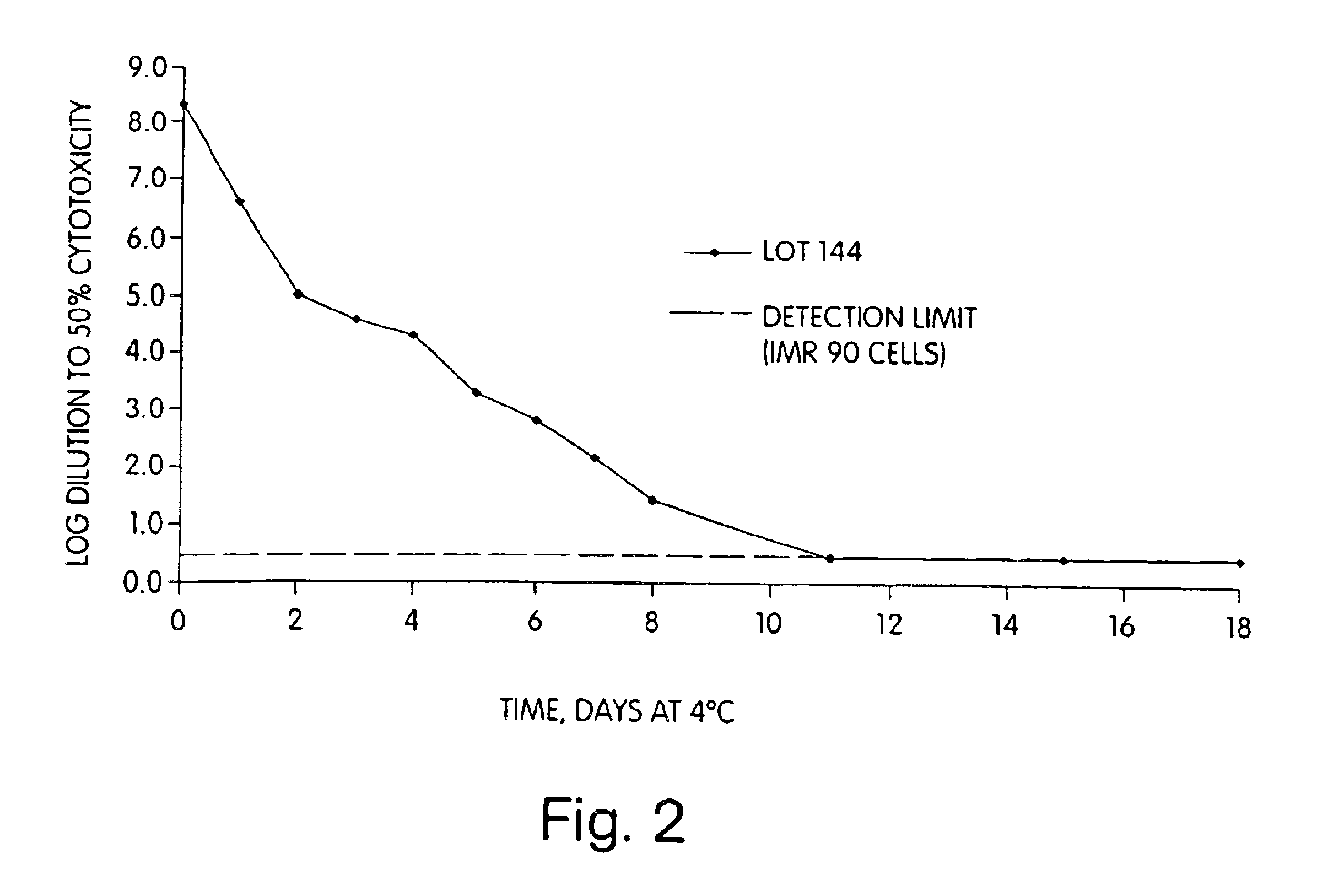
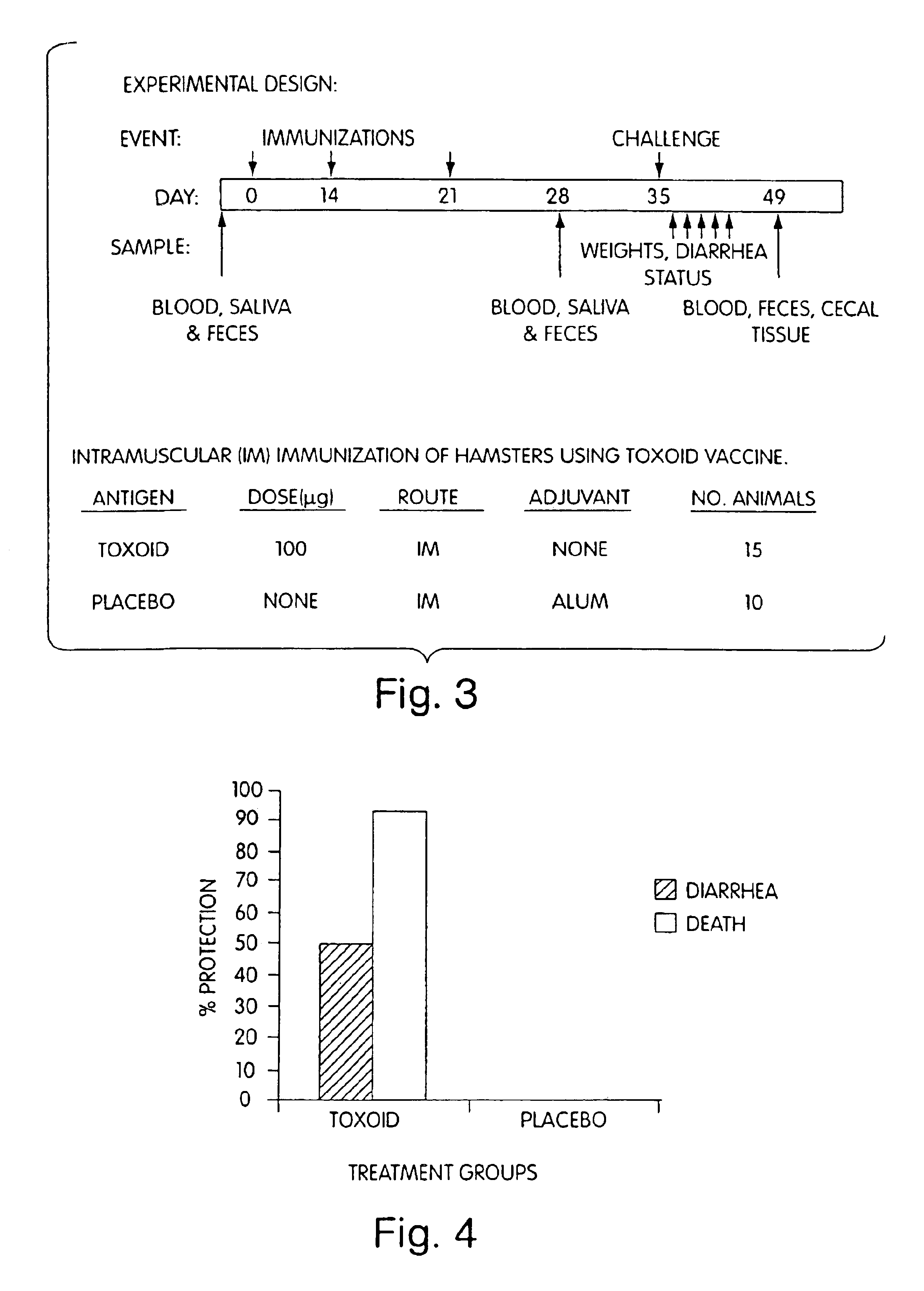
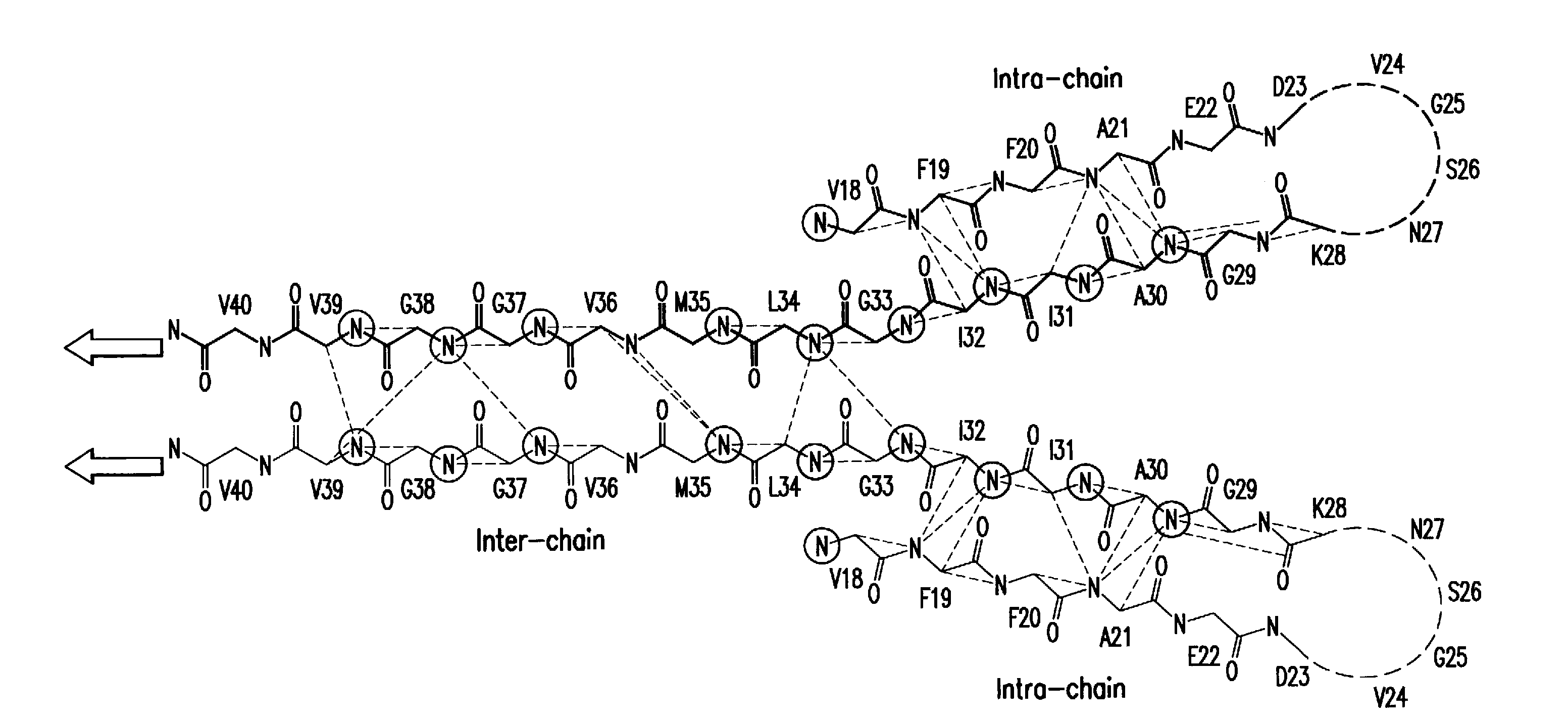
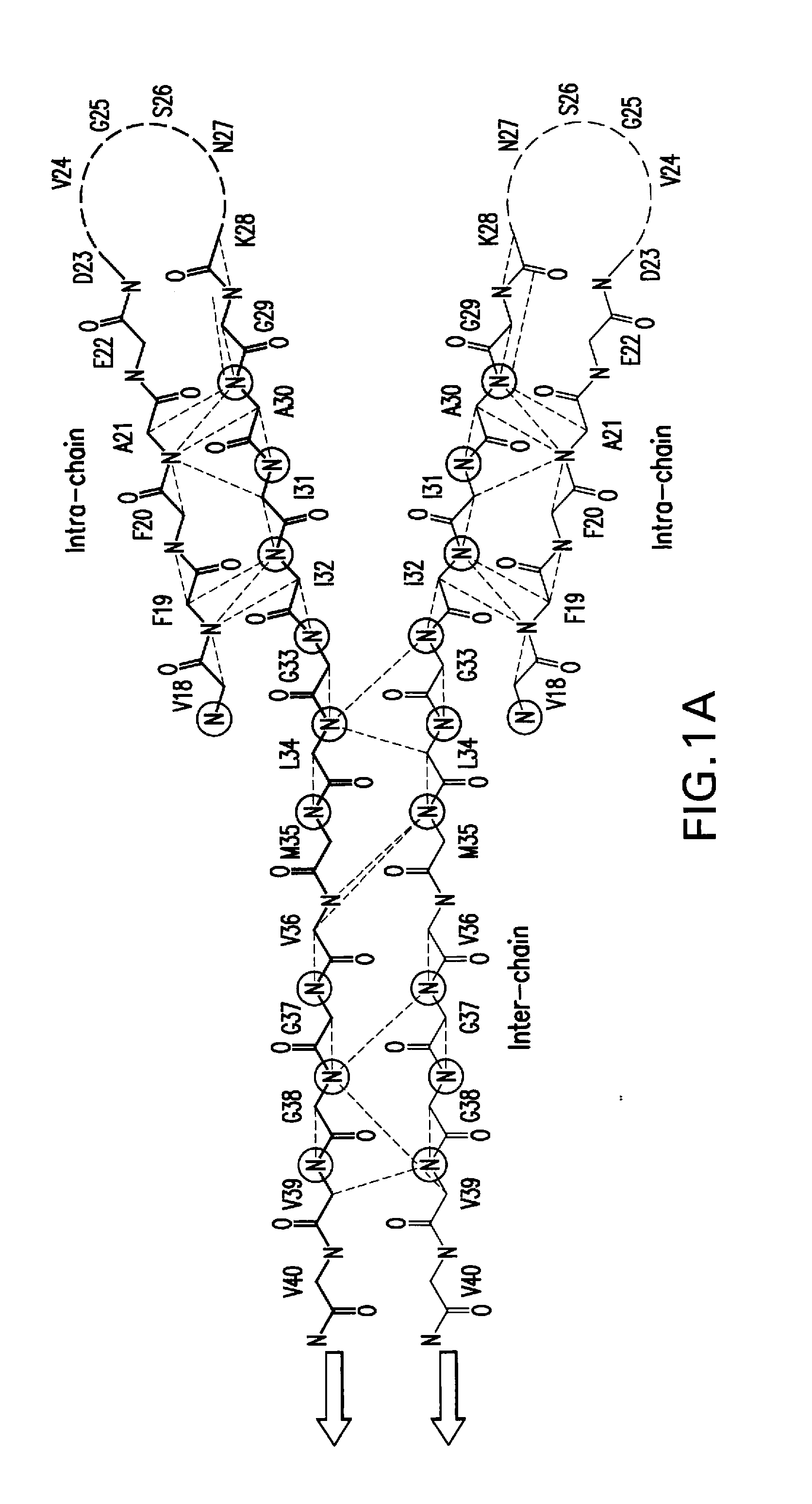
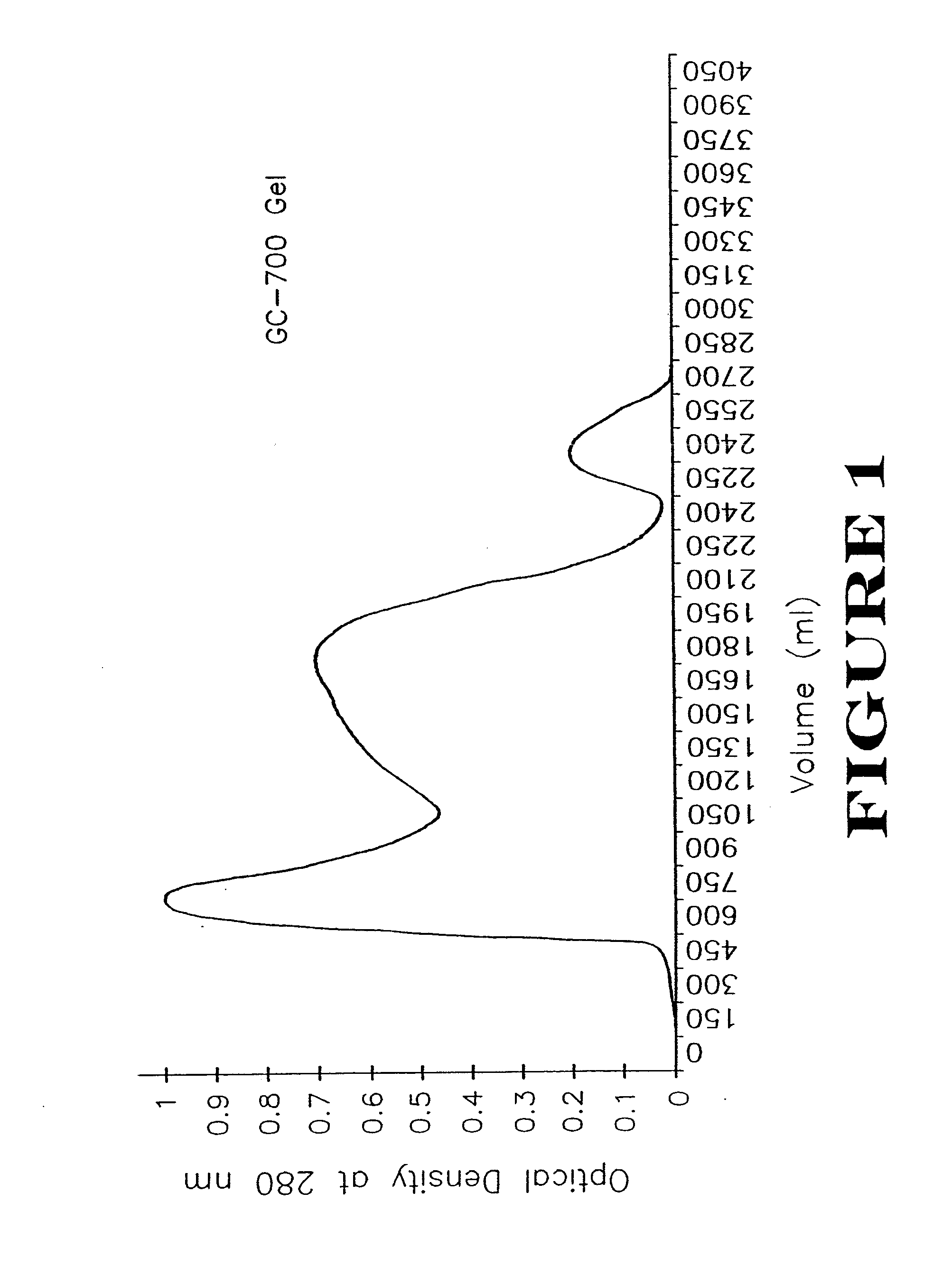
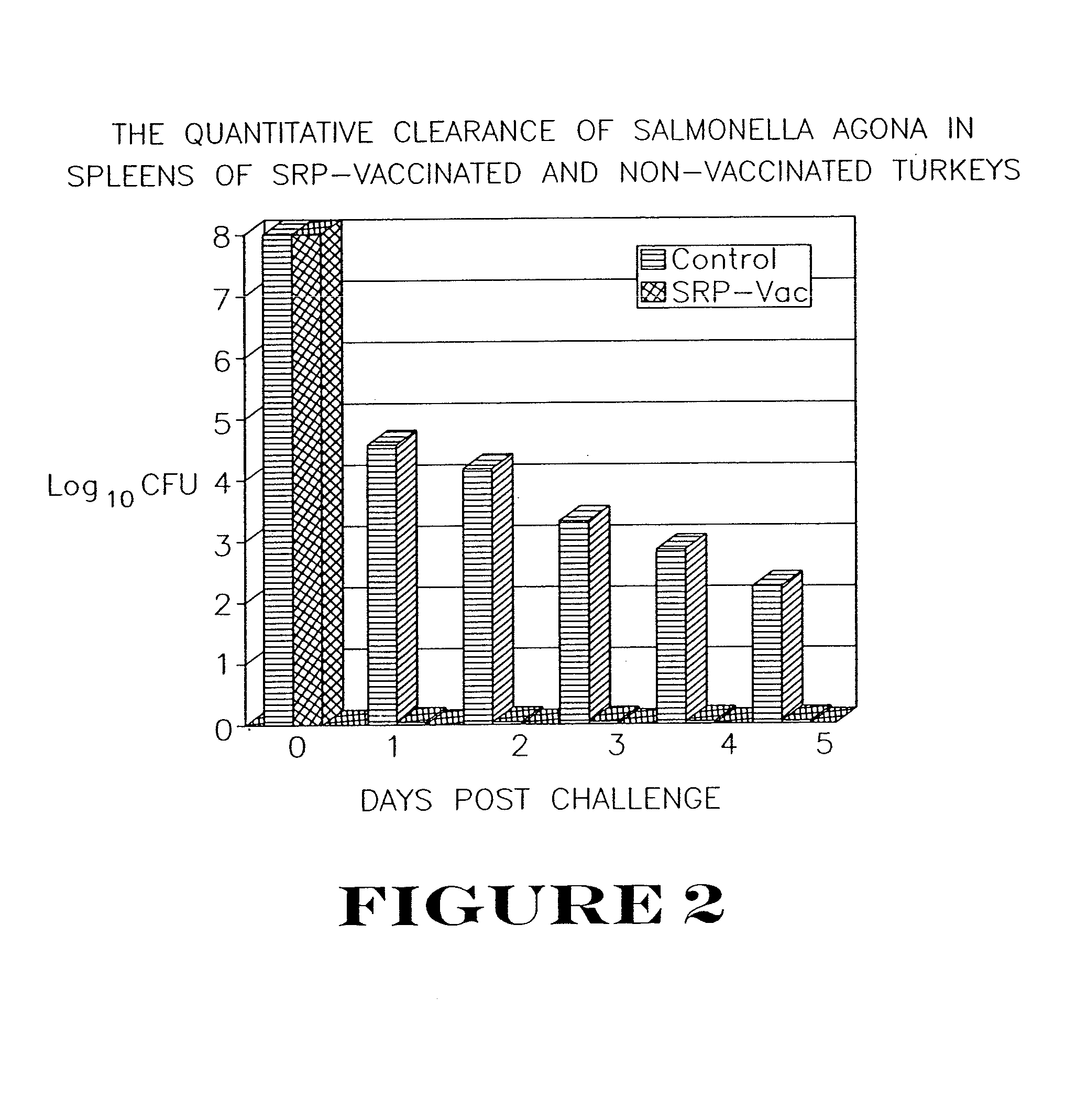
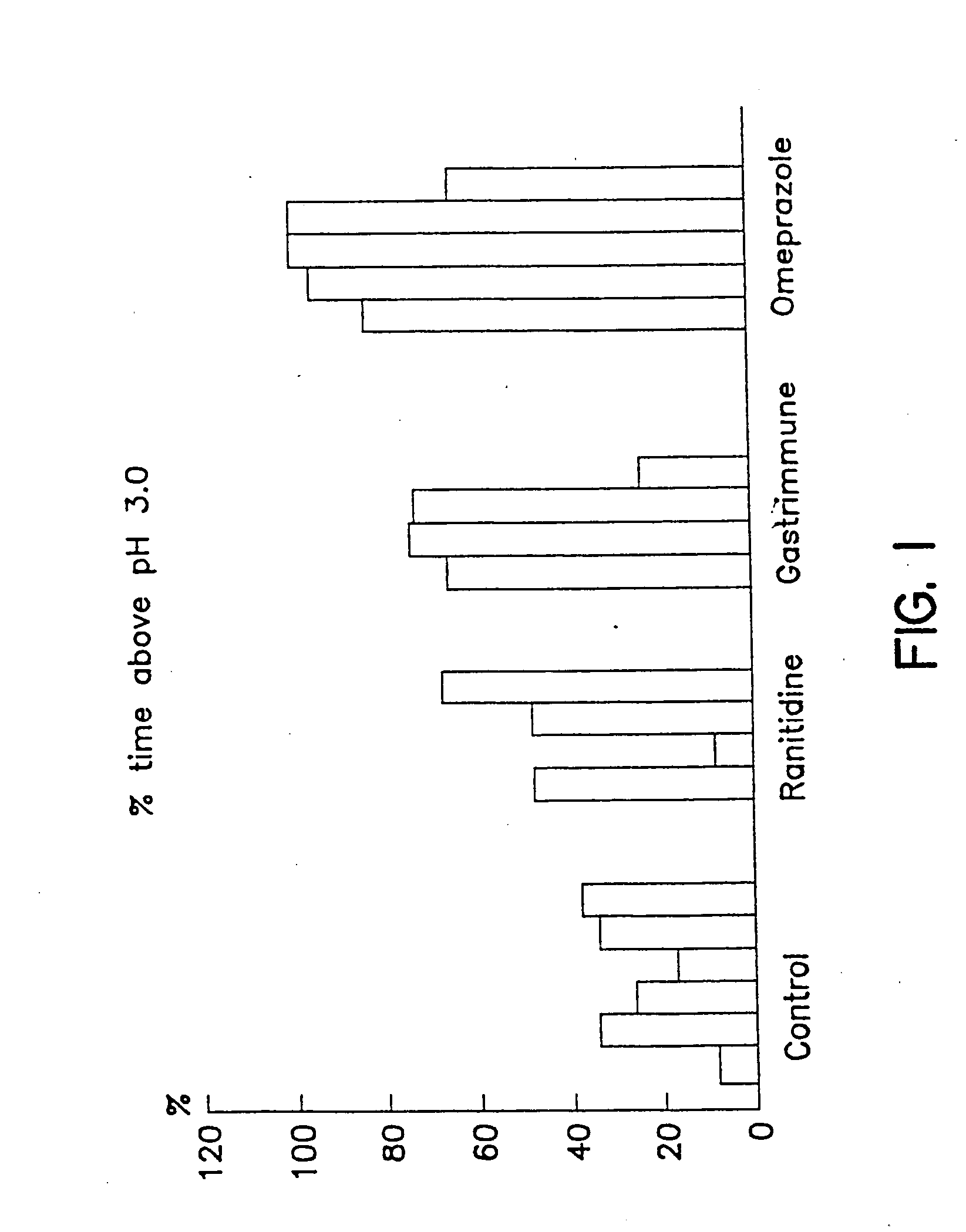
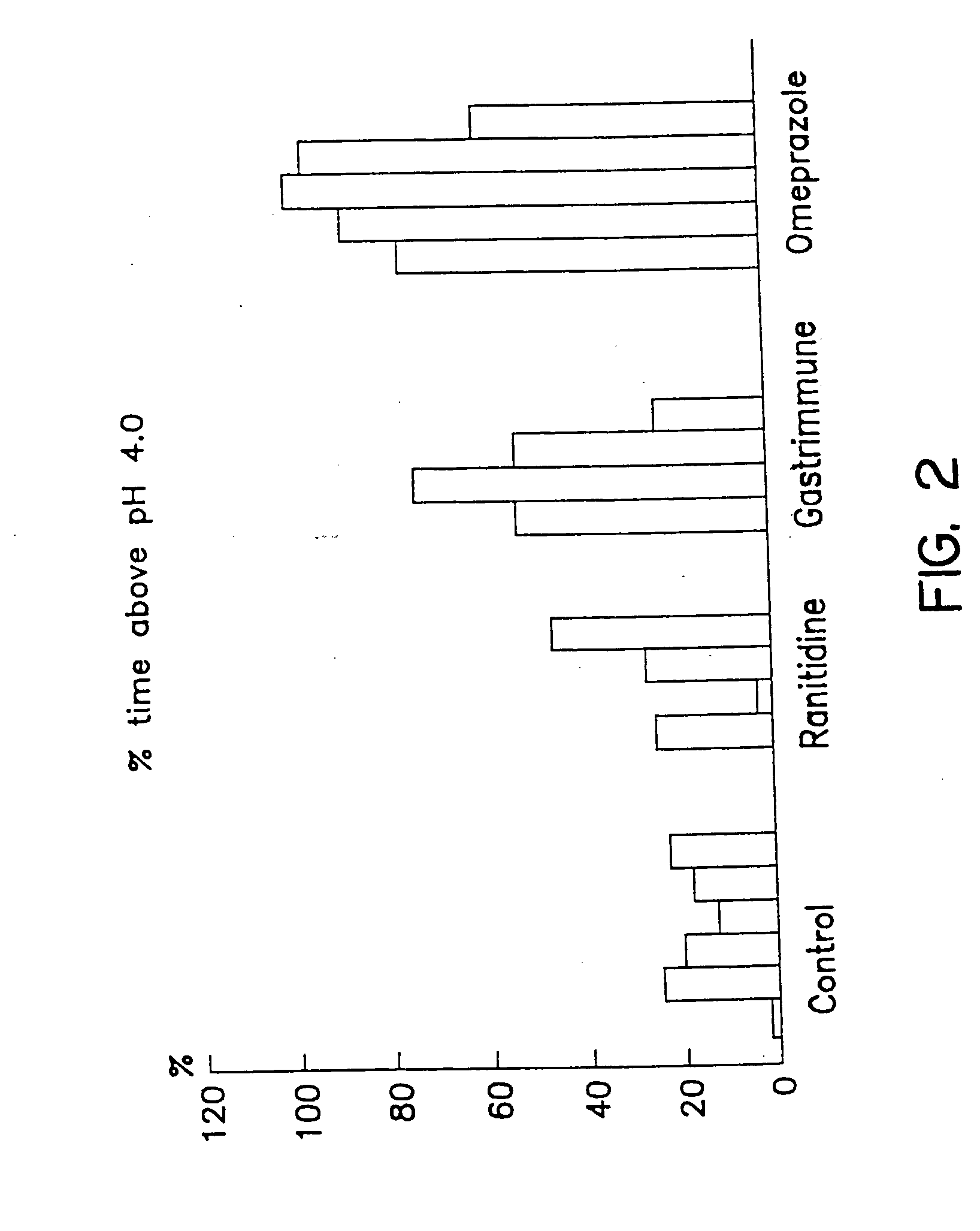
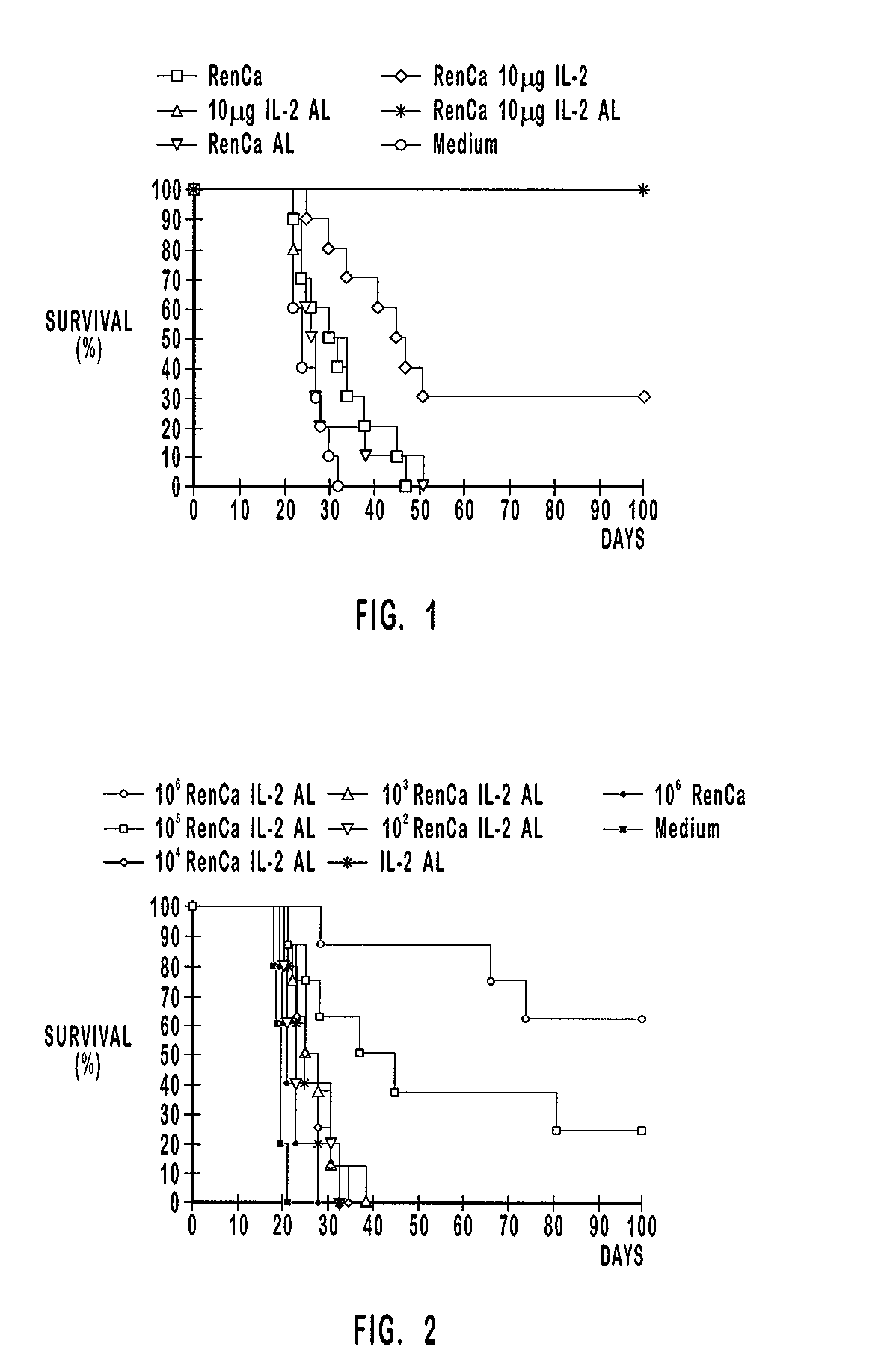
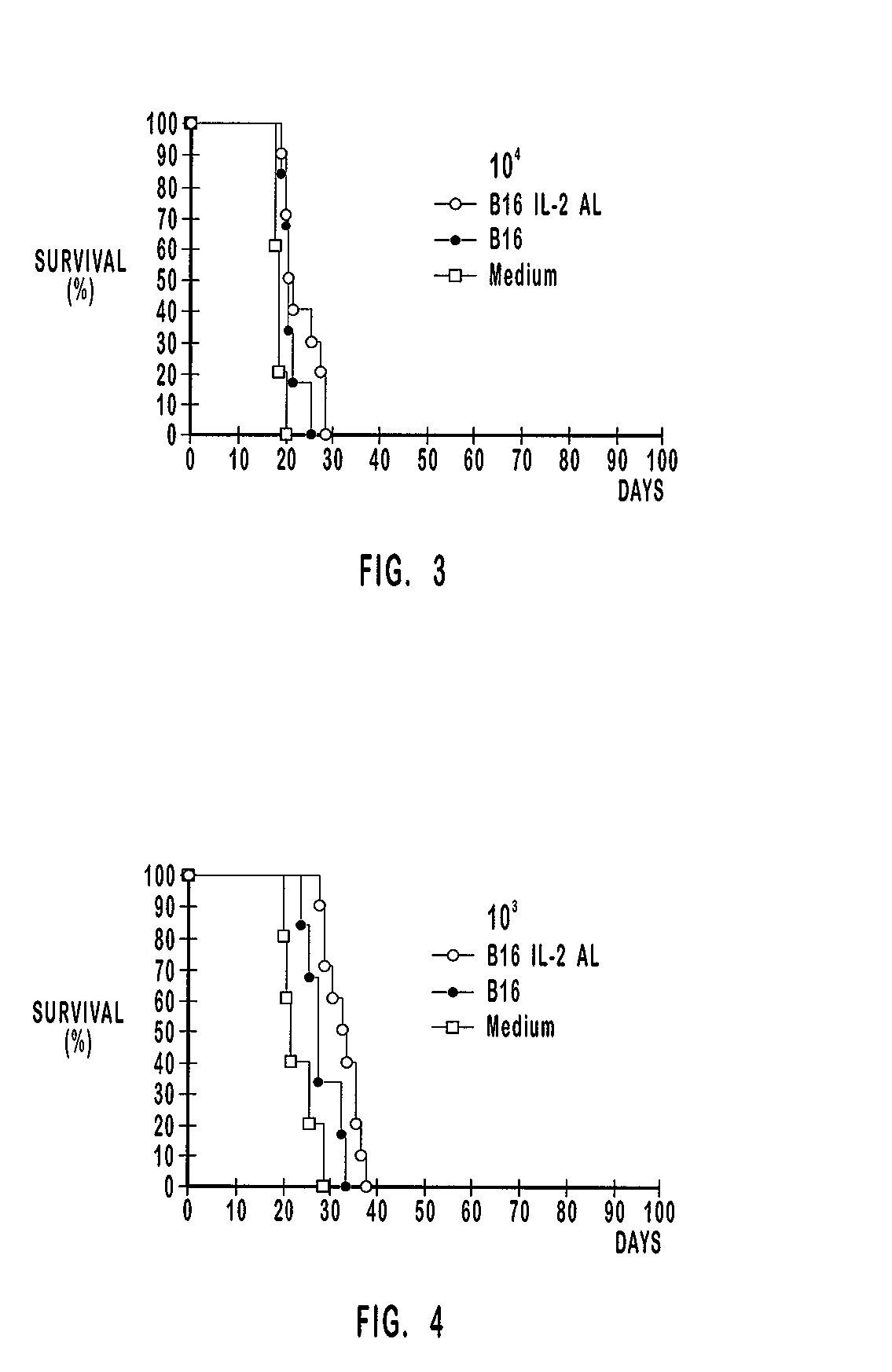
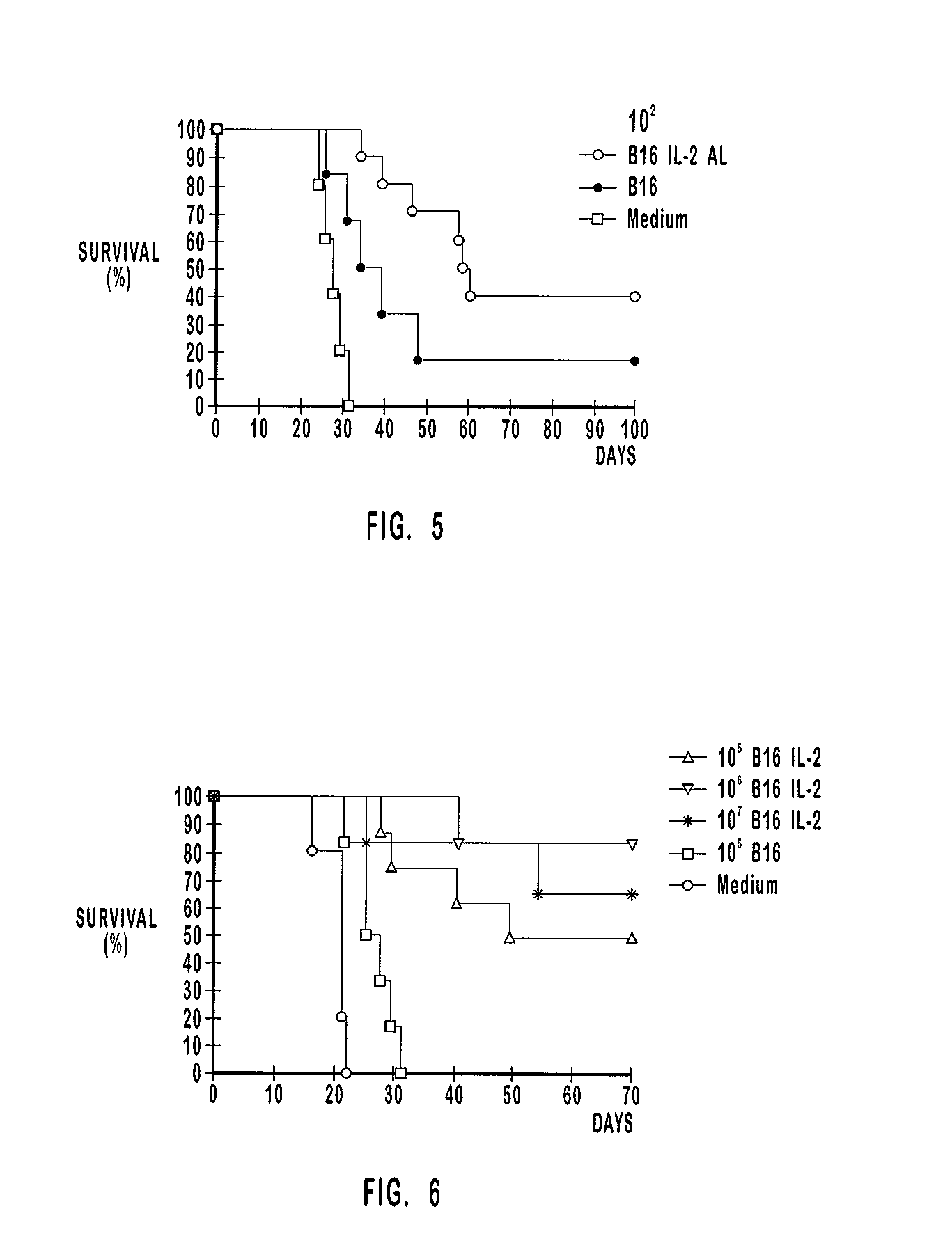
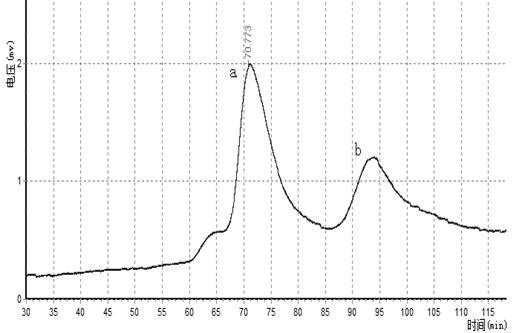
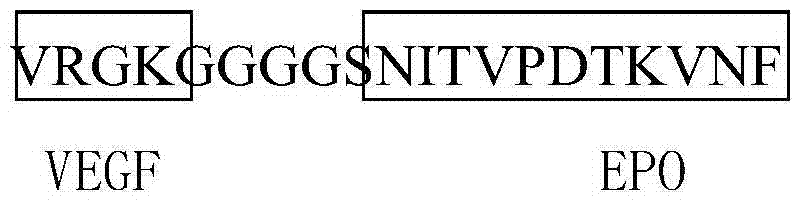Patents
Literature
62 results about "Active immunization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Active immunization is the induction of immunity after exposure to an antigen. Antibodies are created by the recipient and may be stored permanently. Active immunization can occur naturally when a microbe or other antigen is received by a person who has not yet come into contact with the microbe and has no pre-made antibodies for defense. The immune system will eventually create antibodies for the microbe, but this is a slow process and, if the microbe is deadly, there may not be enough time for the antibodies to be used.
