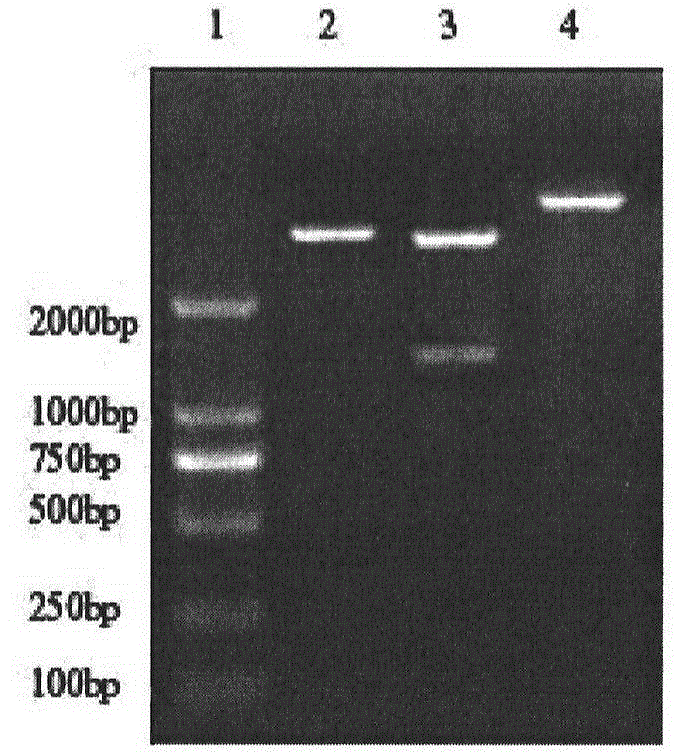
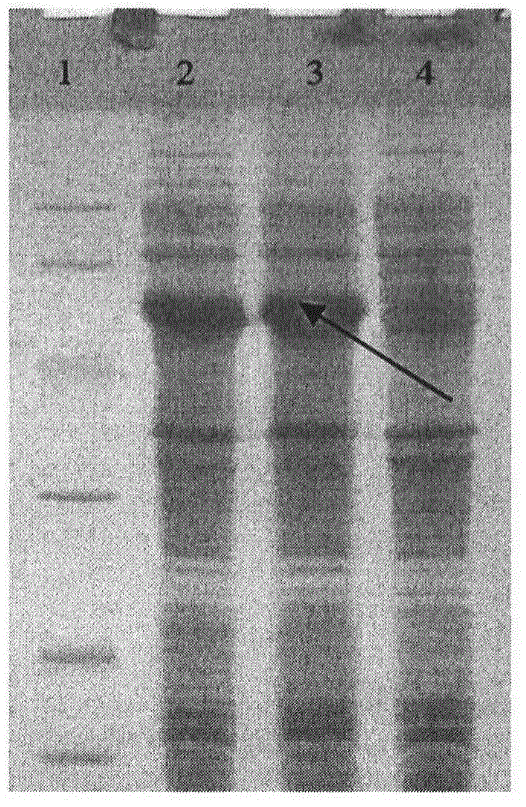
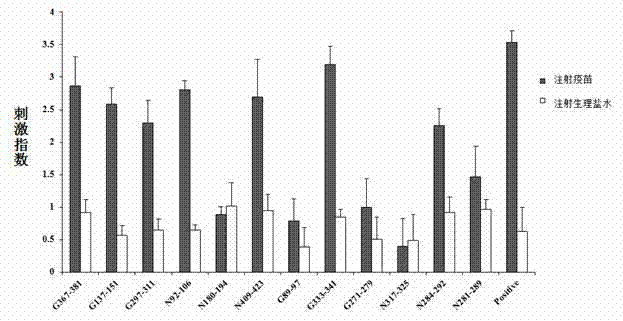
Pseudorabies virus epitope polypeptide gene engineering vaccine
The invention relates to preparation and application of a Pseudorabies virus (PRV) epitope polypeptide gene engineering vaccine. The vaccine uses PRV as the main glycoprotein, and B cell neutralizing epitope of gB, gC and gD and T cell immune epitope as the vaccine frame structure. The vaccine is prepared by the following steps: connecting the main glycoprotein with the vaccine frame structure through a flexible linker, connecting in series with a molecular adjuvant Bovine Herpesvirus 1 (BHV-1) envelope protein VP22, cloning a pRSETB vector, transforming Escherichia coli, fermenting, purifying, emulsifying and the like to obtain the PRV epitope polypeptide gene engineering vaccine. The invention also relates to an application method of the vaccine. The animal experiment indicates that the PRV epitope polypeptide gene engineering vaccine has equivalent effect to the live virus vaccine in the aspect of humoral immunity and cellular immunity level, and can be stimulated to generate T lymphopoiesis immunoreaction on the cellular level and generate antibody immunoreaction with virus neutralizing activity on the humoral level.
Owner:QINGDAO MINGQIN BIOLOGICAL TECH CO LTD
Rabies virus glycoprotein and nucleoprotein antigen epitope polypeptides, and screening and identification method and application thereof
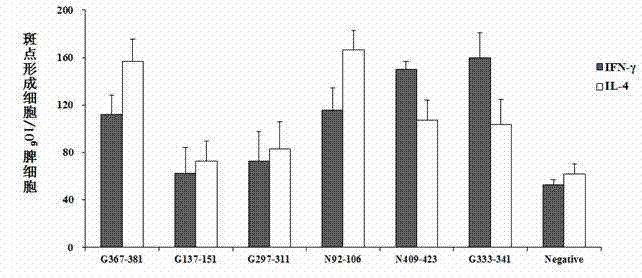
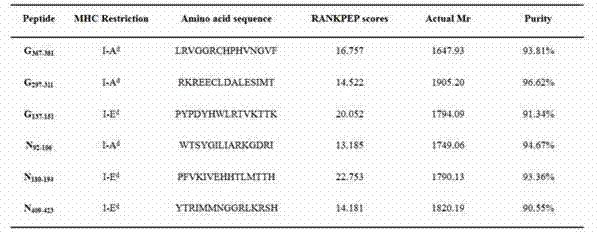
The invention belongs to the technical field of biology, and particularly relates to screening and identification of antigen epitope polypeptides. The invention discloses screening, identification and application of a series of rabies virus glycoprotein and nucleoprotein antigen epitope polypeptides. The rabies virus glycoprotein and nucleoprotein are predicted by biological information means to obtain the candidate epitope polypeptides; and a lymphopoiesis experiment, ELISPOT experiment and a stream-type cell method are utilized to carry out in-vitro experimental verification on the subsequent epitope polypeptides to obtain the four rabies virus protein antigen epitope polypeptides. The invention is characterized in that the antigen epitope polypeptides respectively comprise a Th epitope and a CTL epitope, can stimulate the lymphopoiesis of the vaccine-immunized mouse in vitro and induce the cells to secrete related cell factors, and have the functions of killing virus-infected cells and stimulating the generation of the antibody. The invention can be used for developing rabies virus epitope vaccines and detecting the vaccine effect, and has important value for developing and producing immunologic function detection kits for rabies virus vaccines.
Owner:FUDAN UNIV
Androstane-4, 6, 8 (9), 13 (14)-tetraene-3, 11, 16-triketone and application thereof
InactiveCN103073608AGood inhibitory effectEnhanced inhibitory effectOrganic active ingredientsSteroidsBALB/cPhysiology
The invention discloses a polyene high-oxidation androstane compound, i.e., androstane-4, 6, 8 (9), 13 (14)-tetraene-3, 11, 16-triketone. The compound is extracted from epigynum auritum plant. Experiments prove that the compound has an obvious suppression effect on Balb / c mouse spleen lymphopoiesis stimulated by ConA (concanavalin) and has activity equivalent to that of the conventional immunosuppression active substances, i.e., triptolide and dexamethasone.
Owner:KUNMING UNIV OF SCI & TECH
Method for enhancing proliferation or differentiation of a cell using OB protein
InactiveUS20050272656A1Easy to implantFacilitates mobilizationPeptide/protein ingredientsDepsipeptidesMyelopoiesisIn vivo
Uses for WSX ligands in hematopoiesis are disclosed. In particular, in vitro and in vivo methods for stimulating hematopoiesis (e.g., myelopoiesis, erythropoiesis and especially, lymphopoiesis) using a WSX ligand (e.g., anti-WSX receptor agonist antibodies or OB protein), and optionally another cytokine, are described.
