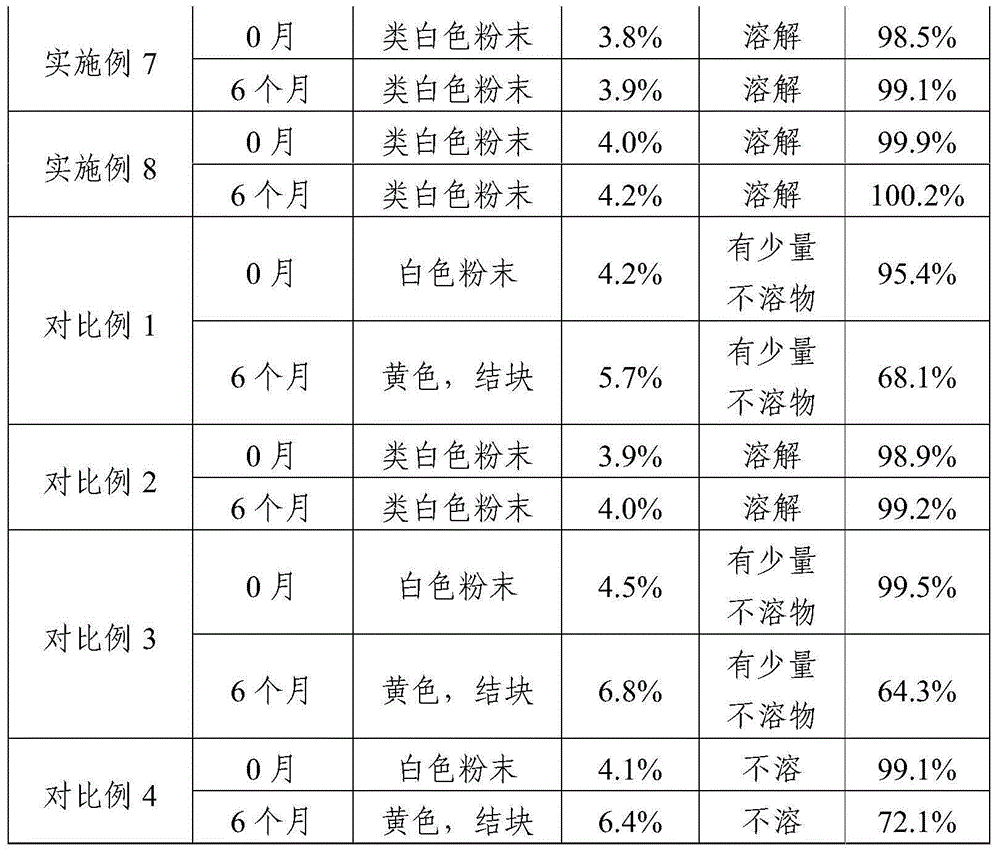
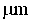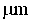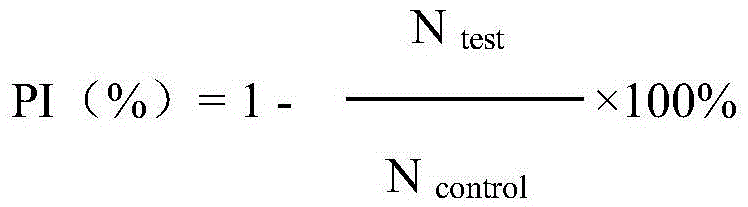Patents
Literature
218 results about "Coliform bacilli" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Coliform bacilli gram-negative bacilli found in the intestinal tract that resemble Escherichia coli, particularly in the fermentation of lactose with gas.
Peptide for high performance inhibition of angiogenesis and method for preparing same and use thereof
ActiveCN1699408AImprove and enhance growthImprove and enhance the anti-tumor effectPeptide/protein ingredientsSkeletal disorderEscherichia coliInclusion bodies
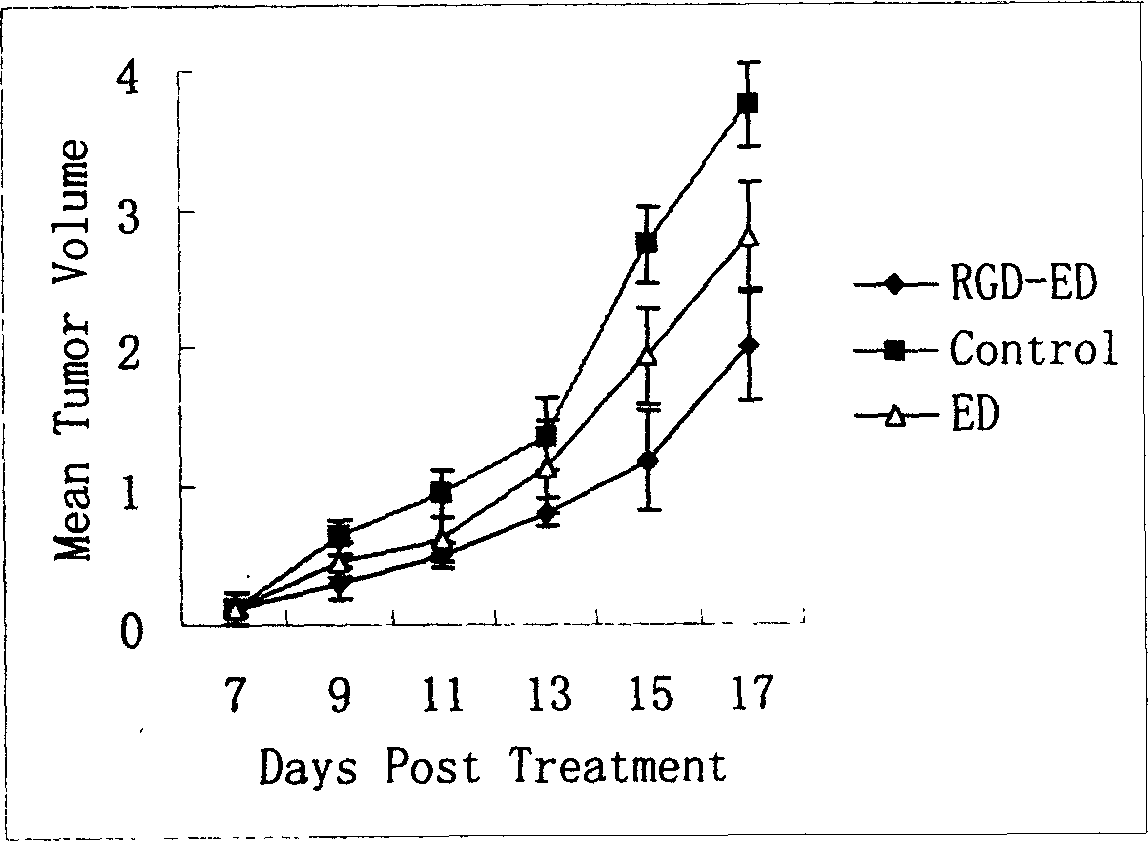
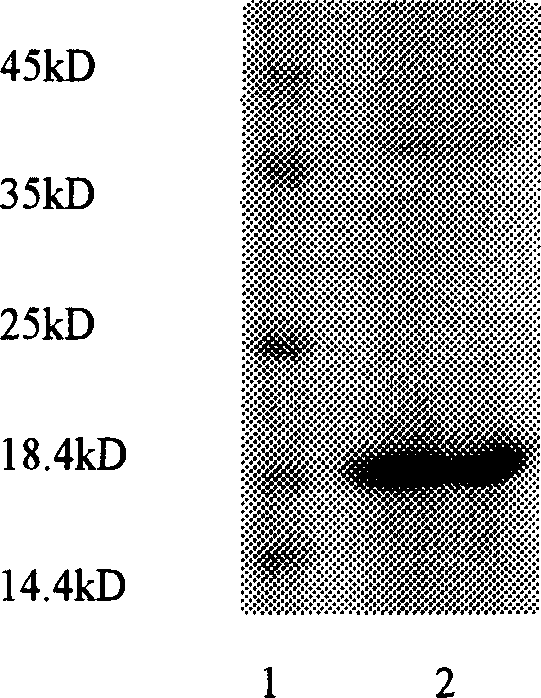
The invention relates to a peptide for high performance inhibition of angiogenesis and method for preparing same and use, wherein high performance blood vessel production inhibiting agent RGD-ED with integration compatibility is designed, the inhibiting agent comprises polypeptide polypeptide-valine-arginine-arginine-alanine-aspartate-arginine-alanine-alanine-valine-praline, its one or two ends are connected with polypeptides containing arginine-glycine-aspartic acid sequence. The RGD-ED provided by the invention can be synthesized. The invention also discloses the expression of one RGD-ED in bacillus coli through gene engineering method, wherein the RGD-ED is prepared through the steps of inclusion body protein segregation, dissolution and renaturation, and ion-exchange chromatography segregation and purification.
Owner:CHINA PHARM UNIV
Method for preparing nanofibre membrane
InactiveCN101638830AHas antibacterial functionPromote wound healing functionFilament/thread formingAbsorbent padsComposite nanofibersNanofiber
The invention discloses a method for preparing a nanofibre membrane, belonging to a technique for preparing a nanofibre compound membrane. The method comprises the following steps: preparing curcuminethanol solution and chitosan acetum, mixing the curcumin ethanol solution and the chitosan acetum by the volume ratio thereof to prepare spinning solution which is injected into an injector of an electrostatic spinning device for electrostatic spinning to form a compound nanofibre membrane and to obtain a curcumin / chitosan nanofibre membrane of antibacterial property, wherein the diameter of thecurcumin / chitosan nanofibre membrane is 200-400 nm. The invention has the advantages that the preparation process is simple, the prepared membrane material heals wound, has broad spectrum bactericidalproperty and has higher bacterial inhibition rate to colibacillus and staphylococcus aureus for 24h.
Owner:JIANGNAN UNIV +1
Preparation method of 3'-sialic acid lactose
InactiveCN102154163AChange the status quo of less ingredientsEfficient additionBacteriaMicroorganism based processesEscherichia coliSialic acid
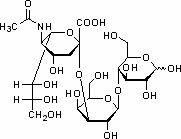
The invention provides a preparation method of 3'-sialic acid lactose, belonging to the technical field of biological engineering. The preparation method comprises the following steps of: taking colibacillus CCTCC (colibacillus China center for type culture collection) NO: M208088 as a starting strain, adding a fermentation medium into a fermentation tank, fermenting for 2-6h, then adding a fed batch culture medium, and adding lactose in a flowing way at the mid-later period of the fermentation; and centrifuging the fermentation liquid to obtain liquid supernatant 1 and a thallus, diluting the centrifuged thallus by adding water and splitting in a heating way, recentrifuging to obtain liquid supernatant 2, merging the liquid supernatant 1 and the liquid supernatant 2, and absorbing, desorbing and vacuum drying by ion exchange resin to obtain the 3'-sialic acid lactose. A mass of the 3'-sialic acid lactose can be prepared by the CCTCC NO: M208088. The 3'-sialic acid lactose can change the current situation that the sialyloligosaccharide component is less in exogenous adding substances in conventional baby milk, and can provide the guarantee to add more efficient sialic acid trophic factors into the baby milk.
Owner:朱莉
Plant lactobacillus strain and its application
InactiveCN1888051AImprove cleanlinessImprove adhesionBacteriaBacteria material medical ingredientsEscherichia coliStaphylococcus cohnii
The present invention discloses one plant lactobacillus strain and its application. The plant lactobacillus, Lactobacillus plantarum L323 CGMCC No. 1329, is separated from Chinese pregnant woman's vaginal secretion, and has relatively high bacteriotasis on common vaginal pathogens, such as staphylococcus aureus, candida albicans, colibacillus and vaginal Gardnar bacillus, and acid producing and H2O2 producing capacity higher than other lactobacillus. The plant lactobacillus, Lactobacillus plantarum L323 CGMCC No. 1329, may be used in preparing vaginal microbial preparation for preventing and / or treating vaginal infectious diseases.
Owner:TIANYOUDA BIO ENG SCI & TECH BEIJING +1
O-type foot-and-mouth disease virus multi-epitope mucous membrane immunization vaccine and use
This invention relates to a fusion protein used for preventing aftosa, its preparation method and application. This fusion protein contains O type foot-and-mouth disease virus main cytomembrane protein VP1 epitope, colibacillus thermolability toxin B subunit, thymus derived cell epitope and purification label.