Compositions and methods for treatment of tumors and metastatic diseases
InactiveUS6406689B1Stimulate immune responseSimple and reliable to useBiocideSnake antigen ingredientsDiseaseActive immunization
Owner:FALKENBERG FR W
Active immunization against clostridium difficile disease
InactiveUS6969520B2Prevent relapseImmune responseAntibacterial agentsBacterial antigen ingredientsDiseasePassive Immunizations
The invention provides active and passive immunization methods for preventing and treating Clostridium difficile infection, which involve percutaneous administration of C. difficile toxin-neutralizing polyclonal immune globulin, C. difficile toxoids, or combinations thereof. Also provided by the invention are C. difficile toxoids, C. difficile toxin-neutralizing polyclonal immune globulin, and methods of identifying subjects that produce C. difficile toxin-neutralizing polyclonal immune globulin.
Owner:SANOFI PASTEUR BIOLOGICS CO
Amyloid ß peptide analogues, oligomers thereof, processes for preparing and composi-tions comprising said analogues or oligomers, and their uses
InactiveUS20110092445A1Easy to measureReversing cognitive defectsCompound screeningNervous disorderPassive ImmunizationsOligomer
The present invention relates to an amyloid β peptide analogues comprising an amino acid sequence or a peptidomimetic thereof, wherein the sequence (i) forms a loop, (ii) has at least 66% identity to the amino acid sequence of native Aβ peptide or a portion thereof, (iii) comprises at least 6 contiguous amino acid residues and (iv) has at least 2 non-contiguous amino acid residues which are covalently linked with each other, oligomers comprising a plurality of said amyloid β peptide analogues, processes for preparing the amyloid β peptide analogues or oligomers, compositions comprising the amyloid β peptide analogues or oligomers, and uses of the amyloid β peptide analogues or oligomers such as their use for treating or preventing an amyloidosis (e.g. by active immunization), for diagnosing an amyloidosis, and for providing agents that are capable of binding to the amyloid β peptide analogues or oligomers. The subject invention also describes agents that are capable of binding to the amyloid β peptide analogues or oligomers, e.g. antibodies, compositions comprising the agents, and uses of the agents such as their use for treating or preventing an amyloidosis (e.g. by passive immunization) and for diagnosing an amyloidosis.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG +1
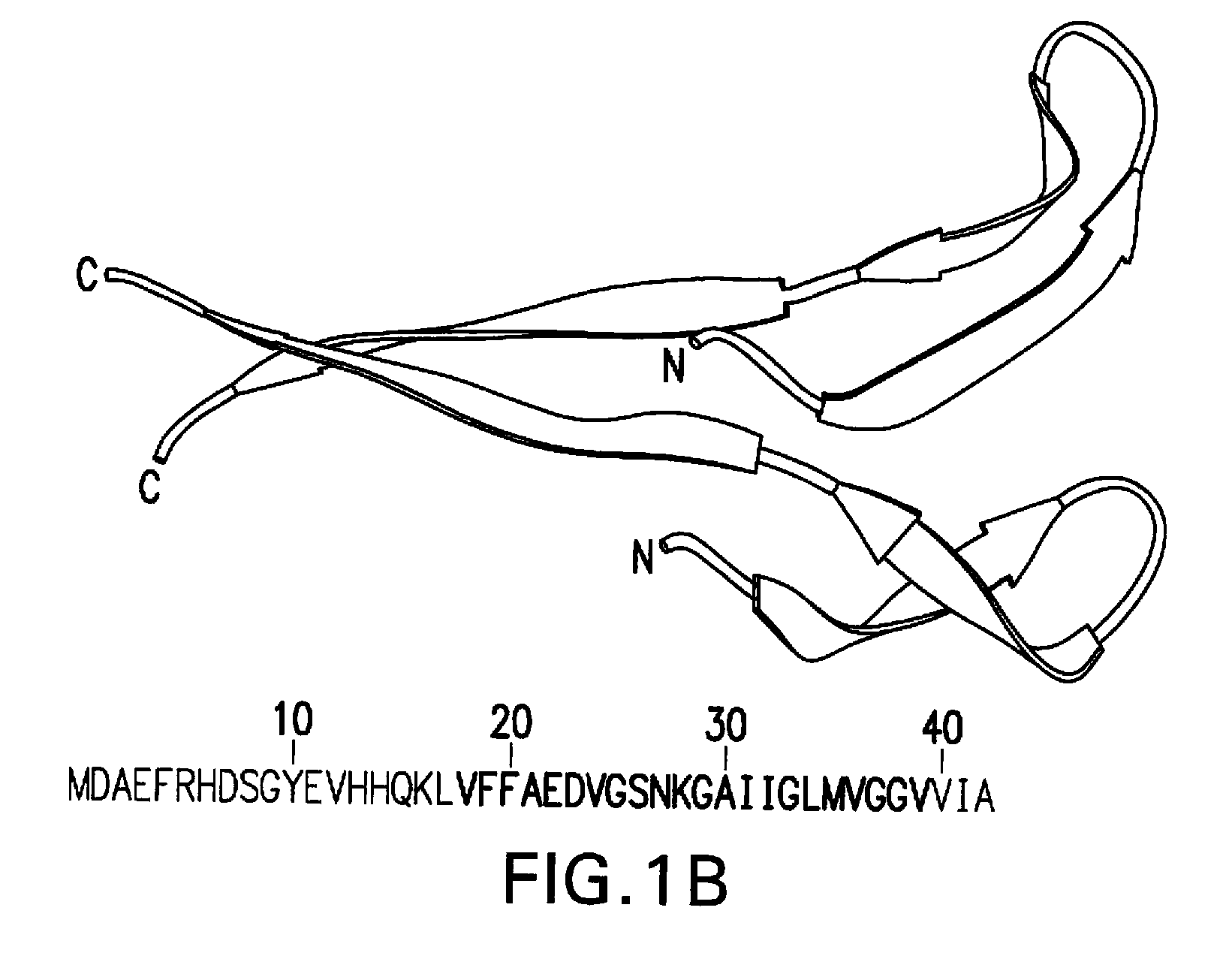
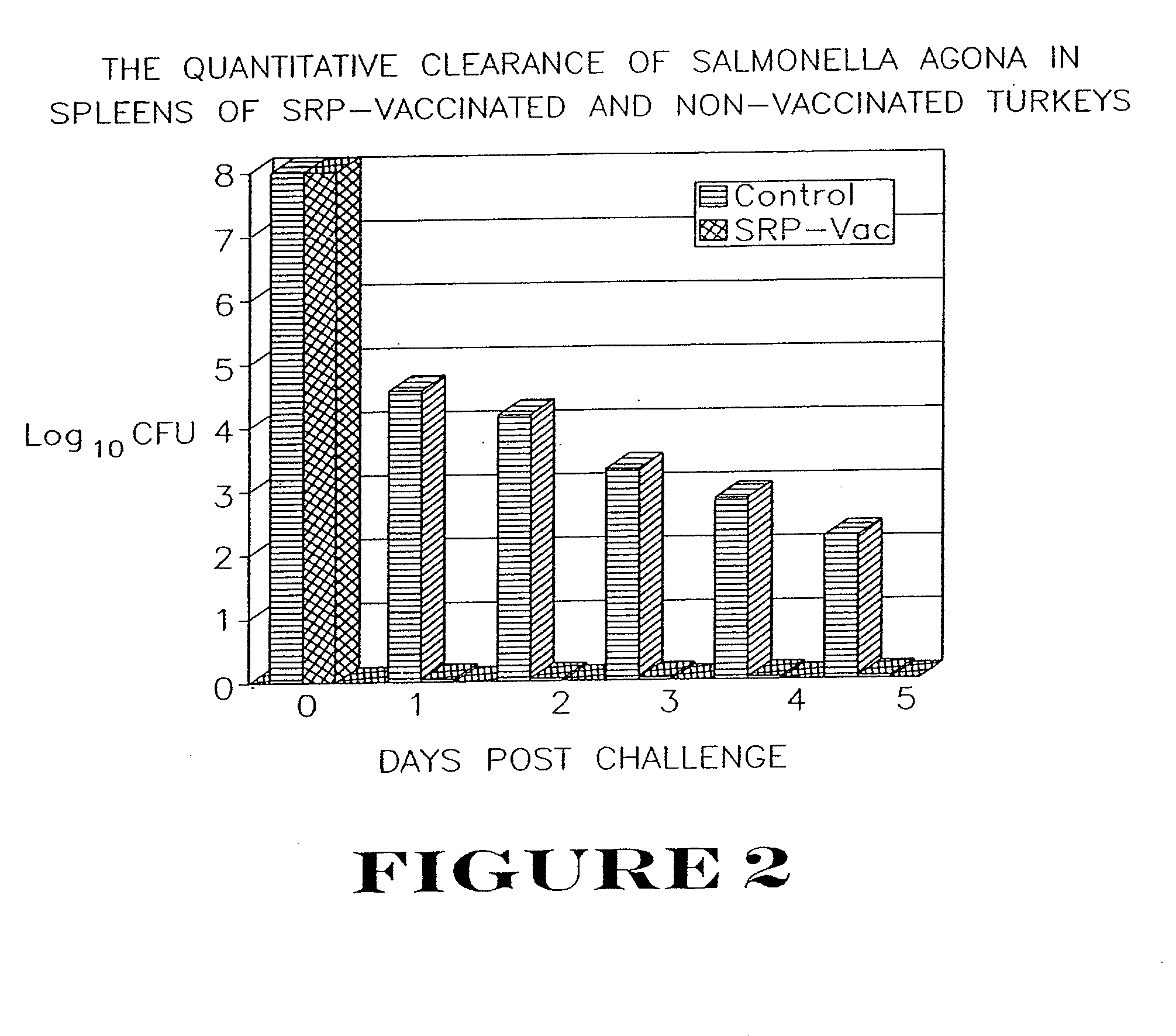
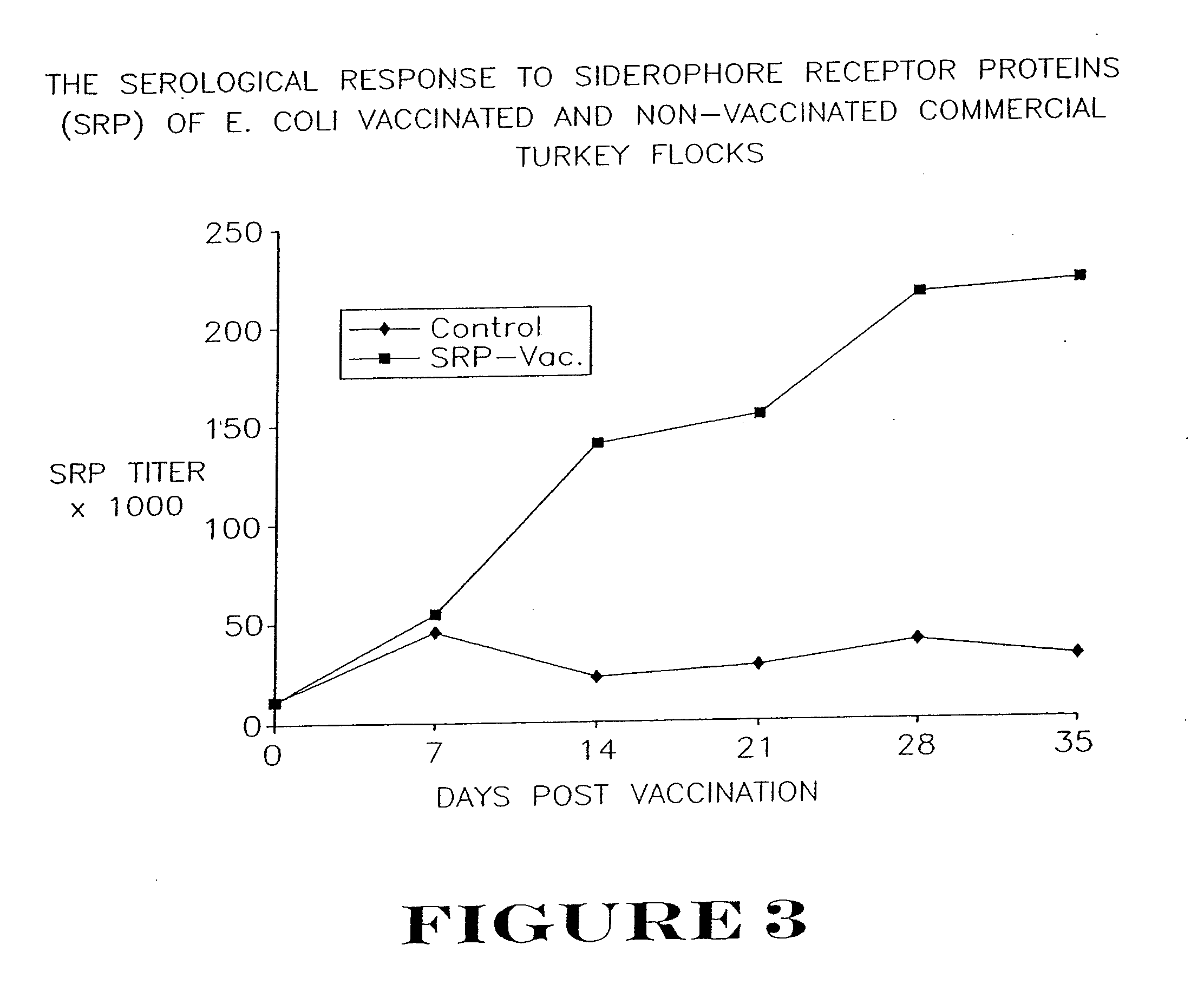
Active immunization using a siderophore receptor protein
InactiveUS20090123500A1Easy to produceInhibition capacityAntibacterial agentsBacterial antigen ingredientsADAMTS ProteinsActive immunization
Owner:EPITOPIX LLC
Immunogenic CEA
InactiveUS20050063952A1Effective immune responseBiocideGenetic material ingredientsVaccinationCarcinoembryonic antigen
The present invention provides for methods for immunizing actively against autologous carcinoembryonic antigen (CEA). The method encompasses that the immune system is engaged with variant CEA which is either administered as a protein vaccine, or is effected expressed by nucleic acid vaccination or live-viral vaccination. Preferred embodiments include immunization with variants that include at least one foreign T-helper epitope introduced in the CEA sequence. Also disclosed is variant proteins, DNA, vectors, and host cells useful for practising the method of the invention.