Owner:GENENTECH INC
Screening method of immunologic function evaluation indexes of anthropopathic nose-only rat
ActiveCN102735804AReflect immune dysfunctionSimple and sensitive immune function evaluation indexMaterial analysisLymphocyte proliferationSerum ige
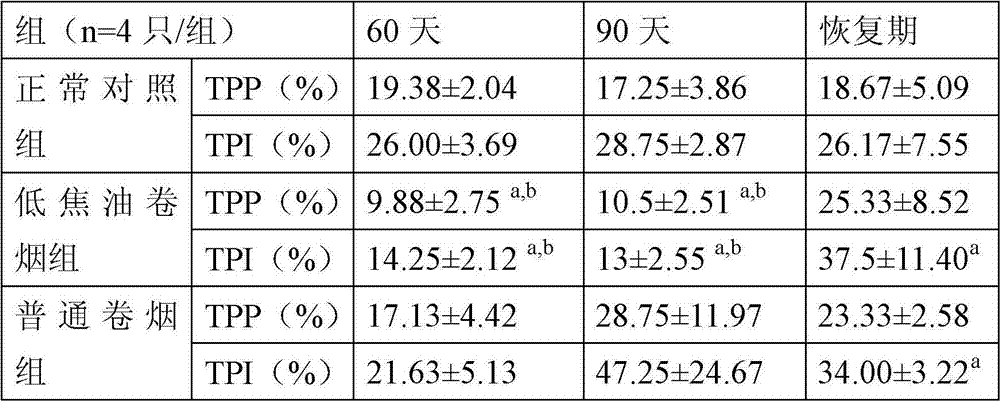
The invention discloses a screening method of immunologic function evaluation indexes of an anthropopathic nose-only rat. The screening method comprises the following steps: taking serum, splenic lymphocyte, splenic lymphocyte culture supernate, peritoneal macrophage and blood of an air breathing rat as a normal contrast group, and taking serum, splenic lymphocyte, splenic lymphocyte culture supernate, peritoneal macrophage and blood of a rat continuously smoking cigarettes through the nose as a smoking group; detecting the multiplication capacities of the lymphocyte, the swallowing conditions of the peritoneal macrophage, the contents of IL-6, TNF-alpha, Ig, IgG and IgE and the differential counting of leukocyte of each group; and finally, screening out five indexes of lymphopoiesis stimulation indexes, the contents of the IL-6, the IgG and the IgE and the differential counting of leukocyte as sensitive and stable evaluation indexes for reflecting the changes of the immunologic functions of the smoking rat. The method is scientific and comprehensive, and the screened evaluation indexes have good sensitive stability.
Owner:CHINA TOBACCO ZHEJIANG IND
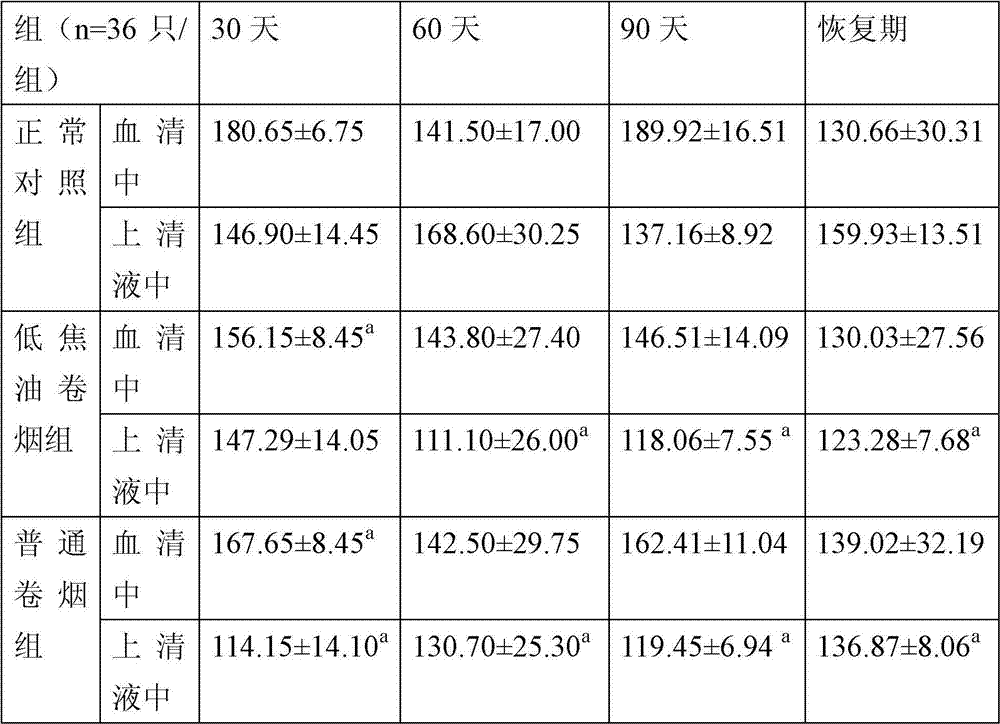
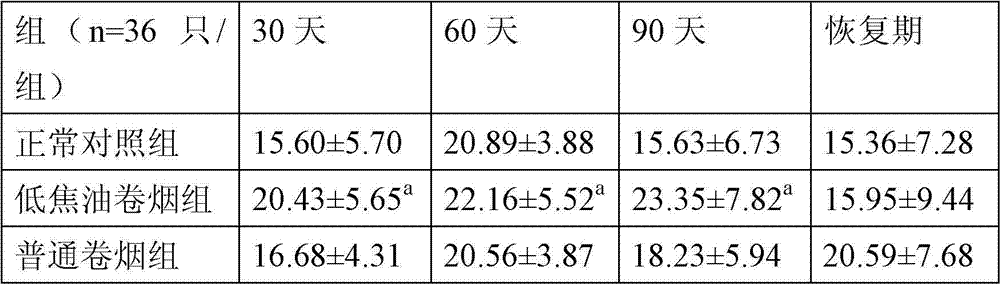
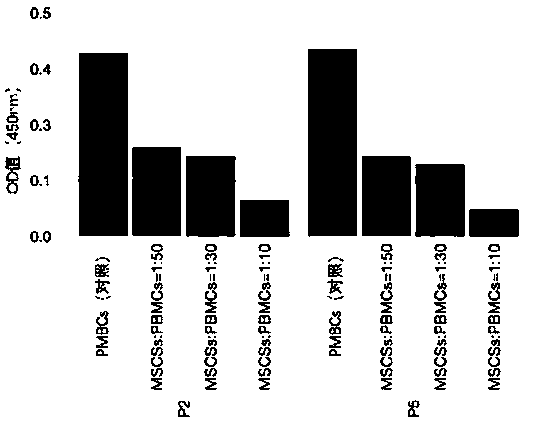
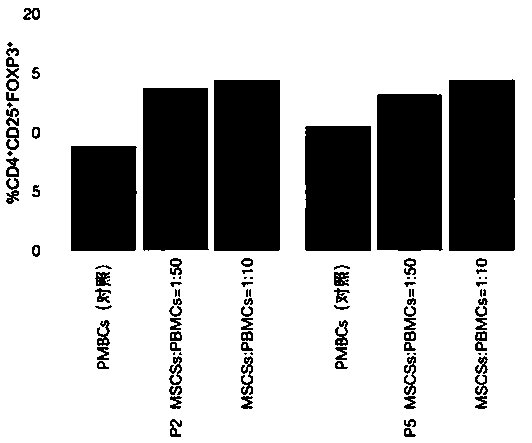
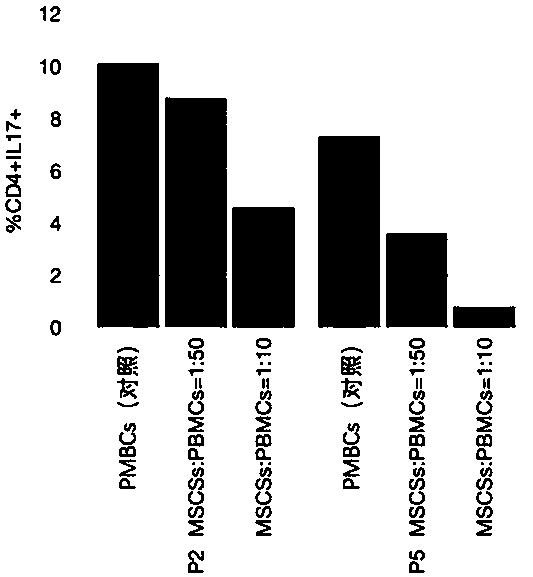
Detection method of immune functions of mesenchymal stem cells
PendingCN111154828AStrong immune functionQuality improvementMicrobiological testing/measurementMaterial analysisPeripheral blood mononuclear cellLymphocyte
The invention provides a detection method of immune functions of mesenchymal stem cells. The detection method of the immune functions of the mesenchymal stem cells comprises the following steps: (1) co-culturing umbilical cord mesenchymal stem cells and peripheral blood mononuclear cells so as to obtain a sample to be tested; and (2) detecting proliferative activity of lymphocytes and proportion of lymphocyte subsets in the sample to be tested. The detection method of the immune functions of the mesenchymal stem cells is suitable for non-diagnostic purposes. According to the detection method of the immune functions of the mesenchymal stem cells provided by the embodiment of the invention, the mesenchyma stem cells and the human peripheral blood mononuclear cells are co-cultured so as to obtain the sample to be tested; and then, the proportion of the lymphocyte subsets and the proliferative activity of the lymphocytes in the sample to be tested are detected so as to evaluate the immunoregulatory capacity of the mesenchymal stem cells. The greater the changes of the proportion of the lymphocyte subsets and the proliferative activity of the lymphocytes are, the stronger the immune functions and the better the quality of the mesenchymal stem cells are.