Owner:GUANGZHOU PUTAI BIOTECH
Recombined collagen and synthesizing and expressing purifying process thereof
InactiveCN1793177ALow immune responseLower immune responseConnective tissue peptidesFungiBacteria coliformsMacromolecule
The invention discloses a rebuilt collagen and the compounding and expression purifying method. The main body of the collagen amino acid sequence is triad Gly-Xaa-Yaa, of which Xaa and Yaa at least have an electrified amino acid residue. The N end and C end contains the sequence of cysteine residue; the Arg-Gly-Asp residue combination is contained in the entire amino acid sequence. Using the compounded oligonucleotide to take annealing treatment to form double helical target DAN monomer that takes repeatedly polymerizing to form macromolecule DNA which would transfer into coliform bacteria, thus, the rebuilt collagen would be gained. The invention has good application value and could be used in biology medicine material, cosmetic and food fields.
Owner:ZHEJIANG SCI-TECH UNIV
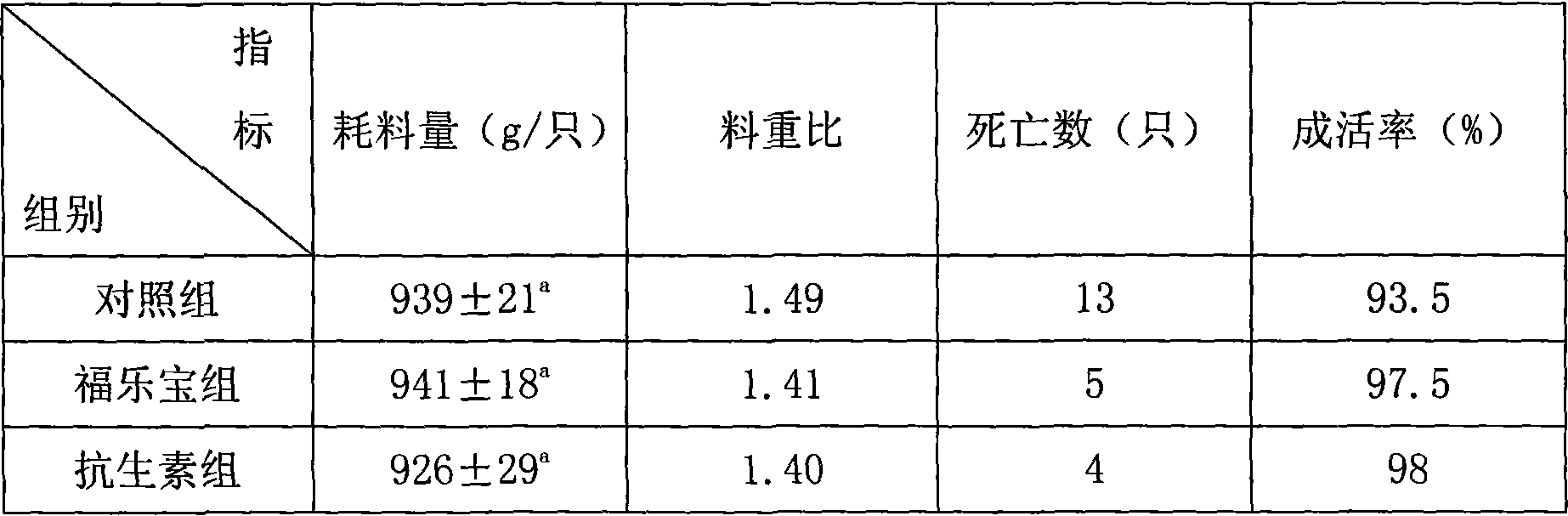
Compound premix feed of 1% green laying hens in laying period
ActiveCN103053831AImprove immunityIncrease production capacityAnimal feeding stuffBiotechnologyIntestinal microorganisms
The invention relates to the field of feed, in particular to a compound premix feed of 1% green laying hens in laying period. The compound premix feed of 1% green laying hens in laying period is formed by uniformly mixing micronutrient substances of trace elements, vitamins, synthetic amino acids and the like, feed additives and powdered grain feed as a carrier, and is characterized by comprising active dry yeast, lactic acid bacteria, bacillus and digestive ferment. In the compound premix feed of 1% green laying hens in laying period, active dry yeast, growing element for poultry containing lactic acid bacteria and bacillus can inhibit propagation of pathogen; in wet mixed feed, active dry yeast and the like can be propagated and fermented, and partial pathogen can be inhibited and killed; and the palatability and digestibility of the feed can be improved in a certain time range. Microzyme, lactic acid bacteria and bacillus enter an alimentary tract for quick propagation, and can inhibit the growth of pathogen such as colon bacillus, salmonella and the like, thereby reducing diseases caused by pathogen, generating the effect similar to antibiotics, eliminating the damage of antibiotics to enteric microorganisms, better balancing intestinal flora, improving the immunizing power of laying hens, and improving the health level of laying hens.
Owner:玉溪持久生物科技有限公司
Superoxide dismutase and preparation method thereof
InactiveCN101633916ARealize the industrialization of productionGood pH stabilityFungiBacteriaDismutaseNucleotide
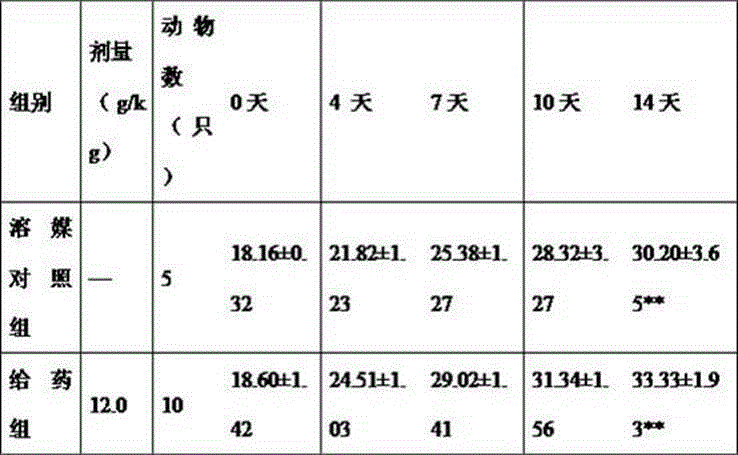
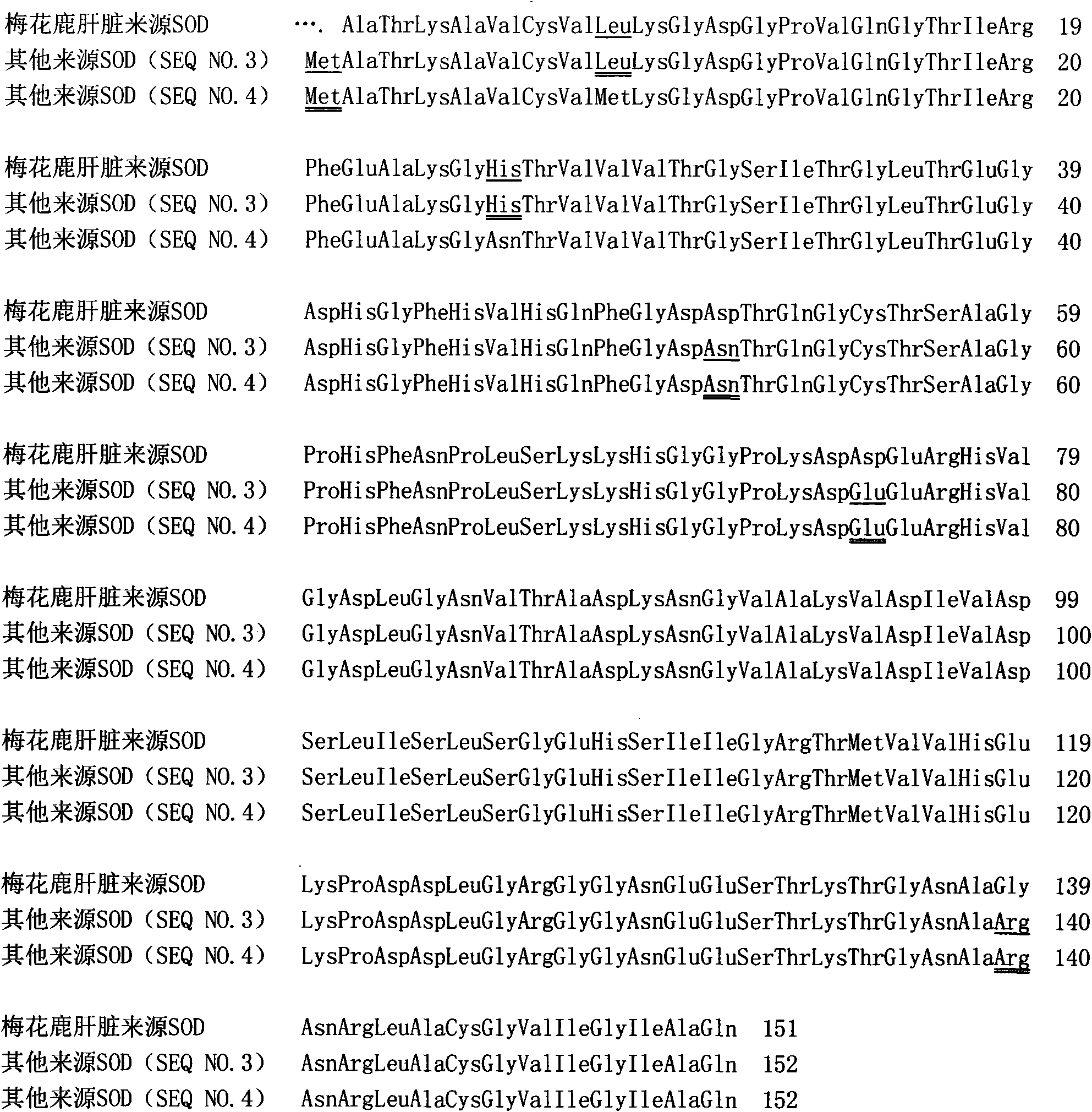
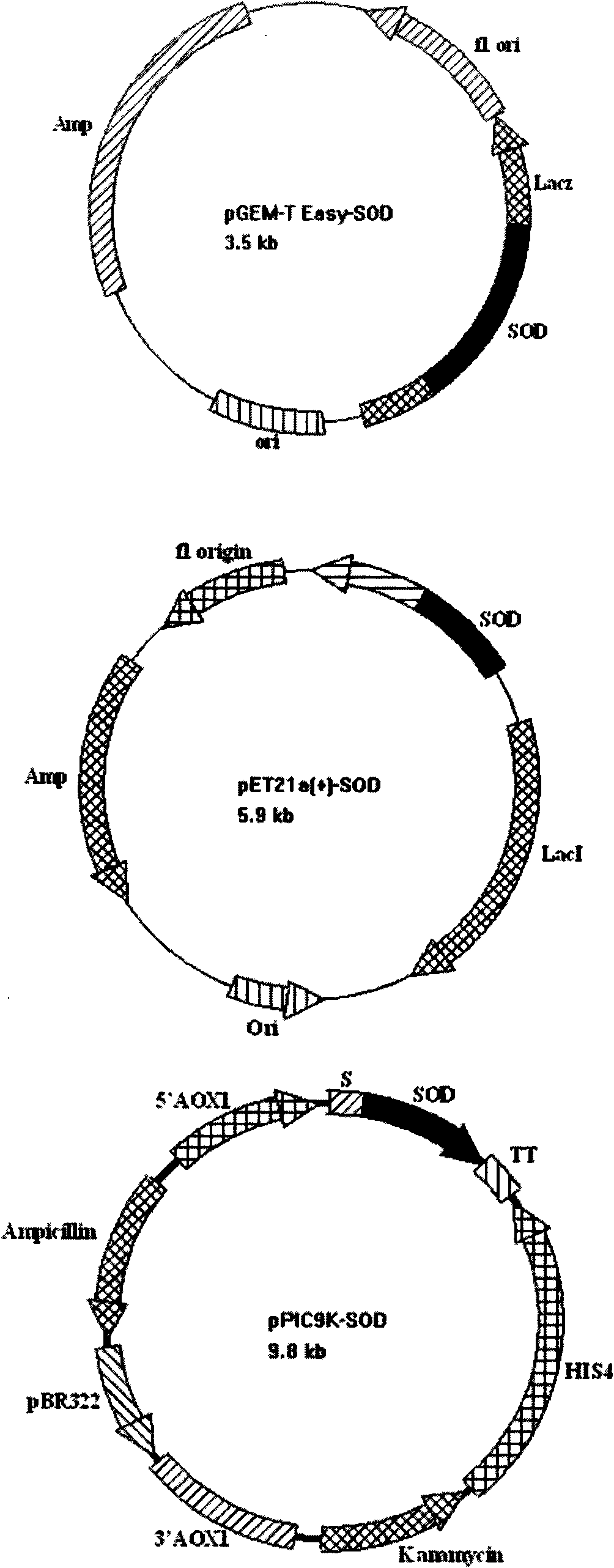
The invention provides a SOD gene from animal and a method for cloning and expressing the SOD gene in colibacillus and yeast cells, wherein a nucleotide sequence of superoxide dismutase is shown as SEQ NO.1; an amino acid sequence of the superoxide dismutase is shown as SEQ NO.2; carriers of nucleotide molecules are colibacillus plasmids or yeast plasmids; cells of the nucleotide molecules are formed by carrier conversion; and cells of the nucleotide molecules of the superoxide dismutase contain colibacillus containing the nucleotide molecules or converted by the carriers or pichia yeast containing the nucleotide molecules or converted by the carriers. The invention can prepare recombinant production strains which can efficiently express and secrete Cu / Zn-SOD, realizes the production industrialization of the Cu / Zn-SOD, and achieves good pH stability, favorable thermal stability and Cu / Zn-SOD products with anti-protease hydrolyzation capacity.
