Acid-proof and high-temperature resistant alpha-amylase and production thereof
A technology of amylase and high temperature resistance, which is applied in the field of acid-resistant and high-temperature-resistant α-amylase and its preparation. It can solve the problems of α-amylase acid resistance, high temperature resistance and application limitations, and achieve a wide pH range. , the effect of good acid stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Amplification of the Precursor Alpha-Amylase Gene
[0032] Chromosomal DNA of Bacillus licheniformis [China Industrial Microorganism Collection Center (CICC) 10181] was extracted. Design the following primers (the primers are commissioned to be synthesized by Shanghai Bioengineering Co., Ltd.):
[0033] Upstream primer F1: 5'-AGGATCCCTTGAAGAAGTGAAGAAGCAGAGAGG
[0034] Downstream primer R1: 5'-AAAAGCTTCCTGAGGGCTGATGATGACACTTTG
[0035] The 5' end of the upstream primer F1 contains a BamHI restriction site, and the 5' end of the downstream primer R1 contains a HindIII restriction site. Perform PCR amplification using the chromosomal DNA of Bacillus licheniformis 020401 as a template, and mix the components in a sterilized thin-walled centrifuge tube in the following order: Use 50L amplification system: ddH 2 O 41.5L, 10×buffer 5L, dNTP (2.5mmol / L each) 1L, upstream primer F1 (20mol / L) 0.5L, downstream primer R1 (20mol / L) 0.5L, DNA template 1L, TaqDNA polymer...
Embodiment 2
[0036] Example 2: Site-directed mutagenesis of precursor alpha-amylases
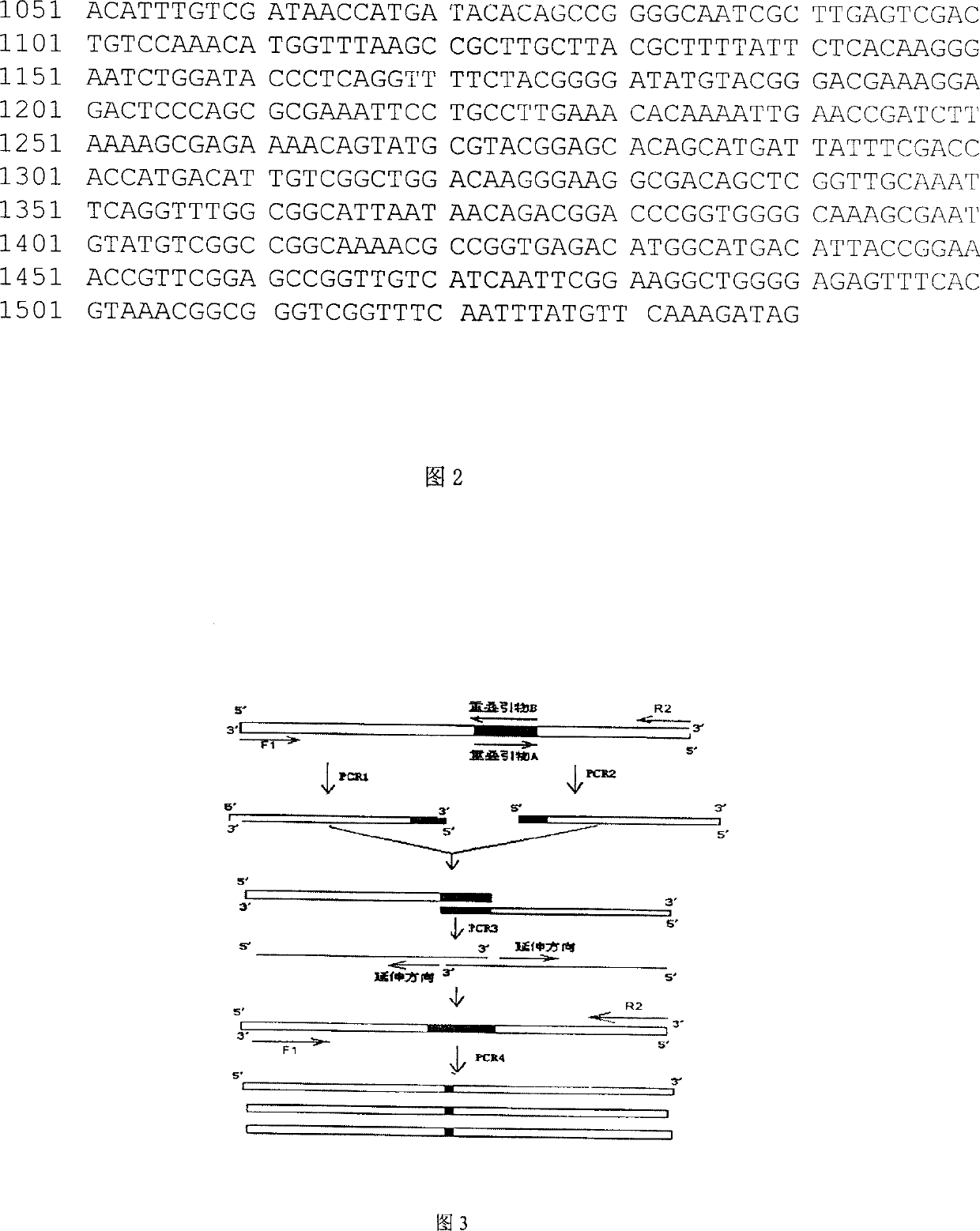
[0037] The schematic diagram of the mutation of α-amylase is shown in Fig. 3 . Design overlapping primers as follows:
[0038] Overlap Primer A: 5'-GAGAACACCGCATTAAAGCCTGGAC-3'
[0039] Overlap Primer B: 5'-GTCCAGGCTTTAATGCGGTGTTCTC-3'
[0040] Overlapping primer C: 5'-AAGCATCCGTTGAAAGCGGTTACAT-3'
[0041] Overlapping primer D: 5'-ATGTAACCGCTTTCAACGGATGCTT-3'
[0042] Overlapping primer A is complementary to overlapping primer B, and overlapping primer C is complementary to overlapping primer D. Overlapping primers A and B contained a mutation at amino acid 134, while overlapping primers C and D contained a mutation at amino acid 320. Use the recombinant plasmid pUCA as a template for PCR amplification, and mix the components in a sterilized thin-walled centrifuge tube in the following order: PCR1: use 50 μL amplification system, ddH 2 O 38.5 μL, 10× buffer 5 μL, dNTP (2.5 mmol / L each) 2 μL, upstre...
Embodiment 3
[0043] Embodiment 3: Preparation of expression vector
[0044] Escherichia coli JM109 strain carrying the plasmid pBCH (purchased from Treasure Biotech Co., Ltd.) was inoculated in LB medium containing ampicillin (50 μg / mL), and cultured overnight at 37° C. with shaking. Transfer 1.5 mL of bacterial liquid into a microcentrifuge tube, centrifuge at 12,000 rpm for 30 seconds to collect bacterial cells, discard the supernatant, and empty the residual liquid. Resuspend the pellet in 100 μL of pre-cooled solution I (50 mmol sucrose, 25 mmol Tris, 10 mmol EDTA, pH 8.0), and mix well. Add 200 μL of newly prepared solution II (0.2mol NaOH, 1% SDS), cap the tube tightly, shake gently, and place it on ice for 1-2 minutes until the liquid becomes clear. Add 150 μL of pre-cooled solution III (3 mol potassium acetate, pH 4.8), gently rotate the centrifuge tube to mix solution III evenly in the viscous bacterial lysate, and place it on ice for 3-5 minutes. Centrifuge at 12,000 rpm for 5 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com