Owner:深圳市芙丽嘉科技有限公司
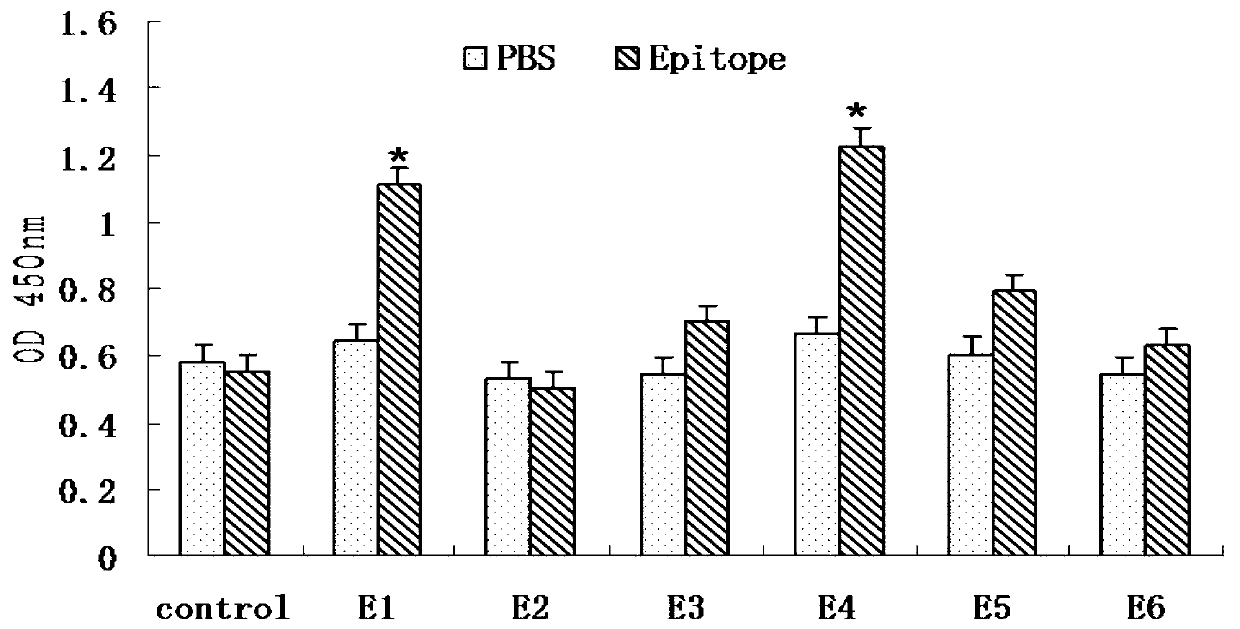
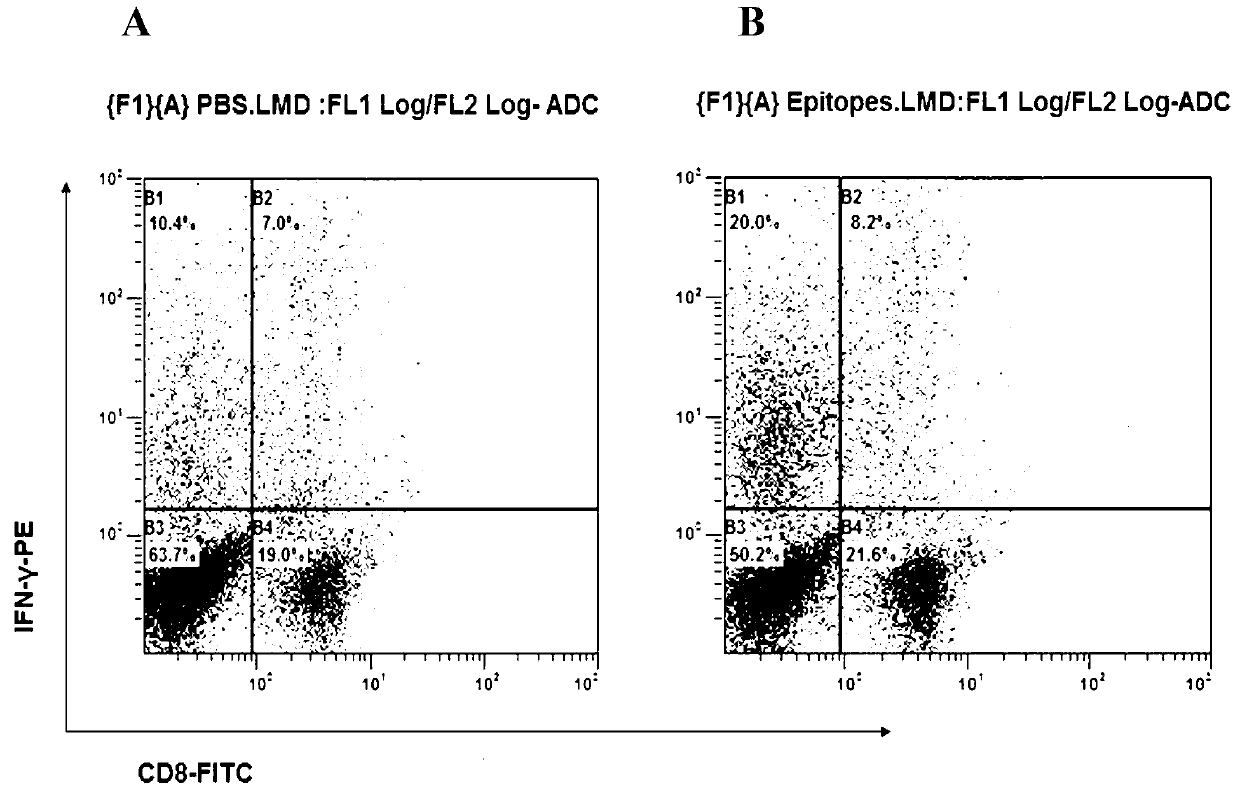
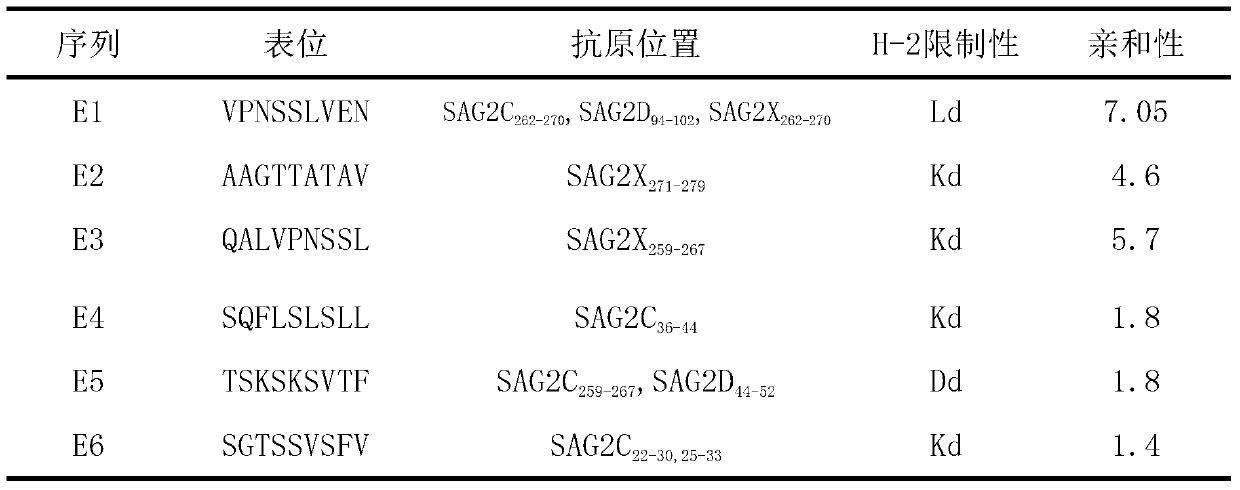
CD8+T cell dominant epitopes based on toxoplasmagondii bradyzoite antigens
ActiveCN103275182AProtozoa antigen ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsBALB/cActive immunization
The invention discloses two CD8+T cell dominant epitopes of VPNSSLVEN and SQFLSLSLL based on toxoplasmagondii bradyzoite specific surface antigens, wherein six CD8+T cell epitopes with H-2 restriction and high affinity are screened according to the protein sequences of the bradyzoite specific surface antigens of SAG2C, -2C and -2X; BALB / c mice are actively immunized by the screened epitope polypeptides; determinations for a lymphopoiesis level and a cell factor content, and an immune protection evaluation for epitope vaccines are performed after the immunization. The result indicates that powerful cell immunization can be generated by inducing the mice via the epitope vaccinesm, wherein the two antigen peptides of VPNSSLVEN and SQFLSLSLL are provided to be great in the capacity of stimulating T cell proliferation, as well as capable of inducing the mice to secrete protective cell factors and immune protection, being used as the epitope vaccines effectively resisting toxoplasmagondii infection, and reducing an encystations rate in the brain tissue of a host.
Owner:SHANDONG UNIV
Compound Chinese herbal medicine immunopotentiator for animal and preparation method thereof
InactiveCN101474272AEnhance immune functionSimple recipeImmunological disordersPlant ingredientsSide effectHigh pressure
The invention discloses a formulation of compound Chinese herbal medicine immunopotentiator used for animals and a preparation method thereof. The compound immunopotentiator is mainly prepared by raw material compositions with the following parts by weight: 5-15 parts of oldenlandia diffusa, 30-50 parts of astragalus root and 30-50 parts of dangshen. The preparation method comprises the steps as follows: firstly, the raw material compositions are crushed, sieved and evenly mixed according to the parts by weight, de-ionized water of 8-15 weight times is added into the mixture, then the mixture is decocted, after the mixture is sieved while hot, the filtrate is combined and separately packaged after vacuum concentration, therefore, the immunopotentiator is prepared by high pressure sterilization. The immunopotentiator is a pure Chinese herbal medicine, has no toxic side effect or harmful residue and does not produce the three wastes or pollutants to the environment; the formulation is simple and the effect is excellent, the immunopotentiator can obviously facilitate lymphopoiesis, greatly improve the humoral immunity function of animals, promote the secretion of cytokines in blood such as IL-2, IFN-gamma, and the like, thus various immunologic functions of the organism can be comprehensively improved.
