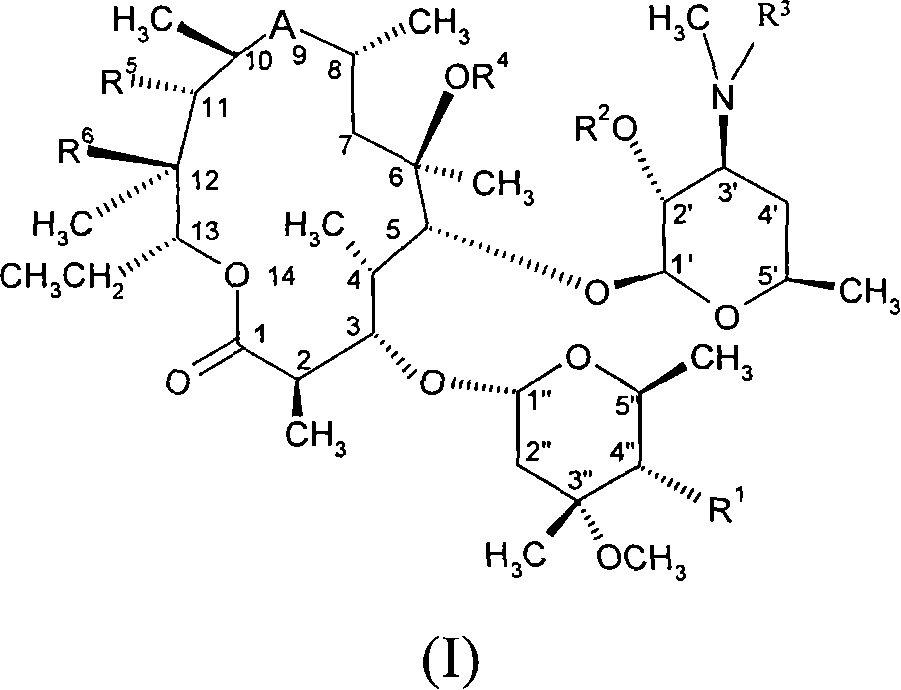
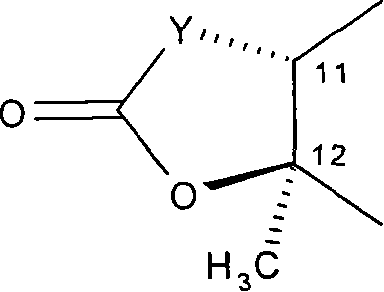
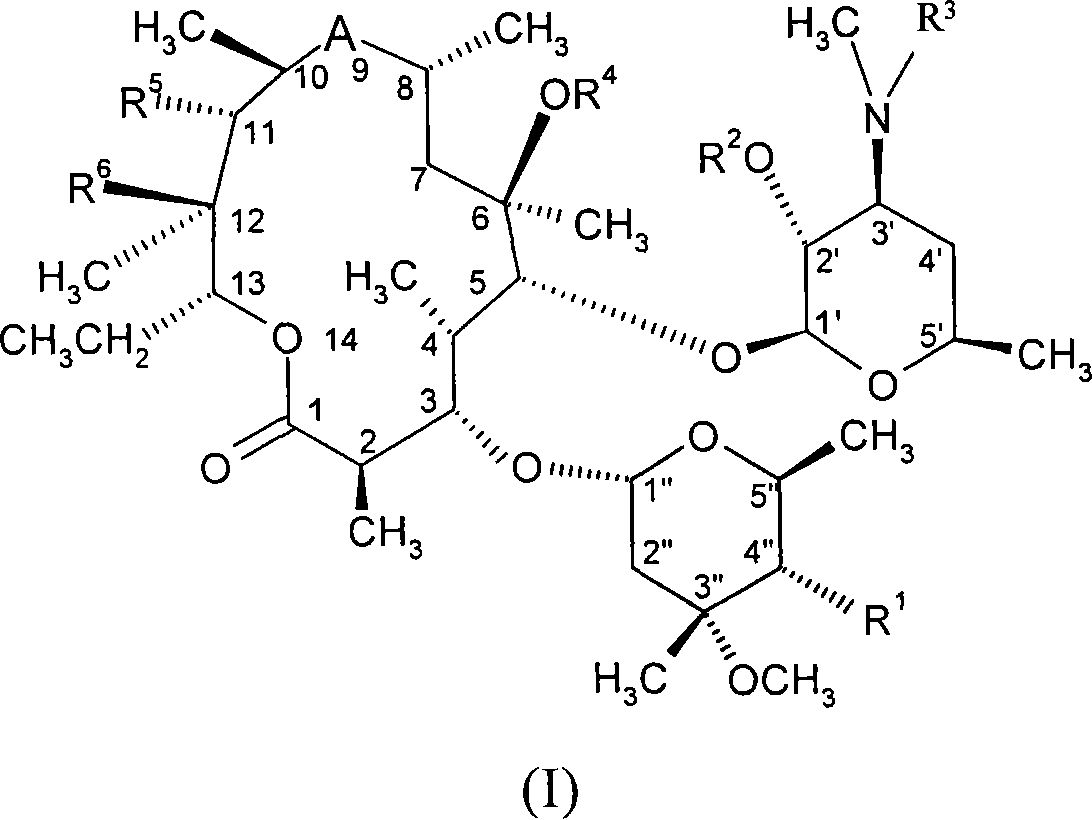
Macrolides with anti-inflammatory activity
An aromatic heterocycle, selected technology, applied in the field of substituted macrolides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0296] Example 1: 11,12-Carbonate-11,12-dideoxy-4"-O-(3-diethylamino-propionyl)-Aqi Mycin
[0297]
[0298] The 11,12-carbonate-11,12-dideoxy-4"-O-acryloyl-azithromycin (0.5g, 0.6mmol) obtained as described in the international patent application WO 03 / 042228, intermediate 50 and diethyl The amine (0.72 mL, 7 mmol) was dissolved in anhydrous methanol (60 mL), and the mixture was stirred overnight at 40° C. The methanol was removed by evaporation under reduced pressure, and the crude product was purified by column chromatography (DCM / MeOH / NH 4 OH=90:9:0.5) to obtain the title compound.
[0299] MS(ES+)m / z: [MH] + =902.46
[0300] 13 C-NMR(125MHz, CDCl 3 )δ: 177.1, 172.1, 153.3, 102.8, 95.3, 85.9, 85.1, 79.0, 78.0, 76.3, 73.3, 73.0, 70.7, 68.2, 67.7, 65.5, 62.9, 61.2, 49.5, 48.2, 46.7, 45.3, 43.0, 41.9 , 40.4, 35.3, 34.4, 29.3, 26.8, 26.2, 22.1, 22.0, 21.6, 21.2, 17.8, 14.9, 14.2, 10.5, 10.4, 5.5.
Embodiment 2
[0301] Example 2: 4″-O-(3-Diethylamino-propionyl)-8a-aza-8a-homoerythromycin A
[0302]
