Active immunization using a siderophore receptor protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production and Purification of Siderophore Receptor Proteins
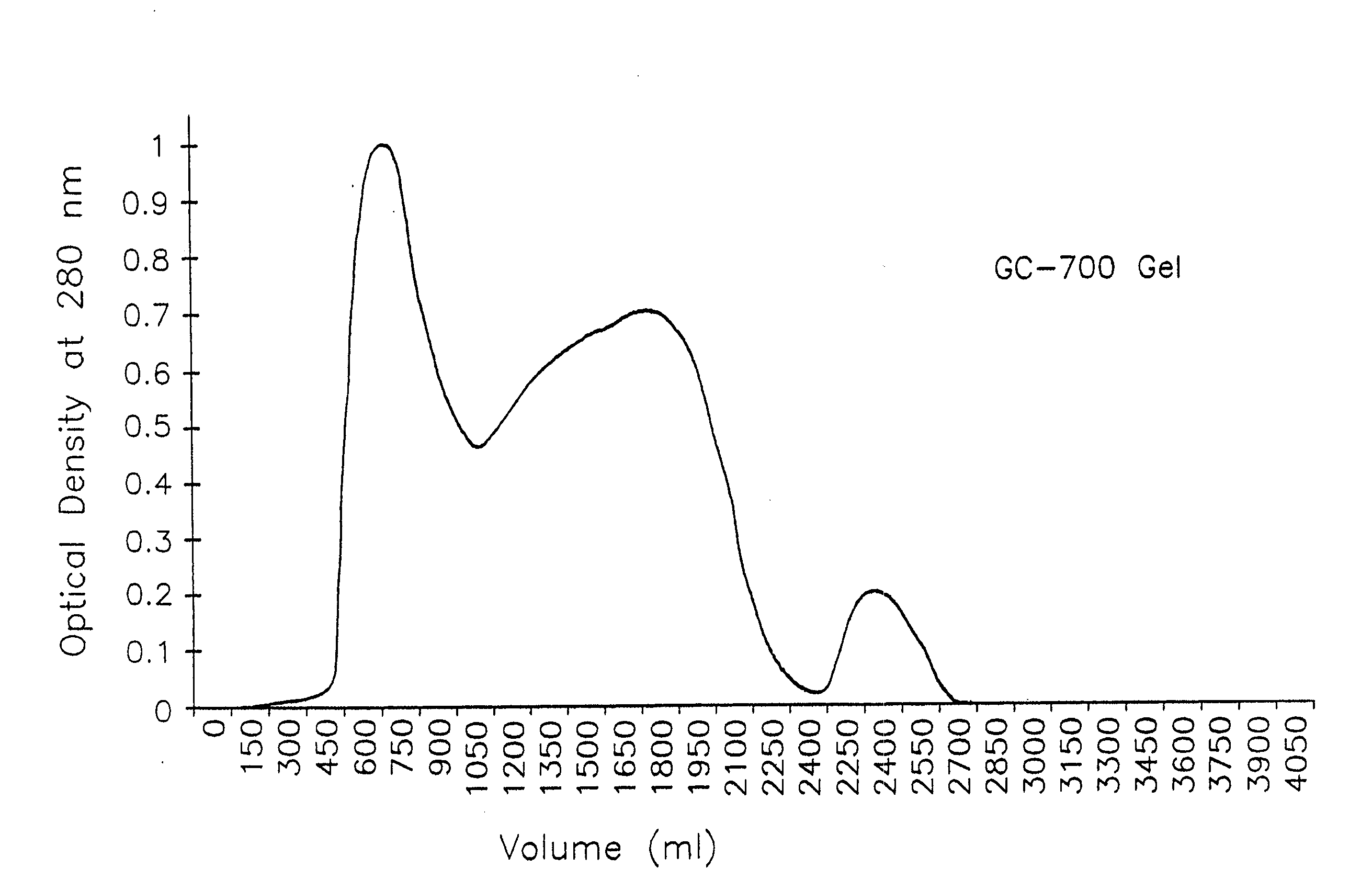
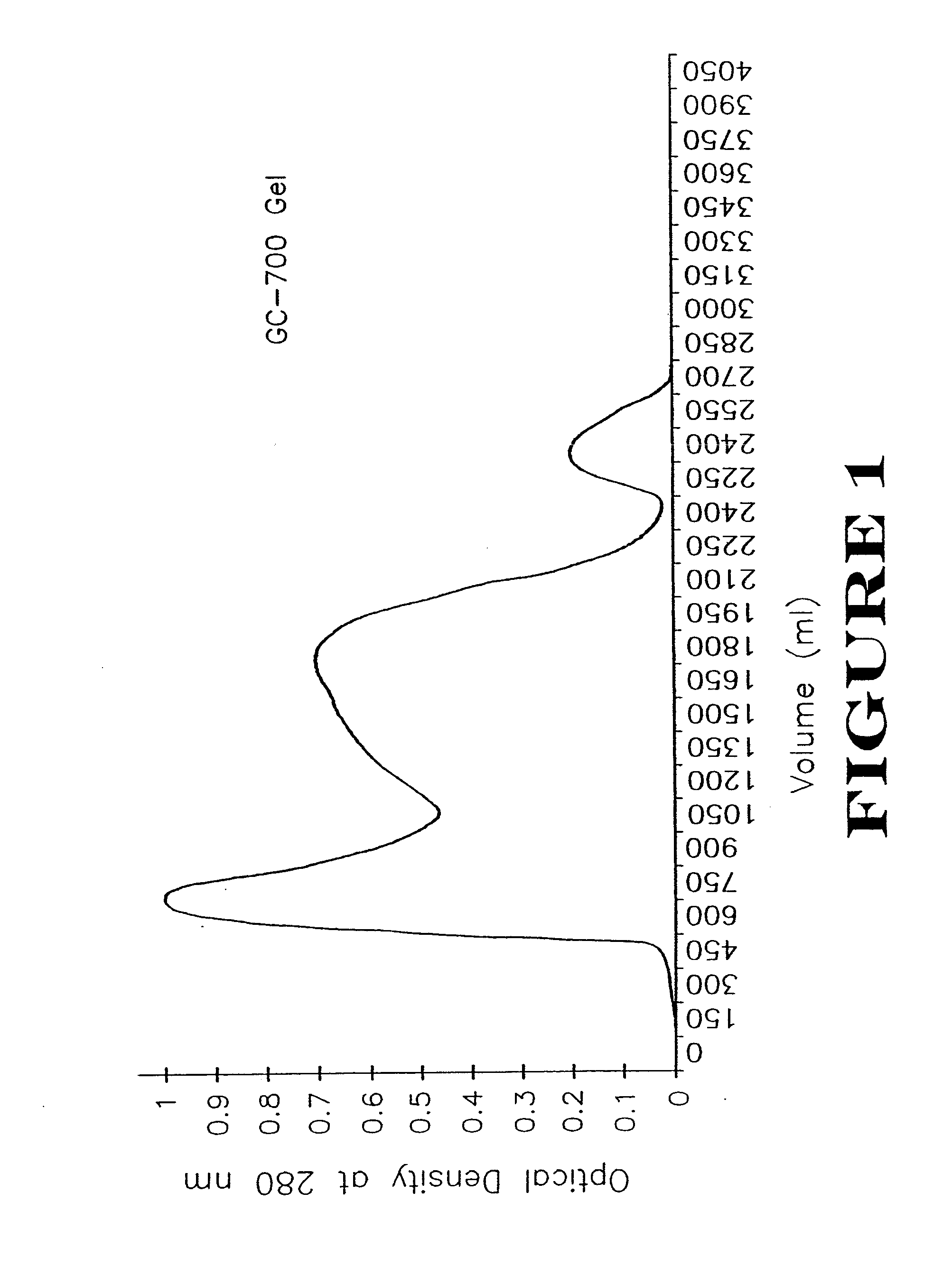
[0070]Escherichia coli serotype 078 (turkey isolate; serotyped by Pennsylvania State University, deposited with the American Type Culture Collection (ATCC), Bethesda, Md., U.S.A., as ATCC #55652, on Jan. 3, 1995) (700 ml at 108 colonies / ml) was inoculated into a Virtis bench-top fermenter (Virtis, Inc., Gardiner, NY), charged with 20-L of brain-heart infusion (BHI, Difco Laboratories, Detroit, Mich.) containing 50 μgrams / ml of dipyridyl (Sigma Chemical Co., St. Louis, Mo.) at 41° C. This isolate has been shown to produce four siderophore receptor proteins for (MW 89 kDa, 84 kDa, 78 kDa, 72 kDa) under iron-restrictive conditions. The pH was held constant at 7.4 by automatic titration with 5N NaOH. The fermenter was stirred at 400 rpm. The culture was grown continuously for 18 hours after which the bacteria were removed by continuous-flow centrifugation at 20,000×g at 4° C. using a Beckman (Model J2-21M) centrifuge (Beckman I...
example 2
Preparation of Vaccine with Siderophore Receptor Proteins
[0074]The precipitate from Example 1, hereinabove, containing siderophore receptor proteins of E. coli serotype 078, were resuspended in physiological saline (0.85%) containing 0.1% formalin as a preservative. The protein concentration was 300 μg / ml. The aqueous protein suspension (1,000 ml) was emulsified in a water-in-mineral oil adjuvant containing 972 ml Drakeol 6 mineral oil and 28 ml of Anlacel A as an emulsifier. The mixture was emulsified using an Ultra-Turnax T50 emulsifier (KIKA Works, Inc., Cincinnati, Ohio) at 4° C. The water-in-oil emulsion was stored at 4° C.