Owner:广东省农业科学院兽医研究所
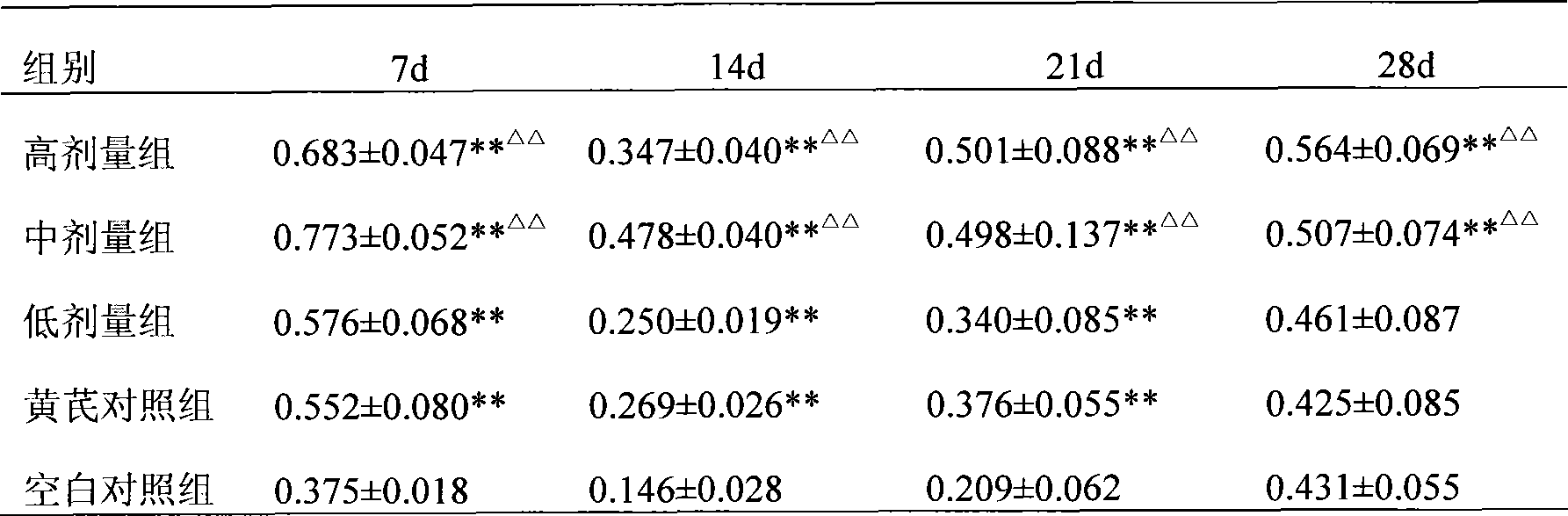
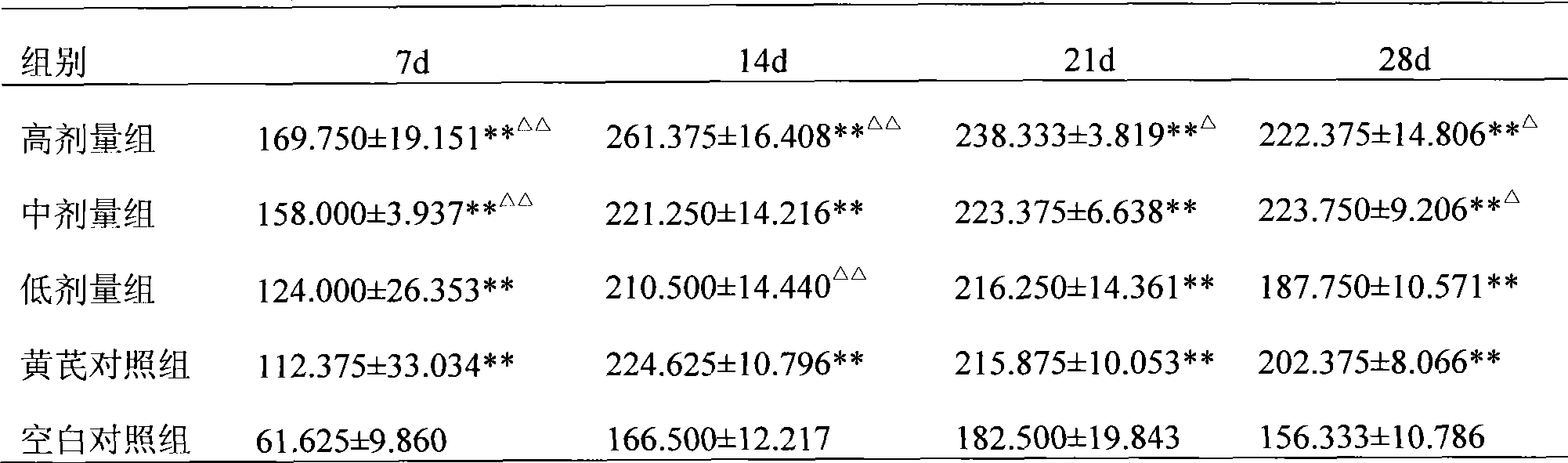
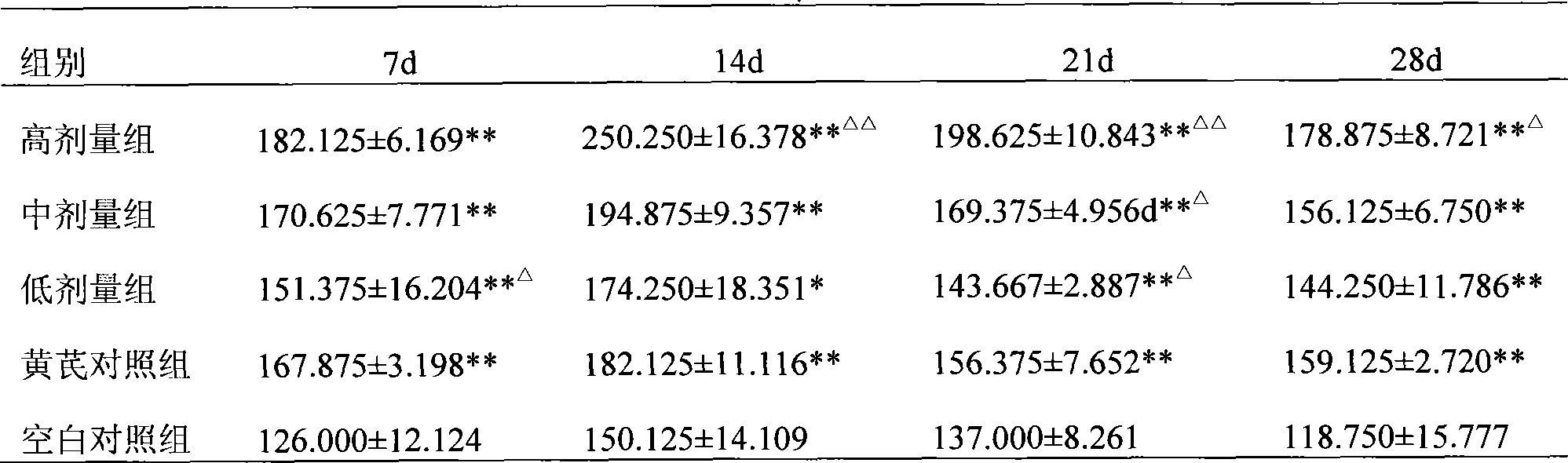
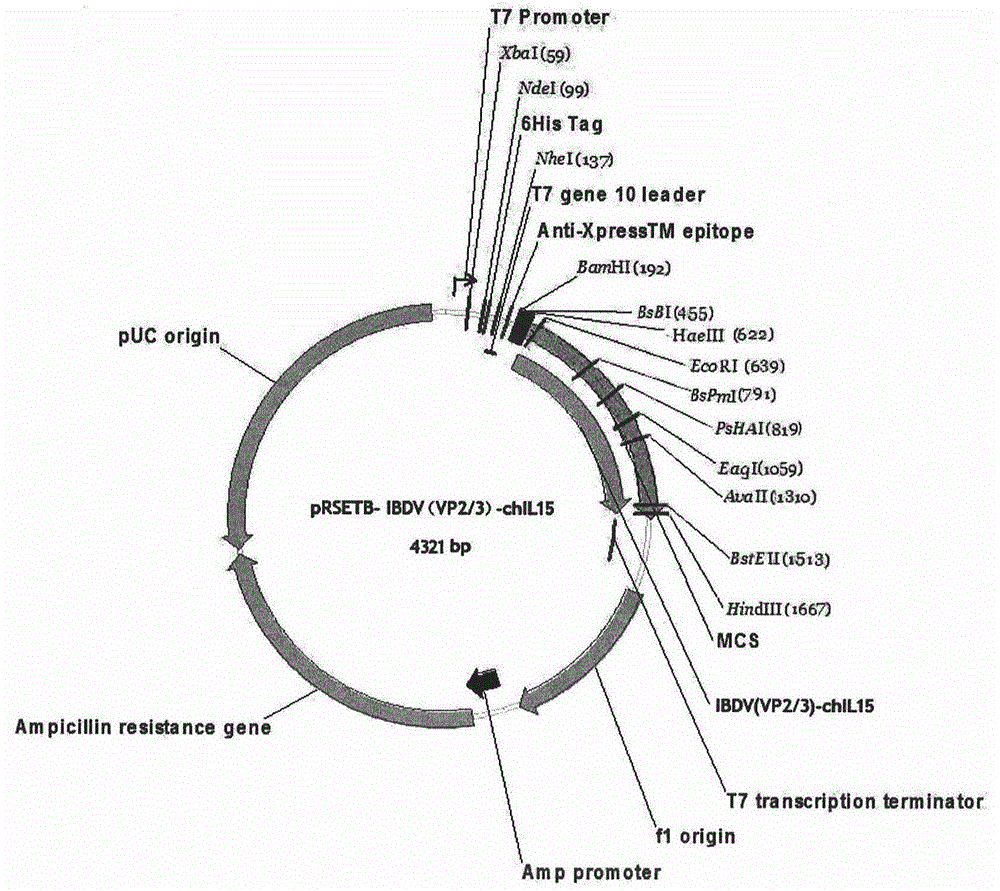
Preparation of infectious bursal disease (IBD) protein engineering vaccine
The invention relates to preparation and application of an infectious bursal disease (IBD) protein engineering vaccine. The preparation method comprises the following steps: connecting B cell neutralizing epitope and T cell immunizing epitope of IBDV (infectious bursal disease virus) primary structure proteins VP2 and VP3 as a vaccine frame structure through a flexible linker, connecting with cell factor poultry interleukin 15(chIL-15) in series, cloning into a pRSETB vector, transforming Escherichia coli, fermenting, purifying, emulsifying and the like to obtain the IBD protein engineering vaccine with ideal immunogenicity. The invention also relates to an application method of the vaccine. The animal experiment indicates that the IBD protein engineering vaccine has equivalent effects to the live virus vaccine on the humoral immunity and cellular immunity level, and can stimulate the generation of T lymphopoiesis immunoreaction on the cellular level and the generation of antibody immunoreaction with virus neutralizing activity on the humoral level. The attacking protection test indicates that the IBD protein engineering vaccine group has obviously better effects than the live vaccine group.