Owner:FUZHOU UNIV
Pig breeding and respiratory syndrome recombined adenovirus and vaccine
InactiveCN1554766ALittle changeChange propertiesGenetic material ingredientsInactivation/attenuationEscherichia coliBiotechnology
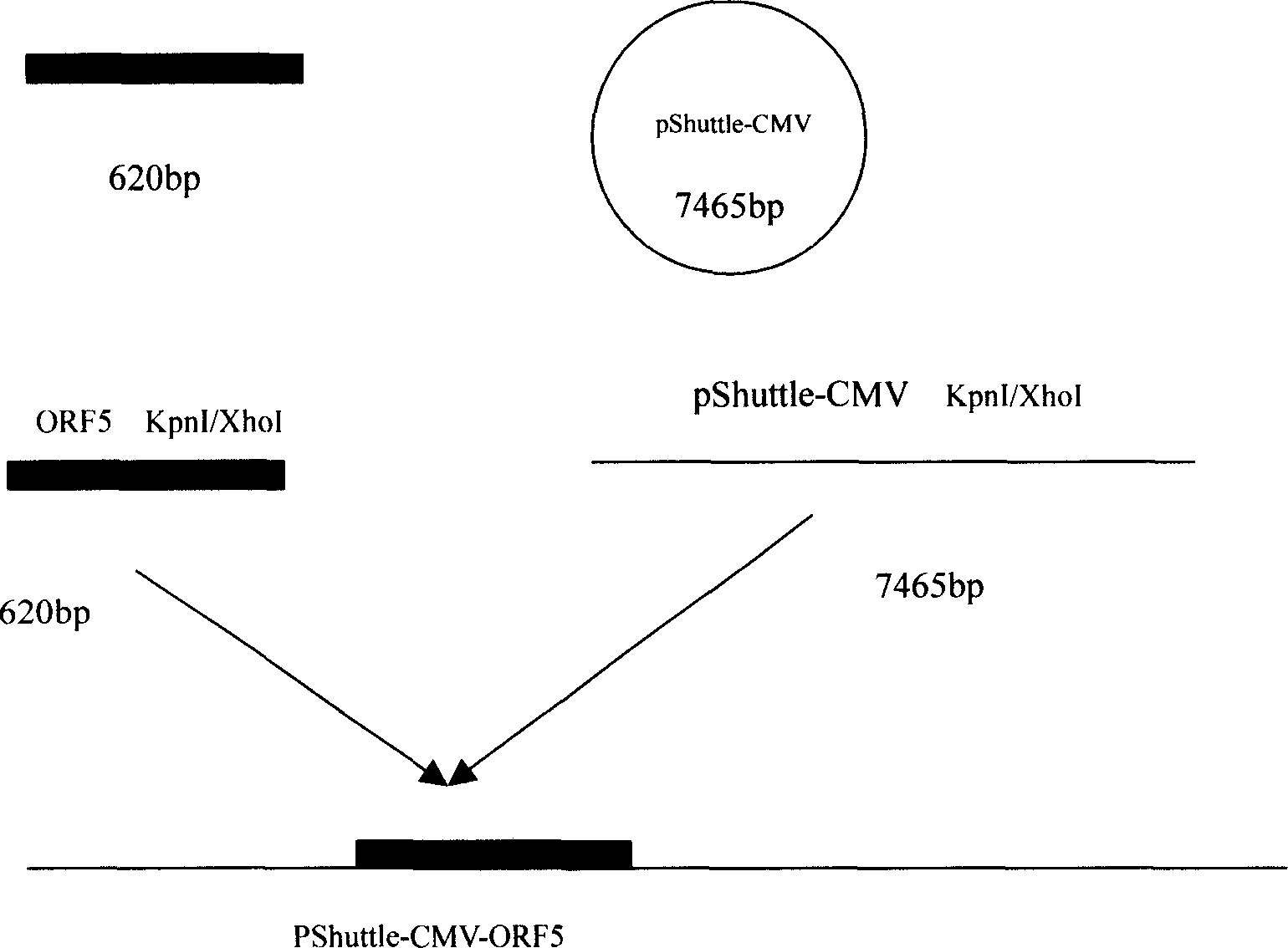
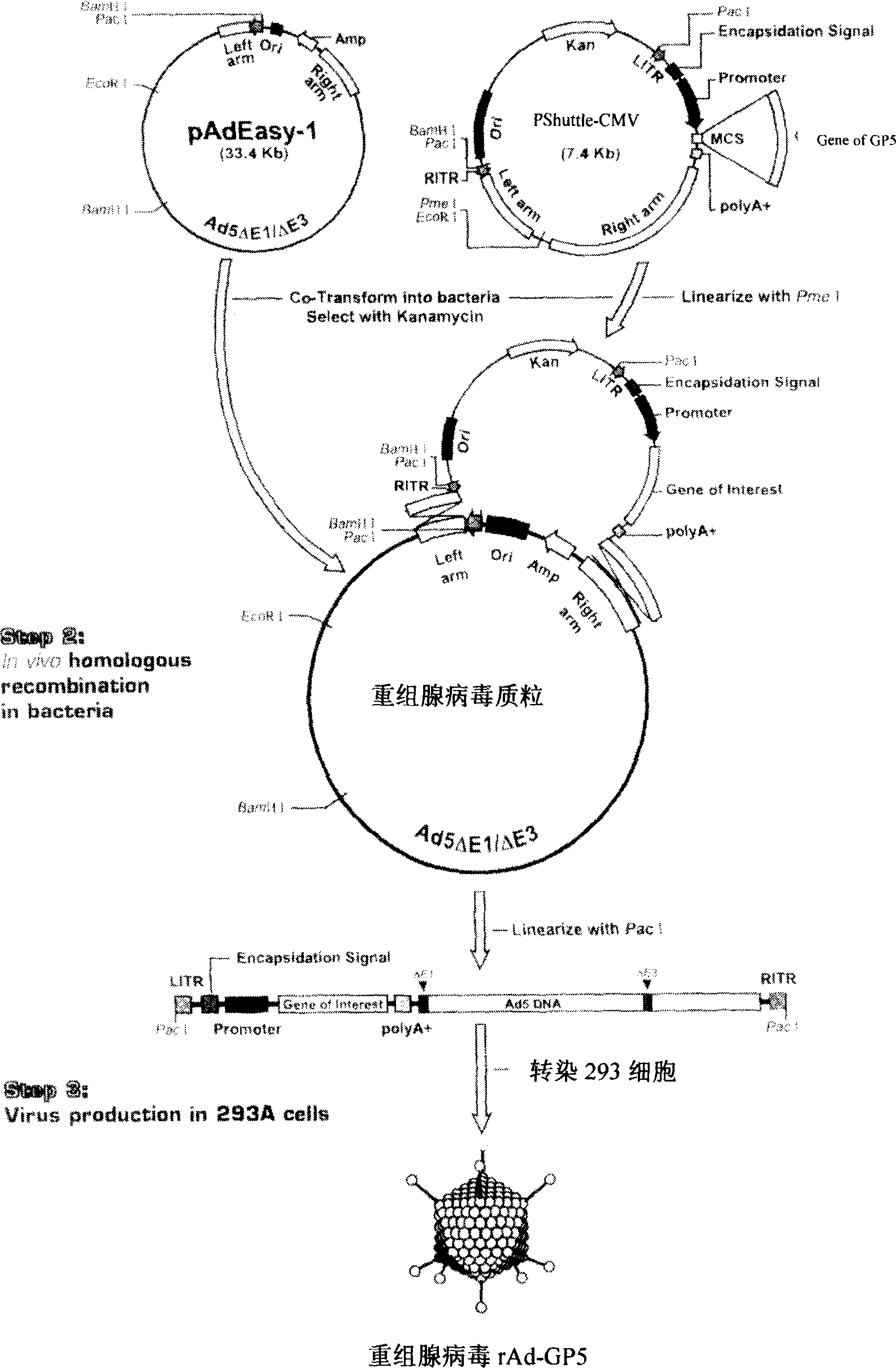
The present invention relates to pig reproduction and respiratory syndrome virus recombined adenovirus and vaccine, and belongs to the field of biological high-tech. Through RT-PCR process to proliferate whole PRRSV GP5 sequence, cloning the gene sequence to the shuttle vector pShuttle-CMV of adenovirus carrier system, cotransforming colibacillus BJ5183 strain together with the skeleton vector of adenovirus carrier system to obtain recombinant plasmid, transfecting HEK293-A cell to obtain recombinant adenovirus and plaque purification, and RT-PCR and indirect immunofluorescence technique inspection, the recombinant adenovirus rAd-GP5 expressing PRRSV GP5 protein is constituted. The recombinant adenovirus can set ahead the expression of PRRSV GP5 protein and raise the expression amount to simulate the immune protecting reaction of body effectively.
Owner:NANJING AGRICULTURAL UNIVERSITY
Amoxicillin preparation and preparation method thereof
ActiveCN105168143AImprove solubilityMeet the use requirementsAntibacterial agentsPowder deliverySolubilityTherapeutic effect
The invention relates to an amoxicillin preparation and a preparation method thereof. The amoxicillin preparation comprises 5-50 parts of amoxicillin, 3-38 parts of a stabilizer, 0.2-10 parts of a solubilizer, 0.1-1 part of a complexing agent and 1-91.7 parts of an auxiliary material. The preparation method comprises the following steps: the stabilizer and the solubilizer are ground and sieved by a sieve with 80-120 meshes and fed into a mixing tank according to a formula to be uniformly mixed with amoxicillin; the auxiliary material is ground and sieved by a sieve with 80-100 meshes and fed into the mixing tank according to a formula to be uniformly mixed, and a finished product is obtained through sub-packaging. The amoxicillin preparation has high water solubility and high stability, and according to 6-mouth acceleration test, the content of amoxicillin is in a prescribed range all along; the use requirement of a doser of a large-scale farm is met; an aqueous solution of the amoxicillin preparation is not prone to be influenced by objective factors such as the temperature, metal ions, ph and the like, the content of effective components can keeps unchanged for 24 hours, and the effect of treatment of livestock colibacillosis caused by Gram-negative bacteria is improved substantially.