Owner:PHARMEXA
Active immunization using a siderophore receptor protein
InactiveUS20080293080A1Easy to produceBlock iron binding capacityAntibacterial agentsImmunological disordersActive immunizationBiological species
The invention provides a vaccine for immunizing poultry and other animals against infection by a gram-negative bacteria, and a method of immunizing an animal using the vaccine. The vaccine may contain purified siderophore receptor proteins derived from a single strain or species of gram-negative bacteria or other organism, which are cross-reactive with siderophores produced by two or more strains, species or genera of gram-negative bacteria. The invention further provides a process for isolating and purifying the siderophore receptor proteins, and for preparing a vaccine containing the proteins. Also provided is a method for diagnosing gram-negative sepsis.
Owner:EPITOPIX LLC
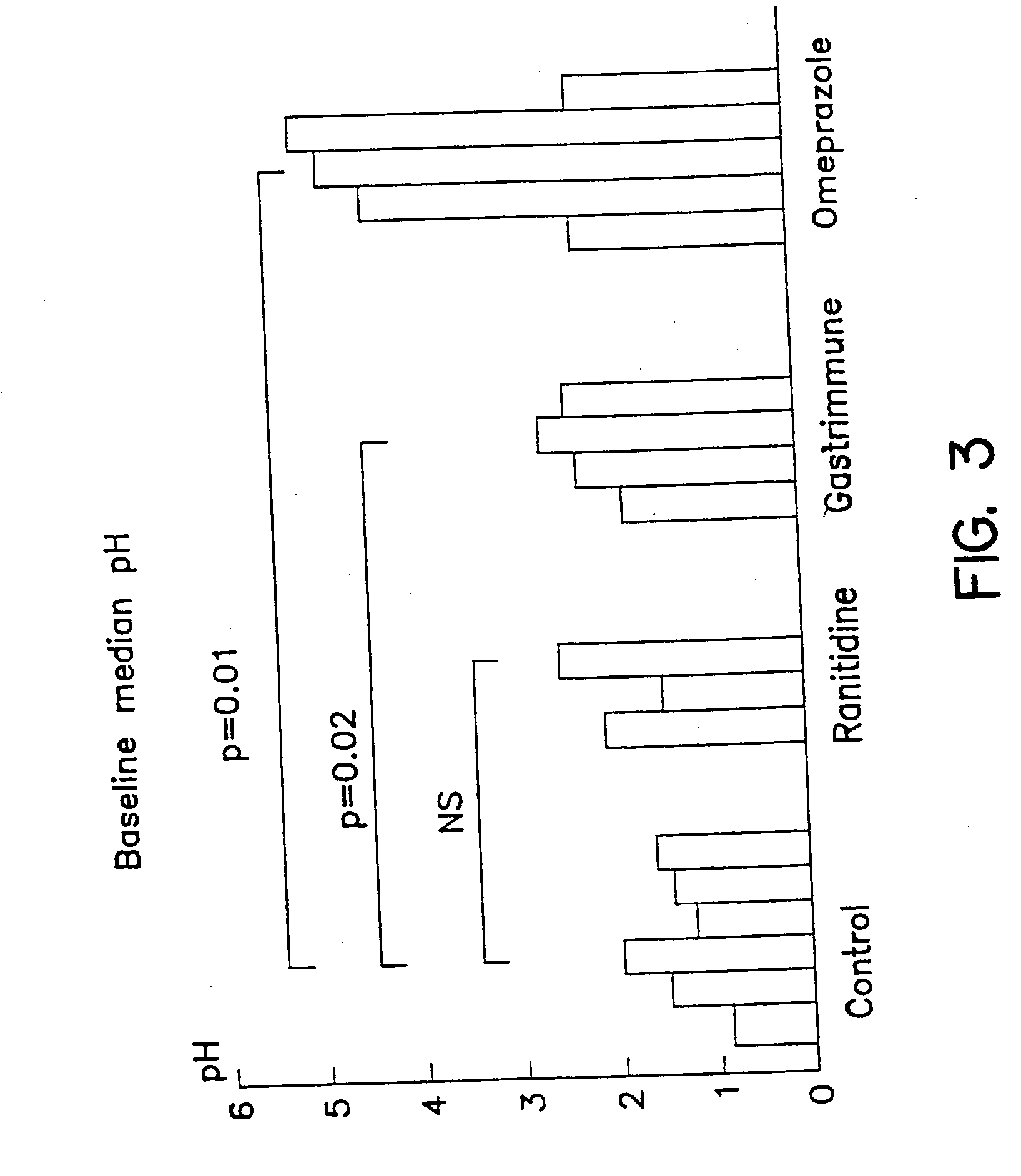
Method for the treatment of gastroesophageal reflux disease
InactiveUS20060039911A1Reducing and preventing increasePreventing elevated levelPeptide/protein ingredientsDigestive systemRefluxPump activity
A method for the treatment of gastroesophageal reflux disease comprising a combination of active immunization with an anti-gastrin immunogenic composition with an antagonist that blocks or inhibits gastric acid pump activity; or alternatively administering purified anti-gastrin antibodies with an H2 antagonist or proton pump inhibitor of the gastric acid producing enzyme system.
Owner:CANCER ADVANCES INC
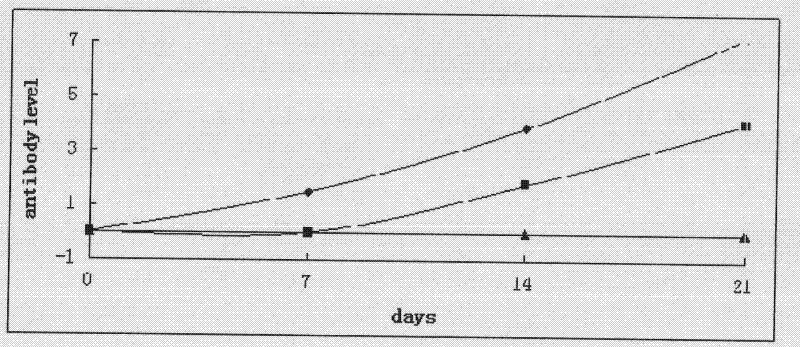
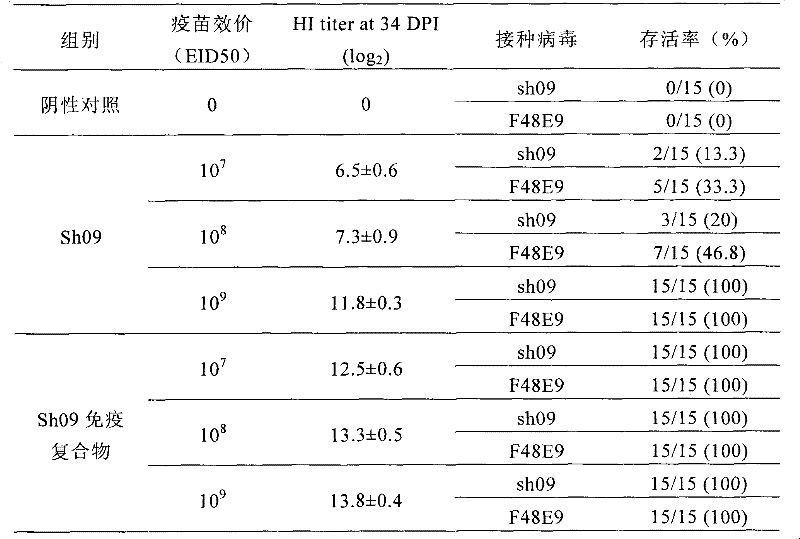
Preparation method and application of Newcastle disease virus infected immune complex vaccines
InactiveCN102233133AAvoid infectionSame characteristicsViral antigen ingredientsAntiviralsYolkMicroorganism
The invention discloses a preparation method and application of Newcastle disease virus infected immune complex vaccines. The preparation method comprises the following steps of: (1) preparing antigens and inactivated vaccines by using Newcastle disease virus Shanghai strains as seed viruses; (2) transferring antibodies in chicken blood serum to yolk to form yolk antibodies IgY; and (3) mixing the prepared inactivated vaccines and the yolk antibodies IgY in equal volume, and hatching to obtain the immune complex vaccines. By combining passive immune (vaccine) and active immune (specific antibody) measures, animals are protected from the infection of pathogenic microbes in time for a long time.
Owner:SHANGHAI ACAD OF AGRI SCI
Compositions and methods for treatment of tumors and metastatic diseases
InactiveUS20020176845A1Effective treatmentStimulate immune responsePeptide/protein ingredientsCancer antigen ingredientsDiseaseActive immunization
Compositions and methods are provided which can be utilized in active immunization as a prophylactic treatment or a therapeutic treatment for tumors. The compositions are employed as injectable tumor vaccines or as preparations for intratumoral administration and are capable of stimulating immune responses to specific tumor antigens. The tumor vaccines are composed of an antigenic cellular material including a plurality of inactivated tumor cells or tumor cell portions, a depot material, and an immunostimulant adsorbed to the depot material. The depot material with absorbed immunostimulant is mixed with the tumor cells or tumor cell portions to form the vaccine compositions. The preparations for intratumoral administration include the depot material adsorbed immunostimulant without the antigenic cellular material. The immunostimulant adsorbed to the depot material permits release of biologically active quantities of the immunostimulant over a period of time rather than all at once.
Owner:FRANK W FALKENBERG
Method for quantitatively detecting allergen alpha-lactalbumin based on quantum dot fluorescence
InactiveCN102680705AIncreased sensitivitySimple and fast operationBiological testingFluorescence/phosphorescenceBALB/cFluorescence
The invention discloses a method for quantitatively detecting allergen alpha-lactalbumin based on quantum dot fluorescence and application of the allergen alpha-lactalbumin. The method comprises the following steps of: preparing a monoclonal antibody from alpha-lactalbumin as active immunization BALB / c mice; carrying out conjugation labeling on the alpha-lactalbumin monoclonal antibody by using a fluorescent quantum dot; forming an immunofluorescence complex by adopting a competitive immunosorbent assay; then detecting a fluorescent signal under a full-wavelength multifunctional ELIASA (Enzyme-Linked Immunosorbent Assay Apparatus); and quantitatively detecting the allergen alpha-lactalbumin in the food by establishing a standard curve. The method constructed by the invention can be widely applied to detection of relevant allergens in various powders and liquid milk products, has the characteristics of quickness, accuracy, high sensitivity, favorable repeatability, excellent specificity and the like, provides an effective means for high-throughput detection of relevant allergens in various foods as well as has a favorable popularization and application prospect.
Owner:NANCHANG UNIV
Compositions and methods for treatment of tumors and metastatic diseases
InactiveUS20020039571A1Stimulate immune responseSimple and reliable to useBiocideGenetic material ingredientsDiseaseActive immunization
Compositions and methods are provided which can be utilized in active immunization as a prophylactic treatment or a therapeutic treatment for tumors. The compositions are employed as injectable tumor vaccines or as preparations for intratumoral administration and are capable of stimulating immune responses to specific tumor antigens. The tumor vaccines are composed of an antigenic cellular material including a plurality of inactivated tumor cells or tumor cell portions, a depot material, and an immunostimulant adsorbed to the depot material. The depot material with absorbed immunostimulant is mixed with the tumor cells or tumor cell portions to form the vaccine compositions. The preparations for intratumoral administration include the depot material adsorbed immunostimulant without the antigenic cellular material. The immunostimulant adsorbed to the depot material permits release of biologically active quantities of the immunostimulant over a period of time rather than all at once.