Owner:QINGDAO MINGQIN BIOLOGICAL TECH CO LTD
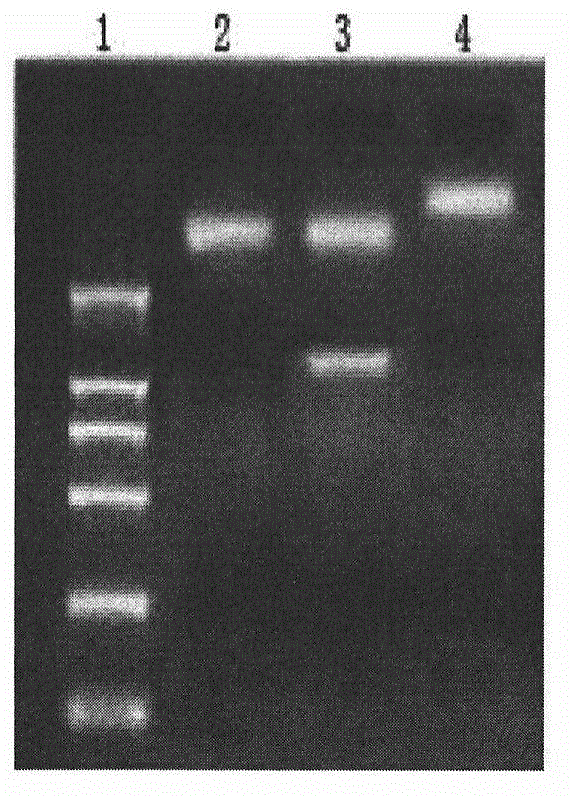
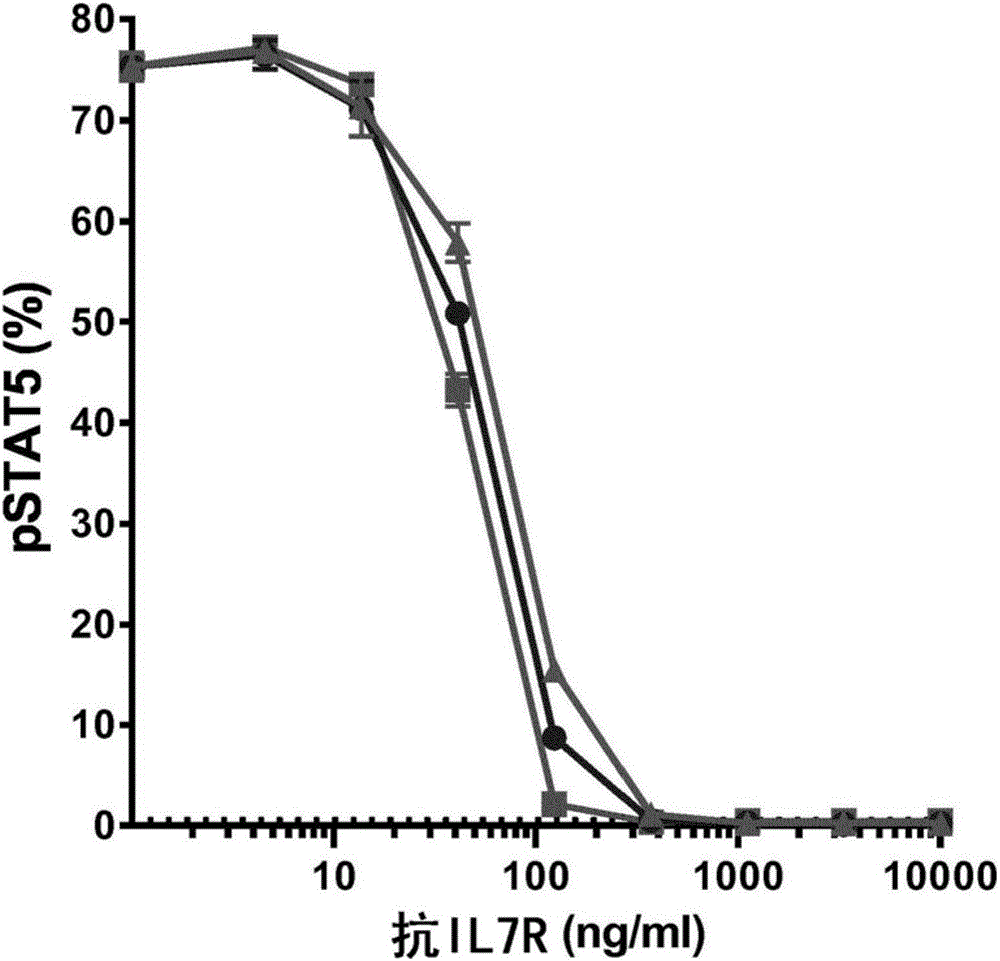
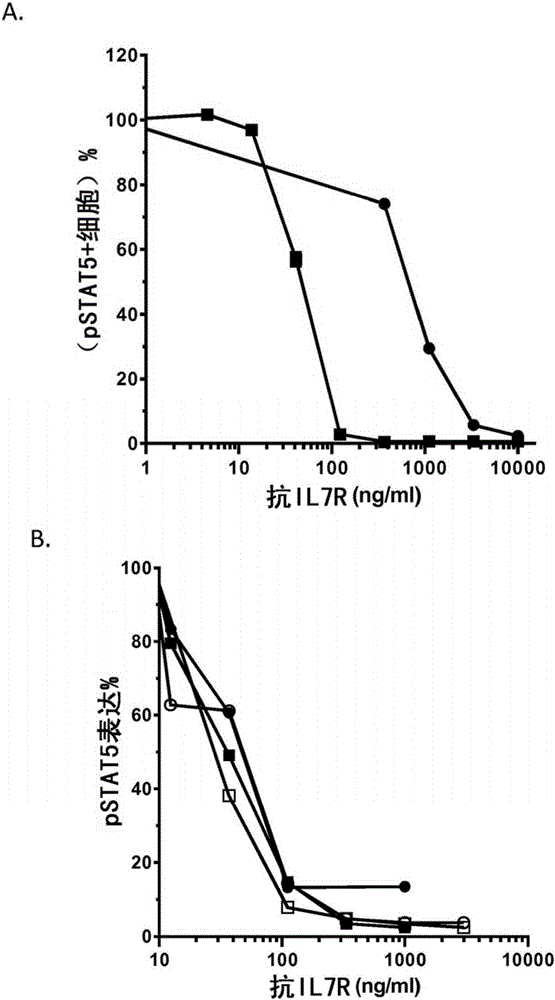
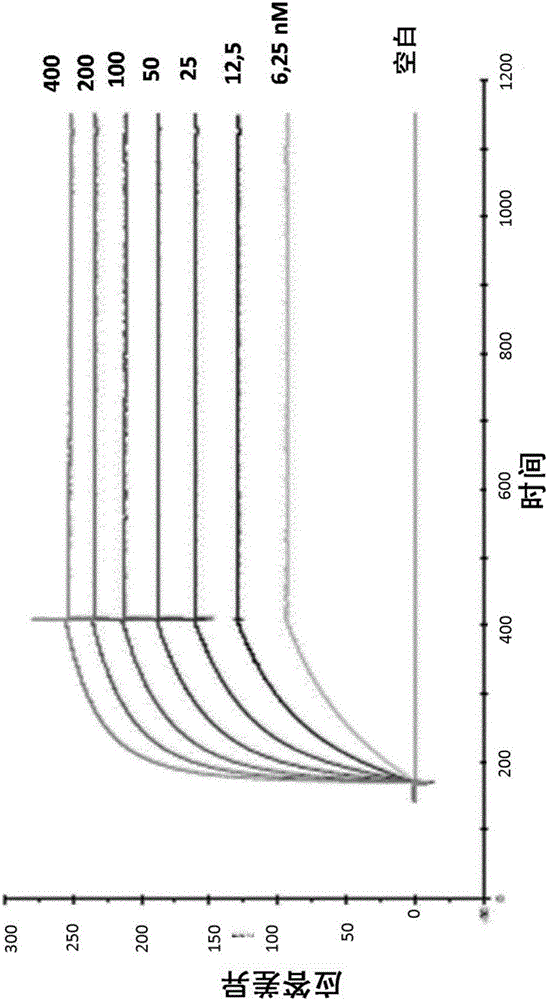
Antibodies directed against cd127
The invention relates to antibodies directed against CD127, the alpha chain of the interleukin 7 (IL-7) receptor IL-7R), and which have antagonist properties for IL-7-IL-7R interaction, may present cytotoxic activity against CD127 positive cells but do not increase the maturation of dendritic cells (DCs) induced by TSLP, a cytokine also using CD127 as part of its receptor. Alternatively, or in addition, these antibodies do not induce the internalization of CD127 and / or inhibit the IL7-induced internalization of CD127. According to another aspect of the invention antibodies are provided which recognize a human CD127 epitope comprising sequences from the 2b site of CD127, in particular the epitope comprising comprises the human CD127 sequences of domain D1 and of the 2b site of CD127, in particular the epitope comprises at least one sequence from D1 comprising SEQ ID No: 115 (in particular comprising SEQ ID No: 10) and / or SEQ ID No: 111 and / or a sequence from the 2b site comprising the sequence of SEQ ID No: 116 and optionally also comprises SEQ ID No: 117 (in particular comprises SEQ ID No: 111).The antibodies of the invention are suitable for use inorder to remedy to a condition diagnosed in a human patient which results from pathogenesis related to lymphopoiesis, when IL-7 signalling pathways provide contribution to said pathogenesis, especially when an increase in the maturation, more precisely the upregulation of costimulatory molecules, of dendritic cells is undesirable.