example 3
Vaccination of Poultry with Siderophore Receptor Protein Vaccine
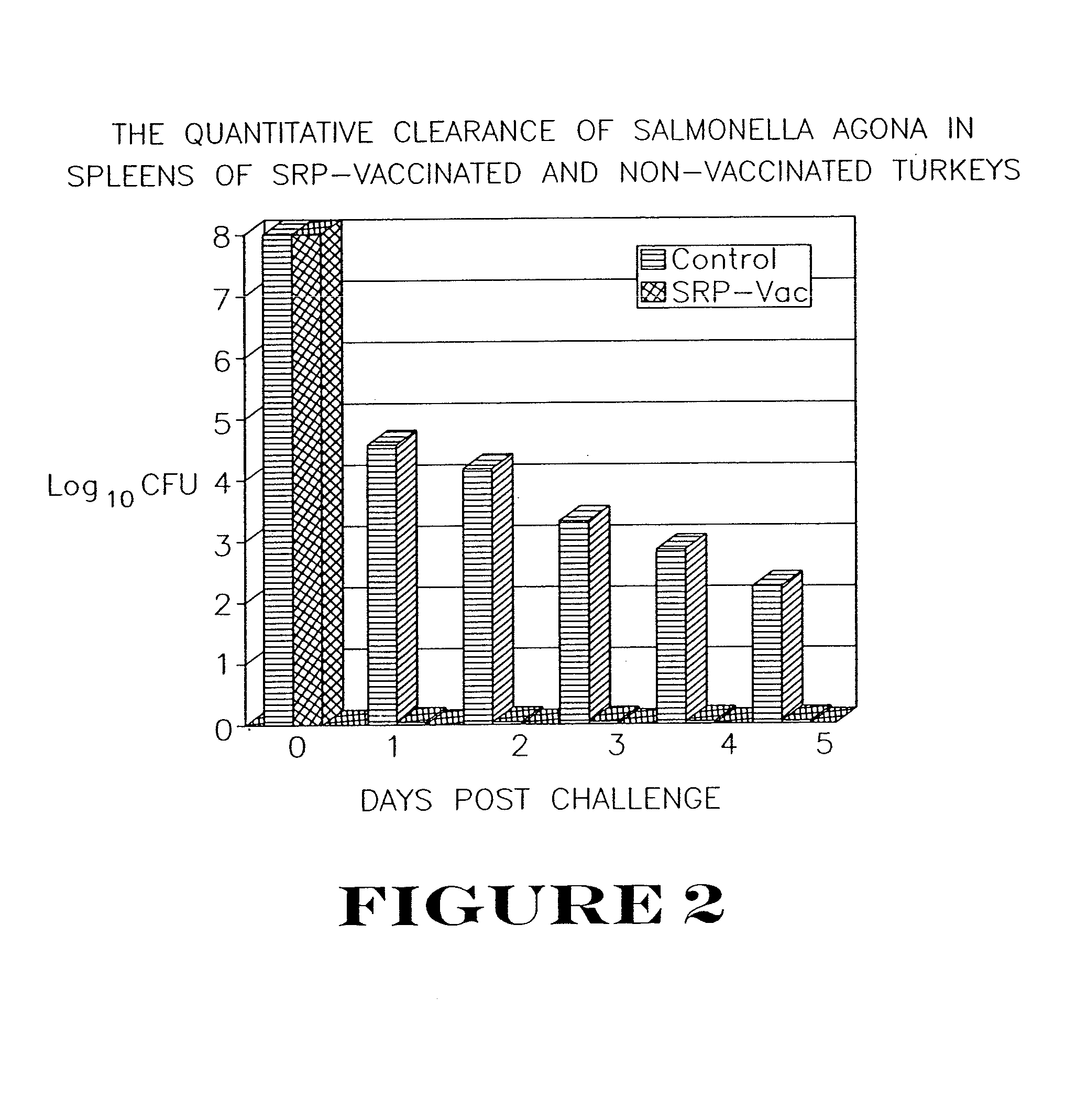
[0075]Seventy-two turkey poults were raised in isolation from one day of age. At three weeks of age, the birds were divided into two equal groups. Group 1 was vaccinated subcutaneously with the vaccine from Example 2 above, at a dosage level of 150 μg of siderophore receptor protein per bird. Group 2 remained as non-vaccinated controls. Group 1 was given a booster vaccination with the vaccine at a dosage level of 250 μg siderophore receptor protein per bird at 18 days after the first vaccination.
[0076]The vaccinated and non-vaccinated birds were equally divided among four isolation rooms. Rooms A and B contained the vaccinated birds, and Rooms C and D contained the non-vaccinated controls. At seven weeks of age, birds in Groups A and C were challenged subcutaneously with Salmonella agona at 1.0×108 cfu / bird. At 24, 48, 72, 96 and 120 hours post-challenge, two controls and two vaccinated birds were killed. The spleens we...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Atomic weight | aaaaa | aaaaa |
| Atomic weight | aaaaa | aaaaa |
| Atomic weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com