Owner:FALKENBERG FR W
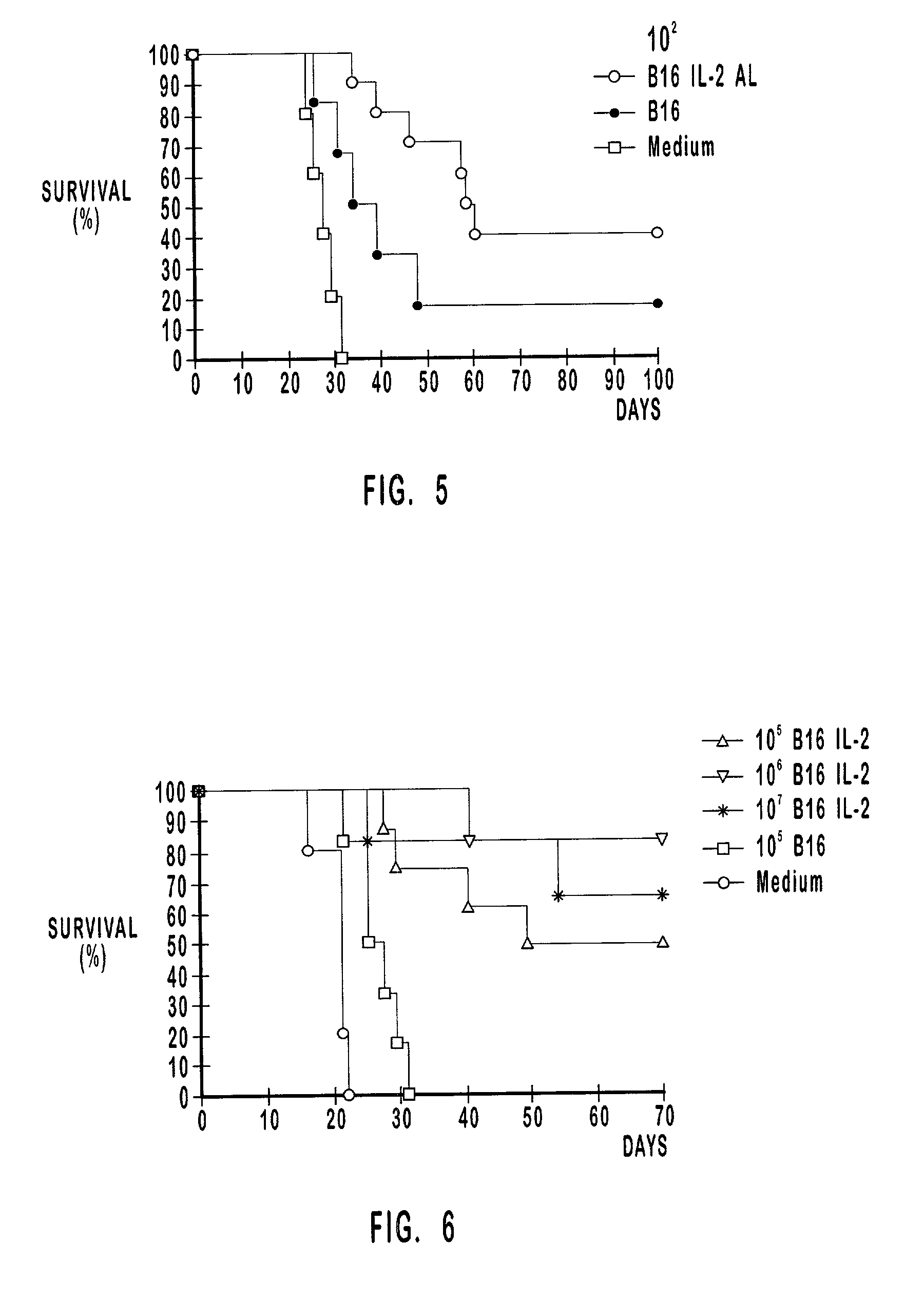
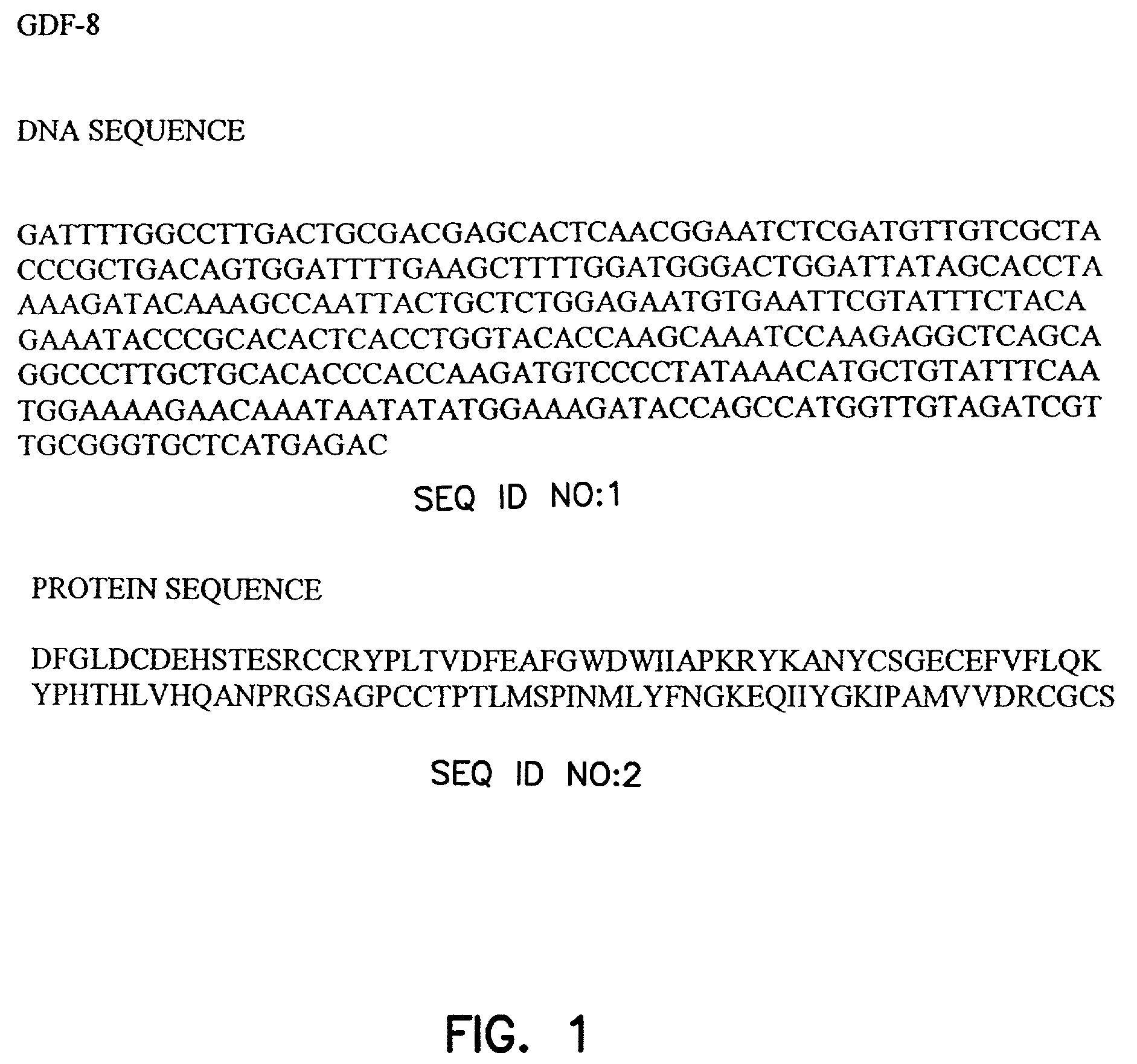
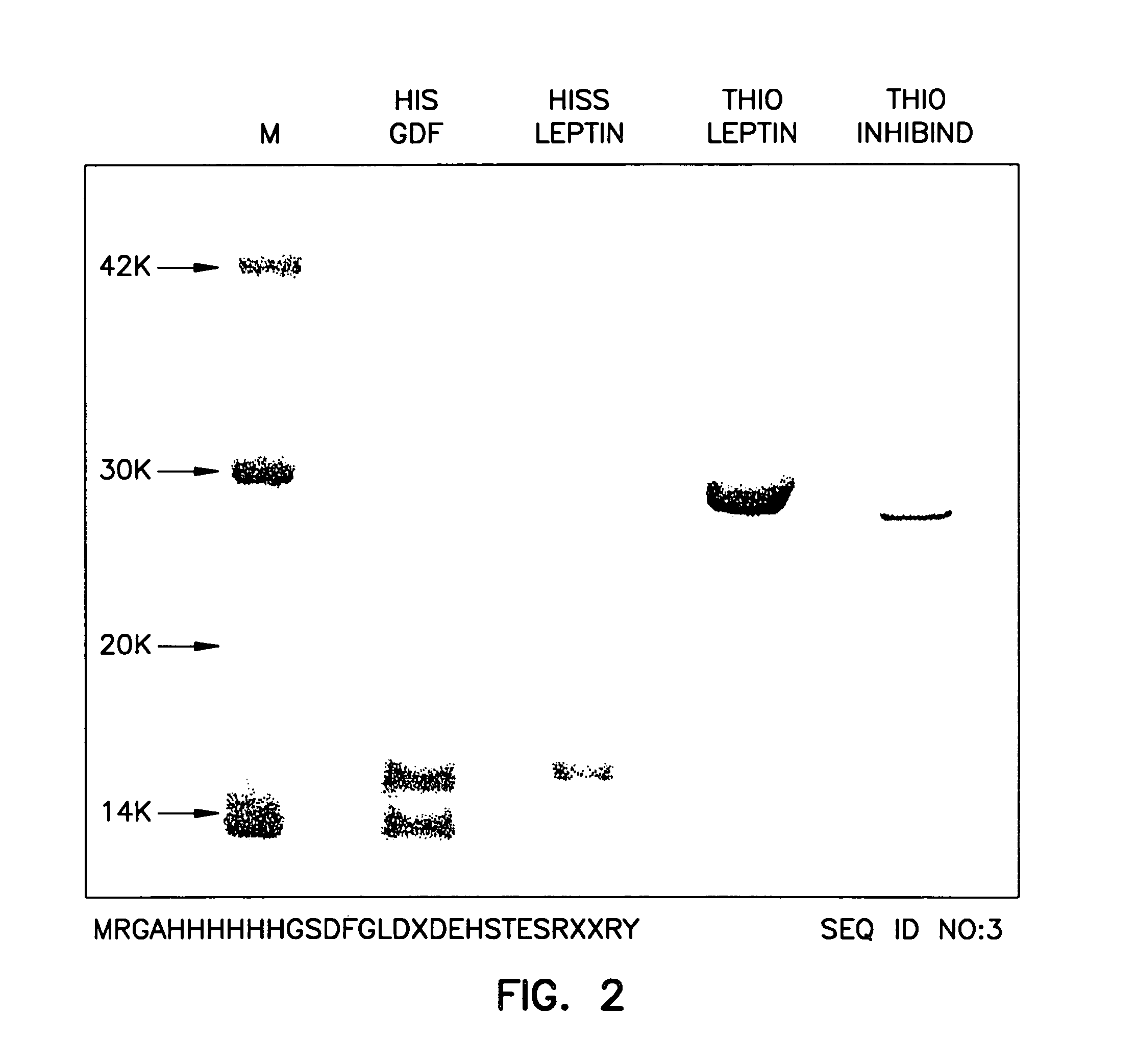
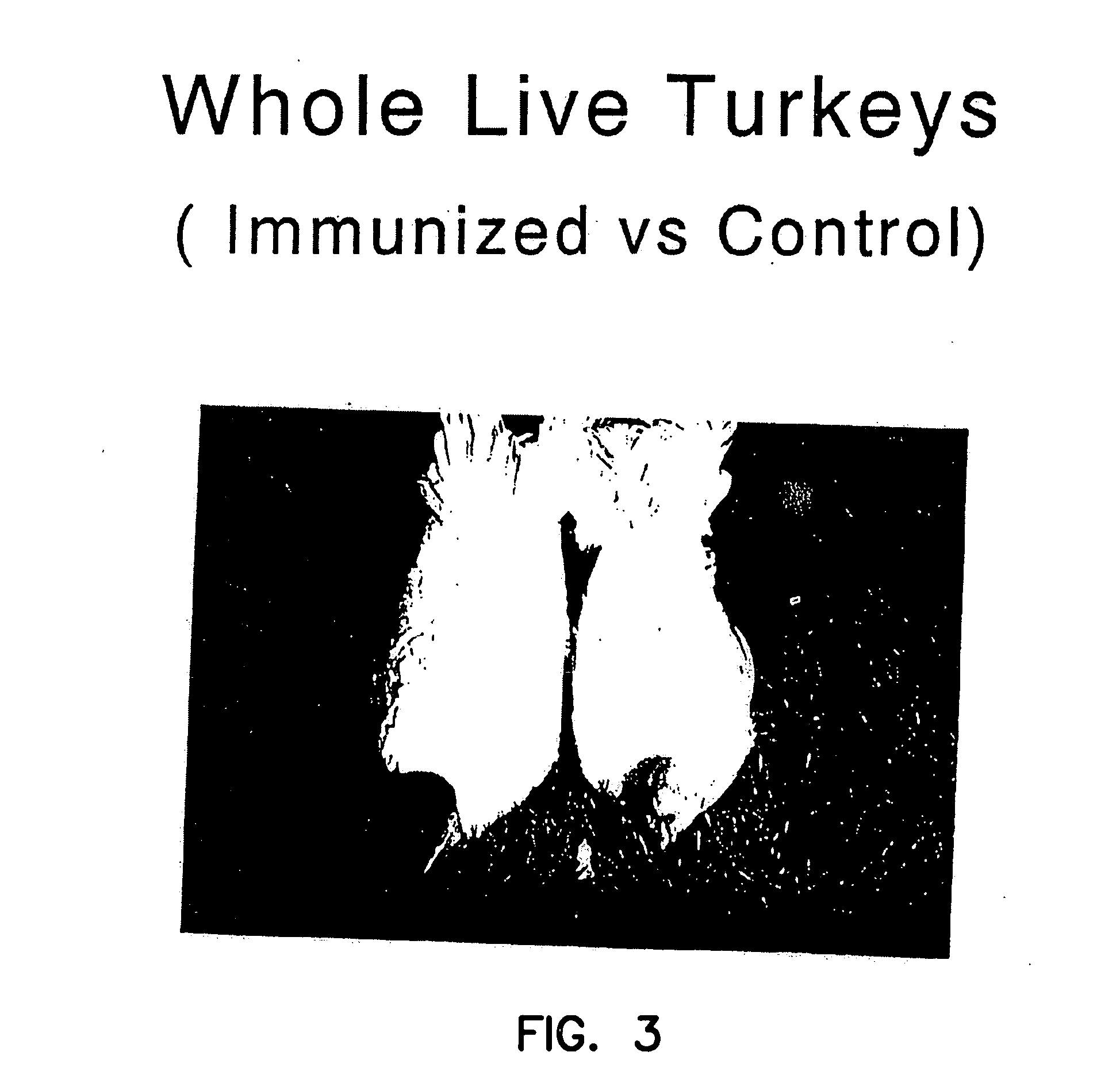
Myostatin immnoconjugate
InactiveUS7037501B2Increase in breast muscle and thigh muscle and testis and heart weightPromote growth ratePeptide/protein ingredientsVertebrate antigen ingredientsMyostatinFowl
A method to alter the phenotype of animals, e.g., avians, which employs passive and active immunization is provided.
Owner:RGT UNIV OF MINNESOTA
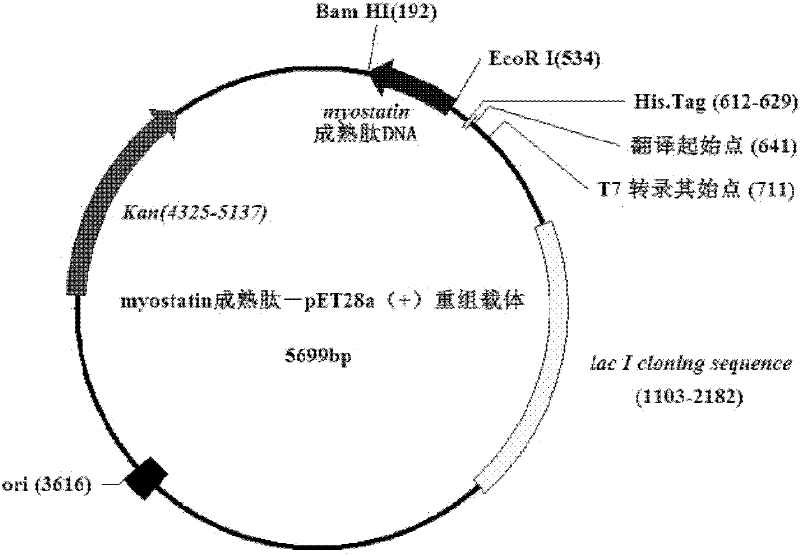
Tibetan sheep myostatin recombinant expression protein
InactiveCN102286513AImprove meat performanceRegulate nutrient metabolismBacteriaMicroorganism based processesEscherichia coliMyostatin
The invention discloses a recombinant Tibetan sheep myostatin protein. In the invention, a Tibetan sheep myostatin (MSTN) coding gene is cloned, the gene is used as a template for polymerase chain reaction (PCR) amplification, the bioactive region of the MSTN coding gene is cloned, a recombinant expression vector is constructed, the cloned bioactive region is transferred into Escherichia coli to construct gene engineering bacteria, and the gene engineering bacteria are subjected to isopropyl thiogalactoside (IPTG) induced expression and purification of MSTN protein. Then the MSTN protein is used to actively immunize a domestic rabbit to prepare polyclonal antibody serum, and an indirect enzyme-linked immuno sorbent assay (ELISA) is used to detect the immunogenicity of the MSTN protein. The recombinant Tibetan sheep MSTN protein is used to immunize a mouse actively so as to detect the influence of the recombinant MSTN protein on the growth of the mouse. The purity and immunogenicity of the recombinant Tibetan sheep MSTN protein provided by the invention are high, and the actively immunizing MSTN protein can obviously increase the diameter and area of muscle fibers of the mouse and promote the muscle fibers to increase. Thus, the recombinant Tibetan sheep MSTN protein can be used for active immunizing of Tibetan sheep and help to accelerate the growth of Tibetan sheep and to improve meat performance.
Owner:SOUTHWEST UNIVERSITY FOR NATIONALITIES
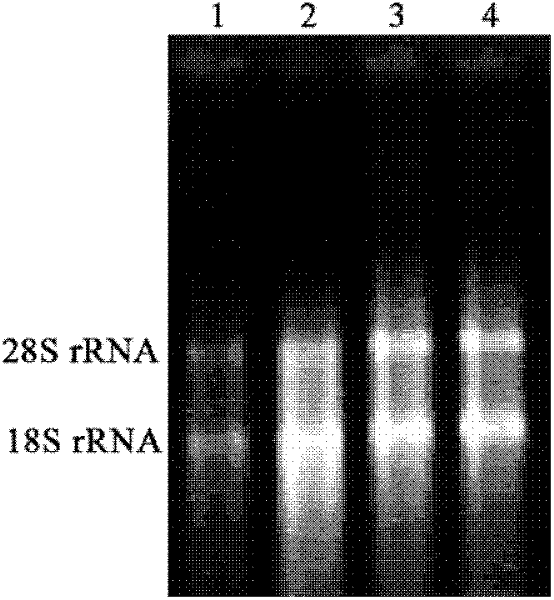
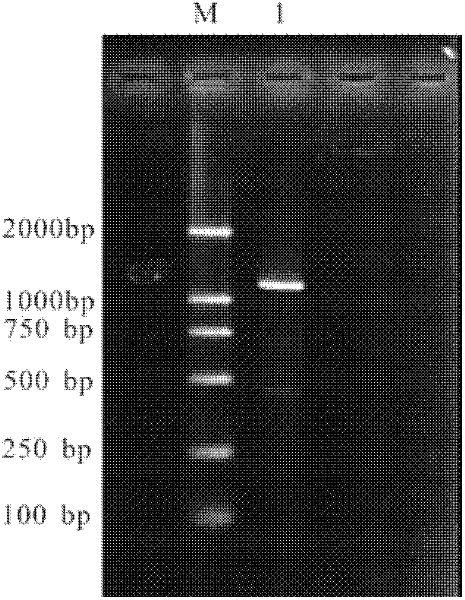
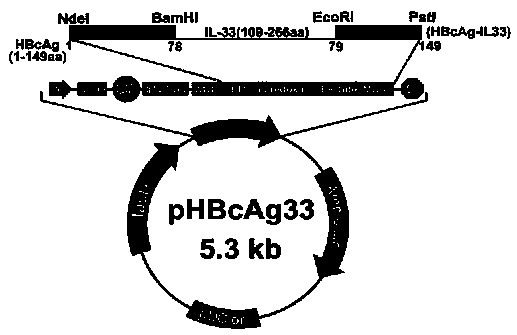
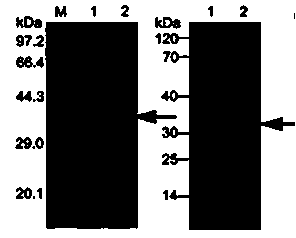
Method for constructing IL-33 presentation VLP (Virus-Like Particle) vaccine used in active immunotherapy of chronic asthma
ActiveCN104001168AEasy to makeEfficient purificationPeptide/protein ingredientsCarrier-bound antigen/hapten ingredientsDiseaseHepatitis B virus core Antigen
The invention provides a method for constructing an IL-33 presentation VLP (Virus-Like Particle) vaccine used in active immunotherapy of chronic asthma. The method comprises the following steps: extracting IL-33 total RNA (Ribonucleic Acid) from a mouse; performing reverse transcription to obtain IL33 total cDNA (complementary deoxyribonucleic acid); performing PCR (Polymerase Chain Reaction) amplification on the obtained total cDNA with a designed specific primer to obtain a coded IL-33 mature segment gene; inserting the gene between 78-bit amino acid and 79-bit amino acid of a hepatitis B virus core antigen HBcAg to obtain a recombinant plasmid pHBcAg33; transferring the plasmid onto escherichia coli DH5alpha or a BL21 competent cell; inducing by using IPTG (isopropyl-beta-d-thiogalactoside) and purifying to obtain the IL-33 presentation VLP vaccine. A strong neutralizing antibody which is specific to own molecules and has a durable action can be induced by inoculating the vaccine repeatedly in order to regulate and control immune response, thereby fulfilling the aim of regulating and controlling the progress of asthma.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Treatment of glioma by Anti-angiogenic active immunization for direct tumor inhibition and augmentation of chemotherapy, immunotherapy and radiotherapy efficacy
InactiveUS20170100438A1Well formedArtificial cell constructsMammal material medical ingredientsActive immunizationMalignancy
Disclosed are compositions of matter, therapeutic protocols, and immunization means to induce an active immune response to vasculature feeding glioma or other brain neoplasia. In one embodiment the invention provides administration of placental derived endothelial cells at concentrations of 10 million to 50 million administered in a manner to stimulate immunity toward blood vessels supplying glioma or other brain neoplastic malignancies. The invention provides means of blocking augmenting efficacy of immunotherapy, chemotherapy, and radiotherapy.
Owner:BATU BIOLOGICS
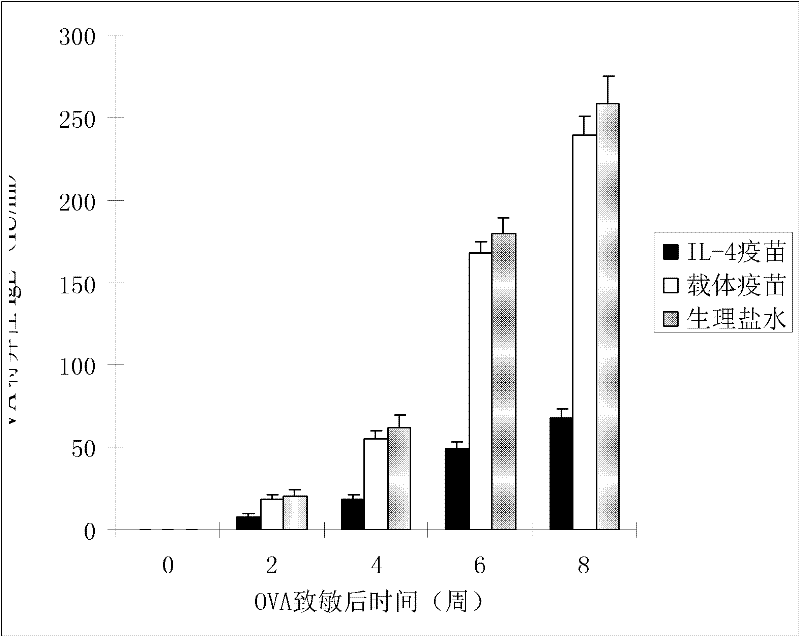
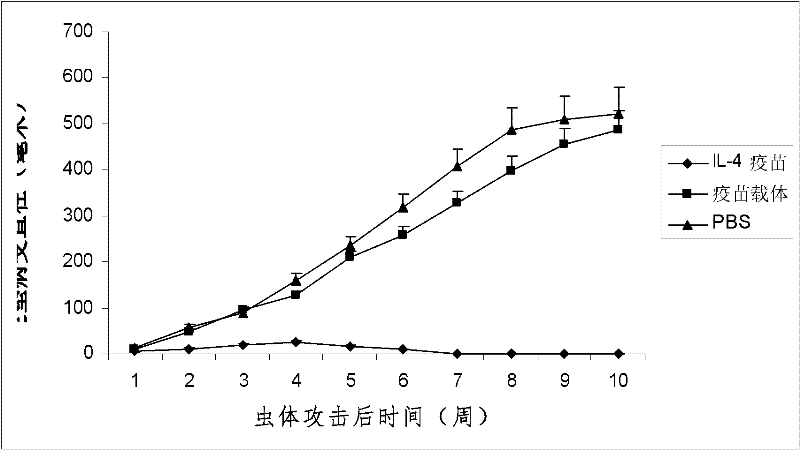
Interleukin-4 Therapeutic Vaccines for the Treatment of Human or Animal Immune-Related Diseases
ActiveCN102266551AProne to allergic reactionsFew applicationsGenetic material ingredientsImmunological disordersDiseaseActive immunization
The invention discloses an interleukin-4 therapeutic vaccine for treating immune related diseases of humans or animals. The interleukin-4 therapeutic vaccine is a protein vaccine or coupling protein vaccine of any form prepared by taking the natural or artificially synthesized complete protein or protein fragment of interleukin-4 as an antigen; or the interleukin-4 therapeutic vaccine is a gene vaccine or fusion gene vaccine of any form prepared by taking the complete gene or gene fragment of interleukin-4 as the antigen gene or the major antigen gene. The IL (interleukin)-4 vaccine is used for performing active immunotherapy on a host, generally, the effective time lasts for about 2-3 months by immunization for the first time, the effective therapeutic time lasts for about half a year by secondary immunization, and recovery can be achieved by 1-3 times of immunotherapy. Compared with direct application of anti-IL-4 antibody for treatment, the invention has the characteristics of few times of application, low dose and the like, thereby greatly reducing the therapeutic cost, and also greatly reducing the possibility of generating allergic reaction.