Owner:OSE IMMUNOTHERAPEUTICS
Controlled live tumor cell vaccine and preparation method and application thereof
InactiveCN101559220ASubmit fullyHigh potencyAntibody medical ingredientsFermentationAbnormal tissue growthSpecific immunity
The invention relates to a controlled live tumor cell vaccine and a preparation method and application thereof. The preparation method is characterized in that a suicide gene thymidine kinase TK and an immunoregulatory gene are allowed to transfect tumor cells by slow virus infection and liposome mediation to perform transgene, cells stably expressing the target gene are screened for cloning culture and expansion to obtain a double-gene modified tumor cell vaccine. The controlled live tumor cell vaccine can help activate lymphopoiesis of an immune system, generate a specific CTL killing efficacy and / or a non-specific NK killing efficacy and generate significantly enhanced anti-tumor immune response in a host. The controlled live tumor cell vaccine has higher titer, more complete tumor antigen presentation, and increased and stable expression of exogenous immunoregulatory genes, and better transgene efficacy, thus enhancing specific immune reaction induced by the tumor cell vaccine and increasing efficacy of the tumor cell vaccine.
Owner:GUANGZHOU GENERAL HOSPITAL OF GUANGZHOU MILITARY COMMAND
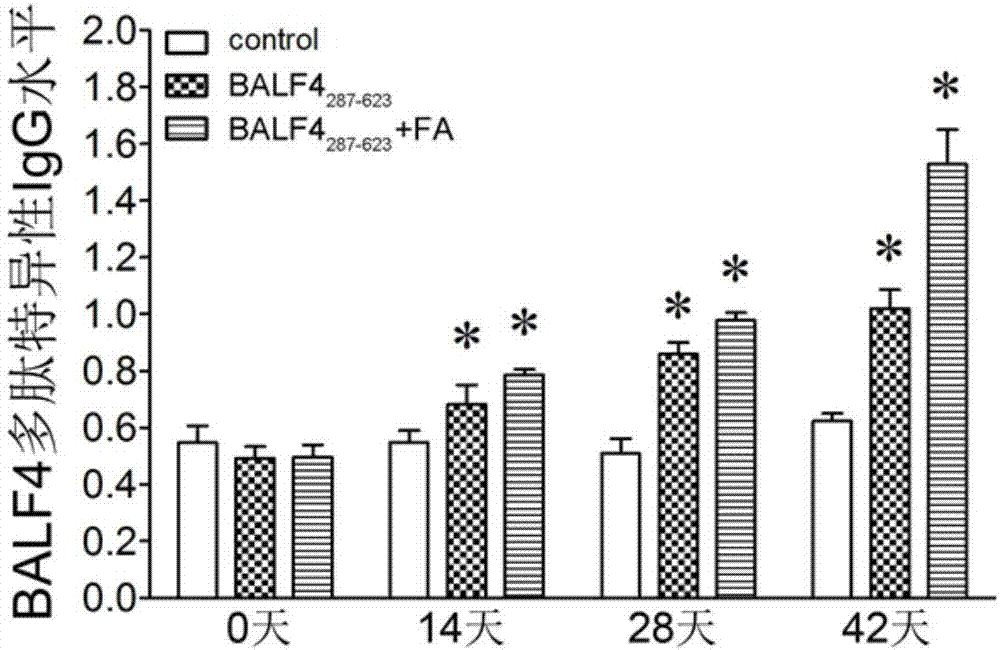
Clone, expression and application of BALF4 polypeptide
InactiveCN107163109ASimple purification methodStimulate the immune systemFungiViral antigen ingredientsYeastLymphocyte proliferation
The invention provides clone, expression and application of BALF4 polypeptide and relates to the technical field of molecular biology. According to the clone, the expression and the application, through gene optimization, an optimized nucleotide sequence encoding the BALF4 polypeptide is obtained, high-expression yeast cells for recombining the BALF4 polypeptide are constructed, a purification method is optimized, and the BALF4 polypeptide is obtained finally. The BALF4 polypeptide provided by the invention can stimulate production of a high-level antigen specific antibody, induces mouse splenic lymphocyte proliferative response, can effectively stimulate a body immune system, has stronger immunogenicity, can be used as an immune adjuvant and is used for improving the prevention effect on an EB virus vaccine so as to reach the purpose of effectively preventing EB virus infection.
Owner:QINGDAO UNIV
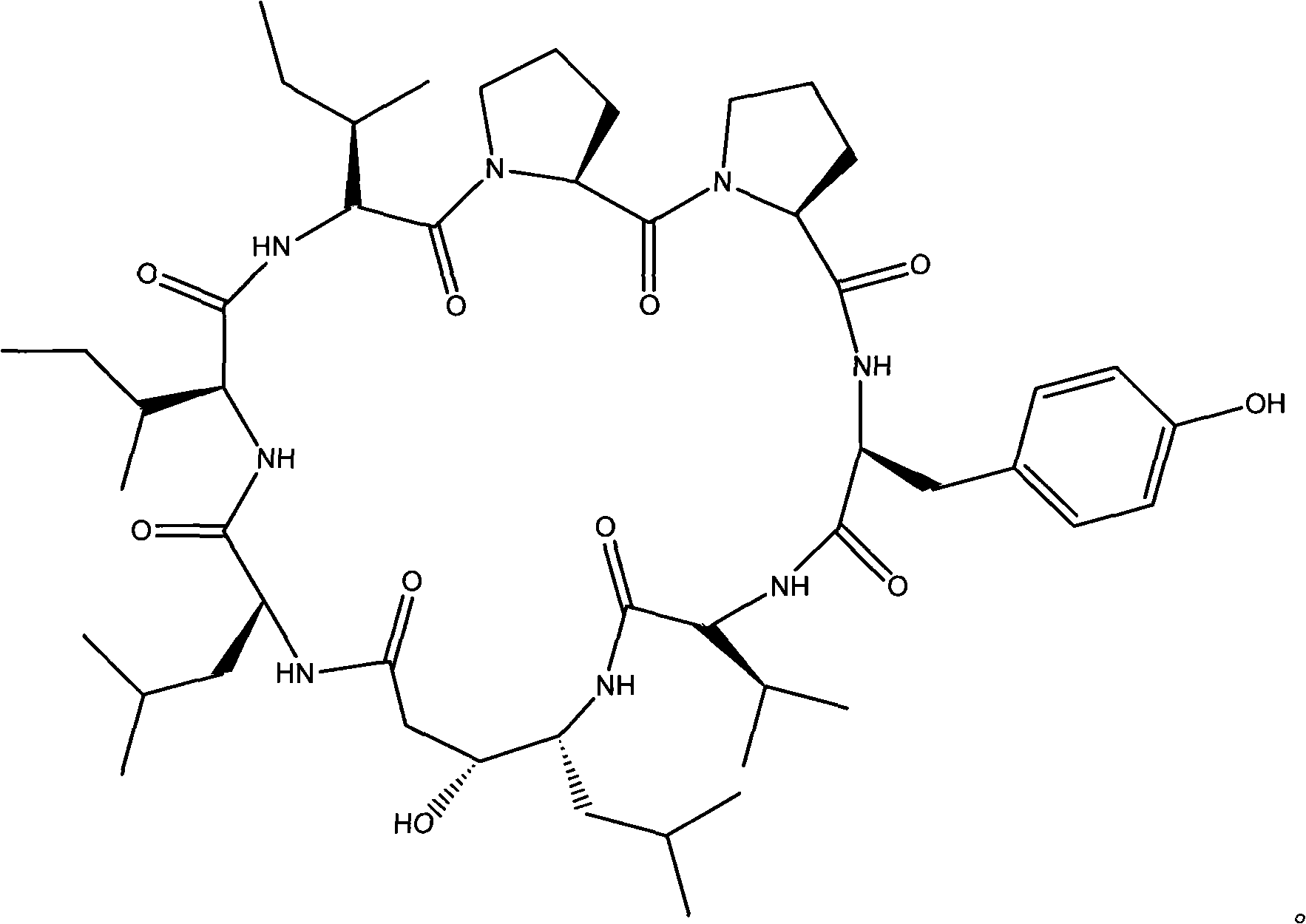
Cyclic peptide with -val-sta-leu- residue segment and used as immunity inhibitor and synthetic process thereof
ActiveCN101289498AVerify correctnessHigh yieldPeptide preparation methodsImmunological disordersCyclic peptideLymphocyte proliferation
The invention relates to a cyclic peptide which is provided with a residue fragment of -Val-Sta-Leu- and is used as immunosuppressive agents, and the synthesis process of the cyclic peptide (Ile-Ile-Pro-Pro-Tyr-Val-Sta-Leu) consists of 1) removing amino-protecting groups, 2) transpeptidase reaction, 3) the cutting of a peptide chain, 4) cyclization of the peptide chain. The cyclic peptide compounds which are provided with the residue fragment of Val-Sta-Leu- and used as immunosuppressive agents and have a high yield and a high purity can be obtained, and the immunosuppressive activity of the cyclic peptide compounds is higher. Through the experiments of the retarding effect of A3HS1 towards the delayed hypersensitivity of mice, the influence of phagocytic function of macrophages, and the retarding effect of the proliferation of lymphocytes, the A3HS1 shows a stronger immunosuppressive effect than HS-1, which reaches or surpasses the immunosuppressive of the positive contrast (cyclosporine).