Owner:QINGDAO KDN BIOTECH +1
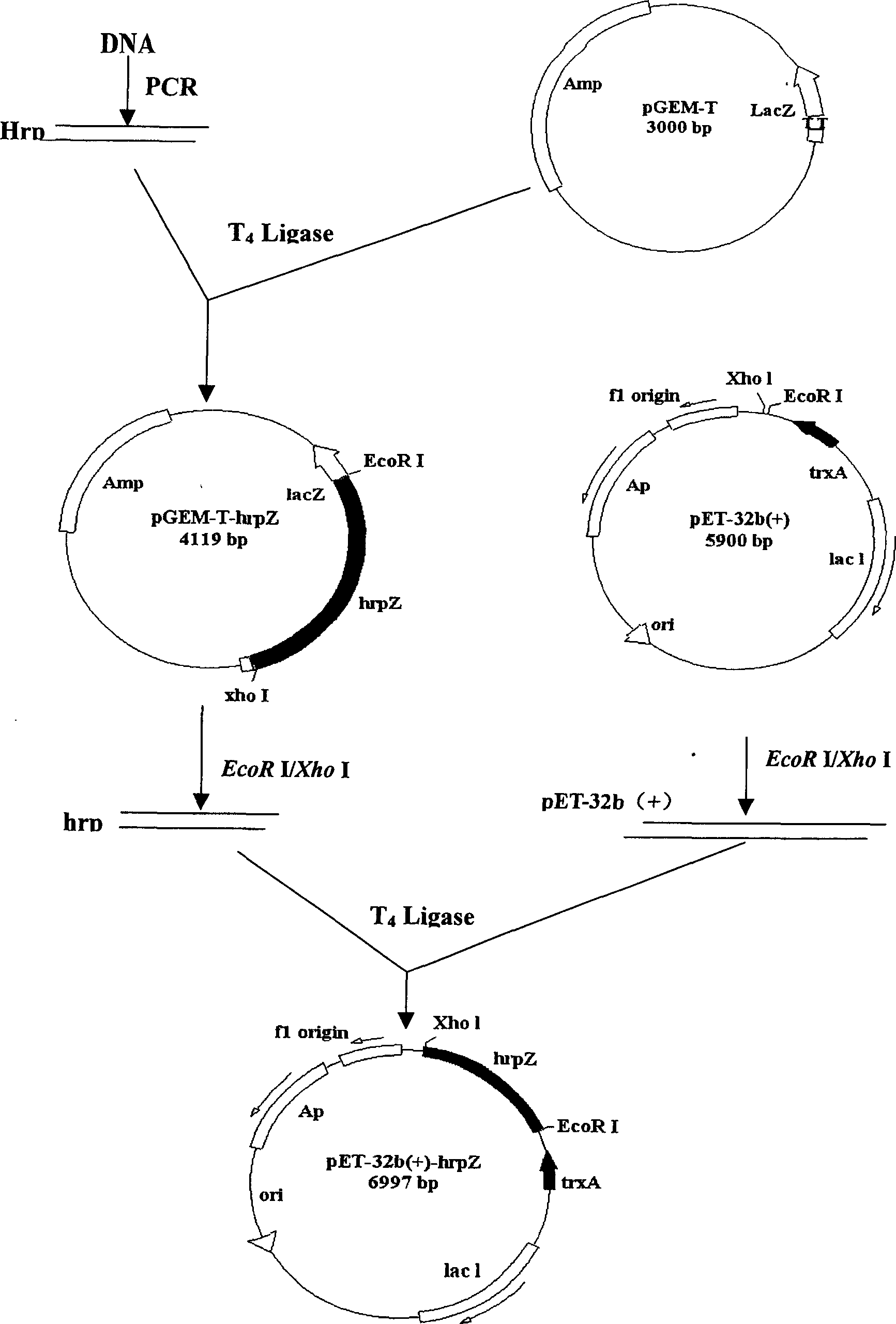
Non-inducing expressing gene engineering strain and structural process and application thereof
The invention discloses a non-induced expression genetic engineering strain and the constructing method and the application. The host cell of the strain is enteric bacilli E.coliBL21, CCTCC M205136, PCR expanding hrp Z gene section to clone to pGEM-T carrier, ferment cutting and bonding by EcoR I / Xho I, inserting the hrp Z gene into the enteric bacilli expression carrier pET-32b(+) lower reaches of thioredoxin, constructing rebuilding expression particle pET-32b-hrp, and transferring into E.coliBL21, filtering to gain positive clone. The non-induced expression rebuilt albumen HrpZ, SDS-PAGE shows that high efficiently expresses APDZ albumen. The rebuilt albumen has good prevention effect to plant disease and could improves the yield.
Owner:WUHAN UNIV
Skin mucous membrane disinfectants and preparation method thereof
ActiveCN101642449AEasy to prepareReduce manufacturing costAntibacterial agentsBiocideDisinfectantAdditive ingredient
The invention provides various skin mucous membrane disinfectants which are prepared by adopting double-chain quaternary ammonium salt and benzalkonium bromide (C20H40BrN) as main germicidal ingredients and using triclosan (C12H7Cl3O2), polyethylene glycol (HOCH2(CH2OCH2)nCH2OH), glycerol (C3H8O3), Tween 80, menthol, borneol, medical alcohol and the like as adjuvant ingredients, can be used in thefields such as the disinfection of skin and mucosa, the disinfection of surgical hand washing and the disinfection of medical apparatus and instruments in the hospital, the disinfection of tools anddevices in public places and different production industries, the mold prevention of industrial products and agricultural grains, the health disinfection of poultry houses, the aquatic product field,the aquaculture, the killing of alga, the preparation of plastic antibacterial agent, the preparation of compound disinfectant and the like and has effective bactericidal action to staphylococcus aureus, colibacillus, candida albicans, HIV and the like ; in addition, the preparation method is simple and the production cost is lower.
Owner:CHENGDU SHUNFA DISINFECTANT & WASHING TECH
Acid-proof and high-temperature resistant alpha-amylase and production thereof
A fire-resistant and acid-proof alpha-amylase and its production are disclosed. The process is carried out by separating precursor-alpha-amylase gene from lichenized bacillosporin, mutating for L134 and S320 amino acid residues and high-efficient expressing alpha-amylase mutant in bacterium. It achieves better safety, expression and acid stability, and more yield.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
High-strength antimicrobial dialdehyde starch crosslinked chitosan membane and preparation method and use thereof
InactiveCN1434072AGood mechanical strengthGood flexibilityPharmaceutical containersMedical packagingPliabilityChemistry
The present invention relates to a high-strength antimirobial dialdehyde starch cross-linked chitosan film, its production method and application. It is made up by using 1-3 wt% of chitosan acetic acid aqueos solution and 2-7 wt% of dialdehyde starch and adopting the processes of fully stirring, mixing, centrifugal defoaming, film-making, washing with acid, washing with water, drying by airing toobtain the invented product dried film. Said chitosan cross-linked film has excellent mechanical strength, flexibility and good antibacterial function, and has no toxic side effect for human body. Said chitosan cross-linked film has good bacteria-inhibiting action, can be used as clinical medical material for reducing infection of wound.