Owner:潍坊康奥思生物技术有限公司
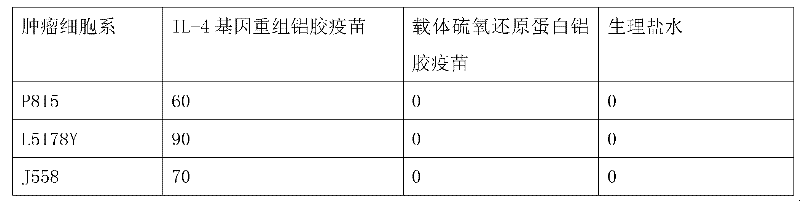
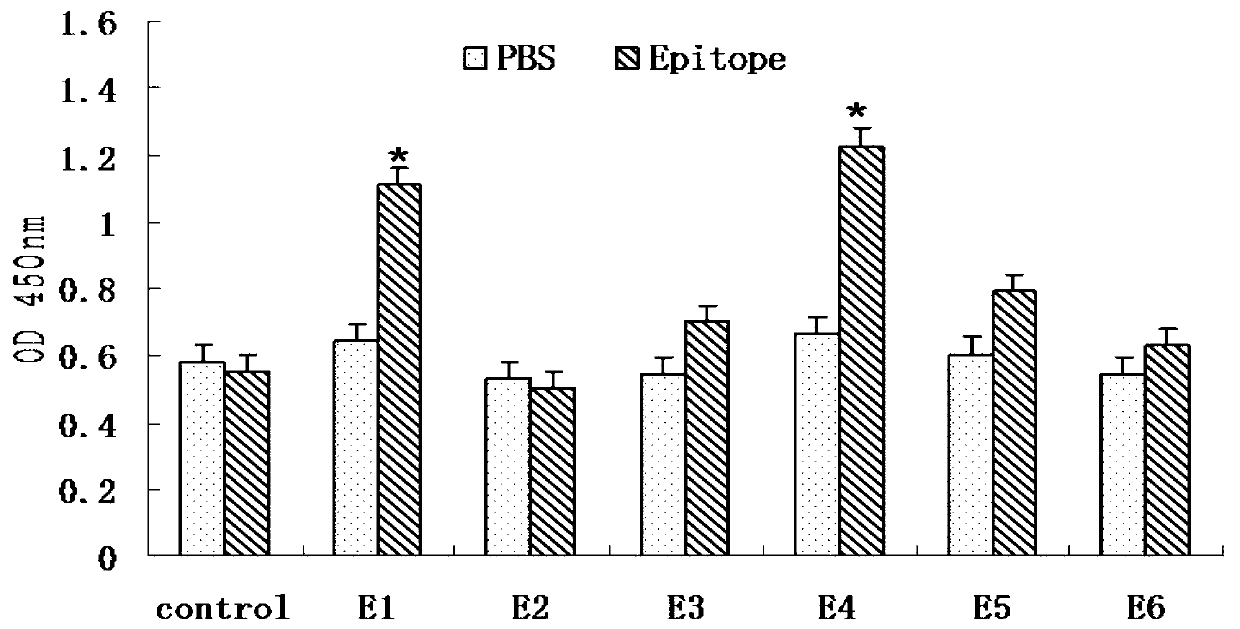
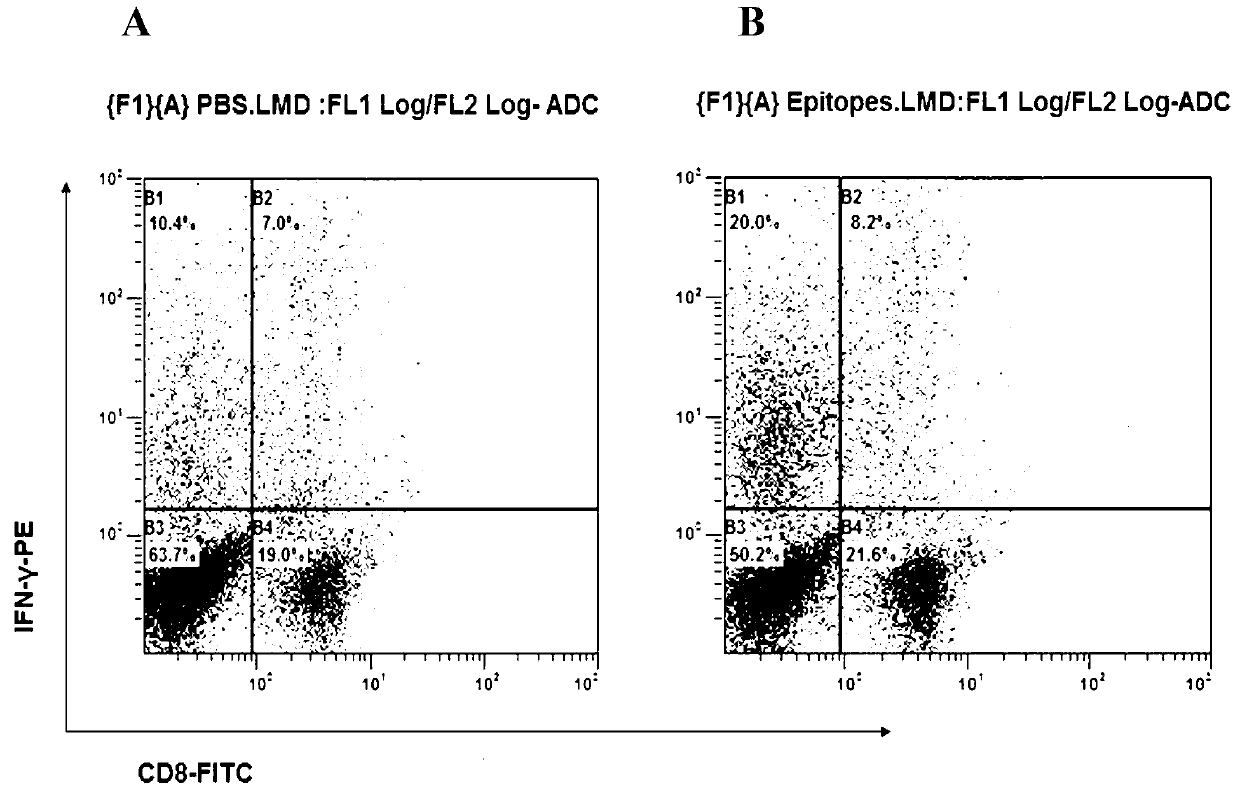
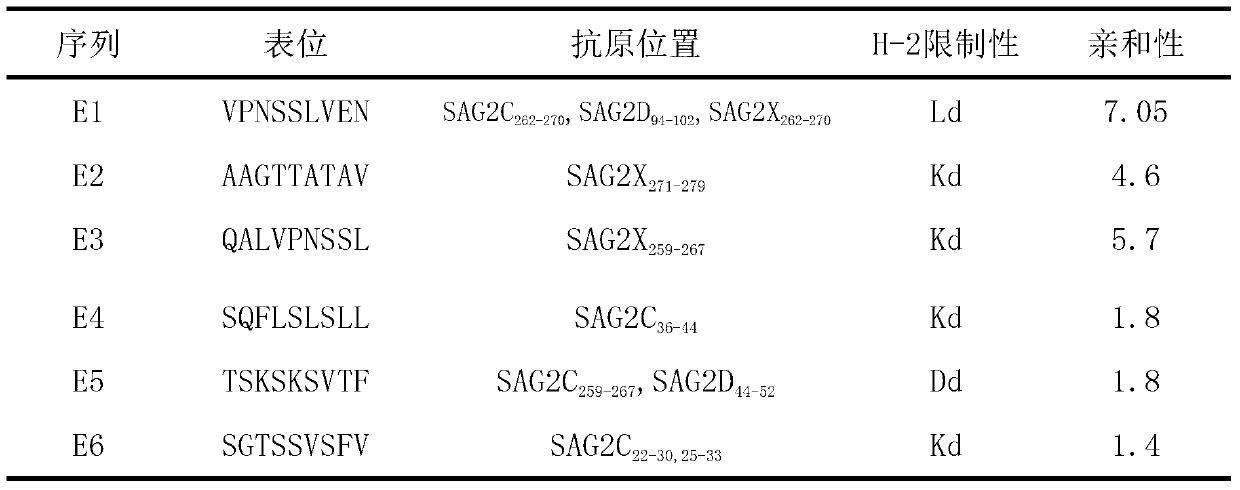
CD8+T cell dominant epitopes based on toxoplasmagondii bradyzoite antigens
ActiveCN103275182AProtozoa antigen ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsBALB/cActive immunization
The invention discloses two CD8+T cell dominant epitopes of VPNSSLVEN and SQFLSLSLL based on toxoplasmagondii bradyzoite specific surface antigens, wherein six CD8+T cell epitopes with H-2 restriction and high affinity are screened according to the protein sequences of the bradyzoite specific surface antigens of SAG2C, -2C and -2X; BALB / c mice are actively immunized by the screened epitope polypeptides; determinations for a lymphopoiesis level and a cell factor content, and an immune protection evaluation for epitope vaccines are performed after the immunization. The result indicates that powerful cell immunization can be generated by inducing the mice via the epitope vaccinesm, wherein the two antigen peptides of VPNSSLVEN and SQFLSLSLL are provided to be great in the capacity of stimulating T cell proliferation, as well as capable of inducing the mice to secrete protective cell factors and immune protection, being used as the epitope vaccines effectively resisting toxoplasmagondii infection, and reducing an encystations rate in the brain tissue of a host.
Owner:SHANDONG UNIV
DNA vaccine for preventing toxoplasmosis of humans or animals
InactiveCN105561343AImprove responseImprove survival rateGenetic material ingredientsNucleic acid vectorBALB/cCyst
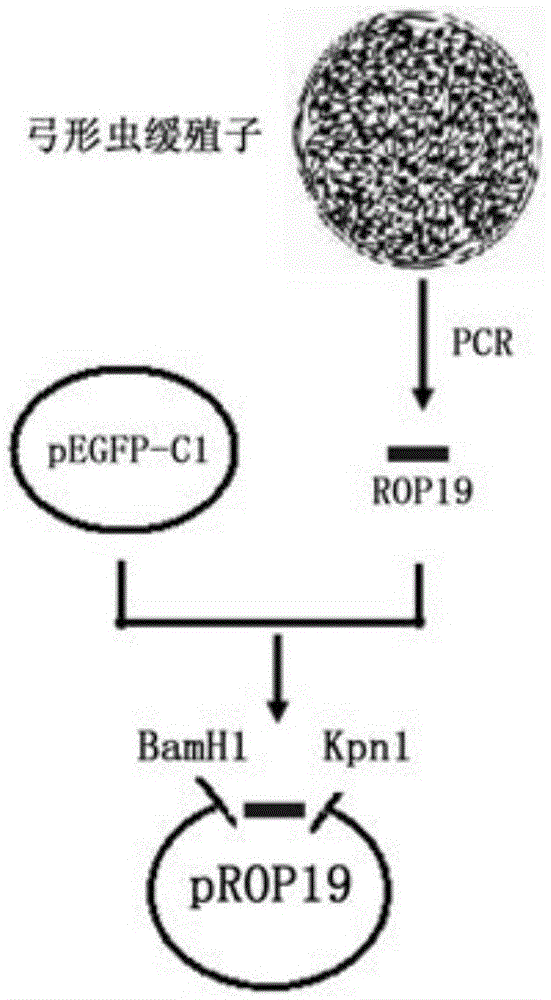
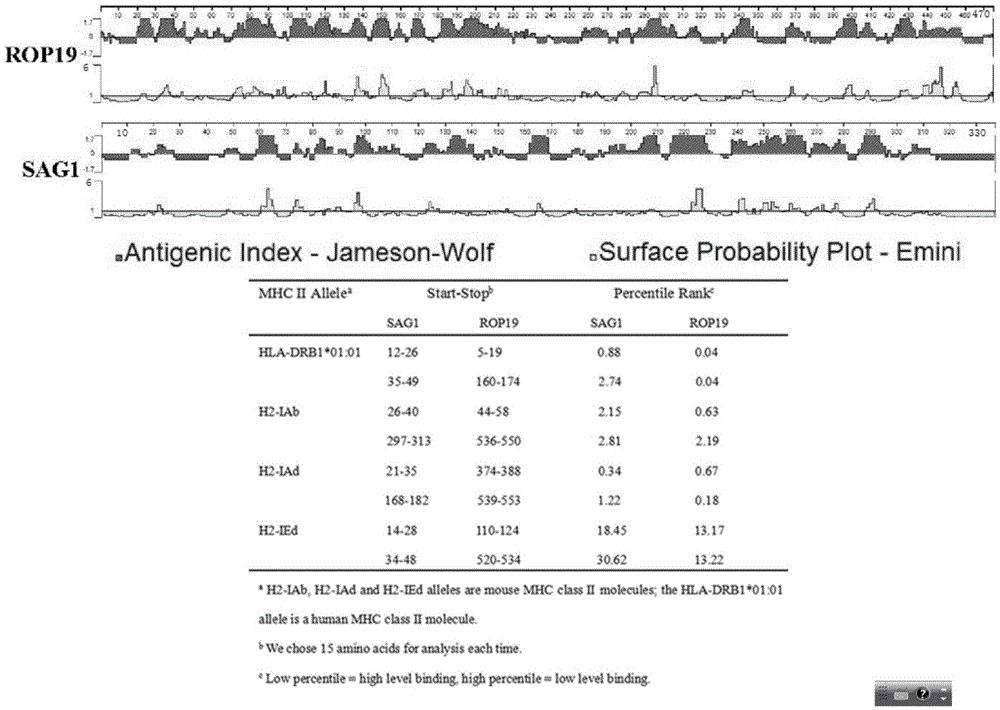
The invention discloses a DNA vaccine for preventing toxoplasmosis of humans or animals. An ROP19 gene is amplified through PCR, the amplified ROP19 gene is inserted into a eukaryotic expression vector pEGFP-C1 to build recombinant plasmid pEGFP-C1-ROP19 (pROP19), the recombinant plasmid containing a target gene is extracted, and the toxoplasma gondii ROP19DNA vaccine is obtained. According to the DNA vaccine for preventing the toxoplasmosis of the humans or the animals, active immunization is conducted on BALB / c mice through the DNA vaccine, the immunogenicity of the vaccine is evaluated by determining cells and humoral immunity indexes of immunized mice, and the immunoprotectivity of the vaccine is estimated by counting the number of cysts in brains of the mice and the survival rate of the mice after attack experiments of toxoplasma gondii are conducted. By means of the DNA vaccine for preventing the toxoplasmosis of the humans or the animals, humoral immunity and cellular immune responses can be effectively enhanced, the formation rate of the brain cysts of the immune mice attacked by a toxoplasma gondii PRU strain (II type) is effectively decreased, and the survival time of the mice attacked by a toxoplasma gondii RH stain (I type) is prolonged.