Owner:CHINESE PEPTIDE CO
Preparation of gene immunization treating tumor used for vein
InactiveCN101940796AHigh transfection efficiencyGood immune regulationOrganic active ingredientsGenetic material ingredientsDendritic cellReverse transcriptase
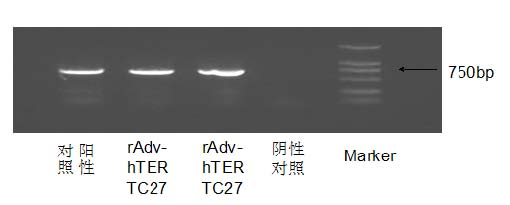
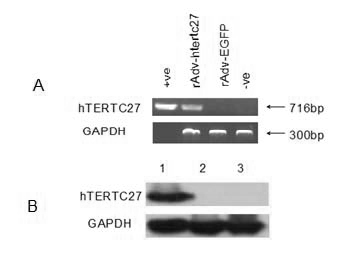
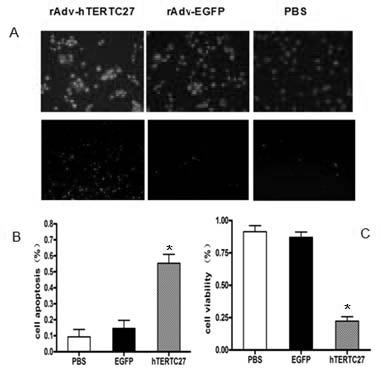
The invention discloses a preparation of gene immunization treating tumor used for veins, which is composed of replication deficient adenovirus loading human telomerase reverse transcriptase C-terminal polypeptide hTERTC27 and an acceptable pharmaceutic adjuvant. The anti-tumor gene preparation rAdv-hTERTC27 of the invention has high transfection efficiency, and is more effective on the increment and the apoptosis regulation of the tumor cell. The anti-tumor gene preparation of the invention can effectively lead the peptides transfection to enter into the dendritic cell DCs, so as to activate the DCs, secrete IFN-gamma, IL-2, increase and further stimulate T lymphopoiesis, promote the T lymphocyte to generate the special killing effect CTL corresponding to the tumor cell, and immunoregulation is carried out on the tumor.
Owner:SUN YAT SEN MEMORIAL HOSPITAL SUN YAT SEN UNIV
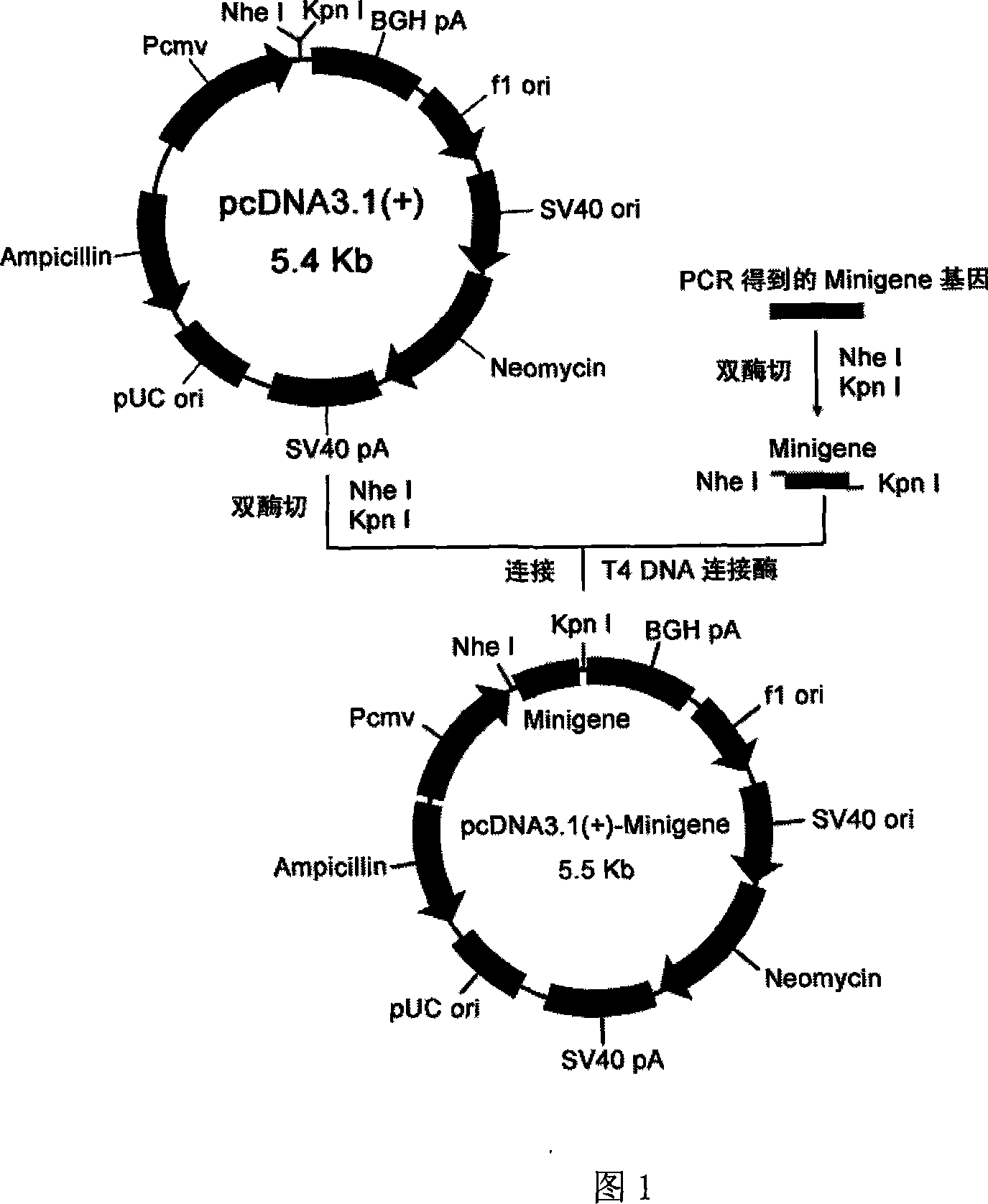
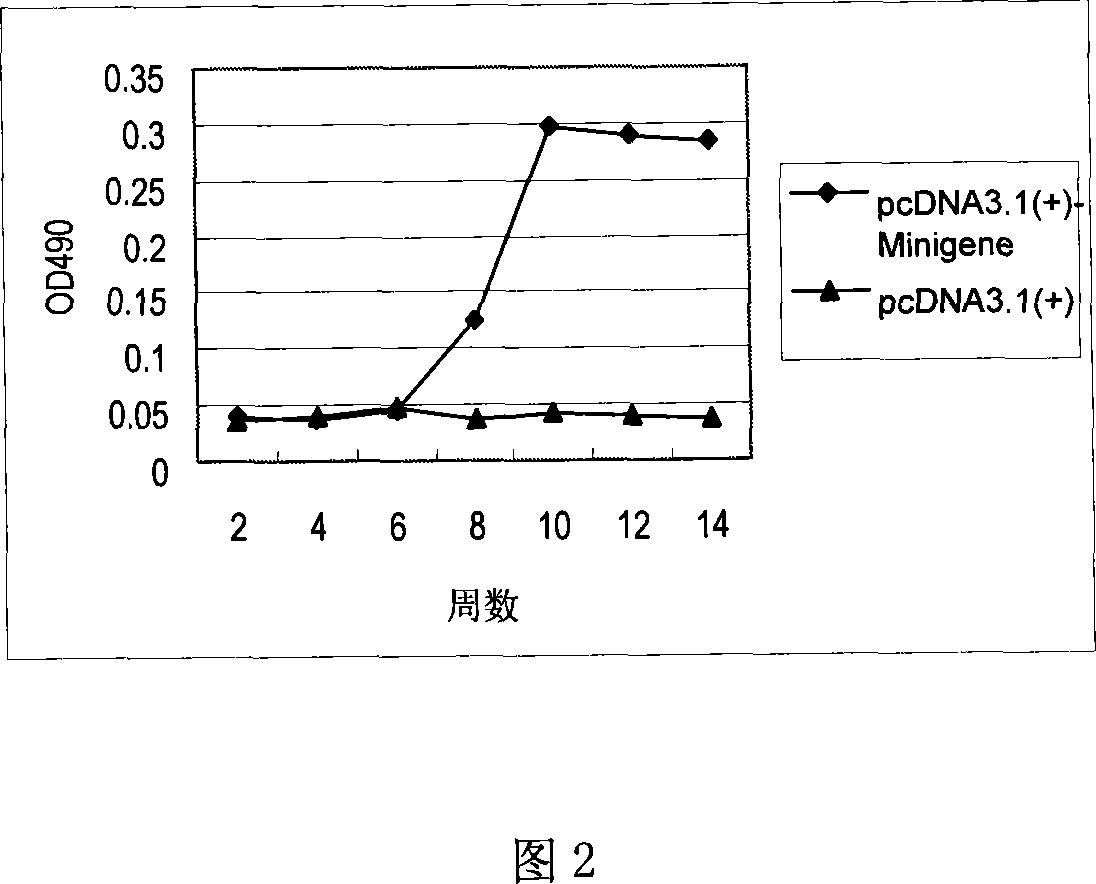
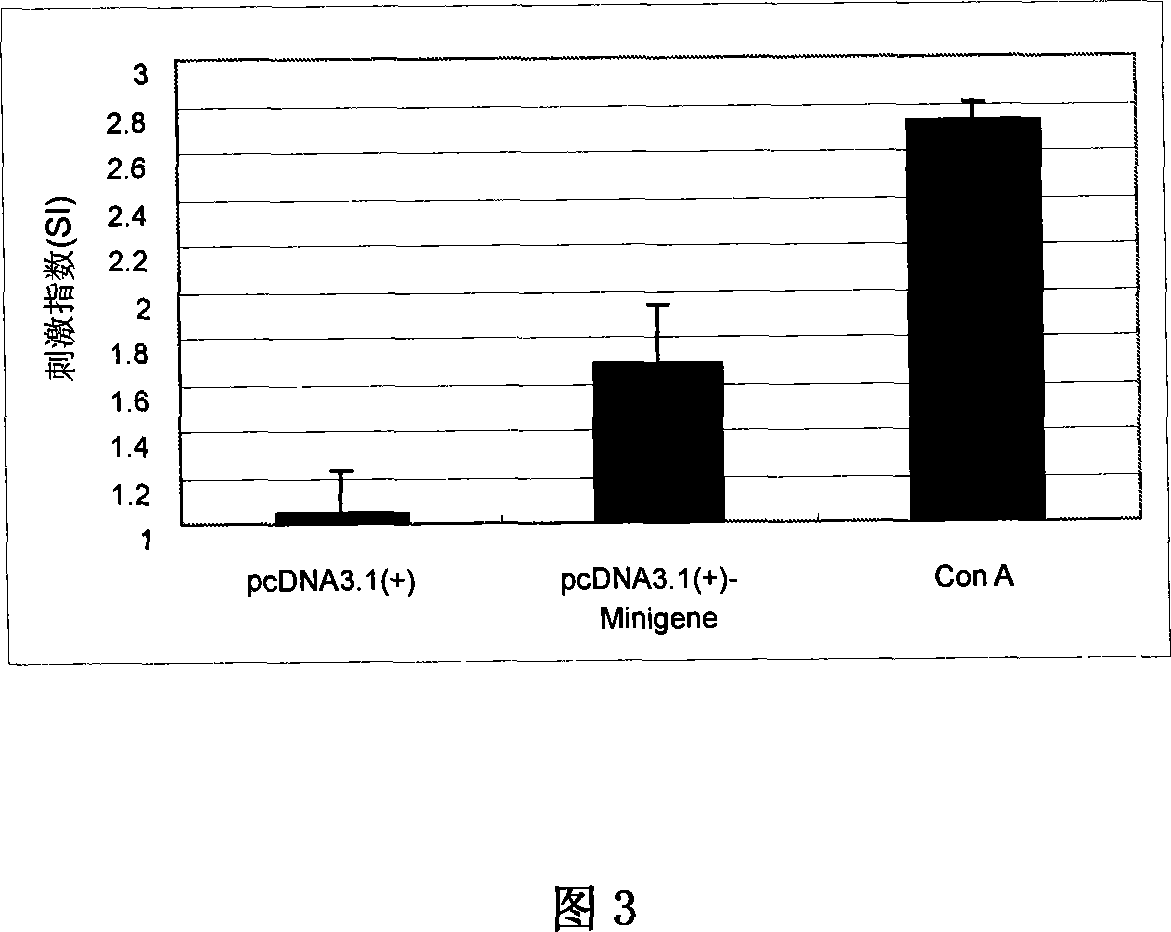
Staphylococcus aureau DNA vaccine pcDNA3.1(+)-Minigene and its prepn process
InactiveCN101066463AGood immune protectionEasy to makeAntibacterial agentsGenetic material ingredientsLymphocyte proliferationHumoral immune reaction
The present invention relates to one kind of staphylococcus aureus DNA vaccine pcDNA3.1(+)-Minigene and its preparation process. Immunological mouse test shows that the staphylococcus aureus DNA vaccine pcDNA3.1(+)-Minigene of the present invention can stimulate mouse body to generate obvious and lasting specific lymphopoiesis reaction and humoral immunity in certain level and in obvious difference (P<0.05) to that of pcDNA3.1 inoculating negative control group. In addition, the toxicity antagonizing test shows that pcDNA3.1(+)-Minigene plasmid can induce mouse to generate staphylococcus aureus resisting antibody to protect the immunized mouse.
Owner:JIANGSU FEIYA CHEM IND
Cyclic peptides with ¿CPro-Sta-Tyr- residue fragment as immunity inhibitor and synthesis process thereof
ActiveCN101284871AVerify correctnessHigh yieldPeptide preparation methodsImmunological disordersCyclic peptideLymphocyte proliferation
The invention relates to cyclic peptide used as immunosuppressant and carried with -Pro-Sta-Tyr- residue fragment as well as ring (Ile-Ile-Pro-Sta-Tyr-Val-Pro-Leu). The synthesis process of the invention is as follows: (1) amino protecting group is removed; (2) synthesized peptide is completed; (3) peptide chain cutting is carried out; (4) peptide chain cyclization is completed. Therefore, a cyclic peptide compound, which has high yield, high purity and -Pro-Sta-Tyr- residue fragment and can be used as immunosuppressant, can be obtained; moreover, the compound has higher immunosuppression activity. The experiments on the inhibition of A2HS1 on mouse delayed hypersensitivity, the influence on the phagocytic capability of macrophage and the inhibition on lymphopoiesis show that the A2HS1 represents stronger natural immunosuppression than HS-1 and reaches or exceeds positive contrast (cyclosporin) immunosuppression.