Owner:WUHAN UNIV
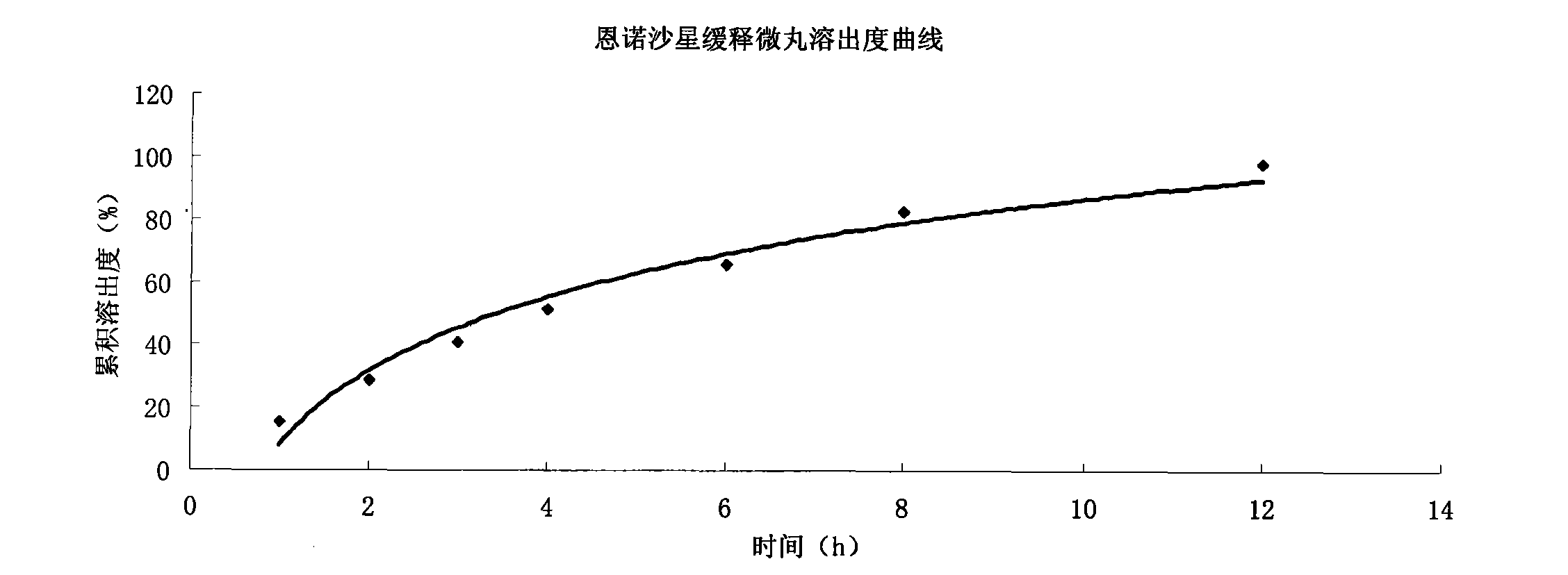
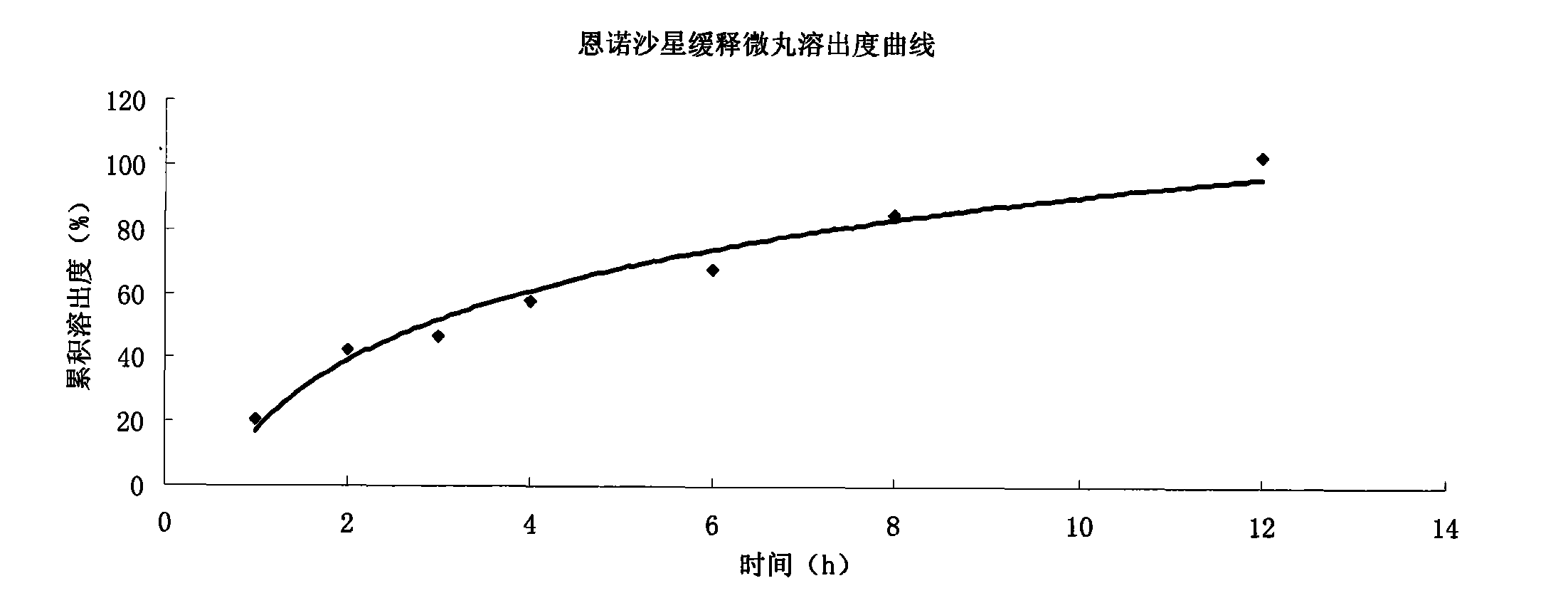
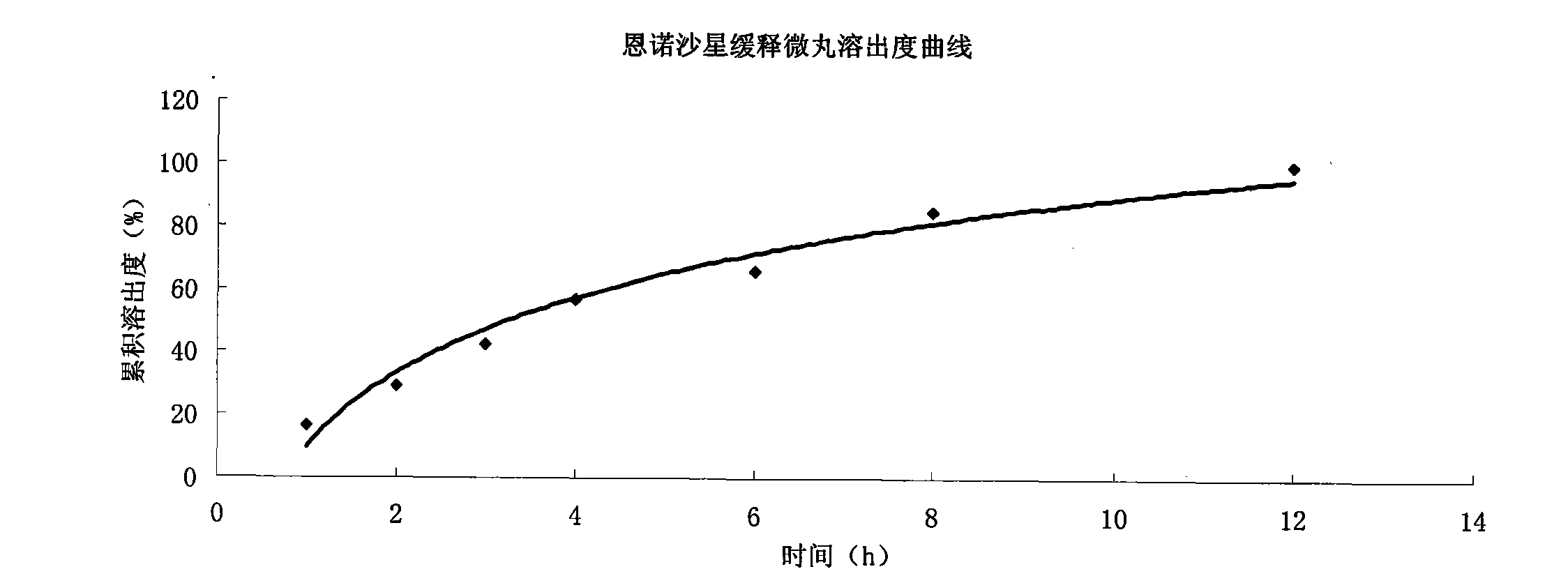
Enrofloxacin slow-release micropill for livestocks, and preparation method of same
InactiveCN102648896AExpand the scope of clinical applicationImprove the characteristics of easy color change when exposed to lightAntibacterial agentsOrganic active ingredientsPharmaceutical formulationSodium carboxymethyl starch
The invention relates to the field of pharmaceutical preparations and particularly relates to a slow-release micropill preparation containing enrofloxacin and a preparation method of the preparation. The slow-release micropill provided by the invention is formed by coating an enrofloxacin micropill; the enrofloxacin micropill comprises enrofloxacin and auxiliary material and is formed through extruding and rounding; the auxiliary material is any one or more of microcrystalline cellulose, starch, cane sugar, artificial gum, lactose and sodium carboxymethyl starch; according to weight percent, the auxiliary material in the micropill accounts for 70% to 95%; and coating is made of high-molecular enteric material, film forming material, opaquer and the like. The slow-release micropill preparation has the characteristics of slow release and high bioavailability, can be used for treating bacteria and mycoplasma infection of livestocks, and has better curative effect on chronic respiratory diseases, colibacillosis and salmonellosis, and the frequency of medicine taking can be reduced; and in addition, the slow-release micropill provided by the invention has the advantages that the stability of medicine is improved, and peculiar bitter of enrofloxacin can be covered completely, so that feeding intake of the animals is not influenced, and the recovery rate is improved.
Owner:ZHENGZHOU FUYUAN ANIMAL PHARMA
Anti-bacterial carboxy apatite composite coating, its preparing method and use
InactiveCN101070441AImprove biological activityImprove antibacterial propertiesAntifouling/underwater paintsPaints with biocidesApatiteAdditive ingredient
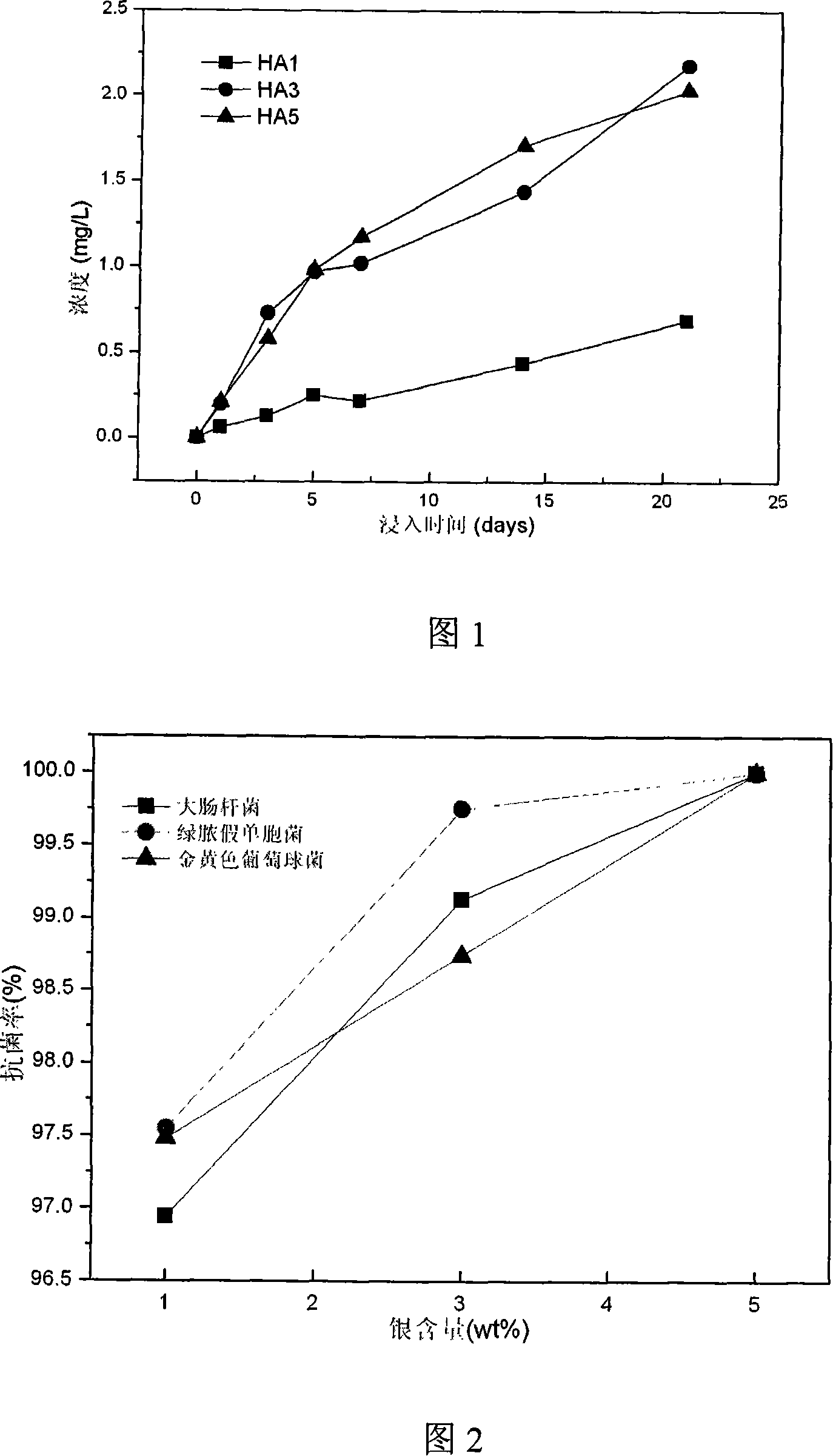
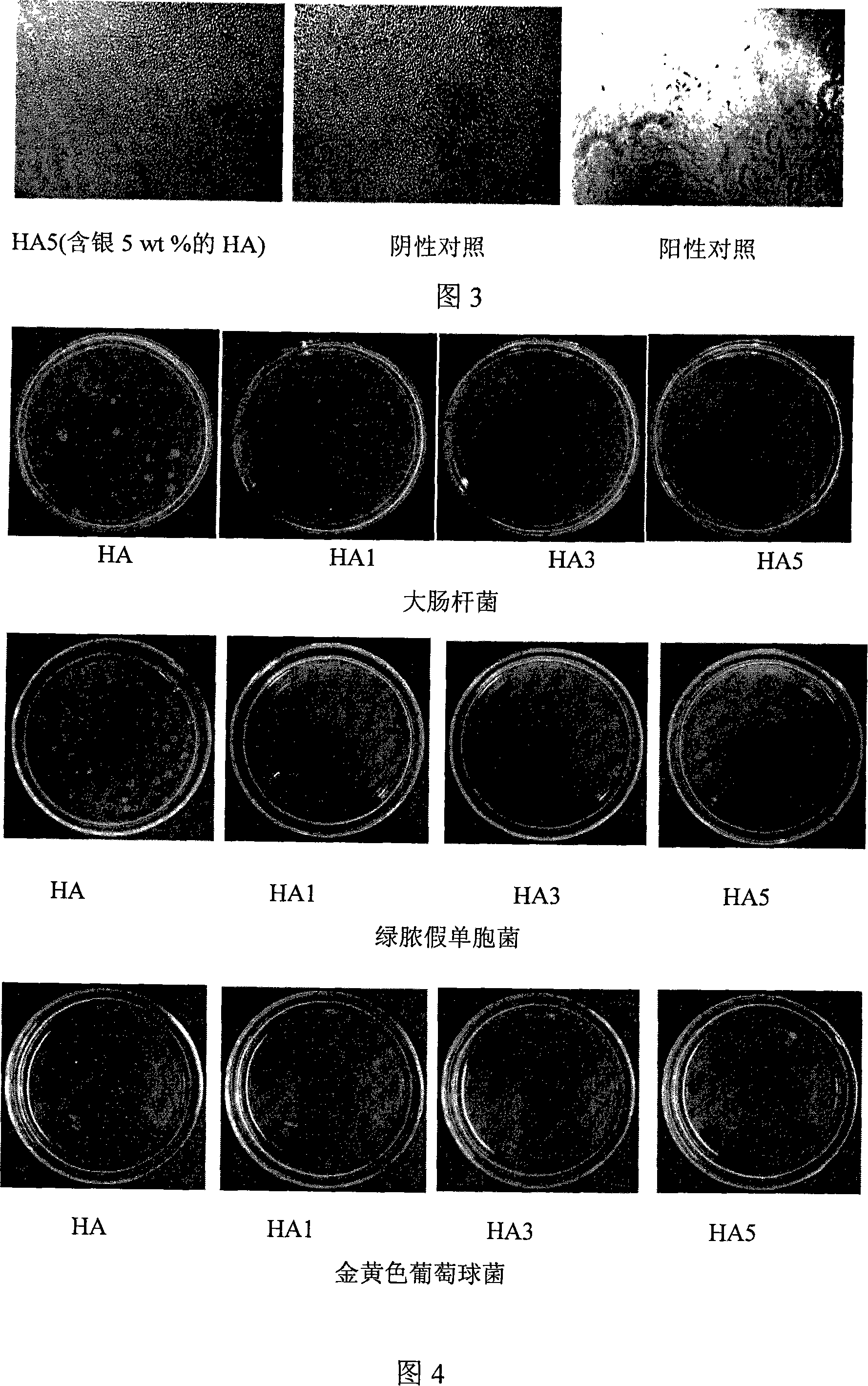
This invention relates to one kind of the antibacterial hydroxyl apatite compound coat, the preparation method and the application, its characteristic is the related compound coat is composed of the metal silver powder and the hydroxyl apatite powder, the metal silver powder is treated as the antibacterial increase ingredient, the silver powder' quality accounts for 1-5% of the compound coat, the particle size of the silver powder is 20-100mum, the particle size of the hydroxyl apatite powder is 10-100mum. This invention making the compound coat is realized by using the spray technics of the vacuum plasma. The provided compound coat has an above 95% excellent antibacterial effect on the coliform, the green pus pseudomonad and the golden yellow staphylococcus, and the cytotoxic rank in vitro of the compound coat is zero, has no cytotoxicity.