Owner:SHANDONG UNIV
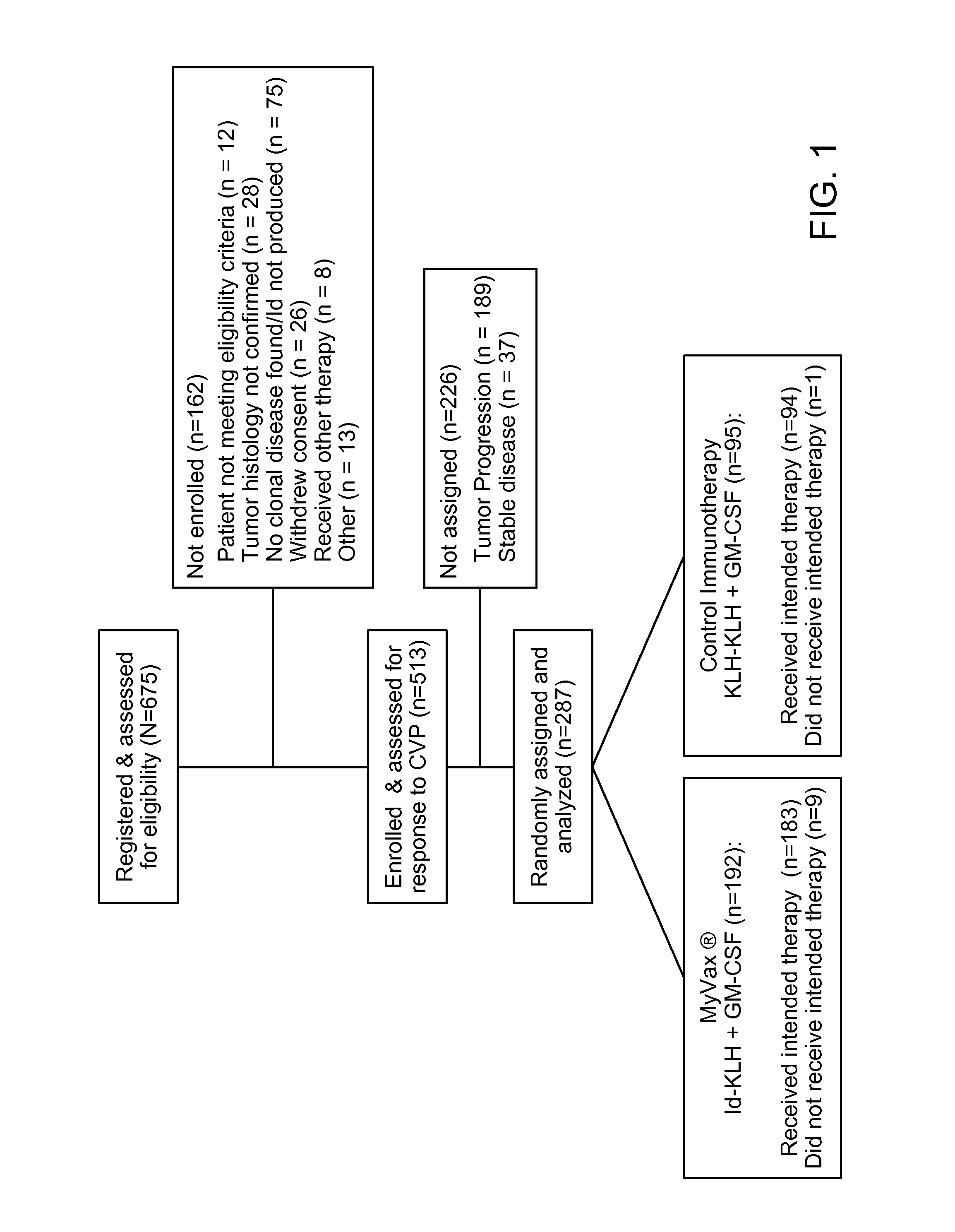
Method of Predicting Responsiveness of B Cell Lineage Malignancies to Active Immunotherapy
InactiveUS20140220562A1Microbiological testing/measurementDisease diagnosisTumor responseActive immunization
Predictive biomarkers identify those patients suffering from immunoglobulin positive (Ig+) B lineage malignancies that are responsive to active immunotherapy, where the active immunotherapy comprises vaccination with a tumor-specific idiotype-immunogen. It is shown herein that patient responsiveness to the idiotype-immunogen is dependent upon the sequence of the immunogen, where an immunogen having a low number of tyrosine residues in the CDR1 (herein termed CDR1-Y10) regions of one or both of the immunogen heavy and light chains is predictive of a positive anti-tumor response, while a high number of CDR1 tyrosine residues (herein termed CDR1-Yhi) is predictive of a low anti tumor response.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
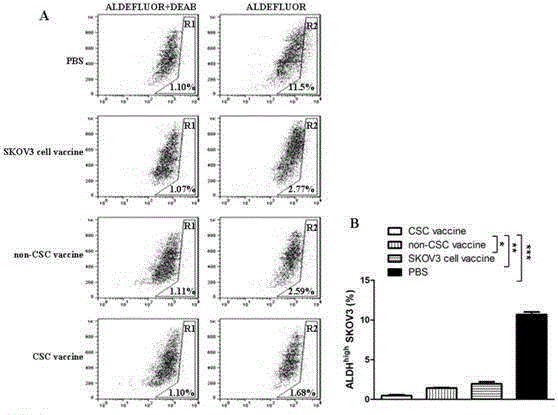
Ovarian cancer stem cell vaccine and preparation method thereof
InactiveCN105219731AHigh activityHigh tumorigenicityTumor/cancer cellsAntibody medical ingredientsCancer cellNatural Killer Cell Inhibitory Receptors
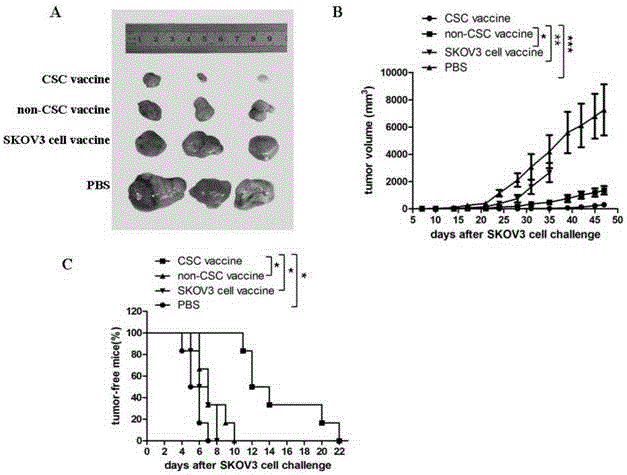
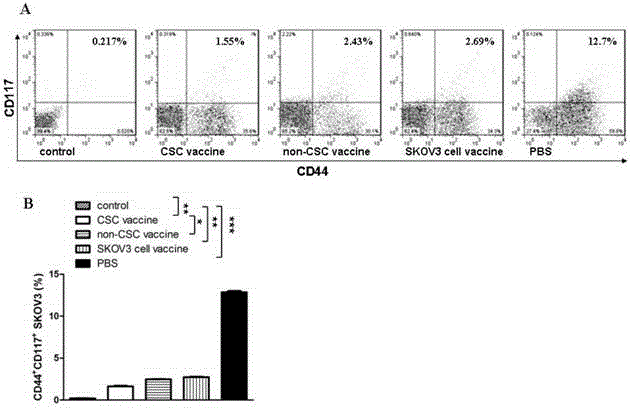
The invention relates to the field of molecular biology and discloses an ovarian cancer stem cell vaccine and a preparation method and application thereof. Cancer stem cells are insensitive to radiotherapy and chemotherapy, which is the root of cancer metastasis and recurrence. Aiming at active immunotherapy of the cancer stem cells, satisfactory therapeutic effect can be gained only on the condition that cancer 'seed' cells, namely the cancer stem cells are eliminated. The ovarian cancer stem cell CD117+CD44+ vaccine is capable of improving blood serum IFN (interferon)-gamma level, lowering TGF (transforming growth factor)-beta expression, enhancing NK cell activity, decreasing the number of ovarian cancer stem cells CD117+CD44+ remarkably and inhibiting cancer growth in animal experiments.
Owner:SOUTHEAST UNIV +1
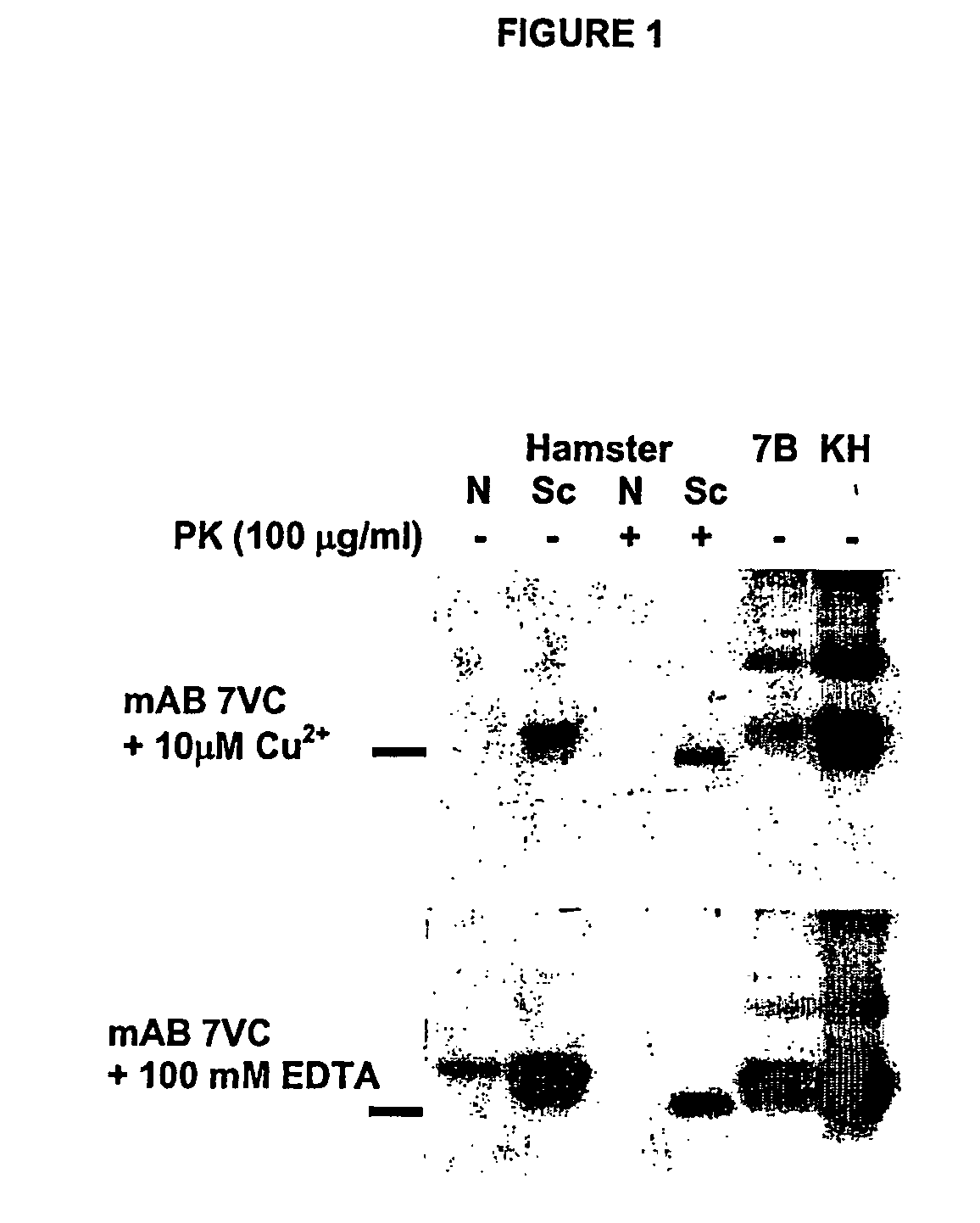
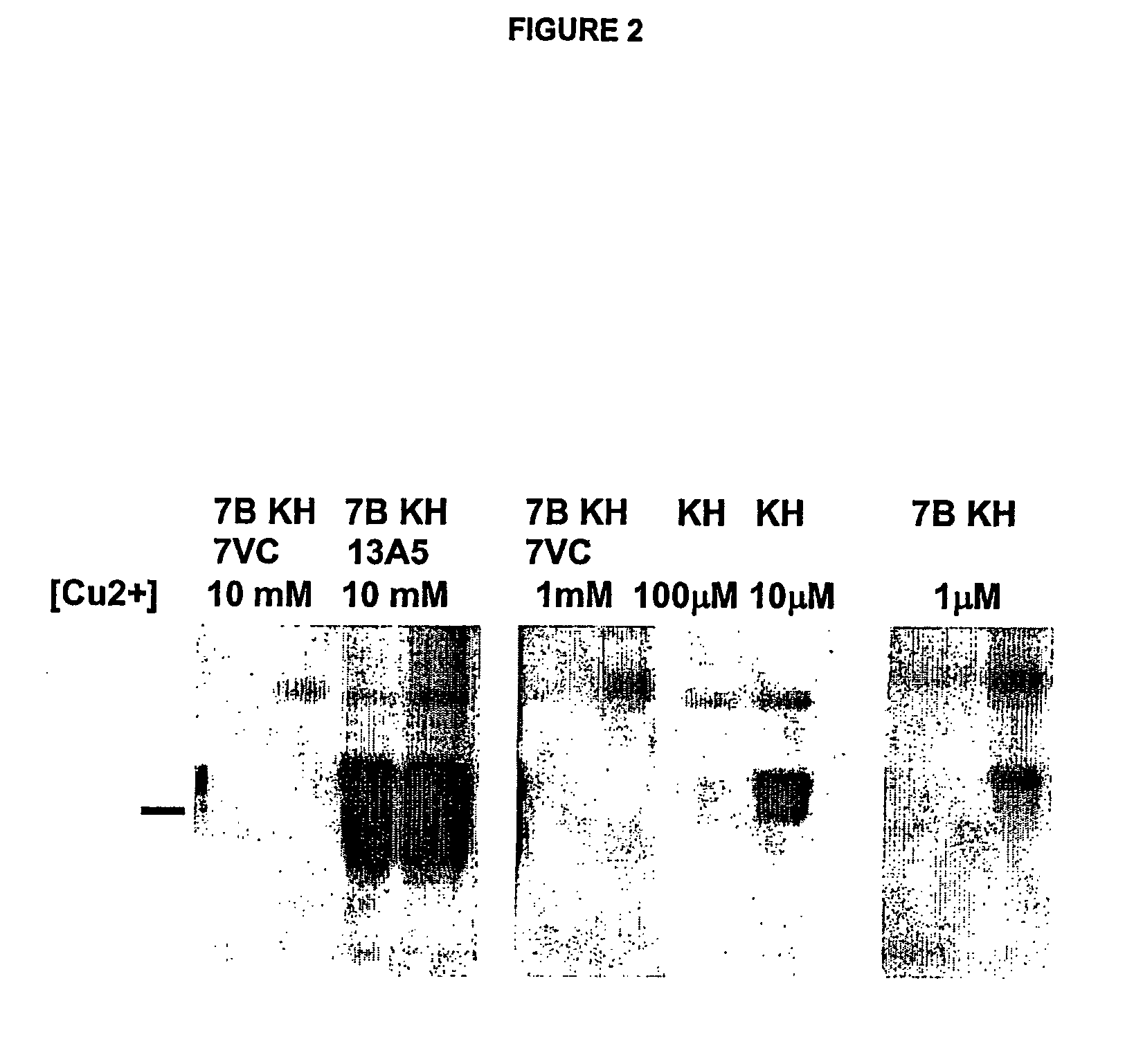
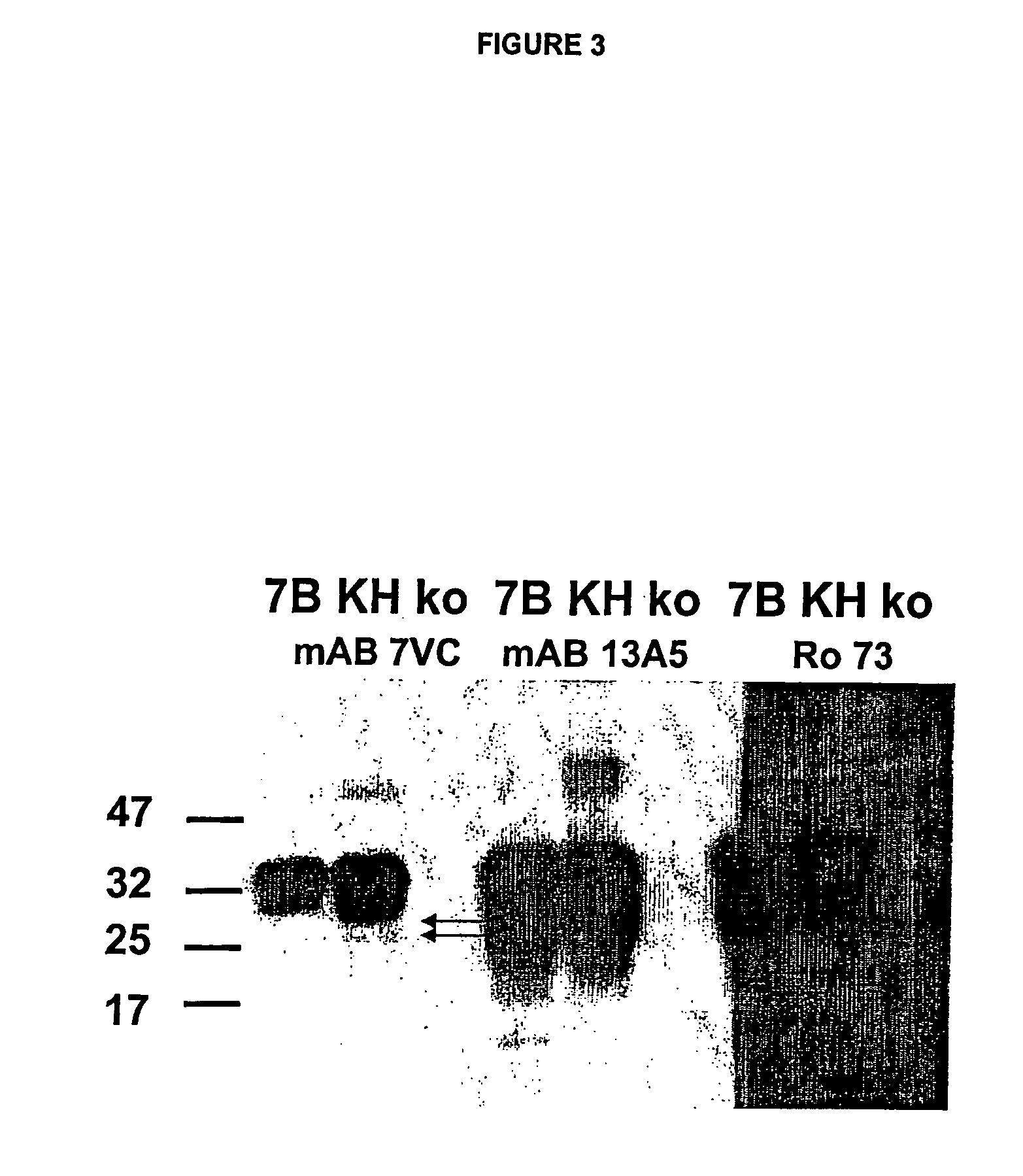
Use of monoclonal antibodies to distinguish protein conformational isoforms
InactiveUS20070281318A1Promote differentiationInhibit PrP expressionImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsScreening techniquesActive immunization
Methods of preparing monoclonal antibodies that differentially bind to a single conformer of a protein of interest are described. Passive immunization using these antibodies as well as use of conformer-specific antibodies as diagnostic reagents for the purpose of stratification of patient populations with regards to disease outcome, drug efficacy or drug sensitivity is also disclosed as well as active immunization with the protein conformer. In the screening techniques, detection can be for example by tissue immunostaining, western blotting or solution IP. A specific mab termed 7VC which shows conformation specificity to CtmPrP, a prion protein conformer that triggers neurodegeneration under specific assay conditions of pH and copper concentration, is described. A second specific antibody termed 19B10 shows conformation specificity for NtmPrP, a prion protein conformer that downregulates total PrP expression and effects cell differentiation.