[0303] 4"-O-acryloyl-8a-aza-8a-homoerythromycin A (0.34g, 0.42mmol) obtained as described in Example 59 of International Patent Application WO 02 / 32917 (incorporated by reference) and two Ethylamine (0.56mL, 5.4mmol) was dissolved in anhydrous methanol (50mL), and the mixture was stirred overnight at 40°C. The methanol was removed by evaporation under reduced pressure, and the crude product was purified by column chromatography (DCM / MeOH / NH 4 OH=90:9:1.5) to obtain the title compound.
[0304] MS(ES+)m / z: [MH] + =876.48
[0305] 13 C-NMR (125MHz, DMSO) δ: 177.2, 174.6, 171.8, 102.2, 93.9, 82.1, 77.9, 76.5, 75.7, 74.6, 73.2, 72.5, 70.7, 70.4, 65.8, 64.7, 62.1, 48.7, 48.2, 45.9, 44.8, 42.2, 40.7, 40.2, 34.2, 32.7, 30.3, 27.2, 23.5, 21.5, 21.4, 20.5, 17.6, 17.2, 14.4, 11.5, 11.3, 9.2, 8.8.
Embodiment 3
[0306] Example 3: 4"-O-(3-Diethylamino-propionyl)-6-O-methyl-8a-aza-8a-homomycin A
[0307]
[0308] Diethylamine (80μl, 0.95mol) was added to the 4″-O-acryl-6-O-methyl-8a-aza-8a-high obtained as described in Example 9 of International Patent Application WO 02 / 32917 In a solution of erythromycin A (0.15g, 0.19mmol) in isopropanol (2mL), the reaction mixture was stirred in a tube overnight at 70°C. The isopropanol was removed by evaporation, and the residue was purified by an SP column (DCM / MeOH / NH 4 OH=90:9:0.5) to obtain the title compound (34mg).
[0309] MS(ES)m / z: [MH] + = 890 (95%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com