Owner:SHANGHAI INST OF CERAMIC CHEM & TECH CHINESE ACAD OF SCI
Low-temperature resistant lactobacillus with ACE (angiotensin converting enzyme) inhibitory activity
ActiveCN102994420AHas inhibitory activityGood antibacterial effectBacteriaMicroorganism based processesBiotechnologyStaphyloccocus aureus
The invention relates to a lactobacillus and in particular relates to a low-temperature resistant lactobacillus with ACE (angiotensin converting enzyme) inhibitory activity. The low-temperature resistant lactobacillus with the ACE inhibitory activity is lactobacillus plantarum I1 which is preserved in the China general microbiological culture collection center of which the preservation address is No.3, No1. yard, Beichen west road, Chaoyang District of Beijing, the preservation data is September 18th, 2012, and the preservation number is CGMCC No.6575; the lactobacillus plantarum I1 has the ACE inhibitory rate being 66%, can tolerate 6% of NaCl and 100mg / kg of nitrite, and can fast generate acid; the growing temperature of the lactobacillus plantarum I1 is 4-40 DEG C, and is suitable for low-temperature fermentation; and the low-temperature resistant lactobacillus has obvious protease activity and lipase activity, can not generate mucus, has an obvious antibacterial effect on staphylococcus aureus and colon bacillus, and can be used as a meat product leavening agent strain under a low-temperature condition.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Blood vessel formation inhibitor IIM3 and its preparation method and application
InactiveCN1830487AImprove and enhance growthImprove and enhance the anti-tumor effectPeptide/protein ingredientsAntineoplastic agentsEscherichia coliInclusion bodies
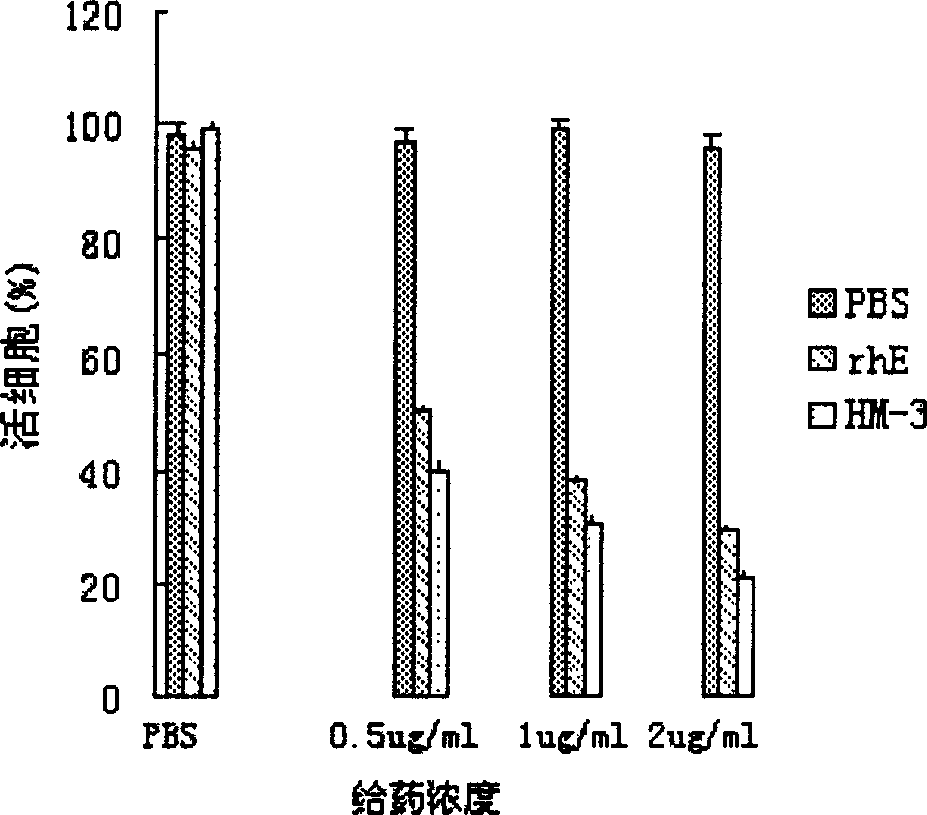
An efficient angiogenesis depressant HM-3 for treating the solid tumors including stomach cancer, lung cancer and liver cancer is prepared through expressing in colibacillus by genetic engineering method, separating the protein of inclusion body, dissolving, re-naturalizing, and separating-purifying by ion change and chromatography.
Owner:徐寒梅
Novel isolated bacteriophage having e. coli-specific bactericidal activity and antibacterial composition comprising the same
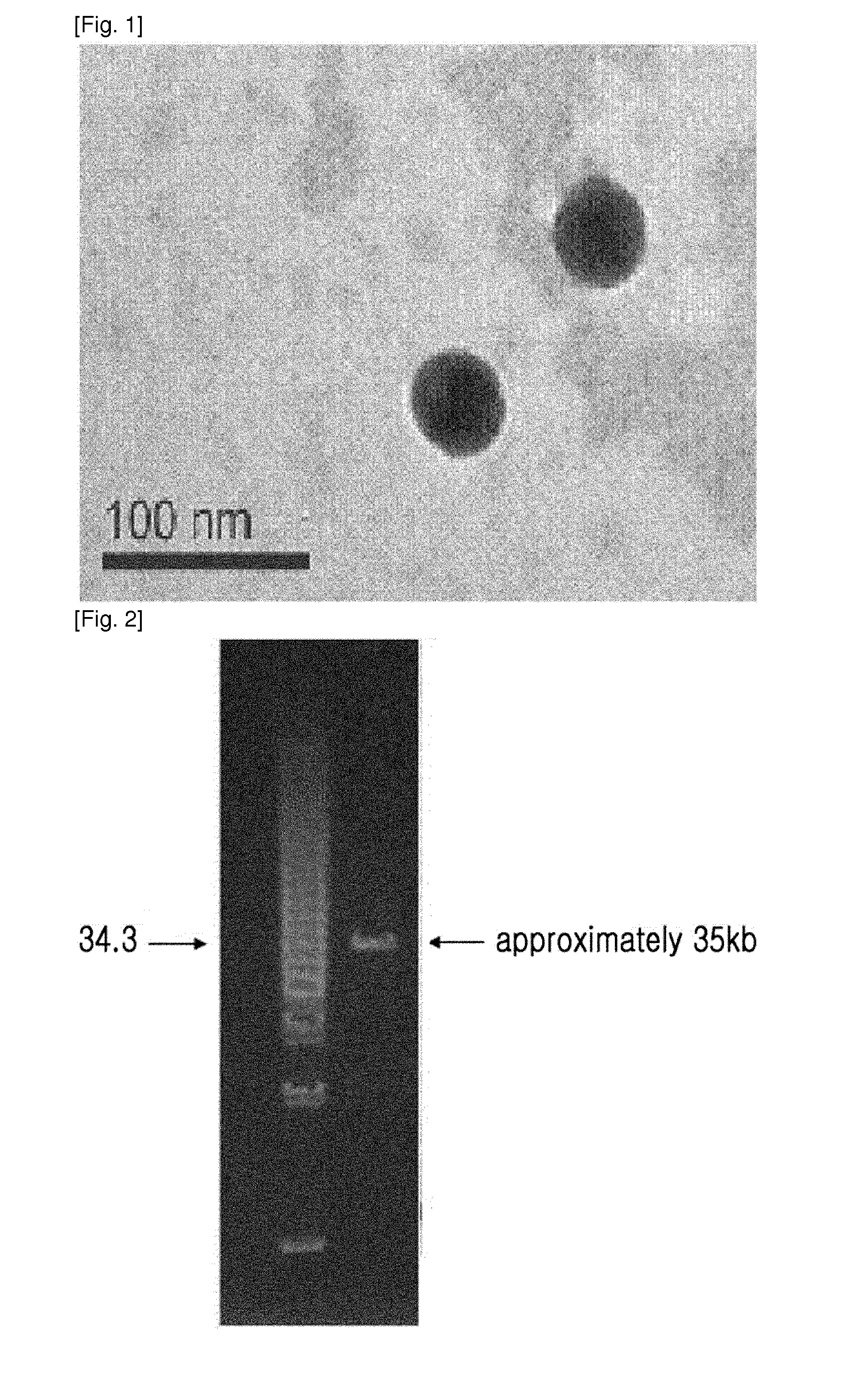
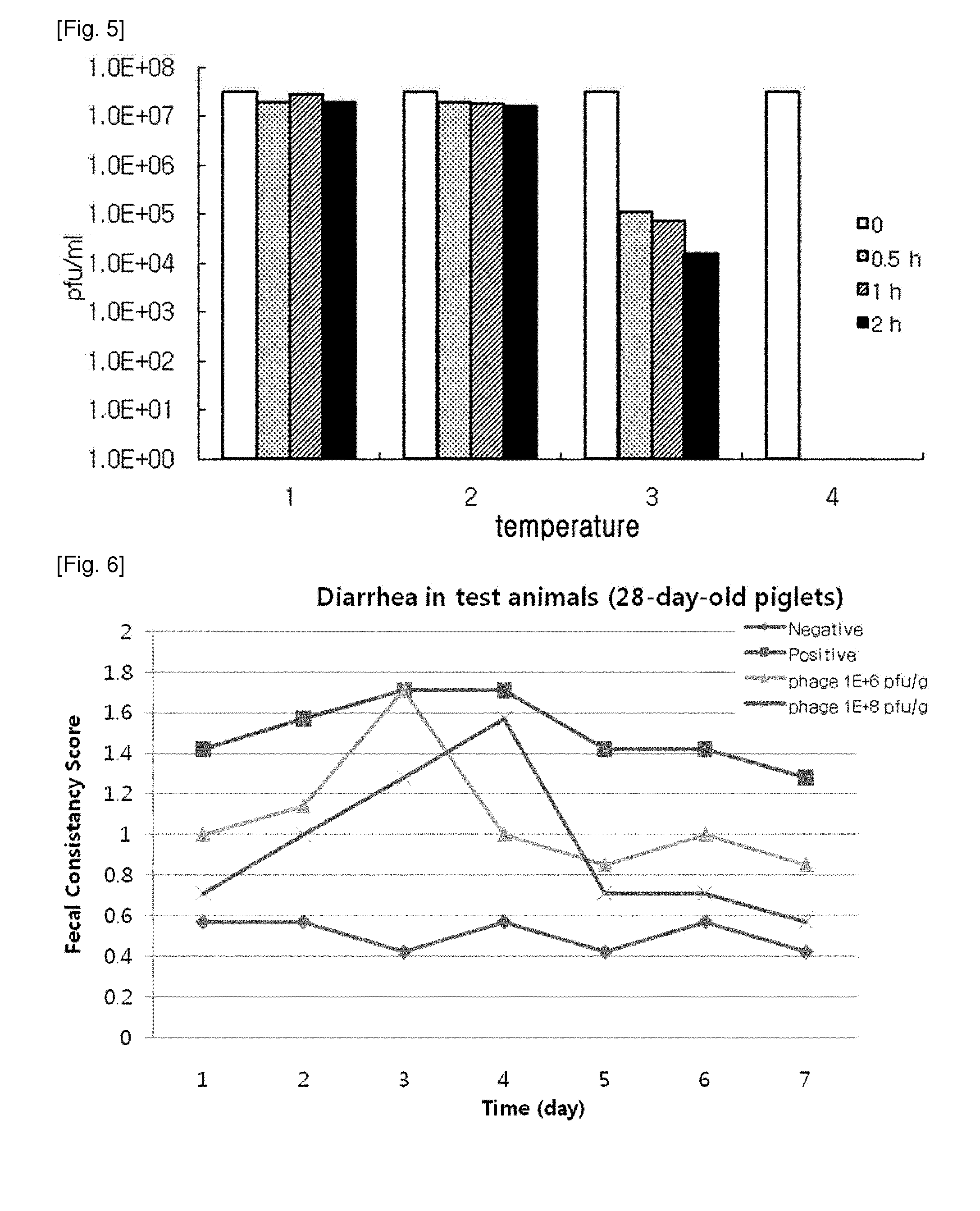
ActiveUS20140356330A1Good acidExcellent heat-resistanceAntibacterial agentsBiocideEscherichia coliPhage therapy
The present invention relates to a novel bacteriophage having an E.-coli-specific bactericidal activity, a composition for the prevention or treatment of infectious diseases caused by Enterotoxigenic E.-coli comprising the bacteriophage as an active ingredient, an antibiotic comprising the bacteriophage as an active ingredient, a feed additive composition comprising the bacteriophage as an active ingredient, a sanitizer or cleaner comprising the bacteriophage as an active ingredient, and a method for treating colibacillosis using the bacteriophage. The novel bacteriophage of the present invention has a specific bactericidal activity against pathogenic E. coli, and excellent acid- and heat-resistance. Therefore, the novel bacteriophage can be used for the prevention or treatment of swine colibacillosis, which is an infectious disease caused by pathogenic E.-coli, and can also be widely used in animal feed additive compositions, sanitizers, and cleaners.