Owner:CHINESE PEPTIDE CO
Immunoregulation peptide, and preparation method and application thereof
InactiveCN104530212AStimulates the proliferation of T lymphocytesLow immunityMilk preparationPeptide/protein ingredientsImmunomodulatory peptideMedicine
The invention belongs to the fields of medicine and bioengineering, and discloses an immunoregulation peptide, and a preparation method and application thereof. The amino acid sequence of the immunoregulation is disclosed as SEQ ID NO:1. The immunoregulation peptide has high T lymphopoiesis promoting function, and can be used for preparing T lymphopoiesis promoting drugs.
Owner:NANJING MATERNITY & CHILD HEALTH CARE HOSPITAL
NK/T cell line of human
ActiveCN108220237AGood antitumor activityMicrobiological testing/measurementMammal material medical ingredientsDiseaseAbnormal tissue growth
The invention relates to a cell line, and specifically relates to a NK / T cell line of the human. The conservation number is CGMCC No. 14892. The NK / T cell line of the human provided by the invention can be passaged for a long time and greatly amplified, and has stronger anti-tumor activity, and provides a new experimental model for deeply development chronic activity EBV infectious disease mechanism research, screening chronic activity EBV infection, diagnosis molecular marker of EBV related lymphocytosis and the like, treating prognosis maker and treatment medicine, CD8+T cell, natural killercell anti-tumor action mechanism and clinic adoptive immunity treatment for a researcher.
Owner:INST OF GENETICS & DEVELOPMENTAL BIOLOGY CHINESE ACAD OF SCI
Compound Chinese traditional medicine preparation for inducing immune tolerance and preparing method and application thereof
ActiveCN105031555ASuppress immune rejectionEffective induction ofImmunological disordersPlant ingredientsMotilityCD86
The invention discloses a compound Chinese traditional medicine preparation for inducing immune tolerance and preparing method and application thereof. The compound Chinese traditional medicine preparation comprises 2-30 parts of roots of Chinese thorowax, 3-31 parts of moutan bark, 4-32 parts of radix paeoniae alba, 5-33 parts of radix curcumae and 6-35 parts of liquorice. The above components are prepared according to the proportion, to be smashed, soaked with water, decocted, filtered, centrifuged and dried to be made into capsules, oral liquid, tablets and the like. The compound Chinese traditional medicine preparation can induce generation of DC regs of high-expression CD11b and low-expression HLA-DR / CD86 / CD80, the DCregs generated through inducing can effectively inhibit DC-mediated lymphopoiesis and promote secretion of cytokines IL-10; a receptor mice is induced to tolerate the iso-allotransplantation skin; no influence on the motility rate of DC cells and the hepatorenal function of the receptor mice is generated, and the compound Chinese traditional medicine preparation is effective and low-toxic and induces immune tolerance.
Owner:TIANJIN MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com