Owner:HEINRICH HEINE UNIV OF DUSSELDORF +1
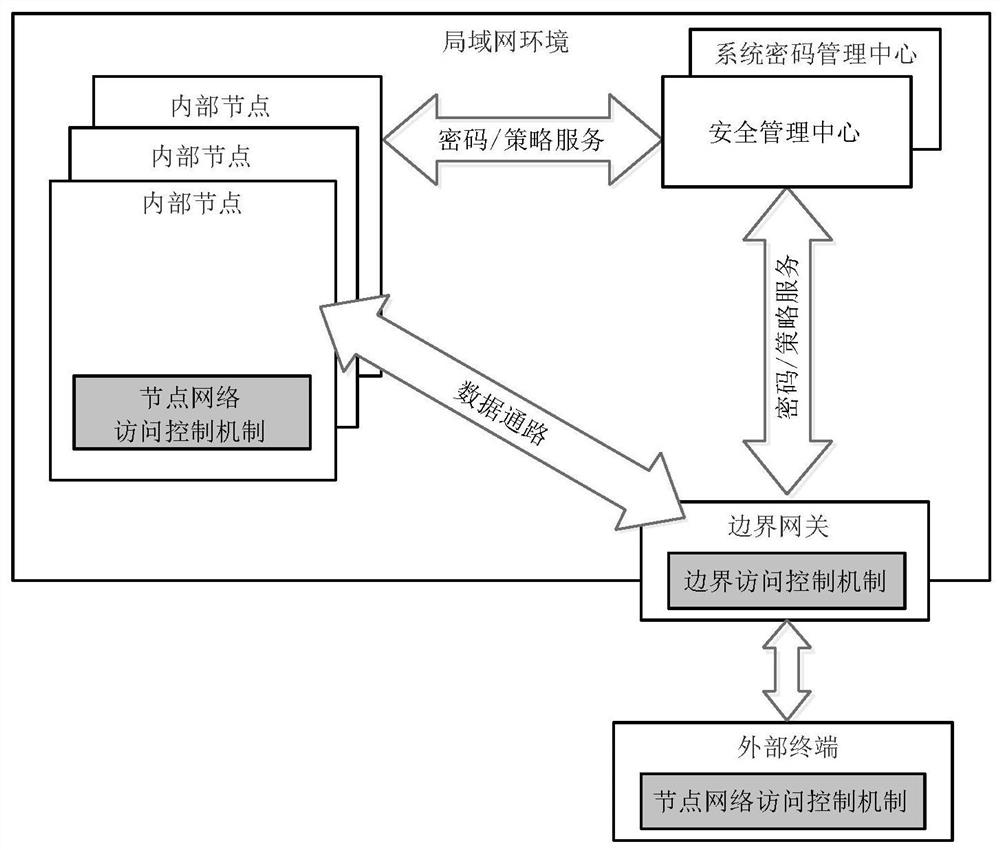
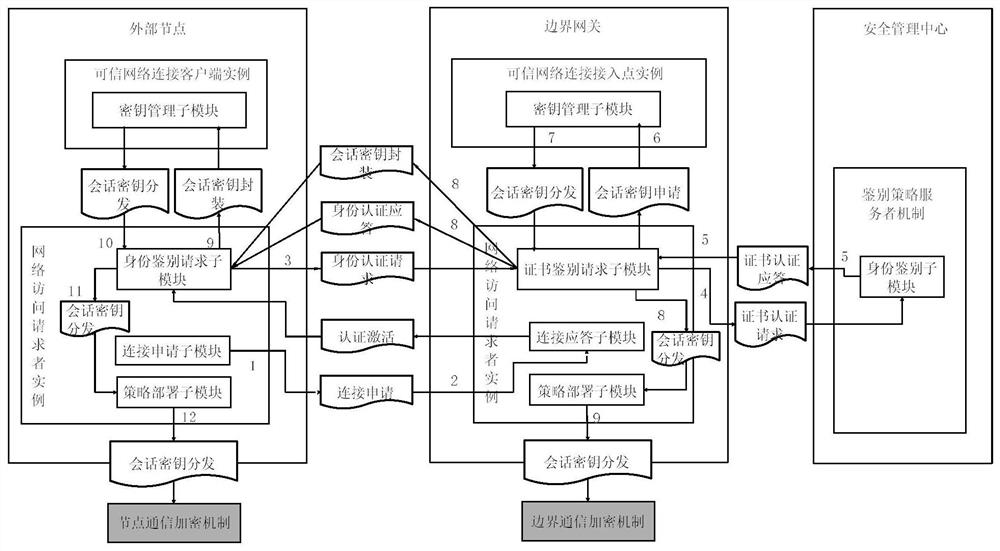
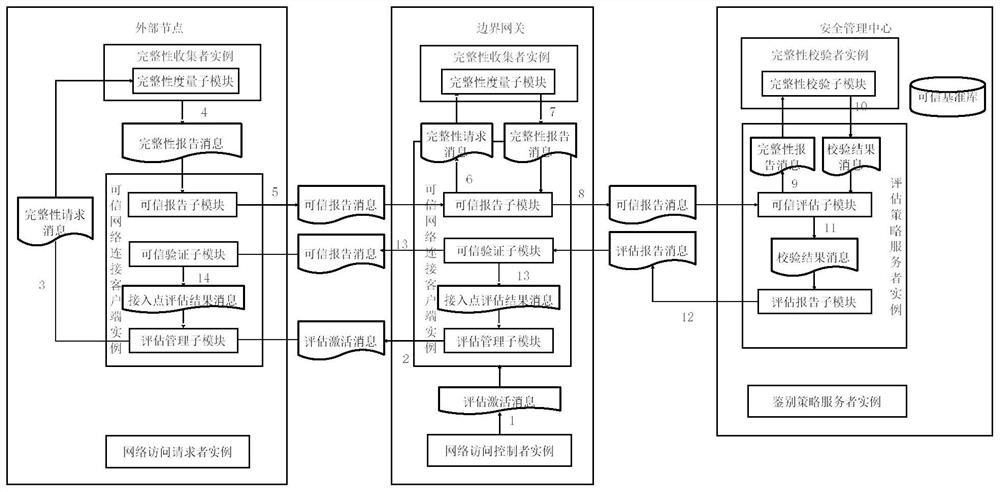
Industrial control equipment safety protection system and method based on active immune mechanism
ActiveCN112015111AContinuously workingWork normallyProgramme controlComputer controlActive immunizationInformation systems security
The invention relates to an industrial control equipment safety protection framework and method based on an active immune mechanism. The method specifically comprises the following steps that under atrusted computing architecture based on active immunization, an information system safety protection system is divided into a trusted node computing environment, a trusted area boundary and a trustedcommunication network; based on the trusted computing theory of active immunization, overall design is carried out from two aspects of related safety technologies and management means, and an active immunization protection system framework adaptive to an industrial communication network scene is established by combining field industrial control system equipment. According to the active immune industrial control safety protection architecture, a safe and efficient cryptographic algorithm is adopted, and a trusted control chip and trusted software are connected with a trusted network; an activesafety policy management and control system is integrated to form a set of active immune information safety protection scheme suitable for an industrial control site, so that a trusted and safe operation environment is provided for key equipment in an industrial control system network.
Owner:SHENYANG INST OF AUTOMATION - CHINESE ACAD OF SCI
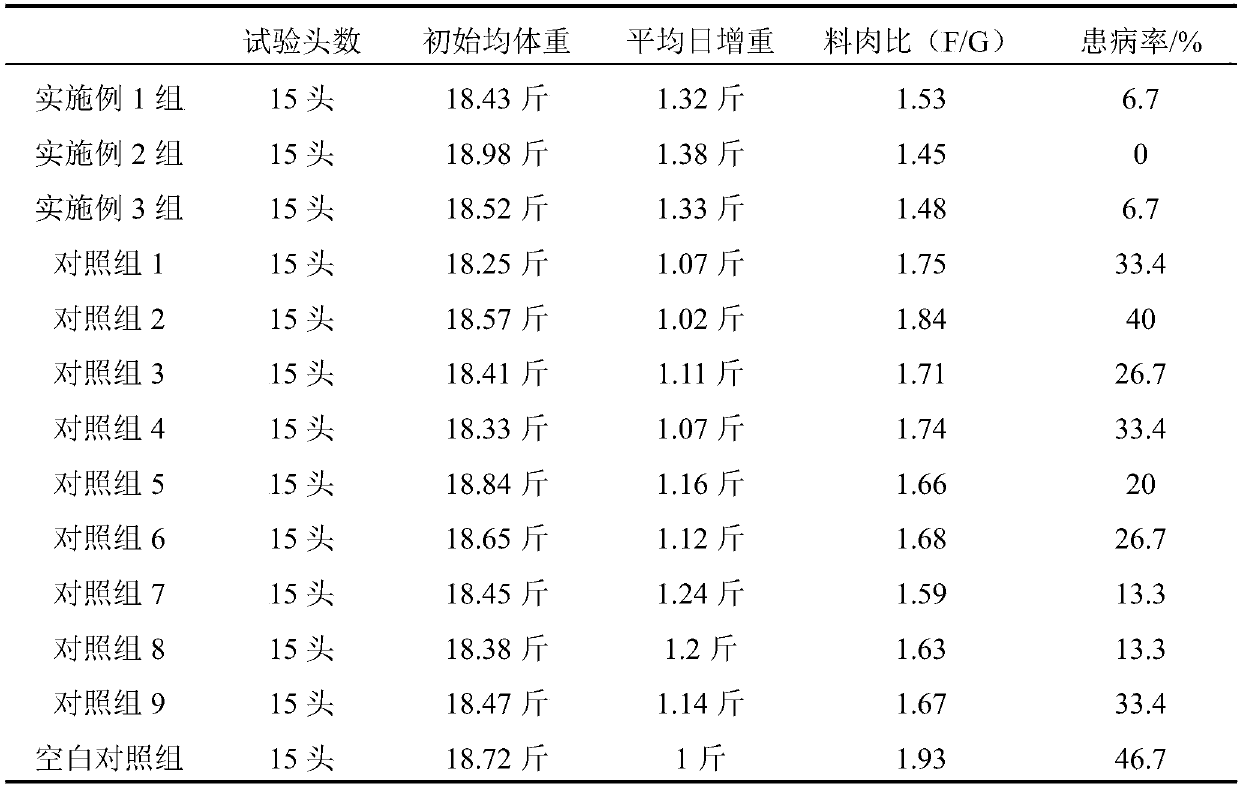
Medicine for promoting growth and development of pigs as well as preparation method and application of medicine
ActiveCN107898955AImprove conversion rateEmission reductionDigestive systemImmunological disordersDiseaseFeed conversion ratio
The invention discloses a medicine for promoting growth and development of pigs as well as a preparation method and application of medicine and belongs to the technical field of livestock and poultrymedicines. The medicine disclosed by the invention is prepared from the following components in percentage by weight: 99 percent to 99.5 percent of a traditional Chinese medicine composition and 0.5 percent to 1 percent of bacillus subtilis, wherein the traditional Chinese medicine composition is prepared from radix astragali seu hedysari, folium eucommiae extract, radix codonopsis, bighead atractylodes rhizome, rhizoma dioscoreae, scorched fructus crataegi, scorched malt, scorched medicated leaven, orange peel and liquorice root according to the weight ratio of (4 to 15) to (2 to 5) to (4 to10) to (3 to 8) to (2 to 8) to (2 to 6) to (1 to 4) to (1 to 4) to (2 to 5) to (0.1 to 2). The medicine disclosed by the invention can be used for strengthening a gastrointestinal function and improving the feed conversion rate; less excrement is formed and the emission of waste gas is reduced. The medicine can be used for stimulating immune organ functions and improving the active immunization level. The medicine is used for promoting repairing and secondary development of internal organs, increasing the disease resistance and reducing the morbidity and the death.
Owner:山东迅达康生物科技有限公司 +1
Amyloid ss peptide analogues, oligomers thereof, processes for preparing and compositions comprising said analogues or oligomers, and their uses
InactiveCN102203124AWell defined and reproducibleImprove hydrodynamic propertiesCompound screeningNervous disorderPassive ImmunizationsAntiendomysial antibodies
The present invention relates to relates to an amyloid ss peptide analogues comprising an amino acid sequence or a peptidomimetic thereof, wherein the sequence (i) forms a loop, (ii) has at least 66 % identity to the amino acid sequence of native Ass peptide or a portion thereof, (iii) comprises at least 6 contiguous amino acid residues and (iv) has at least 2 non-contiguous amino acid residues which are covalently linked with each other, oligomers comprising a plurality of said amyloid ss peptide analogues, processes for preparing the amyloid ss peptide analogues or oligomers, compositions comprising the amyloid ss peptide analogues or oligomers, and uses of the amyloid ss peptide analogues or oligomers such as their use for treating or preventing an amyloidosis (e.g.; by active immunization), for diagnosing an amyloidosis, and for providing agents that are capable of binding to the amyloid ss peptide analogues or oligomers. The subject invention also describes agents that are capable of binding to the amyloid ss peptide analogues or oligomers, e.g. antibodies, compositions comprising the agents, and uses of the agents such as their use for treating or preventing an amyloidosis (e.g. by passive immunization) and for diagnosing an amyloidosis.
Owner:ABBOTT LAB INC +1
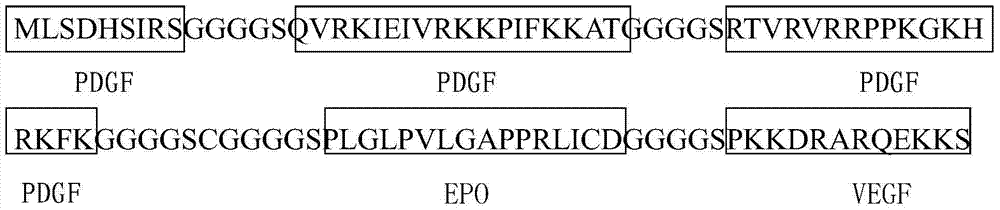
Anti-tumor angiogenesis immune composite peptide, and preparation method and application thereof
InactiveCN104744593AGrowth inhibitionSuppress generationPeptide/protein ingredientsPeptide preparation methodsVascular endotheliumActive immunization
The invention provides an anti-tumor angiogenesis immune composite peptide. The anti-tumor angiogenesis immune composite peptide includes an immunogenicity epitope of a vascular endothelial growth factor (VEGF), an immunogenicity epitope of platelet-derived growth factor (PDGF) and an immunogenicity epitope of erythropoietin EPO. The anti-tumor angiogenesis immune composite peptide has the capability of targeting three target sites, VEGF, PDGF and EPO, thus effectively inhibiting tumor growth; in addition, the anti-tumor angiogenesis immune composite peptide can be used as an active immunization vaccine, and effectively reduce the antibody drug immunogenic response. The invention also provides a preparation method and application of the anti-tumor angiogenesis immune composite peptide.
Owner:SHENZHEN INST OF ADVANCED TECH
Vaccine against Gram-negative bacterial infections
InactiveUS7025963B1Bacterial antigen ingredientsAntibody ingredientsPassive ImmunizationsColiform bacilli
A vaccine, effective in inducing the production of antibodies with which to immunize a second subject passively against infection by Gram-negative bacteria and LPS-mediated pathology, comprises a non-covalent polyvalent complex formed between purified, detoxified LPS derived from E. coli and purified outer membrane protein derived from N. meningitidis. The same vaccine will also actively immunize a host subject against Gram-negative bacterial infections and LPS-mediated pathology. Meningococcal infections are included among those Gram-negative bacterial infections protected against by the vaccine.
Owner:US ARMY MEDICAL RES MATERIEL COMMAND USAMRMC
Pure natural traditional Chinese medicine and marine organism active immunization bone
InactiveCN106421926AAvoid deformationImprove antibacterial propertiesHeavy metal active ingredientsAnthropod material medical ingredientsBone densityActive immunization
The invention discloses a pure natural traditional Chinese medicine and marine organism active immunization bone. The active immunization bone provided by the invention is prepared from bone protein and a concentrated traditional Chinese medicinal solution. The pure natural traditional Chinese medicine and marine organism active immunization bone provided by the invention can rapidly stop bleeding in a part with fracture within a short time, prevent deformation, repair bone cells and promote rapid healing of a wound, enhance an antibacterial effect, nourish bones and muscles as well as tendons and periostea, and enhance a bone density. The pure natural traditional Chinese medicine and marine organism active immunization bone provided by the invention, when applied to osteosynthesis, can avoid pain in a patient who accepts a secondary operation to take out a steel body which is used for setting the fracture due to injury, atrophy and deformation on wound healing as well as complication infection, and can avoid the phenomenon that the patient becomes disabled due to a failed operation.
Owner:范玉梅
Use of passive myostatin immunization
InactiveUS20060088543A1Increase in breast muscle and thigh muscle and testis and heart weightPromote growth rateHormone peptidesPeptide/protein ingredientsMyostatinActive immunization
A method to alter the phenotype of animals, e.g., avians, which employs passive and active immunization is provided.
Owner:RGT UNIV OF MINNESOTA
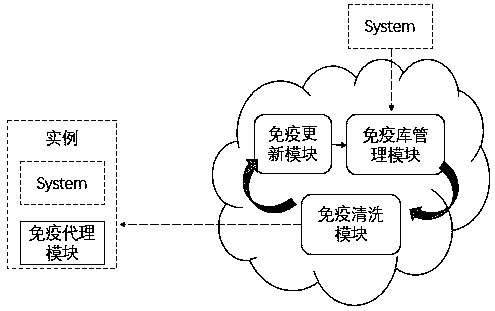
Cloud-based active immune security defense method and device
PendingCN111309450AActive immunity achievedImprove securityPlatform integrity maintainanceSoftware simulation/interpretation/emulationActive immunizationMirror image
The invention relates to a cloud-based active immune security defense method and device. The method mainly comprises the following steps: an immune library management module establishes a mirror imagevirtual machine system which is completely consistent with an off-cloud instance system in an initial state in cloud; the immune cleaning module pushes the in-cloud mirror image system to the out-of-cloud instance system for replacement, the operation and maintenance instance system and the in-cloud mirror image system are kept completely consistent, and meanwhile, inconsistent heterogeneous software is identified; the immune updating module carries out security upgrading on the in-cloud mirror image system; and the immune agent module operates in a hardware remote guidance state in the cloudexternal instance system, receives a cleaning instruction issued by the cloud immune cleaning module, and executes a cleaning action. According to the invention, high security of the system can be realized, risks of continuous penetration and invasion of advanced threats on a system attack surface exposed in a network environment and an operation environment for a long time are reduced, white lists of abnormal programs such as backdoors, Trojans and the like are identified, the system is periodically restored to an initial state, cloud and terminals are isolated in a one-way manner, and active immunization is realized.
Owner:中科天御(苏州)科技有限公司
Vaccine compositions for the treatment of dengue fever and uses thereof
InactiveUS20110262472A1SsRNA viruses positive-senseViral antigen ingredientsVirus-like particleActive immunization
The invention provides compositions, vaccine compositions and pharmaceutical compositions for the treatment, amelioration and / or prevention of dengue fever. The compositions, vaccine compositions and pharmaceutical compositions of the invention comprise a virus-like particle of an RNA bacteriophage and at least one antigen, wherein said at least one antigen is a dengue antigen. When administered to an animal, preferably to a human, said compositions, vaccine compositions and pharmaceutical compositions induce efficient immune responses, in particular antibody responses, wherein typically and preferably said antibody responses are directed against dengue virus, preferably against dengue virus of any one of serotypes 1 to 4. Thus, the invention further provides methods of treating, ameliorating and / or preventing dengue virus infection by way of active immunization against domain III of the dengue virus envelope protein E, or against antigenic fragments thereof.
Owner:CYTOS BIOTECHNOLOGY AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com