Owner:CJ CHEILJEDANG CORP
Culture method engineering bacterium capable of producing 5-amino acetyl propionic acid in high yield
The present invention relates to a method for cultivating engineering bacterium capable of high yielding 5-amino acetylpropionic acid. The engineering bacterium is colibacillus containing 5-amino acetylpropionic acid synthetase gene of Rhodoblastus acidophilus PSB-1 strain in photosynthetic bacteria. The engineering bacterium is firstly cultured in the culture medium containing peptone, yeast extract and inorganic salt as well as glycine, succinic acid and levulic acid for 2-4 hr and then further cultured in the culture medium with inducing agent added for additional 5-14 hr to reach the maximum output of 5-amino acetylpropionic acid. The present invention has not only high 5-amino acetylpropionic acid output, but also simple technological process, environment friendship and capacity of meeting industrial production requirement.
Owner:CHANGSHA AGREEN BIO TECH LTD CO
Antibacterial intelligent thermal-storage nano fibre
Said invention relates to a nano antibiotic intelligent heat storage fiber and preparation and use thereof, which is composed of polypropylene, polyester, polyamide, polycarylonitrile and viscose fiber wherein the nano antibiotic intelligent heat storage function powder of 0.5-4 % of total fiber weight is uniformly distributed in fiber and surface layer. Said invention can absorb visible light source and convert into heat energy, also can absorb human body heat and store in fiber fabric then warm human body. Said invention has bacteriostasis and antibiotic effect to colibacillus, candida albicans, staphylococcus, gonorrhea, hepatitis etc.
Owner:王开利
Beta-agaropectinase gene aga, its preparation method and application
The invented beta-gelase gene agaA is the gene of beta-gelase of coded pseudoallomonad CY24, the recombinant beta-glase produced by using it has good temp. and pH stability, high activity, can degrade agarose, and its degraded product mainly is new agar oligose whose degree of polymerization is 2-6, so that it can be used for preparing new agar aligose and recovering DNA from agarose gel. The recombinant beta-gelase expressed by said invented colibacillus recombinant strain pEAG / BL 21 is 24% of total protein. Its expression level is high. It is easy to purity. After continuous passage of 20 generations its expression amount is not reduced, therefore said invention can be used for large scale production of recombinant beta-gelase.
Owner:OCEAN UNIV OF CHINA
Composition of Chinese traditional medicine for treating yellow / white flux of baby pig, and colibacillosis of birds
InactiveCN101049375AEnhance immune functionAntibacterialAntibacterial agentsClimate change adaptationFowlRose hip
A composite Chinese veterinary medicine for preventing and treating the dysentery of piglet and the colibacillosis of fowls is prepared from 8 Chinese-medicinal materials including scutellaria root, cherokee rose-hip, sanguisorba root, lucid ligustrum fruit, etc through extracting in water, decocting twice, adsorbing and drying.
Owner:WUXI ZHENGDA POULTRY
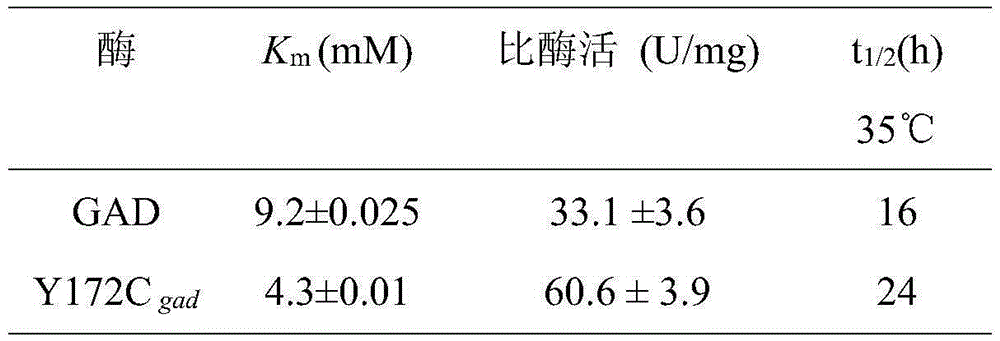
Glutamate decarboxylase mutant establishment improving enzyme activity and application thereof
ActiveCN105255849AIncreased potential for industrial applicationsBacteriaFermentationGlutamate decarboxylaseTyrosine
The invention discloses a glutamate decarboxylase mutant improving enzyme activity and an establishment method thereof, and belongs to the field of gene engineering. On the basis of an amino acid shown as SEQ ID NO.1, a 172 tyrosine is mutated to form cysteine. The obtained mutant is expressed in colibacillus, after being fermented for 24h in a shake flask, the enzyme activity is 28.6U / mL, the mutant enzyme activity is improved by 81 percent, compared with the original enzyme, the substrate affinity is reduced by 53 percent, the enzyme activity is improved by 83 percent, and the half-time period of the enzyme at 35 DEG C is increased from 16h to 24h. The recombinase is expressed in the colibacillus, and the glutamic acid is converted in a total cell manner for 18h to obtain 283.8g / L gamma-aminobutyric acid; the recombinase is expressed in glutamic acid coryneform bacteria, the glutamic acid is converted for 18h in a total cell manner to obtain 126.7g / L gamma-aminobutyric acid. The result shows that the 172 amino acid residue can severely influence the catalytic effect and stability of the enzyme, a foundation is set for researching the catalytic mechanism of the enzyme, and the industrial application potential of the enzyme is improved.
Owner:JIANGNAN UNIV
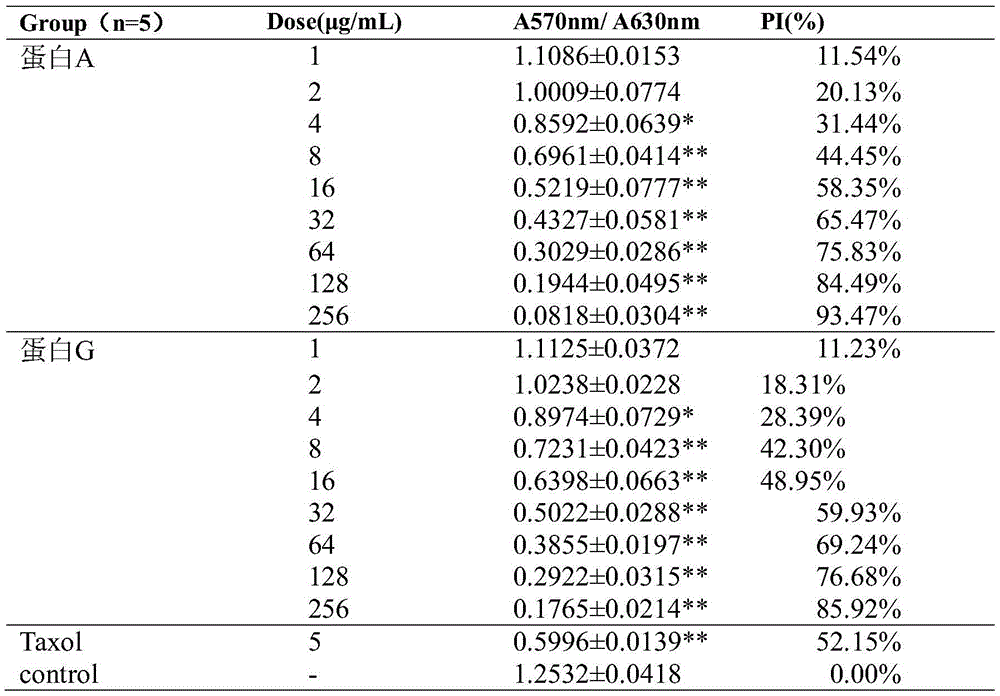
Fusion protein with anti-tumor, anti-inflammation and oculopathy-treatment functions and preparation method and application thereof
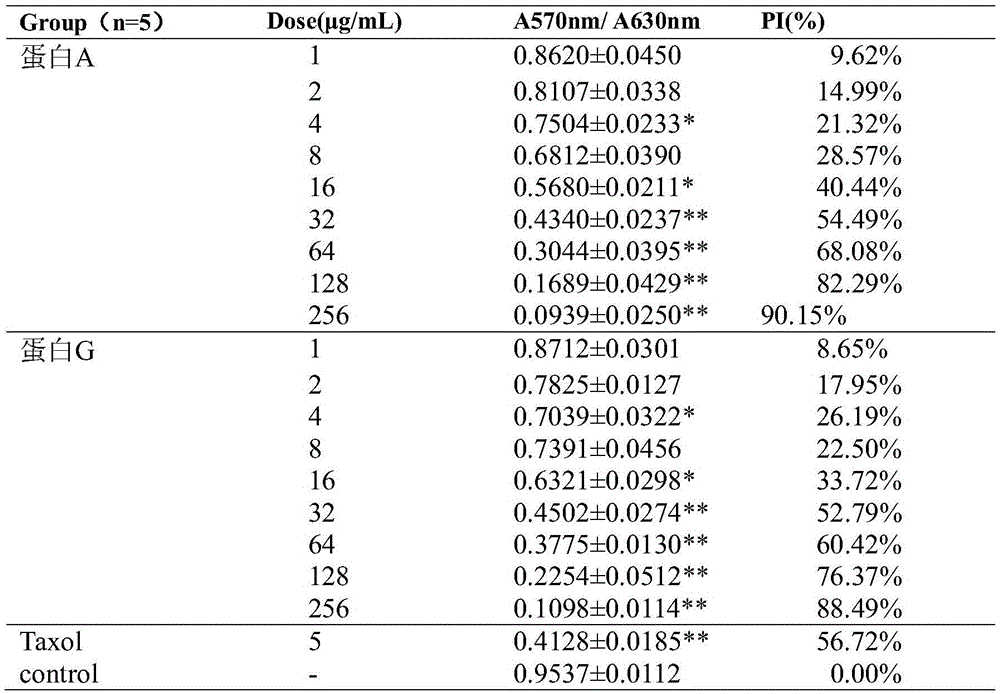
ActiveCN105418769AInhibition of newbornsImprove ductilitySenses disorderPeptide/protein ingredientsTumor angiogenesisHalf-life
The invention discloses fusion protein with anti-tumor, anti-inflammation and oculopathy-treatment functions and a preparation method and application thereof, which belong to the technical field of biological pharmacy. Specifically, the invention relates to the fusion protein of an integrin blocking agent, which has the function of inhibiting tumor angiogenesis and has the integrin affinity and the combining capacity. A rigid (R) or flexible (F) linker is adopted to fuse two polypeptides, so as to respectively obtain protein A and protein G, the medicine effect can be increased, the half-life period can be extended, the stability can be enhanced, and the fusion protein has the characteristics of strong action effect, low toxicity and the like and can be used for preventing and treating solid tumors, all kinds of inflammation and angiogenesis oculopathy. The fusion protein structurally comprises two angiogenesis inhibiting polypeptides and the amino acid linker between the two angiogenesis inhibiting polypeptides, and the fusion protein is expressed in colibacillus or eukaryocyte by using a genetic engineering method and is obtained through separation and purification of a GST (Glutathione S Transferase) affinity column.
Owner:CHINA PHARM UNIV
Protein powder for fodder, and preparing method and use thereof
InactiveCN1507790AReduce energy consumptionLow costEgg immunoglobulinsProtein composition from eggsEscherichia coliAnimal science
The present invention relates to a preparation method of feed protein powder containing colibacillus yolk antibody whose active valence is greater than or equal to 1:64 and its application. Its preparation method includes the following steps: preparing serotype colibacillus immunogen, using said immunogen to immunize health egg-laying hen, immunogen injection dose is 0.5-3.0 ml / every hen, collecting the egg laid by hen after which is immunized for 7-10 days, remaining the egg containing colibacillus yolk antibody whose active valence is greater than 1:64 for stand-by, using whole egg liquor to make pasteurization and spray-drying.
Owner:北京同力兴科农业科技有限公司
Method for screening single chain antibodies of Microcystin-LR and verification thereof
InactiveCN101857866AEasy to filterEasy to identifyImmunoglobulins against fungi/algae/lichensBiological testingEscherichia coliEnzyme digestion
The invention relates to a method for screening single chain antibodies of Microcystin-LR and verification thereof. The method comprises the following steps: carrying out two rounds of affinity screening on the biotinylated Microcystin-LR in a human source synthetic antibody library by using an avidin labeled magnetic bead and a negative screening method; extracting total plasmid DNA from phage colonies produced in the second round, carrying out enzyme digestion with enzyme Sfi I, recycling gel to obtain single chain antibody genes, connecting the single chain antibody genes with soluble expression carrier pAK100CL which is processed by enzyme digestion in the same way, and electrically transforming the connected carrier into colibacillus XL1-Blue to obtain soluble expression single chain antibodies; and verifying the soluble expression single chain antibodies by using a competitive time-resolved fluorescence immune analytical method. The invention has the advantage of quick, simple and convenient screening, and can well expose the Microcystin-LR three-dimensional structure into the incubation system. The verification on the screening result has the advantages of high detection signal and strong anti-interference capacity against stroma.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Sanitary material
InactiveCN101612416AImprove comfortGood hygroscopicityAbsorbent padsNon-woven fabricsTriclosanDry weight
The invention relates to a sanitary material. The sanitary material is silk water-jetting non-woven fabric which uses silk short fibers as raw materials and is prepared by the water-jetting technology. wherein in the after-finishing process of the silk water-jetting non-woven fabric, an organic antibacterial agent which contains at least one of double-chain quaternary ammonium salt, biguanides and triclosan is coated on the silk water-jetting non-woven fabric by the on-line coating technology, wherein the dry weight ratio of the consumption of the antibacterial agent to that of the silk water-jetting non-woven fabric is 0.5:1000-5:1000. The silk water-jetting non-woven fabric has the advantages of good comfort property, fineness, smoothness, lightness, softness, good hygroscopic property and ventilation property and refined pearly luster and good safety and stability; in addition ,the 20-minute antibacterial ratio of the silk water-jetting non-woven fabric against bacteria, such as coliform bacteria and candida albicans, is larger than 90-95 percent, has quick, durable, high-efficiency and broad-spectrum antibacterial effects, and has no irritation, no allergic reaction and no corrosion.
Owner:FUJIAN HENGAN HLDG CO LTD +2
Beta-agaropectionase gene aga B, its preparation method and application
InactiveCN1460717AMake up for the shortcomings of low polymerization degree (generally 2-6 sugars)Low costBacteriaEnzymesEscherichia coliBiotechnology
The present invention relates to a beta-gelase gene agaB. It is a gene of beta-gelase of coded pseudoallomonad CY24. Said beta-gelase coded by beta-gelase gene agaB can specifically degrade agar to produce new agar oligose whose degree of polymerization is 8-14, said new agar oligose content content in the degraded product is greater than 20%. The recombinant beta-glase expressed by colibacillus recombinant strain BL21-pET24-agaB is 25% of total protein, its expressing level is high, and its purification is easy, so that it can be used for large-scale production of beta-gelase.
Owner:青岛中国海洋大学控股有限公司
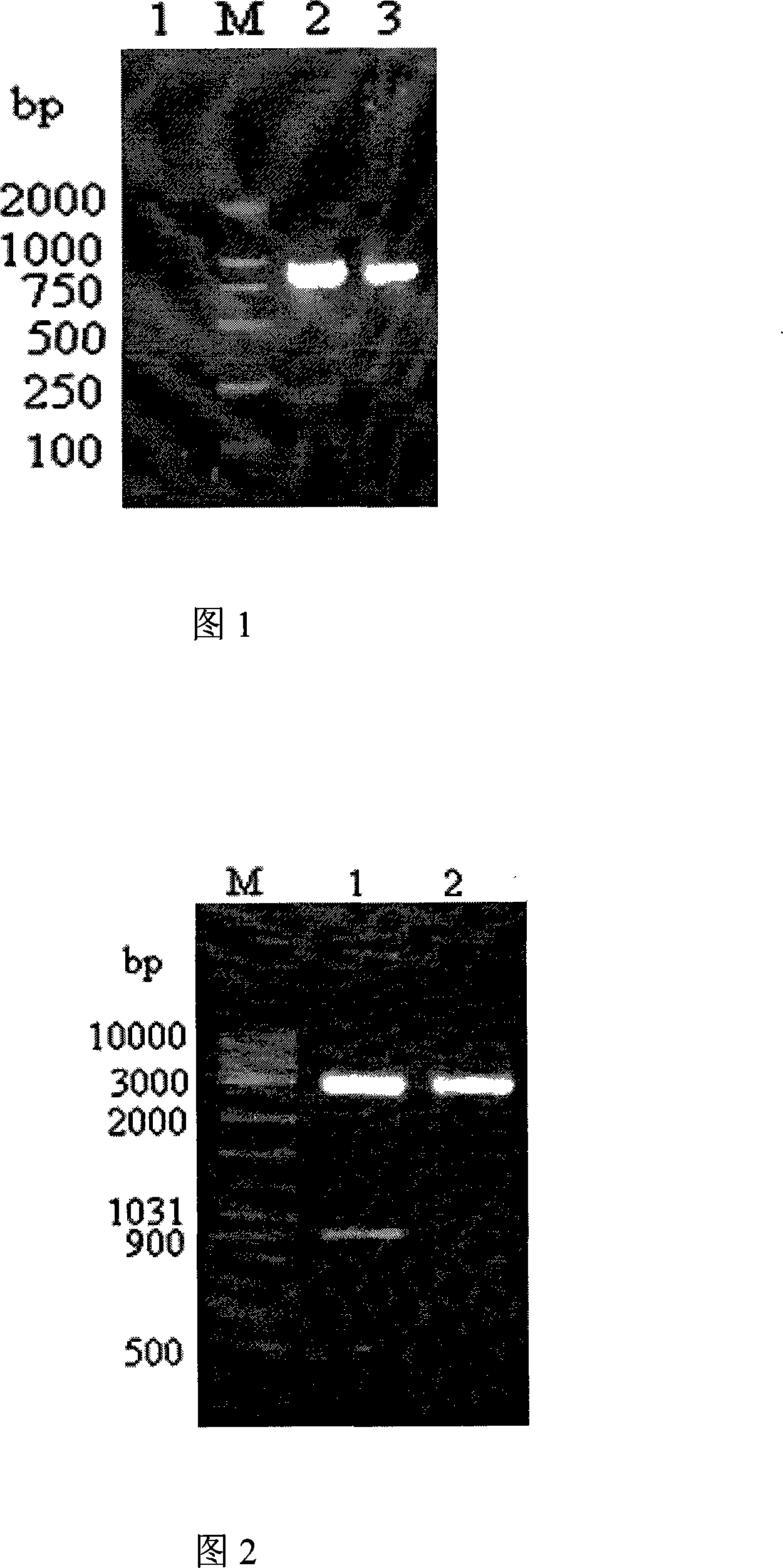
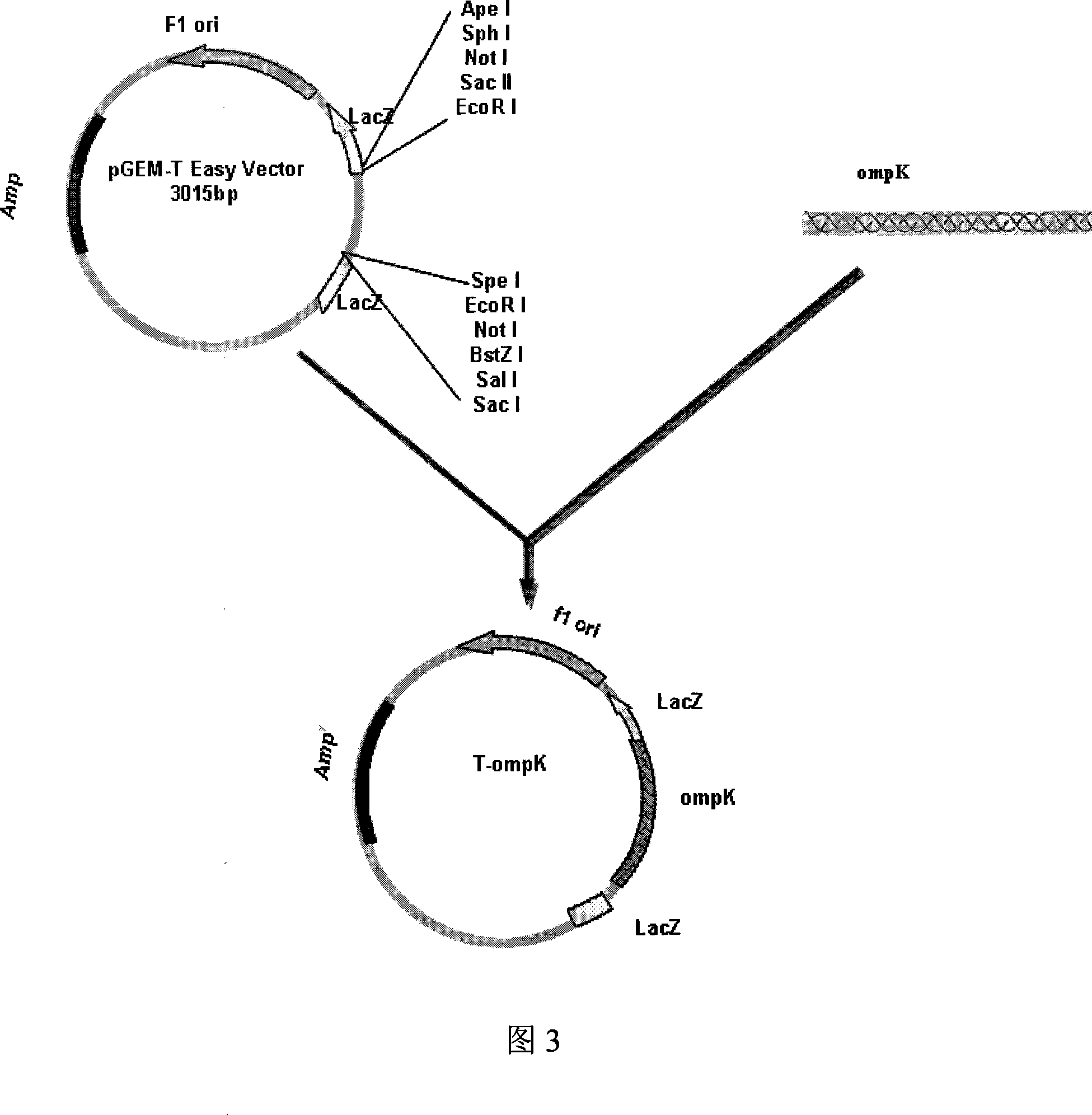
Vibrio parahaemolyticus tunica externa protein ompK subunit vaccine and preparation method thereof
InactiveCN101172157APreserve immunogenicityDefense against invasionAntibacterial agentsDigestive systemEscherichia coliHaemolysis
The invention discloses protein ompK subunit vaccine of assistant haemolysis vibrio extine and a preparation method thereof. The vaccine is PBS solution which converts the recombination protein of the coliform bacteria of recombination prokaryon expression plasmid pET30a-ompK expressed by inducing and after being purified; the concentration of the PBS solution is 0.25-0.5mg / ml. The method has the steps that: firstly, the extraction of an assistant haemolysis vibrio full gene group, the overall length of extine protein ompK DNA and the clone of a mature peptide coded sequence; secondly, the construction of a prokaryon expression plasmid of the ompK mature peptide coded sequence, thirdly, the obtaining way of the recombination ompK protein; fourthly, the detection to the immunity way and the immunity effect of a large yellow croaker with recombination ompK protein. The invention provides the preparation method of the assistant haemolysis vibrio ompK protein subunit vaccine, and simultaneously provides the detection method of the immunity effect of the large yellow croaker with recombination ompK protein, the preparation method is simple, and the usage is convenient.
Owner:ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com